Abstract
Objective
We aimed to determine whether enhanced physical rehabilitation following intensive care unit (ICU) discharge improves activities-of-daily-living function, quality of life (QOL) and mortality among patients who received mechanical ventilation in the ICU.
Design
Systematic review and meta-analysis using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Data sources
MEDLINE, Embase, CENTRAL, PEDro and WHO International Clinical Trials Registry Platform searched through January 2019.
Eligibility criteria for selecting studies
We included randomised controlled trials assessing the effect of post-ICU rehabilitation designed to either commence earlier and/or be more intensive than the protocol employed in the control group. Only adults who received mechanical ventilation for >24 hours were included.
Data extraction and synthesis
Two independent reviewers extracted data and assessed risk of bias. Standard mean differences (SMDs) with 95% CIs were calculated for QOL, and pooled risk ratios (RRs) with 95% CIs are provided for mortality. We assessed heterogeneity based on I² and the certainty of evidence based on the GRADE approach.
Results
Ten trials (enrolling 1110 patients) compared physical rehabilitation with usual care or no intervention after ICU discharge. Regarding QOL, the SMD (95% CI) between the intervention and control groups for the physical and mental component summary scores was 0.06 (–0.12 to 0.24) and −0.04 (−0.20 to 0.11), respectively. Rehabilitation did not significantly decrease long-term mortality (RR 1.05, 95% CI 0.66 to 1.66). The analysed trials did not report activities-of-daily-living data. The certainty of the evidence for QOL and mortality was moderate.
Conclusions
Enhanced physical rehabilitation following ICU discharge may make little or no difference to QOL or mortality among patients who received mechanical ventilation in the ICU. Given the wide CIs, further studies are needed to confirm the efficacy of intensive post-ICU rehabilitation in selected populations.
PROSPERO registration number
CRD42017080532.
Keywords: rehabilitation, critical illness, post-intensive care syndrome, exercise, quality of life, mortality
Strengths and limitations of this study.
This is the first meta-analysis focused on enhanced physical rehabilitation to review randomised controlled trials in which the study intervention was conducted only after intensive care unit discharge.
The conclusions are based on moderate-certainty evidence.
The main limitations of this meta-analysis are that (i) few studies had a follow-up >6 months and (ii) medical resources and costs associated with each intervention were not considered.
We employed rigorous methodology that followed a protocol developed a priori according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, and used the Grading of Recommendations Assessment, Development and Evaluation approach in the review process.
Introduction
In critically ill patients, rehabilitation mainly aims to enhance quality of life (QOL) by improving activities-of-daily-living (ADL) function,1 2 which may be severely impaired also due to postintensive care syndrome (PICS).3–5 According to the guidelines issued by the National Institute for Health and Care Excellence, provision of rehabilitation should be seamlessly integrated with the patient’s transition from the intensive care unit (ICU) to the ward and then to out-of-hospital care.6 However, at the time the guidelines were issued, there was little evidence from clinical trials to support the use of enhanced physical rehabilitation following ICU discharge. Some experts do recommend physical rehabilitation following ICU discharge to improve ADL function and QOL.7 With regard to sepsis survivors, the findings of a large observational study suggested that physical rehabilitation following ICU discharge improves long-term mortality.8 9
A recent systematic review by Connolly et al 10 focused on randomised controlled trials (RCTs) regarding the effectiveness of enhanced exercise rehabilitation following ICU discharge in adult ICU survivors who had been mechanically ventilated for longer than 24 hours in the ICU. Despite the comprehensive search, this previous systematic review included only six RCTs with conflicting results, and no clear effect of the intervention on QOL, mortality, functional exercise capacity or incidence of adverse events could be established at the time. Additionally, ADL, pain, return-to-work rate, muscle strength and duration of delirium were not considered in that review.10 Several RCTs assessing the effect of enhanced physical rehabilitation following ICU discharge on clinically relevant outcomes11–15 have been published since Connolly et al conducted their Cochrane review.10 Therefore, in the present study, we aimed to re-evaluate the available literature and determine whether enhanced physical rehabilitation following ICU discharge improves clinically relevant outcomes among critically ill adults who received mechanical ventilation.
Materials and methods
Compliance with reporting guidelines
Using a prespecified protocol (PROSPERO registry ID: CRD42017080532),16 we conducted a systematic review of the relevant literature in agreement with the recommendations listed in the Cochrane Handbook17 and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.18 We confirmed that this systematic review was PRISMA-compliant by consulting the PRISMA 2009 checklist19 (details provided in online supplementary file 1).
bmjopen-2018-026075supp001.pdf (75.4KB, pdf)
Research question and eligibility criteria
The research question addressed in this study was: ’Does enhanced physical rehabilitation following ICU discharge result in improved QOL, ADL function and mortality (compared with those achievable with usual care) among patients who received mechanical ventilation in the ICU?’ We included all published and unpublished prospective RCTs involving adult human subjects (age ≥18 years) who had been discharged from an ICU or critical care environment after a stay of at least 48 hours during which mechanical ventilation was provided for at least 24 hours. Crossover trials, as well as cluster-randomised, quasi-randomised and non-randomised trials were excluded. Studies were included regardless of the intervention setting (in-hospital or out-of-hospital), follow-up duration and country of origin. We included patients of any sex and race, but excluded those receiving palliative care and those with head or spinal cord injuries, or unstable fracture diminishing mobility.
Intervention was defined as any protocolised rehabilitation following ICU discharge, designed to either commence earlier and/or be more intensive than the care received by the control group. To determine whether enhanced physical rehabilitation following ICU discharge improved clinically relevant outcomes, we excluded studies in which the patients in the intervention group received earlier and/or more intensive physical rehabilitation (compared with the care received by the control group) during their stay in the ICU. However, while we excluded studies in which enhanced rehabilitation was provided in the ICU, we did not exclude studies in which the same rehabilitation programme was provided in the ICU as standard care for both the intervention group and the control group. Protocolised rehabilitation consisting of one or more of the following activities was considered as a form of enhanced physical rehabilitation: neuromuscular stimulation, inspiratory or respiratory muscle training, passive range-of-motion exercise, cycle ergometer exercise, active-assisted exercises, active range-of-motion exercises, bed mobility activities (eg, bridging, rolling, lying-to-sitting exercise), ADL training, transfer training, pregait exercises (including marching in place) and walking exercise.
Outcomes of interest
The primary outcomes were QOL, ADL function and mortality. Secondary outcomes included functional exercise capacity, pain, return-to-work rate, muscle strength, duration of delirium and incidence of adverse events (defined by the trialists). We defined the intervention outcomes according to the timing of their evaluation postintervention, as short-term (evaluated at 28–35 days) or long-term (evaluated at 6 months).
Search strategy and selection of studies
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE via PubMed, Excerpta Medica Database (EMBASE) via Elsevier, the Physiotherapy Evidence Database (PEDro) and the WHO International Clinical Trials Registry Platform (WHO ICTRP) via their dedicated search portal. The search, which employed a set of suitable search terms (details provided in online supplementary file 2), was performed in December 2017 and updated in January 2019. We hand-searched reference lists for the guidelines for rehabilitation after critical illness.6 We attempted to identify other relevant research by hand-searching the reference lists of the studies returned by the search and those of articles citing such studies (based on citation information from the Web of Science). If the database entry for a candidate study did not contain the necessary information, we contacted the study authors. Two reviewers (ST and KY) independently screened the title and abstract of each study returned by the search to determine whether the inclusion criteria were met. The two reviewers performed a full-text review to assess the eligibility of each candidate study. Disagreement was resolved by discussion between the two reviewers, occasionally with arbitration by a third reviewer (YK).
bmjopen-2018-026075supp002.pdf (132.2KB, pdf)
Data abstraction and quality assessment
Two reviewers (ST and KY) independently abstracted trial-level data using prespecified forms. Disagreements regarding data extraction were resolved through discussions. Where necessary, we contacted the authors of studies that did not provide sufficient information. The risk of bias in each study was assessed independently by two reviewers (ST and KY) using the Cochrane risk-of-bias assessment tool.17 Differences in opinion regarding the assessment of risk of bias were resolved through discussion between the two reviewers, occasionally with arbitration by a third reviewer (KY).
Data analysis
All analyses were conducted using the Cochrane Review Manager software (RevMan V.5.3; Cochrane Collaboration, Copenhagen, Denmark). For the dichotomous variables of mortality and return-to-work rate, pooled risk ratios (RRs) with 95% CIs are provided. For continuous outcomes including QOL scores, ADL function scores, pain, muscle strength and duration of delirium (expressed in days of ICU or hospital stay), the standardised mean differences or the mean differences with 95% CIs were calculated, as recommended by the Cochrane Handbook.17 Adverse events were narratively summarised because their definition often varies across studies. We used the random-effects models for all analyses.
We calculated I² as a measure of variation across studies that is due to heterogeneity rather than chance, and interpreted the values as follows: 0%–40%, negligible heterogeneity; 30%–60%, mild-to-moderate heterogeneity; 50%–90%, moderate-to-substantial heterogeneity; 75%–100%, considerable heterogeneity. If heterogeneity was identified for an outcome (I² >50%), we investigated the underlying reasons and conducted the χ² test, with a p value of <0.10 being considered to indicate statistical significance. We investigated reporting bias by checking the WHO ICTRP to detect trials that had been completed but not published at the time of the review.
We planned the following prespecified sensitivity analyses for the primary outcomes: (i) exclusion of studies using imputed statistics and (ii) exclusion of studies with high or unclear risk of bias. We also carried out prespecified subgroup analyses according to the type of rehabilitation involved (neuromuscular stimulation vs other types of rehabilitation), rehabilitation provision in the ICU (received vs did not receive protocolised physical rehabilitation in the ICU), timing of commencement of the intervention (in-hospital or after hospital discharge), intervention duration (≤8 vs >8 weeks), treatment frequency (<5 vs ≥5 times/week) and type of control (no intervention vs standard rehabilitation). Statistical significance was also set at p<0.05. We created a summary-of-findings table that included an overall grading of the certainty of evidence for each of the main outcomes, which was evaluated using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.20 21
Patient and public involvement
The patients or public were not involved in this meta-analysis.
Results
Characteristics of trials on rehabilitation in ICU survivors
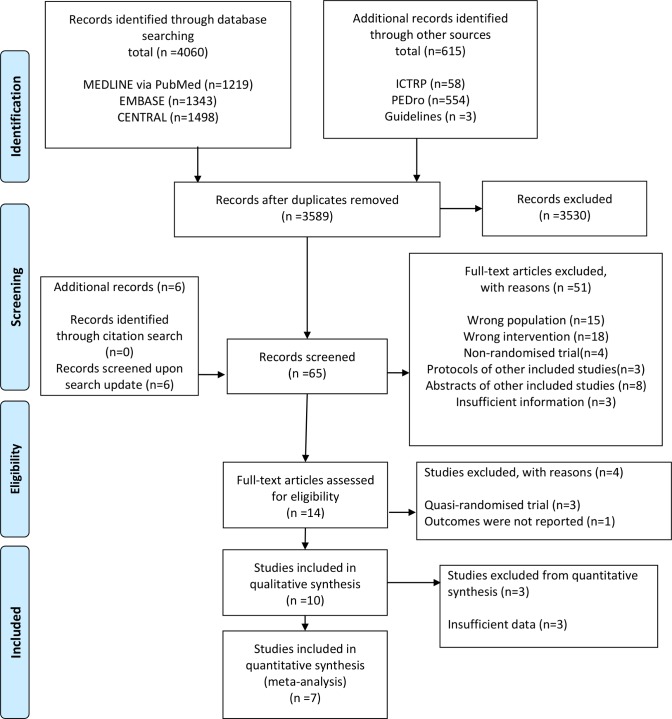
After removing duplicates, we identified 3589 records during the search conducted in December 2017 and updated the electronic searches in January 2019. We identified 10 unique RCTs11–13 15 22–27 that fulfilled all eligibility criteria and were included in the qualitative synthesis (figure 1; details provided in online supplementary file 3). The 10 RCTs provided a pooled sample of 1110 critically ill patients with an ICU stay of >48 hours during which mechanical ventilation was provided for at least 24 hours. Eight studies were performed in the UK, one in Australia and one in India. The mean or median age in the analysed studies ranged from 40.5 to 68.5 years, while the mean or median Acute Physiology and Chronic Health Evaluation II score ranged from 15.2 to 31. Only one RCT included participants with PICS symptoms or ICU-acquired weakness.11 Three RCTs25–27 did not have sufficient outcome data for meta-analysis (details provided in online supplementary file 4), leaving a total pooled sample of 1000 patients (506 patients in the intervention groups; 494 controls) represented across seven studies to be included in the quantitative synthesis. Of the 10 trials analysed, 6 evaluated the effect of physical rehabilitation including self-directed exercise and/or supervised exercise following hospital discharge, while 412 22–24 focused on rehabilitation started during hospitalisation. The duration of intervention ranged from 6 weeks to 3 months, while the frequency of intervention ranged from three times per week to once daily. No study considered intensive intervention (>30 min of active rehabilitation daily) or intervention with neuromuscular stimulation. Two studies12 23 had a follow-up >6 months. We did not identify any ongoing studies.
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses flow diagram.
bmjopen-2018-026075supp003.pdf (93.6KB, pdf)
bmjopen-2018-026075supp004.pdf (63KB, pdf)
Most studies were at high or unclear risk of bias, as determined using the Cochrane risk-of-bias assessment tool17 (details provided in online supplementary file 5). All 10 studies demonstrated adequate random sequence generation and allocation concealment, but participants and personnel were not blinded to the intervention. One study11 demonstrated a high risk of detection bias for all outcomes except mortality, and another study27 did not report whether or not the outcome assessor was aware of group allocation. Five studies had high risk of incomplete outcome data. Four studies had high risk of selective reporting bias, and two studies had unclear risk of bias because the protocols were not published. High or unclear risk of other bias was noted for all studies because of insufficient information regarding the intervention and control protocols.
bmjopen-2018-026075supp005.pdf (17.4KB, pdf)
Primary outcomes
QOL was measured in nine trials (see online supplementary file 3), but the short-term and long-term QOL scores were only available in four trials,12 22–24 whereas the other five trials measured these outcomes at a different time or had insufficient outcome data for meta-analysis. ADL function was measured in one trial,11 but the short-term and long-term data were not available. Short-term mortality was reported in two trials,11 13 while long-term mortality was reported in five trials.12 15 22–24
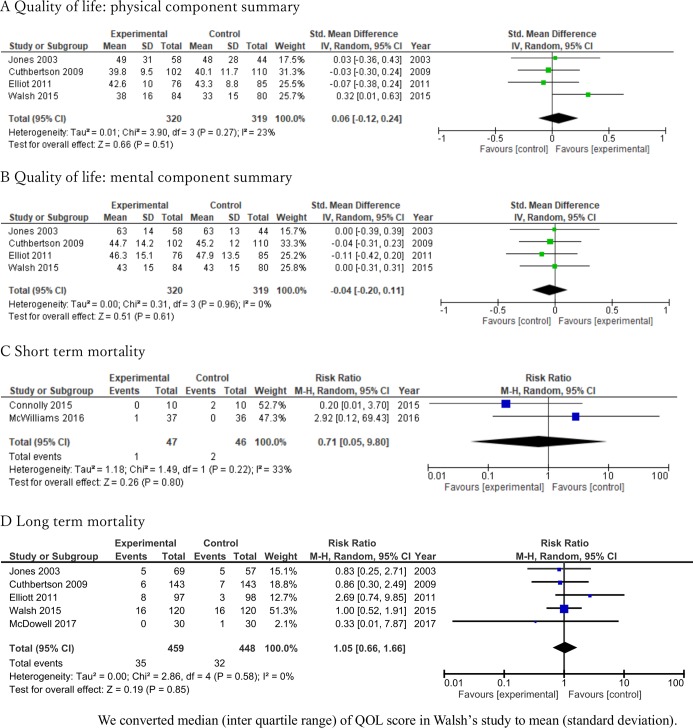
The standard mean deviation between intervention and control regarding the physical and mental component summary scores measured using QOL questionnaires (Short Form 36 or Short Form 12) were 0.06 (95% CI −0.12 to 0.24) and −0.04 (95% CI −0.20 to 0.11), respectively (figure 2A,B respectively). Rehabilitation did not significantly decrease short-term mortality (RR 0.71; 95% CI 0.05 to 9.80, I2=33%; n=93) (figure 2C) or long-term mortality (RR 1.05; 95% CI 0.66 to 1.66, I2=0%; n=907) (figure 2D). The certainty of evidence for QOL and long-term mortality was moderate, while that for short-term mortality was low (table 1). The lack of benefit of enhanced physical rehabilitation after ICU discharge was confirmed on additional analysis of QOL scores and mortality at 12 months postintervention (see details provided in online supplementary file 6).
Figure 2.
Forest plot for quality of life and mortality.
Table 1.
Findings from 10 trials focused on post-ICU rehabilitation of critically ill patients who received mechanical ventilation
| Overview of study design | ||||||
|
Patients or study population: adult patients who have been discharged from an ICU or critical care environment during which mechanical ventilation was provided for at least 24 hours. Setting: any. Intervention: protocolised physical rehabilitation following ICU discharge, designed to be more intensive than the care received by the control group. Comparison: no intervention or usual care. | ||||||
| Outcome | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention | |||||
|
Quality of life
Physical component summary score (6 months) |
Study population | 639 (4 RCTs) | ⊕⊕⊕⊝ Moderate† |
|||
| SMD: 0.06 (−0.12 to 0.24) | ||||||
|
Quality of life
Mental component summary score (6 months) |
Study population | 639 (4 RCTs) | ⊕⊕⊕⊝ Moderate† |
|||
| SMD: −0.04 (−0.20 to 0.11) | ||||||
|
Mortality
Short term (28–35 days) |
Study population | RR: 0.71 (0.05 to 9.80) | 93 (2 RCTs) | ⊕⊕⊝⊝ Low‡§ |
||
| 43 per 1000 | 31 per 1000 (2 to 426) | |||||
|
Mortality
Long term (6 months) |
Study population | RR: 1.05 (0.66 to 1.66) | 907 (5 RCTs) | ⊕⊕⊕⊝ Moderate¶ |
||
| 71 per 1000 | 75 per 1000 (47 to 119) | |||||
| Adverse events | Study population | 153 (3 RCTs) | ⊕⊕⊝⊝ Low**†† |
|||
| Two studies reported no adverse events. One study reported 18 and 5 events in the intervention and control groups, respectively. | ||||||
GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
*The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect (and its 95% CI) estimated for the intervention group.
†Downgraded one point because of high risk of bias associated with the lack of information regarding the dose of physical rehabilitation and adherence in the intervention group (other bias).
‡Downgraded one point because of high risk of bias associated with the fact that the intervention included nutritional therapy but the study provided very little detail regarding the therapy received in the control group (other bias).
§Downgraded because of imprecision (only two small studies).
¶Downgraded one point because of high risk of bias associated with incomplete outcome data and lack of information regarding the dose of physical rehabilitation and adherence in the intervention group, as well as with the fact that the intervention included nutritional therapy but the study provided very little detail regarding the therapy received in the control group (other bias).
**Downgraded one point because of high risk of bias associated with the fact that very little detail was given regarding the therapy received in the control group, and the adherence in the intervention group was 70% (other bias).
††Downgraded because of imprecision (only three small studies).
GRADE, Grading of Recommendations Assessment, Development and Evaluation; ICU, intensive care unit; RCT, randomised controlled trial; RR, risk ratio; SMD, standardised mean difference.
bmjopen-2018-026075supp006.pdf (73.5KB, pdf)
We could not carry out all prespecified sensitivity analyses because there was no study using imputed statistics, and we judged that the risk of bias of all included studies was similar in terms of random sequence generation, allocation concealment, incomplete outcome data and other bias. The prespecified subgroup analyses for the primary outcomes revealed no significant differences among subgroups (see details provided in online supplementary file 7).
bmjopen-2018-026075supp007.pdf (1MB, pdf)
Secondary outcomes
Functional exercise capacity was measured in two trials,11 24 pain was measured in one trial12 and muscle strength was measured in one trial,11 but short-term and long-term data were not available. No trials evaluated return-to-work rate or incidence of delirium.
Adverse events were measured in three trials.11 13 15 Two studies11 13 reported no adverse events. One study15 reported 18 events in the intervention group and 5 events in the control group. Among the 18 adverse events reported in the intervention group, 12 were mild or moderate (musculoskeletal pain higher than expected or muscle soreness potentially indicating injury, 3 cases; any pain higher than expected, 1 case; cardiac symptoms or chest pain, 1 case; any other event considered by the researcher to be of concern, 7 cases; 6 of 12 events were considered to be related or possibly related to study participation), while 6 were serious (hospitalisation or prolonged hospitalisation, with one event related/possibly related to study participation). In the control group, there was one adverse event (musculoskeletal pain higher than expected, muscle soreness potentially indicating injury, related/possibly related to study participation) and four serious adverse events (hospitalisation or prolonged hospitalisation, with one event related/possibly related to study participation). The certainty of evidence for adverse events was low (table 1).
Discussion
The results of this up-to-date review covering 10 RCTs and 1110 patients suggest that enhanced rehabilitation following ICU discharge might not improve QOL or reduce mortality at 6 or 12 months postintervention among patients who received mechanical ventilation in the ICU. We could not confirm the effect of enhanced physical rehabilitation even though all included studies exhibited performance bias potentially increasing the observed effect of the intervention. Furthermore, despite the large sample size in the meta-analysis for QOL and long-term mortality, limited data for these outcomes were available, and the certainty of evidence was only low or moderate.
Furthermore, subgroup meta-analyses revealed no differences among subgroups defined according to the nature or timing of the intervention. The previous review by Connolly et al 10 did not conduct meta-analysis due to the limited number of included studies. A recent systematic review of ICU rehabilitation28 29 also reported no significant difference in QOL between the intervention and control groups. Thus, neither enhanced rehabilitation in the ICU nor rehabilitation following ICU discharge appear to be superior to standard care in terms of QOL outcomes. In addition, we found no benefit in terms of short-term or long-term mortality regardless of timing of commencement, which is consistent with previous findings that ICU rehabilitation did not decrease mortality at ICU discharge, at hospital discharge or at 6 months after discharge.28 30 On the other hand, rehabilitation may be detrimental in acute conditions. Specifically, intensive physical rehabilitation started within 48 hours of admission for exacerbations of chronic respiratory disease increased mortality at 12 months,31 and higher-dose physical rehabilitation very early after stroke decreased favourable outcomes at 3 months.32 Thus, implementation of an intensive rehabilitation programme might not be indicated in all patients who received mechanical ventilation in the ICU.
Subgroup analysis in a previous systematic review28 indicated that, compared with low-dose rehabilitation, high-dose active rehabilitation for >30 min daily was associated with significantly higher QOL. Dose-response analysis of early physical rehabilitation33 in patients with stroke enrolled in A Very Early Rehabilitation Trial32 determined that intervention in such acute cases improved the odds of a favourable outcome with each episode of activity per day. Our present review did not include studies comparing high-dose rehabilitation and usual care, and thus the QOL effect of high-dose rehabilitation remains unclear. Additionally, we could not perform subgroup analysis for PICS symptoms with effect on QOL3–5 or for sepsis, which is a risk factor for PICS.34 35 It remains unclear which population of critically ill patients may truly benefit from intensive physical rehabilitation.
The studies included in our review did not cover all important outcomes included in the core outcome set of rehabilitation after critical illness,7 including ADL function, functional exercise capacity, pain, return-to-work rate, muscle strength or delirium incidence. Nonetheless, our findings regarding QOL and mortality suggest that, even if future studies report improvement in these other aspects, the amount of improvement would likely be too small to affect QOL.
The present review has several strengths. First, we employed rigorous methodology that followed a written protocol developed a priori according to the PRISMA statement, including a comprehensive search for evidence. Second, we performed duplicate assessment of eligibility, risk of bias and data abstraction. Third, we used the GRADE approach for assessing the certainty of evidence. In addition, we only included RCTs, most of which were multicentre studies. We could thus conduct an intention-to-treat analysis to understand the effect of intensive physical rehabilitation or standard care, which gives a pragmatic estimate of the benefit of a change in treatment policy. Fourth, the cohorts of ICU survivors are heterogeneous in terms of demographics and pathologies. To confirm the effect of enhanced physical rehabilitation for a particular group, we selected studies including only participants with an ICU stay of >48 hours during which mechanical ventilation was provided for at least 24 hours.
This systematic review has several potential limitations. First, few studies12 23 had a follow-up >6 months, and thus we could not consider longer follow-up data for primary analysis. The meta-analysis should be updated as the outcomes of further studies with follow-up beyond 6 months become available. Second, none of the studies included in our meta-analysis reported mortality outcomes as time-to-event data, which is the preferred approach for reporting mortality data. Future studies should report time-to-event data for mortality. Third, we could not take into account the medical resources and costs associated with each intervention. However, since studies included in this review compare rehabilitation intervention against standard care or no intervention, it is obvious that intensive physical rehabilitation would be associated with increased medical resources and costs. Fourth, the outcome measures might be not sufficiently sophisticated. For example, the RECOVER (Evaluation of a Rehabilitation Complex Intervention for Patients Following Intensive Care Discharge) trial12 did not demonstrate an improvement in the primary quantitative outcome, but showed evidence of benefit of the intervention in a parallel qualitative evaluation.36 Fifth, we could not consider the psychological aspects that are likely to affect the outcomes of rehabilitation. While our findings indicate a lack of benefit of enhanced post-ICU rehabilitation in the evaluated population, highly self-motivated individuals might have derived benefit from such therapies. Further studies should collect data on motivation and engagement, which are crucial in maximising the benefits of rehabilitation.37 Lastly, the patient characteristics, follow-up timing and types of outcomes reported might exhibit substantial heterogeneity across trials and within each individual trial, an aspect we did not examine in the present analysis. However, on reviewing the best available evidence based on a standardised approach, we confirmed that the direction of the effect and the effect size of enhanced post-ICU physical rehabilitation were similar in pooled studies, as reflected in the forest plots (see details in online supplementary file 7).
Taken together, the findings of the present meta-analysis indicate that enhanced physical rehabilitation following ICU discharge may make little or no difference to QOL or mortality among patients who received mechanical ventilation in the ICU. Given the wide CIs, further studies are needed to determine the efficacy of enhanced rehabilitation in selected populations of ICU survivors.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Avelino C. Verceles (Division of Pulmonary, Critical Care and Sleep Medicine, University of Maryland School of Medicine), Ms Bernie Bissett (Canberra Hospital), Dr Bronwen Connolly (St Thomas’ Hospital), Dr Yen-Huey Chen (Department of Respiratory Therapy, Chang Gung University), Dr Christina Jones (Whiston Hospital), Mr Danny Martin (Department of Physical Therapy, University of Florida), Dr Jennifer Paratz (Burns, Trauma & Critical Care Research Centre, School of Medicine, University of Queensland), Dr Kensuke Nakamura (Hitachi General Hospital), Kirstine Sibilitz (Department of Cardiology, Hvidovre University Hospital), Ms Ling Ling Chiang (School of Respiratory Therapy, Taipei Medical University), Ms Lisa Salisbury (Dietetics, Nutrition & Biological Sciences, Physiotherapy, Podiatry & Radiography Division, Queen Margaret University), Dr Michele Vitacca (Istituti Clinici Scientifici Maugeri), Dr Richard D Griffiths (Institute of Ageing and Chronic Disease, University of Liverpool), Mr Rik Gosselink (Faculty of Kinesiology and Rehabilitation Science, University of Leuven), Ms Seher Özyürek (School of Physical Therapy and Rehabilitation, Dokuz Eylul University), Ms Sunita Mathur (Department of Physical Therapy, University of Toronto) and Dr Timothy S. Walsh (Anaesthesia, Critical Care and Pain Medicine, University of Edinburgh) for providing us with additional information regarding their studies. The authors would like to thank Editage (http://www.editage.jp) for English language editing.
Footnotes
Contributors: ST and KY designed the study, were involved in the systematic review process, analysed and interpreted the data and drafted the manuscript. MB, HT, YK and YT participated in the systematic review process, critically reviewed the initial manuscript and approved the final manuscript as submitted. All authors read and approved the final manuscript.
Funding: This work was supported by JSPS KAKENHI Grant Number JP18K17719.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data associated with this manuscript are included in the main text and supplementary materials.
Patient consent for publication: Obtained.
References
- 1. World Health Organization. International classification of functioning, disability and health (ICF). 2001. http://www.who.int/classifications/icf/en/ [Accessed 24 May 2018].
- 2. Hodgson CL, Udy AA, Bailey M, et al. The impact of disability in survivors of critical illness. Intensive Care Med 2017;43:992–1001. 10.1007/s00134-017-4830-0 [DOI] [PubMed] [Google Scholar]
- 3. Fan E, Dowdy DW, Colantuoni E, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 2014;42:849–59. 10.1097/CCM.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hermans G, Van Mechelen H, Clerckx B, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2014;190:410–20. 10.1164/rccm.201312-2257OC [DOI] [PubMed] [Google Scholar]
- 5. Naidech AM, Beaumont JL, Rosenberg NF, et al. Intracerebral hemorrhage and delirium symptoms. Length of stay, function, and quality of life in a 114-patient cohort. Am J Respir Crit Care Med 2013;188:1331–7. 10.1164/rccm.201307-1256OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Institute for Health and Care Excellence. Rehabilitation after critical illness. 2008. https://www.nice.org.uk/guidance/cg83 [Accessed 24 May 2018]. [PubMed]
- 7. Major ME, Kwakman R, Kho ME, et al. Surviving critical illness: what is next? An expert consensus statement on physical rehabilitation after hospital discharge. Crit Care 2016;20:354 10.1186/s13054-016-1508-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA 2018;319:62–75. 10.1001/jama.2017.17687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chao PW, Shih CJ, Lee YJ, et al. Association of postdischarge rehabilitation with mortality in intensive care unit survivors of sepsis. Am J Respir Crit Care Med 2014;190:1003–11. 10.1164/rccm.201406-1170OC [DOI] [PubMed] [Google Scholar]
- 10. Connolly B, Salisbury L, O’Neill B, et al. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database Syst Rev 2015:CD008632 10.1002/14651858.CD008632.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Connolly B, Thompson A, Douiri A, et al. Exercise-based rehabilitation after hospital discharge for survivors of critical illness with intensive care unit-acquired weakness: a pilot feasibility trial. J Crit Care 2015;30:589–98. 10.1016/j.jcrc.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walsh TS, Salisbury LG, Merriweather JL, et al. Increased hospital-based physical rehabilitation and information provision after intensive care unit discharge: the RECOVER randomized clinical trial. JAMA Intern Med 2015;175:901–10. 10.1001/jamainternmed.2015.0822 [DOI] [PubMed] [Google Scholar]
- 13. McWilliams DJ, Benington S, Atkinson D. Outpatient-based physical rehabilitation for survivors of prolonged critical illness: a randomized controlled trial. Physiother Theory Pract 2016;32:179–90. 10.3109/09593985.2015.1137663 [DOI] [PubMed] [Google Scholar]
- 14. Patsaki I, Gerovasili V, Sidiras G, et al. Effect of neuromuscular stimulation and individualized rehabilitation on muscle strength in intensive care unit survivors: a randomized trial. J Crit Care 2017;40:76–82. 10.1016/j.jcrc.2017.03.014 [DOI] [PubMed] [Google Scholar]
- 15. McDowell K, O’Neill B, Blackwood B, et al. Effectiveness of an exercise programme on physical function in patients discharged from hospital following critical illness: a randomised controlled trial (the REVIVE trial). Thorax 2017;72:594.1–5. 10.1136/thoraxjnl-2016-208723 [DOI] [PubMed] [Google Scholar]
- 16. Taito S, Yamauchi K, Tsujimoto Y, et al. Systematic review and meta-analysis of physical rehabilitation following intensive care unit discharge. 2018. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=80532 [Accessed 24 May 2018]. [DOI] [PMC free article] [PubMed]
- 17. Higgins JPT, Green S, eds Cochrane handbook for systematic reviews of interventions, version 5.1.0. 2011. http://handbook-5-1.cochrane.org/ [Accessed 24 May 2018].
- 18. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 22. Jones C, Skirrow P, Griffiths RD, et al. Rehabilitation after critical illness: a randomized, controlled trial. Crit Care Med 2003;31:2456–61. 10.1097/01.CCM.0000089938.56725.33 [DOI] [PubMed] [Google Scholar]
- 23. Cuthbertson BH, Rattray J, Campbell MK, et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ 2009;339:b3723 10.1136/bmj.b3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elliott D, McKinley S, Alison J, et al. Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care 2011;15:R142 10.1186/cc10265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salisbury LG, Merriweather JL, Walsh TS. The development and feasibility of a ward-based physiotherapy and nutritional rehabilitation package for people experiencing critical illness. Clin Rehabil 2010;24:489–500. 10.1177/0269215509360639 [DOI] [PubMed] [Google Scholar]
- 26. Batterham AM, Bonner S, Wright J, et al. Effect of supervised aerobic exercise rehabilitation on physical fitness and quality-of-life in survivors of critical illness: an exploratory minimized controlled trial (PIX study). Br J Anaesth 2014;113:130–7. 10.1093/bja/aeu051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shelly AG, Prabhu NS, Jirange P, et al. Quality of life improves with individualized home-based exercises in critical care survivors. Indian J Crit Care Med 2017;21:89–93. 10.4103/ijccm.IJCCM_433_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tipping CJ, Harrold M, Holland A, et al. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med 2017;43:171–83. 10.1007/s00134-016-4612-0 [DOI] [PubMed] [Google Scholar]
- 29. Fuke R, Hifumi T, Kondo Y, et al. Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: a systematic review and meta-analysis. BMJ Open 2018;8:e019998 10.1136/bmjopen-2017-019998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Girard TD, Alhazzani W, Kress JP, et al. An official American Thoracic Society/American College of Chest Physicians clinical practice guideline: liberation from mechanical ventilation in critically ill adults. Rehabilitation protocols, ventilator liberation protocols, and cuff leak tests. Am J Respir Crit Care Med 2017;195:120–33. 10.1164/rccm.201610-2075ST [DOI] [PubMed] [Google Scholar]
- 31. Greening NJ, Williams JE, Hussain SF, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ 2014;349:g4315 10.1136/bmj.g4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. AVERT Trial Collaboration Group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet 2015;386:46–55. 10.1016/S0140-6736(15)60690-0 [DOI] [PubMed] [Google Scholar]
- 33. Bernhardt J, Churilov L, Ellery F, et al. Prespecified dose-response analysis for A Very Early Rehabilitation Trial (AVERT). Neurology 2016;86:2138–45. 10.1212/WNL.0000000000002459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stevens RD, Dowdy DW, Michaels RK, et al. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med 2007;33:1876–91. 10.1007/s00134-007-0772-2 [DOI] [PubMed] [Google Scholar]
- 35. Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010;304:1787–94. 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramsay P, Huby G, Merriweather J, et al. Patient and carer experience of hospital-based rehabilitation from intensive care to hospital discharge: mixed methods process evaluation of the RECOVER randomised clinical trial. BMJ Open 2016;6:e012041 10.1136/bmjopen-2016-012041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corner EJ, Murray EJ, Brett SJ. Qualitative, grounded theory exploration of patients' experience of early mobilisation, rehabilitation and recovery after critical illness. BMJ Open 2019;9:e026348 10.1136/bmjopen-2018-026348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-026075supp001.pdf (75.4KB, pdf)
bmjopen-2018-026075supp002.pdf (132.2KB, pdf)
bmjopen-2018-026075supp003.pdf (93.6KB, pdf)
bmjopen-2018-026075supp004.pdf (63KB, pdf)
bmjopen-2018-026075supp005.pdf (17.4KB, pdf)
bmjopen-2018-026075supp006.pdf (73.5KB, pdf)
bmjopen-2018-026075supp007.pdf (1MB, pdf)