Abstract
Asthma is defined as a chronic inflammatory condition in the lung and is characterized by episodic shortness of breath with expiratory wheezing and cough. Asthma is a serious public health concern globally with an estimated incidence over 300 million. Asthma is a complex disease in that it manifests as disease of gene and environmental interactions. Sphingolipids are a unique class of lipids involved in a host of biological functions ranging from serving as key cellular membrane lipids to acting as critical signaling molecules. To date sphingolipids have been studied across various human conditions ranging from neurological disorders to cancer to infection to autoimmunity. This review will focus on the role of sphingolipids in asthma development and pathology with particular focus on the role of mast cell sphingolipid biology.
Keywords: Sphingolipid, Asthma, Mast cell
1. Introduction
1.1. Asthma
Asthma is defined as a chronic inflammatory condition characterized by episodic shortness of breath with wheeze. It is characterized by intermittent airflow obstruction, airway hyperreactivity, and increased production of mucus. According to the U.S. Centers for Disease Control and Prevention (CDC), an estimated 24.6 million Americans, including over 6 million children, have asthma. The estimated annual cost of asthma care in the U.S. is roughly $56 billion and asthma remains a leading cause of missed work and school. According the Global Asthma Network (GAN), it is estimated that over 330 million people worldwide have this disease and asthma is ranked in the top 20 of “most important disorders” due to chronicity of illness. Thus, asthma remains a universal and significant public health concern.
Asthma is a complex, heterogeneous disease in which several variants exist, each with a different underlying immunopathology. Historically asthma was characterized as a TH2-centric disease marked by high levels of IgE and eosinophilic infiltration into the lung. While a majority of patients with asthma do have a history of allergic disease, certain groups of patients present clinically with an asthma phenotype in the absence of atopy. The different underlying pathophysiological mechanisms suggest the need for additional molecular and genetic characterizations, the development of targeted, customized treatment modalities, and the value of biomarkers to improve diagnosis and treatment. Recently, work has highlighted different asthma endotypes that are segregated by clinical characteristics (Moore et al., 2010) or through the use of genetic markers found in sputum (Peters et al., 2014). This clinical cluster modeling has enabled us to categorize endotypes of asthmatics into groups and better understand more of the molecular underpinnings of the disease process. The development of these clinical algorithms has advanced the goal of using precision-based medicine approaches for asthma. But despite these efforts, there are still clusters of patients with severe asthma in which drugs are lacking and specific biomarkers are unavailable.
1.2. Sphingolipids
In 1884, Dr. Johann L.W. Thudichum isolated a unique lipid moiety from the brain he called “sphingosine” which he named after the Great Sphinx due to its perplexing chemical nature (Thudichum, n.d.). This fatty amino alcohol serves as the backbone of a ubiquitous class of lipids found across various species called sphingolipids. Living up to the name, sphingolipids remain an enigmatic class of lipids involved in a host of biological functions ranging from serving as key cellular membrane lipids to acting as critical signaling molecules. To date sphingolipids have been studied across various human conditions ranging from neurological disorders to cancer to infection to autoimmunity. Sphingolipids exhibit great structural and functional diversity. The simple sphingolipid ceramide is often referred to as the nexus of sphingolipid metabolism due to its pivotal role in sphingolipid accumulation through de novo synthesis as well as serving as a predecessor for other sphingolipid family members (Hannun and Obeid, 2002). Whereas complex sphingolipids such as cerebrosides are glycosphingolipids and defects in their catabolism, as the name suggests, have significant effects on the nervous system (Futerman and Platt, 2017). Sphingolipid metabolites such as sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P) are potent bioactive mediators that are critical, especially in relation to immune responses and inflammatory processes as they regulate immune cell migration and play a role in cancer immunopathology (Nitai C. Hait and Maiti, 2017; Spiegel and Milstien, 2011).
In addition to structure and function, the regulation of these lipids is also complex. There are multiple enzymatic processes that regulate the interconversion amongst these lipid species and each enzyme serves as another layer of control in maintaining homeostasis. The year 1993 was a pivotal year in sphingolipid research as two seminal papers, both appearing in Nature, showed that sphingolipids could both induce cell death (Obeid et al., 1993) and cell proliferation (Olivera and Spiegel, 1993). These papers highlighted differing and opposing roles of ceramide and S1P respectively. Three years later, the Spiegel laboratory uncovered the molecular cross-talk between these pathways and coined the term the “sphingolipid rheostat” (Cuvillier et al., 1996) which provided further insight into the regulation and activity of this complex class of lipids.
1.3. Sphingolipids in the lung
The notion that sphingolipids could play a role in pulmonary pathophysiology was first alluded to in the 1940s when Thannhauser et al. isolated and described lipid species in various animal lungs (Thannhauser et al., 1946). Almost twenty years later, Matthews et al. showed that the secretions from patients with either cystic fibrosis (CF) or bronchiectasis had a much higher lipid content when compared to controls (Matthews et al., 1963). It was this work that led Harlan et al. to examine pulmonary secretions to better understand the contributions of lipids other than phosphatidylcholine, the major lipid species found in pulmonary surfactant (Harlan WR, Margraf and Said, 1966). Years later Sahu and Lynn showed that the bronchoalvealoar lavage (BAL) fluid from patients with asthma contained sphingomyelin species as well as glycolipids in the form of hexosylceramides which served as some of the first evidence for a role of sphingolipids in asthma (Sahu and Lynn, 1977).
1.4. Environmental causes of asthma
The National Institute of Environmental Health Sciences (NIEHS) has stated that the contribution of environmental allergens to the pathogenesis of asthma is a serious public health concern and research is warranted in the field. Air pollution has been shown to be both a contributing factor to asthma development (Wright and Brunst, 2013) and asthma exacerbations (Guarnieri and Balmes, 2014). Diesel exhaust has been shown to a major player in the environmental influences of asthma and diesel exhaust particles have been shown to upregulate both ceramide production and sphingosine kinase 1 (SphK1) activity in bronchial epithelial cells (Shaheen et al., 2016). Inhalation of carbonaceous pollutants in the form of carbon nanoparticles has also been linked to pulmonary dysfunction and exposure to these xenobiotic agents has been shown to alter ceramide accumulation in lung epithelial cells leading to aberrant inflammatory response generation in the lung (Peuschel et al., 2012). Cigarette smoke either by direct or secondhand exposure have all been linked to asthma development and exacerbation (Thomson, 2004). Both conventional and electronic cigarette (e-cigs) vapors have shown to disrupt pulmonary endothelial barrier integrity by upregulating intracellular ceramide production (Schweitzer et al., 2015) in a neutral sphingomyelinase 2 (nSmase2) dependent fashion (Levy et al., 2009) as well as alter autophagy by inducing accumulation of lactosylceramide in lipid rafts (Bodas et al., 2015).
There is a mounting body of evidence that infectious agents also contribute to asthma incident and illness (Darveaux et al., 2014). Upper respiratory infections are the most common cause of acute illness across the lifespan. Certain pathogens have been strongly linked to the initiation of asthma such as respiratory syncytial virus (RSV) and human rhinovirus (HRV). In fact there is a significant increase in the likelihood that a child with develop asthma if they are infected with both HSV and HRV in the first three years of life (OR = 10.0) (Busse et al., 2010). Both of these viruses rely heavily on sphingolipid species for viral entry into the cell. RSV utilizes ganglioside GM1 for both viral entry and propagation (Sugrue et al., 2002) and has to ability to activate both neutral ceramidase and SphK1 in the lung (Monick et al., 2004). The activation of these enzymes leads to both the downregulation of ceramide production and the upregulation of S1P production which in turns prolongs lung epithelial survival of virally infected cells. HRV also highjacks the host sphingolipid machinery. HRV has been shown to activate the acidic sphingomyelinase and induce the formation of ceramide-rich platforms to facilitate further viral entry (Grassmé et al., 2005). While these two viral culprits are most closely linked to asthma initiation, other viral species such as influenza have been strongly linked with exacerbations of asthma resulting in significant morbidity and mortality (Busse et al., 2010). Interestingly influenza also affects sphingolipid signaling by inhibition of S1P lyase, thus resulting in increased S1P levels in the infected cell (Vijayan and Hahm, 2014).
Bacterial infections have also been linked to asthma. Numerous studies have begun to examine alterations in the host microbiome and microbial exposures as it pertains to asthma development and severity (Scherzer and Grayson, 2018) whereas atypical pulmonary infections have been shown to aggravate established asthma. A major pathogen responsible for community acquired pneumonia, Mycoplasma pneumoniae, induces profound alterations in host cell sphingolipid composition by inducing activation of serine palmitoyltransferase (SPT), the rate limiting step in de novo sphingolipid production (Yu et al., 2009). Fungi are also a significant environmental contributor to the disease burden of asthma and unlike most prokaryotes, multiple fungal species are capable of producing their own sphingolipids. In fact, fungal derived sphingolipids are found to play a role in their pathogenicity as is the case with Aspergillis niger (Sharma and Prakash, 2017) and inhalation of non-pathogenic fungal species such as Alternaria alternata can induce sphingolipid production from host airway epithelial cells (Worgall et al., 2013).
Allergens themselves can also influence sphingolipid signaling and contribute to asthma pathogenesis. In fact, it has been shown that pollen contains a wide array of sphingolipids (Ischebeck, 2016). House dust mite (HDM) is a prevalent antigen and a leading contributor of asthma. HDM is thought to be one of the most significant sources of indoor antigens associated with human asthma (De Alba et al., 2010) and it has been estimated that 50–85% of asthmatics world-wide are HDM allergic (Gregory and Lloyd, 2011). We recently reported that administration of HDM induces ceramide production in the murine lung and results in allergic airway disease (Oyeniran et al., 2015). Lastly, there has been robust research on how exogenous sphingolipids can directly activate immune cells and leads to pulmonary inflammation. Alpha-galactosylceramide (a-GalCer) is a synthetic sphingolipid derivative from sphingolipid species produced from the marine sponge Agelas mauritanianus. A-GalCer is widely used in the laboratory setting as it is a potent activator of invariant natural killer T cells (iNKT cells) and has shown a to play a role in the pathogenesis of asthma (Iwamura and Nakayama, 2010) by exaggerating TH2 immune responses.
Taken together there is mounting evidence demonstrating that environmental triggers can have a profound effect on asthma. The role of sphingolipids in asthma initiation is not well defined however the strong body of literature suggests that it is these early activation events resulting in altered sphingolipid pathways that may be a key aspect of asthma development and severity.
1.5. Genetic causes of asthma
Aside from environmental contributions, there is also a strong genetic component to asthma. Several genome-wide association studies (GWAS) have demonstrated that polymorphisms in a region of chromosome 17q21, which includes the ORM (yeast)-like protein isoform 3 (ORMDL3) gene, contribute to both childhood and adult-onset asthma in a number of ethnically diverse populations (Galanter et al., 2008; Moffatt et al., 2010, 2007; Vercelli, 2008, 2016; Verlaan et al., 2009). In fact, identification of ORMDL3 as an asthma susceptibility gene ignited both a new era of research into genetics as well as sphingolipid regulation in the asthma field. However, despite the enthusiasm, the mechanism by which ORMDL3 contributes to asthma pathogenesis is not well understood. ORMDL3 is the only member of the evolutionary conserved family of three endoplasmic reticulum localized proteins, ORMDL1–3, that has been linked to asthma. In yeast, the ORM proteins negatively regulate sphingolipid homeostasis in response to physiological needs by forming a complex with and inhibiting serine palmitoyltransferase (SPT) (Breslow et al., 2010; Han et al., 2010). Recent studies suggest that ORMDL3 also regulates ceramide biosynthesis in mammalian cells (Siow et al., 2015). Because ORMDL3 polymorphisms are associated with its high expression (Jin et al., 2012; Verlaan et al., 2009), it was suggested that this expression would correlate with decreased sphingolipid biosynthesis in asthma (Worgall et al., 2013). Yet elevations of sphingolipids rather than reductions have been linked to inflammatory responses in vitro. Additionally, elevated levels of ceramide and S1P have been reported in lung diseases, including asthma, thus presenting a biological paradox (Masini et al., 2007; Oskeritzian et al., 2007; Reinke et al., 2017). Work by our group and others imply that genetic regulation of sphingolipid homeostasis in asthma is much more complex than initially thought. We showed that although small increases in ORMDL3 expression decrease ceramide levels, in agreement with its evolutionary conserved role as a negative regulator of SPT, remarkably however, higher expression in lung epithelial cells and macrophages increased ceramide production by the recycling/salvage pathway (Oyeniran et al., 2015). While our group has focused on the ORMDL3 regulation of sphingolipid biosynthesis itself, other groups have highlighted a role for this protein in the unfolded protein response (Löser et al., 2017), airway remodeling (Cheng and Shang, 2017), and airway smooth muscle biology (Chen et al., 2018) in asthma, again emphasizing the enigmatic role that sphingolipids may play in the complex immunopathology of asthma.
1.6. Mast cells
First described in 1878 by Paul Ehrlich, mast cells (MC) are an important immune cell of the myeloid lineage. MCs are key sentinel cells in the lung that release a host of inflammatory mediators including vasoactive amines, lipids, proteoglycans, proteases, and cytokines upon activation. These MC mediators are the culprits for the outward appearances of allergic responses and can be attributed to the major clinical symptoms of asthma including increased vascular permeability, increased airway smooth muscle contractions, and increased mucus production (da Silva et al., 2014). MCs are most notable for their role in asthma given their primary mechanism of action: IgE mediated responses via FcER1, the high affinity IgE receptor. Upon crosslinking of this receptor, MCs degranulate to release preformed mediators such as histamine and become activated to induce the synthesis and release of neoformed mediators responsible for the late phase responses such as IL-4.
Aside from canonical IgE signaling, it is now appreciated that the MCs have a critical and multifaceted role in asthma immunopathology. MCs secrete a host of chemokines and cytokines that recruit effector cells into the lung such CXCL1 (De Filippo et al., 2013) and CCL11 (Hogaboam et al., 1998) to recruit neutrophils and eosinophils respectively. MCs can serve as antigen presenting cells to naïve T cells (Suurmond et al., 2013) and iNKT (Hong et al., 2014) cells in the microenvironment and enhance B cell IgE production via upregulation of CD40L to (Hong et al., 2013) to enhance local TH2 immune responses in the lung. Despite their fundamental role in asthma, effective therapies are lacking to specifically target MCs. Since MCs have historically been studied in the setting of IgE mediated allergic airway disease, Xolair (omalizumab), a monoclonal antibody that blocks IgE binding to its high affinity receptor has been developed and is now available clinically. It has shown promise in patients with severe asthma; however, unfortunately, initial enthusiasm regarding Xolair’s potential to reduce asthma symptomology has diminished due to its effects primarily on extreme cases of atopic asthma and its high cost (Normansell et al., 2014). Furthermore, recent research has highlighted roles for IgE-independent actions of MCs and the role of mast cells in innate immune responses; thus, examining multiple facets of MC function in the context of asthma may yield better therapeutic targets.
1.7. Mast cells and sphingolipids
The interplay between sphingolipid signaling and mast cell biology has long been appreciated, with the first report that sulfatides could be isolated from neoplastic mast cells in 1960 (GREEN & ROBINSON, 1960). Some 20 years later, Curtain et al. demonstrated that sensitizing rat peritoneal mast cells resulted in glycosphingolipid clustering in the plasma membrane which was critical for degranulation (Curtain et al., 1981). Katz et al. extended these studies to show that sphingolipid reorganization was not only a marker of mast cell activation but was useful in discerning subtypes of murine mast cells (Katz and Austen, 1986; Katz et al., 1985). It was later shown that the presence of these surface expressed sphingolipids could act of signaling receptors as well. Activation of gangliosides through binding of a specific monoclonal antibody, mab AA4, resulted in similar morphological and intracellular activation of MCs as compared to canonical IgE signaling (Oliver et al., 1992) and that these two signaling pathways shared Lyn as a common signaling intermediate (Minoguchi et al., 1994). While lipid moieties such as phosphoinositides and diacylglycerols had been implicated as second messengers in cell signaling, some of the first evidence that sphingolipid species could also act as signaling molecules in the immune system came in 1999. Choi et al. showed that FcER1 activation led to activation of SphK1 which in turn led to production of S1P as a primary mechanism for calcium mobilization in mast cells (Hong Choi, Kim and Kinet, 1996).
Sphk1 has been shown to be a fundamental kinase involved in MC biology. SphK1 rapidly phosphorylates sphingosine to form S1P and that S1P can either act as an intracellular switch for the activation of MCs or can be released from the cell to act extracellularly (Prieschl et al., 1999) via ATP binding cassette transporter 1 (ABCC1) (Mitra et al., 2006). With the discovery that S1P can bind a cluster of G protein coupled receptors on the cell surface of various cells (Lee et al., 1998) the concept of “inside-out” S1P signaling emerged (Takabe et al., 2008). S1P has been shown to transactivate MC S1P receptors 1 and 2 (S1PR1 and S1PR2) and be involved in MC migration and degranulation respectively (Jolly et al., 2004). Recent data has highlighted a role for SIPR4 in anaphylactic reactions (Kulinski et al., 2018), again highlighting the many roles of MCs and S1P signaling in immune responses.
While S1P has been shown to act as positive stimuli in terms of MC responses, ceramide and sphingosine function as negative regulators of MC biology, against reiterating the role of the “sphingolipid rheostat” in maintaining homeostasis. The addition of exogenous sphingosine diminished FcER1 mediated SphK1 activation (Prieschl et al., 1999) and along these lines it has been shown that sphingosine directly blocks calcium release-activated calcium current (ICRAC) in RBL cells (Mathes et al., 1998). Exogenous ceramide has been shown to induce MC apoptosis (Itakura et al., 2002), inhibit LPS induced cytokine production (Chiba et al., 2007), and negatively regulate mast cell activation (Izawa et al., 2012).
While clearly other cell types are involved in the immunopathology of asthma, the focus on MCs was shown as an exemplar for the complex role of sphingolipid biology in this disease (Fig. 1). Thus, better understanding of the role of sphingolipid biology in mast cell responses may provide valuable insight into the development of novel therapeutics for disease management.
Fig. 1.
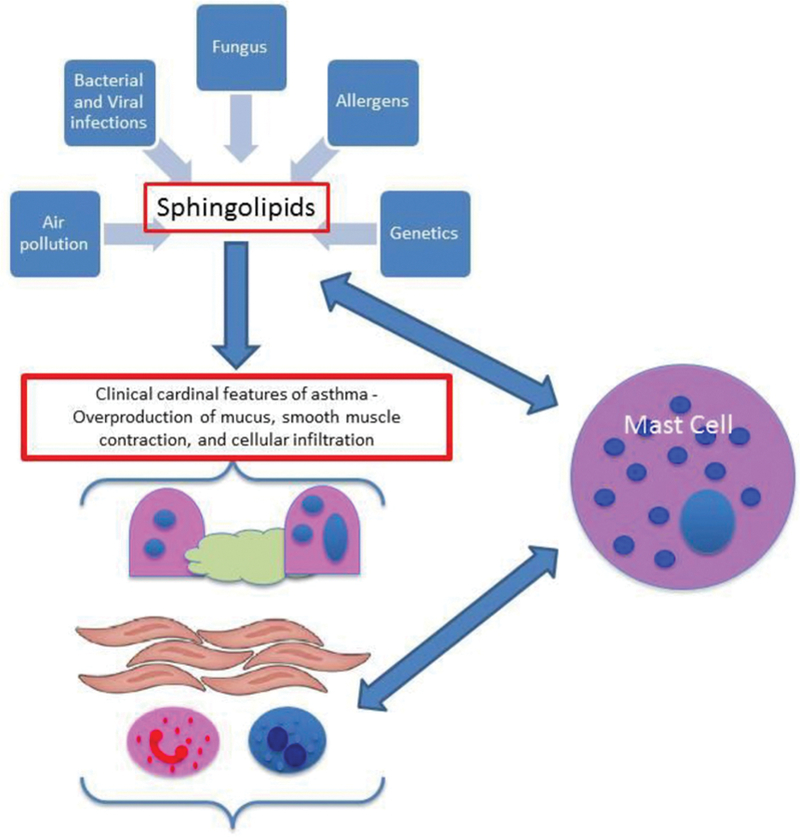
Model.
1.8. Targeting sphingolipids for disease control
While the majority of the data highlighted in this review show a correlative association amongst sphingolipids and asthma disease processes, there is also compelling evidence that sphingolipids exert a direct causative effect in the lung. Both the direct administration of either S1P (Ammit et al., 2001; Fuerst et al., 2014) or ceramide (Masini et al., 2007) induce airway smooth muscle contraction.
The utility of sphingolipid species as viable biomarkers is also an active area of investigation. The Spiegel laboratory has shown that both S1P (Rosenfeldt et al., 2003) and ceramide (Oyeniran et al., 2015) are elevated in the airway of patients with asthma. However, these studies did not take into account endotypes of asthma nor were validated biomarkers of disease such as periostin or FeNO assessed. Other groups have shown plasma S1P to be an asthma biomarker associated with adult disease control (McGeachie et al., 2015) and severity (Reinke et al., 2017). One severe endotype of asthma is aspirin exacerbated respiratory disease (AERD). Recently Trihn et al. demonstrated that S1P is a potential biomarker for AERD (Trinh et al., 2016). Additionally sphingolipid levels in the serum of pediatric patients have also been examined and illustrate an upregulation of various ceramide species associated with childhood asthma (Perzanowski et al., 2017). Taken together, these highlight the importance of better understanding of the role of sphingolipid biology in specific asthma endotypes.
Lastly there have been several studies in animal models that suggest pharmacologic targeting of sphingolipid species may hold promise as a novel class of asthma therapeutics. The use of myriocin, which inhibits SPT, has shown mixed responses in the literature. There have been reports that myriocin exacerbates AHR both in the presence (Edukulla et al., 2016) and absence (Worgall et al., 2013) of allergen challenge. On the other hand Oyeniran et al. showed a significant abrogation of HDM mediated allergic airway disease specifically in relation to AHR (Oyeniran et al., 2015). Part of the discrepancies across these studies could be related to pharmacokinetics of the drug itself and/or sphingolipid signaling. Oyeniran et al. utilized the inhibitor only in the later stages of allergen challenge and the drug was administered I.P. due to drug solubility issues. The two prior studies administered myriocin directly into the lung parenchyma and at different dosing strategies. Thus, targeting SPT as a viable mechanism of asthma control will need further investigation before these findings can be translated from mouse to man.
On the other hand, greater pharmacologic success has been found in relation to targeting either SphKs or S1P itself. FTY720 is a derivative of myriocin and a structural analog of sphingosine. It is a prodrug that is phosphorylated upon entry into the cell by SphK. It is this phosphorylated form that acts as a potent agonist of SIPRs. However newer studies have also shown that FTY720 may exert pleotropic effects on cellular responses by inhibiting ceramide synthases (Berdyshev et al., 2009) or acting as a histone deacyltase inhibitor (N C Hait et al., 2015). Intranasal administration of FTY720 has shown to alter dendritic cell function (Idzko et al., 2006), inhibit airway remodeling (Karmouty-Quintana et al., 2012), and dampen both inflammation and AHR (Oyeniran et al., 2015) in animal models of disease. On the other hand, targeting the kinase responsible for S1P generation has also shown promise. Using a specific inhibitor of SphK1 blocked both IgE production from human and murine B cells (Kim et al., 2014) as well as attenuated MC mediated AHR in vivo (Price et al., 2013).
2. Conclusions
Despite advances in our understanding of disease immunopathology, asthma remains a significant public health burden and a cause for morbidity and mortality worldwide. Sphingolipids play an important role in the susceptibility, initiation, and exacerbation of asthma and may be a key biomarker for identification of certain endotypes and a target for drug discovery. While this review focused on sphingolipid biology in the context of mast cells, sphingolipid have also been appreciated to play a critical role in other cellular mediators of asthma such as T cells, B cells, and airway smooth muscle cells thus better understanding of the complex role of sphingolipids in asthma may prove to yield effective therapeutics that target many facets of disease.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors for the compilation of this review article. This research is supported by National Institutes of Health grant R01AI50094 awarded to Sarah Spiegel.
Footnotes
Disclosures
None to report.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jbior.2018.09.001.
References
- Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, et al. , 2001. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB (Fed. Am. Soc. Exp. Biol.) J.: Off. Pub. Fed. Am. Soc. Exp. Biol 15 (7), 1212–1214. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/11344091 [DOI] [PubMed] [Google Scholar]
- Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, et al. , 2009. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J. Biol. Chem 284 (9), 5467–5477. 10.1074/jbc.M805186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodas M, Min T, Vij N, 2015. Lactosylceramide-accumulation in lipid-rafts mediate aberrant-autophagy, inflammation and apoptosis in cigarette smoke induced emphysema. Apoptosis 20 (5), 725–739. 10.1007/s10495-015-1098-0. [DOI] [PubMed] [Google Scholar]
- Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, et al. , 2010. Orm family proteins mediate sphingolipid homeostasis. Nature 463 (7284), 1048–1053. 10.1038/nature08787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse WW, Lemanske RF, Gern JE, Gern JE, 2010. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 376 (9743), 826–834. 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Miller M, Unno H, Rosenthal P, Sanderson MJ, Broide DH, 2018. Orosomucoid-like 3 (ORMDL3) upregulates airway smooth muscle proliferation, contraction, and Ca 2+ oscillations in asthma. J. Allergy Clin. Immunol 142 (1), 207–218. e6. 10.1016/j.jaci.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Shang Y, 2017. ORMDL3 may participate in the pathogenesis of bronchial epithelial-mesenchymal transition in asthmatic mice with airway remodeling. Mol. Med. Rep 17 (1), 995–1005. 10.3892/mmr.2017.7972. [DOI] [PubMed] [Google Scholar]
- Chiba N, Masuda A, Yoshikai Y, Matsuguchi T, 2007. Ceramide inhibits LPS-induced production of IL-5, IL-10, and IL-13 from mast cells. J. Cell. Physiol 213 (1), 126–136. 10.1002/jcp.21101. [DOI] [PubMed] [Google Scholar]
- Curtain C, Looney FD, Smelstorius JA, 1981. Glycosphingolipid clustering and mast cell degranulation. Int. Arch. Allergy Appl. Immunol 65 (1), 34–41. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/6260692. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind JS, Spiegel S, 1996. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381 (6585), 800–803. 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- da Silva EZM, Jamur MC, Oliver C, 2014. Mast cell function: a new vision of an old cell. J. Histochem. Cytochem.: Off. J. Histochem. Soc 62 (10), 698–738. 10.1369/0022155414545334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveaux JI, Lemanske RF Jr, 2014. Infection-related asthma. The J. Allergy Clin. Immunol. In Practice 2 (6), 658–663. 10.1016/j.jaip.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Alba J, Raemdonck K, Dekkak A, Collins M, Wong S, Nials AT, et al. , 2010. House dust mite induces direct airway inflammation in vivo: implications for future disease therapy? Eur. Respir. J 35 (6), 1377–1387. 10.1183/09031936.00022908. [DOI] [PubMed] [Google Scholar]
- De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. , 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121 (24), 4930–4937. 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- Edukulla R, Rehn KL, Liu B, McAlees JW, Hershey GK, Wang YH, et al. , 2016. Intratracheal myriocin enhances allergen-induced Th2 inflammation and airway hyper-responsiveness. Immun. Inflamm. Dis 4 (3), 248–262. 10.1002/iid3.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst E, Foster HR, Ward JPT, Corrigan CJ, Cousins DJ, Woszczek G, 2014. Sphingosine-1-phosphate induces pro-remodelling response in airway smooth muscle cells. Allergy 69 (11), 1531–1539. 10.1111/all.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, Platt FM, 2017. The metabolism of glucocerebrosides — from 1965 to the present. Mol. Genet. Metabol 120 (1–2), 22–26. 10.1016/j.ymgme.2016.11.390. [DOI] [PubMed] [Google Scholar]
- Galanter J, Choudhry S, Eng C, Nazario S, Rodríguez-Santana JR, Casal J, et al. , 2008. ORMDL3 gene is associated with asthma in three ethnically diverse populations. Am. J. Respir. Crit. Care Med 177 (11), 1194–1200. 10.1164/rccm.200711-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmé H, Riehle A, Wilker B, Gulbins E, 2005. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J. Biol. Chem 280 (28), 26256–26262. 10.1074/jbc.M500835200. [DOI] [PubMed] [Google Scholar]
- Green JP, Robinson JD, 1960. Cerebroside sulfate (sulfatide A) in some organs of the rat and in a mast cell tumor. J. Biol. Chem 235, 1621–1624. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/13851505. [PubMed] [Google Scholar]
- Gregory LG, Lloyd CM, 2011. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri M, Balmes JR, 2014. Outdoor air pollution and asthma. Lancet 383 (9928), 1581–1592. 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Avni D, Yamada A, Nagahashi M, Aoyagi T, Aoki H, et al. , 2015. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERα expression and enhances hormonal therapy for breast cancer. Oncogenesis 4 (6) e156–e156. 10.1038/oncsis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Maiti A, 2017. The role of sphingosine-1-phosphate and ceramide-1-phosphate in inflammation and cancer. Mediat. Inflamm 2017, 1–17. 10.1155/2017/4806541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Lone MA, Schneiter R, Chang A, 2010. Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proc. Natl. Acad. Sci. U.S.A 107 (13), 5851–5856. 10.1073/pnas.0911617107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM, 2002. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem 277 (29), 25847–25850. 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- Harlan WR, Margraf J, Said S, 1966. Pulmonary lipid composition of species with and without surfactant. Am. J. Physiol.-Legacy Content 211 (3), 855–861. 10.1152/ajplegacy.1966.211.3.855. [DOI] [PubMed] [Google Scholar]
- Hogaboam C, Kunkel SL, Strieter RM, Taub DD, Lincoln P, Standiford TJ, Lukacs NW, 1998. Novel role of transmembrane SCF for mast cell activation and eotaxin production in mast cell-fibroblast interactions. J. Immunol 160 (12), 6166–6171. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/9637535. [PubMed] [Google Scholar]
- Hong Choi O, Kim J-H, Kinet J-P, 1996. Calcium mobilization via sphingosine kinase in signalling by the FcɛRI antigen receptor. Nature 380 (6575), 634–636. 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- Hong GU, Kim NG, Kim TJ, Ro JY, 2014. CD1d expressed in mast cell surface enhances IgE production in B cells by up-regulating CD40L expression and mediator release in allergic asthma in mice. Cell. Signal 26 (5), 1105–1117. 10.1016/j.cellsig.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Hong GU, Park BS, Park JW, Kim SY, Ro JY, 2013. IgE production in CD40/CD40L cross-talk of B and mast cells and mediator release via TGase 2 in mouse allergic asthma. Cell. Signal 25 (6), 1514–1525. 10.1016/j.cellsig.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Idzko M, Hammad H, Nimwegen M. van, Kool M, Müller T, Soullié T, et al. , 2006. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J. Clin. Invest 116 (11), 2935–2944. 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck T, 2016. Lipids in pollen — they are different. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1861 (9), 1315–1328. 10.1016/J.BBALIP.2016.03.023. [DOI] [PubMed] [Google Scholar]
- Itakura A, Tanaka A, Aioi A, Tonogaito H, Matsuda H, 2002. Ceramide and sphingosine rapidly induce apoptosis of murine mast cells supported by interleukin-3 and stem cell factor. Exp. Hematol 30 (3), 272–278. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/11882365. [DOI] [PubMed] [Google Scholar]
- Iwamura C, Nakayama T, 2010. Role of NKT cells in allergic asthma. Curr. Opin. Immunol 22 (6), 807–813. 10.1016/j.coi.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Izawa K, Yamanishi Y, Maehara A, Takahashi M, Isobe M, Ito S, et al. , 2012. The receptor LMIR3 negatively regulates mast cell activation and allergic responses by binding to extracellular ceramide. Immunity 37 (5), 827–839. 10.1016/j.immuni.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Jin R, Xu H-G, Yuan W-X, Zhuang L-L, Liu L-F, Jiang L, et al. , 2012. Mechanisms elevating ORMDL3 expression in recurrent wheeze patients: role of Ets-1, p300 and CREB. Int. J. Biochem. Cell Biol 44 (7), 1174–1183. 10.1016/j.biocel.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, et al. , 2004. Transactivation of sphingosine-1–phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J. Exp. Med 199 (7), 959–970. 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmouty-Quintana H, Siddiqui S, Hassan M, Tsuchiya K, Risse P-A, Xicota-Vila L, et al. , 2012. Treatment with a sphingosine-1-phosphate analog inhibits airway remodeling following repeated allergen exposure. Am. J. Physiol. Lung Cell Mol. Physiol 302 (8), L736–L745. 10.1152/ajplung.00050.2011. [DOI] [PubMed] [Google Scholar]
- Katz HR, Austen KF, 1986. Plasma membrane and intracellular expression of globotetraosylceramide (globoside) in mouse bone marrow-derived mast cells. J. Immunol 136 (10), 3819–3824. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/3517161. [PubMed] [Google Scholar]
- Katz HR, Schwarting GA, LeBlanc PA, Austen KF, Stevens RL, 1985. Identification of the neutral glycosphingolipids of murine mast cells: expression of Forssman glycolipid by the serosal but not the bone marrow-derived subclass. J. Immunol 134 (4), 2617–2623. Retrieved: from. http://www.ncbi.nlm.nih.gov/pubmed/3871816. [PubMed] [Google Scholar]
- Kim EY, Sturgill JL, Hait NC, Avni D, Valencia EC, MacEyka M, et al. , 2014. Role of sphingosine kinase 1 and sphingosine-1-phosphate in CD40 signaling and IgE class switching. FASEB (Fed. Am. Soc. Exp. Biol.) J 28 (10), 4347–4358. 10.1096/fj.14-251611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulinski J, Proia R, Larson E, Metcalfe D, Olivera A, 2018. S1P4 regulates passive systemic anaphylaxis in mice but is dispensable for canonical IgE-mediated responses in mast cells. Int. J. Mol. Sci 19 (5), 1279 10.3390/ijms19051279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, et al. , 1998. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279 (5356), 1552–1555. 10.1126/SCIENCE.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Levy M, Khan E, Careaga M, Goldkorn T, 2009. Neutral sphingomyelinase 2 is activated by cigarette smoke to augment ceramide-induced apoptosis in lung cell death. Am. J. Physiol. Lung Cell Mol. Physiol 297 (1), L125–L133. 10.1152/ajplung.00031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löser S, Gregory LG, Zhang Y, Schaefer K, Walker SA, Buckley J, et al. , 2017. Pulmonary ORMDL3 is critical for induction of Alternaria -induced allergic airways disease. J. Allergy Clin. Immunol 139 (5), 1496–1507. e3. 10.1016/j.jaci.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini E, Giannini L, Nistri S, Cinci L, Mastroianni R, Xu W, et al. , 2007. Ceramide: a key signaling molecule in a Guinea pig model of allergic asthmatic response and airway inflammation. J. Pharmacol. Exp. Therapeut 324 (2), 548–557. 10.1124/jpet.107.131565. [DOI] [PubMed] [Google Scholar]
- Mathes C, Fleig A, Penner R, 1998. Calcium release-activated calcium current (ICRAC) is a direct target for sphingosine. J. Biol. Chem 273 (39), 25020–25030. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/9737958. [DOI] [PubMed] [Google Scholar]
- Matthews LW, Spector S, Lemm J, Potter j. L., 1963. Studies on pulmonary secretions. i. the over-all chemical composition of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. Am. Rev. Respir. Dis 88, 199–204. 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- McGeachie MJ, Dahlin A, Qiu W, Croteau-Chonka DC, Savage J, Wu AC, et al. , 2015. The metabolomics of asthma control: a promising link between genetics and disease. Imm. Inflamm. Dis 3 (3), 224–238. 10.1002/iid3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoguchi K, Swaim WD, Berenstein EH, Siraganian RP, 1994. Src family tyrosine kinase p53/56lyn, a serine kinase and Fc epsilon RI associate with alpha-galactosyl derivatives of ganglioside GD1b in rat basophilic leukemia RBL-2H3 cells. J. Biol. Chem 269 (7), 5249–5254. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/8106508. [PubMed] [Google Scholar]
- Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S, 2006. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl. Acad. Sci. Unit. States Am 103 (44), 16394–16399. 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. , 2010. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med 363 (13), 1211–1221. 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. , 2007. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 448 (7152), 470–473. 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- Monick MM, Cameron K, Powers LS, Butler NS, McCoy D, Mallampalli RK, Hunninghake GW, 2004. Sphingosine kinase mediates activation of extracellular signal–related kinase and Akt by respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol 30 (6), 844–852. 10.1165/rcmb.2003-0424OC. [DOI] [PubMed] [Google Scholar]
- Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. , 2010. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am. J. Respir. Crit. Care Med 181 (4), 315–323. 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normansell R, Walker S, Milan SJ, Walters EH, Nair P, 2014. Omalizumab for asthma in adults and children. Cochrane Database Syst. Rev (1), CD003559. 10.1002/14651858.CD003559.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA, 1993. Programmed cell death induced by ceramide. Science 259 (5102), 1769–1771. Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/8456305. [DOI] [PubMed] [Google Scholar]
- Oliver C, Sahara N, Kitani S, Robbins AR, Mertz LM, Siraganian RP, 1992. Binding of monoclonal antibody AA4 to gangliosides on rat basophilic leukemia cells produces changes similar to those seen with Fc epsilon receptor activation. J. Cell Biol 116 (3), 635–646. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/1370498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Spiegel S, 1993. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 365 (6446), 557–560. 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Oskeritzian CA, Milstien S, Spiegel S, 2007. Sphingosine-1-phosphate in allergic responses, asthma and anaphylaxis. Pharmacol. Therapeut 115 (3), 390–399. 10.1016/j.pharmthera.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyeniran C, Sturgill JL, Hait NC, Huang W-C, Avni D, Maceyka M, et al. , 2015. Aberrant ORM (yeast)-like protein isoform 3 (ORMDL3) expression dysregulates ceramide homeostasis in cells and ceramide exacerbates allergic asthma in mice. J. Allergy Clin. Immunol 136 (4). 10.1016/j.jaci.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perzanowski MS, Ono JG, Acosta LM, Kim BI, Divjan A, Miller R, et al. , 2017. Distinct serum sphingolipid profiles among school-aged children with exercise-induced wheeze and asthma persistence. Am. J. Respir. Crit. Care Med 195 (8), 1068–1070. 10.1164/rccm.201609-1884LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV, 2014. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J. Allergy Clin. Immunol 133 (2), 388–394. 10.1016/j.jaci.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuschel H, Sydlik U, Grether-Beck S, Felsner I, Stöckmann D, Jakob S, et al. , 2012. Carbon nanoparticles induce ceramide-and lipid raft-dependent signalling in lung epithelial cells: a target for a preventive strategy against environmentally-induced lung inflammation. Part. Fibre Toxicol 9, 48 10.1186/1743-8977-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MM, Oskeritzian CA, Falanga YT, Harikumar KB, Allegood JC, Alvarez SE, et al. , 2013. A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell–dependent murine model of allergic asthma. J. Allergy Clin. Immunol 131 (2), 501–511. e1. 10.1016/j.jaci.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieschl EE, Csonga R, Novotny V, Kikuchi GE, Baumruker T, 1999. The balance between sphingosine and sphingosine-1-phosphate is decisive for mast cell activation after Fc epsilon receptor I triggering. J. Exp. Med 190 (1), 1–8. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/10429665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke SN, Gallart-Ayala H, Gómez C, Checa A, Fauland A, Naz S, et al. , 2017. Metabolomics analysis identifies different metabotypes of asthma severity. Eur. Respir. J 49 (3), 1601740 10.1183/13993003.01740-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA, Spiegel S, 2003. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. Faseb. J 17 (13), 1789–1799. 10.1096/fj.02-0836com. [DOI] [PubMed] [Google Scholar]
- Sahu S, Lynn WS, 1977. Lipid composition of airway secretions from patients with asthma and patients with cystic fibrosis. Am. Rev. Respir. Dis 115 (2), 233–239. 10.1164/arrd.1977.115.2.233. [DOI] [PubMed] [Google Scholar]
- Scherzer R, Grayson MH, 2018. Heterogeneity and the origins of asthma. Ann. Allergy Asthma Immunol 10.1016/j.anai.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer KS, Chen SX, Law S, Van Demark M, Poirier C, Justice MJ, et al. , 2015. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am. J. Physiol. Lung Cell Mol. Physiol 309 (2), L175–L187. 10.1152/ajplung.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen HM, Onoda A, Shinkai Y, Nakamura M, El-Ghoneimy AA, El-Sayed YS, et al. , 2016. The ceramide inhibitor fumonisin B1 mitigates the pulmonary effects of low-dose diesel exhaust inhalation in mice. Ecotoxicol. Environ. Saf 132, 390–396. 10.1016/j.ecoenv.2016.06.025. [DOI] [PubMed] [Google Scholar]
- Sharma L, Prakash H, 2017. Sphingolipids are dual specific drug targets for the management of pulmonary infections: perspective. Front. Immunol 8, 378 10.3389/fimmu.2017.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow D, Sunkara M, Morris A, Wattenberg B, 2015. Regulation of de novo sphingolipid biosynthesis by the ORMDL proteins and sphingosine kinase-1. Ad. Biol. Reg 57, 42–54. 10.1016/J.JBIOR.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S, 2011. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol 11 (6), 403–415. 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue RJ, Brown G, Rixon HWM, 2002. Respiratory syncytial virus assembly occurs in GM1-rich regions of the host-cell membrane and alters the cellular distribution of tyrosine phosphorylated caveolin-1. J. Gen. Virol 83 (8), 1841–1850. 10.1099/0022-1317-83-8-1841. [DOI] [PubMed] [Google Scholar]
- Suurmond J, van Heemst J, van Heiningen J, Dorjée AL, Schilham MW, van der Beek FB, et al. , 2013. Communication between human mast cells and CD4 + T cells through antigen-dependent interactions. Eur. J. Immunol 43 (7), 1758–1768. 10.1002/eji.201243058. [DOI] [PubMed] [Google Scholar]
- Takabe K, Paugh SW, Milstien S, Spiegel S, 2008. Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev 60 (2), 181–195. 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannhauser SJ, Penotti J, Boncoddo NF, 1946. Isolation and properties of hydrolecithin (dipalmityl lecithin) from lung; its occurrence in the sphingomyelin fraction of animal tissues. J. Biol. Chem 166 (2), 669–675. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/20276181. [PubMed] [Google Scholar]
- Thomson NC, 2004. Asthma and cigarette smoking. Eur. Respir. J 24 (5), 822–833. 10.1183/09031936.04.00039004. [DOI] [PubMed] [Google Scholar]
- Thudichum JLW (n.d.). A treatise on the chemical constitution of the brain: J. L. W Thudichum: Amazon.com: Books. Retrieved from: https://www.amazon.com/treatise-chemical-constitution-brain/dp/B0007E4IMK. [Google Scholar]
- Trinh HKT, Kim S-C, Cho K, Kim S-J, Ban G-Y, Yoo H-J, et al. , 2016. Exploration of the sphingolipid metabolite, sphingosine-1-phosphate and sphingosine, as novel biomarkers for aspirin-exacerbated respiratory disease. Sci. Rep 6 (1), 36599 10.1038/srep36599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercelli D, 2008. Discovering susceptibility genes for asthma and allergy. Nat. Rev. Immunol 8 (3), 169–182. 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- Vercelli D, 2016. A virtuous duplicity: 17q21 variants at the intersection between asthma protection and risk. Am. J. Respir. Crit. Care Med 193 (8), 821–822. 10.1164/rccm.201511-2265ED. [DOI] [PubMed] [Google Scholar]
- Verlaan DJ, Berlivet S, Hunninghake GM, Madore A-M, Larivière M, Moussette S, et al. , 2009. Allele-specific chromatin remodeling in the ZPBP2/GSDMB/ ORMDL3 locus associated with the risk of asthma and Autoimmune disease. Am. J. Hum. Genet 85 (3), 377–393. 10.1016/j.ajhg.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan M, Hahm B, 2014. Influenza viral manipulation of sphingolipid metabolism and signaling to modulate host defense system. Scientifica 2014, 793815 10.1155/2014/793815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worgall TS, Veerappan A, Sung B, Kim BI, Weiner E, Bholah R, et al. , 2013. Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci. Transl. Med 5 (186) 186ra67–186ra67. 10.1126/scitranslmed.3005765. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Brunst KJ, 2013. Programming of respiratory health in childhood. Curr. Opin. Pediatr 25 (2), 232–239. 10.1097/MOP.0b013e32835e78cc. [DOI] [PubMed] [Google Scholar]
- Yu Y, Sun G, Liu G, Wang Y, Shao Z, Chen Z, Yang J, 2009. Effects of Mycoplasma pneumoniae infection on sphingolipid metabolism in human lung carcinoma A549 cells. Microb. Pathog 46 (2), 63–72. 10.1016/j.micpath.2008.10.014. [DOI] [PubMed] [Google Scholar]


