Significance
Body reorganization in eels during gametogenesis can induce undesired side effects with possible pathological significance. This study provides analytical evidence for the maternal transfer of toxic metals from soft and hard tissues to the ovaries of mature females. By illustrating the metabolic fluxes and fate of mobilized minerals and metals in the fishes’ bodies during sexual development, we have identified a previously unreported aspect of anthropogenic impact on endangered anguillid eels. Furthermore, our findings suggest a physiologically connected interplay of energy metabolism and bone resorption in the reproductive strategy of eels. Consequently, we propose the eel as an interesting model organism for investigating the physiological pathways connecting lipid metabolism and mineral retention, known also to affect the health state of humans.
Keywords: eel, maternal transfer, bone loss, metals, spawning migration
Abstract
During their once-in-a-lifetime transoceanic spawning migration, anguillid eels do not feed, instead rely on energy stores to fuel the demands of locomotion and reproduction while they reorganize their bodies by depleting body reserves and building up gonadal tissue. Here we show how the European eel (Anguilla anguilla) breaks down its skeleton to redistribute phosphorus and calcium from hard to soft tissues during its sexual development. Using multiple analytical and imaging techniques, we characterize the spatial and temporal degradation of the skeletal framework from initial to final gonadal maturation and use elemental mass ratios in bone, muscle, liver, and gonadal tissue to determine the fluxes and fates of selected minerals and metals in the eels’ bodies. We find that bone loss is more pronounced in females than in males and eventually may reach a point at which the mechanical stability of the skeleton is challenged. P and Ca are released and translocated from skeletal tissues to muscle and gonads, leaving both elements in constant proportion in remaining bone structures. The depletion of internal stores from hard and soft tissues during maturation-induced body reorganization is accompanied by the recirculation, translocation, and maternal transfer of potentially toxic metals from bone and muscle to the ovaries in gravid females, which may have direct deleterious effects on health and hinder the reproductive success of individuals of this critically endangered species.
Stocks of several eel species of the genus Anguilla have diminished severely in recent decades worldwide, putting them at risk for conservation. The European eel (Anguilla anguilla) is now rated as critically endangered on the red list of the International Union for Conservation of Nature, and the American eel (Anguilla rostrata) and Japanese eel (Anguilla japonica) are rated as endangered. The reason for these declines is not fully understood, however. Anguillid eels undergo a long oceanic larval development before inhabiting inland and coastal waters for their premature growth phase. With the onset of sexual maturation, they change their appearance from resident yellow eel to migratory silver eel to meet the physiological requirements for an up to 6,000-km, once-in-a-lifetime migration back to their oceanic spawning areas. This peculiar changeover, termed “silvering,” involves morphological adaptations, such as increase in eye diameter and fin length, and physiological changes, including the cessation of feeding, degeneration of the gut, and initiation of gonadogenesis (1–3). Consequently, eels rely on the breakdown of their lipid-rich muscle tissue to fuel gonadogenesis and locomotion during their migration (4–6).
No European or American eel in an advanced maturation state has yet been found in the wild, limiting observations to natural initial maturation stages and artificially matured eels. Previous research has shown that the depletion of soft tissues during fasting and maturation is accompanied by the resorption of phosphorus and calcium from the bone, which acts as a mineral reservoir (7, 8). The endoskeleton (plus scales in most bony fish) forms the largest depot for minerals in vertebrates, storing the majority of Ca (∼99%) and P (85–90%) (9–11). It has been postulated that this feature could have promoted the evolution of the early dermal skeleton millions of years ago (12, 13).
The use of the maternal body as a reservoir of nutrients during migration in eels illustrates how bone loss, lipid metabolism, and reproduction can be directly physiologically connected. Among teleosts, diadromous salmonids also use energy reserves and lose bone and scale mass during spawning migration (13, 14). While Pacific salmon die after spawning, Atlantic salmon can restore their skeletons and survive. Depletion and restoration of bone mineral reserves connected to reproduction is also known from mammals, which lose bone during pregnancy and lactation and restore it afterward (10). Not only for this reason, the connection of bone and energy metabolism has received attention in human health sciences (15). Bone metabolism is hormonally linked to fat metabolism (15–20), which can affect several diseases and pathologies, including type 2 diabetes, osteopenia, and osteoporosis (11, 17).
In summary, bone loss in connection with reproduction can be a designated and physiologically purposeful feature, in contrast to the bone loss caused by pathological conditions. Nonetheless, maturation-related and disease-related bone loss may involve similar endocrine mechanisms. In short, appetite, reproduction and bone remodeling are regulated by a complex hormonal system in which leptin and other adipokines control energy homeostasis and modulate bone cells through direct and indirect actions (18–20). At the same time, bone-forming cells (osteoblasts) secrete osteocalcin, which affects lipid storage by regulating insulin (17). The involvement of leptin and its actions in sexual reproduction and energy metabolism seems to be a conserved evolutionary feature found in basal teleosts, such as freshwater eels, as well as in basal osteichthyans, such as gars (20, 21). Interactions between bone and adipose tissue are influenced by an array of hormones, including peripheral hormones and prehormones (18, 19, 22, 23), that have been described in eels (21, 24–26).
Body reorganization during maturation in eels also carries risks. Eels are long-lived, semelparous predators with a high body fat content that are especially prone to the uptake and accumulation of toxic substances during their growth phases (27–29). While many organic compounds are lipophilic and thus concentrate in the eels’ lipid-rich soft tissues, the mineral phase of bone can also act as a sink for metals (23, 30). Some of these elements can exert toxic effects by interfering with nutritionally essential metals, by competing for binding sites, or by inhibiting metabolic pathways (31–33). During migration and sexual maturation, similar to organic xenobiotics, these metals can be further concentrated due to catabolic processes, remobilized, and maternally transferred to the gonads, posing a threat to the reproductive fitness of this endangered species (6, 34–36). However, little is known about the physiological effects and the fate of remobilized substances during the phase of fasting and sexual development.
In this study, we investigated the body reconstructions that may occur during the migration of maturing European eels. Our analyses illustrate details of bone resorption at anatomic, microanatomic, and cellular levels and at the same time draw a comprehensive picture of the remobilization and fate of relevant minerals and potentially toxic metals through different relevant somatic body matrices of eels in several maturation stages.
Results
Female Eels Lose More Bone Than Males During Sexual Maturation.
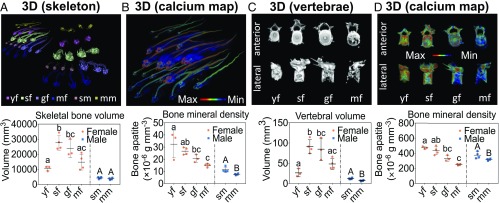
Biological data of individual fish are provided in SI Appendix, Text and Table S1. Demineralization of skeletal structures in premature and artificially matured eels was confirmed by noninvasive whole-body computed tomography (CT) imaging (Fig. 1A, Top). Yellow female eels (yf) exhibited less total bone volume than silver females (sf) and maturing females (gf), and sf had greater skeletal bone volume than the group of fully mature females (mf). No significant differences in skeletal bone volume were found between silver males (sm) and mature males (mm) (Fig. 1A, Bottom). Color-coded Ca maps show differences in bone mineral density (BMD) among the groups (Fig. 1B, Top). Quantitative analyses showed a trend toward decreasing BMD during maturation in both sexes (SI Appendix, Text and Table S2), with significant differences among the groups (Fig. 1B, Bottom). Distinct differences across the progressing maturation stages were also evident in Ca maps of the skull structures (SI Appendix, Fig. S1A). Along with the decrease in BMD, Ca maps of the CT scans also showed signals of elevated mineral density in the body cavities around the developing ovaries, marking the onset of gonadal maturation in sf (SI Appendix, Fig. S1B).
Fig. 1.
Bone loss in skeletons and skeletal elements from eels in different maturation stages. (A, Top) CT-derived 3D renderings of whole eel skeletons depict the total skeletal volumes of female and male eels in different maturation stages. (A, Bottom) Comparative analyses of total bone volume in eel skeletons revealed significant differences among female maturation stages. Sf and gf females showed the greatest skeletal volumes. (B, Top) Ca maps (Imalytics Preclinical software) depict BMD in female and male eels of different maturation stages. (B, Bottom) BMD in skeletal bone from eels of both sexes differed significantly, with an overall trend toward a decrease with progressing maturation. (C, Top) 3D reconstructions of C2 cervical vertebrae bodies of female eels in different maturation stages show the successive decline in bone volume. (C, Bottom) Vertebral volumes in female eels increased during growth phase from yellow stage to silver stage and decreased in both sexes in fully mature stages. (D, Top) Color-coded volume renderings of single skeletal elements of female eels show the decline in BMD during maturation. (D, Bottom) BMD in vertebral bodies from female and male eels decreased from silver stage to fully mature stage. In A–D, different letters indicate statistically significant differences among maturation stages per sex (P < 0.05). yf, n = 3; sf, n = 4; gf, n = 3; mf, n = 4; sm, n = 4; mm, n = 4.
Micro-CT scans and Ca maps of isolated female eel vertebrae of different maturation stages provided insight into spatial differences in bone resorption (Fig. 1 C and D, Top). Bone loss in all areas intensified with progressing maturation. While trabecular bone structures in cervical vertebrae from yf and sf were mostly intact and stable, hormone-treated groups showed signs of progressive bone loss. Vertebrae from hormone-treated groups (gf and mf) had resorbed structures in all parts of the vertebral body (Fig. 1C, Top). Quantitative analyses revealed lower vertebral volumes in yf compared with sf and gf, but no difference between yf and mf (Fig. 1C, Bottom and SI Appendix, Text and Table S2). In contrast, sm had larger vertebral volumes than mm (Fig. 1C, Bottom). In female eels, BMD in vertebrae showed a decreasing trend from yf to mf, in line with maturation (Fig. 1D, Top and Bottom). In male eels, BMD in vertebrae was greater in sf compared with mf (Fig. 1D, Bottom).
Structural Examination of Vertebral Bodies Indicates a Conserved Functionality of the Notochord.
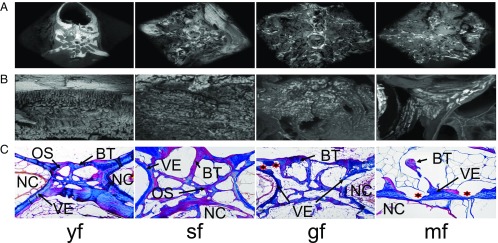
Scanning electron microscopy (SEM) images of vertebral bodies provided details of bone loss in individual bone compartments. While overview images of cut vertebrae (Fig. 2A) depicted a uniform degradation of bone trabeculae, magnified views (Fig. 2B) illustrated microstructural changes in the trabecular bone. Fenestration and increased widths in bone marrow-like structures, lined by exposed collagen fiber bundles, were present in sf and further progressed in the two hormone-treated groups (gf and mf). The loss of trabecular bone structure in male eels was less pronounced compared with that in females. Histological examination of vertebrae (Fig. 2C) revealed abundant bone remodeling. Osteons were clearly visible in yf, sf, and sm. Compared with the other groups, yf had denser bone structures and showed only minor signs of bone resorption. Sf showed a regular amount of bone at the vertebral body end plates with their bone trabeculae connected and the intervertebral space and the notochord still intact. In contrast, both artificially matured female groups (gf and mf) showed progressive bone loss with disconnected bone trabeculae and indications of substantial bone resorption (asterisk) at the vertebral body end plates. In mf, most of the bone was resorbed down to the notochord sheath with no open fenestration, leaving the notochord functional. Traces of previous notochord fenestration with scarring tissue indicated repair of this damage. In male eels (SI Appendix, Fig. S2), maturation-related bone resorption was less pronounced than in females, yet mm showed greater bone resorption compared with untreated sm.
Fig. 2.
Structural aspects of bone loss during sexual maturation in eels. Superior view (A) and 500× magnified (B) SEM images of entire vertebral body end plates of female eels in different maturation stages depict the successive bone loss on a supracellular level. (C) Bone histology based on azan-dyed, parasagittal sections of vertebral bodies illustrates changes in bone structures during the maturation process on a cellular level. Defined structures are marked and labeled: BT, bone trabeculae; NC, notochord; OS, osteon; VE, vertebral body end plate. *Indication of bone resorption.
Eels Use Bone as a Mineral Reservoir to Build up Gonads During Maturation.
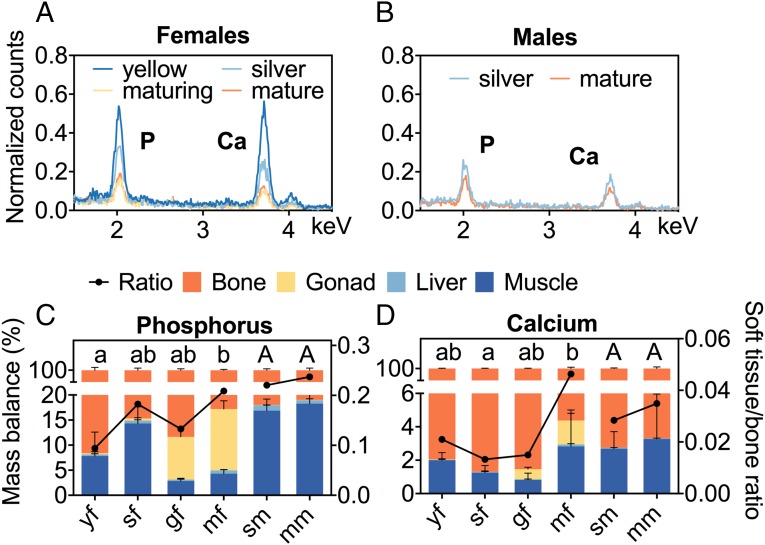
Energy-dispersive X-ray spectroscopy (EDXS) was used to determine emission signals for Ca and P in cervical vertebrae of animals from all the experimental groups. Emission spectra revealed similar Ca:P ratios and thus stable elemental composition (Fig. 3 A and B). In contrast, emission strength and peak areas differed considerably among the groups, indicating decreasing BMD along progressing maturation in both sexes. Integrals of both targeted elements ranged from 0.1 to 0.6 normalized counts, with yf displaying the highest counts for both elements, ranging from 0.55 to 0.59 (Fig. 3A). Ca and P signals in vertebrae from sf showed smaller peaks, and the lowest signals were detected in vertebrae from gf and mf, with counts ranging from 0.10 to 0.20. Vertebrae from male eels also showed differences in emission intensity according to stage (Fig. 3B); however, compared with females, the differences by life stage were not as pronounced.
Fig. 3.
Ca and P fluxes in eels of different maturation stages. SEM-EDXS spectra revealed decreasing bone mineralization in vertebral bones of representative female (A) and male (B) individuals according to maturation stage (colors). Relative mass balances (%) of P (C) and Ca (D) differed among various somatic tissues (colors) as obtained by quantitative ICP-MS. Trend lines illustrate the ratio of total bone-bound analytes in relation to total soft tissue-bound analytes. Different letters indicate significant differences in this ratio across maturation stages by sex (P < 0.05). yf, n = 3; sf, n = 4; gf, n = 3; mf, n = 4; sm, n = 4; mm, n = 4.
Elemental inductively coupled plasma mass spectrometry (ICP-MS) mass-balance analyses of P and Ca in different tissues revealed differences in body composition across the maturation stages (Fig. 3 C and D). P was found mainly in bone (between 82% in mm and 93% in yf), with some (between 2% and 16%) allocated in muscle tissue. With advancing maturation, the soft tissue/bone mass ratio rose, with 9–13% of total allocated P found in gonads of gf and mf. The relative amount of P bound in muscle tissue decreased compared with earlier maturation stages. The ratio of bound P in total soft tissue to bone tissue was significantly greater in mf than in yf (Fig. 3C).
By far the largest share of total Ca mass balance was detected in bones, accounting for ∼95% to >99% of the total estimated mass (Fig. 3D). While Ca was not found in substantial amounts in liver, the amount bound in soft tissue of females rose slightly with onset of hormone treatment, with the highest soft tissue-to-bone mass ratio seen in mf.
Body Reorganization During Maturation Leads to Maternal Transfer of Toxic Metals.
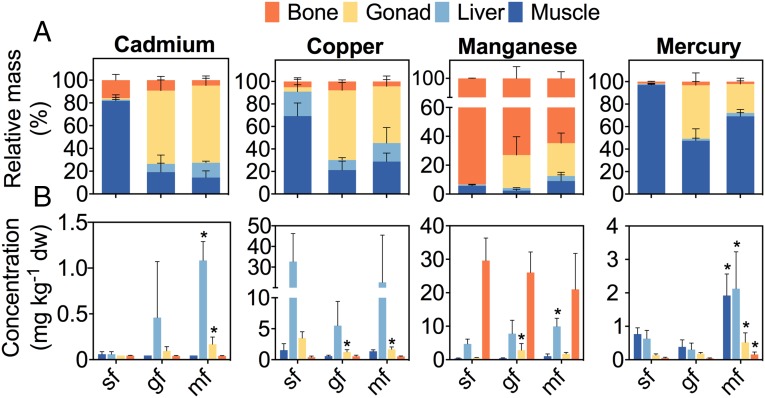
Mass balance analyses (Fig. 4A) and dry weight concentrations (Fig. 4B) were also obtained to analyze the redistribution of different metals in body compartments of relevance in female eels from sf stage to mf stage (SI Appendix, Text and Tables S2 and S4). Cd and Cu exhibited quite similar characteristics. While in sf, both metals accumulated in muscle tissue (60–80%), with low amounts found in bone (5–20%), in gf and mf, 50%–65% of Cd was found in the gonads. Concentrations of Cd were <0.1 mg kg−1 dry weight (dw) in most matrices of all stages, elevated only in livers (>1 mg kg−1 dw) and gonads (>0.2 mg kg−1 dw). Cu in gonads was found in similar ranges but at significantly lower concentrations (<3 mg kg−1 dw) in both gf and mf compared with sf.
Fig. 4.
Mass balance ratios and tissue concentrations of toxic metals in female eels. (A) Relative mass balances of Cd, Cu, and Mn were obtained by quantitative ICP-MS, and Hg was analyzed using TDA-AAS. Metabolic fluxes led to notable relocation of metals from muscle (Cd, Cu, and Hg) and bone (Mn) tissue in sf to gonadal tissue in gf and mf. (B) Metal dry weight concentrations differed among maturation stages and sampled tissues. Metabolic reorganization during fasting and maturation affected dry weight concentrations in several organs. Asterisks indicate significant differences compared with sf. yf, n = 3; sf, n = 4; gf, n = 3; mf, n = 4.
For Mn, the greatest shares were bound in skeletons of sf (90%), with the proportion decreasing with advancing maturation (∼70% in gf and 60% in mf). While the relative mass balance in muscle tissue of all stages remained low (<10%), amounts of Mn in gonadal tissue rose to around 20–30% of total mass balance in the gf and mf. The highest concentrations of Mn were found in bone tissue (20–37 mg kg−1 dw) and revealed a slight decreasing trend during maturation, with no significant differences across the groups. Concentrations in liver and gonads increased during maturation, exceeding 10 mg kg−1 dw in livers of mf and remaining below 5 mg kg−1 dw in gonads of all groups.
For Hg, in untreated sf, almost the entire body burden (∼97%) was found in muscle tissue, while in gf and mf, substantial Hg levels were detected in gonads (Fig. 4A). Total concentrations of Hg in all organs were significantly greater in mf than in females at earlier maturation stages (>0.1 to >3.0 mg kg−1 dw vs. <0.1 to <1.0 mg kg−1 dw) (Fig. 4B).
Discussion
This study demonstrates that body reconstruction in European eels during sexual development can initiate the mobilization and maternal transfer of toxic metals, with possible detrimental consequences on health and reproductive success. By analyzing the changes in relative mass balances of selected elements within different body compartments, we have depicted the dispersal and fate of remobilized minerals and metals during maturation. Our results suggest a reciprocal interrelation between the storage and depletion processes in bone and soft tissues of European eels during their spawning migration. Since the speciation of the genus Anguilla 20–40 Mya (37), the reproductive strategy of these fishes has evolved to include a “programmed death” resulting from use of the parental body as a storehouse to supply the requirements of locomotion, maturation, and successful spawning. The migrating eel’s body provides energy resources, such as lipids and proteins, from muscles (5), as well as minerals from the bones (7, 8).
Eels provide an example of the tight interaction between bone turnover and lipid metabolism in a vertebrate species. As this connection can also be found in extant mammals, the eel may pose an interesting model for generating a better understanding of the physiological pathways involved in associated human pathological conditions and diseases.
In the current study, hormone-treated eels did not constantly swim before being killed. This is important, since with sufficient nutrition, exercise can be beneficial for bone retention and formation in humans and other vertebrates (11). Constant locomotion displayed by nonfeeding, migrating eels however, could amplify bone resorption since muscle mass is continuously depleted during their journey. It has been shown in simulated migration trials that active swimming initiates lipid mobilization and hormonal changes which lead to gonadal maturation in female and male silver eels (5, 38, 39). Due to the lack of feeding during the time of gonadogenesis, especially increasing P requirements would conflict with exercise-triggered bone formation and mineralization. Since the maturing gonads in our sampled fish were supplied with minerals from the bones, we conclude that under natural conditions, the swimming performance necessary for transoceanic migration adds an additional effect to the observed processes.
Ca maps of skeletal structures in this study reveal a corresponding picture. Beginning with initial silvering and thus the start of migration, average mineral content and skeletal mass successively declined in both sexes. This decreasing volume is characterized by the loss of bone tissue in all areas of the vertebrae (Fig. 1 C and D, Top). In fact, single vertebrae in large mf shrunk to the size of those in much smaller, immature yf. This finding is remarkable, since the size of vertebrae in premature eels usually correlates well with their full body size (40). The observed severe bone loss at the vertebral body end plates combined with the disconnection of bone trabeculae until final maturation strongly suggests the loss of mechanical support by vertebral bodies. At the same time, the notochord remained intact and likely functional as axial skeleton (14).
In line with the decline in BMD and bone volume throughout maturation, structural bone loss was more accentuated in female eels compared with males. This was not surprising, since anguillids are sexually dimorphic and females grow larger than males. During oogenesis, female eels also use a greater percentage of their body mass for egg production than male eels do for spermatogenesis (5). To provide sufficient P (and partly Ca) for early embryonic development, egg yolk must be enriched from maternal resources that are stored before migration. Accordingly, CT-derived Ca maps revealed elevated mineral signals located around the gonads of sf (SI Appendix, Fig. S1B, red arrows). At this stage, female gonads are still at an initial maturation stage without further dilution effects initiated by protein metabolism or hydration (41).
EDXS spectra revealed that gross mineral composition in bone remained unchanged during maturation of both sexes. P and Ca persist at the same ratio, which is in line with the observed bone resorption, a process that liberates all bone minerals equally. Yamada et al. (7) found only small amounts of Ca from the bones transferred to the gonads of eels, suggesting discharge of excess Ca from the body. Unlike P, Ca is generally not limited in aqueous marine environments, since fish are usually able to take up Ca2+ from sea water via gill and stomach epithelia (42). At times of extreme Ca demand (e.g., during gonadal maturation), water-derived Ca may be insufficient, and food can provide additional Ca for fish (42, 43). This does not apply to nonfeeding migrating eels (2, 3) and suggests that along with seawater-derived Ca taken up via gills, Ca liberated from the skeleton can be used for oogenesis, muscle function, and other physiological processes.
Elemental analyses also indicated that maturation in eels can induce the redistribution of toxic metals from the animals’ bone and soft tissues. Metals such as Cd, Cu, Mn, and Hg are inducers of oxidative stress and are known to cause toxicity in aquatic organisms. Their toxicity mostly originates from their capacity to bind and interfere with proteins and nucleic acids, and the generation of reactive oxygen and nitrogen species may result in oxidative damage of these biomolecules (44). As reviewed by Jezierska et al. (45), teleost early-life stages are particularly sensitive, and the influence of toxic metals may lead to impaired embryonic development. It was further concluded that exposure of spawning animals to metal might result in contamination of eggs and sperm, with potential adverse effects on fertility and embryogenesis.
How the quality of sperm in eels is affected by paternal metal levels and the extent to which male gametes contribute to the overall transfer of metals into fertilized eggs are unclear at present. In female eels, the distribution of these elements changes substantially with ongoing body restructuring, leading to the incorporation of substantial amounts into the gonads, with possible implications for individual reproductive success. Recirculated and relocated metals are of concern, since their concentration in liver, muscle, or gonads, in line with the metabolic reconstructions, may exceed critical values and produce toxic effects.
Cd has also been shown to interfere with bone turnover (46). Pierron et al. (47) showed that Cd is a strong endocrine disruptor in eels, with the potential to interfere with hormone synthesis, to alter egg quality and quantity, and to lead to exhaustion during migration. Since it can negatively affect the lipid storage capacities of European eels, Cd may impact the interaction of lipid metabolism and bone remodeling once the element is released from muscle during migration (48).
Our results for Cu suggest that this element redistributes similar to Cd. Even though Cu is essential to fish nutrition (49), it can exert genotoxic effects (45) and is known to become toxic to aquatic life at concentrations slightly greater than essentially required concentrations (50).
Knowledge of Mn toxicity in fish is scarce, yet it is of relevance here, as it has been shown to accumulate in vertebrate skeletons (32) and to interfere with bone metabolism (51).
Due to its distinct physiochemistry, body distribution, and mass ratio, Hg differed substantially from Cd, Cu, and Mn. This element is known to produce a range of deleterious effects in vertebrates, including microtubule destruction, mitochondrial damage, and lipid peroxidation (52). It also has been shown to negatively affect reproduction of teleost fish (53) and has specifically been suggested to pose a health risk to eels (36). Although mostly associated with the brain and nervous system, Hg can impair any organ and lead to severe organ malfunction and interference with Ca homeostasis (33). Our results indicate a limited but proportional transfer of Hg from muscle tissue into the ovaries, with elevated concentrations in livers of mature fish, indicating a possible metabolic pathway via the liver. Our results are generally in line with Hg levels in somatic tissues of artificially matured female European eels reported by Nowosad et al. (36).
In conclusion, our findings on the resorption and mineral release from the bones of eels contribute to a better understanding of physiological processes involved in the sexual maturation of European eels. Moreover, this study shows that the metabolic turnover of somatic tissue into gametic tissue can induce mobilization of potentially toxic metals. Proof of maternal transfer of these elements into the ovaries of female eels demonstrates the fairly incalculable challenges that eels encounter in the course of reproduction. For future studies, a desirable focus would be an assessment of specific toxicity biomarkers to generate effect data, to better support the hypothesis that maternal transfer of metals to the gonads represents a specific risk to offspring in eels. Due to the involvement of apparently ancient, congeneric physiological pathways, new knowledge of the pertinent mechanisms in eels may be helpful to better understand the interconnections of lipid metabolism and mineral retention that are also involved in conditions and diseases seen in humans. However, additional investigations are needed to further elucidate the involved endocrine interactions that regulate energy metabolism, storage depletion, and bone remodeling in eels during migration and sexual maturation.
Methods
Female and male European eels in different natural and artificially induced maturation stages were analyzed postmortem for BMD and maturation-related structural changes in skeletal elements using clinical CT and micro-CT. Structural changes on a microanatomic level in single skeletal elements were investigated using histology and SEM. Elemental composition of eel soft and hard tissues was analyzed using EDXS, ICP-MS, and thermal decomposition, amalgamation, and atomic absorption spectrometry (TDA-AAS). Individual elemental tissue burdens were calculated using measured concentrations in the respective tissue multiplied with extrapolated, stage-specific total dry weight of the respective organ, derived from a reference database (SI Appendix, Text and Tables S2 and S4). Further details on biological variables, hormone treatment, sampling and handling, methodological procedures and instrumental settings, and test statistics, as well as the reference database for mass balance calculations, are provided in SI Appendix.
Supplementary Material
Acknowledgments
This work is supported by the German Federal Ministry of Food and Agriculture through the “AalPro” Project (28-1-73.034-10) and by the German Academic Exchange Service/Brazilian National Council for Scientific and Technological Development (Grant 290084/2011-3). M.B. received support through the Canada First Research Excellence Funds Global Water Futures program led by the University of Saskatchewan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817738116/-/DCSupplemental.
References
- 1.Durif C, Dufour S, Elie P (2005) The silvering process of Anguilla anguilla: A new classification from yellow resident to silver migrating stage. J Fish Biol 66:1025–1043. [Google Scholar]
- 2.Chow S, et al. (2010) Japanese eel Anguilla japonica do not assimilate nutrition during the oceanic spawning migration: Evidence from stable isotope analysis. Mar Ecol Prog Ser 402:233–238. [Google Scholar]
- 3.Pankhurst NW, Sorensen PW (1984) Degeneration of the alimentary tract in sexually maturing European Anguilla anguilla (L.) and American eels Anguilla rostrata (LeSeur). Can J Zool 62:1143–1148. [Google Scholar]
- 4.Ozaki Y, Koga H, Takahashi T, Adachi S, Yamauchi K (2008) Lipid content and fatty acid composition of muscle, liver, ovary and eggs of captive-reared and wild silver Japanese eel Anguilla japonica during artificial maturation. Fish Sci 74:362–371. [Google Scholar]
- 5.Palstra AP, Van den Thillart G (2010) Swimming physiology of European silver eels (Anguilla anguilla L.): Energetic costs and effects on sexual maturation and reproduction. Fish Physiol Biochem 36:297–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freese M, et al. (2017) Maternal transfer of dioxin-like compounds in artificially matured European eels. Environ Pollut 227:348–356. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Y, Okamura A, Tanaka S (2002) The roles of bone and muscle as phosphorus reservoirs during the sexual maturation of female Japanese eels, Anguilla japonica Temminck and Schlegel (Anguilliformes). Fish Physiol Biochem 24:327–334. [Google Scholar]
- 8.Rolvien T, et al. (2016) How the European eel (Anguilla anguilla) loses its skeletal framework across lifetime. Proc Biol Sci 283:20161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovell RT. (1989) Nutrition and Feeding of Fish (Van Nostrand Reinhold, New York: ), pp 64. [Google Scholar]
- 10.Kovacs CS, Kronenberg HM (1997) Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 18:832–872. [DOI] [PubMed] [Google Scholar]
- 11.Bartl R, Bartl C (2017) Bone Disorders: Biology, Diagnosis, Prevention, Therapy (Springer International, Cham, Switzerland: ). [Google Scholar]
- 12.Halstead Tarlo BH. (1964). The origin of bone. Bone and Tooth: Proceedings of the First European Bone and Tooth Symposium, ed Blackwood HJJ. (MacMillen, New York: ), pp 3–15. [Google Scholar]
- 13.Witten PE, et al. (2016) A primary phosphorus-deficient skeletal phenotype in juvenile Atlantic salmon Salmo salar: The uncoupling of bone formation and mineralization. J Fish Biol 88:690–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witten PE, Huysseune A (2009) A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol Rev Camb Philos Soc 84:315–346. [DOI] [PubMed] [Google Scholar]
- 15.de Paula FJA, Rosen CJ (2013) Bone remodeling and energy metabolism: New perspectives. Bone Res 1:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen CJ. (2008) Bone remodeling, energy metabolism, and the molecular clock. Cell Metab 7:7–10. [DOI] [PubMed] [Google Scholar]
- 17.de Paula FJA, Horowitz MC, Rosen CJ (2010) Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab Res Rev 26:622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Confavreux CB. (2011) Bone: From a reservoir of minerals to a regulator of energy metabolism. Kidney Int 79121:S14–S19. [DOI] [PubMed] [Google Scholar]
- 19.Karsenty G. (2016) Convergence between bone and energy homeostasis: Leptin regulation of bone mass. Cell Metab 4:341–348. [DOI] [PubMed] [Google Scholar]
- 20.Denver RJ, Bonett RM, Boorse GC (2011) Evolution of leptin structure and function. Neuroendocrinology 94:21–38. [DOI] [PubMed] [Google Scholar]
- 21.Morini M, et al. (2015) Duplicated leptin receptors in two species of eel bring new insights into the evolution of the leptin system in vertebrates. PLoS One 10:e0126008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundy GR, Guise TA (1999) Hormonal control of calcium homeostasis. Clin Chem 45:1347–1352. [PubMed] [Google Scholar]
- 23.Berglund M, Akesson A, Bjellerup P, Vahter M (2000) Metal-bone interactions. Toxicol Lett 112-113:219–225. [DOI] [PubMed] [Google Scholar]
- 24.Olivereau M. (1967) Observations sur l’hypophyse de l’anguille femelle, en particulier lors de la maturation sexuelle. Zellforsch Mikroskop Anat 80:286–306. [PubMed] [Google Scholar]
- 25.Lopez E, Mac Intyre I, Martelly E, Lallier F, Vidal B (1980) Paradoxical effect of 1,25 dihydroxycholecalciferol on osteoblastic and osteoclastic activity in the skeleton of the eel Anguilla anguilla L. Calcif Tissue Int 32:83–87. [DOI] [PubMed] [Google Scholar]
- 26.Sbaihi M, et al. (2007) Thyroid hormone-induced demineralisation of the vertebral skeleton of the eel, Anguilla anguilla. Gen Comp Endocrinol 151:98–107. [DOI] [PubMed] [Google Scholar]
- 27.Belpaire C, Goemans G (2007) Eels: Contaminant cocktails pinpointing environmental contamination. ICES J Mar Sci 67:1423–1436. [Google Scholar]
- 28.Freese M, et al. (2016) A question of origin: Dioxin-like PCBs and their relevance in stock management of European eels. Ecotoxicology 25:41–55. [DOI] [PubMed] [Google Scholar]
- 29.Pannetier P, et al. (2016) A comparison of metal concentrations in the tissues of yellow American eel (Anguilla rostrata) and European eel (Anguilla anguilla). Sci Total Environ 569–570:1435–1445. [DOI] [PubMed] [Google Scholar]
- 30.Bronner F. (2008) Metals in bone: Aluminum, boron, cadmium, chromium, lanthanum, lead, silicon, and strontium. Principles of Bone Biology, eds Bilezikian JP, Raisz LG, Martin TJ (Academic, San Diego: ), 3rd Ed, pp 515–531. [Google Scholar]
- 31.Goyer RA. (1997) Toxic and essential metal interactions. Annu Rev Nutr 17:37–50. [DOI] [PubMed] [Google Scholar]
- 32.O’Neal SL, Zheng W (2015) Manganese toxicity upon overexposure: A decade in review. Curr Environ Health Rep 2:315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geeraerts C, Belpaire C (2010) The effects of contaminants in European eel: A review. Ecotoxicology 19:239–266. [DOI] [PubMed] [Google Scholar]
- 35.Sühring R, et al. (2015) Maternal transfer of emerging brominated and chlorinated flame retardants in European eels. Sci Total Environ 530–531:209–218. [DOI] [PubMed] [Google Scholar]
- 36.Nowosad J, Kucharczyk D, Łuczyńska J (2018) Changes in mercury concentration in muscles, ovaries and eggs of European eel during maturation under controlled conditions. Ecotoxicol Environ Saf 148:857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Ginneken VJT, Maes GE (2005) The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: A literature review. Rev Fish Biol Fish 15:367–398. [Google Scholar]
- 38.Palstra AP, et al. (2007) Swimming stimulates oocyte development in European eel (Anguilla anguilla L.). Aquaculture 270:321–332. [Google Scholar]
- 39.Palstra AP, Schnabel D, Nieveen MC, Spaink HP, van den Thillart GEEJM (2008) Male silver eels mature by swimming. BMC Physiol 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thieren E, Wouters W, Van Neer W, Ervynck A (2012) Body length estimation of the European eel Anguilla anguilla on the basis of isolated skeletal elements. Cybium 36:551–552. [Google Scholar]
- 41.Lubzens E, Young G, Bobe J, Cerdà J (2010) Oogenesis in teleosts: How eggs are formed. Gen Comp Endocrinol 165:367–389. [DOI] [PubMed] [Google Scholar]
- 42.Flik G, Verbost PM (1993) Calcium transport in fish gills and intestine. J Exp Biol 184:17–29. [Google Scholar]
- 43.Sundell K, Björnsson BT (1988) Kinetics of calcium fluxes across the intestinal mucosa of the marine teleost, Gadus morhua, measured using an in vitro perfusion method. J Exp Biol 140:170–186. [Google Scholar]
- 44.Leonard SS, Harris GK, Shi X (2004) Metal-induced oxidative stress and signal transduction. Free Radic Biol Med 37:1921–1942. [DOI] [PubMed] [Google Scholar]
- 45.Jezierska B, Ługowska K, Witeska M (2009) The effects of heavy metals on embryonic development of fish (a review). Fish Physiol Biochem 35:625–640. [DOI] [PubMed] [Google Scholar]
- 46.Youness ER, Mohammed NA, Morsy FA (2012) Cadmium impact and osteoporosis: Mechanism of action. Toxicol Mech Methods 22:560–567. [DOI] [PubMed] [Google Scholar]
- 47.Pierron F, et al. (2008) How cadmium could compromise the completion of the European eel’s reproductive migration. Environ Sci Technol 42:4607–4612. [DOI] [PubMed] [Google Scholar]
- 48.Pierron F, et al. (2007) Impairment of lipid storage by cadmium in the European eel (Anguilla anguilla). Aquat Toxicol 81:304–311. [DOI] [PubMed] [Google Scholar]
- 49.Bury NR, Walker PA, Glover CN (2003) Nutritive metal uptake in teleost fish. J Exp Biol 206:11–23. [DOI] [PubMed] [Google Scholar]
- 50.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crossgrove J, Zheng W (2004) Manganese toxicity upon overexposure. NMR Biomed 17:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patrick L. (2002) Mercury toxicity and antioxidants, Part 1: Role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. Altern Med Rev 7:456–471. [PubMed] [Google Scholar]
- 53.Crump KL, Trudeau VL (2009) Mercury-induced reproductive impairment in fish. Environ Toxicol Chem 28:895–907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.