Abstract
The ethylene response factors have been reported to play critical roles in developmental and environmental responses in plants. In the present study, an ERF transcription factor gene was aimed to be identified from Larix kaempferi. Molecular characteristics and function of this gene were further explored. The result showed that a 1344 bp ERF transcription factor gene containing initiation and termination codon was obtained by RT-PCR and named LkERF-B2. LkERF-B2 gene encoded 447 amino acids containing a typical AP2/ERF domain. Alignment of predicted amino acid sequence of LkERF-B2 in various plant species showed that this ERF transcription factor was highly homologous (79.0%) with that of Picea sitchensi. To elucidate the function of LkERF-B2, LkERF-B2 overexpression vector was successfully constructed and transformed to Arabidopsis thaliana via dip flower. Compared with control plant, LkERF-B2 overexpressed transgenic A. thaliana showed a significantly higher survival rate under cold, heat, NaCl and drought stresses. NaCl stress analysis revealed that control and transgenic Arabidopsis were both flowering earlier under 100 and 150 mM/L NaCl treatment. While under 200–300 mM/L NaCl treatment, the growth of control plant was significantly inhibited compared with transgenic A. thaliana. Salt injury rate and salt injury index of transgenic Arabidopsis were lower than those of the control. Further investigation showed that transgenic Arabidopsis exhibited much higher content of chloroplast pigments under different NaCl concentration. Meanwhile, the activity of SOD and POD was also enhanced in transgenic A. thaliana. These results suggested that LkERF-B2 was a key transcription factor and could lead to enhanced salt stress tolerance.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1793-6) contains supplementary material, which is available to authorized users.
Keywords: Larix kaempferi, LkERF-B2, NaCl tolerance, Functional characterization
Introduction
In long-term evolution, plants have formed a complete and complex mechanism to adapt and resist a variety of abiotic stresses (Liu et al. 2018). Plants resist abiotic stresses at the molecular, cellular, tissue, and whole-plant levels (Bohnert and Jensen 1996; Wang and Altman 2003). At the molecular level, ABA-dependent and -independent pathways participate in stress-responsive (Zhu 2002). However, some scholars believe that the two pathways function either alone or synergistically (Lee et al. 2010). Many genes involved in stress responses have been identified and validated, including functional genes and regulatory genes (Shinozaki et al. 2003; Shinozaki and Yamaguchi-Shinozaki 2007). Transcription factors (TF), which regulate gene expression, are classified into five types: NAC (NAM, ATAF1, ATAF2 and CUC2), MYB (v-myb avian myeloblastosis viral oncogene homolog), WRKY (tryptophan, arginine, lysine and tyrosine), bZIP (basic region/leucine zipper motif) and AP2/ERF (APETALA2/ethylene responsive factor) family (Wang et al. 2016).
AP2/ERF transcription factor plays an important role in plant biotic and/or abiotic stresses (Muhammad et al. 2012; Shu et al. 2016) and plant growth and development (Zhang et al. 2012). AP2/ERF transcription factors are involved in flower development (Elliott et al. 1996), spikelet meristem determinacy (Chuck et al. 1998), leaf epidermal cell identity (Moose and Sisco 1996), embryo development (Boutilier et al. 2002), stresses tolerance (Dubouzet et al. 2003) and so on. Members of AP2/ERF superfamily share a highly conserved DNA-binding domain known as AP2/ERF domain, which possess 60–70 conserved amino acid residues (Nakano et al. 2006). According to different numbers or structures of AP2 and other conserved domains, AP2/ERF family is classified into AP2, RAV (Related to ABI3 and VP1), ERF and Soloist families (Nakano et al. 2006). AP2 TFs contain two AP2 domains or a single AP2 domain, but the single AP2 domain is similar to an AP2 domain in the double-domain groups (Nakano et al. 2006). AP2 TFs were reported to regulate plant organ growth and development, such as flower development and determination of seed size (Elliott et al. 1996; Jofuku et al. 2005). RAV TFs, containing one AP2/ERF domain and a B3 domain, are involved in ethylene response pathway (Alonso 2003) and abiotic/biotic stress (Sohn et al. 2006; Li et al. 2011). Soloist family is a small group with MRG and HLG elements in the AP2/ERF domain (Ma et al. 2017); members of this family strongly diverge in gene sequence from other AP2/ERF members (Rao et al. 2016). ERF TFs, containing one AP2/ERF domain, are involved in both environmental stress responses and hormone regulatory pathways (Yu et al. 2017), such as the ethylene (Fujimoto et al. 2000), salicylic acid (Oñatesánchez and Singh 2002) and jasmonic acid pathways (Mantiri et al. 2008).
Based on differences of conserved residues in DNA binding domain, ERF family is further divided into ERF subfamily and the CBF/DREB subfamily (Nakano et al. 2006; Yu et al. 2017). The differences between CBF/DREB and ERF subfamilies are the 14th and 19th amino acids of AP2/ERF domain. The 14th and 19th amino acids of AP2/ERF domain in CBF/DREB subfamily are valine (V14) and glutamic (E19) acid, while in ERF subfamily the corresponding amino acid are alanine (A14) and aspartic (D19) acid, respectively (Riechmann et al. 2000; Sakuma et al. 2002). It has been reported that many DREB proteins bind to DRE/CRT (drought-responsive/C-repeat) element to activate or suppress gene transcription (Park et al. 2001; Zhao et al. 2012; Zhang et al. 2014). The ERF proteins mainly bind to AGCCGCC of the GCC-boxes (Ohme-Takagi and Shinshi 1995). Recent studies have shown that some ERF proteins also bind to DRE/CRT (Cheng and Lin 2013). In addition to binding to DRE and GCC-box, ERF TFs could also bind to TGG element (Wang et al. 2015). For example, ThERF1 from Tamarix hispida mainly binds to the TTG motif to regulate gene expression, and DRE and GCC box are rarely found in the promoters of ThERF1-regulated genes when exposed to salt stress conditions (Wang et al. 2015). Multiple modulating reactions could be due to their secondary binding to the promoter, which can mediate simultaneous regulation of multiple responses. However, the pathway remains unclear because of different regulatory pathways among plants (Phukan et al. 2017).
Members of ERF TFs were documented in many species, such as Arabidopsis thaliana (Lorenzo et al. 2003; Oñatesánchez et al. 2007; Vogel et al. 2014), Artemisia annua (Yu et al. 2012), Glycine max (Zhang et al. 2009; Hernandezgarcia and Finer 2016), Gossypium barbadense (Zuo et al. 2007), Oryza sativa (Zhao et al. 2015; Lee et al. 2016), Triticum aestivum (Na et al. 2010; Dong et al. 2012; Zhu and Zhang 2014), Nicotiana benthamiana (Todd et al. 2010). Meanwhile ERFs are involved in plant responses to salt (Schmidt et al. 2013; Makhloufi et al. 2014), cold (Ma et al. 2014; Zhuo et al. 2017), heat (Yao et al. 2017), drought (Gao et al. 2008; Yang et al. 2016), pathogen (Zhu and Zhang 2014; Liu et al. 2017) and abscisic acid (Zhu et al. 2010).
Larix kaempferi, one of the most important afforestation and timber species in China, has important economic and ecological value. L. kaempferi is characterized for its fast growth and rapid reproduction, strong adaptability, low vulnerability to pests and diseases (Li et al. 2014). However, owing in part to its long life cycle and complex genetic background, identification and functional characterization of ERF members in L. kaempferi remains largely unexplored. In the present study, a putative ERF gene, named LkERF-B2, was isolated and cloned from L. kaempferi. To investigate the function of LkERF-B2, this gene was transformed into A. thaliana via dip flower to evaluated tolerance ability to abiotic stress.
Materials and methods
Plant materials and treatments
Larix kaempferi planted in Dagujia Forest Farm, Liaoning Province, China, was used in this study. Fresh leaves of L. kaempferi were harvested and frozen in liquid nitrogen and stored at − 80 °C until use.
RNA extraction and cDNA synthesis
Total RNA was extracted from fresh leaves of L. kaempferi with EasyPure RNA Kit (TransGen Biotech, China) according to the manufacturer’s instructions. The concentration and quality of extracted RNA were analyzed by spectrophotometry (ThermoScientific NanoDrop-2000, USA) and 1% gel electrophoresis. TIANScript M-MLV (TIANGEN, China) was used to synthesize first-strand cDNA according to the manufacturer’s instructions.
Isolation and cloning of LkERF-B2
Based on the transcriptome database of L. kaempferi that has been already assembled in our laboratory (Li et al. 2016), the full-length coding sequence of LkERF-B2 was further assembled by the corresponding contigs. Forward primer and reversed primer were designed according to the open reading frame (ORF) of LkERF-B2. LkERF-B2 F: 5′-ATAAGAATGCGGCCGCATGTGTGGAGGTGCTATCATCTC-3′ and LkERF-B2 R: 5′-CCGGAATTCTCAATAAGCAGAATCGGAAATAG-3′. (Underlined are EcoRI and NotI restriction sites). RT-PCR was carried out using first-strand cDNA as template. The RT-PCR cycling conditions were: 94 °C for 2 min, 30 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 2 min, followed by 72 °C for 5 min.
Sequence analysis of LkERF-B2
The homology of the LkERF-B2 protein was identified using protein BLAST tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The second structure of LkERF-B2 was predicted by PredictProtein (https://www.predictprotein.org/). For multiple sequence alignments, LkERF-B2 was aligned with the amino acid sequences of other ERFs using the program Clustal X (Larkin et al. 2007). MEGA 5.0 was used to construct the phylogenetic tree through neighbor-joining method and bootstrap analysis with 1000 replications (Tamura et al. 2011). The theoretical molecular weight and isoelectric point of LkERF-B2 were calculated using expasy (http://web.expasy.org/compute_pi/). The hydrophilicity of LkERF-B2 was predicted using expasy. The transmembrane domains of LkERF-B2 were predicted using TMHMM v2.0 (http://www.cbs.dtu.dk/services/TMHMM/).
Construction of expression vector and plant transformation
The full-length open reading frame of LkERF-B2 was inserted into plant expression vector. Recombinant expression vector was transformed into A. tumefaciens LBA4404 cells by electroporation (conditions: 25 μF, 200 Ω, 2 kV). A. tumefaciens LBA4404 with LkERF-B2 was further introduced into wild-type A. thaliana via floral dip method (Clough and Bent 2010). The seeds of the transgenic plants were seeded on MS medium containing 30 mg/L kanamycin and screened for T3 generations. After screening for kanamycin resistance, positive plants were identified by GUS assay, genomic DNA PCR and RT-PCR.
Treatment of transgenic plants
Arabidopsis thaliana seeds were vernalized at 4 °C for 3 days, surface sterilized, and seeded in MS medium. The culture was carried out in a tissue culture incubator at 22 °C under 16 h light/8 h dark cycle.
Two-week-old A. thaliana seedlings were treated with cold (− 7 °C/5 h and 4 °C/12 h), heat (40 °C/4 h), salt (planted in new MS medium with 200 mM/L NaCl for 1 week), and drought (planted in new MS medium with 400 mM/L mannitol for 1 week) stresses. After cold and heat stresses treatment, the seedlings were transplanted to normal environment for 2 days and survival rate was calculated.
After 25 days of seed germination, A. thaliana plants were treated with different concentrations of NaCl (0, 100, 150, 200, 250, 300 mM/L) for 1 week. Then the physiological indexes in leaves were determined.
Method for determination of physiological indexes
Survival rates are measured by whether plants are alive or not. Survival rate calculation formula:
Salt injury was classified into the following grades: 0, no symptom of salt injury; 1, about 1/5 leaves yellowing; 2, moderate salt injury, about 1/2 leaves yellowing; 3, severe salt injury, most leaves yellowing; and 4, extremely severe salt injury, leaves burning and shedding death. The calculation formula of salt injury rate and salt injury index:
Superoxide dismutase (SOD) activity was determined by the NBT method (Zang et al. 2015). Peroxidase (POD) activity was determined by guaiacol method (Podazza et al. 2012). Soluble protein content was measured through coomassie bright blue colorimetric method G-250 method (Grintzalis et al. 2015). Soluble sugar content was measured using anthrone method (Ibrahim et al. 2012). Chlorophyll content was determined by colorimetry (Pápista et al. 2002).
Statistical analysis
SPSS 17.0 was used in the statistical analyses in the study. One-way analysis of variance (ANOVA) was conducted to determine statistical significance. P < 0.05 was considered statistically significant. All data are shown as mean ± standard error of the mean.
Results
Molecular characterization of LkERF-B2 from L. kaempferi
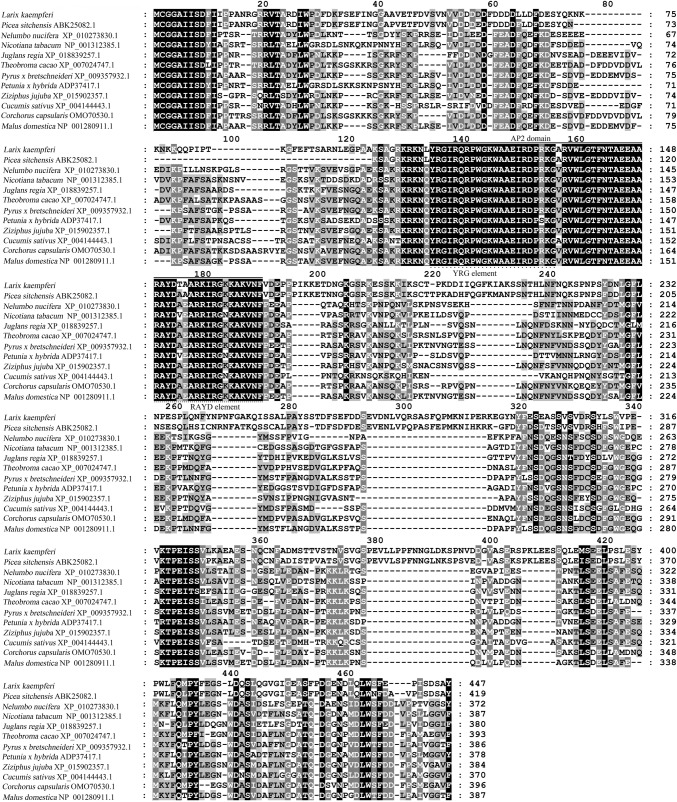
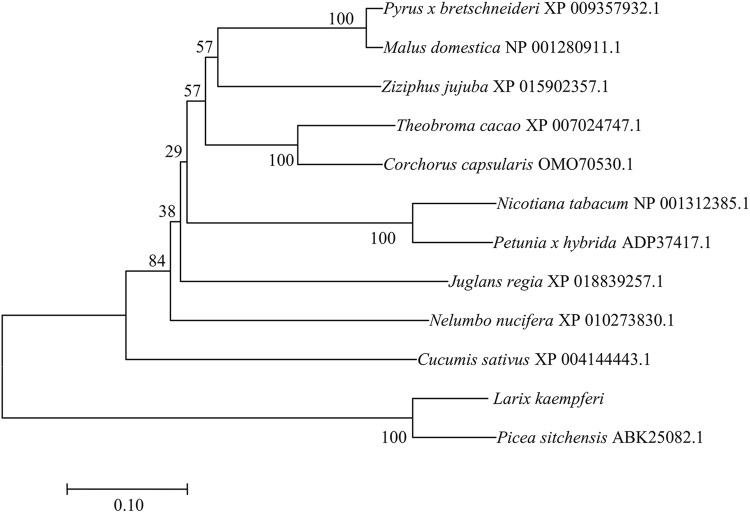
LkERF-B2 gene was isolated from L. kaempferi by RT-PCR based on transcriptome database. Sequence analysis showed that the ORF of LkERF-B2 was 1344 bp, encoding a protein of 447 amino acids. Multiple sequence alignment analysis showed that LkERF-B2 was highly conserved with ERF transcription factors of several other species. In particular, the amino acid sequence of LkERF-B2 showed the highest homology (79.0%) with that of Picea sitchensi (Fig. 1). Phylogenetic analysis indicated that LkERF-B2 mostly closely related to ERF transcription factors products of Picea sitchensis, implying that they have similar origins (Fig. 2).
Fig. 1.
Amino acid sequence alignment of LkERF-B2 with ERFs from other species
Fig. 2.
Phylogenetic tree of LkERF-B2 and ERF sequences from other species
The predicted protein had a calculated molecular weight of 49 KD and an isoelectric point of 4.87. LkERF-B2 was an unstable protein. The amino acid sequence of LkERF-B2 contained a highly conserved 56-residue AP2 domain, which had an YRG and a RAYD element, at the 122nd–167th amino acids. The protein had a three anti-parallel β-sheet and an α-helix. The amino acids in the second β-fold at 14th and 19th were found to be alanine (A) and proline (P), consistent with typical AP2/ERF transcription factors. Secondary structure prediction suggested that the LkERF-B2 contained 5.59% α-helix, 3.58% β-sheet and 90.83% loops and has no transmembrane structure.
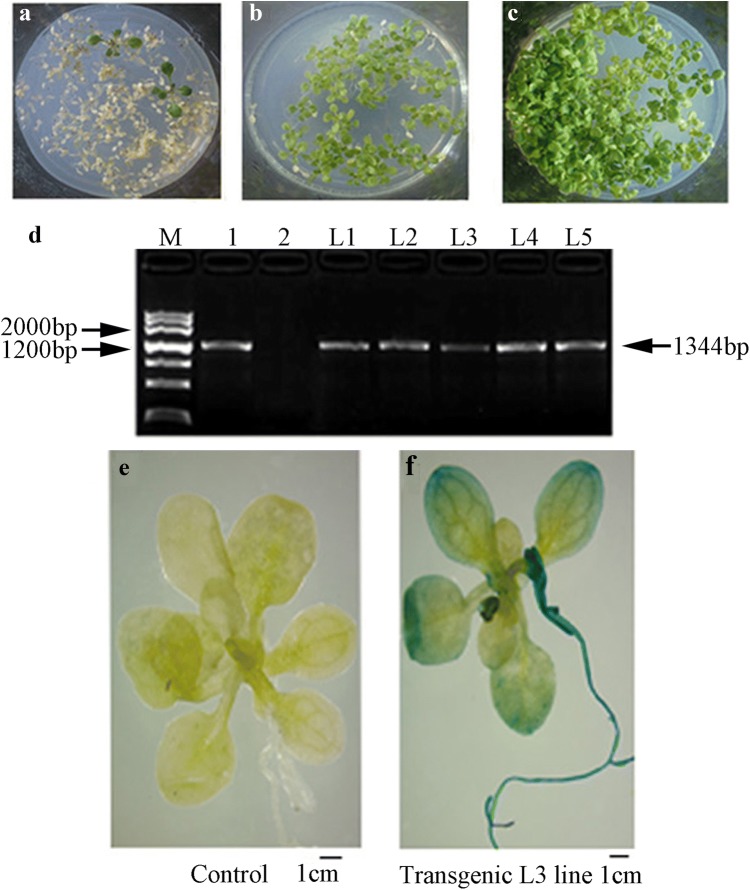
Production and identification of transgenic plants
Five positive transgenic plants, named transgenic plants L1–L5, were selected and identified in T1 generation. Each plant was seeded and harvested separately until T3 generation, which is the homozygous plant (Fig. 3a–c). GUS assay showed GUS activity in the leaf tips and root of transgenic plant. In particular, the GUS activity was very strong in the whole root (Fig. 3e, f). RT-PCR method was used to validate the expression of corresponding transgenic plants (Fig. 3d). This indicates that LkERF-B2 has been expressing into A. thaliana transgene system.
Fig. 3.
Screening and identification of transgenic A. thaliana. a–c T1, T2, T3 generation transgenic plants screening with Kanamycin, d RT-PCR identification of T3 transgenic plants. M: Maker III, 1: RT-PCR products of positive control, 2: RT-PCR products of negative control, L1–L5: RT-PCR products of transgenic plants, e GUS expression in the control, f GUS expression in transgenic L3 line
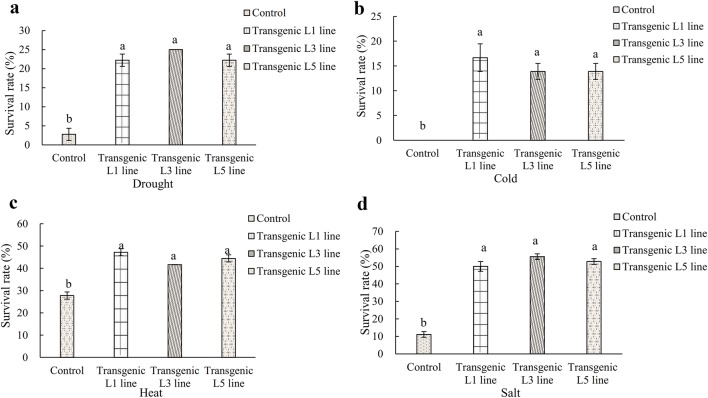
Analysis of survival rate of transgenic plants
Survival rate could effectively reflect the resistance of plants to adverse environments. Transgenic A. thaliana (lines L1, L3 and L5) and control plant were subjected to four abiotic stresses (drought, cold, heat and salt) treatments to analyze their adaptability to environmental stress. Survival rates of L1, L3 and L5 under drought stress were 22.22, 25.00 and 22.22%, respectively, which are significantly higher than that of the control (2.77%) (Fig. 4a). Under cold treatment, the average survival rates of transgenic A. thaliana plants were 14.82%, while all seedlings of control were died (Fig. 4b). Survival rates L1, L3, and L5 after heat stress were 47.22, 41.67, and 44.44%, respectively, which are significantly higher the control (27.78%) (Fig. 4c). The average survival rate of the LkERF-B2 overexpressing plants was 52.77% after salt stress, whereas the control plant rate was only 11.11% (Fig. 4d). These results revealed that overexpression of LkERF-B2 could enhance the adaptability and resistance of plant in various abiotic stresses.
Fig. 4.
Survival rate of control and transgenic A. thaliana seedling under various abiotic stresses. a 400 mM/L Mannitol for 7 days, b − 7 °C for 5 h 2 days later, c 40 °C for 3 h 2 days later, d 200 mM/L NaCl for 7 days
Performance of transgenic plant against salt
The control and transgenic L3 line plant were further treated with different NaCl concentrations (0, 100, 150, 200, 250 and 300 mM/L) to explore the effect of salt on the growth of transgenic A. thaliana. 100 and 150 mM/L NaCl treatment could promote flowering than NaCl-untreated group both in control and transgenic L3 plant (Fig. 5a–c). The earliest bolting was detected in transgenic L3 plant under 150 Mm/L salt stresses (Fig. 5c). While flowering was delayed in both control and transgenic L3 plant and their leaves became yellow or even withered under 200, 250, and 300 mM/L NaCl stresses (Fig. 5d–f). Transgenic L3 line A. thaliana exhibited less damage, larger leaf area and better growth condition than control group. Yellowed leaves were detected earlier in control group than in transgenic L3 line. These results showed that the damage gradually increased with the increase of NaCl concentration (Fig. 5). Interestingly, low concentrations (100 and 150 mM/L) of NaCl promoted the growth of plants, but high concentrations (200, 250 and 300 mM/L) inhibited plant growth and development.
Fig. 5.
Salt treatment of control and transgenic L3 line. a–f 0, 100, 150, 200, 250 and 300 mM/L NaCl
Salt injury status of transgenic L3 line against salt
The salt injury rate and salt damage index of the control and transgenic L3 line increased with increasing NaCl concentration. The salt injury rate was not significantly different between the control and transgenic L3 (Table 1) subjected to high concentrations (200, 250 and 300 mM/L NaCl). The rate even reached 100% in both groups under 300 mM/L NaCl treatment. However, the rate is significantly higher in control group than in the transgenic L3 line under 100 and 150 mM/L NaCl. The salt injury index of the control plant is significantly higher than that of transgenic L3 (Table 2) line under 100–250 mM/L NaCl. However, under 300 mM/L NaCl treatment, there was no significant difference. Under 100 mM/L NaCl, the salt damage rate and index of control plant were 2 and 1.99 times higher than those of transgenic L3 line, respectively.
Table 1.
Salt injury rate in control and transgenic L3 line under different NaCl concentration
| Type NaCl (mM/L) |
Control | Transgenic L3 line |
|---|---|---|
| 0 | 0.00 ± 0.00 e | 0.00 ± 0.00 e |
| 100 | 75.00 ± 7.31 b | 37.50 ± 7.31 d |
| 150 | 87.50 ± 7.31 ab | 50.00 ± 7.31 c |
| 200 | 87.50 ± 7.31 ab | 87.50 ± 0.00 ab |
| 250 | 100.00 ± 0.00 a | 87.50 ± 0.00 ab |
| 300 | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
Same letter means no significant difference according to Duncan’s test at α = 0.05
Table 2.
Salt injury index in control and transgenic L3 line under different NaCl concentration
| Type NaCl (mM/L) |
Control | Transgenic L3 line |
|---|---|---|
| 0 | 0.00 ± 0.00 g | 0.00 ± 0.00 g |
| 100 | 18.75 ± 0.02 e | 9.38 ± 0.02 f |
| 150 | 21.87 ± 0.02 de | 12.50 ± 0.02 f |
| 200 | 36.46 ± 0.04 c | 25.00 ± 0.02 d |
| 250 | 59.38 ± 0.02 b | 38.54 ± 0.01 c |
| 300 | 65.63 ± 0.02 a | 62.50 ± 0.02 ab |
Same letter means no significant difference according to Duncan’s test at α = 0.05
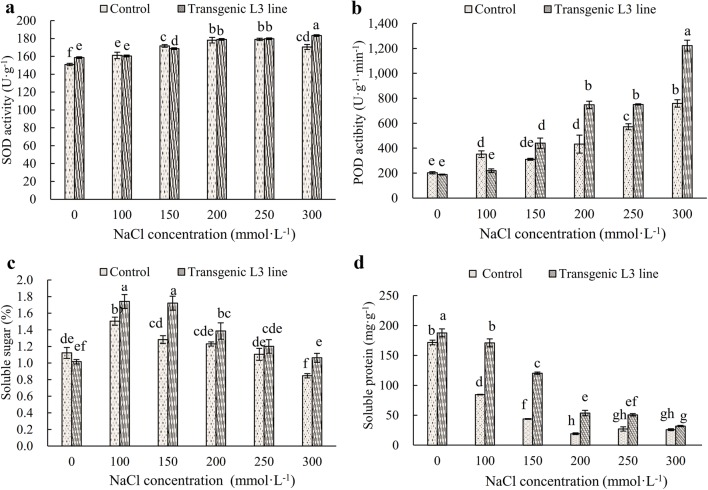
Analysis of SOD and POD of transgenic L3 line
The altered activities of SOD and POD are physiological and biochemical indicators of deterioration conditions in plants exposing to environmental constraints conditions. SOD activity of transgenic L3 line was significantly increased compared with that of the control plant. SOD activity of transgenic L3 line under 300 mM/L NaCl treatment increased by 7.5% compared with that of control plant, but did not increase significantly at other concentrations (Fig. 6a). POD activity had no difference between transgenic A. thaliana and control under 0 mM/L NaCl treatment (Fig. 6b). However, POD activity of transgenic L3 line increased significantly at 200, 250 and 300 mM/L. Overall, SOD and POD activities of transgenic L3 line increased significantly in comparison with those of control plant under 300 mM/L NaCl.
Fig. 6.
Physiological and biochemical characteristics of transgenic L3 line under different NaCl concentration. a SOD activity, b POD activity, c soluble sugar content, d soluble protein content
Analysis soluble sugar and soluble protein content of transgenic L3 line
Soluble sugar content in transgenic L3 line is higher than that in control plant except for untreated (0 mM/L NaCl) plant. Meanwhile, the soluble protein content in transgenic L3 is higher than that in the control plant. The contents of soluble sugars and soluble proteins decreased with increasing NaCl concentration (Fig. 6c, d). However, the soluble protein content was not changed significantly when NaCl concentration reached more than 200 mM/L (Fig. 6d), and the soluble sugar content continuously decreased (Fig. 6c). Under the treatment of 150 mM/L NaCl, the contents of soluble sugars and soluble proteins in transgenic L3 line are 1.34 times and 2.74 times higher than those in the control plant, respectively (Fig. 6c, d).
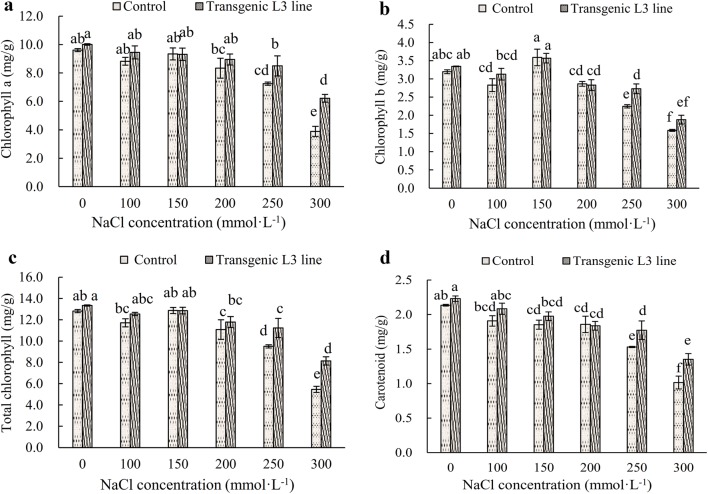
Analysis of chloroplast pigment content of transgenic L3 line
The contents of chloroplast pigments (chlorophyll a, chlorophyll b, total chlorophyll, carotenoid) in transgenic L3 line are higher than those in the control plant (Fig. 7). The contents of chlorophyll a, total chlorophyll, and carotenoid decreased the increasing NaCl concentration (Fig. 7a, c, d). The contents did not decrease significantly under less than 200 mM/L NaCl treatment but decreased significantly under more than 250 mM/L NaCl treatment. Meanwhile, chlorophyll b content showed a trend of increasing first and then decreasing (Fig. 7b). Under 150 mM/L NaCl treatment, chlorophyll b content in control and transgenic L3 line reached the maximum values of 0.41 ± 0.02 and 0.42 ± 0.02 mg/g, respectively. Under 250 mM/L NaCl treatment, the contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids in transgenic L3 line are 1.17, 1.2, 1.18 and 1.15 higher than those in the control group, respectively. Also, under 300 mM/L NaCl treatment, the contents of chlorophyll a, total chlorophyll and carotenoids in transgenic L3 plant are A. thaliana 1.6, 1.37, and 1.33 higher than those in the control group, respectively. At the same concentration, the contents of chlorophyll b in the control plant are higher than those in transgenic L3 line, but the difference was not significant.
Fig. 7.
Chloroplast pigment content in control and transgenic L3 line under different NaCl concentration. a Chlorophyll a content, b chlorophyll b content, c total chlorophyll content, d carotenoid content
Discussion
AP2/ERF family is one of the largest transcription factor families in plants. Among them, the ERF subfamily contained an AP2 domain with typical characteristics. The N-terminal is an alkaline hydrophilic region, and the C-terminal is rich acidic amino acids. The amino acid residues are all composed of three anti-parallel β-sheet and an α-helix (Allen et al. 2014). The 14th and 19th in the second β-fold are conserved, alanine (A) and aspartate (D), respectively. In particular, the 14th alanine plays a key role in determining the specific binding of ERF transcription factor to GCC-box (Ohmetakagi and Shinshi 1990). In this study, the ORF of LkERF-B2 gene from L. kaempferi was obtained by RT-PCR. Analysis of AP2/ERF conserved region in LkERF-B2 by bioinformatics indicated that LkERF-B2 had an AP2 domain, which contained YRG and RAYD element. The secondary structure of LkERF-B2 had three anti-parallel β-sheet and an α-helix. Meanwhile, the 14th is alanine (A) which is absolute conserved, but the 19th is proline (P) which had a slightly difference. These characteristics are basically the same as those of known AP2 conserved regions. Amino acid sequence alignment of LkERF-B2 revealed that LkERF-B2 had the highest homology with P. sitchensis (79%). The homology of ERF transcription factor protein with other species was 35–38%. Hence, LkERF-B2 is a newly discovered sequence that is relatively conserved in evolution and could be a member of ERF subfamily.
Survival rates of transgenic lines and control A. thaliana were analyzed. High survival rate of transgenic plants under abiotic stresses suggested that LkERF-B2 might play an important role in plant abiotic responses. Previous studies revealed that ERFs could improve tolerance ability when it was expressed in transgenic plants (Makhloufi et al. 2014; Phukan et al. 2017). For example, overexpression of ERF1 in rice improved its resistance to salt stresses (Schmidt et al. 2013). Also, overexpression of SpERF1 enhanced drought tolerance of transgenic A. thaliana (Yang et al. 2016). In the present study, transgenic A. thaliana showed higher survival rate than the control plant. Moreover, physiological and biochemical analyses demonstrated that LkERF-B2 could enhance the adaptability of plants to abiotic stress.
Salt stress could adversely influence plant growth and development (Hussain et al. 2017). Previous research reported that treatments with low salt concentration could promote plant growth and development, but high salt concentration could inhibit plant growth. In previous works, treatments with low NaCl concentrations (25 and 50 mM/L NaCl treatment) improved the growth of Citrullus lanatus seedlings. High concentrations of NaCl (75, 100 and 150 mM/L NaCl treatment) obviously inhibited seeding growth (Han et al. 2008). Moreover, low levels of salinity stresses could improve the growth of Medicago sativa, but high levels inhibited seed germination (Gong et al. 2017). In the present study, the growth of A. thaliana (transgenic L3 line and control plant) was promoted by low NaCl concentrations (100 and 150 mM/L NaCl) but inhibited by high concentrations (200, 250 and 300 mM/L NaCl). Moreover, transgenic A. thaliana exhibited obvious growth advantage than control plant under the same NaCl concentration.
Abiotic stress may affect the balance between ROS production and removal in cells, leading to increased ROS. ROS could further destroy membrane lipid, proteins, DNA and RNA, consequently, plant growth and development were inhibited or even death in serious cases (Hossain et al. 2015; Jain and Gould 2015). Antioxidant enzymes (SOD and POD) can be used to effectively deal with ROS in plants. In this study, activities of SOD and POD in transgenic L3 line were significantly higher than those in the control plant. Therefore, LkERF-B2 enhanced plant antioxidant ability in the present study.
Soluble sugars and soluble proteins are solutes that accumulate in plants under abiotic stresses. These substances could regulate osmotic potential and stabilize and protect the structure and function of biological macromolecules. In this study, the contents of soluble sugars and soluble proteins increased increasing NaCl concentration. Moreover, the concentrations of soluble sugars and soluble proteins in the transgenic L3 line plant are significantly higher than those in the control plant under 100 and 150 mM/L NaCl treatment. Therefore, LkERF-B2 increased soluble sugar and soluble protein content in response to NaCl stress.
Content of chloroplast pigment decreased with increasing NaCl concentration. Carotenoids play an important role in plants growth and development. Carotenoids function in two ways: they function as antenna pigments and transmit captured light to chlorophyll; and they act as scavengers of free radicals in plant cells (Polívka et al. 2004; Polívka and Frank 2010). The study on carotenoid content of transgenic L3 showed that LkERF-B2 could increase the carotenoid content and enhance the NaCl tolerance of plant.
Conclusion
The LkERF-B2 was cloned from L. kaempferi. The ORF of LkERF-B2 is 1344 bp, encoding 447 amino acids and containing an AP2/ERF domain. LkERF-B2 has the closest relationship with P. sitchensis (79.0%). LkERF-B2 is a hydrophilic protein with no transmembrane region. The plant expression vector was constructed and LkERF-B2 was transferred into A. thaliana. Five homozygous transgenic lines were obtained. Under various abiotic stresses (cold, heat, salt and drought), survival rate of transgenic A. thaliana was significantly higher than that of control. Under NaCl stress, salt injury rate and salt injury index of transgenic A. thaliana were lower than those of the control, while the activities of SOD, POD and contents of chloroplast pigments were higher than those of control. In conclusion, LkERF-B2 plays a role in abiotic stress, especially salt stress. Further studies on stress tolerance genes are of great significance for improving the yield and quality of L. kaempferi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 11254 kb)
Supplementary material 2 (TIFF 13846 kb)
Supplementary material 3 (TIFF 14624 kb)
Supplementary material 4 (TIFF 12664 kb)
Supplementary material 5 (TIFF 11894 kb)
Acknowledgements
This work was supported by Tianjin Agricultural University Graduate Training Quality Improvement Project (No. 101018), National Natural Science Foundation (No. 31300564, No. 31800572), Tianjin “131” Innovative Talents Training Project, Modern Industrial System Fruit Tree Physiological and Ecological Post (ITTFPRS2018002).
Abbreviations
- GUS
β-Glucuronidase enzyme
- MS
Murashige and Skoog
- NBT
Nitroblue tetrazolium
- SOD
Superoxide dismutase
- POD
Peroxidase
- ROS
Reactive oxygen species
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Beibei Cao, Email: 5786854@qq.com.
Ai Li, Email: lovelee19840204@163.com.
References
- Allen MD, Yamasaki K, Ohme-Takagi M, Tateno M, Suzuki M. A novel mode of DNA recognition by a β-sheet revealed by the solution structure of the GCC-box binding domain in complex with DNA. EMBO J. 2014;17(18):5484–5496. doi: 10.1093/emboj/17.18.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301(5633):653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- Bohnert HJ, Jensen RG. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996;14(3):89–97. doi: 10.1016/0167-7799(96)80929-2. [DOI] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu CM, Lammeren AAM, Miki BLA, Custers JBM, Campagne MML. Ectopic expression of baby boom triggers a conversion from vegetative to embryonic growth. Plant Cell. 2002;14(8):1737–1749. doi: 10.1105/tpc.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MC, Lin TP. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 2013;162(3):1566–1582. doi: 10.1104/pp.113.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck G, Meeley RB, Hake S. The control of maize spikelet meristem fate by the apetala2-like gene indeterminate spikelet1. Genes Dev. 1998;12(8):1145–1154. doi: 10.1101/gad.12.8.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 2010;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Dong W, Ai X, Xu F, Quan T, Liu S, Xia G. Isolation and characterization of a bread wheat salinity responsive ERF transcription factor. Gene. 2012;511(1):38–45. doi: 10.1016/j.gene.2012.09.039. [DOI] [PubMed] [Google Scholar]
- Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-, salt-, and cold-responsive gene expression. Plant J. 2003;33(4):751–763. doi: 10.1046/j.1365-313X.2003.01661.x. [DOI] [PubMed] [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQ, Gerentes D, Perez P, Smyth DR. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell. 1996;8(2):155–168. doi: 10.1105/tpc.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell. 2000;12(3):393–404. doi: 10.1105/tpc.12.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Zhang H, Tian Y, Li F, Zhang Z, Lu X, Chen X, Huang R. Expression of TERF1 in rice regulates expression of stress-responsive genes and enhances tolerance to drought and high-salinity. Plant Cell Rep. 2008;27(11):1787–1795. doi: 10.1007/s00299-008-0602-1. [DOI] [PubMed] [Google Scholar]
- Gong WL, Zhao GQ, Liu H. Comprehensive evaluation on salt tolerance of 22 alfalfa varieties in germination stage. Grassl Turf. 2017;37(5):35–39. [Google Scholar]
- Grintzalis K, Georgiou CD, Schneider YJ. An accurate and sensitive coomassie brilliant blue g-250-based assay for protein determination. Anal Biochem. 2015;480:28–30. doi: 10.1016/j.ab.2015.03.024. [DOI] [PubMed] [Google Scholar]
- Han ZP, Guo SR, Feng JQ, Gao XH. Effect of salinity on plant growth, photosynthetic pigments and proline content in leaves of watermelon seedlings. J Nanjing Agric Univ. 2008;31(2):32–36. [Google Scholar]
- Hernandezgarcia CM, Finer JJ. A novel cis-acting element in the GmERF3 promoter contributes to inducible gene expression in soybean and tobacco after wounding. Plant Cell Rep. 2016;35(2):303–316. doi: 10.1007/s00299-015-1885-7. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Bhattacharjee S, Armin SM, Armin SM, Qian PP, Xin W, Li HY, Burritt DJ, Fujta M, Tran LS. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci. 2015;6:420. doi: 10.3389/fpls.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Zhang JH, Zhong C, Zhu LF, Cao XC, Yu SM, Allen BJ, Hu JJ, Jin QY. Effects of salt stress on rice growth, development characteristics, and the regulating ways: a review. J Integr Agric. 2017;16(11):2357–2374. doi: 10.1016/S2095-3119(16)61608-8. [DOI] [Google Scholar]
- Ibrahim MH, Jaafar HZE, Asmah R, Zaharah AR. Involvement of Nitrogen on flavonoids, glutathione, anthocyanin, ascorbic acid and antioxidant activities of malaysian medicinal plant Labisia pumila Blume (Kacip Fatimah) Int J Mol Sci. 2012;13:393–408. doi: 10.3390/ijms13010393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain G, Gould KS. Are betalain pigments the functional homologues of anthocyanins in plants? Environ Exp Bot. 2015;119:48–53. doi: 10.1016/j.envexpbot.2015.06.002. [DOI] [Google Scholar]
- Jofuku KD, Omidyar PK, Gee Z, Okamuro JK. Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc Natl Acad Sci USA. 2005;102(8):3117–3122. doi: 10.1073/pnas.0409893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, Mcgettigan PA, Mcwilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kang JY, Park HJ, Kim MD, Min SB, Choi HI, Kim SY. DREB2C interacts with ABF2, a bZIP protein regulating abscisic acid-responsive gene expression, and Its overexpression affects abscisic acid sensitivity. Plant Physiol. 2010;153(1):716–727. doi: 10.1104/pp.110.154617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Jung H, Jang G, Jeong JS, Kim YS, Ha SH, Choi YD, Kim JK. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol. 2016;172(1):575–588. doi: 10.1104/pp.16.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CW, Su RC, Cheng CP, Sanjaya You SJ, Hsieh TH, Chao TC, Chan MT. Tomato RAV transcription factor is a pivotal modulator involved in the AP2/EREBP-mediated defense pathway. Plant Physiol. 2011;156(1):213–227. doi: 10.1104/pp.111.174268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Li SJ, Wu GQ, Yan GR. Isolation and functional characterization of APETALA2-Like gene from larix. J Plant Genet Resour. 2014;15(6):1305–1311. [Google Scholar]
- Li A, Wang J, Li H, Chen C, Song W, Wang C. Transcriptome profiling and characterization of gene families with zinc finger and nucleotide binding site (NBS) domains in larix kaempferi. J Plant Biochem Biotechnol. 2016;26(2):1–11. [Google Scholar]
- Liu J, Wang Y, Zhao G, Zhao J, Du H, He X, Zhang H. A novel Gossypium barbadense ERF transcription factor, GbERFb, regulation host response and resistance to Verticillium dahliae in tobacco. Physiol Mol Biol Plants. 2017;23(1):1–10. doi: 10.1007/s12298-016-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang R, Liu W, Zhang H, Guo Y, Wen R. Genome-wide characterization of heat-shock protein 70 s from chenopodium quinoa and expression analyses of cqhsp70 s in response to drought stress. Genes. 2018;9(2):35. doi: 10.3390/genes9020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sánchezserrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15(1):165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zhang L, Zhang J, Chen J, Wu T, Zhu S, Yan S, Zhao X, Zhong G. Expressing a citrus ortholog of Arabidopsis ERF1 enhanced cold-tolerance in tobacco. Sci Hortic. 2014;174(1):65–76. doi: 10.1016/j.scienta.2014.05.009. [DOI] [Google Scholar]
- Ma R, Xiao Y, Lv Z, Tan H, Chen R, Li Q, Chen J, Wang Y, Yin J, Zhang L, Chen W. AP2/ERF transcription factor, li049, positively regulates lignan biosynthesis in isatis indigotica through activating salicylic acid signaling and lignan/lignin pathway genes. Front Plant Sci. 2017;8:1361. doi: 10.3389/fpls.2017.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhloufi E, Yousfi FE, Marande W, Mila I, Hanana M, Bergès H, Bergès H, Mzid R, Bouzayen M. Isolation and molecular characterization of ERF1, an ethylene response factor gene from durum wheat (Triticum turgidum L. subsp. durum), potentially involved in salt-stress responses. J Exp Bot. 2014;65(22):6359–6371. doi: 10.1093/jxb/eru352. [DOI] [PubMed] [Google Scholar]
- Mantiri FR, Kurdyukov S, Lohar DP, Sharopova N, Saeed NA, Wang XD, VandenBosch KA, Rose RJ. The transcription factor MtSERF1 of the ERF subfamily identified by transcriptional profiling is required for somatic embryogenesis induced by auxin plus cytokinin in Medicago truncatula. Plant Physiol. 2008;146(4):1622–1636. doi: 10.1104/pp.107.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moose SP, Sisco PH. Glossy15, an apetala2-like gene from maize that regulates leaf epidermal cell identity. Genes Dev. 1996;10(23):3018–3027. doi: 10.1101/gad.10.23.3018. [DOI] [PubMed] [Google Scholar]
- Muhammad R, He G, Yang G, Javeed H, Yan X. AP2/ERF transcription factor in rice: genome-wide canvas and syntenic relationships between monocots and eudicots. Evol Bioinform Online. 2012;8(4):321–355. doi: 10.4137/EBO.S9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na D, Xin L, Yan L, Du LP, Xu H, Liu HX, Xin ZY, Zhang ZY. Overexpression of TaPIEP1, a pathogen-induced ERF gene of wheat, confers host-enhanced resistance to fungal pathogen Bipolaris sorokiniana. Funct Integr Genom. 2010;10(2):215–226. doi: 10.1007/s10142-009-0157-4. [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006;140(2):411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmetakagi M, Shinshi H. Structure and expression of a tobacco beta-1,3-glucanase gene. Plant Mol Biol. 1990;15(6):941–946. doi: 10.1007/BF00039434. [DOI] [PubMed] [Google Scholar]
- Ohme-Takagi M, Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell. 1995;7(2):173–182. doi: 10.1105/tpc.7.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oñatesánchez L, Singh KB. Identification of Arabidopsis ethylene-responsive element binding factors with distinct induction kinetics after pathogen infection. Plant Physiol. 2002;128(4):1313–1322. doi: 10.1104/pp.010862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oñatesánchez L, Anderson JP, Young J, Singh KB. AtERF14, a member of the ERF family of transcription factors, plays a nonredundant role in plant defense. Plant Physiol. 2007;143(1):400–409. doi: 10.1104/pp.106.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pápista É, Acs E, Böddi B. Chlorophyll-a determination with ethanol—a critical test. Hydrobiologia. 2002;485(1–3):191–198. doi: 10.1023/A:1021329602685. [DOI] [Google Scholar]
- Park JM, Park CJ, Lee SB, Ham BK, Shin R, Paek KH. Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell. 2001;13(5):1035–1046. doi: 10.1105/tpc.13.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phukan UJ, Jeena GS, Tripathi V, Shukla RK. Regulation of apetala2/ethylene response factors in plants. Front Plant Sci. 2017;8:150. doi: 10.3389/fpls.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podazza G, Arias M, Prado FE. Cadmium accumulation and strategies to avoid its toxicity in roots of the citrus rootstock citrumelo. J Hazard Mater. 2012;215–216:83–89. doi: 10.1016/j.jhazmat.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Polívka T, Frank HA. Molecular factors controlling photosynthetic light harvesting by carotenoids. Acc Chem Res. 2010;43(8):1125–1134. doi: 10.1021/ar100030m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polívka T, Pullerits T, Frank HA, Cogdell RJ, Sundström V. Ultrafast formation of a carotenoid radical in LH2 antenna complexes of purple bacteria. J Phys Chem B. 2004;108(39):15398–15407. doi: 10.1021/jp0483019. [DOI] [Google Scholar]
- Rao G, Sui J, Zeng Y, He C, Zhang J. Genome-wide analysis of the AP2/ERF gene family in Salix arbutifolia. Plant Mol Biol Report. 2016;5(1):132–137. doi: 10.1016/j.fob.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Heard J, Martin G, Reuber L, Jiang C, Keddie J, Adam L, Pineda O, Ratcliffe OJ, Samaha RR, Creelman R, Pilgrim M, Broun P, Zhang JZ, Ghandehari D, Sherman BK, Yu GL. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science. 2000;290(5499):2105–2110. doi: 10.1126/science.290.5499.2105. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-shinozaki K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-Inducible gene expression. Biochem Biophys Res Commun. 2002;290(3):998–1009. doi: 10.1006/bbrc.2001.6299. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Mieulet D, Hubberten HM, Obata T, Hoefgen R, Fernie AR, Fisahn J, Segundo BS, Guiderdon E, Schippers JHM, Roeber BM. Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell. 2013;25(6):2115–2131. doi: 10.1105/tpc.113.113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J Exp Bot. 2007;58(2):221. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K, Seki M. Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol. 2003;6(5):410–417. doi: 10.1016/S1369-5266(03)00092-X. [DOI] [PubMed] [Google Scholar]
- Shu Y, Liu Y, Zhang J, Song L, Guo C. Genome-wide analysis of the AP2/ERF superfamily genes and their responses to abiotic stress in Medicago truncatula. Front Plant Sci. 2016;6(676):1247. doi: 10.3389/fpls.2015.01247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn KH, Lee SC, Jung HW, Hong JK, Hwang BK. Expression and functional roles of the pepper pathogen-induced transcription factor RAV1 in bacterial disease resistance, and drought and salt stress tolerance. Plant Mol Biol. 2006;61(8):897–915. doi: 10.1007/s11103-006-0057-0. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AT, Liu E, Polvi SL, Pammett RT, Page JE. A functional genomics screen identifies diverse transcription factors that regulate alkaloid biosynthesis in Nicotiana benthamiana. Plant J Cell Mol Biol. 2010;62(4):589–600. doi: 10.1111/j.1365-313X.2010.04186.x. [DOI] [PubMed] [Google Scholar]
- Vogel MO, Moore M, König K, Pecher P, Alsharafa K, Lee J, Dietz KJ. Fast retrograde signaling in response to high light involves metabolite export, mitogen-activated protein kinase6, and AP2/ERF transcription factors in Arabidopsis. Plant Cell. 2014;26(3):1151–1165. doi: 10.1105/tpc.113.121061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218(1):1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang C, Qin L, Liu W, Wang Y. ThERF1 regulates its target genes via binding to a novel cis-acting element in response to salt stress. J Integr. 2015;57(10):838–847. doi: 10.1111/jipb.12335. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang H, Shao H, Tang X. Recent advances in utilizing transcription factors to improve plant abiotic stress tolerance by transgenic technology. Front Plant Sci. 2016;7(248):67. doi: 10.3389/fpls.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Dong C, Li X, Du J, Qian M, Sun X, Yang Y. A novel Ap2/ERF transcription factor from Stipa purpurea leads to enhanced drought tolerance in Arabidopsis thaliana. Plant Cell Rep. 2016;35(11):1–13. doi: 10.1007/s00299-016-2030-y. [DOI] [PubMed] [Google Scholar]
- Yao Y, He RJ, Xie QL, Zhao XH, Deng XM, He JB, Song L, He J, Marchant A, Chen XY, Wu AM. ETHYLENE RESPONSE FACTOR 74 (ERF74) plays an essential role in controlling a respiratory burst oxidase homolog D (RbohD)-dependent mechanism in response tresses in arabidopsis. New Phytol. 2017;213(4):1667–1681. doi: 10.1111/nph.14278. [DOI] [PubMed] [Google Scholar]
- Yu ZX, Li JX, Yang CQ, Hu WL, Wang LJ, Chen XY. The jasmonate-responsive AP2/ERF transcription factors AaERF1 and AaERF2 positively regulate artemisinin biosynthesis in Artemisia annua L. Mol Plant. 2012;5(2):353–365. doi: 10.1093/mp/ssr087. [DOI] [PubMed] [Google Scholar]
- Yu Y, Duan X, Ding X, Chen C, Zhu D, Yin KD, Cao L, Song XW, Zhu PH, Li Q, Nisa Z, Yu JY, Du JY, Song Y, Li HQ, Liu BD, Zhu YM. A novel AP2/ERF family transcription factor from Glycine soja, GsERF71, is a DNA binding protein that positively regulates alkaline stress tolerance in Arabidopsis. Plant Mol Biol. 2017;94(4–5):509–530. doi: 10.1007/s11103-017-0623-7. [DOI] [PubMed] [Google Scholar]
- Zang D, Wang C, Ji X, Wang Y. Tamarix hispida zinc finger protein thzfp1 participates in salt and osmotic stress tolerance by increasing proline content and sod and pod activities. Plant Sci. 2015;235:111–121. doi: 10.1016/j.plantsci.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Zhang GY, Chen M, Li LC, Xu ZS, Chen XP, Guo JM, Ma YZ. Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J Exp Bot. 2009;60(13):3781–3796. doi: 10.1093/jxb/erp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Wang QJ, Guo ZR. Progresses on plant AP2/ERF transcription factors. Yi chuan = Hereditas. 2012;34(7):835–847. doi: 10.3724/SP.J.1005.2012.00835. [DOI] [PubMed] [Google Scholar]
- Zhang P, Yang PZ, Zhang ZQ, Han B, Wang WD, Wang YF, Cao YM, Hu TM. Isolation and characterization of a buffalograss (Buchloe dactyloides) dehydration responsive element binding transcription factor, BdDREB2. Gene. 2014;536(1):123–128. doi: 10.1016/j.gene.2013.11.060. [DOI] [PubMed] [Google Scholar]
- Zhao T, Liang D, Wang P, Liu J, Ma F. Genome-wide analysis and expression profiling of the DREB transcription factor gene family in Malus under abiotic stress. Mol Genet Genomics. 2012;287(5):423–436. doi: 10.1007/s00438-012-0687-7. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Cheng S, Song Y, Huang Y, Zhou S, Liu X, Zhou DX. The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell. 2015;27(9):2469–2483. doi: 10.1105/tpc.15.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53(53):247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhang Z. The wheat ethylene response factor transcription factor pathogen-induced ERF1 mediates host responses to both the necrotrophic pathogen Rhizoctonia cerealis and freezing stresses. Plant Physiol. 2014;164(3):1499–1514. doi: 10.1104/pp.113.229575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zhang J, Gao X, Tong J, Xiao L, Li W, Zhang H. The Arabidopsis AP2/ERF transcription factor RAP2.6 participates in ABA, salt and osmotic stress responses. Gene. 2010;457(1–2):1–12. doi: 10.1016/j.gene.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Zhuo C, Liang L, Zhao Y, Guo Z, Lu S. A cold responsive ethylene responsive factor from Medicago falcata confers cold tolerance by up-regulation of polyamine turnover, antioxidant protection, and proline accumulation. Plant Cell Environ. 2017;41:2021–2032. doi: 10.1111/pce.13114. [DOI] [PubMed] [Google Scholar]
- Zuo KJ, Qin J, Zhao JY, Ling H, Zhang LD, Cao YF, Tang KX. Over-expression GbERF2 transcription factor in tobacco enhances brown spots disease resistance by activating expression of downstream genes. Gene. 2007;391(1–2):80–90. doi: 10.1016/j.gene.2006.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (TIFF 11254 kb)
Supplementary material 2 (TIFF 13846 kb)
Supplementary material 3 (TIFF 14624 kb)
Supplementary material 4 (TIFF 12664 kb)
Supplementary material 5 (TIFF 11894 kb)