Abstract
In patients with diabetic kidney disease (DKD) plasma renin activity is usually decreased, but there is limited information on urinary renin and its origin.
Urinary renin was evaluated in samples from patients with longstanding type I diabetes and mice with streptozotocin (STZ) –induced diabetes. Renin reporter mouse model (Ren1d-Cre;mT/mG) was made diabetic with STZ to examine whether the distribution of cells of the renin lineage was altered in a chronic diabetic environment.
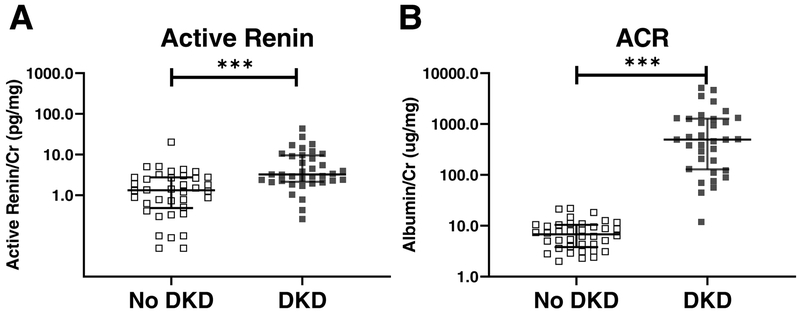
Active renin was increased in urine samples from patients with DKD (n=36), compared to those without DKD (n=38) (3.2 vs. 1.3 pg/mg Cr; p<0.001). In mice with STZ-induced diabetes, urine renin was also increased compared to non-diabetic controls. By immunohistochemistry, in mice with STZ-induced diabetes, juxtaglomerular apparatus (JGA) and proximal tubular renin staining were reduced whereas collecting tubule staining, by contrast, was increased. To examine the role of filtration and tubular reabsorption on urinary renin, mice were either infused with either mouse or human renin (hrRenin) and lysine (a blocker of proximal tubular protein reabsorption). Infusion of either form of renin together with lysine markedly increased urinary renin such that it was no longer different between non-diabetic and diabetic mice. Megalin mRNA was reduced in the kidney cortex of STZ-treated mice (0.70±0.09 vs. 1.01±0.04 in controls, p=0.01) consistent with impaired tubular reabsoprtion. In Ren1d-Cre;mT/mG with STZ – induced diabetes the distribution of renin lineage cells within the kidney, was similar to non–diabetic renin reporter mice. No evidence for migration of cells of renin linage to the collecting duct in diabetic mice could be found. Renin mRNA in microdissected collecting ducts from STZ-treated mice, moreover, was not significantly different than in controls, whereas in kidney cortex, largely reflecting JGA renin, it was significantly reduced.
In conclusion, in urine from patients with type 1 diabetes and DKD and from mice with STZ-induced diabetes, renin is elevated. This cannot be attributed to production from cells of the renin lineage migrating to the collecting duct in a chronic hyperglycemic environment. Rather, the elevated levels of urinary renin found in DKD are best attributed to altered glomerular filteration and impaired proximal tubular reabsorption.
Keywords: Renin, diabetes, juxtaglomerular apparatus, renin paradox, renin reporter mice, collecting duct renin
Introduction
Renin is the key physiologically regulated enzyme of the cascade that culminates in activation of the renin angiotensin system (RAS)1–14. Under normal physiological conditions, juxtaglomerular cells are the main, if not the only soure of circulating, active renin, constituting therefore the endocrine branch of RAS4, 6, 8. Upon reaching the circulation, renin generates angiotensin I by hydrolyzing its uniqe substrate angiotensinogen, which is an indispensable step for the subsequent formation of angiotensin II7, 15, 16. Thus, renin release by secretory cells in the JGA is the rate-limiting step in the activation of systemic RAS. Most RAS components are present at the tissue level and this system moreover is believed to be overactive in kidneys from rodent models and patients with DKD17–26. By contrast, plasma renin activity (PRA) is usually low in patients with diabetic complications27–30 This is known as the renin-paradox27, 31, 32. Despite of the low/normal levels of circulating plasma renin activity, RAS blockers are effective in slowing down the progression of kidney disease26, 33–35. This beneficial effect reflects their direct suppressive actions of the kidney RAS which is in addition to their systemic effects.
The presence of renin was described in proximal and collecting tubules as early as 198236. More recently, there has been an interest in urinary renin and its possible source, which could at least, in part, be of tubular origin19, 37–39. There is limited information on urinary renin in patients with diabetes and renal complications. It is possible that urinary renin, if increased, could contribute to activation of the RAS system, a feature of diabetic and non-diabetic CKD. By cleaving angiotensinongen that is either filtered, locally formed or both, renin could activate RAS locally within the kidney, which is relevant to sodium retention, hypertension, glomerular hemodynamics, inflammation and progression of CKD. We therefore reasoned that the source of urinary renin in DKD deserves further investigation. We hypothesized that urinary renin in DKD is either the result of increased filtration because of alterations to the glomerular permeability or impaired proximal, tubular reabsorption, or both. We also wanted to examine the possibility that urinary renin could be of tubular origin, that is locally produced by tubular segments, namely in the collecting ducts. In this regard it is important to note that the prorenin receptor (PRR), a dual receptor for renin and prorenin is present in collecting duct cells19, 40–43. Genetic ablation of the PRR in collecting duct cells attenuates the hypertensive response to Ang II infusions suggesting that this receptor may act as a positive regulator of the intrarenal RAS40, 44. Augmented renin staining in the collecting duct by angiotensin II and in STZ induced diabetes has been reported19, 36, 45. In rats with Ang II-dependent hypertension with marked suppression of plasma renin activity, urinary levels of renin were increased which was taken as evidence of possible secretion by collecting duct cells into the luminal fluid7. Renin mRNA can be detected not only in the proximal tubules but also in the collecting ducts4, 45. The finding that renin mRNA can be detected in renal medulla, which is devoid of JGA, would be consistent with the notion, that renin secreted in collecting ducts could be a source of urinary renin45. These findings, moreover, could be interpreted to suggest that renin originating from tubular sites can foster kidney RAS activation19, 45. In this scenario increased tubular renin could be reflected by increased urinary renin and be used as a potential marker of increased kidney RAS activity. This would be of interest particularly in the context of diabetic kidney disease where levels of plasma renin activity are usually low27, 28.
The objective of the present study was, therefore, to examine the origin of urinary renin both in patients with type 1 diabetes with or without DKD and in mice with STZ-induced diabetes. The role of proximal tubular reabsorption and tubular formation of renin as a potential source of urinary renin were explored in the setting of DKD by infusing recombinant renin when proximal tubular reabsorption was blocked by L-lysine. Finally, we used a renin reporter mouse model (Ren1d-Cre;mT/mG) made diabetic using STZ to examine whether the distribution of cells of the renin lineage was altered in a chronic diabetic environment.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study cohort
People with type 1 diabetes, who were seen in the Diabetes Care Center (University of Washington) for outpatient endocrinology care, were approached for participation in the Kidney Research Institute Diabetic Kidney Disease Repository (University of Washington) and were enrolled into the repository after providing informed consent. Demographic and clinical information was obtained from the electronic medical records and a questionnaire completed by the participants. DKD was defined as either a urine albumin-to-creatine ratio (ACR) ≥300 mg/g or an estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2 and ACR ≥30 mg/g. People with longstanding diabetes but no evidence of overt DKD were those with ≥25 years of type 1 diabetes, estimated GFR ≥90 mL/min per 1.73 m2, and ACR <30 mg/g. Demographic and clinical data (age, race, sex, diabetes duration and RAS inhibitor use) were obtained from the electronic medical records and confirmed by patient questionnaires (race, diabetes duration and RAS inhibitor use). Diabetes type was extracted from the clinical notes, as ascertained by the endocrinologists caring for the patients. Hemoglobin A1c (HbA1c) and serum creatinine were obtained from the electronic medical records at a time closest to the date of urine sample collection. Glomerular filtration rate was calculated from the serum creatinine using the CKD-EPI formula. A random clean-catch, mid-stream urine sample was collected and stored at 4°C after collection, until processed The use of human samples and data were approved by the Institutional Review Board of the University of Washington. Urine samples used for this study were centrifuged at 4,700g for 15 minutes at 4°C and the supernatant was collected, aliquoted and stored at −80°C. The mean (standard deviation, SD) time from sample collection to storage at −80°C was 5.7 (2.0) hours.
Laboratory measurements in human urine samples
Albumin and creatinine were measured using an immunoturbidometric assay and the modified rate Jaffe reaction, respectively, using a DXC 600 clinical chemistry analyzer (Beckman Coulter, Indianapolis). Active renin was measured using a quantitative solid-phase sandwich ELISA distributed by DRG Instruments (Marburg, Germany) with a minimum detection limit of 0.8 pg/mL and 0.69% crossreactivity with prorenin. Before active renin measurements, each sample was individually concentrated (6 times) using Spin-X 5 kD devices. The coefficients of intra- and inter-assay variation for active renin ELISA in human urine samples were 4.9% and 12.7%, respectively. Internal controls were included (one for high renin and one for low renin values) to assure consistency of inter-assay performance. In addition, ELISA data for urine active renin were compared to those reported in the literature. The geometric mean (range) in our study for all tested urines with detectable active renin were 1.64 (0.14–17.2) pg/ml (n=65). This was in keeping with the geometric mean and range reported in the literature (1.46 (0.13–157) pg/ml using a completely different approach (enzyme-kinetic assay – EKA) in 43 urines from mainly type 2 diabetic subjects46.
In addition, total renin was measured using a quantitative solid-phase sandwich ELISA distributed by R&D Systems (Minneapolis, MN) with a sensitivity of 14.8 pg/ml. According to the manufacturer, this assay recognizes both human recombinant prorenin and renin. The kit for total human renin was tested by the manufacturer specifically for human urine samples.
We also attempted to measure urinary prorenin using a quantitative solid-phase sandwich ELISA distributed by Molecular Innovations (Novi, MI) with a minimum detection limit of 0.016 ng/mL. This assay was reported by the manufacturer to be specific for prorenin only with no detectable crossreactivity with human, mouse and rat renin. However, due to poor reproducibility of prorenin measurements in our samples (Interassay CV=69.5%, n=47) we do not present the data obtained with human prorenin assay as we had to deem the results as questionable.
Experimental animals
Female FVB mice were acquired from Jackson Laboratories (Bar Harbor, ME).
The Ren1d-Cre; mT/mG is a double transgenic reporter mouse model expressing Cre recombinase from the endogenous renin locus (Ren1D) and the mT/mG cassette from the Rosa26 locus10. The 2 reporter genes, membrane-targeted fluorescent tomato protein (mT, red) and membrane-directed enhanced green fluorescent protein (eGFP; mG) were used to trace renin lineage cells (RLCs) in kidneys of diabetic and control mice. While mT is expressed prior to recombination, mG is only expressed in cells post Cre-expression and recombination. Thus, because gene expression from the Rosa26 locus is ubiquitous and gene switch is stably transferred to cell progeny, only RLCs are green (mG positive), while all non-RLCs remain mT positive. These reporter mice were generated as previously described6, 47.
Animals were housed at the Center for Comparative Medicine at Northwestern University Feinberg School of Medicine. Animal care and procedures were approved by the Institutional Animal Care and Use Committee at Northwestern University and in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the institutional, state, and federal guidelines. The total number of animals enrolled was 126.
Diabetes induction using Streptozotocin
Streptozotocin (150 mg/kg, Sigma Chemical, St. Louis, MO) dissolved in sodium citrate buffer pH 4.5 (streptozotocin-treated mice) or sodium citrate buffer pH 4.5 alone (non-diabetic vehicle controls) was injected in two intraperitoneal injections to female FVB mice at 19–20 weeks of age. Spot urines were collected 8 and 20 weeks after the last streptozotocin or vehicle injection (at 28 and 40 weeks of age, respectively) and stored at −80°C. Human recombinant renin (hrRenin, Molecular Innovations, Novi, MI) was administered as a single i.v. bolus injection to female FVB/N mice at the dose of 0.5 ug/g body weight. In order to determine the impact of renin reabsorption in the proximal tubule, lysine, a blocker of proximal tubular reabsorption48, was administered (0.4 mg/g body weight) as a single combination injection together with hrRenin and as a single injection without hrRenin. Urine was collected immediately before (baseline) and within 3 hours after injection. The timeline of the experiment was as follows: mice were weighted; then baseline urines were collected; within 5–10 min. after voiding, mice were administered with lysine, hrRenin or both in a single i.v. injection (0.2 mL/mouse); immediately after i.v. injection to collect urine, mice were placed for 3 hours in urine collection cages with access to water and food.
Laboratory measurements in mouse samples
Ang I and Ang II were extracted and measured by LC-MS/MS as previously described49. Active human renin was measured using a quantitative solid-phase sandwich ELISA distributed by DRG Instruments (Marburg, Germany). Mouse total renin was measured using a quantitative solid-phase sandwich ELISA distributed by RayBiotech (Norcross, GA) with a minimum detection limit of 6 pg/mL. Albumin was measured by a quantitative solid-phase sandwich enzyme-Linked immunosorbent assay (ELISA) (Albuwell M, Exocell, Philadelphia, PA). Creatinine was measured using the Jaffe method (Creatinine Companion, Exocell, Philadelphia, PA).
Western blot of mouse urine samples
For Western blot of urines, proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked in nonfat dry milk solubilized in Tris-buffered saline solution, pH 8.0 containing 0.1 %Tween 20 (7% wt/vol). The PDVF membranes were incubated with primary anti-Renin/Prorenin antibody (Molecular Innovations) and horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology). Bands were visualized using chemiluminescence system (Super Signal Pico, Pierce). For comparisons of urinary renin bands between control and lysine-injected groups, several gels were used in which a similar number of samples from each group was included. The average value of integrated density measured in controls was set as 100% for each gel and the results for injected mice were expressed as percentage of that measured in controls.
Immunohistochemistry
Paraffin blocks were cut at 4 μm and deparaffinized in xylene and rehydrated through graded alcohols. Antigen retrieval was performed with a pressure cooker at 120°C in target retrieval solution (DAKO). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Slides were incubated overnight at 4°C with rabbit anti-mouse renin polyclonal antibody (1:500), washed, and incubated with secondary antibody conjugated with peroxidase-labeled polymer (DAKO). After incubation with 3,3′-diaminobenzidine (DAB) chromogen, slides were counterstained with hematoxylin. Sections were dehydrated and covered with Permount (Fisher Scientific). Coverslips were viewed using a Zeiss microscope. To evaluate renin protein expression in kidneys from STZ-treated and vehicle control mice (n=9 in each group), a semiquantitative analysis of the immunoperoxidase-stained sections was performed. Two masked observers performed evaluation of renin staining within JGA, collecting duct (CD) and proximal tubules (PT). The percentage of JGAs with visible renin staining, the number of renin stained collecting tubules and the intensity of PT staining in the viewing areas (5 viewing fields per slide) were evaluated. The percentage of renin-positive JGA (JGA index) was calculated as (the number of renin-positive JGA in all sections) × 100/(the total number of glomeruli observed)50. Every section was examined observer-blinded using the same magnification (x 200), and only the JGA with a classic donut-shaped outline was evaluated.
Immunofluorescence
Cryosections (7 μm) were cut using a cryostat from the frozen blocks of Ren1d-Cre; mT/mG mouse kidneys and subsequently subjected to the immunostaining for aquaporin 2 (1:200; Santa Cruz Biotechnology) and renin. Kidney sections from Ren1d-Cre;mT/mG mice were screened for the presence of GFP+ in collecting duct epithelial cells based on co-localization with AQP2 immunostaining in both diabetic and non-diabetic anmals using Fiji software (NIH). A Pearson’s correlation coefficient was used to quantify the colocalization parameters. Moreover, a large image analysis of the GFP and AQP2 colocalized area per total area was performed to assess any differences between kidneys of control and diabetic Ren1d-Cre;mT/mG mice.
In addition, kidney sections were assessed for the presence of GFP+ cells in control and diabetic Ren1d-Cre;mT/mG mice within the parietal glomeruli and glomerular tuft and quantified as as percentage of glomerular GFP positivity.
Microdissection of Renal Tubule Segments
STZ treated mice and vehicle controls were euthanized by an overdose of Euthasol (390 mg pentobarbitol-Na and 50 mg phenytoin-Na per ml).
Both kidneys were rapidly removed and transferred into ice-cold HEPES solution. After removal of the capsula and pelvis, the kidneys were cut into 1–2 mm thick coronal slices. Usually three or four slices from the middle of each kidney could be obtained due to the thickness of the cortex and medulla. For the preparation of the tubules, 1- to 2-mm slices of the respective region were prepared under a stereo microscope and transferred into the pre-warmed digestion solution. Cortical and outer medullary tissue was then incubated in a digestion solution containing 4 ml MEM (GibcoBRL), 5 mM glycine, 6 mg/ ml trypsin inhibitor type II-S (Sigma T-9128), and 250 g/ml collagenase (Sigma C-9891), pH 7.4 at 37°C in a water bath for 25 min without shaking. When the medium became cloudy upon gentle shaking, the digestion was stopped by transferring the tubules to ice, carefully removing the supernatant and replacing it with 4 ml ice-cold 1% BSA-HEPES solution. The BSA HEPES solution was replaced with ice-cold HEPES solution after 10 min and tubules were maintained on ice until use. Isolated tubules were selected at 4°C under a stereo microscope after 0.5 ml of the tubule suspension had been diluted in 10 ml of ice-cold HEPES solution. Individual tubule segments were identified by their appearance and topology. For the proximal tubule segments, S1 was identified as the proximal tubule directly attached to the glomerulus, S2 was the straight part in the medullary ray, and S3 was the proximal tubule in the outer medulla. The cortical collecting duct (CCD) was dissected from the medullary rays of the cortex. Around 100 proximal tubule segments and 30 cortical collecting duct segments were dissected from each mouse kidney.
Reverse transcription – polymerase chain reaction analysis
Total RNA from kidney cortex tissue was obtained by using Trizol reagent (Thermo Fischer Scientific, Waltham, MA). Constant amounts of 1 ug of extracted kidney cortex RNA as well as the totality of the total RNA obtained from microdissected tubules (using Arcturus PicoPure RNA kit from Applied Biosystems) were reverse transcribed to synthesize complementary DNA (cDNA). Synthesis of cDNA was performed using Reverse Transcription Kit on a GenAmp PCR System 9700 (both Thermo Fischer Scientific, Waltham, MA) with standard cycling parameters. Quantitative PCR was run on a 96-well plate using a Step One Plus PCR System (Thermo Fischer Scientific, Waltham, MA). GAPDH was used as an internal control for normalization. Each reaction was performed in duplicates. Sequences of the primers for qPCR (IDT, Coralville, IA) are shown in Supplementary table S1.
Statistical Analysis
Shapiro-Wilk was used to test normality. Normally distributed data are reported as the arithmetic mean ± standard error of the mean (SEM). Distribution of non-normally distributed data were described using median and interquartile range (IQR). Differences between two groups with normal distribution were analyzed using a two-tailed Student’s t-test, and for non-normally distributed data (e.g. urinary ACR), using non-parametric Mann-Whitney test. To assess correlation between variables, Spearman correlation coefficient was used for non-normally distributed data, and Pearson correlation coefficient for normally distributed data. Fisher’s exact test was used in the analysis of contingency tables. Interaction between two independent variables was assessed using two-way ANOVA. The nominal significance threshold was set as a two-sided p-value <0.05. The IBM SPSS softare (version 23) and GraphPad Prism software (version 8) were used for the statistical analyses.
Results
Studies in urine samples from patients with type 1 diabetes
Characteristics of the cohort of patients with type 1 diabetes
Urine samples were obtained from a carefully characterized cohort of patients with type 1 diabetes at the University of Washington, Seattle. The majority of patients were Caucasian in both groups. There were no significant differences in age and gender distribution between patients with and without DKD, which both had male predominance (Supplementary table S2). Hemoglobin A1c was significantly higher in the DKD than in the non-DKD group (median 8.3 vs. 7.5%; p= 0.002). Both groups had prolonged disease duration of diabetes, but it was slightly but significantly longer in the group without DKD than in the group with DKD (median 32 vs. 37 years, p=0.032). Therefore the group without DKD had very low risk of developing it after such a long period of disease duration51.
As expected from the selection criteria, the group without DKD had urinary albumin excretion (UAE) in the normal range and an eGFR above 90 mL/min/1.73 m2 whereas the group with DKD had UAE either in the micro- or macroalbuminuric range and an eGFR below 60 mL/min/1.73 m2. The median eGFR was lower (median 39 vs. 101 mL/min/1.73 m2; p<0.001) while ACR was higher (median 497 vs. 7 mg/g Cr; p<0.001) in people with DKD, compared to those without DKD (Supplementary table S2). RAS-blocker use was more prevalent in people with DKD than in those without (86 vs. 50%; p=0.001).
Urine renin and albumin in patients with and without DKD
Active urinary renin was detectable in 94% (34/36) of urines from the cases and 82% (31/38) of urines from controls with no DKD. Total urinary renin was detectable in 56% (20/36) of samples from the cases but only in 9% (5/35) of the urines from the controls with diabetes but no DKD. For subsequent analyses, the values below the lower limit of quantification (LLOQ) of the assays were set to 0.5 × LLOQ (lower limit of quantification). Median creatinine-normalized urine active renin concentrations were significantly higher in people with DKD as compared to those without DKD (3.2; 2.1, 9.5 vs. 1.3; 0.5, 2.8 pg/mg Cr; p<0.001) (Fig. 1A). The urinary albumin/Cr ratio was markedly higher (by study design) in the group with CKD than in the group without (Fig. 1B). A significant positive correlation between active renin and ACR was found (Spearman coefficient: r=0.454, p=0.000062, confidence interval 0.2420 – 0.6247).
Figure 1. Active renin/Cr ratio (A) and albumin/Cr ratio(B) in patients with and without DKD in longstanding type 1 diabetes.
People with no DKD had eGFR ≥90 mL/min/1.73 m2 and ACR <30 mg/g after ≥25 years of type 1 diabetes. Those with DKD had either an ACR ≥300 mg/g or both eGFR <60 mL/min/1.73 m2 and ACR ≥30 mg/g. Panel A: higher active renin/Cr ratio in urines from patients with DKD (black, n=36) compared to those without DKD (white, n=38). Panel B: higher albumin/Cr ratio in urines from patients with DKD (black, n=34) compared to those without DKD (white, n=38) *** p < 0.001
Median creatinine-normalized urine total renin concentrations were also significantly higher in people with DKD than in people without DKD (p=0.023). (Supplement Fig. S1). A positive correlation between creatinine-normalized total urine renin and creatinine normalized urine albumin was found (Spearman coefficient: r=0.410, p=0.0005, confidence interval 0.1851 – 0.5941).
Urinary mouse and plasma renin in experimental DKD caused by STZ in mice
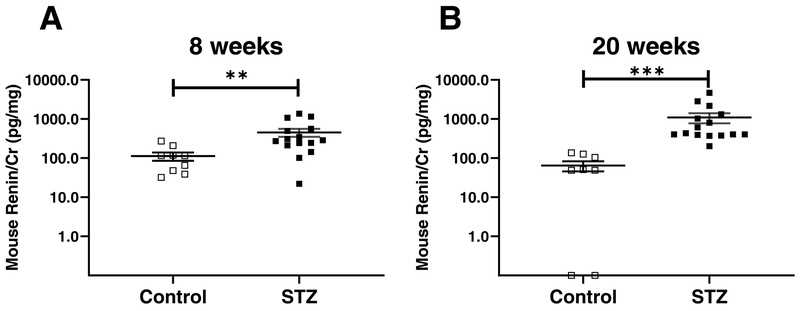
At 8 weeks post-STZ injections, a significant increase in urinary renin normalized for creatinine was found in mice treated with STZ (n=15) compared to those treated with vehicle (n=8) (455 ± 106 vs. 113 ± 27 pg/mg Cr; p= 0.005) (Fig. 2A). Albumin was significantly increased in mice injected with STZ compared to controls (69 ± 23 vs. 11 ± 3 μg/mg Cr; p= 0.006) as was the blood glucose (549 ± 15 vs. 157 ± 6 mg/dl; p < 0.01). Twenty weeks after STZ administration, hyperglycemia and albuminuria also developed and the data on these parameters has been previously reported23. Here, we show urinary data from the same animals, not previously reported. Urinary total mouse renin/creatinine (Cr) ratio was significantly higher in mice treated with STZ (1093 ± 319 pg/mg Cr, n=15) as compared to those treated with vehicle (64 ± 19 pg/mg Cr, n=8, p=0.0001) (Fig. 2B). A significant positive correlation was found between total renin and albumin both at 8 (Spearman coefficient: r=0.477, p=0.018, confidence interval 0.07867 to 0.7441) and 20 post STZ-injection (Spearman coefficient: r=0.704, p=0.0002, confidence interval 0.4006 to 0.8685), respectively. When the two age groups were combined, a significant positive correlation was also found Spearman coefficient: r=0.628, p=0.0000023, confidence interval 0.4089 to 0.7790).
Figure 2. Mouse renin concentrations in non-diabetic and diabetic mice, 8 (A) and 20 weeks after the last STZ injection (B).
Panel A: higher mouse renin/Cr ratio in urines from diabetic FVB mice (STZ, black, n=15) compared to non-diabetic FVB mice (Veh, white, n=9) 8 weeks after the last injection of steptozotocin. Panel B: higher mouse renin/Cr ratio in urines of diabetic FVB mice (STZ, black, n=15) compared to non-diabetic FVB mice (Veh, white, n=8) 20 weeks after the last injection of streptozotocin. Data shown as mean ± SE. Veh: control mice treated with vehicle; STZ: Streptozotocin-treated mice; **p<0.01, ***p<0.001
Since we did not have enough plasma volume for plasma renin activity measurements, we assessed indirectly using an approach recently described by Domenig et al.52. In this approach the summation of angiotensin I (Ang I) and angiotensin II (Ang II) serves as a strong surrogate for PRA52. We applied this concept to our studies and thus inferred PRA from the sum of Ang I and Ang II measured by LC-MS/MS in STZ treated and control mice. The inferred PRA from the sum of plasma Ang I and Ang II was lower in STZ injected mice than in the control group, but the difference was not statistically significant (83±52 vs. 267±179 pg/ml; p=0.230).
Effect of L-lysine infusions on urinary renin
The effect of lysine on total urinary renin excretion was first evaluated in a separate set of control mice using western blots. A renin immunoreactive protein band appeared at around 37 kDa in urines from mice injected with L-Lysine (n=6) which was barely detectable in urines of non-injected control mice (n=7) (Supplementary Fig. S2A). Densitometric analysis showed that the relative abundance of the renin immunoreactive protein band at 37 kDa was significantly increased in urines from mice injected with lysine compared to urines from non-injected controls (1912 ± 890 % of control FVB mice, p=0.001) (Supplementary Fig. S2B).
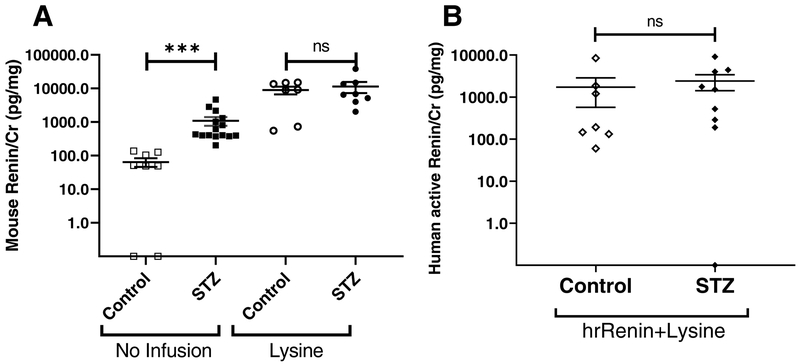
Having shown the increase in renin protein by Western blot we next used ELISA. Urinary creatinine-normalized renin was more than 100-fold increased in mice injected with lysine (n=7) compared to non-injected controls (n=8) (8950 ± 2337 vs. 64 ± 19 pg/mg Cr; p=0.001) (Fig. 3A). This data shows that lysine, by blocking proximal tubular reabsorption of endogenous renin, results in a large increase of urinary renin.
Figure 3. (A) Effect of lysine on urinary mouse renin in control and diabetic mice (B) Human active renin/Cr in non-diabetic and diabetic mice infused with human recombinant renin and lysine.
A: Mouse renin/Cr ratio in urines from control (white) and diabetic (black). Mice were infused with lysine [Lysine (circles); control n=7, diabetic n=8] or did not receive lysine infusion [no infusion (squares); control n=8, diabetic n=15]. In non-infused animals total renin was higher in STZ-treated compared to controls, whereas after lysine infusion no significant differences existed between the two groups. Data shown as mean ± SE. ***p<0.001.
B: In mice infused with both hrRenin and lysine, urinary human active renin/Cr is markedly elevated, but there is no significant difference in STZ treated (closed symbols) vs. non-diabetic mice (Control, open symbols). Human active renin is undetectable in urines from mice infused with lysine only or from mice not infused with hrRenin (not shown, see text). hrRenin: human recombinant renin; Data shown as mean ± SE
In diabetic mice, urinary renin/Cr ratio in mice injected with lysine (n=8) was about 10-fold increased as compared to non-injected mice (n=15) (11372 ± 4126 vs. 1093 ± 319 pg/mg Cr; p<0.001, Fig. 3A). Although the levels of renin were higher in diabetic mice than in controls, the relative renin/Cr increase after lysine injection is actually greater in control mice than in diabetic mice. This suggests a preexisting defect of proximal tubular reabsorption in diabetic mice. In non-injected mice, urinary renin/Cr ratio is significantly increased in diabetic mice compared to control mice (1093 ± 319 vs. 64 ± 19 pg/mg Cr; p<0.001), while in lysine-injected mice, the increase of renin/Cr ratio in diabetic mice compared to control mice no longer is significantly different (11372 ± 4126 vs. 8950 ± 2337 pg/mg Cr; p=0.908, Fig. 3A). Moreover, the effect of lysine administration was not significantly differnet between STZ-treated and control mice when analyzing the interaction between lysine and diabetes status by two-way ANOVA (p=0.4).
Urinary human active renin concentrations after human recombinant renin and lysine infusions to normal mice and STZ-treated mice
Human recombinant renin (hrRenin) was injected to control and STZ-treated mice to examine the renal handling of exogenous renin, distinct from mouse renin. In this model, the renin recovered in the urine provides an estimate of the renal handling of exogenous renin. As expected, human active renin was not detectable in urine samples from non-infused mice and mice infused with lysine only. This shows that the ELISA for human active renin does not recognize mouse renin. Urines of control mice injected with hrRenin (n=12) showed substantial levels of creatinine-normalized human active renin as compared to non-infused control mice in which it was not detectable (n=17) (30±11 vs. 0±0 pg/mg Cr; p<0.001). This demonstrates that human renin can be filtered but does not reveal the extent of how much is reabsorbed. In diabetic mice, injection of hrRenin (n=10) also resulted in an increase in urinary creatinine normalized-human active renin as compared to non-injected diabetic mice (n=15) (779±625 vs. 0±0 pg/mg Cr; p<0.001).
The difference in urinary hrRenin between control and diabetic mice after infusion of hrRenin was large (779±625 vs. 30±11 pg/mg Cr, p=0.032). This reflects either increased filtration, impaired tubular reabsorption or both in diabetic mice.To estimate the effect of tubular reabsorption on urinary renin, lysine was infused together with hrRenin. Urines of control mice injected with a combination of lysine and hrRenin (n=7) had markedly higher urinary creatinine-normalized hrRenin than those of control mice infused with hrRenin only (n=12) (1720±1151 vs. 30±11 pg/mg Cr, p=0.001). In the STZ-treated group, combined injection of lysine and hrRenin (n=9) also resulted in an increase of urinary human active renin compared to injection with hrRenin only (n=10), but it did not reach statistical significance (2418±994 vs. 779±625 pg/mg Cr, p=0.05). There was no apparent interaction between injection of lysine and diabetic status when analyzed by Two-Way ANOVA (p=0.3).
In the STZ-treated group infused with a combination of hrRenin and lysine, urinary human active renin was not significantly different than in controls infused with a combination of hrRenin and lysine (2318±994 vs. 1720±1151 pg/mg Cr, p=0.368, Fig. 3B). Thus, in the presence of lysine induced blockade of proximal tubule reabsorption, the amount of infused human renin that appears in the urine is about the same in diabetic mice as in controls. This suggests the preexistence of a defect in proximal tubular reabsorption of renin in diabetic mice.
Kidney renin immunostaining in STZ-treated mice
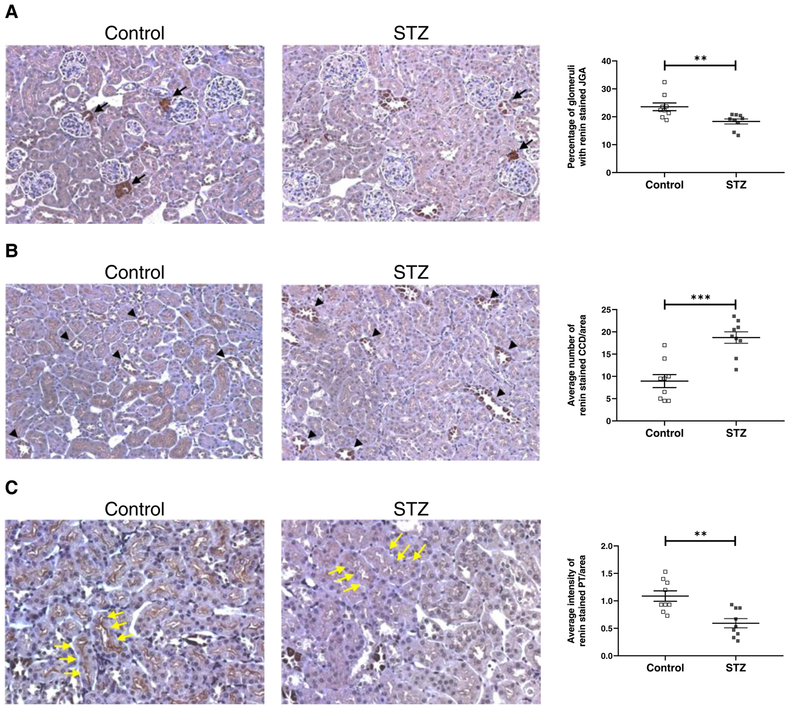
Immunohistochemistry was used to examine renin protein expression in the kidney from control and STZ-treated mice. By immunohistochemistry, the main sites of staining were the juxtaglomerular apparatus (JGA) and kidney collecting tubules. Control animals had strong JGA staining and weak renin staining in collecting tubules. In contrast, in kidneys from STZ treated animals, JGA staining was reduced whereas collecting tubule (CD) staining was increased (Fig. 4A–B). The differences in JGA and CD staining between control and STZ treated mice was statistically significant (p<0.01) based on observations performed by two blinded independent observers. Renin staining was also observed along the brush border of proximal tubules but it appeared to be much weaker than in JGA and in CD. Based on observations performed by two blinded independent observers, proximal tubules renin staining in diabetic mice was significantly less abundant than in control mice (0.59±0.08 vs. 1.09±0.09 average intensity of renin stained PT/area; p=0.0012) (Fig. 4C).
Figure 4. Kidney renin immunostaining in non-diabetic and diabetic FVB mice and semiquantitative analysis of staining in the JGA (A), CCD (B) and PT (C).
A: Immunostainining for renin shows a significant decrease of the percentage of glomeruli with stained JGA (black arrows) in diabetic FVB kidneys (STZ) compared to non-diabetic FVB kidneys (Control). B: Immunostaining for renin shows a significant increase of the average number of stained CCD per area (black tip) in kidneys of diabetic FVB mice compared to non-diabetic FVB mice. C: Immunostaining for renin shows a significant decrease of the average intensity of stained PT per area (yellow arrow) in diabetic FVB kidneys compared to non-diabetic FVB kidneys. STZ: streptozotocin-treated mice; JGA: juxtaglomerular apparatus; CCD: cortical collecting duct; PT: proximal tubule; n=9 in each group;
Kidney renin mRNA expression in kidney cortex and microdissected tubules
Renin/GAPDH mRNA in the kidney cortex, which is largely representative of juxtaglomerular mRNA levels, was significantly lower in diabetic (n=12) as compared to control mice (n=11; 0.78 ± 0.1 vs. 1.39 ± 0.3, p = 0.04, supplementary Table S3). We next investigated whether the decreased renin staining in the PT and increased renin staining in the CD of diabetic mouse kidneys could be a result of different levels of renin mRNA expression in the respective areas. For this, we used microdissected kidney PT and cortical collecting ducts (CCD) from control and diabetic mice. No significant differences were observed in the levels of mouse renin mRNA in either the PT or in the CCD (Supplementary Table S3).
Kidney megalin and mannose-6-phosphate receptor mRNA in experimental DKD
To evaluate receptors that are involved in the reabsorption of renin in the proximal tubule, the mRNA of megalin and mannose-6-phosphate receptor (M6PR) was evaluated. Both receptors are present in the proximal tubule and bind renin and other proteins53. Reverse transcriptase real-time PCR was used to measure megalin and mannose-6-phosphate receptor (M6PR) expression in kidney cortex from control and STZ-treated mice. Mouse megalin mRNA in diabetic mice was detected at a significantly lower level than in control mice in kidney cortex (ratio megalin/GAPDH mRNA, 0.70±0.09 vs. 1.01±0.04, p=0.01, n=6) (Supplementary Fig. S3A). The levels of M6PR mRNA in diabetic mice, however, were not different from control mice in kidney cortex (ratio M6PR/GAPDH mRNA, 1.03±0.06 vs. 0.97±0.07, p=0.573, n=6), (Supplemental Fig. S3B).
Ren1d-Cre;mT/mG with STZ-induced diabetes
A double transgenic mouse model was used to trace renin lineage cells (RLC) in control and diabetic kidneys6. The mT/mG construct switches from membrane-directed fluorescent tomato protein (mT, red) to membrane-directed enhanced green fluorescent protein (eGFP; mG) expression after Cre-mediated recombination. Therefore all RLCs are mG positive, while all non-RLCs remain mT positive6. Ren1d-Cre;mT/mG was made diabetic using STZ and the animals were studied 11–12 weeks after the last STZ injection.
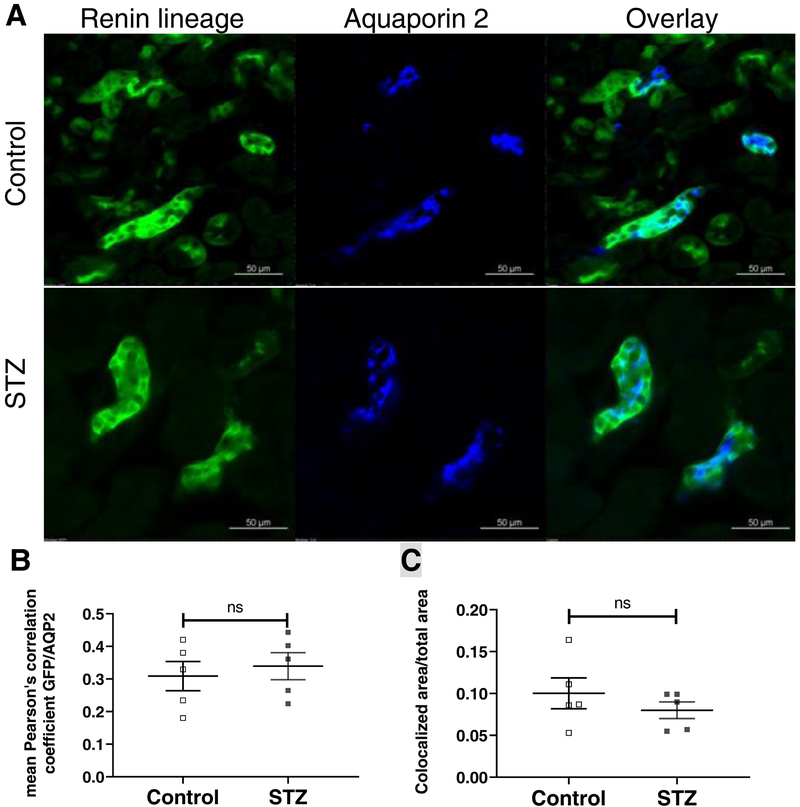
To examine the RLCs in the collecting duct (CD), immunostaining for aquaporin 2 (AQP2) was used as a marker of principal cells. Using confocal laser scanning microscopy, we analyzed the overlay of mG positive RLCs and AQP2 positive CD cells. Colocalization was quantified using Fiji software. We found no significant differences in colocalization at CD sites between kidneys from diabetic (n=5) and control mice (n=5) (Pearson’s correlation coefficient: 0.34±0.04 vs. 0.31±0.04; p=0.632) (Fig. 5). This finding shows that the increased renin staining noted above using IHC (see Fig. 4) can not be attributed to the migration of RLCs to the CD in diabetic mouse kidneys.
Figure 5. Renin lineage and aquaporin 2 expressing cells in non-diabetic and diabetic Ren1d-Cre;mT/mG mouse kidneys (A) and colocalization analysis (B, C).
A: Kidney sections from Ren1d-Cre;mT/mG mice show the presence of GFP+ collecting duct epithelial cells. Immunofluorescence for AQP2 show colocalization with GFP+ collecting duct cells in both diabetic and non-diabetic Ren1d-Cre;mT/mG mice. B: Colocalization analysis of GFP+ cells and immunofluorescence for AQP2 cells using Pearson’s correlation coefficient shows no difference between kidneys of control and diabetic Ren1d-Cre;mT/mG mice. C: A large image analysis of the GFP and AQP2 colocalized area per total area shows no significant difference between kidneys of control and diabetic Ren1d-Cre;mT/mG mice. AQP2: aquaporin 2; STZ: streptozotocin-treated mice; n=5 in each group;
We next evaluated the RLCs in the glomeruli of diabetic and non diabetic renin reporter mice. In both, mG positive cells were found within the Bowman capsule of the glomerulus and to a lesser extent in the intraglomerular compartment namely, mesangial cells (see examples in supplementary Fig. S4A). The quantitation of the frequency mG positive cells revealed that in the parietal epithelium of the glomeruli RLC cells were observed in about 40% of the time while within the intraglomerular mesangium they were found in only 5–10% of the time (Supplementary Fig. S4B). Comparing control and STZ-treated mice in regards to mG positive cells, no significant differences were found in either parietal or intraglomerular staining between both groups (Supplementary Fig. S4B).
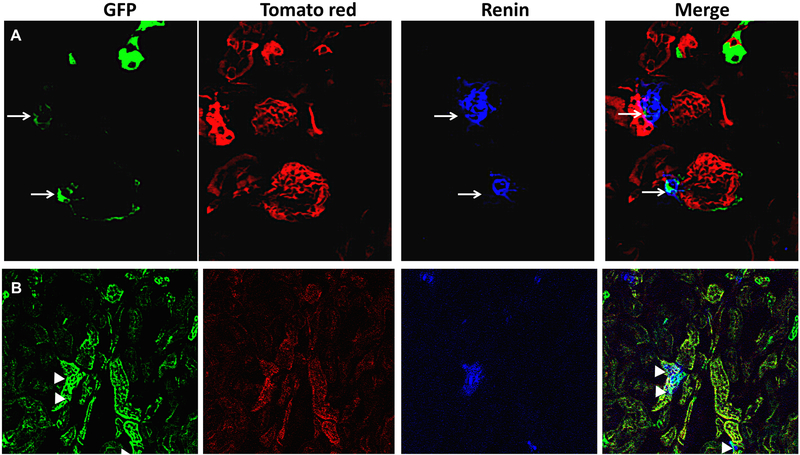
Finally, renin protein staining was performed in renin reporter mice to find out which of the RLCs are associated with renin protein. Positive colocalization of renin with RLC near the vascular pole of the glomerulus was found, reflecting the physiologic site of renin secretion (Fig. 6A). By contrast, only few tubular epithelial structures had a colocalization of renin and RLC (Fig. 6B).
Figure 6. Renin colocalization with renin lineage cells (RLC) near the glomerular pole (A, arrows) and in tubules (B, arrowheads) in kidney sections from renin reporter mice.
Green reflects RLC and tomato red lables the kidney structures. Renin protein staining (blue). The areas of colocalization in turquoise color of renin and renin lineage cells are near the glomerular pole (merged image in panel A, white arrows) and in few areas of tubular epithelium (merged image in panel B, arrowheads).
Discussion
This report shows that in people with type 1 diabetes who have developed DKD, urinary renin is increased as compared to a group who had not developed it after similar disease duration (≥25 years of diagnosis of diabetes). Consistent with the findings in humans with DKD, urinary renin was also markedly increased in STZ-treated diabetic mice, a model of early DKD. The concordant increase in urinary renin in an experimental model of diabetes and in human DKD associated with type 1 diabetes, is in agreement with previous studies where key components of the RAS system including renin, were similarily altered23, 46, 54.
Renin-producing cells in adult life are restricted to the JGA localization whereas in fetal life these cells are also found in large intrarenal arteries, inside the glomeruli and in the interstitium6. As maturation continues, the number of renin-producing cells is reduced as they differentiate into arteriolar smooth muscle and mesangial cells and become progressively restricted to the JGA localization5, 6.In response to a threat to homeostasis, requiring more renin, smooth muscle cells and mesangial cells may acquire the renin phenotype and synthesize renin again until the crisis passes5, 6. For instance, agents that inhibit the response to Ang II (such as the ACE inhibitor captopril) result in an increase in the number of JGA cells that express renin and an accompanying increase in the number (recruitment) of renin expressing cells along the afferent arteriole and sometimes in the glomerulus9. By contrast, ureteral reflux leads to recruitment of renin-expressing mesangial cells and tubular cells55. Despite of its importance as the most frequent cause of CKD, the impact of diabetes on renin lineage cells (RLC) has not been previously studied. To ascertain whether there is an increase in RLC along the nephron and particularly in the collecting duct that could account for increased urinary renin production in STZ-treated mice, we developed a mouse line resulting from the cross of Ren1d-Cre mice with mT/mG reporter mice and used STZ to induce chronic diabetes6. These mice, GFP labels cells (and all their descendants) permanently at the sites where Cre-recombination occurs, enabling tracing of cells of renin lineage, i.e. those cells that either actively express renin or are derived from ureteric bud epithelial cells or Six 2 derived progenitors that expressed renin earlier during development6.
Consistent with previous studies6, we found that in Ren1d-Cre;mT/mG mice the GFP+ cells were present in the renal arterial tree (including JGA cells), collecting duct epithelial cells in the kidney cortex, medulla, and papilla. Immunostaining for aquaporin colocalized with GFP+ collecting duct cells of Ren1d-Cre;mT/mG mice (Fig. 5A). There were, however, no appreciable differences in colocalization pattern or intensity between renin reporter mice with and without diabetes (Fig. 5B). We also observed frequent expression of RLCs in the glomerular parietal epithelium and their occasional expression within the glomerular tuft (Supplementary Fig. S4A). The level of expression of GFP positive cells within either of these glomerular locations was not significantly different between control and STZ-treated mice (Supplementary Fig. S4B). In the aggregate, therefore, data from the renin reporter mice provides no evidence of migration of RLCs to the collecting duct or intraglomerular locations in mice made diabetic by STZ as compared to non-diabetic controls.
The lack of differences in the presence of RLCs at various nephron segments does not inform, however, whether the GFP+ cells at the time after diabetes induction actively produce renin or are dormant. We therefore evaluated renin mRNA expression in microdissected kidney proximal tubules (PT) and cortical collecting ducts (CCD) from control and STZ-treated diabetic mice. No significant differences were noted in the levels of mouse renin mRNA in the PT or in the CCD (Supplementary table S3). This suggests that local renin production at those sites cannot contribute to the markedly increased urinary renin found in STZ-treated mice. In the collecting ducts of STZ-treated diabetic mice, by contrast, the renin immunostaining was increased as previously reported for prorenin19. The possibility that part of the increased urinary renin that we find in diabetes could originate from conversion of prorenin in the collecting tubule, can not be ruled out in our studies. In our study, total renin EIA, that simultaneously detects both active renin and prorenin, yielded 10–100 higher values than the active renin EIA (Fig. 1A and Fig. S1). By simple subtraction of the results of two assays, one could assume that in the final urine the prorenin might predominate. Against this assumption, however, is the evidence presented by Roksnoer et al. who demonstrated that the renin standard included in the total renin EIA assay causes an upward shift resulting in artifcially inflated total renin values56. In addition, studies in humans and rats suggest that urinary renin reflects mostly active renin and not prorenin, since almost no prorenin could be detected in the final urine37, 46.
In the renal cortex renin mRNA was significantly reduced in STZ-treated mice as compared to controls (Supplementary table S3). Most of kidney cortex renin mRNA reflects JGA, the major physiologic site for renin production57. Consistent with this finding, renin staining in the JGA was reduced in STZ-treated diabetic as compared to control mice (Fig. 4). The finding of reduced JGA renin (by immunostaining) and reduced mRNA levels in the renal cortex, alltogether provides, by extrapolation to humans, an explanation for the low levels of plasma renin activity usually seen in patients with diabetic complications26–30.In fact, DKD is the most frequent cause of the syndrome of isolated hyporeninimic hypoaldosteronism58–61. In this syndrome the reduced levels of PRA may be multifactorial but sclerosis of the JGA associated with tubule interstitial fibrosis has been occasionally documented60. Our finding of reduced renin staining in the JGA of diabetic mice is therefore potentially relevant in explaining the known predisposition to the syndrome of hyporenimic hypoaldosteronism, an important cause of hyperkalemia in patients with diabetes58, 59.
Since we found no evidence for migration of RLC to the collecting duct as the potential source of tubular renin the increase in renin staining in collecting ducts observed in this study by us and previously by others19 deserves an alternative explanation. In general, urinary renin can originate from three different sources: from plasma renin that has been fitered and not completely reabsorbed by the proximal tubule, from renin that has been produced and released locally from kidney tubules, or from a conversion of plasma-derived prorenin to renin largely taking place distally in the collecting duct where the prorenin receptor is abundant39, 41–43. Because of the relatively small molecular size, renin is easily filterable and, of course, more in the presence of glomerular pathology due to DKD. Renin staining in the apical part of proximal tubule cells has long been believed to represent filtered and pinocytozed renin62. The proximal tubule is the principal site of uptake of proteins, including renin, a process which appears to be mainly megalin-mediated37, 53. The observed decrease in proximal tubular renin staining (Fig. 4C) suggests that increased urinary renin could be a result of diminished capacity of the proximal tubule to reabsorb renin. Of note, we found in diabetic STZ-treated mice that kidney mRNA expression of megalin, an endocytosis receptor, was decreased (Supplementary Fig. S3A) which is in agreement with previous reports in STZ-treated rats63, 64 and Ove-26 mice65. It has been shown that decreased kidney megalin expression in diabetic rats was associated with reduced reabsorption of albumin63 and increased loss of other urinary proteins, such a transferrin64. Megalin’s major role in proximal tubule renin reabsorption is suggested by studies using megalin knockout53 We therefore surmise that the increase in urinary renin excretion in diabetic mice is due to a reduced kidney expression of megalin causing reduced proximal tubular reabsorption of renin. The lack of differences in renin mRNA between control and diabetic mice at the CCD, noted above, further shows that the source of urinary renin in DKD can not be due to local production at this tubular site.
Our findings in the STZ-model of diabetes are in full agreement with recent work by Danser’s group who have emphasized that normally renin appears in the urine as a result of filtration and only in small amounts because proximal reabsorption retains the majority of the filtered renin37. In a series of elegant experiments using urine samples from patients with Dent disease and Lowe syndrome, entities with impaired proximal tubular reabsorption of low molecular weight proteins, urinary renin was found markedly increased demonstrating the importance of intact tubular processing37. In our studies, urinary albumin was increased in diabetic as compared to control mice likely as the result of increased filtration due to a disturbed filtration barrier in diabetic mice. Likewise, this glomerular alteration can explain increased renin in the urine even if renin secretion from the JGA is reduced. While this undoubtedly contributes to high urinary renin, the finding of reduced expression of megalin in the kidney cortex in diabetic as compared to control mice suggests a likely role of impaired tubular reabsorption as a contributor to high urinary levels of renin in diabetic mice. Our approach to further demonstrate the importance of proximal tubular reabsorption of renin in diabetic mice was based on infusing renin with and without lysine, a blocker of proximal tubular reabsorption. Tubular reabsorption of peptides and proteins can be inhibited by coadministration of cationic amino acids, such as L-Lysine or L-Arginine66, 67. Megalin binds proteins rich in positively charged amino acids and cationic compounds. It is believed that L-Lysine saturate those binding sites and, therefore, interfere with renal reabsorption of proteins. Intravenously infused L-lysine has been previously used to inhibit tubular uptake of renin causing greatly increased urinary renin excretion66. Consistent with previous reports66, 68, we found that L-lysine caused marked increases in urinary excretion of endogenous and exogenous mouse renin. While at baseline, urinary mouse renin was noticeably higher in diabetic than in control mice, after L-lysine injection, the markedly increased urinary mouse renin levels were no longer significantly different statistically between control and diabetic STZ-treated mice (Fig. 3A). Since endogenous mouse renin can originate from plasma or the kidney, we injected i.v. human active renin protein to trace the fate of only plasma renin that has been fitered. For this, human renin protein was infused and detected using an assay which identifies only human active renin and no mouse renin. Injection of human renin alone resulted in a detection of low levels of human active renin in the urine of control mice and a much accentuated urinary levels in diabetic mice. Similar to endogenous mouse renin, co-administration of L-lysine with human renin resulted in marked augmentation in urinary levels of active human renin in both diabetic and non-diabetic mice, such that urinary renin was no longer significanty different (Fig. 3B). The findings of similar levels of both mouse (Fig. 3A) and human renin (Fig. 3B) when proximal tubular reabsorption is blocked by lysine shows that increased urinary renin in diabetes is largely due to impaired proximal tubular reabsorption. It should be noted, however, that L-lysine can increase the GFR, which might contribute to increased levels of renin in the urine69, 70. Decreased expression or “saturation” of megalin receptor in diabetic proximal tubules likely underlies this process. This is further supported by a recent study, reporting a marked increase in urinary renin when the megalin receptor is antagonized54. In DKD, therefore, the source of urinary renin is increased filtered renin, through an abnormal glomerular barrier, which can not efficiently be reabsorbed by the proximal tubule.
In conclusion, in humans with type 1 diabetes and DKD, as well as in mice with STZ-induced diabetes, urinary renin is increased. Our data shows that impaired proximal tubular reabsorption of filtered renin contributes to the increase of urinary renin in DKD likely due to decreased expression of megalin receptor. Studies in the renin renin reporter mice made diabetic by STZ, moreover, provide no evidence for migration of cells of the renin lineage to collecting ducts that could be responsible for renin secretion and thus increased urinary renin in diabetes.
Supplementary Material
Perspectives.
Renin is the key enzyme within the renin-angiotensin system (RAS) that generates Angiotensin I (Ang I) by hydrolyzing its unique substrate, angiotensinogen. As such, activation of the RAS is essentially dependent on circulating levels of renin produced by the juxtaglomerular apparatus. It has been suggested, however, that the kidney collecting ducts may also contribute to renin formation. In patients with diabetic kidney disease (DKD), plasma renin activity is usually decreased, but there is limited information on urinary renin and its origin. In the present study we studied urinary renin from patients with type 1 diabetes and diabetic kidney disease and from mice with STZ-induced diabetes and show that urinary renin is elevated. By immunohistochemistry studies in mice with STZ-induced diabetes it was found that renin staining was reduced in the juxtaglomerular apparatus, but increased in the collecting ducts. Using a renin-reporter mouse model made diabetic by STZ-administration, it is shown that this increase in urinary renin cannot be attributed to production from cells of the renin lineage migrating to the collecting duct in a chronic hyperglycemic environment. The findings obtained by infusing L-lysine to block proximal tubular reabsorption moreover suggest that much of the urinary renin originates from filtered renin that can not be reclaimed by the proximal tubule. Reduced megalin expression in the setting of DKD may account for reduced proximal reabsorption and therefore increase urinary renin. Future studies in patients with DKD should examine if renin staining in the JGA is decreased as demonstrated here in diabetic mice.
Novelty and Significance.
What is new?
Urinary renin is increased in a cohort of patients with type 1 diabetes and chronic kidney disease
Distribution of renin lineage cells were investigated for the first time in a renin reporter mouse model made diabetic.
What is relevant?
This study provides insight on the origin of urinary renin and it’s relevance in the setting of diabetic kidney disease.
This is important because the activation of the renin-angiotensin system within the kidney causes sodium retention, hypertension and progression of chronic kidney disease.
Summary of the conclusions of the study
In humans with DKD and mice with diabetes induced by STZ, urinary renin is increased. The source of this increase is largely attributable to increased filtration and impaired proximal tubular reabsorption. Filtered renin rather than local production of renin from migration of cells of the renin lineage to the renal collecting ducts in a hyperglycemic environment accounts for the bulk of urinary renin in diabetic kidney disease.
Sources of Funding
This work was supported by National Institute of Diabetes and Digestive Kidney Diseases grants R01DK080089 and R01DK104785 as well as by a gift to Northwestern University by the Joseph and Bessie Feinberg Foundation (DB). J.T. had support from the Biomedical Education Program (BMEP).
Footnotes
Disclosures
None
References
- 1.Taugner R, Waldherr R, Hackenthal E. The juxtaglomerular apparatus: Structure and function. Springer; Berlin Heidelberg; 2013. [Google Scholar]
- 2.Schnermann J, Briggs JP. Tubular control of renin synthesis and secretion. Pflugers Archiv : European journal of physiology. 2013;465:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Kim SM, Eisner C, Oppermann M, Huang Y, Mizel D, Li L, Chen M, Sequeira Lopez ML, Weinstein LS, Gomez RA, Schnermann J, Briggs JP. Stimulation of renin secretion by angiotensin ii blockade is gsalpha-dependent. J Am Soc Nephrol. 2010;21:986–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M, Harris MP, Rose D, Smart A, He XR, Kretzler M, Briggs JP, Schnermann J. Renin and renin mrna in proximal tubules of the rat kidney. The Journal of clinical investigation. 1994;94:237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sequeira-Lopez ML, Nagalakshmi VK, Li M, Sigmund CD, Gomez RA. Vascular versus tubular renin: Role in kidney development. Am J Physiol Regul Integr Comp Physiol. 2015;309:R650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starke C, Betz H, Hickmann L, Lachmann P, Neubauer B, Kopp JB, Sequeira-Lopez ML, Gomez RA, Hohenstein B, Todorov VT, Hugo CP. Renin lineage cells repopulate the glomerular mesangium after injury. J Am Soc Nephrol. 2015;26:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Gonzalez AA, McCormack M, Seth DM, Kobori H, Navar LG, Prieto MC. Increased renin excretion is associated with augmented urinary angiotensin ii levels in chronic angiotensin ii-infused hypertensive rats. Am J Physiol Renal Physiol. 2011;301:F1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez ML, Gomez RA. The renin phenotype: Roles and regulation in the kidney. Current opinion in nephrology and hypertension. 2010;19:366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pentz ES, Moyano MA, Thornhill BA, Sequeira Lopez ML, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol. 2004;286:R474–483 [DOI] [PubMed] [Google Scholar]
- 10.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Developmental cell. 2004;6:719–728 [DOI] [PubMed] [Google Scholar]
- 11.Mendez M Renin release: Role of snares. Am J Physiol Regul Integr Comp Physiol. 2014;307:R484–486 [DOI] [PubMed] [Google Scholar]
- 12.Martinez MF, Medrano S, Brown EA, Tufan T, Shang S, Bertoncello N, Guessoum O, Adli M, Belyea BC, Sequeira-Lopez MLS, Gomez RA. Super-enhancers maintain renin-expressing cell identity and memory to preserve multi-system homeostasis. The Journal of clinical investigation. 2018;128:4787–4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowley SD. Atac-ing the mechanisms of renin regulation. The Journal of clinical investigation. 2018;128:4748–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peti-Peterdi J, Kang JJ, Toma I. Activation of the renal renin-angiotensin system in diabetes--new concepts. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:3047–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HS, Maeda N, Oh GT, Fernandez LG, Gomez RA, Smithies O. Homeostasis in mice with genetically decreased angiotensinogen is primarily by an increased number of renin-producing cells. J Biol Chem. 1999;274:14210–14217 [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Regulation of renin in mice with cre recombinase-mediated deletion of g protein gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol. 2007;292:F27–37 [DOI] [PubMed] [Google Scholar]
- 17.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger rna in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. The Journal of clinical investigation. 1990;85:417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson S, Jung FF, Ingelfinger JR. Renal renin-angiotensin system in diabetes: Functional, immunohistochemical, and molecular biological correlations. Am J Physiol. 1993;265:F477–486 [DOI] [PubMed] [Google Scholar]
- 19.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274 [DOI] [PubMed] [Google Scholar]
- 21.Lo C-S, Chang S-Y, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JSD. Heterogeneous nuclear ribonucleoprotein f suppresses angiotensinogen gene expression and attenuates hypertension and kidney injury in diabetic mice. Diabetes. 2012;61:2597–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ubeda M, Matzilevich MM, Atucha NM, Garcia-Estan J, Quesada T, Tang SS, Ingelfinger JR. Renin and angiotensinogen mrna expression in the kidneys of rats subjected to long-term bile duct ligation. Hepatology. 1994;19:1431–1436 [PubMed] [Google Scholar]
- 23.Wysocki J, Goodling A, Burgaya M, Whitlock K, Ruzinski J, Batlle D, Afkarian M. Urine ras components in mice and people with type 1 diabetes and chronic kidney disease. Am J Physiol Renal Physiol. 2017;313:F487–F494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: Implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067–3075 [DOI] [PubMed] [Google Scholar]
- 25.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. Ace and ace2 activity in diabetic mice. Diabetes. 2006;55:2132–2139 [DOI] [PubMed] [Google Scholar]
- 26.Schlueter W, Keilani T, Batlle DC. Tissue renin angiotensin systems: Theoretical implications for the development of hyperkalemia using angiotensin-converting enzyme inhibitors. The American journal of the medical sciences. 1994;307 Suppl 1:S81–86 [PubMed] [Google Scholar]
- 27.Price DA, Porter LE, Gordon M, Fisher ND, De’Oliveira JM, Laffel LM, Passan DR, Williams GH, Hollenberg NK. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol. 1999;10:2382–2391 [DOI] [PubMed] [Google Scholar]
- 28.Christlieb AR, Kaldany A, D’Elia JA. Plasma renin activity and hypertension in diabetes mellitus. Diabetes. 1976;25:969–974 [DOI] [PubMed] [Google Scholar]
- 29.Fisher ND, Price DA, Litchfield WR, Williams GH, Hollenberg NK. Renal response to captopril reflects state of local renin system in healthy humans. Kidney Int. 1999;56:635–641 [DOI] [PubMed] [Google Scholar]
- 30.Miller JA. Renal responses to sodium restriction in patients with early diabetes mellitus. J Am Soc Nephrol. 1997;8:749–755 [DOI] [PubMed] [Google Scholar]
- 31.Burns KD, Lytvyn Y, Mahmud FH, Daneman D, Deda L, Dunger DB, Deanfield J, Dalton RN, Elia Y, Har R, Van JA, Bradley TJ, Slorach C, Hui W, Xiao F, Zimpelmann J, Mertens L, Moineddin R, Reich HN, Sochett E, Scholey JW, Cherney DZ. The relationship between urinary renin-angiotensin system markers, renal function, and blood pressure in adolescents with type 1 diabetes. Am J Physiol Renal Physiol. 2017;312:F335–F342 [DOI] [PubMed] [Google Scholar]
- 32.Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14:274–281 [DOI] [PubMed] [Google Scholar]
- 33.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. The Journal of clinical investigation. 1986;77:1925–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. Journal of Clinical Investigation. 1986;77:1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, Investigators RS. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. The New England journal of medicine. 2001;345:861–869 [DOI] [PubMed] [Google Scholar]
- 36.Taugner R, Hackenthal E, Inagami T, Nobiling R, Poulsen K. Vascular and tubular renin in the kidneys of mice. Histochemistry. 1982;75:473–484 [DOI] [PubMed] [Google Scholar]
- 37.Roksnoer LC, Heijnen BF, Nakano D, Peti-Peterdi J, Walsh SB, Garrelds IM, van Gool JM, Zietse R, Struijker-Boudier HA, Hoorn EJ, Danser AH. On the origin of urinary renin: A translational approach. Hypertension. 2016;67:927–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persson F, Lu X, Rossing P, Garrelds IM, Danser AH, Parving HH. Urinary renin and angiotensinogen in type 2 diabetes: Added value beyond urinary albumin? Journal of hypertension. 2013;31:1646–1652 [DOI] [PubMed] [Google Scholar]
- 39.Roksnoer LC, Verdonk K, van den Meiracker AH, Hoorn EJ, Zietse R, Danser AH. Urinary markers of intrarenal renin-angiotensin system activity in vivo. Current hypertension reports. 2013;15:81–88 [DOI] [PubMed] [Google Scholar]
- 40.Ramkumar N, Stuart D, Mironova E, Bugay V, Wang S, Abraham N, Ichihara A, Stockand JD, Kohan DE. Renal tubular epithelial cell prorenin receptor regulates blood pressure and sodium transport. Am J Physiol Renal Physiol. 2016;311:F186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prieto MC, Botros FT, Kavanagh K, Navar LG. Prorenin receptor in distal nephron segments of 2-kidney, 1-clip goldblatt hypertensive rats. The Ochsner journal. 2013;13:26–32 [PMC free article] [PubMed] [Google Scholar]
- 42.Lu X, Garrelds IM, Wagner CA, Danser AH, Meima ME. (pro)renin receptor is required for prorenin-dependent and -independent regulation of vacuolar h(+)-atpase activity in mdck.C11 collecting duct cells. Am J Physiol Renal Physiol. 2013;305:F417–425 [DOI] [PubMed] [Google Scholar]
- 43.Prieto MC, Reverte V, Mamenko M, Kuczeriszka M, Veiras LC, Rosales CB, McLellan M, Gentile O, Jensen VB, Ichihara A, McDonough AA, Pochynyuk OM, Gonzalez AA. Collecting duct prorenin receptor knockout reduces renal function, increases sodium excretion, and mitigates renal responses in ang ii-induced hypertensive mice. Am J Physiol Renal Physiol. 2017;313:F1243–F1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng K, Lu X, Wang F, Nau A, Chen R, Zhou SF, Yang T. Collecting duct (pro)renin receptor targets enac to mediate angiotensin ii-induced hypertension. Am J Physiol Renal Physiol. 2017;312:F245–F253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin ii-dependent hypertensive rats. Hypertension. 2004;44:223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den Heuvel M, Batenburg WW, Jainandunsing S, Garrelds IM, van Gool JM, Feelders RA, van den Meiracker AH, Danser AH. Urinary renin, but not angiotensinogen or aldosterone, reflects the renal renin-angiotensin-aldosterone system activity and the efficacy of renin-angiotensin-aldosterone system blockade in the kidney. Journal of hypertension. 2011;29:2147–2155 [DOI] [PubMed] [Google Scholar]
- 47.Eng DG, Kaverina NV, Schneider RRS, Freedman BS, Gross KW, Miner JH, Pippin JW, Shankland SJ. Detection of renin lineage cell transdifferentiation to podocytes in the kidney glomerulus with dual lineage tracing. Kidney Int. 2018;93:1240–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thelle K, Christensen EI, Vorum H, Orskov H, Birn H. Characterization of proteinuria and tubular protein uptake in a new model of oral l-lysine administration in rats. Kidney Int. 2006;69:1333–1340 [DOI] [PubMed] [Google Scholar]
- 49.Ye M, Wysocki J, Gonzalez-Pacheco FR, Salem M, Evora K, Garcia-Halpin L, Poglitsch M, Schuster M, Batlle D. Murine recombinant angiotensin-converting enzyme 2: Effect on angiotensin ii-dependent hypertension and distinctive angiotensin-converting enzyme 2 inhibitor characteristics on rodent and human angiotensin-converting enzyme 2. Hypertension. 2012;60:730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castellanos Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez ML, Gomez RA. Transcriptional regulator rbp-j regulates the number and plasticity of renin cells. Physiological genomics. 2011;43:1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Batlle D Clinical and cellular markers of diabetic nephropathy. Kidney Int. 2003;63:2319–2330 [DOI] [PubMed] [Google Scholar]
- 52.Domenig O, Schwager C, van Oyen D, Leitner M, Ziegelmayer C, Fabsich C, Poglitsch M. Abstract p424: Assessment of the raas-status using triple-a-testing: Combining molecular profiling of hypertension with advanced screening for primary aldosteronism in a single blood test. Hypertension. 2017;70:AP424–AP424 [Google Scholar]
- 53.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F. Intrarenal renin angiotensin system revisited: Role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem. 2010;285:41935–41946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye F, Wang Y, Wu C, Howatt DA, Wu CH, Balakrishnan A, Mullick AE, Graham MJ, Danser AHJ, Wang J, Daugherty A, Lu HS. Angiotensinogen and megalin interactions contribute to atherosclerosis-brief report. Arteriosclerosis, thrombosis, and vascular biology. 2019;39:150–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norwood VF, Carey RM, Geary KM, Jose PA, Gomez RA, Chevalier RL. Neonatal ureteral obstruction stimulates recruitment of renin-secreting renal cortical cells. Kidney Int. 1994;45:1333–1339 [DOI] [PubMed] [Google Scholar]
- 56.Roksnoer LC, Verdonk K, Garrelds IM, van Gool JM, Zietse R, Hoorn EJ, Danser AH. Methodologic issues in the measurement of urinary renin. Clinical journal of the American Society of Nephrology : CJASN. 2014;9:1163–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ. Distribution of renin mrna and its protein in the developing kidney. Am J Physiol. 1989;257:F850–858 [DOI] [PubMed] [Google Scholar]
- 58.Perez GO, Lespier L, Jacobi J, Oster JR, Katz FH, Vaamonde CA, Fishman LM. Hyporeninemia and hypoaldosteronism in diabetes mellitus. Archives of internal medicine. 1977;137:852–855 [PubMed] [Google Scholar]
- 59.Batlle DC. Hyperkalemic hyperchloremic metabolic acidosis associated with selective aldosterone deficiency and distal renal tubular acidosis. Seminars in nephrology. 1981;1:260–274 [Google Scholar]
- 60.Schindler AM, Sommers SC. Diabetic sclerosis of the renal juxtaglomerular apparatus. Lab Invest. 1966;15:877–884 [PubMed] [Google Scholar]
- 61.Sebastian A, Schambelan M, Lindenfeld S, Morris RC, Jr. Amelioration of metabolic acidosis with fludrocortisone therapy in hyporeninemic hypoaldosteronism. The New England journal of medicine. 1977;297:576–583 [DOI] [PubMed] [Google Scholar]
- 62.Taugner C, Poulsen K, Hackenthal E, Taugner R. Immunocytochemical localization of renin in mouse kidney. Histochemistry. 1979;62:19–27 [DOI] [PubMed] [Google Scholar]
- 63.Tojo A, Onozato ML, Ha H, Kurihara H, Sakai T, Goto A, Fujita T, Endou H. Reduced albumin reabsorption in the proximal tubule of early-stage diabetic rats. Histochem Cell Biol. 2001;116:269–276 [DOI] [PubMed] [Google Scholar]
- 64.Figueira MF, Castiglione RC, de Lemos Barbosa CM, Ornellas FM, da Silva Feltran G, Morales MM, da Fonseca RN, de Souza-Menezes J. Diabetic rats present higher urinary loss of proteins and lower renal expression of megalin, cubilin, clc-5, and cftr. Physiological reports. 2017;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Day RT, Cavaglieri RC, Feliers D. Apelin retards the progression of diabetic nephropathy. American Journal of Physiology - Renal Physiology. 2013;304:F788–F800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazanti I, Hermann KL, Nielsen AH, Poulsen K. Ultrafiltration of renin in the mouse kidney studied by inhibition of tubular protein reabsorption with lysine. Clin Sci (Lond). 1988;75:331–336 [DOI] [PubMed] [Google Scholar]
- 67.Batlle D, Hays S, Foley R, Chan Y, Arruda JA, Kurtzman NA. Proximal renal tubular acidosis and hypophosphatemia induced by arginine. Adv Exp Med Biol. 1982;151:239–249 [DOI] [PubMed] [Google Scholar]
- 68.Nielsen AH, Hermann KL, Mazanti I, Poulsen K. Urinary excretion of inactive renin during blockade of the renal tubular protein reabsorption with lysine. Journal of hypertension. 1989;7:77–82 [PubMed] [Google Scholar]
- 69.Olsen NV, Hansen JM, Ladefoged SD, Fogh-Andersen N, Nielsen SL, Leyssac PP. Overall renal and tubular function during infusion of amino acids in normal man. Clin Sci (Lond). 1990;78:497–501 [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi H, Yoo TM, Kim IS, Kim MK, Le N, Webber KO, Pastan I, Paik CH, Eckelman WC, Carrasquillo JA. L-lysine effectively blocks renal uptake of 125i- or 99mtc-labeled anti-tac disulfide-stabilized fv fragment. Cancer Res. 1996;56:3788–3795 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.