Abstract
Matrix metalloproteinases 2 and 9 (MMP2/9) have previously been shown to be elevated in serum and amniotic fluid from women undergoing preterm birth. We performed experiments to determine the effects of MMP2/9 on uterine contraction and birth timing. Pregnant mice were injected daily with 50 mg/kg of SB-3CT or vehicle control beginning on gestational day 14–18 to determine if MMP2/9 inhibition would affect parturition timing. MMP2/9 expression in human myometrial tissue was determined by Simple Western (Wes) and semiquantitative western blot. Purified MMP2/9 and SB-3CT inhibitor were added to human myometrial strips to determine the effects of MMP2/9 on oxytocin-induced uterine contraction. Parturition was delayed in mice treated with MMP2/9 inhibitor SB-3CT. MMP2/9 protein levels were elevated in preterm laboring uterine myometrium. Gelatinase activity was confirmed in cell extracts and supernatants from immortalized and primary human uterine myometrial cells in culture. Addition of purified MMP2/9 increased the oxytocin-induced contractile response in myometrial tissue strips from pregnant women. In contrast, addition of the MMP2/9 inhibitor SB-3CT decreased the contractile response to oxytocin in a dose-dependent manner. These results suggest abnormal MMP2/9 expression affects the contractile state of the uterine myometrium to promote parturition and that MMP2/9 inhibition attenuates this effect.
Keywords: human reproduction, labor, myometrium, pregnancy, uterus
Matrix metalloproteinases 2 and 9 are elevated in preterm laboring myometrium, promote myometrial contraction, and may contribute to parturition timing.
Introduction
An estimated 15 million preterm babies (defined as <37 weeks of gestation) are born annually worldwide [1]. Preterm birth is one of the leading causes of neonatal mortality in the United States [2, 3]. Premature babies who survive are more likely to require hospitalization and develop chronic health issues such as cerebral palsy, respiratory problems, feeding difficulties, and developmental delays [4, 5]. A number of genetic, environmental, and disease-state risk factors are thought to promote changes that ultimately activate convergent effector pathways to switch the uterine myometrium from a quiescent to a contractile state [6]. Disorders that can cause spontaneous preterm birth include infection or inflammation responses, vascular disorders, decidual senescence, abnormal uterine distension, disruption of maternal-fetal immune tolerance, and stress [7]. In many spontaneous preterm birth cases, the etiological factors, and therefore molecular mechanisms leading to the onset of preterm uterine contractions, are not known.
Matrix metalloproteinase 2 (MMP2/Gelatinase A) and matrix metalloproteinase 9 (MMP9/Gelatinase B) are traditionally known as enzymes responsible for breakdown of the extracellular matrix [8–10]. MMPs are secreted as inactive proenzymes that can be activated by proteolytic cleavage or chemical modification [10]. MMP2 and MMP9 are known to be involved in the breakdown of the extracellular matrix to allow human cervical ripening [11]. In the mouse uterus, Mmp2 and Mmp9 transcripts decrease as normal pregnancy progresses, with Mmp9 transcript rising again near term [12]. During uncomplicated human pregnancies, MMP9 concentrations remain stable in maternal plasma until labor starts and then increases in women experiencing either term or preterm labor compared to gestational age matched controls [12]. MMP9 activity is increased in the amniotic fluid of patients undergoing spontaneous rupture of membranes (term or preterm), parturition (term or preterm), and with microbial infections [13–15]. MMP2/9 inhibitors rapidly affect the oxytocin-induced contractile response in virgin and pregnant rat myometrium, suggesting MMPs might act via novel mechanisms in this tissue [16] and MMP9 has been shown to regulate contraction in other cell types [17, 18]. These findings led us to investigate the potential role of these matrix metalloproteinases in uterine function at parturition. We performed experiments to determine if inhibition of MMP2/9 affected parturition timing in mice, if MMP2/9 expression was altered in preterm human myometrium, and the effects of MMP2/9 elevation or inhibition on ex vivo human uterine tissue contractility.
Materials and methods
Parturition experiments
The University of Nevada, Reno Institutional Animal Care and Use Committee approved animal experiments. C57BL/6 mice were mated to induce pregnancy. Successful matings were confirmed by the presence of a vaginal plug. Mice were given chow and water ad libitum, and significant differences in maternal body weight between groups were not observed. From gestational days 14–18, 50 mg/kg SB-3CT in normal saline containing 20% dimethylsulfoxide (DMSO) was administered via single daily intraperitoneal injection. This dose was selected because it was the highest previously administered without observed adverse effects [19]. Control mice were injected with vehicle only. Parturition events were monitored from day 17 through birth by observing mice in the morning, afternoon, and in the evening.
Collection of patient uterine tissue
All research was reviewed and approved by the University of Nevada Biomedical Review Committee (Institutional Review Board) for the protection of human participants. Human uterine myometrial biopsies were obtained with written informed consent from women undergoing elective Cesarean section (C/S) at term or preterm. Patient groups collected were women at term not in labor (TNL; scheduled C/S, gestational age 37–40 weeks), women at term in labor at the time of C/S as determined by the surgeon (TL, gestational age 38–40 weeks, cervical dilation >4 cm and or effacement, contractions <10 min apart by tocodynamometry), women in labor preterm (PTL, gestational age 24–36 weeks by ultrasound), and women preterm but not in labor (PTNL, gestational age 27–36 weeks). Gestational timing was based on last menstrual cycle and ultrasound dating [20]. We did not observe significant differences in maternal age or parity between groups. Exclusion criteria included diagnoses such as HIV infection or AIDS, hepatitis C infection. Tissues were transported to the laboratory immediately in cold sterile transport buffer (120 mM NaCl, 5 mM KCl, 0.587 mM KH2PO4, 0.589 mM Na2HPO4, 2.5 mM MgCl2, 20 mM dextrose, 0.5 mM CaCl2, 25 mM Trizma base, 5 mM NaHCO3, pH 7.4) [21]. Uterine samples were dissected to isolate smooth muscle and cut into strips for contractile studies or snap-frozen in liquid nitrogen and stored at −80°C for protein extraction.
MMP expression in human uterine tissue
To assess MMP2 protein expression in human myometrial tissue samples, we used a Wes Simple Western system (ProteinSimple, San Jose, CA), a capillary-based semiquantitative protein analysis tool. This automated machine performs size-based separation after sample loading, immunoprobing, and washing. The Wes system used for MMP2 analysis runs an internal standard and normalizes to total protein. Protein was extracted from frozen myometrial tissue samples in MAPK extraction buffer (60 mM Tris-HCl, 2% SDS, 10% Glycerol, 1 mM EGTA, 1 mM Na2EDTA, pH 7.4) containing 1X Halt Protease and Phosphatase Inhibitor Cocktail, EDTA-free (Thermo Fisher Scientific, Waltham, MA). Samples were centrifuged, and the supernatant was collected. Extracted protein was quantified by EZQ Protein Quantification according to the manufacturer's instructions (Molecular Probes, Eugene, OR). Samples were reduced in 400 mM dithiothreitol and denatured by boiling. A biotinylated ladder (12, 40, 66, 90, 116, and 180 kDa) was used for molecular weight determination. Myometrial lysates (1 μg per lane) were loaded into each capillary and run together with fluorescent standards, which permit molecular weight normalization to the ladder. MMP2 was identified using a specific primary antibody (sc-13594; Santa Cruz Biotechnology, Santa Cruz, CA) probed with goat anti-mouse secondary HRP conjugate (042–205; ProteinSimple, San Jose, CA), and detected by chemiluminescence.
To assess MMP9 protein expression in human myometrial samples, we performed semiquantitative western blot. Protein was extracted from frozen myometrial tissue samples in RIPA buffer (50 mM Tris-HCl, 5 mM Na2EDTA, 150 mM NaCl, 1% Nonidet P-40, pH 7.4) containing 1X Halt Protease and Phosphatase Inhibitor Cocktail, EDTA-free (Thermo Fisher Scientific). Samples were centrifuged and the supernatant was collected. Extracted protein was quantified by EZQ Protein Quantification according to the manufacturer's instructions (Molecular Probes). A primary antibody to MMP9 (04–1150; MilliporeSigma, Burlington, MA) was used, followed by a fluorescent secondary antibody (IRDye 680 goat anti-rabbit, LI-COR, Lincoln, NE). Signal was normalized to the GAPDH control (#2118; Cell Signaling Technology, Danvers, MA) using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE).
MMP protein purification
Human embryonic kidney cells (HEK 293) were transfected with plasmids encoding histidine-tagged human Mmp2 or Mmp9 cDNA (Sino Biological, Beijing, China). Stably transfected cells were allowed to proliferate in DMEM supplemented with 10% fetal bovine serum containing 50 U/ml penicillin, 50 μg/ml streptomycin, and 250 μg/ml hygromycin B. The MMP-containing medium was collected and concentrated with 10 kDa Amicon Ultra-15 Centrifugal Filters (Millipore Sigma, St. Louis, MO). Histidine-tagged MMP protein was purified using a PureProteome Nickel Magnetic Bead System (Millipore Sigma) according to the manufacturer's instructions. Purified MMP proteins were dialyzed into phosphate-buffered saline, quantitated by EZQ, and subjected to gelatinase zymography to confirm metalloproteinase activity. Purified MMP2 and MMP9 proteins were stored at −80°C in aliquots until use in tissue bath experiments. Purified MMPs were assessed for purity by Coomassie staining using SimplyBlue SafeStain (ThermoFisher Scientific, Grand Island, NY) according to the manufacturer's instructions (Supplemental Figure S1A). Gelatinase activity of purified enzymes was confirmed by zymography as described above (Supplemental Figure S1B). Gelatinase activity for MMP9 was observed at the predicted monomer size. Gelatinase activity for MMP2 was observed at the expected monomer and dimer sizes [22]. Coomassie stained purified proteins matched the zymography bands with the exception of a band at approximately 50 kDa, which corresponds to albumin.
Contractile experiments
Contractile studies were performed on uterine myometrial tissues obtained from nonlaboring patients undergoing C/S. Clinical indications for C/S were previous C/S or breech presentation. Maternal age ranged from 22 to 38 with a mean of 31 years. Gestational ages ranged from 37 to 40 weeks, with a mean of 39.1 weeks. Freshly isolated myometrial tissue was dissected, and uterine strips (1 mm × 1 mm × 10 mm) from each patient were mounted vertically in tissue baths and tested for the ability to contract in response to 50 mM KCl. Contracting tissue was stimulated with 8 nM oxytocin (MilliporeSigma), and baseline contractions were established as described previously [23]. Purified MMP2 and MMP9 proteins were combined and added to the bath to a final concentration of 1.2 μg/ml each (∼17 nM MMP2 and 13 nM MMP9). These concentrations produced contractile effects in rat tissue [16] and are in the range observed in plasma from women undergoing preterm labor [24]. Changes in the contractile response were then recorded and analyzed by the change in area under the curve over time.
We determined the effect of SB-3CT (Selleckchem, Houston, TX), a potent pharmacological inhibitor of MMP2/9, on uterine contraction. Myometrial strips were dissected, mounted, and stimulated as described above. Once baseline responses to 8 nM oxytocin were established, SB-3CT (Selleckchem) was added in increasing concentrations at 10-min intervals, followed by wash out and re-addition addition of oxytocin to 8 nM. The changes in the contractile response were recorded and analyzed as described above. In a separate series of experiments, myometrial strips were treated with 8 nM oxytocin for 15 min followed by addition of 1 μM SB-3CT and contractile response was analyzed as described above.
Statistical analyses
All data were analyzed using GraphPad PRISM (PRISM software version 8.0 GraphPad Software, La Jolla, CA) with a P-value of <0.05 considered significant. Data comparing two groups or conditions were analyzed by the Student t-test. Data comparing multiple group values were compared by one-way ANOVA followed by the Tukey HSD post hoc test.
Results
Effects of SB-3CT administration on parturition in mice
To determine if MMPs affect parturition timing, SB-3CT was administered systemically to pregnant C57BL/6 mice. Pregnant females were given daily intraperitoneal injections of 50 mg/kg SB-3CT or vehicle control for days 14–18 of pregnancy. SB-3CT significantly delayed parturition by an average of 0.5 days compared to vehicle-treated mice (Figure 1).
Figure 1.
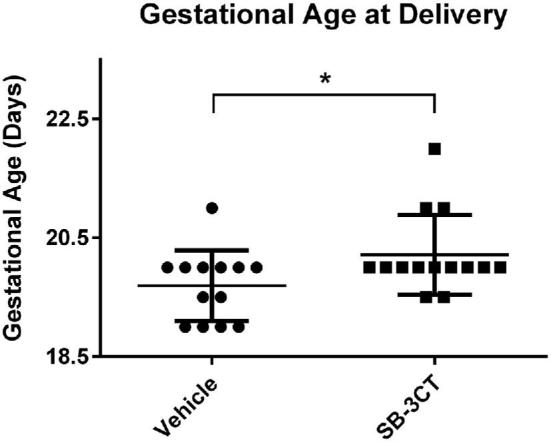
Systemic MMP2/9 inhibition delays parturition in mice. Pregnant female mice were injected daily with SB-3CT or vehicle control on days 14–18 of pregnancy, and delivery times in days were recorded. Mice treated with the inhibitor (n = 14) gave birth an average of 0.5 days later than vehicle-treated controls (n = 13, *P < 0.05). Scatter dot plot shows mean +/– standard deviation.
MMP2 and MMP9 expression in human myometrial tissue samples
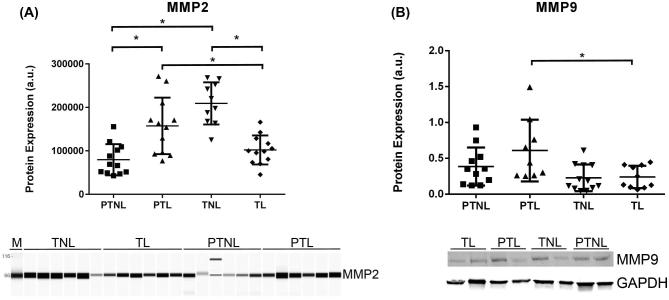
To determine if MMP protein expression levels were altered in human preterm laboring myometrium, we performed semiquantitative Simple Western (Wes) and western blot experiments to compare MMP2 and MMP9 expression in uterine myometrium from the different pregnancy groups (Figure 2). Indication for C/S varied widely. Average gestational parity was higher in the term nonlaboring group. There were no significant differences in maternal age or ethnicity between groups. Gestational ages were similar in the preterm laboring and nonlaboring groups; patients were approximately equally distributed between early and late preterm according to recent guidelines [20]. Detailed patient demographic information is outlined (Supplementary Table S1). Women undergoing preterm labor (PTL) had higher MMP2 expression than gestational age-matched nonlaboring controls (PTNL) and term laboring (TL) controls (P < 0.05). MMP9 protein levels were higher in the preterm laboring group when compared to the term laboring group (P < 0.05) (Figure 2). Therefore, MMP2/9 expression is elevated in preterm laboring tissue compared to nonlaboring samples of similar gestational age or to normal laboring tissue at term.
Figure 2.
MMP2 and MMP9 expression is elevated in preterm laboring myometrial tissue. Wes (MMP2 n = 6 patients/group in duplicate) or Western (MMP9 n = 9–12 patients/group) analysis of samples from women in different states of pregnancy and labor (PTNL = preterm not in labor, PTL = preterm labor, TNL = term not in labor, and TL = term in labor). Women undergoing spontaneous PTL had higher MMP2 expression than gestational age matched and term laboring controls (P < 0.05). MMP9 expression was higher in myometrial tissue from women undergoing spontaneous preterm labor than term laboring controls (P < 0.05). Representative images are shown. a.u. = arbitrary units. Scatter dot plots show mean +/– standard deviation.
Effect of SB-3CT on the oxytocin-induced contractile response in term human myometrial tissue
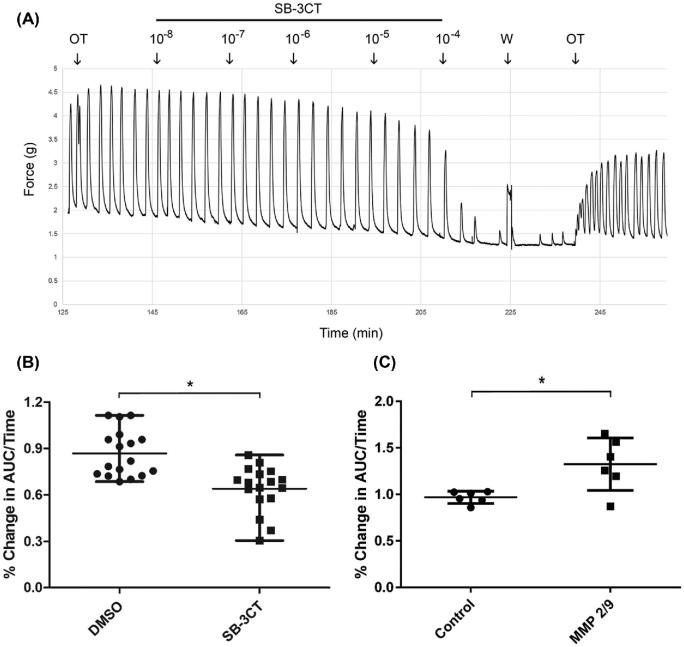
In order to determine if elevated MMP2/9 levels could affect myometrial contractility in pregnant women, uterine strips from term nonlaboring patients were stimulated with oxytocin followed by the addition of the MMP2/9 inhibitor SB-3CT or vehicle control. We observed a dose-dependent and reversible effect of SB-3CT on the oxytocin-induced contractile response (Figures 3A and Supplemental Figure S2). We observed a 34% reduction in the contractile response as measured by area under the curve over time in tissue treated with 1 μM SB-3CT compared to a 13% reduction in the vehicle-treated controls (Figure 3B).
Figure 3.
MMP2/9 inhibition reduces oxytocin-induced contraction in myometrial tissue from pregnant women, while addition of purified MMP2/9 enhances contraction. (A) Freshly isolated term nonlaboring myometrium was dissected, and uterine strips were tested for the ability to contract in response to 60 mM KCl. Tissues were then stimulated with 8 nM oxytocin (OT), and baseline contractions were recorded. Increasing concentrations of SB-3CT were added, followed by washout (W), and tissue was re-stimulated with 8 nM OT. (B) Vehicle or 1 μM SB-3CT was added to tissue baths and contractile responses were compared (n = 7 patients in duplicate or triplicate); SB-3CT reduced mean contractile force by 34%. (C) Purified MMP2/9 (17 and 13 nM, respectively) or vehicle control was added to tissue baths, and changes in the contraction profiles were recorded (n = 3 patients in duplicate); MMP2/9 increased mean contractile force by 36%. Data in B and C are presented as the percent change in the area under the curve over time, *P < 0.05. Scatter dot plots show mean +/– standard deviation.
Effect of purified MMP2/9 on the oxytocin-induced contractile response in term human myometrial tissue
In order to determine if the observed elevated MMP2/9 levels affect myometrial contractility in pregnant women, uterine strips from term nonlaboring patients were stimulated with oxytocin followed by the addition of purified MMP2/9 protein. We observed a 36% increase in the contractile response as measured by area under the curve over time in MMP2/9-treated samples compared to vehicle controls (Figure 3C).
Discussion
The roles of MMP2/9 during pregnancy have previously been examined in mice, but the results have been complicated by functional compensation of other MMP family members [25, 26]. Mmp2 null mice exhibit normal fertility [27]. Mmp9 null mice were initially reported to be fertile [28]; however, further examination revealed that some Mmp9 null strains were either infertile or produced small litters [29, 30]. These Mmp9 null females exhibited defects in vascularization, placentation, implantation, and embryo development [30]. To our knowledge, the role of MMP9 during labor and parturition has not been examined in these animals, as such analyses would likely be complicated by the problems incurred during early pregnancy [29, 30].
We used SB-3CT to systemically inhibit MMP2/9 action during pregnancy in mice resulting in delayed parturition. Circulating MMPs may affect multiple tissues, perhaps the most relevant of which is the uterine cervix. Cervical epithelial cells produce and release MMP2 and MMP9 to promote collagen degradation and cervical dilation [31, 32]. Although our subsequent data indicate SB-3CT suppresses myometrial contraction, it is unclear whether the parturition delay stems from effects on uterine contraction, effects on remodeling of cervical, amniochorion, or uterine tissue, or a combination of factors.
Several laboratories have observed elevated MMP levels in amniotic fluid, placental tissue, cervical mucus plugs, and serum from preterm laboring patients [33–37]. We have shown that MMP2 and MMP9 are present in pregnant human myometrial tissue and that protein levels are elevated in preterm laboring samples. Variation between human tissue samples that could affect MMP expression may arise from a number of factors including indication for C/S, medications administered, gestational parity, and fetal sex [20]. Some of the MMP2/9 may be derived from vascular or immune cells; however, gelatinase activity was detected in extracts from isolated myometrial cell cultures (data not shown). Together these data indicate increased MMP2/9 expression is characteristic of preterm laboring myometrium and that some of this expression can be attributed to the myometrial cells.
Hormonal changes during pregnancy likely influence MMP levels [38]. Overexpression of MMPs before labor results in matrix breakdown and premature rupture of membranes [39, 40]. Administration of lipopolysaccharides or a progesterone receptor inhibitor to pregnant mice can be used to model preterm birth. These agents activate the complement cascade and induce MMP9 release from macrophages [41]. Pretreatment with supplementary progesterone reduces MMP9 release and preterm birth in these animals [41]. Progesterone promotes myometrial quiescence, and one of the contributing mechanisms may be suppression of the inflammatory response and MMP secretion. Estrogen treatment increases MMP9, but not MMP2, activity in the mouse uterus [42]. Stress hormones such as corticotropin releasing hormone and urocortin increase MMP9 secretion in trophoblast, amnion, and syncytiotrophoblast cells [43]. These and other factors could individually or synergistically contribute to increased MMP expression in preterm laboring myometrium.
Both term and preterm parturition can be viewed in part as inflammatory processes [44], and infection is a major cause of spontaneous preterm birth [6, 13, 45]. In fetal membranes, MMP activity is upregulated by inflammatory cytokines [46], and cytokine levels are increased in tissue extracts from preterm labor patients [46–48]. Inflammatory cytokines initiate prostaglandin production leading to myometrial contraction [49–51]. PGF2α stimulation also increases MMP expression and activity in decidual tissue from term patients [52]. Together, these observations suggest that inflammation may underlie increased myometrial MMP2/9 expression in the preterm laboring uterus and drive a more contractile phenotype.
In our experiments, MMP2/9 inhibition reduced the contractile response in a dose-dependent and reversible manner. At the concentration closest to the Ki for MMP9, SB-3CT reduced the oxytocin-induced contractile response while addition of physiologically relevant concentrations of purified MMP2/9 increased the contractile response (Figure 3) [53]. In contrast, pretreatment of pregnant rat myometrial tissue with MMP inhibitors enhanced oxytocin-induced contraction while addition of MMP2 or MMP9 caused relaxation [16]. It is not clear why MMP2/9 and SB-3CT treatment appear to have opposite effects in rat vs. human tissues. These observed differences might be attributed experimental method. Yin et al. administered MMP2/9 inhibitors to tissue during the tonic phase of contraction and observed an immediate response. In contrast, we measured the contractile response over several minutes. Most data indicate MMP2/9 have procontractile effects in other cell and tissue types [54–57], although some studies have found the opposite effect [58, 59]. In one study, MMP9 was found to have a biphasic effect on contraction, which might explain these apparently contradictory observations [18]. Our data indicate that, at physiologically relevant concentrations, MMP2/9 enhance the oxytocin-induced contractile response in human uterine tissue.
Our data indicate that elevated MMP levels enhance myometrial contractility and may contribute to the onset of labor. In addition to their traditional role in matrix remodeling, MMPs can act on signaling molecules to affect their activity and mediate cellular responses [32, 60]. MMPs can also cleave cytoskeletal substrates, resulting in increased force during muscle contraction [61]. The rapid change in contractile response suggests that MMP2/9 act via novel mechanisms in uterine tissue [16].
Supplementary data
Supplemental Figure S1. Purified HIS-MMP2 (solid arrows) or and HIS-MMP9 (dashed arrows) were subjected to electrophoresis and then stained for total protein (A) or subjected to gelatin zymography (B). Gelatinase of purified proteins was compared to that of endogeneous activity in human myometrial tissue (myo). Both enzymes were relatively pure (with the exception of bands corresponding to albumin) and displayed gelatinase activity as expected.
Supplemental Figure S2. Freshly isolated term nonlaboring myometrium was dissected, and uterine strips were tested for the ability to contract in response to KCl. Contracting tissue was then stimulated with oxytocin, and baseline contractions were recorded. Increasing concentrations of SB-3CT (10−8–10−4) were added at 10-min intervals, and the percent change in the area under the curve over time was determined.
Supplemental Table s1. Protein Ex pression Patient Information.
Acknowledgments
We are grateful to the following Renown Hospital physicians for their commitment and contributions to this research: Dr Holly T. Ashley Cothern, Dr Myron Bethel, Dr Corinne E. Capurro, Dr Samuel R. Chacon, Dr Martin E. Dennis, Dr Bruce E. Farringer, Dr Amy Forsberg Condon, Dr Rafaela G. Hernandez, Dr Randall E. Jack, Dr Scott E. Jacobs, Dr Larry D. Klaich, Dr Leah J. Najima, Dr Susan D. Perry, Dr Laura B. Thompson, Dr Vickie L. Tippett, Dr Maria Shiela S. Torres, Dr Arathy Veeraswamy, Dr Morgan Walker, and Dr Alison S. Westfall. We thank Lily Burkin and Janet Lambert for proofreading the manuscript.
Notes
Conference Presentation: Presented in part at the Society for Reproductive Investigation 2015, the Mountain West Clinical and Translational Research Infrastructure Network (2016), and the 49th and 50th meetings of the Society for the Study of Reproduction (2016 and 2017).
Footnotes
Grant Support: This work was supported by a Mountain West Clinical Translational Research-Infrastructure Network subaward (Burkin) under a grant from the National Institute of General Medical Sciences (NIH 1U54GM104944), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Burkin, NIH R00HD067342), and a grant from the University of Nevada Women's Health Initiative (Burkin). These agencies were not involved in the study design, data collection, analysis and interpretation of data, the writing of this report, or the decision to submit this article for publication.
References
- 1. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385:430–440. [DOI] [PubMed] [Google Scholar]
- 2. Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379:2151–2161. [DOI] [PubMed] [Google Scholar]
- 3. Rossen L. Quarterly provisional estimates for infant mortality, 2015-Quarter 3, 2017. National Center for Health Statistics, National Vital Statistics System, Vital Statistics Rapid Release Program. 2018. [Google Scholar]
- 4. Wu P, Gulati M, Kwok CS, Wong CW, Narain A, O’Brien S, Chew-Graham CA, Verma G, Kadam UT, Mamas MA. Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc 2018; 7:e007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ray KN, Lorch SA.. Hospitalization of early preterm, late preterm, and term infants during the first year of life by gestational age. Hosp Pediatr 2013; 3:194–203. [DOI] [PubMed] [Google Scholar]
- 6. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014; 345:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014; 71:330–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagase H, Woessner JF.. Matrix Metalloproteinases. Am Soc Biochem Mol Biol 1999; 274:21491–21494. [DOI] [PubMed] [Google Scholar]
- 9. Sternlicht M, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001; 17:463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Visse R, Nagase H.. Matrix metalloproteinases and tissue inhibitors of metalloproteinases. Circ Res 2003; 92:827–839. [DOI] [PubMed] [Google Scholar]
- 11. Stygar D, Wang H, Vladic YS, Ekman G, Eriksson H, Sahlin L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod 2002; 67:889–894. [DOI] [PubMed] [Google Scholar]
- 12. Lombardi A, Makieva S, Rinaldi SF, Arcuri F, Petraglia F, Norman JE. Expression of matrix metalloproteinases in the mouse uterus and human myometrium during pregnancy, labor, and preterm labor. Reprod Sci 2018; 25:938–949. [DOI] [PubMed] [Google Scholar]
- 13. Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006; 11:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol 2000; 183:887–894. [DOI] [PubMed] [Google Scholar]
- 15. Di Ferdinando A, Patacchiola F, Perilli MG, Amicosante G, Carta G. Expression of matrix metalloproteinase-9 (MMP-9) in human midpregnancy amniotic fluid and risk of preterm labor. Clin Exp Obstet Gynecol 2010; 37:193–196. [PubMed] [Google Scholar]
- 16. Yin Z, Sada AA, Reslan OM, Narula N, Khalil RA. Increased MMPs expression and decreased contraction in the rat myometrium during pregnancy and in response to prolonged stretch and sex hormones. Am J Physiol Endocrinol Metab 2012; 303:E55–E70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kobayashi T, Kim H, Liu X, Sugiura H, Kohyama T, Fang Q, Wen FQ, Abe S, Wang X, Atkinson JJ, Shipley JM, Senior RM et al.. Matrix metalloproteinase-9 activates TGF-beta and stimulates fibroblast contraction of collagen gels. Am J Physiol Lung Cell Mol Physiol 2014; 306:L1006–L1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Defawe OD, Kenagy RD, Choi C, Wan SY, Deroanne C, Nusgens B, Sakalihasan N, Colige A, Clowes AW. MMP-9 regulates both positively and negatively collagen gel contractionA nonproteolytic function of MMP-9. Cardiovasc Res 2005; 66:402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kruger A, Arlt MJ, Gerg M, Kopitz C, Bernardo MM, Chang M, Mobashery S, Fridman R. Antimetastatic activity of a novel mechanism-based gelatinase inhibitor. Cancer Res 2005; 65:3523–3526. [DOI] [PubMed] [Google Scholar]
- 20. Myatt L, Eschenbach DA, Lye SJ, Mesiano S, Murtha AP, Williams SM, Pennell CE. A standardized template for clinical studies in preterm birth. Reprod Sci 2012; 19:474–482. [DOI] [PubMed] [Google Scholar]
- 21. Bradley KK, Buxton IL, Barber JE, McGaw T, Bradley ME. Nitric oxide relaxes human myometrium by a cGMP-independent mechanism. Am J Physiol 1998; 275:C1668–C1673. [DOI] [PubMed] [Google Scholar]
- 22. Koo BH, Kim YH, Han JH, Kim DS. Dimerization of matrix metalloproteinase-2 (MMP-2). J Biol Chem 2012; 287:22643–22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barnett SD, Smith CR, Ulrich CC, Baker JE, Buxton ILO. S-nitrosoglutathione reductase underlies the dysfunctional relaxation to nitric oxide in preterm labor. Sci Rep 2018; 8:5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, Mazor M, Maymon E, Berry S. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol 2002; 187:1125–1130. [DOI] [PubMed] [Google Scholar]
- 25. Hu J, Zhang X, Nothnick WB, Spencer TE. Matrix metalloproteinases and their tissue inhibitors in the developing neonatal mouse uterus. Biol Reprod 2004; 71:1598–1604. [DOI] [PubMed] [Google Scholar]
- 26. Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod 1997; 3:27–45. [DOI] [PubMed] [Google Scholar]
- 27. Caterina JJ, Yamada S, Caterina NC, Longenecker G, Holmback K, Shi J, Yermovsky AE, Engler JA, Birkedal-Hansen H. Inactivating mutation of the mouse tissue inhibitor of metalloproteinases-2(Timp-2) gene alters proMMP-2 activation. J Biol Chem 2000; 275:26416–26422. [DOI] [PubMed] [Google Scholar]
- 28. Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz Ma, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci 2000; 20:7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dubois B, Arnold B, Opdenakker G. Gelatinase B deficiency impairs reproduction. J Clin Invest 2000; 106:627–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K, Ni W, Kalluri R, Werb Z. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci USA 2013; 110:11109–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gonzalez JM, Dong Z, Romero R, Girardi G. Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PLoS One 2011; 6:e26877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nissinen L, Kähäri VM.. Matrix metalloproteinases in inflammation. Biochim Biophys Acta 2014; 1840:2571–2580. [DOI] [PubMed] [Google Scholar]
- 33. Sundrani D, Chavan-Gautam P, Pisal H, Mehendale S, Joshi S. Matrix metalloproteinases-2, -3 and tissue inhibitors of metalloproteinases-1, -2 in placentas from preterm pregnancies and their association with one-carbon metabolites. Reproduction 2013; 145:401–410. [DOI] [PubMed] [Google Scholar]
- 34. Xu P, Alfaidy N, Challis JR. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab 2002; 87:1353–1361. [DOI] [PubMed] [Google Scholar]
- 35. Tency I. Inflammatory response in maternal serum during preterm labour. Facts Views Vis Obgyn 2014; 6:19–30. [PMC free article] [PubMed] [Google Scholar]
- 36. Tency I, Verstraelen H, Kroes I, Holtappels G, Verhasselt B, Vaneechoutte M, Verhelst R, Temmerman M. Imbalances between matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) in maternal serum during preterm labor. PLoS One 2012; 7:e49042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Becher N, Hein M, Danielsen CC, Uldbjerg N. Matrix metalloproteinases in the cervical mucus plug in relation to gestational age, plug compartment, and preterm labor. Reprod Biol Endocrinol 2010; 8:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Geng J, Huang C, Jiang S. Roles and regulation of the matrix metalloproteinase system in parturition. Mol Reprod Dev 2016; 83:276–286. [DOI] [PubMed] [Google Scholar]
- 39. Arechavaleta-Velasco F, Mayon-Gonzalez J, Gonzalez-Jimenez M, Hernandez-Guerrero C, Vadillo-Ortega F. Association of type II apoptosis and 92-kDa type IV collagenase expression in human amniochorion in prematurely ruptured membranes with tumor necrosis factor receptor-1 expression. J Soc Gynecol Investig 2002; 9:60–67. [DOI] [PubMed] [Google Scholar]
- 40. Parry S, Strauss JF.. Premature rupture of the fetal membranes. N Engl J Med 1998; 338:663–670. [DOI] [PubMed] [Google Scholar]
- 41. Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol 2011; 179:838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang X, Christenson LK, Nothnick WB. Regulation of MMP-9 expression and activity in the mouse uterus by estrogen. Mol Reprod Dev 2007; 74:321–331. [DOI] [PubMed] [Google Scholar]
- 43. Li W, Challis JRG.. Corticotropin-releasing hormone and urocortin induce secretion of matrix metalloproteinase-9 (MMP-9) without change in tissue inhibitors of MMP-1 by cultured cells from human placenta and fetal membranes. J Clin Endocrinol Metab 2005; 90:6569–6574. [DOI] [PubMed] [Google Scholar]
- 44. Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update 2016; 22:535–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol 2008; 79:50–57. [DOI] [PubMed] [Google Scholar]
- 46. Arechavaleta-Velasco F, Ogando D, Parry S, Vadillo-Ortega F. Production of matrix metalloproteinase-9 in lipopolysaccharide-stimulated human amnion occurs through an autocrine and paracrine proinflammatory cytokine-dependent system. Biol Reprod 2002; 67:1952–1958. [DOI] [PubMed] [Google Scholar]
- 47. Keelan JA, Blumenstein M, Helliwell RJA, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition—a review. Placenta 2003; 24:S33–S46. [DOI] [PubMed] [Google Scholar]
- 48. Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol 1999; 181:1530–1536. [DOI] [PubMed] [Google Scholar]
- 49. Chiossi G, Costantine MM, Bytautiene E, Kechichian T, Hankins GD V, Sbrana E, Saade GR, Longo M. The effects of prostaglandin E1 and prostaglandin E2 on in vitro myometrial contractility and uterine structure. Amer J Perinatol 2012; 29:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iliodromiti Z, Antonakopoulos N, Sifakis S, Tsikouras P, Daniilidis A, Dafopoulos K, Botsis D, Vrachnis N. Endocrine, paracrine, and autocrine placental mediators in labor. Horm 2012; 11:397–409. [DOI] [PubMed] [Google Scholar]
- 51. Dittrich R, Ph D, Mueller A, Oppelt PG, Hoffmann I, Beckmann MW, Maltaris T. Differences in muscarinic-receptor agonist-, oxytocin-, and prostaglandin-induced uterine contractions. Fertil Steril 2009; 92:1694–1700. [DOI] [PubMed] [Google Scholar]
- 52. Ulug U, Goldman S, Ben-Shlomo I, Shalev E. Matrix metalloproteinase (MMP)-2 and MMP-9 and their inhibitor, TIMP-1, in human term decidua and fetal membranes: the effect of prostaglandin F2alpha and indomethacin. Mol Hum Reprod 2001; 7:1187–1193. [DOI] [PubMed] [Google Scholar]
- 53. Brown S, Bernardo MM, Li ZH, Kotra LP, Tanaka Y, Fridman R, Mobashery S. Potent and selective mechanism-based inhibition of gelatinases. J Am Chem Soc 2000; 122:6799–6800. [Google Scholar]
- 54. Eldred JA, Hodgkinson LM, Dawes LJ, Reddan JR, Edwards DR, Wormstone IM. MMP2 activity is critical for TGFβ2-induced matrix contraction-implications for fibrosis. Investig Ophthalmol Vis Sci 2012; 53:4085–4098. [DOI] [PubMed] [Google Scholar]
- 55. Prado AF, Pernomian L, Azevedo A, Costa RAP, Rizzi E, Ramos J, Paes Leme AF, Bendhack LM, Tanus-Santos JE, Gerlach RF. Matrix metalloproteinase-2-induced epidermal growth factor receptor transactivation impairs redox balance in vascular smooth muscle cells and facilitates vascular contraction. Redox Biol 2018; 18:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wilkinson JM, Davidson RK, Swingler TE, Jones ER, Corps AN, Johnston P, Riley GP, Chojnowski AJ, Clark IM. MMP-14 and MMP-2 are key metalloproteases in Dupuytren's disease fibroblast-mediated contraction. Biochim Biophys Acta - Mol Basis Dis 2012; 1822:897–905. [DOI] [PubMed] [Google Scholar]
- 57. Townley WA, Cambrey AD, Khaw PT, Grobbelaar AO. Matrix metalloproteinase inhibition reduces contraction by Dupuytren fibroblasts. J Hand Surg Am 2008; 33:1608–1616. [DOI] [PubMed] [Google Scholar]
- 58. Raffetto JD, Barros YVR, Wells AK, Khalil RA. MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res 2010; 159:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chew DKW, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg 2004; 40:1001–1010. [DOI] [PubMed] [Google Scholar]
- 60. Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 2013; 13:649–665. [DOI] [PubMed] [Google Scholar]
- 61. Cauwe B, Opdenakker G. Intracellular substrate cleavage: a novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol 2010; 45:351–423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.