Abstract
Objective:
HIV-uninfected pregnant and breastfeeding women are at high risk of HIV acquisition, contributing to vertical transmission. Pre-exposure prophylaxis (PrEP) is safe in pregnancy, but PrEP in pregnancy is not policy in many countries including South Africa (SA). We evaluated the potential impact of providing PrEP for pregnant/breastfeeding women using a HIV model for SA.
Methods:
Our model considers two scenarios: a conservative scenario that matches the experience reported in the Kenyan PrEP programme for pregnant women (probability of uptake=32% and 11% in high-risk and low-risk women, respectively); and an optimistic scenario with PrEP initiated by 80% of all pregnant women. We compared this with PrEP for female sex workers (FSWs), men who have sex with men (MSM), and adolescent girls/young women (AGYW). Women are assumed to remain on PrEP throughout pregnancy and breastfeeding, and an equivalent average PrEP duration (2 years) is assumed in other scenarios.
Results:
Between 2020–2030, if PrEP is provided to pregnant/breastfeeding mothers, we project a 2.5% reduction in total HIV transmission (95%CI:2.4–2.6%) in the conservative scenario and 7.2% (95%CI:6.8–7.5%) in the optimistic scenario, which is similar to that in the FSW and MSM PrEP scenarios (1.9% and 3.0% respectively). Without PrEP, 76,000 (95%CI: 64,000–90,000) new cases of vertical transmission are expected; PrEP provision may reduce these infections by 13% (95%CI:13–14%) in the conservative scenario and 41% (95%CI:39–44%) in the optimistic scenario.
Conclusion:
High levels of uptake of and adherence to PrEP among pregnant/breastfeeding women could substantially reduce maternal and infant HIV acquisition in SA.
Keywords: PMTCT, pregnant women, breastfeeding women, South Africa, pre-exposure prophylaxis, PrEP
Background:
Pregnant and breastfeeding women are at high risk of HIV acquisition [1–6] due to a combination of increased biological and behavioral risks [2, 5, 7]. Pre-exposure prophylaxis (PrEP) is one of the few female-controlled HIV prevention methods that could contribute to the elimination of HIV incidence in this group [8–10]. The WHO released guidelines in July 2017 supporting the delivery of PrEP to prevent HIV in pregnant/breastfeeding women [11]. There are limited data on the potential impact of PrEP initiation, retention, and adherence on HIV prevention in women and vertical transmission in high HIV incidence communities [8, 9]. Pregnant women are ineligible to start PrEP in most countries, with most national policies limiting eligibility to female sex workers (FSWs), adolescent girls and young women (AGYW) and men who have sex with men (MSM). In South Africa (SA), which is one of the countries most affected by HIV, the 2016 PrEP policy does not include pregnant/breastfeeding women. To help understand the potential impact of PrEP in SA, we used a mathematical model to estimate the potential impact of providing PrEP to pregnant/breastfeeding women on HIV incidence in women and infants. We compared the impact of PrEP in pregnancy with providing PrEP to AGYW, FSWs and MSM in SA.
Methods
We used the Thembisa model (version-4.1 [16]), a demographic and HIV model developed for SA, to estimate the potential impact of introducing PrEP for pregnant/breastfeeding women. The model was used previously to estimate the potential impact of targeting PrEP to sex workers [17] and youth [18] in SA. A detailed description of the model structure and assumptions is provided elsewhere [16]. The model stratifies the SA population by individual age, sex, risk group, marital status, sexual experience and uptake of PrEP, simulating the change in the SA population profile using assumptions about sexual behavior patterns, HIV transmission and HIV disease progression to estimate the numbers of individuals moving between the different model compartments over each time step. Vertical transmission is modelled based on assumptions about rates of perinatal and postnatal transmission, which depend on maternal HIV disease stage and the form of antiretroviral treatment/prophylaxis received by the mother-child pair. Perinatal and postnatal transmission both depend on the rate of maternal HIV acquisition during pregnancy/breastfeeding, with high transmission risk assumed during acute maternal HIV infection [5, 16].
We consider two possible scenarios for modelling PrEP uptake during pregnancy/breastfeeding. In the ‘conservative’ scenario, we set the model assumptions to match the experience reported in the Kenyan PrEP program for pregnant women [19] where 16% of all HIV-negative women initiated PrEP during pregnancy, and the odds of PrEP initiation in women who had been diagnosed with a sexually transmitted infection (STI) in the last 6-months was 4-times that in women without an STI diagnosis. Based on these data, we assume a probability of PrEP uptake during pregnancy of 32.3% and 10.6% among high-risk and low-risk women respectively (see supplementary material for details). In the second ‘optimistic’ scenario, we consider the potential impact if PrEP is initiated by 80% of all pregnant women (high and low-risk). In both scenarios, women are assumed to remain on PrEP for an average duration of 2-years (average time from the first antenatal visit to delivery, 0.4-years [20], plus the average duration of breastfeeding, 1.4-years [21]). PrEP use is conservatively assumed to be associated with a 10% reduction in condom use [7, 25]. Women who take PrEP are assumed to be at a 65% lower risk of HIV acquisition per act of condomless sex [26]. Perfect use of PrEP would be associated with a higher reduction in risk of HIV acquisition, but prior studies in women have shown low adherence and imperfect use [27–30]. Women receiving PrEP are assumed to be tested for HIV at regular intervals, and pregnant women are assumed to be offered HIV testing at their first antenatal visit, at 34-weeks gestation and at 6-weeks postpartum [31]. Women who acquire HIV while on PrEP are assumed to be diagnosed on average 3-months after HIV acquisition. In both scenarios, we consider the impact of providing PrEP over the period from 2020 to 2030 in SA.
We compared the effectiveness of PrEP in pregnant/breastfeeding women with three alternative PrEP promotion scenarios: PrEP for FSWs, MSM and AGYW. For the sake of consistency, the same average duration of PrEP (2 years) is assumed in all scenarios. The same PrEP efficacy and reduction in condom use is assumed in all scenarios, except for the MSM scenario, where a higher efficacy (85%) is assumed [32]. In all alternative scenarios, the annual rate of PrEP uptake in the target population is set such that a PrEP coverage of 50% is achieved in the target group, except in the AGYW scenario, where the 50% coverage applies only to sexually active high-risk women aged 15–24 (the annual rate of PrEP uptake in low-risk women is assumed to be 0.329 times that in high-risk women, to be consistent with the assumptions made in the ‘conservative’ PrEP scenario for pregnant/breastfeeding women). Sensitivity analyses were conducted to assess the effect of assuming lower PrEP efficacy and different average PrEP durations (see supplementary material for details).
Scenarios are compared in terms of reduction in total HIV incidence, and reduction in vertical transmission, relative to a baseline scenario in which there is minimal PrEP uptake by FSWs and MSM [16]. Scenarios are also compared in terms of the numbers of HIV infections averted per 100-person years of PrEP provision, as a measure of PrEP efficiency. All outputs are calculated for the period from 2020 to 2030. Means and 95% credibility intervals (CI) are calculated from 1000 different model simulations, each with a different set of assumptions about HIV transmission probabilities, HIV testing and disease progression; these parameter combinations are sampled from the distributions that give the best model fits to SA HIV prevalence, mortality and HIV testing data [16].
Results:
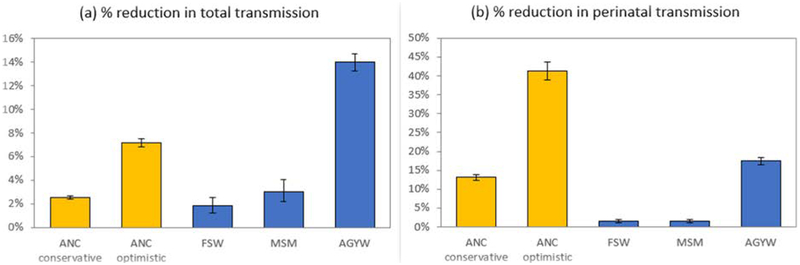
In the absence of PrEP, 1.90 million new HIV infections (95%CI: 1.76–2.06 million) are expected over the 2020–2030 period. The projected total number of HIV infections averted in SA as a result of providing PrEP during pregnancy/breastfeeding is 48,000 (95%CI: 43,000–52,000) in the conservative scenario and 136,000 (95%CI: 124,000–149,000) in the optimistic scenario. This is equivalent to 2.5% (95%CI: 2.4–2.6%) and 7.2% (95%CI: 6.8–7.5%) reductions respectively in the total projected number of HIV infections over the 2020–2030 period (Figure-1a). Without PrEP, 76,000 (95%CI: 64,000–90,000) new cases of vertical transmission are expected over the 2020–2030 period. This total is expected to be reduced by 13.2% (95%CI: 12.5–14.0%) in the conservative scenario and 41.4% (95%CI: 39.1–43.8%) in the optimistic scenario (Figure-1b). The reduction in HIV incidence in the conservative scenario is similar to the FSW and MSM PrEP scenarios, but the reduction in vertical transmission is greater in the conservative PrEP in pregnancy scenario (Figure-1). Under the optimistic scenario PrEP would have a proportionally greater impact on breastfeeding transmission (47% reduction;95%CI:44–49%) vs. in utero and intrapartum transmission (23% reduction;95%CI:18–27%). The reduction in HIV incidence in the optimistic scenario is projected to be less than in the AGYW scenario, but the reduction in vertical transmission is projected to be greater in the former scenario.
Figure 1:
Expected reductions in HIV incidence due to PrEP, 2020–2030, under different enhanced PrEP promotion scenarios
AGYW = adolescent girls and young women (sexually active, aged 15–24); ANC = antenatal care (includes breastfeeding women); FSW = female sex worker; MSM = men who have sex with men.
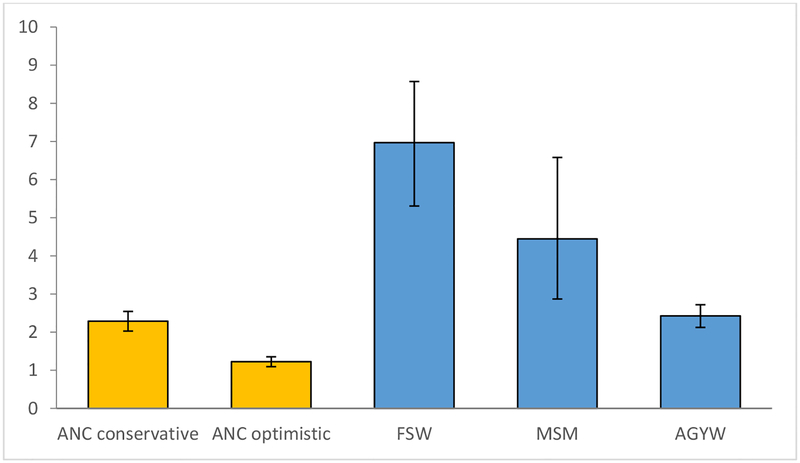
The scenarios differ in terms of efficiency of PrEP provision. The number of HIV infections averted per 100-person years of PrEP is expected to be greatest in the case of PrEP promoted to FSWs (7.0, 95%CI: 5.3–8.6) and MSM (4.4, 95%CI: 2.9–6.6), though credibility intervals are wide due to the scarcity of HIV prevalence data for these populations (Figure-2). The number of infections averted per 100-person years of PrEP is expected to be similar in the AGYW scenario (2.4, 95%CI: 2.1–2.7) and the conservative scenario in which PrEP is promoted to pregnant/breastfeeding women (2.3, 95%CI: 2.0–2.5). If PrEP uptake is high in both high- and low-risk pregnant/breastfeeding women, in the optimistic scenario, PrEP efficiency is expected to be almost half of that in the conservative scenario (1.2, 95%CI: 1.1–1.4). In the sensitivity analysis in which lower PrEP efficacy was assumed, PrEP impact and efficiency were estimated to be substantially lower than in the main analysis, although the relative ranking of different targeting strategies remained unaltered (Figures-S4 and S5). Setting PrEP retention assumptions for key populations to be more consistent with recent SA data led to lower estimates of PrEP impact for FSWs and AGYW but marginally higher estimates of PrEP impact for MSM (Figure-S6), as the latter group appears to have relatively high PrEP adherence.
Figure 2:
HIV infections averted per 100-person years of PrEP, 2020–2030
AGYW = adolescent girls and young women (sexually active, aged 15–24). ANC = antenatal care (includes breastfeeding women). FSW = female sex worker. MSM = men who have sex with men.
Discussion:
This analysis demonstrates that PrEP to pregnant/breastfeeding women in SA may have significant impact on prevention of HIV acquisition and vertical transmission. The greatest impact of PrEP in pregnancy is in vertical HIV transmission, with projected reductions between 13% in the conservative scenario and 41% in the optimistic scenario. The reduction in total new infections with PrEP to pregnant/breastfeeding women is similarly high in the optimistic PrEP scenario, with more infections averted compared to scenarios providing PrEP to either FSWs or MSM.
The effectiveness of PrEP in pregnancy is heavily dependent on the proportion of pregnant women who take PrEP, their risk factors for HIV acquisition, and adherence to PrEP during pregnancy/breastfeeding periods. There is a dearth of data on PrEP uptake and adherence in pregnant/breastfeeding women [9, 19]. A recent study in pregnant and postpartum SA women found that there was a high potential interest in PrEP during pregnancy, though baseline knowledge was low [36].
Although the efficiency of promoting PrEP to pregnant/breastfeeding women is not as great as the efficiency of promoting PrEP to MSM and FSWs, this does not necessarily mean that promoting PrEP to these groups is significantly less cost-effective. There are significant demand creation and outreach costs associated with reaching high-risk groups [35]. Further, the number of life-years saved per infant HIV infection averted is likely to be greater than the number of life-years saved per adult HIV infection averted, given the longer life expectancy of infants compared to adults. Considering our findings, we recommend that PrEP policies be updated to include pregnant/breastfeeding women, especially high-risk pregnant women, which was one of the most efficient strategies in our analysis.
A limitation of our model is the assumption that women who initiate PrEP during pregnancy remain on PrEP throughout the pregnancy/breastfeeding period. Although we lack data on retention rates when PrEP is initiated during pregnancy, early data from the PrEP program in FSWs show a worryingly low rate of retention [33], suggesting that our assumptions about retention in pregnant/breastfeeding women may be optimistic. Interventions to support PrEP retention and adherence in this population are essential.
Conclusion:
High levels of uptake of and adherence to PrEP among pregnant/breastfeeding women could fundamentally alter maternal and infant HIV acquisition in SA. There is an urgent need for implementation research to identify interventions that will facilitate PrEP use during pregnancy/breastfeeding in SA.
Supplementary Material
References
- 1.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med. 2014. February 25;11(2):e1001608 doi: 10.1371/journal.pmed.1001608. eCollection 2014 Feb. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomson KA, Hughes J, Baeten JM, John-Stewart G, Celum C, Cohen CR, Ngure K, Kiarie J, Mugo N, Heffron R; Partners in Prevention HSV/HIV Transmission Study and Partners PrEP Study Teams.. Increased Risk of HIV Acquisition Among Women Throughout Pregnancy and During the Postpartum Period: A Prospective Per-Coital-Act Analysis Among Women With HIV-Infected Partners. J Infect Dis. 2018. June 5;218(1):16–25. doi: 10.1093/infdis/jiy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009. June 19;23(10):1255–9. doi: 10.1097/QAD.0b013e32832a5934. [DOI] [PubMed] [Google Scholar]
- 4.Moodley D, Esterhuizen T, Reddy L, Moodley P, Singh B, Ngaleka L, Govender D. Incident HIV infection in pregnant and lactating women and its effect on mother-to-child transmission in South Africa. J Infect Dis. 2011. May 1;203(9):1231–4. doi: 10.1093/infdis/jir017. Epub 2011 Mar 11. [DOI] [PubMed] [Google Scholar]
- 5.Johnson LF, Stinson K, Newell ML, Bland RM, Moultrie H, Davies MA, Rehle TM, Dorrington RE, Sherman GG. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2012. April 1;59(4):417–25. doi: 10.1097/QAI.0b013e3182432f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinh T-H, Delaney KP, Goga A, Impact of Maternal HIV Seroconversion during Pregnancy on Early Mother to Child Transmission of HIV (MTCT) Measured at 4–8 Weeks Postpartum in South Africa 2011–2012: A National Population-Based Evaluation. Davies M-A, ed. PLoS ONE. 2015;10(5):e0125525 doi: 10.1371/journal.pone.0125525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph Davey D, Farley E, Gomba Y, Coates T, Myer L. Sexual risk during pregnancy and postpartum periods among HIV-infected and -uninfected South African women: Implications for primary and secondary HIV prevention interventions. PLoS One. 2018;13(3):e0192982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mofenson LM, Baggaley RC, Mameletzis I. Tenofovir Disoproxil Fumarate Safety for Women and their Infants during Pregnancy and Breastfeeding: Systematic Review. AIDS. 2016. November 7 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Joseph Davey DL, Bekker L-G, Gorbach P, Coates T, Myer L. Delivering PrEP to pregnant and breastfeeding women in Sub-Saharan Africa: the implementation science frontier. AIDS. 2017;31(16):2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mugwanya KK, Hendrix CW, Mugo NR, Marzinke M, Katabira ET, Ngure K. (2016) Pre-exposure Prophylaxis Use by Breastfeeding HIV-Uninfected Women: A Prospective Short-Term Study of Antiretroviral Excretion in Breast Milk and Infant Absorption. PLoS Med 13(9): e1002132 doi: 10.1371/journal.pmed.1002132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection—recommendations for a public health approach—second edition, 2016. Geneva, Switzerland: World Health Organization, 2016. http://www.who.int/hiv/pub/arv/arv-2016/en/. [Google Scholar]
- 12.Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’reilly KR, Koechlin FM, Rodolph M, Hodges-Mameletzis I, Grant RM. Effectiveness and safety of oral HIV pre-exposure prophylaxis (PrEP) for all populations: A systematic review and meta-analysis. AIDS. 2016. May 5 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirochnick M, Best BM, Clarke DF. Antiretroviral pharmacology: special issues regarding pregnant women and neonates. Clinics in perinatology. Dec 2010;37(4):907–927. [DOI] [PubMed] [Google Scholar]
- 14.M le Roux S, Jao J, Brittain K, Phillips TK, Olatunbosun S, Ronan A, Zerbe A, Abrams EJ, Myer L. Tenofovir exposure in utero and linear growth in HIV exposed, uninfected infants: a prospective study. AIDS. 2016. October 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.South African National Department of Health, “National Policy on HIV Pre-exposure Prophylaxis (PrEP) and Test and Treat (T&T).” Final Draft; 5 May 2016. [Google Scholar]
- 16.Johnson LF, Dorrington RE. Thembisa version 4.1: A model for evaluating the impact of HIV/AIDS in South Africa. 2018. www.thembisa.org
- 17.Bekker LG, Johnson L, Cowan F, Overs C, Besada D, Hillier S, et al. Combination HIV prevention for female sex workers: what is the evidence? Lancet 2015; 385:72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson LF, Chiu C, Myer L, Davies MA, Dorrington RE, Bekker LG, et al. Prospects for HIV control in South Africa: a model-based analysis. Global Health Action 2016; 9:30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinuthia J, Pintye J, Mugwanya K, Serede M, Sila J, Abuna F, et al. High PrEP uptake among Kenyan pregnant women offered PrEP during antenatal care [Abstract 1047] Conference on Retroviruses and Opportunistic Infections. Boston, USA; 2018. [Google Scholar]
- 20.Massyn N, Padarath A, Peer N, Day C. District Health Barometer 2016/17. Durban: Health Systems Trust; 2017. Available: http://www.hst.org.za/publications/Pages/District-Health-Barometer-201617.aspx. Accessed 3 April 2018 [Google Scholar]
- 21.Department of Health. South Africa Demographic and Health Survey 1998: Full Report. 1999.
- 22.Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 2016; 30:1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. New England Journal of Medicine 2012; 367:423–434. [DOI] [PubMed] [Google Scholar]
- 24.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New England Journal of Medicine 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mugwanya KK, Donnell D, Celum C, Thomas KK, Ndase P, Mugo N, et al. Sexual behaviour of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: a longitudinal analysis. Lancet Infectious Diseases 2013; 13:1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanscom B, Janes HE, Guarino PD, et al. Preventing HIV-1 infection in women using oral preexposure prophylaxis: a meta-analysis of current evidence. Journal of Acquired Immune Deficiency Syndrome 2016;73(5):606–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koss CA, Bacchetti P, Hillier SL, Livant E, Horng H, Mgodi N, Mirembe BG, Gomez Feliciano K, Horn S, Liu AY, Glidden DV, Grant RM, Benet LZ, Louie A, van der Straten A, Chirenje ZM, Marrazzo JM, Gandhi M. Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, iPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations. AIDS Res Hum Retroviruses. 2017. March 2 doi: 10.1089/aid.2016.0202. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corneli A, Perry B, McKenna K, Agot K, Ahmed K, Taylor J, Malamatsho F, Odhiambo J, Skhosana J, Van Damme L. Participants’ Explanations for Nonadherence in the FEM-PrEP Clinical Trial. J Acquir Immune Defic Syndr. 2016. April 1;71(4):452–61. doi: 10.1097/QAI.0000000000000880. [DOI] [PubMed] [Google Scholar]
- 29.Corneli AL, Deese J, Wang M, Taylor D, Ahmed K, Agot K, Lombaard J, Manongi R, Kapiga S, Kashuba A, Van Damme L; FEM-PrEP Study Group.. FEM-PrEP: adherence patterns and factors associated with adherence to a daily oral study product for pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2014. July 1;66(3):324–31. doi: 10.1097/QAI.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haberer JE, Bangsberg DR, Baeten JM, Curran K, Koechlin F, Amico KR, Anderson P, Mugo N, Venter F, Goicochea P, Caceres C, O’Reilly K. Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm. AIDS. 2015. July 17;29(11):1277–85. doi: 10.1097/QAD.0000000000000647. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Department of Health. National HIV counselling and testing policy guidelines. 2015. https://www.scribd.com/doc/268967963/Guidelines-National-HIV-Counselling-and-Testing-HCT-Policy-Guidelines-2015#download. Accessed 10 Aug 2015
- 32.Molina JM, Charreau I, Spire B, Cotte L, Chas J, Capitant C, Tremblay C, Rojas-Castro D, Cua E, Pasquet A, Bernaud C, Pintado C, Delaugerre C, Sagaon-Teyssier L, Mestre SL, Chidiac C, Pialoux G, Ponscarme D, Fonsart J, Thompson D, Wainberg MA, Doré V, Meyer L; ANRS IPERGAY Study Group.. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV. 2017. September;4(9):e402–e410. doi: 10.1016/S2352-3018(17)30089-9. Epub 2017 Jul 23. [DOI] [PubMed] [Google Scholar]
- 33.Eakle R, Gomez GB, Naicker N, Bothma R, Mbogua J, Cabrera Escobar MA, et al. HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project. PLoS Medicine 2017; 14:e1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heffron R, Mugo N, Hong T, Celum C, Marzinke MA, Ngure K, Asiimwe S, Katabira E, Bukusi EA, Odoyo J, Tindimwebwa E, Bulya N, Baeten JM; Partners Demonstration Project and the Partners PrEP Study Teams.. Pregnancy outcomes and infant growth among babies with in-utero exposure to tenofovir-based preexposure prophylaxis for HIV prevention. AIDS. 2018. July;32(12):1707–1713. doi: 10.1097/QAD.0000000000001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu J, Zinsou C, Parkhurst J, N’Dour M, Foyet L, Mueller DH. Comparative costs and cost-effectiveness of behavioural interventions as part of HIV prevention strategies. Health Policy Plan. 2013. January;28(1):20–9. doi: 10.1093/heapol/czs021. Epub 2012 Mar 12. [DOI] [PubMed] [Google Scholar]
- 36.Joseph Davey D, Farley E, Towriss C, Gomba Y, Bekker LG, Gorbach P, Shoptaw S, Coates T, Myer L. Risk perception and sex behaviour in pregnancy and breastfeeding in high HIV prevalence settings: Programmatic implications for PrEP delivery. PLoS One. 2018. May 14;13(5):e0197143 doi: 10.1371/journal.pone.0197143. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.