Abstract
This work examined the degree of influence of apple variety, apple ripening stage, and yeast strain on the volatile composition of apple cider. Four apple varieties grown in Estonia were selected for the study – Antei, Melba, Kulikovskoye, and Orlovski Sinap. The must from the apples at various stages of ripening (unripe, ripe, overripe) underwent alcoholic fermentation using commercially available yeast strains. Gas chromatography - mass spectrometry was employed to assess the differences in volatile composition between the samples. Out of the variables analyzed in this work, apple variety turned out to be the primary attribute influencing the quality and aroma properties of apple cider. The effect of yeast strain and the maturity of the fruit was variety-specific where the volatile profiles of ciders made with Melba variety were the least influenced by the ripening stage of apples and yeast strains used for the fermentation.
Keywords: Food Analysis, Food technology, Apple cider, Hard cider, Gas chromatography, Volatile composition
1. Introduction
Сider is a fermented alcoholic beverage made from apples. Despite holding a smaller position on a global scale, cider production is common throughout Europe and has also spread to other Western markets (Northern America, Australia) (Nogueira and Wosiacki, 2012). Technological advances made in other parts of the fermented beverage industry strongly influence cider production. Because of that, a limited amount of published information exists regarding the aroma of cider and factors affecting it the most.
No conclusive information is available on the effect of different apple varieties on the volatile composition of cider. However, some apple varieties themselves have been distinguished from one another based on their chemical composition (El Hadi et al., 2013). As far as non-volatile composition is concerned, aside from apparent fluctuations in sugar and acid ratios, the primary varietal differences occur in the phenolic content (Blanco-Gomisa et al., 1998; Wu et al., 2007; Kalinowska et al., 2014). Phenolic compounds, in turn, have been reported to contribute to the odor profiles of alcoholic beverages such as beer, wine, sherry, and whiskey (Vanbeneden et al., 2008).
Some information is available on how the level of apple fruit ripeness impacts the aroma profile of cider. For example, one of the latest researches conducted on this subject by Alberti et al. (2016) has provided some insight into cider aroma based on the ripeness of the apples used. The cider made from senescent apples was 24–52 % (depending on the variety) more abundant in different volatile compounds than the counterparts made from unripe apples.
The yeast used in the production of fermented beverages contributes to the final aroma profile mainly by elevating the levels of higher alcohols and esters (McKay et al., 2011). The relative concentrations of the fermentation products, however, may depend on the strain. Many yeast strains have already been investigated in another fermented beverage – wine. The application of different yeasts in the wine industry and the impact on the sensory properties have been described by Henick-Kling et al. (1998), Soden et al. (2000), Cadez et al. (2002), Becker Whitener et al. (2014), and Synos et al. (2015), to name a few. For example, Synos et al. (2015) have demonstrated the influence of the yeast strain on the formation of aroma compounds in Cabernet icewines. The yeasts differed not only in the diversity of generated odor-active volatile compounds but also in the amounts generated. According to the results, yeast EC1118 displayed the highest amounts of various alcohols, esters, furfural, hexanoic acid, and β-damascenone. Becker Whitener et al. (2014) observed the fermentation of red and white grape musts with non-Saccharomyces yeasts. They found that the majority of the investigated non-Saccharomyces yeasts provided lower levels of alcohols, esters, and terpenes, except for Kazachstania gamospora, which produced more total esters than Saccharomyces cerevisiae. Less information is known on the effects of different yeast strains on aroma development in cider. The fermentation of industrial apple pomaces with three indigenous yeasts (Saccharomyces cerevisiae, Hanseniaspora valbyensis, and Hanseniaspora uvarum) was described by Rodríguez Madrera et al. (2015). Suárez Valles et al. (2005) have inoculated Asturian apple juice with three strains of cider making isolates (S. cerevisiae r. cerevisiae SSA, S. cerevisiae r. uvarum SSB, S. cerevisiae r. cerevisiae SSC) and a commercial wine yeast (S. cerevisiae r. bayanus UVA-PM). The compounds that were found to differentiate the strains were ethyl acetate, acetaldehyde, and isobutanol.
Additional insights need to be gained regarding the impact of cider production at the molecular level so that the industry can understand and control the character of the final product. Given the knowledge, it should be possible to maintain and expand the diversity in the cider market and influence the economics of cider production. The objective of this study was to examine the degree of influence of apple variety, apple ripeness, and yeast strain on the volatile composition of apple cider.
2. Materials and methods
2.1. Chemicals and reagents
Distilled water was obtained using Elix 5 UV Water Purification system (Merck Millipore, Billerica, MA, USA). 2-chloro-6-methylphenol, as an internal standard, was obtained from Sigma–Aldrich (St. Louis, MO, USA).
2.2. Cider preparation in a laboratory environment
Four autumn or winter apple varieties, ‘Antei’, ‘Kulikovskoye’, ‘Melba’, and ‘Orlovski Sinap’ grown in South Estonia, at a private orchard in Valgjärve (58°8′ N, 26°66′ E) were used in the study. Apples were selected at three different stages of ripening: unripe, ripe, and overripe. The estimation of the ripening stage was based on the iodine starch test (Travers et al., 2002). All apples were first harvested at the ‘unripe’ stage (0 weeks, starch index 1) of their maturity and left to ripen at +4 °C. This is a common practice in Northern-Europe region because some of the autumn and most winter varieties do not reach their maturity before the first frost. The ripe apples (starch index 3) were collected after 2–8 weeks (depending on the variety: ‘Melba’ 2 weeks, ‘Kulikovskoye’ 3 weeks, ‘Antei’ 6 weeks and ‘Orlovski Sinap’ 8 weeks) in storage, and the overripe (starch index 5) – after 6–12 weeks (approx. one month after the ripe stage: 6, 8, 10 and 12 weeks, respectively). Apples were washed with tap water and drained. Apples with visible defects (e.g., rotting, mold) were excluded; however, no further selection was made according to the size or appearance. Before pressing, 20 kg of apples of each variety were randomly selected at each ripening stage. The juice was prepared in two batches of 10 kg using a centrifugal juice press (Vita Pro-Active JE810; Kenwood). The resulting batches were first combined and then distributed immediately into 1 L bottles for fermentation. Malic acid content and sugar profile of the musts are provided in Table 1. The commercial starter cultures used in this study were as follows: Biodiva (Torulaspora delbrueckii), C1108 (Saccharomyces bayanus), EC1118 (Saccharomyces cerevisiae), OKAY (Saccharomyces cerevisiae), OPALE (Saccharomyces cerevisiae), and QA23 (Saccharomyces bayanus). The inoculation and fermentation steps were followed according to the procedure previously described by Laaksonen et al. (2017). The number of cider samples was 144 (4 varieties × 3 ripening stages × 6 yeasts × 2 replicates). A representative sample was taken of each cider and stored at −20 °C in 10 mL plastic tubes.
Table 1.
Malic acid content and sugar profile of apple musts obtained from different apple varieties at diferent stages of maturity.
| Must | Fructose, % | Glucose, % | Disaccharides, % | Malic acid, % |
|---|---|---|---|---|
| Unripe Melba | 4.5 ± 0.1 | 0.5 ± 0.1 | 3.8 ± 0.3 | 1.2 ± 0.1 |
| Ripe Melba | 5.3 ± 0.1 | 0.7 ± 0.1 | 4.9 ± 0.1 | 0.9 ± 0.1 |
| Overripe Melba | 6.3 ± 0.5 | 1.2 ± 0.1 | 5.0 ± 0.1 | 0.8 ± 0.1 |
| Unripe Antei | 5.9 ± 0.2 | 1.8 ± 0.1 | 1.3 ± 0.1 | 0.8 ± 0.1 |
| Ripe Antei | 6.5 ± 0.1 | 1.8 ± 0.1 | 2.8 ± 0.1 | 0.6 ± 0.1 |
| Overripe Antei | 6.9 ± 0.2 | 2.2 ± 0.1 | 2.3 ± 0.1 | 0.5 ± 0.1 |
| Unripe Orlovski Sinap | 5.2 ± 0.3 | 1.1 ± 0.1 | 2.4 ± 0.1 | 1.3 ± 0.1 |
| Ripe Orlovski Sinap | 6.4 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.1 | 0.8 ± 0.1 |
| Overripe Orlovski Sinap | 6.9 ± 0.2 | 2.0 ± 0.1 | 3.0 ± 0.1 | 0.5 ± 0.1 |
| Unripe Kulikovskoye | 4.9 ± 0.4 | 1.1 ± 0.1 | 1.6 ± 0.1 | 0.7 ± 0.1 |
| Ripe Kulikovskoye | 6.4 ± 0.2 | 1.3 ± 0.1 | 2.0 ± 0.1 | 0.5 ± 0.1 |
| Overripe Kulikovskoye | 6.5 ± 0.3 | 1.6 ± 0.1 | 1.8 ± 0.1 | 0.4 ± 0.1 |
2.3. Extraction of cider volatiles
The extraction of cider volatiles was carried out using headspace – solid-phase microextraction (HS-SPME). 300 μL of the sample was measured into a 20 mL glass autosampler vial capped with a PTFE/silicone septum and diluted with 700 μL of distilled water. 7.5 ppb of 2-chloro-6-methylphenol was added as an internal standard. Vials were pre-incubated at 45 °C for 5 minutes. SPME fiber (30/50μm DVB/Car/PDMS Stableflex, length 2 cm; Supelco, Bellefonte, PA, USA) recommended by Villière et al. (2012) was used to extract the volatile compounds from the headspace for 20 minutes under stirring at 45 °C.
2.4. GC-TOF-MS analysis of volatiles
Identification and relative quantitation of cider volatiles were performed using a Micromass GCT Premier gas chromatograph system (Waters, Milford, MA, USA) coupled with CombiPAL autosampler (CTC Analytics AG, Lake Elmo, MN, USA). After the SPME procedure, the volatile compounds were desorbed in splitless mode into a GC injection port equipped with a 0.75 mm internal diameter liner at 250 °C for 10 minutes. A DB5-MS column (30 m length × 0.25 mm i.d. × 1.0 μm film thickness; J&W Scientific, Folsom, CA, USA) was used with helium as a carrier gas at a flow rate of 1.0 mL min−1. The oven was programmed to ramp up from 45 °C at a rate of 10 °C min−1 to a final temperature of 280 °C with an additional holding time of one minute (total run time 24.50 min). Mass spectra were obtained at ionization energy of 70 eV and a scan speed of 10 scans s−1, with a mass range of 35–350. Each cider sample was analyzed in three analytical replicates.
Non-targeted identification of volatile compounds was carried out using ChromaLynx application (MassLynx software; Waters, Milford, MA, USA) and theoretical calculation of retention indices (RI). Theoretical retention indices were calculated using the retention times of the eluting compounds normalized to the retention times of adjacent n-alkanes. Accurate identification of the compounds was verified by comparing theoretical retention indices to the NIST database (US Department of Commerce, Gaithersburg, MD, USA). Semi-quantitative approach against an internal standard (2-chloro-6-methylphenol) was used for quantitation purposes – the amounts of identified volatile compounds were expressed in internal standard equivalents.
2.5. Statistical analysis and data processing
The results of GC-TOF-MS analysis were statistically evaluated by partial least square discriminant analysis (PLS-DA) (mixOmics package, R software 3.4.0; Boston, MA, USA). For evaluation of the classification power of each treatment, cross-validation of PLS-DA results was carried out by calculating classification error using Mahalanobis distance (10 times repetition) – the low numerical value of classification error signifies the statistical importance of the clustering seen on the plot.
The correlation of cider samples with identified volatile compounds was observed using principal component analysis (PCA) (factoextra package, R software 3.4.0; Boston, MA, USA). Each volatile compound is represented on the PCA biplot by a vector. The length of any given vector illustrates the level of correlation between the compounds and the samples – the longer the vector, the stronger the correlation. Before the application of PCA and PLS-DA, the quantitation results were autoscaled.
3. Results and discussion
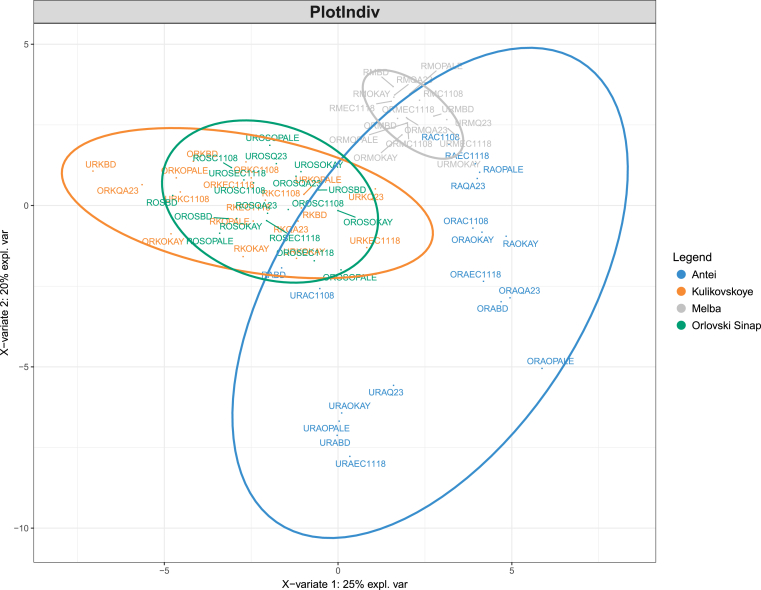
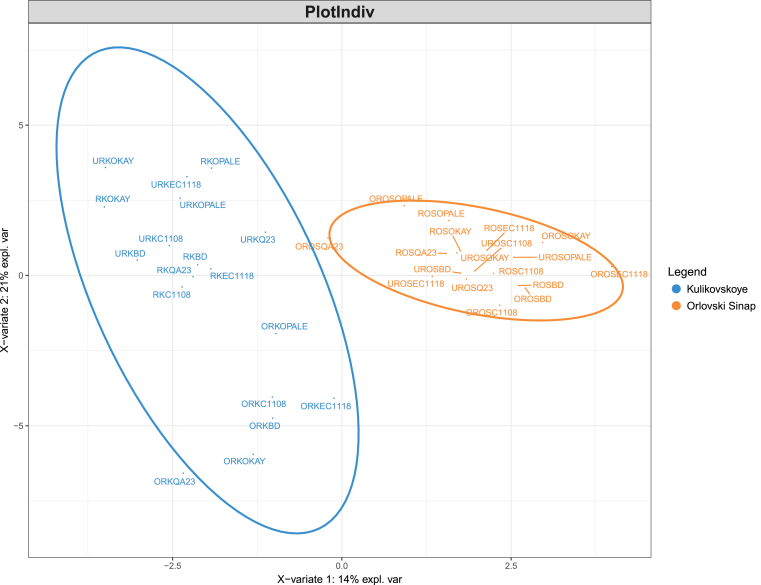
In total, 37 volatile compounds were identified in the cider samples (Table 2). Partial least square-discriminant analysis (PLS-DA) was applied to evaluate the influence of each treatment (apple variety, maturity level, and yeast strain) on the volatile composition of the samples. For that, one treatment at a time was taken as a predicted variable with the other two acting as replicates. The best visual separation of the samples was achieved when using apple variety as a predicted variable (Fig. 1). Statistical significance of the separation was observed using classification power of each treatment. Based on calculated PLS-DA classification error rates, apple variety also showed the best classification capacity followed by maturity and then yeast strain (Table 3). Since there was no clear grouping of the samples based on the maturity level of apples or yeast strains used for fermentation, no conclusive evidence could be drawn on their significance in terms of the volatile composition of the final product. Based on the grouping according to the apple variety, cider samples obtained with Melba apple variety were found to possess similar volatile profiles due to the proximity of the samples on the plot. Thus, the profiles of ciders made with Melba were influenced the least by apple maturity and yeast strain. This may indicate either a specific dominant aroma profile of Melba apple variety making it difficult to influence the cider by using different yeast strains or a lack of certain nutrients (e.g., amino acids) to be used as metabolic precursors in the formation of volatile compounds. As a contrast to the samples made with Melba variety, Antei variety showed a wide spread of the samples on the plot allowing additional subclustering based on the maturity level of apples. The samples made with unripe apples formed a clear subcluster, whereas the samples made with ripe and overripe samples were more similar to each other. The clusters formed by the samples made with Kulikovskoye and Orlovski Sinap varieties overlapped due to similarities in volatile composition. New PLS-DA analysis was carried out with these samples to get a better insight on clustering patterns (Fig. 2). As seen from Fig. 2, better separation of the samples on the plot was achieved. The samples made with Orlovski Sinap variety were relatively similar to each other. However, with the samples of Kulikovskoye variety, a specific clustering based on the maturity level of apples could be observed – the samples made with overripe apples were separate from the samples made with unripe and ripe apples. In terms of the effect of the maturity level of apples, the samples made with Kulikovskoye variety resulted differently to Antei variety where the samples from unripe apples were more different from the other stages of maturity.
Table 2.
Identified volatile compounds.
| Compound name | Odor descriptionb | RT | RIexp | RIlita | Concentration range in the samples, ppb in IS equivalents |
|---|---|---|---|---|---|
| 1-propanol | Fermented, fruity, apple, pear | 2.60 | 589 | 595 | 1.67–91.47 |
| 2-methyl-1-propanol | Wine, whiskey | 3.10 | 627 | 635 | 0.00–233.15 |
| 1-butanol | Balsamic | 3.54 | 657 | 660 | 20.24–411.62 |
| 3-methyl-1-butanol | Cognac, banana, fruity | 4.85 | 735 | 730 | 0.00–3631.49 |
| 2-methyl-1-butanol | Wine, fruity | 5.01 | 743 | 740 | 319.31–3541.74 |
| 2-hexen-1-ol, (E) | Green, leafy | 7.51 | 861 | 865 | 12.61–124.72 |
| 1-hexanol | Green, pungent | 7.67 | 868 | 865 | 283.49–4659.29 |
| 1-octen-3-ol | Earthy, vegetative, mushroom | 10.48 | 990 | 1000 | 0.00–83.00 |
| 2-ethyl-1-hexanol | Citrus, floral | 11.38 | 1030 | 1030 | 0.01–51.97 |
| 1-octanol | Citrus, floral, fatty | 12.06 | 1061 | 1070 | 0.00–2.87 |
| 2-phenylethanol | Floral, rose | 13.25 | 1115 | 1110 | 0.00–598.83 |
| Ethyl acetate | Fruity, green | 2.74 | 603 | 610 | 10.27–713.71 |
| Methyl-2-methylpropanoate | Fruity, ether | 4.02 | 690 | 684 | 0.06–0.92 |
| Ethyl propionate | Fruity, grape, pineapple, rum | 4.13 | 697 | 705 | 1.55–109.10 |
| Methyl butanoate | Pungent, fermented | 4.38 | 711 | 720 | 1.19–19.85 |
| Ethyl-2-methylpropanoate | Ether, pungent, fruity | 5.18 | 752 | 760 | 0.49–24.63 |
| Methyl-2-methylbutanoate | Fruity, ripe, fatty | 5.73 | 780 | 775 | 0.53–6.35 |
| Ethyl butanoate | Pineapple, cognac | 6.08 | 798 | 800 | 3.24–150.82 |
| Butyl acetate | Solvent, banana | 6.58 | 820 | 815 | 0.17–12.26 |
| Ethyl-3-methylbutanoate | Fruity, pineapple, apple, orange | 7.37 | 855 | 855 | 0.12–4.90 |
| Isoamyl acetate | Banana, pear | 7.76 | 872 | 875 | 3.91–252.74 |
| Ethyl-2-methylbutanoate | Fruity, apple | 8.91 | 891 | 895 | 0.22–5.98 |
| Ethyl pentanoate | Fruity, berry, tropical | 9.09 | 930 | 930 | 0.07–2.50 |
| Ethyl hexanoate | Fruity, pineapple, banana | 10.62 | 996 | 1000 | 10.34–254.60 |
| Hexyl acetate | Fruity, green apple, banana | 10.93 | 1010 | 1010 | 0.50–165.57 |
| Hexyl butanoate | Green, fruity, vegetative | 14.70 | 1084 | 1190 | 0.00–51.86 |
| Ethyl octanoate | Fruity, wine, banana, brandy | 14.80 | 1189 | 1190 | 1.41–837.17 |
| 2-phenylethyl acetate | Honey, rose | 16.22 | 1260 | 1260 | 0.00–24.34 |
| Ethyl decanoate | Waxy, fruity, apple, grape | 18.54 | 1382 | 1380 | 0.01–174.10 |
| 3-methylbutyl octanoate | Waxy, fruity, pineapple, coconut | 19.73 | 1449 | 1450 | 0.00–2.11 |
| Butanoic acid | Cheesy | 6.85 | 832 | 840 | 0.00–27.24 |
| 2-methylbutanoic acid | Cheesy, fermented | 7.72 | 870 | 870 | 0.00–13.71 |
| Benzaldehyde | Almond, cherry | 9.79 | 960 | 955 | 0.00–11.78 |
| 3-octanone | Herbal, lavender, mushroom | 10.20 | 978 | 980 | 0.00–0.91 |
| Octanal | Waxy, citrus | 10.75 | 1002 | 1005 | 0.07–150.04 |
| Phenylacetaldehyde | Honey, rose | 11.71 | 1045 | 1045 | 0.00–2.99 |
| Vanillin | Vanilla | 18.78 | 1395 | 1400 | 0.00–0.05 |
Approximate average value according to NIST database (US Department of Commerce, Gaithersburg, MD, USA).
According to www.thegoodscentscompany.com.
Fig. 1.
PLS-DA plot for the cider samples. Samples made with Antei apples are represented with color blue, Kulikovskoye – orange, Melba – gray, Orlovski Sinap – green. Samples are coded according to the preparation: maturity level (UR – Unripe, R – Ripe, OR – Overripe) – apple cultivar (A – Antei, M – Melba, OS – Orlovski Sinap, K – Kulikovskoye) – yeast strain. Thus, sample coded ROSOKAY, for example, represents a cider made with ripe Orlovski Sinap apples and fermented with OKAY commercial yeast strain.
Table 3.
Classification error rates of PLS-DA for different cider treatments (Mahalanobis distance, 10 times repetition).
| No. of components | Maturity | Variety | Yeast |
|---|---|---|---|
| 1 component | 0.37 | 0.44 | 0.81 |
| 2 components | 0.31 | 0.34 | 0.78 |
| 3 components | 0.23 | 0.13 | 0.79 |
| 4 components | 0.21 | 0.16 | 0.73 |
| 5 components | 0.17 | 0.07 | 0.74 |
Fig. 2.
PLS-DA plot for the cider samples made with Kulikovskoye (color blue) and Orlovski Sinap (color orange) apples. Samples are coded according to the preparation: maturity level (UR – Unripe, R – Ripe, OR – Overripe) – apple cultivar (A – Antei, M – Melba, OS – Orlovski Sinap, K – Kulikovskoye) – yeast strain. Thus, sample coded ROSOKAY, for example, represents a cider made with ripe Orlovski Sinap apples and fermented with OKAY commercial yeast strain.
According to overall results of PLS-DA analysis, ciders were grouped based on the apple variety first and only then based on the maturity level or yeast type. These results indicate that the apple variety has a significant influence on the technological aspects of cider production. As was shown with Melba variety, the extent to which any given yeast could potentially influence the volatile composition of the final product will depend on the apple variety used. Similarly, the degree of influence of the maturity stage of apples on the volatile composition of the final product is closely related to an apple variety picked for processing.
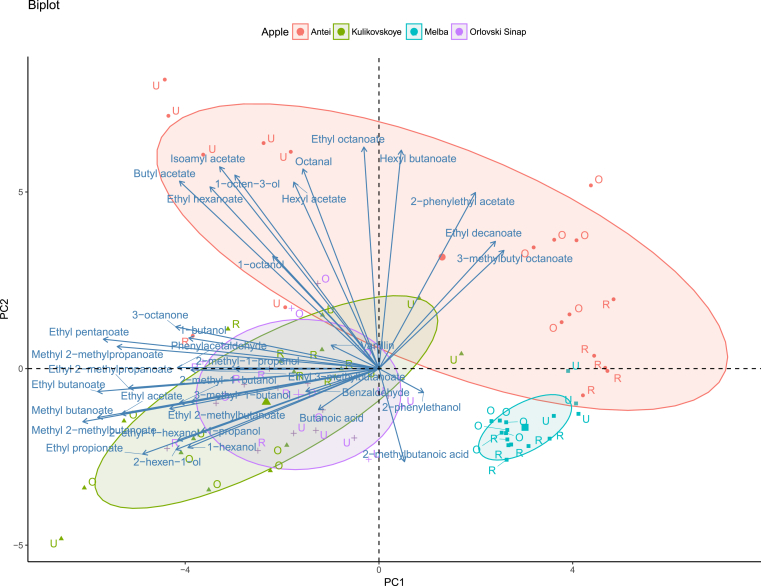
To evaluate the correlations between volatile compounds and apple variety, principal component analysis (PCA) was carried out (Fig. 3). According to the PCA biplot, ciders from Melba apple variety had the least diverse volatile composition; the samples formed a tight cluster with a substantial similarity in the volatile profiles which corresponds to the results obtained using PLS-DA approach. Ciders made with Kulikovskoye, and Orlovski Sinap varieties had similar but the most diverse volatile compositions with different alcohols, aldehydes, and esters contributing to the aroma. As per PLS-DA results, subclustering of the samples made with unripe Antei apples was also observed on the upper left corner of Fig. 3. These samples had higher contents of different acetates like isoamyl acetate, hexyl acetate, and butyl acetate.
Fig. 3.
Principal component analysis biplot. Samples made with Antei apples are represented with color red, Kulikovskoye apples – color green, Melba apples – color blue, Orlovski Sinap apples – color purple. Letter U represents samples made with underripe apples, letter R – ripe apples, letter O – overripe apples.
The influence of apple variety, maturity level, and yeast strain on the relative content of identified volatile compounds was observed using p-values (Table 4). According to the results, most of the compounds that distinguished the samples from one another were associated with apple variety, which corresponded to the conclusions made based on the results of PLS-DA. The apple variety and juice obtained from it can be viewed as a nutritional base for fermentation that directly affects the volatile composition and properties of cider. The difference between the apple varieties could, for example, come at the expense of initial nitrogen content. According to Santos et al. (2015), the initial content of nitrogen-containing compounds in apples is mainly composed of amino acids, especially aspartic acid, glutamic acid, asparagine, serine, and proline. The content of amino acids and yeast assimilable nitrogen can, in turn, be tied to the production of desired volatile compounds (e.g., esters) (Lambrechts and Pretorius, 2000; Bell and Henschke, 2005; Belda et al., 2017). Most of the alcohols identified in the samples, some esters (butyl acetate, ethyl-3-methylbutanoate, and isoamyl acetate), 3-methylbutanoic acid, 3-octanone, benzaldehyde, and phenylacetaldehyde were associated with the maturity stage of the apples used in processing. Some of the compounds (2-methylbutanol, 3-methylbutanol and hexanol) were previously detected by Sapers et al. (1977) in ripe McIntosh apples as indicators of ripeness. The mentioned compounds could have either originated from the biochemical changes during ripening or formed from specific precursors developed during ripening (e.g., 3-methylbutanol can be utilized as a precursor in the formation of isoamyl acetate) (Eden et al., 1996; Osorio and Fernie, 2013). The number of volatiles significantly influenced (significance level of more than 95%) by the yeast strain used for fermentation was the lowest when compared to the other treatments. Compounds 2-methyl-1-propanol, ethyl propionate, ethyl-2-methyl butanoate, ethyl-3-methyl butanoate, butanoic acid, and 2-methylbutanoic acid were found to be associated with the yeast strain used for fermentation and are products of metabolic activity. For example, 2-methyl-1-propanol is a product of amino acid (valine) catabolism (Bigelis et al., 1983). Acids (butanoic acid, 2-methylbutanoic acid) are formed as a by-product of either fatty acid metabolism or oxidation of intermediate compounds in amino acid catabolism (Alexandre and Charpentier, 1998). Fatty acid esters are reported to be the result of enzymatic activity during lipid biosynthesis (Suomalainen, 1981).
Table 4.
Statistical importance (p-values) of the identified volatile compounds in differentiating the sampling according to variety, maturity level, and yeast used. The compounds with significant statistical importance across each viewed variable are marked in bold.
| Compound name | p-value |
||
|---|---|---|---|
| Maturity | Variety | Yeast | |
| 1-propanol | 0.001860 | 0.000050 | 0.624665 |
| 2-methyl-1-propanol | 0.004188 | 0.048784 | 0.018511 |
| 1-butanol | 0.433735 | 0.000000 | 0.775817 |
| 3-methyl-1-butanol | 0.005499 | 0.000004 | 0.700366 |
| 2-methyl-1-butanol | 0.001963 | 0.000000 | 0.849864 |
| 2-hexen-1-ol, (E) | 0.067941 | 0.020647 | 0.308530 |
| 1-hexanol | 0.007116 | 0.028643 | 0.390312 |
| 1-octen-3-ol | 0.002924 | 0.000270 | 0.676520 |
| 2-ethyl-1-hexanol | 0.020197 | 0.000000 | 0.719560 |
| 1-octanol | 0.183813 | 0.000117 | 0.541413 |
| 2-phenylethanol | 0.000015 | 0.000206 | 0.583034 |
| Ethyl acetate | 0.093975 | 0.000004 | 0.165060 |
| Methyl-2-methylpropanoate | 0.083557 | 0.000088 | 0.237205 |
| Ethyl propionate | 0.589052 | 0.000162 | 0.003749 |
| Methyl butanoate | 0.220832 | 0.000000 | 0.948923 |
| Ethyl-2-methylpropanoate | 0.038091 | 0.000005 | 0.027933 |
| Methyl-2-methylbutanoate | 0.169946 | 0.000000 | 0.899196 |
| Ethyl butanoate | 0.185900 | 0.000007 | 0.991370 |
| Butyl acetate | 0.034334 | 0.000061 | 0.677873 |
| Ethyl-3-methylbutanoate | 0.014082 | 0.000005 | 0.001300 |
| Isoamyl acetate | 0.009240 | 0.000662 | 0.546437 |
| Ethyl-2-methylbutanoate | 0.618731 | 0.000052 | 0.999389 |
| Ethyl pentanoate | 0.481229 | 0.000000 | 0.446490 |
| Ethyl hexanoate | 0.099934 | 0.000735 | 0.747280 |
| Hexyl acetate | 0.122128 | 0.001101 | 0.556462 |
| Hexyl butanoate | 0.469554 | 0.000000 | 0.825437 |
| Ethyl octanoate | 0.183897 | 0.000000 | 0.797699 |
| 2-phenylethyl acetate | 0.125416 | 0.000008 | 0.384849 |
| Ethyl decanoate | 0.054354 | 0.000007 | 0.620213 |
| 3-methylbutyl octanoate | 0.237256 | 0.000014 | 0.313501 |
| Butanoic acid | 0.108986 | 0.236146 | 0.000084 |
| 2-methylbutanoic acid | 0.016010 | 0.001982 | 0.009141 |
| Benzaldehyde | 0.038750 | 0.007941 | 0.890327 |
| 3-octanone | 0.000041 | 0.015216 | 0.881535 |
| Octanal | 0.102392 | 0.000253 | 0.446443 |
| Phenylacetaldehyde | 0.006131 | 0.000000 | 0.837806 |
| Vanillin | 0.188694 | 0.235916 | 0.670147 |
4. Conclusions
Apple variety was the primary attribute influencing the volatile composition of apple cider. The effect of yeast strains and the maturity of apples was highly variety-specific. Ripe and overripe apples imparted mostly similar aroma profiles; however, with Kulikovskoye variety, the cider from overripe apples differed the most from the others. The volatile profiles of the samples made with Melba variety were the least influenced by the maturity level of apples and yeast strains used for the fermentation.
Declarations
Author contribution statement
Julia Rosend: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Rain Kuldjärv: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Sirli Rosenvald: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Enterprise Estonia project EU48667.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors would like to thank OÜ Siidrikoda for providing the apples, and Lallemand Inc. for providing the yeast cultures.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Alberti A., Machado dos Santos T.P., Ferreira Zielinski A.A., Eleutério dos Santos C.M., Braga C.M., Demiate I.M., Nogueira A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. LWT - Food Sci. Technol. 2016;65:436–443. [Google Scholar]
- Alexandre H., Charpentier C. Biochemical aspects of stuck and sluggish fermentation in grape must. J. Ind. Microbiol. Biotechnol. 1998;20:20–27. [Google Scholar]
- Beckner Whitener M.E., Carlin S., Jacobson D., Weighill D., Divol B., Conterno L., Du Toit M., Vrhovsek U. Early fermentation volatile metabolite profile of non-Saccharomyces yeasts in red and white grape must: a targeted approach. LWT - Food Sci. Technol. 2014;64:412–422. [Google Scholar]
- Belda I., Ruiz J., Esteban-Fernandez A., Navascues E., Marquina D., Santos A., Moreno-Arribas M.V. Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules. 2017;22:189. doi: 10.3390/molecules22020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S.J., Henschke P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005;11:242–295. [Google Scholar]
- Bigelis R., Weir P.D., Jones R.R.M., Umbarger H.E. Exogenous valine reduces conversion of leucine to 3-Methyl-l-Butanol in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1983;45:658–664. doi: 10.1128/aem.45.2.658-664.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Gomisa D., Herrero-Sáncheza I., Mangas Alonso J.J. Characterisation of apple cider cultivars by chemometric techniques using data from high-performance liquid chromatography and flow-injection analysis. Analyst. 1998;123:1187–1191. [Google Scholar]
- Cadez N., Raspor P., de Cock A., Boekhout W.A.M., Smith M. Molecular identification and genetic diversity within species of the genera Hanseniaspora and Kloeckera. FEMS Yeast Res. 2002;1:279–289. doi: 10.1111/j.1567-1364.2002.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Eden A., Simchen G., Benvenisty N. Two yeast homologs of ECA39, a target for c-Myc regulation, code for cytosolic and mitochondrial branched chain amino acid aminotransferases. J. Biol. Chem. 1996;271:20242–20425. doi: 10.1074/jbc.271.34.20242. [DOI] [PubMed] [Google Scholar]
- El Hadi M.A.M., Zhang F.-J., Wu F.-F., Zhou C.-H., Tao J. Advances in fruit aroma volatile research. Molecules. 2013;18:8200–8229. doi: 10.3390/molecules18078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henick-Kling T., Edinger W.D., Daniel P., Monk P. Selective effects of sulfur dioxide and yeast starter culture addition on indigenous yeast populations and sensory characteristics of wine. J. Appl. Microbiol. 1998;84:865–876. [Google Scholar]
- Kalinowska M., Bielawska A., Lewandowska-Siwkiewicz H., Priebe W., Lewandowski W. Apples: content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014;84:169–188. doi: 10.1016/j.plaphy.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Laaksonen O., Kuldjärv R., Paalme T., Virkki M., Yang B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017;233:29–37. doi: 10.1016/j.foodchem.2017.04.067. [DOI] [PubMed] [Google Scholar]
- Lambrechts M.G., Pretorius I.S. Yeast and its importance to wine aroma. S. Afr. J. Enol. Vitic. 2000;21:97–129. [Google Scholar]
- McKay M., Buglass A.J., Lee C.G. Fermented beverages: beers, ciders, wines and related drinks. In: Buglass A.J., editor. Handbook of Alcoholic Beverages: Technical, Analytical and Nutritional Aspects. Wiley; Chichester: 2011. pp. 63–454. [Google Scholar]
- Nogueira A., Wosiacki G. Apple cider fermentation. In: Hui Y.H., Özgül E.E., editors. Handbook of Plant-Based Fermented Food and Beverage Technology. CRC Press; Boca Raton: 2012. pp. 209–236. [Google Scholar]
- Osorio S., Fernie A.R. Biochemistry of fruit ripening. In: Seymour G.B., editor. The Molecular Biology and Biochemistry of Fruit Ripening. Wiley-Blackwell; Ames: 2013. pp. 1–20. [Google Scholar]
- Rodríguez Madrera R., Pando Bedriñana R., Suárez Valles B. Production and characterization of aroma compounds from apple pomace by solid-state fermentation with selected yeasts. LWT - Food Sci. Technol. 2015;64:1342–1353. [Google Scholar]
- Santos C.M.E., Pietrowski G.A.M., Braga C.M., Rossi M.J., Ninow J., Santos T.P.M., Wosiacki G., Jorge R.M.M., Nogueira A. Apple amino acid profile and yeast strains in the formation of fusel alcohols and esters in cider production. J. Food Sci. 2015;80:1170–1177. doi: 10.1111/1750-3841.12879. [DOI] [PubMed] [Google Scholar]
- Sapers G.M., Abbott J., Massie O., Watada A., Finney E.E., Jr. Volatile composition of McIntosh apple juice as a function of maturity and ripeness indices. J. Food Sci. 1977;41:44–47. [Google Scholar]
- Soden A., Francis I.L., Oakley H., Henschke P.A. Co-fermentation of chardonnay. Aust. J. Grape Wine Res. 2000;6:21–30. [Google Scholar]
- Suárez Valles B., Pando Bedriñana R., Fernández Tascón N., González Garcia A., Rodríguez Madrera R. Analytical differentiation of cider inoculated with yeast (Saccharomyces cerevisiae) isolated from Asturian (Spain) apple juice. LWT - Food Sci. Technol. 2005;38:455–461. [Google Scholar]
- Suomalainen H. Yeast esterases and aroma esters in alcoholic beverages. J. Inst. Brew. 1981;87:296–300. [Google Scholar]
- Synos K., Reynolds A.G., Bowen A.J. Effect of yeast strain on aroma compounds in Cabernet franc icewines. LWT - Food Sci. Technol. 2015;64:227–235. [Google Scholar]
- Travers I., Jacquet A., Brisset A., Maite C. Relationship between the enzymatic determination of starch and the starch iodine index in two varieties of cider apple. J. Sci. Food Agric. 2002;82:983–989. [Google Scholar]
- Vanbeneden N., Van Roey T., Willems F., Delvaux F.R. Release of phenolic flavour precursors during wort production: influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008;111:83–91. [Google Scholar]
- Villière A., Arvisenet G., Lethuaut L., Prost C., Sérot T. Selection of a representative extraction method for the analysis of odourant volatile composition of French cider by GC–MS–O and GC × GC–TOF-MS. Food Chem. 2012;4:1561–1568. [Google Scholar]
- Wu J., Gao H., Zhao L., Hu X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007;103:88–93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.