Abstract
Background
Disease progression and delayed neurological complications are common after aneurysmal subarachnoid hemorrhage (aSAH). We explored the potential of quantitative blood-brain barrier (BBB) imaging to predict disease progression and neurological outcome.
Methods
Data were collected as part of the Co-Operative Studies of Brain Injury Depolarizations (COSBID). We analyzed retrospectively, blinded and semi-automatically magnetic resonance images from 124 aSAH patients scanned at 4 time points (24–48 h, 6–8 days, 12–15 days and 6–12 months) after the initial hemorrhage. Volume of brain with apparent pathology and/or BBB dysfunction (BBBD), subarachnoid space and lateral ventricles were measured. Neurological status on admission was assessed using the World Federation of Neurosurgical Societies and Rosen-Macdonald scores. Outcome at ≥6 months was assessed using the extended Glasgow outcome scale and disease course (progressive or non-progressive based on imaging-detected loss of normal brain tissue in consecutive scans). Logistic regression was used to define biomarkers that best predict outcomes. Receiver operating characteristic analysis was performed to assess accuracy of outcome prediction models.
Findings
In the present cohort, 63% of patients had progressive and 37% non-progressive disease course. Progressive course was associated with worse outcome at ≥6 months (sensitivity of 98% and specificity of 97%). Brain volume with BBBD was significantly larger in patients with progressive course already 24–48 h after admission (2.23 (1.23–3.17) folds, median with 95%CI), and persisted at all time points. The highest probability of a BBB-disrupted voxel to become pathological was found at a distance of ≤1 cm from the brain with apparent pathology (0·284 (0·122–0·594), p < 0·001, median with 95%CI). A multivariate logistic regression model revealed power for BBBD in combination with RMS at 24-48 h in predicting outcome (ROC area under the curve = 0·829, p < 0·001).
Interpretation
We suggest that early identification of BBBD may serve as a key predictive biomarker for neurological outcome in aSAH.
Fund
Dr. Dreier was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (DFG DR 323/5-1 and DFG DR 323/10–1), the Bundesministerium für Bildung und Forschung (BMBF) Center for Stroke Research Berlin 01 EO 0801 and FP7 no 602150 CENTER-TBI.
Dr. Friedman was supported by grants from Israel Science Foundation and Canada Institute for Health Research (CIHR). Dr. Friedman was supported by grants from European Union's Seventh Framework Program (FP7/2007–2013; grant #602102).
Keywords: Aneurysmal subarachnoid hemorrhage, Blood-brain barrier, Extended Glasgow outcome scale (eGOS), Magnetic resonance imaging (MRI), Lesion, Long term output
Research in context.
Evidence before this study
We searched PubMed for articles published in any language about the effect of blood-brain barrier (BBB) damage seen on magnetic resonance imaging (MRI) of aneurysmal subarachnoid hemorrhage (aSAH) patients on risk for long-term neurological deficit. We used the search terms “blood-brain barrier”, “BBB”, “Magnetic Resonance Imaging”, “MRI”, “subarachnoid hemorrhage”, “SAH”, “lesion”, “edema”, “ischemia”, “infarction”, “outcome”, “eGOS”, “GOS”, “Glasgow Coma Scale”, and “Glasgow Outcome Scale”. Our last search was done on September 18, 2018. We found only one pilot work that was published on 6 March 2018. It reported that in a small cohort of patients (n = 16), blood-brain barrier (BBB) permeability imaging could predict the occurrence of delayed cerebral ischemia within 14 days of aSAH. No quantitative assessment of delayed cerebral ischemia was provided. Overall, we found no reports that provide quantitative characterization of the dynamics of BBB dysfunction together with quantified dynamics of brain pathology and clinically-based assessment of disease progression in patients with acute aSAH.
Added value of this study
This study used a large multicenter cohort of 1643 brain MR images from 124 aSAH patients imaged sequentially for over 6 months, to test the potential of quantitative BBB imaging as a prognostic and diagnostic biomarker for disease progression and neurological outcome. The strength of our study is the use of imaging-based quantitative approaches for a better understanding of the pathogenesis of neurological complications following brain injury within the clinical setting. For the first time, we identify apparently normal brain voxels that show a leaky barrier and are at risk to become pathological. We further show that a significant BBB pathology can be detected as early as 24–48 h after the acute injury. BBB pathology was most likely found in the apparently normal brain tissue surrounding the pathological brain, indicating that this adjacent tissue is at the highest risk for delayed injury. Importantly, we found BBB pathology to persist for months after the insult and was significantly more extensive in patients with a progressive disease, anatomically (MRI-based) and functionally (clinical outcome). This is the first study to propose a clinically applicable, quantitative approach for MRI imaging that detects microvascular pathology for early prediction of patients' outcome with high sensitivity and specificity. Since similar pathogenic mechanisms are involved in other brain injuries, we suggest imaging of BBB pathology as a potential novel diagnostic and predictive biomarker in brain injuries.
Implications of all the available evidence
Due to high mortality and morbidity, accurate prognosis of aSAH patients is crucial for the identification of patients at high risk for delayed neurological complications. Specifically, there is an unmet need for biomarkers that are related to disease pathophysiological mechanisms and may be monitored during treatment. We describe, for the first time, that a leaky BBB, detected as early as 24–48 h after the acute event, is associated with disease progression. We propose BBB pathology as a potential early biomarker for the identification of patients at high risk for disease complications and progression. Our study lays the foundation for a novel prognostic utility, employing MRI-based quantitative analysis, to significantly improve the capacity of the widely used grading systems to identify patients at high risk for disease progression. Our study suggests investigating microvascular pathology a leaky BBB as potential novel therapeutic targets in brain injury.
Alt-text: Unlabelled Box
1. Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating brain insult associated with high morbidity and mortality. The incidence of aSAH is at 9·1 cases per 100,000 patient years [1] with a death rate of 40–50% [2]. Of those who survive, >40% will have long-term neurological sequelae, and less than a fifth will have no residual symptoms [3]. Due to the high mortality and morbidity rates, understanding the pathophysiology of delayed neurological complications following aSAH and identification of measurable associated biomarkers are crucial for the detection of patients at high risk for such complications, testing novel treatments and improving outcome [4].
Cerebral edema is often seen in CT or MR images of patients with aSAH and other types of brain injuries. Brain edema is a predominant cause of poor clinical outcome. The two main types are cytotoxic and vasogenic edema [5]; the latter is primarily attributed to breakdown and dysfunction of the blood-brain barrier (BBB) [6]. BBB dysfunction (BBBD) has been described within hours after aSAH and has been linked with pathologic changes such as brain edema, thrombosis, inflammation and abnormal cerebral metabolism [[7], [8], [9], [10]]. BBBD has also been associated with delayed ischemia in aSAH patients [11], and the development of epilepsy and neurodegeneration after traumatic brain injury [12,13]. Accumulating pre-clinical data show that prolonged breakdown of the BBB underlies astroglial activation, neuroinflammation, neural network reorganization, dysfunction and degeneration [12,14,15]. These data suggest BBBD as a potential early prognostic biomarker after brain injury. Previously, BBB function was assessed either using statistical comparison between images acquired before and after injection of the normally non-permeable gadolinium-based contrast agent [16,17], or by dynamic studies following the kinetics of gadolinium brain concentrations [[18], [19], [20], [21]]. However, such assessment has not yet been introduced in the routine clinical setting, and the hypothesis on the role of BBBD in complications after injury has not been challenged.
Along with BBBD, MR imaging in brain injured patients is often characterized by the appearance of abnormal brain tissue (ABT), which reflects cytotoxic or vasogenic edemas, gliosis or hemorrhage. Our main goal in the present study was to quantitatively characterize the dynamics of BBB dysfunction following aSAH, and assess its prognostic value using imaging- and clinically-based measures for disease progression. To this end, we developed an MRI-based quantitative approach for the identification of brain voxels with apparent ABT and BBBD. We implemented our approach in 1643 brain MR images from 124 aSAH patients. To our knowledge, this is the first study to quantitatively assess ABT dynamics and the potential of BBB dysfunction as a predictive biomarker.
2. Materials and methods
2.1. Study design
The study was designed and performed as a longitudinal sub-study within the framework of the Co-Operative Studies of Brain Injury Depolarizations (COSBID). Patients with aSAH were selected from a prospectively collected database using pre-specified criteria and endpoints, as described below. The protocol was approved by the ethics committees of the Charité University Medicine Berlin, the Goethe-University Frankfurt, the University Hospital Heidelberg and the University of Cologne. Research was conducted in accordance with the Declaration of Helsinki. Patients or their legal representative gave written consent for study inclusion. Results are reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.
Prospective inclusion criteria for aSAH patients in COSBID have been described elsewhere [22]: (i) age (≥18 years); (ii) World Federation of Neurosurgical Societies (WFNS) grade I–V; (iii) ruptured saccular aneurysm proven by computed tomography (CT)-angiography or digital subtraction angiography; (iv) symptom onset within the preceding 72 h; and (v) either surgical treatment of the aneurysm via craniotomy or, in coiled patients, burr hole trepanation for placement of a ventricular drain or oxygen sensor, which allows the simultaneous placement of a subdural electrode strip [23]. Exclusion criteria were subarachnoid hemorrhage due to other causes (e.g., trauma, fusiform or mycotic aneurysm), admission in a clinical state with unfavorable prognosis (e.g., wide, nonreactive pupils for >1 h), bleeding diasthesis or pregnancy, unavailability of the monitoring equipment and refusal of the patient or legal representative to participate in the study.
For the present study, patients were retrospectively screened for the availability of at least two serial MRI scans including a T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence, a T1-weighted (T1w) 3D high-resolution sequence (i.e. magnetization prepared rapid gradient echo), a diffusion weighted imaging (DWI) sequence, and T1w sequences before and 5 min after the injection of gadolinium-DTPA (Gd-DTPA 0·5 M, 0·1 mmol/kg).
One hundred twenty-four patients enrolled between January 2007 and March 2016 met criteria, including 50 at Campus Virchow Klinikum, 44 at Campus Benjamin Franklin (Charité University Medicine Berlin), 18 at the Goethe-University Frankfurt, 6 at the University of Cologne and 6 at the University Hospital Heidelberg.
Retrospective analysis of MR images was performed blindly on 377 examinations from the 124 aSAH patients, as well as on images from 21 healthy controls. Each patient had between two and four MRI examination visits and was imaged sequentially for >6 months. Details of the sequences are provided in appendix Table 1.
Since the longitudinally collected imaging datasets of aSAH patients accommodated an unequal number of follow-up scans per subject and unequal time intervals between scans, we categorized scans according to the time windows after aneurysm event as follows: acute: 24–48 h (t1), sub-acute: 3–9 days (t2), delayed: 10–19 days (t3), and late, around one year (267·6(196·6–358·2); range of 59–1472 days) (t4) after the initial hemorrhage. Availability of clinical data and the number of imaging sets available for analysis at each time point are presented in Table 1, Table 2.
Table 1.
Clinical data: Demographic data, characteristics of disease and interventiona.
| eGOS ≤ 5 (n = 69, 61·6%)b | eGOS > 5 (n = 43, 38·4%)b | OR (95% CI) | p-value | PC (n = 78, 62·9%) | NPC (n = 46 37·1%) | OR (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|---|
| Sex | 0·6757 | 0·5524 | ||||||
| Female | 46 | 31 | .. | 51 | 33 | .. | ||
| Male | 23 | 12 | .. | 27 | 13 | .. | ||
| Neurological score at admission | ||||||||
| WFNS ≥ 4 | 46 | 14 | 4·14 (1·84–9·32) | 0·0005 | 49 | 17 | 3·31 (1·46–6·52) | 0·0018 |
| WFNS < 4 | 23 | 29 | 29 | 31 | ||||
| RMS > 4 | 63 | 23 | 9·13 (3·26–25·57) | 0·0000 | 68 | 27 | 4·79 (1·97–11·61) | 0·0004 |
| RMS≤4 | 6 | 20 | 10 | 19 | ||||
| Aneurysm locationc | 0·7695 | 0·8350 | ||||||
| ACA | 3 | 2 | .. | 3 | 2 | .. | ||
| MCA | 26 | 15 | .. | 29 | 18 | .. | ||
| ICA | 3 | 4 | .. | 4 | 4 | .. | ||
| ACoP | 11 | 6 | .. | 12 | 6 | .. | ||
| ACoA | 21 | 14 | .. | 25 | 14 | .. | ||
| PeriA | 0 | 1 | .. | 0 | 1 | .. | ||
| BCA | 3 | 0 | .. | 3 | 0 | .. | ||
| PICA | 2 | 1 | .. | 2 | 1 | .. | ||
| Type of intervention | 0·7631 | 0·5683 | ||||||
| Clipping | 60 | 39 | 0·68 (0·20–2·37) | 68 | 42 | 0·65 (0·19–2·20) | ||
| Coiling | 9 | 4 | 10 | 4 | ||||
| Presence of vasospasm | 0·2946 | 0·1347 | ||||||
| V- | 17 | 12 | .. | 19 | 16 | .. | ||
| V−/+ | 21 | 18 | .. | 23 | 17 | .. | ||
| V+ | 31 | 13 | .. | 36 | 13 | .. | ||
| Arterial hypertensiond | 0·0013 | 0·0153 | ||||||
| True | 40 | 13 | 3·69 (1·63–8·39) | 42 | 16 | 2·46 (1·15–5·27) | ||
| Not true | 25 | 30 | 32 | 30 | ||||
| Anemiad | 0·0266 | 0·0309 | ||||||
| True | 17 | 3 | 3·98 (1·06–15·00) | 18 | 3 | 3·77 (1·01–14·01) | ||
| Not true | 37 | 26 | 43 | 27 | ||||
| Abnormal liver enzymesd | 0·4688 | 0·3716 | ||||||
| True | 19 | 8 | .. | 23 | 8 | .. | ||
| Not true | 38 | 19 | .. | 41 | 19 | .. | ||
| Uric acidd | 0·5021 | 0·2708 | ||||||
| Below normal | 24 | 11 | .. | 29 | 9 | .. | ||
| Normal | 19 | 10 | .. | 20 | 10 | .. | ||
| Total cholesterold | 0·9686 | 0·9811 | ||||||
| Evaluated | 1 | 2 | .. | 1 | 2 | .. | ||
| Normal | 38 | 17 | .. | 43 | 15 | .. | ||
| Previous aSAH | 1·0000 | 1·0000 | ||||||
| True | 0 | 1 | .. | 0 | 1 | .. | ||
| Not true | 69 | 42 | .. | 78 | 45 | .. | ||
OR = odds ratio. PC = progressive course of aSAH. NPC = non-progressive course of aSAH.
Only significant OR values were presented in the table.
Neurological outcome (eGOS) was available for 112 (90%) patients.
Aneurysms were assessed using four-vessel digital subtraction angiography (DSA).
Clinical data were not available for all of the patients.
Table 2.
Quantitative imaging data as related to clinical outcome‡.
| eGOS ≤ 5 | eGOS > 5 | n of eGOS ≤ 5, n of eGOS > 5 | OR (95% CI) | p-value | AUC (95% CI) | PC | NPC | n of PC, n of NC | OR (95% CI) | p-value | AUC (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | 57·00 (48·00–65·00) | 52·00⁎ (44·00–59·00) | 69, 43 | 1·06 (1·02–1·10) | 0·0261 | 0·62 (0·51–0·73) | 57·00 (48·00–65·00) | 52·00⁎ (44·00–60·00) | 78, 46 | 1·04 (1·00–1·07) | 0·0351 | 0·62 (0·51–0·73) |
| WFNS | 4 (2–5) | 2⁎ (1–4·5) | 69, 43 | 1·60 (1·22–2·11) | 0·0007 | 0·66 (0·55–0·77) | 4 (2–5) | 2 (1–4·5)⁎ | 78, 46 | 1·56 91·21–2·01) | 0·0005 | 0·65 (0·55–0·75) |
| RMS | 7 (5–8) | 5⁎ [[3], [4], [5], [6], [7]] | 69, 43 | 1·73 (1·33–2·24) | 0·0002 | 0·75 (0·65–0·85) | 7 (5–8) | 5⁎ [[3], [4], [5], [6], [7]] | 78, 46 | 1·52 (1·22–1·90) | 0·0002 | 0·71 (0·62–0·81) |
| ABT volume, ml | ||||||||||||
| ABT1 | 85·24 (58·95–131·88) | 55·82 (34·79–98·11) | 69, 43 | 1·01 (1·00–1·02) | 0·0401 | 0·67 (0·58–0·79) | 84·31 (54·69–131·57) | 56·25 (35·19–102·02) | 78, 46 | 1·01 (1·00–1·02) | 0·0410 | 0·66 (0·57–0·79) |
| ABT2 | 111·46† (80·47–219·23) | 68·05⁎ (33·05–105·38) | 42, 24 | 1·02 (1·01–1·04) | 0·0040 | 0·74 (0·62–0·88) | 109·88† (80·47–219·23) | 67·46⁎ (33·05–115·15) | 47, 25 | 1·02 (1·01–1·03) | 0·0045 | 0·74 (0·62–0·87 |
| ABT3 | 134·98† (91·94–199·37) | 59·75⁎ (28·68–93·24) | 62, 37 | 1·02 (1·01–1·04 | 0·0002 | 0·83 (0·74–0·92) | 134·55† (91·42–205·27) | 61·39⁎ (30·71–102·64) | 71, 40 | 1·02 (1·01–1·03) | 0·0003 | 0·80 (0·71–0·89 |
| ABT4 | 138·71† (102·79–204·39) | 29·55⁎† (15·78–73·55) | 36, 33 | 1·03 (1·02–1·05) | 0·0002 | 0·88 (0·81–0·97) | 138·66† (99·23–204·39) | 29·55⁎† (16·92–71·70) | 37, 33 | 1·03 (1·02–1·05) | 0·0002 | 0·88 (0·80–0·96 |
| NBT volume, ml | ||||||||||||
| NBT1 | 1496·3‡ (1359·1–1568·5) | 1461·8‡ (1370·5–1530·8) | 69, 43 | ·· | 0·7218 | ·· | 1503·0‡ (1401·2–1575·0) | 1455·6‡ (1364·4–1522·0) | 78, 46 | ·· | 0· 4101 | ·· |
| NBT2 | 1425·2‡ (1349·7–1534·9) | 1452·0‡ (1384·1–1515·1) | 42, 24 | ·· | 0·4159 | ·· | 1425·7‡ (1350·1–1534·9) | 1440·8‡ (1377·1–1515·1) | 47, 25 | ·· | 0·5738 | ·· |
| NBT3 | 1383·3†‡ (1276·6–1480·7) | 1451·4‡ (1373·2–1531·6) | 62, 37 | ·· | 0·0869 | ·· | 1390·0 †‡ (1287·5–1495·1) | 1442·5‡ (1366·6–1521·4) | 71, 40 | ·· | 0·0908 | ·· |
| NBT4 | 1347·4†‡ (1198·6–1411·8) | 1475·5⁎‡ (1410·8–1542·3) | 36, 33 | 0·99 (0·98–1·00) | 0·0025 | 0·81 (0·70–0·91) | 1351·8† ‡ (1198·6–1437·0) | 1469·3⁎‡ (1389·9–1537·0) | 37, 33 | 0·99 (0·99–1·00) | 0·0055 | 0·77 (0·66–0·89) |
| LV volume, ml | ||||||||||||
| LV1 | 36·59‡ (22·59–57·57) | 35·13‡ (23·18–48·08) | 69, 43 | ·· | 0·7216 | ·· | 33·81‡ (20·36–51·44) | 35·18‡ (23·81–49·51) | 78, 46 | ·· | 0·9792 | ·· |
| LV2 | 34·30‡ (18·44–50·59) | 27·86 (21·31–37·19) | 42, 24 | ·· | 0·8668 | ·· | 30·47‡ (17·92–49·00) | 27·52‡ (21·31–37·19) | 47, 25 | ·· | 0·9723 | ·· |
| LV3 | 40·04‡ (22·00–60·38) | 35·87‡ (22·00–54·46) | 62, 37 | ·· | 0·2754 | ·· | 38·29‡ (23·10–60·27) | 36·32‡ (27·60–54·46) | 71, 40 | ·· | 0·8910 | ·· |
| LV4 | 68·29†‡ (47·98–108·98) | 45·62⁎†‡ (32·07–69·85) | 36, 33 | 1·02 (1·00–1·03) | 0·0153 | 0·67 (0·54–0·80 | 71·51†‡ (50·90–109·39) | 44·69⁎‡ (30·77–56·16) | 37, 33 | 1·03 (1·01–1·05) | 0·0012 | 0·74 (0·62–0·86) |
| SAS volume, ml | ||||||||||||
| SAS1 | 267·08‡ (154·80–379·21) | 228·55‡ (150·32–344·57) | 69, 43 | ·· | 0·2901 | ·· | 257·66‡ (148·10–354·18) | 246·45‡ (160·75–377·70) | 78, 46 | ·· | 0·8161 | ·· |
| SAS2 | 159·26† (107·35–296·91) | 195·45 (147·68–298·13) | 42, 24 | ·· | 0·3408 | ·· | 157·28† (104·13–296·91) | 196·00 (147·69–319·27) | 47, 25 | ·· | 0·2162 | ·· |
| SAS3 | 231·66‡ (154·20–337·51) | 233·05‡ (145·36–349·60) | 62, 37 | ·· | 0·6926 | ·· | 221·41‡ (148·19–333·90) | 257·89‡ (187·68–401·95) | 71, 40 | ·· | 0·5036 | ·· |
| SAS4 | 447·19†‡ (272·10–564·83) | 361·93⁎†‡ (281·57–481·77) | 36, 33 | ·· | 0·1223 | ·· | 450·92†‡ (272·10–564·83) | 363·43⁎†‡ (281·57–497·71) | 37, 33 | ·· | 0·1584 | ·· |
| ABTBBBD volume, ml | ||||||||||||
| ABTBBBD1 | 19·23 (9·53–24·57) | 11·72⁎ (9·08–22·17) | 49, 30 | 1·06 (1·01–1·11) | 0·0109 | 0·68 (0·55–0·80) | 23·02 (12·01–31·08) | 12·13⁎ (9·23–22·83) | 59, 34 | 1·05 (1·01–1·09) | 0·0177 | 0·66 (0·54–0·78) |
| ABTBBBD2 | 27·60† (14·83–48·23) | 19·20⁎ (8·68–25·24) | 32, 18 | 1·05 (1·01–1·10) | 0·0184 | 0·69 (0·52–0·86) | 39·11† (19·06–60·50) | 18·80⁎ (7·82–24·73) | 35, 20 | 1·06 (1·01–1·11) | 0·0088 | 0·72 (0·56–0·88) |
| ABTBBBD3 | 35·88† (23·77–56·47) | 14·55⁎ (8·84–36·32) | 50, 28 | 1·05 (1·02–1·08) | 0·0004 | 0·79 (0·68–0·90) | 44·14† (28·15–71·10) | 15·54⁎ (8·83–37·41) | 58, 30 | 1·05 (1·02–1·08) | 0·0002 | 0·78 (0·67–0·89) |
| ABTBBBD4 | 19·91 (13·12–43·18) | 5·90⁎† (2·89–13·87 | 23, 21 | 1·07 (1·02–1·13) | 0·0071 | 0·85 (0·73–0·97) | 24·38 (18·23–50·99) | 5·84⁎† (2·89–11·33) | 24, 22 | 1·08 (1·02–1·14) | 0·0077 | 0·85 (0·73–0·97) |
| NBTBBBD volume, ml | ||||||||||||
| NBTBBBD1 | 127·05‡ (109·78–160·60) | 106·70⁎‡ (62·94–128·60) | 49, 30 | 1·02 (1·01–1·04) | 0·0017 | 0·73 (0·61–0·82) | 129·47‡ (111·38–161·72) | 102·08⁎‡ (65·53–127·64) | 59, 34 | 1·03 (1·01–1·04) | 0·0003 | 0·73 (0·62–0·84) |
| NBTBBBD2 | 135·41a (90·94–180·24) | 90·94⁎‡ (64·84–115·12) | 32, 18 | 1·03 (1·01–1·05) | 0·0086 | 0·80 (0·64–0·94) | 140·32‡ (104·34–191·58) | 90·23⁎‡ (62·70–102·74) | 35, 20 | 1·04 (1·02–1·07) | 0·0019 | 0·84 (0·71–0·97) |
| NBTBBBD3 | 127·72‡ (102·66–167·98) | 94·63⁎‡ (72·22–110·45) | 50, 28 | 1·02 (1·01–1·04) | 0·0016 | 0·77 (0·65–0·88) | 127·72‡ (102·66–167·98) | 94·63⁎‡ (70·52–109·71) | 58, 30 | 1·03 (1·01–1·05) | 0·0002 | 0·77 (0·66–0·88) |
| NBTBBBD4 | 105·61†‡ (79·18–146·66) | 69·87⁎†‡ (54·99–97·62) | 23, 21 | 1·03 (1·01–1·05) | 0·0114 | 0·75 (0·61–0·90) | 110·68†‡ (79·21–147·64) | 67·52⁎†‡ (54·99–92·29) | 24, 22 | 1·04 (1·01–1·07) | 0·0031 | 0·79 (0·65–0·93) |
| ABTBBBD10volume, ml | ||||||||||||
| ABTBBBD110 | 42·19 (28·51–60·71) | 29·19⁎ (17·29–46·95) | 49, 30 | 1·04 (1·01–1·06) | 0·0092 | 0·66 (0·53–0·78) | 45·78 (28·68–65·18) | 29·63⁎ (18·58–45·26) | 59, 34 | 1·04 (1·01–1·06) | 0·0077 | 0·67 (0·54–0·79) |
| ABTBBBD210 | 51·74 (38·48–72·96) | 28·42⁎ (15·92–47·61) | 32, 18 | 1·05 (1·01–1·08) | 0·0076 | 0·77 (0·62–0·93) | 55·58 (40·57–74·87) | 28·42⁎ (13·77–43·44) | 35, 20 | 1·09 (1·04–1·16) | 0·0001 | 0·81 (0·67–0·95) |
| ABTBBBD310 | 52·14 (34·98–74·48) | 28·11⁎ (17·88–38·96) | 50, 28 | 1·04 (1·01–1·06) | 0·0022 | 0·76 (0·65–0·88) | 52·14 (34·98–74·48) | 28·11⁎ (17·82–39·98) | 58, 30 | 1·05 (1·02–1·07) | 0·0004 | 0·76 (0·64–0·87) |
| ABTBBBD410 | 36·67† (24·35–54·67) | 9·98⁎† (6·65–23·52) | 23, 21 | 1·08 (1·03–1·14) | 0·0012 | 0·85 (0·73–0·97) | 36·66† (24·74–54·31) | 9·47⁎† (6·65–20·89) | 24, 22 | 1·09 (1·04–1·16) | 0·0009 | 0·87 (0·76–0·98) |
| ABTBBBD20volume, ml | ||||||||||||
| ABTBBBD120 | 38·89 (29·32–47·82) | 27·50 (20·35–48·14) | 49, 30 | 1·02 (0·99–1·05) | 0·0824 | 0·61 (0·48–0·74) | 39·16 (30·12–48·89) | 30·41⁎ (21·07–42·53) | 59, 34 | 1·03 (1·00–1·06) | 0·0264 | 0·64 (0·52–0·76) |
| ABTBBBD220 | 35·51 (30·21–50·70) | 28·61⁎ (16·60–34·74) | 32, 18 | 1·06 (1·01–1·12) | 0·0225 | 0·76 (0·53–0·87) | 35·59 (27·83–50·98) | 25·02⁎ (15·84–34·51) | 35, 20 | 1·09 (1·03–1·16) | 0·0040 | 0·77 (0·60–0·91) |
| ABTBBBD320 | 33·71 (26·32–44·09) | 25·64⁎ (17·03–32·99) | 50, 28 | 1·06 (1·02–1·11) | 0·0049 | 0·74 (0·58–0·83) | 33·71 (25·42–44·35) | 25·64⁎ (16·70–30·37) | 58, 30 | 1·07 (1·03–1·12) | 0·0013 | 0·74 (0·59–0·83) |
| ABTBBBD420 | 34·60 (29·37–50·75) | 18·39⁎ (7·76–30·01) | 23, 21 | 1·08 (1·02–1·14) | 0·0039 | 0·79 (0·65–0·92) | 34·69 (29·73–50·28) | 17·94⁎† (7·76–28·12) | 24, 22 | 1·10 (1·03–1·17) | 0·0017 | 0·82 (0·67–0·95) |
OR = odds ratio. PC = progressive course of aSAH. NPC = non-progressive course of aSAH.
Significant difference ≤ 0·05 between outcome groups (eGOS ≤ 4, vs eGOS > 4, or PC vs NC) with Wilcoxon sum-rank test⁎. Significant difference ≤ 0·05 between different time points with Friedman test†. Significant difference ≤ 0·05 between control and a measurement at a single time point with Wilcoxon sum-rank test‡. The area under the ROC curve (AUC) results were considered “excellent” for AUC values between 0·9–1, “good” for AUC values between 0·8–0·9, “fair” for AUC values between 0·7–0·8, “poor” for AUC values between 0·6–0·7 and failed for AUC values between 0·5–0·6.
Only significant OR values were presented in the table.
2.2. Clinical data
Obtained data included location of aneurysm; type of intervention; vasospasm identification; history of arterial hypertension, anemia on admission anemia on admission defined as total hemoglobin <14 g/dl in males and < 12 g/dl in females, abnormal liver enzymes on admission defined as alanine aminotransferase (ALT) >45/34 U/L (male/female), aspartate aminotransferase (AST) >50/35 U/L or gammaglutamyl transferase (GGT) >55/38 U/L; history of previous aSAH; World Federation of Neurosurgical Societies (WFNS) scale [24] and Rosen-Macdonald Score (RMS) [25] at admission and extended Glasgow Outcome Scale (eGOS) measured at t4, as mentioned above. Significant proximal vasospasm (V+) was defined using Doppler sonography, if mean velocity was >200 cm/s in at least one middle cerebral artery (MCA). Vasospasm (V-) was excluded if the MCA mean velocities remained <120 cm/s and was defined as possible/not excluded (V−/+) when mean velocity was between 120 and 200 cm/s [26]. WFNS and RMS data on admission were categorized into favorable (WFNS<4, RMS ≤ 4) or poor (WFNS≥4, RMS > 4). Poor outcome was defined as eGOS≤5 (severe disability or death) and favorable outcome as eGOS>5 (no or moderate disability).
2.3. Image analysis
MR images (including T1w, FLAIR and DWI sequences) were automatically segmented into apparently normal brain tissue (NBT) in the combined gray matter (GM) and white matter (WM), lateral ventricles (LV) and subarachnoid space (SAS), or ABT comprising apparently abnormal signal, reflecting cytotoxic or vasogenic edema, gliosis or hemorrhage. Image processing methods, including multimodal normal tissue and ABT segmentation, BBB permeability evaluation, and ABT vicinity analysis, are provided in the appendix. Prediction of ABT volume at long-term outcome, assessment of BBBD persistency over time, and evaluation of BBBD predictive properties in ABT progression and long-term clinical outcomes are provided in the appendix.
2.4. Patient classification based on imaging-detected loss of normal brain tissue in consecutive scans
Because intracranial volume is fixed, the increase in ABT and/or enlargement of LV and SAS were associated with decrease in NBT volume. Such decrease in consecutive scans was attributed to disease “progressive course” (PC), while no change, or increase in NBT volume was considered as a “non-progressive course” (NPC) (see appendix).
2.5. Statistical analysis
Quantitative and ordinal data are presented as median with interquartile range and categorical variables are presented as values and percentages. The χ2 and Fisher's exact (employing Monte Carlo method) tests (for contingency table >2 × 2) were used to analyze categorical variables. The Wilcoxon rank-sum test was used to analyze continuous independent variables between two groups. A non-parametric Kruskal–Wallis test was performed for continuous independent variables to test the overall multiple group (>2) differences. The Wilcoxon sign-rank test and Friedman test were used for comparison of dependent variables. When the overall comparisons between different time points were significant, post-hoc tests for multiple comparison correction were performed using Bonferroni procedure. Univariate-logistic regression analysis was used to identify possible risk factors for outcomes of aSAH and odds ratios including 95% confidence intervals were presented. The possible risk factors were included in the multivariate logistic regression analysis for outcomes prediction. Variable selection (forward, inclusion p = .05, exclusion p = .10) was applied. Receiver operating characteristic (ROC) analysis was performed to assess the diagnostic accuracy of outcome prediction models [27]. The level of significance was 0.05 (two-sided) in all analyses.
3. Results
3.1. Clinical findings, demographics and outcome of aSAH patients
Overall, clinical, demographic and imaging data were analyzed from 124 aSAH patients, including 84 women (67·7%) and 40 men, admitted to 5 medical centers between 2007 and 2016 (appendix, Table 1). Mean age of patients was 55·2 ± 11·3 years (range, 22–79 years). We also scanned 21 healthy control subjects, 28·1 ± 14·7 years (range, 19–46 years). Females were significantly older at the time of admission compared to males (56 (49–64) vs. 50 (44–60·5) years, p < 0·001, Wilcoxon test). The most frequent locations of aneurysm were middle cerebral artery (MCA) (47/124, 37·9%), anterior communicating artery (ACoA) (n = 39, 31·5%) and posterior communicating artery (n = 18, 14·5%). Among females, most were along the MCA (45·2% compared to 22·5% in males, p = 0·025, χ2 test), while ACoA aneurysms were more common in males (50·0% compared to 22·6% in females, p = 0·004, χ2 test). Other locations showed no statistically significant difference between both genders (p > 0·4, χ2 test). Near-significant association was found between age and aneurysm location (p = 0·063, Kruskal-Wallis, patients with MCA aneurysm were younger 51 (46–59) years, than patients with anterior communicating artery (ACA) aneurysm 69 (59–72) years, n = 5). Patients were either treated with surgical clipping (110, 88·7%) or coiling (14, 11·3%). No statistically significant relationships were found between the intervention type and age (p > 0·4), gender (p = 0·377, χ2 test), aneurysm location (p > 0·7, Fisher's test) or presence of vasospasm (p > 0·4, Fisher's test).
No statistically significant relationships were found between WFNS score on admission and gender (p = 0·294, χ2 test), age (p = 0·305, Wilcoxon test), aneurysm location (p = 0·680, Fisher test), type of treatment (p = 0·333, χ2 test) or presence of vasospasm (p = 0·264, Fisher test). Similarly, no statistically significant relationships were found between RMS score on admission and gender (p = 0·771, χ2 test), aneurysm location (p = 0·299, Fisher test), or presence of vasospasm (p = 0·472, Fisher test). However, RMS was related to patient age (p < 0·001, Wilcoxon test).
Patients with a poor outcome (eGOS) were older than those with a favorable outcome (57 (48–65) years vs 52 (44–59) years; p = 0·009). No statistically significant relationships were found between eGOS category and aneurysm location (p = 0·762, Fisher's test), gender (p = 0·604, χ2 test), intervention (p = 0·660, χ2 test) or presence of vasospasm (p = 0·743, Fisher's test) (Table 1).
A significant association was found between presence of arterial hypertension and anemia on admission and a poor outcome (p = 0·0013 and p = 0·0266, χ2 test, correspondently, Table 1).
3.2. Dynamics of intracranial measures
Volume of ABT was measured longitudinally in 377 imaging sets from 124 patients (Fig. 1 and appendix, Table 2). ABT volume in the early scan (t1, 24–48 h after event) was similar in females and males; increased with patient age; largest in patients with MCA aneurysms; significantly larger in patients with poor WFNS (p = 0·003, Wilcoxon rank-sum test) and in patients with poor outcome (Table 1, Table 2). ABT volume at t4 (>6 months after event) was not significantly associated with age or aneurysm location and was significantly larger in patients with poor WFNS and with poor eGOS (p = 0·0002, Table 2). Overall, ABT volume significantly increased with time (Fig. 1f). As expected, change in ABT volume was inversely correlated with the volume of NBT (p < 0·006). Interestingly, volume of CSF compartments (lateral ventricles and SAS) significantly increased only at t4 (p < 0·001, Fig. 1d-e). No significant differences were found between patients who were surgically clipped or coiled (p > 0·6, Wilcoxon rank-sum test).
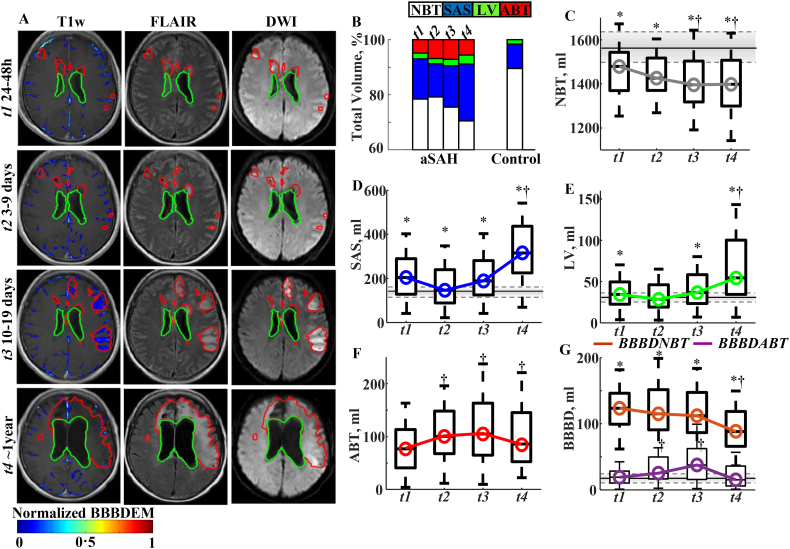
Fig. 1.
Dynamics of brain MR findings during follow-up.
a. Representing MR scans during follow-up. Detected abnormal brain tissue (ABT) in red contour. BBBD enhancement maps (BBBDEM) were created by reassigning each voxel with BBBD its enhancement level. The final BBBDEM was normalized to 0–1 range (minimal to maximal enhancement level).
b. Bar graph showing composition of skull-peeled volume of interest at different time point during follow-up. Normal brain tissue (NBT - white); CSF sub-arachnoid space (SAS - blue); Lateral ventricles (LV -green); ABT - red. Note the gradual reduction in NBT during follow-up.
c. Box plot showing mean NBT volumes in aSAH patients over time. Note a significant decrease beginning ~2 weeks after the event (p = 0·002, Friedman).
d, e. Volume of LV (D) and SAS (E) showing a significant increase at t4 (p < 0·001, Friedman).
f. ABT volume significantly increased in aSAH patients at all time intervals in comparison to t1 (p < 0·02). NBT and ABT volumetric changes followed a logarithmic pattern (R2adj = 0·82(0·76–0·99) for brain volume and R2adj = 0·81(0·78–0·97) for ABT volume (p < 0·01); correlation between model intercept (Co) and NBT and ABT sizes were correspondently: R2 = 0·96 (p = 0·02) and R2 = 0·88 (p = 0·01)). Dynamic of ABT and BBBD in a subgroup with all four time points is presented in Supplementary Fig. 3.
g. Brain volume with BBBD within the “abnormal” brain (ABTBBBD) significantly increased in t2 and t3 compared to t1 followed by a significant reduction at t4 (lilac color). BBBD within the NBT (brown color) was 3·2 (0·8–10·8) fold greater compared to that in healthy controls at all the investigated time intervals t1-t4 (p < 0·001). Notably, brain volume with BBBD within the NBT persisted during the first two week after the acute bleeding event and was significantly reduced only at t4 (p < 0·01). Overall, NBT brain volume with BBBD was larger compared to that measured in controls at all-time points.
Control data are represented by horizontal solid (median) and dash (third and first quartiles) lines.
†Significant difference (p ≤ 0·05) between time points (Friedman test followed the Bonferroni procedure).
*Significant difference (p ≤ 0·05) between control and aSAH patients at a single time point (Wilcoxon rank sum test).
ABT = abnormal brain tissue; aSAH = aneurysmal subarachnoid hemorrhage; BBBD = blood brain barrier damage; ABTBBBD = blood brain barrier damage measured in abnormal brain tissue volume; NBTBBBD = blood brain barrier damage measured in normal brain tissue volume; LV = lateral ventricles; NBT = normal brain tissue; SAS = subarachnoid space.
3.3. BBB dysfunction
Contrast-enhanced MRIs from 104 patients (total of 282 scans) were used to detect brain voxels with BBBD (Supp. Figure and Fig. 1, Fig. 2a). Interestingly, most voxels with BBBD were found within the apparently healthy brain (Fig. 1g), indicating that despite abnormal contrast enhancement, this brain tissue does not show a pathological signal in routine MR sequences (including T1w, FLAIR and DWI). BBBD within the apparently healthy brain voxels was most commonly found within a 1-cm ring surrounding lesion cores (Fig. 2m).
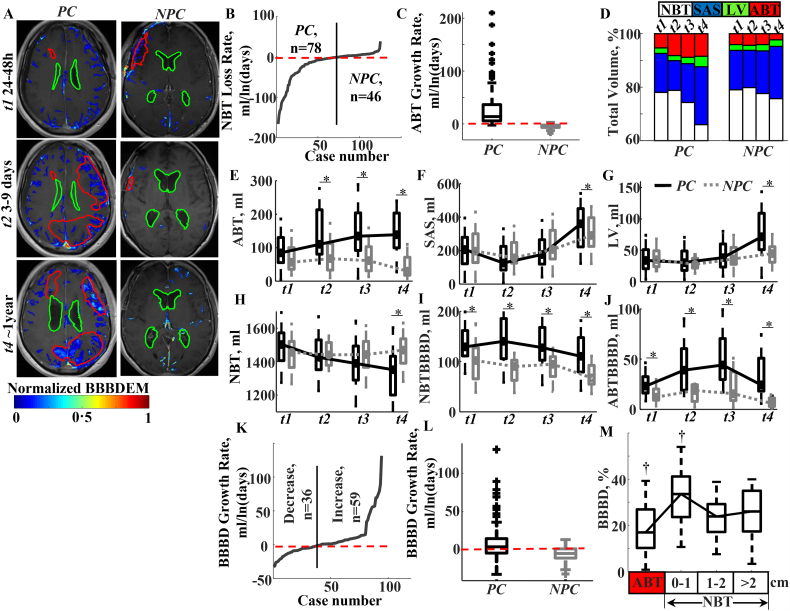
Fig. 2.
Different BBB dynamics in patients with progressive vs non-progressive disease course.
a. Representing MR images from aSAH patients with a progressive (PC) and non-progressive (NPC) disease course. BBBD enhancement maps (BBBDEM, color bar) are superimposed. Red contour demarcates detected abnormal brain tissue (ABT) and green contour demarcates lateral ventricles (LV). BBBDEM were created by reassigning each voxel with BBBD its enhancement level. The final BBBDEM was normalized to 0–1 range (minimal to maximal enhancement level).
b. Based on measurements of changes in normal brain tissue (NBT) volume over time, patients were classified as having either a “progression course” (PC), in which NBT decreased with time (t2-t4) compared to the first, acute scan (t1) (median slope: −18·75 (−48·92–9·07) ml/ln(days)), or a “non-progression course” (NPC), characterized by NBT volume change slope of 0·73 (0·06–3·58) ml/ln(days).
c. Box plot showing a significant difference in ABT growth rate between PC and NPC groups: 13·99 (5·90–36·68) ml/ln(days) vs −3·50 (−6·45–2·09) ml/ln(days); p < 0·001, Wilcoxon test.
d. Bar graph showing the distribution in brain volume of NBT (white), subarachnoid space (SAS, blue), LV (green) and ABT (red) over time in the two patient groups. The increase in ABT and/or enlargement of LV and SAS were associated with decrease in NBT volume. The relative contributions of ABT growth vs CSF enlargement (LV + SAS) to NBT atrophy was about 1:2 for PC and 1:4 for NPC patients.
e. A significant increase in ABT volume during consecutive scans in PC patients (ABTi/ABT1 > 1, for i = 2÷4, p < 0·001; see also Table 2, appendix: Table 2B). In contrast, NPC patients displayed non-significant change in ABT volume during the first 2 weeks (3 scans) after the acute bleeding event and a significant decrease in ABT volume by t4 (p < 0·007; see also appendix Table 2B).
f, g. Significant differences between PC and NPC groups in both SAS volume (f) and LV volume (g) were found only in the late (t4) scans (p < 0·001, Wilcoxon test; see also Table 2). Note lack of significantly differences between the groups in none of the measures (ABT, LV or SAS volume) during the acute stage.
h. Significant difference between the groups in NBT volume were seen only in the late (t4) scans (p < 0·001; Table 2). In the PC group, a significant decrease in NBT was detected from ~2 weeks after the event (p < 0·01, Friedman).
i, j. Volumes of BBBD in both NBT (i, NBTBBBD) and ABT (i, ABTBBBD) were persistently and significantly greater in the PC group relative to NPC group in all time points (p < 0·001, Wilcoxon test, see also Table 2). In both groups, a significant decrease in NBTBBBD was seen only by t4 (p < 0·001, Friedman), whereas ABTBBBD increased significantly between t2-t3 in PC group, and decreased significantly by t4 in NPC group (p < 0·001, Friedman). Resolution of BBBD to 95% CI values of “healthy controls” (<47·5 ml) was found only in three patients. All these three patients were in NPC group and had ABT size significantly smaller than patients with non-resolved BBBD throughout the investigation. Overall, NBTBBBD volume was larger in both groups compared to that measured in controls at all time points (Table 2).
k. Overall, in 62% of aSAH patients brain volume with BBBD (NBTBBBD + ABTBBBD) increased with time (slope: 8·57(2·02–24·64) ml/ln(days)) and in 38% of patients BBBD decreased with time (slope: −8·95(−16·87–5·07) ml/ln(days)).
l. Box plot showing a significant difference in BBBD growth rate between PC and NPC groups: 3·93 (−4·44–16·78) ml/ln(days) vs −4·73 (−10·74–1·32) ml/ln(days), p = 0·001, Wilcoxon test). Interestingly, while in 64% of the patients from the PC group brain volume with BBBD increased with time, in 76% of patients in the NPC group BBBD decreased with time (p = 0·022, χ2 test).
m. Box plot of the distribution of BBBD in abnormal and apparently normal brain tissue, where it was measured in three regions of interest based on the distance to ABT border (in cm). BBBD voxels were not distributed equally through all regions (p < 0·001). The highest content of BBBD voxels was found within 1 cm of ABT (33·58 (23·66–41·18) % of entire BBBD volume), whereas the lowest content of BBBD voxels was located in ABT (16·98 (10·25–26·92) % of entire BBBD volume). No significant difference was found in content of BBBD between the two remaining regions (1–2 cm and > 2 cm from ABT border): 23·90 (17·17–29·27) % and 25·16 (17·34–35·01) %, correspondently.
*Significant difference (p ≤ 0·05) between outcome groups (PC vs NPC), Wilcoxon sum-rank test.
†Significant difference (p ≤ 0·05) between time points, Friedman test followed the Bonferroni procedure.
ABT = abnormal brain tissue; aSAH = aneurysmal subarachnoid hemorrhage; ABTBBBD = blood brain barrier damage measured in abnormal brain tissue volume; NBTBBBD = blood brain barrier damage measured in normal brain tissue volume; CI = confidence interval; CSF = cerebrospinal fluid; LV = lateral ventricles; NBT = normal brain tissue; SAS = subarachnoid space.
The extent and volume of brain regions with BBBD were not significantly associated with gender (p > 0·09, the Mann-Whitney test), aneurysm location (p > 0·3, Kruskal-Wallis test), or treatment (clipped vs coiled, p > 0·7). BBBD within the ABT was significantly increased at t2 and t3 compared to t1 and was significantly reduced only in delayed scans (t4). Within the apparently normal brain tissue, BBBD did not change during the first two weeks after the event (t1-t3) and significantly declined only in the late scan (t4, p < 0·01, Friedman test, Fig. 1g). To examine the time course of BBBD in specific brain voxels, we calculated the probability of a voxel with BBBD to remain permeable. Interestingly, the probability of any brain voxel to display BBBD during the first week after the acute bleeding (P1, 2BBBD=0·370(0·274–0·587)) was not significantly different from that measured during the second week (P2, 3BBBD=0·382(0·311–0·412)) and significantly lower only at delayed time points, t3 and t4 (P3, 4BBBD=0·238(0·176–0·365)), p = 0·005, Friedman test).
To test the predictive value of BBBD, we examined the outcome of a BBB-disrupted voxel within healthy brain tissue. Results showed that a NBT voxel with BBBD had a significantly higher probability to become abnormal (ABT) compared to a voxel with intact BBB (p < 0·001, Wilcoxon rank-sum test, Table 3). These results highlight BBBD as a potential early predictive biomarker for brain tissue at risk. While BBBD could be found in brain regions distant to the ABT, the highest probability of a BBB-disrupted voxel (comparably to non-BBB-disrupted) to turn into ABT was found at a distance of ≤1 cm from the ABT (p < 0·001, Kruskal-Wallis, Table 3). In contrast, the probability of a non-BBBD voxel to turn into ABT was significantly lower compared to a voxel with BBB and was not related to distance from ABT (Table 3).
Table 3.
Investigation of a voxel fate:
| Probability of turnover of a normal brain tissue voxel at t1 to turn into ABT at t4: |
||||
|---|---|---|---|---|
| ABT10 | ABT20 | ABT>20 | n | |
| NBTBBBD1 | 0·284⁎,⁎⁎⁎ (0·122–0·594) | 0·196⁎ (0·106–0·412) | 0·212⁎ (0·099–0·464) | 70 |
| 0·089 (0·068–0·141) | 0·063 (0·045–0·101) | 0·069 (0·052–0·117) | 70 | |
| Probability of persistence of an abnormal brain tissue voxel with BBBD to remain ABT at t4: | ||
|---|---|---|
| Total | n | |
| ABTBBBD1 | 0·697 (0·584–0·841)⁎⁎ | 70 |
| ABTBBBD2 | 0·721 (0·596–0·838)⁎⁎ | 61 |
| ABTBBBD3 | 0·740 (0·630–0·937)⁎⁎ | 64 |
| Probability of persistence of an abnormal brain tissue voxel without BBBD to remain ABT at t4: | ||
|---|---|---|
| Total | n | |
| 0·244 (0·102–0·348) | 70 | |
| 0·252 (0·121–0·377) | 61 | |
| 0·242 (0·114–0·353) | 64 | |
⁎Significant difference between probabilities of a normal brain tissue (NBT) voxel with blood brain barrier damage (BBBD) (NBTBBBD1) and without BBBD () to become an abnormal brain tissue (ABT4) voxel at t4 (p < 0·05, Wilcoxon sum-rank test).
⁎⁎Significant difference between probabilities of ABT voxel with BBBD (ABTBBBDj) and without BBBD () to remain an ABT4 voxel at t4 (where j = 1, …,3 denotes time interval t1-t3) (p < 0·05, Wilcoxon sum-rank test).
⁎⁎⁎Significant difference between different regions: ABT10, ABT20 and ABT>20 (p < 0·05, Kruskal-Wallis test).
3.4. Dynamics of intracranial measures reveals a progressive or non-progressive disease course
According to the dynamic changes found in tissue characteristics over time, patients were grouped into a “progressive” and “non-progressive” disease course (PC and NPC, respectively). PC was attributed to patients in whom the healthy brain tissue (NBT) volume decreased between the initial and last scan, while NPC was attributed to patients in whom the abnormal tissue volume decreased (Fig. 2b-c). In our cohort, 63% (78/124) of patients were classified as having a PC and 37% (46/124) displayed a NPC. No differences were found between the groups in distributions of gender, aneurysm location and type of intervention (Table 2). Importantly, the initial early scan (t1) did not show differences between the groups in volume of ABT, LV or SAS.
Imaging-based categorization of disease course highly correlated with clinical outcome, as determined by eGOS, with a sensitivity of 98% and specificity of 97%: Forty-two (97·7%) patients with a non-progressive course had favorable eGOS, whereas 67 (97·1%) patients with a progressive course had poor eGOS. Thus, out of 112 patients, only three patients (2·68%) were misclassified: one patient with a gradual increase in the volume of apparently abnormal tissue showed a good outcome (eGOS = 6), and two patients with a decrease in measured abnormal tissue had a poor outcome (eGOS = 5). In contrast, disease course correlated poorly with clinical status on admission (sensitivity = 0·674, specificity = 0·654, appendix Table 3). The correspondence between aSAH course (PC/NPC groups) and WFNS categories was 64·5% (80/124 matched), and to RMS categories it was 70·2% (87/124 matched, sensitivity = 0·655, specificity = 0·716).
3.5. BBBD dynamics and clinical course
Interestingly, already at the very early scan (t1) and in all investigation time points, brain volume with BBBD was significantly larger in the progressive course compared to the non-progressive disease course group (p = 0·001, Wilcoxon rank-sum test). When correlated with clinical status on admission (WFNS), we found that during all scanning time points, the extent of BBBD in abnormal brain tissue (ABT-BBBD) was associated with poor WFNS (p < 0·041, Wilcoxon rank-sum test). Notably, at all time points and in both NBT and ABT, BBBD was significantly larger in patients within the poor eGOS group (p < 0·001, Wilcoxon rank-sum test, Table 2).
3.6. Predictive value of clinical and imaging biomarkers with ROC analysis
We next used logistic regression models in search of clinical and imaging biomarkers that best predict aSAH outcome. Model fit and ROC analysis results for predicting long-term eGOS are shown in Table 4 and Fig. 3. When only clinical data were used, ROC analysis revealed a “fair” area under curve (AUC) for both models, consisting of either age and WFNS or RMS score only. Prediction was improved with addition of extent of BBBD in apparently normal brain tissue (BBBD-NBT) and volume of ABT.
Table 4.
Models for long-term outcome prognoses:
| Model | Coefficient | Coefficient Value (SE) | OR (95% CI) | p | n | AUC (SE) | |
|---|---|---|---|---|---|---|---|
| A. Models for prediction course of aSAHa | |||||||
| Ψ0WFNS | β1*WFNS | β1 | −0·445 (0·129) | 0·64 (0·50-0·83) | 0·001 | 124 | 0·673 (0·052) |
| Ψ0Age&WFNS | β1*Age + β2*WFNS | β1 | −0·034 (0·018) | 0·97 (0·93-1·00) | 0·060 | 124 | 0·712 (0·049) |
| β2 | −0·434 (0·130) | 0·65 (0·50-0·84) | 0·001 | ||||
| Ψ0RMS | β1*RMS | β1 | −0·431 (0·113) | 0·65 (0·52-0·81) | 0·000 | 124 | 0·719 (0·048) |
| Ψ1 | β1*NBTBBBD1 + | β1 | −0·035 (0·010) | 0·97 (0·95-0·98) | 0·000 | 93 | 0·829 (0·043) |
| β2 *RMS | β2 | −0·348 (0·149) | 0·71 (0·53-0·95) | 0·020 | |||
| Ψ2 | β1*NBTBBBD2 + | β1 | −0·057 (0·018) | 0·55 (0·33-0·94) | 0·030 | 55 | 0·905 (0·042) |
| β2*WFNS | β2 | −0·590 (0·272) | 0·95 (0·91-0·98) | 0·001 | |||
| Ψ3 | β1*NBTBBBD3 | β1 | −51·697 (13·931) | 0·00 (0·00-0·00) | 0·000 | 88 | 0·793 (0·049) |
| Ψ4 | β1*ABT4 + | β1 | −0·138 (0·060) | 0·87 (0·77-0·98) | 0·022 | 46 | 0·979 (0·017) |
| β2*NBTBBBD4 + | β2 | −0·059 (0·027) | 0·94 (0·89-0·99) | 0·027 | |||
| β3*ABTBBBD4 | β3 | 0·298 (0·148) | 1·35 (1·01-1·80) | 0·044 | |||
| B. Models for prediction of eGOS status of aSAHa: | |||||||
| Ψ0WFNS | β1*WFNS | β1 | −0·471 (0·138) | 0·63 (0·48-0·82) | 0·001 | 112 | 0·676 (0·055) |
| Ψ0Age&WFNS | β1*Age + β2*WFNS | β1 | −0·062 (0·021) | 0·94 (0·90-0·98) | 0·003 | 112 | 0·750 (0·050) |
| β2 | 0·484 -(0·144) | 0·62 (0·47-0·82) | 0·001 | ||||
| Ψ0RMS | β1*RMS | β1 | −0·564 (0·132) | 0·57 (0·44-0·74) | 0·000 | 112 | 0·757 (0·048) |
| Ψ1 | β1*NBTBBBD1 + | β1 | −0·027 (0·009) | 0·97 (0·96-0·99) | 0·003 | 79 | 0·827 (0·051) |
| β2 *RMS | β2 | −0·491 (0·168) | 0·61 (0·44-0·85) | 0·004 | |||
| Ψ2 | β1*ABT2 + β2*NBTBBBD2 | β1 | −0·013 (0·007) | 0·99 (0·97-1·00) | 0·073 | 50 | 0·871 (0·052) |
| β2 | −0·030 (0·012) | 0·97 (0·95-0·99) | 0·012 | ||||
| Ψ3 | β1*ABTBBBD3 + | β1 | −0·044 (0·014) | 0·96 (0·93-0·98) | 0·002 | 78 | 0·830 (0·048) |
| β2*RMS | β2 | −0·382 (0·169) | 0·68 (0·49-0·95) | 0·023 | |||
| Ψ4 | β1*ABT4 + | β1 | −0·087 (0·030) | 0·92 (0·86-0·97) | 0·004 | 44 | 0·942 (0·035) |
| β2*ABTBBBD4 | β2 | 0·156 (0·075) | 1·17 (1·01-1·36) | 0·037 | |||
SE = standard error.
Uncategorized WFNS and RMS grades were used in the logistic model. Since the neurological grades on admission RMS and WFNS are dependent [25] variables, only one of them can be included in the multivariate model at the time.
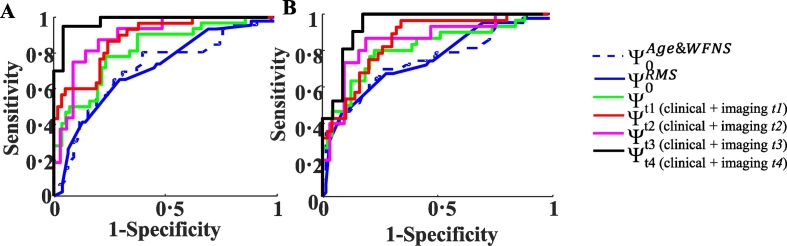
Fig. 3.
Analysis of the predictive value of BBBD measurements in aSAH patients.
Receiver operating characteristic (ROC) analysis showing specificity and sensitivity for prediction of aSAH course (a) and of long-term eGOS category (b). Whereas Ψ0Age&WFNS (blue dashed line) and Ψ0RMS (blue solid line) models contain only clinical data, the remaining models combine clinical data with imaging results at the different time points (t1-t4). When only clinical data was used, ROC analysis revealed a “fair” area under the curve (AUC) for the models consisting of either patient age and WFNS score (Ψ0Age&WFNS: AUC = 0·712 for (a) and AUC = 0·750 for (b)), or of RMS score only (Ψ0RMS: AUC = 0·719 for (a) and AUC = 0·757 for (b)). Prediction was improved with addition of t1 time–restricted imaging predictor, i.e. the extent of BBBD in apparently normal brain tissue (NBTBBBD1) and in volume of ABT1 (ABTBBBD1) (Ψt1: AUC = 0·829 for (a) and AUC = 0·827 for (b)). A better yet prediction was achieved with t2 time-restricted models (Ψt2: correspondently: AUC = 0·905 and 0·871 for (a) and AUC = 0·855 and 0·887 for (b)). The best prediction was achieved with t4 time-restricted model (Ψt4: AUC = 0·979 for (a) and AUC = 0·942 for (b)). While BBBD imaging alone revealed a “fair” prediction at t1 (AUC = 0·724(0·059) for (a)AUC = 0·726(0·061) for (b)) and a “good” prediction at t2 (AUC = 0.867(0.062) for (a)AUC = 0·816(0·073) for (b)).
ABT = abnormal brain tissue; AUC = area under the curve; aSAH = aneurysmal subarachnoid hemorrhage; ABTBBBD = blood brain barrier damage measured in abnormal brain tissue volume; NBTBBBD = blood brain barrier damage measured in normal brain tissue volume.
4. Discussion
Cerebral ischemia after aSAH is a cause of substantial morbidity and mortality [28]. In the present study, we examined the potential of quantitative imaging, including imaging of BBBD, in predicting delayed tissue damage and clinical outcome. In this patient population with severe aSAH [29], we found that: (1) Disease progression occurred mostly during the first week, but the volume of abnormal tissue may continue to progress for months after the acute hemorrhagic insult; (2) Imaging-based measurements showing a progressive disease were associated with a worse clinical outcome at >6 months; (3) Significant BBB pathology can be detected as soon as 24–48 h after the acute bleeding, and was found within the apparently normal and abnormal brain tissue; (4) BBB pathology was widespread but most likely found in the apparently normal brain tissue surrounding the abnormal brain tissue; (5) BBB pathology may persist for months after the insult and was larger in patients with a progressive course; (6) Voxels with BBBD within the apparently healthy brain, especially those located in close proximity to the ABT, were likely to become pathological; (7) Multi-linear regression model revealed a significant power for early detection of BBBD in predicting clinical outcome.
Demographic characteristics of the present aSAH cohort were consistent with previous studies. While females were twice more prone to aSAH, they were affected later in life compared to males [[30], [31], [32], [33]]. MCA and anterior communicating artery (ACoA) were the most frequent aneurysm locations, with ACoA aneurysms more frequent in males and MCA aneurysms more frequent in females [31,34,35]. Although coiling has gained acceptance as an alternative to clipping for aSAH treatment, it remains elusive how the two procedures are compared in terms of outcomes [36]. In the present cohort, no differences were found between the interventions in imaging- or clinical-based outcome. Investigation of clinical data revealed, that history of hypertension (~3.7 times is more likely), anemia (~4 times) and neurological scores WFNS (~4 times) and RMS (~9 times) at admission were detected as predictors for a poor clinical outcome.
We present here two novel, independent, semi-automatic and objective image analysis approaches for the identification of brain pathologies. The first was used for the detection and monitoring of brain lesions (ABT). Interpretation of abnormal brain tissue after aSAH is particularly challenging because of the multitude of pathological processes. For instance, cytotoxic edema after aSAH may result from the initial global ischemia, intracerebral hemorrhage, early or delayed focal cerebral ischemia, brain retraction or extra-ventricular drainage. In a similar fashion, findings of BBBD/vasogenic edema can be the radiological correlate of edema caused by the initial ischemia and reperfusion injury leading to global cerebral edema, subarachnoid blood products, brain retraction during aneurysmal surgery, early or delayed focal cerebral ischemia, chronic white matter lesions or transependymal edema due to hydrocephalus. Therefore, based on multimodal signal intensities, anatomical location, and morphology, we used a general assessment of ABT to measure a sum of different pathogenic processes. We validated our approach by comparison to manual segmentation in a subset of aSAH patients (see appendix). Furthermore, the excellent correlation of disease progression with patients' clinical outcome confirms the validity of our analysis approach. It further suggests that, while most of the tissue damage occurs during the first week after bleeding, disease continues to progress for weeks and months.
Studies aimed at determining reliable biomarkers for the identification of patients at high risk for delayed complications following aSAH have been scarce. DeRooij and collagues [37] developed a practical risk chart based solely on easily obtainable admission characteristics. Good clinical condition on admission (WFNS), small amount of extravasated blood and younger age were found to be associated with a low risk of delayed cerebral ischemia. However, the prediction model had poor specificity and sensitivity (AUC < 0·70). Ayling and colleagues [28] found that presence of an early cerebral infarct, poor WFNS and greater subarachnoid clot were associated with poor outcome. However, the ability of early infarction to predict outcomes was also poor (AUC = 0·62). In accordance with previous studies, we found that poor clinical condition on admission and advanced age are the earliest predictors of delayed increase in ABT [28,[37], [38], [39], [40], [41]]. In the present study, the predictive value of a model consisting of the significant predictor RMS was fair (AUC = 0·71), greater than WFNS, and comparable to prognostic accuracy of a model combining WFNS with age. We show that MR-based quantitative analysis significantly improves the capacity to identify patients with disease progression (i.e. decline in NBT) and poor outcome. Importantly, we found that detection of BBBD as early as 24–48 h after the acute event improves model prediction. BBBD after aSAH has been described in human [42] and in a number of animal studies [[43], [44], [45]]. Based on animal experiments, BBBD has been suggested to play a key role in neuro-inflammation, astroglial activation and network modification, epileptogenesis and neurodegeneration [12,46,47]. These data suggest a direct link between BBBD and long-term outcome.
This is the first clinical study testing the potential of quantitative BBBD imaging in identifying patients at-risk for disease progression. We report that BBBD is common in aSAH patients and is found in both the apparently healthy and abnormal brain tissue. Our findings are in agreement with pre-clinical experiments showing that disease progression overlaps spatially and follows temporally BBB pathology [48]. A substantial part of lesions observed after aSAH involves the cerebral cortex [49], and lesions typically develop adjacent to subarachnoid blood clots in both animals and humans [50,51]. Therefore, it has been suggested that subarachnoid blood products are an important pathophysiological elements, likely through constrictive effects on small cortical arteries, depolarizing effects on cortical neurons and astrocytes and disturbed functional coupling between the different cellular elements within the neurovascular unit [52]. An additional effect of blood might be BBB opening through factors such as potassium and/or hemoglobin released from erythrocytes [53]. Thus, as supported by the present study, BBBD may serve as an indicator for tissue at risk to develop ischemia in response to subarachnoid blood clots.
We thus suggest BBBD imaging as a potential novel diagnostic and predictive biomarker for tissue at risk to develop ischemia in response to subarachnoid hemorrhage for the following reasons: (1) BBB permeability increase can be detected as early as 24 h after the acute insult; (2) Brain volume with BBBD is found in patients with a progressive disease, and was the only differentiating factor between the groups at the early time point (t1); (3) Apparently normal brain tissue with BBBD is likely to become abnormal with time; and (4) Inclusion of early BBBD within the normal brain tissue in a regression model improves prediction of outcome. With the accumulation of data on vascular protecting therapy, it is not unlikely that microvascular pathology will become a target for early preventive treatment of patients after brain injury, further stressing the importance of quantitative diagnosis of vascular integrity.
This study had several limitations. First, ABT, detected by our algorithm, represents a mixture of different pathophysiological processes, including edema, hemorrhage and scar tissue. Future developments and identification of specific pathologies is expected to increase imaging sensitivity in predicting outcome, shed light on pathophysiological mechanisms in individual patients and offer specific treatment targets. Second, the study design posed difficulties such as unavailability of imaging data for all of the patients at all time points. Third, eGOS data were missing for ~10% of the patients. Only differences in frequencies of about 26% or more could be detected with sufficient power in this study. Nevertheless, despite of small sample size (n = 124) this is the first large-scale study within the research field on BBB spatiotemporal course and lesion progression in patients with brain injury, offering new quantitative neuroimaging analysis methods that are informative for patient monitoring and outcome prediction. To conclude, we highlighted micro-vascular pathology and specifically a leaky BBB as a potential mechanism underlying disease progression as well as a diagnostic and predictive biomarker. Future prospective studies are awaited to test and validate our approach following traumatic and ischemic brain injuries before contrast-enhanced MRI should be recommended as routine test in patients after brain injuries.
Declaration of interests
All authors declare no competing interests.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and corresponding author had final responsibility for the decision to submit for publication.
Contributors
All authors contributed to the idea for design of the study and data acquisition, reviewed and approved the final manuscript. SL had responsibility for data analysis and writing of the first draft of the manuscript. SL and PM performed statistical analysis of the data. AF and JPD came up with the concept and design for the study, and were responsible for study supervision and funding.
Acknowledgments
The study was designed and performed as a sub-study within the Co-Operative Study of Brain Injury Depolarizations (COSBID) study group. JPD was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (DFG DR 323/5-1), the Bundesministerium für Bildung und Forschung (BMBF) Center for Stroke Research Berlin 01 EO 0801 and FP7 no 602150 CENTER-TBI. AF was supported by grants from the DFG (DFG DR 323/5-1), Israel Science Foundation, Canada Institute for Health Research (CIHR) and the European Union’s Seventh Framework Program (FP7/2007–2013; grant #602102). JW was supported by grants from the DFG DR 323/5-1 and DFG WO 1704/1-1.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.04.054.
Appendix A. Supplementary data
Supplementary Fig. 1. Abnormal brain tissue quantification.
Flowchart describing steps 2 and 3 of the segmentation algorithm (see text for details). The final labeling overlaid T1w image (green, gray matter; blue, white matter; yellow, cerebrospinal fluid; red, abnormal brain tissue (ABT)). GMM = Gaussian mixture model.
Supplementary Fig. 2. Quantitative approach for the assessment of BBB pathology.
a. Enhancement distribution histograms of three representative regions, namely background (green), muscle (black) and vessel (red), were used to define BBB breakdown enhancement range of τ1 - τ2.;b. BBB damage enhancement map of brain tissue (gray matter + white matter) and lesion (excluding pixels relating to ventricles and subarachnoid space) as defined by enhancement range of τ1 - τ2.;C. Increased sensitivity BBB damage enhancement map. Initial BBB damage was iteratively expended by appending the connected neighbouring voxels that have enhancement in the extended range 0·5τ1−1·5τ2. BBB = blood brain barrier.
Supplementary Fig. 3. Dynamic of ABT and BBBD in a subgroup with all four time points.
a. ABT volume was not significantly in subgroup of aSAH patients (with all four time points MRI data, n = 76) in comparison to entire cohort - 124 aSAH patients (p > 0·11, Wilcoxon sign-rank test, Fig. 1f).;b. BBBD volume was not significantly in subgroup of aSAH patients (with all four time points MRI data, n = 61) in comparison to entire cohort - 124 aSAH patients (p > 0·16, Wilcoxon sign-rank test).
ABT = abnormal brain tissue; aSAH = aneurysmal subarachnoid hemorrhage; BBBD = blood brain barrier damage.
Supplementary material 1
Supplementary material 2
References
- 1.van Gijn J., Kerr R.S., Rinkel G.J. Subarachnoid haemorrhage. Lancet. Jan 27, 2007;369(9558):306–318. doi: 10.1016/S0140-6736(07)60153-6. http://www.ncbi.nlm.nih.gov/pubmed/17258671 Internet. cited 2017 Jul 9. Available from. [DOI] [PubMed] [Google Scholar]
- 2.Feigin V.L., Rinkel G.J.E., Lawes C.M.M., Algra A., Bennett D.A., van Gijn J. Risk factors for subarachnoid hemorrhage. Stroke. 2005;36(12) doi: 10.1161/01.STR.0000190838.02954.e8. http://stroke.ahajournals.org/content/36/12/2773.full Internet. cited 2017 Jul 9. Available from. [DOI] [PubMed] [Google Scholar]
- 3.Hong C.M., Tosun C., Kurland D.B., Gerzanich V., Schreibman D., Simard J.M. Biomarkers as outcome predictors in subarachnoid hemorrhage—a systematic review. Biomarkers. Mar, 2014;19(2):95–108. doi: 10.3109/1354750X.2014.881418. http://www.ncbi.nlm.nih.gov/pubmed/24499240 [Internet]. NIH Public Access. cited 2017 Jul 9. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dreier J.P., Fabricius M., Ayata C., Sakowitz O.W., William Shuttleworth C., Dohmen C. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J Cereb Blood Flow Metab. 2017;37(5) doi: 10.1177/0271678X16654496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreier J.P., Lemale C.L., Kola V., Friedman A., Schoknecht K. Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology. May 15, 2018;134(Pt B):189–207. doi: 10.1016/j.neuropharm.2017.09.027. http://www.ncbi.nlm.nih.gov/pubmed/28941738 Internet. cited 2018 Jun 2. Available from: [DOI] [PubMed] [Google Scholar]
- 6.Claassen J., Carhuapoma J.R., Kreiter K.T., Du E.Y., Connolly E.S., Mayer S.A. Global cerebral edema after subarachnoid hemorrhage. Stroke. 2002;33(5) doi: 10.1161/01.str.0000015624.29071.1f. http://stroke.ahajournals.org/content/33/5/1225.long Internet. cited 2017 Jul 9. Available from. [DOI] [PubMed] [Google Scholar]
- 7.Ostrowski R.P., Colohan A.R., Zhang J.H. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. Jun 19, 2006;28(4):399–414. doi: 10.1179/016164106X115008. http://www.tandfonline.com/doi/full/10.1179/016164106X115008 [Internet]. Taylor & Francis. cited 2017 Jul 9. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Song J.-N., Chen H., Zhang M., Zhao Y.-L., Ma X.-D. Dynamic change in cerebral microcirculation and focal cerebral metabolism in experimental subarachnoid hemorrhage in rabbits. Metab Brain Dis. Mar 12, 2013;28(1):33–43. doi: 10.1007/s11011-012-9369-8. http://link.springer.com/10.1007/s11011-012-9369-8 [Internet]. Springer US. cited 2017 Jul 9. Available from: [DOI] [PubMed] [Google Scholar]
- 9.Gu H., Fei Z.-H., Wang Y.-Q., Yang J.-G., Zhao C.-H., Cai Y. Angiopoietin-1 and angiopoietin-2 expression imbalance influence in early period after subarachnoid hemorrhage. Int Neurourol J. Dec, 2016;20(4):288–295. doi: 10.5213/inj.1632692.346. http://www.ncbi.nlm.nih.gov/pubmed/28043115 [Internet]. Korean Continence Society. cited 2017 Jul 9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanidze J., Ferraro R.A., Giambrone A.E., Segal A.Z., Gupta A., Sanelli P.C. Blood-brain barrier permeability in aneurysmal subarachnoid hemorrhage: correlation with clinical outcomes. Am J Roentgenol. Oct 7, 2018;211(4):891–895. doi: 10.2214/AJR.17.18237. https://www.ajronline.org/doi/10.2214/AJR.17.18237 [Internet]. American Roentgen Ray Society. cited 2018 Oct 20. Available from: [DOI] [PubMed] [Google Scholar]
- 11.Russin J.J., Montagne A., D'Amore F., He S., Shiroishi M.S., Rennert R.C. Permeability imaging as a predictor of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. Jun 3, 2018;38(6):973–979. doi: 10.1177/0271678X18768670. http://www.ncbi.nlm.nih.gov/pubmed/29611451 Internet. cited 2018 Jul 9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman A., Kaufer D., Heinemann U. Blood–brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. Aug, 2009;85(2–3):142–149. doi: 10.1016/j.eplepsyres.2009.03.005. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3615244&tool=pmcentrez&rendertype=abstract Internet. cited 2015 Apr 7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar-Klein G., Lublinsky S., Kamintsky L., Noyman I., Veksler R., Dalipaj H. Imaging blood–brain barrier dysfunction as a biomarker for epileptogenesis. Brain. Jun 1, 2017;140(6):1692–1705. doi: 10.1093/brain/awx073. http://www.ncbi.nlm.nih.gov/pubmed/28444141 Internet. cited 2017 Jul 10. Available from: [DOI] [PubMed] [Google Scholar]
- 14.Shlosberg D., Benifla M., Kaufer D., Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. Jul 15, 2010;6(7):393–403. doi: 10.1038/nrneurol.2010.74. http://www.nature.com/doifinder/10.1038/nrneurol.2010.74 Internet. cited 2017 Aug 7. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomkins O., Friedman O., Ivens S., Reiffurth C., Major S., Dreier J.P. Blood–brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. Feb, 2007;25(2):367–377. doi: 10.1016/j.nbd.2006.10.006. http://www.ncbi.nlm.nih.gov/pubmed/17188501 Internet. cited 2017 Aug 7. Available from. [DOI] [PubMed] [Google Scholar]
- 16.Tomkins O., Feintuch A., Benifla M., Cohen A., Friedman A., Shelef I. Blood-brain barrier breakdown following traumatic brain injury: a possible role in posttraumatic epilepsy. Cardiovasc Psychiatry Neurol. Jan, 2011;2011:765923. doi: 10.1155/2011/765923. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3056210&tool=pmcentrez&rendertype=abstract Internet. cited 2015 Feb 18. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chassidim Y., Vazana U., Prager O., Veksler R., Bar-Klein G., Schoknecht K. Analyzing the blood-brain barrier: the benefits of medical imaging in research and clinical practice. Semin Cell Dev Biol. Nov 29, 2014 doi: 10.1016/j.semcdb.2014.11.007. http://www.ncbi.nlm.nih.gov/pubmed/25455024 Internet. [cited 2015 Feb 18]; Available from: [DOI] [PubMed] [Google Scholar]
- 18.Tofts P.S. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. Jan, 1997;7(1):91–101. doi: 10.1002/jmri.1880070113. http://www.ncbi.nlm.nih.gov/pubmed/9039598 Internet. cited 2015 Apr 5. Available from: [DOI] [PubMed] [Google Scholar]
- 19.Tofts P.S., Kermode A.G. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. Feb, 1991;17(2):357–367. doi: 10.1002/mrm.1910170208. http://www.ncbi.nlm.nih.gov/pubmed/2062210 Internet. cited 2017 Aug 7. Available from: [DOI] [PubMed] [Google Scholar]
- 20.Chassidim Y., Veksler R., Lublinsky S., Pell G.S., Friedman A., Shelef I. Quantitative imaging assessment of blood-brain barrier permeability in humans. Fluids Barriers CNS. Jan, 2013;10(1):9. doi: 10.1186/2045-8118-10-9. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3570379&tool=pmcentrez&rendertype=abstract Internet. cited 2015 Jan 23. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weissberg I., Reichert A., Heinemann U., Friedman A. Blood-brain barrier dysfunction in epileptogenesis of the temporal lobe. Epilepsy Res Treat. 2011;2011:1–10. doi: 10.1155/2011/143908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkler M.K., Dengler N., Hecht N., Hartings J.A., Kang E.J., Major S. Oxygen availability and spreading depolarizations provide complementary prognostic information in neuromonitoring of aneurysmal subarachnoid hemorrhage patients. J Cereb Blood Flow Metab. May 20, 2017;37(5):1841–1856. doi: 10.1177/0271678X16641424. http://www.ncbi.nlm.nih.gov/pubmed/27025768 Internet. cited 2018 Jun 2. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreier J.P., Major S., Manning A., Woitzik J., Drenckhahn C., Steinbrink J. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. Jul 1, 2009;132(7):1866–1881. doi: 10.1093/brain/awp102. http://www.ncbi.nlm.nih.gov/pubmed/19420089 Internet. cited 2018 Dec 16. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drake C.G. Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. Jun, 1988;68(6):985–986. doi: 10.3171/jns.1988.68.6.0985. http://www.ncbi.nlm.nih.gov/pubmed/3131498 Internet. cited 2017 Aug 7. Available from. [DOI] [PubMed] [Google Scholar]
- 25.Rosen D.S., Macdonald R.L. Grading of subarachnoid hemorrhage: modification of the world World Federation of Neurosurgical Societies scale on the basis of data for a large series of patients. Neurosurgery. Mar, 2004;54(3):566–575. http://www.ncbi.nlm.nih.gov/pubmed/15028129 Internet. cited 2018 Feb 7. discussion 575-6. Available from: [PubMed] [Google Scholar]
- 26.Vora Y.Y., Suarez-Almazor M., Steinke D.E., Martin M.L., Findlay J.M. Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. Jun, 1999;44(6):1237–1247. http://www.ncbi.nlm.nih.gov/pubmed/10371622 Internet. cited 2018 Jan 24. discussion 1247-8. Available from: [PubMed] [Google Scholar]
- 27.Safari S., Baratloo A., Elfil M., Negida A. Evidence based emergency medicine; part 5 receiver operating curve and area under the curve. Emerg (Tehran, Iran) 2016;4(2):111–113. http://www.ncbi.nlm.nih.gov/pubmed/27274525 [Internet]. Shahid Beheshti University of Medical Sciences; [cited 2018 Dec 27]. Available from: [PMC free article] [PubMed] [Google Scholar]
- 28.Ayling O.G.S., Ibrahim G.M., Alotaibi N.M., Gooderham P.A., Macdonald R.L. Dissociation of early and delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. Stroke. Dec, 2016;47(12):2945–2951. doi: 10.1161/STROKEAHA.116.014794. http://www.ncbi.nlm.nih.gov/pubmed/27827324 Internet. cited 2017 Sep 16. Available from. [DOI] [PubMed] [Google Scholar]
- 29.Dreier J.P., Woitzik J., Fabricius M., Bhatia R., Major S., Drenckhahn C. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. Jun 9, 2006;129(12):3224–3237. doi: 10.1093/brain/awl297. http://www.ncbi.nlm.nih.gov/pubmed/17067993 Internet. cited 2018 Jun 2. Available from. [DOI] [PubMed] [Google Scholar]
- 30.Park S.K., Kim J.M., Kim J.H., Cheong J.H., Bak K.H., Kim C.H. Aneurysmal subarachnoid hemorrhage in young adults: a gender comparison study. J Clin Neurosci. Apr, 2008;15(4):389–392. doi: 10.1016/j.jocn.2007.04.007. http://www.ncbi.nlm.nih.gov/pubmed/18242092 Internet. cited 2017 Aug 24. Available from. [DOI] [PubMed] [Google Scholar]
- 31.Ghods A.J., Lopes D., Chen M. Gender differences in cerebral aneurysm location. Front Neurol. 2012;3(78) doi: 10.3389/fneur.2012.00078. http://www.ncbi.nlm.nih.gov/pubmed/22661965 [Internet]. Frontiers Media SA. cited 2017 Aug 24. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eden S.V., Meurer W.J., Sánchez B.N., Lisabeth L.D., Smith M.A., Brown D.L. Gender and ethnic differences in subarachnoid hemorrhage. Neurology. Sep 2, 2008;71(10):731–735. doi: 10.1212/01.wnl.0000319690.82357.44. http://www.ncbi.nlm.nih.gov/pubmed/18550859 [Internet]. American Academy of Neurology. cited 2017 Aug 24. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horiuchi T., Tanaka Y., Hongo K. Sex-related differences in patients treated surgically for aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo) Jul, 2006;46(7):328–332. doi: 10.2176/nmc.46.328. http://www.ncbi.nlm.nih.gov/pubmed/16861825 Internet. cited 2017 Aug 24. discussion 332. Available from: [DOI] [PubMed] [Google Scholar]
- 34.Lindner S.H., Bor A.S.E., Rinkel G.J.E. Differences in risk factors according to the site of intracranial aneurysms. J Neurol Neurosurg Psychiatry. Jan 1, 2010;81(1):116–118. doi: 10.1136/jnnp.2008.163063. http://www.ncbi.nlm.nih.gov/pubmed/20019231 Internet. cited 2017 Sep 16. Available from. [DOI] [PubMed] [Google Scholar]
- 35.Wermer M.J.H., van der Schaaf I.C., Algra A., Rinkel G.J.E. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics. Stroke. 2007;38(4) doi: 10.1161/01.STR.0000260955.51401.cd. http://stroke.ahajournals.org/content/38/4/1404 Internet. cited 2017 Sep 16. Available from. [DOI] [PubMed] [Google Scholar]
- 36.Yoon W. Current update on the randomized controlled trials of intracranial aneurysms. Neurointervention. Feb 1, 2011;6(1):1. doi: 10.5469/neuroint.2011.6.1.1. https://synapse.koreamed.org/DOIx.php?id=10.5469/neuroint.2011.6.1.1 Internet. cited 2017 Sep 24. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Rooij N.K., Greving J.P., Rinkel G.J.E., Frijns C.J.M. Early prediction of delayed cerebral ischemia after subarachnoid hemorrhage: development and validation of a practical risk chart. Stroke. May 1, 2013;44(5):1288–1294. doi: 10.1161/STROKEAHA.113.001125. http://www.ncbi.nlm.nih.gov/pubmed/23512975 Internet. cited 2017 Sep 2. Available from. [DOI] [PubMed] [Google Scholar]
- 38.Adams H.P., Kassell N.F., Torner J.C., Haley E.C. Predicting cerebral ischemia after aneurysmal subarachnoid hemorrhage: influences of clinical condition, CT results, and antifibrinolytic therapy. A report of the cooperative aneurysm study. Neurology. Oct, 1987;37(10):1586–1591. doi: 10.1212/wnl.37.10.1586. http://www.ncbi.nlm.nih.gov/pubmed/3658161 Internet. cited 2017 Sep 2. Available from. [DOI] [PubMed] [Google Scholar]
- 39.Fisher C.M., Kistler J.P., Davis J.M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. Jan, 1980;6(1):1–9. doi: 10.1227/00006123-198001000-00001. http://www.ncbi.nlm.nih.gov/pubmed/7354892 Internet. cited 2017 Sep 2. Available from. [DOI] [PubMed] [Google Scholar]
- 40.Lanzino G., Kassell N.F., Germanson T.P., Kongable G.L., Truskowski L.L., Torner J.C. Age and outcome after aneurysmal subarachnoid hemorrhage: why do older patients fare worse? J Neurosurg. Sep, 1996;85(3):410–418. doi: 10.3171/jns.1996.85.3.0410. http://www.ncbi.nlm.nih.gov/pubmed/8751625 Internet. cited 2017 Sep 24. Available from. [DOI] [PubMed] [Google Scholar]
- 41.Degos V., Gourraud P.-A., Tursis V.T., Whelan R., Colonne C., Korinek A.M. Elderly age as a prognostic marker of 1-year poor outcome for subarachnoid Hemorrhage patients through its interaction with admission hydrocephalus. Anesthesiology. Dec, 2012;117(6):1289–1299. doi: 10.1097/ALN.0b013e318267395b. http://www.ncbi.nlm.nih.gov/pubmed/22854979 Internet. cited 2017 Sep 24. Available from. [DOI] [PubMed] [Google Scholar]
- 42.Dóczi T. The pathogenetic and prognostic significance of blood-brain barrier damage at the acute stage of aneurysmal subarachnoid haemorrhage. Clinical and experimental studies. Acta Neurochir. 1985;77(3–4):110–132. doi: 10.1007/BF01476215. http://www.ncbi.nlm.nih.gov/pubmed/4072781 Internet. cited 2017 Sep 24. Available from: [DOI] [PubMed] [Google Scholar]
- 43.Trojanowski T. Blood-brain barrier changes after experimental subarachnoid haemorrhage. Acta Neurochir. Mar, 1982;60(1–2):45–54. doi: 10.1007/BF01401749. http://link.springer.com/10.1007/BF01401749 [Internet]. Springer-Verlag. cited 2017 Sep 24. Available from: [DOI] [PubMed] [Google Scholar]
- 44.Germanò A., d'Avella D., Imperatore C., Caruso G., Tomasello F. Time-course of blood-brain barrier permeability changes after experimental subarachnoid haemorrhage. Acta Neurochir. 2000;142(5):575–580. doi: 10.1007/s007010050472. http://www.ncbi.nlm.nih.gov/pubmed/10898366 Internet. cited 2017 Sep 24. discussion 580-1. Available from. [DOI] [PubMed] [Google Scholar]
- 45.Schöller K., Trinkl A., Klopotowski M., Thal S.C., Plesnila N., Trabold R. Characterization of microvascular basal lamina damage and blood–brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. Apr 20, 2007;1142:237–246. doi: 10.1016/j.brainres.2007.01.034. http://www.ncbi.nlm.nih.gov/pubmed/17303089 Internet. cited 2017 Sep 24. Available from. [DOI] [PubMed] [Google Scholar]
- 46.Friedman A., Kaufer D. Blood–brain barrier in health and disease. Semin Cell Dev Biol. Feb, 2015;38(1) doi: 10.1016/j.semcdb.2015.03.006. http://linkinghub.elsevier.com/retrieve/pii/S108495211500049X Internet. cited 2017 Jul 10. Available from. [DOI] [PubMed] [Google Scholar]
- 47.Lippmann K., Kamintsky L., Kim S.Y., Lublinsky S., Prager O., Nichtweiss J.F. Epileptiform activity and spreading depolarization in the blood–brain barrier-disrupted peri-infarct hippocampus are associated with impaired GABAergic inhibition and synaptic plasticity. J Cereb Blood Flow Metab. May 1, 2017;37(5):1803–1819. doi: 10.1177/0271678X16652631. http://journals.sagepub.com/doi/10.1177/0271678X16652631 [Internet]. SAGE Publications Sage UK: London, England. cited 2017 Jul 10. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoknecht K., Prager O., Vazana U., Kamintsky L., Harhausen D., Zille M. Monitoring stroke progression: in vivo imaging of cortical perfusion, blood-brain barrier permeability and cellular damage in the rat photothrombosis model. J Cereb Blood Flow Metab. 2014;34:1791–1801. doi: 10.1038/jcbfm.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neil-Dwyer G., Lang D.A., Doshi B., Gerber C.J., Smith P.W. Delayed cerebral ischaemia: the pathological substrate. Acta Neurochir. 1994;131(1–2):137–145. doi: 10.1007/BF01401464. http://www.ncbi.nlm.nih.gov/pubmed/7709776 Internet. cited 2018 Jun 2. Available from: [DOI] [PubMed] [Google Scholar]
- 50.Hartings J.A., York J., Carroll C.P., Hinzman J.M., Mahoney E., Krueger B. Subarachnoid blood acutely induces spreading depolarizations and early cortical infarction. Brain. Oct 1, 2017;140(10):2673–2690. doi: 10.1093/brain/awx214. https://academic.oup.com/brain/article/140/10/2673/4102114 Internet. cited 2018 Jun 2. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoltenberg-Didinger G., Schwarz K. Brain lesions secondary to subarachnoid hemorrhage due to ruptured aneurysms. In: Cervos-Navarro J., Ferszt R., editors. Stroke and microcirculation. Raven Press; New York: 1987. pp. 471–480. [Google Scholar]
- 52.Dreier J.P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. Apr 7, 2011;17(4):439–447. doi: 10.1038/nm.2333. http://www.ncbi.nlm.nih.gov/pubmed/21475241 Internet. cited 2018 Jun 2. Available from. [DOI] [PubMed] [Google Scholar]
- 53.Ohta O., Osaka K., Siguma M., Yamamoto M., Shimizu K., Toda N. Cerebral vasospasm following ruptured intracranial aneurysms, especially some contributions of potassium ion released from subarachnoid hematoma to delayed cerebral vasospasm. In: Bevan S.A., editor. Vascular neuroeffector mechanisms. Raven Press; New York: 1983. p. 535–358. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Abnormal brain tissue quantification.
Flowchart describing steps 2 and 3 of the segmentation algorithm (see text for details). The final labeling overlaid T1w image (green, gray matter; blue, white matter; yellow, cerebrospinal fluid; red, abnormal brain tissue (ABT)). GMM = Gaussian mixture model.
Supplementary Fig. 2. Quantitative approach for the assessment of BBB pathology.
a. Enhancement distribution histograms of three representative regions, namely background (green), muscle (black) and vessel (red), were used to define BBB breakdown enhancement range of τ1 - τ2.;b. BBB damage enhancement map of brain tissue (gray matter + white matter) and lesion (excluding pixels relating to ventricles and subarachnoid space) as defined by enhancement range of τ1 - τ2.;C. Increased sensitivity BBB damage enhancement map. Initial BBB damage was iteratively expended by appending the connected neighbouring voxels that have enhancement in the extended range 0·5τ1−1·5τ2. BBB = blood brain barrier.
Supplementary Fig. 3. Dynamic of ABT and BBBD in a subgroup with all four time points.
a. ABT volume was not significantly in subgroup of aSAH patients (with all four time points MRI data, n = 76) in comparison to entire cohort - 124 aSAH patients (p > 0·11, Wilcoxon sign-rank test, Fig. 1f).;b. BBBD volume was not significantly in subgroup of aSAH patients (with all four time points MRI data, n = 61) in comparison to entire cohort - 124 aSAH patients (p > 0·16, Wilcoxon sign-rank test).
ABT = abnormal brain tissue; aSAH = aneurysmal subarachnoid hemorrhage; BBBD = blood brain barrier damage.
Supplementary material 1
Supplementary material 2