Abstract
Background
There is a global obesity crisis, particularly among women and disadvantaged populations. Early-life intervention to prevent childhood obesity is a priority for public health, global health, and clinical practice. Understanding the association between childhood obesity and maternal pre-pregnancy weight status would inform policy and practice by allowing one to estimate the potential for offspring health gain through channelling resources into intervention. This systematic review and meta-analysis aimed to examine the dose–response association between maternal body mass index (BMI) and childhood obesity in the offspring.
Methods and findings
Searches in MEDLINE, Child Development & Adolescent Studies, CINAHL, Embase, and PsycInfo were carried out in August 2017 and updated in March 2019. Supplementary searches included hand-searching reference lists, performing citation searching, and contacting authors. Two researchers carried out independent screening, data extraction, and quality assessment. Observational studies published in English and reporting associations between continuous and/or categorical maternal and child BMI or z-score were included. Categorical outcomes were child obesity (≥95th percentile, primary outcome), overweight/obesity (≥85th percentile), and overweight (85th to 95th percentile). Linear and nonlinear dose–response meta-analyses were conducted using random effects models. Studies that could not be included in meta-analyses were summarised narratively. Seventy-nine of 41,301 studies identified met the inclusion criteria (n = 59 cohorts). Meta-analyses of child obesity included 20 studies (n = 88,872); child overweight/obesity, 22 studies (n = 181,800); and overweight, 10 studies (n = 53,238). Associations were nonlinear and there were significantly increased odds of child obesity with maternal obesity (odds ratio [OR] 3.64, 95% CI 2.68–4.95) and maternal overweight (OR 1.89, 95% CI 1.62–2.19). Significantly increased odds were observed for child overweight/obesity (OR 2.69, 95% CI 2.10–3.46) and for child overweight (OR 1.80, 95% CI 1.25, 2.59) with maternal obesity. A limitation of this research is that the included studies did not always report the data in a format that enabled inclusion in this complex meta-analysis.
Conclusions
This research has identified a 264% increase in the odds of child obesity when mothers have obesity before conception. This study provides substantial evidence for the need to develop interventions that commence prior to conception, to support women of childbearing age with weight management in order to halt intergenerational obesity.
Nicola Heslehurst and colleagues highlight the association between childhood obesity and maternal pre-pregnancy weight status.
Author summary
Why was this study done?
Obesity is a leading cause of lifelong poor health globally, and is significantly associated with inequalities.
Capitalising on opportunities for early-life prevention of obesity is a priority for public health, global health, and clinical practice.
This research aimed to estimate the extent to which mothers’ pre-pregnancy body mass index was associated with the weight status of their children, and hence the extent to which this life course stage may be an opportunity for obesity prevention.
What did the researchers do and find?
This systematic review included 79 studies from international settings that investigated maternal pre-pregnancy body mass index and childhood weight status.
Meta-analysis identified that maternal pre-pregnancy obesity was significantly associated with child obesity (OR 3.64, 95% CI 2.68–4.95; n = 88,872), child overweight/obesity combined (OR 2.69, 956% CI 2.10–3.46; n = 181,800), and child overweight (OR 1.80, 95% CI 1.25–2.59; n = 53,238).
Meta-regression found increasing odds of child obesity with increasing child age.
What do these findings mean?
Our finding of 264% increased odds of child obesity when mothers have pre-pregnancy obesity suggests that commencing obesity prevention interventions prior to conception would be beneficial.
This research adds to the substantial evidence base that the causes of obesity involve a complex interplay between in utero and life course exposures, which may unequally affect those with a predisposition to obesity.
Little attention has been given to the preconception period among obesity prevention interventions to date; attention to this period may help to address the complex early-life inequalities associated with obesity development.
Introduction
Halting childhood obesity is essential to tackle the global obesity crisis and for the future health of the population. Childhood obesity increases the risk of hypertension, cardiovascular disease, diabetes, reduced lung function, mental health conditions, and obesity in adulthood [1,2]. The prevalence of adult obesity has almost tripled over 40 years, with an estimated 13% of the world’s population having obesity, and with the highest prevalence being among women [2–4]. An estimated 41 million children aged 0–5 years and over 340 million aged 5–19 years have overweight or obesity [2,5]. The alarming increase in extreme obesity [6] demonstrates the limited impact of interventions to date in halting or reversing the obesity trend. Prevalence is particularly increasing in low- and middle-income countries, with almost half of overweight and obesity in children under 5 years occurring in Asia, and with a 50% increase in child overweight and obesity in Africa between 2000 and 2016 [2,3]. The cost of treating obesity and related comorbidities was estimated to be 76% higher than healthcare costs for patients with a recommended body mass index (BMI) in the US [7], further demonstrating the need for preventative action.
Prevention of childhood obesity is a priority for public health, global health, and clinical practice, yet interventions to date have produced disappointing results. A key question that remains unanswered is: When is the best life course stage to intervene? There are multiple published studies reporting associations between maternal pre-pregnancy weight status and offspring BMI, with some conflicting and inconsistent results on the extent to which these factors are associated. Understanding this association would inform public health policy and practice by allowing estimation of the potential for offspring health gain through channelling resources into early-life intervention. This systematic review and meta-analysis aimed to determine the dose–response association between maternal pre-pregnancy BMI and offspring obesity.
Methods
This study is reported as per the MOOSE Checklist for Meta-analyses of Observational Studies (S1 Text). A 5-stage search strategy was implemented to limit the effect of publication bias, as database searches alone are insufficiently rigorous [8]. (1) The MEDLINE, CINAHL, Embase, Child Development & Adolescent Studies, and PsycInfo databases were searched using keywords and MeSH headings developed by an information scientist (S. Robalino) (S1 Fig). Searches were restricted to human studies published in English. No date restrictions were applied. (2) The reference lists of all studies that met the inclusion criteria and all related systematic reviews were hand searched. (3) Citation searches for all studies that met the inclusion criteria and all related systematic reviews were performed using Google Scholar Citations. (4) Any additional studies identified in stages 2 and 3 that met the inclusion criteria were subject to further reference list and citation searching. Stages 2–4 were repeated until no further new studies were identified. (5) Authors of included studies were contacted for additional data when required for inclusion in the meta-analyses. Database searches were completed in August 2017 and updated in March 2019.
Inclusion criteria were peer-reviewed studies reporting both the exposure variable (maternal pre- or early-pregnancy BMI) and the outcome variable (offspring BMI or z-score) among children aged 1–18 years. We did not restrict to continuous or categorical exposure or outcome data. Four combinations of data were reported in the included studies: (1) categorical maternal BMI and continuous child BMI/z-score, (2) continuous maternal BMI and continuous child BMI/z-score, (3) continuous maternal BMI and categorical child BMI/z-score, and (4) categorical maternal BMI and categorical child BMI/z-score.
Studies reporting duplicate data from the same cohort were excluded, except when data were reported as different combinations and were included in separate analyses. When multiple studies reported data from the same cohort, 3 authors (NH, ZA, and RV) selected which to include using a priority list based on study characteristics: all data required for meta-analysis present, a greater number of maternal BMI categories, child ages not combined, larger sample size, and adjusted analyses. Data extractions and quality assessments were carried out independently by 2 researchers for each included study (NH, RV, ZA, ES, HB, LN, JR, and AP) using a standardised protocol and the Newcastle–Ottawa scale, which assesses information bias, selection bias, and confounding in cohort studies (S2 Table; S2 Fig).
Analysis of categorical outcomes
The primary outcome was childhood obesity. For the purposes of this systematic review, we categorised 3 outcome variables using BMI percentiles (or equivalent z-score categories): obese (≥95th percentile), overweight or obese (≥85th percentile), and overweight (85th to 95th percentile). If data were reported for the same children at multiple ages, then these were related and could not be included in the same analysis; the decision was made to use the oldest age in the meta-analyses and narratively report the younger ages. Dose–response meta-analyses were conducted to investigate the association between maternal and child BMI. When maternal BMI was reported in a continuous form, the reported study-specific linear trends (odds ratios [ORs]) for continuous BMI were used (assuming linearity). For categorical maternal BMI, the study-specific linear trends were derived using the method by Greenland and Longnecker [9], which requires the ORs, confidence intervals (CIs), and number of cases and participants for at least 2 exposure categories. If the adjusted ORs and CIs were not available, the respective unadjusted parameters were derived from the data. The maternal BMI exposure categories were underweight (BMI < 18.5 kg/m2), recommended BMI (18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), and obese (BMI ≥ 30 kg/m2). For each category, the midpoint was calculated as the average of the lower and upper bound, and the respective OR was assigned to each midpoint. As the BMI midpoint was required for these analyses, upper and lower cut-offs were applied to open-ended BMI categories. For underweight, a lower limit of 13.5 kg/m2 was applied; the respective midpoint was 17 kg/m2. For obese, the midpoint was selected as being 35 kg/m2, reflecting that the majority of pregnant women with obesity have class I (BMI 30–34.9 kg/m2) or class II (BMI 35–39.9 kg/m2) obesity [10]. The summary ORs were calculated using the random effects model by DerSimonian and Laird [11].
A 2-stage, random effects, nonlinear dose–response meta-analysis [12–14] was conducted to assess potential nonlinear associations, using cubic splines regression to model maternal BMI (S1 Text). Studies reporting continuous maternal BMI or only 2 categories were excluded from the nonlinear analyses.
Analysis of continuous outcomes
A dose–response meta-analysis was used to analyse continuous child BMI and z-score outcomes with categorical maternal BMI exposures, using maternal recommended BMI (18.5–24.9 kg/m2) as the reference group. As child BMI and z-scores are 2 different scales, we computed the standardised mean differences (SMDs) as effect sizes, which were combined using the method described by Crippa and Orsini [15]. This consisted of the estimation of flexible dose–response models within each study considering the covariance of the SMDs. A multivariate random effects model was used to combine the parameters describing the study-specific curves to address heterogeneity across studies.
Publication bias was tested for using Egger’s test [16]. A 2-sided p-value < 0.05 was considered statistically significant. Sensitivity analyses were performed by excluding 1 study at a time from each meta-analysis. Meta-regressions were carried out to explore additional factors identified a priori as being potentially important sources of heterogeneity. Heterogeneity among studies was evaluated using the I2 statistic [17] with a threshold of >75% representing considerable heterogeneity [18]. For those factors identified in the meta-regression as statistically significant sources of heterogeneity, subgroup meta-analyses were performed, and pooled ORs were reported for each group. For continuous variables, a linear prediction model was built, and the association between the OR and the continuous variable was plotted. The statistical analyses were conducted using dosresmeta [15] and metafor [19] packages for R version 3.4.1. Studies that met the inclusion criteria but did not present data suitable for inclusion in the meta-analyses, studies where duplicate data were reported for the same children at different ages, and studies identified in the updated search were summarised narratively and compared to the meta-analysis results. The systematic review was registered on the PROSPERO database (reference CRD42016035599).
Results
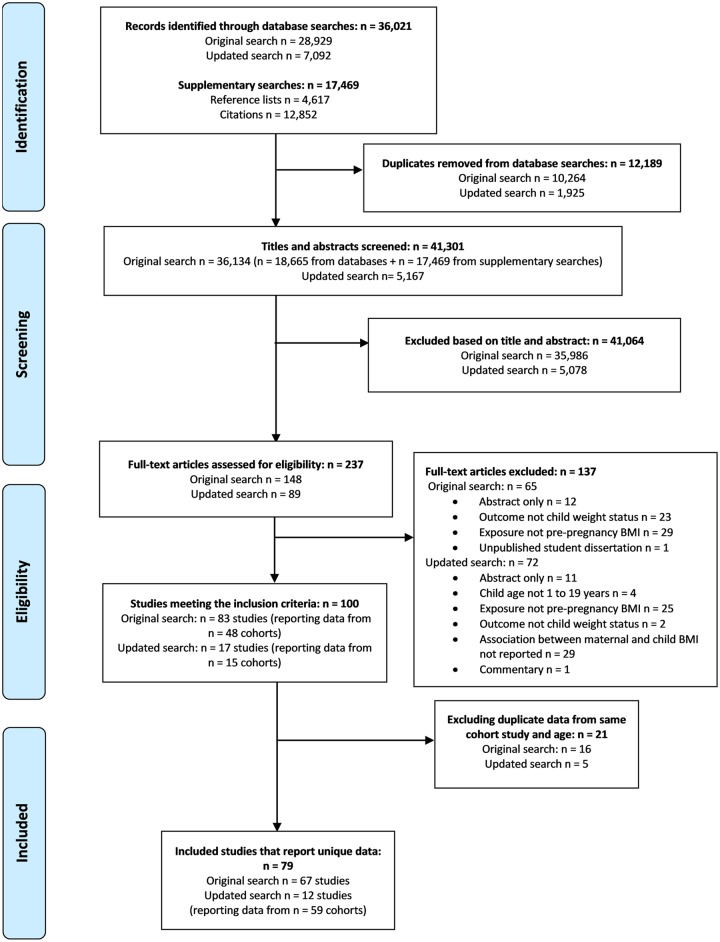
A total of 79 studies reporting data from 59 cohorts are reported in this review (Fig 1). The searches identified 41,301 studies, of which 100 studies met the inclusion criteria (Fig 1; S3 Table). Following exclusion of 21 studies that reported duplicate data (S4 Table), 79 studies remained [20–98], with sample sizes ranging from 70 to 100,612 (S5 Table); 67 studies were identified in the original searches [20,33–98], and a further 12 studies were identified in the updated searches that reported unique data not already included in the review or meta-analysis [21–32] (Fig 1; S5 Table). Of these studies, 56 were prospective, 21 reported national-level data, and the majority (n = 63) were published since 2010. Studies were predominantly from the US (n = 32), followed by the Netherlands (n = 8), UK (n = 6), China (n = 6), Australia (n = 5), Denmark (n = 3), Greece (n = 3), Norway (n = 3), Finland (n = 2), Canada (n = 2), Malaysia/Singapore (n = 2), and Chile, France, Japan, Spain, Sweden, and Sri-Lanka (n = 1 each); 1 study included populations from multiple European countries (S5 Table). Of the 9 studies from Asian countries, all except 1 [20] used Asian-specific BMI criteria. The quality score of studies ranged from 3 to 8; no studies were rated low quality, 26 medium quality, and 53 high quality (S6 Table). Additional information was requested from the authors of 56 studies: 8 authors provided additional data, 6 informed us they were unable to provide the data, 41 did not respond, and we were unable to contact the authors of 1 study (S7 Table).
Fig 1. PRISMA flow diagram.
From the original searches, 26 studies reported data for the primary outcome of child obesity (≥95th percentile); 20 of these could be pooled for meta-analysis [33–52]. Twenty-nine studies reported data for childhood overweight or obesity (≥85th percentile); 22 of these reported data that could be pooled for meta-analysis [20,33,34,36,38,40–44,46,52,56–61,70–73]. Fourteen studies reported data for child overweight (85th to 95th percentile); 10 of these could be pooled for meta-analysis [33,34,36,38,40–44,46]. Twenty-three studies reported data for continuous child BMI or z-score outcomes, 18 of which could be pooled for meta-analysis [33,36,38,41,70,71,73,76–86]. Some studies reported multiple outcomes and are included in multiple meta-analyses.
Primary outcome: Child obesity (≥95th percentile)
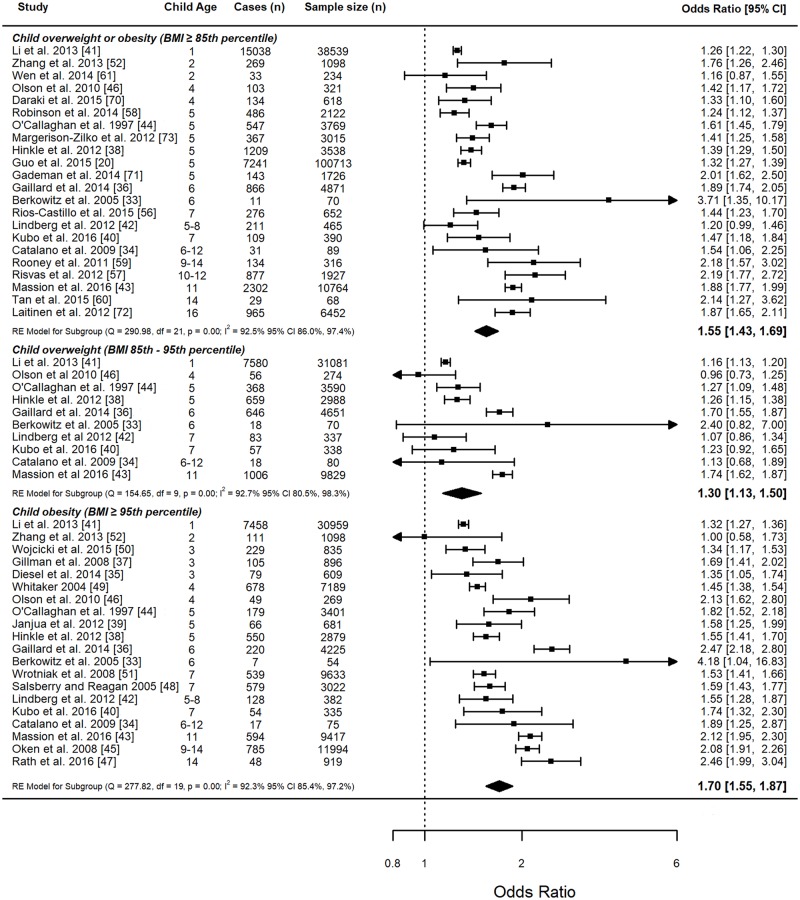
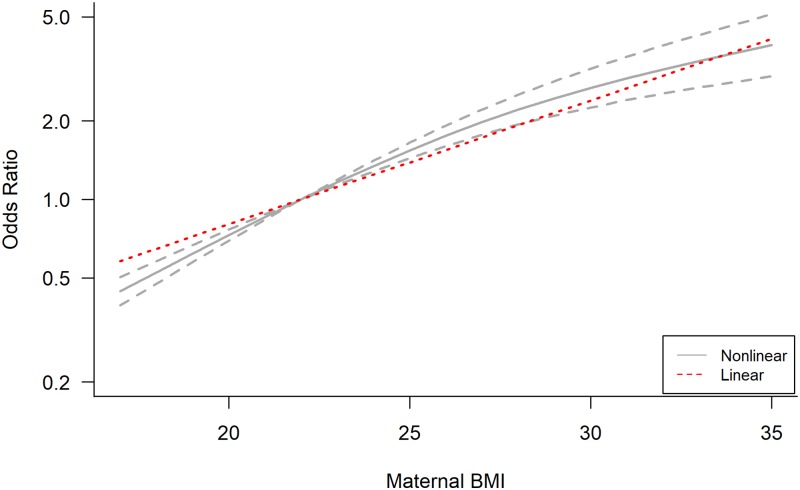
The 20 studies with data that could be pooled for meta-analysis included 12,475 cases of obesity among 88,872 children aged between 1 and 14 years. In the linear dose–response meta-analysis, the OR for each 5-kg/m2 increase in maternal BMI was 1.70 (95% CI 1.55–1.87) (Fig 2). Linearity of association between maternal BMI and child BMI was rejected (p < 0.001; S8 Table), although linear and nonlinear effect size estimates for each maternal BMI category were of a similar magnitude. Assuming a nonlinear association, there was a statistically significant decrease in the odds of child obesity when mothers had an underweight BMI compared with the reference group, and an increase in odds of 89% (OR 1.89, 95% CI 1.62–2.19) with maternal overweight and 264% (OR 3.64, 95% CI 2.68–4.95) with maternal obesity (Table 1; Fig 3). There was no evidence of publication bias in the analyses of children with obesity (p = 0.53; S3 Fig).
Fig 2. Linear meta-analysis of odds ratios and 95% confidence intervals for child weight status categories.
Meta-analysis by child weight status categories: child obesity (≥95th percentile), overweight or obesity (≥85th percentile), and overweight (85th–95th percentile). Pooled summary data for each child weight status category represent the odds ratio and 95% CI for each 5-kg/m2 increase in maternal BMI. The size of the data markers indicates the weight assigned to each study in the meta-analysis. Squares represent the odds ratio, bars represent the 95% confidence interval, and diamonds represent the pooled analysis for each child BMI category. RE, random effects.
Table 1. Linear and nonlinear dose–response meta-analyses for maternal and child BMI.
| Outcome | Model | Maternal underweight (BMI 17 kg/m2)a | Maternal reference (BMI 22 kg/m2)a | Maternal overweight (BMI 27 kg/m2)a | Maternal obesity (BMI 35 kg/m2)a |
|---|---|---|---|---|---|
| Child obesity (BMI ≥ 95th percentile) | Linear OR (95% CI) | 0.60 (0.53, 0.67) | 1.00 | 1.68 (1.50, 1.89) | 3.68 (2.85, 5.21) |
| Nonlinear OR (95% CI) | 0.47 (0.39, 0.57) | 1.00 | 1.89 (1.62, 2.19) | 3.64 (2.68, 4.95) | |
| Child overweight/obesity (BMI ≥ 85th percentile) | Linear OR (95% CI) | 0.65 (0.60, 0.71) | 1.00 | 1.54 (1.41, 1.67) | 3.05 (2.45, 3.81) |
| Nonlinear OR (95% CI) | 0.51 (0.44, 0.60) | 1.00 | 1.65 (1.47, 1.85) | 2.69 (2.10, 3.46) | |
| Child overweight (BMI 85th to 95th percentile) | Linear OR (95% CI) | 0.77 (0.67, 0.88) | 1.00 | 1.30 (1.13, 1.50) | 1.99 (1.39, 2.85) |
| Nonlinear OR (95% CI) | 0.64 (0.53, 0.78) | 1.00 | 1.41 (1.19, 1.67) | 1.80 (1.25, 2.59) | |
| Child continuous BMI and z-score | Linear SMD (95% CI) | −0.48 (−0.83, −0.13) | 0.00 | 0.48 (0.13, 0.83) | 1.24 (0.33, 2.15) |
| Nonlinear SMD (95% CI) | −0.50 (−0.65, −0.35) | 0.00 | 0.45 (0.31, 0.59) | 0.99 (0.62, 1.36) |
aBMI represents the maternal pre-/early-pregnancy BMI category midpoint estimate.
BMI, body mass index; CI, confidence interval; OR, odds ratio; SMD, standardized mean difference.
Fig 3. Comparison of linear and nonlinear association between maternal BMI and child obesity (≥95th percentile).
Pooled dose–response association between maternal BMI and odds of child obesity. Maternal BMI was modelled with restricted cubic splines in a random effects dose–response model (grey line). Grey dashed lines represent the 95% confidence interval for the spline model. The red dotted line represents the linear trend. The value of 22 kg/m2 served as referent. The odds ratios are plotted on the log scale.
Additional data were available for child obesity between ages 1 and 13 and were not included in the meta-analysis [23,26,30,47–49,53–55,67–69] (S9 Table). All additional ORs for maternal obesity and child obesity were statistically significant, ranging from 1.37 to 5.58. The majority of additional ORs reported for maternal overweight and child obesity were statistically significant, ranging from 1.04 to 3.36.
Secondary outcomes
Child overweight or obesity (≥85th percentile)
The 22 studies [20,33,34,36,38,40–44,46,52,56–61,70–73] with data available to be pooled for meta-analysis included 31,328 cases of overweight or obesity among 181,800 children aged 1 to 16 years. In the linear dose–response analysis, the OR for each 5-kg/m2 increase in maternal BMI was 1.55 (95% CI 1.43–1.69) (Fig 2). There was evidence of a nonlinear association (p < 0.001; S8 Table), with a statistically significant decrease in odds of child overweight or obesity when mothers had an underweight BMI compared with the reference group, and an increase in odds of child overweight or obesity of 65% (OR 1.65, 95% CI 1.47–1.85) for maternal overweight and 169% (OR 2.69, 95% CI 2.10–3.46) for maternal obesity (Table 1; S4 Fig). There was no evidence of publication bias in the analyses of overweight or obese children (p > 0.05; S3 Fig).
Additional data were available for child overweight or obesity between ages 1 and 14 years and were not included in the meta-analysis [21,22,25,27,29,31,53,59,62–66,69,74,97,98] (S10 Table). All additional ORs were statistically significant for all types of maternal BMI exposure: For maternal obesity, ORs ranged from 1.58 to 4.59; for maternal overweight, ORs ranged from 1.3 to 2.35; and for maternal overweight or obesity (BMI ≥ 25 kg/m2), ORs ranged from 1.13 to 4.00. The ORs for continuous maternal BMI and child overweight/obesity ranged between 1.09 and 1.60.
Child overweight (BMI 85th to 95th percentile)
The 10 studies [33,34,36,38,40–44,46] with data available to pool for meta-analysis included 10,491 cases of child overweight among 53,238 children aged 1 to 11 years. In the dose–response analysis, the OR for each 5-kg/m2 increase in maternal BMI was 1.30 (95% CI 1.13–1.50) (Fig 2). There was evidence of a nonlinear association (p < 0.001; S8 Table), with a statistically significant decrease in odds of child overweight for underweight maternal BMI compared with the reference group, and an increase in odds of child overweight of 41% (OR 1.41, 95% CI 1.19–1.67) for maternal overweight and 80% (OR 1.80, 95% CI 1.25–2.59) for maternal obesity (Table 1; S5 Fig). There was no evidence of publication bias in the analyses of child overweight (p = 0.71; S3 Fig).
Additional data were available for child overweight for children between ages 4 and 13 years and were not included in the meta-analysis [39,54,69,75] (S11 Table). All reported significantly increased odds of child overweight with maternal obesity, with ORs ranging between 1.26 and 2.29.
Continuous child BMI and z-score
The 18 studies [33,36,38,41,70,71,73,76–86] with data for meta-analysis of continuous child BMI (n = 11 studies) and z-score (n = 7 studies) outcomes and categorical maternal BMI exposures included data on 90,580 children (n = 43,877 for BMI; n = 46,703 for BMI z-score). Linear meta-analyses for BMI and BMI z-score showed a SMD of 0.09 (95% CI 0.01–0.17) for child BMI for every 1-kg/m2 increase in maternal BMI, and a SMD of 0.10 (95% CI –0.02 to 0.23) for child BMI z-score (S6 Fig). Linearity was not rejected for either measure when analysed separately (S8 Table). However, when analysing the pooled BMI and z-score data, linearity was rejected, and the nonlinear analysis showed that the SMD in child BMI was significantly decreased for maternal underweight, and increased for maternal overweight and obesity (Table 1; S7 Fig). There was no evidence of publication bias for the continuous outcomes (p = 0.995; S3 Fig).
Additional data were available for associations between categorical maternal BMI and continuous child BMI or z-score for children aged 1 to 9 years, but were not included in the meta-analysis [28,32,33,61,76,82,84,87–91] (S12 Table), and for associations between continuous maternal BMI and continuous child BMI or z-score for children aged 1 to 18 years [24,36,60,61,71,75,80,85,86,88,92–97] (S13 Table). All except 1 showed a significant association between increasing maternal BMI and increasing child BMI or z-score.
Heterogeneity and sensitivity analyses
Sensitivity analyses for linear and nonlinear meta-analyses did not show any 1 study to be substantially influencing the overall direction of association, effect size, statistical significance, or heterogeneity (S14–S17 Tables). Heterogeneity was present in all analyses (I2 92.3%–99.9%; S18 Table). Meta-regression identified child age and continent of study to contribute to heterogeneity for all categorical child BMI outcomes, but no factors substantially reduced heterogeneity between studies for continuous outcomes (S18 Table). Univariate adjustment for child age decreased the I2 to 81.2% for obesity analyses, 83.2% for overweight/obesity analyses, and 83.0% for overweight analyses; continent of study decreased the I2 to 76.4%, 80.3%, and 0.08%, respectively (S18 Table). When adjusting for both factors, the overall I2 decreased to 62.6%, 72.9%, and 25.4%, respectively. Subgroup meta-analysis for continent of study identified that ORs for child obesity and overweight were consistently highest in studies from Europe (S19 Table), and plots show that the predicted average OR for child obesity and overweight increases with increasing child age (S8–S10 Figs).
Discussion
This systematic review aimed to determine the dose–response association between maternal pre-pregnancy BMI and child obesity. The meta-analyses identified significantly increased odds of child obesity with increasing maternal BMI; this association was strongest with maternal obesity, which increased the odds of child obesity by 264%, followed by maternal overweight, which increased the odds by 89%. Similar patterns were observed for the secondary categorical and continuous child BMI and z-score outcomes. Meta-regression found an association between child obesity and increasing child age, which may reflect the combination of in utero and child life course exposures. The development of obesity involves a complex interplay between physiological, environmental, psychological, social, and behavioural exposures [99]. For example, there is evidence of epigenetic processes in utero that contribute to offspring obesity, including alterations in DNA methylation and the gut microbiome [100]. Additional life course exposures include socio-economic status, food production and marketing, food insecurity, and obesogenic environments, which promote unhealthy lifestyles to which some individuals are genetically more susceptible [99,101–103]. If mothers were exposed to these complex factors, contributing to their own obesity development, then their children are also likely to be exposed to the same complex factors, which exacerbate in utero development and predisposition to obesity.
This systematic review has strengths and limitations. The rigorous search strategy involved an experienced information scientist, database searches were supplemented with additional searches, and we contacted authors in an attempt to maximise the number of studies included in the meta-analyses. Procedures to minimise human error and subjectivity included duplicate independent screening, data extraction, and quality assessment. The meta-analyses were complex given the inclusion of both categorical and continuous exposure and outcome data, and the fact that we did not restrict the outcome to either child BMI or z-score. The decision was made to include all combinations of data based on pre-existing knowledge of the variability in how data are reported in the published literature; had we restricted the inclusion criteria to facilitate a more straightforward meta-analysis, we would have incurred bias by excluding a well-established body of evidence. However, the complex analytical approach employed required specific data to be reported in the studies, which were not always available, and the efforts to contact authors had limited return on time invested. Future research should ensure that full data are reported in the publications to enable inclusion in more complex meta-analyses. There was substantial heterogeneity between studies. Meta-regression explored maternal and child clinical, socio-demographic, behavioural, and study design factors, yet only child age and the continent of study significantly contributed towards heterogeneity between studies. It must be noted that the meta-regression relied on the primary studies accounting for these factors in their analyses; for example, paternal BMI, gestational weight gain, and gestational diabetes were rarely considered. Future research using individual participant data would be an opportunity to further explore the complex picture of child obesity development to inform targeted interventions. Few studies reported maternal obesity classes; rather, there was a tendency to group all obesity as BMI ≥ 30 kg/m2. This resulted in wider confidence intervals and less certainty of the true effect size at the upper ends of maternal BMI. Obesity is not a homogeneous group and in order to better understand the differences within obesity, future research should use obesity classes when defining categories. Disappointingly, we identified limited data from low- and middle-income countries. Our inclusion criteria restricted to studies published in English, and we excluded 1 non-English-language study at the title and abstract stage that appeared to otherwise meet the inclusion criteria [104]. This study from Brazil identified a 10% increase in adolescent obesity per 1-kg/m2 increase in maternal pre-pregnancy BMI: OR 1.09 (95% CI 1.01–1.19) for males and OR 1.16 (95% CI 1.04–1.30) for females. These results are similar to our pooled linear meta-analysis result (OR 1.70 per 5-kg/m2 increase in maternal BMI). Given the global inequalities associated with childhood obesity, this is an important area for future research.
This research has identified the need for early intervention in the prevention of childhood obesity, starting before conception. For many years, obesity prevention interventions have targeted environmental settings, such as schools [105]. However, increasing obesity prevalence in preschool age children highlights the importance of earlier prevention, in the first 1,000 days of life, from conception to 2 years old. Considering the evidence on developmental origins of health and disease, it could be argued that the first 1,000 days is not early enough. Obesity prevention must start with women of childbearing age [106], and preconception has been identified as a critical life course time period for ending child obesity [107]. Little attention has been given to the preconception period among interventions to date [108]. This attention is essential for future public health and clinical research, policy, and practice, given the inequalities associated with obesity. The failure to implement preventative action increases intergenerational life course inequalities.
This systematic review and meta-analysis identified significantly increased odds of child obesity when mothers have obesity before conception. This study provides substantial evidence for the need to develop interventions commencing preconception, to support women of childbearing age with weight management to contribute to the prevention of childhood obesity. Given the complex interplay between physiological, social, economic, environmental, and behavioural factors in the development of obesity, multifactorial interventions targeting women of childbearing age are likely to be required to halt intergenerational obesity.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to acknowledge Shannon Robalino (information scientist) for support in developing search strategies for database searches, and the authors who responded to our correspondence about additional data required for the meta-analysis. Authors who responded to our correspondence and provided the data requested were Dr Aline Andres, Professor Patrick Catalano, Dr Romy Gaillard, Professor Dana Dabelea, Dr Ai Kubo, Professor David Taylor-Robinson, Dr Sophie Wickham, Professor Christine Olson, and Dr Stephen Weng. Authors who responded to our correspondence but were unable to provide data were Zoe Bider-Canfield, Dr Claire Margerison-Zilko, Professor Juliana Kain, Dr Marieke de Hoog, and Professor Aaron Caughey.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- OR
odds ratio
- SMD
standardised mean difference
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
ES is a PhD student funded by the Medical Research Council (reference MR/K501396/1, https://mrc.ukri.org/). AP is a PhD student funded by the Economic and Social Research Council (reference 160149300, https://www.ninedtp.ac.uk/). ZA is a PhD student funded by Newcastle University Research Excellence Academy (https://www.ncl.ac.uk). NH is funded by a National Institute of Health Research Career Development Fellowship (reference CDF-2018-11-ST2-011, https://www.nihr.ac.uk/our-research-community/NIHR-academy). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ekström S, Hallberg J, Kull I, Protudjer JLP, Thunqvist P, Bottai M, et al. Body mass index status and peripheral airway obstruction in school-age children: a population-based cohort study. Thorax. 2018;73(6):538–45. 10.1136/thoraxjnl-2017-210716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Overweight and obesity. Geneva: World Health Organization; 2018. [cited 2018 Jul 2]. http://www.who.int/mediacentre/factsheets/fs311/en/. [Google Scholar]
- 3.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the united states, 2005 to 2014. JAMA. 2016;315(21):2284–91. 10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston EH. Reimagining obesity in 2018: a JAMA theme issue on obesity. JAMA. 2018;319(3):238–40. 10.1001/jama.2017.21779 [DOI] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–9. 10.1001/jama.2016.6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biener AI, Decker SL. Medical care use and expenditures associated with adult obesity in the United States. JAMA. 2018;319(3):218 10.1001/jama.2017.21063 [DOI] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin J, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 9.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 10.Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989–2007. Int J Obes (Lond). 2010;34(3):420–8. [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 12.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. STATA J. 2006;6(1):40–57. [Google Scholar]
- 13.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2011;175(1):66–73. 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57. 10.1002/sim.3841 [DOI] [PubMed] [Google Scholar]
- 15.Crippa A, Orsini N. Dose-response meta-analysis of differences in means. BMC Med Res Methodol. 2016;16(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S, editors. Cochrane Handbook for systematic reviews of interventions. Version 5.1.0. Cochrane Collaboration; 2011 [cited 2019 May 10]. http://handbook-5-1.cochrane.org/.
- 19.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 20.Guo L, Liu J, Ye R, Liu J, Zhuang Z, Ren A. Gestational weight gain and overweight in children aged 3–6 years. J Epidemiol. 2015;25(8):536–43. 10.2188/jea.JE20140149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Androutsos O, Moschonis G, Ierodiakonou D, Karatzi K, De Bourdeaudhuij I, Iotova V, et al. Perinatal and lifestyle factors mediate the association between maternal education and preschool children’s weight status: the ToyBox study. Nutrition. 2018;48:6–12. 10.1016/j.nut.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 22.Aris IM, Bernard JY, Chen LW, Tint MT, Pang WW, Soh S-E, et al. Modifiable risk factors in the first 1000 days for subsequent risk of childhood overweight in an Asian cohort: significance of parental overweight status. Int J Obes (Lond). 2018;42(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bridgman SL, Azad MB, Persaud RR, Chari RS, Becker AB, Sears MR, et al. Impact of maternal pre-pregnancy overweight on infant overweight at 1 year of age: associations and sex-specific differences. Pediatr Obes. 2018;13(10):579–89. 10.1111/ijpo.12291 [DOI] [PubMed] [Google Scholar]
- 24.Fujita Y, Kouda K, Nakamura H, Iki M. Relationship between maternal pre-pregnancy weight and offspring weight strengthens as children develop: a population-based retrospective cohort study. J Epidemiol. 2018;28(12):498–502. 10.2188/jea.JE20170137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iguacel I, Escartín L, Fernández-Alvira JM, Iglesia I, Labayen I, Moreno LA, et al. Early life risk factors and their cumulative effects as predictors of overweight in Spanish children. Int J Public Health. 2018;63(4):501–12. 10.1007/s00038-018-1090-x [DOI] [PubMed] [Google Scholar]
- 26.Kjaer TW, Faurholt-Jepsen D, Medrano R, Elwan D, Mehta K, Christensen VB, et al. Higher birthweight and maternal pre-pregnancy BMI persist with obesity association at age 9 in high risk Latino children. J Immigr Minor Health. 2019;21(1):89–97. 10.1007/s10903-018-0702-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao G, Nachman RM, Sun Q, Zhang X, Koehler K, Chen Z, et al. Individual and joint effects of early-life ambient PM 2.5 exposure and maternal prepregnancy obesity on childhood overweight or obesity. Environ Health Perspect. 2017;125(6):067005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mintjens S, Gemke RJBJ, van Poppel MNM, Vrijkotte TGM, Roseboom TJ, van Deutekom AW. Maternal Prepregnancy overweight and obesity are associated with reduced physical fitness but do not affect physical activity in childhood: the Amsterdam Born Children and Their Development Study. Child Obes. 2018;15(1):31–9. 10.1089/chi.2018.0171 [DOI] [PubMed] [Google Scholar]
- 29.Toftemo I, Jenum AK, Lagerløv P, Júlίusson PB, Falk RS, Sletner L. Contrasting patterns of overweight and thinness among preschool children of different ethnic groups in Norway, and relations with maternal and early life factors. BMC Public Health. 2018;18(1):1056 10.1186/s12889-018-5952-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallby T, Lagerberg D, Magnusson M. Relationship between breastfeeding and early childhood obesity: results of a prospective longitudinal study from birth to 4 years. Breastfeed Med. 2017;12(1):48–53. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Niu F, Ren X. Association of maternal pre-pregnancy body mass index and gestational weight gain with Chinese infant growth. J Paediatr Child Health. 2018. October 30 10.1111/jpc.14274 [DOI] [PubMed] [Google Scholar]
- 32.Zheng M, Bowe SJ, Hesketh KD, Bolton K, Laws R, Kremer P, et al. Relative effects of postnatal rapid growth and maternal factors on early childhood growth trajectories. Paediatr Perinat Epidemiol. 2019;33(2):172–80. 10.1111/ppe.12541 [DOI] [PubMed] [Google Scholar]
- 33.Berkowitz RI, Stallings VA, Maislin G, Stunkard AJ. Growth of children at high risk of obesity during the first 6 y of life: implications for prevention. Am J Clin Nutr. 2005;81(1):140–6. 10.1093/ajcn/81.1.140 [DOI] [PubMed] [Google Scholar]
- 34.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–13. 10.3945/ajcn.2008.27416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diesel JC, Eckhardt CL, Day NL, Brooks MM, Arslanian SA, Bodnar LM. Is gestational weight gain associated with offspring obesity at 36 months? Pediatr Obes. 2014;10(4):305–10. 10.1111/ijpo.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaillard R, Steegers EA, Duijts L, Felix JF, Hofman A, Franco OH, et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension. 2014;63(4):683–91. 10.1161/HYPERTENSIONAHA.113.02671 [DOI] [PubMed] [Google Scholar]
- 37.Gillman MW, Rifas-Shiman SL, Kleinman K, Oken E, Rich-Edwards JW, Taveras EM. Developmental origins of childhood overweight: potential public health impact. Obesity (Silver Spring). 2008;16(7):1651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinkle SN, Sharma AJ, Swan DW, Schieve LA, Ramakrishnan U, Stein AD. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J Nutr. 2012;142(10):1851–8. 10.3945/jn.112.161158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janjua NZ, Mahmood B, Islam MA, Goldenberg RL. Maternal and early childhood risk factors for overweight and obesity among low-income predominantly black children at age five years: a prospective cohort study. J Obes. 2012;2012:457173 10.1155/2012/457173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubo A, Ferrara A, Laurent CA, Windham GC, Greenspan LC, Deardorff J, et al. Associations between maternal pregravid obesity and gestational diabetes and the timing of pubarche in daughters. Am J Epidemiol. 2016;184(1):7–14. 10.1093/aje/kww006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Liu E, Guo J, Pan L, Li B, Wang P, et al. Maternal prepregnancy body mass index and gestational weight gain on offspring overweight in early infancy. PLoS ONE. 2013;8(10):e77809 10.1371/journal.pone.0077809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindberg SM, Adams AK, Prince RJ. Early predictors of obesity and cardiovascular risk among American Indian children. Matern Child Health J. 2012;16(9):1879–86. 10.1007/s10995-012-1024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massion S, Wickham S, Pearce A, Barr B, Law C, Taylor-Robinson D. Exploring the impact of early life factors on inequalities in risk of overweight in UK children: findings from the UK Millennium Cohort Study. Arch Dis Child. 2016;101(8):724–30. 10.1136/archdischild-2015-309465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Callaghan MJ, Williams GM, Andersen MJ, Bor W, Najman JM. Prediction of obesity in children at 5 years: a cohort study. J Paediatr Child Health. 1997;33(4):311–6. [DOI] [PubMed] [Google Scholar]
- 45.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008;112(5):999–1006. 10.1097/AOG.0b013e31818a5d50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olson CM, Demment MM, Carling SJ, Strawderman MS. Associations between mothers’ and their children’s weights at 4 years of age. Child Obes. 2010;6(4):201–7. 10.1089/chi.2010.0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rath SR, Marsh JA, Newnham JP, Zhu K, Atkinson HC, Mountain J, et al. Parental pre-pregnancy BMI is a dominant early-life risk factor influencing BMI of offspring in adulthood. Obes Sci Pract. 2016;2(1):48–57. 10.1002/osp4.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salsberry PJ, Reagan PB. Dynamics of early childhood overweight. Pediatrics. 2005;116(6):1329–38. 10.1542/peds.2004-2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114(1):e29–36. [DOI] [PubMed] [Google Scholar]
- 50.Wojcicki JM, Young MB, Perham-Hester KA, de Schweinitz P, Gessner BD. Risk factors for obesity at age 3 in Alaskan children, including the role of beverage consumption: results from Alaska PRAMS 2005–2006 and its three-year follow-up survey, CUBS, 2008–2009. PLoS ONE. 2015;10(3):e0118711 10.1371/journal.pone.0118711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wrotniak BH, Shults J, Butts S, Stettler N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am J Clin Nutr. 2008;87(6):1818–24. 10.1093/ajcn/87.6.1818 [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Himes JH, Guo Y, Jiang J, Yang L, Lu Q, et al. Birth weight, growth and feeding pattern in early infancy predict overweight/obesity status at two years of age: a birth cohort study of Chinese infants. PLoS ONE. 2013;8(6):e64542 10.1371/journal.pone.0064542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond). 2011;35(4):522–9. [DOI] [PubMed] [Google Scholar]
- 54.Birbilis M, Moschonis G, Mougios V, Manios Y, Healthy Growth Study Group. Obesity in adolescence is associated with perinatal risk factors, parental BMI and sociodemographic characteristics. Eur J Clin Nutr. 2013;67(1):115–21. 10.1038/ejcn.2012.176 [DOI] [PubMed] [Google Scholar]
- 55.Salsberry PJ, Reagan PB. Taking the long view: the prenatal environment and early adolescent overweight. Res Nurs Health. 2007;30(3):297–307. 10.1002/nur.20215 [DOI] [PubMed] [Google Scholar]
- 56.Rios-Castillo I, Cerezo S, Corvalan C, Martinez M, Kain J. Risk factors during the prenatal period and the first year of life associated with overweight in 7-year-old low-income Chilean children. Matern Child Nutr. 2015;11(4):595–605. 10.1111/mcn.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Risvas G, Papaioannou I, Panagiotakos DB, Farajian P, Bountziouka V, Zampelas A. Perinatal and family factors associated with preadolescence overweight/obesity in Greece: the GRECO study. J Epidemiol Glob Health. 2012;2(3):145–53. 10.1016/j.jegh.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson CA, Cohen AK, Rehkopf DH, Deardorff J, Ritchie L, Jayaweera RT, et al. Pregnancy and post-delivery maternal weight changes and overweight in preschool children. Prev Med. 2014;60:77–82. 10.1016/j.ypmed.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rooney BL, Mathiason MA, Schauberger CW. Predictors of obesity in childhood, adolescence, and adulthood in a birth cohort. Matern Child Health J. 2011;15(8):1166–75. 10.1007/s10995-010-0689-1 [DOI] [PubMed] [Google Scholar]
- 60.Tan HC, Roberts J, Catov J, Krishnamurthy R, Shypailo R, Bacha F. Mother’s pre-pregnancy BMI is an important determinant of adverse cardiometabolic risk in childhood. Pediatr Diabetes. 2015;16(6):419–26. 10.1111/pedi.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen LM, Baur LA, Rissel C, Xu H, Simpson JM. Correlates of body mass index and overweight and obesity of children aged 2 years: findings from the healthy beginnings trial. Obesity (Silver Spring). 2014;22(7):1723–30. [DOI] [PubMed] [Google Scholar]
- 62.Bider-Canfield Z, Martinez MP, Wang X, Yu W, Bautista MP, Brookey J, et al. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatr Obes. 2017;12(2):171–8. 10.1111/ijpo.12125 [DOI] [PubMed] [Google Scholar]
- 63.Pham MT, Brubaker K, Pruett K, Caughey AB. Risk of childhood obesity in the toddler offspring of mothers with gestational diabetes. Obstet Gynecol. 2013;121(5):976–82. 10.1097/AOG.0b013e31828bf70d [DOI] [PubMed] [Google Scholar]
- 64.Rathnayake KM, Satchithananthan A, Mahamithawa S, Jayawardena R. Early life predictors of preschool overweight and obesity: a case-control study in Sri Lanka. BMC Public Health. 2013;13:994 10.1186/1471-2458-13-994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mamun AA, Lawlor DA, O’Callaghan MJ, Williams GM, Najman JM. Family and early life factors associated with changes in overweight status between ages 5 and 14 years: findings from the Mater University Study of Pregnancy and its outcomes. Int J Obes (Lond). 2005;29(5):475–82. [DOI] [PubMed] [Google Scholar]
- 66.Olson CM, Strawderman MS, Dennison BA. Maternal weight gain during pregnancy and child weight at age 3 years. Matern Child Health J. 2009;13(6):839–46. 10.1007/s10995-008-0413-6 [DOI] [PubMed] [Google Scholar]
- 67.Li C, Kaur H, Choi WS, Huang TT, Lee RE, Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res. 2005;13(2):362–71. 10.1038/oby.2005.48 [DOI] [PubMed] [Google Scholar]
- 68.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330(7504):1357 10.1136/bmj.38470.670903.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weng SF, Redsell SA, Nathan D, Swift JA, Yang M, Glazebrook C. Estimating overweight risk in childhood from predictors during infancy. Pediatrics. 2013;132(2):e414–21. 10.1542/peds.2012-3858 [DOI] [PubMed] [Google Scholar]
- 70.Daraki V, Georgiou V, Papavasiliou S, Chalkiadaki G, Karahaliou M, Koinaki S, et al. Metabolic profile in early pregnancy is associated with offspring adiposity at 4 years of age: the Rhea pregnancy cohort Crete, Greece. PLoS ONE. 2015;10(5):e0126327 10.1371/journal.pone.0126327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gademan MG, Vermeulen M, Oostvogels AJ, Roseboom TJ, Visscher TL, van Eijsden M, et al. Maternal prepregancy BMI and lipid profile during early pregnancy are independently associated with offspring’s body composition at age 5–6 years: the ABCD study. PLoS ONE. 2014;9(4):e94594 10.1371/journal.pone.0094594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laitinen J, Jaaskelainen A, Hartikainen AL, Sovio U, Vaarasmaki M, Pouta A, et al. Maternal weight gain during the first half of pregnancy and offspring obesity at 16 years: a prospective cohort study. BJOG. 2012;119(6):716–23. 10.1111/j.1471-0528.2012.03319.x [DOI] [PubMed] [Google Scholar]
- 73.Margerison-Zilko CE, Shrimali BP, Eskenazi B, Lahiff M, Lindquist AR, Abrams BF. Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child Health J. 2012;16(6):1215–23. 10.1007/s10995-011-0846-1 [DOI] [PubMed] [Google Scholar]
- 74.Kitsantas P, Pawloski LR, Gaffney KF. Maternal prepregnancy body mass index in relation to Hispanic preschooler overweight/obesity. Eur J Pediatr. 2010;169(11):1361–8. 10.1007/s00431-010-1230-7 [DOI] [PubMed] [Google Scholar]
- 75.Durmus B, Arends LR, Ay L, Hokken-Koelega AC, Raat H, Hofman A, et al. Parental anthropometrics, early growth and the risk of overweight in pre-school children: the Generation R Study. Pediatr Obes. 2012;8(5):339–50. 10.1111/j.2047-6310.2012.00114.x [DOI] [PubMed] [Google Scholar]
- 76.Andres A, Hull HR, Shankar K, Casey PH, Cleves MA, Badger TM. Longitudinal body composition of children born to mothers with normal weight, overweight, and obesity. Obesity (Silver Spring). 2015;23(6):1252–8. [DOI] [PubMed] [Google Scholar]
- 77.Deierlein AL, Siega-Riz AM, Chantala K, Herring AH. The association between maternal glucose concentration and child BMI at age 3 years. Diabetes Care. 2011;34(2):480–4. 10.2337/dc10-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eisenman JC, Sarzynski MA, Tucker J, Heelan KA. Maternal prepregnancy overweight and offspring fatness and blood pressure: role of physical activity. Pediatr Exerc Sci. 2010;22(3):369–78. [DOI] [PubMed] [Google Scholar]
- 79.Fleten C, Nystad W, Stigum H, Skjaerven R, Lawlor DA, Davey Smith G, et al. Parent-offspring body mass index associations in the Norwegian Mother and Child Cohort Study: a family-based approach to studying the role of the intrauterine environment in childhood adiposity. Am J Epidemiol. 2012;176(2):83–92. 10.1093/aje/kws134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacota M, Forhan A, Saldanha-Gomes C, Charles MA, Heude B, EDEN Mother-Child Cohort Study Group. Maternal weight prior and during pregnancy and offspring’s BMI and adiposity at 5–6 years in the EDEN mother–child cohort. Pediatr Obes. 2017;12(4):320–9. 10.1111/ijpo.12145 [DOI] [PubMed] [Google Scholar]
- 81.Kaar JL, Crume T, Brinton JT, Bischoff KJ, McDuffie R, Dabelea D. Maternal obesity, gestational weight gain, and offspring adiposity: the exploring perinatal outcomes among children study. J Pediatr. 2014;165(3):509–15. 10.1016/j.jpeds.2014.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leng J, Li W, Zhang S, Liu H, Wang L, Liu G, et al. GDM women’s pre-pregnancy overweight/obesity and gestational weight gain on offspring overweight status. PLoS ONE. 2015;10(6):e0129536 10.1371/journal.pone.0129536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Makela J, Lagstrom H, Kaljonen A, Simell O, Niinikoski H. Hyperglycemia and lower diet quality in pregnant overweight women and increased infant size at birth and at 13 months of age—STEPS study. Early Hum Dev. 2013;89(6):439–44. 10.1016/j.earlhumdev.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 84.Toemen L, Gishti O, Van Osch-Gevers L, Steegers EAP, Helbing WA, Felix JF, et al. Maternal obesity, gestational weight gain and childhood cardiac outcomes: role of childhood body mass index. Int J Obes (Lond). 2016;40(7):1070–8. [DOI] [PubMed] [Google Scholar]
- 85.Zalbahar N, Jan Mohamed HJB, Loy SL, Najman J, McIntyre HD, Mamun A. Association of parental body mass index before pregnancy on infant growth and body composition: evidence from a pregnancy cohort study in Malaysia. Obes Res Clin Pract. 2016;10:S35–47. 10.1016/j.orcp.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 86.Mesman I, Roseboom TJ, Bonsel GJ, Gemke RJ, van der Wal MF, Vrijkotte TGM. Maternal pre-pregnancy body mass index explains infant’s weight and BMI at 14 months: results from a multi-ethnic birth cohort study. Arch Dis Child. 2009;94(8):587–95. 10.1136/adc.2008.137737 [DOI] [PubMed] [Google Scholar]
- 87.Basatemur E, Gardiner J, Williams C, Melhuish E, Barnes J, Sutcliffe A. Maternal prepregnancy BMI and child cognition: a longitudinal cohort study. Pediatrics. 2013;131(1):56–63. 10.1542/peds.2012-0788 [DOI] [PubMed] [Google Scholar]
- 88.Ehrenthal DB, Maiden K, Rao A, West DW, Gidding SS, Bartoshesky L, et al. Independent relation of maternal prenatal factors to early childhood obesity in the offspring. Obstet Gynecol. 2013;121(1):115–21. 10.1097/AOG.0b013e318278f56a [DOI] [PubMed] [Google Scholar]
- 89.Jin WY, Lv Y, Bao Y, Tang L, Zhu ZW, Shao J, et al. Independent and combined effects of maternal prepregnancy body mass index and gestational weight gain on offspring growth at 0–3 years of age. Biomed Res Int. 2016;2016:4720785 10.1155/2016/4720785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stamnes Kopp UM, Dahl-Jorgensen K, Stigum H, Frost Andersen L, Naess O, Nystad W. The associations between maternal pre-pregnancy body mass index or gestational weight change during pregnancy and body mass index of the child at 3 years of age. Int J Obes (Lond). 2012;36(10):1325–31. [DOI] [PubMed] [Google Scholar]
- 91.Jharap VV, Santos S, Steegers EAP, Jaddoe VWV, Gaillard R. Associations of maternal obesity and excessive weight gain during pregnancy with subcutaneous fat mass in infancy. Early Hum Dev. 2017;108:23–8. 10.1016/j.earlhumdev.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li A, Teo KK, Morrison KM, McDonald SD, Atkinson SA, Anand SS, et al. A genetic link between prepregnancy body mass index, postpartum weight retention, and offspring weight in early childhood. Obesity (Silver Spring). 2017;25(1):236–43. [DOI] [PubMed] [Google Scholar]
- 93.Sorensen TIA, Ajslev TA, Angquist L, Morgen CS, Ciuchi IG, Smith GD. Comparison of associations of maternal peri-pregnancy and paternal anthropometrics with child anthropometrics from birth through age 7 y assessed in the Danish National Birth Cohort. Am J Clin Nutr. 2016;104(2):389–96. 10.3945/ajcn.115.129171 [DOI] [PubMed] [Google Scholar]
- 94.Davey Smith G, Steer C, Leary S, Ness A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC). Arch Dis Child. 2007;92(10):876–80. 10.1136/adc.2006.104869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Terry MB, Wei Y, Esserman D, McKeague IW, Susser E. Pre- and postnatal determinants of childhood body size: cohort and sibling analyses. J Dev Orig Health Dis. 2011;2(2):99–111. 10.1017/S2040174411000067 [DOI] [PubMed] [Google Scholar]
- 96.Knight B, Shields BM, Hill A, Powell RJ, Wright D, Hattersley AT. The impact of maternal glycemia and obesity on early postnatal growth in a nondiabetic Caucasian population. Diabetes Care. 2007;30(4):777–83. 10.2337/dc06-1849 [DOI] [PubMed] [Google Scholar]
- 97.Morgen C, Angquist L, Baker J, Andersen A, Michaelsen K, SoRensen T. Prenatal risk factors infuencing childhood BMI and overweight independent of birth weight and infancy BMI—a path analysis within the Danish national birth cohort. Obes Facts. 2017;10:21–2. [DOI] [PubMed] [Google Scholar]
- 98.de Hoog ML, van Eijsden M, Stronks K, Gemke RJ, Vrijkotte TG. Overweight at age two years in a multi-ethnic cohort (ABCD study): the role of prenatal factors, birth outcomes and postnatal factors. BMC Public Health. 2011;11(1):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Butland B, Jebb S, Kopelman P, McPherson K, Thomas S, Mardell J, et al. Foresight. Tackling obesities: future choices—project report. 2nd edition London: Government Office for Science; 2007. [Google Scholar]
- 100.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VWV, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5(1):53–64. 10.1016/S2213-8587(16)30107-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nettle D, Andrews C, Bateson M. Food insecurity as a driver of obesity in humans: the insurance hypothesis. Behav Brain Sci. 2016;40:e105 10.1017/S0140525X16000947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Llewellyn CH. Genetic susceptibility to the “obesogenic” environment: the role of eating behavior in obesity and an appetite for change. Am J Clin Nutr. 2018;108(3):429–30. 10.1093/ajcn/nqy210 [DOI] [PubMed] [Google Scholar]
- 103.Bush NR, Allison AL, Miller AL, Deardorff J, Adler NE, Boyce W. Socioeconomic disparities in childhood obesity risk: association with an oxytocin receptor polymorphism. JAMA Pediatr. 2017;171(1):61–7. 10.1001/jamapediatrics.2016.2332 [DOI] [PubMed] [Google Scholar]
- 104.Monteiro P, Victora C, Barros F. [Social, familial, and behavioral risk factors for obesity in adolescents]. Revista Panam Salud Publica. 2004;16(4):250–8. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y, Cai L, Wu Y, Wilson RF, Weston C, Fawole O, et al. What childhood obesity prevention programmes work? A systematic review and meta-analysis. Obes Rev. 2015;16(7):547–65. 10.1111/obr.12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zylke JW, Bauchner H. The unrelenting challenge of obesity. JAMA. 2016;315(21):2277–8. 10.1001/jama.2016.6190 [DOI] [PubMed] [Google Scholar]
- 107.World Health Organization. Report of the Commission on Ending Childhood Obesity. Geneva: World Health Organization; 2016. [Google Scholar]
- 108.Stephenson J, Heslehurst N, Hall J, Schoenaker DAJM, Hutchinson J, Cade JE, et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet. 2018;391(10132):1830–41. 10.1016/S0140-6736(18)30311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.