Abstract
Background
The live respiratory syncytial virus (RSV) candidate vaccine LIDcpΔM2-2 is attenuated through deletion of M2-2 and 5 cold-passage mutations.
Methods
RSV-seronegative children aged 6–24 months received a single intranasal dose of 105 plaque-forming units (PFU) of LIDcpΔM2-2 or placebo. RSV serum antibodies, vaccine infectivity, and reactogenicity were assessed.
Results
Four of 11 (36%) vaccinees shed vaccine virus with median peak titers of 1.6 log10 PFU/mL by quantitative culture and 4.5 log10 copies/mL by polymerase chain reaction; 45% had ≥4-fold rise in serum-neutralizing antibodies. Respiratory symptoms or fever were common in vaccinees (64%) and placebo recipients (6/6, 100%).
Conclusions
RSV LIDcpΔM2-2 is overattenuated.
Clinical Trial Numbers. NCT02890381, NCT02948127.
Keywords: immunogenicity, live-attenuated viral vaccine, neutralizing antibodies, pediatric RSV vaccine, respiratory syncytial virus, RNA regulatory protein M2-2
Respiratory syncytial virus (RSV) causes about 33 million episodes of lower respiratory illness (LRI), 3.2 million hospital admissions, and up to 118 200 deaths annually [1]. A substantial burden of RSV disease occurs in children >6 months of age. Passive immune prophylaxis with monoclonal antibodies is effective for high-risk infants [2] but is not feasible for general use. Also, passive protection eventually wanes. Thus, there is a strong rationale for development of pediatric RSV vaccines for active immunization [3]. Live-attenuated RSV vaccines for intranasal application are attractive because they have the potential to induce a spectrum of protective immune responses, including mucosal immune responses. Unlike formalin-inactivated or other nonreplicating RSV vaccines, they are not associated with a risk of priming for enhanced RSV disease [4, 5].
Use of reverse genetics [6] and improved understanding of RSV gene function allow for rational design of attenuated vaccine candidates for children as young as 4 weeks of age [7, 8]. A promising attenuation strategy employs deletion of most of the open reading frame (ORF) encoding the RNA synthesis regulatory protein M2-2. Deletion of M2-2 results in a shift in the viral RNA synthesis program such that gene transcription and antigen expression are increased, whereas genome replication is decreased [9], which might lead to greater immunogenicity per infectious unit.
A candidate vaccine, MEDI∆M2-2, was highly restricted in replication yet more immunogenic than prior RSV vaccine candidates in a previous study of RSV-seronegative children [10]. A second candidate with M2-2 deletion, LID∆M2-2 [11], based on a different laboratory isolate of RSV strain A2, seemed less restricted in replication. The current study evaluated LIDcp∆M2-2, a version of LID∆M2-2 that was further attenuated by an additional set of 5 well-characterized attenuating mutations derived from cold-passaged (cp) RSV [12, 13] in RSV-seronegative children aged 6–24 months.
METHODS
Vaccine
LIDcpΔM2-2 is based on LIDΔM2-2 (described in detail in McFarland et al. [11]). Both viruses are derived from a recombinant version of wild-type (wt) RSV subgroup A strain A2 (GenBank accession number KT992094) and are attenuated by a 241 nucleotide deletion of the M2-2 ORF (nt 8189–8429). LIDcpΔM2-2 differs from LIDΔM2-2 only by a set of 5 amino acid substitutions in 3 RSV proteins that in aggregate are called the “cp” mutations (V267I in N, E218A and T523I in F, and C319Y and H1690Y in the L protein) [12, 13]. LIDcpΔM2-2 was generated by reverse genetics on World Health Organization Vero cells. Clinical trial material was manufactured at Charles River Laboratories (Malvern, PA). The live vaccine had a potency of 5.6 log10 plaque-forming units (PFU)/mL, was stored at –80°C, and was diluted with Leibovitz L15 medium to a dose of 105 PFU in a 0.5-mL volume. The vaccine was administered intranasally as nosedrops in a single dose divided between the nostrils. Leibovitz L15 medium was used as placebo.
Study Design
This randomized (2:1 vaccine to placebo), double-blind, placebo-controlled study (https://clinicaltrials.gov: NCT02890381/NCT02948127) was conducted at 5 clinical trials sites with accrual between October 5 and October 26, 2016. Eligible children were ≥6 and <25 months of age, healthy, with no history of lung disease, and were RSV-seronegative at screening, defined as having a serum RSV 60% plaque-reduction neutralizing titer (PRNT60) ≤1:40.
Clinical assessments and nasal washes (NWs) were performed on study days 0 (before inoculation), 3, 5, 7, 10, 12, 14, 17, and 28, with telephone contact on intervening days. Additional physical examinations and NWs were obtained in the event of respiratory illness (upper respiratory illness [URI; rhinorrhea, pharyngitis, or hoarseness]; cough; acute otitis media [OM]; LRI) or fever. Adverse events were recorded through day 28; serious adverse events (SAEs) were recorded until day 56. Surveillance for medically attended respiratory illness associated with naturally occurring RSV was conducted from November 1, 2016, through March 31, 2017, by weekly contacts. Within 3 days of each illness episode, a clinical assessment was performed and NW was obtained and tested for the presence of viral pathogens as described below. Sera to measure antibodies to RSV were obtained before inoculation, 56 days after inoculation, and after the surveillance period.
Written informed consent was obtained from parents/guardians, and human experimentation guidelines by the US Department of Health and Human Services were followed. Studies were approved by each site’s institutional review board and monitored by the Independent Data Safety and Monitoring Board of the National Institute of Allergy and Infectious Diseases, Division of Clinical Research.
Laboratory Assays
Vaccine virus in NWs was quantified by immuno-plaque assay on Vero cells and by reverse transcription quantitative polymerase chain reaction (RT-qPCR) specific to the RSV matrix protein as previously described [11], and the genetic stability of vaccine isolates was determined as previously described [11]. NW specimens from days of illness were evaluated for the presence of adventitious respiratory agents by multiplex RT-PCR (FTD Respiratory pathogens 21, Fast-track Diagnostics, Esch-sur-Alzette, Luxembourg).
Serum RSV PRNT60 were determined by complement-enhanced 60% plaque reduction neutralization assay, and serum IgG antibody titers to the RSV F glycoprotein (anti-RSV F IgG) were determined by an IgG-specific enzyme-linked immunosorbent assay (ELISA) as previously described [10, 11, 14].
Statistical Analysis
Medians and interquartile ranges (IQRs) were used to summarize peak NW titers and serum antibody titers. Mean and standard deviation values are presented to allow descriptive comparisons with other studies. The summaries of vaccine virus shed in NW detected by culture and RT-qPCR were restricted to the vaccine recipients who were infected with vaccine (defined as detection of vaccine virus by culture and/or RT-qPCR and/or a ≥4-fold rise in serum RSV PRNT60 or anti-RSV F IgG). Analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC), and graphs were produced using R software.
RESULTS
The study accrued 11 vaccinees and 6 placebo recipients. The distribution of sex, age, ethnicity, and racial characteristics was similar for vaccine and placebo recipients (Supplementary Table 1). All children were assigned study treatment. One placebo recipient did not have serum obtained at the postinoculation visit.
During the 28 days postinoculation, respiratory and/or febrile illnesses were frequent in both vaccine and placebo recipients, with 7/11 (64%; 90% confidence interval [CI], 35%–86%) and 6/6 (100%; 90% CI, 61%–100%) having 1 or more illnesses, respectively (Table 1). Six of these 7 vaccinees had respiratory agents detected in NWs, including rhinovirus (n = 5), adenovirus (n = 2), and parainfluenza 3 (n = 1), with (n = 2) or without detection of vaccine virus. Similarly, 4/6 symptomatic placebo recipients had 1 or more viruses isolated, including rhinovirus (n = 4), parainfluenza 2 and 3, and enterovirus (1 each). Two placebo recipients had documented, symptomatic wt RSV infections during this period. There were 2 adverse events that were of Grade 3 severity: Fever (39.2°C) occurred in a vaccinee without vaccine virus or other agents detected, and fever (39.3°C) occurred in a placebo recipient, concurrent with isolation of rhinovirus and RSV subgroup B. All other reactogenicity events in both groups were of Grade 2 severity or less. Two vaccinees had Grade 2 LRI, 1 noted on Day 21 with bronchitis and the other on day 28 with wheezing; both were accompanied by acute OM and URI symptoms. Both children had rhinovirus detected at the time of symptoms without detection of vaccine virus. No SAEs occurred.
Table 1. .
Vaccine Virus Shedding, Peak Virus Titers, and Clinical Assessment During the First 28 Days After Inoculation
| Viral Detectiona | No. (%) With Indicated Symptomb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | No. of Children | No. (%) Shedding Vaccine Virusc | Plaque Assay, Log10 PFU/mLd | RT-qPCR, Log10 Copies/mLe | Fever | URI | LRI | Cough | OM | Respiratory or Febrile Illness |
| Vaccine | 11 | 4 (36%) | 1.6 (0.5–3.4) | 4.5 (1.7–6.8) | 2 (18) | 6 (55) | 2 (18) | 4 (36) | 3 (27) | 7 (64) |
| Placebo | 6 | 0 | 0.5 (0.5–0.5) | 1.7 (1.7–1.7) | 4 (67) | 6 (100) | 0 (0) | 4 (67) | 0 (0) | 6 (100) |
Abbreviations: LRI, lower respiratory illness, defined as wheezing, rhonchi, or rales, or having been diagnosed with pneumonia or laryngotracheobronchitis (croup); NW, nasal wash; OM, acute otitis media; PFU, plaque-forming unit; RT-qPCR, reverse transcription quantitative polymerase chain reaction; RSV, respiratory syncytial virus; URI, upper respiratory illness, defined as rhinorrhea, pharyngitis, or hoarseness.
aMedian (25th–75th percentile) peak viral titers detected in nasal washes. For the vaccine group, these summaries were calculated only for the 6 children who were infected with vaccine virus; infection was defined as the detection of vaccine virus by culture and/or RT-qPCR and/or a ≥4-fold rise in RSV serum-neutralizing antibody titer and/or serum anti-RSV F antibody titer. As expected, no placebo recipients shed vaccine virus.
bNumber (%) of children with indicated respiratory symptoms occurring in the 28 days after inoculation.
cPercentage of children with vaccine virus detected in NW by culture and/or RT-qPCR. Three children had vaccine virus detected by both culture and PCR, and 1 only by RT-qPCR.
dFor each child, the individual peak (highest) titer, irrespective of day, was selected from among all titers measured in the NW by viral culture and expressed as log10 PFU/mL. The lower limit of detection was 0.5 log10 PFU/mL.
eFor each participant, the individual peak (highest) titer, irrespective of day, was selected from among all titers measured in NW by RT-qPCR and expressed as log10 copies/mL. The lower limit of detection was 1.7 log10 copies/mL.
Six of 11 (55%) vaccinated children met the definition of infection with vaccine virus. Four of 11 (36%; 90% CI, 14%–65%) vaccinees had vaccine virus detected by quantitative culture or RT-qPCR (Table 1; Supplementary Table 2). Two additional vaccinees had seroconversion without detection of vaccine virus. Among those 6 vaccinees, vaccine virus was shed for a median duration (IQR) of 6 (0–12) days by culture and 12 (0–15) days by RT-qPCR. Median (IQR) peak titers of shed vaccine virus were 1.6 (0.5–3.4) log10 PFU/mL and 4.5 (1.7–6.8) log10 copies/mL for the 6 vaccinees meeting vaccine infection criteria (Table 1). Sequence analysis of LIDcp∆M2-2 isolates from which sequence analysis data could be obtained confirmed that the shed vaccine stably retained the cp codons (isolates from 3 participants) and the M2-2 deletion (isolates from 4 participants).
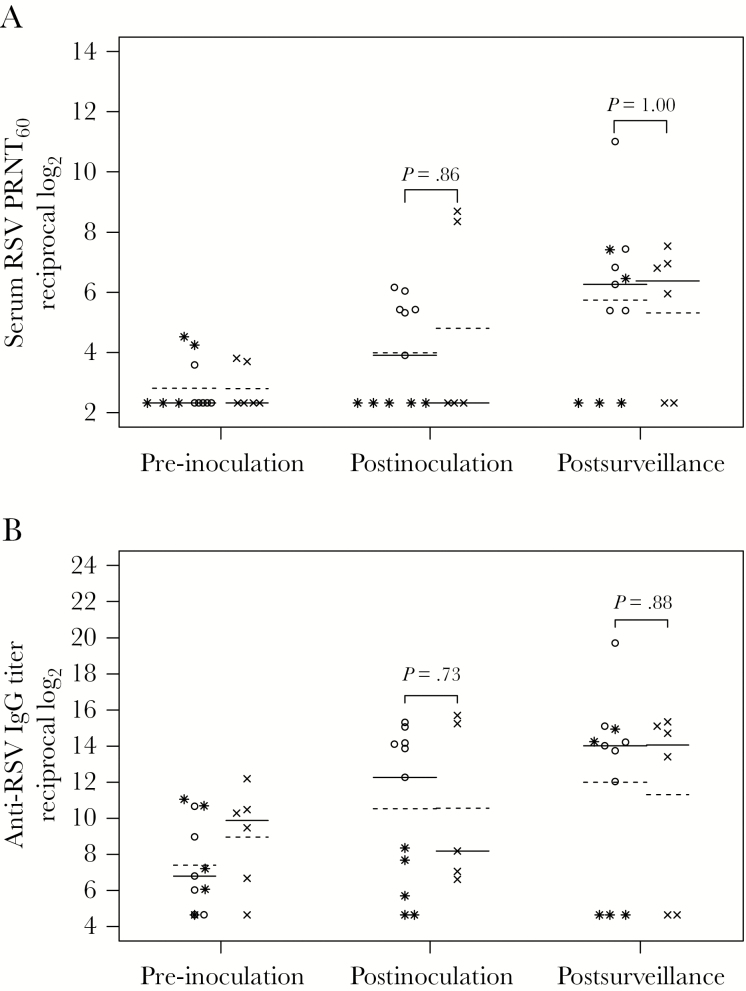
Changes in serum antibody titers are shown in Figure 1. Four-fold or greater rises in serum RSV PRNT60 and anti-RSV F IgG titers at day 56 (Supplementary Table 3) were present in 5/11 (45%) vaccinees and 2/5 (40%) placebo recipients (1-tailed P = .64). One vaccinee with virus identified by RT-qPCR but not culture did not seroconvert. Both placebo sero-converters had wt RSV isolated at the time of clinical symptoms on study day 27. Among vaccinees, only 18% achieved a serum RSV PRNT60 ≥ 6.0 log2 (1:64). Antibody responses at day 56 to RSV F glycoprotein were similar in vaccine and placebo recipients (Supplementary Table 3).
Figure 1.
Serum respiratory syncytial virus (RSV) antibody titers in vaccine and placebo recipients. Serum RSV 60% plaque reduction neutralizing titers (PRNT60) (A) and anti-RSV F IgG titers (B) were determined by complement-enhanced 60% plaque reduction neutralization assay and IgG-specific enzyme-linked immunosorbent assay against purified baculovirus-expressed F protein (provided by Novavax, Inc., Gaithersburg, MD), respectively, for vaccine (open circles and stars) and placebo (x’s) recipients in sera collected at pre-inoculation (screening), postinoculation (study day 56), and postsurveillance (after the RSV season, April 1 to 30 in the calendar year after the inoculation). Titers are expressed as the reciprocal log2. The lines indicate median (solid line) and mean (dashed line) values. P values were determined by Wilcoxon rank-sum test. Five vaccine recipients who did not shed vaccine virus are indicated with the star symbol. The data from the postinoculation visit are missing for 1 placebo recipient.
RSV Surveillance
During the RSV surveillance period, rates of MAARI were similar between the vaccine and placebo groups (5/11, 45%; 90% CI, 20%–73%; vs 3/6, 50%; 90% CI, 15%–85%), respectively). Three vaccinees (2 without vaccine take and 1 vaccinee with shedding detected by PCR but without seroconversion) and 1 placebo recipient experienced RSV-associated MAARI during the surveillance period, including 1 vaccinee with bronchiolitis, wheezing, rhonchi, rhinorrhea, OM, and cough (RSV type A), 1 with croup and cough (RSV type A), and a third with bronchiolitis, dyspnea, OM, fever, rhinorrhea, and cough (RSV type B). One of the placebo recipients had cough and nasal congestion (RSV type A). A second placebo recipient demonstrated a rise in RSV titer during the season without evidence of a MAARI.
Assessment after the RSV season showed that serum antibody responses to vaccine in 5 vaccinees who did not have a boosted response and thus were presumed not to have been exposed to RSV during the surveillance period were unchanged, indicating that the serum antibody response to vaccine was durable.
DISCUSSION
The RSV vaccine candidate LIDcp∆M2-2 is overattenuated and not suitable for further development. A related product, LID∆M2-2, was previously shown to have excellent infectivity and immunogenicity in RSV-seronegative children aged 6–24 months, inducing 4-fold increases in serum RSV-neutralizing antibodies in 90% of vaccinees [11]. The cp mutations were added with the expectation of slightly reducing the level of replication of LID∆M2-2. However, the results of the present study suggest that adding the additional 5 cp mutations significantly increased attenuation, exceeding the moderately restrictive effects observed in preclinical studies [13] and resulting in a candidate vaccine with suboptimal vaccine take and immunogenicity. In preclinical studies in African Green Monkeys, LIDcpΔM2-2 at doses of 1 and 2 × 106 PFU demonstrated low or undetectable levels of replicating virus and excellent antibody responses (Investigator’s Brochure, version 29 July 2016). Cold-passaged RSV was originally derived by 52 sequential cell culture passages at low temperatures. Earlier studies of cpRSV demonstrated that the vaccine was underattenuated, and subsequent studies evaluating cpRSV with additional attenuating mutations have shown over- [14] or underattenuation [15].
The median peak titer of vaccine virus shed among those vaccinees who shed virus (1.6 log10 PFU/mL by culture, 4.5 log10 copies/mL by qRT-PCR) was lower than that of LIDΔM2-2, which produced median peak titers of 3.8 log10 PFU/mL and 6.2 log10 copies/mL, respectively [11]. However, the peak titers were higher than those seen with at least 1 other product, RSVcps2, which produced a median peak titer of 0.5 log10 PFU/mL by culture and 2.9 log10 copies/mL by RT-qPCR [15]. Despite the low magnitude of vaccine virus detected in RSV-seronegative children, 77% of recipients of RSVcps2 shed vaccine virus, whereas in the present study, only 36% of recipients of RSV LIDcp∆M2-2 had demonstrable viral replication.
The rates of respiratory events after inoculation were high among both vaccine and placebo recipients, and there was no evidence that the vaccine virus was causally associated with any of these events. Further, there was no evidence of enhanced RSV illness when participants subsequently acquired wt RSV infection.
This study has several limitations. The small sample size precludes firm conclusions regarding rates of vaccine-associated events and the precision of the point estimate of the rate of immune response. Further, the timing of enrollment and overlap of the first 56 days of follow-up and RSV season resulted in some children acquiring RSV infection before the collection of the day 56 serum sample. Future studies will measure antibody response 28 days after immunization, rather than 56 days, to minimize the overlap with RSV season. Nevertheless, the very low incidence of vaccine virus replication as measured by culture and RT-qPCR indicates that this vaccine is overattenuated.
In summary, the LIDcpΔM2-2 vaccine is not a candidate for further development. RSV ΔNS2/Δ1313/I1314L, attenuated by the deletion of the viral interferon antagonist NS2, and RSV 276, attenuated by deletion of the M2-2 ORF but without additional cp mutations, are proceeding to larger clinical trials (ClinicalTrials.gov: NCT03422237/NCT03227029). It is expected that both of these candidate vaccines will demonstrate viral replication in a higher proportion of children immunized, with improved vaccine immune response rates.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the children and families for their commitment to advancing research in the prevention of RSV. We acknowledge members of the protocol team for their expert contributions, including Data Managers Linda Marillo, BS, Benjamin Johnston, BS, and Andee Fox, MPH, at Frontier Science & Technology Research; Protocol Pharmacist Lynette Purdue, PharmD, at the Pharmaceutical Affairs Branch, Division of AIDS (DAIDS); Laboratory Technologist Paul Harding, MS, at the University of Colorado School of Medicine; Network Laboratory Center Specialist Dale Dayton, RN, CCRA, at the Children’s Hospital of Los Angeles; Westat Clinical Research Associate Scott Watson, RN, BS, at Westat, Inc.; the companion protocol (CIR 312) Medical Monitor Shirley Jankelevich, MD, and the CIR 312 Clinical Research Oversight Managers Kelly Cahill, RN, CCRC, RAC, Susan Vogel, RN, BSN, and John Tierney RN, MPM, of the Regulatory Compliance & Human Subjects Protection Branch, Division of Clinical Research, NIAID, NIH. We thank the members of the NIAID, Division of Clinical Research, Data and Safety Monitoring Board. Dr. Gregory Glenn, Novavax, graciously provided the gift of baculovirus-expressed RSV F protein used as an ELISA antigen. Finally, we thank the dedicated site investigators and research professionals at the following institutions, in alphabetical order: Children’s Hospital of Philadelphia: Steven Douglas, MD. David Geffen School of Medicine at University of California Los Angeles: Michele F. Carter, RN; Christina S. Shin, PharmD; and Ruth Cortado. Johns Hopkins University Center for Immunization Research: Jennifer Oliva, MS; Jocelyn San Mateo, NP; Kristi Herbert, CRNP. Rush University: Maureen McNichols, RN, CCRC; Ixchell Ortiz-Estes, APN; Kenneth Boyer, MD. University of Colorado Denver: D’Andra Mixon-Walker, BS; Hannah Bernath, MPH; Carrie Chambers, RN, BSN; Suzanne Paul FNP, MSN.
Financial support. This work was supported by the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network, funded by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH; UM1AI068632 [IMPAACT LOC], UM1AI068616 [IMPAACT SDMC], and UM1AI106716 [IMPAACT LC]), and by the NICHD (HHSN275201800001I). R.A.K., B.T., and E.S. were supported by NIAID contract (HHSN272200900010C). This work also received support from Sanofi Pasteur Inc. through a Cooperative Research And Development Agreement (CRADA) with the NIAID. L.Y., C.L., P.L.C., and U.J.B. were supported by the Intramural Program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. This publication resulted in part from research supported by the Duke University Center for AIDS Research (CFAR), an NIH-funded program (5P30 AI064518), to C.K.C. and the Colorado Clinical and Translational Science Award (CTSA) from the National Center for Advancing Translational Science (NCATS; UL1 TR001082) to E.J.M.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Potential conflicts of interest. U.J.B., C.L., and P.L.C. are listed as inventors on patents related to live-attenuated RSV vaccines. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentations. Study results were presented in part at the Pediatric Academic Society (PAS) 2018 Conference; May 5–8, 2018; Toronto, ON, Canada (poster abstract 1457, board 498).
References
- 1. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Groothuis JR, Simoes EA, Levin MJ, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The respiratory syncytial virus immune globulin study group. N Engl J Med 1993; 329:1524–30. [DOI] [PubMed] [Google Scholar]
- 3. Giersing BK, Vekemans J, Nava S, et al. Report from the World Health Organization’s third Product Development For Vaccines advisory committee (PDVAC) meeting, Geneva, 8-10th June 2016. Vaccine 2017. In Press: doi:10.1016/j.vaccine.2016.10.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Acosta PL, Caballero MT, Polack FP. Brief history and characterization of enhanced respiratory syncytial virus disease. Clin Vaccine Immunol 2015; 23:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wright PF, Karron RA, Belshe RB, et al. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine 2007; 25:7372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins PL, Hill MG, Camargo E, et al. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5’ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci U S A 1995; 92:11563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karron RA, Wright PF, Belshe RB, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis 2005; 191:1093–104. [DOI] [PubMed] [Google Scholar]
- 8. Collins PL, Murphy BR. Vaccines against human respiratory syncytial virus. In: Cane PA, ed. Respiratory Syncytial Virus. Amsterdam: Elsevier; 2007:233–77. [Google Scholar]
- 9. Bermingham A, Collins PL. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc Natl Acad Sci U S A 1999; 96:11259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karron RA, Luongo C, Thumar B, et al. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Science Translational Medicine 2015; 7:312ra175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McFarland EJ, Karron RA, Muresan P, et al. ; International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) 2000 Study Team Live-attenuated respiratory syncytial virus vaccine candidate with deletion of RNA synthesis regulatory protein M2-2 is highly immunogenic in children. J Infect Dis 2018; 217:1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedewald WT, Forsyth BR, Smith CB, et al. Low-temperature-grown RS virus in adult volunteers. JAMA 1968; 204:690–4. [PubMed] [Google Scholar]
- 13. Whitehead SS, Juhasz K, Firestone CY, et al. Recombinant respiratory syncytial virus (RSV) bearing a set of mutations from cold-passaged RSV is attenuated in chimpanzees. J Virol 1998; 72:4467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buchholz UJ, Cunningham CK, Muresan P, et al. ; International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1114 Study Team Live respiratory syncytial virus (RSV) vaccine candidate containing stabilized temperature-sensitivity mutations is highly attenuated in RSV-seronegative infants and children. J Infect Dis 2018; 217:1338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol 2013; 372:259–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.