Abstract
Objective:
To develop treatment recommendations for children with juvenile idiopathic arthritis manifesting with non-systemic polyarthritis, sacroiliitis, or enthesitis.
Methods:
PICO (population/intervention/comparator/outcome) questions were developed and refined by members of the guideline development teams. A systematic review was conducted to compile evidence for the benefits and harms associated with treatments for these conditions. Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was used to rate the quality of evidence. A group consensus process was conducted among the Voting Panel to generate the final recommendations and grade their strength. A Parent and Patient Panel used a similar consensus approach to provide patient/caregiver preferences for key questions.
Results:
39 recommendations were developed (8 strong and 31 conditional). The quality of supporting evidence was “very low” or “low” for 90% of recommendations. Recommendations are provided for the use of nonsteroidal anti-inflammatory drugs (NSAIDs), disease modifying anti-rheumatic drugs (DMARDs), biologics, and intraarticular and oral glucocorticoids. Recommendations for the use of physical and occupational therapy are also provided. Specific recommendations for polyarthritis address general medication use, initial and subsequent treatment, and adjunctive therapies. Good disease control, with therapeutic escalation to achieve low disease activity, was recommended. The sacroiliitis and enthesitis recommendations primarily address initial therapy and adjunctive therapies.
Conclusion:
This guideline provides direction for clinicians, caregivers, and patients making treatment decisions. Clinicians, caregivers, and patients should use a shared decision-making process that accounts for patients’ values, preferences, and comorbidities. These recommendations should not be used to limit or deny access to therapies.
Keywords: Juvenile idiopathic arthritis, enthesitis, polyarticular JIA, sacroiliitis, DMARDs, glucocorticoids, biologics, NSAIDs
INTRODUCTION
Juvenile arthritis is one of the most common chronic diseases of childhood, with an estimated prevalence of 1 per 1,000 children.1–3 The term juvenile idiopathic arthritis (JIA) defines a heterogeneous collection of inflammatory arthritides of unknown etiology with onset prior to age 16 years and a minimum of 6 weeks duration, following the exclusion of other known causes of synovitis.4 Current International League of Associations for Rheumatology (ILAR) classification criteria divide JIA into 7 mutually exclusive categories defined by the number of joints involved, presence or absence of extraarticular manifestations, and presence or absence of additional markers including rheumatoid factor (RF) and HLA-B27.4
All forms of JIA are associated with decreased health-related quality of life and risk for permanent joint damage, and the disease may persist into adulthood causing ongoing significant morbidity and impaired quality-of-life.5–13 A number of treatments are available, including non-steroidal anti-inflammatory drugs (NSAIDs), systemic and intraarticular glucocorticoids, and non-biologic and biologic disease modifying antirheumatic drugs (DMARDs). Prompt initiation of appropriate therapy is of critical importance in preventing permanent damage and improving outcomes. While earlier disease recognition and expanded treatment options have made good disease control a possibility for many patients, they have also made the decision-making around treatments more complex for physicians, caregivers, and patients.
The American College of Rheumatology (ACR) published initial recommendations for JIA in 2011 that provided guidance for the treatment of JIA and for the monitoring of select medical therapies, and an update in 2013 focused on the treatment of systemic arthritis.14, 15 The ACR has subsequently transitioned from the RAND/UCLA Appropriateness Method used to generate these prior recommendations to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, which has the advantages of a more transparent decision-making process and well-defined criteria for moving from evidence to recommendation, including balancing benefits and harms and consideration of patients values and preferences, while maintaining methodological rigor.16
The goal of this guideline project was to provide updated recommendations for non-systemic polyarthritis, sacroiliitis, and enthesitis, incorporating recently published data and utilizing the GRADE methodology. Recommendations for the treatment of chronic and acute JIA-associated uveitis were developed concomitantly and are presented separately (REF when available).
METHODS
Methodology Overview.
This guideline followed the American College of Rheumatology (ACR) guideline development process (http://www.rheumatology.org/Practice-Quality/Clinical-Support/Clinical-Practice-Guidelines). This process includes using the GRADE methodology (www.gradeworkinggroup.org) to rate the quality of the available evidence and to develop the recommendations.16–18 ACR policy guided disclosures and the management of conflicts of interest (insert link here to full participant disclosure list just before publication). Supplementary Appendix 1 describes the methods in detail.
Guideline Development Teams.
This work involved five teams: 1) a Core Leadership Team, consisting of 4 pediatric rheumatologists, that supervised and coordinated the project and assisted with developing the scope of the project and initial PICO [population/intervention/comparator/outcomes] questions and drafting the manuscript; 2) a Literature Review Team, led by an experienced literature review consultant, which completed the literature search and data abstraction, and rated the quality of evidence; 3) an Expert Panel, composed of 9 pediatric rheumatologists, which assisted with developing the scope of the project, and drafting and refining the PICO questions; 4) a Voting Panel, consisting of 15 pediatric rheumatologists and 2 adult patients with JIA, which assisted with developing the scope of the project and refining the PICO questions, and voted on the recommendations; and 5) a Parent and Patient Panel, consisting of 9 adult patients with JIA and 2 parents of children with JIA, which reviewed the collated evidence and provided input on their values and preferences within the context of a separate meeting. Supplementary Appendix 2 presents rosters of the team and panel members. In accordance with ACR policy, the principal investigators and the literature review consultant were free of potential conflicts of interest, and all teams had >50% members free of potential conflicts of interest.
PICO Question Development and Importance of Outcomes.
The Core Leadership Team drafted the initial project scope, key principles and examples of relevant PICO questions. The following topics were proposed to the guideline development groups for consideration: acute and chronic anterior uveitis, oligoarthritis, polyarthritis, systemic arthritis, sacroiliitis, enthesitis, and temporomandibular joint arthritis. PICO questions for each topic were developed and discussed at a face-to-face meeting during which the topics were refined. The project scope was subsequently limited to patients with non-systemic polyarthritis, sacroiliitis, and enthesitis as they were deemed to be the most high impact areas. The PICO questions for these topics were subsequently reviewed and further refined by the Expert and Voting Panels via email.
Populations (Table 1).
Table 1.
Terms and Definitions*
| Term | Definition |
|---|---|
| Polyarthritis population | Children with JIA and non-systemic polyarthritis (≥ 5 joints ever involved); may include children from ILAR JIA categories of polyarticular (rheumatoid factor positive or negative), extended oligoarticular, enthesitis related arthritis, psoriatic arthritis and undifferentiated arthritis. |
| Risk Factors | One or more of the following: positive rheumatoid factor (RF), positive anti-cyclic citrullinated peptide (CCP) antibodies, joint damage. |
| Moderate/High Disease Activity† | Clinical Juvenile Disease Activity Score based on 10-joints (cJADAS-10) > 2.5† |
| Low Disease Activity† | Clinical Juvenile Disease Activity Score based on 10-joints (cJADAS-10) ≤ 2.5 and ≥ 1 active joint |
| Sacroiliitis population | Patients with active sacroiliitis who will most likely be classified within the ILAR categories of enthesitis related arthritis, psoriatic arthritis, and undifferentiated arthritis, but may include patients in any of the ILAR JIA categories. |
| Active sacroiliitis | Prior or current MRI findings consistent with sacroiliitis along with clinical examination findings consistent with sacroiliitis (e.g. pain with direct palpation of the SI joints) and/or patient-reported symptoms of inflammatory back pain. |
| Enthesitis population | Patients with enthesitis (tendon to bone insertion sites) who will most likely be from the ILAR categories of enthesitis-related arthritis, psoriatic arthritis, and undifferentiated arthritis, but may include patients from any of the ILAR JIA categories. |
| Active enthesitis | Tenderness and/or swelling of the entheses determined to require medical treatment per the treating provider. |
JIA = juvenile idiopathic arthritis; ILAR = International League Against Rheumatism.
The cJADAS-10 was used to define low disease activity (≤ 2.5 and ≥ 1 active joint) versus high/moderate disease activity (> 2.5). While this is provided as a general parameter, the cJADAS-10 should be interpreted within the clinical context.
While the current ILAR classification criteria have been useful for identifying homogeneous groups of patients for research, more recent data suggest that these categories may not entirely reflect the underlying genetic and clinical heterogeneity of the disease or be relevant for guiding treatment decisions.19–21 For this reason, it was decided to base the current guideline on broad clinical phenotypes, rather than ILAR categories, similar to the approach of the 2011 guideline.15 The patient populations addressed in this guideline are defined below. The present recommendations are intended to address typical patients with the phenotype, and may not be applicable to patients with uncommon features or highly refractory disease.
1). Polyarthritis.
This group includes children with JIA and polyarthritis (≥ 5 joints ever involved), and may include children from different ILAR JIA categories, but excludes children with systemic arthritis or sacroiliitis. These guidelines are not intended to be applicable to children with associated extra-articular manifestations (e.g., psoriasis, uveitis, inflammatory bowel disease) that may also influence treatment decisions. Given the heterogeneity of patients with JIA and polyarthritis, the Expert and Voting Panels initially categorized patients into treatment groups using combinations of the following categories: 1) presence or absence of risk factors for disease severity and potentially a more refractory disease course, and 2) low disease activity versus moderate/high disease activity.
Risk factors were defined as the presence of one or more of the following: positive rheumatoid factor (RF), positive anti-cyclic citrullinated peptide (CCP) antibodies, or joint damage. The Juvenile Arthritis Disease Activity Score (JADAS) was proposed as a means of categorizing disease activity, with the acknowledgement that a number of versions exist, and that validation is not fully complete and cut-off scores may change.22–26 An active joint was defined using the ACR definition: presence of swelling [not due to currently inactive synovitis or to bony enlargement] or, if swelling is not present, limitation of motion accompanied by pain, tenderness, or both.27, 28 The Voting Panel used the clinical JADAS based upon 10 joints (cJADAS-10) and a cut off of ≤2.5 versus >2.5 to define low versus high/moderate disease activity. Low disease activity was further defined as cJADAS-10 ≤2.5 and ≥ 1 active joint to ensure that active arthritis was also present. Moderate and high disease activity were considered together as it was felt that treatment approaches would be similar.29 The cJADAS-10 is a sum of total active joint count (to a maximum of 10), physician global assessment of disease activity (0–10), and parent/patient global assessment of well-being (0–10). It was also acknowledged that one of the limitations of the JADAS is the lack of standardization of the physician and parent global assessments. It is therefore recommended that the JADAS be interpreted within the context of the clinical presentation, rather than considered an absolute determinant of disease activity.
Because there were ultimately few data available to support different treatment approaches based upon the risk factors and disease activity categories, patients were often grouped together to provide a recommendation. The few instances where the Voting Panel made differing recommendations based upon disease activity or risk factors are explicitly noted.
2). Sacroiliitis.
This group includes patients with active sacroiliitis who will most likely be classified within the ILAR categories of enthesitis related arthritis, psoriatic arthritis, and undifferentiated arthritis, but may include patients in any of the ILAR JIA categories. In addition to active sacroiliitis, patients may or may not have active peripheral joint disease and/or enthesitis to be included in this population. It is anticipated that patients with peripheral spondyloarthritis and no sacroiliitis would be treated using the polyarthritis recommendations included in this update or existing JIA oligoarthritis treatment recommendations from the 2011 ACR JIA Guideline, depending upon numbers of joints involved. For the purposes of these guidelines, patients were considered to have active sacroiliitis if they had prior or current MRI findings consistent with sacroiliitis along with clinical examination findings consistent with sacroiliitis (e.g. pain with direct palpation of the SI joints) and/or patient-reported symptoms of inflammatory back pain.
3). Enthesitis.
This group is intended to include patients with enthesitis (tendon to bone insertion sites) who will also most likely be from the ILAR categories of enthesitis-related arthritis, psoriatic arthritis, and undifferentiated arthritis, but may include patients from any of the ILAR JIA categories. Patients may or may not have concomitant active peripheral arthritis or sacroiliitis to be included in these guidelines, but the recommendations for enthesitis are intended to apply to patients with isolated enthesitis or with active enthesitis despite adequate control of their other disease manifestations. For the purposes of these guidelines, active enthesitis is tenderness and/or swelling of the entheses determined to require medical treatment per the treating provider.
Interventions.
The pharmacologic and non-pharmacologic therapies considered are listed in Table 2. Both intraarticular and oral glucocorticoids were considered. For the purposes of the recommendations, a bridging course of oral glucocorticoids was defined as a short course (< 3 months) of oral glucocorticoid intended to control disease activity quickly during escalation of DMARD or biologic therapy, using the shortest possible duration and the lowest dose needed to control symptoms. The duration of bridging therapy would likely to be primarily determined by the anticipated timing of onset of action of the other DMARD or biologic treatment(s). An optimal trial of methotrexate was considered to be 3 months; however, if no or minimal response was observed after 6–8 weeks, it was agreed that changing or adding therapy may be appropriate.
Table 2.
Interventions Included in the Literature Review
| Intervention | |
|---|---|
| Medications | Medication Names |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) | Any |
| Disease modifying anti-rheumatic drugs (DMARDs) | Leflunomide*, Methotrexate,
Sulfasalazine Triple non-biologic DMARD* (methotrexate, sulfasalazine, hydroxychloroquine) |
| Biologics | Tumor necrosis factor alpha inhibitors
(TNFi)*: Adalimumab,
Etanercept, Infliximab, Golimumab Non-TNFi Biologics†: Abatacept (CTLA4-Ig), Tocilizumab (anti-IL-6R), Rituximab (anti-CD20) |
| Glucocorticoids | Oral: Any Intraarticular: Triamcinolone Acetonide, Triamcinolone Hexacetonide, Methylprednisolone Acetate |
| Other Interventions | |
| Physical Therapy Occupational Therapy* |
Certolizumab was not included in the recommendations as there were no pediatric data yet available.
Evaluated for polyarthritis only.
Outcomes.
Outcomes were selected during the initial face-to-face scoping meeting and subsequently refined by online vote (Supplementary Appendix 3). Critical outcomes included disease activity, quality of life, joint damage, and serious adverse events. Pain was selected as an important outcome. While these outcomes were each felt to be important in decision-making by the guideline development teams, they were not routinely reported across studies. Disease activity and serious adverse events were the most consistently reported outcomes.
Literature searches, data abstraction, and rating the quality of evidence.
Systematic searches of the published English-language literature included OVID Medline, PubMed, Embase, and the Cochrane Library (including Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, and Health Technology Assessments) from the beginning of each database through June 12, 2017 (Supplementary Appendix 4); updated searches were conducted on October 13, 2017. DistillerSR software (https://distillercer.com/products/distillersr-systematic-reviewsoftware/) facilitated duplicate screening of literature search results (citation flow diagram, Supplementary Appendix 5). Reviewers entered extracted data into RevMan software (http://tech.cochrane.org/revman), and evaluated the risk of bias of primary studies using the Cochrane risk of bias tool (http://handbook.cochrane.org/). RevMan files were exported into GRADEpro software to formulate a GRADE summary of findings table (Supplementary Appendix 6) for each PICO question.30
When available, the evidence summaries included the benefits and harms for outcomes of interest across studies, the relative effect (95% CI), the number of participants, and the absolute effects. GRADE criteria provided the framework for judging the overall quality of evidence.16 The literature review team rated the quality of evidence for each critical and important outcome as high, moderate, low, or very low quality, taking into account limitations of study design, risk of bias, inconsistency, indirectness, imprecision, and other considerations. The overall quality listed in this report for a body of evidence (i.e., either an individual paper or a group of papers) is not a statement about the methodological quality of the study (or studies). Rather, the intention was to rate the paper(s) in relation to the PICO question under consideration. As a result, a very well conducted study might be rated lower in the evidence report. For example, if the population or intervention being studied does not completely match the population or intervention being examined by the PICO question, the evidence is downgraded for indirectness. The overall quality of evidence may also be downgraded due to imprecision in the effect estimate (e.g., wide confidence intervals or a low number of patients or events).
During the Voting Panel meeting, the panel also considered relevant data from adult studies, but these studies were not systematically searched or compiled in the evidence report. The Voting Panel was provided with a copy of the evidence report from the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis as a reference (https://www.rheumatology.org/Portals/0/Files/Axial-SpA-Guideline-Supplement-E.pdf).31
Moving from Evidence to Recommendations.
Each recommendation was made based on a consideration of the balance of relative benefits and harms of the treatment options under consideration, the quality of the evidence (e.g., confidence in the effect estimates), and patients’ values and preferences, as per GRADE methodology. Discussion points and voting results from the Parent and Patient Panel meeting were presented during the Voting Panel meeting as relevant. When the literature did not clearly guide recommendations, recommendations were based on the experience of the Voting Panel members (including physicians and the two patients present) as well as the results from the Parent and Patient Panel. Financial costs were not formally considered during the voting process.
Consensus Building.
The Voting Panel voted on the direction and strength of the recommendation related to each PICO question. Recommendations required a 70% level of agreement; if 70% agreement was not achieved during an initial vote, the Panel members held additional discussions before re-voting, including rewording of recommendations if needed, until consensus was attained.32 Discussion and iterative voting occurred until consensus was achieved. An additional round of voting was conducted online after the Voting Panel meeting to address questions that arose during the preparation of the final recommendations. For each recommendation, a written explanation is provided, describing the reasons for this decision and conditions under which the alternative choice may be preferable when relevant.
Moving from Recommendations to Practice.
These recommendations are designed to help health care providers, caregivers, and patients engage in shared decision-making regarding treatment choices. Health care providers, caregivers, and patients must take into consideration not only clinical phenotype and level of disease activity, but also comorbidities, response and tolerance of prior therapies, patient’s values and preferences, and patient’s functional status and functional goals in choosing the optimal therapy for an individual patient at the given point in treatment.
RESULTS/RECOMMENDATIONS
How to Interpret the Recommendations16–18
A strong recommendation means that the Voting Panel was confident that the desirable effects of following the recommendation outweigh the undesirable effects (or vice versa), so the course of action would apply to all or almost all patients, and only a small proportion would not want to follow the recommendation. In some cases, strong recommendations were made even in the absence of moderate- or high-quality evidence based on Voting Panel experience and data from adult studies.
A conditional recommendation means that the Voting Panel believed that the desirable effects of following the recommendation probably outweigh the undesirable effects, so the course of action would apply to the majority of the patients, but some may not want to follow the recommendation. Because of this, conditional recommendations are particularly preference-sensitive and warrant a shared decision-making approach. Conditional recommendations were generally based on low- to very-low-quality evidence. Most recommendations in this guideline are conditional.
For each recommendation, Supplementary Appendix 6 provides details regarding the PICO questions and the GRADE evidence tables. PICO questions were combined when possible to create simplified recommendations.
General recommendations for patients with JIA and polyarthritis (Table 3)
Table 3.
General Medication Recommendations for Children and Adolescents with JIA and Polyarthritis*
| Recommendation | Level of Evidence |
|---|---|
| Each recommendation is preceded with the phrase: “In children and adolescents with JIA and polyarthritis…” | |
| Non-Steroidal Anti-inflammatory Drugs (NSAIDs) | |
|
Very low |
| Disease Modifying Anti-Inflammatory Drugs (DMARDs) | |
| Moderate (leflunomide); Very low (sulfasalazine) | |
|
Very low |
| Glucocorticoids | |
|
Very low |
|
Moderate |
|
Very low |
|
Very low |
|
Very low |
| Biologic DMARDs | |
|
Very low (etanercept, golimumab); low
(abatacept, tocilizumab); moderate (adalimumab) Low |
| Physical Therapy & Occupational Therapy | |
| Low (PT); Very low (OT) |
TNFi = tumor necrosis factor alpha inhibitor (etanercept, adalimumab, infliximab, golimumab).
A bridging course of oral glucocorticoids was defined a short course (< 3 months) of oral glucocorticoid intended to control disease activity quickly during the initiation or escalation of therapy. An adequate trial of methotrexate was considered to be 3 months. If no or minimal response is observed after 6–8 weeks, it was agreed that changing or adding therapy may be appropriate.
For this population, an initial set of general recommendations was made regarding NSAID, DMARD, intraarticular glucocorticoid, and biologic use. These general recommendations are intended to apply to the subsequent specific polyarthritis recommendations addressing these medications, as the recommendations were not anticipated to differ based on initial versus subsequent therapy, level of disease activity, or presence or absence of risk factors. For example, in PICO A.2 and A.3, methotrexate is conditionally recommended over leflunomide and sulfasalazine. It is intended that this recommendation apply to subsequent recommendations referring to DMARD therapy.
Each recommendation in this section is prefaced with the statement “In children and adolescents with JIA and active polyarthritis…”
PICO A.1. NSAIDs are conditionally recommended as adjunct therapy.
This recommendation is conditional based on the very low quality of evidence and incorporation of patient and caregiver preferences, particularly concerns regarding medication adverse effects. In general, NSAIDs were felt to be appropriate for symptom management, particularly during initiation or escalation of therapy with DMARDs or biologics.33–35 It was acknowledged that NSAIDs are not appropriate as monotherapy for chronic, persistent synovitis.
PICO A.2–A.3. Using methotrexate is conditionally recommended over leflunomide or sulfasalazine.
Leflunomide.
The quality of supporting evidence for this recommendation was moderate. The recommendation to favor methotrexate over leflunomide is due to the greater volume of data supporting the effectiveness of methotrexate. The Voting Panel also specifically noted the lack of data for the dosing, safety and effectiveness of leflunomide in children younger than age 3 years, and lack of long-term safety data for leflunomide in general for children with polyarthritis.36, 37 In addition, there is no liquid form of this medication currently which may make administration difficult, particularly for younger children.
Sulfasalazine.
The recommendation for methotrexate over sulfasalazine is conditional because the supporting evidence is of very low quality, there are no head to head comparison studies, and there are more data supporting the effectiveness of methotrexate. There were also concerns raised by the Voting Panel regarding the safety of sulfasalazine as compared to methotrexate, specifically the risk of Stevens-Johnson syndrome and bone marrow suppression.34, 35
Although methotrexate is conditionally recommended over each of these therapies, it is important to note that at the Parent and Patient Voting Panel participants stated that they would want to be made aware of available alternatives to methotrexate because methotrexate adverse effects, particularly gastrointestinal intolerance, are very limiting for some children.
PICO A.4. Using subcutaneous methotrexate is conditionally recommended over oral methotrexate.
This recommendation is conditional because the supporting evidence is of very low quality and patient preferences may guide choice of route of administration.38–45 The strength of recommendation also reflects Voting Panel experience, lack of certainty regarding differences in adverse event rates between the 2 routes of administration, consideration of data suggesting variable bioavailability of oral methotrexate (particularly at higher doses), and the goal of optimizing methotrexate effectiveness prior to escalating therapy.46, 47
PICO A.5. Intraarticular glucocorticoids are conditionally recommended as adjunct therapy.
This recommendation is conditional given that the supporting evidence is very low quality and primarily generated in children with oligoarthritis, and given the variable parent and patient experiences and preferences regarding a procedure that may require sedation or be painful.48 In addition, intraarticular glucocorticoid injections may not be an appropriate treatment approach for large numbers of joints or joints that have been injected multiple times; escalation of systemic therapy may be preferred in these situations. The Voting Panel also suggested that intraarticular glucocorticoid injections be more strongly considered when arthritis is preventing ambulation or otherwise interfering with important daily activities and more prompt disease control is needed.
PICO A.6. Triamcinolone hexacetonide is strongly recommended over triamcinolone acetonide for intraarticular glucocorticoid injections.
The quality of supporting evidence for this recommendation was moderate.49 This recommendation was further supported by observational studies showing improved outcomes with triamcinolone hexacetonide in oligoarticular JIA and RA.50, 51 Voting panel members specifically noted their consistent and repeated observation of more complete and longer duration of clinical response without increased adverse effects with triamcinolone hexacetonide versus triamcinolone acetonide. The Parent and Patient Voting Panel also supported this judgment on the strength of recommendation.
PICO A.7. Bridging therapy with a limited course of oral glucocorticoid (< 3 months) during initiation or escalation of therapy in patients with high or moderate disease activity is conditionally recommended.
This recommendation is conditional based on very low quality of supporting evidence and known risks associated with systemic glucocorticoid use. Parents and patients agreed that bridging therapy was acceptable in this setting. Bridging glucocorticoids may have most utility in the setting of high disease activity, limited mobility, and/or significant symptoms.
PICO A.8. Conditionally recommend against bridging therapy with a limited course of oral glucocorticoid (< 3 months) in patients with low disease activity.
The quality of evidence for this recommendation was very low. Intraarticular glucocorticoid injection was considered preferable in this setting.
PICO A.9. Strongly recommend against adding chronic low dose glucocorticoid, irrespective of risk factors or disease activity.
The quality of supporting evidence for this recommendation was very low. The recommendation was strong, however, given the known adverse effects of long-term systemic glucocorticoid use in children, particularly growth suppression, weight gain, osteopenia and cataracts, and availability of other treatment options. The Voting Panel agreed that in the setting of low disease activity, targeted joint injections may be more appropriate (PICO A.5). In the setting of moderate or high disease activity, escalating DMARD or biologic therapy is likely more appropriate.
PICO A.10-A.14. In children and adolescents with JIA and polyarthritis receiving treatment with a DMARD, combination therapy with a biologic (etanercept, adaliumab, golimumab, abatacept or tocilizumab) is conditionally recommended over biologic monotherapy.
This recommendation is intended for patients adding biologics for additional disease control and not intended for patients who may be tapering therapy due to inactive disease for whom tapering or removal of the DMARD while continuing biologic therapy may be an appropriate strategy. The available evidence addresses combination therapy with methotrexate, with no evidence identified for other DMARDs. There was variability in the quality of supporting evidence for the medications ranging from very low (etanercept, golimumab), to low (abatacept, tocilizumab) and moderate (adalimumab).28, 52–66 The potential benefit of methotrexate use for prevention of anti-drug antibodies to adalimumab was included in the discussion for concomitant DMARD use with that medication.60 The overall recommendation is conditional based upon the quality of supporting evidence and variable parent and patient preferences given the burden of taking multiple medications and concerns regarding methotrexate intolerance. The Voting Panel recognized that there may be situations in which biologic monotherapy is also acceptable, particularly in the setting of adequate disease control or methotrexate intolerance.
PICO A.15. Combination therapy with a DMARD is strongly recommended for infliximab.
Using infliximab in combination with a DMARD was a strong recommendation despite the low quality of evidence, primarily given more extensive experience with the need for combination therapy to reduce the risk of anti-drug antibody formation.67, 68
PICO A.16, A.17. In children and adolescents with JIA and polyarthritis who have or are at risk for functional limitations, using PT and/or OT is conditionally recommended.
This recommendation is conditional based on the low quality of supporting evidence for PT, very low level of evidence for OT, and Voting Panel experience.69, 70
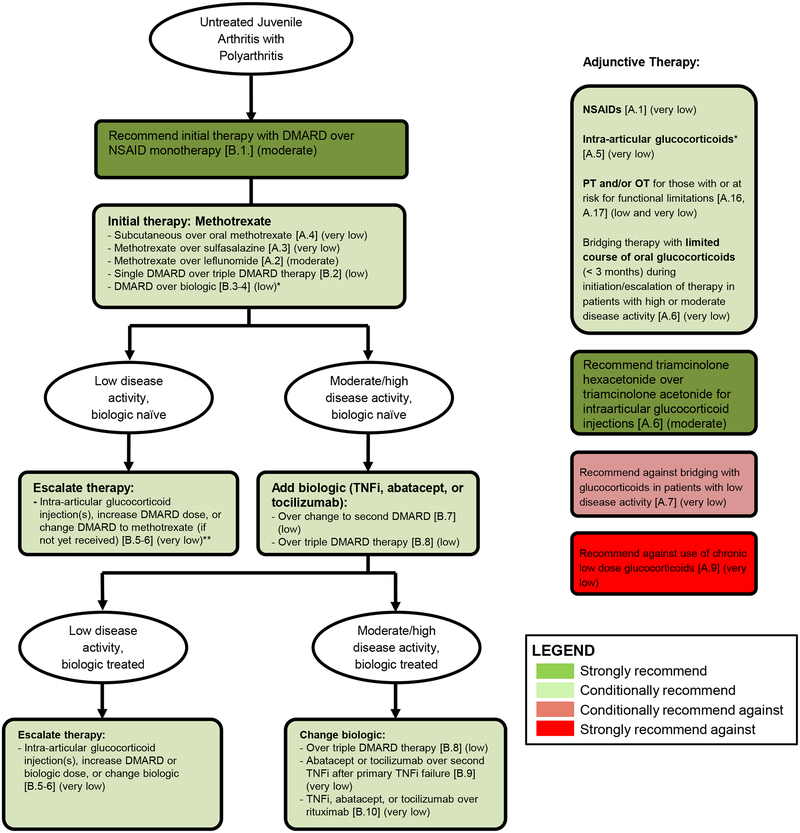
Recommendations for the initial and subsequent treatment of JIA and polyarthritis (Table 4, Figure 1)
Table 4.
General Guidelines for the Initial and Subsequent Treatment of Children and Adolescents with JIA and Polyarthritis*
| Recommendation |
Level of Evidence |
|---|---|
| Each recommendation is preceded with the phrase: “In children and adolescents with JIA and active polyarthritis…” | |
| Initial Therapy | |
| All patients | |
|
Moderate |
|
Low |
| Patients without risk factors†: | |
|
Low |
| Patients with risk factors: | |
|
Low |
| Subsequent Therapy - Low Disease Activity (cJADAS10 ≤ 2.5 and at least 1 active joint) | |
| For children receiving a DMARD and/or biologic: | |
| Very low | |
| Subsequent Therapy – Moderate/High Disease Activity (cJADAS10 > 2.5) | |
| If patient is receiving DMARD monotherapy: | |
|
Low |
|
Low |
| If patient is receiving first TNFi (+/− DMARD): | |
|
Very low |
| If patient is receiving second biologic: | |
|
Very low |
TNFi = tumor necrosis factor alpha inhibitor (etanercept, adalimumab, infliximab, golimumab).
Risk factors include presence of any of the following: positive anti-cyclic citrullinated peptide antibodies, positive rheumatoid factor, or presence of joint damage. The clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS-10) was used to define low disease activity (≤ 2.5 with ≥ 1 active joint) versus high/moderate disease activity (> 2.5). This is suggested as a general parameter, and the cJADAS-10 value should always be interpreted within the clinical context. An adequate trial of methotrexate was considered to be 3 months. If no or minimal response is observed after 6–8 weeks, it was agreed that changing or adding therapy may be appropriate. For the purposes of these recommendations, triple DMARD therapy is methotrexate, sulfasalazine, and hydroxychloroquine. The term biologic refers to TNFi, abatacept, or tocilizumab for each of the recommendations, with the exception of PICO B.10, which includes rituximab. Shared decision-making between the physician, parents, and child, including discussion of recommended treatments and potential alternatives, is recommended when initiating or escalating treatment.
Figure 1: Summary of the primary recommendations for the initial and subsequent treatment of children with JIA and active polyarthritis (see also Tables 3 and 4; for patients with sacroiliitis and/or enthesitis, see also Tables 5 and 6).
PICO questions in brackets, quality of evidence in parentheses. Strength of recommendation indicated by colors (see legend). The clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS-10) was used to define low disease activity (≤ 2.5 with ≥ 1 active joint) versus high/moderate disease activity (> 2.5). While this is provided as a general parameter, the cJADAS-10 should be interpreted within the clinical context. An adequate trial of methotrexate was considered to be 3 months. If no or minimal response is observed after 6–8 weeks, it was agreed that changing or adding therapy may be appropriate. Shared decision-making between the physician, parents, and patient, including discussion of recommended treatments and potential alternatives, is recommended when initiating or escalating treatment.
* DMARD over biologic recommended for patients without and with risk factors, although initial biologic therapy may be appropriate for some patients with risk factors and involvement of high risk joints, high disease activity, and/or those judged by their physician to be at high risk of disabling joint damage.
** Adding a biologic may be considered in biologic naïve patients with continued low disease activity after escalating therapy (not formally addressed in the guidelines)
DMARD = non-biologic disease modifying anti-rheumatic drug (methotrexate, leflunomide or sulfasalazine).
TNFi = tumor necrosis factor inhibitor, triple DMARD therapy = methotrexate, sulfasalazine, and hydroxychloroquine, NSAID = non-steroidal anti-inflammatory drug, PT = physical therapy, OT = occupational therapy.
While the initial set of PICO questions for polyarthritis also included comparisons between specific biologics, these questions were discarded at the in-person Voting Panel meeting due to lack of evidence to guide decision-making in those scenarios. Although there is most experience with TNFi as initial biologic, the class of initial biologic is not specified in the recommendations again due to lack of comparative data and the consideration that non-TNFi biologics may be preferred in certain scenarios based on patient-level factors (e.g., family history of demyelinating disease) and preferences. In the recommendations below, biologic therapy refers to TNFi, abatacept, or tocilizumab with the exception of PICO B.9, in which rituximab is also addressed.
Each recommendation in this section is prefaced with the statement “In children and adolescents with JIA and active polyarthritis…”
Initial Therapy
PICO B.1 Initial therapy with a DMARD is strongly recommended over NSAID monotherapy.
This recommendation is strong based upon moderate quality of supporting evidence, known risk of permanent joint damage associated with ongoing, active disease, and Voting Panel experience.34, 35, 40
PICO B.2. Using methotrexate monotherapy as initial therapy is conditionally recommended over triple DMARD therapy.
The quality of evidence for this recommendation was low, based on the available trial being relatively small and not blinded.71 Parents and patients also expressed concerns about the burden of taking the 3 different medications, but they did state a preference to be informed about this treatment option.
PICO B.3. For patients without risk factors, initial therapy with a DMARD is conditionally recommended over a biologic.
This recommendation is conditional based upon low quality of supporting evidence and parent and patient differing preferences around the risks and benefits of DMARDs and biologics. While initial treatment with a biologic has been studied in TREAT-JIA and ACUTE-JIA, the results were not felt to be conclusive enough to support biologics as initial therapy for low risk patients.39, 71 Of note, the majority of the Parent and Patient Panel voted against DMARDS as initial therapy as a number of the participants had considerable adverse effects with methotrexate and had experienced better outcomes with biologics.
PICO B.4. For patients with risk factors, initial therapy with a DMARD is conditionally recommended over a biologic, recognizing that there are situations where initial therapy that includes a biologic may be preferred.
This recommendation is conditional based upon low quality of supporting evidence and parent and patient differing preferences around the risks and benefits of DMARDs and biologics. While initial treatment with a biologic has been studied in TREAT-JIA and ACUTE-JIA, the results were not felt to be conclusive enough to support biologics as initial therapy.39, 71 However, the voting panel acknowledged that biologics may be appropriate initial therapy for some patients with risk factors and involvement of high risk joints (e.g. cervical spine, hip, and wrist), high disease activity, and/or those judged by their physician to be at high risk of disabling joint damage. Of note, the majority of the parent and patient group voted against DMARDs as initial therapy as a number of the participants had considerable adverse effects with methotrexate and had experienced better outcomes with biologics.
Subsequent Therapy - Low Disease Activity (cJADAS10 ≤ 2.5 and at least 1 active joint)
PICO B.5, B.6. In patients with JIA and polyarthritis and low disease activity (cJADAS10 ≤ 2.5 and at least 1 active joint) despite a DMARD or biologic, escalating therapy is conditionally recommended over no escalation of therapy.
This recommendation is conditional based upon the very low quality of supporting evidence and parent and patient preferences regarding risks and benefits of the treatment options. For this recommendation, escalating therapy is defined as any of the following: intraarticular glucocorticoid injection, increasing the DMARD or biologic dose (if not at optimal dosage), or changing biologic. Changing to an alternate DMARD (methotrexate) was suggested primarily for patients who had not yet received methotrexate and had not yet escalated to a biologic. Additional considerations included degree of improvement on current therapy and the specific joint that was active. Synovitis preventing ambulation or otherwise interfering with important daily activities was identified as a factor that would guide more aggressive intervention (e.g., intraarticular glucocorticoid injection or adding/changing biologic).
Subsequent Therapy - Moderate or High Disease Activity (cJADAS10 > 2.5)
PICO B.7. In patients with JIA and polyarthritis and moderate or high disease activity despite DMARD monotherapy, adding a biologic to the original DMARD is conditionally recommended over changing to a second DMARD.
This recommendation was conditional based upon low quality of supporting evidence and parent and patient preferences around the risks and benefits of DMARDs and biologic medications.28, 57, 60, 61, 63, 64, 66, 72–81
PICO B.8. In patients with JIA and polyarthritis and moderate or high disease activity receiving DMARD monotherapy, adding a biologic is conditionally recommended over changing to triple DMARD therapy.
The quality of evidence for this recommendation was low, based on the published pediatric trial being relatively small and not blinded.71 Although studies in RA have suggested that triple therapy is non-inferior to biologic therapy, the pediatric study showed improved outcomes for biologic therapy over triple DMARD therapy.82, 83 This recommendation was also supported by Voting Panel concerns about adherence to the regimen and tolerability, and parent and patient concerns about the burden of taking the 3 different medications.
PICO B.9. In patients with JIA and polyarthritis and moderate or high disease activity receiving a first TNFi (with or without DMARD), switching to a non-TNFi biologic (tocilizumab or abatacept) is conditionally recommended over switching to a second TNFi.
This recommendation was conditional based upon very low quality of supporting evidence and parent and patient preferences around the risks and benefits of biologics with different mechanisms of action.78, 84 In making this recommendation, the Voting Panel also considered data from rheumatoid arthritis that have suggested better outcomes with switching to a non-TNFi biologic.85–87 The Voting Panel agreed that a second TNFi may be appropriate for patients who had a good initial response to their first TNFi (i.e., secondary failure), particularly failure due to the presence of suspected or measured anti-drug antibodies to the first TNFi.
PICO B.10. In patients with JIA and polyarthritis and moderate or high disease activity despite a second biologic, using TNFi, abatacept, or tocilizumab (depending upon prior biologics received) is conditionally recommended over rituximab.
This recommendation was conditional based upon very low quality of supporting evidence, Voting Panel member experience, and parent and patient preferences around the risks and benefits of biologics with different mechanisms of action. This recommendation was also supported by the availability of data from randomized clinical trials of tocilizumab and abatacept establishing their efficacy in JIA, which is lacking for rituximab.63, 64, 66 In addition, the article identified for the evidence report showed a higher rate of serious adverse events for rituximab compared to other biologics.77 The Voting Panel did discuss that rituximab may be considered earlier for RF positive children based on data from rheumatoid arthritis, although the other 3 classes of biologics would still be primarily recommended.88
Recommendations for the treatment of JIA and sacroiliitis (Table 5)
Table 5.
Recommendations for the Initial and Subsequent Treatment of Children and Adolescents with JIA and Sacroiliitis*
| Recommendation | Level of Evidence |
|
Very low |
| In children and adolescents with active sacroiliitis despite NSAIDs: | |
|
Low |
|
Low |
|
Very low |
| Glucocorticoids | |
| In children and adolescents with active sacroiliitis despite treatment with NSAIDs: | |
| Very low | |
|
Very low |
| Physical Therapy | |
|
Very low |
TNFi = tumor necrosis factor alpha inhibitor (etanercept, adalimumab, infliximab, golimumab).
A bridging course of oral glucocorticoids was defined a short course (< 3 months) of oral glucocorticoid intended to control disease activity quickly during the initiation or escalation of therapy.
PICO C.1. In children and adolescents with JIA and active sacroiliitis, treatment with an NSAID is strongly recommended over no treatment with an NSAID.
This recommendation was strong despite very low quality of supporting evidence in children given the established utility of NSAIDs in adult spondyloarthritis, and the analgesic effects of NSAIDs in children with other forms of arthritis. This recommendation is in alignment with the treatment recommendations for ankylosing spondylitis co-developed by the American College of Rheumatology, Spondylitis Association of America, and Spondyloarthritis Research and Treatment Network.31
PICO C.2. In children and adolescents with active sacroiliitis despite NSAIDs, adding TNFi is strongly recommended over continued NSAID monotherapy.
Although the quality of supporting evidence in pediatrics for this recommendation was low, this recommendation is based on evaluation of both pediatric data, and data from adult spondyloarthritis that includes randomized controlled trials showing benefit.79, 89–94,95–98
PICO C.3. In children and adolescents with active sacroiliitis despite NSAIDs, using sulfasalazine for patients who have contraindications to TNFi or have failed more than one TNFi is conditionally recommended.
This recommendation was conditional based on low quality of supporting evidence, particularly the relatively limited efficacy of sulfasalazine demonstrated in a randomized controlled trial of juvenile spondyloarthritis.99 However, it was considered as a potential option for patients with contraindications to TNFi and based on parent and patient considerations of the risks and benefits of biologics versus sulfasalazine. Sulfasalazine was also considered as an option for patients with adverse events associated with their initial TNFi that would be considered a class effect and who would therefore not be able to receive additional TNFi. Non-TNFi biologics (e.g., interleukin-17 inhibition) were not considered by the Voting Panel because there are no published pediatric studies.
PICO C.4. In children and adolescents with active sacroiliitis despite NSAIDs, strongly recommend against using methotrexate monotherapy.
Although the quality of supporting evidence for this recommendation was very low, this recommendation is based on data from adult spondyloarthritis suggesting lack of effectiveness.91, 100–102 While we recommend against using methotrexate monotherapy as a treatment for sacroiliitis, methotrexate may have utility as adjunct therapy in patients with concomitant peripheral polyarthritis or to prevent the development of anti-drug antibodies against monoclonal TNFis.
PICO C.5. In children and adolescents with active sacroiliitis despite treatment with NSAIDs, bridging therapy with a limited course of oral glucocorticoid (< 3 months) during initiation or escalation of therapy is conditionally recommended.
This recommendation is conditional based upon very low quality of supporting evidence and known risks of glucocorticoid use. Bridging oral glucocorticoids may have most utility in the setting of high disease activity, limited mobility, and/or significant symptoms.
PICO C.6. In children and adolescents with active sacroiliitis despite treatment with NSAIDs, intraarticular glucocorticoid injections of the sacroiliac joints as adjunct therapy is conditionally recommended.
This recommendation was conditional based on very low quality of evidence and based upon varying parent and patient preferences regarding the procedure.
PICO C.7. In children and adolescents with sacroiliitis who have or are at risk for functional limitations, using PT is conditionally recommended.
This recommendation was conditional based on the very low quality of supporting evidence and Voting Panel experience. It was also discussed that there may be a role for PT and activity modification in specifically identifying and reducing mechanical factors contributing to microtrauma and repetitive stress that could potentially contribute to disease activity in these patients.103
Recommendations for the treatment of JIA and enthesitis (Table 6)
Table 6.
Recommendations for the Initial and Subsequent Treatment of Children and Adolescents with JIA and Enthesitis*
| Recommendation | Level of Evidence |
|---|---|
| In children and adolescents with active enthesitis, we strongly recommend NSAID over no treatment with an NSAID (PICO D.1). | Very low |
| In children and adolescents with active enthesitis despite treatment with NSAIDs: | |
| Low | |
| Very low | |
| Physical Therapy | |
|
Very low |
TNFi = tumor necrosis factor alpha inhibitor (etanercept, adalimumab, infliximab, golimumab).
A bridging course of oral glucocorticoids was defined a short course (< 3 months) of oral glucocorticoid intended to control disease activity quickly during the initiation or escalation of therapy.
PICO D.1. In children and adolescents with JIA and active enthesitis, we strongly recommend NSAID over no treatment with an NSAID.
This recommendation is strong despite the very low quality of supporting evidence based upon Voting Panel experience, established analgesic effects, and data from adult disease showing benefit.104
PICO D.2, D.3. In children and adolescents with JIA and active enthesitis despite treatment with NSAIDs, we conditionally recommend using TNFi over methotrexate or sulfasalazine.
This recommendation is conditional based upon the low quality of supporting evidence. While TNFi is preferred, the voting panel discussed that a trial of methotrexate or sulfasalazine may be warranted for patients with contraindications to TNFi, patients with mild enthesitis, and patients with concomitant active peripheral polyarthritis.79, 89–94, 99
PICO D.4. In children and adolescents with JIA and chronic active enthesitis despite treatment with NSAIDs, we conditionally recommend bridging therapy with a limited course of oral glucocorticoid (< 3 months) during initiation or escalation of therapy.
This recommendation is conditional based upon very low quality of supporting evidence and known risks of glucocorticoid use in the pediatric population. Bridging glucocorticoids may have most utility in the setting of high disease activity, limited mobility, and/or significant symptoms.
PICO D.5. In children and adolescents with JIA and enthesitis who have or are at risk for functional limitations, we conditionally recommend using PT.
This recommendation was conditional based on very low quality of evidence and Voting Panel experience.
DISCUSSION
This guideline includes 39 recommendations for the treatment of children with JIA and non-systemic polyarthritis, sacroiliitis, and enthesitis. Most of the available evidence was low or very low quality in relation to the relevant clinical PICO questions, resulting in 31 of the recommendations being conditional.
These recommendations provide an updated approach to the treatment of children with non-systemic polyarthritis, sacroiliitis, and enthesitis. These populations were chosen for this guideline as they have been the focus of significant recent research, with better delineation of the underlying biology and additional treatments available since the 2011 ACR recommendations for JIA. Similar to the 2011 recommendations, this guideline defined patient populations by clinical phenotypes, rather than ILAR categories. This decision was made as data continue to suggest that current JIA categories may not accurately reflect the underlying biology and anticipated treatment responses in juvenile arthritis.
This guideline differs from the 2011 recommendations in the definitions of risk factors and disease activity assessment used to generate patient scenarios. While PICO questions were initially stratified by risk factors and disease activity, the Voting Panel ultimately determined that in most scenarios there were not sufficient data to recommend different treatments for these patients and recommendations were frequently combined. Another important difference from the 2011 recommendations is that initial NSAID monotherapy for polyarthritis is no longer recommended, given the established benefits of early initiation of DMARD treatment. Individual PICO questions for each biologic were initially considered but subsequently dropped by the Voting Panel given mostly equivalent data for safety and efficacy between the biologics and lack of head to head comparisons. The exceptions were that TNFi are specifically recommended for sacroiliitis and rituximab is only considered after TNFi, abatacept, and tocilizumab have been tried. This approach has resulted in a simplified treatment algorithm. Lastly this guideline also includes recommendations for escalating care in the setting of low disease activity, highlighting the importance of achieving and maintaining complete disease control, which was not previously addressed.
The current recommendations also differ from the 2011 recommendations in that they were developed using the GRADE methodology instead of the RAND/UCLA Appropriateness Method. The systematic, transparent, and explicit process of developing recommendations through GRADE is a major feature accelerating its adoption by professional groups internationally (www.gradeworkinggroup.org). Important features of this method are 1) specification of the patient groups, interventions, competing alternatives, and outcomes so that each recommendation is clearly focused on a particular clinical situation; 2) grading of the quality of evidence; and 3) basing the strength of recommendations on the quality of evidence, balance of benefits and harms, and patient values and preferences for different treatment options. This guideline considered parent and patient preferences assessed by a separate Parent and Patient Panel. Primary themes that emerged from that discussion were: 1) the importance of shared-decision making; 2) the importance of parents and patients receiving information regarding not only the preferred medication or intervention, but also the alternatives and 3) parent/patient support of earlier consideration of biologics given their experiences with decreased adverse effects and improved quality of life with the use of these medications relative to their experiences with methotrexate. Although this was a select group of parents and patients and their experiences may not be representative of all patients, their discussion provided an important perspective that was incorporated into the Voting Panel discussion and voting.
A topic of particular debate among the Voting Panel was the appropriateness of the use of biologics as initial therapy in children with polyarthritis, particularly for those with risk factors. Ultimately, non-biologic DMARD therapy was recommended, but it was noted that there may be some patients for whom initial biologic therapy is indicated. This remains an area of active research and currently ongoing studies may better clarify which patients are most likely to benefit from initial biologic therapy. Importantly, studies in pediatrics are underway or planned for a number of new medications, including Janus kinase inhibitors and interleukin-17 and interleukin-12/23 inhibitors, and these medications may become useful additions as treatment options for JIA, particularly for patients with sacroiliitis for whom limited options exist. Other studies currently underway in parallel adult diseases may also inform the optimal treatment of enthesitis and the treatment of peripheral spondyloarthritis. Future guidelines efforts may determine where these treatments fit into the treatment algorithm and will incorporate the results from the ongoing studies once complete.
Non-pharmacologic interventions addressed in this guideline included PT and OT. In each case very limited data were identified and future research in these modalities will be helpful in identifying patients most likely to benefit from these interventions and which modalities have most effectiveness for particular clinical scenarios.
The cJADAS-10 was used to provide general disease activity parameters for defining low and moderate/high disease activity. However, this is not intended to be prescriptive and should be interpreted within the overall clinical context. Furthermore, as the JADAS is a relatively new disease activity measurement tool, new cutoffs may be proposed as additional data are generated that may accommodate different numbers of active joints or other levels of physician or parent global. The identification of valid and practical disease activity measures in JIA remains an important research agenda in pediatric rheumatology. Nevertheless, it was agreed by the Voting Panel that treatment should be escalated in patients with even one active joint. Formal recommendations regarding disease activity measurement tools, cut-offs, and monitoring intervals were not specifically addressed in this guideline. Lastly, the management of inactive disease and the tapering and withdrawal of medications for patients with inactive disease are not addressed in this guideline but will be important for future guidelines.
As the quality of evidence was overall low and most recommendations were conditional, clinicians, caregivers, and patients should use a shared decision-making process in considering these recommendations. While these recommendations are intended to address common clinical situations, all treatment decisions must be individualized, with consideration of the unique aspects of each patient’s presentation, medical history, and preferences.
Supplementary Material
Guidelines and recommendations developed and/or endorsed by the American College of Rheumatology (ACR) are intended to provide guidance for patterns of practice and not to dictate the care of a particular patient. The ACR considers adherence to the recommendations within this guideline to be voluntary, with the ultimate determination regarding their application to be made by the physician in light of each patient’s individual circumstances. Guidelines and recommendations are intended to promote beneficial or desirable outcomes, but cannot guarantee any specific outcome. Guidelines and recommendations developed and endorsed by the ACR are subject to periodic revision, as warranted by the evolution of medical knowledge, technology, and practice. ACR recommendations are not intended to dictate payment or insurance decisions. These recommendations cannot adequately convey all uncertainties and nuances of patient care.
The American College of Rheumatology is an independent, professional, medical and scientific society that does not guarantee, warrant, or endorse any commercial product or service.
SIGNIFICANCE & INNOVATION.
Children with non-systemic polyarthritis, sacroiliitis, and enthesitis are at risk for permanent joint damage and decreased health related quality of life.
Early initiation of DMARD therapy in children with juvenile idiopathic arthritis is important for optimal disease outcomes.
In children with low disease activity, escalation of therapy may be needed for complete disease control.
ACKNOWLEDGMENTS
We thank Alexei Grom, MD, Ron Laxer, MD, FRCP, Mindy Lo, MD, PhD, Sampath Prahalad, MD, MSc, Meredith Riebschleger, MD, Angela Byun Robinson, MD, MPH, Grant Schulert, MD, PhD, Heather Tory, MD, and Richard Vehe, MD, for serving on the Expert Panel. We thank Ms. Suzanne Schrandt with the AF for her involvement throughout the guideline development process. We thank our patient representative for adding valuable perspectives. We thank Dr. Liana Frankel for leading the Patient Panel meeting, as well the patients and parents who participated in this meeting – Linda Aguiar, Jake Anderson, Samantha Bell, Julianne Capron, Stephanie Dodunski, Holly Dwyer, Stephanie Kweicein, Carolina Mejia Pena, and Nikki Reitz, LCSW. We thank the ACR staff, including Ms. Regina Parker for assistance in organizing the face-to-face meeting and coordinating the administrative aspects of the project, and Ms. Robin Lane for assistance in manuscript preparation. We thank Ms. Janet Waters for help in developing the literature search strategy and performing the literature search and updates, and Ms. Janet Joyce for peer-reviewing the literature search strategy.
Grant support: This material is the result of a project supported by the American College of Rheumatology (ACR) and the Arthritis Foundation (AF). Dr. Angeles-Han was supported by Award Number K23EY021760 from the National Eye Institute, the Rheumatology Research Foundation, and the Cincinnati Children’s Hospital Medical Center Research Innovation and Pilot fund. Drs. Colbert (AR041184) and Ombrello (AR041198) were supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. Dr. Nigrovic was supported by the Fundación Bechara.
Footnotes
Financial Conflict: Forms submitted as required.
IRB approval: This study did not involve human subjects and, therefore, approval from Human Studies Committees was not required.
REFERENCES
- 1.Mielants H, Veys EM, Maertens M, Goemaere S, De Clercq L, Castro S, Praet J. Prevalence of inflammatory rheumatic diseases in an adolescent urban student population, age 12 to 18, in Belgium. Clin Exp Rheumatol. 1993;11(5):563–567. [PubMed] [Google Scholar]
- 2.Danner S, Sordet C, Terzic J, Donato L, Velten M, Fischbach M, Sibilia J. Epidemiology of juvenile idiopathic arthritis in Alsace, France. J Rheumatol. 2006;33(7):1377–1381. [PubMed] [Google Scholar]
- 3.Hanova P, Pavelka K, Dostal C, Holcatova I, Pikhart H. Epidemiology of rheumatoid arthritis, juvenile idiopathic arthritis and gout in two regions of the Czech Republic in a descriptive population-based survey in 2002–2003. Clin Exp Rheumatol. 2006;24(5):499–507. [PubMed] [Google Scholar]
- 4.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur AM, Suarez-Almazor ME, Woo P. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. [PubMed] [Google Scholar]
- 5.Gutierrez-Suarez R, Pistorio A, Cespedes Cruz A, Norambuena X, Flato B, Rumba I, Harjacek M, Nielsen S, Susic G, Mihaylova D, Huemer C, Melo-Gomes J, Andersson-Gare B, Balogh Z, De Cunto C, Vesely R, Pagava K, Romicka AM, Burgos-Vargas R, Martini A, Ruperto N. Health-related quality of life of patients with juvenile idiopathic arthritis coming from 3 different geographic areas. The PRINTO multinational quality of life cohort study. Rheumatology (Oxford). 2007;46(2):314–320. [DOI] [PubMed] [Google Scholar]
- 6.Seid M, Opipari L, Huang B, Brunner HI, Lovell DJ. Disease control and health-related quality of life in juvenile idiopathic arthritis. Arthritis Rheum. 2009;61(3):393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minden K, Niewerth M, Listing J, Biedermann T, Bollow M, Schontube M, Zink A. Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46(9):2392–2401. [DOI] [PubMed] [Google Scholar]
- 8.Oen K, Malleson PN, Cabral DA, Rosenberg AM, Petty RE, Cheang M. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002;29(9):1989–1999. [PubMed] [Google Scholar]
- 9.Zak M, Pedersen FK. Juvenile chronic arthritis into adulthood: a long-term follow-up study. Rheumatology (Oxford). 2000;39(2):198–204. [DOI] [PubMed] [Google Scholar]
- 10.Schanberg LE, Anthony KK, Gil KM, Maurin EC. Daily pain and symptoms in children with polyarticular arthritis. Arthritis Rheum. 2003;48(5):1390–1397. [DOI] [PubMed] [Google Scholar]
- 11.Schanberg LE, Gil KM, Anthony KK, Yow E, Rochon J. Pain, stiffness, and fatigue in juvenile polyarticular arthritis: contemporaneous stressful events and mood as predictors. Arthritis Rheum. 2005;52(4):1196–1204. [DOI] [PubMed] [Google Scholar]
- 12.Ringold S, Wallace CA, Rivara FP. Health-related quality of life, physical function, fatigue, and disease activity in children with established polyarticular juvenile idiopathic arthritis. J Rheumatol. 2009;36(6):1330–1336. [DOI] [PubMed] [Google Scholar]
- 13.Ringold S, Ward TM, Wallace CA. Disease activity and fatigue in juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ringold S, Weiss PF, Beukelman T, DeWitt EM, Ilowite NT, Kimura Y, Laxer RM, Lovell DJ, Nigrovic PA, Robinson AB, Vehe RK. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum. 2013;65(10):2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, Ilowite NT, Kimura Y, Laxer RM, Lovell DJ, Martini A, Rabinovich CE, Ruperto N. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011;63(4):465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, Nasser M, Meerpohl J, Post PN, Kunz R, Brozek J, Vist G, Rind D, Akl EA, Schunemann HJ. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719–725. [DOI] [PubMed] [Google Scholar]
- 18.Andrews JC, Schunemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, Rind D, Montori VM, Brito JP, Norris S, Elbarbary M, Post P, Nasser M, Shukla V, Jaeschke R, Brozek J, Djulbegovic B, Guyatt G. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66(7):726–735. [DOI] [PubMed] [Google Scholar]
- 19.Hinks A, Cobb J, Marion MC, Prahalad S, Sudman M, Bowes J, Martin P, Comeau ME, Sajuthi S, Andrews R, Brown M, Chen WM, Concannon P, Deloukas P, Edkins S, Eyre S, Gaffney PM, Guthery SL, Guthridge JM, Hunt SE, James JA, Keddache M, Moser KL, Nigrovic PA, Onengut-Gumuscu S, Onslow ML, Rose CD, Rich SS, Steel KJ, Wakeland EK, Wallace CA, Wedderburn LR, Woo P, Boston Children’s JIAR, British Society of P, Adolescent Rheumatology Study G, Childhood Arthritis Prospective S, Childhood Arthritis Response to Medication S, German Society for Pediatric R, Study JIAGE, Registry NJG, Study T, United Kingdom Juvenile Idiopathic Arthritis Genetics C, Bohnsack JF, Haas JP, Glass DN, Langefeld CD, Thomson W, Thompson SD. Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat Genet. 2013;45(6):664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinks A, Bowes J, Cobb J, Ainsworth HC, Marion MC, Comeau ME, Sudman M, Han B, Juvenile Arthritis Consortium for I, Becker ML, Bohnsack JF, de Bakker PI, Haas JP, Hazen M, Lovell DJ, Nigrovic PA, Nordal E, Punnaro M, Rosenberg AM, Rygg M, Smith SL, Wise CA, Videm V, Wedderburn LR, Yarwood A, Yeung RS, Prahalad S, Langefeld CD, Raychaudhuri S, Thompson SD, Thomson W. Fine-mapping the MHC locus in juvenile idiopathic arthritis (JIA) reveals genetic heterogeneity corresponding to distinct adult inflammatory arthritic diseases. Ann Rheum Dis. 2017;76(4):765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nigrovic PA, Raychaudhuri S, Thompson SD. Review: Genetics and the Classification of Arthritis in Adults and Children. Arthritis Rheumatol. 2018;70(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, Malattia C, Viola S, Martini A, Ravelli A. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61(5):658–666. [DOI] [PubMed] [Google Scholar]
- 23.Consolaro A, Bracciolini G, Ruperto N, Pistorio A, Magni-Manzoni S, Malattia C, Pederzoli S, Davi S, Martini A, Ravelli A. Remission, minimal disease activity, and acceptable symptom state in juvenile idiopathic arthritis: defining criteria based on the juvenile arthritis disease activity score. Arthritis Rheum. 2012;64(7):2366–2374. [DOI] [PubMed] [Google Scholar]
- 24.Consolaro A, Negro G, Chiara Gallo M, Bracciolini G, Ferrari C, Schiappapietra B, Pistorio A, Bovis F, Ruperto N, Martini A, Ravelli A. Defining criteria for disease activity states in nonsystemic juvenile idiopathic arthritis based on a three-variable juvenile arthritis disease activity score. Arthritis Care Res (Hoboken). 2014;66(11):1703–1709. [DOI] [PubMed] [Google Scholar]
- 25.Bulatovic Calasan M, de Vries LD, Vastert SJ, Heijstek MW, Wulffraat NM. Interpretation of the Juvenile Arthritis Disease Activity Score: responsiveness, clinically important differences and levels of disease activity in prospective cohorts of patients with juvenile idiopathic arthritis. Rheumatology (Oxford). 2014;53(2):307–312. [DOI] [PubMed] [Google Scholar]
- 26.Backstrom M, Tynjala P, Ylijoki H, Aalto K, Karki J, Pohjankoski H, Keskitalo P, Sard S, Hietanen M, Lehto H, Kauko T, Vahasalo P. Finding specific 10-joint Juvenile Arthritis Disease Activity Score (JADAS10) and clinical JADAS10 cut-off values for disease activity levels in non-systemic juvenile idiopathic arthritis: a Finnish multicentre study. Rheumatology (Oxford). 2016;55(4):615–623. [DOI] [PubMed] [Google Scholar]
- 27.Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40(7):1202–1209. [DOI] [PubMed] [Google Scholar]
- 28.Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Whitmore J, Finck BK. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342(11):763–769. [DOI] [PubMed] [Google Scholar]
- 29.Magni-Manzoni S, Ruperto N, Pistorio A, Sala E, Solari N, Palmisani E, Cugno C, Bozzola E, Martini A, Ravelli A. Development and validation of a preliminary definition of minimal disease activity in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2008;59(8):1120–1127. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, Alderson P, Glasziou P, Falck-Ytter Y, Schunemann HJ. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400. [DOI] [PubMed] [Google Scholar]
- 31.Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, Gensler LS, Smith JA, Borenstein D, Hiratzka J, Weiss PF, Inman RD, Majithia V, Haroon N, Maksymowych WP, Joyce J, Clark BM, Colbert RA, Figgie MP, Hallegua DS, Prete PE, Rosenbaum JT, Stebulis JA, Van Den Bosch F, Yu DT, Miller AS, Reveille JD, Caplan L. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Care Res (Hoboken). 2016;68(2):151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaeschke R, Guyatt GH, Dellinger P, Schunemann H, Levy MM, Kunz R, Norris S, Bion J, Group GW. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:a744. [DOI] [PubMed] [Google Scholar]
- 33.Sobel RE, Lovell DJ, Brunner HI, Weiss JE, Morris PW, Gottlieb BS, Chalom EC, Jung LK, Onel KB, Petiniot L, Goldsmith DP, Nanda K, Shishov M, Abramsky S, Young JP, Giannini EH, Pediatric Rheumatology Collaborative Study G. Safety of celecoxib and nonselective nonsteroidal anti-inflammatory drugs in juvenile idiopathic arthritis: results of the Phase 4 registry. Pediatr Rheumatol Online J. 2014;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rossum MA, Fiselier TJ, Franssen MJ, Zwinderman AH, ten Cate R, van Suijlekom-Smit LW, van Luijk WH, van Soesbergen RM, Wulffraat NM, Oostveen JC, Kuis W, Dijkstra PF, van Ede CF, Dijkmans BA. Sulfasalazine in the treatment of juvenile chronic arthritis: a randomized, double-blind, placebo-controlled, multicenter study. Dutch Juvenile Chronic Arthritis Study Group. Arthritis Rheum. 1998;41(5):808–816. [DOI] [PubMed] [Google Scholar]
- 35.van Rossum MA, van Soesbergen RM, Boers M, Zwinderman AH, Fiselier TJ, Franssen MJ, ten Cate R, van Suijlekom-Smit LW, Wulffraat NM, van Luijk WH, Oostveen JC, Kuis W, Dijkmans BA, Dutch Juvenile Idiopathic Arthritis Study g. Long-term outcome of juvenile idiopathic arthritis following a placebo-controlled trial: sustained benefits of early sulfasalazine treatment. Ann Rheum Dis. 2007;66(11):1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silverman E, Mouy R, Spiegel L, Jung LK, Saurenmann RK, Lahdenne P, Horneff G, Calvo I, Szer IS, Simpson K, Stewart JA, Strand V, Leflunomide in Juvenile Rheumatoid Arthritis Investigator G. Leflunomide or methotrexate for juvenile rheumatoid arthritis. N Engl J Med. 2005;352(16):1655–1666. [DOI] [PubMed] [Google Scholar]
- 37.Silverman E, Spiegel L, Hawkins D, Petty R, Goldsmith D, Schanberg L, Duffy C, Howard P, Strand V. Long-term open-label preliminary study of the safety and efficacy of leflunomide in patients with polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2005;52(2):554–562. [DOI] [PubMed] [Google Scholar]
- 38.Klein A, Kaul I, Foeldvari I, Ganser G, Urban A, Horneff G. Efficacy and safety of oral and parenteral methotrexate therapy in children with juvenile idiopathic arthritis: an observational study with patients from the German Methotrexate Registry. Arthritis Care Res (Hoboken). 2012;64(9):1349–1356. [DOI] [PubMed] [Google Scholar]
- 39.Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O’Neil KM, Zeft AS, Szer IS, Ringold S, Brunner HI, Schanberg LE, Sundel RP, Milojevic D, Punaro MG, Chira P, Gottlieb BS, Higgins GC, Ilowite NT, Kimura Y, Hamilton S, Johnson A, Huang B, Lovell DJ, Childhood A, Rheumatology Research A. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum. 2012;64(6):2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giannini EH, Brewer EJ, Kuzmina N, Shaikov A, Maximov A, Vorontsov I, Fink CW, Newman AJ, Cassidy JT, Zemel LS. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.A.-U.S.S.R. double-blind, placebo-controlled trial. The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children’s Study Group. N Engl J Med. 1992;326(16):1043–1049. [DOI] [PubMed] [Google Scholar]
- 41.Bulatovic M, Heijstek MW, Verkaaik M, van Dijkhuizen EH, Armbrust W, Hoppenreijs EP, Kamphuis S, Kuis W, Egberts TC, Sinnema G, Rademaker CM, Wulffraat NM. High prevalence of methotrexate intolerance in juvenile idiopathic arthritis: development and validation of a methotrexate intolerance severity score. Arthritis Rheum. 2011;63(7):2007–2013. [DOI] [PubMed] [Google Scholar]
- 42.Franova J, Fingerhutova S, Kobrova K, Srp R, Nemcova D, Hoza J, Uher M, Saifridova M, Linkova L, Dolezalova P. Methotrexate efficacy, but not its intolerance, is associated with the dose and route of administration. Pediatr Rheumatol Online J. 2016;14(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Dijkhuizen EH, Pouw JN, Scheuern A, Hugle B, Hardt S, Ganser G, Kummerle-Deschner JB, Horneff G, Holzinger D, Bulatovic Calasan M, Wulffraat NM. Methotrexate intolerance in oral and subcutaneous administration in patients with juvenile idiopathic arthritis: a cross-sectional, observational study. Clin Exp Rheumatol. 2016;34(1):148–154. [PubMed] [Google Scholar]
- 44.Zuber Z, Turowska-Heydel D, Sobczyk M, Banach-Gornicka M, Rusnak K, Piszczek A, Mezyk E. Methotrexate efficacy and tolerability after switching from oral to subcutaneous route of administration in juvenile idiopathic arthritis. Reumatologia. 2016;54(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravelli A, Gerloni V, Corona F, Falcini F, Lepore L, De Sanctis R, Zulian F, Buoncompagni A, Sardella ML, Strano CG, Alessio M, Fantini F, Bardare M, Martini A. Oral versus intramuscular methotrexate in juvenile chronic arthritis. Italian Pediatric Rheumatology Study Group. Clin Exp Rheumatol. 1998;16(2):181–183. [PubMed] [Google Scholar]
- 46.Tukova J, Chladek J, Nemcova D, Chladkova J, Dolezalova P. Methotrexate bioavailability after oral and subcutaneous dministration in children with juvenile idiopathic arthritis. Clin Exp Rheumatol. 2009;27(6):1047–1053. [PubMed] [Google Scholar]
- 47.Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses >/=15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73(8):1549–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papadopoulou C, Kostik M, Gonzalez-Fernandez MI, Bohm M, Nieto-Gonzalez JC, Pistorio A, Lanni S, Consolaro A, Martini A, Ravelli A. Delineating the role of multiple intraarticular corticosteroid injections in the management of juvenile idiopathic arthritis in the biologic era. Arthritis Care Res (Hoboken). 2013;65(7):1112–1120. [DOI] [PubMed] [Google Scholar]
- 49.Zulian F, Martini G, Gobber D, Plebani M, Zacchello F, Manners P. Triamcinolone acetonide and hexacetonide intra-articular treatment of symmetrical joints in juvenile idiopathic arthritis: a double-blind trial. Rheumatology (Oxford). 2004;43(10):1288–1291. [DOI] [PubMed] [Google Scholar]
- 50.Zulian F, Martini G, Gobber D, Agosto C, Gigante C, Zacchello F. Comparison of intra-articular triamcinolone hexacetonide and triamcinolone acetonide in oligoarticular juvenile idiopathic arthritis. Rheumatology (Oxford). 2003;42(10):1254–1259. [DOI] [PubMed] [Google Scholar]
- 51.Blyth T, Hunter JA, Stirling A. Pain relief in the rheumatoid knee after steroid injection. A single-blind comparison of hydrocortisone succinate, and triamcinolone acetonide or hexacetonide. Br J Rheumatol. 1994;33(5):461–463. [DOI] [PubMed] [Google Scholar]
- 52.Giannini EH, Ilowite NT, Lovell DJ, Wallace CA, Rabinovich CE, Reiff A, Higgins G, Gottlieb B, Singer NG, Chon Y, Lin SL, Baumgartner SW, Pediatric Rheumatology Collaborative Study G. Long-term safety and effectiveness of etanercept in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 2009;60(9):2794–2804. [DOI] [PubMed] [Google Scholar]
- 53.Horneff G, Ebert A, Fitter S, Minden K, Foeldvari I, Kummerle-Deschner J, Thon A, Girschick HJ, Weller F, Huppertz HI. Safety and efficacy of once weekly etanercept 0.8 mg/kg in a multicentre 12 week trial in active polyarticular course juvenile idiopathic arthritis. Rheumatology (Oxford). 2009;48(8):916–919. [DOI] [PubMed] [Google Scholar]
- 54.Beukelman T, Xie F, Baddley JW, Chen L, Mannion ML, Saag KG, Zhang J, Curtis JR. The risk of hospitalized infection following initiation of biologic agents versus methotrexate in the treatment of juvenile idiopathic arthritis. Arthritis Res Ther. 2016;18(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barthel D, Ganser G, Kuester RM, Onken N, Minden K, Girschick HJ, Hospach A, Horneff G. Inflammatory Bowel Disease in Juvenile Idiopathic Arthritis Patients Treated with Biologics. J Rheumatol. 2015;42(11):2160–2165. [DOI] [PubMed] [Google Scholar]
- 56.Papsdorf V, Horneff G. Complete control of disease activity and remission induced by treatment with etanercept in juvenile idiopathic arthritis. Rheumatology (Oxford). 2011;50(1):214–221. [DOI] [PubMed] [Google Scholar]
- 57.Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Chon Y, Lin SL, Baumgartner SW, Giannini EH, Pediatric Rheumatology Collaborative Study G. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008;58(5):1496–1504. [DOI] [PubMed] [Google Scholar]
- 58.Davies R, Southwood TR, Kearsley-Fleet L, Lunt M, Hyrich KL, British Society for P, Adolescent Rheumatology Etanercept Cohort S. Medically Significant Infections Are Increased in Patients With Juvenile Idiopathic Arthritis Treated With Etanercept: Results From the British Society for Paediatric and Adolescent Rheumatology Etanercept Cohort Study. Arthritis Rheumatol. 2015;67(9):2487–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klotsche J, Niewerth M, Haas JP, Huppertz HI, Zink A, Horneff G, Minden K. Long-term safety of etanercept and adalimumab compared to methotrexate in patients with juvenile idiopathic arthritis (JIA). Ann Rheum Dis. 2016;75(5):855–861. [DOI] [PubMed] [Google Scholar]
- 60.Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, Nemcova D, Mouy R, Sandborg C, Bohnsack J, Elewaut D, Foeldvari I, Gerloni V, Rovensky J, Minden K, Vehe RK, Weiner LW, Horneff G, Huppertz HI, Olson NY, Medich JR, Carcereri-De-Prati R, McIlraith MJ, Giannini EH, Martini A, Pediatric Rheumatology Collaborative Study G, Pediatric Rheumatology International Trials O. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359(8):810–820. [DOI] [PubMed] [Google Scholar]
- 61.Brunner HI, Ruperto N, Tzaribachev N, Horneff G, Chasnyk VG, Panaviene V, Abud-Mendoza C, Reiff A, Alexeeva E, Rubio-Perez N, Keltsev V, Kingsbury DJ, Del Rocio Maldonado Velazquez M, Nikishina I, Silverman ED, Joos R, Smolewska E, Bandeira M, Minden K, van Royen-Kerkhof A, Emminger W, Foeldvari I, Lauwerys BR, Sztajnbok F, Gilmer KE, Xu Z, Leu JH, Kim L, Lamberth SL, Loza MJ, Lovell DJ, Martini A, Paediatric Rheumatology International Trials O, the Pediatric Rheumatology Collaborative Study G. Subcutaneous golimumab for children with active polyarticular-course juvenile idiopathic arthritis: results of a multicentre, double-blind, randomised-withdrawal trial. Ann Rheum Dis. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruperto N, Lovell DJ, Li T, Sztajnbok F, Goldenstein-Schainberg C, Scheinberg M, Penades IC, Fischbach M, Alcala JO, Hashkes PJ, Hom C, Jung L, Lepore L, Oliveira S, Wallace C, Alessio M, Quartier P, Cortis E, Eberhard A, Simonini G, Lemelle I, Chalom EC, Sigal LH, Block A, Covucci A, Nys M, Martini A, Giannini EH, Paediatric Rheumatology International Trials O, Pediatric Rheumatology Collaborative Study G. Abatacept improves health-related quality of life, pain, sleep quality, and daily participation in subjects with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2010;62(11):1542–1551. [DOI] [PubMed] [Google Scholar]
- 63.Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, Abud-Mendoza C, Burgos-Vargas R, Gerloni V, Melo-Gomes JA, Saad-Magalhaes C, Chavez-Corrales J, Huemer C, Kivitz A, Blanco FJ, Foeldvari I, Hofer M, Horneff G, Huppertz HI, Job-Deslandre C, Loy A, Minden K, Punaro M, Nunez AF, Sigal LH, Block AJ, Nys M, Martini A, Giannini EH, Paediatric Rheumatology International Trials O, the Pediatric Rheumatology Collaborative Study G. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62(6):1792–1802. [DOI] [PubMed] [Google Scholar]
- 64.Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, Abud-Mendoza C, Burgos-Vargas R, Gerloni V, Melo-Gomes JA, Saad-Magalhaes C, Sztajnbok F, Goldenstein-Schainberg C, Scheinberg M, Penades IC, Fischbach M, Orozco J, Hashkes PJ, Hom C, Jung L, Lepore L, Oliveira S, Wallace CA, Sigal LH, Block AJ, Covucci A, Martini A, Giannini EH, Paediatric Rheumatology ITO, Pediatric Rheumatology Collaborative Study G. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372(9636):383–391. [DOI] [PubMed] [Google Scholar]
- 65.Lovell DJ, Ruperto N, Mouy R, Paz E, Rubio-Perez N, Silva CA, Abud-Mendoza C, Burgos-Vargas R, Gerloni V, Melo-Gomes JA, Saad-Magalhaes C, Chavez-Corrales J, Huemer C, Kivitz A, Blanco FJ, Foeldvari I, Hofer M, Huppertz HI, Job Deslandre C, Minden K, Punaro M, Block AJ, Giannini EH, Martini A, Pediatric Rheumatology Collaborative Study G, the Paediatric Rheumatology International Trials O. Long-term safety, efficacy, and quality of life in patients with juvenile idiopathic arthritis treated with intravenous abatacept for up to seven years. Arthritis Rheumatol. 2015;67(10):2759–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brunner HI, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, Lu P, Cuttica R, Keltsev V, Xavier RM, Calvo I, Nikishina I, Rubio-Perez N, Alexeeva E, Chasnyk V, Horneff G, Opoka-Winiarska V, Quartier P, Silva CA, Silverman E, Spindler A, Baildam E, Gamir ML, Martin A, Rietschel C, Siri D, Smolewska E, Lovell D, Martini A, De Benedetti F. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, Wouters C, Silverman ED, Balogh Z, Henrickson M, Apaz MT, Baildam E, Fasth A, Gerloni V, Lahdenne P, Prieur AM, Ravelli A, Saurenmann RK, Gamir ML, Wulffraat N, Marodi L, Petty RE, Joos R, Zulian F, McCurdy D, Myones BL, Nagy K, Reuman P, Szer I, Travers S, Beutler A, Keenan G, Clark J, Visvanathan S, Fasanmade A, Raychaudhuri A, Mendelsohn A, Martini A, Giannini EH, Paediatric Rheumatology International Trials O, Pediatric Rheumatology Collaborative Study G. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56(9):3096–3106. [DOI] [PubMed] [Google Scholar]
- 68.Ruperto N, Lovell DJ, Cuttica R, Woo P, Meiorin S, Wouters C, Silverman ED, Balogh Z, Henrickson M, Davidson J, Foeldvari I, Imundo L, Simonini G, Oppermann J, Xu S, Shen YK, Visvanathan S, Fasanmade A, Mendelsohn A, Martini A, Giannini EH, Paediatric Rheumatology ITO, Pediatric Rheumatology Collaborative Study G. Long-term efficacy and safety of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis: findings from an open-label treatment extension. Ann Rheum Dis. 2010;69(4):718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eid MA, Aly SM, El-Shamy SM. Effect of Electromyographic Biofeedback Training on Pain, Quadriceps Muscle Strength, and Functional Ability in Juvenile Rheumatoid Arthritis. Am J Phys Med Rehabil. 2016;95(12):921–930. [DOI] [PubMed] [Google Scholar]
- 70.Klepper SE. Effects of an eight-week physical conditioning program on disease signs and symptoms in children with chronic arthritis. Arthritis Care Res. 1999;12(1):52–60. [DOI] [PubMed] [Google Scholar]
- 71.Tynjala P, Vahasalo P, Tarkiainen M, Kroger L, Aalto K, Malin M, Putto-Laurila A, Honkanen V, Lahdenne P. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (ACUTE-JIA): a multicentre randomised open-label clinical trial. Ann Rheum Dis. 2011;70(9):1605–1612. [DOI] [PubMed] [Google Scholar]
- 72.Lovell DJ, Giannini EH, Reiff A, Jones OY, Schneider R, Olson JC, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Lange M, Finck BK, Burge DJ, Pediatric Rheumatology Collaborative Study G. Long-term efficacy and safety of etanercept in children with polyarticular-course juvenile rheumatoid arthritis: interim results from an ongoing multicenter, open-label, extended-treatment trial. Arthritis Rheum. 2003;48(1):218–226. [DOI] [PubMed] [Google Scholar]
- 73.Hissink Muller PC, Brinkman DM, Schonenberg D, Koopman-Keemink Y, Brederije IC, Bekkering WP, Kuijpers TW, van Rossum MA, van Suijlekom-Smit LW, van den Berg JM, Allaart CF, Ten Cate R. A comparison of three treatment strategies in recent onset non-systemic Juvenile Idiopathic Arthritis: initial 3-months results of the BeSt for Kids-study. Pediatr Rheumatol Online J. 2017;15(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kearsley-Fleet L, Davies R, Lunt M, Southwood TR, Hyrich KL. Factors associated with improvement in disease activity following initiation of etanercept in children and young people with Juvenile Idiopathic Arthritis: results from the British Society for Paediatric and Adolescent Rheumatology Etanercept Cohort Study. Rheumatology (Oxford). 2016;55(5):840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verazza S, Davi S, Consolaro A, Bovis F, Insalaco A, Magni-Manzoni S, Nicolai R, Marafon DP, De Benedetti F, Gerloni V, Pontikaki I, Rovelli F, Cimaz R, Marino A, Zulian F, Martini G, Pastore S, Sandrin C, Corona F, Torcoletti M, Conti G, Fede C, Barone P, Cattalini M, Cortis E, Breda L, Olivieri AN, Civino A, Podda R, Rigante D, La Torre F, D’Angelo G, Jorini M, Gallizzi R, Maggio MC, Consolini R, De Fanti A, Muratore V, Alpigiani MG, Ruperto N, Martini A, Ravelli A, Italian Pediatric Rheumatology Study G. Disease status, reasons for discontinuation and adverse events in 1038 Italian children with juvenile idiopathic arthritis treated with etanercept. Pediatr Rheumatol Online J. 2016;14(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Otten MH, Anink J, Prince FH, Twilt M, Vastert SJ, ten Cate R, Hoppenreijs EP, Armbrust W, Gorter SL, van Pelt PA, Kamphuis SS, Dolman KM, Swart JF, van den Berg JM, Koopman-Keemink Y, van Rossum MA, Wulffraat NM, van Suijlekom-Smit LW. Trends in prescription of biological agents and outcomes of juvenile idiopathic arthritis: results of the Dutch national Arthritis and Biologics in Children Register. Ann Rheum Dis. 2015;74(7):1379–1386. [DOI] [PubMed] [Google Scholar]
- 77.Tarkiainen M, Tynjala P, Vahasalo P, Lahdenne P. Occurrence of adverse events in patients with JIA receiving biologic agents: long-term follow-up in a real-life setting. Rheumatology (Oxford). 2015;54(7):1170–1176. [DOI] [PubMed] [Google Scholar]
- 78.Schmeling H, Minden K, Foeldvari I, Ganser G, Hospach T, Horneff G. Efficacy and safety of adalimumab as the first and second biologic agent in juvenile idiopathic arthritis: the German Biologics JIA Registry. Arthritis Rheumatol. 2014;66(9):2580–2589. [DOI] [PubMed] [Google Scholar]
- 79.Minden K, Niewerth M, Zink A, Seipelt E, Foeldvari I, Girschick H, Ganser G, Horneff G. Long-term outcome of patients with JIA treated with etanercept, results of the biologic register JuMBO. Rheumatology (Oxford). 2012. [DOI] [PubMed] [Google Scholar]
- 80.Halbig M, Horneff G. Improvement of functional ability in children with juvenile idiopathic arthritis by treatment with etanercept. Rheumatol Int. 2009;30(2):229–238. [DOI] [PubMed] [Google Scholar]
- 81.Prince FH, Twilt M, ten Cate R, van Rossum MA, Armbrust W, Hoppenreijs EP, van Santen-Hoeufft M, Koopman-Keemink Y, Wulffraat NM, van Suijlekom-Smit LW. Long-term follow-up on effectiveness and safety of etanercept in juvenile idiopathic arthritis: the Dutch national register. Ann Rheum Dis. 2009;68(5):635–641. [DOI] [PubMed] [Google Scholar]
- 82.Moreland LW, O’Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, Bridges SL Jr., Zhang J, McVie T, Howard G, van der Heijde D, Cofield SS, Investigators T. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum. 2012;64(9):2824–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, Warren SR, Lew RA, Cannella AC, Kunkel G, Phibbs CS, Anis AH, Leatherman S, Keystone E, Investigators CR. Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med. 2013;369(4):307–318. [DOI] [PubMed] [Google Scholar]
- 84.Horneff G, Klein A, Klotsche J, Minden K, Huppertz HI, Weller-Heinemann F, Kuemmerle-Deschner J, Haas JP, Hospach A. Comparison of treatment response, remission rate and drug adherence in polyarticular juvenile idiopathic arthritis patients treated with etanercept, adalimumab or tocilizumab. Arthritis Res Ther. 2016;18(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gottenberg JE, Brocq O, Perdriger A, Lassoued S, Berthelot JM, Wendling D, Euller-Ziegler L, Soubrier M, Richez C, Fautrel B, Constantin AL, Mariette X, Morel J, Gilson M, Cormier G, Salmon JH, Rist S, Liote F, Marotte H, Bonnet C, Marcelli C, Sellam J, Meyer O, Solau-Gervais E, Guis S, Ziza JM, Zarnitsky C, Chary-Valckenaere I, Vittecoq O, Saraux A, Pers YM, Gayraud M, Bolla G, Claudepierre P, Ardizzone M, Dernis E, Breban MA, Fain O, Balblanc JC, Aberkane O, Vazel M, Back C, Candon S, Chatenoud L, Perrodeau E, Sibilia J, Ravaud P. Non-TNF-Targeted Biologic vs a Second Anti-TNF Drug to Treat Rheumatoid Arthritis in Patients With Insufficient Response to a First Anti-TNF Drug: A Randomized Clinical Trial. JAMA. 2016;316(11):1172–1180. [DOI] [PubMed] [Google Scholar]
- 86.Li N, Betts KA, Messali AJ, Skup M, Garg V. Real-world Effectiveness of Biologic Disease-modifying Antirheumatic Drugs for the Treatment of Rheumatoid Arthritis After Etanercept Discontinuation in the United Kingdom, France, and Germany. Clin Ther. 2017;39(8):1618–1627. [DOI] [PubMed] [Google Scholar]
- 87.Wei W, Knapp K, Wang L, Chen CI, Craig GL, Ferguson K, Schwartzman S. Treatment Persistence and Clinical Outcomes of Tumor Necrosis Factor Inhibitor Cycling or Switching to a New Mechanism of Action Therapy: Real-world Observational Study of Rheumatoid Arthritis Patients in the United States with Prior Tumor Necrosis Factor Inhibitor Therapy. Adv Ther. 2017;34(8):1936–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, Racewicz AJ, van Vollenhoven RF, Li NF, Agarwal S, Hessey EW, Shaw TM, Group DS. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54(5):1390–1400. [DOI] [PubMed] [Google Scholar]
- 89.Horneff G, Burgos-Vargas R, Constantin T, Foeldvari I, Vojinovic J, Chasnyk VG, Dehoorne J, Panaviene V, Susic G, Stanevica V, Kobusinska K, Zuber Z, Mouy R, Rumba-Rozenfelde I, Breda L, Dolezalova P, Job-Deslandre C, Wulffraat N, Alvarez D, Zang C, Wajdula J, Woodworth D, Vlahos B, Martini A, Ruperto N, Paediatric Rheumatology International Trials O. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann Rheum Dis. 2014;73(6):1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Horneff G, Fitter S, Foeldvari I, Minden K, Kuemmerle-Deschner J, Tzaribacev N, Thon A, Borte M, Ganser G, Trauzeddel R, Huppertz HI. Double-blind, placebo-controlled randomized trial with adalimumab for treatment of juvenile onset ankylosing spondylitis (JoAS): significant short term improvement. Arthritis Res Ther. 2012;14(5):R230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weiss PF, Xiao R, Brandon TG, Pagnini I, Wright TB, Beukelman T, Morgan-DeWitt E, Feudtner C. Comparative Effectiveness of Tumor Necrosis Factor Agents and Disease-modifying Antirheumatic Therapy in Children with Enthesitis-related Arthritis: The First Year after Diagnosis. J Rheumatol. 2018;45(1):107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burgos-Vargas R, Tse SM, Horneff G, Pangan AL, Kalabic J, Goss S, Unnebrink K, Anderson JK. A Randomized, Double-Blind, Placebo-Controlled Multicenter Study of Adalimumab in Pediatric Patients With Enthesitis-Related Arthritis. Arthritis Care Res (Hoboken). 2015;67(11):1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Windschall D, Muller T, Becker I, Horneff G. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis. Clin Rheumatol. 2015;34(1):61–69. [DOI] [PubMed] [Google Scholar]
- 94.Constantin T, Foeldvari I, Vojinovic J, Horneff G, Burgos-Vargas R, Nikishina I, Akikusa JD, Avcin T, Chaitow J, Koskova E, Lauwerys BR, Calvo Penades I, Flato B, Gamir ML, Huppertz HI, Jaller Raad JJ, Jarosova K, Anton J, Macku M, Otero Escalante WJ, Rutkowska-Sak L, Trauzeddel R, Velez-Sanchez PJ, Wouters C, Wajdula J, Zang C, Bukowski J, Woodworth D, Vlahos B, Martini A, Ruperto N, Paediatric Rheumatology International Trials O. Two-year Efficacy and Safety of Etanercept in Pediatric Patients with Extended Oligoarthritis, Enthesitis-related Arthritis, or Psoriatic Arthritis. J Rheumatol. 2016;43(4):816–824. [DOI] [PubMed] [Google Scholar]
- 95.Benhamou M, Gossec L, Dougados M. Clinical relevance of C-reactive protein in ankylosing spondylitis and evaluation of the NSAIDs/coxibs’ treatment effect on C-reactive protein. Rheumatology (Oxford). 2010;49(3):536–541. [DOI] [PubMed] [Google Scholar]
- 96.Dougados M, Gueguen A, Nakache JP, Velicitat P, Veys EM, Zeidler H, Calin A. Ankylosing spondylitis: what is the optimum duration of a clinical study? A one year versus a 6 weeks non-steroidal anti-inflammatory drug trial. Rheumatology (Oxford). 1999;38(3):235–244. [DOI] [PubMed] [Google Scholar]
- 97.Dougados M, Behier JM, Jolchine I, Calin A, van der Heijde D, Olivieri I, Zeidler H, Herman H. Efficacy of celecoxib, a cyclooxygenase 2-specific inhibitor, in the treatment of ankylosing spondylitis: a six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum. 2001;44(1):180–185. [DOI] [PubMed] [Google Scholar]
- 98.van der Heijde D, Baraf HS, Ramos-Remus C, Calin A, Weaver AL, Schiff M, James M, Markind JE, Reicin AS, Melian A, Dougados M. Evaluation of the efficacy of etoricoxib in ankylosing spondylitis: results of a fifty-two-week, randomized, controlled study. Arthritis Rheum. 2005;52(4):1205–1215. [DOI] [PubMed] [Google Scholar]
- 99.Burgos-Vargas R, Vazquez-Mellado J, Pacheco-Tena C, Hernandez-Garduno A, Goycochea-Robles MV. A 26 week randomised, double blind, placebo controlled exploratory study of sulfasalazine in juvenile onset spondyloarthropathies. Ann Rheum Dis. 2002;61(10):941–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haibel H, Brandt HC, Song IH, Brandt A, Listing J, Rudwaleit M, Sieper J. No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann Rheum Dis. 2007;66(3):419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roychowdhury B, Bintley-Bagot S, Bulgen DY, Thompson RN, Tunn EJ, Moots RJ. Is methotrexate effective in ankylosing spondylitis? Rheumatology (Oxford). 2002;41(11):1330–1332. [DOI] [PubMed] [Google Scholar]
- 102.Gonzalez-Lopez L, Garcia-Gonzalez A, Vazquez-Del-Mercado M, Munoz-Valle JF, Gamez-Nava JI. Efficacy of methotrexate in ankylosing spondylitis: a randomized, double blind, placebo controlled trial. J Rheumatol. 2004;31(8):1568–1574. [PubMed] [Google Scholar]
- 103.Van Mechelen M, Lories RJ. Microtrauma: no longer to be ignored in spondyloarthritis? Curr Opin Rheumatol. 2016;28(2):176–180. [DOI] [PubMed] [Google Scholar]
- 104.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, Bautista-Molano W, Boehncke WH, Campbell W, Cauli A, Espinoza LR, FitzGerald O, Gladman DD, Gottlieb A, Helliwell PS, Husni ME, Love TJ, Lubrano E, McHugh N, Nash P, Ogdie A, Orbai AM, Parkinson A, O’Sullivan D, Rosen CF, Schwartzman S, Siegel EL, Toloza S, Tuong W, Ritchlin CT. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 Treatment Recommendations for Psoriatic Arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.