ABSTRACT
Visual analog scale (VAS) questionnaires are widely used in nutrition research to assess appetite and subsequent food intake. However, a number of methodological considerations exist. The study aims were to test whether 1) appetite VASs alter subsequent food intake and 2) viewing previous appetite responses influences subsequent responses. Twelve healthy adults (age: 22 ± 3 y; BMI: 22.0 ± 3.1 kg/m2) completed the randomized crossover study. On separate days, participants were provided breakfast preloads and completed appetite VASs every 30 min for 4 h or did not complete the questionnaires. When completing VASs, the participants were shown their previous responses (PR) or were not (No-PR). After 4 h, an ad libitum lunch was provided. Hunger, fullness, desire to eat, and prospective food consumption were not different between PR and No-PR. All data are reported as mean ± SEM. VASs did not affect lunch intake (484 ± 50 kcal) compared with no VASs (500 ± 53 kcal, nonsignificant). Thus, neither past appetite responses nor the use of appetite VASs influenced ingestive behavior in healthy adults. This trial was part of a larger acute, randomized crossover trial registered at clinicaltrials.gov as NCT03154606.
Keywords: appetite, methodology, energy intake, visual analog scales, ingestive behavior
Introduction
Uses for visual analog scale (VAS) questionnaires in ingestive behavior research include the assessment of pre- and postprandial hunger, fullness, desire to eat, and prospective food consumption (PFC) before and during acute, tightly controlled studies (1, 2). This approach has been validated in the literature as the best strategy to assess appetite and satiety (1–4). Further, analyses by Flint et al. (3) suggest that a 10% difference in appetite over a 4-h postprandial period is considered “reasonable and realistic” to predict subsequent energy intake. In addition, previous data from our lab show that the magnitude of postprandial hunger predicts eating initiation (5). However, a recent systematic review challenges the application of these questionnaires owing to the discrepancies in findings between studies (6). Thus, the broad aim of this article is to explore methodological procedures that may alter appetitive responses and subsequent eating behavior.
The first consideration addresses a fundamental question as to whether the repetitive use of these questions alters subsequent eating behavior. As supported by the current literature, the thought of eating food, associated hunger sensations, etc., generate a cephalic response which primes the body to consume food (7). The release of hormones generally accompanies this response, potentially affecting subsequent food intake (8). In addition, according to studies examining behavioral or environmental cuing on food choices, ingestive behavior, and weight management (9–11), it is possible, albeit untested, that the continual acknowledgment (or cuing) of one's perceived hunger or fullness state might influence subsequent food intake—in either direction. For example, repeated questions on a hunger state might cause an individual to become more attentive to his/her cues, potentially leading to a reduction in food intake. Alternatively, repetitive prompting of a hungry state might elicit an exaggerated hunger response that could
actually increase subsequent food intake. Thus, the primary aim of this study was to test whether the repetitive use of VAS appetite questionnaires alters subsequent food intake in healthy young adults.
Another consideration stems from the methodological or administrative differences when using VAS questionnaires in current studies as compared with past studies. Originally, the questionnaires were provided using the “pen and paper” method (3). In this approach, the participant had the ability to see and reflect on previous answers. However, with the implementation of computerized platforms, this no longer occurs. Previous research by Brunstrom et al. (12) suggests memory of previous meal size drives an “expected satiation” even ≤24 h later. This “expected satiation” decreases subsequent meal energy intake not based on the energy content of the meal but instead based on a recall of the perceived satiation of the meal. However, it is unclear whether the “expected satiety” based on previous appetitive responses drives subsequent appetitive sensations. Thus, the second aim of the study is to assess whether the ability to see previous responses influences subsequent responses.
Methods
Study participants
Healthy young men and women were recruited from the Columbia, MO area using word-of-mouth and snowball sampling. Eligibility was determined through the following inclusion criteria: 1) healthy; 2) aged 18–35 y; 3) normal weight to overweight [BMI (in kg/m2): 18.5–29.9]; 4) no metabolic, psychological, or neurological diseases/conditions; 5) weight stable (no weight loss/gain within the past 6 mo); 6) not currently consuming a weight-loss or other special diet (in the past 6 mo); 7) not a vegetarian; 8) not pregnant; and 9) nonsmoker.
The study included 12 healthy young adults (age: 22 ± 3 y; BMI: 22.0 ± 3.1). All participants were informed of the study purpose, procedures, and risks and signed the consent/assent forms. The study was approved by the university Institutional Review Board and all procedures were followed in accordance with the ethical standards of the board.
Experimental procedures
This study was conducted within a larger acute, randomized crossover design study (NCT03154606) assessing the consumption of preloads varying in protein source on postprandial appetite and satiety. The larger study included 5 separate days. Four of the 5 d included appetite and satiety responses and 1 d did not assess these responses.
For the current study, the participants reported to the research facility, on 2 separate days in a randomly assigned order, between 0700 and 0900, after an overnight fast, to complete a 4-h testing day. Upon arrival at the facility, each participant was familiarized with the testing day procedures. At the start of the day, a 130-kcal (30 g protein; 1 g carbohydrates) preload beverage breakfast was provided and the participants had 20 min to consume the beverage. During 1 of the testing days, the participants were asked to complete a set of computerized VAS questionnaires before breakfast and every 30 min afterwards over the 4-h period, whereas during the other testing day, no VAS questionnaires were completed. After the 4 h, the participants were given an ad libitum pasta meal (77% carbohydrates, 12% protein, and 11% fat). The participants were given 30 min to “consume as much or as little as possible until feeling comfortably full.” After these procedures, the participants were allowed to leave the facility. The testing days were separated by ≥48 h.
Before each testing day, the participants were provided with a standardized dinner (∼530 kcal, 55% carbohydrates, 27% fat, and 18% protein) to consume, at home, between 1700 and 1900 on the night before each testing day.
VAS questionnaires
VAS questionnaires were completed on a smartphone and included questions assessing “how strong is your feeling of” hunger, fullness, and desire to eat or “how much food can you eat right now” with anchors of “not (much) at all” to “extremely/an extreme amount” (3). To test the secondary aim, participants were given 2 questionnaires at each time point with a vertical line that either contained their previous response (PR) or were void of their response (No-PR). Each questionnaire (PR compared with No-PR) contained an identical set of questions. Each testing day that assessed the aforementioned questions included the completion of the PR and No-PR procedures in a randomly assigned order.
Data and statistical analysis
Summary statistics (sample means and SEMs) were computed for all data. Total net incremental area under the curve (niAUC) was calculated from the postprandial time points for the appetite responses. Paired-samples t tests were applied to compare PR with No-PR for all appetite data and VASs with no VASs for subsequent lunch intake.
All data are reported as means ± SEMs. Analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 24.0 (SPSS Inc.). P < 0.05 was considered statistically significant.
Results
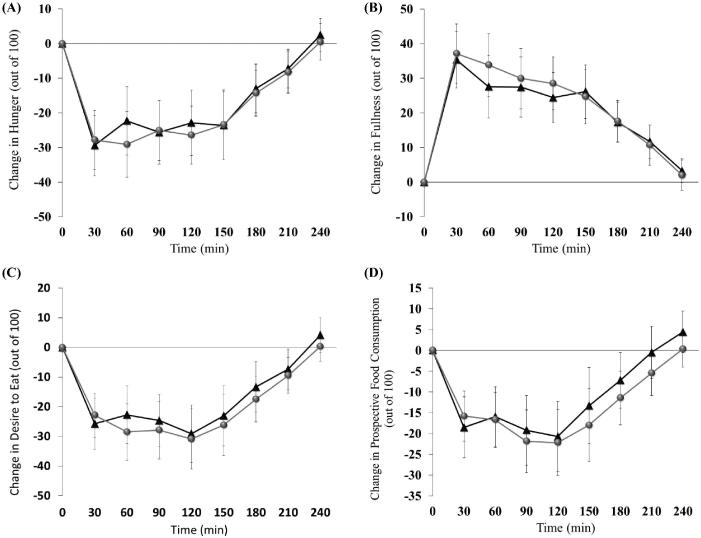
Figure 1 depicts the hunger, fullness, desire to eat, and PFC responses completed for PR and No-PR. No differences between PR and No-PR in 4-h hunger (−4797 ± 1621 compared with −4600 ± 1387 mm × min, respectively), fullness (5902 ± 1419 compared with 5479 ± 1134 mm × min, respectively), desire to eat (−4946 ± 1497 compared with −4495 ± 1482 mm × min, respectively), and PFC niAUC (−3425 ± 1118 compared with −2997 ± 1253 mm × min, respectively) were detected (all, P > 0.05).
FIGURE 1.
The hunger (A), fullness (B), desire to eat (C), and prospective food consumption (D) responses from visual analog scale questionnaires that contained the previous response (black triangles) compared with no previous response (grey circles). Data are means ± SEMs.
When comparing subsequent (lunch) food intake after testing days that contained VAS questionnaires with intake after those that did not, no differences were detected (VASs: 484 ± 50 kcal, compared with no VASs: 500 ± 53 kcal, P > 0.05).
Discussion
For >50 y (13), VAS questionnaires have been utilized to determine perceived appetite sensations in responses to diet, exercise, and sleep interventions (1, 14, 15). Further, most of the appetite indexes have been shown to predict subsequent food intake and/or daily food intake (16, 17). Despite the wide range of uses, very few considerations have been proposed to assess several aspects of these questionnaires.
In the current study, we sought to test whether the repetitive use of VAS appetite questionnaires over a 4-h period influences subsequent food intake and to determine whether the acknowledgment of one's previous appetite influences subsequent responses. Appetite responses were not different between the PR and No-PR, and subsequent food intake was not different between the VASs and no VASs testing days.
The strength of this study lies within the null findings. This is the first study, to our knowledge, to test whether the ability to see one's past hunger, fullness, desire to eat, or PFC responses influences subsequent responses. This is a critical question given the different methodologies utilized in the past compared with the present. The majority of studies in the past utilized the “pen and paper” approach causing previous responses to be seen; however, with the more recent development of computerized questionnaires, participants typically do not see their responses. The findings from the current study suggest that the ability to see previous responses does not significantly affect subsequent responses.
In addition, we proposed that the repetitive use of appetite questionnaires would affect subsequent food intake given that provoking thoughts of food consumption increases food intake (18, 19). The thoughts of food experienced when considering perceived hunger and fullness (part of the cephalic phase) may evoke a cephalic phase response and initiate a cascade of preabsorptive physiological responses to initiate feeding behavior. Thus, to the best of our knowledge, the results from the current study are the first to suggest the repetitive use of appetite questionnaires does not influence subsequent intake. However, it is important to note that a number of factors may have also influenced the study findings. Specifically, the lack of variety provided during the lunch meal may have led to sensory-specific satiety, which is a perceived boredom with a particular product’s taste, or sensory-mediated satiety, which is a learned/conditioned response driven by prior eating occasions with the food (1). Thus, participants in studies that utilize a single food for an ad libitum meal may experience sensory-specific satiety and feel “bored with the taste,” leading to premature eating cessation. Alternatively, participants may experience sensory-mediated satiety driven by a food commonly consumed by participants. To avoid the aforementioned issues, future research evaluating this question should provide a variety of commonly consumed foods (i.e., in a novel ad libitum buffet) in order to rule out the possibilities that sensory-specific satiation led to a “capped” response or that a sensory-mediated response was driven by prior experiences. In addition, lack of differences may also be due to the relatively low caloric content of the breakfast (i.e., 130 kcal). It's possible that the initial satiety effect of the beverage preload was reduced to the baseline state before lunch, thus masking differences mitigated by the use of appetite questionnaires.
In conclusion, in exploring several methodological procedures when utilizing VAS appetite questionnaires for eating behavior research, we found that neither past appetite responses nor the repetitive use of the VAS questionnaires influences subsequent appetite or food intake in healthy adults.
Acknowledgments
The authors’ responsibilities were as follows—SMD: conducted the research, analyzed the data, and wrote the first draft of the paper; HJL: reviewed and edited the paper; and both authors: designed the research, had primary responsibility for the final content, and read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: SMD and HJL, no conflicts of interest.
Abbreviations used: niAUC, net incremental area under the curve; No-PR, questionnaires did not contain participant's previous response; PFC, prospective food consumption; PR, questionnaires contained participant's previous response; VAS, visual analog scale.
References
- 1. Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, van der Knaap H et al.. Appetite control: methodological aspects of the evaluation of foods. Obes Rev 2010;11(3):251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hill AJ, Rogers PJ, Blundell JE. Techniques for the experimental measurement of human eating behaviour and food intake: a practical guide. Int J Obes Relat Metab Disord 1995;19(6):361–75. [PubMed] [Google Scholar]
- 3. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000;24(1):38–48. [DOI] [PubMed] [Google Scholar]
- 4. Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, Stratton R, Delargy H, King N, Blundell JE. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr 2000;84(4):405–15. [DOI] [PubMed] [Google Scholar]
- 5. Ortinau LC, Culp JM, Hoertel HA, Douglas SM, Leidy HJ. The effects of increased dietary protein yogurt snack in the afternoon on appetite control and eating initiation in healthy women. Nutr J 2013;12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holt GM, Owen LJ, Till S, Cheng Y, Grant VA, Harden CJ, Corfe BM. Systematic literature review shows that appetite rating does not predict energy intake. Crit Rev Food Sci Nutr 2017;57(16):3577–82. [DOI] [PubMed] [Google Scholar]
- 7. Mattes RD. Physiologic responses to sensory stimulation by food: nutritional implications. J Am Diet Assoc 1997;97(4):406–13. [DOI] [PubMed] [Google Scholar]
- 8. Faipoux R, Tome D, Gougis S, Darcel N, Fromentin G. Proteins activate satiety-related neuronal pathways in the brainstem and hypothalamus of rats. J Nutr 2008;138(6):1172–8. [DOI] [PubMed] [Google Scholar]
- 9. Mason AE, Jhaveri K, Cohn M, Brewer JA. Testing a mobile mindful eating intervention targeting craving-related eating: feasibility and proof of concept. J Behav Med 2018;41(2):160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papies EK. Health goal priming as a situated intervention tool: how to benefit from nonconscious motivational routes to health behaviour. Health Psychol Rev 2016;10(4):408–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stampfli AE, Stockli S, Brunner TA. A nudge in a healthier direction: how environmental cues help restrained eaters pursue their weight-control goal. Appetite 2017;110:94–102. [DOI] [PubMed] [Google Scholar]
- 12. Brunstrom JM, Burn JF, Sell NR, Collingwood JM, Rogers PJ, Wilkinson LL, Hinton EC, Maynard OM, Ferriday D. Episodic memory and appetite regulation in humans. PloS One 2012;7(12):e50707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silverstone JT, Stark JE, Buckle RM. Hunger during total starvation. Lancet 1966;287(7451):1343–4. [DOI] [PubMed] [Google Scholar]
- 14. Gwin JA, Leidy HJ. Breakfast consumption augments appetite, eating behavior, and exploratory markers of sleep quality compared with skipping breakfast in healthy young adults. Curr Dev Nutr 2018;2(11):nzy074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laan DJ, Leidy HJ, Lim E, Campbell WW. Effects and reproducibility of aerobic and resistance exercise on appetite and energy intake in young, physically active adults. Appl Physiol Nutr Metab 2010;35(6):842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drapeau V, Blundell J, Therrien F, Lawton C, Richard D, Tremblay A. Appetite sensations as a marker of overall intake. Br J Nutr 2005;93(2):273–80. [DOI] [PubMed] [Google Scholar]
- 17. Drapeau V, King N, Hetherington M, Doucet E, Blundell J, Tremblay A. Appetite sensations and satiety quotient: predictors of energy intake and weight loss. Appetite 2007;48(2):159–66. [DOI] [PubMed] [Google Scholar]
- 18. Haasova S, Elekes B, Missbach B, Florack A. Effects of imagined consumption and simulated eating movements on food intake: thoughts about food are not always of advantage. Front Psychol 2016;7:1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smeets PA, Erkner A, de Graaf C. Cephalic phase responses and appetite. Nutr Rev 2010;68(11):643–55. [DOI] [PubMed] [Google Scholar]