Significance
The design of cell-penetrating long peptidomimetic scaffolds for interacting with large protein binding interfaces is challenging because they involve various necessary functional groups. Here we report the first series of helical sulfono-γ-AApeptides that disrupt protein–protein interactions. Specifically, we discovered that these helical mimetics can structurally and functionally mimic the B Cell Lymphoma 9 (BCL9) helix and disrupt cancer-related β-catenin/BCL9 protein–protein interaction in cells with excellent potency and specificity. Enzymatic stability studies demonstrate the remarkable stability of the helical sulfono-γ-AApeptides, augmenting their biological potential. This strategy could be adapted to target a myriad of other disease-related protein–protein interactions.
Keywords: α-helix mimetics, β-catenin, B-cell lymphoma 9, protein–protein interactions, inhibitors
Abstract
The rational design of α-helix–mimicking peptidomimetics provides a streamlined approach to discover potent inhibitors for protein−protein interactions (PPIs). However, designing cell-penetrating long peptidomimetic scaffolds equipped with various functional groups necessary for interacting with large protein-binding interfaces remains challenging. This is particularly true for targeting β-catenin/BCL9 PPIs. Here we designed a series of unprecedented helical sulfono-γ-AApeptides that mimic the binding mode of the α-helical HD2 domain of B Cell Lymphoma 9 (BCL9). Our studies show that sulfono-γ-AApeptides can structurally and functionally mimic the α-helical domain of BCL9 and selectively disrupt β-catenin/BCL9 PPIs with even higher potency. More intriguingly, these sulfono-γ-AApeptides can enter cancer cells, bind with β-catenin and disrupt β-catenin/BCL9 PPIs, and exhibit excellent cellular activity, which is much more potent than the BCL9 peptide. Furthermore, our enzymatic stability studies demonstrate the remarkable stability of the helical sulfono-γ-AApeptides, with no degradation in the presence of pronase for 24 h, augmenting their biological potential. This work represents not only an example of helical sulfono-γ-AApeptides that mimic α-helix and disrupt protein–protein interactions, but also an excellent example of potent, selective, and cell-permeable unnatural foldameric peptidomimetics that disrupt the β-catenin/BCL9 PPI. The design of helical sulfono-γ-AApeptides may lead to a new strategy to modulate a myriad of protein–protein interactions.
The development of peptidomimetic helical foldamers for applications in chemical biology and drug discovery has attracted much interest in the field of medicinal chemistry. Helical foldamers are commonly featured with many attractive merits, such as enhanced resistance to proteolytic degradation and high sequence diversity (1–8), and have been explored extensively for inhibition of protein–protein interactions (PPIs) (9–13). Nevertheless, the targeting of intracellular proteins still entails significant challenges (14–16), largely due to the limited availability of molecular frameworks of peptidomimetic peptides.
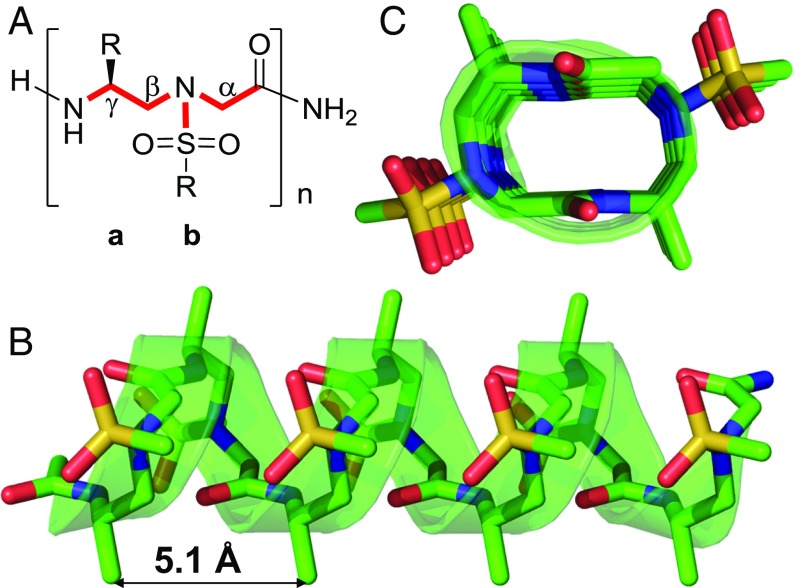
As a new class of proteolytically stable peptidomimetics, γ-AApeptides have emerged as effective peptidomimetics that play important roles in chemical biology and biomedical sciences (17–19). Specifically, sulfono-γ-AApeptides have been shown to have excellent folding stability to adopt a series of helical structures with a well-defined hydrogen-bonding pattern (20–24). In sulfono-γ-AApeptides, one-half of the side chains are introduced by sulfonyl chlorides, providing enormous chemical diversity (Fig. 1A) (17–19). In particular, we recently reported the X-ray crystal structures of a series of homogeneous l-sulfono-γ-AA foldamers (24), which form well-defined left-handed 414 helices (Fig. 1 B and C). Intriguingly, the side chains of sulfono-γ-AApeptides are aligned perfectly on the top of one another with a pitch of 5.1 Å. Based on the precise 3D arrangement of their side functional groups, their close similarity in helical pitch compared with that of α-helix (5.4 Å), as well as the remarkable stability of sulfono-γ-AApeptides, we envisioned that helical sulfono-γ-AApeptides could be adapted to develop a new class of helical mimetics that disrupt α-helix-mediated protein–protein interactions.
Fig. 1.
(A) The chemical structure of sulfono-γ-AApeptides; a and b denote the chiral side chain and the sulfonamido side chain from the building block, respectively. (B) The crystal structure of a sulfono-γ-AApeptide. (C) Top view of B.
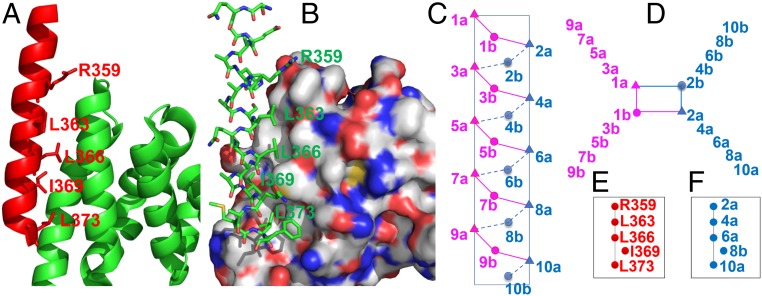
With the aim of developing new PPIs for various biological targets, we wondered whether B Cell Lymphoma 9 (BCL9) (25–39), previously shown to engage its α-helical HD2 domain to interact with β-catenin (Fig. 2), could be used to develop peptidomimetic helical foldamers based on sulfono-γ-AApeptides. The Wnt/β-catenin signaling pathway plays important roles in embryonic development and tissue homeostasis, as well as in several types of human cancer, such as colorectal cancer, breast cancer, melanoma, prostate cancer, and others (40–43).
Fig. 2.
(A and B) The α-helical HD2 domain of BCL9, which directly engages a surface groove of β-catenin, provided the template for structural stabilization by hydrocarbon stapling [Protein Data Bank (PDB) ID code 2GL7]. (A) Cartoon representation of the residues of BCL9 (red) critical for binding to β-catenin, shown as sticks. (B) BCL9 shown as sticks, and β-catenin represented with the surface model. (C–F) Schematic representation of distribution of side chains from sulfono-γ-AApeptides. (C) Side view. (D) Top view, helical wheel. (E) Position map of critical residues of the BCL9 helix. (F) Position map of side chains of sulfono-γ-AApeptides designed to mimic residues in E.
As a central mediator of the signaling, β-catenin controls the expression of several key genes that regulate the cell cycle and apoptosis. As the matter of fact, transcriptional activation of Wnt/β-catenin signaling pathway is dependent on formation of the β-catenin supercomplex involving BCL9 or BCL9-like (B9L), as well as the T cell factor (Tcf)/lymphoid enhancer-binding factor (Lef) family of transcriptional factors (37). Specifically, BCL9 functions as a scaffolding structure of the Wnt enhanceosome and brings β-catenin to TCF/LEF to transcribe specific Wnt target genes, leading to cell growth, proliferation, and differentiation (44). As such, molecules that disrupt BCL9/β-catenin protein–protein interaction could inhibit Wnt/β-catenin signaling transduction and thus could be developed as novel anticancer agents.
The crystal structure of the β-catenin/BCL9/TCF-4 ternary complex (37) revealed that the helical domain of BCL9 (351-374) interacts with a binding groove in β-catenin (Fig. 2 A and B). The critical residues of BCL9, R359, L363, L366, I369, and L373, which are on the one face of the BCL9 helix, form both hydrophilic and hydrophobic contacts with the binding surface of β-catenin. Despite an unambiguous mechanism of action, the design of potent intracellular inhibitors to block this PPI remains a challenge, attributed mainly to the interaction between BCL9 and β-catenin, which is mediated by an ∼25-residue helical segment from BCL9. While several small-molecule inhibitors have been designed to disrupt β-catenin/BCL9 PPIs, peptide inhibitors are scarce (25, 27–30, 34). Wang (33, 35) and Takada (32) reported triazole-stapled and olefin-stapled BCL9 L351−F374 α-helical peptides, respectively.
While the cell permeability of triazole-stapled peptides was not discussed, the olefin-stapled BCL9 peptide was found to pass the cell membrane, disrupt the β-catenin/BCL9 PPI, and selectively suppress the transcription of Wnt target genes (32). This olefin-stapled peptide also inhibited cancer cell growth, angiogenesis, and metastasis without any evident damage to normal tissues in mouse xenograft models for colorectal carcinoma and multiple myeloma. It is envisioned that unnatural peptidomimetic inhibitors of β-catenin/BCL9 PPI would be appealing because they can mimic peptide helices while being highly resistant to proteolytic degradation. However, to the best of our knowledge, to date there has been no report on the development of potent unnatural peptidomimetic inhibitors to disrupt β-catenin/BCL9 PPI. This is possibly due to the fact that known peptidic foldamers do not efficiently mimic the long α-helix, as well as to the limited availability of scaffolds and molecular frameworks.
With the availability of helical sulfono-γ-AApeptide scaffold, we questioned whether sulfono-γ-AApeptides can be designed to effectively disrupt the β-catenin/BCL9 PPI. If so, this would offer a new template for generating potent helical peptidomimetics that inhibit a variety of medicinally relevant PPIs. In view of the folding pattern (Fig. 2 C–F), we believe that our sulfono-γ-AApeptides can effectively mimic BCL9, because the side chains of sulfono-γ-AApeptides could be designed to mimic the critical residues of the α-helix of BCL9 HD2 domain. Here we report the development of some unnatural peptidomimetics that are highly effective for inhibiting β-catenin/BCL9 PPI.
Results and Discussion
Design and Biological Activity of Sulfono-γ-AApeptides.
Our design was straightforward. As shown in Fig. 2 C and D, the chiral side chains 2a, 4a, 6a, 8a, and 10a are on the same face of the helical scaffold of sulfono-γ-AApeptides, and thus this face was chosen to mimic those critical residues of the BCL9 helical domain. The position map of those residues (Fig. 2E) on the α-helical scaffold demonstrates that R359, L363, L366, and L373 are almost on the same line, while I369 is not. A close comparison of helical scaffolds of the sulfono-γ-AApeptide and the BCL9 peptide reveals that 8b, rather than 8a, could closely mimic I369 (Fig. 2F). As 8b is the sulfonyl side chain, we hypothesized that a methyl sulfonyl group would be sufficient, because the sulfonyl group sticks out more than chiral side chains on helical sulfono-γ-AApeptides.
We thus designed and synthesized a panel of sulfono-γ-AApeptides (Fig. 3). The first sulfono-γ-AApeptide sequence that we designed, sequence 2 (Fig. 3), contains only 10 sulfono-γ-AA building blocks (comparable in length to a 20-mer peptide), as the crystal structure (Fig. 2 A and B) shows that the first few residues of BCL9 do not interact with β-catenin directly.
Fig. 3.
Structures of sulfono-γ-AApeptides investigated for the disruption of β-catenin–BCL9 interaction. The critical side chains are shown in red.
We next measured the binding affinity of sulfono-γ-AApeptide 2 toward β-catenin using a fluorescence polarization assay. In our assay, the binding affinity of BCL9 peptide 1 exhibited a Kd value of 0.97 µM (Table 1), consistent with previous reports (19, 29). Excitingly, the first sulfono-γ-AApeptide sequence 2 that we designed, with a shorter length than the BCL9 peptide 1, had a Kd value of 0.43 µM, which is already twofold more affinitive to β-catenin than BCL9 peptide 1.
Table 1.
Activity of peptides for the disruption of β-catenin and BCL9 interaction
| Peptide | Kd, µM | IC50, µM, ± SD | Ki, µM, ± SD |
| 1 | 0.97 | 1.28 ± 0.29 | 1.13 ± 0.24 |
| 2 | 0.43 | 0.74 ± 0.15 | 0.64 ± 0.11 |
| 3 | 0.86 | 1.04 ± 0.21 | 0.92 ± 0.17 |
| 4 | 0.82 | 1.10 ± 0.14 | 0.98 ± 0.11 |
| 5 | 1.97 | 2.71 ± 0.37 | 2.43 ± 0.32 |
| 6 | 0.16 | 0.54 ± 0.13 | 0.46 ± 0.10 |
| 7 | 0.70 | 1.00 ± 0.10 | 0.89 ± 0.08 |
| 8 | 3.89 | 8.01 ± 0.82 | 7.20 ± 0.72 |
| 9 | 5.14 | 12.9 ± 2.15 | 11.6 ± 1.92 |
| 10 | 2.51 | 4.07 ± 0.59 | 3.65 ± 0.51 |
| 11 | 8.26 | 17.4 ± 3.35 | 15.7 ± 3.00 |
| 12 | >10 | >10 | >10 |
The ability of the sulfono-γ-AApeptide 2 to act as a functional mimic of BCL9 peptide 1 to disrupt BCL9/β-catenin PPI was then examined by AlphaScreen assays (29, 30). As shown in Table 1, the helical BCL9 peptide 1 could disrupt β-catenin/BCL9 PPI, with Ki and IC50 values of 1.13 µM and 1.28 µM, respectively, which again are in very good agreement with the literature (25, 27–30). To our delight, the sulfono-γ-AApeptide 2 was found to disrupt β-catenin/BCL9, with Ki and IC50 values of 0.64 µM and 0.74 µM, respectively, making it almost twofold more potent than BCL9 peptide 1. This initial success demonstrates the potential of sulfono-γ-AApeptides to mimic the long α-helix, as well as their robust helical folding propensity (24).
To investigate the importance of the side chains in the sulfono-γ-AApeptide sequence, alanine scanning studies were carried out based on sulfono-γ-AApeptide sequence 2 (Fig. 3, sequences 3–8). We replaced each key residue with an Ala side chain at positions 1b, 2a, 6a, 8a, and 10a (marked with an asterisk). It appears that aminoethane (position 1b), Arg (position 2a), and Leu (position 10a) have important roles in inhibiting β-catenin/BCL9 PPI, as an approximate 1.5-fold decrease in binding affinity relative to sequence 2 was caused by each Ala substitution (Table 1, sequences 3, 4, and 7). The results for sulfono-γ-AApeptide sequence 3 indicate that positively charged side chains could affect binding activity even if they were not involved in direct contact with the β-catenin/BCL9-binding pocket.
It appears that Leu (position 6a) is the most critical group for inhibiting β-catenin/BCL9 PPI (Fig. 3 and Table 1, sequence 5), as the Ala substitution resulted in decreased binding affinity, with Kd, Ki, and IC50 values of 1.97 µM, 2.43 µM, and 2.71 µM, respectively. Interestingly, mutation of Ile (position 8a) to Ala in sequence 6 further improved the binding affinity and inhibitory activity, with Kd, Ki, and IC50 values of 0.16 µM (approximately sixfold greater), 0.46 µM (approximately threefold greater), and 0.54 µM (approximately threefold greater), respectively, compared with the values for peptide 1 (Table 1). We hypothesized that although the critical residue for interaction is the side chain 8b, the less bulkier methyl group at 8b is expected to have less impact on steric hindrance than Ile side chain on the neighboring 8b, which may ensure a closer interaction of the sequence with β-catenin. Indeed, a bulkier group at 8b led to decreased binding activity (sequence 10).
It seems that strong hydrophobic interaction near the C-terminal region is critical, as a change in the groups at positions 7b, 8a, and 9a to less hydrophobic groups or cationic groups led to sequences 8, 9, and 11, which show inferior binding activity. The importance of these key side chains was further manifested by sulfono-γ-AApeptide sequence 12 (Fig. 3 and Table 1), which lacks several key side chains at positions 1b, 2a, 6a, and 10a, resulting in complete loss of the ability to inhibit β-catenin/BCL9 PPIs. It should be noted that the binding affinity, Kd, from fluorescence anisotropy studies is highly consistent with the Ki and IC50 values obtained from AlphaScreen assays that measure the direct competition of all tested sequences for the interaction of BCL9 peptide with β-catenin, suggesting that these sequences bind to the same site on β-catenin.
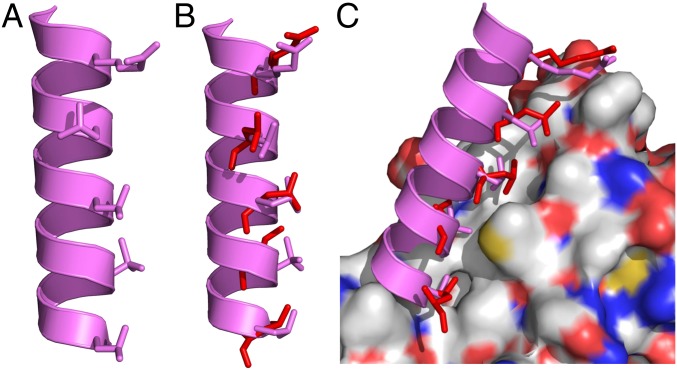
The excellent binding activity of sulfono-γ-AApeptides was further rationalized by the modeling studies. The structure of sequence 6 was built on the scaffold of the crystal structure (24) and then superimposed onto the BCL9 helical domain by overlaying the helical backbone orientations using PyMOL software (Fig. 4A) (45). As shown in Fig. 4 B and C, the side chains of critical residues of the BCL9 peptide overlap very well with the side chains of 2a, 4a, 6a, 8b, and 10a of sulfono-γ-AApeptide 6. As a result, the helical sulfono-γ-AApeptide 6 could tightly bind to the groove of β-catenin through both hydrophilic and hydrophobic interactions using these side chains.
Fig. 4.
(A) Proposed structure of sulfono-γ-AApeptide 6 with critical side chains 2a, 4a, 6a, 8b, and 10a shown in stick representation. (B) Overlay of 6 with critical residues of the BCL9 helical peptide using PyMOL software. (C) Overlay of 6 with critical residues of BCL9 on the binding surface of β-catenin (PDB ID code 2GL7) using PyMOL software.
Circular Dichroism Measurements.
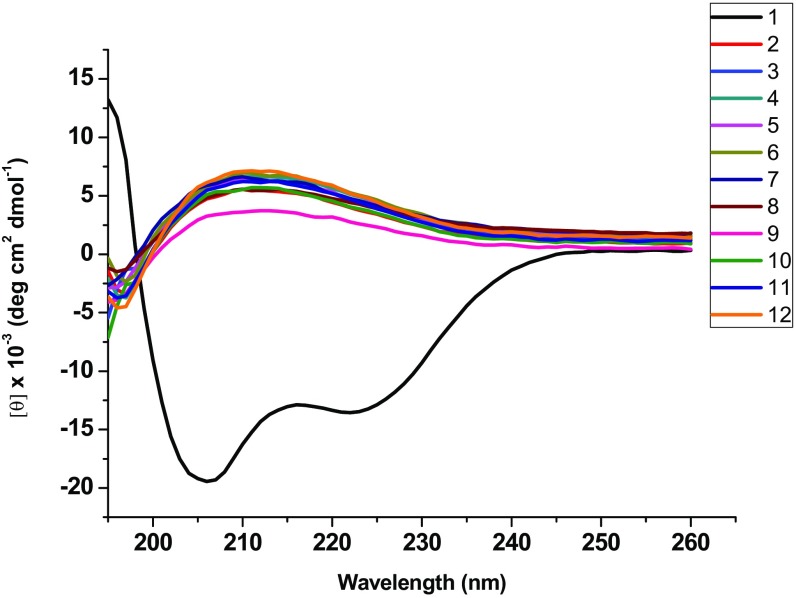
We hypothesized that sulfono-γ-AApeptides should adopt well-defined helices in solution because they generally have better activity than the natural BCL9 peptide 1, the natural binding partner of β-catenin. Thus, we performed circular dichroism (CD) spectroscopic studies to investigate the helicity of regular peptide 1 and homogeneous sulfono-γ-AApeptides 2–12. The CD studies were performed in PBS buffer between 190 nm and 260 nm. As shown in Fig. 5, each sulfono-γ-AApeptide showed a marked Cotton effect, with a strong positive maximum around 210 nm, which is consistent with the previously reported CD spectra of helical sulfono-γ-AApeptides (24), suggesting that sequences 2–12 adopt a similar left-handed helical conformation. As anticipated, peptide 1, with a length of 23 residues, also adopted a helical conformation in solution.
Fig. 5.
CD spectra of BCL9 peptide 1 and sulfono-γ-AApeptides 2–12 (100 μM) measured at room temperature in PBS buffer.
Cell Permeability Test.
The inhibition of intracellular PPIs remains challenging in chemical biology and drug discovery (14–16). An intracellular PPI inhibitor has to penetrate and cross the cell membrane, which may also be why developing peptidomimetic-based inhibitors for β-catenin/BCL9 PPI, which has extremely long sequences, is so challenging.
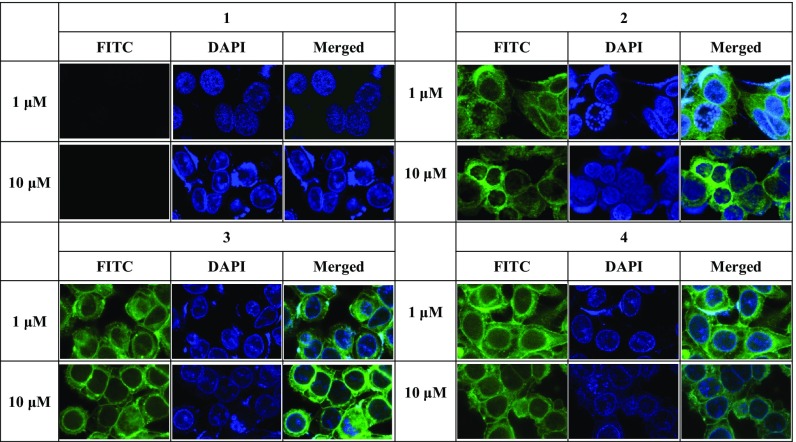
To determine whether our newly developed sulfono-γ-AApeptide inhibitors could permeate Wnt/β-catenin–dependent cancer cells, we next examined the cellular uptake of fluorescein isothiocyanate (FITC)-labeled derivatives of our sulfono-γ-AApeptides 2, 3, 4, 6, and 9 and the BCL9 peptide 1 in SW480 cells by confocal fluorescence microscopy (Fig. 6 and SI Appendix, Fig. S5). When SW480 cells were treated with FITC-labeled peptide 1, negligible green fluorescence was observed at 1 μM and 10 μM for 2 h, consistent with the observation that peptide 1 has poor cell permeability and exhibits no cellular activity (30). However, it is surprising that when SW480 cells were treated with FITC-labeled sulfono-γ-AApeptides 2, 3, 4, 6, and 9 at the same concentrations for 2 h (Fig. 6 and SI Appendix, Fig. S5), strong and evenly diffused intracellular green fluorescence was noted in cytoplasm, even at concentrations as low as 1 µM. These results suggest that sulfono-γ-AApeptides are highly cell-permeable, possibly due to the existence of multisulfonamide groups on the molecular scaffold. As such, although the BCL9 peptide 1 is known for its cell impermeability as well as poor cellular activity toward Wnt/β-catenin–dependent cancer cells, we hypothesized that our sulfono-γ-AApeptides could cross membranes and gain access to targets within the cytoplasm of living cells.
Fig. 6.
Confocal fluorescence microscopy images of SW480 cells treated with 1 μM and 10 μM of the FITC-labeled peptide 1 and sulfono-γ-AApeptides 2–4 for 2 h. (Magnification, 630×.)
MTS Cell Viability Assay.
MTS tetrazolium cell viability assays were performed to assess the effect of β-catenin/BCL9 inhibitors on the cell proliferation of colorectal cancer cells (SW480), which exhibit hyperactive Wnt/β-catenin signaling (Table 2). Consistent with previous reports, the regular BCL9 peptide 1 showed very poor activity, with an IC50 of >200 µM. Intriguingly, the MTS assays of sulfono-γ-AApeptides 2–7 showed that the sulfono-γ-AApeptides inhibited cancer cell proliferation in a dose-dependent manner. Sequences 2–4 exhibited excellent inhibitory activity, with IC50 values of ∼12 µM toward SW480 cells (Table 2). Note that these sequences were roughly threefold more selective toward SW480 cells than A549 cells that have normal β-catenin signaling, suggesting good specificity of the tested sulfono-γ-AApeptides. Interestingly, sequence 6, which was most active in vitro, displayed relatively weak activity, possibly due to some unknown side interactions. This initial encouraging result prompted us to further study the effects on Wnt/β-catenin signaling by TOPFlash and FOPFlash luciferase reporter assays (25, 28, 30).
Table 2.
MTS assay to monitor the inhibitory activities of sulfono-γ-AApeptides on the viability of cancer cells
| Peptide | MTS IC50, µM, ± SD | |
| Hyperactive β-catenin– signaling SW480 cell | Normal β-catenin– signaling A549 cell | |
| 1 | >200 | >200 |
| 2 | 12.5 ± 2.86 | 39.9 ± 4.75 |
| 3 | 12.8 ± 2.90 | 43.7 ± 3.62 |
| 4 | 16.4 ± 4.40 | 43.2 ± 5.60 |
| 5 | 132 ± 18.9 | 230 ± 27.5 |
| 6 | 65.4 ± 7.06 | 150 ± 19.2 |
| 7 | 46.6 ± 5.57 | 120 ± 13.6 |
TOPFlash/FOPFlash Luciferase Reporter Assays.
Wnt-specific TOPFlash/FOPFlash luciferase reporter assays were used to evaluate the effects of these compounds on β-catenin–dependent transcription. For the TOPFlash reporter construct, the firefly luciferase reporter gene was placed downstream of three wild-type Tcf-binding sites. For the FOPFlash reporter construct, the firefly luciferase reporter gene was placed downstream of three mutant Tcf-binding sites. The high expression of firefly luciferase in TOPFlash assays was controlled by tandem Tcf-binding sites. The Renilla luciferase reporter construct (pCMV-RL) served as the internal control to normalize luciferase reporter signals and eliminate systematic errors such as cell viability, transfection effect, and others. The TOPFlash luciferase reporter assay was performed on the most potent sulfono-γ-AApeptides 2–4. Consistent with cell proliferation studies, sulfono-γ-AApeptide inhibitors 2–4 suppressed the TOPFlash luciferase activity in SW480 in a dose-dependent manner (Fig. 7A), with estimated IC50 values of 35.4, 26.5, and 20.6 µM, respectively (SI Appendix, Fig. S6). At 50 µM, each compound inhibited >90% of luciferase activity. As expected, the regular BCL9 peptide 1 did not show any activity.
Fig. 7.
(A) Wnt-responsive TOPFlash luciferase reporter assay results of inhibitors 1–4 in β-catenin–activated SW480 cells. (B) Wnt-responsive FOPFlash luciferase reporter assay results of inhibitors 1–4 in β-catenin–activated SW480 cells.
Interestingly, in the follow-up FOPFlash luciferase reporter assay, the inhibitory activity of sulfono-γ-AApeptides 2–4 dropped significantly (Fig. 7B). At a concentration of 50 µM, luciferase retained >85% of its activity. The Renilla internal control values were constant in all TOPFlash/FOPFlash assays (SI Appendix, Table S2). Taken together, these results suggest that sulfono-γ-AApeptide inhibitors 2–4 selectively inhibit Wnt/β-catenin signaling transactivation while not generally inhibiting other transcriptional pathways.
Cellular Target Engagement.
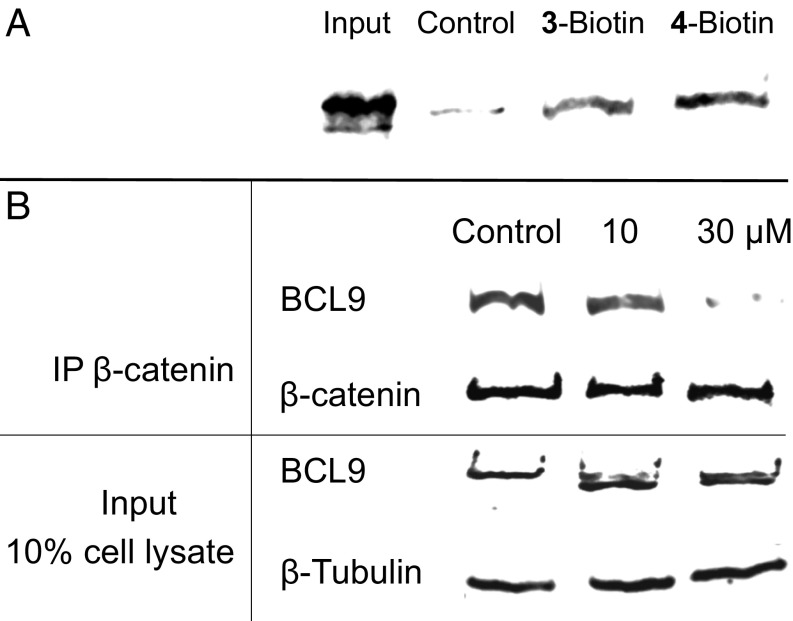
We conducted two experiments to examine whether these sulfono-γ-AApeptides can engage β-catenin in the cellular context. Biotinylated 3 (3-Biotin) and biotinylated 4 (4-Biotin) (the best two compounds in luciferase reporter assays) were synthesized and incubated with SW480 cell lysates. The proteins that bind with these two sequences were then pulled down by streptavidin-conjugated beads and examined by Western blot analyses using β-catenin–specific antibody. As shown in Fig. 8A, both 3-Biotin and 4-Biotin could effectively bind with β-catenin in SW480 cell lysates at a concentration of 1 µM.
Fig. 8.
(A) SW480 cell lysate was incubated with 3-Biotin or 4-Biotin, followed by streptavidin pull-down experiments. The levels of β-catenin associated with 3-Biotin and 4-Biotin were analyzed by Western blot analysis. Input: 5% of cell lysate. (B) Co-IP experiments to evaluate the disruption of the β-catenin/BCL9 PPI by 4 in Wnt/β-catenin hyperactive cancer cells. IP, immunoprecipitation; Input, 10% of the cell lysate. Each experiment was performed in duplicate.
Coimmunoprecipitation (co-IP) experiments were also performed with Wnt/β-catenin hyperactive HCT116 cancer cells to evaluate the effects of 4 on disruption of the β-catenin/BCL9 PPI in cells. As shown in Fig. 8B, inhibitor 4 disrupted the β-catenin/BCL9 PPI in a dose-dependent manner, while the input and immunoprecipitation controls were constant in the different experiments.
Enzymatic Stability Study.
Along with cell permeability, protease stability of the sequences is critical for their biological activity. We further evaluated the proteolytic stability of helical sulfono-γ-AApeptides 2–4 and BCL9 peptide 1 in pronase, a mixture of broad scope endopeptidases and exopeptidases isolated from Streptomyces griseus (46). The assays were conducted by incubating 0.1 mg/mL of three lead compounds 2–4 and the regular peptide 1 with 0.1 mg/mL of pronase in 100 mM ammonium bicarbonate buffer (pH 7.8) at 37 °C for 24 h. The stability of the examined compounds was analyzed by HPLC-MS (SI Appendix, Figs. S12–S15). The control peptide 1 was completely degraded by pronase, with no intact peptide remaining (SI Appendix, Fig. S12), which may explain why peptide 1 showed weak cell permeability and completely abandoned its cellular activity. Strikingly, our linear sulfono-γ-AApeptides showed no detectable degradation (SI Appendix, Figs. S13–S15), demonstrating extraordinarily high stability against enzymatic degradation, augmenting their potential in therapeutic applications.
In summary, we report a series of unprecedented helical sulfono-γ-AApeptides that mimic α-helix and disrupt PPIs. These unnatural helical peptidomimetics are able to disrupt cancer-related β-catenin/BCL9 PPIs with excellent potency and specificity. The cell-based studies indicated that sulfono-γ-AApeptides are cell-permeable and can effectively inhibit the growth of cancer cells with hyperactive Wnt/β-catenin signaling. The TOPFlash/FOPFlash luciferase reporter assays demonstrated that sulfono-γ-AApeptides can selectively suppress transactivation of Wnt/β−catenin signaling. The protein pull-down and co-IP experiments demonstrated that these sulfono-γ-AApeptides can bind with β-catenin and disrupt β-catenin/BCL9 PPIs in cells. This work also represents the successful application of unnatural peptidomimetics in disrupting β-catenin/BCL9 PPIs, which has long been considered a challenging target, providing a practical method for the development of novel foldameric peptidomimetics that serve as proteolytically stable and cell-penetrating inhibitors for a myriad of PPIs. We believe this work can expand the utility of sulfono-γ-AApeptides in the preparation of potent and cell-permeable peptidomimetic agents that will find many applications in chemical biology and biomedical sciences.
Materials and Methods
Building blocks and sulfono-γ-AApeptides were synthesized following previously reported methods. All other chemicals and solvents were purchased from commercial sources and used as received. 1H and 13C NMR spectra were recorded on a Varian INOVA 400 spectrometer. High-resolution mass spectra were obtained on an Agilent 6220 using electrospray ionization time-of-flight. Synthesis, characterization, and biological experiments are described in detail in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation CAREER Award 1351265 (to J.C.) and National Institutes of Health Grant 1R01 GM112652-01A1 (to J.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819663116/-/DCSupplemental.
References
- 1.Jones J. E., et al. , Length-dependent formation of transmembrane pores by 310-helical α-Aminoisobutyric acid foldamers. J. Am. Chem. Soc. 138, 688–695 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collie G. W., et al. , Shaping quaternary assemblies of water-soluble non-peptide helical foldamers by sequence manipulation. Nat. Chem. 7, 871–878 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Cheloha R. W., Maeda A., Dean T., Gardella T. J., Gellman S. H., Backbone modification of a polypeptide drug alters duration of action in vivo. Nat. Biotechnol. 32, 653–655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buratto J., et al. , Structure of a complex formed by a protein and a helical aromatic oligoamide foldamer at 2.1 Å resolution. Angew. Chem. Int. Ed. Engl. 53, 883–887 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Mayer C., Müller M. M., Gellman S. H., Hilvert D., Building proficient enzymes with foldamer prostheses. Angew. Chem. Int. Ed. Engl. 53, 6978–6981 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Wang P. S. P., Nguyen J. B., Schepartz A., Design and high-resolution structure of a β3-peptide bundle catalyst. J. Am. Chem. Soc. 136, 6810–6813 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Wolffs M., et al. , Helical aromatic oligoamide foldamers as organizational scaffolds for photoinduced charge transfer. J. Am. Chem. Soc. 131, 4819–4829 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Hamuro Y., Schneider J. P., DeGrado W. F., De novo design of antibacterial β-Peptides. J. Am. Chem. Soc. 121, 12200–12201 (1999). [Google Scholar]
- 9.Barnard A., et al. , Selective and potent proteomimetic inhibitors of intracellular protein-protein interactions. Angew. Chem. Int. Ed. Engl. 54, 2960–2965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azzarito V., Long K., Murphy N. S., Wilson A. J., Inhibition of α-helix-mediated protein-protein interactions using designed molecules. Nat. Chem. 5, 161–173 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Pelay-Gimeno M., Glas A., Koch O., Grossmann T. N., Structure-based design of inhibitors of protein-protein interactions: Mimicking peptide binding epitopes. Angew. Chem. Int. Ed. Engl. 54, 8896–8927 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs S., et al. , Proline primed helix length as a modulator of the nuclear receptor-coactivator interaction. J. Am. Chem. Soc. 135, 4364–4371 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Ernst J. T., Becerril J., Park H. S., Yin H., Hamilton A. D., Design and application of an α-helix-mimetic scaffold based on an oligoamide-foldamer strategy: Antagonism of the Bak BH3/Bcl-xL complex. Angew. Chem. Int. Ed. Engl. 42, 535–539 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Wilson A. J. Inhibition of protein-protein interactions using designed molecules. Chem. Soc. Rev. 38, 3289–3300 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Nero T. L., Morton C. J., Holien J. K., Wielens J., Parker M. W., Oncogenic protein interfaces: Small molecules, big challenges. Nat. Rev. Cancer 14, 248–262 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Arkin M. R., Tang Y., Wells J. A., Small-molecule inhibitors of protein-protein interactions: Progressing toward the reality. Chem. Biol. 21, 1102–1114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y., et al. , γ-AApeptides: Design, structure, and applications. Acc. Chem. Res. 49, 428–441 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng P., Shi Y., Sang P., Cai J., γ-AApeptides as a new class of peptidomimetics. Chemistry 22, 5458–5466 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y., et al. , One-bead-two-compound thioether Bridged macrocyclic γ-AApeptide screening library against EphA2. J. Med. Chem. 60, 9290–9298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H., et al. , The synthesis of head-to-tail cyclic sulfono-γ-AApeptides. Org. Biomol. Chem. 13, 672–676 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Wu H., Teng P., Cai J., Rapid access to multiple classes of peptidomimetics from common γ-AApeptide building blocks. Eur. J. Org. Chem. 2014, 1760–1765 (2014). [Google Scholar]
- 22.Teng P., et al. , Right-handed helical foldamers consisting of De novo d-AApeptides. J. Am. Chem. Soc. 139, 7363–7369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng P., et al. , Hydrogen-bonding-driven 3D supramolecular assembly of peptidomimetic zipper. J. Am. Chem. Soc. 140, 5661–5665 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.She F., et al. , De novo left-handed synthetic peptidomimetic foldamers. Angew. Chem. Int. Ed. Engl. 57, 9916–9920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M., Wang Z., Zhang Y., Guo W., Ji H., Structure-based optimization of small-molecule inhibitors for the β-Catenin/B-cell lymphoma 9 protein-protein interaction. J. Med. Chem. 61, 2989–3007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan R., et al. , SOX7 suppresses Wnt signaling by disrupting β-Catenin/BCL9 interaction. DNA Cell Biol. 37, 126–132 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Teuscher K. B., Zhang M., Ji H., A versatile method to determine the cellular bioavailability of small-molecule inhibitors. J. Med. Chem. 60, 157–169 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wisniewski J. A., Yin J., Teuscher K. B., Zhang M., Ji H., Structure-based design of 1,4-dibenzoylpiperazines as β-Catenin/B-cell lymphoma 9 protein-protein interaction inhibitors. ACS Med. Chem. Lett. 7, 508–513 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M., Wisniewski J. A., Ji H., AlphaScreen selectivity assay for β-catenin/B-cell lymphoma 9 inhibitors. Anal. Biochem. 469, 43–53 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Hoggard L. R., et al. , Rational design of selective small-molecule inhibitors for β-catenin/B-cell lymphoma 9 protein-protein interactions. J. Am. Chem. Soc. 137, 12249–12260 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Zhao J.-J., et al. , miR-30-5p functions as a tumor suppressor and novel therapeutic tool by targeting the oncogenic Wnt/β-catenin/BCL9 pathway. Cancer Res. 74, 1801–1813 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada K., et al. , Targeted disruption of the BCL9/β-catenin complex inhibits oncogenic Wnt signaling. Sci. Transl. Med. 4, 148ra117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawamoto S. A., et al. , Design of triazole-stapled BCL9 α-helical peptides to target the β-catenin/B-cell CLL/lymphoma 9 (BCL9) protein-protein interaction. J. Med. Chem. 55, 1137–1146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de la Roche M., et al. , An intrinsically labile α-helix abutting the BCL9-binding site of β-catenin is required for its inhibition by carnosic acid. Nat. Commun. 3, 680 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamoto S. A., et al. , Analysis of the interaction of BCL9 with β-catenin and development of fluorescence polarization and surface plasmon resonance binding assays for this interaction. Biochemistry 48, 9534–9541 (2009). [DOI] [PubMed] [Google Scholar]
- 36.de la Roche M., Worm J., Bienz M., The function of BCL9 in Wnt/beta-catenin signaling and colorectal cancer cells. BMC Cancer 8, 199 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sampietro J., et al. , Crystal structure of a β-catenin/BCL9/Tcf4 complex. Mol. Cell 24, 293–300 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Adachi S., et al. , Role of a BCL9-related β-catenin-binding protein, B9L, in tumorigenesis induced by aberrant activation of Wnt signaling. Cancer Res. 64, 8496–8501 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., et al. , The Wnt/β-catenin pathway is required for the development of leukemia stem cells in AML. Science 327, 1650–1653 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nusse R., Clevers H., Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Anastas J. N., Moon R. T., WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13, 11–26 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Grossmann T. N., et al. , Inhibition of oncogenic Wnt signaling through direct targeting of β-catenin. Proc. Natl. Acad. Sci. USA 109, 17942–17947 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietrich L., et al. , Cell permeable stapled peptide inhibitor of Wnt signaling that targets β-catenin protein-protein interactions. Cell Chem. Biol. 24, 958–968.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 44.van Tienen L. M., Mieszczanek J., Fiedler M., Rutherford T. J., Bienz M., Constitutive scaffolding of multiple Wnt enhanceosome components by Legless/BCL9. eLife 6, e20882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The PyMOL Molecular Graphics System, version 2.1.1 (Schrödinger LLC, 2015).
- 46.Hook D. F., et al. , The proteolytic stability of “designed” β-peptides containing α-peptide-bond mimics and of mixed α,β-peptides: Application to the construction of MHC-binding peptides. Chem. Biodivers. 2, 591–632 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.