Abstract
We report a 58-year-old male with sequelae of polio who presented with low back and left buttock pain, and pitting oedema of both legs for four months. The patient had a history of poliomyelitis at the age of 1 year which resulted in bilateral lower leg weakness, particularly on the left side. Magnetic resonance imaging showed cervical spinal stenosis secondary to posterior osteophyte formation, left paracentral disc extrusion at L2/L3 and L3/L4 levels with compression of the traversing L4 nerve root. The findings confirmed a diagnosis of lumbar radiculopathy caused by a herniated disc. The patient subsequently underwent a chiropractic treatment. The painful symptoms and pitting oedema in this case resolved with spinal adjustment in addition to scraping therapy to strengthen bilateral low back and the gluteal muscles. This case provides circumstantial evidence of a scarcely mentioned association between pitting oedema and lumbar radiculopathy caused by disc herniation. The pathophysiological mechanism is elusive, but might involve a complexity of cytokine-mediated inflammation and interconnection between somatic and autonomic nervous systems.
Keywords: Chiropractic, disc herniation, lumbar radiculopathy, pitting oedema, poliomyelitis
Introduction
Decades after partial or complete functional recovery from acute poliomyelitis, a consequence of biomechanical alterations, leg length inequality, muscle atrophy, joint stiffness, and age-relative changes may cause the late effects of polio.[1] Long-termed overloading of the intervertebral disc (IVD) may induce inflammation by activating components of the innate immune system. Inflammatory cytokines are produced by immune cells and also by the cells of a degenerative IVD. The degenerative materials can protrude through the weakened annulus and are referred to as disc herniation. A herniated disc is the most common cause of pinching spinal nerve root, called radiculopathy. Despite the common notion that removing a putative insult can alleviate pain, there remains uncertainty about whether mechanical or chemical factors that contribute more to pain from a disc herniation.[2] In fact, no evidence supports a long-term benefit for surgery to be superior comparing to conservative management.[3]
Case Report
A 59-year-old man with sequelae of poliomyelitis complained of bilateral leg swelling, low back and left buttock pain for 4 months. Paralytic polio occurred at the age of 1 and left the patient with bilateral leg weakness. It affected mainly the left leg. The patient had been walking with the aid of crutches. However, over the past year, he started noticing progressive leg weakness and fatigue. His symptoms failed to improve despite treatment with acupuncture. He was diagnosed with cervical degenerative spondylosis and lumbar disc herniation, which was present in two segments of the lumbar spine (L3/L4 and L4/L5), and was taking Ibuprofen for pain on an as-necessary basis over the last 4 months.
The patient walked into our clinic with crutches. On examination, his left leg was atrophic and hypotonic. There was bilateral leg swelling to the knees, more prominent on the right side [Figure 1]. Spinal palpation revealed spinal restriction at C4-7, T5-7, and L1-3. There was muscle weakness resulting in an inability to move the left leg. Neurological examinations revealed hypoesthesia in right C6 and left L4 dermatomes. Deep tendon reflex was absent in the left lower limb. Strength grading of the lower limbs were 0/5 at left L3-S1 and 4/5 at right L3-S1; grading of the upper limbs were 5/5 at C7-T1 and 4/5 at right C5-C7. MRI taken from an antecedent orthopedic consultation showed narrowing of the spinal canal at C5/6 to C6/C7 levels secondary to posterior osteophyte formation, left paracentral disc extrusion at L2/L3 and L3/L4 levels with compression of the traversing L4 nerve root. Surface electromyography (MyoVision®) revealed severe muscle spasm in the nuchal, upper back, and lumbosacral regions [Figure 2]. This patient was diagnosed as having cervical spondylotic myelopathy and lumbar disc herniation complicated by C7 and L4 radiculopathy. Additionally, the muscle weakness and pitting oedema of legs were suspected of being caused by post-polio syndrome.
Figure 1.
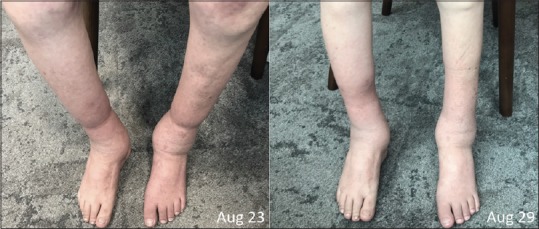
The patient presented with bilateral swelling of the legs to knees, more prominent on the right side (left). Oedema was reduced in both legs after a few treatment sessions (right)
Figure 2.
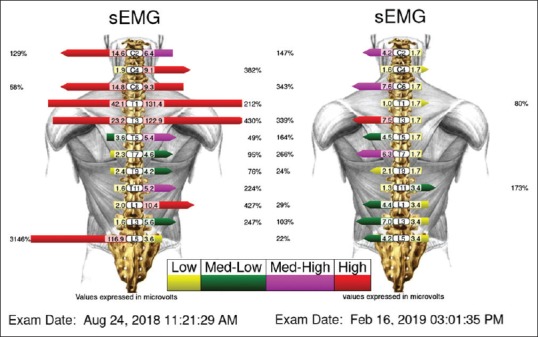
The initial surface EMG (left) revealed multiple levels of paraspinal muscle spasm. The post-treatment surface EMG (right) showed almost normalization of the paraspinal condition. At this time, his spinal complaints had resolved along with the disappearance of the leg oedema
A course of manual spinal mobilization and pinched nerve releasing, progressing to chiropractic adjustment (diversified technique) of his cervical and lumbar spine, was recommended. Manipulations were utilized in conjunction with scraping therapy (Graston® technique) to strengthen bilateral quadratus lumborum and gluteal max muscles on a twice weekly schedule. The patient noticed a reduction of pain and leg oedema after a few visits. He stated a complete relief from oedema, muscle cramps, and neck and back pain after 3-month treatment. At six months after his initial visit, the patient presented with a full recovery. The post-treatment surface EMG showed normalization of the paraspinal muscle strain [Figure 2].
Discussion
Degenerative disc disease is a common consequence of biomechanical alterations that occur as a result of polio-related muscle weakness, anisomelia (unequal length of limbs) and skeletal misalignment. Mechanical overloading of the IVD may induce inflammation by activating components of the innate immune system. Disc tissue, particularly the nucleus pulposus, releases inflammatory cytokines which trigger a range of pathogenic responses to initiate autophagy, senescence, and apoptosis.[4] Inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6),[2] interleukin-1β (IL-1β),[5] and nerve growth factor (NGF),[6] are key mediators of disc degeneration. Expression of these cytokines is not only correlated with disc degeneration but also believed to be the cause of disease. Emerging evidence suggests that even transient exposure to the cytokines may cause long-lasting effects on the disc.[5] Degenerative disc material, referred to as herniated disc, may protrude and compress on the spinal nerve of relevant segment resulting in painful radiculopathy.
Radiculopathy, commonly referred to as a pinched nerve, describes a conduction block of spinal nerve roots. Rather than a simple mechanical nerve root compression, the pathophysiological mechanism of radiculopathy caused by a herniated disc has recently evolved into a more complex mechanism that involves both mechanical and biochemical mechanisms. Histopathologically, herniated IVD tissues exhibit a high inflammatory feature by the presence of infiltrating macrophages and lymphocytes.[7] The dorsal root ganglion (DRG), located between the dorsal root and the spinal nerve, is critical in carrying the periphery sensory signal to the central nervous system (the spinal cord and brain).[8] In this inflammatory milieu, neurotrophic factors released from the herniated disc and immune cells induce expression of hyperalgesia associated cation channels in DRGs. Depolarization of these ion channels is likely to promote discogenic and radicular pain, and reinforce the cytokine-mediated degenerative cascade.[4]
Despite the common notion that removing the herniated disc can alleviate pain,[2] a substantial portion (23%−28%) of patients will continue to have pain after surgery (level of evidence: 4, case-series).[9] Since the prime pathophysiology of a painful radiculopathy due to disc herniation is an inflammatory process,[10] surgical treatment did not show a benefit over conservative treatment in mid-term and long-term follow-up.[11] This is in line with the results of an earlier investigation in 126 adult patients with radicular pain and confirmed lumbar disc herniation.[12] The researcher concluded that although open discectomy may enhance pain relief initially, no evidence supports a long-term benefit for surgery over conservative management (strength of recommendation: A, multiple randomized controlled trials).[3] There uncertainty remains in regard to whether mechanical or chemical factors contribute more to radicular pain from a disc herniation.[2] Currently, surgical intervention is reserved for patients with severe symptoms related to lumbar disc herniation.[9] The new endoscopic discectomy technique may allow treating a herniated disk more safely and in a more tissue-preserving manner.
Spinal manipulation is commonly used for treating individuals experiencing musculoskeletal pain. According to the NASS (North American Spine Society) 2014 guidelines, spinal manipulation is an option for symptomatic relief in patients with radiculopathy caused by disc herniation (grade of recommendation: C).[9,13] It has been reported that a single spinal adjustment may reduce the level of circulating inflammatory cytokines (TNF-α andIL-1β) via a central yet unknown mechanism.[14] Even if chiropractic manipulation does not target the immune system for modulating specific cytokines, the nature of manual intervention can be effective in strengthening muscles and minimizing functional decline through a variety of hands-on treatments, and potentially alleviating inflammation and associated symptoms. Although oral medication (such as analgesics, anti-inflammatories, etc.) is commonly used in clinical practice, there continues to be low to very low quality evidence available for their benefits and risks in pain relief.[15] In addition, limited data from low to very low grade evidence suggest that there may be a benefit in the use of the current medicinal injections.[15]
Leg oedema is often caused by a localized problem with the venous return, or a systemic presentation reflecting the heart, kidneys, or liver functions. Both were ruled out in this case. Theoretically, disc herniation never directly interferes the fluid balance between intravascular (the vessel) and extravascular spaces (the interstitium) governed by the Starling law. One explanation for oedema being bilateral in our case could be due to a lack of movement in acute low back pain. Another explanation could be a dysfunction of the complex interconnections between the somatic and autonomic nervous systems. The somatic senses (mechanoreception, thermoreception, and pain) are the defense mechanisms for collecting sensory information from the body. Persistent inflammatory hypernociception may cause an aberrant autonomic response. The autonomic dysfunction representing in the form of abnormal vasomotor, pilomotor, or sudomotor activity is occasionally related to lumbar disc herniation.[16,17] Moreover, individuals with compromised motor neurons, as seen in polio survivors, are predisposed to developing leg oedema. Oedema may occur when the weakened polio-affected muscles fail to pump the blood that has pooled in the legs, and the fluid is forced out of the vessels into the surrounding interstitium. The mechanisms underlying pitting oedema are often multifactorial.
Conclusion
This case provides circumstantial evidence of a scarcely mentioned association between pitting oedema and lumbar radiculopathy caused by disc herniation. Bilateral leg oedema could be derived from the complex interconnections between somatic and autonomic nervous systems.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Farbu E. Update on current and emerging treatment options for post-polio syndrome. Ther Clin Risk Manag. 2010;6:307–13. doi: 10.2147/tcrm.s4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothman SM, Winkelstein BA. Chemical and mechanical nerve root insults induce differential behavioral sensitivity and glial activation that are enhanced in combination. Brain Res. 2007;21(1181):30–43. doi: 10.1016/j.brainres.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorensen B, Hulkower S, Stigleman S. Does surgery relieve the pain of a herniated disc? J Fam Pract. 2010;59:228–33. [PubMed] [Google Scholar]
- 4.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson ZI, Schoepflin ZR, Choi H, Shapiro IM, Risbud MV. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104–17. doi: 10.22203/ecm.v030a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sainoh T, Inage K, Orita S, Koda M, Furuya T, Yamauchi K, et al. Correlation among inflammatory cytokine expression levels, degree of disk degeneration, and predominant clinical symptoms in patients with degenerated intervertebral discs. Asian Spine J. 2017;11:472–7. doi: 10.4184/asj.2017.11.3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: A study in surgical specimen and autopsy controls. Spine. 2005;30:44–53. doi: 10.1097/01.brs.0000149186.63457.20. [DOI] [PubMed] [Google Scholar]
- 8.Ahimsadasan N, Kumar A Neuroanatomy, dorsal root ganglion. StatPearls [Internet] Treasure Island, FL: StatPearls Publishing; 2019. Updated 2018 Oct 27. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532291/ [PubMed] [Google Scholar]
- 9.Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. 2014;14:180–91. doi: 10.1016/j.spinee.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami M, Tamaki T, Hayashi N, Hashizume H, Nishi H. Possible mechanism of painful radiculopathy in lumbar disc herniation. Clin Orthop Relat Res. 1998;351:241–51. [PubMed] [Google Scholar]
- 11.Gugliotta M, da Costa BR, Dabis E, Theiler R, Jüni P, Reichebach S, et al. Surgical versus conservative treatment for lumbar disc herniation: A prospective cohort study. BMJ Open. 2016;6:e012938. doi: 10.1136/bmjopen-2016-012938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber H. Lumbar disc herniation. A controlled, prospective study with 10 years of observation. Spine. 1983;8:131–40. [PubMed] [Google Scholar]
- 13.Santilli V, Beghi E, Finucci S. Chiropractic manipulation in the treatment of acute back pain and sciatica with disc protrusion: A randomized double-blind clinical trial of active and simulated spinal manipulations. Spine J. 2006;6:131–7. doi: 10.1016/j.spinee.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Teodorczyk-Injeyan JA, Injeyan HS, Ruegg R. Spinal manipulative therapy reduces inflammatory cytokines but not substance P production in normal subjects. J Manipulative Physiol Ther. 2006;29:14–21. doi: 10.1016/j.jmpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Peloso PM, Khan M, Gross AR, Carlesso L, Santaguida L, Lowcock J, et al. Pharmacological interventions including medical injections for neck pain: An overview as part of the ICON project. Open Orthop J. 2013;7(Suppl 4):473–93. doi: 10.2174/1874325001307010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jinkins JR, Whittemore AR, Bradley WG. The anatomic basis of vertebrogenic pain and the autonomic syndrome associated with lumbar disk extrusion. AJR Am J Roentgenol. 1989;152:1277–89. doi: 10.2214/ajr.152.6.1277. [DOI] [PubMed] [Google Scholar]
- 17.Yildiz N, Güngen GÖ, Yaylali O, Ardiç F. Bilateral complex regional pain syndrome associated with lumbar disc herniation. Arch Rheumatol. 2011;26:66–7. [Google Scholar]


