Abstract
Aims:
The purpose of our study is to assess the role of high-resolution real-time gray-scale ultrasonography with ultrasound-guided fine-needle aspiration cytology (FNAC) in detecting malignant nodules in the thyroid gland.
Settings and Design:
It is a retrospective study of 25 patients (aged 16–63 years) who underwent high-resolution ultrasound (HRUSG) of the thyroid gland and ultrasound-guided FNAC from February 2017 till November 31, 2017.
Materials and Methods:
A total of 25 patients with thyroid nodules detected at ultrasound were included in this study. The characteristics of each nodule were determined. The results were then compared with FNAC (ultrasound-guided) findings.
Statistical Analysis Used:
Sensitivity, specificity, positive predictive value, negative predictive value and accuracy were used.
Results:
Of the 25 nodules examined, 5 (20%) were found to be malignant on cytology.
Conclusions
Gray-scale ultrasound features of thyroid nodules are useful to distinguish malignant thyroid nodules from those with benign nodules. From our study, it is obvious that the HRUSG findings of hypoechogenicity, microcalcification, and poorly defined margins have high diagnostic accuracy for identifying malignant thyroid nodules.
Keywords: Nodule, thyroid gland, ultrasonography
Introduction
Nodular thyroid disease is detected in 3–7% of the adult population worldwide. The majority of thyroid nodules are readily detected by high-resolution ultrasound (HRUSG).[1,2,3] HRUSG is used for evaluation of the thyroid gland morphology and pathologic conditions of the thyroid gland.
The purpose of our study is to assess the accuracy of HRUSG in determining malignant nodules. The nodules were evaluated based on their margins, shape, echogenicity, presence of calcification, presence of circumferential halo, and internal composition [Tables 1 and 2]. The advantages of HRUSG are that it is an easily available, inexpensive, noninvasive, and highly sensitive imaging modality for the depiction of morphology and distinguishing cystic from solid lesions.
Table 1.
Distribution of HRUSG characteristics in benign and malignant thyroid nodules
| HRUSG findings of thyroid nodules | Histopathology | ||||
|---|---|---|---|---|---|
| Benign | Malignant | ||||
| Reading | Percentage | Reading | Percentage | ||
| 1 | Single/Multiple nodules | ||||
| Single | 4 | 16 | 1 | 4 | |
| Multiple | 16 | 64 | 4 | 16 | |
| 2 | Echogenicity | ||||
| Hyperechoic | 2 | 8 | 1 | 4 | |
| Hypoechoic | 10 | 40 | 4 | 16 | |
| Mixed echoic | 8 | 32 | - | - | |
| 3 | Halo | ||||
| Present | 7 | 28 | - | - | |
| Absent | 13 | 52 | 5 | 20 | |
| 4 | Calcification | ||||
| Present | 2 (Macro) | 8 | 1 (Micro) 2 (Macro) |
12 | |
| Absent | 18 | 72 | 2 | 8 | |
| 5 | Component | ||||
| Solid | 12 | 48 | 5 | 20 | |
| Solid and cystic | 8 | 32 | - | - | |
| 6 | Margins | ||||
| Well defined | 12 | 48 | 1 | 4 | |
| Ill defined | 8 | 32 | 4 | 16 | |
Table 2.
Sonographic features of various thyroid lesions
| Sonographic features | Benign | Malignant papillary CA | Total | ||
|---|---|---|---|---|---|
| Goiter | Thyroiditis | Follicular adenoma | |||
| Hyperechoic | 2 | - | - | 1 | 3 |
| Hypoechoic | 5 | 2 | 3 | 4 | 14 |
| Mixed echoic | 8 | - | - | - | 8 |
| Single nodule | 1 | - | 3 | 1 | 5 |
| Multiple nodules | 14 | 2 | - | 4 | 20 |
| Perilesional halo | 7 | - | - | - | 7 |
| Calcification | 2 | - | - | 3 | 5 |
| Solid | 7 | 2 | 3 | 5 | 17 |
| Solid and cystic | 8 | - | - | - | 8 |
| Well defined | 10 | 1 | 1 | 1 | 13 |
| Ill defined | 5 | 1 | 2 | 4 | 12 |
Materials and Methods
A retrospective study of 25 patients (aged 16–63 years) who underwent HRUSG of the thyroid gland and ultrasound-guided fine-needle aspiration cytology (FNAC) from February 2017 till November 31, 2017 was included.
Inclusion criteria: (1) Patients with thyroid nodule detected by ultrasound. (2) Patients with thyroid nodules suspicious of malignancy.
Exclusion criteria: Patients with diffuse thyroid enlargement with no nodules.
Equipment: Gray-scale real-time ultrasound examination was done using 7.5–13 MHz linear array transducer. Ultrasound machine used was GE Voluson E-8.
The needle used for thyroid FNAC is standard 25-ga, lumbar puncture needle. After introducing the needle, the needle is moved gently into the solid area of the nodule under ultrasound guidance. Then, the stillette alone is withdrawn by around 2–4 cm. To and fro movement of the needle is done so that the tip moves within the nodule. The stillette is withdrawn first. The operator's thumb is used to close the hub before withdrawing the needle. A 10-ml syringe was attached to the hub of the needle, and the content of the needle was spread on slides and then slides were prepared for cytology. Advantages of using the 25-ga lumbar puncture needle are that there is no need to give local anesthesia with less chance of aspirating blood.
Results
In HRUSG, out of the 25 patients, nodules of 4 patients (16%) were malignant, 13 patients (48%) were benign, and 8 patients (36%) were indeterminate on HRUSG. Pathologically, 20 (80%) were benign and 5 (20%) were malignant [Table 3]. All the four pathologically proved malignant nodules were correctly diagnosed as malignant nodules by HRUSG. Out of the 13 benign nodules, 1 proved to be malignant on pathology as papillary carcinoma. That case on HRUSG was presented as a hyperechoic lesion with well-defined margin and macrocalcification. It was diagnosed as benign on HRUSG but turned out to be papillary carcinoma on pathology. Out of the eight indeterminate nodules on ultrasound (all were hypoechoic), all proved to be benign on pathology.
Table 3.
Correlation of radiological diagnosis with pathological diagnosis
| Radiological diagnosis | No. of cases | Pathological diagnosis | |
|---|---|---|---|
| Benign | Malignant | ||
| Benign | 13 | 12 | 1 |
| Indeterminate | 8 | 8 | 0 |
| Malignant | 4 | 0 | 4 |
| Total | 25 | 21 | 4 |
Discussion
In our study, most of the patients (52%) were in 40–49-year age group, the youngest being 16 years old and the eldest, 63 years old. The mean age was 39 years. There were 22 (88%) females and 3 males (12%). Studies performed by other workers also reported high percentage of female patients.[4]
Out of the 25 patients, 80% (20) were benign and 20% (5) were malignant by cytology. Out of the five malignant patients, one (4%) was that of a male patient and four (58.4%) were seen in female patients with age distribution between 20 and 44 years. Percentage of malignancy in male is 1 (33.3%) out of 3 and in female is 4 (18%) out of 22.
Most common benign pathology in our study was benign colloid goiter [Figure 1] seen in 60% cases. Follicular adenoma was seen in 12% and thyroiditis in 8% of patients. In a study by Bumiya and Roopa, benign pathology was observed in 90% cases, amongst which the commonest was goiter (66%) patients.[5]
Figure 1.

Colloid goiter: scattered and sheets of thyroid follicular epithelial cells lying in colloid
By HRUSG, 40% lesions were hypoechoic, 32% showed mixed echogenicity, and 8% were hyperechoic [Figure 2]. Perilesional halo was seen in 28% cases and calcification in 20% cases. Single nodule noted in 20% cases and multiple nodules in 80%. The margin was well defined in 52% and ill defined in 48%. Sixty-eight percent of the lesions were solid, 32% had solid-cystic components [Figures 3 and 4]. Similar studies show that sonographic criteria for predicting benign nature of thyroid nodule are well-defined margins, well-defined thin peripheral halo, and wider than tall in shape, and absence of microcalcification.[6,7]
Figure 2.
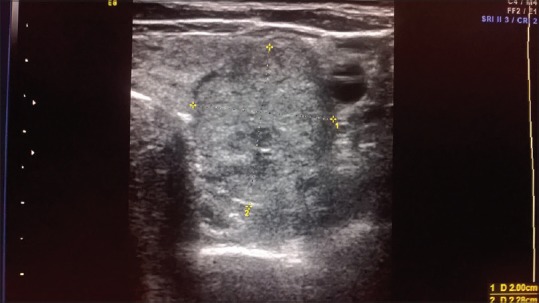
HRUSG thyroid – hyperechoic solid nodule with hypoechoic halo
Figure 3.
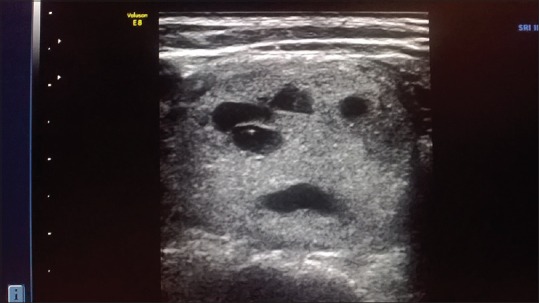
HRUSG thyroid – hyperechoic solid nodule with cystic degeneration
Figure 4.

Colloid goiter with cystic degeneration – scattered and sheets of thyroid follicular epithelial cells and hemosiderin laden macrophages
In the nodules pathologically diagnosed as follicular adenoma, all the three cases were hypoechoic in ultrasound. Two showed ill-defined margins and one case showed well-defined margin.
In pathologically proved thyroiditis, the nodules were hypoechoic in two cases. One showed well-defined border and the other one showed ill-defined border.
The five patients with malignant thyroid nodules were proved as papillary thyroid carcinoma [Figure 5] and were in the age group of 20–44 years. Four nodules were hypoechoic and one nodule was hyperechoic. In our study, out of the malignant lesions, one (20%) had a single nodule and four (80%) had multiple nodules. Three malignant nodules (60%) had calcification within the nodules (1 microcalcification, 2 macrocalcification). Margins of the nodules were well defined in 20% of malignant cases and ill defined in 80% of malignant cases. None of the malignant nodules showed perilesional halo. In a study conducted by Samghabadi et al., the absence of halo was most predictive of malignancy on conventional ultrasound.[8] In a study by Hoang et al., microcalcification and hypoechogenicity are predictive of malignancy.[9] In a study by Sharma et al., predominantly solid composition and hypoechogenicity of the nodules are predictive of malignancy.[10]
Figure 5.

Papillary thyroid carcinoma (Bethesda VI): follicular epithelial cells lying as sheets, papillary clusters, and scattered manner. Cells show nuclear groove and intranuclear inclusion
In our study, ultrasound has sensitivity of 80%, specificity of 75%, positive predictive value of 44%, negative predictive value of 93%, and accuracy of 76%. In a study by Popli et al., the sensitivity was 81.8% and specificity was 87.2%.[6]
Because of its easy availability and low cost, ultrasound-guided FNAC can be done even at primary care level.
Conclusion
HRUSG is a sensitive modality in the assessment of thyroid nodules with good overall accuracy. Various sonographic features of thyroid nodules in combination can be used to predict malignancy in thyroid nodules. Using these multiple features, HRUSG has accuracy of 76% with sensitivity of 80% and specificity of 75% for detecting thyroid malignancy, considering ultrasound-guided FNAC as the standard. FNAC is always suggested for the confirmation of diagnosis in suspicious thyroid nodule detected by HRUSG.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lee MJ, Hong SW, Chung WY, Kwak JY, Kim MJ, Kim EK. Cytological results of ultrasound-guided fine-needle aspiration cytology for thyroid nodules: Emphasis on correlation with sonographic findings. Yonsei Med J. 2011;52:838–44. doi: 10.3349/ymj.2011.52.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung MJ, Serpell JW. Management of the solitary thyroid nodule. Onchologist. 2008;13:105–12. doi: 10.1634/theoncologist.2007-0212. [DOI] [PubMed] [Google Scholar]
- 3.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 4.Ashwini BR, Vernekar S, Kulkarni MH, Kiran T. Comparative study of conventional and ultrasound-guided fine needle aspiration cytology of thyroid in a tertiary care center of North Karnataka. Int J Cur Res Rev. 2012;4:64–9. [Google Scholar]
- 5.Bumiya RG, Roopa Ultrasonography of the thyroid lesions correlated with FNAC. Int J of Sci Res. 2018;7:33–5. [Google Scholar]
- 6.Popli MB, Rastogi A, Bhalla PJS, Solanki Y. Utility of gray-scale ultrasound to differentiate benign from malignant thyroid nodules. Indian J Radiol Imaging. 2012;22:63–8. doi: 10.4103/0971-3026.95407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YH, Kim DW, In HS, Park JS, Kim SH, Eom JW, et al. Differentiation between benign and malignant solid thyroid nodules using an US classification system. Korean J Radiol. 2011;12:559–67. doi: 10.3348/kjr.2011.12.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samghabadi MA, Rahmani M, Saberi H, Behjati J, Firouznia K, Ghasemian A, et al. Sonography and color Doppler in the evaluation of cold thyroid nodules. Iran J Radiol. 2004;2:12–7. [Google Scholar]
- 9.Hoang JK, Lee WK, Lee M, Johnson D, Farrell S. US features of thyroid malignancy: Pearls and pitfalls. Radiographics. 2007;27:847–60. doi: 10.1148/rg.273065038. [DOI] [PubMed] [Google Scholar]
- 10.Sharma G, Keshava GH, Hanchinal V. Ultrasonographic evaluation of thyroid nodules with pathologic correlation. IJARS. 2017;6:53–7. [Google Scholar]


