Abstract
Objective:
Genetic variation in the first intron of FTO (e.g., SNP rs9939609) is strongly associated with adiposity. This effect is thought to be mediated – at least in part - via increasing caloric intake, although the precise molecular genetic mechanisms are not fully understood. Prior pediatric studies of FTO have included youth with overweight and obesity; however, they do not inform whether a genotypic effect on ingestive behavior is present prior to obesity onset.
Methods:
We therefore investigated the association between FTO and caloric intake in children aged 5-10 without obesity (adiposity ≤ 95%ile).
Results:
122 children were genotyped for rs9939609 and ate ad libitum from a laboratory lunch buffet following a standardized breakfast. Linear regressions adjusting for body mass were used to examine the association between FTO “dose” (number of copies of snp rs9939609) and intake variables. There was a significant association between FTO and total intake. Each risk allele predicted an additional 64 calories, accounting for 3% of the variance. There were no associations between FTO and macronutrient preference, energy density, or diet variety. Results were influenced by race.
Conclusion:
Results corroborate and extend prior work by showing a dose-dependent effect on food intake in children without obesity.
Keywords: pediatric obesity, FTO, eating behavior
Introduction
Obesity is a complex disease reflecting interactions between an increasingly permissive environment (more calorically dense food and sedentary lifestyle availabilities) and multiple known obesity-risk allelic variants (1). Obesity currently affects approximately 35% of adults, and nearly 20% of children in the United States (2). The increasing prevalence of obesity (2, 3) and the parallel increases in adiposity-related co-morbdities (4, 5) disprortionately affect African American, Asian, Hispanic, and Native American populations (6, 7).
Although body fatness (adiposity) is a continuous quantitative trait reflecting the interaction of environment, genotype, and development (8), the biological and behavioral mechanisms by which genetic factors produce positive energy balance (weight gain) may be less evident at maximal adiposity (energy balance) (9-12). Therefore, studying eating behavior in youth who are already overweight may not capture the behaviors that are premonitory of excess weight gain. Relatedly, studies of the causes of weight gain in a mixed population of individuals with and without obesity may be confounded by the metabolic effects (e.g., hyperinsulinemia) of excess weight (9, 12). Studying children at varying risk for subsequent weight gain and who do not yet have obesity is one strategy for addressing these confounders.
Family and twin studies suggest that body mass index (BMI, kg/m2) is 40–70% heritable (13), yet genome-wide association studies (GWAS) for BMI have identified only a small fraction of the implied genetic substrate for human obesity (1). In GWAS studies, rs9939609 in the first intron of FTO on chromosome 16p12.2 accounts for 0.34% of BMI variance, (14) with each minor allele associated with +0.39 BMI units and 1.20 fold greater risk of obesity (1). Approximately 14–18% of the population is homozygous for the obesity risk allele (AA) of rs9939609, 29–50% is heterozygous (AT) and 30–35% is homozygous for the T allele (15). These effect sizes are small but constitute the largest and statistically most significant GWAS signal for adiposity and represent a common quantifiable genetic risk factor for subsequent weight gain.
The most common behavioral phenotype associated with relevant FTO snps in adults and children is increased caloric intake (1, 15) (16), though one pediatric laboratory meal study reported that the FTO snp rs9939609 was associated with increased preference for calorically dense (higher fat) foods without a significant increase in total caloric intake (17). A limitation common to each of these studies was the inclusion of overweight and obese children which may have diminished the sensitivity to detect gene effects predisposing to subsequent weight gain.
The aim of the current study was to examine the association between FTO snp rs9939609 and total calorie intake during a laboratory lunch meal in children 5–10 years old whose BMI for age and sex was between the 15th – 95th percentile. We hypothesized a positive dose-dependent relationship (AA>AT>TT) between number of copies of the A allele of rs9939609 and caloric intake and proportional selection of calorie-dense, high fat foods during the laboratory lunch meal. Although prior studies of FTO and intake in children examined a dominant model (two-group, A/X > TT), mounting evidence suggest that an additive model (three group, AA > AT > TT) may more accurately describe the association between FTO rs9939609 and adiposity in children (18). Finally, data suggest that rs9939609 genotype is not associated with adiposity in individuals of African descent (19), thus, we also conducted an exploratory analysis of the associations between genotype and intake in non-African American children.
Methods
Participants
Participants were recruited via print and online advertisements targeting parents of 5 – 10 year-old children in the greater New York City metropolitan area. Children whose parent-reported BMI (by telephone) was near or below the 95th percentilewere invited for further screening. Exclusion criteria included medical conditions known to affect eating behavior (e.g., diabetes, eating disorders), regular use of medication known to affect eating behavior (e.g., anorexiants, catecholamines, corticosteroids), severe food allergy, or disliking ≥ 50% of foods presented during the laboratory meal (17). Because BMI is considered an imprecise indicator of adiposity (20), participants with body fat < 95th percentile by bioimpedance were included and those with body fat < 95th percentile were excluded regardless of BMI.
All participants and their parents signed assent and consent forms respectively after reviewing the study with a PhD or MD clinician (AB, LESM, LMR) with ample opportunity to ask questions. The study was approved by the Institutional Review Board at the New York State Psychiatric Institute/Columbia University Department of Psychiatry.
Procedures
Parents of potential participants were provided a brief description of the study and asked a series of preliminary screening questions to determine eligibility (e.g., age, height, weight, and presence of major medical or psychiatric illness). Families who were both interested and potentially eligible were scheduled to attend an in-person evaluation.
During the initial visit (Visit 1), height, weight and body composition were measured by a research assistant (see below). Parent-child dyads participated in a clinical interview with an MD- or PhD-clinician in which general medical, psychiatric, and dietary (food allergies and restrictions) histories were obtained. To obtain material for genotyping, children were offered lollipops and then asked to spit into a salivette saliva collection tube, collecting at least 2 ml of saliva. Finally, to ensure that a minimum number of foods presented during the laboratory meal would be acceptable to the child, children were asked to provide verbal liking ratings for a series of foods, including those presented during the meal. If a child was still eligible after review of screening data, the child and parent were invited to return to the clinic to complete the laboratory test meal.
For the study day (Visit 2), parents were told that children should not eat or drink anything except for water after 10 PM the night before. Upon arrival to the clinic in the morning, the child’s weight and height were measured. Children were then provided a standardized breakfast consisting of cereal (Cheerios™ or Kellogg’s Cornflakes®) and whole milk. Portion size was adjusted for calculated energy expenditure (see below) and was consumed in 10 minutes or less. Children remained in the clinic waiting area or general hospital vicinity during the 3 ½ hours between breakfast and lunch; parents were instructed that their children must refrain from having anything to eat or drink during this time, except for water.
Approximately ten minutes before the lunch meal, families were brought to the Eating Behavior Laboratory of the Research Nutrition Kitchen on the Biological Studies Unit of NYSPI. While seated at the table, but prior to eating, children were instructed “This is your lunch for today. You may eat as much as you would like, but you do not have to eat anything you do not like.” For all participants, a series of cartoons were played on a portable DVD player during the meal to address the concern that the young children might feel anxious about eating alone in a novel environment and to help children feel more comfortable and complete the meal.
Children had up to 60 minutes to eat, and they were asked to indicate when they were finished eating by pressing a doorbell that was placed on the table. The meal was monitored by research staff via closed-circuit television to ensure that the child was safe (e.g., not choking) and did not dispose of food by any means other than eating it (e.g., putting food in his/her pockets).
Measures
Anthropometrics
Weight was measured to the nearest 0.1 pound using a calibrated scale (Detecto); height was measured to the nearest 0.1 inch using a calibrated wall-mount mechanical stadiometer (Seca). Body fat percentage was measured by bioimpedance using a single-frequency pediatric Tanita body fat scale (Model BF-689). BMI, BMI percentile, and Z-scores were calculated according to CDC growth charts (21). Adiposity percentiles were calculated according to published norms for U.S. children (22).
Genotyping
Saliva was collected and DNA was extracted using DNA Genotek™ kit. Children were genotyped for the A/T rs9939609 SNP of FTO by the pyrosequencing (PSQ96 Biotage, LLC. Westborough, MA). PCR reactions consisted of 6 pmol of each of the appropriate forward and reverse primer, 0.75 U GoTaq, 1xGoTaq buffer, 0.2 mM dNTP’s and 50ng of genomic DNA in a 30 μλ reaction volume for 35 cycles at an annealing temperature of 50°C.
Total intake and macronutrient composition
Breakfast:
On the morning of testing, children were asked to consume a standardized breakfast equivalent to 20% of their estimated daily energy requirement (EER), calculated based upon age, height and weight using equations from the Institute of Medicine Dietary Reference Intakes (23). Breakfast food items were weighed before and after consumption in order to calculate caloric intake. After the first year of the study, it was noted that the majority of children were unable to complete breakfast, consuming 12.34±5.36% of EER; therefore, an alteration was made to the procedure such that subsequent participants were served 10% of their EER. Children were instructed not to eat or drink anything other than water between breakfast and lunch.
Lunch:
The laboratory lunch meal consisted of 28 food and beverage items thought to be palatable to children, such as traditional lunch entrees (e.g., items for making a sandwich, chicken nuggets) sides (e.g., fruits and vegetables, salty snacks and desserts), and beverages (Figure S1). The protocol was adapted from prior laboratory meal studies effectively carried out in children ages 6–12 (24). All foods were of known macronutrient composition and were weighed on an electronic balance to the nearest gram pre- and post-meal. A Diet Energy Density score (DEDS), defined as intake (kcal) divided by weight (g) of food and beverage consumed, was calculated. A Diet Variety Score (DVS) was also calculated, consisting of the total number of different caloric foods and beverages eaten during the meal (25). Duration of eating was measured from the time the door of the eating lab closed until the child pressed the doorbell indicating (s)he had finished eating.
Race/Ethnicity
Participants’ parents were asked to report their child’s race (Black/African American, Caucasian, Asian, “Other”) and ethnicity (Hispanic or Non-Hispanic) separately, per the existing National Institute of Health recommendation at the time the project was initiated (26).
Analyses
Descriptive statistics
Descriptive statistics were used to examine baseline characteristics. Data were screened for normality. There were no influential outliers. Demographic and anthropometric characteristics of children by genotype were compared using Chi-Square (for categorical variables including sex, race, and ethnicity) and ANOVAs (for continuous variables including age, height, weight, BMI indices, and body fat). Body composition was measured at the evaluation visit only, and therefore, analyses that included fat free mass are based on the child’s anthropometrics measured during the evaluation visit.
Primary analyses
Multiple linear regressions were used to evaluate the associations between FTO genotype at rs9939609 and total caloric intake and proportional fat intake during the lunch meal. The primary independent variable was dose of the ‘A’ allele (0, 1 or 2 copies). Covariates considered for the primary analyses were age, sex, race (White, Black, Asian, Other, not reported), ethnicity (Hispanic, non-Hispanic), body weight, fat free mass, and calories consumed during breakfast. A linear regression (for continuous variables) or a univariate ANOVA (for categorical variables) was conducted for each covariate to test for significant associations between the respective independent variable and total caloric intake. In the final models, only variables that were individually associated with intake were considered as covariates. To address multicollinearity of predictor variables, variable pairs that were highly correlated with each other (e.g., age and height) were not included in the same model.
Secondary analyses
If there was a significant association between genotype and total caloric intake, the relationship between genotype and additional intake variables (percentage intake from carbohydrate and protein, diet energy density, diet variety score) was examined in secondary analyses to elucidate potential mechanisms for increased caloric intake. In analyses of macronutrient composition, total energy intake was included as a covariate.
Analyses of Race/Ethnicity
In exploratory analyses of race/ethnicity, the effect of genotype on intake was examined in children with parent-reported child race of non-African American.
Analyses were done using Statistical Package for the Social Sciences (SPSS) version 22.0 (IBM corp). Means±SD are presented. Statistical significance was prospectively defined as Pα≤0.05. All p values greater than 0.06 but less than 0.10 were considered marginal and are noted as results that might be the focus of future investigations.
Results
Descriptive data
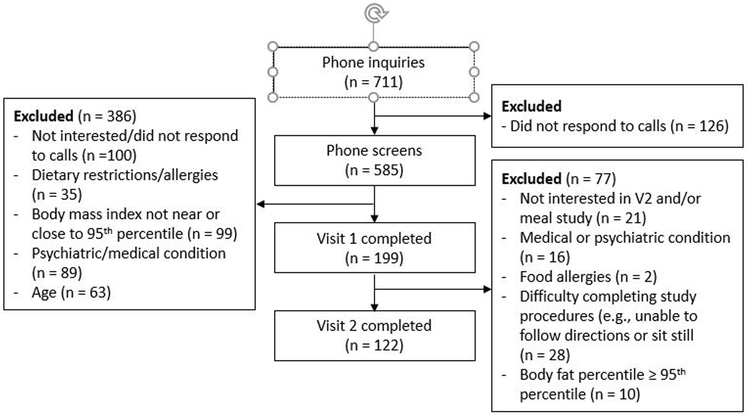
Out of 711 inquiries, 585 telephone screenings were conducted with parents (n = 126 did not return phone calls, see Figure 1). Of these, 199 attended an initial visit; 386 were excluded for the following reasons: Not interested/did not respond to calls (n = 100), dietary restrictions (n = 35), BMI well above the 95th percentile (n = 99), psychiatric or medical condition (n = 89), age (<5y, >10y, n = 63). Reasons for exclusion after visit one were: not interested in or unlikely to complete (e.g. difficulty following directions, unable to stay seated) day 2 of study (n = 49), obesity (defined as percent body fat ≥ 95th percentile, n = 10), concurrent medical or psychiatric diagnosis (n = 16), and significant food allergies (n = 2). Data from 122 children (age 8.66±1.38 years; BMI Z-score 0.23±0.87) are presented.
Figure 1.
Consort diagram of participant flow
Consistent with Hardy-Weinberg predictions, 25 (20.5%) were AA, 53 (43.4%) were AT and 44 (36.1 %) were TT. In the subset of children who were not African American, 21 (23.6%) were AA, 34 (38.6%) were AT, and 33 (37.5%) were TT, also consistent with Hardy-Weinberg predictions. Table 1 presents children’s baseline characteristics by genotype. There were no between-genotype group differences in age, height, weight, BMI, BMI percentile, BMI Z-score, or percent body fat (all p’s ≥ 0.11). Prior research suggests that FTO A allele is less common in Hispanic populations (27). Consistent with these data, there was a lower proportion of Hispanic children in the AA group compared to the proportion of Hispanic children in the TT group (AA=28.0% Hispanic, AT = 43.3% Hispanic, TT = 56.8% Hispanic (p = 0.053)). On average, children completed their meal visit within 30.23±33.44 days (1 month) of their evaluation visit. However, AA subjects had more time between their visits (44.64±42.76 days) than AT subjects (23.55±26.02 days, p = 0.01) and marginally more time between visits than TT subjects (30.09±33.64 days, p = 0.08). Age, body mass (weight), fat free mass, and calories consumed during breakfast (all p’s ≤ 0.01) were significantly associated with total intake, while sex (p = 0.52), race (p = 0.16), and ethnicity (p = 0.22) were not. Given the high level of multicollinearity among age, total body mass, and fat free mass (r’s = 0.65 – 0.95, all p’s ≤ 0.001), final models only included a single covariate (i.e., total body mass or fat free mass).
Table 1.
Baseline participant demographic characteristics by genotype from meal visit (Visit 2) or initial visit (Visit 1)*
| AA (n = 25) M±SD or % |
AT (n = 54) M±SD or % |
TT (n = 44) M±SD or % |
p/Chi-square | |
|---|---|---|---|---|
| Age (years) | 8.73±1.34 | 8.91±1.41 | 8.54±1.40 | 0.42 |
| Sex | 44.0% Female | 56.6% Female | 52.3% Female | 0.58 |
| Height (cm) | 132.49±10.19 | 133.35±9.48 | 129.64±8.86 | 0.15 |
| Weight (kgs) | 30.80±7.22 | 30.75±7.67 | 28.77±7.09 | 0.36 |
| BMI | 17.30±2.51 | 17.03±2.31 | 16.82±2.21 | 0.71 |
| BMI percentile | 57.64±29.81 | 55.02±28.81 | 55.82±25.68 | 0.93 |
| BMI Z-score | 0.26±0.97 | 0.16±0.93 | 0.19±0.78 | 0.89 |
| % body fat* | 19.85±5.49 | 19.45±5.58 | 20.48±4.95 | 0.64 |
| Fat free mass (kg)* | 24.21±4.72 | 24.44±4.95 | 22.48±4.50 | 0.11 |
| Race | 60.0% C | 37.7% C | 34.1% C | 0.12 |
| 16.0% AA | 30.2% AA | 15.9% AA | ||
| 24.0% Other | 5.7% Asian | 13.6% Asian | ||
| 20.8% Other | 27.3% Other | |||
| 5.7% Missing | 9.1% Missing | |||
| Ethnicity | 28.0% Hispanic | 43.4% Hispanic | 56.8% Hispanic | 0.053 |
| 72.0% Non-Hispanic | 54.7% Non-Hispanic | 40.9% Non-Hispanic | ||
| 1.9% Not known | 2.3% Not known |
C = Caucasian; AA = African American
Primary analyses: Impact of genotype on caloric intake
Adjusting for body weight and the number of calories consumed during breakfast, there was a significant effect of genotype on total caloric intake (unstandardized beta±SE = 64.15±31.53, p = 0.04, R2 = 0.16, ΔR2 = 0.03), Figure 2. For each A allele, the children consumed 64.15±31.53more calories than predicted based upon body weight and calories eaten during breakfast. FTO A allele dose explained approximately 3% of the variance in calories consumed during lunch. There was a marginally significant effect of genotype (unstandardized beta±SE = 57.86±31.26, p = 0.07, R2 = 0.18, ΔR2 = 0.02) on intake adjusted for fat-free mass and breakfast intake at visit 1. Results were unaffected by controlling for days between visit 1 and visit 2.
Figure 2.
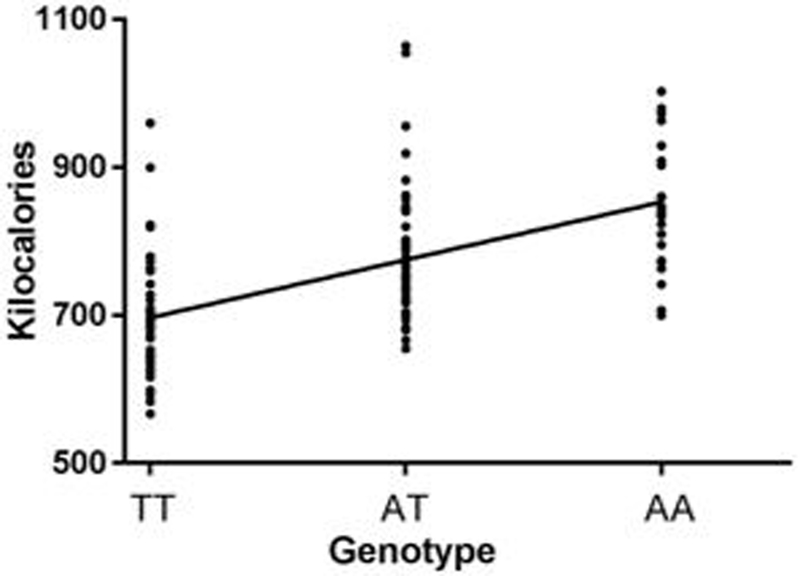
Linear regression predicting intake from genotype, controlling for body weight
Impact of genotype on proportion of calories consumed as fat
There was no association between genotype and proportion intake from dietary fat when controlling for body weight (p = 0.70) or for fat free mass (p = 0.62). All results were unaffected by controlling for days between visit 1 and visit 2.
Secondary analyses
There were no significant associations between genotype and carbohydrate or protein intake (p’s > 0.22). There were no significant associations between genotype and either energy density (p = 0.41) or diet variety score (p = 0.26).
Race and ethnicity
Eighty-eight (AA = 21, AT = 34, TT = 33) children’s parents reported the child’s race as Caucasian, Asian, or “other;” 27 parents reported the child’s race as African American; the remainder did not report the child’s race and these participants were excluded from the sub-analysis. In the non-African American children, controlling for body weight and calories eaten during breakfast, each copy of the ‘A’ allele predicted an additional 84.92±35.74 calories eaten (p = 0.02). Because of the small sample of children whose race was African American (n=27, AA=4, AT=16, TT=7), an analysis was not conducted in this sub group.
Discussion
The aim of the current study was to evaluate the association of FTO adiposity-related genotype (rs 9939609) and food intake during a laboratory meal in children without, but at genetic risk for, obesity. The specific and novel question was whether the rs9939609 SNP-associated increases in energy intake or preference for calorically dense foods reported in previous studies of children across the weight spectrum, including obesity, would be evident in children who did not have obesity. Adjusting for body weight, there was a significant effect of the adiposity risk ‘A’ allele of rs9939609 on total caloric intake, with each copy of the A allele associated with an additional approximately 65 calories consumed, explaining 3% of variance in intake. Adjusting for fat free mass, there was a near significant effect of the risk allele in the same direction.
Our primary significant finding that the FTO risk allele dose is associated with overall caloric intake during a laboratory meal in children replicates prior child (15, 16) and adult (28) studies and extends existing knowledge to a pediatric sample without obesity. It demonstrates that rs9939609 is associated with biological processes and behaviors essential to the development of excess weight gain, rather than those resulting from a state of obesity. Among studies of youth, both the Cecil (16) and Wardle (15) samples included a broad weight/BMI range, and did not exclude participants with overweight and obesity. The Tanofsky-Kraff et al. (17) study specifically recruited a sample enriched for overweight and obese youth. The explained variance of 3.0% is also consistent with the small but consistent effects of rs9939609 allele dose on body mass (with each A allele predicting only a few extra pounds (1)).
Consistent with one prior study in children (16) but in contrast to others (17), our data did not suggest that allelic dose affects food choices (proportional consumption of fat calories, dietary energy density, or diet variety). A similar study in an adult population, most of whom had obesity (28), also reported no difference in dietary macronutrient composition consumed despite a 350 kcal difference in overall intake predicted by AA genotype compared to X/T. Other recent studies of self-reported dietary intake in adults suggest a small but significant impact of the risk allele on protein intake (29, 30). However, in contrast to the majority of laboratory studies, these studies reported that each risk allele predicted lower caloric intake, which the authors hypothesize resulted from under- or mis-reporting intake by individuals with higher BMI (who may also be more likely to carry the risk allele).
On a behavioral level, our data suggest that the potential mechanism of weight gain associated with the FTO gene relates to an increase in overall intake, and not a preference for highly palatable foods. However, it does not define potential underlying mechanisms such as alterations in reward value of food, general increased impulsivity or impulsivity specifically to food. The neuro-molecular mechanisms underlying genotypic effects are under intense scrutiny in cell-based and animal models, and by use of functional magnetic resonance imaging in humans. There is some evidence of genotypic differences in central nervous system reward/satiety pathways during exposure to highly-palatable food pictures (e.g., amygdala, medial orbitofrontal cortex, ventral tegmental area, striatum, nucleus accumbens) (28, 31) suggesting hyper-responsivity in those with the obesity-promoting allele. Melhorn et al. (28) describe that AA adults self-reported less fullness and rated highly-palatable food pictures as more appealing than X/T. In terms of molecular mechanisms, the intronic sequence variants in FTO have been shown to affect the expression of numerous genes and possibly FTO itself. Some of these genes affect brain development and function (IRX3, IRX5, RPGRIP1L) (32-34), and may implicate reward and/or cognitive control neural circuits. Others influence energy expenditure by adipocytes (ARID5B) (32) or the development of adipocytes (35, 36).
There are varying reports as to whether FTO effects on food intake follow a dominant model (A/X>TT) (15, 16), a recessive model (AA>X/T) (28), or an additive model (AA>AT>TT) (31). Our findings suggest that the dose-dependent additive model (AA > AT > TT) explains more of the variance in energy intake. The “best model” may depend on the dependent variable of interest and may explain why the optimal genetic model for eating behavior may be different from that for weight outcome.
Consistent with previous studies indicating that rs993609 is not associated with adiposity in African American individuals (19), the statistical strength of the effect of genotype on intake in the subset of non-African American children was greater than that for the full sample. It is possible that differences in the molecular mechanisms by which race-related FTO snps influence adiposity (1) accounts for some of the complexities of the epidemiology of FTO associations with adiposity. Increased understanding of the neural and biological pathways by which FTO influences food intake will help to clarify genetic findings related to dose-dependence and race.
Strengths of the present study include the use of well-validated laboratory measures of food intake and the assessment of body composition in addition to body mass. Limitations include the small sample size and laboratory setting which may not be generalizable to children’s typical meal environment. In spite of possibly limited generalizability, laboratory test meals are an accepted paradigm designed to improve the rigor and reproducibility with which food intake can be assessed (37, 38). It is possible that intake was influenced by the short videos that were played during the meal. This could either lead to decreased intake by distracting attention away from the food or increased intake secondary to mindless eating. Although our method was adapted from a laboratory meal paradigm previously shown to be effective in youth in this age range (24), results might have differed if no other activities were offered and analyses might have been improved if the extent to which children attended to the video could be quantified.
Although breakfast intake was included as a covariate in the primary analysis, the fact that some children were served 10% TEE for breakfast while others were served 20% is a limitation; however, among children served 20% of their daily TEE, mean intake was only 12%. In addition, exercise/physical activity was not measured, and studies suggest that physical activity may attenuate the effects of FTO genotype on obesity risk in adults though not in children (39). Regarding the sample, we did not account for possible effects of socioeconomic status on the relationship between genotype and measured intake, and future studies should measure and control for this potential confounder. Self-reported race and ethnicity is an imprecise measure and future research may require genotyping to more objectively categorize individuals in this regard (40, 41). Finally, results should not be generalized to youth with adiposity greater than the 95th percentile because these children were not included in analyses.
In conclusion, consistent with and extending prior work, FTO genotype at rs9939609 is associated with increased food intake in a sample of children without obesity. The influence of genotype dose on intake adds support to the hypothesis that FTO genotypic associations with body weight are mediated by effects on food intake more so than on energy expenditure.
Supplementary Material
Figure S1. Laboratory meal paradigm
Study Importance Questions:
What is known about the subject?
Alleles of the FTO gene (e.g., SNP rs9939609) influence body weight in children and adults.
In children of a broad weight range, including those with obesity, children with A/X at rs9939609 genotype eat more than children with TT genotype.
What does your study add?
In our sample of 5–10 y old, children without obesity (body adiposity ≤ 95th percentile for age and sex), results support an effect of FTO (snp rs9939609) on intake, suggesting that the genotype/phenotype correlation may be detectable before the onset of obesity.
The effect of FTO (snp rs9939609) on measured caloric intake may be dose dependent.
The increase in intake does not appear to be driven by diet composition or variety.
Acknowledgements
We wish to thank Patricia Lanzano and Liyong Deng for performing genetic analyses and all the children and their families for contributing their time and energy to the study.
Funding: This work was supported by NIH grants R56/R01 DK097399, UL1TR000040, K23 DK110539
Footnotes
Disclosures: None
References
- 1.Loos RJ, Yeo GS. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol. 2014;10(1):51–61. Epub 2013/11/20. doi: 10.1038/nrendo.2013.227. PubMed PMID: 24247219; PubMed Central PMCID: PMCPMC4188449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. Journal of the American Medical Association. 2002;288:1728–32. [DOI] [PubMed] [Google Scholar]
- 4.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377(1):13–27. Epub 2017/06/13. doi: 10.1056/NEJMoa1614362. PubMed PMID: 28604169; PubMed Central PMCID: PMCPMC5477817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadde KM, Martin CK, Berthoud H, Heymsfield SB. Obesity pathophysiology and management. Journal of the American College of Cardiology. 2018;71(1):E pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–55. Epub 2006/04/06. doi: 10.1001/jama.295.13.1549. PubMed PMID: 16595758. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum M Epidemiology of pediatric obesity. Pediatr Ann. 2007;36(2):89–95. Epub 2007/03/03. PubMed PMID: 17330571. [DOI] [PubMed] [Google Scholar]
- 8.Rankinen T, Bouchard C. Genetics of food intake and eating behavior phenotypes in humans. Annu Rev Nutr. 2006;26:413–34. Epub 2006/07/20. doi: 10.1146/annurev.nutr.26.061505.111218. PubMed PMID: 16848714. [DOI] [PubMed] [Google Scholar]
- 9.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–8. Epub 1995/03/09. doi: 10.1056/NEJM199503093321001. PubMed PMID: 7632212. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115(12):3579–86. Epub 2005/12/03. doi: 10.1172/JCI25977. PubMed PMID: 16322796; PubMed Central PMCID: PMCPMC1297250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. The American journal of clinical nutrition. 2008;88(4):906–12. Epub 2008/10/10. PubMed PMID: 18842775. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. The American journal of clinical nutrition. 2000;71(6):1421–32. Epub 2000/06/06. PubMed PMID: 10837281. [DOI] [PubMed] [Google Scholar]
- 13.Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJ, et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol (Lausanne). 2012;3:29. Epub 2012/05/31. doi: 10.3389/fendo.2012.00029. PubMed PMID: 22645519; PubMed Central PMCID: PMCPMC3355836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–48. Epub 2010/10/12. doi: 10.1038/ng.686. PubMed PMID: 20935630; PubMed Central PMCID: PMCPMC3014648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. International journal of obesity. 2009;33(1):42–5. Epub 2008/10/08. doi: 10.1038/ijo.2008.174. PubMed PMID: 18838977. [DOI] [PubMed] [Google Scholar]
- 16.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359(24):2558–66. Epub 2008/12/17. doi: 10.1056/NEJMoa0803839. PubMed PMID: 19073975. [DOI] [PubMed] [Google Scholar]
- 17.Tanofsky-Kraff M, Han JC, Anandalingam K, Shomaker LB, Columbo KM, Wolkoff LE, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. The American journal of clinical nutrition. 2009;90(6):1483–8. doi: 10.3945/ajcn.2009.28439. PubMed PMID: 19828706; PubMed Central PMCID: PMC2777464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan M, Thompson JMD, Mitchell EA, Murphy R, McCowan LME, Shelling AN, et al. Analysis of association of gene variants with obesity traits in New Zealand European children at 6 years of age. Molecular bioSystems. 2017;13(8):1524–33. Epub 2017/06/22. doi: 10.1039/c7mb00104e. PubMed PMID: 28636007. [DOI] [PubMed] [Google Scholar]
- 19.McCormack S, Grant SF. Genetics of obesity and type 2 diabetes in African Americans. Journal of obesity. 2013;2013:396416. Epub 2013/04/12. doi: 10.1155/2013/396416. PubMed PMID: 23577239; PubMed Central PMCID: PMC3614120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009;124 Suppl 1:S23–34. Epub 2009/09/16. doi: 10.1542/peds.2008-3586E. PubMed PMID: 19720664. [DOI] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002(246):1–190. Epub 2002/06/05. PubMed PMID: 12043359. [PubMed] [Google Scholar]
- 22.Laurson KR, Eisenmann JC, Welk GJ. Body fat percentile curves for U.S. children and adolescents. Am J Prev Med. 2011;41(4 Suppl 2):S87–92. Epub 2011/10/14. doi: 10.1016/j.amepre.2011.06.044. PubMed PMID: 21961617. [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Otten JJ, Hellwig JP, Meyers LD, editors. Washington, D.C.: The National Academies Press; 2006. [Google Scholar]
- 24.Mirch MC, McDuffie JR, Yanovski SZ, Schollnberger M, Tanofsky-Kraff M, Theim KR, et al. Effects of binge eating on satiation, satiety, and energy intake of overweight children. The American journal of clinical nutrition. 2006;84(4):732–8. PubMed PMID: 17023698; PubMed Central PMCID: PMC1864961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schebendach JE, Mayer LE, Devlin MJ, Attia E, Contento IR, Wolf RL, et al. Dietary energy density and diet variety as predictors of outcome in anorexia nervosa. The American journal of clinical nutrition. 2008;87(4):810–6. Epub 2008/04/11. PubMed PMID: 18400701. [DOI] [PubMed] [Google Scholar]
- 26.Office of Management and Budget. Revisions to the standards for the classification of Federal data on race and ethnicity. In: Affairs OoIaR, editor. 1997. [Google Scholar]
- 27.Wing MR, Ziegler JM, Langefeld CD, Roh BH, Palmer ND, Mayer-Davis EJ, et al. Analysis of FTO gene variants with obesity and glucose homeostasis measures in the multiethnic Insulin Resistance Atherosclerosis Study cohort. International journal of obesity. 2011;35(9):1173–82. Epub 2010/11/26. doi: 10.1038/ijo.2010.244. PubMed PMID: 21102551; PubMed Central PMCID: PMCPMC4068260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melhorn SJ, Askren MK, Chung WK, Kratz M, Bosch TA, Tyagi V, et al. FTO genotype impacts food intake and corticolimbic activation. The American journal of clinical nutrition. 2018;107(2):145–54. Epub 2018/03/13. doi: 10.1093/ajcn/nqx029. PubMed PMID: 29529147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardy DS, Racette SB, Hoelscher DM. Macronutrient Intake as a Mediator with FTO to Increase Body Mass Index. J Am Coll Nutr. 2014;33(4):256–66. doi: 10.1080/07315724.2013.879458. PubMed PMID: WOS:000342139900002. [DOI] [PubMed] [Google Scholar]
- 30.Qi QB, Kilpelainen TO, Downer MK, Tanaka T, Smith CE, Sluijs I, et al. FTO genetic variants, dietary intake and body mass index: insights from 177 330 individuals. Human Molecular Genetics. 2014;23(25):6961–72. doi: 10.1093/hmg/ddu411. PubMed PMID: WOS:000347923000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert-Diamond D, Emond JA, Lansigan RK, Rapuano KM, Kelley WM, Heatherton TF, et al. Television food advertisement exposure and FTO rs9939609 genotype in relation to excess consumption in children. International journal of obesity. 2017;41(1):23–9. doi: 10.1038/ijo.2016.163. PubMed PMID: WOS:000394143100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N Engl J Med. 2015;373(10):895–907. Epub 2015/08/20. doi: 10.1056/NEJMoa1502214. PubMed PMID: 26287746; PubMed Central PMCID: PMCPMC4959911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gomez-Marin C, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507(7492):371–5. Epub 2014/03/22. doi: 10.1038/nature13138. PubMed PMID: 24646999; PubMed Central PMCID: PMCPMC4113484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stratigopoulos G, Padilla SL, LeDuc CA, Watson E, Hattersley AT, McCarthy MI, et al. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1185–96. Epub 2008/02/08. doi: 10.1152/ajpregu.00839.2007. PubMed PMID: 18256137; PubMed Central PMCID: PMCPMC2808712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carli JFM, LeDuc CA, Zhang Y, Stratigopoulos G, Leibel RL. The role of Rpgrip1l, a component of the primary cilium, in adipocyte development and function. FASEB J. 2018;32(7):3946–56. Epub 2018/02/22. doi: 10.1096/fj.201701216R. PubMed PMID: 29466054; PubMed Central PMCID: PMCPMC5998974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin Carli JF, LeDuc CA, Zhang Y, Stratigopoulos G, Leibel RL. FTO mediates cell-autonomous effects on adipogenesis and adipocyte lipid content by regulating gene expression via 6mA DNA modifications. J Lipid Res. 2018;59(8):1446–60. Epub 2018/06/24. doi: 10.1194/jlr.M085555. PubMed PMID: 29934339; PubMed Central PMCID: PMCPMC6071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sysko R, Steinglass J, Schebendach J, Mayer L, Walsh BT. Rigor and Reproducibility via Laboratory Studies of Eating Behavior. A Focused Update and Conceptual Review. International Journal of Eating Disorders. 2018; In press. [DOI] [PubMed] [Google Scholar]
- 38.Tanofsky-Kraff M, Haynos AF, Kotler LA, Yanovski SZ, Yanovski JA. Laboratory-Based Studies of Eating among Children and Adolescents. Current nutrition and food science. 2007;3(1):55–74. PubMed PMID: 19030122; PubMed Central PMCID: PMC2585783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilpelainen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS medicine. 2011;8(11):e1001116. Epub 2011/11/10. doi: 10.1371/journal.pmed.1001116. PubMed PMID: 22069379; PubMed Central PMCID: PMC3206047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian C, Kosoy R, Nassir R, Lee A, Villoslada P, Klareskog L, et al. European population genetic substructure: further definition of ancestry informative markers for distinguishing among diverse European ethnic groups. Mol Med. 2009;15(11–12):371–83. Epub 2009/08/27. doi: 10.2119/molmed.2009.00094. PubMed PMID: 19707526; PubMed Central PMCID: PMCPMC2730349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, et al. The genetic structure and history of Africans and African Americans. Science. 2009;324(5930):1035–44. Epub 2009/05/02. doi: 10.1126/science.1172257. PubMed PMID: 19407144; PubMed Central PMCID: PMCPMC2947357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Laboratory meal paradigm