Abstract
Objective:
Osteoclastogenic activation of macrophages (OCG) occurs in human abdominal aortic aneurysms (AAAs) and in calcium chloride-induced degenerative AAAs in mice, which have increased matrix metalloproteinase activity. As the activity of OCG in dissecting aneurysms is not clear, we tested the hypothesis that OCG contributes to angiotensin II (Ang II)-induced dissecting aneurysm (Ang II-induced AAA) in apolipoprotein E knockout mice.
Methods:
AAAs were produced in apolipoprotein E knockout mice via the administration of Ang II. Additionally, receptor activator of nuclear factor kB ligand (RANKL)-neutralizing antibody (5 mg/kg) was administered to one group of mice 7 days prior to Ang II infusion. Aneurysmal sections were probed for presence of RANKL and tartrate-resistant acid phosphatase via immunohistochemistry and immunofluorescence staining. Mouse aortas were also examined for RANKL and matrix metalloproteinase 9 expression via Western blot. In vitro murine vascular smooth muscle cells (MOVAS) and murine macrophages (RAW 264.7) were analyzed for the expression of osteogenic factors via Western blot, qPCR, and flow cytometry in response to Ang II or RANKL stimulation. The signaling pathway that mediates Ang II-induced RANKL expression in MOVAS cells was also investigated via application of TG101348, a Janus kinase 2 (JAK2) inhibitor, and Western blot analysis.
Results:
Immunohistochemical staining of Ang II-induced AAA sections revealed OCG as evidenced by increased RANKL and tartrate-resistant acid phosphatase expression compared with control mice. Immunofluorescence staining of AAA sections revealed co-localization of vascular smooth muscle cells and RANKL, revealing vascular smooth muscle cells as one potential source of RANKL. Systemic administration of RANKL-neutralizing antibody suppressed Ang II-induced AAA, with significant reduction of the maximum diameter of the abdominal aorta compared with vehicle controls (1.5 ± 0.4 mm vs 2.2 ± 0.2 mm). Ang II (1 μM) treatment induced a significant increase in RANKL messenger RNA expression levels in MOVAS cells compared with the vehicle control (1.0 ± 0.2 vs 2.8 ± 0.2). The activities of JAK2 and signal transducer and activator of transcription 5 (STAT5) were also significantly increased by Ang II treatment. Inhibition of JAK2/STAT5 suppressed Ang II-induced RANKL expression, suggesting the involvement of the JAK2/STAT5 signaling pathway.
Conclusions:
OCG with increased RANKL expression was present in Ang II-induced AAA, and neutralization of RANKL suppressed AAA formation. As neutralization of RANKL has been used clinically to treat osteoporosis and other osteoclast-related diseases, additional study of the effectiveness of RANKL neutralization in AAA is warranted. (J Vasc Surg 2018;68:48S-59S.)
Clinical Relevance:
We previously demonstrated that osteoclastogenic differentiation of macrophages (OCG) plays an important role in the development of human abdominal aortic aneurysms and murine calcium chloride-induced degenerative abdominal aortic aneurysms. In angiotensin II-induced dissecting aneurysm, we demonstrated the presence of OCG and its induction by receptor activator of nuclear factor κB ligand, which is a stimulator of osteoclast formation in apolipoprotein E knockout mice. Neutralization of receptor activator of nuclear factor κB ligand significantly suppressed the development of angiotensin II-induced dissecting aneurysm, suggesting that targeting of OCG could be an effective therapeutic approach to dissecting aneurysm.
Abdominal aortic aneurysm (AAA) is among the 20 leading causes of death in the United States. Currently, open surgical repair and endovascular placement of a stent graft are the only proven treatments for AAA. The significant morbidity and mortality associated with treatment emphasize the need for alternative therapeutic strategies.1,2
Studies have demonstrated the involvement of balanced mineralization in diseased arteries through the tight control of calcification by osteoblast-like and osteoclast-like cells (OCLs).3,4 OCLs are similar to osteoclasts but occur in tissues other than bone, differentiate from monocyte-macrophages, and, histologically, are multinucleate cells positive for tartrate-resistant acid phosphatase (TRAP) staining. We previously demonstrated the role of the receptor activator of nuclear factor κB ligand (RANKL) in stimulating the differentiation of macrophages into TRAP-positive OCLs in vitro.5 RANKL is generally expressed in the bone and is essential for formation of mature osteoclasts, which express matrix metalloproteinase (MMP) 9 that facilitates migration of osteoclasts to resorption sites through the extracellular matrix.6,7
We previously demonstrated that osteoclastogenic differentiation of macrophages (OCG) plays an important role in the development of aneurysms through stimulation of tumor necrosis factor α plus calcium phosphate,5 but the involvement of OCG in dissecting AAA is still unclear. Dissecting aneurysm is distinguished from degenerative aneurysm as occurring after the aortic dissection that induces tearing of the medial layer of the aorta, resulting in the pooling of blood within the vessel layers and subsequent hematoma formation. Angiotensin II (Ang II)-infused apolipoprotein E-deficient (apoE−/−) mice are accepted mouse models of dissecting AAA. Medial accumulation of macrophages and dissection are early events in Ang II-induced AAA.8 Gavrila et al9 suggested that this model more closely resembles aortic dissection than common aneurysm formation in humans. Here, we tested the hypothesis that OCG contributes to Ang II-induced dissecting aneurysm in apoE−/− mice.
METHODS
Materials.
RANKL-neutralizing antibody was purchased from Oriental Yeast (Tokyo, Japan). TG101348, a selective inhibitor of Janus kinase 2 (JAK2) tyrosine kinase, was purchased from Santa Cruz Biotechnology (Dallas, Tex). Ang II and RANKL antibodies for immunohistochemical staining were purchased from Millipore (Billerica, Mass) and Cell Signaling Technology (Danvers, Mass). All chemicals used in this study were of the highest purity available.
Cell culture.
Murine vascular smooth muscle cells (MOVAS cells) were purchased from American Type Culture Collection (Manassas, Va) and maintained in Dulbecco modified Eagle medium containing 10% fetal bovine serum (FBS) and 1% G418 (Cellgro Mediatech, Herndon, Va). The murine macrophage cell line RAW 264.7 (American Type Culture Collection) was maintained in high-glucose (4.5 g/L) Dulbecco modified Eagle medium supplemented with 10% FBS and 100 IU/mL penicillin/100 μg/mL streptomycin. MOVAS cells (passage number 5–10) were seeded in multiwell plates at a density of 1.0 × 104 cells/cm2. At 100% confluence, the medium was supplemented with 0.1 to 10 μM of Ang II for 0 to 72 hours. Cells were incubated at 37°C in a humidified atmosphere of 95% air/5% carbon dioxide; the culture medium was changed every second or third day.
Animals and treatments.
Retired male breeder apoE−/− mice >6 months of age were obtained from the Jackson Laboratory (Bar Harbor, Me). All Ang II-treated mice received Ang II (1000 ng/min/kg) through continuous infusion by an osmotic pump (Alzet model 1004; Durect Corporation, Cupertino, Calif) implanted in the back of the mouse. Mice receiving either normal rat immunoglobulin G (control, n = 6) or RANKL-neutralizing antibody mg/kg; anti-RANKL group, n = 5) were administered a subcutaneous injection, once, 7 days before Ang II infusion. After 28 days, the abdominal aorta was fixed by perfusion of 4% paraformaldehyde and harvested. The maximum diameter of the abdominal aorta was measured ex vivo with digital calipers to evaluate aneurysm formation. All animal procedures were conducted in accordance with experimental protocols that were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin, Madison (protocol M02394).
Histologic, immunohistochemical, and immunofluorescence evaluation.
Mouse aortas were embedded with OCT compound (Sakura Tissue Tek, Torrance, Calif), frozen, and sectioned at 6 μm. Enzymatic TRAP staining was performed with an acid phosphatase kit (Sigma Chemical, St. Louis, Mo). Immunohistochemical staining was done using a commercially available polymer-based detection system (ImmPACT and ImmPRESS; Vector Laboratories, Inc, Burlingame, Calif). To retrieve antigen activity, sections were microwaved in 0.1 mM citrate buffer (pH 6.0) for 5 minutes, and after blocking with 0.8% bovine serum albumin in tris-buffered saline (TBS) for 1 hour, the tissue sections were incubated with the mouse anti-RANKL primary antibody (ab45039; Abcam, Cambridge, United Kingdom) overnight at 4°C. After washing with TBS, the sections were incubated with secondary antibody for 30 minutes at room temperature. Immunofluorescence staining involved antigen retrieval by submersion of sections in tris-based buffer (pH 9.0) maintained at 95°C to 10°C on a hotplate for 15 minutes. Sections were then rinsed in deionized water and permeabilized for 20 minutes in TBS-Tween 20 (TBST). After blocking with 1% bovine serum albumin for 1 hour, tissue sections were incubated with the primary antibodies overnight at 4°C. The primary antibodies used were mouse anti-smooth muscle actin (sc-53142; Santa Cruz Biotechnology), mouse anti-RANKL (ab45039; Abcam), and rat anti-CD68 (ab53444; Abcam). After washing with TBST, the sections were incubated with fluorochrome-conjugated secondary antibodies for 1 hour. The secondary antibodies used were goat anti-mouse Alexa488, goat anti-mouse Alex568, and donkey anti-mouse Alexa488 (Life Technologies, San Diego, Calif). Sections were then washed in TBST and counter-stained with 4′,6-diamidino-2-phenylindole for 10 minutes. Finally, the sections were washed with TBST and mounted with Vectashield HardSet Antifade mounting medium (Vector Laboratories). The stained tissue sections were then examined with a BX53 microscope (Olympus, Tokyo, Japan).
Western blotting.
Protein extraction from cultured cells and isolated aortas was performed at 0°C to 4°C with a radioimmunoprecipitation assay buffer and protease inhibitor cocktail (Cell Signaling Technology). Twenty-microgram samples were separated by electrophoresis on 8% to 12% polyacrylamide gels in Laemmli buffer and transferred onto polyvinylidene difluoride membranes. Primary antibodies used for Western blotting included rabbit RANKL antibody (sc-9073; Santa Cruz Biotechnology), rabbit Mmp9 antibody (ab38898; Abcam), rabbit p-Jak2 antibody (sc-21870; Santa Cruz Biotechnology), rabbit JAK2 antibody (#3230; Cell Signaling Technology), rabbit p-signal transducer and activator of transcription 5 (Stat5) antibody (sc-11761; Santa Cruz Biotechnology), rabbit Stat5 antibody (sc-74442; Santa Cruz Biotechnology), and mouse α-tubulin antibody as a positive reference control (sc-23948; Santa Cruz Biotechnology). Primary antibodies were detected using a horseradish peroxidase-conjugated secondary antibody and visualized with an enhanced chemiluminescence kit (Thermo Fisher Scientific, Rockford, Ill).
Quantitative real-time polymerase chain reaction (qPCR).
Total RNA was extracted from MOVAS cells using an RNeasy Plus Mini Kit (Qiagen, Valencia, Calif) following the manufacturer’s instructions. qPCR was performed with SYBR Green in a real-time PCR instrument (ABI, Foster City, Calif). The Gapdh primers for qPCR were purchased from Qiagen (PPM02946E). Sequences of the other primers used were as follows. Rankl, F: 5′-GTCTGTAGGTACGCTTCCCG-3′; and R: 5′-CATTTGCACACCTCACCATCAAT-3′; Mmp9, F: 5′-CATTCGCGTGGATAAGGAGT-3′, and R: 5′-GTTCACCTCATGGTCCACCT-3′. The expression level for each gene was normalized to Gapdh expression in the same sample.
Methylthiazol tetrazolium (MTT) assay.
MOVAS and RAW 264.7 cells were cultured to confluence in a 96-well plate as previously described in 100 μL of media per well. Cells were then treated with 1 μM Ang II, 1 μM Ang II plus 100 ng/mL RANKL-neutralizing antibody, 100 ng/mL RANKL-neutralizing antibody alone, or left untreated for 48 hours. Five wells per treatment condition were prepared. After 2 days of incubation, cell proliferation was analyzed with the MTT cell proliferation assay kit (Cayman Chemical, Ann Arbor, Mich). Briefly, 10 μL of MTT reagent was added to each well, mixed gently, and allowed to incubate for 1 hour at 37°C in a carbon dioxide incubator. Next, 100 μL of dimethyl sulf-oxide (Sigma) was added to each well, and the absorbance of each well was read at 570 nm using a FlexStation 3 microplate reader (Molecular Devices, Sunnyvale, Calif).
JC-1 assay.
MOVAS and RAW 264.7 cells were cultured to confluence in a 96-well black culture plate as previously described in 100 μL of media per well. Cells were then treated with 1 μM Ang II, 1 μM Ang II plus 100 ng/mL RANKL-neutralizing antibody, 100 ng/mL RANKL-neutralizing antibody alone, or left untreated for 48 hours. Five wells per treatment condition were prepared. After 2 days of incubation, apoptosis was analyzed with the JC-1 mitochondrial membrane potential assay kit according to the manufacturer’s instructions (Cayman Chemical). Briefly, 10 μL of JC-1 staining solution was added to each well and allowed to incubate for 30 minutes at 37°C in a carbon dioxide incubator. The plate was then centrifuged for 5 minutes at 400 g, and the supernatant was removed. The wells were then washed twice with 200 μL of phosphate-buffered saline (PBS) and centrifuged for 5 minutes at 400 g, and the supernatant was removed. Last, 100 μL of PBS was added to each well, and the plate was read on a FlexStation 3 microplate reader. The fluorescence intensities of each well were recorded for the 535/595 nm and 485/535 nm excitation/emission pairs. Data are reported as the ratio of green fluorescence at 535 nm to red fluorescence at 595 nm.
Intracellular protein staining.
MOVAS and RAW 264.7 cells (3 × 105) were cultured as previously described, in quadruplicate, and treated with 1 μM Ang II, 1 μM Ang II plus 100 ng/mL RANKL-neutralizing antibody, 100 ng/mL RANKL-neutralizing antibody alone, or left untreated for 48 hours. All cells were then treated with 1.5 μM monensin (Calbiochem, San Diego, Calif) and allowed to incubate at 37°C for an additional 4 hours. Cells were washed with fluorescence-activated cell sorting wash buffer (PBS, 3% FBS) and live/dead stained with Ghost 780 (Tonobo, San Diego, Calif) for 30 minutes at 4°C. Cells were then washed and resuspended in fixation buffer (Invitrogen, San Diego, Calif) and allowed to fix for 15 minutes at room temperature. Cells were washed in permeabilization buffer (Invitrogen) and blocked with avidin for 15 minutes at room temperature, washed, and blocked with biotin for 15 minutes at room temperature. Cells were resuspended in permeabilization buffer containing the following primary antibodies: anti-transforming growth factor β (TGF-β)-Alexa Fluor 700 (IC1835N; R&D Systems, Minneapolis, Minn), anti-bone morphogenetic protein 4 (BMP4)-Alexa Fluor 647 (sc-12721; Santa Cruz Biotechnology), anti-MMP-2-phycoerythrin (sc-13594; Santa Cruz Biotechnology), anti-tissue inhibitor of metalloproteinase 1 (TIMP1)-biotin (BAF980; R&D Systems), anti-RANKL-Alexa Fluor 405 (NB100–56593AF405; Novus Biologicals, Littleton, Colo), and anti-Mmp9- peridinin-chlorophyll (SAB5200309; Sigma-Aldrich). Primary antibodies and cells were incubated for 30 minutes at room temperature. Cells were then washed and resuspended in permeabilization buffer plus avidin-fluorescein isothiocyanate (Invitrogen) and incubated at room temperature for 30 minutes. After incubation, the cells were washed with permeabilization buffer and resuspended in fluorescence-activated cell sorting wash buffer. Cells were analyzed on an Attune N×T Flow Cytometer (Invitrogen). Fluorescence minus one controls were prepared and analyzed for each fluorochrome used.
Statistical analysis.
Data were reported as means ± standard deviations. Statistical analysis was performed with GraphPad Prism, version 4.00 (GraphPad Software, San Diego, Calif). In comparisons between groups at a single time, the normality of the data was first assessed using the Shapiro-Wilk test. After the Shapiro-Wilk test, normally distributed data were compared by the Student t-test and non-normally distributed data by the Mann-Whitney U test. Multiple comparisons among treatments were performed by one-way analysis of variance followed by Tukey multiple comparison test. P values of <.05 were accepted as statistically significant.
RESULTS
OCG in Ang II-induced AAA in ApoE−/− mice.
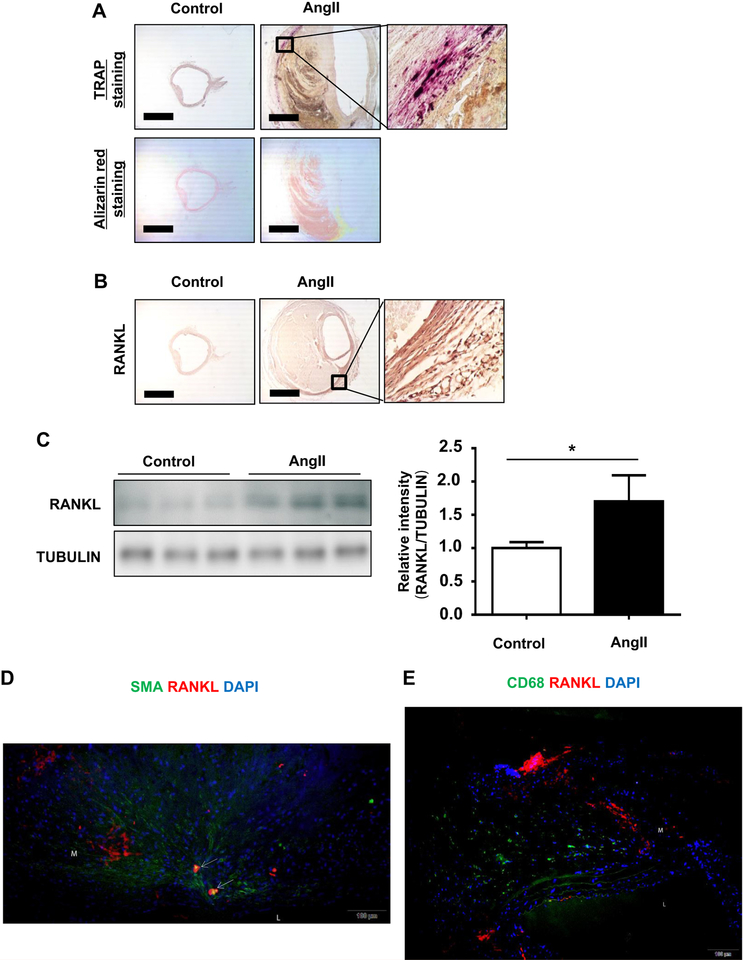
We found aneurysms in all mice after 4 weeks of Ang II infusion and found purple-stained TRAP-positive multinuclear cells in the aneurysmal arteries of Ang II-induced mice (100%; N = 10) but not in the control mice (N = 5; Fig 1, A). Next, we evaluated RANKL expression in tissues of Ang II-induced AAA by immunohistochemistry. RANKL expression was significantly increased in Ang II-induced AAA tissue compared with control aortas (Fig 1, B). Analysis of Western blots showed that RANKL expression was increased 1.7-fold in Ang II-induced AAA compared with the control aortas (Fig 1, C). The results indicate that Ang II infusion induced RANKL expression in dissecting AAA. In addition, some costaining with the macrophage marker MOMA-2 and a marker of OCL, calcitonin receptor, was observed in Ang II-induced AAA tissue, further indicating the differentiation of macrophages in AAA (data not shown). We then investigated the location of RANKL expression in relation to smooth muscle actin and CD68 expression, which served as markers for vascular smooth muscle cells and macrophages, respectively. We found co-localization of RANKL- and smooth muscle actin-expressing cells in the medial layer of AAA tissue (Fig 1, D). We also observed CD68 expression in the medial layer of aneurysmal tissue, confirming TRAP staining; however, CD68 does not appear to co-localize with RANKL (Fig 1, E).
Fig 1.
Osteoclastogenic differentiation of macrophages (OCG) in angiotensin II (Ang II)-induced dissecting abdominal aortic aneurysm (Ang II-induced AAA) in apolipoprotein E knockout (apoE−/−) mice. Ang II (1000 ng/min/kg) was continuously infused by an osmotic pump implanted in the back of apoE−/−mice. After 28 days, the abdominal aortas were fixed by perfusion of 4% paraformaldehyde and harvested. The aortas were embedded in OCT, and frozen sections were cut for histologic, immunohistochemical, and immunofluorescence evaluation. A, Representative images of arteries stained with tartrate-resistant acid phosphatase (TRAP) and alizarin red in Ang II-infused mice. TRAP-positive cells (purple) were seen only in the medial layer of the Ang II-induced AAA. Alizarin red staining showed no positivity in either group. Scale bar = 0.5 mm. B, Representative immunohistochemical images of the abdominal aorta of Ang II-infused apoE−/− mice. Increased receptor activator of nuclear factor κB ligand (RANKL) expression (brown staining) is seen in the medial layer. Scale bar = 0.5 mm. C, Representative Western blot and densitometric analysis of RANKL expression in the aortas. RANKL expression was significantly increased in the Ang II group compared with controls. Values are means ± standard deviations of three replicates (n = 3). *P < .05. Relative expression levels for Western blotting were quantified using the ImageJ program. D, Location of RANKL expression relative to vascular smooth muscle cells in the aorta of Ang II-infused mice. Representative immunofluorescence images of sequential aortic sections labeled with fluorescent antibodies specific for RANKL and smooth muscle actin (SMA) and nuclear staining with 4′.6-diamidino-2-phenylindole (DAPI). The arrows indicate areas of RANKL and SMA co-localization. E, Location of RANKL expression relative to macrophages in the aorta of Ang II-infused mice. Representative immunofluorescence image of an aortic section double-stained with antibodies specific for RANKL and CD68 and nuclear staining with DAPI. L, Lumen; M, media.
Ang II-induced RANKL expression in smooth muscle cells.
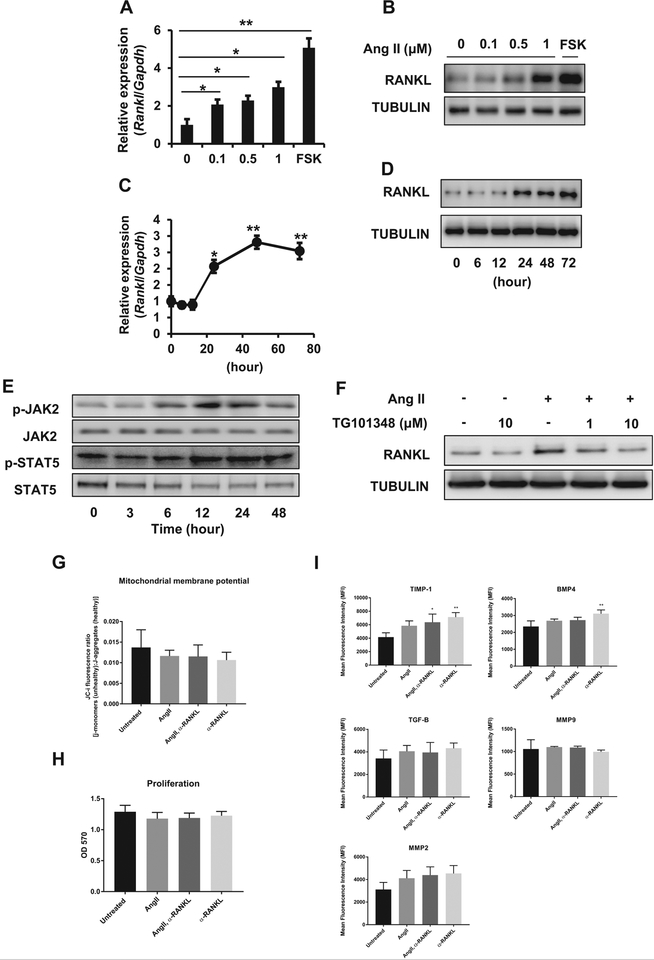
To investigate the mechanism of macrophage differentiation into OCLs in Ang II-induced AAA, MOVAS cells (a murine aortic smooth muscle cell line) were treated with Ang II. Forskolin was used as a positive control for RANKL expression. As shown in Fig 2, A and B, Ang II treatment for 48 hours resulted in a dose-dependent increase in Rankl messenger RNA (mRNA) and protein expression in MOVAS cells. As shown in Fig 2, C, Ang II induced a significant increase in Rankl mRNA expression starting at 12 hours after stimulation and continuing through 48 hours (0.9 ± 0.1 at 12 hours vs 2.8 ± 0.2 at 48 hours). Expression remained constant from 48 to 72 hours after stimulation. The same trend was observed in RANKL protein expression (Fig 2, D). Densitometry analysis of Western blots indicated a 1.8-fold increase of RANKL expression at 48 hours after Ang II treatment.
Fig 2.
Angiotensin II (Ang II)-induced receptor activator of nuclear factor κB ligand (RANKL) expression in smooth muscle cells. Murine vascular smooth muscle cells (MOVAS cells) were stimulated with Ang II. Expression of RANKL messenger RNA (mRNA) and protein was quantified by quantitative real-time polymerase chain reaction (qPCR) and Western blotting. Forskolin (FSK) was used as a positive control. Ang II dose dependently induced Rankl mRNA (A) and RANKL protein expression (B). A time-course experiment showed increased Rankl mRNA (C) and RANKL protein (D) from 24 to 72 hours with a maximum at around 48 hours. Relative expression of Western blots was quantified using ImageJ. E and F, Increased RANKL expression through the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 5 (STAT5) pathway in smooth muscle cells. MOVAS cells were stimulated with 1 μM Ang II. JAK2 and STAT5 activities were assayed in Western blots. E, JAK2 and STAT5 phosphorylation was increased at 12 hours. F, Effect of TG101348, a selective inhibitor of JAK2, on Ang II-induced RANKL expression. TG101348 suppressed Ang II-induced RANKL expression in MOVAS cells. G-I, Analysis of the effects of Ang II and RANKL-neutralizing antibody on MOVAS cells. Cells were cultured in growth medium alone or media containing Ang II (1 μM), RANKL-neutralizing antibody (100 ng/mL), or both for 48 hours. G, Mitochondrial membrane potential of MOVAS cells cultured with Ang II, RANKL-neutralizing antibody, or both for 48 hours. H, Proliferation of MOVAS cells cultured with Ang II, RANKL-neutralizing antibody, or both for 48 hours. OD, optical density. I, Flow cytometric analysis of MOVAS cells cultured with Ang II, RANKL-neutralizing antibody, or both for 48 hours. Cells were analyzed for the expression of tissue inhibitor of metalloproteinase 1 (TIMP1), transforming growth factor β (TGF-β), bone morphogenetic protein 4 (BMP4), matrix metalloproteinase (MMP) 9, and MMP2. Flow cytometric data are represented as the mean fluorescence intensity ± standard deviation of four replicates.
*P < .05. **P < .01.
Increased RANKL expression in smooth muscle cells through the JAK2/STAT5 pathway.
We investigated JAK2/STAT5 activity as a possible signaling mechanism in MOVAS cells treated with Ang II. Phospho-JAK2 and STAT5 levels were increased by Ang II treatment (Fig 2, E), and inhibition of JAK2 by TG101348 suppressed Ang II-induced RANKL expression (Fig 2, F). The results suggest that the JAK2/STAT5 pathway is involved in the increase in RANKL expression in response to Ang II in MOVAS cells.
Treatment of MOVAS cells with Ang II and RANKL-neutralizing antibody altered expression of osteogenic modulating proteins.
We demonstrated the importance of Ang II in stimulation of MOVAS cells to produce RANKL; therefore, we were interested in the effect of RANKL-neutralizing antibody on apoptosis, proliferation, and expression of proteins involved in the progression of vascular disease. To investigate effects of Ang II and RANKL-neutralizing antibody on apoptosis and proliferation of cells, we used the JC-1 mitochondrial membrane potential (ΔψM) and MTT assays, respectively. Ang II treatment of MOVAS cells alone or in combination with RANKL-neutralizing antibody did not have significant effects on apoptosis or proliferation in vitro (Fig 2, G and H). Treatment of MOVAS cells with Ang II plus RANKL-neutralizing antibody resulted in significant stimulation of TIMP1 expression, and treatment with RANKL-neutralizing antibody alone stimulated the expression of TIMP1 and BMP4 (Fig 2, I). This suggests that in the absence of stimulation by RANKL, vascular smooth muscle cells may be involved in creation of an osteogenic vascular environment rather than the osteoclastogenic environment promoted by RANKL expression. In addition, we demonstrated the ability of Ang II to stimulate RANKL expression, confirming our previous observations (Supplementary Fig, online only).
RANKL stimulation of macrophages resulted in increased MMP9 expression.
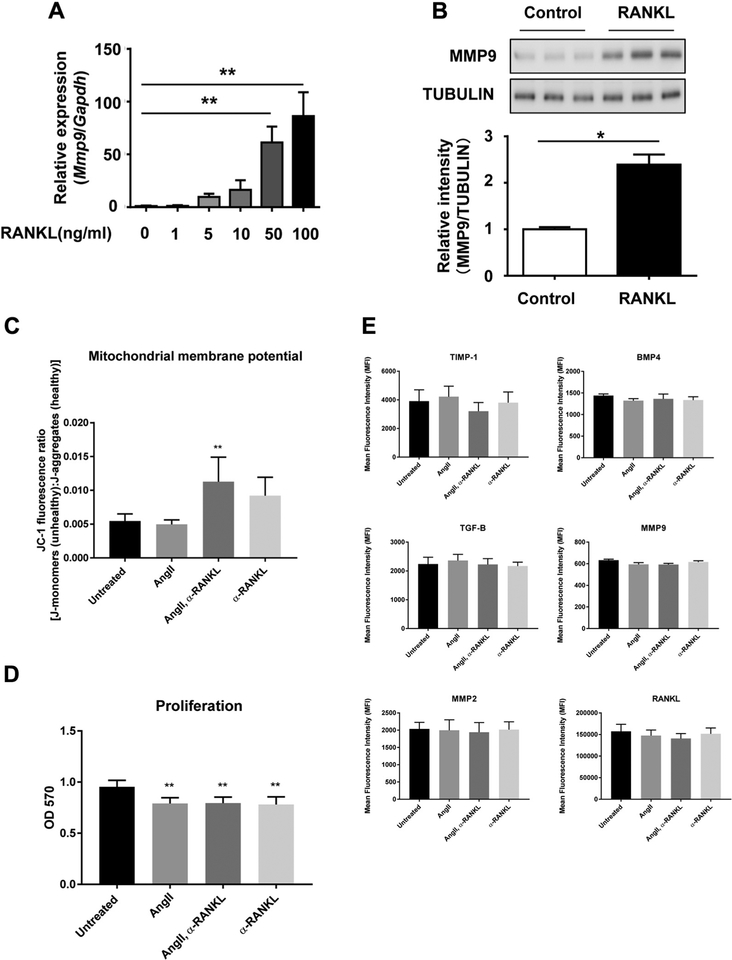
As MMP9 is secreted by macrophages and is involved in the development of aneurysms,10 we assayed MMP9 expression in RAW 264.7 murine macrophage cells after RANKL stimulation. The RANKL treatment significantly increased both Mmp9 mRNA expression and protein secretion (Fig 3, A and B). The results suggest that Ang II-induced RANKL expression in vascular smooth muscle cells may stimulate MMP9 expression in macrophages.
Fig 3.
Receptor activator of nuclear factor κB ligand (RANKL)-mediated stimulation of macrophages. RAW 264.7 macrophages were cultured with RANKL for 2 days, and matrix metalloproteinase 9 (MMP9) messenger RNA (mRNA) and protein expression was determined in the cell lysates by quantitative real-time polymerase chain reaction (qPCR) and Western blotting. A, Mmp9 mRNA expression was dose dependently increased by RANKL stimulation. B, Representative Western blot and densitometric analysis of MMP9 protein expression in cell lysates of RAW264.7 cells treated with RANKL. Relative expression of Western blots was quantified using ImageJ. C-E, Analysis of the effects of angiotensin II (Ang II) and RANKL-neutralizing antibody on RAW 264.7 cells. Cells were cultured in growth medium alone or media containing Ang II (1 μM), RANKL-neutralizing antibody (100 ng/mL), or both for 48 hours. C, Mitochondrial membrane potential of RAW 264.7 cells cultured with Ang II, RANKL-neutralizing antibody, or both for 48 hours. D, Proliferation of RAW 264.7 cells cultured with Ang II, RANKL-neutralizing antibody, or both for 48 hours. OD, optical density. E, Flow cytometric analysis of RAW 264.7 cells cultured with Ang II, RANKL-neutralizing antibody, or both for 48 hours. Cells were analyzed for the expression of tissue inhibitor of metalloproteinase 1 (TIMP1), transforming growth factor β (TGF-β), bone morphogenetic protein 4 (BMP4), MMP9, MMP2, and RANKL. Flow cytometric data are represented as the mean fluorescence intensity ± standard deviation of four replicates.
*P < .05. **P < .01.
RANKL neutralization-induced apoptosis is associated with decreased cell proliferation in RAW 264.7 macrophages.
Given the importance of RANKL stimulation in osteoclastogenesis and MMP9 expression in macrophages, we examined the effect of treatment of RAW 264.7 cells with Ang II and RANKL-neutralizing antibody, alone or in combination. We observed that the combined treatment of Ang II and RANKL-neutralizing antibody significantly increased the ratio of apoptotic cells to healthy cells in vitro and resulted in decreased cell proliferation (Fig 3, C and D). In addition, we examined the expression of various modulators of vascular disease by RAW 264.7 cells under the same culture conditions described previously. Ang II and RANKL-neutralizing antibody, alone or in combination, did not significantly alter the expression of TIMP1, BMP4, TGF-β, MMP9, MMP2, or RANKL (Fig 3, E).
The development of aneurysms in Ang II-induced AAA in apoE−/− mice was suppressed by RANKL-neutralizing antibody.
A total of 11 mice received Ang II infusion in the experiment depicted in Fig 4. Five mice received injection of RANKL-neutralizing antibody and six mice received normal rat immunoglobulin G as a control. However, during the first 2 weeks of Ang II infusion, two mice in the control group and one mouse in the anti-RANKL group died because of a ruptured aneurysm, confirmed by necropsy.8 As shown in Fig 4, A and B, administration of RANKL-neutralizing antibody significantly suppressed aneurysm formation, indicated by the decrease in the maximum diameter of the aorta at the time of sacrifice (2.2 ± 0.2 mm vs 1.5 ± 0.4 mm; P < .05). The suppression of OCG development through RANKL neutralization was evaluated by TRAP staining, which confirmed a significant decrease of TRAP-positive cells in mice that received RANKL-neutralizing antibody compared with controls (Fig 4, C). The RANKL-neutralizing antibody also significantly decreased Ang II-induced MMP9 expression in the aorta (Fig 4, D). These results suggest that RANKL-neutralizing antibody suppressed Ang II-induced aneurysm through inhibition of OCG development.
Fig 4.
Angiotensin II (Ang II)-induced aneurysm development was suppressed by receptor activator of nuclear factor κB ligand (RANKL)-neutralizing antibody. RANKL (5 mg/kg)-neutralizing antibody was subcutaneously administered to apoE−/− mice. After 7 days, Ang II (1000 ng/min/kg) was continuously infused by an osmotic pump. The maximum diameter of the abdominal aortas was measured to evaluate the severity of aneurysm formation. A, Images of Ang II-induced dissecting abdominal aortic aneurysm (Ang II-induced AAA). B, Images of Ang II-induced AAA after administration of RANKL-neutralizing antibody. C, Maximum diameter of abdominal aortas in response to RANKL-neutralizing antibody (n = 4). Anti-RANKL antibody significantly suppressed Ang II-induced AAA formation. D, Representative images of tartrate-resistant acid phosphatase (TRAP)-positive staining of Ang-II induced AAA tissues. Anti-RANKL antibody suppressed TRAP-positive staining. Scale bar = 0.5 mm. E, Representative Western blot and densitometry analysis of matrix metalloproteinase 9 (MMP9) expression in aortas. Anti-RANKL antibody significantly suppressed MMP9 expression. Values are means ± standard deviations of three replicates.
*P < .05. Relative expression was quantified in Western blots using ImageJ.
DISCUSSION
We confirmed the presence of OCG in mice with Ang II-induced dissecting aneurysms as a result of Ang II-induced RANKL expression by vascular smooth cells. This study and our previous work showed the involvement of OCG in both degenerative and dissecting aneurysms, but the stimulation of OCG proceeded by distinct mechanisms. In degenerative aneurysms, OCG was induced through costimulation by calcium phosphate and tumor necrosis factor α.5 In this study, RANKL induced OCG in dissecting aneurysms, and immunohistochemical analysis of Ang II-induced AAA confirmed increased RANKL expression compared with control aortas. Our hypothesis was that macrophages are stimulated to transition into OCLs in aneurysmal tissues. Therefore, we examined dissecting aneurysmal sections for TRAP- and CD68-positive cells and found the presence of both markers in the medial layer. Because CD68 is found on both recruited monocyte precursor cells and differentiated macrophage populations at the site of diseased vessels, it is possible that cells from both populations may mature into OCLs.11
Based on the results from co-localization staining, we focused on vascular smooth muscle cells as a source of RANKL, although we could not exclude other cell types as a potential source. We found that Ang II treatment of MOVAS cells induced significant increases in Rankl mRNA and protein expression, which corresponds to the previous findings of Osako et al.12
JAK2 and STAT5 were studied as possible mediators of Ang II-induced RANKL expression in vascular smooth muscle cells and were found to be significantly activated by Ang II. Ang II-induced JAK2/STAT5 activation in MOVAS cells was suppressed by a selective inhibitor of JAK2, TG101348, which inhibits polycythemia vera progenitor erythroid differentiation in mice,13 suggesting that Ang II-induced RANKL expression in MOVAS cells proceeds through the JAK2/STAT5 pathway. When we investigated the effect of TG101348 inhibition of JAK2/STAT5 in Ang II-induced AAA in mice, we found that the diameter of the aorta was not affected (data not shown). Therefore, further study with different dosages and methods of administration is needed.
The involvement of RANKL in dissecting aneurysm formation was further indicated by the suppression of Ang II-induced AAA progression in mice after administration of RANKL-neutralizing antibody. Furthermore, we showed that RANKL-neutralizing antibody significantly reduced MMP9 expression in the aorta. This finding corroborates the importance of RANKL in stimulating MMP9 expression by macrophages in vitro and provides a link between RANKL stimulation and the production of an agent of aneurysm formation.
Suppression of aneurysm formation by RANKL-neutralizing antibody prompted us to examine the role of Ang II and RANKL neutralization on vascular smooth muscle cells and macrophages in vitro. In vascular smooth muscle cells, RANKL neutralization increased expression of TIMP1 and BMP4 on RANKL neutralization. This increase is intuitive in light of the vital role RANKL plays in OCG aneurysm ontology that we exhibited and considering the role of TIMP1 in suppression of MMP function and the osteogenic function of BMP4. Furthermore, this result is consistent with previous findings that demonstrated decreased BMP4 expression in the murine Ang II model of AAA.14 Our observation of increased apoptosis and decreased cell proliferation in macrophages cultured with RANKL-neutralizing antibody is consistent with the role of RANKL-induced OCG we demonstrated in these studies. This finding also concurs with previous studies that revealed the antiapoptotic effect of RANKL on osteoclasts and the pathway by which this signal is propagated.15 As such, RANKL plays a role in maintenance of macrophage populations that are well known to contribute to the inflammatory environment associated with aneurysmal disease.16,17 Thus, the activation and survival of macrophages through RANKL signaling appear vital to development of aneurysmal disease.
As RANKL neutralization has been used clinically to treat osteoporosis and other osteoclast-related diseases, further study of the effectiveness of RANKL neutralization on AAA is warranted. The results of studies of the effects of bisphosphonates and osteoclast inhibitors on mouse aneurysms are inconsistent. Tsai et al18 reported suppression of Ang II-induced AAA by daily administration of zoledronic acid. We have also studied the effect of zoledronic acid on Ang II-induced AAA. Only a single yearly intravenous injection is required for the treatment of osteoporosis in humans. Therefore, we evaluated the effect of a single injection of zoledronic acid in AngII-induced AAA but did not find a suppressive effect.5 As bisphosphonates act by binding to calcium phosphate to exert its suppressive effect on osteoclastogenesis,19 we speculated that the positive effect reported by Tsai et al was systemic rather than local because Ang II-induced AAA did not show arterial calcification, whereas degenerative aneurysms developed calcification.5
CONCLUSIONS
We demonstrated the presence of OCG associated with increased RANKL expression in Ang II-induced AAA. In vitro, Ang II induced RANKL expression by vascular smooth muscle cells through the JAK2/STAT5 signaling pathway, and RANKL-neutralizing antibody suppressed AAA formation. Although further studies are needed, these data suggest that RANKL-neutralizing antibody may be useful as a therapeutic agent to treat dissecting aneurysms.
We thank Jay Yang, MD, PhD, professor of the Department of Anesthesiology at the University of Wisconsin-Madison, for valuable comments and support. We are also grateful to our colleague Chitaru Kurihara, MD, for technical assistance.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: Experimental study using the angiotensin II-induced dissecting abdominal aortic aneurysm (AAA) in a male mouse model
Take Home Message: In angiotensin II-induced AAA, osteoclasts existed and were induced by receptor activator of nuclear factor κB ligand (RANKL), a stimulator of osteoclast formation in apolipoprotein E knockout mice. Inhibition of RANKL was associated with decreased AAA development.
Recommendation: RANKL inhibition may be a potential target for medical therapy for AAA in humans.
Acknowledgments
This work was supported by a Scientist Development Grant from the American Heart Association (12SDG9120024), Grant-in-Aid from the American Heart Association (17GRNT33671064), and T32 Vascular Surgery Research Training Grant (T32HL110853).
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest. 0741–5214
Additional material for this article may be found online at www.jvascsurg.org.
REFERENCES
- 1.Kurosawa K, Matsumura JS, Yamanouchi D. Current status of medical treatment for abdominal aortic aneurysm. Circ J 2013;77:2860–6. [DOI] [PubMed] [Google Scholar]
- 2.Takayama T, Yamanouchi D. Aneurysmal disease: the abdominal aorta. Surg Clin North Am 2013;93:877–891, viii. [DOI] [PubMed] [Google Scholar]
- 3.Yamanouchi D, Takei Y, Komori K. Balanced mineralization in the arterial system: possible role of osteoclastogenesis/osteoblastogenesis in abdominal aortic aneurysm and stenotic disease. Circ J 2012;76:2732–7. [DOI] [PubMed] [Google Scholar]
- 4.Liberman M, Pesaro AE, Carmo LS, Serrano CV Jr. Vascular calcification: pathophysiology and clinical implications. Einstein (Sao Paulo) 2013;11:376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takei Y, Tanaka T, Kent KC, Yamanouchi D. Osteoclastogenic differentiation of macrophages in the development of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2016;36:1962–71. [DOI] [PubMed] [Google Scholar]
- 6.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev 1999;13:2412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishibashi O, Niwa S, Kadoyama K, Inui T. MMP-9 anti-sense oligodeoxynucleotide exerts an inhibitory effect on osteoclastic bone resorption by suppressing cell migration. Life Sci 2006;79:1657–60. [DOI] [PubMed] [Google Scholar]
- 8.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2003;23:1621–6. [DOI] [PubMed] [Google Scholar]
- 9.Gavrila D, Li WG, McCormick ML, Thomas M, Daugherty A, Cassis LA, et al. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2005;25:1671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest 2000;105:1641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iqbal AJ, McNeill E, Kapellos TS, Regan-Komito D, Norman S, Burd S, et al. Human CD68 promoter GFP transgenic mice allow analysis of monocyte to macrophage differentiation in vivo. Blood 2014;124:e33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osako MK, Nakagami H, Shimamura M, Koriyama H, Nakagami F, Shimizu H, et al. Cross-talk of receptor activator of nuclear factor-κB ligand signaling with renin-angiotensin system in vascular calcification. Arterioscler Thromb Vasc Biol 2013;33:1287–96. [DOI] [PubMed] [Google Scholar]
- 13.Geron I, Abrahamsson AE, Barroga CF, Kavalerchik E, Gotlib J, Hood JD, et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell 2008;13:321–30. [DOI] [PubMed] [Google Scholar]
- 14.Spin JM, Hsu M, Azuma J, Tedesco MM, Deng A, Dyer JS, et al. Transcriptional profiling and network analysis of the murine angiotensin II-induced abdominal aortic aneurysm. Physiol Genomics 2011;43:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda F, Matsubara T, Tsurukai T, Hata K, Nishimura R, Yoneda T. JNK/c-Jun signaling mediates an anti-apoptotic effect of RANKL in osteoclasts. J Bone Miner Res 2008;23:907–14. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2006;26:987–94. [DOI] [PubMed] [Google Scholar]
- 17.Golledge AL, Walker P, Norman PE, Golledge J. A systematic review of studies examining inflammation associated cytokines in human abdominal aortic aneurysm samples. Dis Markers 2009;26:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai SH, Huang PH, Peng YJ, Chang WC, Tsai HY, Leu HB, et al. Zoledronate attenuates angiotensin II-induced abdominal aortic aneurysm through inactivation of Rho/ROCK-dependent JNK and NF-κB pathway. Cardiovasc Res 2013;100:501–10. [DOI] [PubMed] [Google Scholar]
- 19.Schindeler A, Little DG. Osteoclasts but not osteoblasts are affected by a calcified surface treated with zoledronic acid in vitro. Biochem Biophys Res Commun 2005;338:710–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.