Key Points
Question
Are there differences in mortality and stroke between patients who undergo transcatheter aortic valve replacement (TAVR) for bicuspid compared with tricuspid aortic stenosis?
Findings
In this registry-based cohort study that included 2691 propensity-score matched pairs of patients undergoing TAVR for bicuspid vs tricuspid aortic stenosis, there was no statistically significant difference in 30-day mortality (2.6% vs 2.5%; respectively) or 1-year mortality (10.5% vs 12.0%). However, the 30-day risk of stroke was significantly greater among those with bicuspid aortic stenosis (2.5% vs 1.6%).
Meaning
Patients who underwent TAVR for bicuspid aortic stenosis compared with tricuspid aortic stenosis had no significant difference in mortality, but had increased 30-day risk of stroke; because of the potential for selection bias, randomized trials would be needed to adequately assess the efficacy and safety of TAVR for bicuspid aortic stenosis.
Abstract
Importance
Transcatheter aortic valve replacement (TAVR) indications are expanding, leading to an increasing number of patients with bicuspid aortic stenosis undergoing TAVR. Pivotal randomized trials conducted to obtain US Food and Drug Administration approval excluded bicuspid anatomy.
Objective
To compare the outcomes of TAVR with a balloon-expandable valve for bicuspid vs tricuspid aortic stenosis.
Design, Setting, and Participants
Registry-based prospective cohort study of patients undergoing TAVR at 552 US centers. Participants were enrolled in the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapies Registry from June 2015 to November 2018.
Exposures
TAVR for bicuspid vs tricuspid aortic stenosis.
Main Outcomes and Measures
Primary outcomes were 30-day and 1-year mortality and stroke. Secondary outcomes included procedural complications, valve hemodynamics, and quality of life assessment.
Results
Of 81 822 consecutive patients with aortic stenosis (2726 bicuspid; 79 096 tricuspid), 2691 propensity-score matched pairs of bicuspid and tricuspid aortic stenosis were analyzed (median age, 74 years [interquartile range {IQR}, 66-81 years]; 39.1%, women; mean [SD] STS-predicted risk of mortality, 4.9% [4.0%] and 5.1% [4.2%], respectively). All-cause mortality was not significantly different between patients with bicuspid and tricuspid aortic stenosis at 30 days (2.6% vs 2.5%; hazard ratio [HR], 1.04, [95% CI, 0.74-1.47]) and 1 year (10.5% vs 12.0%; HR, 0.90 [95% CI, 0.73-1.10]). The 30-day stroke rate was significantly higher for bicuspid vs tricuspid aortic stenosis (2.5% vs 1.6%; HR, 1.57 [95% CI, 1.06-2.33]). The risk of procedural complications requiring open heart surgery was significantly higher in the bicuspid vs tricuspid cohort (0.9% vs 0.4%, respectively; absolute risk difference [RD], 0.5% [95% CI, 0%-0.9%]). There were no significant differences in valve hemodynamics. There were no significant differences in moderate or severe paravalvular leak at 30 days (2.0% vs 2.4%; absolute RD, 0.3% [95% CI, −1.3% to 0.7%]) and 1 year (3.2% vs 2.5%; absolute RD, 0.7% [95% CI, −1.3% to 2.7%]). At 1 year there was no significant difference in improvement in quality of life between the groups (difference in improvement in the Kansas City Cardiomyopathy Questionnaire overall summary score, −2.4 [95% CI, −5.1 to 0.3]; P = .08).
Conclusions and Relevance
In this preliminary, registry-based study of propensity-matched patients who had undergone transcatheter aortic valve replacement for aortic stenosis, patients with bicuspid vs tricuspid aortic stenosis had no significant difference in 30-day or 1-year mortality but had increased 30-day risk for stroke. Because of the potential for selection bias and the absence of a control group treated surgically for bicuspid stenosis, randomized trials are needed to adequately assess the efficacy and safety of transcatheter aortic valve replacement for bicuspid aortic stenosis.
This registry-based cohort study compared mortality and stroke rates among patients with bicuspid and tricuspid aortic stenosis who had undergone TAVR to determine whether advances in technology and in valve devices improved outcomes for patients with bicuspid aortic anatomy.
Introduction
Bicuspid aortic valve was estimated to have a prevalence of 1% (in 2004)1,2 and was more prone to early degeneration and accounted for up to 50% of patients requiring surgery in the younger population.3 Transcatheter aortic valve replacement (TAVR) has become the established treatment for aortic stenosis in patients at increased risk of surgery based on multiple randomized clinical trials and registries.4,5,6,7,8,9,10 These early pivotal studies primarily enrolled elderly patients with degenerative tricuspid aortic valve disease and excluded patients with bicuspid anatomy. Since the initial US Food and Drug Administration approval of TAVR in 2011, improvement in the safety profile of TAVR compared with surgery has led to further clinical trials and off-label use in younger patients who are at low risk of open heart surgery, especially outside the United States.11 The further expansion of TAVR indication in the large number of patients who are currently undergoing surgery may be limited since even the most contemporary low-risk trials comparing TAVR vs surgery excluded patients with bicuspid anatomy.12,13
Although bicuspid anatomy has been considered to be a relative contraindication to TAVR, a limited number of patients who are at high risk of surgery have been treated with TAVR.14 The previously published small series of TAVR for bicuspid aortic stenosis demonstrated limited success with the use of older generation valves but also improved outcomes with the newer generation devices.15 Nevertheless, these previous registries comprised elderly patients treated in select centers, and it is unknown if these findings can be applied to contemporary clinical practice. The Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapies (TVT) Registry includes all consecutive TAVR procedures performed in the United States. In this study, the outcomes of TAVR for bicuspid aortic stenosis were evaluated in this largest national registry.10
Methods
The STS and ACC developed the collaborative clinical registry program in response to the Centers for Medicare & Medicaid Services (CMS) national coverage decisions (May 2012) requirement for national registry participation of all US TAVR centers. The study was approved by the Registry Scientific and Strategic Committee. Participating centers used standardized definitions to collect clinical information—including patient demographics, comorbidities, functional status, quality-of-life indexes, and procedural details and outcomes—from consecutive TAVR cases using commercially approved devices. The registry protocol was granted a waiver of informed consent by the Chesapeake Research Review Incorporated and the Duke University institutional review boards. Data were obtained from the registry for all 92 236 patients undergoing TAVR with the third-generation balloon-expandable Sapien 3 transcatheter heart valve since commercial approval in June 2015 to November 2018. The analyses were performed on data downloaded by Edwards Lifesciences from the STS/ACC TVT Registry. Patients with previous surgical aortic valve replacement or TAVR, unicuspid, quadricuspid, or uncertain valve types were excluded from the present analysis. The primary imaging modality for the determination of aortic valve morphology was echocardiography. This study sought to compare the baseline and procedural characteristics as well as in-hospital, 30-day, and 1-year clinical outcomes between patients with bicuspid and tricuspid aortic stenosis.
Outcomes
The primary objective of the study was to evaluate the short-term and long-term outcomes of TAVR in bicuspid aortic stenosis, in terms of death and stroke at 30 days and 1 year. The secondary outcomes included procedural complications, in-hospital adverse events, postprocedural echocardiographic assessment of the valve, functional status (New York Heart Association [NYHA] heart failure class), and health status (the Kansas City Cardiomyopathy Questionnaire overall summary score [KCCQ-OS]) at 30 days and 1 year.16,17 The KCCQ-OS score ranges from 0 to 100 (higher scores indicate less symptom burden and better quality of life). In accordance with prior studies, the KCCQ-OS scores were categorized as very poor (<25), poor (25-49), fair (50-74), and good (≥75) quality of life.18,19 Changes in the KCCQ-OS of 5, 10, and 20 points correspond to small, moderate, or large clinical improvements, respectively.20 All adverse outcomes were defined using Valve Academic Research Consortium-2 definitions.21
Statistical Analysis
Continuous variables were presented as mean SD or median interquartile range (IQR) and were compared between groups using the 2-sample t tests or Wilcoxon rank sums tests. Categorical variables were given as frequencies and percentages and were compared using χ2 or 2-tailed Fisher exact test. The 30-day and 1-year adverse event rates were based on Kaplan-Meier estimates and all comparisons were made using the log-rank test.
It was anticipated that patients with bicuspid and tricuspid aortic stenosis would have significantly different baseline and procedural characteristics.22 To avoid confounding due to these differences, propensity score–based matching was used. Propensity scores were calculated using a logistic regression model based on 25 relevant baseline patient characteristics (covariates) with aortic valve type (bicuspid or tricuspid aortic stenosis) as the dependent variable. The covariates were age, sex (male), body mass index, access site, prior percutaneous coronary intervention, prior coronary artery bypass graft surgery, prior stroke, carotid stenosis, peripheral arterial disease, hypertension, diabetes, chronic lung disease, immunocompromise, porcelain aorta, atrial fibrillation, serum creatinine level, hemoglobin level, estimated glomerular filtration ratio, aortic valve mean gradient, left ventricular ejection fraction, mitral regurgitation, tricuspid regurgitation, NYHA functional class III/IV, 5-meter walk test, and KCCQ-OS score. Missing baseline values were imputed using the Markov Chain Monte Carlo method prior to modeling. Missing baseline characteristic values were included in the eTable 1 in the Supplement. The missing clinical follow-up was not imputed. Patients with bicuspid aortic stenosis were matched 1-to-1 to those with tricuspid aortic stenosis using a greedy matching strategy with caliper of 0.01, producing 2 patient cohorts. Balance between the groups was assessed by calculating standardized differences for which a difference of less than 0.10 was considered to indicate good balance.
This is an ongoing registry enrolling all commercial TAVRs in the United States. Thus, at any given time point, not all patients would have reached the 1-year end point. In addition, clinical follow-up in the registry is lacking in a fraction of patients who have reached the 1-year end point but not followed up at the index hospital performing the TAVR procedure. To overcome these limitations, mortality and stroke data between the study cohort and CMS were linked to compare longitudinal outcomes between bicuspid and tricuspid cohorts. Patient survival and other clinical events were determined with CMS linkage, irrespective of patients following up at the hospital where the TAVR procedure was performed (eFigure 1 in the Supplement). The follow-up in the TVT registry ended in November 2018. CMS-linked data were available from 2015 through 2017. The mortality and stroke follow-up data presented in this study represent data pooled from the TVT registry as well as from CMS linkage. Because CMS linkage was only available until 2017, further sensitivity analyses were performed by comparing death and stroke in propensity-matched cohorts only including patients for whom CMS data were available for linkage; as well as for those who had CMS linkage and had completed the 1-year end point.
Cox regression model using stepwise selection was performed to assess the adjusted hazard ratio (HR) of patients with bicuspid aortic stenosis vs those with tricuspid aortic stenosis on coprimary end points. The stepwise selection consisted of entering in the model covariates with P ≤.10 and removing covariates with P >.10. The candidate covariates were identical to those used in the propensity-score matching analysis. The model was checked for violation of the proportional hazards assumption by Kolmogorov-type supremum test. All P values were 2-sided, and P < .05 was considered significant for all tests. No adjustment for multiple testing was undertaken. Because of the potential for type I error due to multiple comparisons, all findings of this study should be interpreted as exploratory. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). SAS Proc MI was used for multiple imputation, Proc Phreg for the Cox regression model, and Proc logistics for propensity score calculation.
Results
Baseline Characteristics
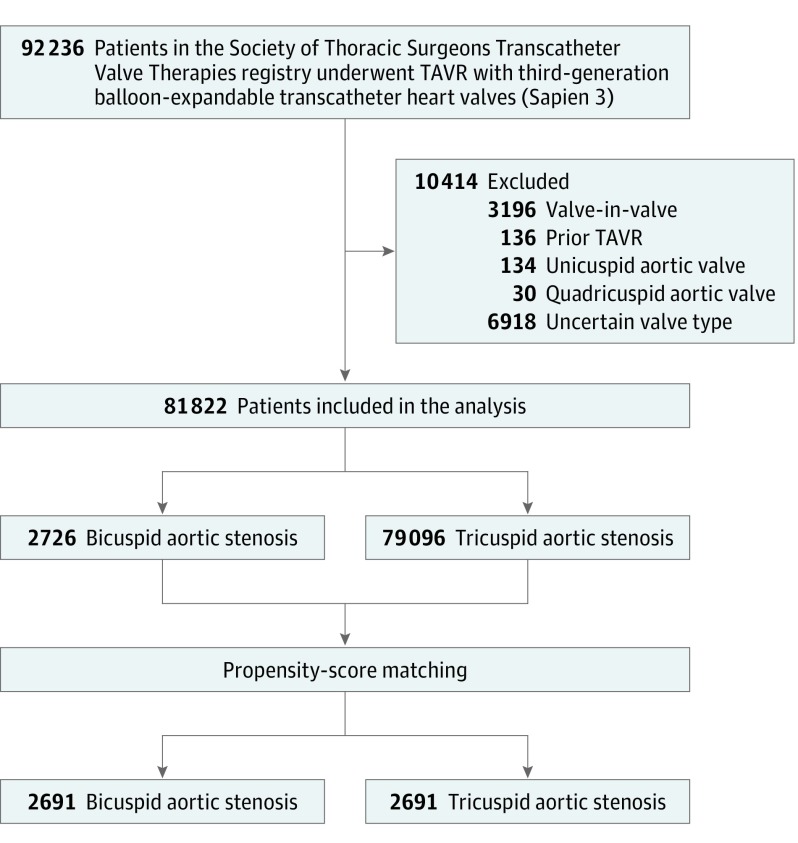
A total of 92 236 patients underwent a TAVR procedure with the third-generation balloon-expandable transcatheter heart valves between June 2015 and November 2018 at 552 institutions in the United States. Participant follow-up ended on November 20, 2018. A total of 81 822 patients (2726 patients with bicuspid aortic stenosis and 79 096 patients with tricuspid aortic stenosis) were included in the present analysis, producing 2691 propensity-matched pairs of patients with bicuspid and tricuspid aortic stenosis (Figure 1). CMS-linked data were available for 32 346 patients (836 bicuspid, 31 510 tricuspid), creating 784 propensity-matched pairs of patients for whom CMS data were available for linkage and 469 pairs of patients who had CMS linkage and had completed the 1-year end point.
Figure 1. Study Flowchart of Patients Who Underwent Transcatheter Aortic Valve Replacement Therapy.
TAVR indicates transcatheter aortic valve replacement.
In the unadjusted cohort, patients with bicuspid aortic stenosis were younger (median age, 73 years [IQR, 66-81 years] vs 82 years [IQR, 76-87 years]; P < .001); more likely to be men (60.4% vs 55.1%; P < .001); had lower STS-predicted risk of mortality (4.9% [4.0%] vs 6.5% [4.6%]; P < .001); and had fewer comorbidities. After adjusting with propensity-score matching, baseline characteristics were not significantly different (Table 1). The median procedure dates in the matched cohort were July 6, 2017, for bicuspid aortic stenosis cohort and June 3, 2017, for tricuspid aortic stenosis cohort. All patients in both cohorts completed follow-up at discharge.
Table 1. Baseline Characteristics of Patients Who Underwent Transcatheter Aortic Valve Replacement.
| Characteristic | Unadjusted Cohort | Propensity-Score Matched Cohorta | ||||
|---|---|---|---|---|---|---|
| No./Total (%) of Patients With Aortic Valve Stenosis | ASD | No./Total (%) of Patients With Aortic Valve Stenosis | ASD | |||
| Bicuspid (n = 2726) | Tricuspid (n = 79 096) | Bicuspid (n = 2691) | Tricuspid (n = 2691) | |||
| Demographics | ||||||
| Age, median (IQR), y | 73 (66-81) | 82 (76-87) | 0.839 | 74 (66-81) | 74 (66-81) | 0.02 |
| Men | 1647/2725 (60.4) | 43 582/79 081 (55.1) | 0.108 | 1621/2690 (60.3) | 1655/2691 (61.5) | 0.025 |
| Women | 1078/2725 (39.6) | 35 499/79 081 (44.9) | 1069/2690 (39.7) | 1036/2691 (38.5) | ||
| Body mass index, mean (SD)b | 29.2 (7.6) | 29.0 (7.3) | 0.031 | 29.2 (7.6) | 29.4 (7.4) | 0.028 |
| NYHA class III/IV heart failurec | 2008/2702 (74.3) | 59 147/78 460 (75.4) | 0.025 | 1983/2667 (74.4) | 1974/2664 (74.1) | 0.006 |
| Society of Thoracic Surgeons Predicted Risk of Mortality score, mean (SD)d | 4.9 (4.0) | 6.5 (4.6) | 0.373 | 4.9 (4.0) | 5.1 (4.2) | 0.047 |
| Creatinine, median (IQR), mg/dL | 1.0 (0.8-1.3) | 1.1 (0.9-1.4) | 0.065 | 1.0 (0.8-1.3) | 1.0 (0.8-1.3) | 0.025 |
| Estimated GFR, mean (SD), mL/min/1.73 m2 | 65.3 (28.7) | 59.3 (24.5) | 0.225 | 65.0 (28.4) | 64.4 (27.2) | 0.023 |
| Hemoglobin level, mean (SD), g/dL | 12.6 (2.0) | 12.1 (2.0) | 0.253 | 12.6 (2.0) | 12.6 (2.4) | 0.011 |
| Albumin level, mean (SD), g/dL | 3.8 (0.5) | 3.7 (0.5) | 0.066 | 3.8 (0.5) | 3.8 (0.5) | 0.033 |
| Comorbidities | ||||||
| Hypertension | 2287/2721 (84.1) | 72 073/78 985 (91.2) | 0.218 | 2269/2686 (84.5) | 2263/2687 (84.2) | 0.007 |
| Diabetes mellitus | 970/2720 (35.7) | 30 597/78 935 (38.8) | 0.064 | 961/2685 (35.8) | 989/2686 (36.8) | 0.021 |
| Current dialysis | 77/2719 (2.8) | 3153/78 974 (4.0) | 0.064 | 77/2684 (2.9) | 139/2688 (5.2) | 0.021 |
| Atrial fibrillation or flutter | 783/2719 (28.8) | 30525/78 932 (38.7) | 0.210 | 779/2684 (29.0) | 790/2683 (29.4) | 0.009 |
| Prior stroke | 278/2720 (10.2) | 9096/78 976 (11.5) | 0.042 | 275/2685 (10.2) | 274/2685 (10.2) | 0.001 |
| Prior transient ischemic attack | 176/2716 (6.1) | 6684/78 850 (8.5) | 0.090 | 167/2681 (6.2) | 177/2680 (6.6) | 0.015 |
| Carotid stenosis | 307/2069 (14.8) | 15 712/62 332 (25.2) | 0.261 | 307/2043 (15.0) | 331/2127 (15.6) | 0.015 |
| Prior coronary stenting | 684/2718 (25.1) | 26 853/78 919 (34.0) | 0.195 | 683/2683 (25.5) | 714/2685 (26.6) | 0.026 |
| Prior coronary bypass graft surgery | 426/2718 (15.7) | 16435/78951 (20.6) | 0.133 | 426/2683 (15.9) | 463/2688 (17.2) | 0.036 |
| Peripheral vascular disease | 655/2719 (24.1) | 21 779/78 943 (27.6) | 0.08 | 653/2684 (24.3) | 657/2684 (24.5) | 0.003 |
| Chronic lung disease | 1123/2706 (41.5) | 31 482/78 600 (40.1) | 0.029 | 1113/2672 (41.7) | 1125/2678 (42.0) | 0.007 |
| Immunocompromised | 259/2716 (9.5) | 6256/78 928 (7.9) | 0.057 | 258/2681 (9.6) | 250/2683 (9.3) | 0.010 |
| Hostile cheste | 198/2719 (7.3) | 4836/78 967 (6.1) | 0.046 | 197/2684 (7.3) | 240/2687 (8.9) | 0.058 |
| Porcelain aortae | 73/2719 (2.7) | 2657/78 924 (3.4) | 0.040 | 72/2684 (2.7) | 83/2682 (3.1) | 0.025 |
| KCCQ-OS score, median (IQR)f | 44.8 (27.1-65.6) | 43.8 (26.6-63.5) | 0.049 | 44.8 (27.1-65.6) | 45.8 (27.1-66.7) | 0.005 |
| Five-m walk test, mean (SD), s | 7.5 (4.1) | 8.4 (5.4) | 0.169 | 7.6 (4.2) | 7.6 (3.9) | 0.008 |
| Echocardiographic findings | ||||||
| Aortic valve area, mean (SD), cm2 | 0.7 (0.2) | 0.7 (0.2) | 0.163 | 0.7 (0.2) | 0.7 (0.2) | 0.018 |
| Mean gradient, mean (SD), mm Hg | 45.3 (15.1) | 42.9 (14.0) | 0.163 | 45.2 (15.0) | 44.9 (15.2) | 0.018 |
| Ejection fraction, mean (SD), % | 53.4 (14.8) | 55.2 (13.1) | 0.128 | 53.5 (14.7) | 52.5 (15.0) | 0.065 |
| Annulus size, mean (SD), mm | 25.1 (3.2) | 24.3 (2.9) | 0.261 | 25.1 (3.2) | 24.6 (3.0) | 0.142 |
| Mitral insufficiency (moderate/severe) | 431/2109 (20.4) | 18 705/65 773 (28.4) | 0.187 | 429/2081 (20.6) | 474/2186 (21.7) | 0.026 |
| Tricuspid insufficiency (moderate/severe) | 377/2705 (13.9) | 15 776/78 442 (20.1) | 0.165 | 373/2670 (14.0) | 378/2673 (14.1) | 0.005 |
Abbreviations: ASD, absolute standardized difference; GFR, glomerular filtration rate; IQR, interquartile range; KCCQ-OS, Kansas City Cardiomyopathy Questionnaire overall summary; NYHA, New York Heart Association.
SI conversion factor: To convert creatinine from mg/dL to μmol/L, multiply by 88.4.
To account for baseline differences between patients with bicuspid and tricuspid aortic valve stenosis, propensity score–based matching was used. Propensity scores were calculated using a logistic regression model based on 25 relevant baseline patient characteristics (covariates) with aortic valve type (bicuspid or tricuspid aortic valve stenosis) as the dependent variable. The covariates in the model included baseline characteristics and demographics, echocardiographic findings, health status, and functional status. Patients with bicuspid aortic valve stenosis were matched 1-to-1 to those with tricuspid aortic valve stenosis, producing 2 balanced patient cohorts (n = 2691 for each group). The entire list of covariates and details of matching appears in the Statistical Analysis in the Supplement.
Calculated as weight in kilograms divided by height in meters squared.
Categorizes patients based on how much they are limited during physical activity (I, no limitation; IV, symptoms at rest).
Estimates the potential risk of operative mortality of isolated aortic valve replacement (range, 0% to 100%); a higher score indicates an increased risk. The risk models were developed and validated using Society of Thoracic Surgeons data from 2002 to 2006.
Defined according to the Valve Academic Research Consortium-2 consensus document. The detailed definition appears in the Supplement.
Health status was evaluated with the 12-item KCCQ-12, a patient-reported disease specific status survey used to describe symptoms, functional status, and quality of life in patients with heart failure. The KCCQ-12 is collected by sites at baseline, at 30 days, and at 1 year after transcatheter aortic valve replacement and assesses 4 domains (physical limitation, symptoms, quality of life, and social limitation), which are combined into an overall summary score (KCCQ-OS) score (range, 0 to 100, with higher scores indicating less symptom burden and better quality of life).
Procedural Characteristics and In-Hospital Outcomes
Among the propensity-score matched patients, procedural characteristics did not significantly differ between cohorts except for more frequent use of the largest-size prostheses in the bicuspid group than in the tricuspid group (35.2% vs 26.4%; P < .001) (Table 2; eTable 2 in the Supplement). There were no significant differences between groups in implant success (99.0% vs 99.0%; absolute risk difference [RD], 0% [95% CI, −0.6% to 0.6%]) or device success (96.5% vs 96.6%; absolute RD, 0.1% [95% CI, −1.1% to 0.9%]). Conversion to surgery (0.9% vs 0.4%; absolute RD, 0.5% [95% CI, 0% to 0.9%]) and annulus rupture (0.3% vs 0%; absolute RD, 0.3% [95% CI, 0% to 0.5%]) occurred more frequently in the bicuspid group than in the tricuspid group. There were no significant differences between the 2 groups in procedure complications or in-hospital events including all-cause death (1.7% vs 1.6%; absolute RD, 0.1% [95% CI, −0.6% to 0.8%]) whereas the bicuspid group had higher rate of in-hospital stroke (2.1% vs 1.2%; absolute RD, 0.9% [95% CI, 0.2% to 1.6%]).
Table 2. Procedural Characteristics and In-Hospital Outcomes.
| Total/No. (%) of Patients With Aortic Valve Stenosis | Absolute Difference (95% CI), % | P Value | ||
|---|---|---|---|---|
| Bicuspid (n = 2691) | Tricuspid (n = 2691) | |||
| Procedural Characteristics | ||||
| Procedure statusa | ||||
| Electiveb | 2431/2689 (90.4) | 2423/2689 (90.1) | 0.3 (−1.3 to 1.9) | .51 |
| Urgentc | 248/2689 (9.2) | 259/2689 (9.6) | 0.4 (−2.0 to 1.2) | |
| Emergentd | 8/2689 (0.3) | 7/2689 (0.3) | 0 (−0.3 to 0.4) | |
| Salvagee | 2/2689 (0.1) | 0/2689 (0) | 0.1 (−0.1 to 0.2) | |
| Cardiopulmonary bypass | 38/2687 (1.4) | 26/2686 (1.0) | 0.4 (−0.2 to 1.1) | .13 |
| Access sitea | ||||
| Transfemoral | 2518/2690 (93.6) | 2526/2690 (93.9) | 0.3 (−1.6 to 1.0) | .74 |
| Subclavianf | 75/2690 (2.8) | 65/2690 (2.4) | 0.1 (−0.6 to 0.9) | |
| Transapicalg | 45/2690 (1.7) | 36/2690 (1.3) | 0.3 (−0.4 to 1.0) | |
| Transaortich | 27/2690 (1.0) | 37/2690 (1.4) | 0.4 (−1.0 to 0.2) | |
| Prosthesis size, mma | ||||
| 20 | 72/2691 (2.7) | 84/2691 (3.1) | 0.4 (−1.4 to 0.5) | <.001 |
| 23 | 620/2691 (23.0) | 767/2691 (28.5) | 5.5 (3.1 to 7.8) | |
| 26 | 1052/2691 (39.1) | 1129/2691 (42.0) | 2.9 (0.2 to 5.5) | |
| 29 | 947/2691 (35.2) | 711/2691 (26.4) | 8.8 (6.3 to 11.3) | |
| Secondary outcomes | ||||
| Implant successi | 2663/2689 (99.0) | 2662/2688 (99.0) | 0 (−0.6 to 0.6) | >.99 |
| Device successj | 2577/2671 (96.5) | 2586/2678 (96.6) | 0.1 (−1.1 to 0.9) | .87 |
| Conversion to open heart surgery | 24/2689 (0.9) | 11/2683 (0.4) | 0.5 (0 to 0.9) | .03 |
| Annulus rupture | 7/2689 (0.3) | 0/2683 (0) | 0.3 (0 to 0.5) | .02 |
| Ventricular rupture | 2/2689 (0.1) | 1/2683 (<0.1) | 0 (−0.1 to 0.2) | >.99 |
| Device embolization to left ventricle | 3/2689 (0.1) | 2/2683 (0.1) | 0 (−0.2 to 0.2) | >.99 |
| Coronary occlusion | 3/2689 (0.1) | 4/2683 (0.1) | 0 (−0.3 to 0.2) | .73 |
| Other | 9/2689 (0.3) | 4/2683 (0.1) | 0.2 (−0.1 to 0.5) | .17 |
| Procedure complications | ||||
| Annular dissection | 9 (0.3) | 3 (0.1) | 0.2 (−0.1 to 0.5) | .08 |
| Aortic dissection | 7 (0.3) | 3 (0.1) | 0.1 (−0.1 to 0.4) | .34 |
| Coronary compression or obstruction | 11 (0.4) | 7 (0.3) | 0.1 (−0.2 to 0.5) | .34 |
| Device embolization to aorta | 0 | 3 (0.1) | 0.1 (−0.3 to 0.1) | .25 |
| Device embolization to left ventricle | 3 (0.1) | 1 (<0.1) | 0.1 (−0.1 to 0.3) | .62 |
| Perforation with or without tamponadek | 25 (0.9) | 15 (0.6) | 0.4 (−0.1 to 0.9) | .11 |
| Need for second valve | 12 (0.4) | 6 (0.2) | 0.2 (−0.1 to 0.6) | .16 |
| In-Hospital Event | ||||
| Death | 45 (1.7) | 42 (1.6) | 0.1 (−0.6 to 0.8) | .75 |
| Stroke | 56 (2.1) | 32 (1.2) | 0.9 (0.2 to 1.6) | .01 |
| Death or stroke | 92 (3.4) | 70 (2.6) | 0.8 (−0.1 to 1.8) | .08 |
| Myocardial infarction | 8 (0.3) | 7 (0.3) | 0 (−0.3 to 0.4) | .80 |
| Life-threatening bleeding | 0 | 0 | >.99 | |
| Major vascular complication | 23 (0.9) | 24 (0.9) | 0 (−0.6 to 0.5) | .88 |
| New requirement for dialysis | 12 (0.4) | 14 (0.5) | 0.1 (−0.5 to 0.3) | .69 |
| New permanent pacemaker | 196 (7.3) | 160 (5.9) | 1.3 (−0.0 to 2.7) | .05 |
| New-onset atrial fibrillation | 45 (1.7) | 48 (1.8) | 0.1 (−0.8 to 0.6) | .75 |
P values were calculated with χ2 test for an overall test of the 4 categories.
Cardiac function stable days or weeks before the operation; procedure could be deferred without increased risk of cardiac compromise.
Procedure required during same hospitalization to minimize further clinical deterioration .
Ongoing, refractory unrelenting cardiac compromise, with or without hemodynamic instability, and not responsive to any therapy but cardiac intervention and should not be delayed.
Cardiopulmonary resuscitation en route to the procedure, before anesthesia, or ongoing extracorporeal membrane oxygenation to maintain life.
Performed from subclavian/axillary artery when femoral access is prohibitive.
Procedure performed from left ventricle apex with the anterolateral minithoracotomy when femoral access is prohibitive.
Procedure performed from ascending aorta with ministernotomy or right thoracotomy when femoral access is prohibitive.
Correct positioning of a single prosthetic heart valve.
Composite end point (successful vascular access, delivery, and deployment of the device and successful retrieval of the delivery system; correct position of the device in the proper anatomical location; intended performance of the prosthetic heart valve (aortic valve area >1.2 cm2 and mean aortic valve gradient <20 mm Hg or peak velocity <3 m per second, without moderate or severe prosthetic valve aortic regurgitation); only 1 valve implanted in the proper anatomical location.
Perforation of the myocardium, aortic annulus, or aorta, with or without tamponade associated with the perforation.
30-Day and 1-Year Clinical Outcomes
Adverse clinical outcomes in the propensity-score matched cohorts are shown in Table 3. There were no significant differences in 30-day all-cause mortality between the propensity-matched bicuspid and tricuspid aortic stenosis groups (2.6% vs 2.5%; absolute RD, 0.09% [95% CI, 0.08%-0.1%]; HR, 1.04 [95% CI, 0.74-1.47]). The 30-day stroke rate was significantly higher among patients in the bicuspid cohort than among patients in the tricuspid cohort (2.5% vs 1.6%; absolute RD, 0.89% [95% CI, 0.88%-0.9%]; HR, 1.57 [95% CI, 1.06-2.33]). There were no significant differences between the 2 cohorts in other 30-day outcomes except a higher rate of permanent pacemaker implants in the bicuspid aortic stenosis cohort (9.1% vs 7.5%; absolute RD, 1.65% [95% CI, 1.63%-1.66%]; HR, 1.23 [95% CI, 1.02-1.49]). Unadjusted clinical outcomes are summarized in eTable 3 in the Supplement.
Table 3. Thirty-Day and 1-Year Clinical Outcomesa.
| No. (%) of Patients With Aortic Valve Stenosis | Absolute Difference (95% CI), % | Hazard Ratio (95% CI) | Log-Rank P Value | ||
|---|---|---|---|---|---|
| Bicuspid (n = 2691) | Tricuspid (n = 2691) | ||||
| Primary Outcomes | |||||
| At 30 d | |||||
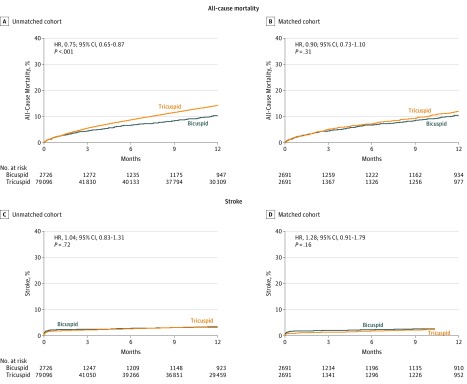
| Mortality | 66 (2.6) | 63 (2.5) | 0.09 (0.08-0.1) | 1.04 (0.74-1.47) | .82 |
| Stroke | 64 (2.5) | 41 (1.6) | 0.89 (0.88-0.90) | 1.57 (1.06-2.33) | .02 |
| At 1 y | |||||
| Mortality | 171 (10.5) | 200 (12.0) | 1.48 (1.45-1.50) | 0.90 (0.73-1.10) | .31 |
| Stroke | 76 (3.4) | 61 (3.1) | 0.34 (0.32-0.35) | 1.28 (0.91-1.79) | .16 |
| Secondary Outcomes | |||||
| At 30 d | |||||
| Mortality or stroke | 115 (4.5) | 98 (3.8) | 0.65 (0.64-0.66) | 1.19 (0.91-1.55) | .21 |
| Aortic valve reintervention | 10 (0.4) | 10 (0.4) | 0 (0.00-0.01) | 1.01 (0.42-2.42) | .99 |
| New pacemaker | 236 (9.1) | 194 (7.5) | 1.65 (1.63-1.66) | 1.23 (1.02-1.49) | .03 |
| Valve-related readmissionsb | 15 (0.6) | 18 (0.7) | 0.12 (0.12-0.13) | 0.84 (0.42-1.67) | .62 |
| At 1 y | |||||
| Mortality or stroke | 228 (12.9) | 246 (14.1) | 1.18 (1.16-1.21) | 0.97 (0.81-1.16) | .74 |
| Aortic valve reintervention | 14 (0.7) | 13 (0.6) | 0.09 (0.09-0.10) | 1.10 (0.52-2.35) | .80 |
| New pacemaker | 247 (10.0) | 209 (8.6) | 1.38 (1.37-1.40) | 1.20 (1.00-1.45) | .05 |
| Valve-related readmissionsb | 28 (1.6) | 37 (2.2) | 0.65 (0.64-0.66) | 0.79 (0.48-1.29) | .34 |
Event rates were calculated by Kaplan-Meier methods.
Readmissions related to aortic valve disease were determined locally at each site.
Cumulative incidences for all-cause mortality and stroke at the 1-year follow-up are depicted in Figure 2 and eFigure 2 in the Supplement. There was no significant difference in 1-year mortality between the propensity-score matched bicuspid and tricuspid groups (10.5% vs 12.0%; absolute RD, 1.48% [95% CI, 1.45%-1.5%]; HR, 0.90 [95% CI, 0.73-1.10]). There was no significant difference in 1-year stroke rate between the 2 groups (3.4% vs 3.1%; absolute RD, 0.34% [95% CI, 0.32%-0.35%]; HR, 1.28 [95% CI, 0.91-1.79]). The results were consistent when analyzed using 784 propensity-matched pairs of patients for whom data from CMS linkage were available, or 469 propensity-matched pairs of patients who had reached the 1-year end-point and had data from CMS linkage available (eFigure 3 in the Supplement). By multivariable analysis, there were no significant differences between the bicuspid and tricuspid groups in 1-year all-cause mortality (HR, 1.01 [95% CI, 0.83-1.23]; P = .93) and stroke (HR, 1.23 [95% CI, 0.94-1.62]; P = .14) (eTables 4 through 8 in the Supplement).
Figure 2. One-Year Cumulative Event Rates of All-Cause Mortality or Stroke Among Patients With Bicuspid and Tricuspid Aortic Stenosis in Unadjusted and Propensity-Matched Cohorts.
The P values were obtained from Cox proportional hazards models. In the unadjusted cohort, the median follow-up for the bicuspid group was 44 days (interquartile range [IQR], 31-365 days) and for the tricuspid group, 55 days (IQR, 32-365 days). In the propensity score–matched cohort, the median follow-up for the bicuspid group was 44 days (IQR, 31-365 days) and for the tricuspid, 53 days (IQR, 32-365 days). This is a continuous registry in which all patients will not have reached the 1-year follow-up at any given time point. At 1 year, there were missing data from 1586 patients in the bicuspid group (660 had not completed their first year of follow-up at the time of the analysis; 926, unknown) and 1514 patients in the tricuspid group (684 had not completed their first year of follow-up at the time of the analysis; 830, unknown), which were further assessed in the Centers for Medicare & Medicaid Services linked sensitivity analyses (eFigure 3 in the Supplement).
Valve Hemodynamics and Functional Status
Valve function significantly improved after TAVR and was maintained at 30 days and 1 year in both groups (eTable 9 in the Supplement). At discharge, there were no significant differences between the bicuspid and tricuspid cohorts in the mean aortic valve area (1.8 cm2 vs 1.8 cm2, respectively; absolute RD, 0 cm2 [95% CI, 0 to 0.1 cm2]), valve gradient (11.6 vs 11.8 mm Hg; absolute RD, 0.2 mm Hg [95% CI, −0.5 to 0.1 mm Hg]), the rate of mean gradient of 20 mm Hg or more (6.9% vs 8.2%, respectively; absolute RD, 1.2% [95% CI, −2.8% to 0.3%]) and prosthesis patient mismatch (moderate, 28.1% vs 27.5%; absolute RD, 0.6% [95% CI, −2.3% to 3.4%]; severe, 13.7% vs 13.6%; absolute RD, 0.1% [95% CI, −2.0% to 2.3%]). Moderate or severe paravalvular leak was more frequent in the bicuspid group than in the tricuspid group at discharge (1.5% vs 0.8%; absolute RD, 0.7% [95% CI, 0% to 1.3%]), whereas there were no significant differences between the 2 groups at 30 days (2.0% vs 2.4%; absolute RD, 0.3% [95% CI, −1.3% to 0.7%]) or 1 year (3.2% vs 2.5%; absolute RD, 0.7% [95% CI, −1.3% to 2.7%]). Increase of mean gradient of 10 mm Hg or more compared with discharge was more frequently observed in the bicuspid group than in the tricuspid group at 30 days (3.8% vs 2.5%; absolute RD, 1.4% [95% CI, 0.1% to 2.6%]) but was not significantly different between the 2 groups at 1 year (5.7% vs 5.2%; absolute RD, 0.4% [95% CI, −2.2% to 3.1%]).
Both bicuspid and tricuspid groups had improvement of functional status without significant difference in NYHA functional class I or II symptom at 30 days (93.2% vs 92.5%, respectively; absolute RD, 0.8% [95% CI, −0.9% to 2.4%]) and 1 year (92.0% vs 93.5%; absolute RD, 1.5% [95% CI, −4.2% to 1.2%]) (eTable 10 in the Supplement). Both bicuspid and tricuspid groups showed improved health status at 30 days (median KCCQ-OS score, 83.3 vs 83.3; difference in improvement, −0.1 [95% CI, −1.8 to 1.6]; P < .89), which persisted up to 1 year without significant difference between the groups (median KCCQ-OS score, 86.5 vs 87.5; difference in improvement, −2.4 [95% CI, −5.1 to 0.3]; P = .08).
Discussion
In this registry-based study of propensity-matched patients who had undergone TAVR for aortic stenosis, patients who had bicuspid aortic stenosis, compared with tricuspid aortic stenosis, had no significant difference in 30-day or 1-year mortality. The stroke rate was higher in patients with bicuspid aortic stenosis at 30 days but did not significantly differ at 1 year between the 2 groups. There were no significant differences in valve hemodynamics (aortic valve gradients and areas) and paravalvular aortic regurgitation between the 2 groups at 30 days and 1 year. Both groups had significant and comparable improvement in functional and health status after TAVR.
Initially, transcatheter aortic valve replacement was performed in patients with tricuspid anatomy. Bicuspid aortic stenosis was an exclusion criterion in the randomized clinical trials of TAVR. TAVR for bicuspid aortic stenosis presents both anatomic and clinical challenges. The concern for suboptimal valve expansion in an orifice with 2 commissures (in lieu of 3 commissures) and the resultant paravalvular regurgitation was the main reason for exclusion of bicuspid aortic stenosis from TAVR trials. In addition, these valves are often heavily calcified, accompanied by raphe (fusion between adjacent cusps) and have concomitant aortopathy (dilatation of the ascending aorta), which may require additional surgical treatment of the aorta. The early experience with the first-generation TAVR devices was limited by higher incidence of paravalvular leak, aortic root injury, and high pacemaker implant rates.23,24,25 The current analysis showed the contemporary outcomes of a third-generation balloon-expandable TAVR in bicuspid aortic stenosis and represents not only the evolution of device technology but also likely operator experience, improved imaging, and procedural advancements.
This study included all consecutive patients with bicuspid anatomy undergoing TAVR with the third-generation balloon-expandable valves in the United States since commercialization and as such, represents a generalized experience. In the present analysis, while only 3% of patients undergoing TAVR had bicuspid anatomy, the prevalence of bicuspid anatomy among all patients with aortic stenosis, especially in younger patients with lower surgical risk, has been reported to be as high as 50%.3 The procedural and clinical outcomes after TAVR for bicuspid aortic stenosis are critical to the expansion of TAVR as an alternative to surgery in younger patients with lower surgical risk.
Mortality in the propensity-matched patients with bicuspid and tricuspid aortic stenosis was not significantly different at discharge, 30 days, and 1 year. The 1-year mortality among patients with tricuspid aortic stenosis was higher in the unadjusted cohort than in the matched cohort, while the 1-year mortality among those with bicuspid aortic stenosis remained unchanged with propensity-matching, which may reflect the lower-risk population of this study. The higher 30-day stroke rate among patients with bicuspid anatomy may be due to multiple factors. The bicuspid valve anatomy is more often accompanied by greater calcium burden and may have required more frequent balloon dilation before and after transcatheter aortic valve replacement. Although these procedural data are unavailable in this study, the complexity of the procedure may be responsible for the increased cerebral embolization of debris during the procedure. Data presented in this analysis largely represent patients who underwent TAVR without the use of cerebral embolic protection. The use of embolic protection devices during TAVR has been shown to reduce the incidence of periprocedural strokes.26 Routine use of these devices during TAVR in bicuspid anatomy may provide an opportunity to reduce procedure-related stroke rates.
The rates of aortic root injury, aortic dissection, and conversion to surgery were higher in the bicuspid cohort than in the tricuspid aortic stenosis cohort. Nevertheless, the combined incidence of these complications was less than 1%, which may be acceptable for patients at elevated risk of surgery. Patients with bicuspid aortic stenosis represent various phenotypes (Sievers type 0, 1, and 2 depending on the number of raphe),27,28 with varying degrees of aortic root and leaflet calcification. The procedural outcomes of TAVR may be affected by the bicuspid phenotype and the extent and distribution of calcification, as well as the valve sizing criteria used for bicuspid anatomy. Because preprocedural computed tomography (CT) is routinely performed and provides good information on the type of bicuspid morphology, further research evaluating the association between CT phenotype and the procedural and clinical outcomes is needed to define the most optimal candidates for TAVR. The results of this study may not be applicable to all patients with bicuspid aortic stenosis, since the operators may have excluded patients with challenging anatomical features that may increase the risk of procedural complications.
Despite theoretical concerns of suboptimal expansion of bioprostheses within bicuspid aortic stenosis, the valve hemodynamics (aortic valve area and mean gradient) were comparable with the tricuspid aortic stenosis cohort and sustained up to 1 year. A greater proportion of patients in the bicuspid cohort experienced a rise in gradients of more than 10 mm Hg at 30 days compared with the tricuspid cohort. Whether this is secondary to valve recoil, valve thrombosis,29,30 valve underexpansion, or a chance finding will need to be determined. The small but significant increase in new permanent pacemaker implants in the bicuspid aortic stenosis cohort was similar to other observed registries.24,31 The mechanism of higher pacemaker rate is unclear and needs to be investigated in future studies.
Surgical aortic valve replacement is associated with low rates of procedural complications and long-term mortality.32 The lack of data regarding use of TAVR in bicuspid anatomy due to exclusion from pivotal studies represents a significant challenge in further expansion of the application of TAVR to younger patients who have bicuspid anatomy. Even the recently completed clinical trials involving young patients excluded patients with bicuspid anatomy, thus limiting the application of results to young patients with bicuspid aortic stenosis.12,13 Until data from randomized clinical trials are available, these registry data may be able to guide clinical practice. Even though not randomized, these represent generalized outcomes not restricted to highest-volume or most experienced TAVR centers.
Limitations
This study has several limitations. First, it had the inherent limitations of an observational study, including lack of center-independent adjudication of adverse events, lack of an independent imaging core laboratory to confirm bicuspid anatomy and potential underreporting of adverse events. Second, bicuspid aortic stenosis represents a heterogeneous anatomic cohort, with varying degrees of calcification. It is possible that the operators selected the most favorable anatomic subsets of bicuspid aortic stenosis for TAVR while patients with highest-risk anatomical features were treated surgically. Propensity-score matching was used to adjust for differences in baseline characteristics; however, it does not address this anatomic selection bias in the study. Third, aortopathy is often seen in patients with bicuspid valves, but due to lack of data, the association between aortopathy and procedural complications such as aortic root rupture and aortic dissection was not assessed. Fourth, this study included only patients treated with the contemporary balloon-expandable valves; thus, the results cannot be generalized to other valve types.
Conclusions
In this preliminary, registry-based study of propensity-matched patients who had undergone transcatheter aortic valve replacement for aortic stenosis, patients with bicuspid aortic stenosis had no significant difference in 30-day or 1-year mortality but had increased 30-day risk of stroke. Because of the potential for selection bias and the absence of a control group treated surgically for bicuspid stenosis, randomized trials are needed to adequately assess the efficacy and safety of transcatheter aortic valve replacement for bicuspid aortic stenosis.
eAppendix. Statistical Analysis
eTable 1. Missing Baseline Characteristic Values
eTable 2. Procedural Characteristics and In-Hospital Outcomes in Unadjusted Cohort
eTable 3. 30-Day and 1-Year Clinical Outcomes in Unadjusted Cohort
eTable 4. Adjusted Hazard Ratios for Adverse Outcomes of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 5. Adjusted Hazard Ratios for 30 Days Mortality of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 6. Adjusted Hazard Ratios for 30 Days Stroke of TAVR in Bicuspid AS Compared With Tricuspid
eTable 7. Adjusted Hazard Ratios for 1 Year Mortality of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 8. Adjusted Hazard Ratios for 1 Year Stroke of TAVR in Bicuspid AS Compared With Tricuspid
eTable 9. Postprocedural Echocardiographic Data in Matched Cohort
eTable 10. Functional and Health Status in Matched Cohort
eFigure 1. Numbers of 1-Year Follow-up Status
eFigure 2. Cumulative Event Rates of All-Cause Mortality or Stroke After Transcatheter Aortic Valve Replacement in Patients with Bicuspid and Tricuspid Aortic Stenosis
eFigure 3. Cumulative Event Rates of All-Cause Mortality or Stroke Among Patients with Bicuspid and Tricuspid Aortic Stenosis in Propensity-Matched Cohort with CMS Linkage
References
- 1.Nistri S, Basso C, Marzari C, Mormino P, Thiene G. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am J Cardiol. 2005;96(5):718-721. doi: 10.1016/j.amjcard.2005.04.051 [DOI] [PubMed] [Google Scholar]
- 2.Basso C, Boschello M, Perrone C, et al. . An echocardiographic survey of primary school children for bicuspid aortic valve. Am J Cardiol. 2004;93(5):661-663. doi: 10.1016/j.amjcard.2003.11.031 [DOI] [PubMed] [Google Scholar]
- 3.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111(7):920-925. doi: 10.1161/01.CIR.0000155623.48408.C5 [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack MJ, et al. ; PARTNER 2 Investigators . Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609-1620. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 5.Leon MB, Smith CR, Mack M, et al. ; PARTNER Trial Investigators . Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607. doi: 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 6.Smith CR, Leon MB, Mack MJ, et al. ; PARTNER Trial Investigators . Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198. doi: 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 7.Adams DH, Popma JJ, Reardon MJ, et al. ; US CoreValve Clinical Investigators . Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790-1798. doi: 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 8.Reardon MJ, Van Mieghem NM, Popma JJ, et al. ; SURTAVI Investigators . Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321-1331. doi: 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 9.Holmes DR Jr, Brennan JM, Rumsfeld JS, et al. ; STS/ACC TVT Registry . Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313(10):1019-1028. doi: 10.1001/jama.2015.1474 [DOI] [PubMed] [Google Scholar]
- 10.Mack MJ, Brennan JM, Brindis R, et al. ; STS/ACC TVT Registry . Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310(19):2069-2077. doi: 10.1001/jama.2013.282043 [DOI] [PubMed] [Google Scholar]
- 11.Durko AP, Osnabrugge RL, Van Mieghem NM, et al. . Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. 2018;39(28):2635-2642. doi: 10.1093/eurheartj/ehy107 [DOI] [PubMed] [Google Scholar]
- 12.Mack MJ, Leon MB, Thourani VH, et al. ; PARTNER 3 Investigators; the PARTNER 3 Investigators . Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695-1705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 13.Popma JJ, Deeb GM, Yakubov SJ, et al. ; Evolut Low Risk Trial Investigators . Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706-1715. doi: 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 14.Hira RS, Vemulapalli S, Li Z, et al. . Trends and outcomes of off-label use of transcatheter aortic valve replacement: insights from the NCDR STS/ACC TVT Registry. JAMA Cardiol. 2017;2(8):846-854. doi: 10.1001/jamacardio.2017.1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon SH, Bleiziffer S, De Backer O, et al. . Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol. 2017;69(21):2579-2589. doi: 10.1016/j.jacc.2017.03.017 [DOI] [PubMed] [Google Scholar]
- 16.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. doi: 10.1016/S0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 17.Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City Cardiomyopathy Questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7(2):235-242. doi: 10.1016/j.ejheart.2004.05.012 [DOI] [PubMed] [Google Scholar]
- 18.Arnold SV, Spertus JA, Lei Y, et al. . Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6(1):61-67. doi: 10.1161/CIRCHEARTFAILURE.112.970053 [DOI] [PubMed] [Google Scholar]
- 19.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110(5):546-551. doi: 10.1161/01.CIR.0000136991.85540.A9 [DOI] [PubMed] [Google Scholar]
- 20.Spertus J, Peterson E, Conard MW, et al. ; Cardiovascular Outcomes Research Consortium . Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707-715. doi: 10.1016/j.ahj.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 21.Kappetein AP, Head SJ, Généreux P, et al. . Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33(19):2403-2418. doi: 10.1093/eurheartj/ehs255 [DOI] [PubMed] [Google Scholar]
- 22.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281. doi: [DOI] [PubMed] [Google Scholar]
- 23.Yoon SH, Lefèvre T, Ahn JM, et al. . Transcatheter aortic valve replacement with early- and new-generation devices in bicuspid aortic valve stenosis. J Am Coll Cardiol. 2016;68(11):1195-1205. doi: 10.1016/j.jacc.2016.06.041 [DOI] [PubMed] [Google Scholar]
- 24.Yoon SH, Whisenant BK, Bleiziffer S, et al. . Transcatheter mitral valve replacement for degenerated bioprosthetic valves and failed annuloplasty rings. J Am Coll Cardiol. 2017;70(9):1121-1131. doi: 10.1016/j.jacc.2017.07.714 [DOI] [PubMed] [Google Scholar]
- 25.Mylotte D, Lefevre T, Søndergaard L, et al. . Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol. 2014;64(22):2330-2339. doi: 10.1016/j.jacc.2014.09.039 [DOI] [PubMed] [Google Scholar]
- 26.Seeger J, Kapadia SR, Kodali S, et al. . Rate of peri-procedural stroke observed with cerebral embolic protection during transcatheter aortic valve replacement: a patient-level propensity-matched analysis. Eur Heart J. 2019;40(17):1334-1340. doi: 10.1093/eurheartj/ehy847 [DOI] [PubMed] [Google Scholar]
- 27.Michelena HI, Prakash SK, Della Corte A, et al. ; BAVCon Investigators . Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation. 2014;129(25):2691-2704. doi: 10.1161/CIRCULATIONAHA.113.007851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133(5):1226-1233. doi: 10.1016/j.jtcvs.2007.01.039 [DOI] [PubMed] [Google Scholar]
- 29.Makkar RR, Fontana G, Jilaihawi H, et al. . Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. 2015;373(21):2015-2024. doi: 10.1056/NEJMoa1509233 [DOI] [PubMed] [Google Scholar]
- 30.Chakravarty T, Søndergaard L, Friedman J, et al. ; RESOLVE; SAVORY Investigators . Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389(10087):2383-2392. doi: 10.1016/S0140-6736(17)30757-2 [DOI] [PubMed] [Google Scholar]
- 31.Perlman GY, Blanke P, Dvir D, et al. . Bicuspid aortic valve stenosis: favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv. 2016;9(8):817-824. doi: 10.1016/j.jcin.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 32.Goland S, Czer LS, De Robertis MA, et al. . Risk factors associated with reoperation and mortality in 252 patients after aortic valve replacement for congenitally bicuspid aortic valve disease. Ann Thorac Surg. 2007;83(3):931-937. doi: 10.1016/j.athoracsur.2006.10.047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Statistical Analysis
eTable 1. Missing Baseline Characteristic Values
eTable 2. Procedural Characteristics and In-Hospital Outcomes in Unadjusted Cohort
eTable 3. 30-Day and 1-Year Clinical Outcomes in Unadjusted Cohort
eTable 4. Adjusted Hazard Ratios for Adverse Outcomes of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 5. Adjusted Hazard Ratios for 30 Days Mortality of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 6. Adjusted Hazard Ratios for 30 Days Stroke of TAVR in Bicuspid AS Compared With Tricuspid
eTable 7. Adjusted Hazard Ratios for 1 Year Mortality of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 8. Adjusted Hazard Ratios for 1 Year Stroke of TAVR in Bicuspid AS Compared With Tricuspid
eTable 9. Postprocedural Echocardiographic Data in Matched Cohort
eTable 10. Functional and Health Status in Matched Cohort
eFigure 1. Numbers of 1-Year Follow-up Status
eFigure 2. Cumulative Event Rates of All-Cause Mortality or Stroke After Transcatheter Aortic Valve Replacement in Patients with Bicuspid and Tricuspid Aortic Stenosis
eFigure 3. Cumulative Event Rates of All-Cause Mortality or Stroke Among Patients with Bicuspid and Tricuspid Aortic Stenosis in Propensity-Matched Cohort with CMS Linkage