Abstract
Receptor-mediated cell mechanosensing plays critical roles in cell spreading, migration, growth, and survival. Dynamic force spectroscopy (DFS) techniques have recently been advanced to visualize such processes, which allow the concurrent examination of molecular binding dynamics and cellular response to mechanical stimuli on single living cells. Notably, the live-cell DFS is able to manipulate the force “waveforms” such as tensile versus compressive, ramped versus clamped, static versus dynamic, and short versus long lasting forces, thereby deriving correlations of cellular responses with ligand binding kinetics and mechanical stimulation profiles. Here, by differentiating extracellular mechanical stimulations into two major categories, tensile force and compressive force, we review the latest findings on receptor-mediated mechanosensing mechanisms that are discovered by the state-of-the-art live-cell DFS technologies.
Keywords: Mechanosensing, Receptor–ligand interactions, Dynamic force spectroscopy, Force waveform
Introduction
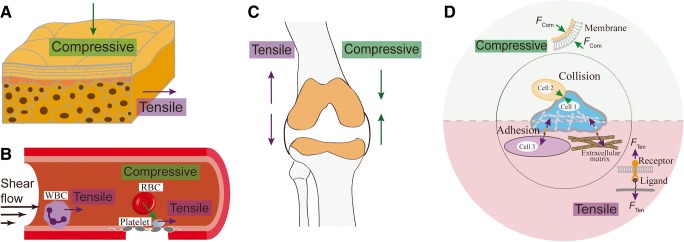
Mechanical force has been long recognized for its versatile roles in countless physiological processes. For example, it triggers the touch and pain sensation through the skin cells (Fig. 1a) (Maksimovic et al. 2014; Orr et al. 2006). The contractile forces between endothelial cells tighten cell–cell junction for the maintenance of vessel integrity (Charras and Yap 2018; Hoffman and Yap 2015). Adhesion forces enable leukocyte migration and trafficking in inflammation and innate immune response (Nordenfelt et al. 2016; Yeh et al. 2018) and platelet attachment to the vascular surface in hemostasis and thrombosis under dynamic blood flow (Fig. 1b) (Feghhi et al. 2016; Kim et al. 2017; Lam et al. 2011). Furthermore, the adaptation of local bone mass and architecture is also driven by mechanical loading (Bacabac et al. 2004) (Fig. 1c).
Fig. 1.
Mechanical forces in the cell physiological environments and their molecular mechanisms. a Skin cells experience compressive forces from the external touching and tensile forces pulled by adjacent cells. b White blood cells and platelets form adhesions in blood vessels, where tensile dragging force is generated by dislodging blood flow. Collision of blood cells against the vessel wall or with each other generates compressive force. c Osteocytes inside the bone are constantly subject to both tensile and compressive force. d Tensile force is generated in cell–cell or cell–matrix adhesions, which is transmitted via receptor–ligand bonds, whereas compressive force is generated onto the cell membrane by cell–cell or cell–matrix collision
External mechanical stimuli onto cells are received by certain receptors or molecular assemblies associated with cell membrane (Tarbell et al. 2014) and subsequently converted into biochemical signals to trigger cellular responses and alter cellular behaviors (Chen et al. 2017a). In such mechanosensing processes, the molecular assembly responsible for the presentation, reception, transmission, and transduction of the force signal can be regarded as a nano-machine (Chen et al. 2017a), and the force signal is regarded as the input, which correlates with the output—the triggered intracellular signaling.
Based on the form of external mechanical force at the single-molecule level, such “mechanosensing” processes can be categorized, in general, into two forms (Fig. 1d):
Tensile force exerted by pulling of cell surface receptors via engaged ligands (Brockman et al. 2018). For example, platelets sense hemodynamic tensile force in blood flow and initiate thrombus formation, where platelet adhesion and aggregation process is mediated by the molecular interactions between platelet receptors (e.g., GPIb, integrin αIIbβ3) and plasma ligands (e.g., von Willebrand factor (VWF), fibrinogen). In certain circumstances, the tensile force extrudes cell membrane into tethers, which can stabilize cell adhesion under high shear condition (Jackson et al. 2009; Roest et al. 2011; Sundd et al. 2012).
Compressive force that applies tension to the membrane, which can be exerted by cell–cell collision (Ju et al. 2018) or cell compression onto the extracellular matrix (ECM) (Pagliara et al. 2014).
DFS techniques, such as atomic force microscopy (AFM), biomembrane force probe (BFP), and optical tweezer (OT), have been invented to examine protein dynamics including receptor–ligand interactions, protein conformational changes and enzymatic cleavage (Chaudhuri et al. 2016; Neuman and Nagy 2008). By utilizing an ultra-sensitive force transducer (e.g., a cantilever in AFM, an aspirated red blood cell (RBC) in BFP or a laser-induced gradient force trap in OT), DFS can visualize single molecular behaviors under controlled mechanical stimulation waveforms (Chen et al. 2017a).
The investigation of cell mechanosensing requires the manipulation of mechanical stimuli to cells and simultaneous readout of the cells’ behavior change. In this regard, DFS techniques that were widely used to study purified proteins have been upgraded with the capability to manipulate single living cells, enabling the examination of live-cell dynamics in response to mechanical and biochemical stimulations (Su and Ju 2018). In this review, we aim to discuss how the latest live-cell DFS techniques enable us to examine the tensile and compressive force-induced cell mechanosensing at the molecular scale.
Tensile force-mediated cell mechanosensing
Receptor–ligand bonds under tensile force
The application of extracellular tensile force mainly relies on the association of cell receptors with surface-immobilized ligands. Their relative movement produces the dragging tensile force on the molecular bond (Fig. 1d). For instance, neutrophils and platelets adhere to the vascular surface via the binding of selectins, integrins, and other glycoproteins, which bear dislodging forces from the arterial or venous blood flow. In the absence of external force, a migrating cell exerts endogenous tensile forces on the ECM via receptors (e.g., integrins) binding to immobilized ligands (Chen et al. 2017a; Fournier et al. 2010; Valignat et al. 2013). Even when the cell remains static, actin retrograde flow could still mobilize adhesion receptors for spatial reorganization (Comrie et al. 2015; Li et al. 2010; Swaminathan et al. 2017), exerting tensile forces on the bonds. The best example for this cell behavior can be observed in the cadherin-based adherent junctions in epithelia and endothelia, where cell–cell adhesion couples the contractile actomyosin cytoskeletons of cells together to generate tensile force and tissue-scale tension (Charras and Yap 2018).
In the DFS systems, the application of tensile force was achieved by the programmed pulling of a formed receptor–ligand bond, where the deformation of the elastic force transducer measures the force amplitude (Roca-Cusachs et al. 2017; Su and Ju 2018). By tuning the ligand coating density and controlling the adhesion frequency below 20%, DFS is able to, most likely, probe one bond at a time, thereby measure the binding kinetics on single-molecule level (Liu et al. 2015).
Dynamic bonds and their roles in cell mechanosensing
A molecular bond can be regulated by mechanical force to manifest catch (bond lifetime increases as force increases), slip (bond lifetime decreases as force increase), and ideal (bond lifetime is indifferent to force change) bond behaviors (Liu et al. 2015). As a counter-intuitive phenomenon, catch bond has been displayed by many adhesion receptors such as selectins (Marshall et al. 2003), GPIb (Ju et al. 2013), integrins (Chen et al. 2017b; Choi et al. 2014; Fiore et al. 2014; Kong et al. 2009; Rosetti et al. 2015), Notch receptors (Luca et al. 2017), and cadherins (Manibog et al. 2014). While the existence of catch bond is still being identified more in the intracellular protein systems (Akiyoshi et al. 2010; Huang et al. 2017; Lee et al. 2013) and its molecular mechanisms being modeled, recent studies started to unravel its physiological and pathological relevance:
The interactions of T cell receptor (TCR) with self-peptide major histocompatibility complex (pMHC) ligands to induce decision-making of “kill” and “survival” have been linked to their catch and slip bonds (Liu et al. 2014; Sibener et al. 2018). Negative selection (“kill”) ligands were found to form cooperative trimolecular catch bonds (“dynamic catch”) with TCR and the co-receptor CD8 and stimulate T cell to exert force for bond strengthening, whereas positive selection (“survival”) ligands can only form weak slip bonds with either TCR or CD8 (Hong et al. 2018). Such a difference in the bond strength, reflecting the ligand discriminative power of TCR, has been proposed to affect the downstream signaling with the final decision of thymocyte selection. In adaptive immunity, cancer-associated somatic mutations of HLA-A2 suppress the TCR–pMHC catch bond, suggesting a functional contribution of TCR–pMHC catch bond to T cell immunological signaling and functioning (Wu et al. 2019).
l-selectin on neutrophils interacts with E-selectin expressed on endothelial cells via a catch bond. This interaction triggers mechano-signaling that induces the activation and clustering of β2 integrins on the neutrophil surface (Block et al. 2012; Kuwano et al. 2010), whereas inhibition of the catch bond avidly suppresses both the activation of β2 integrins and the assembly of focal adhesions (Morikis et al. 2017). In the context of platelet adhesion under high shear condition, eliminating the GPIb catch bond with the type 2B von Willebrand disease (VWD) mutations in VWF ligand suppresses GPIb mechano-signaling (Ju et al. 2016), suggesting an emerging concept that VWD-caused bleeding disorder is likely contributed by the compromised platelet mechanosensing in addition to the altered binding kinetics. For integrin-mediated mechanosensing scenarios, the endothelial surface molecule Thy-1 (CD90) forms a slip bond with integrin α5β1 or syndecan-4 alone, but a trimolecular dynamic catch bond in the presence of both receptors, the inhibition of which suppresses FAK- and myosin II-mediated cell mechano-signaling at focal adhesions (Fiore et al. 2014). Besides, the abrogation of leukocyte integrin αMβ2 catch bonds has been suggested as a potential cause of systemic lupus erythematosus (SLE), as it dysregulates αMβ2 signaling and impairs the negative regulations of autoimmune responses (Rosetti et al. 2015).
Cell mechanosensing by distinct force waveforms
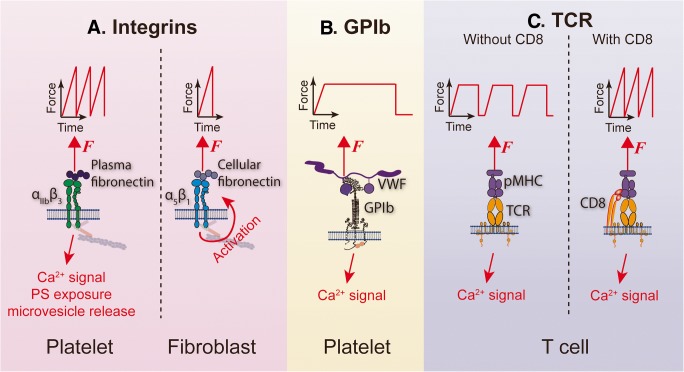
The tensile force applied to a cell receptor can adopt various waveforms. The two most commonly used force waveforms in DFS experiments are ramped and clamped forces. For a ramped force waveform, the force is linearly loaded till bond rupture without any durability (Fig. 2a), whereas for a clamped force waveform, the force is linearly loaded but then sustained at a constant level (Fig. 2b) (Chen et al. 2017a).
Fig. 2.
Distinct force waveform-mediated mechanosensing mechanisms in various molecular systems. a Repeated ramped forces on platelet integrin αIIbβ3 can trigger platelet-activating signaling including intracellular Ca2+ signal (Chen et al. 2019), phosphatidylserine (PS) exposure, and microvesicle release (Pang et al. 2018), whereas on fibroblasts, a single ramped force on α5β1 is sufficient to trigger intracellular activating signals (Strohmeyer et al. 2017). b A durable clamped force event triggers GPIb-mediated platelet mechanosensing, leading to intracellular Ca2+ mobilization (Ju et al. 2016). c A TCR requires repeated durable bindings to pMHC and accumulated clamped forces to trigger intracellular Ca2+ (Liu et al. 2014); however, the co-binding of T cell surface CD8 to pMHC changes the requirement, allowing repeated ramped forces to trigger Ca2+ as well (Pryshchep et al. 2014)
As a unique feature of DFS, it can apply various tensile force waveforms on a living cell and examine the distinct cellular response accordingly. Integrins appear to, in general, allow cell activation by ramped forces (Fig. 2a). For single integrin αIIbβ3-mediated platelet mechanosensing, repeated and intermittent ramped force induces intermediate state integrin affinity maturation towards full activation (Chen et al. 2019). The repeated pulling of αIIbβ3 ensuing platelet activation by thrombin can even trigger the procoagulant functions of platelets with phosphatidylserine exposure and microvesicle release (Pang et al. 2018) (Fig. 2a, left). However, in the context of focal adhesions, the binding of fibroblast α5β1 integrins to fibronectin can be avidly reinforced within a single-cycled pulling in less than 1 s (Strohmeyer et al. 2017), suggesting that a single ramped force on integrin is sufficient to trigger cell mechano-signaling (Fig. 2a, right). Possibly as part of the mechanism, the high forces reached by ramping can induce the unfolding of integrin-linked cytoplasmic proteins like talin, vinculin, and kindlin, thereby re-organizing the actin cytoskeleton, which leads to integrin clustering and downstream biochemical signals (Elosegui-Artola et al. 2016; Holle et al. 2013; Sun et al. 2019).
Distinct from integrin-mediated cell mechanosensing, a single GPIb bond under a clamped force of > 2-s duration triggers intracellular Ca2+ flux and induces integrin activation, while ramped forces fail so (Ju et al. 2016) (Fig. 2b). For the TCR system, in the absence of CD8 binding, the accumulation of repeated clamped force cycles is required to trigger T cell Ca2+ signaling (Liu et al. 2014) (Fig. 2c, left); however, when CD8 is allowed to form trimolecular complex with TCR and pMHC, repeated ramped forces are sufficient to trigger Ca2+ as well (Pryshchep et al. 2014) (Fig. 2c, right).
These observations indicate that each receptor-mediated mechanosensing system has distinct force waveform sensitivity, which might be relevant to their respective physiological roles. The requirement of a single durable bond for GPIb mechanosensing ensures rapid hemostatic function of platelets at sites of vascular injury. The immediate signaling process of fibroblast α5β1 integrins may serve as a mechanism for the quick development of stable focal adhesions. By comparison, the accumulation of multiple bonds in TCR triggering, which reviews the binding kinetics in a comprehensive fashion, ensures maximal accuracy in pMHC recognition and right decision for immune response.
Membrane compressive force-mediated cell mechanosensing
In contrast to tensile forces, which are far more widely and extensively studied in the mechanobiology field, the biological effects of compressive force and the mechanisms of its reception, transmission, and transduction in cells are less defined. Yet at the cellular level, the significant role of compressive force has been demonstrated in several biological scenarios (Fig. 3a). For example, in developmental biology, compression caused by normal morphogenetic movements during mesoderm invagination induces signaling to control the formation of the dorso-ventral axis in the early gastrula-stage Drosophila melanogaster embryo (Farge 2003). In oncology, compressive force on spheroids of murine mammary carcinoma cells regulates their proliferation and death (Cheng et al. 2009). In stem cell biology, compressive force on naive mouse embryonic stem cells undergoing a transition towards differentiation expands their nuclei (Pagliara et al. 2014). In plant biology, compressive stress orients microtubules in Arabidopsis leaves (Jacques et al. 2013) and prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells (Sampathkumar et al. 2014). In dental bone biology, compressive stress which constantly exerts on periodontal ligament cells triggers a series of biochemical activities to support osteoclastogenesis, such as an increase of prostaglandin E2 production and cyclo-oxygenase 2 expression (Kanzaki et al. 2002; Nakajima et al. 2008). Similar compressive stresses also induce the production of inflammatory cytokines and their receptors in osteoblasts (Koyama et al. 2008).
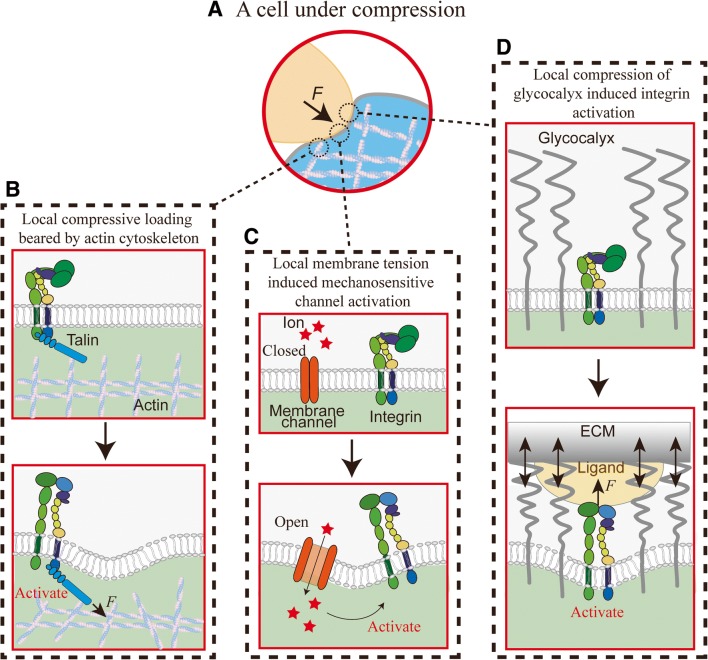
Fig. 3.
Membrane compressive force induced integrin activation. a A cell under compressive force. b Local compressive force rearranges the actin cytoskeleton, via the integrin cytoplasmic adaptor protein talin, propagating lateral force to induce integrin tail separation and activation. c Local membrane tension induced mechanosensitive ion channel opening, leading to Ca2+ influx and subsequent intracellular signals that activate the integrin. d Local compression of glycocalyx by extracellular matrix (ECM) enables ligand engagement of nearby integrin receptors. It also creates opposing elastic force, which transmits to pull on the integrin via a bound ligand and induces integrin activation
In this context, we have recently used BFP to provide the first evidence demonstrating that compressive force can be sensed by platelets to upregulate integrin αIIbβ3 binding (Ju et al. 2018). These experimental results support the previous rheological and modeling studies demonstrating that RBCs push and subject platelets to collision forces (compression), thereby promoting platelet thrombus formation (Tokarev et al. 2011; Tovar-Lopez et al. 2013). However, the exact mechanism of how compressive forces exerted on the platelet membrane lead to integrin αIIbβ3 activation remains elusive.
The first possibility may be related to the force-through-filament principle as the compression force is sensed by the cytoskeleton rather than plasma membrane (Fletcher and Mullins 2010). In response to the external compression force, the platelet cytoskeleton might undergo local remodeling, leading to integrin activation (Fig. 3b). Indeed, compressive force has been shown to alter the growth of branched actin filaments at the leading edge of crawling cells (Chaudhuri et al. 2007).
The second possibility is in accordance with the force-through-lipid model which has been established for mechanosensitive ion channels, i.e., MscL (Cox et al. 2017). In this scenario, compression force normal to the membrane is converted into tension in the membrane, which may trigger the opening of Ca2+ ion channels and induce integrin activation (Fig. 3c). This is consistent with the observation that chelating extracellular calcium reduced αIIbβ3-dependent compressive force sensing on platelets (Ju et al. 2018).
The third possibility is demonstrated in endothelial mechanotransduction that the glycocalyx, a layer of glycoprotein–polysaccharide complex on the cell surface, can be compressed by RBCs and leukocytes (Weinbaum et al. 2007). Glycocalyx can extend > 100 nm from the cell surface (Hattrup and Gendler 2008), a distance far exceeding the axial length of bent (< 11 nm) and extended integrins (> 20 nm) (Chen et al. 2012; Ye et al. 2010), thereby burying the ligand binding site of integrins. Therefore, it is likely that compressive force compresses glycocalyx on platelets and exposes αIIbβ3 for adhesive function. Moreover, considering that the external compressive force is most likely dynamic, the length of the compressed glycocalyx would consistently fluctuate, which can exert pulling force on the ligand-engaged integrin (Fig. 3d) to accelerate its extension and activation (Chen et al. 2012, 2017b). Recently, it has been demonstrated that local compression of the glycocalyx near integrin adhesive contacts promotes integrin clustering and focal adhesion maturation (Paszek et al. 2009, 2014). The current single-cell glycocalyx imaging technique can be utilized to examine this mechanism for future studies (Scrimgeour et al. 2017).
Conclusion
The new biomechanical nanotools prompted the field of mechanobiology into a new era, which allow the researchers to investigate cell mechanosensing at the single-cell and single-molecule level. Under this background, combining live-cell DFS analysis with intracellular signaling readouts can reveal the inner-working of each mechanosensing nano-machine, which will undoubtedly expand our knowledge of many physiological and pathological processes. Ultimately, under the concept of “mechanomedicine”, the mechanics/engineering-based principles and technologies, such as live-cell DFS, and its discovered molecular insights, could all be repurposed to the diagnosis, treatment, control, and cure of various human diseases (Guo et al. 2018; Naruse 2018).
Acknowledgments
We thank Prof. Cheng Zhu for helpful discussion. This work was supported by the Cardiac Society of Australia and New Zealand BAYER Young Investigator Research Grant (L.A.J.). L.A.J. is an Australian Research Council DECRA Fellow (DE190100609) and a former National Heart Foundation of Australia postdoctoral fellow (101798). Y.C. is a MERU (Medolago-Ruggeri) Foundation post-doctoral awardee. Z.L. is an Australian Research Council Future Fellow (FT140101152).
Abbreviations
- DFS
Dynamic force spectroscopy
- AFM
Atomic force microscopy
- BFP
Biomembrane force probe
- OT
Optical tweezers
- TCR
T cell receptor
- pMHC
Peptide major histocompatibility complex
- VWF
von Willebrand factor
Compliance with ethical standards
Conflict of interest
Yunfeng Chen declares that he has no conflict of interest. Zhiyong Li declares that he has no conflict of interest. Lining Arnold Ju declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yunfeng Chen and Lining Arnold Ju contributed equally to this work.
References
- Akiyoshi B, Sarangapani KK, Powers AF, Nelson CR, Reichow SL, Arellano-Santoyo H, Gonen T, Ranish JA, Asbury CL, Biggins S. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature. 2010;468:576–579. doi: 10.1038/nature09594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacabac RG, Smit TH, Mullender MG, Dijcks SJ, Van Loon JJ, Klein-Nulend J. Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem Biophys Res Commun. 2004;315:823–829. doi: 10.1016/j.bbrc.2004.01.138. [DOI] [PubMed] [Google Scholar]
- Block H, Herter JM, Rossaint J, Stadtmann A, Kliche S, Lowell CA, Zarbock A. Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury. J Exp Med. 2012;209:407–421. doi: 10.1084/jem.20111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman JM, Blanchard AT, Pui-Yan VM, Derricotte WD, Zhang Y, Fay ME, Lam WA, Evangelista FA, Mattheyses AL, Salaita K. Mapping the 3D orientation of piconewton integrin traction forces. Nat Meth. 2018;15:115–118. doi: 10.1038/nmeth.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras G, Yap AS. Tensile forces and mechanotransduction at cell-cell junctions. Curr Biol. 2018;28:R445–R457. doi: 10.1016/j.cub.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Chaudhuri O, Parekh SH, Fletcher DA. Reversible stress softening of actin networks. Nature. 2007;445:295–298. doi: 10.1038/nature05459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15:326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Lou J, Evans EA, Zhu C. Observing force-regulated conformational changes and ligand dissociation from a single integrin on cells. J Cell Biol. 2012;199:497–512. doi: 10.1083/jcb.201201091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ju L, Rushdi M, Ge C, Zhu C. Receptor-mediated cell mechanosensing. Mol Biol Cell. 2017;28:3134–3155. doi: 10.1091/mbc.E17-04-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lee H, Tong H, Schwartz M, Zhu C. Force regulated conformational change of integrin alphaVbeta3. Matrix Biol. 2017;60-61:70–85. doi: 10.1016/j.matbio.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ju LA, Zhou F, Liao J, Xue L, Su QP, Jin D, Yuan Y, Lu H, Jackson SP et al (2019) An integrin alphaIIbbeta3 intermediate affinity state mediates biomechanical platelet aggregation. Nat Mater In press [DOI] [PMC free article] [PubMed]
- Cheng G, Tse J, Jain RK, Munn LL. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS One. 2009;4:e4632. doi: 10.1371/journal.pone.0004632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YI, Duke-Cohan JS, Chen W, Liu B, Rossy J, Tabarin T, Ju L, Gui J, Gaus K, Zhu C, et al. Dynamic control of β1 integrin adhesion by the plexinD1-sema3E axis. Proc Natl Acad Sci U S A. 2014;111:379–384. doi: 10.1073/pnas.1314209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comrie WA, Babich A, Burkhardt JK. F-actin flow drives affinity maturation and spatial organization of LFA-1 at the immunological synapse. J Cell Biol. 2015;208:475–491. doi: 10.1083/jcb.201406121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CD, Bavi N, Martinac B. Origin of the force: the force-from-lipids principle applied to piezo channels. Curr Top Membr. 2017;79:59–96. doi: 10.1016/bs.ctm.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Elosegui-Artola A, Oria R, Chen Y, Kosmalska A, Perez-Gonzalez C, Castro N, Zhu C, Trepat X, Roca-Cusachs P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol. 2016;18:540–548. doi: 10.1038/ncb3336. [DOI] [PubMed] [Google Scholar]
- Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- Feghhi S, Tooley WW, Sniadecki NJ (2016) Nonmuscle myosin IIA regulates platelet contractile forces through rho kinase and myosin light-chain kinase. J Biomech Eng 138 [DOI] [PMC free article] [PubMed]
- Fiore VF, Ju L, Chen Y, Zhu C, Barker TH. Dynamic catch of a Thy-1-alpha5beta1+syndecan-4 trimolecular complex. Nat Commun. 2014;5:4886. doi: 10.1038/ncomms5886. [DOI] [PubMed] [Google Scholar]
- Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier MF, Sauser R, Ambrosi D, Meister JJ, Verkhovsky AB. Force transmission in migrating cells. J Cell Biol. 2010;188:287–297. doi: 10.1083/jcb.200906139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo XE, Hung CT, Sandell LJ, Silva MJ. Musculoskeletal mechanobiology: a new era for mechanomedicine. J Orthop Res. 2018;36:531–532. doi: 10.1002/jor.23789. [DOI] [PubMed] [Google Scholar]
- Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- Hoffman BD, Yap AS. Towards a dynamic understanding of cadherin-based Mechanobiology. Trends Cell Biol. 2015;25:803–814. doi: 10.1016/j.tcb.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Holle AW, Tang X, Vijayraghavan D, Vincent LG, Fuhrmann A, Choi YS, del Alamo JC, Engler AJ. In situ mechanotransduction via vinculin regulates stem cell differentiation. Stem Cells. 2013;31:2467–2477. doi: 10.1002/stem.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Ge C, Jothikumar P, Yuan Z, Liu B, Bai K, Li K, Rittase W, Shinzawa M, Zhang Y, et al. A TCR mechanotransduction signaling loop induces negative selection in the thymus. Nat Immunol. 2018;19:1379–1390. doi: 10.1038/s41590-018-0259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DL, Bax NA, Buckley CD, Weis WI, Dunn AR. Vinculin forms a directionally asymmetric catch bond with F-actin. Science. 2017;357:703–706. doi: 10.1126/science.aan2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Nesbitt WS, Westein E. Dynamics of platelet thrombus formation. J Thromb Haemost. 2009;7:17–20. doi: 10.1111/j.1538-7836.2009.03401.x. [DOI] [PubMed] [Google Scholar]
- Jacques E, Verbelen JP, Vissenberg K. Mechanical stress in Arabidopsis leaves orients microtubules in a ‘continuous’ supracellular pattern. BMC Plant Biol. 2013;13:163. doi: 10.1186/1471-2229-13-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju L, Dong J-F, Cruz MA, Zhu C. The N-terminal flanking region of the A1 domain regulates the force-dependent binding of von Willebrand factor to platelet glycoprotein Ibα. J Biol Chem. 2013;288:32289–32301. doi: 10.1074/jbc.M113.504001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju L, Chen Y, Xue L, Du X, Zhu C. Cooperative unfolding of distinctive mechanoreceptor domains transduces force into signals. Elife. 2016;5:e15447. doi: 10.7554/eLife.15447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju L, McFadyen JD, Al-Daher S, Alwis I, Chen Y, Tonnesen LL, Maiocchi S, Coulter B, Calkin AC, Felner EI, et al. Compression force sensing regulates integrin alphaIIbbeta3 adhesive function on diabetic platelets. Nat Commun. 2018;9:1087. doi: 10.1038/s41467-018-03430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res. 2002;17:210–220. doi: 10.1359/jbmr.2002.17.2.210. [DOI] [PubMed] [Google Scholar]
- Kim OV, Litvinov RI, Alber MS, Weisel JW. Quantitative structural mechanobiology of platelet-driven blood clot contraction. Nat Commun. 2017;8:1274. doi: 10.1038/s41467-017-00885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Mitsui N, Suzuki N, Yanagisawa M, Sanuki R, Isokawa K, Shimizu N, Maeno M. Effect of compressive force on the expression of inflammatory cytokines and their receptors in osteoblastic Saos-2 cells. Arch Oral Biol. 2008;53:488–496. doi: 10.1016/j.archoralbio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Kuwano Y, Spelten O, Zhang H, Ley K, Zarbock A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood. 2010;116:617–624. doi: 10.1182/blood-2010-01-266122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam WA, Chaudhuri O, Crow A, Webster KD, Li T-D, Kita A, Huang J, Fletcher DA. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat Mater. 2011;10:61–66. doi: 10.1038/nmat2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-Y, Lou J, Wen K-k, McKane M, Eskin SG, Ono S, Chien S, Rubenstein PA, Zhu C, Mcintire LV. Actin depolymerization under force is governed by lysine 113:glutamic acid 195-mediated catch-slip bonds. Proc Natl Acad Sci U S A. 2013;110:5022–5027. doi: 10.1073/pnas.1218407110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bhimalapuram P, Dinner AR. Model for how retrograde actin flow regulates adhesion traction stresses. J Phys Condens Matter. 2010;22:194113. doi: 10.1088/0953-8984/22/19/194113. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen W, Evavold BD, Zhu C. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell. 2014;157:357–368. doi: 10.1016/j.cell.2014.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Chen W, Zhu C. Molecular force spectroscopy on cells. Annu Rev Phys Chem. 2015;66:427–451. doi: 10.1146/annurev-physchem-040214-121742. [DOI] [PubMed] [Google Scholar]
- Luca VC, Kim BC, Ge C, Kakuda S, Wu D, Roein-Peikar M, Haltiwanger RS, Zhu C, Ha T, Garcia KC. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science. 2017;355:1320–1324. doi: 10.1126/science.aaf9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509:617–621. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manibog K, Li H, Rakshit S, Sivasankar S. Resolving the molecular mechanism of cadherin catch bond formation. Nat Commun. 2014;5:3941. doi: 10.1038/ncomms4941. [DOI] [PubMed] [Google Scholar]
- Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- Morikis VA, Chase S, Wun T, Chaikof EL, Magnani JL, Simon SI. Selectin catch-bonds mechanotransduce integrin activation and neutrophil arrest on inflamed endothelium under shear flow. Blood. 2017;130:2101–2110. doi: 10.1182/blood-2017-05-783027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R, Yamaguchi M, Kojima T, Takano M, Kasai K. Effects of compression force on fibroblast growth factor-2 and receptor activator of nuclear factor kappa B ligand production by periodontal ligament cells in vitro. J Periodontal Res. 2008;43:168–173. doi: 10.1111/j.1600-0765.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- Naruse K. MECHANOMEDICINE: applications of mechanobiology to medical sciences and next-generation medical technologies. J Smooth Muscle Res. 2018;54:83–90. doi: 10.1540/jsmr.54.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman KC, Nagy A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods. 2008;5:491–505. doi: 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenfelt P, Elliott HL, Springer TA. Coordinated integrin activation by actin-dependent force during T-cell migration. Nat Commun. 2016;7:13119. doi: 10.1038/ncomms13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Pagliara S, Franze K, McClain CR, Wylde GW, Fisher CL, Franklin RJ, Kabla AJ, Keyser UF, Chalut KJ. Auxetic nuclei in embryonic stem cells exiting pluripotency. Nat Mater. 2014;13:638–644. doi: 10.1038/nmat3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang A, Cui Y, Chen Y, Cheng N, Delaney MK, Gu M, Stojanovic-Terpo A, Zhu C, Du X. Shear-induced integrin signaling in platelet phosphatidylserine exposure, microvesicle release, and coagulation. Blood. 2018;132:533–543. doi: 10.1182/blood-2017-05-785253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Boettiger D, Weaver VM, Hammer DA. Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS Comput Biol. 2009;5:e1000604. doi: 10.1371/journal.pcbi.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Hudak JE, Lakins JN, Wijekoon AC, Cassereau L, et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511:319–325. doi: 10.1038/nature13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryshchep S, Zarnitsyna VI, Hong J, Evavold BD, Zhu C. Accumulation of serial forces on TCR and CD8 frequently applied by agonist antigenic peptides embedded in MHC molecules triggers calcium in T cells. J Immunol. 2014;193:68–76. doi: 10.4049/jimmunol.1303436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Cusachs P, Conte V, Trepat X. Quantifying forces in cell biology. Nat Cell Biol. 2017;19:742–751. doi: 10.1038/ncb3564. [DOI] [PubMed] [Google Scholar]
- Roest M, Reininger A, Zwaginga JJ, King MR, Heemskerk JW, Biorheology Subcommittee of the, S.S.C.o.t.I Flow chamber-based assays to measure thrombus formation in vitro: requirements for standardization. J Thromb Haemost. 2011;9:2322–2324. doi: 10.1111/j.1538-7836.2011.04492.x. [DOI] [PubMed] [Google Scholar]
- Rosetti F, Chen Y, Sen M, Thayer E, Azcutia V, Herter JM, Luscinskas FW, Cullere X, Zhu C, Mayadas TN. A lupus-associated Mac-1 variant has defects in integrin allostery and interaction with ligands under force. Cell Rep. 2015;10:1655–1664. doi: 10.1016/j.celrep.2015.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A, Krupinski P, Wightman R, Milani P, Berquand A, Boudaoud A, Hamant O, Jonsson H, Meyerowitz EM. Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. eLife. 2014;3:e01967. doi: 10.7554/eLife.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimgeour J, McLane LT, Chang PS, Curtis JE. Single-molecule imaging of proteoglycans in the pericellular matrix. Biophys J. 2017;113:2316–2320. doi: 10.1016/j.bpj.2017.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibener LV, Fernandes RA, Kolawole EM, Carbone CB, Liu F, McAffee D, Birnbaum ME, Yang X, Su LF, Yu W, et al. Isolation of a structural mechanism for uncoupling T cell receptor signaling from peptide-MHC binding. Cell. 2018;174:672–687 e627. doi: 10.1016/j.cell.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmeyer N, Bharadwaj M, Costell M, Fassler R, Muller DJ. Fibronectin-bound alpha5beta1 integrins sense load and signal to reinforce adhesion in less than a second. Nat Mater. 2017;16:1262–1270. doi: 10.1038/nmat5023. [DOI] [PubMed] [Google Scholar]
- Su QP, Ju LA. Biophysical nanotools for single-molecule dynamics. Biophys Rev. 2018;10:1349–1357. doi: 10.1007/s12551-018-0447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Costell M, Fassler R. Integrin activation by Talin, kindlin and mechanical forces. Nat Cell Biol. 2019;21:25–31. doi: 10.1038/s41556-018-0234-9. [DOI] [PubMed] [Google Scholar]
- Sundd P, Gutierrez E, Koltsova EK, Kuwano Y, Fukuda S, Pospieszalska MK, Groisman A, Ley K. ‘Slings’ enable neutrophil rolling at high shear. Nature. 2012;488:399–403. doi: 10.1038/nature11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan V, Kalappurakkal JM, Mehta SB, Nordenfelt P, Moore TI, Koga N, Baker DA, Oldenbourg R, Tani T, Mayor S, et al. Actin retrograde flow actively aligns and orients ligand-engaged integrins in focal adhesions. Proc Natl Acad Sci U S A. 2017;114:10648–10653. doi: 10.1073/pnas.1701136114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu Rev Biomed Eng. 2014;16:505–532. doi: 10.1146/annurev-bioeng-071813-104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarev AA, Butylin AA, Ataullakhanov FI. Platelet adhesion from shear blood flow is controlled by near-wall rebounding collisions with erythrocytes. Biophys J. 2011;100:799–808. doi: 10.1016/j.bpj.2010.12.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Lopez FJ, Rosengarten G, Nasabi M, Sivan V, Khoshmanesh K, Jackson SP, Mitchell A, Nesbitt WS. An investigation on platelet transport during thrombus formation at micro-scale stenosis. PLoS One. 2013;8:e74123. doi: 10.1371/journal.pone.0074123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valignat MP, Theodoly O, Gucciardi A, Hogg N, Lellouch AC. T lymphocytes orient against the direction of fluid flow during LFA-1-mediated migration. Biophys J. 2013;104:322–331. doi: 10.1016/j.bpj.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- Wu P, Zhang T, Liu B, Fei P, Cui L, Qin R, Zhu H, Yao D, Martinez RJ, Hu W, et al. Mechano-regulation of peptide-MHC class I conformations determines TCR antigen recognition. Mol Cell. 2019;73:1015–1027 e7. doi: 10.1016/j.molcel.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Hu G, Taylor D, Ratnikov B, Bobkov AA, McLean MA, Sligar SG, Taylor KA, Ginsberg MH. Recreation of the terminal events in physiological integrin activation. J Cell Biol. 2010;188:157–173. doi: 10.1083/jcb.200908045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh YT, Serrano R, Francois J, Chiu JJ, Li YJ, Del Alamo JC, Chien S, Lasheras JC. Three-dimensional forces exerted by leukocytes and vascular endothelial cells dynamically facilitate diapedesis. Proc Natl Acad Sci U S A. 2018;115:133–138. doi: 10.1073/pnas.1717489115. [DOI] [PMC free article] [PubMed] [Google Scholar]