Abstract
Calcium-dependent protein kinases (CDPKs) are found in various subcellular localizations, which suggests that this family of serine/threonine kinases may be involved in multiple signal transduction pathways. CDPKs are believed to be involved in the response of plants to low temperatures, but the precise role in the signal transduction pathway is largely unknown. Previous reports described changes in CDPKs' mRNA levels in response to cold treatment, but whether these changes are accompanied by increases in protein level and/or kinase activities is unknown. In the present study, we identify in rice (Oryza sativa L. cv Don Juan) plants a 56-kD membrane-bound CDPK that is activated in response to cold treatment. Immunoblot analysis of the enzyme preparations from control and cold-treated plants showed that the kinase level was similar in both preparations. However, both kinase and autophosphorylating activities of the enzyme prepared from cold-treated plants were significantly higher than that obtained from control plants. The activation of the CDPK is detected after 12 to 18 h of cold treatment, which indicates that the kinase does not participate in the initial response to low temperature but in the adaptative process to adverse conditions. To our knowledge, this is the first demonstration of a CDPK that is posttranscriptionally activated in response to low temperature.
Low temperature is one of the most important environmental factors limiting the geographic distribution of plants and accounts for significant reductions in the yield of agriculturally important crops (Boyer, 1982). To respond to cold stress, plants must perceive low temperature signals and transduce them into biochemical responses. After several primary transient responses to cold, such as membrane depolarization and increase in cytosolic calcium concentration, there is an orchestration of subsequent events in plant physiology. These events include protein phosphorylation, altered gene activity, and changes in secondary metabolism (Monroy et al., 1993; Berbich and Kusano, 1997; Monroy et al., 1997; Shinozaki and Yamaguchi-Shinozaki, 2000).
Several lines of evidence lead to propose calcium as second messenger in response to chilling (Minorsky, 1989; Minorsky and Spanswick, 1989; Knight et al., 1991) and cold acclimation (Ding and Pickard, 1993; Monroy et al., 1993). These studies reported transient changes of cytoplasmic calcium concentrations and modulation of channel activity by low temperature, as well as the observation that calcium chelators, calcium channel blockers, and inhibitors of calcium-dependent protein kinases (CDPKs) prevent cold acclimation.
CDPKs are a family of Ser/Thr protein kinases first discovered in plants and also found in protozoa (Harmon, 1991). CDPKs are biochemically distinct from other calcium-regulated kinases such as protein kinase C and calcium/calmodulin-dependent protein kinases. The basic structural features of CDPKs are conserved. Within a single polypeptide chain, these kinases contain three functional domains: catalytic, autoinhibitory, and calcium binding (Roberts and Harmon, 1992; Harmon et al., 2000). CDPKs are found in several subcellular localizations; cytoplasm, membranes, and some isoforms have been localized in both compartments (Putnam-Evans et al., 1990; Morello et al., 1993; Abo-El-Saad and Wu, 1995; Barker et al., 1998). A subset of these kinases contains an src homology domain (SH4) at the N-terminal portion of the molecule that targets them to the membrane fraction through lipid modifications (Martín and Busconi, 2000). The SH4 domain has a consensus sequence for myristoylation, which is an irreversible cotranslational lipid modification, and one or two Cys residues that can be reversibly and posttranslationally modified by palmitoylation (Resh, 1994). Although CDPKs have been often proposed to play a central role in calcium-dependent pathways, the collection of direct evidence linking CDPKs to these pathways has been challenging. Transient expression of genes encoding CDPKs in maize (Zea mays) protoplasts showed for the first time the connection of particular CDPKs to specific signal/response pathways (Sheen, 1996).
There is little information concerning the regulation of CDPKs in response to low temperature and most of the data is related to changes in RNA levels. In alfalfa (Medicago sativa), the transcript levels of two CDPKs are differentially regulated by low temperature (Monroy and Dhindsa, 1995), but it is not known if the increases in mRNA levels are accompanied by increases in protein levels and/or kinase activities. In rice (Oryza sativa L. cv Don Juan), the gene encoding rice CDPK7 is induced by cold and salt stresses in both shoots and roots (Saijo et al., 1998). It has been reported more recently that overexpression of the OsCDPK7 protein conferred both cold and salt/drought tolerance in rice plants. The OsCDPK7 protein was expressed in transgenic plants at similar levels both in the presence or absence of stress stimuli, thus suggesting that a posttranslational mechanism(s) should regulate the kinase activity in plant cells (Saijo et al., 2000).
In the present study, we searched for CDPKs possibly involved in the response of rice plants to low temperatures. We found a 56-kD membrane-bound CDPK whose kinase and autophosphorylating activities increased after several hours of cold treatment. The protein level of the enzyme remained constant with the cold treatment, which indicates that the enzyme is posttranslationally activated.
RESULTS
Calcium-Dependent Kinase Activity from Soluble and Membrane Fractions in Response to Low Temperatures
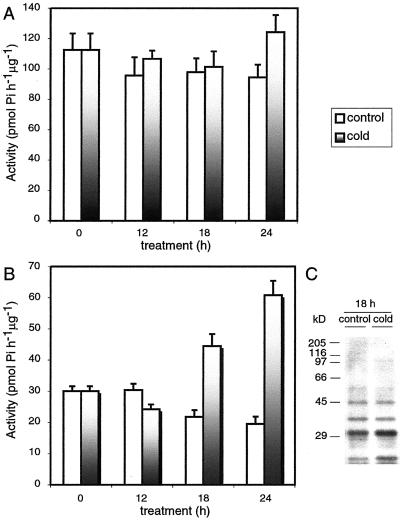
CDPKs are known to be involved in calcium signaling. These kinases are found in cytoplasm, membranes, and some isoforms in both compartments. To test a possible involvement of CDPKs in response to low temperatures, rice plants were grown at 27°C for 2 weeks and then either kept at 27°C (control plants) or shifted to 12°C (cold-treated plants). Shoots were harvested at different periods of time of cold treatment, and soluble and membrane fractions were prepared. Membranes were solubilized with a buffer containing 1% (v/v) Nonidet P-40, and calcium-dependent kinase activity was assayed in both fractions using the synthetic peptide, syntide-2, as a substrate. Figure 1, A and B, shows that calcium-dependent kinase activity did not change in the cytosolic fraction but markedly increased in the membrane fraction as a consequence of the cold treatment. Eighteen to 24 h after the beginning of the cold treatment, the calcium-dependent kinase activity of membranes from cold-treated plants was 3-fold higher than that of control membranes.
Figure 1.
Time course of calcium-dependent kinase activity from soluble and membrane fractions from cold-treated rice plants. Rice plants were grown at 27°C for 2 weeks and then either kept at 27°C (control plants) or shifted to 12°C (cold-treated plants). Shoots were harvested at different periods of time and soluble and membrane fractions were prepared. The kinase activity was assayed using syntide-2 as substrate (see “Materials and Methods”) in soluble (A) and membrane (B) fractions. C, Phosphorylation of endogenous proteins present in membranes prepared from control and cold-treated plants. The intensity of the bands was quantified using the TN Image software (version 2.13, T.J. Nelson, Rockville, MD). The experiment was repeated four times with similar results.
We next investigated whether the increase in calcium-dependent kinase activity in membranes from cold-treated plants would be reflected in changes in the in vitro phosphorylation pattern of membrane proteins. Figure 1C shows that when membrane fractions from control plants or plants subjected to 18 h of cold treatment were incubated in the presence of [32P]ATP and calcium and analyzed by SDS-PAGE, several endogenous proteins became phosphorylated. It is interesting that membranes from cold-treated plants showed a specific increase of 40% in the degree of phosphorylation of two polypeptides with molecular masses of 31 and 21 kD with respect to membranes from control plants. A similar effect was observed after 24 h of cold treatment (not shown). Protein phosphorylation was calcium dependent because no phosphorylation was observed in the presence of EGTA (data not shown).
Isolation of a Membrane-Bound Calcium- Dependent Kinase
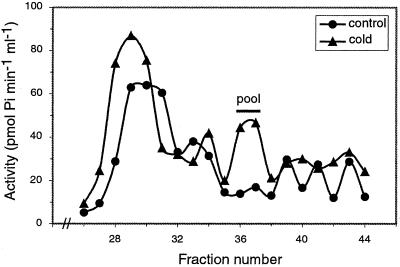
CDPKs are monomeric enzymes with molecular masses ranging between 50 and 90 kD. To characterize the kinase activity (or activities) responsible for the increase of the calcium-dependent syntide-2 phosphorylating activity observed after cold treatment, we analyzed solubilized membranes from control and cold-treated (18 h at 12°C) plants by gel filtration chromatography over a Sephadex G-100 column (Pharmacia, Uppsala; Fig. 2). Aliquots of the column fractions were assayed for kinase activity using syntide-2 as substrate in the absence (EGTA) or presence of calcium. Figure 2 shows the profile corresponding to the calcium-dependent kinase activity after substrating the calcium-independent activity. Several peaks corresponding to calcium-dependent kinase activities were observed in membranes from control and cold-treated plants. However, we consistently observed a marked increase in the kinase activity present in fractions 28 through 30 and 35 through 38 in the elution profile of membranes from cold-treated plants. We decided to further characterize the kinase activity present in fractions 35 through 38 because the extent of its activation in cold-treated plants with respect to control plants was consistently higher than that of the kinase activity present in fractions 28 through 30.
Figure 2.
Gel filtration chromatography of solubilized membranes from control and cold-treated rice plants. Solubilized membranes prepared from shoots obtained from control (27°C) and cold-treated (12°C) plants were chromatographed on a Sephadex G-100 column. The kinase activity was assayed using syntide-2 as substrate (see “Materials and Methods”). The molecular mass markers used were: myosin (205 kD), β-galactosidase (116 kD), phosphorylase b (97 kD), ovoalbumin (45 kD), and carbonic anhydrase (29 kD). Similar results were obtained in three different experiments.
Properties of the Membrane-Bound Calcium-Dependent Kinase Activity Present in Membranes from Cold-Treated Plants
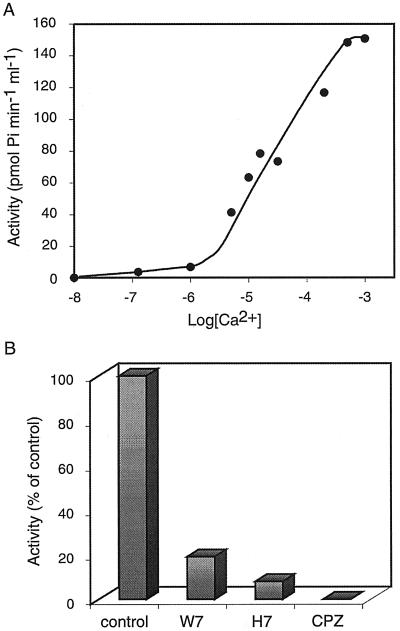
Fractions 36 and 37 from the Sephadex column were pooled and used for further characterization of the kinase activity. We first studied the calcium requirements of the enzyme and its response to different inhibitors. Figure 3A shows that the half-maximal calcium concentration of the kinase activity was 15 μm. The activity was inhibited by H7, an inhibitor of protein kinase C that also affects the activity of CDPKs, and by two calmodulin antagonists: CPZ {2-chloro-10-[3-(dimethylamino)propyl]phenothiazine, HCl} and W-7 (Fig. 3B; Roberts and Harmon 1992; Ritchie and Gilroy, 1998). Taken together, these results strongly suggest that the kinase belongs to the CDPK family. Similar results were obtained when the kinase was further purified by Affigel-blue affinity chromatography (data not shown). However, this enzyme preparation could not be used for the experiments described below because the purified enzyme was highly unstable.
Figure 3.
Characterization of the membrane-bound calcium-dependent kinase from cold-treated plants. The fractions pooled from the Sephadex G-100 column were used to study the effect of calcium concentration (A) and the effect of inhibitors on the kinase activity (B). The activity was assayed using syntide-2 as substrate (see “Materials and Methods”). Similar results were obtained in three different experiments.
Characterization of the Membrane-Bound CDPK by Immunoblot and In-Gel Kinase Assays
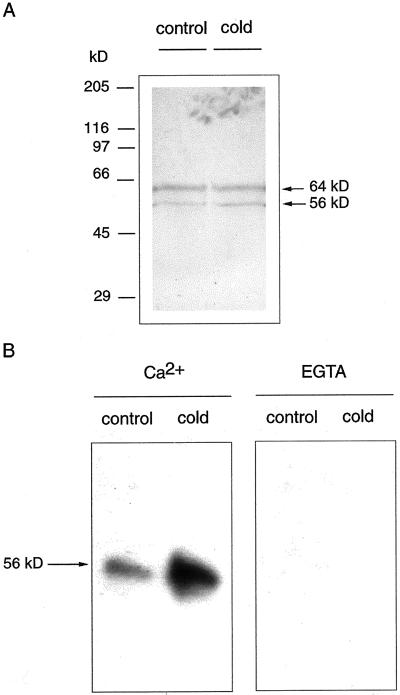
To confirm that the kinase is a CDPK, the pooled fractions from Sephadex G-100 chromatography were subjected to immunoblot analysis using a polyclonal anti-CDPK antibody made against the calmodulin-like domain of soybean (Glycine max) CDPK. Figure 4A shows that the antibody recognized two polypeptides with molecular masses of 56 and 64 kD in enzyme fractions from both control and cold-treated plants. The levels of these polypeptides were similar in both enzyme preparations. This immunoblot experiment did not allow us to inequivocally identify the band corresponding to the CDPK whose activity increased by cold treatment because the antibody recognized two polypeptides. To solve this problem we performed an in-gel kinase assay: We electrophoresed the enzyme fractions from control and cold-treated plants on SDS-polyacrylamide gels to which histone III-S had been added to the polymerization mixture as kinase substrate. After protein renaturation, the gel was incubated with [32P]ATP to allow the detection of histone phosphorylation. The results depicted in Figure 4B show that only the polypeptide with a molecular mass of 56kD had kinase activity, and that this activity was significantly higher in enzyme fractions from cold-treated plants than in equivalent fractions from control plants. Shorter exposure times of the autoradiograms (not shown) allowed us to confirm that only the 56-kD and not the 64-kD polypeptide underwent autophosphorylation under these conditions. These results, together with the immunoblot analysis shown in Figure 4A, indicate that the protein level of the 56-kD CDPK did not change upon the cold treatment, but there was an increase in the activity of the preexisting enzyme. The absence of kinase activity of the 64-kD polypeptide recognized by the anti-CDPK antibody suggests that the band corresponded either to a non-CDPK calmodulin-like domain containing protein or to an unsuccessfully renatured CDPK.
Figure 4.
Characterization of the membrane-bound CDPK by immunoblot and in-gel kinase assay. Partially purified CDPK prepared from shoots from control (27°C) and cold-treated (12°C) plants was analyzed by immunostaining (A) and in-gel kinase activity (B). A, Samples were electrophoresed in 8% (w/v) SDS-polyacrylamide gels, electroblotted to nitrocellulose, and probed with a polyclonal antibody against the calmodulin-like domain of soybean CDPK. B, Samples were electrophoresed in 8% (w/v) SDS-polyacrilamide gel containing Histone III-S and incubated with [32P]ATP as described in “Materials and Methods.” The prestained molecular mass markers used were: myosin (202 kD), β-galactosidase (133 kD), bovine serum albumin (71 kD), carbonic anhydrase (41.8 kD), soybean trypsin inhibitor (30.6 kD), lysozyme (17.8 kD), and aprotinin (6.9 kD)
Autophosphorylation of the Membrane-Bound CDPK
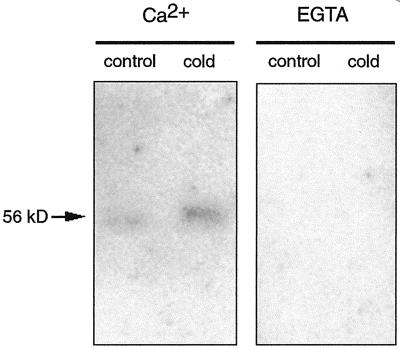
Autophosphorylation is a common regulatory mechanism of protein kinases, which leads to changes in their activities and/or dependence on activators. We decided to investigate if the higher activity observed in the membrane-bound CDPK in response to low temperatures paralleled a higher autophosphorylation activity. To this end, the same enzyme preparations used in the experiments shown in Figure 4 were subjected to SDS-PAGE, proteins were renatured in the gel, and the gel was incubated with [32P]ATP to allow the detection of autophosphorylation. Figure 5 shows that the level of autophosphorylation of the enzyme obtained from cold-treated plants was higher than that obtained from control plants. This self-catalyzed phosphate incorporation was inhibited in the presence of 1 mm EGTA, which indicates that the reaction was calcium dependent.
Figure 5.
Autophosphorylation of the CDPK isolated from control and cold-treated plants. Partially purified CDPK prepared from shoots from control (27°C) and cold-treated (12°C) plants was electrophoresed in 8% (w/v) SDS-polyacrylamide gel and incubated with [32P]ATP as described in “Materials and Methods.”
DISCUSSION
The mechanisms by which low-temperature signals are perceived and transduced into biochemical responses are poorly understood. When plants are exposed to low temperatures they first respond with an early set of events that include a shift in membrane fluidity and cold-induced calcium influx, followed by a secondary response in which calcium- and cold-regulated protein kinases and phosphatases are believed to be involved (Monroy et al., 1998). Calcium acts as a second messenger in response to chilling and in cold acclimation processes. CDPKs, the most abundant Ser/Thr kinases present in plants, are believed to participate in transducing the signal.
CDPKs are localized in cytoplasm, membranes, or in both compartments. Thus, in an effort to identify CDPKs potentially involved in the response to low temperatures, we examined the activity of calcium-dependent kinases in soluble and membrane fractions prepared from shoots of rice plants that had been shifted from 27°C to 12°C. Twelve degrees lies within the range of temperatures that triggers calcium influx into the cytoplasm and induces changes in membrane fluidity in chilling-sensitive plants (Levitt, 1980; Monroy and Dhindsa, 1995). Our results show that the activity of calcium-dependent kinases in response to low temperatures was unchanged in the soluble fraction, whereas the activity associated to membranes increased after 18 h of cold treatment. In some experiments, the increase was observed as early as 12 h after the temperature shift (data not shown). These results led us to characterize the calcium-dependent kinase whose activity increased with the cold treatment. We partially purified a 56-kD kinase that, according to its calcium requirements, the effect of various inhibitors on its activity and its recognition by a polyclonal antibody against a soybean CDPK, was identified as a membrane-bound CDPK.
Cold stress-induced gene expression of some CDPKs has been reported in several plant species. However, whether the increases in mRNA levels are accompanied by increases in protein levels and/or kinase activities is largely unknown. To try to understand the mechanism by which the activity of the membrane-bound CDPK increased with the cold treatment, we analyzed if its protein level and/or its enzyme activity were affected in response to low temperature. Taken together, our results of the western-blot analysis and the in-gel kinase assay clearly indicate that the 56-kD kinase was a preexisting membrane-bound CDPK whose intrinsic activity increased in response to cold treatment. The fact that the activation of the kinase was observed when similar amounts of the enzyme from control and cold-treated plants were subjected to SDS-PAGE rules out the possibility that the activation could be caused by dissociation from macromolecular complexes or endogenous inhibitors during the extraction and/or purification procedures.
Autophosphorylation is a common regulatory property of protein kinases, leading to changes in their activity (Chaudhuri et al., 1999). It is interesting that we observed that the autophosphorylating activity of the kinase was higher when the enzyme was obtained from cold-treated plants than when it was isolated from control plants. A likely interpretation of our results is that the CDPK's activation by the cold treatment might be due to conformational changes caused by cold-induced posttranslational modifications, which might also affect its autophosphorylating activity. We have recently reported that a rice membrane-bound CDPK, OsCPK2, contains an SH4 domain located at the N-terminal portion of the molecule. This domain, which serves as a myristate and palmitate acceptor, is responsible for targeting OsCPK2 to membranes (Martín and Busconi, 2000). In a similar manner, it seems possible that the 56-kD CDPK, which is also membrane bound, might have myristoylation and palmitoylation sites at the N-terminal portion of the molecule. Because palmitoylation is a lipid modification that can be reversibly regulated by different stimuli (Robinson et al., 1995; Wedegaertner et al., 1995), it is interesting to speculate that palmitoylation may cause a conformational change in the CDPK affecting its autophosphorylating activity. In any case, cloning of the 56-kD CDPK will be necessary to understand in detail its mechanism of activation and in vivo experiments will be required to analyze if this increase in the enzyme's autophosphorylating activity has a regulatory role. We cannot rule out that other posttranslational modifications such as phosphorylation by other kinases or limited proteolysis could be responsible for the observed enzyme activation.
Rice seeds contain a 58-kD membrane-bound CDPK whose kinase and autophosphorylating activities are posttranslationally stimulated by the hormone gibberellin (Abo-El-Saad and Wu, 1995). This CDPK has remarkable similarities with the CDPK described in this paper, suggesting that there may be a common mechanism of regulation of membrane-bound CDPKs by different stimuli.
To our knowledge this is the first identification by biochemical analysis of a membrane-bound CDPK that is posttranscriptionally activated in response to low temperatures. It is important to mention that the activation of the CDPK is detected after 12 to 18 h of cold treatment, which indicates that the kinase does not participate in the initial response to low temperature but in the adaptative process to adverse conditions. The upstream events responsible for this activation are currently being investigated.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Rice (Oryza sativa L. cv Don Juan) plants were grown at 27°C for 2 weeks with a photoperiod of 14 h and then either kept at 27°C (control plants) or shifted to 12°C (cold-treated plants). The cold treatment did not have any effect on the shoot growth during the time course of the experiment (up to 24 h at 12°C). Shoots from both sets of plants were harvested at the indicated time points and inmediately frozen in liquid nitrogen.
Chemicals
W7, H7, and CPZ were purchased from Calbiochem (San Diego), [32P]ATP was purchased from DuPont-New England Nuclear (Boston), prestained electrophoresis markers were purchased from Bio-Rad (Hercules, CA), and Syntide-2, Histone III-S, and all other chemicals were purchased from Sigma Chemical (St. Louis) unless mentioned otherwise.
Preparation of Soluble and Membrane Fractions
All procedures were performed at 4°C. To prepare soluble and membrane fractions, 5 g of shoot tissue was ground in a chilled mortar with 2.5 vol of buffer A (50 mm Tris-HCl [pH 8.0], 50 μm EDTA, 2 mm dithiothreitol [DTT], 5 mm NaF, 1 mm NaVO4, 20 mm β-glicerophosphate, 1 mg mL−1 leupeptin, and 2 mg mL−1 aprotinin). The crude extract was filtered through cheesecloth and centrifuged at 3,000g for 10 min to remove cell debris. The homogenate was centrifuged at 100,000g for 1 h obtaining a soluble fraction (kept for protein kinase activity assays) and a pellet. The pellet was washed in buffer A and the membrane fraction was obtained by resuspending the pellet during 1 h at 4°C in buffer A containing 1% (v/v) Nonidet P-40. When membranes were prepared for further purification steps, a similar procedure was used but starting from 30 g of shoots. Protein concentration was determined using the Bradford method.
Purification Procedures
Membrane fractions prepared from control and cold-treated plants (3 mg of protein) were applied to a Sephadex G-100 filtration column (60 × 1 cm) equilibrated with buffer B (50 mm Tris-HCl [pH 7.5], 10 mm MgCl2, 2 mm DTT, 0.1 mm EDTA, and 100 mm NaCl containing 1% [v/v] Nonidet P-40). Fractions 36 and 37 shown in Figure 2 were pooled, diluted with buffer B to obtain a final Nonidet P-40 concentration of 0.1% (v/v), and used in further characterization experiments.
Kinase Activity Assay
The kinase activity was determined by phosphate incorporation into a synthetic peptide, syntide-2 (Abdel-Ghany et al., 1989). The reaction was carried out in a final volume of 30 μL and aliquots of the different column fractions (10 μL) were assayed in a reaction mixture containing 20 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 50 μm [32P]ATP (specific activity 100 cpm pmol−1), 10 mm β-mercaptoethanol, and 25 μm syntide-2 with the addition of 1 μm CaCl2 or 1 mm EGTA. After incubating at 30°C for 10 min, samples were placed on P81 phosphocellulose squares (Whatman, Springfield Mill, Maidstone, UK) and the reaction was stopped by immersion in 0.5% (v/v) orthophosphoric acid. The paper squares were washed, dried, and counted as described (Ulloa et al., 1991). The kinase activity was measured in the absence (EGTA) or presence of calcium and the calcium-dependent kinase activity was expressed after substrating the calcium-independent activity.
The activity was monitored at different concentrations of free Ca2+ by using Ca2+/EGTA buffers in standard reaction mixtures (Bartfai, 1979).
To determine the effect of inhibitors on the kinase activity, aliquots of fractions pooled from the Sephadex G-100 column were pre-incubated with different inhibitors (3 mm CPZ, 1 mm W7, and 100 μm H7) during 10 min at 30°C and then the kinase activity was assayed as described above in the presence of 1 μm CaCl2 or 1 mm EGTA.
In Vitro Phosphorylation of Endogenous Proteins
Aliquots of membrane fractions (10 μg) were incubated in a final volume of 20 μL in a reaction mixture containing 25 mm Tris-HCl (pH 8.0), 1 mm DTT, 2.5 mm NaF, 0.5 mm NaVO4, 10 mm β-glicerophosphate, 10 μm phenylmethylsulfonyl fluoride, 0.5 mg mL−1 Leupeptin, 1 mg mL−1 aprotinin, 10 mm MgCl2, and 50 μm [32P]ATP (specific activity 3,000 cpm pmol−1) in the presence of 1 μm CaCl2 or 1 mm EGTA. The reaction was incubated for 6 min at 30°C and stopped by the addition of 5 μL of 5× Laemmli sample buffer and heating for 3 min in boiling water. The sample buffer contained DTT (the final concentration in the sample was 5 mm) instead of mercaptoethanol. Proteins were resolved in 10% (w/v) SDS-PAGE and analyzed by autoradiography.
In-Gel Kinase Assay
The in-gel kinase assay was performed according to Carter (1999). Aliquots of pooled fractions from the Sephadex G-100 column were subjected to 8% (w/v) SDS-PAGE previously polymerized with 1 mg mL−1 Histone III-S. After protein renaturation, the gel was incubated for 60 min at room temperature in a buffer containing 25 mm Tris-HCl (pH 8.0), 50 μm EDTA, 50 μm EGTA, 1 mm DTT, 2.5 mm NaF, 0.5 mm NaVO4, 10 mm β-glicerophosphate, 10 μm phenylmethylsulfonyl fluoride, 0.5 mg mL−1 Leupeptin, 1 mg mL−1 aprotinin, 10 mm MgCl2, and 50 μm [32P]ATP (specific activity 880 cpm pmol−1) in the presence of 50 μm CaCl2 or 1 mm EGTA, washed, dried, and analyzed by autoradiography.
Autophosphorylation was performed as described above except that the gel was polymerized in the absence of histone and the incubation was done using 50 μm [32P]ATP (specific activity 440 cpm pmol−1) and 1 μm CaCl2.
Western-Blot Analysis
Aliquots from pooled fractions from the Sephadex G-100 column were resolved in 8% (w/v) SDS-PAGE and electroblotted onto nitrocellulose membranes (Amersham, Buckinghamshire, UK). Mr markers were identified by staining the membrane with Red Ponceau. We used as primary antibody a polyclonal antibody against the calmodulin-like domain of soybean (Glycine max) CDPK (dilution 1/1000). The kinase was visualized using the 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium substrate system according to the manufacturer's directions.
ACKNOWLEDGMENTS
We thank Dr. E. Blumwald (University of California, Davis) for his encouragement. We thank Dr. Alice H. Harmon (University of Florida, Gainesville) for generously providing the polyclonal antibody against the calmodulin-like domain of a soybean CDPK and Dr. Eduardo Folco (Harvard Medical School, Boston) for his comments and critical reading of the manuscript.
Footnotes
L.B. is a Career Investigator of Consejo Nacional de Investigaciones Científicas y Technológicas (CONICET) and the recipient of a grant from Fundación Antorchas. M.L.M. is a recipient of a Research Fellowship from CONICET.
LITERATURE CITED
- Abdel-Ghany M, Kole H, Abo-El-Saad M, Racker E. Stimulation of phosphorylation of lipocortin at threonine residues by EGF and the EGF-receptor. Proc Natl Acad Sci USA. 1989;86:6072–6076. doi: 10.1073/pnas.86.16.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo-El-Saad M, Wu R. A rice calcium-dependent protein kinase is induced by gibberellin. Plant Physiol. 1995;108:787–793. doi: 10.1104/pp.108.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker LDP, Templeton MD, Ferguson IB. A 67-kDa plasma membrane-bound Ca2+-stimulated protein kinase active in sink tissue of higher plants. Planta. 1998;205:197–204. [Google Scholar]
- Bartfai T. Preparation of metal chelate complex and the design of steady-state kinetics experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Berbich T, Kusano T. Cycloheximide induces a subset of low-temperature inducible genes in maize. Mol Gen Genet. 1997;254:275–283. doi: 10.1007/s004380050416. [DOI] [PubMed] [Google Scholar]
- Boyer JS. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Carter AN. Analysis of protein phosphorylation. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl E, editors. Short Protocols in Molecular Biology. New York: Wiley & Sons, Inc; 1999. pp. 24–25. [Google Scholar]
- Chaudhuri S, Seal A, DasGupta M. Autophosphorylation-dependent activation of a calcium-dependent protein kinase from groundnut. Plant Physiol. 1999;120:859–866. doi: 10.1104/pp.120.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JP, Pickard BG. Modulation of the mechanosensitive calcium-selective cation channels by temperature. Plant J. 1993;3:713–720. [PubMed] [Google Scholar]
- Harmon AC. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science. 1991;252:951–954. doi: 10.1126/science.1852075. [DOI] [PubMed] [Google Scholar]
- Harmon AC, Gribskov M, Harper JF. CDPKs: a kinase for every Ca2+ signal? Trends Plant Sci. 2000;5:154–159. doi: 10.1016/s1360-1385(00)01577-6. [DOI] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Levitt J. Responses of plants to environmental stresses. In: Kozlowski TT, editor. Physiological Ecology: A Series of Monographs, Texts, and Treatises. 1: Chilling, Freezing, and High Temperature Stresses. New York: Academic Press; 1980. pp. 23–64. [Google Scholar]
- Martín ML, Busconi L. Membrane localization of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J. 2000;24:1–7. doi: 10.1046/j.1365-313x.2000.00889.x. ) [DOI] [PubMed] [Google Scholar]
- Minorsky PV. Temperature sensing by plants: a review and hypothesis. Plant Cell Environ. 1989;12:119–135. [Google Scholar]
- Minorsky PV, Spanswick RM. Electrophysiological evidence for a role of calcium in temperature sensing by roots of cucumber seedlings. Plant Cell Environ. 1989;12:137–143. [Google Scholar]
- Monroy AF, Dhindsa RS. Low-temperature signal transduction: induction of cold acclimation-specific genes of alfalfa by calcium at 25°C. Plant Cell. 1995;7:321–331. doi: 10.1105/tpc.7.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy AF, Labbé E, Dhindsa RS. Low-temperature perception in plants: effects of cold on protein phosphorylation in cell-free extracts. FEBS Lett. 1997;410:206–209. doi: 10.1016/s0014-5793(97)00589-9. [DOI] [PubMed] [Google Scholar]
- Monroy AF, Sangwan V, Dhindsa RS. Low temperature signal transduction during cold acclimation: protein phosphatase 2A as an early target for cold-inactivation. Plant J. 1998;13:653–660. [Google Scholar]
- Monroy AF, Sarhan F, Dhindsa RS. Cold-induced changes in freezing tolerance, protein phosphorylation, and gene expression: evidence for a role of calcium. Plant Physiol. 1993;102:1227–1235. doi: 10.1104/pp.102.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello L, Giani S, Coraggio I, Breviario D. Rice membranes contain a calcium-dependent protein kinase activity with biochemical features of animal protein kinase C. Biochem Biophys Res Commun. 1993;197:55–61. doi: 10.1006/bbrc.1993.2440. [DOI] [PubMed] [Google Scholar]
- Putnam-Evans CL, Harmon AC, Cormier MJ. Purification and characterization of a novel calcium-dependent protein kinase from soybean. Biochemistry. 1990;29:2488–2495. doi: 10.1021/bi00462a008. [DOI] [PubMed] [Google Scholar]
- Resh MD. Myristoylation and palmitoylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Ritchie S, Gilroy S. Calcium-dependent protein phosphorylation may mediate the gibberellic acid response in barley aleurone. Plant Physiol. 1998;116:765–776. doi: 10.1104/pp.116.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Harmon AC. Calcium-modulated proteins: targets of intracellular calcium signals in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:375–414. [Google Scholar]
- Robinson LJ, Busconi L, Michel T. Agonist-modulated palmitoylation of endothelial nitric oxide synthase. J Biol Chem. 1995;270:995–998. doi: 10.1074/jbc.270.3.995. [DOI] [PubMed] [Google Scholar]
- Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000;23:319–327. doi: 10.1046/j.1365-313x.2000.00787.x. [DOI] [PubMed] [Google Scholar]
- Saijo Y, Hato S, Izui K. Characterization of a rice cold-stress-inducible calcium-dependent protein kinase 475(H1p18) Plant Cell Physiol Suppl. 1998;39:s127. [Google Scholar]
- Sheen J. Ca2+-dependent protein kinases and stress signal transduction in plants. Science. 1996;274:1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: cross-talk between two stress signaling pathways. Curr Opin Plant Biol. 2000;3:217–223. [PubMed] [Google Scholar]
- Ulloa RM, Torres HN, Ochatt CM, Téllez-Iñon MT. Ca2+ calmodulin-dependent protein kinase activity in the ascomycetes Neurospora crassa. Mol Cell Biochem. 1991;102:155–163. doi: 10.1007/BF00234573. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Wilson PT, Bourne HR. Lipid modifications of trimeric G proteins. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]