Summary
Background
Cerebral microbleeds are a neuroimaging biomarker of stroke risk. A crucial clinical question is whether cerebral microbleeds indicate patients with recent ischaemic stroke or transient ischaemic attack in whom the rate of future intracranial haemorrhage is likely to exceed that of recurrent ischaemic stroke when treated with antithrombotic drugs. We therefore aimed to establish whether a large burden of cerebral microbleeds or particular anatomical patterns of cerebral microbleeds can identify ischaemic stroke or transient ischaemic attack patients at higher absolute risk of intracranial haemorrhage than ischaemic stroke.
Methods
We did a pooled analysis of individual patient data from cohort studies in adults with recent ischaemic stroke or transient ischaemic attack. Cohorts were eligible for inclusion if they prospectively recruited adult participants with ischaemic stroke or transient ischaemic attack; included at least 50 participants; collected data on stroke events over at least 3 months follow-up; used an appropriate MRI sequence that is sensitive to magnetic susceptibility; and documented the number and anatomical distribution of cerebral microbleeds reliably using consensus criteria and validated scales. Our prespecified primary outcomes were a composite of any symptomatic intracranial haemorrhage or ischaemic stroke, symptomatic intracranial haemorrhage, and symptomatic ischaemic stroke. We registered this study with the PROSPERO international prospective register of systematic reviews, number CRD42016036602.
Findings
Between Jan 1, 1996, and Dec 1, 2018, we identified 344 studies. After exclusions for ineligibility or declined requests for inclusion, 20 322 patients from 38 cohorts (over 35 225 patient-years of follow-up; median 1·34 years [IQR 0·19–2·44]) were included in our analyses. The adjusted hazard ratio [aHR] comparing patients with cerebral microbleeds to those without was 1·35 (95% CI 1·20–1·50) for the composite outcome of intracranial haemorrhage and ischaemic stroke; 2·45 (1·82–3·29) for intracranial haemorrhage and 1·23 (1·08–1·40) for ischaemic stroke. The aHR increased with increasing cerebral microbleed burden for intracranial haemorrhage but this effect was less marked for ischaemic stroke (for five or more cerebral microbleeds, aHR 4·55 [95% CI 3·08–6·72] for intracranial haemorrhage vs 1·47 [1·19–1·80] for ischaemic stroke; for ten or more cerebral microbleeds, aHR 5·52 [3·36–9·05] vs 1·43 [1·07–1·91]; and for ≥20 cerebral microbleeds, aHR 8·61 [4·69–15·81] vs 1·86 [1·23–2·82]). However, irrespective of cerebral microbleed anatomical distribution or burden, the rate of ischaemic stroke exceeded that of intracranial haemorrhage (for ten or more cerebral microbleeds, 64 ischaemic strokes [95% CI 48–84] per 1000 patient-years vs 27 intracranial haemorrhages [17–41] per 1000 patient-years; and for ≥20 cerebral microbleeds, 73 ischaemic strokes [46–108] per 1000 patient-years vs 39 intracranial haemorrhages [21–67] per 1000 patient-years).
Interpretation
In patients with recent ischaemic stroke or transient ischaemic attack, cerebral microbleeds are associated with a greater relative hazard (aHR) for subsequent intracranial haemorrhage than for ischaemic stroke, but the absolute risk of ischaemic stroke is higher than that of intracranial haemorrhage, regardless of cerebral microbleed presence, antomical distribution, or burden.
Funding
British Heart Foundation and UK Stroke Association.
Introduction
A central challenge in stroke prevention after ischaemic stroke or transient ischaemic attack is to predict the risk of intracranial haemorrhage and to differentiate this from the risk of recurrent ischaemic stroke in patients treated with antithrombotic therapy—usually antiplatelet drugs or, in patients with atrial fibrillation, oral anticoagulants.1 Cerebral microbleeds are a radiological finding of small (<10 mm), hypointense (black), ovoid or rounded regions on T2*-weighted gradient-recalled echo (GRE) or susceptibility-weighted imaging (SWI).2 Cerebral microbleeds mostly correspond pathologically to haemosiderin-laden macrophages close to arterioles affected by small vessel diseases;3, 4 strictly lobar cerebral microbleeds suggest cerebral amyloid angiopathy (CAA), whereas deep patterns probably indicate arteriolosclerosis and mixed patterns probably indicate mixed pathologies.5, 6, 7, 8 Cerebral microbleeds might result from red blood cell leakage from arterioles and capillaries, raising clinical concerns that they herald an increased risk of potentially devastating intracranial haemorrhage, particularly in patients treated with antithrombotic drugs.9 However, cerebral microbleeds signal small vessel diseases that can also cause ischaemic stroke, and might result from non-haemorrhagic mechanisms.10, 11, 12, 13 In ischaemic stroke cohorts, cerebral microbleeds are associated with the risks of both subsequent intracranial haemorrhage and recurrent ischaemic stroke.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 As the number of cerebral microbleeds increases, the risk of intracranial haemorrhage seems to rise more steeply than that of ischaemic stroke, and having five or more cerebral microbleeds has been reported to be associated with similar absolute risks of intracranial haemorrhage and ischaemic stroke.28, 29
Because previous studies had small sample sizes and few intracranial haemorrhage outcome events, they could not reliably answer the important clinical question of whether many cerebral microbleeds, or patterns (distributions) of cerebral microbleeds, indicate a higher risk of intracranial haemorrhage than of recurrent ischaemic stroke. We established the Microbleeds International Collaborative Network30 to undertake large-scale pooled analyses of prospective observational cohort studies. We tested the hypothesis that a large burden of cerebral microbleeds, or their anatomical patterns, can identify ischaemic stroke or transient ischaemic attack patients at higher absolute risk of intracranial haemorrhage than ischaemic stroke.
Research in context.
Evidence before this study
We searched Medline and EMBASE from Jan 1, 1996, to Dec 1, 2018 (search strategy: “cerebral adj2 micro*” OR “CMB” OR “microbleed.mp” AND [“stroke.mp” OR “stroke/” OR “intracerebral h?emorr*” OR “intracranial h?emorr*” OR “isch?emic stroke” OR “isch?emic infarct*”]) for studies in English that included patients with ischaemic stroke or transient ischaemic attack in whom the presence and anatomical distribution of cerebral microbleeds were measured at baseline, with at least 90 days of follow-up. An aggregate level meta-analysis (n=5068) showed that cerebral microbleeds were associated with both intracranial haemorrhage (risk ratio [RR] 3·8 [95% CI 3·5–11·4]) and ischaemic stroke (RR 1·8 [1·4–2·5]); this pooled analysis, and another study in two cohorts (one including 1003 mainly Chinese participants and the other including 1080 mainly white participants) reported that five or more cerebral microbleeds were associated with similar absolute risks of intracranial haemorrhage and ischaemic stroke. However, small sample sizes and few intracranial haemorrhage outcome events in previous studies did not provide enough statistical power and precision to establish whether a large cerebral microbleed burden or distribution pattern is associated with a higher absolute risk of intracranial haemorrhage than ischaemic stroke in patients with recent ischaemic stroke or transient ischaemic attack treated with antithrombotic drugs.
Added value of this study
Our pooled analysis of individual data from 20 322 patients shows that regardless of cerebral microbleed burden and distribution (ie, mixed, deep, or lobar), or the type of antithrombotic treatment received (oral anticoagulants or antiplatelet therapy), the absolute rate of ischaemic stroke is consistently substantially higher than that of intracranial haemorrhage. By contrast with previous studies, the large number of participants provided more precise estimates of stroke recurrence rates and risks, while inclusion of individual patient data allowed adjustment for potential confounding factors. Our study adds new data for patients with many (eg, ≥20) cerebral microbleeds, which cause the most clinical concern regarding intracranial bleeding.
Implications of all the available evidence
Although cerebral microbleeds can inform regarding the hazard for intracranial haemorrhage in patients with recent ischaemic stroke or transient ischaemic attack treated with antithrombotic drugs, the absolute risk of ischaemic stroke is much higher than that of intracranial haemorrhage, regardless of cerebral microbleed presence, burden, or pattern. The available evidence does not support witholding antithrombotic treatment because of cerebral microbleeds, but to definitively answer this question requires data from randomised controlled trials.
Methods
Study design
For this pooled analysis of individual patient data, we identified cohorts by searching Medline and EMBASE (search terms “cerebral adj2 micro*” OR “CMB” OR “microbleed.mp” AND “stroke.mp” OR “stroke/” OR “intracerebral h?emorr*” OR “intracranial h?emorr*” OR “isch?emic stroke” OR “isch?emic infarct*”), clinical trial databases (clinicaltrials.gov and strokecenter.org), and scientific meeting abstracts. We invited members of the METACOHORTS consortium;31 an international database of more than 90 studies of small vessel disease, including 660 000 patients. Two authors (DW and DJWe) independently did the search and reviewed all titles and abstracts; they also did an independent risk of bias assessment for all included studies. Cohorts were eligible for inclusion if they prospectively recruited adult participants with ischaemic stroke or transient ischaemic attack; included at least 50 participants; collected data on stroke events over at least 3 months follow-up; used an appropriate MRI sequence that is sensitive to magnetic susceptibility (GRE or SWI); and documented the number and anatomical distribution of cerebral microbleeds reliably using consensus criteria and validated scales. Each patient was only included in one cohort. We assessed all studies for risk of bias (including selection bias) and quality using the Cochrane Collaboration tool.32 All cohorts obtained ethical approval as required by local regulations to allow data sharing. All data reviewed by the co-ordinating centre was fully anonymised. The project was approved by the Health Research Authority of the UK (REC reference: 8/HRA/0188). The Microbleeds International Collaborative Network protocol and statistical analysis plan were registered with PROSPERO on April 5, 2016 (CRD42016036602).
Outcomes
Our prespecified primary outcomes were a composite of any symptomatic intracranial haemorrhage (confirmed radiologically, including subdural, extradural, and subarachnoid haemorrhage, and excluding intracranial haemorrhages attributed to intravenous thrombolysis or trauma) or ischaemic stroke (acute or subacute neurological symptoms lasting >24 h and attributed to cerebral ischaemia, diagnosed clinically, with or without radiological confirmation); symptomatic intracranial haemorrhage; and symptomatic ischaemic stroke. Secondary outcome events were death (all cause) and vascular death. All events were adjudicated according to individual cohort protocols.
Statistical analysis
As per our prespecified protocol, a single dataset was created by combining individual participant data from the 38 cohorts. We compared baseline demographic and risk factor profiles between patients with and without cerebral microbleeds and between patients with and without outcome events using the Mann-Whitney test if not normally distributed or the t test if normally distributed; we compared categorical variables between groups with the χ2 test or Fisher's exact test. We censored patients at the last available follow-up (truncated to 5 years) or at the time of the prespecified outcome event. When a patient had multiple events of the same type, we censored follow-up at the first event. We calculated absolute event rates per 1000 patient-years for primary outcomes in patients with and without cerebral microbleeds. We assessed the proportional hazards assumption through visual inspection of (log–log) plots of log cumulative hazard against time and tested for a non-zero slope in a regression of scaled Schoenfeld residuals against time. We calculated univariate Kaplan-Meier survival probabilities in patients with and without cerebral microbleeds to estimate event rates and used the log-rank test to compare groups. We did multivariable Cox regression adjusting for the following prognostic and confounding variables (selected by consensus based on availability, biological plausibility, and known associations with cerebral microbleeds and outcomes): age, sex, presentation with transient ischaemic attack or ischaemic stroke, history of hypertension, previous stroke, known atrial fibrillation, antithrombotic use after index event, and type of MRI sequence used to detect cerebral microbleeds (T2*-weighted GRE or SWI). We investigated the effect of predefined cerebral microbleed burden categories (one, two to four, five or more, ten or more, and 20 or more). When investigating cerebral microbleed distribution, we adjusted for number of cerebral microbleeds. We added a shared frailty term33 to account for patients being nested in individual studies (thus potentially having correlated data). We performed subanalyses for patients treated with oral anticoagulants and antiplatelet drugs and added interaction terms between antithrombotic therapy and presence of cerebral microbleeds. We categorised ethnicity (when available) as white or Asian (Japanese, Chinese, Malays, Indian, Pakistani, or Korean) to investigate the interaction between ethnicity and cerebral microbleed presence. We performed two prespecified sensitivity analyses: the first exploring time-varying risks within the Cox model to investigate later events (beyond the first year) accounting for death as a competing risk (using the Fine-Gray subdistribution hazard model), calculating subdistribution hazard ratios (sHRs); and the second, a two-stage individual-patient meta-analysis to quantify between-study heterogeneity using the inverse-variance method (which fits a separate survival model for each cohort then pools and displays estimates in a forest plot). We did three post-hoc analyses as follows: (1) we added white matter hyperintensities (another common marker of cerebral small vessel disease, rated using the Fazekas scale34 and considered severe if rated two or greater in the periventricular of deep white matter) into our multivariable model; (2) we included only intracerebral haemorrhage, convexity subarachnoid haemorrhage, and subdural haemorrhage, because these bleeding events are the most likely to be associated with cerebral microbleeds; and (3) we investigated the interaction between cerebral microbleeds and age (<80 years or ≥80 years). In sensitivity analyses, if data for a variable of interest was not sufficiently available in a cohort, the cohort was excluded. We did all statistical analysis using STATA, version 15.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, or data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
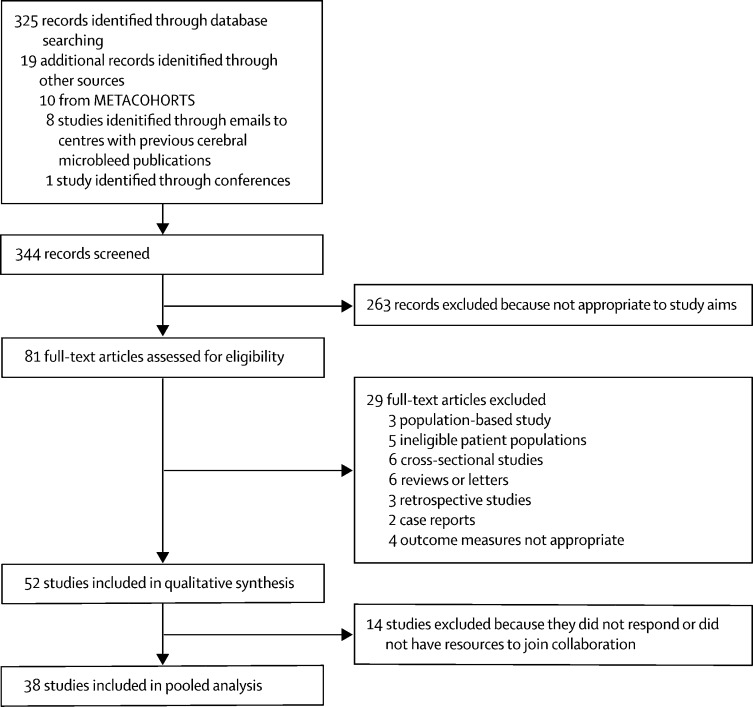
Between Jan 1, 1996, and Dec 1, 2018, we identified and screened 344 records (325 from database search and 19 from other sources; figure 1). 263 records were excluded because they were not full-text articles, and then a further 29 full-text articles were excluded because they did not meet study inclusion criteria. The remaining 52 studies were included in our qualitative analyses, but 14 of these were excluded from the meta-analysis because they did not respond to requests for individual patient data or declined to join the collaboration (reasons included a lack of resources or because of data sharing policies). From the 38 remaining cohorts (23 published and 15 unpublished studies), we included 20 322 participants (table 1). Although more than half of participants and outcome events came from the six largest cohorts, no major risk of bias was detected for any included cohort (appendix). The mean age of participants was 70 years (SD 13); 8593 (42%) of the 20 322 were women. Cerebral microbleeds were present in 5649 (28%) patients (appendix), including 2415 (12%) with one cerebral microbleed, 1990 (10%) with two to four cerebral microbleeds, and 1244 (6%) with five or more cerebral microbleeds. Over the 35 225 patient-years of follow-up (median 1·34 years [IQR 0·19–2·44]), 1474 composite events occurred: 189 intracranial haemorrhages; 1113 ischaemic strokes; and 172 composite events of unknown type from one cohort of 3355 participants, which did not subclassify composite outcomes as intracranial haemorrhage or ischaemic stroke. Characteristics between patients with and without events are in the appendix. Visual assessment of the log-log plots and the results of testing the Schoenfeld residuals suggest that the proportional hazards assumption was not violated in any of the following analyses.
Figure 1.
Study selection profile
Table 1.
Demographics, risk factors, and outcome events for each cohort
| Total partici-pants | Taking oral anticoagu-lants | Transient ischaemic attack | Mean age (SD), years | Proportion women | Hyper-tension | Atrial fibrillation | Previous stroke | Ischaemic heart disease | Any cerebral microbleed | Susceptibility-weighted imaging | Median follow-up, days (IQR) | Patients with composite events | Participants with intracranial haemorrhage events | Participants with ischaemic stroke events | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CROMIS-227 | 1490 | 1436 (96%) | 238 (16%) | 76 (10) | 631 (42%) | 930 (63%) | 1490 (100%) | 148 (10%) | 243 (16%) | 311 (21%) | 0 | 774 (705–974) | 70 (5%) | 14 (1%) | 56 (4%) |
| HBS | 660 | 114 (17%) | 60 (9%) | 69 (15) | 289 (44%) | 498 (75%) | 194 (29%) | 156 (24%) | 138 (21%) | 98 (15%) | 2 (<1%) | 90 (90–90) | 4 (1%) | 0 | 4 (1%) |
| IPAAC-Warfarin35 | 182 | 173 (95%) | 27 (15%) | 73 (9) | 84 (46%) | 158 (87%) | 179 (98%) | 36 (20%) | 17 (9%) | 68 (37%) | 182 (100%) | 738 (191–812) | 7 (4%) | 1 (1%) | 6 (3%) |
| Bern36 | 392 | 74 (19%) | 0 | 68 (14) | 169 (43%) | 249 (64%) | 142 (46%) | 59 (15%) | 69 (18%) | 90 (23%) | 392 (100%) | 93 (5–106) | 16 (4%) | 0 | 16 (4%) |
| CU-STRIDE37 | 536 | 24 (4%) | 81 (15%) | 67 (11) | 227 (42%) | 373 (70%) | 38 (7%) | 80 (15%) | 35 (7%) | 124 (23%) | 238 (44%) | 524 (472–557) | 17 (3%) | 2 (<1%) | 15 (3%) |
| TABASCO38 | 436 | 33 (8%) | 213 (28%) | 67 (9) | 184 (43%) | 250 (59%) | 33 (8%) | 0 | 60 (14%) | 64 (15%) | 0 | 1825 (1164–1825) | 57 (13%) | 0 | 57 (13%) |
| Graz | 460 | 78 (17%) | 48 (10%) | 67 (13) | 179 (39%) | 359 (78%) | 115 (25%) | 102 (22%) | 95 (21%) | 88 (19%) | 0 | 117 (87–973) | 65 (14%) | 13 (3%) | 54 (12%) |
| PERFORM-MRI39 | 1056 | 0 | 127 (12%) | 68 (8) | 370 (35%) | 887 (84%) | 16 (2%) | 120 (11%) | 69 (7%) | 381 (36%) | 0 | 774 (701–1042) | 104 (10%) | 10 (1%) | 94 (9%) |
| PARISK40 | 228 | 0 | 127 (56%) | 71 (9) | 67 (29%) | 156 (68%) | 0 | 66 (29%) | 50 (22%) | 61 (27%) | 0 | 786 (757–819) | 10 (4%) | 0 | 10 (4%) |
| SAMURAI NVAF41 | 1103 | 1039 (94%) | 45 (4%) | 78 (10) | 480 (44%) | 1027 (93%) | 1103 (100%) | 246 (22%) | 101 (9%) | 265 (24%) | 817 (74%) | 723 (758–818) | 82 (7%) | 10 (1%) | 72 (7%) |
| RUNDMC42 | 179 | 19 (11%) | 89 (50%) | 65 (9) | 63 (35%) | 145 (81%) | 18 (10%) | 47 (26%) | 31 (17%) | 35 (20%) | 0 | 1825 (1825–1825) | 25 (14%) | 2 (1%) | 23 (13%) |
| Wuerzburg | 358 | 122 (34%) | 77 (22%) | 71 (13) | 158 (44%) | 287 (80%) | 105 (29%) | 91 (25%) | 38 (11%) | 87 (24%) | 160 (45%) | 95 (89–103) | 22 (6%) | 1 (<1%) | 21 (6%) |
| Monash Stroke43 | 359 | 356 (99%) | 52 (15%) | 75 (11) | 173 (48%) | 285 (79%) | 359 (100%) | 101 (28%) | 122 (34%) | 154 (43%) | 339 (94%) | 530 (280–898) | 14 (4%) | 7 (2%) | 9 (3%) |
| Basel TIA18 | 192 | 33 (17%) | 192 (100%) | 69 (13) | 73 (38%) | 137 (71%) | 26 (14%) | 13 (7%) | 38 (20%) | 21 (11%) | 0 | 90 (90–90) | 26 (14%) | 0 | 26 (14%) |
| Yonsei44 | 504 | 487 (97%) | 28 (6%) | 70 (11) | 288 (57%) | 392 (78%) | 504 (100%) | 101 (20%) | 109 (22%) | 155 (31%) | 0 | 849 (393–1398) | 56 (11%) | 7 (1%) | 49 (10%) |
| SNUBH Stroke Cohort45, 46 | 3355 | 625 (19%) | 368 (11%) | 67 (13) | 1347 (40%) | 2324 (69%) | 630 (19%) | 487 (15%) | 284 (8%) | 1166 (35%) | 1 (<1%) | 355 (340–365) | 172 (5%) | NA | NA |
| BIOSTROKE/TIA47 | 260 | 73 (28%) | 160 (62%) | 68 (13) | 95 (37%) | 150 (59%) | 77 (31%) | 21 (8%) | 57 (22%) | 24 (9%) | 0 | 90 (90–365) | 14 (5%) | 0 | 14 (5%) |
| Kushiro City48 | 784 | 63 (8%) | 0 | 72 (11) | 330 (42%) | 498 (64%) | 104 (13%) | 142 (18%) | 89 (11%) | 320 (41%) | 0 | 1008 (105–1825) | 139 (18%) | 22 (3%) | 119 (15%) |
| Soo49 | 81 | 81 (100%) | 16 (20%) | 72 (9) | 40 (49%) | 56 (69%) | 81 (100%) | 25 (31%) | 8 (10%) | 24 (30%) | 71 (88%) | 737 (641–794) | 8 (10%) | 3 (4%) | 5 (6%) |
| CASPER50 | 135 | 18 (13%) | 0 | 66 (11) | 39 (29%) | 96 (71%) | 13 (10%) | 10 (7%) | 29 (21%) | 79 (59%) | 135 (100%) | 453 (444–465) | 3 (2%) | 0 | 3 (2%) |
| HERO51 | 937 | 933 (>99%) | 122 (13%) | 78 (7) | 488 (52%) | 694 (74%) | 468 (50%)* | 248 (27%) | 146 (16%) | 248 (26%) | 0 | 737 (641–794) | 49 (5%) | 18 (2%) | 32 (3%) |
| HAGAKURE | 426 | 157 (37%) | 35 (8%) | 74 (13) | 174 (41%) | 320 (76%) | 135 (32%) | 76 (18%) | 45 (11%) | 158 (37%) | 39 (9%) | 748 (350–1040) | 34 (8%) | 9 (2%) | 25 (6%) |
| Leuven14 | 487 | 133 (27%) | 133 (27%) | 72 (9) | 192 (39%) | 313 (64%) | 103 (21%) | 61 (13%) | 112 (23%) | 129 (26%) | 0 | 804 (686–968) | 36 (7%) | 4 (1%) | 32 (7%) |
| NOACISP | 306 | 286 (93%) | 30 (10%) | 73 (19) | 139 (45%) | 240 (79%) | 306 (100%) | 60 (20%) | 83 (27%) | 87 (28%) | 300 (98%) | 735 (417–836) | 28 (9%) | 10 (3%) | 19 (6%) |
| Min Lou52 | 126 | 14 (11%) | 0 | 65 (13) | 46 (37%) | 94 (75%) | 25 (20%) | 18 (14%) | 4 (3%) | 42 (33%) | 126 (100%) | 92 (87–218) | 2 (2%) | 0 | 2 (2%) |
| MICRO21 | 397 | 40 (10%) | 362 (91%) | 65 (12) | 165 (42%) | 218 (55%) | 30 (8%) | 35 (9%) | 24 (6%) | 72 (18%) | 0 | 1212 (579–1825) | 30 (8%) | 11 (3%) | 21 (5%) |
| Orken53 | 454 | 454 (100%) | 20 (4%) | 72 (12) | 233 (51%) | 258 (79%) | 296 (65%) | 123 (27%) | 79 (32%)† | 134 (30%) | 250 (55%) | 575 (228–1825) | 11 (2%) | 3 (1%) | 8 (2%) |
| CATCH54 | 416 | 67 (16%) | 173 (42%) | 67 (14) | 164 (39%) | 226 (54%) | 27 (6%) | 0 | NA | 65 (16%) | 0 | 88 (80–100) | 14 (3%) | 1 (<1%) | 13 (3%) |
| MSS255 | 263 | 24 (9%) | 0 | 67 (12) | 109 (41%) | 190 (72%) | 25 (10%) | 32 (12%) | 53 (20%) | 44 (17%) | 251 (95%) | 368 (253–403) | 31 (12%) | 0 | 31 (12%) |
| Sainte-Anne (Paris) | 385 | 302 (78%) | 0 | 80 (11) | 204 (53%) | 277 (72%) | 358 (100%) | 61 (16%) | 72 (19%) | 99 (26%) | 0 | 440 (163–733) | 25 (6%) | 5 (1%) | 23 (6%) |
| STROKDEM | 181 | 48 (27%) | 0 | 64 (13) | 69 (38%) | 100 (55%) | 12 (7%) | 20 (11%) | 17 (9%) | 24 (13%) | 0 | 1150 (420–1820) | 17 (9%) | 0 | 17 (9%) |
| Singapore (Chen) | 45 | 15 (33%) | 0 | 67 (10) | 13 (29%) | 34 (76%) | 11 (24%) | 3 (7%) | 4 (9%) | 25 (56%) | 45 (100%) | 1057 (703–1199) | 6 (13%) | 0 | 6 (13%) |
| FUTURE Study | 19 | 0 | 7 (37%) | 44 (6) | 10 (53%) | 8 (42%) | 0 | 0 | 0 | 1 (5%) | 19 (100%) | 164 (131–242) | 4 (21%) | 0 | 4 (21%) |
| Heidelberg56 | 650 | 119 (18%) | 109 (17%) | 64 (14) | 240 (37%) | 496 (76%) | 115 (18%) | 107 (17%) | NA | 155 (24%) | 650 (100%) | 1534 (1271–1825) | 34 (5%) | 4 (1%) | 30 (5%) |
| NNI | 184 | 32 (17%) | 0 | 58 (11) | 57 (31%) | 143 (78%) | 28 (15%) | 27 (15%) | NA | 50 (27%) | 0 | 251 (86–477) | 0 | 0 | 0 |
| OXVASC29 | 1080 | 118 (11%) | 572 (52%) | 68 (14) | 514 (48%) | 588 (55%) | 167 (15%) | 201 (19%) | 146 (13%) | 157 (15%) | 0 | 1271 (681–1825) | 90 (8%) | 11 (1%) | 79 (7%) |
| HKU29 | 1003 | 104 (10%) | 0 | 69 (12) | 402 (40%) | 657 (66%) | 130 (13%) | 116 (12%) | 92 (9%) | 450 (45%) | 1003 (100%) | 1005 (599–1549) | 112 (11%) | 20 (2%) | 92 (9%) |
| SIGNaL | 213 | 43 (20%) | 22 (10%) | 72 (14) | 88 (41%) | 152 (71%) | 67 (32%) | 60 (28%) | 49 (23%) | 94 (44%) | 144 (68%) | 225 (202–249) | 27 (13%) | 1 (<1%) | 26 (12%) |
| Total | 20 322 | 7737/20 319 (38%) | 3443/20 311 (17%) | 70 (13) | 8593/20 314 (42%) | 14 365/20 271 (71%) | 7557/20 207 (37%) | 3299/20 290 (16%) | 2608/18 842 (14%) | 5649/20 322 (28%) | 5164/20 284 (25%) | 534 (243–928) | 1461/20 322 (7%) | 189/16 967 (1%) | 1113/16 967 (7%) |
Data are n (%) or n/N (%) unless otherwise stated. Studies without references are unpublished. FUTURE study=Follow-Up of Transient ischemic attack and stroke patients and Unelucidated Risk factor Evaluation study. HAGAKURE=Hypertension, Amyloid, and aGe Associated Kaleidoscopic brain lesions on CT/MRI Undertaken with stroke REgistry. HBS=Heart Brain Interactions Study. NNI=National Neuroscience Institute, Singapore. NOACISP=Novel Oral Anticoagulants in Stroke Patients, Basel; NCT02353585. SIGNaL=Stroke Investigation in North and Central London. STROKDEM=Study of Factors Influencing Post-stroke Dementia.
Denominator for this result is 932.
Denominator for this result is 250.
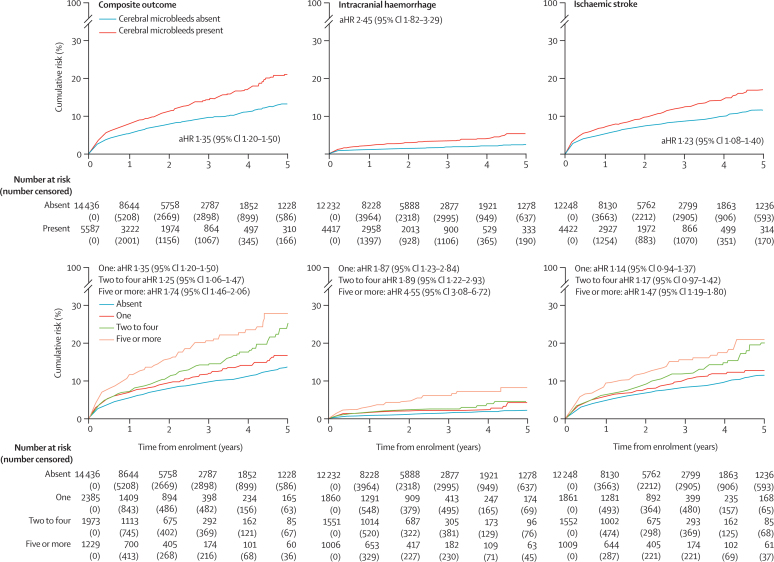
The composite outcome of any intracranial haemorrhage or ischaemic stroke (aHR 1·35 [95% CI 1·20–1·50], p<0·0001; log-rank test), symptomatic intracranial haemorrhage (2·45 [1·82–3·29], p<0·0001), and symptomatic ischaemic stroke (1·23 [1·08–1·40], p<0·0001) were more frequent in patients with cerebral microbleeds than those without (figure 2; appendix).
Figure 2.
Kaplan-Meier estimates for the primary outcomes in all patients (n=20 322)
The incidence of all composite events in patients with any cerebral microbleed was 59 per 1000 patient-years (95% CI 54–64) compared with 35 per 1000 patient-years (33–38) in those without cerebral microbleeds, an absolute increased incidence of 24 per 1000 patient-years (21–26; table 2). The aHR for a composite event became larger with increased cerebral microbleed burden (figure 2, table 2; ptrend<0·0001). aHRs were similar across different cerebral microbleed anatomical distributions (table 2).
Table 2.
Rate and risk of outcome events according to number (burden) and anatomical distribution of baseline cerebral microbleeds in all patients (n=20 322)
|
Composite of intracranial haemorrhage and ischaemic stroke (n=19 816 for multivariable model) |
Symptomatic intracranial haemorrhage (n=16 447 for multivariable model) |
Symptomatic ischaemic stroke (n=16 464 for multivariable model) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate, per 1000 patient-years* | Absolute rate increase, per 1000 patient-years | Adjusted hazard ratio | Rate, per 1000 patient-years | Absolute rate increase, per 1000 patient-years | Adjusted hazard ratio | Rate, per 1000 patient-years | Absolute rate increase, per 1000 patient-years | Adjusted hazard ratio | ||
| None | 35 (33–38) | .. | 1 (ref) | 4 (3–5) | .. | 1 (ref) | 30 (28–33) | .. | 1 (ref) | |
| Any | 59 (54–64) | 24 (21–26) | 1·35 (1·20–1·50) | 12 (10–14) | 8 (7–9) | 2·45 (1·82–3·29) | 46 (42–51) | 16 (14–18) | 1·23 (1·08–1·40) | |
| One | 46 (40–53) | 11 (7–15) | 1·21 (1·03–1·42) | 8 (5–12) | 4 (2–7) | 1·87 (1·23–2·84) | 37 (31–44) | 7 (3–11) | 1·14 (0·94–1·37) | |
| Number | ||||||||||
| Two to four | 58 (50–67) | 23 (17–29) | 1·25 (1·06–1·47) | 9 (6–14) | 5 (3–9) | 1·89 (1·22–2·93) | 48 (40–56) | 18 (12–23) | 1·17 (0·97–1·42) | |
| Five or more† | 85 (73–99) | 50 (40–61) | 1·74 (1·46–2·06) | 23 (16–31) | 19 (13–26) | 4·55 (3·08–6·72) | 64 (53–77) | 34 (25–43) | 1·47 (1·19–1·80) | |
| Ten or more† | 91 (73–113) | 56 (40–75) | 1·82 (1·44–2·29) | 27 (17–41) | 23 (14–36) | 5·52 (3·36–9·05) | 64 (48–84) | 34 (20–51) | 1·43 (1·07–1·91) | |
| 20 or more† | 118 (86–160) | 83 (53–122) | 2·61 (1·90–3·57) | 39 (21–67) | 35 (18–62) | 8·61 (4·69–15·81) | 73 (46–108) | 43 (18–75) | 1·86 (1·23–2·82) | |
| Anatomical distribution | ||||||||||
| Mixed | 80 (68–94) | 45 (35–56) | 1·28 (1·06–1·54) | 20 (14–28) | 16 (11–23) | 2·38 (1·55–3·65) | 60 (49–73) | 30 (21–40) | 1·12 (0·88–1·41) | |
| Deep | 73 (65–82) | 38 (32–44) | 1·29 (1·12–1·48) | 17 (13–22) | 13 (10–17) | 2·57 (1·78–3·70) | 57 (49–66) | 27 (21–33) | 1·14 (0·96–1·36) | |
| Lobar | 60 (53–67) | 25 (20–29) | 1·22 (1·06–1·41) | 13 (9–16) | 9 (6–9) | 1·87 (1·29–2·71) | 48 (42–56) | 18 (14–23) | 1·17 (0·99–1·40) | |
| Probable cerebral amyloid angiopathy | 55 (40–73) | 20 (7–35) | 1·21 (0·90–1·64) | 9 (4–18) | 5 (1–13) | 1·29 (0·60–2·77) | 48 (34–66) | 18 (6–33) | 1·31 (0·94–1·83) | |
Ranges in brackets are 95% CIs. Cerebral microbleed location hazard ratios are versus patients without cerebral microbleeds in each location and are adjusted for cerebral microbleed number and our prespecified variables.
Number of patients and time at risk are shown in the appendix.
Overlapping categories.
189 patients had a symptomatic intracranial haemorrhage over 32 847 patient-years of follow-up (151 intracerebral haemorrhages, 31 subdural haemorrhages, eight subarachnoid haemorrhages [four of which were cortical], and three extradural haemorrhages; four patients had more than one type of intracranial haemorrhage). The incidence of intracranial haemorrhage was 12 per 1000 patient-years (95% CI 10–14) in those with cerebral microbleeds compared with 4 per 1000 patient-years (3–5) in those without cerebral microbleeds, an absolute increased incidence of 8 per 1000 patient-years (7–9; table 2). The rate of intracranial haemorrhage increased with increasing cerebral microbleed burden, but was consistently lower than the rate of ischaemic stroke (table 2). The aHR for symptomatic intracranial haemorrhage was 2·45 (95% CI 1·82–3·29) for patients with cerebral microbleeds versus those without, and became larger with increased cerebral microbleed burden (ptrend <0·0001; figure 2; table 2); aHRs did not significantly differ between different cerebral microbleed anatomical distributions. Patients with multiple strictly lobar cerebral microbleeds (fulfilling the Boston criteria5 for probable CAA) did not have a significantly higher aHR for symptomatic intracranial haemorrhage than those without multiple strictly lobar cerebral microbleeds (1·29 [95% CI 0·60–2·77]; table 2). No interaction was detected between cerebral microbleeds and antiplatelet medication (pinteraction=0·358), oral anticoagulants (pinteraction=0·717), or combined oral anticoagulants and antiplatelet medication (pinteraction=0·163) for intracranial haemorrhage risk.
1113 patients had a symptomatic ischaemic stroke over 32 293 patient-years of follow-up. The incidence of symptomatic ischaemic stroke in patients with cerebral microbleeds was 46 per 1000 patient-years (95% CI 42–51) compared with 30 per 1000 patient-years (28–33) in those without, with an absolute increased incidence of 16 per 1000 patient-years (14–18; table 2). The rate of ischaemic stroke became greater with an increasing burden of cerebral microbleeds, and for each burden category substantially exceeded the rate of intracranial haemorrhage (table 2). The aHR for symptomatic ischaemic stroke was 1·23 (95% CI 1·08–1·40) for patients with cerebral microbleeds versus those without, and the aHR became larger with increasing cerebral microbleed burden (ptrend=0·0053; figure 2; table 2). Cerebral microbleed anotomical distribution had little effect on ischaemic stroke risk (table 2). No interaction was detected between cerebral microbleeds and antiplatelet medication (pinteraction=0·943) or oral anticoagulants (pinteraction=0·408) for ischaemic stroke risk, but there was weak evidence for an interaction between cerebral microbleeds and combined use of oral anticoagulants and antiplatelet medication (pinteraction=0·047).
There were 2148 deaths, 484 of which were due to vascular causes. In multivariable analyses, cerebral microbleed presence was not associated with all-cause death (aHR 1·03 [95% CI 0·94–1·12]) or vascular death (aHR 0·97 [0·79–1·19]). No interaction was detected between cerebral microbleeds and ethnicity (n=15 123; 6743 white and 8380 Asian) for the risks of the composite outcome of intracranial haemorrhage or ischaemic stroke (pinteraction=0·707); intracranial haemorrhage (pinteraction=0·537); or ischaemic stroke (pinteraction=0·654). No interaction was detected between cerebral microbleed and older age (4376 patients older than 80 years) for the risk of the composite outcome (pinteraction=0·538); intracranial haemorrhage (pinteraction=0·219); or ischaemic stroke (pinteraction=0·286).
Using a two-stage meta-analysis, the estimated risks associated with cerebral microbleed presence were consistent with our main model for the composite outcome (heterogeneity [I2=31·7%]; intracranial haemorrhage [I2=0%]; and ischaemic stroke [2=24·2%]; appendix).
23 cohorts, including 10 235 patients, provided ratings for white matter hyperintensities, which were moderate to severe (Fazekas grade ≥2) in 3105 (30%) patients. Including white matter hyperintensities in multivariable models did not substantially change the aHR associated with the presence of cerebral microbleeds for the composite outcome (aHR 1.30 [95% CI 1.12–1.52]); intracranial haemorrhage (aHR 2.44 [1.68–3.53]); or for ischaemic stroke (aHR 1.16 [0.98–1.37]).
In our sensitivity analysis including only intracerebral, convexity subarachnoid, and subdural intracranial haemorrhages, 183 patients had a symptomatic intracranial haemorrhage over 32 847 patient-years of follow-up. The aHR for symptomatic intracranial haemorrhage was 2·59 (95% CI 1·91–3·50) for patients with cerebral microbleeds versus patients without, and became larger with increasing burden. Compared with no cerebral microbleeds, aHRs were 1·92 (95% CI 1·25–2·94) for one cerebral microbleed; 2·02 (1·30–3·16) for two to four cerebral microbleeds; 4·88 (3·29–7·25) for five or more cerebral microbleeds; 5·87 (3·56–9·66) for ten or more cerebral microbleeds; and 9·32 (5·06–17·16) for 20 or more cerebral microbleeds. These results are consistent with our primary findings.
There were 102 symptomatic intracranial haemorrhages over 12 794 patient-years of follow-up within the first year, and 87 over 31 059 patient-years of follow-up after the first year. In patients with cerebral microbleeds, the rate of intracranial haemorrhage was 18 per 1000 patient-years (95% CI 14–23) within the first year, and 5 per 1000 patient-years (3–6) after the first year.
696 ischaemic strokes were recorded over 12 873 patient-years of follow-up within the first year and 417 symptomatic ischaemic strokes during 30 447 patient-years of follow-up after the first year. In patients with cerebral microbleeds, the rate of symptomatic ischaemic stroke within the first year was 70 (95% CI 62–80), then 18 (15–21) after the first year.
Accounting for death as a competing risk, we found no evidence for a change in risk over time associated with cerebral microbleed presence for intracranial haemorrhage (sHR 4·96 [95% CI 3·18–7·74] at day 0 vs 4·81 [3·15–7·35] after 1 year) or ischaemic stroke (sHR 1·46 [1·23–1·73] at day 0 vs 1·49 [1·27–1·75] after 1 year).
In those treated with oral anticoagulants after their index ischaemic stroke or transient ischaemic attack (n=7737; vitamin K antagonist=5253, non-vitamin K oral anticoagulant=2484), 91 intracranial haemorrhages occurred over 13 942 patient-years of follow-up, and 384 ischaemic strokes occurred over 13 737 patient-years of follow-up. For patients with cerebral microbleeds, the rate of intracranial haemorrhage was 12 per 1000 patient-years (95% CI 9–16); the rate of ischaemic stroke was 32 per 1000 patient-years (26–39; table 3). The rate of ischaemic stroke was much higher than that of intracranial haemorrhage for all cerebral microbleed burden and anatomical distribution categories; the aHR for intracranial haemorrhage for patients with cerebral microbleeds (vs those without) rose more steeply than that of ischaemic stroke with increasing cerebral microbleed burden. Mixed and deep cerebral microbleed distributions had similar aHRs for intracranial haemorrhage, but patients with lobar cerebral microbleeds had a lower risk of intracranial haemorrhage (table 3). Cerebral microbleeds were not significantly associated with ischaemic stroke risk. We found no evidence of an interaction between oral anticoagulants type (vitamin K antagonist vs direct oral anticoagulant) and cerebral microbleed presence for intracranial haemorrhage (pinteraction=0·4) or ischaemic stroke (pinteraction=0·61).
Table 3.
Rate and risk of outcome events according to baseline cerebral microbleeds in patients treated with oral anticoagulants with or without antiplatelet drugs (n=7737)
|
Composite of intracranial haemorrhage and ischaemic stroke (n=7582 for multivariable model) |
Symptomatic intracranial haemorrhage (n=6942 for multivariable model) |
Symptomatic ischaemic stroke (n=6958 in multivariable models) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate, per 1000 patient-years* | Absolute rate increase, per 1000 patient-years | Adjusted hazard ratio | Rate, per 1000 patient-years | Absolute rate increase, per 1000 patient-years | Adjusted hazard ratio | Rate, per 1000 patient-years | Absolute rate increase, per 1000 patient-years | Adjusted hazard ratio | ||
| None | 31 (28 to 35) | .. | 1 (ref) | 5 (3 to 6) | .. | 1 (ref) | 27 (23 to 30) | .. | 1 (ref) | |
| Any | 46 (39 to 53) | 15 (11 to 18) | 1·30 (1·07 to 1·57) | 12 (9 to 16) | 7 (6 to 10) | 2·49 (1·64 to 3·79) | 32 (26 to 39) | 5 (3 to 9) | 1·07 (0·86 to 1·35) | |
| One | 38 (30 to 49) | 7 (2 to 14) | 1·19 (0·91 to 1·56) | 10 (6 to 17) | 5 (3 to 11) | 2·15 (1·23 to 3·75) | 26 (19 to 35) | −1 (−4 to 5) | 0·96 (0·69 to 1·33) | |
| Number | ||||||||||
| Two to four | 47 (36 to 60) | 16 (8 to 25) | 1·23 (0·93 to 1·62) | 11 (6 to 19) | 6 (3 to 13) | 2·22 (1·21 to 4·06) | 36 (26 to 48) | 11 (3 to 18) | 1·10 (0·80 to 1·52) | |
| Five or more† | 62 (45 to 84) | 31 (17 to 49) | 1·69 (1·22 to 2·35) | 20 (11 to 34) | 15 (8 to 28) | 3·91 (2·08 to 7·34) | 40 (26 to 59) | 13 (3 to 29) | 1·27 (0·84 to 1·91) | |
| Ten or more† | 75 (46 to 116) | 44 (18 to 81) | 2·15 (1·35 to 3·43) | 23 (8 to 50) | 18 (5 to 44) | 4·63 (1·92 to 11·22) | 46 (24 to 81) | 19 (1 to 51) | 1·52 (0·84 to 2·67) | |
| Anatomical distribution | ||||||||||
| Mixed | 58 (42 to 77) | 27 (14 to 42) | 1·43 (1·02 to 2·00) | 15 (7 to 26) | 10 (4 to 20) | 2·21 (1·09 to 4·47) | 42 (29 to 60) | 15 (6 to 30) | 1·28 (0·85 to 1·94) | |
| Deep | 52 (42 to 63) | 21 (14 to 28) | 1·43 (1·11 to 1·84) | 14 (9 to 21) | 9 (6 to 15) | 2·71 (1·61 to 4·59) | 35 (27 to 46) | 8 (4 to 16) | 1·16 (0·85 to 1·59) | |
| Lobar | 41 (32 to 51) | 10 (4 to 16) | 1·13 (0·87 to 1·47) | 10 (6 to 16) | 5 (3 to 10) | 1·63 (0·94 to 2·83) | 29 (22 to 38) | 2 (−1 to 8) | 1·00 (0·73 to 1·38) | |
| Probable cerebral amyloid angiopathy | 27 (13 to 47) | −4 (−15 to 12) | 0·76 (0·41 to 1·39) | 10 (3 to 25) | 5 (0 to 19) | 1·29 (0·47 to 3·57) | 17 (7 to 35) | −10 (−16 to 5) | 0·64 (0·30 to 1·37) | |
Ranges in brackets are 95% CIs. Cerebral microbleed location hazard ratios are versus patients without cerebral microbleeds in each location and are adjusted for cerebral microbleed number and our prespecified variables.
Number of patients and time at risk are shown in the appendix.
Overlapping categories.
In patients treated with antiplatelet drugs only (n=11 520), 93 intracranial haemorrhages occurred over 18 059 patient-years of follow-up and 664 ischaemic strokes occurred over 17 731 patient-years of follow-up. The rate of ischaemic stroke remained higher than that of intracranial haemorrhage for all cerebral microbleed burden and anatomical distribution categories (appendix); aHRs for intracranial haemorrhage and ischaemic stroke in patients with versus without cerebral microbleeds were similar to those in the full cohort, with little variation according to cerebral microbleed anatomical distribution (appendix).
Compared with patients who received antithrombotic treatment (oral anticoagulants or antiplatelets), those not treated with antithrombotic drugs (n=1065) were older (mean age 72 years [SD 14] for those not treated with antithrombotic drugs vs 70 years [SD 13] for those treated with antithrombotic drugs), a greater proportion were women (46% vs 42%), more had ischaemic stroke (91% vs 83%), more had a previous intracranial haemorrhage (6% vs 2%), more had atrial fibrillation (44% vs 37%), fewer had been taking regular antiplatelet drugs before the qualifying event (27% vs 34%), and more had been taking regular oral anticoagulants before the qualifying event (13% vs 8%). No difference in the prevalence of cerebral microbleeds was observed based on receiving antithrombotic treatment (29% vs 28%). In those not treated with any antithrombotic drugs, five had intracranial haemorrhages over 846 patient-years and 65 had ischaemic strokes over 825 patient-years. The aHRs associated with cerebral microbleed presence were 1·10 (95% CI 0·17–7·34) for intracranial haemorrhage and 1·51 (0·87–2·65) for ischaemic stroke.
Discussion
Our large-scale pooled analysis of individual patient data confirms that, in patients with recent ischaemic stroke or transient ischaemic attack treated with antithrombotic drugs, cerebral microbleeds are associated with the subsequent risks of symptomatic intracranial haemorrhage and ischaemic stroke; as cerebral microbleed burden increases, the relative risk (aHR) of intracranial haemorrhage rises more steeply than that of ischaemic stroke. Our most important new finding is that, regardless of cerebral microbleed burden and distribution (ie, mixed, deep, or lobar), or the type of antithrombotic treatment received (oral anticoagulants or antiplatelet therapy), the absolute risk of ischaemic stroke is consistently substantially higher than that of intracranial haemorrhage.
As well as confirming the association between cerebral microbleeds and both recurrent ischaemic stroke and symptomatic intracranial haemorrhage found in smaller cohorts of patients with ischaemic stroke and transient ischaemic attack treated with antiplatelet drugs28 or oral anticoagulants,27, 57, 35 the large number of participants has improved the precision of our estimates of stroke recurrence rates and relative hazards, while the inclusion of individual patient data allowed adjustment for potential confounding factors. Our study also adds new data for the important subgroups of patients with many (eg, ≥20) cerebral microbleeds, which cause the most clinical concern and could not be addressed by any of the previously published meta-analyses. The association of cerebral microbleeds with a consistently higher rate of ischaemic stroke than intracranial haemorrhage suggests that cerebral microbleeds are a marker for cerebral small vessel diseases that can cause not only intracranial haemorrhage, but also ischaemic stroke. Although it has been inferred that cerebral microbleeds are a marker of direct extravasation of red blood cells from arterioles and capillaries damaged by bleeding-prone arteriopathies, alternative non-haemorrhagic mechanisms include ischaemia-mediated iron store release by oligodendrocytes10 or phagocytosis of red cell microemboli into the perivascular space.11 A report of haemorrhagic transformation of small acute microinfarcts into cerebral microbleeds provides direct evidence that cerebral microbleeds can result from ischaemic mechanisms.13 These varied mechanisms underlying cerebral microbleeds might explain why even patients at the highest risk of intracranial haemorrhage still have a higher absolute risk of ischaemic stroke. Moreover, patients with cerebral microbleeds often have multiple vascular risk factors, so are at risk of not only small vessel ischaemic stroke but also other ischaemic stroke subtypes.58 Patients with cerebral microbleeds usually also have white matter hyperintensities, which are associated with the risk of recurrent stroke, death, and poor functional outcome after ischaemic stroke59 and might also contribute to the increased risk of ischaemic stroke associated with cerebral microbleeds.
We found no evidence that a strictly lobar pattern of cerebral microbleeds (fulfilling the Boston criteria for probable CAA,5 causing clinical concern for intracranial bleeding risk35) is associated with the risk of intracranial haemorrhage or ischaemic stroke. These findings might reflect low diagnostic accuracy when using cerebral microbleeds for diagnosis of CAA in patients without intracerebral haemorrhage or dementia,60 rather than a true absence of any association of CAA with intracranial haemorrhage. Furthermore, the aHRs for intracranial haemorrhage associated with lobar cerebral microbleeds (compared with patients without lobar cerebral microbleeds [including none]) were closer to those associated with deep or mixed cerebral microbleeds (compared with patients without deep or mixed cerebral microbleeds [including none]).
Our results differ from some previous observations in smaller cohorts. First, in contrast to a smaller two-centre study,29 we did not find that the risk of intracranial haemorrhage approached the risk of ischaemic stroke after 1 year. Rather, we found that the rate of ischaemic stroke was consistently higher than that of intracranial haemorrhage, and the aHRs associated with cerebral microbleeds for both ischaemic stroke and intracranial haemorrhage remained stable over time. Second, our data indicate a smaller increase in the relative risk of intracranial haemorrhage for patients with five or more cerebral microbleeds than reported in a previous smaller meta-analysis,28 but our much larger individual participant sample size allowed us to investigate high cerebral microbleed burdens (five or more, ten or more, and 20 or more) with adjustment for confounders and greater statistical precision and power.
The comparatively low frequency of symptomatic intracranial haemorrhage after ischaemic stroke or transient ischaemic attack and the consistently higher risk of recurrent ischaemic stroke make randomised controlled trials of antithrombotic treatment (themselves proven in large randomised trials) guided by cerebral microbleeds challenging. However, ongoing and future randomised controlled trials should provide further insights. The MRI substudy in the RESTART trial61 of antiplatelet therapy after intracerebral haemorrhage excluded all but a very modest harmful effect of antiplatelet therapy on recurrent intracerebral haemorrhage in the presence of cerebral microbleeds, but also illustrates how very large sample sizes are probably required to identify statistically significant interactions in smaller cerebral microbleed subgroups in current (eg, the MRI substudy of NAVIGATE ESUS [NCT02313909]) and future randomised controlled trials. Nevertheless, our large collaborative pooled analysis provides the best available evidence on the associations of cerebral microbleeds with subsequent intracranial haemorrhage and ischaemic stroke after ischaemic stroke or transient ischaemic attack.
We included data from a worldwide collaborative network, making our results globally generalisable. The large individual patient dataset provides high statistical power and precision for risk estimates, allowing us to explore associations with several clinically important primary outcomes, while adjusting for important prognostic variables to minimise confounding. Included cohorts used validated rating instruments for cerebral microbleeds, and we adjusted for the use of different MRI sequences (T2* GRE or SWI) to detect cerebral microbleeds, which accounts for the higher sensitivity of SWI for detecting cerebral microbleeds compared with T2* GRE.62 We followed a published statistical analysis plan and confirmed our findings in a two-stage meta-analysis, indicating the robustness of our results.
In terms of limitations, our observational design has potential for selection bias and confounding of antithrombotic therapy by indication or unmeasured physician factors; thus, the relative hazards (aHRs) for intracranial haemorrhage and ischaemic stroke must be interpreted with caution. To definitively establish whether cerebral microbleeds modify the net clinical benefit of antithrombotic drugs would require a randomised controlled trial. Many of the included studies did not formally adjudicate events. The requirement for MRI-suitable patients probably led to the inclusion of less severe strokes than an unselected population. Even with the many individual patients included, we could not precisely estimate risks associated with an extremely large number of cerebral microbleeds (eg, ≥50), but such patients are very rare in clinical practice. Although we adjusted for known prognostic variables, residual confounding secondary to unknown or uncontrolled factors such as stroke mechanism could still have affected our results. Furthermore, we were unable to include some candidate variables in our multivariable models because they were not sufficiently widely available across all participating cohorts (eg, white matter hyperintensities, MRI field strength, diabetes, ischaemic heart disease, renal function, and statin use on discharge). Our analyses did not formally assess net clinical benefit, accounting for the greater severity of intracranial haemorrhage compared with recurrent ischaemic stroke.
In summary, our large-scale pooled analysis in patients with recent ischaemic stroke or transient ischaemic attack found that the absolute risk of ischaemic stroke is consistently higher than that of intracranial haemorrhage, regardless of the number or anatomical distribution of cerebral microbleeds. However, cerebral microbleeds are associated with a greater relative hazard (aHR) for intracranial haemorrhage than ischaemic stroke; further studies are needed to establish the usefulness of neuroimaging biomarkers, including cerebral microbleeds, in improving risk prediction scores for intracranial haemorrhage and ischaemic stroke.
This online publication has been corrected. The corrected version first appeared at thelancet.com/neurology on July 12, 2019, with subsequent corrections on January 3, 2020
Acknowledgments
Acknowledgments
Funding for the included cohort studies was provided by the British Heart Foundation, Stroke Association, UCLH National Institute of Health Research (NIHR) Biomedical Research Centre, Wellcome Trust, Health Research Board Ireland, NIHR Biomedical Research Centre (Oxford, UK), Canadian Institutes of Health Research, Pfizer Cardiovascular Research award, Basel Stroke Funds, Science Funds Rehabilitation Felix-Platter-Hospital, Neurology Research Pool University Hospital Basel, Bayer AG, Fondo de Investigaciones Sanitarias Instituto de Salud Carlos III (FI12/00296; RETICS INVICTUS PLUS RD16/0019/0010; FEDER), Imperial College London NIHR Biomedical Research Centre, Dutch Heart Foundation, Servier, Association de Recherche en Neurologie Vasculaire and RHU TRT_cSVD (ANR-16-RHUS-004), Vidi innovational grant from The Netherlands ZonMw, Chest Heart Stroke Scotland, Medical Research Council, Fondation Leducq, The Row Fogo Charitable Trust, National Institute of Health (USA), Adriana van Rinsum-Ponsen Stichting, Japan Agency for Medical Research and Development (AMED), Ministry of Health, Labour and Welfare (Japan), and National Cerebral and Cardiovascular Center, Health and Medical Research Grant, Singapore National Medical Research Council, and Dutch Heart Foundation.
Contributors
DJWe, DW, GA, and JM-F drafted the initial protocol, which was reviewed with critical revisions and approval by all authors. DW and GA did the statistical analysis. DW, DJW, and GA wrote the first draft of the manuscript. All authors contributed to data acquisition, management, and brain imaging analyses. All authors contributed to critical revision of the manuscript and approved the final manuscript for submission.
Declaration of interests
MK reports grants from the Ministry of Health, Labour and Welfare, Japan, and from the National Cerebral and Cardiovascular Center during the conduct of the study; and speaker honoraria from Bayer Yakuhin, Daiichi-Sankyo Company, and Bristol-Myers Squibb (BMS)/Pfizer. HC reports participation in the steering committee for a clinical trial supported by Servier and was a consultant for Hovid Inc. EMA reports personal fees from Pfizer, Boehringer Ingelheim, Nutricia, Abbott, and Sanofi, outside the submitted work. JP reports personal fees from Boehringer Ingelheim and Akcea and personal fees and non-financial support from Pfizer outside the submitted work. EBA reports grants from US–Israel Bi-national Science Foundation, The American Federation for Aging Research, and The Israeli Chief Scientist, Ministry of Health, during the conduct of the study. SBC reports grants from the Canadian Institute of Health Research and a Pfizer Cardiovascular award during the conduct of the study. DJS reports other funding from Bayer and from BMS/Pfizer outside the submitted work. PL reports other funding from Daiichi-Sankyo, Bayer, and Boehringer Ingelheim, outside the submitted work. RA-SS reports grants from the British Heart Foundation, The Stroke Association, and Chest Heart & Stroke Scotland outside the submitted work. GYHL reports consultancy for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Microlife, and Daiichi-Sankyo; and speaker honoraria from Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche, and Daiichi-Sankyo. HPM reports personal fees from Neuravi/Cerenovus, Medtronic, Bayer, Daiichi-Sankyo, and Servier outside the submitted work. DH reports grants from University College Dublin Newman Fellowship supported Bayer during the conduct of the study. MEK reports grants from the Center for Translational Molecular Medicine during the conduct of the study. AMT reports grants from the Dutch Heart Foundation during the conduct of the study. AvdL reports grants from the Center for Translation Molecular Medicine and Dutch Heart Foundation during the conduct of the study. JMW reports grants from Wellcome Trust, Chest Heart Stroke Scotland, and Row Fogo Charitable Trust during the conduct of the study. YS reports a grant from Health and Medical Research Fund. VIHK reports grants from the Netherlands Heart Foundation (grant 2001B071) during the conduct of the study. STE reports grants from Daiichi-Sankyo, Bayer, Pfizer, and Swiss Heart Foundation during the conduct of the study; other funding from Daiichi-Sankyo, Mindmaze, and Stago; and grants from the Swiss National Science Foundation outside the submitted work. NP reports other funding from Daiichi-Sankyo, Bayer, and Boehringer Ingelheim outside the submitted work. EES reports personal fees from Portola Pharmaceuticals and Alnylam Pharmaceuticals outside the submitted work. VT reports personal fees and non-financial support from Boehringer Ingelheim and personal fees from Bayer, Pfizer/BMS, and Amgen and Medtronic outside the submitted work. RV reports grants and personal fees from Bayer, Boehringer Ingelheim, BMS, Daiichi-Sankyo, and Medtronic; and personal fees from Morphosys and Amgen outside the submitted work. HA reports grants from National Institutes of Health during the conduct of the study. PMR reports personal fees from Bayer outside the submitted work. KT reports personal fees from Daiichi-Sankyo, Bayer Yakuhin, BMS, and Nippon Boehringer Ingelheim outside the submitted work. DJWe reports personal fees from Bayer outside the submitted work. All other authors declare no competing interests.
Contributor Information
David J Werring, Email: d.werring@ucl.ac.uk.
Microbleeds International Collaborative Network:
Kirsty Harkness, Louise Shaw, Jane Sword, Azlisham Mohd Nor, Pankaj Sharma, Deborah Kelly, Frances Harrington, Marc Randall, Matthew Smith, Karim Mahawish, Abduelbaset Elmarim, Bernard Esisi, Claire Cullen, Arumug Nallasivam, Christopher Price, Adrian Barry, Christine Roffe, John Coyle, Ahamad Hassan, Jonathan Birns, David Cohen, Lakshmanan Sekaran, Adrian Parry-Jones, Anthea Parry, David Hargroves, Harald Proschel, Prabel Datta, Khaled Darawil, Aravindakshan Manoj, Mathew Burn, Chris Patterson, Elio Giallombardo, Nigel Smyth, Syed Mansoor, Ijaz Anwar, Rachel Marsh, Sissi Ispoglou, Dinesh Chadha, Mathuri Prabhakaran, Sanjeevikumar Meenakishundaram, Janice O'Connell, Jon Scott, Vinodh Krishnamurthy, Prasanna Aghoram, Michael McCormick, Nikola Sprigg, Paul O'Mahony, Martin Cooper, Lillian Choy, Peter Wilkinson, Simon Leach, Sarah Caine, Ilse Burger, Gunaratam Gunathilagan, Paul Guyler, Hedley Emsley, Michelle Davis, Dulka Manawadu, Kath Pasco, Maam Mamun, Robert Luder, Mahmud Sajid, Ijaz Anwar, James Okwera, Elizabeth Warburton, Kari Saastamoinen, Timothy England, Janet Putterill, Enrico Flossman, Michael Power, Krishna Dani, David Mangion, Appu Suman, John Corrigan, Enas Lawrence, Djamil Vahidassr, Clare Shakeshaft, Martin Brown, Andreas Charidimou, Hannah Cohen, Gargi Banerjee, Henry Houlden, Mark White, Tarek Yousry, Kirsty Harkness, Enrico Flossmann, Nigel Smyth, Louise Shaw, Elizabeth Warburton, Keith Muir, Marwan El-Koussy, Pascal Gratz, Jeremy Molad, Amos Korczyn, Efrat Kliper, Philippe Maeder, Achim Gass, Chahin Pachai, Luc Bracoub, Marie-Yvonne Douste-Blazy, Marie Dominique Fratacci, Eric Vicaut, Shoichiro Sato, Kaori Miwa, Kyohei Fujita, Toshihiro Ide, Henry Ma, John Ly, Shahoo Singhal, Ronil Chandra, Lee-Anne Slater, Cathy Soufan, Christopher Moran, Christopher Traenka, Sebastian Thilemann, Joachim Fladt, Henrik Gensicke, Leo Bonati, Beom Joon Kim, Moon-Ku Han, Jihoon Kang, Eunbin Ko, Mi Hwa Yang, Myung Suk Jang, Sean Murphy, Fiona Carty, Layan Akijian, John Thornton, Mark Schembri, Elles Douven, Raquel Delgado-Mederos;, Rebeca Marín, Pol Camps-Renom, Daniel Guisado-Alonso, Fidel Nuñez, Santiago Medrano-Martorell, Elisa Merino, Kotaro Iida, Syuhei Ikeda, Masashi Nishihara, Hiroyuki Irie, Derya Selcuk Demirelli, Jayesh Modi Medanta, Charlotte Zerna, Maria Valdés Hernández, Paul Armitage, Anna Heye, Susana Muñoz-Maniega, Eleni Sakka, Michael Thrippleton, Martin Dennis, Ysoline Beigneux, Mauro Silva, Narayanaswamy Venketasubramanian, Shu Leung Ho, Raymond Tak Fai Cheung, Koon Ho Chan, Kay Cheong Teo, Edward Hui, Joseph Shiu Kwong Kwan, Richard Chang, Man Yu Tse, Chu Peng Hoi, Chung Yan Chan, Oi Ling Chan, Ryan Hoi Kit Cheung, Edmund Ka Ming Wong, Kam Tat Leung, Suk Fung Tsang, Hing Lung Ip, Sze Ho Ma, Karen Ma, Wing Chi Fong, Siu Hung Li, Richard Li, Ping Wing Ng, Kwok Kui Wong, Wenyan Liu, Lawrence Wong, Lino Ramos, Els De Schryver, Joost Jöbsis, Jaap van der Sande, Paul Brouwers, Yvo Roos, Jan Stam, Stef Bakker, Henk Verbiest, Wouter Schoonewille, Cisca Linn, Leopold Hertzberger, Maarten van Gemert, Paul Berntsen, Jeroen Hendrikse, Paul Nederkoorn, Werner Mess, Peter Koudstaal, Alexander Leff, Nicholas Ward, Parashkev Nachev, Richard Perry, Hatice Ozkan, and John Mitchell
Supplementary Material
References
- 1.Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision-making. Thromb Haemost. 2017;117:1230–1239. doi: 10.1160/TH16-11-0876. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg SM, Vernooij MW, Cordonnier C. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fazekas F, Kleinert R, Roob G. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 4.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis. 2011;32:528–534. doi: 10.1159/000331466. [DOI] [PubMed] [Google Scholar]
- 5.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 6.Fisher CM. Hypertensive cerebral hemorrhage. Demonstration of the source of bleeding. J Neuropathol Exp Neurol. 2003;62:104–107. doi: 10.1093/jnen/62.1.104. [DOI] [PubMed] [Google Scholar]
- 7.Pasi M, Charidimou A, Boulouis G. Mixed-location cerebral hemorrhage/microbleeds: Underlying microangiopathy and recurrence risk. Neurology. 2018;90:e119–e126. doi: 10.1212/WNL.0000000000004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai HH, Pasi M, Tsai LK. Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds: A PiB-PET study. Neurology. 2019;92:e774–e781. doi: 10.1212/WNL.0000000000006953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charidimou A, Shakeshaft C, Werring DJ. Cerebral microbleeds on magnetic resonance imaging and anticoagulant-associated intracerebral hemorrhage risk. Front Neurol. 2012;3:133. doi: 10.3389/fneur.2012.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janaway BM, Simpson JE, Hoggard N, the MRC Cognitive Function and Ageing Neuropathology Study Brain haemosiderin in older people: pathological evidence for an ischaemic origin of magnetic resonance imaging (MRI) microbleeds. Neuropathol Appl Neurobiol. 2014;40:258–269. doi: 10.1111/nan.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grutzendler J, Murikinati S, Hiner B. Angiophagy prevents early embolus washout but recanalizes microvessels through embolus extravasation. Sci Transl Med. 2014;6:226ra31. doi: 10.1126/scitranslmed.3006585. [DOI] [PubMed] [Google Scholar]
- 12.Tanskanen M, Mäkelä M, Myllykangas L. Prevalence and severity of cerebral amyloid angiopathy: a population-based study on very elderly Finns (Vantaa 85+) Neuropathol Appl Neurobiol. 2012;38:329–336. doi: 10.1111/j.1365-2990.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- 13.Ito AO, Shindo A, Ii Y. Small cortical infarcts transformed to lobar cerebral microbleeds: A Case Series. J Stroke Cerebrovasc Dis. 2019;28:e30–e32. doi: 10.1016/j.jstrokecerebrovasdis.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 14.Thijs V, Lemmens R, Schoofs C. Microbleeds and the risk of recurrent stroke. Stroke. 2010;41:2005–2009. doi: 10.1161/STROKEAHA.110.588020. [DOI] [PubMed] [Google Scholar]
- 15.Boulanger JM, Coutts SB, Eliasziw M, the VISION Study Group Cerebral microhemorrhages predict new disabling or fatal strokes in patients with acute ischemic stroke or transient ischemic attack. Stroke. 2006;37:911–914. doi: 10.1161/01.STR.0000204237.66466.5f. [DOI] [PubMed] [Google Scholar]
- 16.Bokura H, Saika R, Yamaguchi T. Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke. 2011;42:1867–1871. doi: 10.1161/STROKEAHA.110.601922. [DOI] [PubMed] [Google Scholar]
- 17.Akoudad S, Ikram MA, Koudstaal PJ. Cerebral microbleeds are associated with the progression of ischemic vascular lesions. Cerebrovasc Dis. 2014;37:382–388. doi: 10.1159/000362590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fluri F, Jax F, Amort M. Significance of microbleeds in patients with transient ischaemic attack. Eur J Neurol. 2012;19:522–524. doi: 10.1111/j.1468-1331.2011.03522.x. [DOI] [PubMed] [Google Scholar]
- 19.Imaizumi T, Inamura S, Kohama I, Yoshifuji K, Nomura T, Komatsu K. Antithrombotic drug uses and deep intracerebral hemorrhages in stroke patients with deep cerebral microbleeds. J Stroke Cerebrovasc Dis. 2013;22:869–875. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Jeon SB, Kang DW, Cho AH. Initial microbleeds at MR imaging can predict recurrent intracerebral hemorrhage. J Neurol. 2007;254:508–512. doi: 10.1007/s00415-006-0406-6. [DOI] [PubMed] [Google Scholar]
- 21.Kwa VI, Algra A, Brundel M, Bouvy W, Kappelle LJ, the MICRO Study Group Microbleeds as a predictor of intracerebral haemorrhage and ischaemic stroke after a TIA or minor ischaemic stroke: a cohort study. BMJ Open. 2013;3:e002575. doi: 10.1136/bmjopen-2013-002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim JS, Hong KS, Kim GM. Cerebral microbleeds and early recurrent stroke after transient ischemic attack: results from the Korean Transient Ischemic Attack Expression Registry. JAMA Neurol. 2015;72:301–308. doi: 10.1001/jamaneurol.2014.3958. [DOI] [PubMed] [Google Scholar]
- 23.Mok VC, Lau AY, Wong A. Long-term prognosis of Chinese patients with a lacunar infarct associated with small vessel disease: a five-year longitudinal study. Int J Stroke. 2009;4:81–88. doi: 10.1111/j.1747-4949.2009.00262.x. [DOI] [PubMed] [Google Scholar]
- 24.Naka H, Nomura E, Takahashi T. Combinations of the presence or absence of cerebral microbleeds and advanced white matter hyperintensity as predictors of subsequent stroke types. AJNR Am J Neuroradiol. 2006;27:830–835. [PMC free article] [PubMed] [Google Scholar]
- 25.Ueno H, Naka H, Ohshita T. Association between cerebral microbleeds on T2*-weighted MR images and recurrent hemorrhagic stroke in patients treated with warfarin following ischemic stroke. AJNR Am J Neuroradiol. 2008;29:1483–1486. doi: 10.3174/ajnr.A1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregoire SM, Brown MM, Kallis C, Jäger HR, Yousry TA, Werring DJ. MRI detection of new microbleeds in patients with ischemic stroke: five-year cohort follow-up study. Stroke. 2010;41:184–186. doi: 10.1161/STROKEAHA.109.568469. [DOI] [PubMed] [Google Scholar]
- 27.Wilson D, Ambler G, Shakeshaft C, the CROMIS-2 collaborators Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol. 2018;17:539–547. doi: 10.1016/S1474-4422(18)30145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson D, Charidimou A, Ambler G. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: a meta-analysis. Neurology. 2016;87:1501–1510. doi: 10.1212/WNL.0000000000003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau KK, Lovelock CE, Li L. Antiplatelet treatment after transient ischemic attack and ischemic stroke in patients with cerebral microbleeds in 2 large cohorts and an updated systematic review. Stroke. 2018;49:1434–1442. doi: 10.1161/STROKEAHA.117.020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Microbleeds International Collaborative Network Worldwide collaboration in the Microbleeds International Collaborative Network. Lancet Neurol. 2016;15:1113–1114. doi: 10.1016/S1474-4422(16)30213-7. [DOI] [PubMed] [Google Scholar]
- 31.Dichgans M, Wardlaw J, Smith E. METACOHORTS for the study of vascular disease and its contribution to cognitive decline and neurodegeneration: an initiative of the Joint Programme for Neurodegenerative Disease Research. Alzheimers Dement. 2016;12:1235–1249. doi: 10.1016/j.jalz.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cochrane Tool to assess risk of bias in cohort studies. http://methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/Tool%20to%20Assess%20Risk%20of%20Bias%20in%20Cohort%20Studies.pdf
- 33.Collett D. 3rd edn. Chapman and Hall; London: 2015. Modelling survival data in medical research. [Google Scholar]
- 34.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 35.Soo Y, Abrigo JM, Leung KT, the IPAAC study group Risk of intracerebral haemorrhage in Chinese patients with atrial fibrillation on warfarin with cerebral microbleeds: the IPAAC-Warfarin study. J Neurol Neurosurg Psychiatry. 2019;90:428–435. doi: 10.1136/jnnp-2018-319104. [DOI] [PubMed] [Google Scholar]
- 36.Gratz PP, El-Koussy M, Hsieh K. Preexisting cerebral microbleeds on susceptibility-weighted magnetic resonance imaging and post-thrombolysis bleeding risk in 392 patients. Stroke. 2014;45:1684–1688. doi: 10.1161/STROKEAHA.114.004796. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Wong A, Wang Z. Risk factors for incident dementia after stroke and transient ischemic attack. Alzheimers Dement. 2015;11:16–23. doi: 10.1016/j.jalz.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Ben Assayag E, Korczyn AD, Giladi N. Predictors for poststroke outcomes: the Tel Aviv Brain Acute Stroke Cohort (TABASCO) study protocol. Int J Stroke. 2012;7:341–347. doi: 10.1111/j.1747-4949.2011.00652.x. [DOI] [PubMed] [Google Scholar]
- 39.Chabriat H, Maeder P, Gass A, Michel P, Bracoud L, Hennerici M. Results of the PERFORM magnetic resonance imaging study. J Neurol. 2013;260:3071–3076. doi: 10.1007/s00415-013-7111-z. [DOI] [PubMed] [Google Scholar]
- 40.Truijman MT, Kooi ME, van Dijk AC. Plaque At RISK (PARISK): prospective multicenter study to improve diagnosis of high-risk carotid plaques. Int J Stroke. 2014;9:747–754. doi: 10.1111/ijs.12167. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimura S, Koga M, Sato S. Two-year outcomes of anticoagulation for acute ischemic stroke with nonvalvular atrial fibrillation—SAMURAI-NVAF Study. Circ J. 2018;82:1935–1942. doi: 10.1253/circj.CJ-18-0067. [DOI] [PubMed] [Google Scholar]
- 42.van Leijsen EMC, van Uden IWM, Ghafoorian M. Nonlinear temporal dynamics of cerebral small vessel disease: The RUN DMC study. Neurology. 2017;89:1569–1577. doi: 10.1212/WNL.0000000000004490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karayiannis C, Soufan C, Chandra RV. Prevalence of brain MRI markers of hemorrhagic risk in patients with stroke and atrial fibrillation. Front Neurol. 2016;7:151. doi: 10.3389/fneur.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song TJ, Kim J, Song D. Association of cerebral microbleeds with mortality in stroke patients having atrial fibrillation. Neurology. 2014;83:1308–1315. doi: 10.1212/WNL.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 45.Jeong HG, Kim BJ, Yang MH, Han MK, Bae HJ. Neuroimaging markers for early neurologic deterioration in single small subcortical infarction. Stroke. 2015;46:687–691. doi: 10.1161/STROKEAHA.114.007466. [DOI] [PubMed] [Google Scholar]
- 46.Kim BJ, Park JM, Kang K. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke-fifth division registry in South Korea. J Stroke. 2015;17:38–53. doi: 10.5853/jos.2015.17.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Callaly E, Ni Chroinin D, Hannon N. Rates, predictors, and outcomes of early and late recurrence after stroke: the north Dublin population stroke study. Stroke. 2016;47:244–246. doi: 10.1161/STROKEAHA.115.011248. [DOI] [PubMed] [Google Scholar]
- 48.Imaizumi T, Inamura S, Nomura T. The severities of white matter lesions possibly influence the recurrences of several stroke types. J Stroke Cerebrovasc Dis. 2014;23:1897–1902. doi: 10.1016/j.jstrokecerebrovasdis.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Soo Y, Abrigo J, Leung KT. Correlation of non-vitamin K antagonist oral anticoagulant exposure and cerebral microbleeds in Chinese patients with atrial fibrillation. J Neurol Neurosurg Psychiatry. 2018;89:680–686. doi: 10.1136/jnnp-2017-317151. [DOI] [PubMed] [Google Scholar]
- 50.Douven E, Schievink SHJ, Verhey FRJ. The cognition and affect after stroke—a prospective evaluation of risks (CASPER) study: rationale and design. BMC Neurol. 2016;16:1–11. doi: 10.1186/s12883-016-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martí-Fàbregas J, Medrano-Martorell S, Merino E. MRI predicts intracranial hemorrhage in patients who receive long-term oral anticoagulation. Neurology. 2019 doi: 10.1212/WNL.0000000000007532. published online April 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan S, Jin X, Zhang X, Zhang S, Liebeskind DS, Lou M. Extensive cerebral microbleeds predict parenchymal haemorrhage and poor outcome after intravenous thrombolysis. J Neurol Neurosurg Psychiatry. 2015;86:1267–1272. doi: 10.1136/jnnp-2014-309857. [DOI] [PubMed] [Google Scholar]
- 53.Orken DN, Uysal E, Timer E, Kuloglu-Pazarcı N, Mumcu S, Forta H. New cerebral microbleeds in ischemic stroke patients on warfarin treatment: two-year follow-up. Clin Neurol Neurosurg. 2013;115:1682–1685. doi: 10.1016/j.clineuro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Zerna C, Modi J, Bilston L, Shoamanesh A, Coutts SB, Smith EE. Cerebral microbleeds and cortical superficial siderosis in patients presenting with minor cerebrovascular events. Stroke. 2016;47:2236–2241. doi: 10.1161/STROKEAHA.116.013418. [DOI] [PubMed] [Google Scholar]
- 55.Wardlaw J, Makin S, Valdés Hernández M. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimers Dement. 2017;13:634–643. [Google Scholar]
- 56.Horstmann S, Möhlenbruch M, Wegele C. Prevalence of atrial fibrillation and association of previous antithrombotic treatment in patients with cerebral microbleeds. Eur J Neurol. 2015;22:1355–1362. doi: 10.1111/ene.12608. [DOI] [PubMed] [Google Scholar]
- 57.Charidimou A, Karayiannis C, Song TJ, the International META-MICROBLEEDS Initiative Brain microbleeds, anticoagulation, and hemorrhage risk: meta-analysis in stroke patients with AF. Neurology. 2017;89:2317–2326. doi: 10.1212/WNL.0000000000004704. [DOI] [PubMed] [Google Scholar]
- 58.Song TJ, Kim J, Lee HS. The frequency of cerebral microbleeds increases with CHADS(2) scores in stroke patients with non-valvular atrial fibrillation. Eur J Neurol. 2013;20:502–508. doi: 10.1111/ene.12003. [DOI] [PubMed] [Google Scholar]
- 59.Georgakis MK, Duering M, Wardlaw JM, Dichgans M. WMH and long-term outcomes in ischemic stroke: a systematic review and meta-analysis. Neurology. 2019;92:e1298–e1308. doi: 10.1212/WNL.0000000000007142. [DOI] [PubMed] [Google Scholar]
- 60.Martinez-Ramirez S, Romero JR, Shoamanesh A. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Dement. 2015;11:1480–1488. doi: 10.1016/j.jalz.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Shahi Salman R, Minks D, Mitra D. Effects of antiplatelet therapy on stroke risk by brain imaging features of intracerebral haemorrhage and cerebral small vessel diseases: sub-group analyses of the RESTART randomised, open-label trial. Lancet Neurol. 2019 doi: 10.1016/S1474-4422(19)30184-X. published online May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shams S, Martola J, Cavallin L. SWI or T2*: which MRI sequence to use in the detection of cerebral microbleeds? The Karolinska Imaging Dementia Study. AJNR Am J Neuroradiol. 2015;36:1089–1095. doi: 10.3174/ajnr.A4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.