Significance
Mirabegron as a β3-adrenoceptor agonist is a routinely prescribed drug for treating overactive bladder syndrome. However, the effect of this drug on off-targeted tissues and organs is largely unknown. In this study, we provide mechanistic insights on mirabegron-triggered atherosclerosis in causing potential cardiovascular and cerebrovascular diseases by activation of brown fat. Given that mirabegron can induce brown fat activation in adult human subjects, these findings are clinically relevant. Moreover, genetic mutations of low-density lipoprotein receptor (LDLR) frequently occur in human populations (1 in 300 humans carries LDLR mutations). If this population of patients receive mirabegron therapy, it would be of a high risk. This important issue has not been explored in clinical studies, and warrants further investigation.
Keywords: mirabegron, atherosclerosis, plaque instability, lipolysis, adipose tissue
Abstract
Mirabegron (Myrbetriq) is a β3-adrenoreceptor agonist approved for treating overactive bladder syndrome in human patients. This drug can activate brown adipose tissue (BAT) in adult humans and rodents through the β3-adrenoreceptor-mediated sympathetic activation. However, the effect of the mirabegron, approved by the US Food and Drug Administration, on atherosclerosis-related cardiovascular disease is unknown. Here, we show that the clinical dose of mirabegron-induced BAT activation and browning of white adipose tissue (WAT) exacerbate atherosclerotic plaque development. In apolipoprotein E−/− (ApoE−/−) and low-density lipoprotein (LDL) receptor−/− (Ldlr−/−) mice, oral administration of clinically relevant doses of mirabegron markedly accelerates atherosclerotic plaque growth and instability by a mechanism of increasing plasma levels of both LDL-cholesterol and very LDL-cholesterol remnants. Stimulation of atherosclerotic plaque development by mirabegron is dependent on thermogenesis-triggered lipolysis. Genetic deletion of the critical thermogenesis-dependent protein, uncoupling protein 1, completely abrogates the mirabegron-induced atherosclerosis. Together, our findings suggest that mirabegron may trigger cardiovascular and cerebrovascular diseases in patients who suffer from atherosclerosis.
Atherosclerosis, the thickening, hardening, and loss of elasticity of the arterial vessel wall, is the major cause of morbidity and mortality worldwide (1–4). Atherosclerotic lesions containing fatty plaques, cholesterol, and inflammatory cells are the primary causes of cardiovascular disease and stroke (5, 6). Atherosclerosis as a chronic and progressive disease usually starts with damage of the endothelial layer of an artery. Hypertension, hyperglycemia, hyperlipidemia especially hypercholesterolemia, smoking, obesity and diabetes, certain inflammatory disorders such as arthritis, infections, and drugs are the common risk factors of atherosclerotic plaque formation (7, 8). Of interest, high incidences of myocardial infarction and stroke have been associated with colder ambient temperature and cold seasons (9, 10). Although the exact mechanism underlying low ambient temperature has not been elucidated, our recent findings show that cold-induced lipolysis in brown adipose tissue (BAT) and browning of white adipose tissue (WAT) may partly explain the high incidences of cardiovascular disease and stroke (11).
The balance between energy deposition and dissipation is regulated by multiple factors, including the central nervous system, the endocrine system, food intake, physical exercise, pathological disease, and medications (12, 13). Although WAT stores excessive energy in its lipid form, activated BAT specializes energy consumption by producing heat (14, 15). Under certain conditions such as cold exposure, WAT, especially that located in the s.c. region, undergoes the brown-like transition, a process named browning WAT (16, 17). Instead of storing energy, adipocytes of browning WAT consume energy by thermogenesis. Breakdown of lipids in adipocytes (i.e., lipolysis) is achieved by hydrolysis of triglycerides into glycerol and free fatty acid (FFA). Lipoproteins are particles consisting of triacylglycerol, cholesterol, phospholipids, and amphipathic proteins called apolipoproteins (18, 19). On the basis of the ratio between lipids and amphipathic proteins, lipoproteins can be divided into four major types: chylomicrons; very low density lipoprotein (VLDL), LDL, and high-density lipoprotein (HDL) (20). VLDL is synthesized in the liver and delivers energy-rich triacylglycerol to cells in the body (21). Triacylglycerol is stripped from chylomicrons and VLDL through the action of lipoprotein lipase, an enzyme that is found on the surface of endothelial cells and digests the triacylglycerol into fatty acids and monoglycerides. After VLDL particles are stripped of triacylglycerol, they become denser particles, which are remodeled in the liver and transformed into LDL. The function of LDL is to deliver cholesterol to cells, where it is used in membranes, or for the synthesis of steroid hormones. Cells take up cholesterol by receptor-mediated endocytosis. LDL binds to a specific LDL receptor (LDLR) and is internalized in an endocytic vesicle (22). Receptors are recycled to the cell surface, whereas hydrolysis in an endolysosome releases cholesterol for the lipid composition in cells (22). The serum levels of VLDL-C and LDL-C have been directly associated with risk for atherosclerotic cardiovascular disease (23).
Mirabegron (Myrbetriq) is an orally active β3-adrenergic receptor agonist approved for treating the symptoms of overactive bladder (24). Many patients stop anticholinergic medication because of the adverse effects (25). About 50% of patients with overactive bladder discontinue treatment in 3 mo because of adverse effects, including dry eyes, dry mouth, blurred vision, and constipation (26, 27). More recently, a causal link between anticholinergic burden and the development of dementia has been established (28–30), raising further concerns about the safety issue in human patients. Activation of the β3-adrenergic receptor also induces BAT activation and browning of WAT (31, 32). In a recent pilot human study, it has been shown that a clinical dose of mirabegron can activate BAT and browning WAT in human subjects (32).
In this study, we investigate the mirabegron-induced BAT activation and WAT browning in relation to atherosclerotic plaque development in preclinical animal models. In ApoE−/− and Ldlr−/− mice, we show that a clinically equivalent dose of mirabegron accelerates atherosclerotic plaque development through a thermogenesis-dependent mechanism of lipolysis. These findings demonstrate a causal link of mirabegron to atherosclerosis-related cardiovascular disorder and perhaps stroke. These preclinical findings warrant future validation in human patients.
Materials and Methods
Animals.
Male 8-wk-old C57BL/6 mice were purchased from the Beijing SPF Bioscience Co. Male 8-wk-old ApoE−/− and Ldlr−/− mice were purchased from the Nanjing BioMedical Research Institute of Nanjing University (Nanjing, China). Mice were kept at room temperature (22 °C) before random grouping. All animal studies complied with the Management Rules of the Chinese Ministry of Health and were approved by the Local Ethical Committee of Qilu Hospital, Shandong University.
Mirabegron.
Mirabegron was purchased form BOC sciences (223673-61-8) and was dissolved in polyethylene glycol (91893; Sigma-Aldrich) as a stock, which was further diluted in PBS upon use. Detailed information was described in the SI Appendix.
Antibodies, histology and immunohistochemistry, blood sample collection, real-time PCR, Western blot analysis, indirect calorimetry, serum lipid analysis and FPLC chromatography, blood glucose, insulin, insulin tolerance test, and glucose tolerance test were described in the SI Appendix.
Statistics.
All data were presented as the mean ± SEM. Data for insulin and glucose tolerance tests were analyzed by two-way ANOVA. Data analysis involved unpaired Student’s t-test for two groups and one-way ANOVA for more than two groups. P < 0.05 was considered statistically significant.
Results
A Clinical Dose of Mirabegron Induces BAT Activation and WAT Browning.
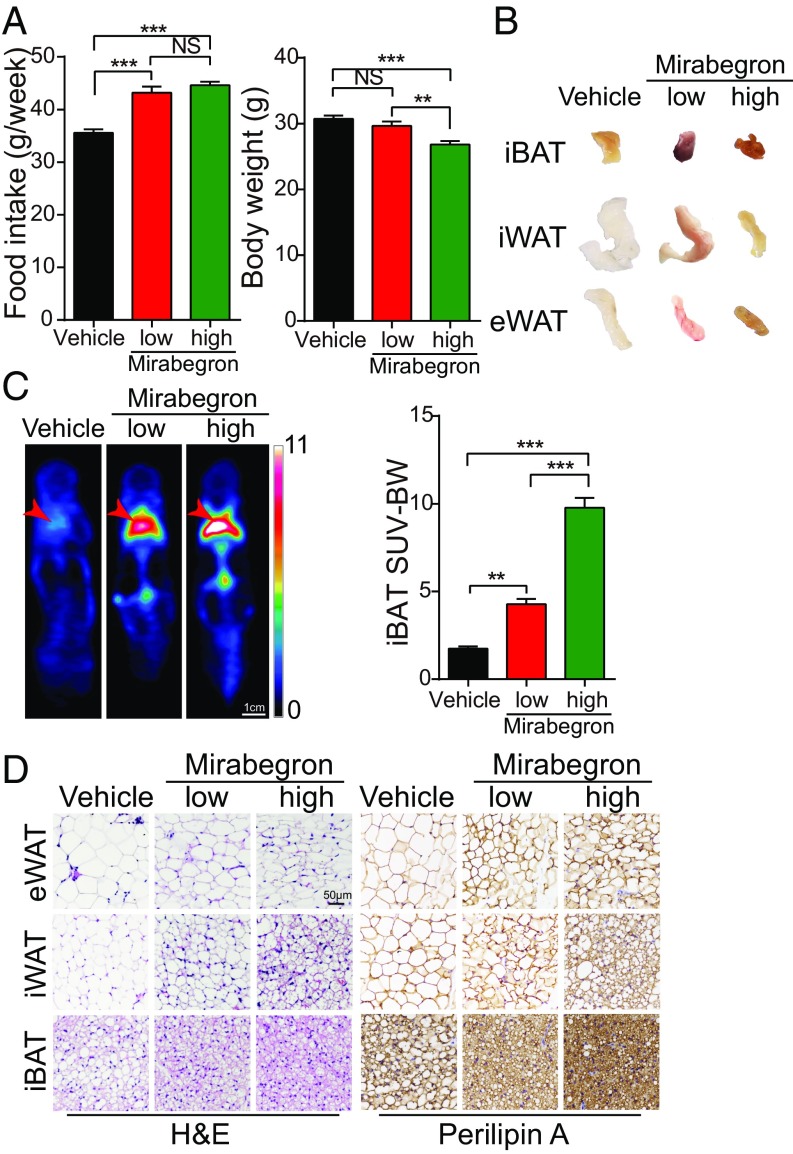
To study the effect of mirabegron on adipose tissues, a clinical dose of 0.8 mg/kg, equivalent to 50 mg/person used for treating human patients with overactive bladder, and a high dose of 8 mg/kg was administrated once daily in wt, ApoE−/−, and Ldlr−/− adult mice. Interestingly, both a low and a high dose of mirabegron significantly increased food intake (Fig. 1A). Despite the increase of food intake, mirabegron, especially at the high dose, reduced the body weight and body mass index (SI Appendix, Fig. S1A). The mirabegron-treated interscapular BAT (iBAT), inguinal WAT (iWAT), and epididymal WAT (eWAT) exhibited more brownish color relative to their respective controls (Fig. 1B), suggesting activation of these adipose depots. In support of this notion, position emission tomography-computed tomography examination showed marked increases of 18F-fluorodeoxyglucose uptake in BAT and browning WAT of the mirabegron-treated mice (Fig. 1C). In addition, the weights of mirabegron-treated adipose depots were also markedly decreased (SI Appendix, Fig. S1B).
Fig. 1.
The effect of mirabegron on food intake, body weight, adipose deposition, and browning of WAT and BAT. (A) The effect of 6 wk of treatment with mirabegron at low and high doses on food intake (n = 5 animals per group) and body weight (n = 15 animals per group). NS, not significant; **P < 0.01; ***P < 0.001. (B) Adipose tissue morphology of 6-wk mirabegron-treated iBAT, iWAT, and eWAT. (C) Representative position emission tomography-computed tomography images of mirabegron-treated mice at day 4. Red arrows indicate the position of iBAT. (D) H&E and perilipin A staining of 6-wk mirabegron-treated eWAT, iWAT, and iBAT. (Scale bar, 50 μm.)
Histological analysis of various adipose depots supported an activated phenotype in mirabegron-treated BAT and WATs, which comprised smaller adipocytes relative to their vehicle-treated controls (Fig. 1D). Quantitative analysis showed that the average adipocyte sizes were significantly smaller in mirabegron-treated adipose tissues compared with their controls (SI Appendix, Fig. S1C). Particularly, adipocytes in visceral WAT (visWAT) appeared as high-density multilocular structures, a characteristic for browning WAT. Mirabegron markedly increased the mitochondrial contents and UCP1 expression in BAT, s.c. WAT (subWAT), and visWAT (Fig. 2 and SI Appendix, Fig. S1 D and E). These findings show that both low and high doses of mirabegron augment BAT activation and browning of WAT.
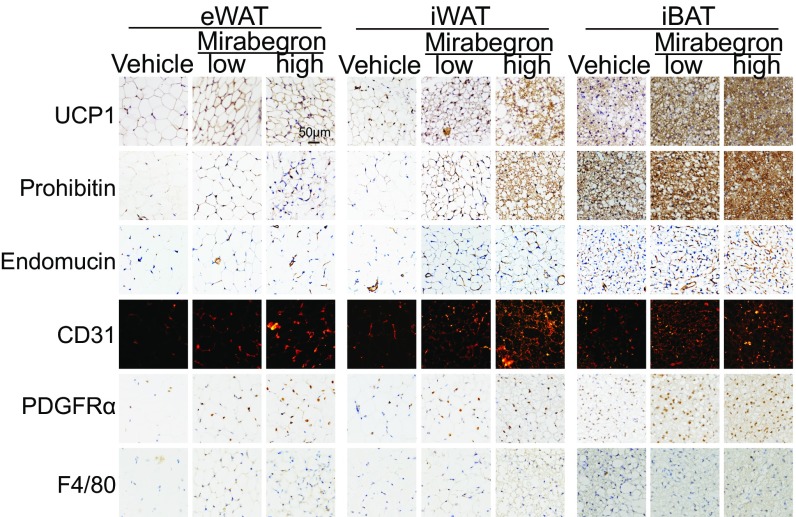
Fig. 2.
Immunohistologic analysis of mirabegron-treated adipose tissues. Histological micrographs of UCP1, prohibitin, endomucin, CD31, PDGFRα, and F4/80 staining of mirabegron-treated eWAT, iWAT, and iBAT. (Scale bar, 50 μm.)
Modulation of the Cellular and Molecular Compositions in the Adipose Microenvironment.
Along activation of BAT and browning of WAT, nonadipocyte stromal cellular contents increased. The number of endomucin+ and CD31+ microvessels in mirabegron-treated adipose depots was significantly increased (Fig. 2 and SI Appendix, Fig. S1 F and G). In addition, PDGFRα+ stromal cell components markedly increased in browning WAT. As PDGFRα+ stromal cells serve as adipocyte stem cells (33), expansion of the PDGFRα+ population suggests that differentiation of stem cells and preadipocytes is a crucial process of WAT browning. Similarly, the PDGFRα+ cellular population in BAT was also expanded (Fig. 2 and SI Appendix, Fig. S1H). In contrast, the number of F4/80+ macrophages remained unchanged in the vehicle- and mirabegron-treated adipose tissues (Fig. 2 and SI Appendix, Fig. S1I).
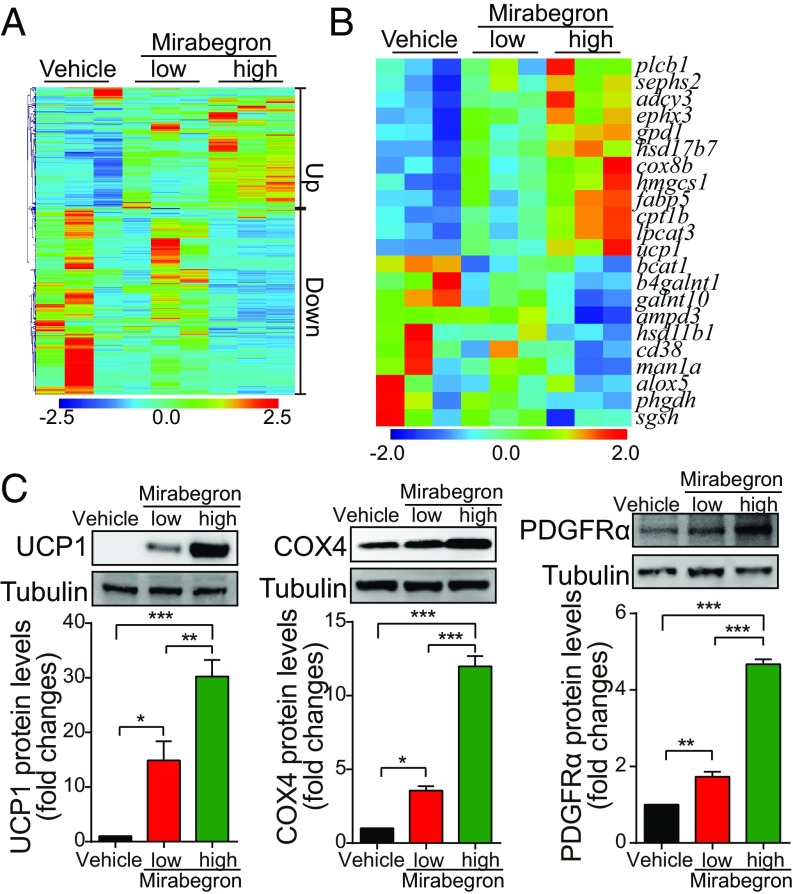
To explore molecular mechanisms, whole-genome expression profiling in subWAT was analyzed. Treatment with mirabegron resulted in noticeable alterations of gene expression patterns with up- and down-regulations of numerous genes (Fig. 3 A and B and SI Appendix, Figs. S2A and S3). Among them, a nearly 50-fold increase of UCP1 expression was detected in mirabegron-treated subWAT compared with the nontreated sample (SI Appendix, Fig. S2B). Dose escalation of mirabegron further boosted the UCP1 expression level. Interestingly, leptin, the crucial feeding hormone derived from adipocytes, was markedly down-regulated, partly explaining the increase of food intake in mirabegron-treated mice (SI Appendix, Fig. S2C). Adiponectin as an adipose tissue-specific adipokine was also significantly down-regulated on mirabegron treatment (SI Appendix, Fig. S2C). Expectedly, expression levels of WAT browning-related genes including Cox7a1, Dio2, Pgc1α, and Cidea were significantly up-regulated (SI Appendix, Fig. S2D). Conversely, Ebf2 and Prdm16 expression levels were down-regulated in mirabegron-treated subWAT (SI Appendix, Fig. S2B). Consistent with alteration of the mRNA expression levels, protein expression levels of UCP1, COX4, and PDGFRα were significantly increased in the mirabegron-treated subWAT (Fig. 3C). These data show that mirabegron treatment significantly modulate the cellular and molecular components in the adipose microenvironment.
Fig. 3.
Genome-wide expression profiling of mirabegron-treated iWAT and browning markers. (A and B) Heat map analysis of up- and down-regulated genes and lipolysis-related genes. (C) Western blot analyses of UCP1, COX4, and PDGFRα protein expression levels in mirabegron-treated WAT (n = 3 samples per group). *P < 0.05; **P < 0.01; ***P < 0.001.
Mirabegron Accelerates Atherosclerotic Plaque Growth and Instability in ApoE-Deficient Mice.
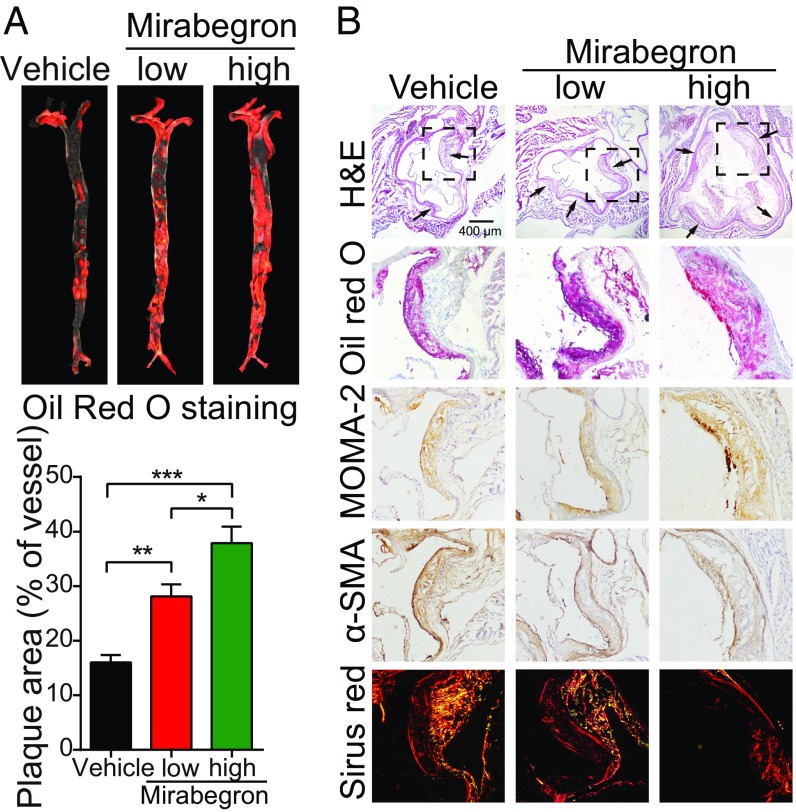
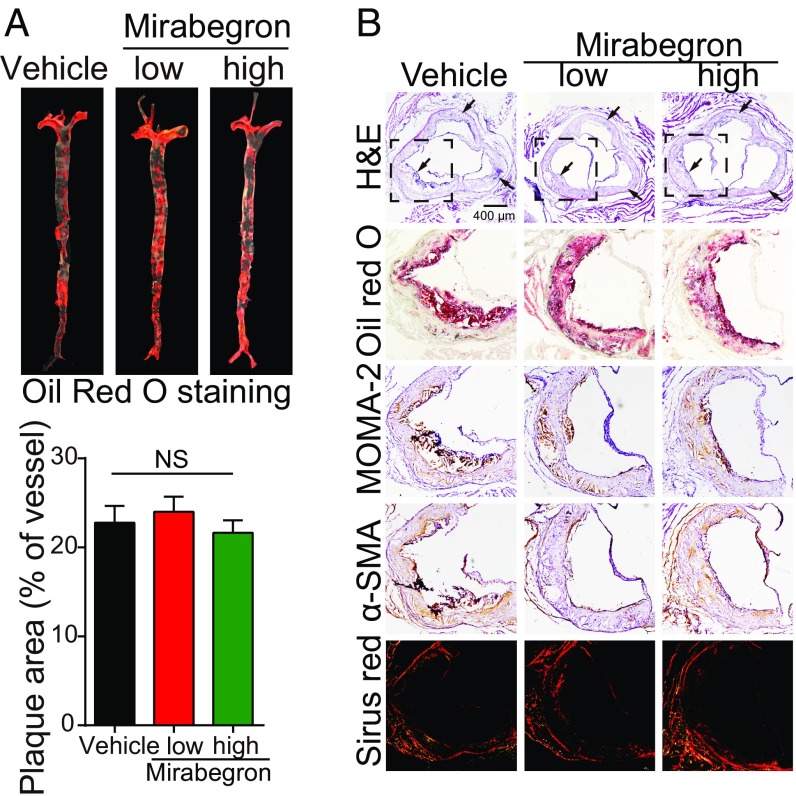
We next investigated the effect of mirabegron on atherosclerotic plaque formation in ApoE−/− mice. Oil red O staining showed that mirabegron markedly increased the atherosclerotic plaque formation in the aorta (Fig. 4A). Histologic examination validated the existence of larger plaques in the mirabegron-treated mice (Fig. 4B and SI Appendix, Fig. S4A). A significant increase of infiltration of inflammatory macrophages in the atherosclerotic plaques was found in the mirabegron-treated mice compared with controls (Fig. 4B and SI Appendix, Fig. S4A). In contrast, the α-smooth muscle actin (α-SMA+) and collagen+ components within plaques were markedly decreased in the mirabegron-treated animals (Fig. 4B and SI Appendix, Fig. S4A). As a consequence, the plaque instability index {calculated according to the following formula: [oil red O+ area + macrophages/monocytes monoclonal antibody-2 (MOMA-2+) area]/[α-SMA+ area + collagen I+ area]} significantly increased in mirabegron-treated groups compared with controls (SI Appendix, Fig. S4A).
Fig. 4.
Functional effects of mirabegron on atherosclerotic plaque development in ApoE−/− mice. (A) Gross examination and quantification of oil red O-stained aorta stem from mirabegron-treated and vehicle-treated ApoE−/− mice (n = 6 samples per group). (B) Histologic examination of a cross-section of aorta roots from mirabegron-treated and vehicle-treated ApoE−/− mice. Aorta sections were stained with H&E, oil red O, MOMA-2, α-SMA, or Sirius red. The boxed area the H&E-stained sections were amplified. Arrows point to the plaques. *P < 0.05; **P < 0.01; ***P < 0.001.
The necrotic core size of plaques was markedly enlarged in mirabegron-treated mice, further supporting the instability of atherosclerotic plaques (SI Appendix, Fig. S4 B and C). Additional evidence of plaque instability was obtained by analyzing the fibrous caps, which was markedly decreased in the mirabegron-treated animals. Together, infiltration of an increased number of inflammatory cells, reduction of the α-SMA+ component, decrease of collagen+ component, increase of necrotic area, and decrease of fibrous caps all destabilize the plaques and likely cause rupture.
Mirabegron Accelerates Atherosclerotic Plaque Growth and Instability in Ldlr-Deficient Mice.
To relate our findings to clinical relevance, we employed a Ldlr−/− mouse strain in our study. In humans, LDL receptor mutations are frequently occurring (1 in 300–500 people) and cause familial hypercholesterolemia, manifesting high levels of blood cholesterol and LDL and an early onset of cardiovascular disease (34, 35). The underlying mechanism of familial hypercholesterolemia is the defective clearance of cholesterol and LDL. Similar to ApoE−/− mice, Ldlr-deficient mice developed similar, albeit more severe, atherosclerosis disorders in response to mirabegron treatment (SI Appendix, Fig. S5). Atherosclerotic plaque growth and instability were overwhelmingly increased in Ldlr−/− mice (SI Appendix, Fig. S5 A–C). It should be emphasized that the mirabegron-treated mice exhibited a high degree of plaque instability, which might cause plaque rupture after prolonged experimental duration. In addition, larger areas of the necrotic core within plaques were identified in mirabegron-treated Ldlr−/− mice (SI Appendix, Fig. S5 D and E). These results validated the phenotypic changes in mirabegron-treated ApoE-deficient mice and may recapitulate clinical settings by which patients with LDLR mutations might receive mirabegron treatment.
Elevated Levels of Serum LDL-C, Total Cholesterol, and Metabolism.
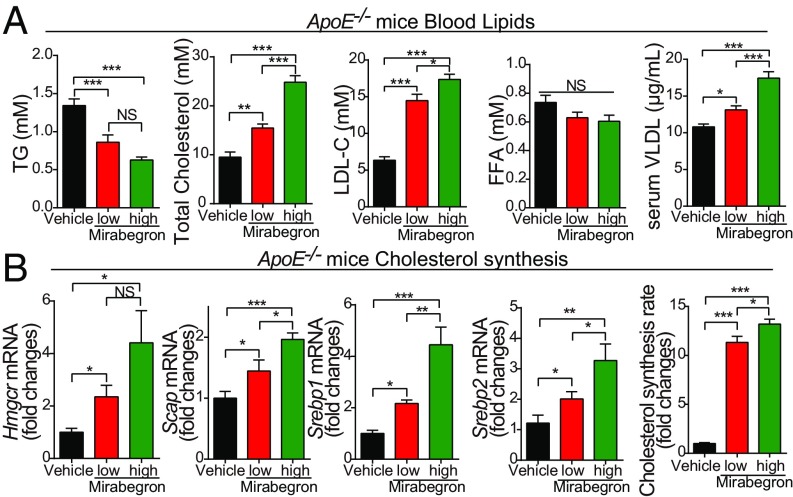
Because atherosclerotic plaque growth is associated with hypercholesterolemia, and in particular increased serum levels of LDL-cholesterol (LDL-C) and VLDL-C, we measured blood lipid and glucose levels. In ApoE−/− and Ldlr−/− mice, treatment with mirabegron elevated serum levels of total cholesterol and LDL-C (Fig. 5A and SI Appendix, Fig. S7A). Serum levels of triglyceride were significantly decreased because of an active phenotype of lipolysis (Fig. 5A and SI Appendix, Fig. S7A). Levels of FFA remained unchanged between the mirabegron- and vehicle-treated groups (Fig. 5A and SI Appendix, Fig. S7A). Quantitative analysis of cholesterol synthesis-related genes, including Hmgcr, Scap, Srebp1, and Srebp2, showed elevated expression levels in low- and high-dose mirabegron-treated liver tissues relative to their controls (Fig. 5B and SI Appendix, Fig. S7B). These results show that mirabegron induced hypercholesterolemia and elevated LDL-C in ApoE- and Ldlr-deficient mice through a possible mechanism of increasing cholesterol synthesis. Despite the changes of blood lipid profiling, glucose tolerance test and insulin tolerance test showed significantly lower blood glucose levels and increased insulin sensitivity (SI Appendix, Fig. S8 A and B).
Fig. 5.
Blood chemistry analysis and lipid profiling of mirabegron-treated ApoE−/− mice. (A) Serum levels of triglyceride, total cholesterol, LDL-C, FFA, and VLDL in mirabegron- and vehicle-treated ApoE−/− mice (n = 8 animals per group). (B) Analysis of cholesterol synthesis by qPCR quantification of liver Hmgcr, Scap, Srebp1, and Srebp2 expression levels in mirabegron- and vehicle-treated ApoE−/− mice (n = 6 animals per group). NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Mirabegron significantly increased oxygen consumption and release of carbon dioxide, indicating an elevated rate of metabolism in the treated animals (SI Appendix, Fig. S8C). These findings demonstrate that mirabegron improves global metabolism of the norepinephrine-triggered nonshivering thermogenesis in ApoE-deficient mice.
UCP1-Dependent Atherosclerotic Plaque Growth and Instability.
Next, we studied the causational mechanisms underlying the browning adipose tissues in development of atherosclerotic plaques. Because UCP1 is the crucial mitochondrial membrane protein for nonshivering thermogenesis, blocking UCP1 might impair the thermogenesis-dependent metabolism. To test this hypothesis, we generated a double-knockout mouse strain that lacked ApoE and Ucp1 (ApoE−/−:Ucp1−/−). ApoE−/−:Ucp1−/− were fed with a high-fat diet to induce the formation of atherosclerotic plaques. Notably, mirabegron-induced accelerated plaque growth was completely abrogated in ApoE−/−:Ucp1−/−, which was indistinguishable from the vehicle-treated animals (Fig. 6). The plaque formation and sizes were similar in the vehicle- and mirabegron-treated groups (Fig. 6). Inflammatory cell infiltration, α-SMA+ and collagen+ components, and plaque instability indexes remain indistinguishable in mirabegron-treated and control groups (SI Appendix, Fig. S6A). Similarly, the necrotic core, necrotic area, and fibrous caps are virtually identical in mirabegron- and vehicle-treated plaques (SI Appendix, Fig. S6 B and C). Genetic deletion of Ucp1 gene also normalized blood lipids profile and cholesterol synthesis (SI Appendix, Fig. S9). These findings provide compelling evidence that UCP1-mediated lipolysis is a causative factor for intensifying atherosclerotic plaque growth and instability.
Fig. 6.
Genetic deletion of Ucp1 ablates the mirabegron-accelerated atherosclerotic plaque growth and instability. (A) Gross examination and quantification of oil red O-stained aorta stem from mirabegron-treated and vehicle-treated ApoE−/−:Ucp1−/− double-knockout mice (n = 6 samples per group). (B) Histological examination of cross-section of aorta roots from mirabegron-treated and vehicle-treated ApoE−/−:Ucp1−/− double-knockout mice. Aorta sections were stained with H&E, oil red O, MOMA-2, α-SMA, or Sirius red. The boxed area the H&E-stained sections were amplified. Arrows point to the plaques. NS, not significant.
FPLC Analysis of Blood Lipid Components and Lipoproteins.
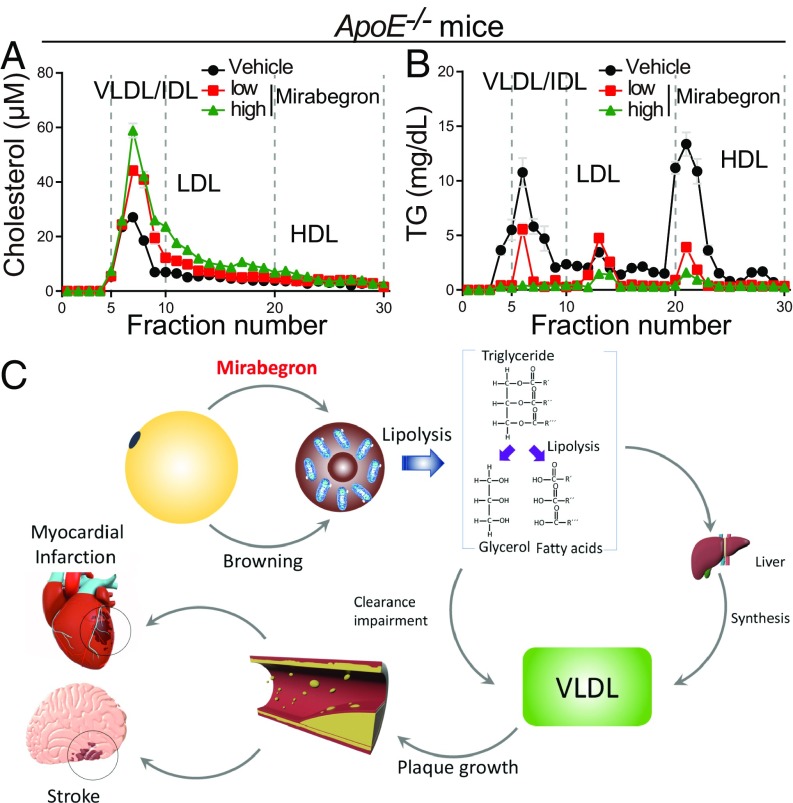
To further analyze lipoproteins in different fractions of chylomicrons, FPLC was applied for fractionation. As shown in Fig. 7 A and B, in mirabegron-treated ApoE−/− mice, the cholesterol-chylomicrons contained significantly higher LDL and VLDL. In contrast, HDL-C levels were insignificantly altered in these particles. The high levels of LDL and VLDL contents were specific to cholesterol-chylomicrons because the triglyceride-containing particles did not exhibit the elevated LDL and VLDL remnants. Similarly, Ldlr−/− mice also showed high levels of serum LDL-C and VLDL-C remnants (SI Appendix, Fig. S10 A and B). These results further validate the high circulating levels of LDL-C and VLDL-C in mirabegron-treated ApoE−/− and Ldlr−/−mice.
Fig. 7.
FPLC analysis of plasma cholesterols and schematic diagram of mechanisms underlying the mirabegron-accelerated atherosclerotic plaque growth and instability. (A and B) Plasma cholesterol and triglyceride levels of the mirabegron- and vehicle-treated ApoE−/− mice (n = 6 animals per group). Values from fractions 5–10 were used for calculation of VLDL/IDL, whereas fractions 11–19 and fractions 20–30 represent LDL and HDL, respectively. (C) Mirabegron induces browning of WAT and activation of BAT, which undergo thermogenesis by the UCP1-dependent lipolysis. FFA promotes biosynthesis of VLDL remnants to facilitate the atherosclerotic plaque growth and instability. Accelerated plaque growth and instability increase the cardiovascular and cerebrovascular events.
Discussion
Numerous risk factors have been associated with high incidence of atherosclerotic cardiovascular and cerebrovascular diseases, including hypertension, high-fat and high-calorie diet, hyperlipidemia, lack of exercise, overweight and obesity, smoking, overdose of alcohol, stress, genetic factors, low temperature exposure, and suffering from other diseases such as diabetes, infections, and inflammation. However, little is known about the effect of drugs on cardiovascular events. Our study provides an example that drugs used for treating noncardiovascular and noncerebrovascular diseases have a profound effect on the development of atherosclerosis. The clinical risk of mirabegron-triggered atherosclerotic plaque growth and instability has been overlooked, and the underlying mechanism is obscure.
Dietary restriction of fat intake, increasing physical exercise, and reducing body weight prevent the cardiovascular and cerebrovascular events (36, 37). Lipolysis is often triggered by fasting, exercise, and cold exposure, which mobilize the stored excessive energy for coping with increased metabolic rates. As a β3-adrenoreceptor agonist, mirabegron has been shown in humans to be able to activate BAT and thermogenesis (31, 32). In this work, we show that a clinical relevant dose of mirabegron can activate BAT and induce a browning phenotype in subWAT and visWAT in mice. In both ApoE- and Ldlr-deficient mice, mirabegron accelerates atherosclerotic plaque growth and instability through elevating serum levels of LDL-C and VLDL-C remnants, plaque inflammation, and reduction of fibrous components. Although the detailed molecular mechanism needs to be elucidated, multifaceted mechanisms might be involved (Fig. 7C), including lipolysis, which triggers increased biosynthesis of cholesterol and mobilization of LDL-C, manifesting the high levels of blood LDL-C and VLDL-C. In both ApoE- and Ldlr-deficient mice, we provide evidence that the elevation of LDL-C and VLDL-C is dependent on lipolysis. Genetic deletion of UCP1 in ApoE−/− mice completely ablates the elevated levels of LDL and atherosclerotic plaque growth. As UCP1 is essentially required for nonshivering thermogenesis, these findings indicate that the mirabegron-triggered atherosclerotic plaque growth is dependent on thermogenic metabolism in adipose tissues. Lipolysis also triggered plaque inflammation. Treatment with mirabegron significantly augments infiltration inflammatory cells in both ApoE- and Ldlr-deficient mice. Browning of adipose tissues releases an array of adipokines and adipocytokines that might contribute to plaque instability. Finally, the increases of lipid and inflammatory components within the plaque reduced the ratio of fibrous and smooth muscle cell contents, leading to increased plaque instability.
Impairment of LDL-C clearance is commonly seen in patients with cardiovascular disease. In addition to dietary factors, genetic mutations of LDLR frequently occur in human populations (34, 38). In fact, more than 1,700 mutations have been identified in the Ldlr gene for causing familial hypercholesterolemia, and 1 in 300 humans carries LDLR mutations (39, 40). Assuming this population of people receives mirabegron treatment because of their bladder disorder, mirabegron might increase their cardiovascular and cerebrovascular risks as a result of lipolysis in browning WAT and BAT. In particular, it is known that mirabegron can induce a browning phenotype of adipose tissues in human subjects. In this regard, our findings obtained from the Ldlr-deficient mice are highly relevant to clinical situations. Similar to this study, our recent work shows that exposure of experimental animals to low ambient temperature accelerates atherosclerotic plaque growth and instability. Again, cold-triggered lipolysis is responsible for accelerated atherosclerosis (11), which further supports the general conclusion of lipolysis-triggered high risks of cardiovascular and cerebrovascular diseases. For those who do not carry LDLR mutations, perhaps the elevated levels of LDL-C and VLDL-C will be cleared. The effect of mirabegron on atherosclerotic plaque development warrants further investigation. Some studies show that activation of β3-adrenoreceptor significantly increases apoA-1, a major protein component of HDL in plasma (41). HDL promotes cholesterol efflux from tissues to the liver for excretion. In our study, we did not observe significant differences of HDL in mirabegron-treated and control mice.
Together, our findings raise a concern of clinically approved mirabegron that might increase the cardiovascular and cerebrovascular diseases through a mechanism of the lipolysis-triggered atherosclerotic plaque growth and instability. This issue warrants a clinical study to validate the preclinical findings.
Supplementary Material
Acknowledgments
We thank Dr. Jianping Zhang in Fudan University Shanghai Cancer Center for the position emission tomography-computed tomography imaging analysis. Y. Cao’s laboratory is supported through research grants from the European Research Council advanced Grant ANGIOFAT (Project 250021), the Swedish Research Council, the Swedish Cancer Foundation, the Swedish Children’s Cancer Foundation, the Karolinska Institute Foundation, the Karolinska Institute distinguished professor award, the Torsten Soderbergs Foundation, the Maud and Birger Gustavsson Foundation, the NOVO Nordisk Foundation-Advance grant, and the Knut and Alice Wallenberg’s Foundation. This work is also supported by the Taishan Scholars Program of Shandong Province, China (Y. Cao, M.Z., and C.Z.). Yun Zhang’s laboratory is supported by Program of Introducing Talents of Discipline to Universities (Grant B07035), the State Key Program of National Natural Science of China (Grants 61331001 and 81530014), the International Collaboration and Exchange Program of China (Grant 81320108004), the National Natural Science Foundation of China (Grants 81425004, 81770442, and 31770977), and the Fundamental Research Funds of Shandong University (Grant 2018JC001) and National Key R & D Program of China (Grants 2017YFC0908700 and 2017YFC0908703).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901655116/-/DCSupplemental.
References
- 1.Libby P, Ridker PM, Hansson GK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473:317–325. [DOI] [PubMed] [Google Scholar]
- 2.Borissoff JI, Spronk HM, ten Cate H (2011) The hemostatic system as a modulator of atherosclerosis. N Engl J Med 364:1746–1760. [DOI] [PubMed] [Google Scholar]
- 3.Swirski FK, Nahrendorf M (2013) Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass CK, Witztum JL (2001) Atherosclerosis. The road ahead. Cell 104:503–516. [DOI] [PubMed] [Google Scholar]
- 5.Amarenco P, et al. ; TIAregistry.org Investigators (2018) Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med 378:2182–2190. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg IJ, et al. (2018) Deciphering the role of lipid droplets in cardiovascular disease: A report from the 2017 National Heart, Lung, and Blood Institute Workshop. Circulation 138:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross R. (1993) The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 362:801–809. [DOI] [PubMed] [Google Scholar]
- 8.Pleniceanu O, et al. (2017) Peroxisome proliferator-activated receptor gamma (PPARγ) is central to the initiation and propagation of human angiomyolipoma, suggesting its potential as a therapeutic target. EMBO Mol Med 9:508–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett AG. (2007) Temperature and cardiovascular deaths in the US elderly: Changes over time. Epidemiology 18:369–372. [DOI] [PubMed] [Google Scholar]
- 10.Wolf K, et al. ; Cooperative Health Research in the Region of Augsburg Study Group (2009) Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation 120:735–742. [DOI] [PubMed] [Google Scholar]
- 11.Dong M, et al. (2013) Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab 18:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waterson MJ, Horvath TL (2015) Neuronal regulation of energy homeostasis: Beyond the hypothalamus and feeding. Cell Metab 22:962–970. [DOI] [PubMed] [Google Scholar]
- 13.Morrison SF, Madden CJ, Tupone D (2014) Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 19:741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowell BB, Spiegelman BM (2000) Towards a molecular understanding of adaptive thermogenesis. Nature 404:652–660. [DOI] [PubMed] [Google Scholar]
- 15.Keesey RE, Powley TL (2008) Body energy homeostasis. Appetite 51:442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrovic N, et al. (2010) Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285:7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, et al. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, et al. (2014) Lipoprotein lipase: From gene to atherosclerosis. Atherosclerosis 237:597–608. [DOI] [PubMed] [Google Scholar]
- 19.Stefanutti C, Julius U (2013) Lipoprotein apheresis: State of the art and novelties. Atheroscler Suppl 14:19–27. [DOI] [PubMed] [Google Scholar]
- 20.Utermann G. (1989) The mysteries of lipoprotein(a). Science 246:904–910. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL (1989) Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 320:915–924. [DOI] [PubMed] [Google Scholar]
- 22.Lagace TA. (2014) PCSK9 and LDLR degradation: Regulatory mechanisms in circulation and in cells. Curr Opin Lipidol 25:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nabel EG. (2003) Cardiovascular disease. N Engl J Med 349:60–72. [DOI] [PubMed] [Google Scholar]
- 24.Deeks ED. (2018) Mirabegron: A review in overactive bladder syndrome. Drugs 78:833–844. [DOI] [PubMed] [Google Scholar]
- 25.Moyson J, Legrand F, Vanden Bossche M, Quackels T, Roumeguère T (2017) [Efficacy and safety of available therapies in the management of idiopathic overactive bladder: A systematic review of the literature]. Prog Urol 27:203–228. [DOI] [PubMed] [Google Scholar]
- 26.Andersson KE. (2013) New developments in the management of overactive bladder: Focus on mirabegron and onabotulinumtoxinA. Ther Clin Risk Manag 9:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagg A, Compion G, Fahey A, Siddiqui E (2012) Persistence with prescribed antimuscarinic therapy for overactive bladder: A UK experience. BJU Int 110:1767–1774. [DOI] [PubMed] [Google Scholar]
- 28.Jessen F, et al. (2010) Anticholinergic drug use and risk for dementia: Target for dementia prevention. Eur Arch Psychiatry Clin Neurosci 260:S111–S115. [DOI] [PubMed] [Google Scholar]
- 29.Gray SL, Hanlon JT (2018) Anticholinergic drugs and dementia in older adults. BMJ 361:k1722. [DOI] [PubMed] [Google Scholar]
- 30.Carrière I, et al. (2009) Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: The 3-city study. Arch Intern Med 169:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finlin BS, et al. (2018) Human adipose beiging in response to cold and mirabegron. JCI Insight 3:121510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cypess AM, et al. (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry R, Rodeheffer MS (2013) Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 15:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panach K, Garg A, Ahmad Z (2017) Heterozygous null LDLR mutation in a familial hypercholesterolemia patient with an atypical presentation because of alcohol abuse. Circ Cardiovasc Genet 10:e001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Graaf A, et al. (2011) Molecular basis of autosomal dominant hypercholesterolemia: Assessment in a large cohort of hypercholesterolemic children. Circulation 123:1167–1173. [DOI] [PubMed] [Google Scholar]
- 36.Li B, et al. (2000) Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat Med 6:1115–1120. [DOI] [PubMed] [Google Scholar]
- 37.Schuler G, et al. (1992) Regular physical exercise and low-fat diet. Effects on progression of coronary artery disease. Circulation 86:1–11. [DOI] [PubMed] [Google Scholar]
- 38.Fass D, Blacklow S, Kim PS, Berger JM (1997) Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature 388:691–693. [DOI] [PubMed] [Google Scholar]
- 39.Chora JR, Medeiros AM, Alves AC, Bourbon M (2018) Analysis of publicly available LDLR, APOB, and PCSK9 variants associated with familial hypercholesterolemia: Application of ACMG guidelines and implications for familial hypercholesterolemia diagnosis. Genet Med 20:591–598. [DOI] [PubMed] [Google Scholar]
- 40.Strøm TB, Tveten K, Laerdahl JK, Leren TP (2014) Mutation G805R in the transmembrane domain of the LDL receptor gene causes familial hypercholesterolemia by inducing ectodomain cleavage of the LDL receptor in the endoplasmic reticulum. FEBS Open Bio 4:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao X, Li Y (2017) β3-Adrenergic receptor regulates hepatic apolipoprotein A-I gene expression. J Clin Lipidol 11:1168–1176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.