Abstract
This review integrates scientific knowledge obtained over the past few decades on the biological mechanisms that contribute to the profound association between exposure to early adversity, including childhood trauma and prenatal stress, and the lifelong elevated risk to develop a broad range of diseases. We further discuss insights into gene-environment interactions moderating the association between early adversity and disease manifestation and we discuss the role of epigenetic and other molecular processes in the biological embedding of early adversity. Based on these findings, we propose potential mechanisms that may contribute to the intergenerational transmission of risk related to early adversity from the mother to the fetus. Finally, we argue that basic research knowledge on the biological embedding of early adversity must now be translated into novel intervention strategies that are mechanism-driven and sensitive to developmental timing. Indeed, to date, there are no diagnostic biomarkers of risk or mechanism-informed interventions that we can offer to victims of early adversity in order to efficiently prevent or reverse adverse health outcomes. Such translational efforts can be expected to have significant impact on both clinical practice and the public health system, and will promote precision medicine in pediatrics and across the lifespan.
Keywords: Developmental programming, Early life stress, Prenatal stress, Intergenerational transmission, Targeted intervention
“It is easier to build strong children than to repair broken men.”
Frederick Douglass (1819–1895)
Frederick Douglass’ proclamation of the mid-19th century resonates today in remarkable accordance with insights from modern neuroscience and biomedical research, suggesting that the foundation for health and positive adaptation across the lifespan is laid early in life. During times of developmental plasticity, adverse experiences can cause lifelong “scars” that leave the individual vulnerable to subsequent challenge and at markedly heightened risk to develop a broad spectrum of diseases. We here propose that the same developmental plasticity that enables the lifelong negative effects of early adversity may give rise to the opportunity to intervene early in life in order to prevent or reverse unfavorable outcomes of adversity across the lifespan. By specifically targeting processes of developmental programming, it may even be conceivable to set children on positive trajectories of health and adaptation with equally exponential and lifelong beneficial effects.
Interventions later in life may be less effective in reversing the adverse health outcomes of early adversity and may need to address compensatory mechanisms. Indeed, in adults, consideration of developmental origins of pathology and the corresponding biological phenotypes may be a useful strategy for defining subtypes of disorders and for selecting mechanism-informed treatment strategies.
This review integrates scientific knowledge obtained over the past few decades on the long-term consequences of early adversity, including childhood trauma and prenatal stress. We argue that this basic research knowledge must now be translated into novel intervention strategies that are mechanism-driven and sensitive to developmental timing. Indeed, to date, there are no diagnostic biomarkers of risk or mechanism-driven interventions that we can offer to victims of early adversity in order to efficiently prevent or reverse adverse health outcomes. Such translational efforts can be expected to have significant impact on both clinical practice and the public health system, and will promote precision medicine in pediatrics and across the lifespan. In order to develop such novel approaches, there is a dire need for multilevel investigations of the immediate processes of biological embedding of early adversities in children over time and across systems of regulation. We here discuss our previous findings and describe our integrated current research program which we developed in order to pursue this overarching objective.
This paper is part of a Festschrift in honor of Dirk Hellhammer who, as scientific mentor to each of us, vastly inspired and continuously nurtured our research interests. In the 1990′s, substantial impetus for the development of our research interest arose from observations in studies at the University of Trier suggesting that individuals with various stress-related psychosomatic disorders, including nurses with burnout and women with chronic pelvic pain, exhibited decreased, and not increased, cortisol secretion under basal conditions (Fries et al., 2005; Heim et al., 1998, 2000a; Hellhammer, 1990). At the time, we posited that a relative lack of the regulatory effects of glucocorticoids during stress promotes disinhibition of central stress circuits as well as pain and pro-inflammatory mediators, resulting in a characteristic constellation of symptoms, including fatigue, pain, and stress sensitivity (Heim et al., 2000a). In search of explanations for the origins of this so called hypocortisolism, we considered earlier results from animal models by Seymour Levine and others, suggesting that brief maternal separations and early handling stress in the postnatal period can induce a hypoactive stress-response system in rodents (Levine, 1967). We further considered emerging seminal results by Rachel Yehuda and colleagues reporting low cortisol states in Vietnam veterans with posttraumatic stress disorder (Yehuda, 2000). In consequence, we studied trauma exposure in women with chronic pelvic pain and found that these women reported significantly elevated rates of sexual and physical abuse, including childhood abuse (Heim et al., 1998). In subsequent years, the research group further focused on emerging evidence that prenatal stress exposures can have equally potent and long-term effects on the brain and stress-regulatory systems as well as cellular aging, resulting in disease risk (Buss et al., 2012d; Entringer et al., 2010, 2011b).
Dirk Hellhammer tremendously nourished our academic curiosity and skills as well as our personal and career development, and we are grateful for his outstanding mentorship. Each of us continued research on the developmental programming of disease in the United States (Entringer et al., 2015; Heim and Nemeroff, 2001). We now combine our expertise in an integrated research program that we implemented at Charité Universitätsmedizin Berlin, which we present as part of this Festschrift. Notably, several aspects of our research program strongly support Dirk Hellhammer’s seminal proposition that the definition of “conceptual endophenotypes” may be useful in designing mechanism-based treatments for patients with stress-related disorders (Hellhammer et al., 2018)
1. Early-life stress and disease risk
An unacceptably high number of children in our society experiences adversity while growing up.2 Early-life stress (ELS) encompasses exposure to various forms of severe stressors in childhood, including maltreatment, abuse, violence, neglect, and separation or loss of a parent among other forms of ELS. It is estimated that 2 in 10 girls and boys experience some form of abuse during childhood (Wildeman et al., 2014). When other forms of ELS are considered, this number rises to nearly one in two children affected.3 In a retrospective population-based study in Germany, about one third of adult respondents reported some form of child maltreatment (Hauser et al., 2011), paralleling estimates of the prevalence of child maltreatment in the United States (e.g., Briere and Elliott, 2003; Edwards et al., 2003). A significant proportion of children are victims of more than one form of ELS.
Substantial evidence from epidemiological and clinical studies suggests that exposure to ELS not only strongly and robustly increases the risk for several of the major psychiatric disorders, including depression, anxiety, and substance abuse disorders, but also induces lifelong risk for chronic physical disease outcomes, including cardiovascular disease, obesity, diabetes, lung cancer, chronic pain, headaches, and immune-related diseases, resulting in reduced longevity (e.g., Felitti et al., 1998; Norman et al., 2012; Shonkoff et al., 2012). There are dose-response relationships between the severity of ELS and the risk for later disease, while the association between types of ELS and disease outcomes appears to be nonspecific, perhaps because ELS types often co-occur. ELS-related disorders often occur in comorbidity and manifest or aggravate in response to acute stress, and individuals with ELS appear to have a decreased threshold for developing symptoms in relation to acute stressors in adulthood (Hammen et al., 2000). This would suggest that ELS induces a core dysfunction or biological “scars” at the level of stress-regulatory systems that promote the pathophysiology of various disorders.
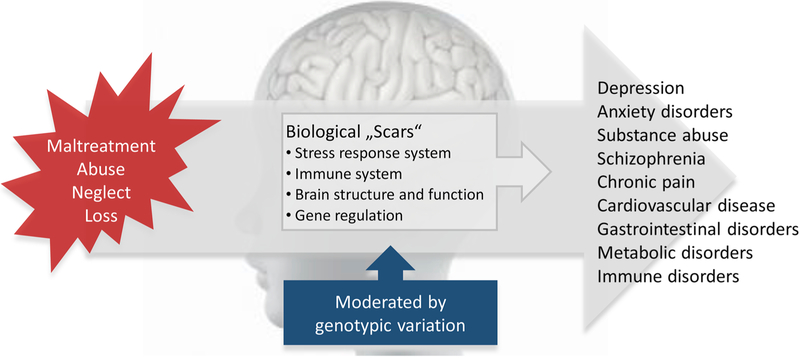
Indeed, the developing brain and its adaptation systems are shaped by experience, and adversity during sensitive periods of developmental plasticity can lead to profound and persistent changes in regulatory systems, including endocrine, autonomic, immune alterations as well as neurostructural and neurofunctional changes, that leave these individuals at lifelong risk to develop a wide range of diseases and adverse developmental outcomes (reviewed in, e.g., Anacker et al., 2014; Lupien et al., 2009; Nemeroff, 2016; see Fig. 1). Of note, the manifestation of symptoms or disease after ELS is moderated by allele variations in stress-regulatory genes and such gene-environment interactions are mediated in part by allelic and environmentally driven epigenetic programming of gene expression, resulting in the manifestation of ELS-related physiological and neural endophenotypes that underlie disease processes (Halldorsdottir and Binder, 2017; Provencal and Binder, 2015).
Fig. 1. Early-Life Stress, Biological Consequences, and Risk for Disease.
Exposure to ELS robustly increases the lifelong risk for psychiatric disorders and chronic physical diseases. A core dysfunction at the neural, stress-physiological, immune and molecular levels promotes the pathophysiology of these disorders. The manifestation of symptoms or disease after ELS is moderated by allele variations in stress-regulatory genes and such gene-environment interactions are mediated in part by allelic and environmentally driven epigenetic programming of gene expression.
The profound impact of ELS on lifelong disease risk is one of the most critical and costly public health problems of our time. Indeed, the CDC estimates a $124 billion aggregate lifetime economic burden incurred by victims of child maltreatment alone in the US (Fang et al., 2012), surpassing the combined economic costs of all other major pediatric health problems, including autism, asthma, pediatric cancers, exposure to environmental toxins, and obesity. Recent evidence suggests that ELS-associated risk can be transmitted into the next generation, thus multiplying the number of affected individuals and public health burden (Buss et al., 2017). Therefore, a precise understanding of the mechanisms of developmental programming of disease risk by ELS as well the moderation of such risk is a critical challenge for current research that will enable the development of biomarkers of risk, resilience, and treatment susceptibility as well as precise targets for novel mechanism-based interventions that mitigate the adverse outcomes of ELS.
2. Long-term biological and neural consequences of childhood trauma
The precise mechanisms that mediate the detrimental and persistent impact of ELS on long-term health and adaptation have been subject to intense inquiry over decades. Advances from neuroscience have provided compelling insights into the enormous plasticity of the developing brain as a function of experience. E.g., visual sensory input early in life is required for normal development of the visual cortex and perception, and disruptive experiences during such critical periods of plasticity lead to lifelong and sometimes irreversible damage (Weiss and Wagner, 1998). The same principle may be applied to early traumatic experiences during critical periods that may permanently impact on the development of brain regions that are implicated in the regulation of emotion and stress responses (Lupien et al., 2009). Enduring effects of ELS on the brain and its regulatory outflow systems, i.e. the autonomic, endocrine and immune systems, may then lead to the development of a vulnerable phenotype with increased sensitivity to stress and risk for a range of psychiatric and somatic disorders.
It is important to note that developmental programming indeed refers to the concept of sensitive periods of plasticity, as opposed to cumulative stress effects or allostatic load. It could be argued that ELS is associated with long-term impact on disease risk simply because stressors start earlier in life and hence accumulate to a greater overall stress load in these individuals as they age, resulting in heightened disease burden. The concept of developmental programming, in the opposite, assumes that a vulnerability is laid early in life, during periods when the brain and physiological systems are malleable, and that this vulnerability persists into adulthood and permanently shifts responsiveness to the environment beyond genetic factors. While some of the adaptations to ELS may indeed be protective, particularly as the child is growing up, these changes may form the basis for maladaptation and disease later in life.
Studies in animal models have provided the direct and causal evidence that ELS, such as prolonged maternal separation or naturally occurring low maternal care, leads to structural, functional and epigenetic changes in a connected network of brain regions that is implicated in neuroendocrine control, autonomic regulation, vigilance, emotional regulation, and fear conditioning. These neural changes converge into increased physiological and behavioral responses to subsequent stress (reviewed, e.g., in Anacker et al., 2014; Heim and Nemeroff, 2001). These effects are present across species and in different models of adversity, with the unifying element across studies being the timing of the stressor early in life.
Accumulating evidence suggests that these preclinical findings can be translated to humans: We demonstrated that women with a history of childhood sexual or physical abuse exhibit markedly increased pituitary-adrenal and autonomic responses to psychosocial laboratory stress, and this effect was most pronounced in those abused women who suffered from current depression (Heim et al., 2000b). We subsequently reported alterations at multiple levels of stress regulation, including reduced adrenal capacity (Heim et al., 2001), relative glucocorticoid resistance as identified with the dexamethasone/corticotropin-releasing hormone (CRH) challenge test (Heim et al., 2008a), and increased inflammatory responses (Pace et al., 2006). At the central nervous system (CNS) level, we measured increased cerebrospinal fluid (CSF) concentrations of the stress-mediating neuropeptide CRH (Heim et al., 2008b) and decreased CSF concentrations of the prosocial and stress-buffering neuropeptide oxytocin (Heim et al., 2009b). These changes conceivably converge into increased stress vulnerability. Similar hypersensitivity to stress after ELS was reported by other groups (Rao et al., 2008), although some studies report hyposensitivity in healthy individuals after ELS (Carpenter et al., 2007, 2011). Notably, systemic inflammation is one of the most replicated correlates of ELS in humans (Baumeister et al., 2016; Danese et al., 2008, 2007). In addition, ELS is associated with metabolic dysregulation and faster deposition of body fat across adolescence (Danese and McEwen, 2012; Noll et al., 2007).
Brain regions that are implicated in the processing and regulation of stress responses and emotion mature during early childhood, and there are differences in developmental trajectories between regions (Lupien et al., 2009; Teicher et al., 2016). During these times of plasticity, ELS may guide the development of these brain regions through experience-dependent plasticity. In addition, elevations of cortisol or inflammatory cytokines that occur as a function of ELS may exert neurotoxic effects on these structures during development and across the lifespan. The hippocampus has been the subject of intense inquiry in relation to ELS. The hippocampus exerts important inhibitory regulation of hypothalamic CRH neurons and plays a critical role in contextual aspects of fear conditioning. Moreover, the hippocampus is one of the most plastic regions within the CNS, with a high degree of synaptic plasticity and neurogenesis occurring throughout life. The hippocampus has a high density of glucocorticoid receptors (GR) and is therefore particularly vulnerable to damaging effects of stress. Several studies of adults have now repeatedly demonstrated small hippocampal volumes after ELS (Frodl et al., 2010; Stein et al., 1997; Vythilingam et al., 2002). Using high-resolution imaging methods to investigate region-specific effects of ELS, Teicher et al. (2012) reported that childhood maltreatment was strongly associated with a volume reduction in the CA3 region, the dentate gyrus, and the left subiculum. However, findings in maltreated children are inconsistent and unaltered hippocampal volume has been reported (De Bellis et al., 2002). This may suggest that hippocampal atrophy develops over time, perhaps in result of repeated glucocorticoid overexposure. Hence, in the Teicher et al. (2012) study, volume loss as a function of ELS occurred precisely in the subfields that are sensitive to stress and glucocorticoids.
Other neuroimaging studies suggest structural and/or functional changes in cortical-limbic circuits as a function of ELS. The prefrontal cortex (PFC) mediates executive functions, regulates goal-directed behavior and is involved in the inhibition of impulses and emotional regulation. In particular, the medial PFC is relevant for emotional regulation, via connections with the cingulate cortex and the amygdala. A decreased volume of PFC areas, including the medial PFC and anterior cingulate cortex, is a consistent finding in adults with histories of ELS (e.g., Tomoda et al., 2009; Treadway et al., 2009; van Harmelen et al., 2010). Volume reductions in frontal cortex have also been reported for maltreated children (de Brito et al, 2013; Hanson et al., 2010). In addition, ELS has been associated with structural and functional changes of the amygdala. The amygdala plays a critical role in evaluating potentially threatening information, fear conditioning, emotional processing, and memory for emotional events. Prolonged institutional rearing characterized by severe deprivation has been associated with greater amygdala volume in these children (Tottenham et al., 2010), although findings are inconsistent (see meta-analysis in Calem et al., 2017). Functional neuroimaging studies indicate that ELS is associated with sustained hyperactivity of the amygdala in response to emotionally threatening stimuli (Dannlowski et al., 2012; Grant et al., 2011, 2014; Tottenham et al., 2011). The PFC exerts inhibitory and the amygdala exerts stimulating effects on hypothalamic CRH neurons via indirect projections (Ulrich-Lai and Herman, 2009) and, hence, the constellation of ELS-related neural alterations appears to promote stress responses. In addition, decreased structural and functional connectivity between the medial PFC and the amygdala has been reported after ELS, and this was predicted by cortisol levels at the age of 4.5 years in a prospective study (Burghy et al., 2012). These findings together may reflect a loss of “top down” control of emotional responses, fear learning, and stress responses, which may converge into heightened disease risk.
Several studies suggest an impact of ELS on sensory representation areas implicated in the perception of the abusive experience. Using whole mantle cortical thickness analysis in adults, we observed pronounced cortical thinning of the somatosensory genital field as a function of childhood sexual abuse. Emotional abuse was specifically associated with cortical thinning in the precuneus, a region that is relevant for self-awareness and self-evaluation, as well as thinning in the anterior cingulate cortex, which is relevant for emotional regulation (Heim et al., 2013). These findings suggest that experience-dependent plasticity leads to effects of ELS on sensory processing areas in a highly region-specific manner. This specific cortical thinning in sensory processing areas may represent the most adaptive and protective response of the developing brain that may “shield” the child living under these conditions from the abusive experience, similar to sensory gating. In later life, these neuroplastic changes may represent a direct biological substrate for behavioral disorders, such as sexual dysfunction. Of note, Teicher and colleagues report similar findings for other sensory modalities, including thinning of the visual cortex after witnessing domestic violence and thinning of the auditory cortex after verbal abuse (see Teicher et al., 2016).
Of note, many of the neuroendocrine, immune and neural changes occurred as a function of ELS in depressed samples and were not present in depressed patients without ELS exposure (e.g., Danese et al., 2008; Grant et al., 2014; Heim et al., 2000b, b; Vythilingam et al., 2002). Conversely, in several of these studies, changes were present in patients with ELS, even in the absence of psychopathology, which might reflect a vulnerability that did not result in a clinical manifestation. These observations led us to suggest that some of the classic features of depression, as described in the literature, may indeed be secondary to ELS and reflect risk to develop depression in response to stress, whereas depression in the absence of ELS might have a differential pathophysiology that does not involve changes in stress regulatory systems (Heim et al., 2004). Such biological subtypes of disease within the same diagnostic category may also be responsive to differential treatments. Indeed, we found that patients with chronic depression were differentially responsive to different types of treatment as a function of exposure to ELS. Patients who had developed chronic depression in relation to ELS were more likely to remit in response to psychotherapy4, whereas chronically depressed patients without ELS showed better remission rates in response to pharamacological treatment or the combination of pharmacological treatment and psychotherapy (Nemeroff et al., 2003). This suggests the existence of differential treatment responder types. More specifically, patients who developed depression in response to stress may also have the capacity for plastic change in response to a positive therapeutic environment. This might also generalize to other disorders that may be differentially responsive to treatment strategies depending on ELS. Such context sensitivity may favor adverse health outcomes under destructive conditions, but may promote favorable outcomes under nurturing or therapeutic conditions, whereas individuals without pronounced con-text sensitivity may be moderately functioning under all conditions. Such differential susceptibility to the environment may be influenced by genes that are implicated in plasticity and learning (Ellis and Boyce, 2011; Pluess and Belsky, 2013).
These findings give rise to the question as to whether or not there are sensitive periods during human childhood where the brain is particularly sensitive to the environment, including the effects of ELS. Little is known to date about such circumscribed time windows for effects of ELS. Teicher and colleagues have conducted statistical analyses to identify sensitive periods for the effects of ELS on brain regional development. They report that the amygdala is particularly sensitive to the effects of abuse at the age of around 10 years, whereas the hippocampus has heightened sensitivity at an earlier age and the prefrontal cortex seems to be particularly amenable around puberty (see Teicher et al., 2016).Whether or not such temporally differential effects of ELS on brain regions are associated with specific symptom constellations remains poorly understood. Of note, rodent models of maternal separation or naturally occurring low care typically focus on the first 2 weeks of life, which developmentally corresponds to fetal life in humans. Hence, it is conceivable that prenatal stress has profound impact on adult health and adaptation in humans, as discussed in the next paragraph.
3. Prenatal stress: disease risk and mechanisms
As discussed above, the long-term consequences of exposure to excess stress, particularly during sensitive developmental windows, on the initiation and progression of diseases are well established. In addition to early postnatal developmental periods, development during intrauterine life represents among the most sensitive of these windows, at which time the effects of stress may be transmitted intergenerationally from a mother to her as-yet-unborn child. As explicated by the concept of fetal programming of health and disease susceptibility, a growing body of evidence supports the notion that disease susceptibility is in part determined by the dynamic interplay between genetic makeup and environment during intrauterine life (Gluckman and Hanson, 2004). Accumulating evidence suggests that maternal psychosocial stress and emotional state during pregnancy represent conditions that adversely affect the developing fetus, including increased risk for a diverse range of physical and mental health outcomes (Entringer et al., 2010, 2015). How is the information about the mother’s emotional state during pregnancy transferred to the fetus? There are no direct vascular or neural connections between the maternal and fetal compartments; all exchange and communication is mediated by biological processes that interface with the placenta. The developing fetus acquires and incorporates information about the nature of its environment in part via the same biological systems that in an already-developed individual mediate adaptation and central and peripheral responses to endogenous and exogenous stress (i.e., the maternal-placental-fetal neuroendocrine, immune, metabolic and oxidative-stress-related systems (Entringer et al., 2015). Thus, these stress-related biological processes appear to play a role as key sensors, transducers and effectors of maternal stress on the developing fetus. In line with this thinking, growing evidence including findings from our own studies (reviewed below) converge to suggest that stress-related maternal-placental-fetal biological perturbations during pregnancy may serve as key physiological pathways mediating the effects of intrauterine perturbations on fetal development and long-term disease risk.
Given that one of the principles of fetal programming is that organs undergoing rapid developmental changes are especially vulnerable to organizing and disorganizing influences of environmental conditions, the brain is a prominent target for such influences because particularly during fetal development growth and differentiation of major brain structures occur. Since brain development represents a cascade of bidirectional interactions with its environment, even small or subtle alterations in brain structure or function during fetal life can become progressively and substantially magnified over time to produce longlasting or permanent deficits (Buss et al., 2012c).
There is substantial empirical evidence from epidemiological studies suggesting that exposure to excess stress in intrauterine life has the potential to adversely impact short- and long-term neurodevelopmental outcomes. Endocrine and immune stress mediators play a critical, obligatory role in neuronal and glial cell migration, differentiation, synaptic maturation, and many other important aspects of brain development, inappropriate levels of these biological mediators can produce detrimental effects on the developing brain (Buss et al., 2012e). Our studies were among the first to characterize alterations in offspring brain anatomy and connectivity in association with maternal stress exposure during pregnancy. Our initial findings were based on a prospective longitudinal cohort of mother-child dyads, for which maternal stress was characterized during pregnancy and child’s neurodevelopmental status was assessed at 7 years age (Buss et al., 2010; Sandman et al., 2015). We found a link between maternal emotional state during pregnancy and changes in offspring brain morphology. Children born to mothers who experienced high levels of pregnancy anxiety had region-specific reductions in gray matter volume (Buss et al., 2010). Furthermore, pregnancy anxiety was associated with impaired executive function in a larger subset of the same cohort of children (Buss et al., 2011), which is interesting in light of the gray matter reductions in prefrontal cortical regions in association with pregnancy anxiety (Buss et al., 2010). In another study, we found evidence for maternal depressive symptoms during pregnancy to be associated with cortical changes in her child at 7 years of age that, in part, mediated the association between maternal depressive symptoms and child externalizing behavior (Sandman et al., 2015). Since cortisol has been suggested as one important mediator of the effects of maternal emotional state (Entringer et al., 2011a), we tested the association between maternal elevated cortisol concentrations during pregnancy and her child’s limbic brain anatomy. Higher maternal cortisol concentration in early pregnancy was associated with larger amygdala volumes in girls, which mediated, in part, the association between high maternal cortisol concentrations and affective symptoms (Buss et al., 2012a). Furthermore, maternal cortisol concentrations during pregnancy were associated with altered neural connectivity and internalizing problems in 7-yr old girls (Kim et al., 2017). Interestingly, we have also shown that moderately elevated maternal cortisol concentrations in late pregnancy are associated with greater child cortical thickness primarily in frontal regions and enhanced child cognitive performance (Davis et al., 2017). These findings point to the importance of considering the moderating role of sex and timing of exposure during pregnancy. In addition to variation in endogenous maternal cortisol concentrations, exogenous glucocorticoid administration during pregnancy which is the clinical standard to promote fetal lung maturation in case of preterm labor, also seems to be associated with brain development later in life, even among children born at term (after 37 weeks gestation, Davis et al., 2013). In these studies, child neurodevelopment was assessed in middle childhood, thus, influences of the postnatal environment on the rapidly changing brain after birth cannot be ruled out. Also, our (Buss et al., 2007) and other findings (Bergman et al., 2010) have suggested that prenatal risk conditions can be modified by the quality of the postnatal environment. Therefore, in our next set of prospective longitudinal studies we adopted a research design that allowed distinguishing the effects of the preversus the postnatal environment by characterizing maternal stress and stress biology during pregnancy and obtaining a first MRI scan in the child shortly after birth, when postnatal influences cannot yet have affected its development and serial follow-up assessments of neurodevelopmental trajectories and important aspects of the postnatal environment. This design allows testing the independent as well as interactive effects of pre- and postnatal conditions.
The first set of findings from this cohort has focused on variation in maternal interleukin 6 (IL-6) concentrations and newborn brain integrity. The pro-inflammatory cytokine interleukin-6 (IL-6) likely constitutes a key biological mediator by acting as a sensor, transducer, and effector of environmental conditions (including maternal stress) on the developing fetal brain (Entringer et al., 2015). While IL-6 plays a requisite role in fetal brain development, it is evident that inappropriately elevated levels may produce perturbations in cellular survival, proliferation and differentiation, axonal growth and synaptogenesis (Buss et al., 2012e). Our findings support a link between maternal systemic inflammation during pregnancy, indexed by IL-6 concentrations, and offspring amygdala volume and functional connectivity (Graham et al., 2018) as well as structural connectivity of the amygdala (i.e., uncinate fasciculus maturity, Rasmussen et al., 2018) already at the time of birth. We recently showed that higher maternal IL-6 concentrations not only are associated with amygdala connectivity but also predict within and between connectivity of functional networks involved in both basic sensory processing and higher order cognition (Rudolph et al., 2018). Importantly, these newborn neurophenotypes that are altered in the context of maternal systemic inflammation predict delayed cognitive development as well as impairments in executive functions in infancy, a phenotype that underlies many emotional and behavioral problems (Graham et al., 2016, 2018; Rasmussen et al., 2018). Our findings are in concordance with animal studies as well as in vitro studies that have demonstrated that a pro-inflammatory milieu can alter specific aspects of brain anatomy and connectivity that result from excessive exposure of the fetal brain to pro-inflammatory cytokines (e.g., Bilbo and Schwarz, 2009). In the context of maternal inflammation-effects on fetal brain development, we have furthermore shown that chorioamnionitis, inflammation of the fetal membranes due to a bacterial infection, is associated with widespread cortical and subcortical changes in children born prematurely (Hatfield et al., 2011) and that maternal preconecptual obesity, a highly prevalent and particularly potent condition that produces an increased inflammatory milieu during gestation, is associated with subsequent child ADHD symptoms at about 7 years age (Buss et al., 2012b). Several other independent research groups have in recent years provided evidence for maternal stress during pregnancy predicting newborn (Posner et al., 2016; Qiu et al., 2017; Rifkin-Graboi et al., 2015; Scheinost et al., 2016), child (El Marroun et al., 2016; Lebel et al., 2016; Soe et al., 2018; van der Knaap et al., 2018), and adult (Mareckova et al., 2018) brain outcomes with behavioral implications suggesting increased risk for mental health disorders in the context of intrauterine stress exposure being mediated by early alterations in brain developmental trajectories.
Accumulating evidence suggests that maternal psychosocial stress and emotional state during pregnancy not only adversely affect fetal brain development and risk for psychiatric disorders, but may also impact risk for a diverse range of physical and somatic health outcomes (Entringer et al., 2012a, 2010). As a first step to addressing this question, we conducted a retrospective case-control study in a sample of healthy young adults born to mothers with healthy pregnancies and normal birth outcomes. One half of the study population of young adults was born to mothers who had experienced a major stressful life event during the index pregnancy (prenatal stress group; PS), whereas the other half was a sociodemographically-matched population with no history of maternal exposure to prenatal stress (comparison group; CG). As study outcomes, we focused on pre-disease markers of physiological dysregulation of the metabolic, endocrine and immune system as early predictors of disease susceptibility. Our results indicated that the young adults exposed during intrauterine life to maternal psychosocial stress consistently exhibited significant dysregulation in key physiological parameters, thereby placing them at increased risk for developing complex common disorders. Specifically, individuals in the PS group exhibited higher BMI and percent body fat, primary insulin resistance, and a lipid profile consistent with the metabolic syndrome (Entringer et al., 2008b); altered immune function with a TH2 shift in the TH1/TH2 balance (consistent with increased risk of asthma and autoimmune disorders (Entringer et al., 2008a); altered endocrine function, with increased ACTH and reduced cortisol levels during pharmacological and psychological stimulation paradigms; accelerated cellular aging (as indexed by shortened leukocyte telomere length (Entringer et al., 2011a,b)); and impairments in working memory performance after hydrocortisone administration (Entringer et al., 2009). We recently published the first study in humans showing a prospective association between maternal cortisol concentrations during pregnancy with infant adiposity. Maternal cortisol production particularly during the third trimester of pregnancy was associated with a greater change in infant percent body fat (%BF) from 1 to 6 months assessed with Dual-energy X-ray absorptiometry (DXA) imaging. Our findings related to prenatal exposure to stress and glucocorticoids on body composition are consistent with reports in large, national cohort samples linking pre-pregnancy and prenatal stress exposure (e.g., maternal bereavement) to risk of offspring overweight, obesity and risk for type-2 diabetes (Hohwu et al., 2014; Li et al., 2018; Virk et al., 2012), and with findings of animal models of stress induction in pregnant females that have found that their offspring were heavier and exhibited greater adiposity (Mueller and Bale, 2006; Paternain et al., 2012a, b; Tamashiro et al., 2009). Taken together, our findings and that of other groups suggest that in utero exposure to prenatal psychosocial stress may confer in- creased long-term risk of a range of negative health-related outcomes in humans that are independent from those of other established obstetric and childhood risk factors, including sociodemographic status, presence of medical complications during pregnancy, adverse birth outcomes and maternal and infant nutritional status.
4. Genetic moderation, epigenetic programming, and telomere biology
Interindividual differences in genetic makeup may account for the fact that individuals differ in the degree to which they are amenable to the modulating role of environmental conditions (Belsky, 2016; Belsky and Hartman, 2014; Ellis et al., 2011). To date there are many examples for genetic susceptibilities to pre- and postnatal stress exposure. The first empirical evidence in support of gene environment (G × E) interactions came from studies focusing on candidate genes (i.e. a single nucleotide polymorphism [SNP] in a specific gene). Among such candidate genes that have been studied, the serotonin transporter gene (5-HTTLPR) has been studied extensively, for which carriers of the short allele have a higher risk to develop depression after exposure to stressful life events, including maltreatment in childhood (Caspi et al., 2003; Karg et al., 2011). Similarly, there is evidence for prenatal stress exposure (e.g. maternal anxiety or depression during pregnancy) to interact with child 5-HTTLPR genotype to predict offspring emotional problems (Tiemeier et al., 2012), behavioral dysregulation (Babineau et al., 2015) and negative emotionality (Green et al., 2017). Besides the significant interest in the 5-HTTLPR gene, other candidate gene studies focusing on the consequences of ELS have studied variants in genes related to the stress hormone system and brain plasticity (Abbott et al., 2018; Heim and Binder, 2012). Polymorphisms in the GR (Bet et al., 2009) and the corticotrophin receptor 1 (CRHR1, Bradley et al., 2008; Heim et al., 2009a) genes for example interact with ELS to predict adult depressive symptoms. Genetic variants of FKBP5, a co-chaperone of the GR, have received significant attention because the polymorphisms interacting with ELS to predict psychiatric disorders have been shown to disrupt an intracellular feedback loop between the GR and FKBP5 that leads to GR resistance and disturbed negative feedback of the stress hormone system (Binder, 2009; Binder et al., 2008; Klengel et al., 2013). In fact, it seems like all polymorphisms in genes regulating the HPA axis (CRHR1, FKBP5 and the GR itself) that confer risk for depression in the light of ELS are associated with GR resistance or enhanced stress hormone system activity. Furthermore, gene by (pre- as well as postnatal) environment (G × E) interactions on risk for neurodevelopmental and affective disorders have been reported for variants of the oxytocin receptor gene (Bradley et al., 2011; Rijlaarsdam et al., 2017), the dopamine receptor gene (Hawi et al., 2015; Hayden et al., 2010), the brain derived neurotrophic factor (BDNF) gene (O’Donnell et al., 2014) as well as the catechol-O-methyltransferase (COMT) gene (O’Donnell et al., 2017), among others. There have been inconsistencies in the observed effects of single SNPs and their interactive effects with the environment. Heterogeneity of results have been specifically addressed in meta-analyses on the 5-HTTLPR because not all studies that focused on this gene variant were able to consistently show a higher risk of mental health problems in short allele carriers exposed to early life stress (for an overview see Sharpley et al., 2014). Indeed, it is unlikely that one SNP, by itself, substantially increases risk for disease but more reasonable that its influence depends on other genetic variants an individual carries. After initial studies with candidate gene approaches, in the past years the field has therefore moved towards studying polygenic risk scores and genome-wide hypotheses-free studies (for a review of the implied challenges and opportunities see Bogdan et al., 2017; Halldorsdottir and Binder, 2017).
The field of epigenetics allows insight into how ELS can have a long-term impact on gene activity without changing DNA sequence (Szyf, 2009). Allele-specific moderation of experience-dependent alteration of epigenetic marks, such as DNA methylation or histone modification may represent the molecular mechanism underlying observed G × E interactions. A number of studies in animals as well as humans have now shown that ELS can leave persistent epigenetic marks in the genome, which alter gene expression and can influence neurobiological substrates until adulthood (McGowan et al., 2009, 2011; Teh et al., 2014). Of these epigenetic modifications, DNA methylation has been the most studied in relationship to ELS. It involves the methylation of cytosines in cytosine-guanine (CpG) dinucleotides, which can reduce access of transcription factors to regulatory elements resulting in transcriptional repression by decreasing the binding of specific transcriptional enhancers (Murgatroyd et al., 2010). Hyper- as well as de-methylation of specific regulatory sites in key genes for stress processing, like the GR, has been shown in association with prenatal (Braithwaite et al., 2015; Oberlander et al., 2008; Radtke et al., 2011) as well as postnatal (McGowan et al., 2009; Weaver et al., 2004) stress experience. In human postmortem brain tissue, a history of child abuse exposure was associated with increased DNA methylation of the GR promoter (NR3C1) as well as reduced levels of GRmRNA. Similar to the findings in rats, this methylation pattern led to a decrease of NGFI-A transcription factor binding (McGowan et al., 2009; Weaver et al., 2004). Interestingly, prenatal exposure to depressed/anxious maternal mood during pregnancy was associated with increased methylation of NR3C1 in cord blood, which in turn was associated with increased salivary cortisol stress responses at 3 months (Oberlander et al., 2008).
Changes in DNA methylation of the FKBP5 gene in the context of ELS have also been under investigation. It has been sown that childhood trauma is associated with alterations in DNA demethylation in functional glucocorticoid response elements of FKBP5 and that this association is moderated by a functional polymorphism of the FKBP5 gene, resulting in an increased risk for developing stress-related psychiatric disorders in adulthood (Klengel et al., 2013). The association between postnatal adverse experience and DNA demethylation of the FKBP5 gene has been replicated in several studies in children and adults (Harms et al., 2017; Non et al., 2016; Parade et al., 2017; Tyrka et al., 2015). To the best of our knowledge only one study has examined the association between prenatal stress and offspring DNA methylation of the FKBP5 gene (Monk et al., 2016). Perceived stress was found to be associated with elevated methylation of CpG sites in the FKBP5 gene in placental tissue. The direction of this effect was unexpected but DNA methylation was examined in placental tissue and consequences for offspring stress regulation were not studied. Overall, empirical evidence demonstrating epigenetic alterations in the context of pre-and postnatal stress exposure provide first insight into the molecular underpinnings of the long-term physiological changes that result from ELS exposure.
As recently reviewed elsewhere (Entringer et al., 2018), the observation that adverse intrauterine and early postnatal conditions simultaneously influence a diverse set of disease risk-related phenotypes, raises the possibility that ELS may exert effects via some common underlying mechanisms (i.e., that are common across different cells, tissues and phenotypes). In this context, we have proposed that telomere biology represents a candidate mechanism of particular interest (Entringer et al., 2012c, 2018). Telomeres, and the activity of the enzyme telomerase, play a central and very fundamental role in maintaining the integrity of the genome and cell. Telomeres are non-coding tandem DNA repeats at the ends of chromosomes that form a protective cap (Blackburn, 2005). Telomeres lose base pairs with each cell division, due in part to incomplete chromosomal end replication (Blackburn, 2005). Eventually telomeres reach a critical short length leading to cellular senescence or apoptosis (Stewart and Weinberg, 2006). Senescent cells produce inflammatory mediators that also affect neighboring cells, leading to further damage within organs and tissues that accumulates over the life course. Thus, as individuals age, they acquire more senescent cells, accompanied by various age-related pathologies (e.g., arteriosclerosis). This is how a reduction of telomere length (TL) and a steeper telomere attrition rate not only relates to longevity, but also to earlier onset and more rapid progression of common chronic diseases.
A substantial body of research has established that shortened telomeres and reduced telomerase expression are linked to several diseases including cardiovascular disease, diabetes, obesity, psychiatric diseases like depression, dementia, and schizophrenia (Armanios and Blackburn, 2012; Haycock et al., 2014; Ma et al., 2011; Zhao et al., 2013) and earlier mortality (Cawthon et al., 2003; Kimura et al., 2008; Rode et al., 2015). Telomere length, at any given age, is a joint function of the initial (early life) setting of TL and the magnitude of TL attrition over time (Entringer et al., 2012b, 2018). It appears that the initial setting and regulation of telomere homeostasis may be plastic and receptive to the influence of conditions during intrauterine or early postnatal life. We have advanced the hypothesis that context- and time-inappropriate exposures to physiological stress mediators during the conceptional, embryonic, fetal, and early postnatal periods of development may alter or program the telomere biology system in a manner that accelerates cellular dysfunction, aging, and disease susceptibility over the lifespan (Entringer et al., 2012b, 2015; Entringer et al., 2018). We have furthermore proposed that the same stress-related biological processes that mediate the effects of a range of unfavorable conditions on telomere biology during adult life may also impact programming of the telomere system during early development (Entringer et al., 2018). These stress-related oxidative, immune endocrine, and metabolic processes may exert stable, long-term effects via epigenetic and other processes on the developing telomere biology system (Entringer et al., 2012b; Wadhwa et al., 2009).
We published the first human study that established an effect of maternal prenatal psychosocial stress (assessed retrospectively) on adult offspring’s TL (Entringer et al., 2011b) and found a significant association with shorter leukocyte TL in young adult offspring (Entringer et al., 2011b). We more recently replicated this finding prospectively with newborn TL (Entringer et al., 2013). Other recent animal studies have found that prenatal administration of the stress hormone cortisol or prenatal exposure to maternal infection produced shorter offspring TL, thus providing the first experimental evidence of a ‘programming’ effect of prenatal stress biology on the telomere system (Asghar et al., 2015; Haussmann et al., 2011).
The influence of early postnatal adverse conditions on the characteristics of the telomere biology system has been described in a growing number of studies (Blaze et al., 2015; Coimbra et al., 2017; Ridout et al., 2015, 2017). For example, several human studies have found that exposure to adverse experiences in infancy and childhood such as abuse and maltreatment, exposure to violence, family disruption, and institutionalized care is associated with child TL or TL attrition rate (Chen et al., 2014; Drury et al., 2014, 2012; Kananen et al., 2010; Kiecolt-Glaser et al., 2011; Mason et al., 2015; O’Donovan et al., 2011; Shalev et al., 2012; Surtees et al., 2011; Tyrka et al., 2010) and PBMC resting telomerase activity (Chen et al., 2014). In animals, induction of stress during the early postnatal period (handling and cortisol exposure (Herborn et al., 2014) and maternal separation in a rhesus monkey model (Schneper et al., 2016)) has been shown to produce higher age-related decline of TL during early life (Herborn et al., 2014) and shorter TL in adult life (Schneper et al., 2016).
It is important to note that the effects of prenatal and postnatal states and conditions on telomere biology may not be mutually exclusive, and that in many instances the effects of postnatal exposures may, in part, be conditioned upon the effects of prenatal exposures. Furthermore, it is very likely that the effects of prenatal conditions may be moderated or compensated by positive experiences in postnatal life such as high maternal and paternal sensitivity and secure attachment patterns.
Taken together, the process of developmental programming of the telomere system may represent an important avenue by which stressful experiences are biologically embedded to influence the health and well-being of individuals and their offspring across the entire life span.
5. Intergenerational transmission
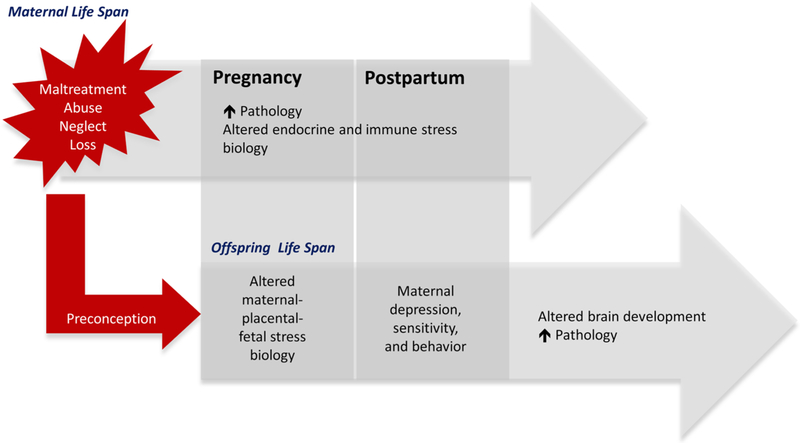
Emerging evidence now suggests that among women, the ELS-associated consequences for mental and physical health outcomes may not be restricted to only their own life span, but also may be transmitted to their children. Several studies have reported that children of mothers with a history of ELS exposure exhibit a higher prevalence of adverse birth outcomes (Smith et al., 2016), neurodevelopmental and behavioral problems (Bouvette-Turcot et al., 2015; Collishaw et al., 2007; Miranda et al., 2013; Myhre et al., 2014; Plant et al., 2013; Racine et al., 2018; Rijlaarsdam et al., 2014), autism (Roberts et al., 2013), obesity (Roberts et al., 2014) and poorer general health (Flory et al., 2011). The majority of the scientific literature on the intergenerational transmission of the effects of ELS has postulated that the mechanism is environmental in nature, and that offspring exposure after birth to suboptimal maternal care (often resulting from maternal ELS-associated depression) represents the primary transmission pathway (Collishaw et al., 2007; Lang et al., 2010; Plant et al., 2013; Rijlaarsdam et al., 2014; Thompson, 2007). While we agree that this scenario likely represents an important pathway, we have suggested that the intergenerational transmission of the effects of maternal ELS exposure may start even earlier, during the highly sensitive period of gestation and fetal development (see Fig. 2, Buss et al., 2017). Our recently published findings showing that maternal ELS predicts offspring brain volume, measured shortly after birth when postnatal influences cannot yet have exerted an effect, support the notion that maternal ELS gets transmitted to her offspring during intrauterine life (Moog et al., 2017a).
Fig. 2. Intergenerational Transmission of the Effects of Early-Life Stress.
The transmission of maternal ELS to her child may occur during the sensitive prenatal and early postnatal periods of her offspring’s development. Maternal exposure to ELS can affect her long-term physical and mental health and these pathological conditions will likely endure when the mother becomes pregnant and after delivery. As a result, maternal ELS may be associated with an altered maternal-placental-fetal stress biology, which has the potential to affect the developing fetal brain. Maternal ELS experience may furthermore increase the risk of developing postpartum depression and parenting difficulties, which additionally impose a risk on the development of her offspring’s brain and increase vulnerability for adverse health outcomes.
Broadly there are three possibilities how an embryo/fetus can obtain information about past events and conditions going back to her or his mother’s childhood (Buss et al., 2017): 1) through epigenetic transmission, 2) through transmission via ELS-associated alterations in her oocyte cytoplasm and 3) through transmission via ELS-associated changes in gestational biology.
Firstly, epigenetic transmission may occur if maternal ELS-related epigenetic alterations in her germ line (oocytes) survive the re-establishment of postconceptional epigenetic marks (Monk et al., 1987; Smith et al., 2012). Animal data support the possibility of inter-and trans-generational transmission of ELS-induced behavioral traits mediated by epigenetic modifications through the paternal germ line (Franklin et al., 2010; Gapp et al., 2014; Pang et al., 2017; Rodgers et al., 2015; Zhang et al., 2018). However, to date, we are not aware of any such evidence for heritable ELS-associated epigenetic marks mediated by the maternal germ line but we have discussed the theoretical plausibility of such transmission (Buss et al., 2017). There is also the possibility for de novo methylation during fetal development that may result from an unfavorable intrauterine environment (Bale, 2015; Bock et al., 2015; Palma-Gudiel et al., 2015; Teh et al., 2014). Thus, even in the instance when mothers and offspring share the same or similar epigenetic alterations (that, for example, are associated in the mother with ELS exposure), it is very challenging to identify the origins of these alterations in the offspring because prenatally de novo obtained epigenetic alterations cannot easily be differentiated from those inherited through the germ line.
Secondly, maternal ELS may produce alterations in her oocyte cytoplasm (such as mitochondria, proteins and RNA molecules). The accumulation of proteins and metabolites in the cytoplasm of maternal oocytes in response to physiological stress and other conditions may also directly influence embryonic and fetal development (Kovalchuk, 2012). While it is plausible that ELS-associated biological alterations may affect the integrity of the oocyte cytoplasm, this, to our knowledge, has not yet been systematically studied.
And thirdly, the mode of transmission may be mediated via gestational biology. As reviewed above biological stress exposure during intrauterine life can result in long-term “programming” consequences by producing changes in developing organs altering their integrity and function in later life and, as also reviewed above ELS is known to produce long-term alterations in endocrine and immune/inflammatory physiology. Recent evidence suggests that these biological alterations persist when the woman becomes pregnant. For example, pregnant women with a history of childhood sexual abuse exhibit a significantly higher cortisol awakening response compared to pregnant women without a history of sexual abuse (Bublitz et al., 2014; Bublitz and Stroud, 2012, 2013). Furthermore, women exposed to physical and/or sexual abuse have elevated hair cortisol concentrations during pregnancy (Schreier et al., 2015). We published the first study that established an association between a woman’s ELS exposure and increased placental corticotrophin-releasing hormone concentrations (CRH, a hormone directly implicated in key developmental processes in the fetal brain and other peripheral systems, Moog et al., 2016). Also, we have recently shown that women with ELS exposure have a higher risk for subclinical hypothyroidism during pregnancy (Moog et al., 2017b), which represents a risk factor for neurodevelopmental disorders in the offspring (Moog et al., 2015). Pregnant women with a history of ELS also are more likely to develop conditions in pregnancy such as depression (Knuesel et al., 2014; Lang et al., 2010; Plant et al., 2013), sleep disturbances (Gelaye et al., 2015), and certain obstetric complications (Cammack et al., 2011; Roberts et al., 2013) that, in turn, are associated with altered maternal-placental-fetal (MPF) endocrine and immune/inflammatory stress biology (Entringer et al., 2010). Such ELS-associated alterations in MPF biology may affect fetal development and predispose offspring for mental and physical health disorders (Entringer et al., 2015).
In addition to and in interaction with the prenatal environment, the quality of the child’s postnatal environment may mediate the inter-generational transmission of the effects of maternal ELS exposure. While a nurturing postnatal environment may exert beneficial effects on the developing brain and even partially mitigate the deleterious effects of a suboptimal intrauterine environment (Buss et al., 2007), it appears that the suboptimal intrauterine environment of children of ELS-ex-posed mothers is likely to be followed by an unfavorable postnatal environment characterized by higher levels of maternal depression (Rijlaarsdam et al., 2014; Thompson, 2007; Wosu et al., 2015), suboptimal parenting (Collishaw et al., 2007; Lang et al., 2010; Plant et al., 2013; Rijlaarsdam et al., 2014; Thompson, 2007) and by abuse experience (Collishaw et al., 2007; Plant et al., 2013), all of which constitute risk factors for child neurodevelopmental and psychiatric (Heim and Binder, 2012; Lupien et al., 2009) as well as somatic disorders (Hughes et al., 2017; Suglia et al., 2018).
The ELS-associated intrauterine alterations discussed above may adversely impact those maternal and child characteristics that determine the quality of the postnatal mother-child relationship, i.e., maternal sensitivity and newborn temperament. Non-human primate (Winslow et al., 2003) as well as human studies (Heim et al., 2009b) have provided empirical evidence for ELS-related oxytocinergic dysregulation, which may mediate the observed ELS-associated suboptimal parenting behavior (Collishaw et al., 2007; Lang et al., 2010; Plant et al., 2013; Rijlaarsdam et al., 2014; Thompson, 2007). Intriguingly, oxytocinergic adaptations in preparation for motherhood get initiated during pregnancy itself and may be altered by ELS-associated gestational changes with important consequences for maternal sensitivity in the postnatal period (Toepfer et al., 2017). Infant temperament, a well-established contributor to the quality of the postnatal mother-child relationship has also been shown to be shaped by the intrauterine environment (Davis et al., 2007), suggesting that ELS-associated alterations of the intrauterine environment may underlie observations between maternal ELS exposure and offspring difficult temperament (Bouvette-Turcot et al., 2015), which, may further elicit suboptimal maternal parenting behavior. In summary, the quality of the postnatal mother-child relationship – a major determinant of healthy child development – may, in part, be conditioned upon the quality of the intrauterine environment via its effects on maternal parenting behavior and on infant temperament.
Furthermore, based on the empirical evidence from animal models demonstrating an effect of maternal stress on breast milk composition (e.g., cortisol concentrations (Hinde et al., 2015)) as well as on the composition and diversity of the microbiome (Jasarevic et al., 2015), there is the possibility for direct postnatal biological transmission from a mother to her child via breast feeding (contents of breast milk) and/or the exchange of microbiota (Pembrey et al., 2014).
As highlighted in a recent review paper (Haussmann and Heidinger, 2015), effects of parental stress exposure on the offspring could also be passed on through alterations in offspring telomere length through the same mechanisms as discussed above – either a) directly by affecting germline telomere length prior to fertilization thereby influencing the telomeres that the offspring inherits, or b) indirectly through effects of parental stress exposure on telomere shortening in offspring tissues through increases in maternally derived biological stress mediators during intrauterine life, or c) through alterations in parental behavior or care, which then affects offspring stress regulation and thereby induces changes in telomere biology (Haussmann and Heidinger, 2015).
6. Knowledge gaps
Many of the findings on the biological correlates of prenatal and childhood stress exposure and their link to the manifestation of disease derive from cross-sectional, short-term, or adult retrospective study designs, severely limiting scientific credibility and causal inferences. The Institute of Medicine released a status report regarding research on the effects of child abuse and neglect and concluded that this research has suffered from fundamental scientific flaws (http://iom.nationalacademies.org/Reports/2013/New-Directions-in-Child-Abuse-and-Neglect-Research.aspx). These flaws include small sample sizes, failure to confirm maltreatment in exposed samples and presumable contamination of comparison samples due to undisclosed maltreatment. Moreover, the report states that there is a lack of longitudinal assessments starting at the time of ELS exposure, which is necessary to infer developmental trajectories of change and to test mediating mechanisms. Most studies fail to examine sex differences and there is inability to test the interplay between environmental, biological and behavioral factors over time that drive the deleterious developmental and health outcomes. Such lack of knowledge contributes to the apparent lack of translation of basic research knowledge into novel guidelines and strategies for clinical care and hinder the ability of the field to derive specific targets for intervention.
Of note, no published study to date has attempted to prospectively map trajectories of biological embedding across multiple levels of regulatory systems in order to understand of risk and resilience for negative mental and physical health outcomes following exposure to a wide range of prenatal and childhood stress exposure types and different exposure ages, and across generations. In particular, the most obvious current gaps of knowledge are:
Longitudinal data on the effects of prenatal and childhood stress exposure on physical health outcomes remains fragmentary;
For any outcome, it remains poorly understood why some individuals are more vulnerable whereas others remain resilient;
n humans, little is known regarding the biological and molecular mechanisms that translate prenatal and childhood stress exposure into disease manifestation. Elucidating these mechanisms will serve to develop biomarkers of risk as well as precise targets of direct mechanism-driven intervention;
Knowledge about biological mechanisms involved in the inter-generational effects of maternal ELS on offspring mental and physical health in humans is still sparse;
No study has attempted to counteract or reverse biological embedding trajectories of prenatal and childhood stress exposure in human children and there is a paucity of data on the reversibility of bio-logical correlates of ELS in later life.
We therefore claim that we must produce rigorous scientific evidence regarding the precise mechanisms of developmental programming that underlie the profound and lifelong adverse health outcomes of ELS, and their transmission into the next generation, in order to be able to derive novel strategies that take advantage of developmental plasticity and translate knowledge on biological mechanisms into early and targeted interventions. Interventions that prevent, compensate or reverse the biological mechanisms may induce equally profound and lifelong beneficial effects in children, thereby programming healthy and successful life trajectories.
7. Current research needs
As noted above, cross-sectional studies do not allow for identification of developmental trajectories of biological embedding of ELS over time nor do they inform about sensitive periods for the effects of ELS. While a handful of longitudinal studies exist, i.e. the E-Risk studies (http://www.scopic.ac.uk/StudiesERisk.html) and the Dunedin (http://dunedinstudy.otago.ac.nz), these were started decades ago and thus were not able to collect cutting-edge neurobiological, neural, molecular or epigenetic data in the direct aftermath of ELS exposure and across development. Most available studies, cross-sectional or longitudinal, did not adopt a multisystem approach. Thus, there is a dire gap in research ascertaining causality and considering complex interactions of multiple systems across levels of regulation. Because of these shortages, there is an even greater gap of translation between basic research in-sights into the biological and molecular mechanisms mediating the link between ELS and long-term disease risk and the use of this knowledge to develop novel diagnostic markers to identify cases at risk and cases susceptible to a specific intervention (personalized or precision medicine), or to develop novel interventions that directly target specific mechanisms of biological embedding.
There is no doubt that prenatal and childhood stress exposure induces fundamental changes in regulatory systems at an early point in development, which programs these systems for life and into the next generation. To achieve critical translation, we now must 1) map the mechanistic and temporal events of biological embedding and transmission of ELS, 2) understand interactions of risk and resilience factors with embedding and transmission trajectories, 3) test the potential of existing interventions to prevent or reverse these biological embedding trajectories, 4) and develop novel pharmacologically-driven interventions that directly manipulate the biological mechanism. Such studies should also combine laboratory research with ecologically momentary assessments in the actual environment to increase validity. To date, no study has attempted to address these gaps in longitudinal cohorts of children with prenatal and childhood stress exposure.
At Charité Universitätsmedizin Berlin, we have built a comprehensive and integrated research program that addresses the current knowledge gaps and research needs. At the core of our research program is the implementation of longitudinal studies designed to precisely understand the immediate early processes of biological embedding of ELS that give rise to long-term biological “scars”, leading to lifelong disease risk. We further aim to mitigate or reverse such biological embedding in proof-of-principle studies.
As part of our ongoing German Ministry of Research and Education-funded program, we have implemented a cohort of 3–5 year old children with verified maltreatment exposure as identified by Child Protection Services and other child welfare institutions in Berlin over the past 4 years. These children underwent clinical, neuroendocrine, immune, neural, and molecular-genetic assessments in short-term intervals (BMBF 01KR1301 to CH). With this cohort, we aim to elucidate immediate process of biological embedding of maltreatment in children over time and link such processes to the manifestation of symptoms. We further aim to uncover factors moderating this ELS-related risk. In addition, with funding from the European research Council, cohorts of pregnant women with and without ELS exposures in their own childhood are followed throughout pregnancy and their newborns are assessed in terms of brain development and metabolic and molecular profiles at birth (ERC Grants to CB and SE). We now were able to continue and extend these cohorts over the next 4 years with new funding from the German Ministry of Research and Education (01GL1743). In this large project, we study four cohorts of children over time, including the aforementioned maltreated children and controls and newborn children of mothers with ELS-exposure in their childhood, as well as a cohort of refugee children with war-and refuge-related trauma. With these cohorts, we aim to further study immediate processes of biological embedding of ELS, compare these processes across different types of ELS and populations, and elucidate preversus postnatal mechanisms of intergenerational transmission of ELS. We further include a cohort of children who manifest a clinical phenotype that has been related to ELS in adults, i.e. clinical obesity, and we study the contribution of ELS to the pathophysiology of obesity in these children. In maltreated and refugee children as well as in obese children with identified ELS exposure, we implement trauma-focused interventions to test the potential of such interventions to reverse or prevent embedding mechanisms through strengthening “top-down” control of regulatory systems. In collaboration with Drs. Binder and Schmidt at the Max Planck Institute of Psychiatry, we consider epigenetic processes and gene-environment interactions across these cohorts. We further test for causality as well as novel “bottom-up” interventions that directly target the mechanisms of biological embedding of ELS in animal models, by blocking specific gene products that are critical for the pathophysiology of ELS-related disorders, i.e. the FKBP51 protein (Gaali et al., 2015).
8. Implications for intervention
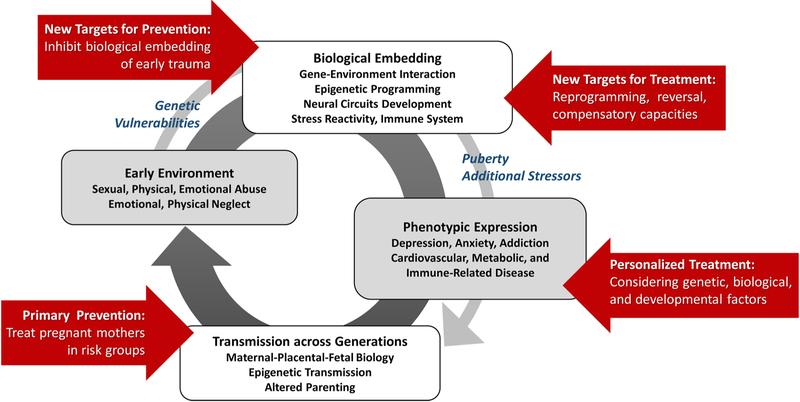
Taken together, ELS is a profound and nonspecific risk factor for a wide range of diseases. Exposure to ELS likely leads to immediate processes of biological embedding. This biological embedding of ELS may involve glucocorticoid-induced epigenetic modifications in stress-regulatory genes, with subsequent dysregulation of stress response systems, metabolic dysregulation, and inflammation, ultimately leading to structural and functional changes in brain regions critical for stress, emotional, and energy homeostatic regulation, as well as accelerated cellular ageing. These biological embedding trajectories will eventually lead to manifestation of clinical phenotypes, including adverse mental and physical health outcomes. Additional stressors or developmental processes during puberty may accentuate risk. Genetic and social-protective factors, such as social support, may moderate outcomes. Such factors may act in developmentally sensitive time windows. The phenotypic manifestation of the biological embedding of ELS may be transmitted into the next generation, perhaps already in the fetal phase, forming a vicious cycle of maltreatment and adverse health outcomes (see Fig. 3).
Fig. 3. A Vicious Cycle of Biological Embedding of Early-Life Stress, Disease Manifestation, and Transgernerational Transmission: Deriving Specific Targets for Intervention.
Exposure to ELS likely leads to immediate processes of biological embedding. This biological embedding of ELS with subsequent dysregulation of stress response systems, metabolic dysregulation, and inflammation, may lead to structural and functional changes in brain regions critical for stress, emotional, and energy homeostatic regulation, as well as accelerated cellular ageing. These biological embedding trajectories will eventually lead to manifestation of clinical phenotypes, including adverse mental and physical health outcomes. Additional stressors or developmental processes during puberty may accentuate risk. Genetic and social-protective factors, such as social support, may moderate outcomes. The phenotypic manifestation of the biological embedding of ELS may be transmitted into the next generation, perhaps already in the fetal phase, forming a vicious cycle of maltreatment and adverse health outcomes. This model may provide a framework for developing and testing novel intervention strategies that target different stages of this vicious cycle.
Research results to date provide an interesting, yet fragmentary framework for developing and testing novel intervention strategies that target different stages of this vicious cycle (see Fig. 3):
1) Our research results suggesting biological subtypes of depression as a function of ELS that are differentially responsive to drug or psychotherapy may inform individual treatment decisions for adult patients. It has long been argued that there is a need for empirically derived predictors of treatment response in psychiatry and that algorithms for differential treatment decision should be developed on the bases of biomarkers, genetic factors, and symptom constellations, leading towards precision medicine in psychiatry or personalized or stratified interventions (Perna et al., 2018). Our results would suggest that developmental factors should be included in such algorithms in order to improve their prognostic validity (Heim et al., 2004).
2) However, it might be even more efficient to intervene earlier in the vicious cycle and try to counteract, reverse or compensate biological “scars” of ELS that ultimately lead to clinical manifestations over time. Here the idea is to treat the mechanism instead of the symptoms. Systematic research is needed to evaluate such approaches of counteraction, reversibility, or compensation. Our above described research program starts to address these needs. We believe that psychotherapeutic interventions may induce “top down” compensatory regulations that eventually help to normalize neural and physiological regulatory systems. Targeted “bottom up” approaches, such as the use of FKBP51 blockers (currently in animal models), represent an attempt of a direct counteraction of the biological embedding effects of ELS (Gaali et al., 2015). Seminal work by Takao Hensch and colleagues would propose that a precise understanding of the molecular mechanisms that determine sensitive periods of brain development may enable the development of entirely novel treatment approaches that remove such “brakes” of plasticity in later life and re-open a sensitive period in order to reverse the programming effects of ELS (see Bavelier et al., 2010).
3) Ideally, interventions should even be given at an earlier time in the vicious cycle in an attempt to prevent those biological effects of ELS that are part of pathophysiological pathways. Here the challenge will be to decide which biological changes are in fact adaptations that may help the system to overcome exposure to ELS and which processes are detrimental. The dimension of timing must further be considered, as certain responses might at first be protective but lead to pathology over time. One example for the preventability of the effects of ELS comes from a study by Brenhouse and Anderson (2011) suggesting that administration of anti-inflammatory agents can indeed prevent the effects of maternal separation on neurochemistry and-function in rodent models.
4) Last but not least, the earliest time-point of intervention may be during pregnancy in an attempt to interrupt intergenerational transmission of ELS-related risk. As noted above, ELS might result in altered MPF physiology that may lead to suboptimal brain development of the fetus, and this system could be a target for intervention. In addition, behavioral interventions could involve stress and trauma coping as well as parenting training for pregnant mothers who have experienced ELS in order to minimize effects on the fetus or offspring.
In sum, we propose that in adult patients we should consider bio-logical phenotypes that arise from ELS exposure, in combination with genetic risk factors, in order to select optimal and mechanism-based treatment strategies. This notion is well in line with an important conceptual model suggested by Dirk Hellhammer and colleagues positing that novel treatment techniques should be derived from so-called “conceptual endophenoytpes” that are informed by theory on the evolutionary purpose and function of specific neurochemical systems and by scientific and clinical observations. The aim is to directly target the individual imbalance or dysregulation of these systems in a given patient and thereby improve treatment success (Hellhammer et al., 2018). One or more ELS-related conceptual endophenotypes may be conceivable in this model. This resonates with the proposition of Martin Teicher termed “ecophenotpyes”, which occur within and across diagnostic entities in psychiatry as a function of adversity in the early environment and that might respond to specific treatments (see Teicher and Samson, 2013).
In addition to these promising approaches, we argue that we must take advantage of the early developmental plasticity (that gives rise to the long-term detrimental outcomes of ELS) and intervene early in life in order to induce equally long-term and exponential beneficial effects in children. Targeted strategies should be developed that mitigate the long-term adverse outcomes of ELS in affected children and hopefully “program” healthy and successful life trajectories in these children. Beyond that, research results will inform novel approaches that make use of developmental plasticity in order to promote optimal development, health, and longevity in all children, with significant impact on the public health system and society.
Acknowledgements
Our current work is funded by the Federal Ministry of Education and Research01KR1301 (CH, CB), 01GL1743 (CH, CB, SE), by U.S. Public Health Service (National Institutes of Health) grants P50 HD-551411 (CH), R01 AG-050455 (SE), R01 HD-060628 (SE), R01 HD-065825 (SE), and R01 MH-105538 (CB), 5UG3OD023349 (CB), by European Research Council ERC 678073 (SE) and ERC 639766 (CB), and by the German Research Foundation DFG EN 851/2-1 (SE) and EXC257 (CH). Previous work covered in this review was funded by NIH MH 58922.
Footnotes
Declarations of interest: none.
There is no uniform definition of early-life stress, adversity or trauma. For the purposes of this paper, we use the term “early-life stress” where appropriate and broadly define the term as any severe stressor that occurs up to the age of 18 years.
Cognitive Behavioral Analysis System of Psychotherapy
References
- Abbott PW, Gumusoglu SB, Bittle J, Beversdorf DQ, Stevens HE, 2018. Prenatal stress and genetic risk: how prenatal stress interacts with genetics to alter risk for psychiatric illness. Psychoneuroendocrinology 90, 9–21. [DOI] [PubMed] [Google Scholar]
- Anacker C, O’Donnell KJ, Meaney MJ, 2014. Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues Clin. Neurosci 16, 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, Blackburn EH, 2012. The telomere syndromes. Nat. Rev. Genet 13,693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S, 2015. Chronicinfection. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. [DOI] [PubMed] [Google Scholar]
- Babineau V, Green CG, Jolicoeur-Martineau A, Bouvette-Turcot AA, Minde K, Sassi R, St-Andre M, Carrey N, Atkinson L, Kennedy JL, Lydon J, Steiner M, Gaudreau H, Levitan R, Meaney M, Wazana A, project M, 2015. Prenatal depression and 5-HTTLPR interact to predict dysregulation from 3 to 36 months–a differential susceptibility model. J. Child Psychol. Psychiatry 56, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, 2015. Epigenetic and transgenerational reprogramming of brain development.Nat. Rev. Neurosci 16, 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V, 2016. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol. Psychiatry 21, 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK, 2010. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J. Neurosci 30, 14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, 2016. The differential susceptibility hypothesis: sensitivity to the environment for better and for worse. JAMA Pediatr. 170, 321–322. [DOI] [PubMed] [Google Scholar]
- Belsky J, Hartman S, 2014. Gene-environment interaction in evolutionary perspective: differential susceptibility to environmental influences. World Psychiatry 13, 87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, Glover V, O’Connor TG, 2010. Maternal prenatal cortisol and infant cognitive development: moderation by infant-mother attachment. Biol. Psychiatry 67, 1026–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bet PM, Penninx BW, Bochdanovits Z, Uitterlinden AG, Beekman AT, van Schoor NM, Deeg DJ, Hoogendijk WJ, 2009. Glucocorticoid receptor gene polymorphisms and childhood adversity are associated with depression: New evidence for a gene-environment interaction. Am. J. Med. Genet. B Neuropsychiatr. Genet 150B, 660–669. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM, 2009. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front. Behav. Neurosci 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, 2009. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 34 (Suppl 1), S186–195. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ, 2008. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299, 1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH, 2005. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 579, 859–862. [DOI] [PubMed] [Google Scholar]
- Blaze J, Asok A, Roth TL, 2015. The long-term impact of adverse caregiving environments on epigenetic modifications and telomeres. Front. Behav. Neurosci 9, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Wainstock T, Braun K, Segal M, 2015. Stress in utero: prenatal programming of brain plasticity and cognition. Biol. Psychiatry 78, 315–326. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Salmeron BJ, Carey CE, Agrawal A, Calhoun VD, Garavan H, Hariri AR, Heinz A, Hill MN, Holmes A, Kalin NH, Goldman D, 2017. Imaging genetics and genomics in psychiatry: a critical review of progress and potential. Biol. Psychiatry 82, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvette-Turcot AA, Fleming AS, Wazana A, Sokolowski MB, Gaudreau H, Gonzalez A, Deslauriers J, Kennedy JL, Steiner M, Meaney MJ, 2015. Maternal childhood adversity and child temperament: an association moderated by child 5-HTTLPR genotype. Genes Brain Behav 14, 229–237. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ, 2008. Influence of child abuse on adult depression: moderation by the corticotropin releasing hormone receptor gene. Arch. Gen. Psychiatry 65, 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, Westen D, Mercer KB, Binder EB, Jovanovic T, Crain D, Wingo A, Heim C, 2011. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: moderation by oxytocin receptor gene. Dev. Psychopathol 23, 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite EC, Kundakovic M, Ramchandani PG, Murphy SE, Champagne FA, 2015. Maternal prenatal depressive symptoms predict infant NR3C1 1F and BDNF IV DNA methylation. Epigenetics 10, 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL, 2011. Nonsteroidal anti-inflammatory treatment pre-vents delayed effects of early life stress in rats. Biol. Psychiatry 70, 434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J, Elliott DM, 2003. Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse Negl. 27, 1205–1222. [DOI] [PubMed] [Google Scholar]
- Bublitz MH, Stroud LR, 2012. Childhood sexual abuse is associated with cortisol awakening response over pregnancy: preliminary findings. Psychoneuroendocrinology 37, 1425–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublitz MH, Stroud LR, 2013. Maternal history of child abuse moderates the association between daily stress and diurnal cortisol in pregnancy: a pilot study. Stress 16, 706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublitz MH, Parade S, Stroud LR, 2014. The effects of childhood sexual abuse on cortisol trajectories in pregnancy are moderated by current family functioning. Biol. Psychol 103, 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, Birn RM, 2012. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat. Neurosci 15, 1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, Pruessner JC, 2007. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J. Neurosci 27, 2592–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, Sandman CA, 2010. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology 35, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Hobel CJ, Sandman CA, 2011. Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress 14, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA, 2012a. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. U. S. A 109, E1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Davis EP, Hobel CJ, Swanson JM, Wadhwa PD, Sandman CA, 2012b. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS One 7, e37758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Swanson JM, Wadhwa PD, 2012c. the role of stress in brain development: the gestational environment’s long-term effects on the brain. Cerebrum 4. [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Wadhwa PD, 2012d. Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Sci. Signal 5, pt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Wadhwa PD, 2012e. Fetal programming of brain development: role of intrauterine stress in susceptibility to psychopathology. Sci. Signal 5 pt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Moog NK, Toepfer P, Fair DA, Simhan HN, Heim CM,Wadhwa PD, 2017. Intergenerational transmission of maternal childhood maltreatment exposure: implications for fetal brain development. J. Am. Acad. Child Adolesc. Psychiatry 56, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calem M, Bromis K, McGuire P, Morgan C, Kempton MJ, 2017. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage Clin. 2017 14, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack AL, Buss C, Entringer S, Hogue CJ, Hobel CJ, Wadhwa PD, 2011. The association between early life adversity and bacterial vaginosis during pregnancy. Am. J. Obstet. Gynecol 204 (431), e431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH, 2007. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry 62, 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH, 2011. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology 214, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R, 2003. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA, 2003. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395. [DOI] [PubMed] [Google Scholar]
- Chen SH, Epel ES, Mellon SH, Lin J, Reus VI, Rosser R, Kupferman E, Burke H, Mahan L, Blackburn EH, Wolkowitz OM, 2014. Adverse childhood experiences and leukocyte telomere maintenance in depressed and healthy adults. J. Affect. Disord 169, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra BM, Carvalho CM, Moretti PN, Mello MF, Belangero SI, 2017. Stress-related telomere length in children: a systematic review. J. Psychiatr. Res 92, 47–54. [DOI] [PubMed] [Google Scholar]
- Collishaw S, Dunn J, O’Connor TG, Golding J, 2007. Maternal childhood abuse and offspring adjustment over time. Dev. Psychopathol 19, 367–383. [DOI] [PubMed] [Google Scholar]
- Danese A, McEwen BS, 2012. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav 106, 29–39. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R, 2007. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. U. S. A 104, 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A, 2008. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry 65, 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H, 2012. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry 71, 286–293. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA, 2007. Prenatal exposure to maternal depression and cortisol influences infant temperament. J. Am. Acad. Child Adolesc. Psychiatry 46, 737–746. [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, Buss C, Wing DA, Head K, 2013. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol. Psychiatry 74, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Head K, Buss C, Sandman CA, 2017. Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology 75, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G, 2002. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol. Psychiatry 52, 1066–1078. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, McCrory EJ, 2013. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J. Child Psychol. Psychiatry 54 105–s12. [DOI] [PubMed] [Google Scholar]
- Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JY, Fox NA,Zeanah CH, Nelson CA, 2012. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol. Psychiatry 17, 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Mabile E, Brett ZH, Esteves K, Jones E, Shirtcliff EA, Theall KP,2014. The association of telomere length with family violence and disruption. Pediatrics 134, e128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards VJ, Holden GW, Felitti VJ, Anda RF, 2003. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am. J. Psychiatry 160, 1453–1460. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Muetzel RL, Thijssen S, van der Knaap NJ, Jaddoe VW, Fernandez G, Verhulst FC, White TJ, 2016. Prenatal exposure to maternal and paternal depressive symptoms and brain morphology: a population-based prospective neuroimaging study in Young children. Depress. Anxiety 33, 658–666. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, 2011. Differential susceptibility to the environment: toward an understanding of sensitivity to developmental experiences and context. Dev. Psychopathol 23, 1–5. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH, 2011. Differential susceptibility to the environment: an evolutionary–neurodevelopmental theory. Dev. Psychopathol 23, 7–28. [DOI] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD, 2010. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr. Opin. Endocrinol. Diabetes Obes 17, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD, 2011a. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr. Opin. Endocrinol. Diabetes Obes 17, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wust S,Wadhwa PD, 2011b. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl. Acad. Sci. U. S. A 108, E513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Swanson JM, Cooper DM, Wing DA, Waffarn F, Wadhwa PD, 2012a. Fetal programming of body composition, obesity, and metabolic function: the role of intrauterine stress and stress biology. J. Nutr. Metab 2012, 632548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD, 2012b. Prenatal stress, telomere biology, and fetalprogramming of health and disease risk. Sci. Signal 5 pt12. [DOI] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD, 2012c. Prenatal stress, telomere biology, and fetal programming of health and disease risk. Sciene Signaling 5 pt12. [DOI] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH, Simhan HN, Wadhwa PD, 2013. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am. J. Obstet. Gynecol 208 (134), e131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD, 2015. Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 curt richter award paper. Psychoneuroendocrinology 62, 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, de Punder K, Buss C, Wadhwa PD, 2018. The fetal programming of telomere biology hypothesis: an update. Philos. Trans. R. Soc. Lond. B Biol. Sci 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Brown DS, Florence CS, Mercy JA, 2012. The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse Negl 36, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 1998. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am. J. Prev. Med 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Flory JD, Bierer LM, Yehuda R, 2011. Maternal exposure to the holocaust and health complaints in offspring. Dis. Markers 30, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM, 2010. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 68, 408–415. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH, 2005. A new view on hypocortisolism. Psychoneuroendocrinology 30, 1010–1016. [DOI] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM, 2010. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J. Psychiatr. Res 44, 799–807. [DOI] [PubMed] [Google Scholar]
- Gaali S, Kirschner A, Cuboni S, Hartmann J, Kozany C, Balsevich G, Namendorf C, Fernandez-Vizarra P, Sippel C, Zannas AS, Draenert R, Binder EB, Almeida OF, Ruhter G, Uhr M, Schmidt MV, Touma C, Bracher A, Hausch F, 2015. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat. Chem. Biol 11, 33–37. [DOI] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM, 2014. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci 17, 667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelaye B, Kajeepeta S, Zhong QY, Borba CP, Rondon MB, Sanchez SE, Henderson DC, Williams MA, 2015. Childhood abuse is associated with stress-related sleep disturbance and poor sleep quality in pregnancy. Sleep Med 16, 1274–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, 2004. Living with the past: evolution, development, and patterns of disease. Science 305, 1733–1736. [DOI] [PubMed] [Google Scholar]
- Graham AM, Buss C, Rasmussen JM, Rudolph MD, Demeter DV, Gilmore JH, Styner M, Entringer S, Wadhwa PD, Fair DA, 2016. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev. Cogn. Neurosci 18, 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M,Potkin SG, Entringer S, Wadhwa PD, Fair DA, Buss C, 2018. Maternal sys-temic interleukin-6 during pregnancy Is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol. Psychiatry 83, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Cannistraci C, Hollon SD, Gore J, Shelton R, 2011. Childhood trauma history differentiates amygdala response to sad faces within MDD. J. Psychiatr. Res 45, 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, White D, Hadley J, Hutcheson N, Shelton R, Sreenivasan K,Deshpande G, 2014. Early life trauma and directional brain connectivity within major depression. Hum. Brain Mapp 35, 4815–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CG, Babineau V, Jolicoeur-Martineau A, Bouvette-Turcot AA, Minde K, Sassi R, St-Andre M, Carrey N, Atkinson L, Kennedy JL, Steiner M, Lydon J, Gaudreau H, Burack JA, Levitan R, Meaney MJ, Wazana A, Maternal Adversity V., Neurodevelopment Research T., 2017. Prenatal maternal depression and child serotonin transporter linked polymorphic region (5-HTTLPR) and dopamine receptor D4 (DRD4) genotype predict negative emotionality from 3 to 36 months. Dev. Psychopathol 29, 901–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldorsdottir T, Binder EB, 2017. Gene x environment interactions: from molecular mechanisms to behavior. Annu. Rev. Psychol 68, 215–241. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD, 2010. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J. Neurosci 2010 (30), 7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MB, Birn R, Provencal N, Wiechmann T, Binder EB, Giakas SW, Roeber BJ, Pollak SD, 2017. Early life stress, FK506 binding protein 5 gene (FKBP5) methylation, and inhibition-related prefrontal function: a prospective longitudinal study. Dev. Psychopathol 29, 1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield T, Wing DA, Buss C, Head K, Muftuler LT, Davis EP, 2011. Magnetic resonance imaging demonstrates long-term changes in brain structure in children born preterm and exposed to chorioamnionitis. Am. J. Obstet. Gynecol 205 (384), e381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W, Schmutzer G, Brahler E, Glaesmer H, 2011. Maltreatment in childhood and adolescence: results from a survey of a representative sample of the German population. Dtsch Arztebl Int. 108, 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Heidinger BJ, 2015. Telomere dynamics may link stress exposure and ageing across generations. Biol. Lett 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM, 2011. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. Biol. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawi Z, Cummins TD, Tong J, Johnson B, Lau R, Samarrai W, Bellgrove MA, 2015. The molecular genetic architecture of attention deficit hyperactivity disorder. Mol. Psychiatry 20, 289–297. [DOI] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P, 2014. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 349, g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Dougherty LR, Olino TM, Laptook RS, Dyson MW,Bufferd SJ, Durbin CE, Sheikh HI, Singh SM, 2010. The dopamine D2 receptor gene and depressive and anxious symptoms in childhood: associations and evidence for gene-environment correlation and gene-environment interaction. Psychiatr. Genet 20, 304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Binder EB, 2012. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol 233, 102–111. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB, 2001. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry 49, 1023–1039. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hanker JP, Hellhammer DH, 1998. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom. Med 60, 309–318. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH, 2000a. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 25, 1–35. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB, 2000b. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 284, 592–597. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB, 2001. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry 158, 575–581. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB, 2004. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 29, 641–648. [DOI] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB, 2008a. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol. Psychiatry 63, 398–405. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB, 2008b. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33, 693–710. [DOI] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB,Ressler KJ, Binder EB, 2009a. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 Gene. Front. Behav. Neurosci 3, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB, 2009b. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol. Psychiatry 14, 954–958. [DOI] [PubMed] [Google Scholar]
- Heim CM, Mayberg HS, Mletzko T, Nemeroff CB, Pruessner JC, 2013. Decreased cortical representation of genital somatosensory field after childhood sexual abuse. Am. J. Psychiatry 170, 616–623. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, 1990. Burnout Bei Pfelgepersonal - Eine PsychoendokrinologischeUntersuchung. Diploma Thesis. Univerdity of Trier, Germany. [Google Scholar]
- Hellhammer D, Meinlschmidt G, Pruessner JC, 2018. Conceptual endophenotypes: a strategy to advance the impact of psychoneuroendocrinology in precision medicine. Psychoneuroendocrinology 89, 147–160. [DOI] [PubMed] [Google Scholar]
- Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P, 2014. Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc. Biol. Sci 281, 20133151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde K, Skibiel AL, Foster AB, Del Rosso L, Mendoza SP, Capitanio JP, 2015Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behav. Ecol 26, 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohwu L, Li J, Olsen J, Sorensen TI, Obel C, 2014. Severe maternal stress exposure due to bereavement before, during and after pregnancy and risk of overweight and obesity in young adult men: a Danish national cohort study. PLoS One 9, e97490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L,Dunne MP, 2017. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2, e356–e366. [DOI] [PubMed] [Google Scholar]
- Jasarevic E, Howerton CL, Howard CD, Bale TL, 2015. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 156, 3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, Ripatti S, Hovatta I, 2010. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One 5, e10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S, 2011. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch. Gen. Psychiatry 68, 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R, 2011. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom. Med 73, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Davis EP, Sandman CA, Sporns O, O’Donnell BF, Buss C, Hetrick WP, 2017. Prenatal maternal cortisol has sex-specific associations with child brain network properties. Cereb. Cortex 27, 5230–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X,Christiansen L, Vaupel JW, Aviv A, Christensen K, 2008. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am. J. Epidemiol 167, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB, 2013. Allele-specific FKBP5 DNA de-methylation mediates gene-childhood trauma interactions. Nat. Neurosci 16, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S,Prinssen EP, 2014. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol 10, 643–660. [DOI] [PubMed] [Google Scholar]
- Kovalchuk I, 2012. Transgenerational epigenetic inheritance in animals. Front. Genet 3, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AJ, Gartstein MA, Rodgers CS, Lebeck MM, 2010. The impact of maternal childhood abuse on parenting and infant temperament. J. Child Adolesc. Psychiatr. Nurs 23, 100–110. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walton M, Letourneau N, Giesbrecht GF, Kaplan BJ, Dewey D, 2016. Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biol. Psychiatry 80, 859–868. [DOI] [PubMed] [Google Scholar]
- Levine S, 1967. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science 156, 258–260. [DOI] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, Obel C, Baker JL, Sorensen TI, 2018. Prenatal stress exposure related to maternal bereavement and risk of childhood overweight. PLoS One 5, e11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C, 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci 10, 434–445. [DOI] [PubMed] [Google Scholar]
- Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, Dunning AM, Svenson U, Roos G,Hosgood HD 3rd, Shen M, Wei Q, 2011. Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One 6, e20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareckova K, Klasnja A, Bencurova P, Andryskova L, Brazdil M, Paus T, 2018Prenatal stress, mood, and gray matter volume in young adulthood. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SM, Prescott J, Tworoger SS, DeVivo I, Rich-Edwards JW, 2015Childhood physical and sexual abuse history and leukocyte telomere length among women in Middle adulthood. PLoS One 10, e0124493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ, 2009. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci 12, 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, Szyf M, 2011. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS One 6, e14739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JK, de la Osa N, Granero R, Ezpeleta L, 2013. Maternal childhood abuse, intimate partner violence, and child psychopathology: the mediator role of mothers’ mental health. Violence Against Women 19, 50–68. [DOI] [PubMed] [Google Scholar]
- Monk M, Boubelik M, Lehnert S, 1987. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development 99, 371–382. [DOI] [PubMed] [Google Scholar]
- Monk C, Feng T, Lee S, Krupska I, Champagne FA, Tycko B, 2016. Distress during pregnancy: epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. Am. J. Psychiatry 173, 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C, 2015Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, Wadhwa PD, 2016. Maternal exposure to childhood trauma Is associated during pregnancy with placental-fetal stress physiology. Biol. Psychiatry 79, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog NK, Entringer S, Rasmussen JM, Styner M, Gilmore JH, Kathmann N,Heim CM, Wadhwa PD, Buss C, 2017a. Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biol. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog NK, Heim CM, Entringer S, Kathmann N, Wadhwa PD, Buss C, 2017bChildhood maltreatment is associated with increased risk of subclinical hypothyroidism in pregnancy. Psychoneuroendocrinology 84, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, Bale TL, 2006. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol. Behav 88, 605–614. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Wu Y, Bockmuhl Y, Spengler D, 2010. Genes learn from stress: how infantile trauma programs us for depression. Epigenetics 5, 194–199. [DOI] [PubMed] [Google Scholar]
- Myhre MC, Dyb GA, Wentzel-Larsen T, Grogaard JB, Thoresen S, 2014. Maternal childhood abuse predicts externalizing behaviour in toddlers: a prospective cohort study. Scand. J. Public Health 42, 263–269. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, 2016. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron 89, 892–909. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP Jr, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB, 2003. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc. Natl. Acad. Sci. U. S. A 100, 14293–14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll JG, Zeller MH, Trickett PK, Putnam FW, 2007. Obesity risk for female victims of childhood sexual abuse: a prospective study. Pediatrics 120, e61–67. [DOI] [PubMed] [Google Scholar]
- Non AL, Hollister BM, Humphreys KL, Childebayeva A, Esteves K, Zeanah CH, Fox NA, Nelson CA, Drury SS, 2016. DNA methylation at stress-related genes is associated with exposure to early life institutionalization. Am. J. Phys. Anthropol 161, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T, 2012. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med 9, e1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KJ, Glover V, Holbrook JD, O’Connor TG, 2014. Maternal prenatal anxiety and child brain-derived neurotrophic factor (BDNF) genotype: effects on internalizing symptoms from 4 to 15 years of age. Dev. Psychopathol 26, 1255–1266. [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, Glover V, Lahti J, Lahti M, Edgar RD, Raikkonen K, O’Connor TG, 2017. Maternal prenatal anxiety and child COMT genotype predict working memory and symptoms of ADHD. PLoS One 12, e0177506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, Metzler T, Lenoci M, Blackburn E, Neylan TC, 2011. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM, 2008Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 3, 97–106. [DOI] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM, 2006. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 163, 1630–1633. [DOI] [PubMed] [Google Scholar]
- Palma-Gudiel H, Cordova-Palomera A, Eixarch E, Deuschle M, Fananas L, 2015. Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: a meta-analysis. Epigenetics 10, 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang TYC, Short AK, Bredy TW, Hannan AJ, 2017. Transgenerational paternal transmission of acquired traits: stress-induced modification of the sperm regulatory transcriptome and offspring phenotypes. Curr. Opin. Behav. Sci 14, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parade SH, Parent J, Rabemananjara K, Seifer R, Marsit CJ, Yang BZ, Zhang H,Tyrka AR, 2017. Change in FK506 binding protein 5 (FKBP5) methylation over time among preschoolers with adversity. Dev. Psychopathol 29, 1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain L, Batlle MA, De la Garza AL, Milagro FI, Martinez JA, Campion J,2012a. Transcriptomic and epigenetic changes in the hypothalamus are involved in an increased susceptibility to a high-fat-sucrose diet in prenatally stressed female rats. Neuroendocrinology 96, 249–260. [DOI] [PubMed] [Google Scholar]
- Paternain L, de la Garza AL, Batlle MA, Milagro FI, Martinez JA, Campion J, 2012b. Prenatal stress increases the obesogenic effects of a high-fat-sucrose diet in adult rats in a sex-specific manner. Stress. [DOI] [PubMed] [Google Scholar]
- Pembrey M, Saffery R, Bygren LO, 2014. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J. Med. Genet 51, 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna G, Grassi M, Caldirola D, Nemeroff CB, 2018. The revolution of personalized psychiatry: will technology make it happen sooner? Psychol. Med 48, 705–713. [DOI] [PubMed] [Google Scholar]
- Plant DT, Barker ED, Waters CS, Pawlby S, Pariante CM, 2013. Intergenerational transmission of maltreatment and psychopathology: the role of antenatal depression. Psychol. Med 43, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, Belsky J, 2013. Vantage sensitivity: individual differences in response to positive experiences. Psychol. Bull 139, 901–916. [DOI] [PubMed] [Google Scholar]
- Posner J, Cha J, Roy AK, Peterson BS, Bansal R, Gustafsson HC, Raffanello E, Gingrich J, Monk C, 2016. Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl. Psychiatry 6, e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencal N, Binder EB, 2015. The effects of early life stress on the epigenome: from the womb to adulthood and even before. Exp. Neurol 268, 10–20. [DOI] [PubMed] [Google Scholar]
- Qiu A, Shen M, Buss C, Chong YS, Kwek K, Saw SM, Gluckman PD, Wadhwa PD, Entringer S, Styner M, Karnani N, Heim CM, O’Donnell KJ, Holbrook JD, Fortier MV, Meaney MJ, the G.s.g, 2017. Effects of antenatal maternal depressive symptoms and socio-economic Status on neonatal brain development are modulated by genetic risk. Cereb. Cortex 27, 3080–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine N, Plamondon A, Madigan S, McDonald S, Tough S, 2018. Maternal adverse childhood experiences and infant development. Pediatrics 141. [DOI] [PubMed] [Google Scholar]
- Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, Elbert T, 2011. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl. Psychiatry 1, e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE, 2008. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol. Psychiatry 64, 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JM, Graham AM, Entringer S, Gilmore JH, Styner M, Fair DA, Wadhwa PD, Buss C, 2018. Maternal interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout SJ, Ridout KK, Kao HT, Carpenter LL, Philip NS, Tyrka AR, Price LH, 2015. Telomeres, early-life stress and mental illness, clinical challenges in the biopsychosocial interface. Update on Psychosomatics for the 21st Century. Adv Psychosom Med. BAsel, Karger, pp 92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, Price LH, Tyrka AR, 2017. Early life adversity and telomere length: a meta-analysis. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A, Meaney MJ, Chen H, Bai J, Hameed WB, Tint MT, Broekman BF, Chong YS, Gluckman PD, Fortier MV, Qiu A, 2015. Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. J. Am. Acad. Child Adolesc. Psychiatry 54 (313–321), e312. [DOI] [PubMed] [Google Scholar]
- Rijlaarsdam J, Stevens GW, Jansen PW, Ringoot AP, Jaddoe VW, Hofman A,Ayer L, Verhulst FC, Hudziak JJ, Tiemeier H, 2014. maternal childhood mal-treatment and offspring emotional and behavioral problems: maternal and paternal mechanisms of risk transmission. Child Maltreat. 19, 67–78. [DOI] [PubMed] [Google Scholar]
- Rijlaarsdam J, van IMH, Verhulst FC, Jaddoe VW, Felix JF, Tiemeier H, Bakermans-Kranenburg MJ, 2017. Prenatal stress exposure, oxytocin receptor gene (OXTR) methylation, and child autistic traits: the moderating role of OXTR rs53576 genotype. Autism Res. 10, 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Lyall K, Rich-Edwards JW, Ascherio A, Weisskopf MG, 2013Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psychiatry 70, 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Galea S, Austin SB, Corliss HL, Williams MA, Koenen KC, 2014. Women’s experience of abuse in childhood and their children ’s smoking and over-weight. Am. J. Prev. Med 46, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L, Nordestgaard BG, Bojesen SE, 2015. Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J. Natl. Cancer Inst 107 djv074. [DOI] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Leu NA, Bale TL, 2015. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. U. S. A 112, 13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph MD, Graham AM, Feczko E, Miranda-Dominguez O, Rasmussen JM, Nardos R, Entringer S, Wadhwa PD, Buss C, Fair DA, 2018. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci 21, 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Buss C, Davis EP, Head K, 2015. Fetal exposure to maternal depressive symptoms Is associated with cortical thickness in late childhood. Biol. Psychiatry 77 (4), 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Kwon SH, Lacadie C, Sze G, Sinha R, Constable RT, Ment LR, 2016. Prenatal stress alters amygdala functional connectivity in preterm neonates. Neuroimage Clin 12, 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneper LM, Brooks-Gunn J, Notterman DA, Suomi SJ, 2016. Early-life experiences and telomere length in adult rhesus monkeys: an exploratory study. Psychosom. Med 78, 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier HM, Enlow MB, Ritz T, Gennings C, Wright RJ, 2015. Childhood abuse is associated with increased hair cortisol levels among urban pregnant women. J. Epidemiol. Commun. Health 69, 1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J,Arseneault L, Caspi A, 2012. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpley CF, Palanisamy SK, Glyde NS, Dillingham PW, Agnew LL, 2014. An update on the interaction between the serotonin transporter promoter variant (5-HTTLPR), stress and depression, plus an exploration of non-confirming findings. Behav. Brain Res 273, 89–105. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Committee on Psychosocial Aspects of, C., Family, H,Committee on Early Childhood, A, Dependent, C, Section on, D, Behavioral, P, 2012. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 129, e232–246. [DOI] [PubMed] [Google Scholar]
- Smith ZD, Chan MM, Mikkelsen TS, Gu H, Gnirke A, Regev A, Meissner A, 2012. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature 484, 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MV, Gotman N, Yonkers KA, 2016. Early childhood adversity and pregnancy outcomes. Matern. Child Health J 20, 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe NN, Wen DJ, Poh JS, Chong YS, Broekman BF, Chen H, Shek LP, Tan KH, Gluckman PD, Fortier MV, Meaney MJ, Qiu A, 2018. Perinatal maternal depressive symptoms alter amygdala functional connectivity in girls. Hum. Brain Mapp 39, 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B, 1997. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 27, 951–959. [DOI] [PubMed] [Google Scholar]
- Stewart SA, Weinberg RA, 2006. Telomeres: cancer to human aging. Annu. Rev. Cell Dev. Biol 22, 531–557. [DOI] [PubMed] [Google Scholar]
- Suglia SF, Koenen KC, Boynton-Jarrett R, Chan PS, Clark CJ, Danese A, Faith MS, Goldstein BI, Hayman LL, Isasi CR, Pratt CA, Slopen N, Sumner JA, Turer A, Turer CB, Zachariah JP, American Heart Association Council on, E, Prevention, Council on Cardiovascular Disease in the, Y, Council on Functional, G, Translational, B., Council on, C, Stroke, N, Council on Quality of, C, Outcomes, R, 2018. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American heart association. Circulation 137, e15–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, Easton DF, Dunning AM, 2011. Life stress, emotional health, and mean telomere length in the European prospective investigation into cancer (EPIC)-Norfolk population study. J. Gerontol. A. Biol. Sci. Med. Sci 66, 1152–1162. [DOI] [PubMed] [Google Scholar]
- Szyf M, 2009. Epigenetics, DNA methylation, and chromatin modifying drugs. Annu.Rev. Pharmacol. Toxicol 49, 243–263. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH, 2009. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes 58, 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh AL, Pan H, Chen L, Ong ML, Dogra S, Wong J, MacIsaac JL, Mah SM, McEwen LM, Saw SM, Godfrey KM, Chong YS, Kwek K, Kwoh CK, Soh SE, Chong MF, Barton S, Karnani N, Cheong CY, Buschdorf JP, Stunkel W, Kobor MS, Meaney MJ, Gluckman PD, Holbrook JD, 2014. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Res. 24, 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, 2013. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am.J. Psychiatry 170, 1114–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A, 2012. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U. S. A 109, E563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K, 2016. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci 17, 652–666. [DOI] [PubMed] [Google Scholar]
- Thompson R, 2007. Mothers’ violence victimization and child behavior problems: examining the link. Am. J. Orthopsychiatry 77, 306–315. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Velders FP, Szekely E, Roza SJ, Dieleman G, Jaddoe VW,Uitterlinden AG, White TJ, Bakermans-Kranenburg MJ, Hofman A, Van Ijzendoorn MH, Hudziak JJ, Verhulst FC, 2012. The generation R study: a review of design, findings to Date, and a study of the 5-HTTLPR by environmental interaction from fetal life onward. J. Am. Acad. Child Adolesc. Psychiatry 51 (1119–1135), e1117. [DOI] [PubMed] [Google Scholar]
- Toepfer P, Heim C, Entringer S, Binder E, Wadhwa P, Buss C, 2017. Oxytocin pathways in the intergenerational transmission of maternal early life stress. Neurosci. Biobehav. Rev 73, 293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A, Suzuki H, Rabi K, Sheu YS, Polcari A, Teicher MH, 2009. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage 47 (Suppl 2), T66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ, 2010. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci 13, 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ, 2011. Elevated amygdala response to faces following early deprivation. Dev. Sci 14, 190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC, 2009. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS One 4, e4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL, 2010Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol. Psychiatry 67, 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Ridout KK, Parade SH, Paquette A, Marsit CJ, Seifer R, 2015Childhood maltreatment and methylation of FK506 binding protein 5 gene (FKBP5). Dev. Psychopathol 27, 1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP, 2009. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap NJF, Klumpers F, El Marroun H, Mous S, Schubert D, Jaddoe V, Hofman A, Homberg JR, Tiemeier H, White T, Fernandez G, 2018. Maternal depressive symptoms during pregnancy are associated with amygdala hyperresponsivity in children. Eur. Child Adolesc. Psychiatry 27, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, van der Wee NJ, Veltman DJ, Aleman A, Spinhoven P, van Buchem MA, Zitman FG, Penninx BW, Elzinga BM, 2010. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol. Psychiatry 68, 832–838. [DOI] [PubMed] [Google Scholar]
- Virk J, Li J, Vestergaard M, Obel C, Kristensen JK, Olsen J, 2012. Prenatal exposure to bereavement and type-2 diabetes: a Danish longitudinal population based study. PLoS One 7, e43508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD, 2002. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. Psychiatry 159, 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, Swanson JM, 2009. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med 27, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR,Dymov S, Szyf M, Meaney MJ, 2004. Epigenetic programming by maternal behavior. Nat. Neurosci 7, 847–854. [DOI] [PubMed] [Google Scholar]
- Weiss MJ, Wagner SH, 1998. What explains the negative consequences of adverse childhood experiences on adult health? Insights from cognitive and neuroscience research. Am. J. Prev. Med 14, 356–360. [DOI] [PubMed] [Google Scholar]
- Wildeman C, Emanuel N, Leventhal JM, Putnam-Hornstein E, Waldfogel J, Lee H, 2014. The prevalence of confirmed maltreatment among US children, 2004 to 2011. JAMA Pediatr 168, 706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR, 2003. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology 28, 910–918. [DOI] [PubMed] [Google Scholar]
- Wosu AC, Gelaye B, Williams MA, 2015. History of childhood sexual abuse and risk of prenatal and postpartum depression or depressive symptoms: an epidemiologic review. Arch. Womens Ment. Health 18, 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, 2000. Biology of posttraumatic stress disorder. J. Clin. Psychiatry 61 (Suppl7), 14–21. [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Shi J, Tuorto F, Li X, Liu Y, Liebers R, Zhang L, Qu Y, Qian J, Pahima M, Liu Y, Yan M, Cao Z, Lei X, Cao Y, Peng H, Liu S, Wang Y, Zheng H, Woolsey R, Quilici D, Zhai Q, Li L, Zhou T, Yan W, Lyko F, Zhang Y, Zhou Q, Duan E, Chen Q, 2018. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat. Cell Biol 20, 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Miao K, Wang H, Ding H, Wang DW, 2013. Association between telomere length and type 2 diabetes mellitus: a meta-analysis. PLoS One 8, e79993. [DOI] [PMC free article] [PubMed] [Google Scholar]