Abstract
Background
Low back pain (LBP) is the leading global cause of disability and is associated with intervertebral disc degeneration (DD) in some individuals. However, many adults have DD without LBP. Understanding why DD is painful in some and not others may unmask novel therapies for chronic LBP. The objectives of this study were to a) identify factors in human cerebrospinal fluid (CSF) associated with chronic LBP and b) examine their therapeutic utility in a proof-of-concept pre-clinical study.
Methods
Pain-free human subjects without DD, pain-free human subjects with DD, and patients with chronic LBP linked to DD were recruited and lumbar MRIs, pain and disability levels were obtained. CSF was collected and analyzed by multiplex cytokine assay. Interleukin-8 (IL-8) expression was confirmed by ELISA in CSF and in intervertebral discs.
The SPARC-null mouse model of progressive, age-dependent DD and chronic LBP was used for pre-clinical validation. Male SPARC-null and control mice received systemic Reparixin, a CXCR1/2 (receptors for IL-8 and murine analogues) inhibitor, for 8 weeks. Behavioral signs of axial discomfort and radiating pain were assessed. Following completion of the study, discs were excised and cultured, and conditioned media was evaluated with a protein array.
Findings
IL-8 was elevated in CSF of chronic LBP patients with DD compared to pain-free subjects with or without DD. Chronic inhibition with reparixin alleviated low back pain behaviors and attenuated disc inflammation in SPARC-null mice.
Interpretation
These studies suggest that the IL-8 signaling pathway is a viable therapy for chronic LBP.
Fund
Supported by NIH, MMF, CIHR and FRQS.
Keywords: Intervertebral disc degeneration, CXCL1, CXCL5, Cerebrospinal fluid, CXCR1/2, Reparixin
Research in context.
Evidence before this study
Chronic low back pain is the leading cause of disability world-wide. While intervertebral disc degeneration contributes to chronic low back pain in some individuals, many adults with disc degeneration do not have chronic low back pain. The reasons why disc degeneration is painful in some and not others are not understood. We and others have previously reported altered protein levels in the cerebral spinal fluid of individuals with chronic low back pain and/or disc degeneration compared to healthy subjects, indicating that differences associated with disc degeneration and pain are reflected in cerebrospinal fluid. Identification of these pain-generating or protective factors could reveal novel therapies for chronic back pain.
Added value of this study
Interleukin-8 (IL-8) is increased in the spinal fluid of individuals with disc degeneration and chronic low back pain compared to individuals with disc degeneration and no pain and individuals with no disc degeneration and no pain. IL-8 is also elevated in degenerating disc tissue compared to non-degenerating disc tissue. Blocking IL-8 receptor signaling in a mouse model of disc degeneration and pain reduces behavioral signs of back pain and biochemical signs of disc degeneration. These data suggest that IL-8 is increased in individuals with painful disc degeneration and that IL-8 signaling may play an important role in chronic low back pain.
Implications of all the available evidence
There is increasing rationale to explore therapeutic strategies that target IL-8 signaling to manage chronic low back pain associated with disc degeneration.
Alt-text: Unlabelled Box
1. Introduction
Chronic pain is debilitating, difficult to treat, and often due to unknown causes. In the US alone, pain is estimated to impact at least 100 million adults, costing $560–635 billion annually [1]. Globally, low back pain (LBP) is the single largest source of years lived with disability [2]. Therapeutic interventions are either ineffective, offer only low to moderate benefits that are generally short-term or are associated with undesired side-effects, and many patients who undergo invasive spinal surgery often continue to have pain [[3], [4], [5]]. Despite enormous efforts, significant advances in pain management continue to be elusive. The urgency of this unmet medical need is amplified by concerns regarding the current opioid crisis. Long term opioid usage for chronic pain management has numerous deleterious effects, thus new non-opioid approaches for the treatment of pain are desperately needed.
Chronic LBP is associated with intervertebral disc degeneration (DD) and inflammation in some individuals [6]. However, the degree of disc degeneration is a poor predictor of pain intensity, and the overall relationship between DD and LBP are weak. This may be due to many factors, including a high rate of asymptomatic (i.e. pain free) disc degeneration in adults [7]. Understanding why chronic LBP is present in some individuals with DD and not others may unmask novel therapies for chronic LBP associated with DD.
The disconnect between LBP and DD could be related to biochemical differences between painful and non-painful degenerating discs, specific structural defects (e.g. Schmorl's nodes) and other risk factors (e.g. psychosocial, environmental, genetic) that influence an individual's susceptibility to chronic pain. As intervertebral discs degenerate, the extracellular matrix of the disc begins to break down, resulting in loss of disc height and biomechanical function [8,9]. In addition, the production of pro-inflammatory cytokines, such as Interleukin-1β (IL-1β) and Tumor Necrosis Factor-α (TNFα), and pro-nociceptive neurotrophins, such as Nerve Growth Factor (NGF), increase in degenerating discs [[10], [11], [12], [13]]. Positive feedback pathways further amplify the degradative and inflammatory processes [[14], [15], [16], [17]]. While cytokines and neurotropins are linked to the DD and LBP in humans [18], many studies are limited to comparisons between surgical samples from LBP patients with DD vs. discs from organ donors or between surgical samples for LBP patients with DD vs. radiculopathy and disc herniation [19]; inclusion of pain-free subjects with DD is needed to determine what additional factors drive discogenic LBP.
Examining the composition of cerebral spinal fluid (CSF) allows for comparison to pain-free individuals with and without DD. Unlike disc tissue, CSF can be collected from pain-free participants that have been diagnosed with DD by magnetic resonance imaging (MRI). CSF from the spinal canal is in proximity to intervertebral discs and differences in CSF composition between LBP and control subjects have been previously identified [[20], [21], [22], [23], [24]], indicating the validity of this approach. For example, using this strategy, we have previously reported an elevation of nerve injury-associated markers in CSF of chronic LBP patients [25]. The identification of potential pain mediators that are differentially expressed in DD-related chronic LBP compared to asymptomatic disc degeneration could provide insights into the pathogenesis of painful disc degeneration.
The goals of the current study were to unmask clinically-relevant new therapeutic avenues for chronic LBP and to provide supporting proof-of-concept pre-clinical data. To accomplish these goals, we first screened for differences in inflammatory mediators in the CSF of individuals with DD and chronic LBP (+DD, +LBP), compared to pain-free subjects with (+DD, -LBP) or without (−DD, -LBP) lumbar disc degeneration using a high-throughput protein screen. IL-8 was identified as being selectively upregulated in subjects with LBP and DD compared to pain-free volunteers with degenerating or non-degenerating discs. We then investigated the potential therapeutic value of inhibiting IL-8 signaling pathways in the SPARC-null mouse model of progressive, disc degeneration-associated chronic back pain [26,27].
2. Methods
2.1. Human studies
2.1.1. Overview
Pain-free participants and patients with chronic LBP associated with intervertebral DD scheduled for spinal fusion surgery were recruited between March 2006 and August 2008.
Exclusion criteria for all participants included complicating medical factors such as previous spine surgery, pregnancy, lactation, meningitis, hepatitis, scoliosis, osteoporosis, neuropathies, and neurological conditions (e.g. psychosis, dementia, Parkinson's, etc.). Cerebrospinal fluid and degenerating, painful IVDs (from surgical patients) were obtained. Questionnaires, physical examination and CSF collection were performed in a single visit to the University of Minnesota General Clinical Research Center. Lumbar MRIs were collected at the Fairview University Medical Center or the Twin Cities Spine Center.
Control, non-degenerating human lumbar IVDs lacking signs of degeneration were harvested from human organ donors via collaboration with Transplant Quebec.
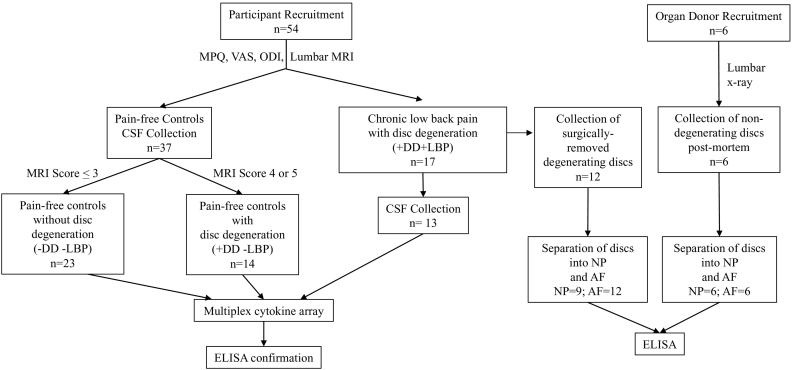
All procedures were approved by the Institutional Review Boards of the University of Minnesota (Protocol #0407 M62061), Allina Health Hospitals & Clinics (Protocol #1885). Informed consent was obtained from each subject. Procedures involving discs from organ donors were approved by and performed in accordance with the Institutional Review Board of McGill University (IRB#s A04-M53-08B and A10-M113-13B). Consent for use of tissue for research was obtained from next of kin. CSF collection, MRI scoring, and all biochemical analyses were performed by individual's blind to experimental group. Fig. 1 provides an overview of all participants. Descriptive statistics are shown in Table 1, Table 2.
Fig. 1.
Illustration showing the number and source of human participants.
Table 1.
Characteristics of the patients and pain-free participants in the CSF analysis.
| N | M:F | Age | Highest Lumbar MRI score (/5) | Total Lumbar MRI Score (/25) | Disability (ODI %) | Pain (VAS/10) | Total MPQ score | |
|---|---|---|---|---|---|---|---|---|
| -DD -LBP |
23 | 11:12 | 32 ± 1 | 2.6 ± 0.1 | 12.0 ± 0.6 | 0.08 ± 0.08 | 0.7 ± 0.5 | 0.04 ± 0.04 |
| +DD -LBP |
14 | 11:3 | 46 ± 4 *** |
4.1 ± 0.1 *** |
17.1 ± 0.8 *** |
0.8 ± 0.5 *** |
3 ± 2 | 0.4 ± 0.2 |
| +DD + LBP | 17 | 6:11 | 46.2 ± 3 *** |
4.2 ± 0.3 *** |
16.4 ± 0.8 *** |
44 ± 2 *** ### |
51 ± 6 *** ### |
15.4 ± 2 *** ### |
All values listed as mean ± SEM.
ODI: Oswestry low back disability index version 2.0.
VAS: Visual analogue scale.
MPQ: McGill Pain Questionnaire.
Compared to pain free controls without disc degeneration (−DD –LBP) * = p < 0.05, *** = p < 00.001).
Compared to pain-free controls with disc degeneration (+DD, -LBP) ### = p < 0.001.
Table 2.
Characteristics of the patient and transplant donors in the IVD analysis.
| N | M:F | Age | # Discs per subject | Distribution by disc level | NP | AF | |
|---|---|---|---|---|---|---|---|
| +DD + LBP | 12 | 5:3 | 47 ± 3 | 2.1 ± 0.3 | L2/3: 1 L3/4: 6 L4/5: 9 L5/S1: 9 Lumbar unknown: 1 |
9 | 12 |
| Transplant Quebec | 6 | 3:3 | 52 ± 7 | 1 | L2/3: 0 L3/4: 1 L4/5: 3 L5/S1: 0 Lumbar unknown: 2 |
6 | 6 |
All values listed as mean ± SEM.
2.1.2. Participants with low back pain and disc degeneration (+DD + LBP)
Subjects aged 21–65 years with chronic LBP associated with diagnosed DD were recruited at the Twin Cities Spine Centre. Patients with a minimum of 6 months of LBP, with self-reported pain scores ≥25/100 and a score of 4 or 5 for at least one lumbar disc on the Pfirrmann scale [28] were recruited. MRI images were used to determine a subject's suitability for surgery and images were re-evaluated by a blind observer together with the MRIs from the pain-free volunteers. Subjects were included if they belonged to one of three medication regiments: non-steroidal anti-inflammatory drugs (NSAIDs) and opioids, NSAIDs and steroids, opioids and steroids. Analgesic use was not withheld. Discs removed during spinal fusion were collected in the operating room, dissected into NP and AF fragments and flash frozen in liquid nitrogen and stored at −80 °C.
2.1.3. Pain-free participants
Healthy male and non-pregnant female volunteers ages 21–65 were recruited by advertisement. Individuals were considered for inclusion if they had no history of chronic pain of any type and no LBP over the last three months. Additional exclusion criteria for pain-free controls were use of prescribed steroids or narcotics for chronic medical conditions, refusal to discontinue anti-inflammatory and analgesic medications for 72 h prior to physical exam and CSF collection, and antidepressant users who had not been on a steady dose for at least 2 months. Pain-free controls were divided into two groups at the completion of the study based on lumbar MRI: those with moderate to severe disc degeneration according to MRI (+DD –LBP), and those with little to no disc degeneration (−DD –LBP).
2.1.4. Non-degenerating discs from organ donors
Lumbar spinal columns were removed from organ donors and imaged radiographically and visually for signs of degeneration (i.e. osteophytes, loss of disc height, herniation). Discs were then dissected from the spinal column and tissue samples from NP and AF tissues were taken using a 4 mm tissue biopsy. Only discs lacking signs of degeneration were used.
2.1.5. T2 Lumbar MRI
T2-weighted lumbar MRIs were scored by a radiologist blind to participant status to determine DD severity. Pain-free participants with all lumbar discs scoring ≤3 on the 5-point Pfirrmann Scale [28] were placed in the DD-free, pain-free (−DD, -LBP) group. Briefly, the Pfirrmann Scale uses T2-weighted MRI and consists of grades 1–5. Each grade is characterized by the overall structure and quality of the disc that is being assessed. For example, a grade 1 disc has a bright homogenous NP structure and a clear distinction between the NP and AF, whereas a grade 4 disc lacks a homogenous NP structure and the distinct definition between AF and NP is lost. For a detailed flow chart to determine the grade see Fig. 2 in Pfirrmann et al [28]. Pain-free participants with at least one lumbar disc scoring 4 or 5 were placed in the +DD –LBP group. Chronic LBP patients with at least one disc scoring 4 or 5 were placed in the +DD, +LBP group.
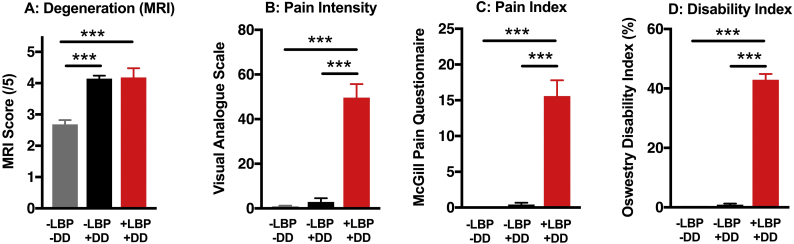
Fig. 2.
Stratification of participants by Low Back Pain (LBP) and Disc Degeneration (DD) severity. A) Individuals with and without LBP were stratified into one of three groups. Dividing the pain-free volunteers by the presence (+DD) or absence (−DD) of at least one lumbar disc with a score of 4 or 5 on the Pffirmann scale revealed a group of pain-free individuals (-LBP) with degeneration scores similar to the +LBP group. Compared to the pain-free volunteers, patients with LBP scored significantly higher on B) the visual analogue scale (VAS), C) the McGill Pain Questionnaire-Short Form (MPQ), and D) the Oswestry Disability Index (ODI). Data is presented as mean ± SEM, n = 14–23, one-way ANOVA followed by Tukey post-hoc test. *** = p < 0.001.
2.1.6. Pain and disability
All subjects were assessed for perceived pain intensity using the short form of the McGill Pain Questionnaire (MPQ) [29,30] and the visual analogue scale (VAS) within the MPQ. The Oswestry low back disability index (ODI) version 2.0 [31] was used to evaluate how chronic LBP affected subjects' perceived ability to perform daily activities.
2.1.7. CSF collection and storage
Subjects were asked to refrain from strenuous exercise 3 days before the pain assessment and CSF collection. CSF was collected with a 25 gauge Whitacre spinal needle under i.v. sedation with midazolam. The needle was introduced to the spinal canal at L3/4 according to standard practice using surface landmarks and CSF was collected by passive drip until either a) the 20 ml cut-off was reached or b) the CSF stopped flowing freely. At the time of collection, the quality of the tap and the visual appearance of the CSF were recorded (clear, cloudy, yellow or bloody). No traumatic taps were recorded and all but 4 samples were clear. One of the 4 was excluded due to high protein content, the remaining 3 became clear after the initial tap and total protein concentration was in range of their respective experimental groups (2 pain-free with no DD and 1 pain-free with DD). Collected CSF was chilled, centrifuged at 250 G for 10 min to remove any cellular or other contamination, and the supernatant flash frozen in liquid nitrogen and stored at −80 °C.
2.1.8. Luminex® Mulitplex assay
The following proteins were measured using a Luminex® multiplex assay according to manufacturer's instructions: Cytokines (Fractalkine, GM-CSF, IL-1β, IL-1ra, IL-4, IL-6, IL-8, IL-10, IL-13, MCP-1, MDC, MIP-1a, TNFα), bone markers (OPG (Osteoprotegrin), OPN (Osteopontin) and OC (Osteocalcin)), extracellular matrix proteases (MMP-3 and MMP-9) and TGFβ. Fractalkine, GM-CSF, IL-1β, IL-4, IL-10, IL-13, and MIP-1a were excluded because most samples were below the detection limit of the assay.
2.1.9. Protein extraction from human discs
Frozen disc samples were manually crushed in liquid nitrogen using a mortar and pestle. 200–400 μL of RIPA buffer (50 ml Tris HCl 1 M, 8.79 g NaCl, 2 ml EDTA 0.5 M and 10 ml Triton-X100 diluted in 100 ml with distilled water) containing 1× protease inhibitor (SIGMAFAST, Sigma-Aldrich) was then added to each set of crushed IVDs in 1 ml test tubes. A pestle grinder was next inserted into each tube for additional grinding. To avoid friction-induced warming, grinding was limited to no more than a few minutes per round, with an average of ~ 20 min of total grinding per disc. Samples were kept on ice throughout the procedure. Tubes were then centrifuged at 8000 G for 15 min at 4 °C. The supernatant was recovered, and protein concentration was determined (Bio-Rad DC TM Protein Assay Kit 1, Bio-Rad Laboratories, 500-0111). Samples were frozen at −80 until use.
2.1.10. Human IL-8 ELISA assay
The Human CXCL8/IL-8 Quantikine HS ELISA Kit (R&D Systems) was used as per manufacturers' instructions. All samples were processed in duplicate using a microplate reader (Spectramax M2E, Molecular Devices).
2.2. Animal studies
2.2.1. Overview
All experiments were approved by the Animal Care Committee of McGill and University following the guidelines of the Canadian Council of Animal Care. SPARC-null mice were developed on C57BL/6x129SVJ background [32], backcrossed to C57BL/6 and bred in-house as previously described [26,27,33]. C57BL/6 mice (Charles River, bred in house) were used as WT controls. Mice were housed in a temperature-controlled room with a 12-h light/dark cycle, 2–5 per ventilated polycarbonate cage (Allentown), and with corncob bedding (Envigo) and cotton nesting squares. Mice were given ab libitum access to food (Global Soy Protein-Free Extruded Rodent Diet, Irradiated) and water. A cohort of 7–9-month-old male and female SPARC-null and age-matched WT mice were used to quantify CXCL1 (KC) and CXCL5 (LIX) in lumbar intervertebral discs and serum (n = 7–13/group). For all subsequent CXCR1/2 inhibition, behavioral, and histological studies a single cohort of 7–9-month-old male mice consisting of SPARC-null (n = 10/group) and age-matched wild-type mice (n = 4–6/group) were used. A subset (n = 8 SPARC-null/group, 5 wild-type/group) of these were included in the ex vivo cytokine secretion analysis.
The cohort used for CXCR1/2 inhibition, behavioral, disc culture and histological studies shared WT and SPARC-null vehicle controls with another study that is now published [16]. These experiments, testing the effects of two different drugs compared to vehicle, were conducted at the same time, in parallel, to reduce the number of mice required in accordance with the three Rs.
2.2.2. Protein extraction from mouse discs
Spinal columns were harvested intact, flash frozen in conical tubes and stored at −80 until use. After thawing on ice, discs were extracted and manually crushed with liquid nitrogen using a mortar and pestle and resuspended in 400 μl of RIPA buffer (50 ml Tris HCl 1 M, 8.79 g NaCl, 2 ml EDTA 0.5 M and 10 ml Triton-X100 diluted in 100 ml with distilled water) and protease inhibitor (SIGMAFAST) in VWR reinformed 2 mL bead mill tubes containing metallic beads (VWR International). Cryolysis was then performed using a precellys 24 tissue homogenizer (Bertin Instruments) in two rounds of 2 x 20s @ 6500 rpm with 15 s breaking time followed by a 5-min ice bath to prevent overheating. The beads were then magnetically removed, the samples centrifuge at 4 °C, 5 min, 13.2 rpm and the supernatant were collected and kept on ice. Samples were re-suspended in a final volume of >300 μl/sample. Protein concentration in the RIPA buffer was quantified using the DC™ protein assay kit (Biorad) and samples were frozen at −80 until use.
2.2.3. Mouse KC and LIX ELISA assay
CXCL1 (KC) and CXCL5 (LIX) ELISA kits (RayBiotech, Cat. #s ELM-KC and ELM-LIX) were used to quantify concentrations in protein extracts from mouse discs and from serum collected from the same mice. ELISAs were performed according to manufactures instructions. All samples were processed in duplicate using a microplate reader (Spectramax M2E, Molecular Devices).
2.2.4. Drug treatment and behavioral testing schedule
Experimenters were blind to treatment group and mice were randomized into equal sized treatment groups. 7–9-month old SPARC-null and WT mice were given i.p. injections of Reparixin (MedChem Express, 20 or 30 mg/kg), a non-competitive allosteric inhibitor of CXCR1 and CXCR2 that prevents downstream signaling [34], or vehicle (n = 5–6 for WT treatment group, n = 10 per SPARC-null treatment group). Reparixin was dissolved in saline with 5% DMSO and 5% Tween 80; saline with 5% DMSO and 5% Tween 80 was also used as the vehicle. Dosage was based on efficacy of reparixin in a mouse lung-injury model [35]. Behavioral indices of axial discomfort have been previously reported [26,27,36]. In the acute study, mice were randomized into reparixin- or vehicle-treated groups and given a single injection. Grip strength, acetone-evoked behavior and mechanical sensitivity to von Frey filaments were assessed 1, 3, 6 and 24 h after injection. Following a 10-day washout period mice were re-randomized for the chronic study and treated 3 times per week for 8 weeks. Grip strength, acetone-evoked behavior and mechanical sensitivity to von Frey filaments were assessed during weeks 1, 2, 4, 6, and 8 on non-treatment days. Distance travelled in an open field was measured in weeks 3 and 7 on non-treatment days.
Testing was carried out between 08:00–12:00 (with the exception of the 6 h time point in the acute study) in a room dedicated for behavioral analysis with regular indoor lighting. Prior to testing mice were habituated to the testing room for 1 h. Prior to mechanical and cold testing, mice were habituated for a second hour in Plexiglas testing boxes on a metal mesh floor. Mechanical and cold sensitivities and grip strength were assessed on the same day. The order of testing was 1) von Frey, 2) acetone-evoked behavior, 3) 1-h rest in home cage and 4) grip force assay. Open field (chronic treatment only) was assessed during separate weeks from other tests.
2.2.5. Von Frey test for mechanical sensitivity
As previously described, calibrated von Frey filaments (Stoelting Co.) were applied to the plantar surface of the left hind paw to the point of bending until either the mouse withdrew from the stimuli or 5 s [26,27]. Filament stimuli intensity ranged from 0.6 to 4.0 g, which corresponds to filament numbers 3.84, 4.08, 4.17, 4.31, 5.46. The 50% withdrawal threshold in grams was calculated using the up-down method adapted from Chaplan et al. [37].
2.2.6. Acetone-evoked behavior test for cold sensitivity
Sensitivity to cold stimuli was used as a measure of radiating leg symptoms; previous studies have reported a consistent increase in cold sensitivity in SPARC-null mice [26,27]. Acetone was applied to the right hind paw and the total time of evoked behavior (paw lifting, shaking and scratching) was recorded.
2.2.7. Grip force
Axial discomfort was measured with a Grip Strength Meter (Stoelting Co) by allowing the mice to grip a bar with their forepaws and gently stretching them by increasing tension on the tail until the bar is released. The force at the time of release was recorded in grams [26]. Grip strength was measured 2–3 times and then averaged per session. Mice were returned to their home cages for approximately 15 min between each measurement. The device was cleaned between measurements.
2.2.8. Open field
Mice were placed in a 24 × 24 cm Plexiglas enclosure for five minutes under normal lighting. The enclosure was cleaned between each mouse. Mice were video recorded from above and the total distance travelled was analyzed using AnyMaze software as previously described [26].
2.2.9. Disc height index
At the completion of the 8-week treatment period, lateral in vivo radiographs were taken using a Faxitron MX-20 (Faxitron X-Ray, LLC) on anesthetized mice. Disc height index was calculated using the formula: DHI = ((D2a + D2b + D2c)/3/(V1a + V1b + V1c + V3a + V3b + V3c)/6)*100 where D represents a disc, V represent the vertebral bodies adjacent to the disc and lower case letters represent the three measurements of the same disc or vertebra [26,27].
2.2.10. Immunofluorescent histochemistry
Spinal cords were harvested following euthanasia and post-fixed in 4% paraformaldehyde for 24 h at 4 °C, followed by cryoprotection using 30% sucrose solution for 24 h at 4 °C. Samples were embedded in blocks of 6–11 spinal cords in optimum cutting temperature medium (OCT, Tissue-Tek). 14 μm cryostat (Leica CM3050S) sections were thaw mounted on gel coated slides and stored at 20 °C until use. Three sections per animal were randomly selected spanning the lumbar spinal cord for each antibody. Sections were incubated for 1 h at room temperature in blocking buffer containing 0.3% Triton X-100, 1% bovine albumin, 1% normal donkey serum and 0.1% sodium azide in PBS. Slides were then incubated with either sheep anti-calcitonin gene-related peptide (CGRP) polyclonal antibody (1:1000; Enzo Life Sciences, catalogue# BML-CA11370100, lot# 0807B74), goat anti-glial fibrillary acidic protein (GFAP) polyclonal antibody (1:1000; Sigma-Aldrich, catalogue# SAB2500462, lot# 747852C2G2), or rat monoclonal anti-CD11b antibody (1:1000; BioRad, catalogue# MCA711G, lot# 0614) in blocking buffer overnight at 4 °C, washed 3 times for 5 min in PBS and incubated for 1.5 h at room temperature with appropriate donkey-derived secondary antibodies from Jackson Immunoresearch; Donkey anti-sheep Cy3, catalogue #713-165-147; Donkey anti-goat AlexaFlour 594, catalogue #705-85-144; Donkey anti-Rat AlexaFluor 488, catalogue# 712-225-153) in blocking buffer. DAPI (1:50000 in water, Sigma-Aldrich) was briefly applied and slides were washed another 3 times for 5 min. Coverslips were mounted using Aqua Polymount (Polysciences Inc.). Images were taken at 10× magnification using an Olympus BX51microscope equipped with an Olympus DP71 camera (Olympus). Using ImageJ, a region of interest was drawn around the dorsal horn and a threshold was established to differentiate between positive immunoreactivity (ir) and background. The % area of the region of interest at, or above, the threshold was quantified to measure CGRP-ir, GFAP-ir or CD11b-ir. The average % area immunoreactivity across the three sections from each animal was averaged and used as the value for that mouse. Image analysis was performed by an experimenter blind to strain and treatment group.
2.2.11. Ex vivo disc culture and cytokine analysis
Following completion of the chronic drug treatment and behavioral testing, the L1/2, L2/3 and L3/4 discs were excised. Discs were washed once with Phosphate Buffered Solution and twice with Hanks Balanced Salt Solution, both supplemented with 20 U/ml penicillin and 20 μg/ml streptomycin for 5 min per wash. Discs were placed in 24 well plates and cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 1X glutamax and 10 U/ml penicillin and 10 μg/ml streptomycin for 48 h. The media was collected and analyzed using the 62 protein RayBiotech Mouse Cytokine Antibody Array C3 according to the manufacturer's instructions (RayBiotech, Cat. # AAM-CYT-3). Arrays were imaged using an ImageQuant LAS4000 Image Analyzer (GE) and analyzed with ImageQuant TL array analysis software (GE). Data were normalized to either WT vehicle-treated mice or SPARC-null vehicle-treated mice to calculate the fold difference of each protein.
2.3. Statistical analysis
All data was analyzed using GraphPad Prism version 7 with p ≤ 0.05 being considered statistically different. Data is presented as mean ± SEM. Outliers in the human data set were identified using the Grubbs' test and removed from the analysis. Results from the mouse study were similarly analyzed and no outliers were identified. Data was analyzed as indicated in figure legends.
3. Results
3.1.1. Stratification of participants by low back pain (LBP) and disc degeneration (DD) severity
Individual participants scheduled for surgery for DD-related chronic LBP formed the +DD, +LBP experimental group. Volunteers without LBP were recruited in parallel. Lumbar T2-MRI scans were obtained from all subjects and scored for DD severity. Subjects without chronic LBP were then stratified into groups with (+DD, -LBP) and without disc degeneration (−DD, -LBP) by the presence of at least one lumbar disc that scored 4 or 5 on the 5-point Pfirrmann scale (Fig. 1) [28].
The severity of DD was similar between the LBP patients and the pain-free individuals with moderate-severe disc degeneration (Fig. 2A) and consistent with previous work reporting a high incidence of moderate to severe disc degeneration in individuals without LBP [38]. In contrast, LBP patients had higher levels of self-reported pain intensity using the Visual Analog Scale (VAS, Fig. 2B) and the McGill Pain Questionnaire (MPQ, Fig. 2C) and higher levels of disability measured by the Oswestry Disability Index (ODI, Fig. 2D), when compared to pain-free individuals, regardless of disc degeneration status. Thus, the study subjects are divided into three groups based on MRI and questionnaire results: disc degeneration-free, pain-free subjects (−DD -LBP), subjects with disc degeneration but no pain (+DD -LPB), and the surgical subjects with degeneration and pain (+DD + LBP). Descriptive statistics of each group are presented in Table 1. As expected, the average age of pain-free participants with DD was higher. These three distinct groups enabled the identification factors unique to painful disc degeneration.
3.1.2. Interleukin-8 (IL-8) is upregulated in cerebrospinal fluid (CSF) from chronic LBP patients
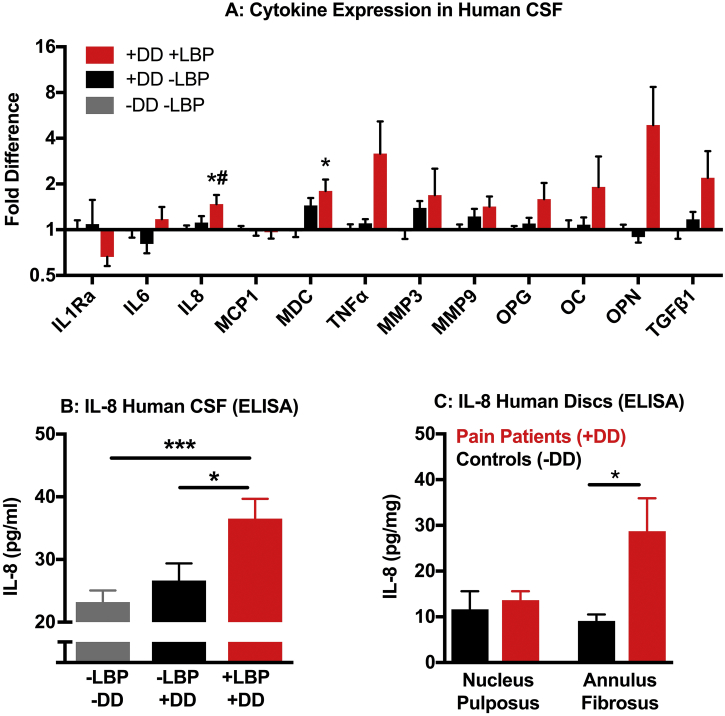
Previous studies have reported on CSF composition in subjects with chronic LBP associated with DD or disc herniation [[20], [21], [22], [23], [24]]. However, because those studies did not include pain-free individuals, it is unclear if any observed changes were due to DD-related inflammation or are specifically associated with pain. We therefore investigated changes in cytokine and chemokine levels in the CSF of +DD + LPB subjects compared to +DD -LBP and -DD -LBP subjects using a Luminex® protein assay approach. Of 19 factors measured, 7 were below the detection threshold of the assay (fractalkine, GM-CSF, IL-1β, IL-4, IL-10, IL-13, and MIP-1α). Of the remaining 12 factors, IL-8 and macrophage-derived chemokine (MDC) were significantly increased in the CSF of +DD + LBP subjects compared to -DD -LBP subjects (Fig. 3A). However, MDC was also upregulated in asymptomatic DD, suggesting a link to disc inflammation, whereas the IL-8 elevation was specific to LBP subjects. We then confirmed elevated levels of IL-8 in the CSF of +DD + LBP subjects compared to pain free subjects by ELISA (Fig. 3B). These results indicate IL-8 is elevated in the CSF of subjects with elevated pain scores, thus suggesting a role for IL-8 in DD-associated chronic LBP.
Fig. 3.
Interleukin-8 (IL-8) is increased in Cerebrospinal Fluid (CSF) and Intervertebral Discs from chronic LBP Patients. A) Cytokine levels in the CSF of subjects with disc degeneration and chronic low back pain (+DD + LBP), disc degeneration without low back pain (+DD –LPB) and no degeneration or pain (−DD –LBP) were quantified using a 19 factor Luminex array. Only factors above the detection threshold of the assay are shown. Fold-difference is normalized to –DD -LBP controls. B) The increase in IL-8 in CSF from the +DD + LBP compared to pain-free individuals with or without DD was confirmed by ELISA. C) IL-8 levels were elevated in the annulus fibrosus (AF) but not the nucleus pulposus (NP) of intervertebral discs surgically removed from chronic LBP patients (+DD + LBP) compared to discs obtained from human organ donors with no visible signs of disc degeneration. In cases where patients had multiple discs removed, IL-8 expression is presented as the average per all discs obtained from that individual. Data is presented as mean ± SEM, n = 14–23 for A&B, n = 6–12 for C. No sex differences were observed, males and females are therefore pooled. One-way ANOVA with a Tukey post-hoc test was used for A&B. A: * = p < 0.05 (vs –DD -LBP), # = p < .05 (vs + DD -LBP); B: * = p < 0.05, *** = p < 0.001 vs. +DD + LBP. The AF and NP were analyzed separately by t-test in part C. IL-1Ra, interleukin-1 receptor antagonist; IL-6, interleukin-6; IL-8, interleukin-8; MCP1, macrophage chemoattractant protein-1; MDC, macrophage-derived cytokine/CCL22; TNFα, tumor necrosis factor-alpha, MMP3, matrix metalloproteinase-3; MMP9, matrix metalloproteinase-9; OPG, Osteopotegrin; OC, Osteocalcin; OPN, Osteopontin; TGFβ1, transforming growth factor- beta 1).
3.1.3. Interleukin-8 (IL-8) is elevated in intervertebral discs in individuals with DD and LBP
The elevation of IL-8 in CSF from chronic LBP patients with DD could be from numerous sources, including degenerating intervertebral discs, increased infiltration of factors from blood into the CSF or neuroinflammation. To test the potential involvement of intervertebral discs, we quantified IL-8 levels in the nucleus pulposus (NP, inner part of the disc) and annulus fibrosus (AF, outer part of the disc) of discs surgically removed from +DD + LBP subjects and from discs obtained from human organ donors that lacked signs of degeneration (Table 2). In cases where multiple discs were surgically removed, IL-8 was measured in each sample and the average per subject was determined. Compared to controls, IL-8 concentrations were elevated in the AF, but not in the NP, of degenerated discs from chronic LBP patients (Fig. 3C). This indicates that degenerating discs are a potential source of IL-8 found in the CSF.
3.1.4. The murine IL-8 analogue, CXCL5 (LIX), is elevated in intervertebral discs from SPARC-null mice
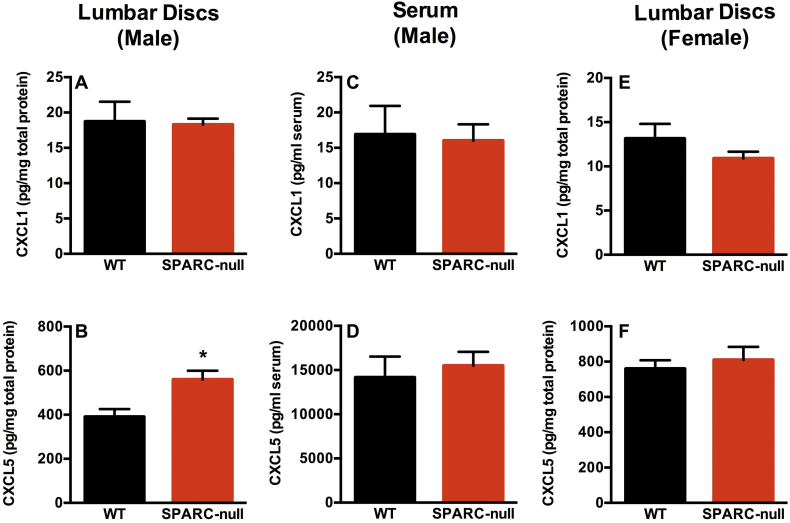
We have previously reported that mice lacking the sparc gene exhibit progressive, age-related intervertebral disc degeneration and behavioral signs of LBP that are sensitive to analgesic and anti-inflammatory treatment [16,26,27,36]. Thus, the SPARC-null mouse was used here as a clinically-relevant model. First, to confirm that IL-8 signaling is similarly altered in degenerating discs from mice as in humans, we measured the mouse functional analogues CXCL1 (KC) and CXCL5 (LIX), which activate the same receptors as IL-8: CXCR1 and 2. CXCL1 and CXCL5 were quantified in lumber discs from 7–9-month-old male and female SPARC-null and WT mice; at this age SPARC-null mice have developed disc degeneration and display behavioral signs of back pain [26]. CXCL5, but not CXCL1, was upregulated in male SPARC-null lumbar discs compared to age-matched WT mice (Fig. 4, A, B). In contrast, neither CXCL1 nor CXCL5 were different between strains in serum, indicating that the increase in discs is not associated with a systemic difference (Fig. 4, C, D). Since no changes were observed in female SPARC-null vs. WT mice in this age range (Fig. 4, E, F), CXCL1 and CXCL5 levels were tested in mice from ages 2–24 months and no differences were uncovered (data not shown). Thus, male SPARC-null mice were selected as a suitable model to investigate the therapeutic potential of CXCR1/2 inhibition in LBP associated DD.
Fig. 4.
The murine IL-8 analogue, CXCL5 (LIX), is elevated in intervertebral discs from male SPARC-null mice. The murine IL-8 analogues CXCL1 (KC, top row, A,C,E) and CXCL5 (LIX, bottom row, B,D,F) were measured by ELISA in male discs (1st column, A,B), male serum (2nd column, C,D) or female discs (3rd column, E,F) from SPARC-null and WT mice. No changes were observed in CXCL1 (KC). In contrast, CXCL5 (LIX) was upregulated in male mouse discs. Data is presented as mean ± SEM, n = 5–8, t-tests were used to analyze the data. * = p < 0.05.
3.1.5. Acute inhibition of CXCR1/2 by Reparixin does not alter behavioral indices of chronic back pain in mice
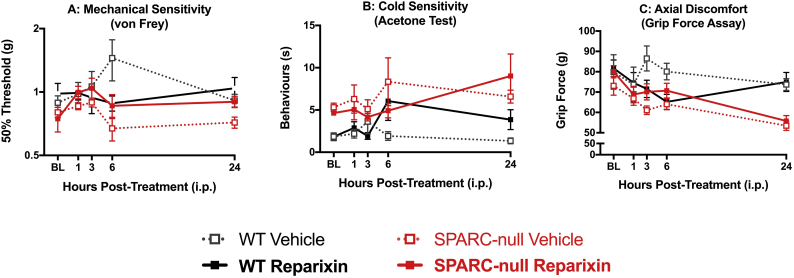
To examine the potential of targeting the IL-8 signaling pathway as a treatment for chronic DD-associated LBP, we first tested the effects of acute systemic reparixin, a small molecule inhibitor of CXCR1/2, in 7–9-month-old male SPARC-null mice. In SPARC-null mice, cold hypersensitivity in the hind paw is used as an indicator of radiating leg pain and resistance to stretch along the axis of the spine is used as a measure of axial discomfort. Following a single i.p injection of reparixin (30 mg/kg, i.p.) or vehicle, SPARC-null and WT animals were tested 1, 3, 6 and 24 h later for hind paw mechanical and cold sensitivity, and grip strength. As previously reported, at baseline there was no difference in mechanical sensitivity to von Frey filaments (Fig. 5A), but SPARC-null mice displayed increased cold sensitivity in the acetone test (Fig. 5B) and decreased grip strength (Fig. 5C) compared to age-matched WT. A single treatment with reparixin had no effects on these behavioral measures.
Fig. 5.
Effect of acute inhibition of CXCR1/2 by reparixin on behavioral indices of chronic back pain in mice. The effects of a single injection of reparixin (30 mg/kg, i.p.) were evaluated 1, 3, 6 and 24 h after treatment. Reparixin had no effect on A) mechanical sensitivity measured with von Frey filaments, B) cold sensitivity measured with acetone-evoked behaviors or C) axial discomfort measured by grip strength in neither SPARC-null nor WT mice. Data is presented as mean ± SEM, n = 9–10 per SPARC-null groups, n = 4–6 per WT groups. Data was analyzed by two-way repeated measures ANOVA followed by Tukey's post hoc test.
3.1.6. Chronic inhibition of CXCR1/2 by Reparixin reduces behavioral indices of chronic back pain
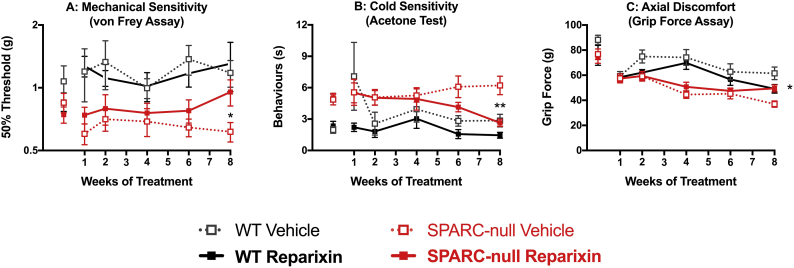
We hypothesized that chronic inhibition might be necessary to reduce ongoing disc inflammation and ultimately lead to reduced pain behavior. Following a ten-day washout, mice were treated with reparixin (20 mg/kg, i.p.) or vehicle 3 times/week for 8 weeks and mechanical sensitivity to von Frey filaments, acetone-evoked behavior and grip strength were evaluated on non-treatment days during weeks 1, 2, 4, 6, and 8. While not different at baseline, sensitivity to von Frey filaments was elevated in SPARC-null mice during the experiment and chronic reparixin decreased von Frey sensitivity in SPARC-null mice at week 8 compared to SPARC-null vehicle treated mice (Fig. 6A). Over the course of 8 weeks CXCR1/2 inhibition led to a progressive trend towards decreased sensitivity to cold in the acetone test that was significantly different from SPARC-null vehicle-treated mice at week 8 (Fig. 6B). Chronic CXCR1/2 inhibition improved grip strength compared to vehicle-treated SPARC-null mice following 8 weeks of treatment (Fig. 6C), suggesting a reduction of axial pain. Taken together, these results indicate chronic CXCR1/2 inhibition progressively decreases behavioral signs of chronic back pain in SPARC-null mice and provide proof-of-concept data for targeting this pathway to treat disc degeneration and chronic low back pain.
Fig. 6.
Effects of chronic inhibition of CXCR1/2 by Reparixin on behavioral indices of chronic back pain in mice. Mice were treated with Reparixin (20 mg/kg, i.p.) or vehicle 3 times/week for 8 weeks and behavioral assays were performed on non-treatment days. At the end of the treatment period, reparixin-treated SPARC-null mice were less sensitive to A) mechanical and B) cold stimuli compared to vehicle-treated controls in the von Frey and acetone tests, respectively. C) At the end of the treatment, an improvement in grip strength (index of axial discomfort) was observed in reparixin-treated SPARC-null mice. Data is presented as mean ± SEM, n = 9–10 per SPARC-null groups, n = 4–6 per WT groups. Data was analyzed by two-way ANOVA with Tukey's post hoc test post-hoc test. *
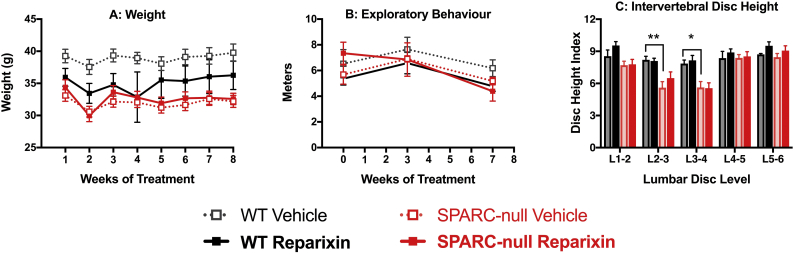
3.1.7. Chronic Reparixin has no effect on body weight, activity level or disc height in SPARC-null or WT mice
Potential adverse effects of reparixin were evaluated by assessing body weight and the distance travelled in an open field. Reparixin treatment had no significant effects on either measure (Fig. 7A, B), suggesting the changes in pain-like behavior are unlikely to be due to systemic adverse effects. As previously reported, the SPARC-null mice used in this study had narrower L2/3 and L3/4 discs compared to WT; reparixin had no restorative effects on disc height (Fig. 7C).
Fig. 7.
Effect of chronic inhibition of CXCR1/2 by Reparixin on body weight, activity level or disc height in SPARC-null or WT mice. Mice were treated with Reparixin (20 mg/kg, i.p.) or vehicle 3 times/week for 8 weeks. A) No statistical differences between either strain or treatment were observed in body weight, although the WT mice tended to be heavier than SPARC-null. B) No strain or treatment effects were observed in overall distance travelled in an open field at baseline or on non-treatment days during weeks 3 or 7. C) Following 8 weeks of chronic CXCR1/2 inhibition lumbar spines were imaged with X-ray and disc height index was determined. While L2/3 and L3/4 disc height was reduced in SPARC-null mice compared to WT, reparixin had no effect in either strain. Data is presented as mean ± SEM, n = 9–10 per SPARC-null groups, n = 4–6 per WT groups. Data was analyzed by two-way repeated measures ANOVA with Tukey's post hoc test post-hoc test. * = p < 0.05, ** = p < 0.01.
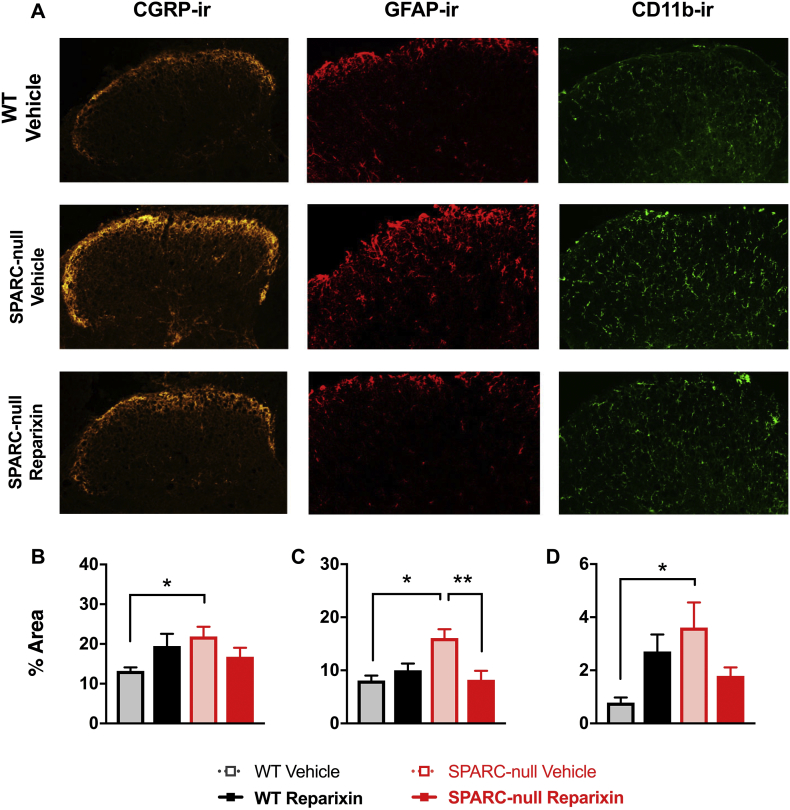
3.1.8. Chronic CXCR1/2 inhibition reduced indicators of astrocyte activity
We have previously reported pain-related neuroplastic changes in the dorsal horn of the spinal cord in SPARC-null mice compared to WT as evidenced by increases in immunoreactivity (−ir) for the sensory neuropeptide CGRP, the astrocyte marker GFAP and the microglia marker CD11b [16,39]. We investigated the effects of 8-weeks of chronic CXCR1/2 inhibition on each of these markers. Similar to previous studies, all three markers were elevated in the lumbar spinal cord dorsal horn in SPARC-null mice compared to WT (Fig. 8). Chronic CXCR1/2 inhibition had no effect on CGRP-ir (Fig. 8B) or CD11b-ir (Fig. 8D) but a significant decrease was observed in GFAP-ir in reparixin-treated vs. vehicle-treated SPARC-null mice (Fig. 8C).
Fig. 8.
Effect of chronic inhibition of CXCR1/2 by Reparixin on markers of spinal cord plasticity. After 8-weeks of chronic reparixin treatment the dorsal horn of the spinal cord was assessed for pain-related changes. Immunoreactivity (ir) for calcitonin gene-related peptide (CGRP, 1st column, a marker for sensory neurons), glia fibrillary acidic protein (GFAP, 2nd column, an astrocyte marker), and CD11b (3rd column, a microglial marker) were all elevated in vehicle-treated SPARC-null (2nd row) compared to vehicle-treated WT mice. A significant decrease in the % area of the dorsal horn positive for GPAP-ir was observed in reparixin-treated SPARC-null compared to vehicle-treated. No treatment effects were observed in WT mice or in CGRP-ir or CD11b-ir. Data is presented as mean ± SEM, n = 9–10 per SPARC-null groups, n = 4–6 per WT groups. Data was analyzed by one-way ANOVA followed by tukey's post-hoc test. * = p < 0.05, ** = p < 0.01.
3.1.9. CXCR1/2 inhibition decreases pro-inflammatory cytokine release from degenerating discs
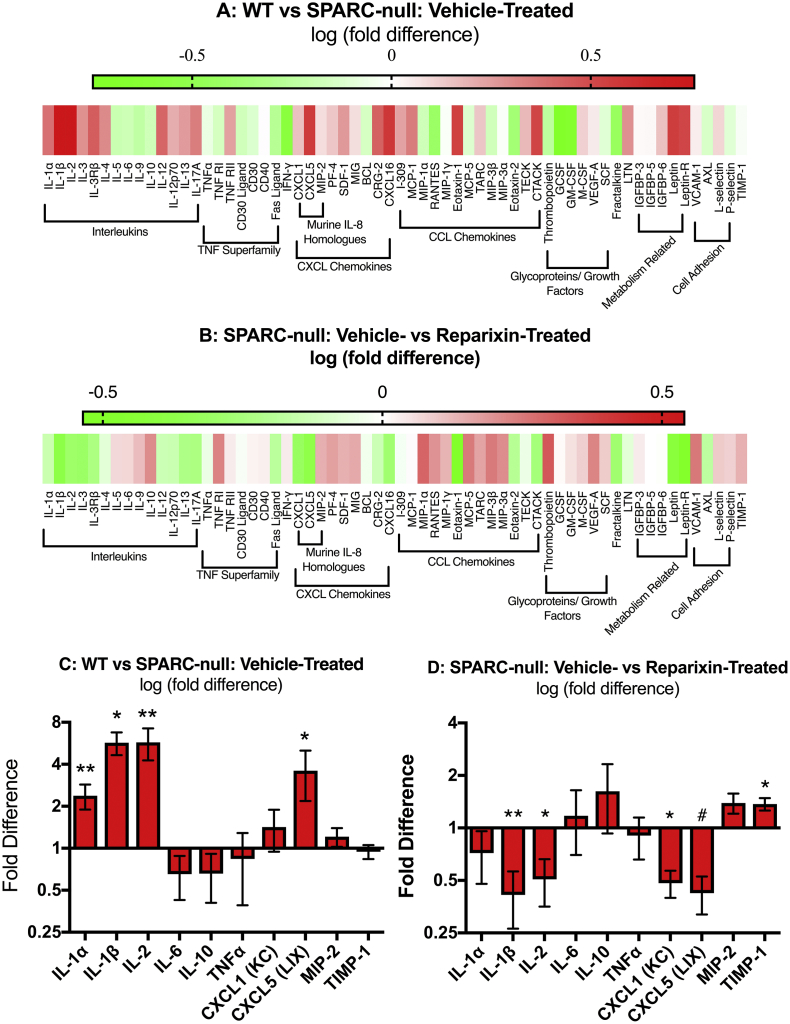
To determine if the therapeutic effects of reparixin could be mediated, in part, by acting on the intervertebral discs to decrease levels of pro-nociceptive factors, cytokine secretion was measured in cultured L1/2, L2/3 and L3/4 discs by protein arrays. As previously reported [16], discs from SPARC-null mice secrete increased levels of many pro-inflammatory cytokines including IL-1β, IL-2, CXCL5 (LIX), and CCL2 compared to WT (Fig. 9A, C). Discs from SPARC-null mice that received chronic reparixin treatment secreted decreased levels of pro-inflammatory and pro-nociceptive cytokines including IL-1β, IL-2, CXCL1(KC), and CXCL5(LIX) compared to vehicle-treated SPARC-null mice (Fig. 9B, D). Interestingly, CXCR1/2 inhibition also increased TIMP-1, an inhibitor of proteases implicated in in disc degeneration. These results demonstrate that chronic CXCR1/2 inhibition decreases overall inflammation in degenerating discs and suggest that reparixin can act directly on discs.
Fig. 9.
Effect of chronic inhibition of CXCR1/2 by Reparixin on cytokine release from degenerating discs. Following 8 weeks of reparixin treatment, the L1/2, 2/3 and 3/4 discs were excised and cultured for 48 h. Conditioned media was analyzed by protein arrays. A) Secretion of cytokines from SPARC-null discs normalized to WT discs and presented as the log of the fold-difference between the two groups. B) Secretion of cytokines from reparixin-treated vs. vehicle-treated SPARC-null discs and presented as the log of the fold-difference between the two groups. Red indicates increased secretion of a factor whereas green indicates a decrease in secretion of a factor. The mean ± SEM fold difference of select cytokines comparing discs from C) vehicle-treated SPARC-null vs. WT and D) reparixin-treated vs. vehicle-treated SPARC-null mice. Note C&D are the fold-change representations of the same data as A&B (log fold-change). n = 8 per SPARC-null groups, n = 5 per WT group. Data was analyzed by one-tail t-tests. * = p < 0.05, ** = p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Despite its enormous cost to individuals and to society, therapeutic options for LBP are limited. This is due, in part, to poor understanding of the relationship between chronic LBP and intervertebral disc degeneration. While some individuals with MRI-identified disc degeneration develop chronic LBP, others do not, suggesting that other factors, undetectable by imaging, are involved. In this study, we used CSF to identify biochemical factors that differentiate between painful and asymptomatic lumbar DD. IL-8 was significantly increased in chronic LBP subjects that report pain and disability compared to human pain-free subjects with or without DD. IL-8 and its mouse homologue, CXCL5 (LIX) were also upregulated in degenerating vs. healthy discs in humans and mice, respectively. We therefore conducted a proof-of-concept study investigating the therapeutic benefit of inhibiting the IL-8 and CXCL5 receptors, CXCR1 and 2, in the SPARC-null mouse model of progressive, disc degeneration-associated chronic back pain [26,27]. Chronic CXCR1/2 inhibition decreased behavioral signs of back pain and reduced disc inflammation following 8 weeks of treatment. These results implicate IL-8 is the pathogenesis of chronic LBP and suggest its receptors as novel therapeutic targets for discogenic low back pain.
4.1.1. Interleukin-8, low back pain and intervertebral disc pathology
Differential expression of CSF proteins could be driven by several potential sources. For example, elevated IL-8 in the CSF could be produced by activity-induced increases in neuroinflammation in dorsal root ganglia, spinal cord or supraspinal structures [[40], [41], [42]]. Alternatively, the blood-spinal cord barrier can break down following nerve injury [43,44], allowing for transport of peripheral cytokines into the CSF. Consistent with this hypothesis, we and others have previously identified markers of nerve-injury in chronic LBP patients with DD [25]. Since the intervertebral discs are adjacent to the CSF, and IL-8 is increased in the annulus fibrosus of degenerating discs from LBP subjects, leakage from painful degenerating discs into the CSF is possible. Alternatively, elevated IL-8 from CSF may diffuse into adjacent degenerating discs, which could account for the higher levels compared to non-degenerating discs.
Previous studies have investigated changes in CSF and intervertebral disc cytokine levels in patients with disc degeneration or disc herniation. In radicular pain patients with disc herniations, Brisby et al. found elevated IL-8 in CSF in a subset of patients and Ahn et al., demonstrated that increased IL-8 mRNA in herniated discs was associated with the emergence of radicular pain evoked by back extension [45]. Comparisons between tissue from degenerating vs herniated discs found IL-8 was higher in DD, leading the authors to suggest that elevated IL-8 might contribute to the more severe pain observed with DD. Most recently, Palada et al., found CSF levels of IL-8 from lumbar disc herniation patients are related to pain intensity and spinal pressure point thresholds [19]. However, these studies were limited by the lack of CSF or discs from participants without pain. Thus, they were unable to dissociate asymptomatic disc pathology from painful DD. The current findings further implicate a role for IL-8 in LBP by demonstrating its elevation in a) CSF of LBP patients vs. pain-free subjects with and without DD and b) in degenerating discs obtained from LBP patients vs. non-degeneration control discs.
The mechanisms that initiate and perpetuate disc degeneration, namely mechanical strain and sterile inflammation, have both been found to regulate IL-8 in discs. We have previously reported that adverse mechanical strain to human NP and AF cells and acute mechanical injury to ex vivo human discs increases IL-8 secretion [46,47]. Other reports of elevated IL-8 following sterile inflammation includes TLR2 activation of NP [17], TNFα treatment of AF cells [48], IL-1β treatment of NP cells [49], and TLR2 activation and TNFα and IL-1β treatment of mixed disc cells [50]. Taken together, these previous studies indicate a variety of pathological mechanisms, including adverse mechanical strain and sterile inflammation, may contribute to the elevated IL-8 levels in degenerating discs that were observed here.
While our data suggests IL-8 is involved in chronic LBP, its role in disc degeneration per se is unclear. The classically described role of IL-8 is to induce neutrophil chemotaxis and activation, and to stimulate vascularization. Infiltrating neutrophils have been found in degenerating discs, where they likely contribute to the pro-inflammatory and catabolic environment [51]. However, in one study exposure of NP cells to IL-8 did not alter expression of genes associated with disc degeneration, including aggrecan, MMP3 or MMP13 [52]. Thus, while DD drives pathways likely to upregulate IL-8, the impact of IL-8 on DD requires further investigation.
4.1.2. Interleukin-8 as a therapeutic target for disc degeneration, inflammation and low back pain
To investigate the role of IL-8 in disc degeneration, disc inflammation and low back pain in vivo we used the SPARC-null mouse model. SPARC-null mice develop progressive, age-dependent disc degeneration that is characterized by a loss of disc height, internal disc disruption and dehydration, and at later stages, disc herniation [26,33]. Behaviourally, SPARC-null mice exhibit signs of axial low back pain and radiating leg pain [26,33]; symptoms which can be attenuated by analgesic drugs, including morphine. Compared to models initiated by acute disc injury, the slow progression in SPARC-null mice more closely models the evolution in humans and is a useful pre-clinical model for long-term studies aimed at attenuating progression of disc degeneration and reducing pain.
In this proof-of-concept study, chronic, but not acute, CXCR1/2 inhibition with reparixin attenuated behavioral signs of axial discomfort and radiating leg pain in SPARC-null mice. The requirement for chronic inhibition is consistent with a disease-modifying mechanism of action, in contrast to a transient inhibitory effect on the sensory nervous system. We therefore examined the impact of chronic reparixin on disc height and cytokine expression. As expected, measurement of disc height by X-ray confirmed previous findings that L2/3 and L3/4 disc height is reduced in SPARC-null mice. The lack of recovery of disc height in the reparixin-treated mice suggests that either recovery of disc height is not necessary for the therapeutic action, the x-ray method used was not sensitive enough to detect changes, or a longer treatment is required. Regardless, the clinical literature suggests disc height is a poor proxy measure for pain intensity [53].
In addition to disc narrowing and dehydration, SPARC-null discs secrete increased levels of pro-inflammatory cytokines [16] including IL-1β and MCP-1, similar to degenerating human discs [10]. Here we show that long-term reparixin treatment reduces pro-inflammatory cytokine release from SPARC-null degenerating discs, suggesting IL-8 is a regulator of disc inflammation. However, it is currently unclear if this effect is direct or indirect. For example, reparixin could act directly on disc cells to decrease production of pro-inflammatory factors, it could reduce immune cell infiltration into discs or attenuate neurogenic inflammation by inhibiting of sensory neuron activity. Regardless, chronic CXCR1/2 inhibition decreases inflammation of the disc by breaking a pro-inflammatory feed-forward loop. The gradual effect of chronic reparixin on SPARC-null mice might reflect the time required to break the pro-inflammatory cycle.
4.1.3. Interleukin-8: possible actions beyond the intervertebral disc
While the reduction in disc inflammation is consistent with a therapeutic action within intervertebral discs, reparixin may attenuate low back pain-like behaviour through a host of other mechanisms. Murine primary nociceptive neurons and dorsal horn neurons express CXCR2 [54,55]. CXCR2 signaling on nociceptors leads to increased nociceptor activity through up regulation of the sodium channels Nav1.1 and Nav1.7 [56] and through sensitisation of TRPV1 [57]. Therefore, reparixin could act directly on primary nociceptors to decrease pain behavior.
Unlike neurons, murine astrocytes and microglia are not thought to express CXCR1/2 [54,55]. SPARC-null mice have increased staining for astrocyte and microglia [39], which we confirm here. Despite not expressing CXCR1 or 2, astrocyte staining decreases following reparixin treatment, whereas microglia are unaffected. This could reflect decreased peripheral nociceptive signaling or a decrease in spinal neuron activity. Immunoactivation of nerve roots, dorsal root ganglia and supraspinal structures have all been associated with chronic LBP in humans and represent potential therapeutic sites of reparixin [40,41]. Finally, chronic LBP is associated with inflammation in other tissues, such as the multifidus spinal muscles, which show abnormal inflammatory markers in SPARC-null mice [58]. While the mechanisms of action of reparixin and their relative impact remain to be fully elucidated; it is our hope that this proof-of-concept study serves as a stimulus for additional studies.
5. Potential limitations and future directions
Chronic pain conditions can affect one sex more than the other and sex-dependent differences in pain mechanisms have been clearly established rodent models [59,60]. While no sex differences in IL-8 content were observed in human CSF or discs, the sample size may have been too small. In mice, neither of the IL-8 homologues differed between SPARC-null and WT females, but CXCL5 (LIX) expression and release were elevated in male SPARC-null discs. Female SPARC-null mice also develop disc degeneration and behavioral signs of LBP and leg pain [33], suggesting that there may be multiple mechanisms driving pain-like behaviour between sexes. Since only male mice were used in the chronic CXCR1/2 inhibition study, future studies with female rodents are needed to determine if the results of CXCR1/2 inhibition are generalizable to females.
In this study, we did not examine the expression of the CXCR1/2 receptors in either humans or rodents, nor was IL-8 mRNA measured. It will be important to understand the cellular sources and relative contribution of both the ligands and their receptors in future studies.
This study is limited by the use of a global deletion of the SPARC gene that can alter other tissues. For example, SPARC-null mice have osteopenia, decreased dermal strength and impaired wound healing and early onset cataractogenesis [32,61]. Interestingly, the SPARC-null mouse model is not associated with knee arthritis and SPARC is increased in arthritic cartilage [62]. This is in contrast to human disc degeneration, where SPARC levels decrease [63]. While we cannot rule out contributions from other tissues, the SPARC-null behavioral phenotype has regional specificity (e.g. increased sensitivity in hindpaw but not in facial area) [26]. Regardless, the contribution of IL-8 should be tested in additional models of disc-related LBP with complementary mechanisms such as the disc puncture models [64,65].
Although disc degeneration is age-dependent, the proof-of-concept study was conducted only in middle-aged rodents. At the age used in the study (7–9 months), SPARC-null mice have at least moderate degeneration in multiple lumbar discs but disc rupture is rare. This models a clinical situation where pain and DD are established but most discs are not severely compromised. It will be interesting to explore the role of and therapeutic value of the IL-8 system earlier in disc pathology as a prophylactic tool as well as in larger animals or in naturally occurring models of degeneration.
6. Conclusions
Here we show that IL-8 is elevated in the CSF of chronic low back pain subjects with disc degeneration compared to subjects without chronic low back pain, regardless of disc degeneration. IL-8 is also increased in degenerating discs obtained from chronic low back pain patients compared to non-degenerating disc tissue. Inhibition of the IL-8 receptor system reduced signs of disc degeneration and chronic back pain in a mouse model. These results suggest that IL-8 may be a key difference between painful and non-painful disc pathology, suggesting this pathway as a therapeutic target to slow the progression of disc degeneration and reduce pain.
Funding sources
The study sponsors(s) had no role in study design, in the collection, analysis or interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
Declaration of interests
None.
Author contributions
Study conception or design – EK, MM, KMA, TER, GLW, KGB, DSB, JO, MRP, LJK, LH, LSS; Funding acquisition – EK, KMA, TER, GLW, KGB, DSB, JO, LJK, LH, LSS; Data acquisition – EK, MM, KMA, AS, TER, PH, YRS, SHJ, KGB, DSB, MRP, LJK, LH, LSS; Data analysis – EK, KMA, AS, TER, PH, YRS, SHJ, LJK, LH, LSS; Interpretation of data – EK, MM, KMA, AS, PH, YRS, SHJ, GW, JO, LJK, LH, LSS; Writing and/or revision – EK, MM, KMA, AS, TER, PH, YRS, SHJ, GLW, KGB, DSB, JO, MRP, LJK, LH, LSS; Study supervision – EK, MM, KMA, TER, JO, MRP, LJK, LH, LSS; Project administration – EK, KMA, TER, GLW, JO, LJK, LH, LSS.
Acknowledgements
This study was supported by grants from the National Institutes for Health (R21 DA020108) and the Minnesota Medical Foundation to LJK and LSS, Canadian Institutes of Health Research (CIHR) operating grants to LSS (MOP-126046), to LH and LSS (PJT-148678 and MOP-142291), and to LSS and MM (MOP-126046), and a Fonds de recherche du Québec – Santé (FRQS) Doctoral Award to EK. The authors would like to thank Ms. Janet Moir and Ms. Lina Naso for technical support throughout the course of this project.
Contributor Information
Emerson Krock, Email: emerson.krock@ki.se.
Magali Millecamps, Email: magali.millecamps@mcgill.ca.
Kathleen M. Anderson, Email: ander646@umn.edu.
Akanksha Srivastava, Email: akanksha.srivastava@mail.mcgill.ca.
Troy E. Reihsen, Email: treihsen@uw.edu.
Pawan Hari, Email: pawan.Hari@nyumc.org.
Yue Ran Sun, Email: yue.ran.sun@mail.mcgill.ca.
Seon Ho Jang, Email: seonho.jang@mail.mcgill.ca.
George L. Wilcox, Email: George@umn.edu.
Kumar G. Belani, Email: belan001@umn.edu.
David S. Beebe, Email: beebe001@umn.edu.
Manuel R. Pinto, Email: mrpinto@tcspine.com.
Lois J. Kehl, Email: kehlx001@umn.edu.
Lisbet Haglund, Email: lisbet.haglund@mcgill.ca.
Laura S. Stone, Email: laura.s.stone@mcgill.ca.
References
- 1.Medicine Io . The National Academies Press; Washington, D.C.: 2011. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study C Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou R., Deyo R., Friedly J. Nonpharmacologic therapies for low Back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166:493–505. doi: 10.7326/M16-2459. [DOI] [PubMed] [Google Scholar]
- 4.Chou R., Deyo R., Friedly J. Systemic pharmacologic therapies for low Back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166:480–492. doi: 10.7326/M16-2458. [DOI] [PubMed] [Google Scholar]
- 5.Deyo R.A., Dworkin S.F., Amtmann D. Focus article: report of the NIH task force on research standards for chronic low Back pain. Eur Spine J. 2014;23:2028–2045. doi: 10.1007/s00586-014-3540-3. [DOI] [PubMed] [Google Scholar]
- 6.Raj P.P. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8:18–44. doi: 10.1111/j.1533-2500.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 7.Boden S.D., Davis D.O., Dina T.S., Patronas N.J., Wiesel S.W. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 8.Le Maitre C.L., Freemont A.J., Hoyland J.A. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 9.Patel K.P., Sandy J.D., Akeda K. Aggrecanases and aggrecanase-generated fragments in the human intervertebral disc at early and advanced stages of disc degeneration. Spine (Phila Pa 1976) 2007;32:2596–2603. doi: 10.1097/BRS.0b013e318158cb85. [DOI] [PubMed] [Google Scholar]
- 10.Krock E., Rosenzweig D.H., Chabot-Doré A.-J.J. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. J Cell Mol Med. 2014;18:1213–1225. doi: 10.1111/jcmm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freemont A.J., Watkins A., Le Maitre C. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286–292. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 12.Le Maitre C.L., Hoyland J.A., Freemont A.J. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purmessur D., Freemont A., Hoyland J. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10 doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seguin C.A., Bojarski M., Pilliar R.M., Roughley P.J., Kandel R.A. Differential regulation of matrix degrading enzymes in a TNFalpha-induced model of nucleus pulposus tissue degeneration. Matrix Biol. 2006;25:409–418. doi: 10.1016/j.matbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Krock E., Currie B.J., Weber M.H. Nerve growth factor is regulated by toll-like receptor 2 in human intervertebral discs. J Biol Chem. 2016;291:3541–3551. doi: 10.1074/jbc.M115.675900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krock E., Millecamps M., Currie J.B., Stone L.S., Haglund L. Low back pain and disc degeneration are decreased following chronic toll-like receptor 4 inhibition in a mouse model. Osteoarthr Cartil. Sep 2018;26(9):1236–1246. doi: 10.1016/j.joca.2018.06.002. (Epub 2018 Jun 18) [DOI] [PubMed] [Google Scholar]
- 17.Krock E., Rosenzweig D.H., Currie J.B., Bisson D.G., Ouellet J.A., Haglund L. Toll-like receptor activation induces degeneration of human intervertebral discs. Sci Rep. 2017;7 doi: 10.1038/s41598-017-17472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krock E., Rosenzweig D.H., Haglund L. The inflammatory milieu of the degenerate disc: is Mesenchymal stem cell-based therapy for intervertebral disc repair a feasible approach? Curr Stem Cell Res Ther. 2015;10(4):317–328. doi: 10.2174/1574888x10666150211161956. [DOI] [PubMed] [Google Scholar]
- 19.Palada V., Ahmed A.S., Finn A., Berg S., Svensson C.I., Kosek E. Characterization of neuroinflammation and periphery-to-CNS inflammatory cross-talk in patients with disc herniation and degenerative disc disease. Brain Behav Immun. 2019;75:60–71. doi: 10.1016/j.bbi.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Brisby H., Olmarker K., Larsson K., Nutu M., Rydevik B. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002;11:62–66. doi: 10.1007/s005860100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brisby H., Olmarker K., Rosengren L., Cederlund C.G., Rydevik B. Markers of nerve tissue injury in the cerebrospinal fluid in patients with lumbar disc herniation and sciatica. Spine (Phila Pa 1976) 1999;24:742–746. doi: 10.1097/00007632-199904150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Liu X.D., Zeng B.F., Xu J.G., Zhu H.B., Xia Q.C. Proteomic analysis of the cerebrospinal fluid of patients with lumbar disk herniation. Proteomics. 2006;6:1019–1028. doi: 10.1002/pmic.200500247. [DOI] [PubMed] [Google Scholar]
- 23.Skouen J.S., Larsen J.L., Gjerde I.O., Hegrestad S.E., Vollset S.E. Cerebrospinal fluid protein concentrations in patients with sciatica caused by lumbar disc herniation: an investigation of biochemical, neurologic, and radiologic predictors of long-term outcome. J Spinal Disord. 1997;10:505–511. [PubMed] [Google Scholar]
- 24.Ohtori S., Suzuki M., Koshi T. Proinflammatory cytokines in the cerebrospinal fluid of patients with lumbar radiculopathy. Eur Spine J. 2011;20:942–946. doi: 10.1007/s00586-010-1595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim T.K.Y., Anderson K.M., Hari P. Evidence for a role of nerve injury in painful intervertebral disc degeneration: a cross-sectional proteomic analysis of human cerebrospinal fluid. J Pain. Oct 2017;18(10):1253–1269. doi: 10.1016/j.jpain.2017.06.002. (Epub 2017 Jun 24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millecamps M., Tajerian M., Naso L., Sage E.H., Stone L.S. Lumbar intervertebral disc degeneration associated with axial and radiating low back pain in ageing SPARC-null mice. Pain. 2012;153:1167–1179. doi: 10.1016/j.pain.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Millecamps M., Tajerian M., Sage E.H., Stone L.S. Behavioral signs of chronic back pain in the SPARC-null mouse. Spine. 2011;36:95–102. doi: 10.1097/BRS.0b013e3181cd9d75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfirrmann C.W., Metzdorf A., Zanetti M., Hodler J., Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 29.Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 30.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 31.Fairbank J.C., Pynsent P.B. The Oswestry disability index. Spine. 2000;25:2940–2952. doi: 10.1097/00007632-200011150-00017. [discussion 52] [DOI] [PubMed] [Google Scholar]
- 32.Norose K., Clark J.I., Syed N.A. SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci. 1998;39:2674–2680. [PubMed] [Google Scholar]
- 33.Millecamps M., Czerminski J.T., Mathieu A.P., Stone L.S. Behavioral signs of axial low back pain and motor impairment correlate with the severity of intervertebral disc degeneration in a mouse model. Spine J. 2015;15:2524–2537. doi: 10.1016/j.spinee.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 34.Bertini R., Allegretti M., Bizzarri C. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:11791–11796. doi: 10.1073/pnas.0402090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarbock A., Allegretti M., Ley K. Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung injury in mice. Br J Pharmacol. 2008;155:357–364. doi: 10.1038/bjp.2008.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tajerian M., Millecamps M., Stone L.S. Morphine and clonidine synergize to ameliorate low back pain in mice. Pain Res Treat. 2012;2012 doi: 10.1155/2012/150842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 38.Jensen M.C., Brant-Zawadzki M.N., Obuchowski N., Modic M.T., Malkasian D., Ross J.S. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 39.Miyagi M., Millecamps M., Danco A.T., Ohtori S., Takahashi K., Stone L.S. ISSLS prize winner: increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine (Phila Pa 1976) 2014;39:1345–1354. doi: 10.1097/BRS.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 40.Loggia M.L., Chonde D.B., Akeju O. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138:604–615. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albrecht D.S., Ahmed S.U., Kettner N.W. Neuroinflammation of the spinal cord and nerve roots in chronic radicular pain patients. Pain. 2018;159:968–977. doi: 10.1097/j.pain.0000000000001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amaya F., Samad T.A., Barrett L., Broom D.C., Woolf C.J. Periganglionic inflammation elicits a distally radiating pain hypersensitivity by promoting COX-2 induction in the dorsal root ganglion. Pain. 2009;142:59–67. doi: 10.1016/j.pain.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim T.K.Y., Shi X.Q., Martin H.C. Blood-nerve barrier dysfunction contributes to the generation of neuropathic pain and allows targeting of injured nerves for pain relief. PAIN®. 2014;155:954–967. doi: 10.1016/j.pain.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 44.Echeverry S., Shi X.Q., Rivest S., Zhang J. Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J Neurosci. 2011;31:10819–10828. doi: 10.1523/JNEUROSCI.1642-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn S.H., Cho Y.W., Ahn M.W., Jang S.H., Sohn Y.K., Kim H.S. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911–917. doi: 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- 46.Gawri R., Rosenzweig D.H., Krock E. High mechanical strain of primary intervertebral disc cells promotes secretion of inflammatory factors associated with disc degeneration and pain. Arthritis Res Ther. 2014;16:R21. doi: 10.1186/ar4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alkhatib B., Rosenzweig D.H., Krock E. Acute mechanical injury of the human intervertebral disc: link to degeneration and pain. Eur Cell Mater. 2014;28:98–111. doi: 10.22203/ecm.v028a08. [DOI] [PubMed] [Google Scholar]
- 48.Likhitpanichkul M., Torre O.M., Gruen J., Walter B.A., Hecht A.C., Iatridis J.C. Do mechanical strain and TNF-α interact to amplify pro-inflammatory cytokine production in human annulus fibrosus cells? J Biomech. 2016;49:1214–1220. doi: 10.1016/j.jbiomech.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniels J., Binch A.A.L., Le Maitre C.L. Inhibiting IL-1 signaling pathways to inhibit catabolic processes in disc degeneration. J Orthop Res. 2017;35:74–85. doi: 10.1002/jor.23363. [DOI] [PubMed] [Google Scholar]
- 50.Klawitter M., Hakozaki M., Kobayashi H. Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells. Eur Spine J. 2014;23:1878–1891. doi: 10.1007/s00586-014-3442-4. [DOI] [PubMed] [Google Scholar]
- 51.Risbud M.V., Shapiro I.M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2013;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips K.L.E., Cullen K., Chiverton N. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: interleukin-1 is a master regulator of catabolic processes. Osteoarthr Cartil. 2015;23:1165–1177. doi: 10.1016/j.joca.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Dabbs V.M., Dabbs L.G. Correlation between disc height narrowing and low-back pain. Spine (Phila Pa 1976) 1990;15:1366–1369. doi: 10.1097/00007632-199012000-00026. [DOI] [PubMed] [Google Scholar]
- 54.Cao D.L., Zhang Z.J., Xie R.G., Jiang B.C., Ji R.R., Gao Y.J. Chemokine CXCL1 enhances inflammatory pain and increases NMDA receptor activity and COX-2 expression in spinal cord neurons via activation of CXCR2. Exp Neurol. 2014;261:328–336. doi: 10.1016/j.expneurol.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z.J., Cao D.L., Zhang X., Ji R.R., Gao Y.J. Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain. 2013;154:2185–2197. doi: 10.1016/j.pain.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J.-G., Strong J.A., Xie W. The chemokine CXCL1/growth related oncogene increases sodium currents and neuronal excitability in small diameter sensory neurons. Mol Pain. 2008;4 doi: 10.1186/1744-8069-4-38. [1744-8069-4-38] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong F., Du Y.R., Xie W., Strong J.A., He X.J., Zhang J.M. Increased function of the TRPV1 channel in small sensory neurons after local inflammation or in vitro exposure to the pro-inflammatory cytokine GRO/KC. Neurosci Bull. 2012;28:155–164. doi: 10.1007/s12264-012-1208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.James G., Millecamps M., Stone L.S., Hodges P.W. Dysregulation of the inflammatory mediators in the Multifidus muscle after spontaneous intervertebral disc degeneration SPARC-null mice is ameliorated by physical activity. Spine. Oct 15 2018;43(20):E1184–E1194. doi: 10.1097/BRS.0000000000002656. [DOI] [PubMed] [Google Scholar]
- 59.Sorge R.E., LaCroix-Fralish M.L., Tuttle A.H. Spinal cord toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woller S.A., Ravula S.B., Tucci F.C. Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: the role of TLR4 in the evolution of a persistent pain state. Brain Behav Immun. 2016;56:271–280. doi: 10.1016/j.bbi.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delany A.M., Amling M., Priemel M., Howe C., Baron R., Canalis E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J Clin Invest. 2000;105:1325. doi: 10.1172/JCI7039C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nanba Y., Nishida K., Yoshikawa T., Sato T., Inoue H., Kuboki Y. Expression of osteonectin in articular cartilage of osteoarthritic knees. Acta Med Okayama. 1997;51:239–243. doi: 10.18926/AMO/30790. [DOI] [PubMed] [Google Scholar]
- 63.Gruber H.E., Ingram J.A., Leslie K., Hanley E.N., Jr. Cellular, but not matrix, immunolocalization of SPARC in the human intervertebral disc: decreasing localization with aging and disc degeneration. Spine (Phila Pa 1976) 2004;29:2223–2228. doi: 10.1097/01.brs.0000142225.07927.29. [DOI] [PubMed] [Google Scholar]
- 64.Mosley G.E., Evashwick-Rogler T.W., Lai A., Iatridis J.C. Looking beyond the intervertebral disc: the need for behavioral assays in models of discogenic pain. Ann N Y Acad Sci. 2017;1409:51–66. doi: 10.1111/nyas.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi C., Qiu S., Riester S.M. Animal models for studying the etiology and treatment of low back pain. J Orthop Res. 2018;36:1305–1312. doi: 10.1002/jor.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]