Abstract
Empathetic verbal feedback from others has been shown to alleviate the intensity of experimental pain. To investigate the brain changes associated with this effect, we conducted 3T-fMRI measurements in 30 healthy subjects who received painful thermal stimuli on their left hand while overhearing empathetic, neutral or unempathetic comments, supposedly made by experimenters, via headsets. Only the empathetic comments significantly reduced pain intensity ratings. A whole-brain BOLD analysis revealed that both Empathetic and Unempathetic conditions significantly increased the activation of the right anterior insular and posterior parietal cortices to pain stimuli, while activations in the posterior cingulate cortex and precuneus (PCC/Prec) were significantly stronger during Empathetic compared to Unempathetic condition. BOLD activity increased in the DLPFC in the Empathetic condition and decreased in the PCC/Prec and vmPFC in the Unempathetic condition. In the Empathetic condition only, functional connectivity increased significantly between the vmPFC and the insular cortex. These results suggest that modulation of pain perception by empathetic feedback involves a set of high-order brain regions associated with autobiographical memories and self-awareness, and relies on interactions between such supra-modal structures and key nodes of the pain system.
Subject terms: Sensory processing, Empathy
Introduction
Humans have the capacity to estimate each others’ pain and to provide adapted care in order to reduce it. It is largely assumed that empathetic skills are crucial for caregivers involved in pain management1–4; consequently, educational programs and theories have emphasized the positive role of empathy to reduce pain intensity. It is also widely assumed that if caregivers lack empathy, they will underestimate pain intensity in their patients and this unempathetic attitude can negatively influence pain intensity perception. In a recent study, we addressed this issue by creating a dedicated setup mimicking a medical environment where people enduring painful stimuli received empathetic or unempathetic comments from others. Positive (empathetic) feedback was able to reduce pain intensity perception, in agreement with previous theoretical models5,6 and experimental studies7, while negative (unempathetic) feedback did not induce consistent changes in pain intensity reports8.
Here, we transposed the experimental setup employed in our preceding behavioral study8 to functional magnetic resonance imaging (fMRI). To heighten the realism, healthy subjects placed in the scanner received thermal noxious stimuli while overhearing positive, neutral or negative comments about them, made by the experimenters, via an ‘inadvertently’ switched-on communication device. To our knowledge, no neuroscientific study has experimentally investigated the neural mechanisms mediating the effects of empathetic or unempathetic feedback on pain. BOLD (Blood Oxygen Level Dependent) activity and functional connectivity (FC) changes related to pain intensity perception were compared according to these three experimental conditions. Our objective was to investigate how empathetic or unempathetic comments could interact with the brain structures underlying the assessment of pain intensity.
The current concepts about cortical pain integration consider that there is, in the conscious appraisal of pain, a need to integrate sensory encoding with stimulus salience, executive control, memory encoding and self-awareness9–13. Thus, the neural mechanism of pain can be conceptualized as a system composed of multiple functional modules of brain regions interacting together to achieve different subgoals9,11,14. A ‘nociceptive subnetwork’ responsible for processing the sensory aspect of pain includes regions receiving input from the ascending spinothalamic tract (e.g., posterior operculo-insular regions and S1). A further set of subnetworks recruited by nociceptive input can support the ‘salience’ attributes of noxious stimuli, trigger top-down cognitive controls, and ensure the conscious evaluation of pain intensity (e.g., anterior insula, mid cingulate cortex, dorsolateral prefrontal and posterior parietal cortices). Affective-cognitive processes including, expectations, beliefs, contextual factors, and self-awareness can still modulate the conscious experience of pain via activity in supramodal regions with widespread cortical projections (e.g., ALPFC, posterior parietal cortex, precuneus, posterior cingulate cortex). Pain modulation by empathy or pleasant/unpleasant contexts tends to change activity in higher order executive and salience networks15,16 and sometimes secondarily in sensory cortices17. A few studies have examined the brain mechanisms associated with receiving social support18 from the participant’s romantic partner (i.e., the effect of viewing pictures19, holding the hand20 or the physical presence in the room21) during a pain experience. Brain activity associated with pain reduction in these conditions was found in areas responding to “safety” (i.e., the ventromedial prefrontal cortex and the posterior cingulate cortex)18 and self-regulatory processes (i.e., dorsolateral prefrontal cortex)20. A recent work by Hein and colleagues22 pointed out that pain relief provided by an outgroup member decreased right anterior insula activity.
Since the pain experience results from the interaction of sensory brain areas with higher-order brain networks9,10,23, we postulated that empathetic feedback would modulate pain-related responses through changes in these high-level brain areas associated with inferring and representing states of self and other awareness24. We hypothesised that brain areas (i) responding to “safety” (i.e., the ventromedial prefrontal cortex and the posterior cingulate cortex); (ii) implicated in self-regulatory processes (i.e., dorsolateral prefrontal cortex) and (iii) contextual processing (i.e., anterior insula) would be involved in the cerebral mechanisms related to the reappraisal of pain perception by others’ empathetic comments.
Results
Brain responses to verbal and pain thermal stimuli over the entire experiment
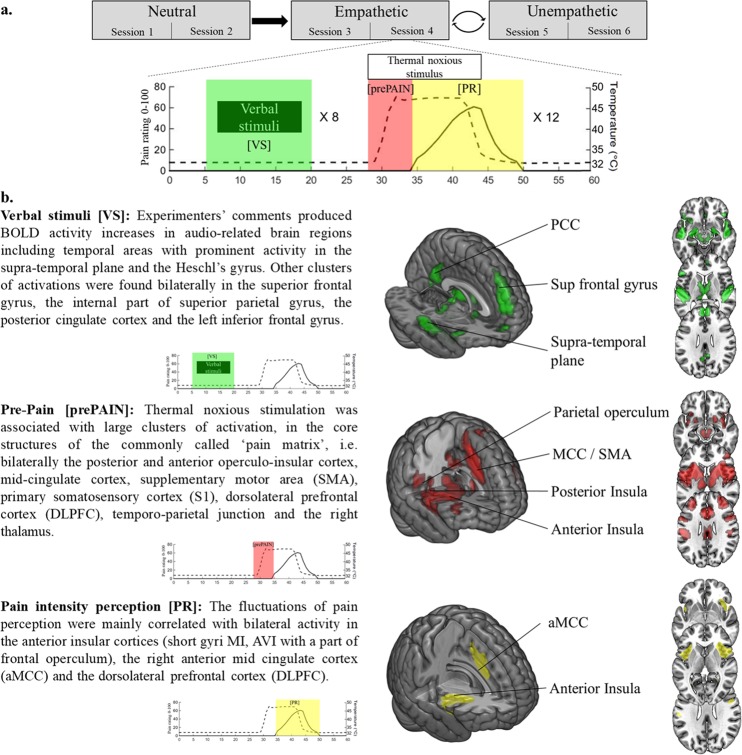
Brain activity related to experimenters’ comments, thermal noxious stimuli, and fluctuation of pain intensity perception were examined for the whole fMRI experiment (Fig. 1a) across all the conditions, and thresholded at p < 0.01 corrected (family-wise error, FWE voxel level). Verbal stimuli [VS] were associated with brain activity in the auditory network. Thermal noxious stimuli [prePAIN] activated cortical areas commonly related to nociceptive stimuli (i.e., the posterior and anterior operculo-insular cortex, mid-cingulate cortex, SMA, primary motor and somatosensory cortex) while conscious pain intensity ratings [PR] were mainly associated with brain activity changes in the anterior mid-cingulate (aMCC), anterior insula and dorsolateral prefrontal cortex (DLPFC). More details are reported in Fig. 1b. For the full list of activated brain regions see Supplementary Table 1.
Figure 1.
Brain activations related-to pain stimulations and audio comments over the whole experiment. (a) The MRI task was split in six sessions of 8 min (i.e., two successive sessions for each experimental condition). Empathetic and Unempathetic conditions were randomly assigned between subjects. Each session was composed of 12 thermal noxious stimuli alternating with 8 verbal stimuli. For fMRI analysis, three regressors were considered: verbal stimuli (green; [VS]), noxious thermal stimulation (red; [prePAIN]) and ratings of pain intensity perception on a scale of 0–100, where 0 is no pain and 100 is maximum imaginable pain (yellow; [PR]). (b) [VS] and [prePAIN] are associated with brain activity in the core areas of the auditory and pain networks. Pain intensity ratings [PR] are mainly associated with brain activity changes in the anterior mid-cingulate (aMCC), anterior insula, and dorsolateral prefrontal cortex (DLPFC). Thresholded at p < 0.01, FWE corrected.
Effect of empathetic conditions on behavioural scores and pain brain activity
Pain perception scores
Pain ratings varied significantly according to experimental conditions (F(2,28) = 3.89; p = 0.026) without interaction between condition and order of presentation (F(2,28) = 0.59; ns). Post-hoc tests showed a significant decrease of pain ratings in the Empathetic condition (PRmax = 50.41) as compared to both the Neutral (PRmax = 58.10; −13% p = 0.007) and Unempathetic conditions (PRmax = 55.23; −9% p = 0.047). No difference was found between Unempathetic and Neutral conditions (p = 0.23). Figure 2a depicts these findings. The latency from the onset of the thermal noxious stimuli to the onset of pain ratings was also computed. No significant influence of experimental conditions (F(2,28) = 2.50; p = 0.08) on subject’s pain perception response latency was found.
Figure 2.
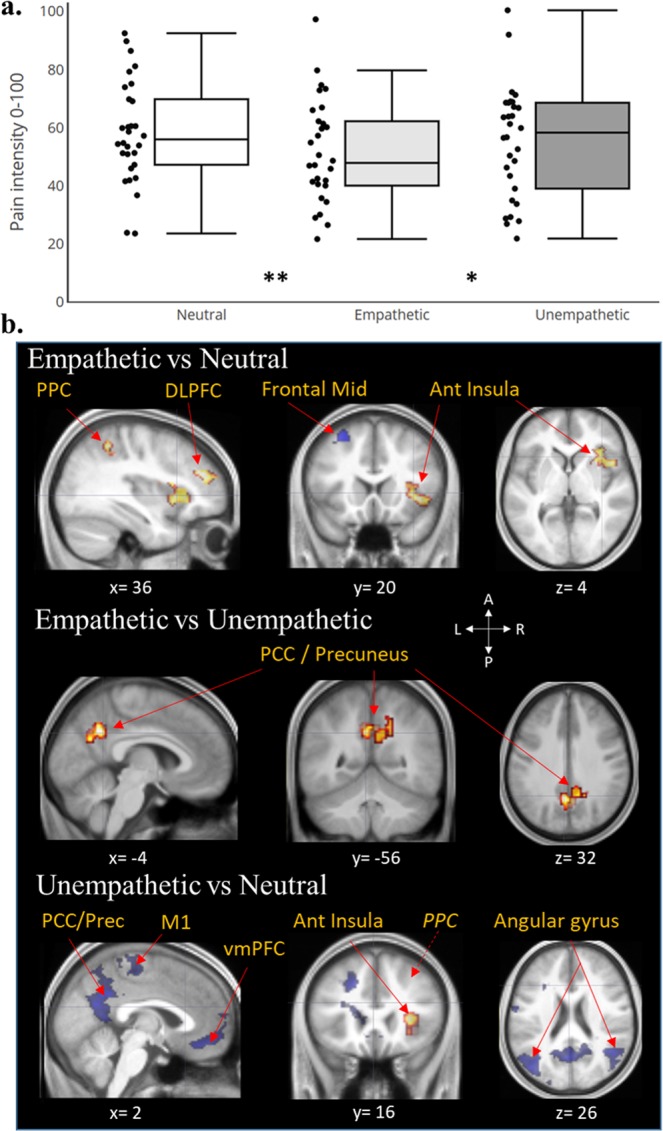
Brain correlates associated to experimental conditions effects on pain intensity perception. (a) Box plots (with median and quartile) and scatter plots of individual pain scores related to experimental conditions. Empathetic condition reduced significantly pain ratings as compared to Neutral (**p < 0.01) and Unempathetic conditions (*p < 0.05). (b) Activations (red: E or U > N) and deactivations (blue: N > E or U) superimposed on the anatomical scan averaged across all subjects. Empathetic and Unempathetic feedbacks from others both activated the right anterior insular cortex and the right posterior parietal cortex (PPC) compared to the Neutral condition. In addition, empathetic comments increased activity in the right dorso-lateral prefrontal cortex (DLPFC) and decreased the middle frontal gyrus whereas an Unempathetic situation induced mainly a deactivation in the default mode network structures. Only, the activity of the Posterior Cingulate Cortex/Precuneus (PCC/Prec) distinguished between these two opposite contexts (E > U). Statistical maps are thresholded at FWE-corrected cluster-based p < 0.05 after voxel threshold at p < 0.001.
Brain activity related to experimental conditions during pain perception
Empathetic feedback
During the Empathetic condition, BOLD activity associated with pain intensity increased significantly in the: right anterior insular cortex, right posterior parietal cortex (PPC) and DLPFC, while it decreased significantly in the left middle frontal gyrus in the Empathetic condition as compared to Neutral control (t(30) > 5.66). Compared to the Unempathetic condition, pain intensity ratings during the Empathetic condition were specifically associated with bilateral activity increases in the ventral posterior cingulate cortex (PCC) and the precuneus (Prec) including a slight overlap with the inferior parietal lobule (IPL) and the temporoparietal junction (TPJ) (t(30) > 3.75; Fig. 2b).
Unempathetic feedback
During the Unempathetic condition, BOLD activity associated with pain intensity increased significantly in the right: anterior insula (short gyrus), the IPL part of the PPC (t(30) > 5.02) and decreased bilaterally in the angular gyrus, precuneus, posterior cingulate cortex, ventro medial prefrontal cortex (orbital and subgenual), hippocampus, the middle part of temporal gyrus and the right primary motor cortex (t(30) > 5.63; Fig. 2b), relative to the Neutral condition. See Supplementary Table 2 for more details on brain activations’ localisations.
Modulation of FC related to pain in experimental conditions
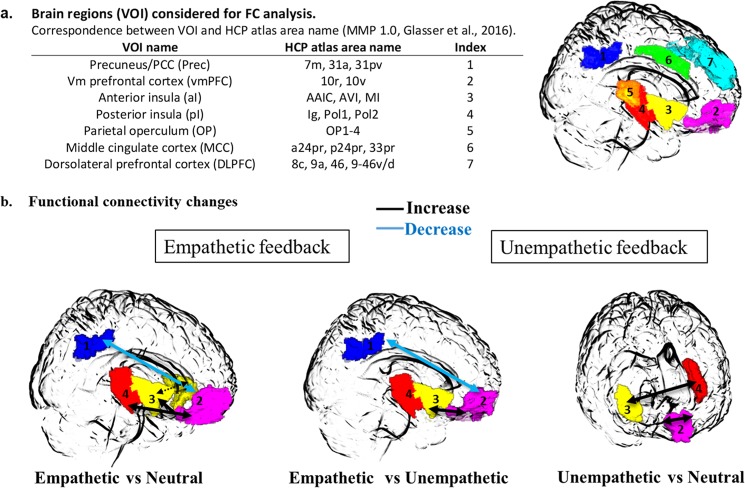
The main activation clusters that differentiated the Experimental conditions (Fig. 2b) were the anterior insula (aI), the posterior cingulate cortex/Precuneus (PCC/Prec), the dorsolateral prefrontal cortex (DLPFC) and the medial prefrontal cortex (vmPFC) including the subgenual part of the anterior cingulate. We also included brain structures associated with pain stimuli that corresponded mainly in our experiment to the mid-cingulate cortex (MCC), the parietal operculum (OP) and the posterior insula (pI). The 7 brain regions (PO, pI, aI, MCC, PCC/Prec, vmPFC, DLPFC) considered bilaterally for FC analysis are depicted on Fig. 3a.
Figure 3.
Functional connectivity (FC) changes according to experimental conditions during pain stimulation. (a) List of brain regions considered for FC comparison analysis projected on a glass brain with HCP correspondences for pain-stimulation based pairwise correlation matrix (14 × 14). (b) The three 3D-glass brains represent the FC changes for the paired comparisons of experimental conditions. Empathetic feedback induced significant increase of connectivity between vmPFC and both posterior and anterior insula, between both anterior insular cortices (dotted line) and a FC decrease between vmPFC and PCC/Prec relative to the Neutral and Unempathetic conditions. Only FC changes between vmPFC and pI was not significant as compared to the Unempathetic condition. Unempathetic feedbacks induced only inter-hemispheric increases of FC between both vmPFC, and between aI and pI (p < 0.05, FP-corrected).
Empathetic feedback
Most of the changes in FC concerned the comparisons between Empathetic minus Neutral conditions: In the Empathetic condition time-series correlations increased between right pI and bilateral vmPFC areas, between both anterior insular cortices, between right anterior insula and right vmPFC (t(30) > 2.90, p < 0.007). The FC between left vmPFC and right PCC/Prec (t(30) = −3.27, p = 0.003; Fig. 3b).
FC increased significantly in empathetic conditions between left anterior insula and right vmPFC and decreased between right PCC/Prec and left vmPFC (t(30) = 3.51, p < 0.001) as compared to the Unempathetic conditions.
Unempathetic feedback
Comparing the Unempathetic and Neutral conditions, time-series correlation increased significantly between bilateral left and right vmPFC and between right anterior insula and left posterior insula (t(30) > 2.85, p < 0.008; Fig. 3b).
Discussion
On behavioral grounds, this study confirms previous results indicating that receiving empathetic feedback from others can decrease the perceived intensity of pain, whereas unempathetic feedback appeared less powerful to enhance pain ratings, at least in our experimental conditions. The average magnitude of pain relief (−12%) is also consistent with results reported in our previous behavioral study8 and gives further evidence for the beneficial effects of empathetic support during acute pain. Furthermore, the present study provides some insight on the neural mechanisms by which empathetic support may modulate pain perception.
The key cerebral areas of the pain network10,12,25,26 and auditory network27 were vigorously activated by, respectively, the thermal noxious stimuli and the verbal comments (Fig. 1b & Supplementary Table 1). The introduction of two separate regressors, one corresponding to the period between stimulus onset and pain threshold [prePAIN], and the other restricted to the window where subjective pain ratings were produced [PR], permitted identification of brain regions associated with the initial reception of the stimulus, the subjective pain perception, or both (Fig. 1b). Thus, areas receiving spinothalamic input such as the posterior operculo-insular cortex and posterior MCC were active during the first portion of the evaluation ([prePAIN], stimulus detection) but were not significantly activated in connection with the regressor strictly associated with the conscious assessment of pain [PR], which in turn yielded significant bilateral activation of a smaller network comprising the anterior insula (a-Ins), the dorsolateral and anterolateral PFC, and the anterior mid-cingulate cortex (aMCC). This result is consistent with previous studies28–30 indicating that the coding of the conscious sensation of pain may be dissociated from the initial “nociceptive network” composed of sensory and motor/premotor areas9,10,31. This is highlighted by the results of contrast: [prePAIN] > [PR], and shown in Supplementary Fig. 4. These ‘later acting’ cerebral regions are not only associated with working memory, but also appear to be involved in the cognitive act of rating pain magnitude, and may participate in pain control32. The neural mechanism underlying conscious pain can be conceptualized as a fluid system composed of several interacting networks with different temporal dynamics9–11,23. The a-Ins, DLPFC, aMCC and PPC are activated later than the first somatosensory network, which receives the noxious inputs from the periphery14. These brain regions also support the transition to conscious awareness, transforming nociception into a pain sensation that may be influenced by multiple cognitive modulations (e.g., attentional, anticipative, and contextual-such as receiving empathetic support) from supramodal structures outside this network9.
The activity of the right a-Ins and right PPC (including the inferior parietal lobe and temporo-parietal junction) increased during both the Empathetic and Unempathetic conditions. Hence, these structures appeared to be globally influenced by the emotional context and did not dissociate positive from negative feedback, in accordance with their participation to a non-specific ‘salience’ network33–35. The engagement of the PPC may support the subject’s attentional shift to relevant (positive or negative) feedbacks36. The aI is a multimodal cortex37, generally recruited by components of the environment that are behaviorally relevant. Accordingly, neuroimaging studies have repeatedly shown enhancement of aI activity by the presentation of emotional stimuli (e.g. pictures, sounds, etc.) either positive38–40 or negative41,42. Although, this region is considered to play an important role in pain processing43, its activity is certainly not specific to pain10,35,37, and in our experiments did not reflect the modulation of pain scores by empathetic comments. Unchanged aI activity despite profound changes in subjective pain rating was previously reported during tasks involving highly emotional contexts; e.g., where pain rating was changed by religious beliefs44 or through observation of pain in others (compassional hyperalgesia)16.
In our experiments, the enhanced BOLD activity in the PCC/Precuneus, vmPFC and DLPFC was related to a decrease of pain intensity ratings in the Empathetic condition, whereas activities in the same regions decreased in the Unempathetic condition, as compared to Neutral control. These structures, in particular the two former, have been associated with different functions involved in the processing of self-oriented attention and autobiographical recall45–47, and are also known to play a central role in our ability to represent others’ emotional state48–51 (e.g., perspective taking and “theory of mind”). In addition, engagement of PCC/Prec and vmPFC has previously been shown in subjects receiving support from others during negative situations such as stress, threat or pain18,20. The opposite behavior of these regions in the Empathetic and Unempathetic conditions may suggest a differential integration of the experimenters’ comments, according to their valence52, in processes associated with the self and participants’ memories. This differential activity in fronto-parietal brain structures appears to be involved to the reappraisal of pain experience.
One intriguing, albeit robust, result was the decrease of functional connectivity (FC) between posterior and anterior midline regions (PCC/Prec and vmPFC) during pain stimulation in the Empathetic condition only. These are key nodes of the brain ‘default mode network’ (DMN) and recent work has described the importance of dynamic communication amongst DMN subnetworks in the pain experience23. Disengagement of attention from pain (i.e., mind-wandering) has also been associated with downplay of pain-induced DMN deactivation, that may prompt the engagement of pain control systems53. On the contrary, enhanced functional connectivity between vmPFC and PCC/Prec was observed in chronic pain patients, and was correlated with the degree to which patients ruminated54. One hypothesis could be that, in our experiments, empathetic feedback facilitated the detachment of subjects’ attention from thermal painful stimulation, reducing pain ratings to the same degree distraction does (around −10%)55,56.
A concomitant enhancement of FC was observed during the Empathetic condition between the above midline structures (PCC/Prec, vmPFC) and the insular cortex (both anterior and posterior parts) contralateral to the stimulation side, whereas FC increase was limited to inter-hemispheric connections in the Unempathetic condition. Although any concrete explanation can only be speculative at this point, these results support the notion that changes in pain-related behavioral responses may be under the influence of top-down control in synchrony between poly-supramodal areas which instantiate the “self” (i.e., PCC/Prec, DLPFC, vmPFC) and insular cortices (i.e., a-Ins and p-Ins).
A number of limitations should be taken into consideration when interpreting our results. First of all, participants passively listened to the experimenters’ conversation while lying inside the MRI scanner and did not participate in real exchanges. Our results cannot therefore provide a full picture of the neural processes involved during patient-caregiver interactions. Also, the lack of behavioural metrics of attentional focus and mood ratings during the different conditions, due to the experiment and the fMRI constraints, restricts the interpretation of some of the neural mechanisms proposed here. However, the results are consistent with changes in mood found in previous psychophysical studies8,57. Interestingly, such changes appeared to be especially linked to the empathetic comments which were unanimously considered as “positive” and “pleasant” on debriefing8, whereas less than one third of subjects considered unempathetic comments as”harmful” or “injurious”. It is therefore likely that the brain activity changes observed are related to the subjective impression of mood change. However, in the absence of any quantitative evaluation it was not possible to assess this relationship. Our hypothesis based on a top-down control of the insular cortex by supramodal midline networks is consistent with the results but should be considered with caution. Although the pI undoubtedly contains networks highly sensitive to thermal and/or painful events58–61, this cortex is also involved in the processing of other sensory inputs62, not only somatosensory but also vestibular and auditory, in particular related to temporal and spatial context analysis63,64. Since a fine-grained temporal dynamics of insular responses is not accessible to fMRI30, there are certainly temporal modulations of responses between posterior and anterior insular cortices, that could not be observed here65.
In conclusion, we show that empathetic and unempathetic comments appear to be integrated differently in high-level brain structures (i.e., PCC/Prec, vmPFC and DLPFC) generally involved in the processing of other’s attitudes, self-awareness and autobiographical recall. The subjective perception of pain intensity is the result of an interaction between different brain regions, and a number of networks appear to be tightly coupled. The increase of functional connectivity of the insular cortex in Empathetic condition may reflect a control process on this central region of pain processing, inducing a reappraisal of pain intensity perception. We show here that the recruitment of these brain areas by hearing empathetic feedback from others is involved in pain reduction. A better understanding of this neural system in the pain experience may allow us to identify new anatomical targets and non-pharmacologic methods for inducing effective pain relief.
Material and Methods
Participants
Thirty-six right-handed healthy subjects naive to the experiment participated to the study that was approved by the local Ethics Committee (CHU Saint-Étienne, Comité de Protection des Personnes, Sud-Est 1, France). They gave written informed consent according to the Declaration of Helsinki and received a compensation for their participation. Of them, 30 participants (16/30 males; mean age ± SD = 25.5 ± 6.0 years) completed the study. Six were excluded from analyses, because they either did not believe in the scenario (i.e., they considered the comments as not credible) or identified that the comments were a part of the experimental manipulation. Participants had no depression or anxiety according to the Beck questionnaire66 and the State-Trait Anxiety Inventory67, respectively. Cardiac abnormality, neurological, psychiatric, chronic pain or mood disorders were exclusion criteria; all subjects had similar socioeconomic and ethnic/cultural background.
Experimental procedures and materials
The audio-scenarios and experimental setup were those validated in a previous psychophysical study8. The participants were unaware that this was an empathy-related experiment and were informed that the study’s goal was to collect brain data associated with their pain perception. The full aim of the study was revealed to them only at the end of the MRI session. Briefly, participants received noxious heat stimuli on their left hand and could inadvertently overhear discussions coming from the imaging staff, who commented on the participants’ attitude towards pain with different degrees of empathy. Subjects were scanned during three conditions (i.e., Neutral, Unempathetic and Empathetic), identical in terms of pain stimuli, but during which the degree of empathetic feedback expressed by the observers was changed. The neutral (N) comments were always heard at the beginning of the experiment, and concerned irrelevant discussion to make the participants familiar with the special situation of overhearing what should normally not be heard in an fMRI experiment (e.g., the conversation between the radiological staff outside the magnet room). Gradually, the conversations came to discuss the experiment, the volunteers, and their pain. However, keywords and sentences were selected to keep the neutrality of all comments8. This neutral exchange at the beginning of the experiment allowed a progressive and credible shift to distinct Empathetic/Unempathetic conversations. It also served as a control condition in the fMRI analysis. Then, the order of conditions with Empathetic (E) or Unempathetic (U) comments was counterbalanced across subjects (‘N-E-U’ or ‘N-U-E’) to avoid an order effect. The conversations were pre-recorded to ensure repeatability of the experiment. Participants and experimenters were introduced face-to-face to each other just before the test (without verbal exchanges), so that participants were able to attribute discussions to a group of persons they had previously met.
Each of the three experimental conditions consisted of two successive sessions, each lasting 8 min (Fig. 1a). The sessions were made of 12 thermal noxious stimuli alternated with 8 verbal stimuli. The term “verbal stimuli” (VS) refers to short verbal exchanges between experimenters (comments lasting 27.4 ± 14.3 s, range 10–50 s), interleaved with silences and background noise. Verbal stimuli were delivered through high-quality headsets (Nordic Neuro Lab fMRI audio system, Neuro Device, Poland) with a pseudo-randomized Inter-Stimulus Interval (mean ISI = 51.7 s ± 33.1 s). Thermal noxious stimuli were delivered through a 30 × 30 contact probe (Pathway Pain & Sensory Evaluation System, Medoc Ltd., Advanced Medical System, Israel) on the back of the left hand and alternated between a baseline temperature of 32 °C and a target noxious temperature determined individually for each participant just before the test8. Target temperatures were maintained for 10 seconds (+5 s of ascending and descending ramps) and were set in order to obtain a stable pain perception, rated around 60/100 by the participants (mean temperature of stimulation 46.8 ± 1.1 °C, range 44–49). Noxious stimuli were delivered during the silent interval between verbal stimuli, with a pseudo-randomized inter-stimulus interval (mean ISIp = 23.0 s ± 12.9 s) to avoid anticipation effects. The time period between the end of noxious stimuli and the onset of verbal stimuli was also variable and ranged from 12 to 20 seconds. A computer with E-Prime 2.0 software (Psychology Software Tools, Inc.) was used to ensure the synchronization of stimuli onset (noxious and verbal) and the online acquisition of pain ratings. At the end of the MRI acquisition, each volunteer was interviewed during a debriefing conversation with the aim to assess whether they had believed to the scenario and had perceived the different contexts. If they had, their data were considered for analysis. If not, they were excluded.
Behavioral data
The volunteers rated pain intensity continuously by means of two response buttons (model Lumina LU400-PAIR; Cedrus Corporation, USA), one to decrease and the other to increase ratings on a VAS scale going from 0 (i.e., no pain) to 100 (i.e., worst imaginable pain). Rating was recorded in continuous which allowed to define a curve indicating pain intensity ratings (PR) as a function of time. In addition, for each noxious stimulus, the peak pain score (i.e., [PR]max) were assessed in order to test the effect of condition on pain ratings. Statistical analysis was performed using two-way, mixed design analysis of variance (ANOVA) with experimental conditions (N/E/U) as within factor, and the order of conditions (‘N-E-U’ or ‘N-U-E’) as between factor. Greenhouse-Geisser correction and Tukey post-hoc tests were applied following significant main effects or interactions (p < 0.05). Statistics were calculated with SPSS (Statistical Package for the Social Sciences, SPSS Statistics 20 Inc, Chicago USA).
EPI images data acquisition, processing and analyses
Subjects were scanned in a 3T MR system (Prisma Siemens, Erlangen, Germany, EU) with a 64 channel head/neck coil. Structural T1-weighted images were acquired for anatomical reference during the first session of the Neutral condition with a 3D rapid gradient-echo sequence (MP-RAGE; TR = 2000 ms; TE = 2.24 ms, flip angle 8°, 192 sagittal slices, FOV = 230 mm, voxel size = 0.9 × 0.9 × 0.9 mm, matrix 256 × 256). Changes in blood oxygen level dependent (BOLD) T2* weighted MR signal were measured, using an interleaved gradient echo-planar imaging (EPI) sequence (TR = 2200 ms, TE = 30 ms, voxel size = 3.0 × 3.0 × 3.0 mm, flip angle 90°, slices/volume 40). A total of 220 EPI volumes were acquired in each functional session. The Statistical Parametric Mapping software (SPM12, Welcome Trust Centre for Neuroimaging, UK) was used for image processing and analyses. The first two scans of each session were removed, then images were slice-time corrected, spatially realigned, co-registered with the MNI152 brain using nonlinear warping, segmented using masks derived from the anatomical scan, and smoothed with a 6-mm FWHM Gaussian kernel. A high-pass temporal filter with a cutoff of 128 s was then applied.
First-level individual analyses
Multiple brain regions are activated by a nociceptive stimuli and reflect different processes leading to the final pain perception9,10,12,25. Most previous neuroimaging studies on pain did not distinguish brain responses related to the sensory representation of the nociceptive stimuli from those corresponding to the conscious perception of pain intensity. Recording continuous ratings of pain perception during the experiment provides a solution to separate these distinct activations28. Here, the pain stimulation was split into two periods (Fig. 1a) according to the onset of pain intensity ratings by the subject. The aim was to capture specifically the brain activity correlated to the magnitude of pain ratings, and to assess the effect of Empathetic condition on it.
A within-subject general linear model (GLM) design matrix was built and incorporated 5 functional sessions corresponding to experimental conditions; each included three regressors of interest (see Fig. 1a). The first regressor described Verbal Stimuli [VS], i.e., the short phrases with different empathetic value. The second regressor captured the brain activity corresponding to the period between the onset of thermal noxious stimulation and the onset of pain perception ratings, called “pre-pain” [prePAIN] i.e., the beginning of the nociceptive stimulation that was not yet judged as painful by the subjects. The third regressor, successive to the second, described the window where pain perception ratings [PR] were produced; i.e., the time period when the subjects felt the stimulus as painful (pain ratings ≠0) until they felt no pain and came back the scale’s cursor to 0. [prePAIN] and [PR] regressors were orthogonalized to minimize the occurrence of collinearity in the model68, and convolved with the canonical hemodynamic response function (hrf) and its time-derivative. Six time-series of head movement parameters were added in the linear model as covariates of no interest to remove residual variance.
Second-level group effect analyses
Group analyses were performed to assess (1) the brain activity related to regressors for all stimuli delivered during the experiment, whatever the conditions (Fig. 1b), in order to show the brain network associated with them compared to the baseline (thresholded at p < 0.01 FWE corrected voxel level); and (2) the effects of the experimental conditions (N, E, U) on the behavioural pain scores [PRmax] and on the brain activity related to conscious pain perception [PR]. These BOLD contrasts between conditions aim to reveal the neural activations and deactivations associated with the effect of Empathetic and Unempathetic conditions controlled for the Neutral condition during pain assessment. For each voxel, experimental conditions were compared using paired sample t-tests. The resulting SPM {z} maps were thresholded at p < 0.001 and clusters that survived FWE correction for multiple comparisons p < 0.05 were reported, revealing the brain activities associated with empathetic and unempathetic feedback effects on pain perception. Anatomical regions were labeled using both the Hammersmith69,70 and the Human Connectome Project atlases (HCP MMP 1.0)71.
Functional connectivity (FC) analyses
Connectivity analysis aimed at investigated the between-areas relationships (i.e., BOLD signal synchrony) modulated by experimental conditions. Based on previous literature, we selected a subset of brain regions that could plausibly mediate the effect on pain perception, from the set of brain regions (i.e., main clusters) that were significantly activated in the task analysis. We utilized that subset to define volumes of interest (VOI) for the connectivity analysis. These brain regions represented a short part of the whole functional network involved but focused on the main areas that specifically contributed to our effect. VOI were defined on the basis of their activation peaks and corresponding HCP brain atlas71. Figure 3a depicts these VOI.
Time-series extraction, correlation matrices, weighted signed modularity
FC analysis design was achieved under the open-source python package nipype72. The Neuropycon project was used for extracting time-series over VOI and computing FC matrices. Time-series averaged over the voxels within the VOI were extracted for each of the participants. Head movement parameters (rotation and translation), as well as average signals in the white matter and CSF were regressed out from VOI time series, and the residuals of the regression were kept. Residuals were then high-pass filtered (>0.01 Hz) to remove the scanner drift component of the signal and normalized (Z-score) for each experimental condition.
Task-based FC
We computed FC relative to pain stimulation using weighted correlations for each experimental condition73. This corresponds to computing Pearson correlation between two time-series over the whole session, weighting the contributions of each time points by the pain stimuli, giving more influence to periods where the regressor is high, and lower influence when the regressor is low or null. The pain stimulation was convolved with a canonical hrf and only the positive and null parts of the hrf were used to compute weighted correlations of VOI structure time-series pairs. This resulted in a set of correlation matrices (14 × 14) for each condition.
FC statistic comparisons
Weighted correlation matrices of each experimental condition were compared with paired sample T tests. A false positive correction for multiple comparisons, limiting the risk of type II errors, was retained, because it has been demonstrated to be a suitable threshold in order to determine the significance of connectivity results74.
Supplementary information
Supplementary information: brain activations tables and figure 4
Acknowledgements
This work was supported by a PhD grant from the Academic Research Community (ARC-2) of Region Rhône-Alpes, France; the Institut UPSA de la douleur; the Labex CORTEX (ANR-11-LABX-0042, ANR-11-IDEX-0007); and Novartis France. We thank N.R. Osborne and G. Pope for reviewing the manuscript.
Author Contributions
C.F., I.F. and R.P. developed the study concept and design. Testing and collection were performed by C.F., I.F., D.M. and C.Q. C.F., I.F., D.M., F.C., L.G.-L. and R.P. drafted the manuscript. All authors approved the final version of the manuscript for submission in Scientific Reports.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44879-9.
References
- 1.Decety J, Fotopoulou A. Why empathy has a beneficial impact on others in medicine: unifying theories. Front. Behav. Neurosci. 2015;8:457. doi: 10.3389/fnbeh.2014.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton BW. Walking a fine line: is it possible to remain an empathic physician and have a hardened heart? Front. Hum. Neurosci. 2013;7:233. doi: 10.3389/fnhum.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevenson ACT. Compassion and patient centred care. Aust. Fam. Physician. 2002;31:1103–1106. [PubMed] [Google Scholar]
- 4.Tait RC. Empathy: necessary for effective pain management? Curr. Pain Headache Rep. 2008;12:108–112. doi: 10.1007/s11916-008-0021-6. [DOI] [PubMed] [Google Scholar]
- 5.Edmond SN, Keefe FJ. Validating pain communication: current state of the science. PAIN. 2015;156:215–219. doi: 10.1097/01.j.pain.0000460301.18207.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vangronsveld KL, Linton SJ. The effect of validating and invalidating communication on satisfaction, pain and affect in nurses suffering from low back pain during a semi-structured interview: The effect of validating and invalidating communication on satisfaction, pain and affect. Eur. J. Pain. 2012;16:239–246. doi: 10.1016/j.ejpain.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Krahé C, Springer A, Weinman JA, Fotopoulou A. The Social Modulation of Pain: Others as Predictive Signals of Salience – a Systematic Review. Front. Hum. Neurosci. 2013;7:386. doi: 10.3389/fnhum.2013.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fauchon C, et al. Does an observer’s empathy influence my pain? Effect of perceived empathetic or unempathetic support on a pain test. Eur. J. Neurosci. 2017;46:2629–2637. doi: 10.1111/ejn.13701. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Larrea L, Bastuji H. Pain and consciousness. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;87:193–199. doi: 10.1016/j.pnpbp.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: A review. PAIN. 2013;154:S29–S43. doi: 10.1016/j.pain.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Lichtner G, et al. Effects of propofol anesthesia on the processing of noxious stimuli in the spinal cord and the brain. Neuro Image. 2018;172:642–653. doi: 10.1016/j.neuroimage.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Tracey I, Mantyh PW. The Cerebral Signature for Pain Perception and Its Modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Wiech K. Deconstructing the sensation of pain: The influence of cognitive processes on pain perception. Science. 2016;354:584. doi: 10.1126/science.aaf8934. [DOI] [PubMed] [Google Scholar]
- 14.Bastuji H, Frot M, Perchet C, Magnin M, Garcia-Larrea L. Pain networks from the inside: Spatiotemporal analysis of brain responses leading from nociception to conscious perception: Pain Networks From the Inside. Hum. Brain Mapp. 2016;37:4301–4315. doi: 10.1002/hbm.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuro Image. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Godinho F, et al. How the pain of others enhances our pain: Searching the cerebral correlates of ‘compassional hyperalgesia’: Cerebral correlates of CH. Eur. J. Pain. 2012;16:748–759. doi: 10.1002/j.1532-2149.2011.00039.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Yang C-Y, Cheng Y. Sensorimotor resonance is an outcome but not a platform to anticipating harm to others. Soc. Neurosci. 2012;7:578–590. doi: 10.1080/17470919.2012.686924. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberger NI. An Empirical Review of the Neural Underpinnings of Receiving and Giving Social Support: Implications for Health. Psychosom. Med. 2013;75:545–556. doi: 10.1097/PSY.0b013e31829de2e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing Pictures of a Romantic Partner Reduces Experimental Pain: Involvement of Neural Reward Systems. PLoS ONE. 2010;5:e13309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychol. Sci. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- 21.Krahé C, et al. Attachment style moderates partner presence effects on pain: a laser-evoked potentials study. Soc. Cogn. Affect. Neurosci. 2015;10:1030–1037. doi: 10.1093/scan/nsu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hein G, Engelmann JB, Tobler PN. Pain relief provided by an outgroup member enhances analgesia. Proc. R. Soc. B Biol. Sci. 2018;285:20180501. doi: 10.1098/rspb.2018.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci. 2015;38:86–95. doi: 10.1016/j.tins.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Kawamichi H, et al. Increased frequency of social interaction is associated with enjoyment enhancement and reward system activation. Sci. Rep. 2016;6:24561. doi: 10.1038/srep24561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005;9:463–463. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Wager TD, et al. An fMRI-Based Neurologic Signature of Physical Pain. N. Engl. J. Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alho K, Rinne T, Herron TJ, Woods DL. Stimulus-dependent activations and attention-related modulations in the auditory cortex: A meta-analysis of fMRI studies. Hear. Res. 2014;307:29–41. doi: 10.1016/j.heares.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Baliki MN, Geha PY, Apkarian AV. Parsing Pain Perception Between Nociceptive Representation and Magnitude Estimation. J. Neurophysiol. 2008;101:875–887. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain Mechanisms Supporting Discrimination of Sensory Features of Pain: A New Model. J. Neurosci. 2009;29:14924–14931. doi: 10.1523/JNEUROSCI.5538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pomares FB, Faillenot I, Barral FG, Peyron R. The ‘where’ and the ‘when’ of the BOLD response to pain in the insular cortex. Discussion on amplitudes and latencies. Neuro Image. 2013;64:466–475. doi: 10.1016/j.neuroimage.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 31.Peyron, R. & Fauchon, C. The posterior insular-opercular cortex: An access to the brain networks of thermosensory and nociceptive processes? Neurosci. Lett. in press (2018). [DOI] [PubMed]
- 32.Lorenz J, Casey KL. Imaging of acute versus pathological pain in humans. Eur. J. Pain. 2005;9:163–165. doi: 10.1016/j.ejpain.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Craig, A. D. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10 (2009). [DOI] [PubMed]
- 34.Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back) Exp. Brain Res. 2010;205:1–12. doi: 10.1007/s00221-010-2340-1. [DOI] [PubMed] [Google Scholar]
- 35.Wiech K, et al. Anterior Insula Integrates Information about Salience into Perceptual Decisions about Pain. J. Neurosci. 2010;30:16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr. Opin. Neurobiol. 2004;14:212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuro Image. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 39.Johnstone T, van Reekum CM, Oakes TR, Davidson RJ. The voice of emotion: an FMRI study of neural responses to angry and happy vocal expressions. Soc. Cogn. Affect. Neurosci. 2006;1:242–249. doi: 10.1093/scan/nsl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koelsch S, Fritz TV, Cramon DY, Müller K, Friederici AD. Investigating emotion with music: An fMRI study. Hum. Brain Mapp. 2006;27:239–250. doi: 10.1002/hbm.20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu X, et al. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135:2726–2735. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuro Image. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Starr CJ, et al. Roles of the Insular Cortex in the Modulation of Pain: Insights from Brain Lesions. J. Neurosci. 2009;29:2684–2694. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiech K, et al. An fMRI study measuring analgesia enhanced by religion as a belief system. Pain. 2008;139:467–476. doi: 10.1016/j.pain.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 45.Boehme S, Miltner WHR, Straube T. Neural correlates of self-focused attention in social anxiety. Soc. Cogn. Affect. Neurosci. 2015;10:856–862. doi: 10.1093/scan/nsu128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson SC, et al. Neural correlates of self‐reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 47.Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 48.Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the ‘Self’ is Processed in the Posterior Cingulate Cortex? Front. Hum. Neurosci. 2013;7:647. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demertzi A, Soddu A, Laureys S. Consciousness supporting networks. Curr. Opin. Neurobiol. 2013;23:239–244. doi: 10.1016/j.conb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 50.DeWitt SJ, Ketcherside A, McQueeny TM, Dunlop JP, Filbey FM. The hyper-sentient addict: an exteroception model of addiction. Am. J. Drug Alcohol Abuse. 2015;41:374–381. doi: 10.3109/00952990.2015.1049701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Che X, et al. Synchronous activation within the default mode network correlates with perceived social support. Neuropsychologia. 2014;63:26–33. doi: 10.1016/j.neuropsychologia.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 53.Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc. Natl. Acad. Sci. 2013;110:18692–18697. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kucyi A, et al. Enhanced Medial Prefrontal-Default Mode Network Functional Connectivity in Chronic Pain and Its Association with Pain Rumination. J. Neurosci. 2014;34:3969–3975. doi: 10.1523/JNEUROSCI.5055-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peyron R, et al. Haemodynamic brain responses to acute pain in humans Sensory and attentional networks. Brain. 1999;122:1765–1780. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- 56.Valet M, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 57.Fauchon C, et al. Contextual modulation of autonomic pain reactivity. Auton. Neurosci. Basic Clin. 2018;212:28–31. doi: 10.1016/j.autneu.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Frot M, Magnin M, Mauguiere F, Garcia-Larrea L. Human SII and Posterior Insula Differently Encode Thermal Laser Stimuli. Cereb. Cortex. 2007;17:610–620. doi: 10.1093/cercor/bhk007. [DOI] [PubMed] [Google Scholar]
- 59.Mazzola L, Faillenot I, Barral F-G, Mauguière F, Peyron R. Spatial segregation of somato-sensory and pain activations in the human operculo-insular cortex. Neuro Image. 2012;60:409–418. doi: 10.1016/j.neuroimage.2011.12.072. [DOI] [PubMed] [Google Scholar]
- 60.Peyron R. Functional brain imaging: what has it brought to our understanding of neuropathic pain? A special focus on allodynic pain mechanisms. PAIN. 2016;157:S67–S71. doi: 10.1097/j.pain.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 61.Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nat. Neurosci. 2015;18:499–500. doi: 10.1038/nn.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazzola L, Isnard J, Peyron R, Mauguiere F. Stimulation of the human cortex and the experience of pain: Wilder Penfield’s observations revisited. Brain. 2012;135:631–640. doi: 10.1093/brain/awr265. [DOI] [PubMed] [Google Scholar]
- 63.Bamiou D-E, et al. Auditory temporal processing deficits in patients with insular stroke. Neurology. 2006;67:614–619. doi: 10.1212/01.wnl.0000230197.40410.db. [DOI] [PubMed] [Google Scholar]
- 64.Griffiths TD, et al. Spatial and temporal auditory processing deficits following right hemisphere infarction. A psychophysical study. Brain J. Neurol. 1997;120:785–794. doi: 10.1093/brain/120.5.785. [DOI] [PubMed] [Google Scholar]
- 65.Bastuji H, Frot M, Perchet C, Hagiwara K, Garcia-Larrea L. Convergence of sensory and limbic noxious input into the anterior insula and the emergence of pain from nociception. Sci. Rep. 2018;8:13360. doi: 10.1038/s41598-018-31781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beck AT, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988;8:77–100. doi: 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- 67.Spielberger, C. D., Gorsuch, R. L. & Lushene, R. E. Manual for the State-Trait Anxiety Inventory. Paolo Alto Calif Consult. Psychol. Press (1970).
- 68.Mumford JA, Poline J-B, Poldrack RA. Orthogonalization of Regressors in fMRI Models. PLOS ONE. 2015;10:e0126255. doi: 10.1371/journal.pone.0126255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faillenot I, Heckemann RA, Frot M, Hammers A. Macroanatomy and 3D probabilistic atlas of the human insula. Neuro Image. 2017;150:88–98. doi: 10.1016/j.neuroimage.2017.01.073. [DOI] [PubMed] [Google Scholar]
- 70.Hammers A, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glasser MF, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gorgolewski K, et al. Nipype: A Flexible, Lightweight and Extensible Neuroimaging Data Processing Framework in Python. Front. Neuroinformatics. 2011;5:13. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meunier D, et al. Modular structure of functional networks in olfactory memory. Neuro Image. 2014;95:264–275. doi: 10.1016/j.neuroimage.2014.03.041. [DOI] [PubMed] [Google Scholar]
- 74.Bassett, D. S. & Lynall, M.-E. Network methods to characterize brain structure and function. Cogn. Neurosci. Biol. Mind 5th edn (eds Gazzaniga, M. S. & Mangun, G. R.) Ch. 79 (2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: brain activations tables and figure 4
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.