Abstract
With the advent of antibiotics, bacterial infections were supposed to be a thing of past. However, this instead led to the selection and evolution of bacteria with mechanisms to counter the action of antibiotics. Antibiotic efflux is one of the major mechanisms, whereby bacteria pump out the antibiotics from their cellular interior to the external environment using special transporter proteins called efflux pumps. Inhibiting these pumps seems to be an attractive strategy at a time when novel antibiotic supplies are dwindling. Molecules capable of inhibiting these pumps, known as efflux pump inhibitors (EPIs), have been viewed as potential therapeutic agents that can rejuvenate the activity of antibiotics that are no longer effective against bacterial pathogens. EPIs follow some general mechanisms of efflux inhibition and are derived from various natural as well as synthetic sources. This review focuses on EPIs and identifies the challenges that have kept these futuristic therapeutics away from the commercial realm so far.
Keywords: Antibiotics, efflux pumps, multiple drug resistance, pathogens, therapeutics
Introduction
With the discovery of penicillin and streptomycin in the early 20th century, we entered the antibiotic era where previously considered deadly bacterial infections could be easily treated. The mid-decades of the 20th century witnessed the ‘golden age’ of antibiotic discovery as about half of the antibiotics in use today were discovered during that period1. However, the rampant use, misuse and abuse of antibiotics accelerated the evolution of bacteria, resulting in selection of antibiotic-resistant bacteria2. The Centers for Disease Control and Prevention (CDC), USA, estimate states that about 30 per cent of the antibiotics prescribed to the outpatients are unnecessary3. The reckless use of broad-spectrum antibiotics as growth promoters in animal farming has also aggravated the problem. The gravity of the situation can be understood by the fact that in a developed nation like the USA, nearly two million people develop hospital-acquired infections from drug-resistant bacteria that leave about a hundred thousand dead4. Estimates on medical expense per patient with antibiotic-resistant infections vary from $18,588 to $29,069 which ultimately amounts to a healthcare loss as high as $20 billion and a productivity loss of $35 billion every year5. The situation is much worse in economically backward countries that are generally plagued by poor sanitary, health and medical conditions. The increasing incidence of multidrug-resistant (MDR), extensively drug-resistant (XDR, resistant to all but one or two classes of antibiotics) and pan-drug-resistant (PDR, resistant to all classes of antibiotics) microbes has brought us to the brink of the ‘post-antibiotic era’ where no antibiotics will be effective any longer and even slightest of infections would prove deadly6. The serious threat this situation poses was recognized by the UN General Assembly that drew a framework for all the nations to co-operate and work in the direction of combating antimicrobial resistance7.
Bacteria develop resistance to antibiotics through four major mechanisms (Fig. 1): (i) altering the cellular permeability to avoid the entry of antibiotics into the cells, (ii) modifying the molecular targets of the antibiotics so that they can no longer act on them, (iii) enzymatic modification of antibiotics to render them inactive, and (iv) expression of efflux pumps to pump out antibiotics from the cellular milieu8. These factors responsible for resistance could be intrinsic or acquired through various mechanisms. The presence of determinants of resistance on mobile genetic elements such as plasmids and transposons combined with the free mobility of human carriers has resulted in dissemination of drug resistance to a wide variety of bacterial genera and geographical locations. The research endeavour that previously had the sole aim of discovery of novel antibiotics now has added burden of understanding the development of resistance and strategies to reverse them. This review focuses on one of the causes of antibiotic resistance, the efflux pumps, and deals with the current progress in inhibiting these determinants of resistance.
Fig. 1.
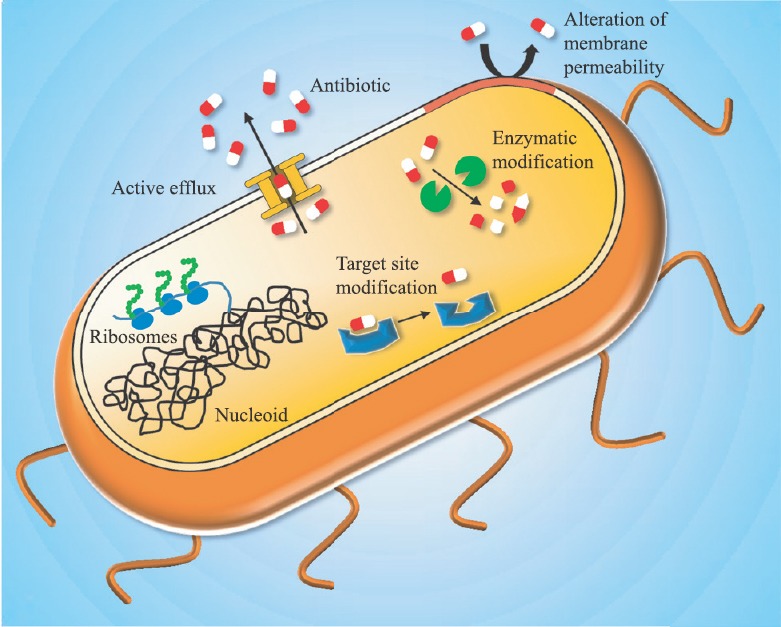
The four major mechanisms by which the bacterial cells develop multiple drug resistance. (i) Altering the cellular permeability to avoid the entry of antibiotics into the cells, (ii) modifying the targets of the antibiotics so that they can no longer act on them, (iii) enzymatic modification of antibiotics to render them inactive, and (iv) expression of efflux pumps to pump out antibiotics from cell interior.
Bacterial efflux systems as determinants of multidrug resistance
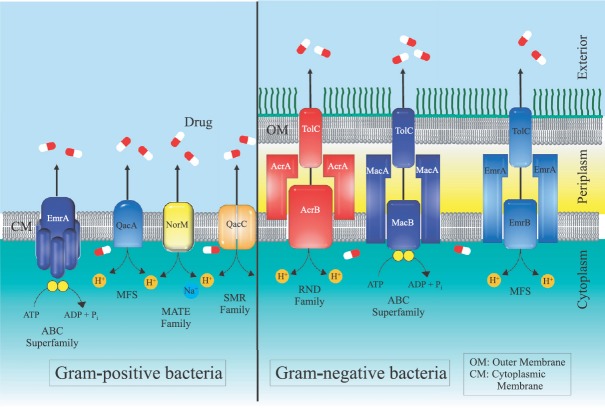
Efflux pumps are bacterial transport proteins which are involved in extrusion of substrates from the cellular interior to the external environment. These substrates are often antibiotics, imparting the efflux pump expressing bacteria antibiotic resistant phenotype9. From the first drug-resistant efflux pump discovered in the 1990s, the development in molecular microbiology has led to the characterization of many efflux pumps in Gram-positive bacteria (GPB) including methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pneumoniae, Clostridium difficile, Enterococcus spp. and Listeria monocytogenes and Gram-negative bacteria (GNB) such as Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, Stenotrophomonas maltophilia, Campylobacter jejuni, Pseudomonas aeruginosa, Neisseria gonorrhoeae, Vibrio cholerae and Salmonella spp.10,11. Since these transport substrates against a concentration gradient, these efflux pumps are energy dependent. Based on the mechanism by which these derive this energy, the efflux pumps are broadly classified into two categories. The primary efflux pumps draw energy from active hydrolysis of ATP, whereas the secondary efflux pumps draw energy from chemical gradients formed by either protons or ions such as sodium. Five major families of efflux pumps have been described in the prokaryotes (Fig. 2), namely (i) ATP binding cassette (ABC), which are primary active transporters, (ii) small multidrug resistance family, (iii) multidrug and toxin extrusion (MATE) family, (iv) major facilitator superfamily (MFS) and (v) resistance nodulation cell division (RND) family, which are all secondary active transporters12. The complexity with which these pump proteins are organized has also provided insight into their structure and molecular mechanism of substrate transport. The drug resistance in GPB is mainly mediated by cytoplasmic membrane located efflux transporters, while the efflux pumps in GNB are more complex due to their multi-layered cell envelop: the inner or cytoplasmic membrane and the outer membrane, which are separated by the periplasmic space that combines to form a tripartite protein channel through which the drug is effluxed. RND family efflux pumps have tripartite organization and are the major contributors to intrinsic antibiotic resistance in GNB, which expel a broad spectrum of antibiotics and biocides, including fluoroquinolones, β-lactams, tetracycline and linezolid. However, in GPB, MFS transporters are predominant including NorA of S. aureus, PmrA of S. pneumoniae and EmeA of E. faecalis that extrude a large number of antibiotics belonging to different classes10,11.
Fig. 2.
The five classes of efflux pumps in bacteria, (i) ATP-binding cassette superfamily, (ii) major facilitator superfamily, (iii) multidrug and toxic compound extrusion family, (iv) small multidrug resistance family, and (v) resistance nodulation division family. The organization of these efflux pumps is different in Gram-positive and Gram-negative bacteria.
Efflux pumps, unlike most other determinants of resistance, are more often intrinsic. The genes coding for these transporters are found in susceptible as well as resistant bacteria13 and are often parts of an operon whose expression is regulated at the transcriptional level. The mutations in the regulatory proteins or the mutations at the promoters result in overexpression of these efflux pumps, resulting in drug resistance13. Bacterial efflux system can be either specific, extruding only one or a single class of antibiotics (such as TetA and AbaF that selectively exclude specific antibiotics such as tetracycline and fosfomycin, respectively)14 or capable of pumping out several classes of antibiotics (such as MexAB-OprM, NorA and BmrA that extrude distinct class of antibiotics, disinfectant, dyes and detergents) being designated as MDR efflux pumps. Most of the MDR efflux pumps are chromosomally encoded including NorA, NorB, MepA and MdeA of S. aureus that are responsible for intrinsic resistance in bacteria to several antibiotics, while some of the pumps are encoded on plasmids (QacA/B of S. aureus) or transposons (MefA and MefB of Streptococcus spp.) that provide the transferable mode of resistance15,16.
Apart from drug resistance, the physiological role of efflux pumps in bacteria extends to bile tolerance in enteric bacteria, leading to colonization, increase in virulence, biofilm secretion and bacterial survival in the host17.
Efflux pump inhibitors as new therapeutic agents
Considering the importance of efflux in mediating antibiotic resistance, it is worthwhile to expect that circumventing these determinants of resistance could potentiate the activity of substrate antibiotics. Abolishment the efflux could be achieved by different ways namely, (i) downregulating the expression of efflux pump genes by interfering in genetic regulation, (ii) redesigning antibiotics that are no longer recognized as substrates, (iii) inhibiting the assembly of functional efflux pumps, (iv) blocking the pump to avoid substrate binding to the active site, and (v) collapsing the energy mechanism responsible for energizing these pumps18. This review mainly focuses on the last two categories that attempt to inhibit the efflux pumps using chemical entities called efflux pump inhibitors (EPIs). EPIs are the molecules that inhibit efflux pumps by one or more mechanisms, leading to inactive drug transport. Since this could eventually lead to successful build-up of an antibiotic inside the cell, these EPIs can be used as adjuncts in combination with antibiotics to enhance their activity against bacteria expressing efflux pumps. The possibility of using EPIs to rejuvenate the activity of antibiotics has been at an experimental stage since the beginning of this century. MC-207,110 [phenylalanyl arginyl β-naphthylamide (PAβN)], a peptidomimetic EPI, was the first to be discovered in 2001. It potentiates the antibacterial activity of levofloxacin and erythromycin against MexAB-OprM-overexpressing clinical isolates of P. aeruginosa19. However, the success has been limited, and no EPI has made it to the commercial realm so far.
A chemical entity would have to go through a stringent checklist to make it as a successful EPI. First, the molecule must not be antibacterial per se. An antibacterial molecule would ultimately lead to selection of mutants resistant to its action that will severely impact its utility as an EPI. Second, the molecule should be selective and not target any eukaryotic efflux pumps. Since efflux pumps are ubiquitous and their basic functional aspects tend to be similar across the life forms, selective inhibition of bacterial efflux pumps becomes a difficult task. Third, it should possess ideal pharmacological features such as non-toxicity, high therapeutic and safety indices, good ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) profile and serum stability. Finally, to be successful at a commercial level, the production of the EPI must be economically feasible18.
Types of EPIs based on their mechanism of action
The EPIs in laboratories have shown good promise as therapeutic adjuvants. Although a multitude of EPIs have been reported with different modes of action, these can be broadly characterized into two categories:
Energy dissipation
Since efflux pumps are dependent on cellular energy, the decoupling of the energy and efflux activity presents an interesting approach to efflux inhibition. The proton gradient or the ATPase that supplies energy to these pumps has been tried as targets of various EPIs. Such an inhibition scheme does not require any direct interaction of the inhibitor with the efflux pump itself. This approach appears to be advantageous as many efflux pumps are dependent on the proton gradient, making this a universal scheme for inhibiting them.
Carbonyl cyanide-m-chlorophenylhydrazone (CCCP) is perhaps the most well-known laboratory EPI. It is an ionophore that disrupts the proton motive force (PMF) by affecting both its components, Δψ and ΔpH20. This also makes the bacterial cells metabolically inactive giving rise to the debate whether the synergistic effect that CCCP shows with a range of antibiotics is actually a consequence of efflux pump inactivity or metabolic inactivity of the cells. The CCCP has been reported to revive the activity of tetracycline in Helicobacter pylori and Klebsiella spp.21,22. Synergy between carbapenems and CCCP was also reported, which was independent of the efflux inhibition activity of CCCP, supporting the previous hypothesis that CCCP leads to metabolically inactive cells giving rise to synergistic effect with antibiotics23. This combined with the cellular toxicity towards mammalian cells has kept CCCP limited to laboratory use only.
Our group has also reported a synthetic EPI, IITR08027, from a library of 8000 synthetic molecules that was screened for potentiators of ciprofloxacin20. The molecule was found to be very effective at reversing the resistance against fluoroquinolones in both recombinant E. coli and clinical strains of A. baumannii overexpressing the MATE efflux pump AbeM. IITR08027 disturbs the proton gradient that is necessary for energizing the pump. Since it had a little impact on the Δψ component of PMF, it did not have any antibacterial effect of its own and displayed low toxicity towards animal cells. These qualities of IITR08027 make it very close to an ideal EPI and it is being assessed for its preclinical potential.
Inhibition by direct binding
Another mechanism of efflux pump inhibition is the binding of the EPIs to functional efflux pumps, resulting in reduced ability of the pumps to interact with their substrates. This binding could be competitive, where the EPI competes with the substrates for the same binding site; or non-competitive, where the binding of EPI to the pump causes decrease in the affinity of pump towards its substrates. However, bacteria can always mutate their efflux pumps to modify the target sites of these inhibitors, rendering them useless.
PAβN (or MC-207,110) is a paradigm in synthetic EPIs as it was the first inhibitor of the RND family pumps. Screened from a synthetic library as a potentiator of levofloxacin against P. aeruginosa cells expressing MexAB, MexCD and MexEF pumps, this molecule also potentiates erythromycin and chloramphenicol19. Since it is a substrate for the RND pumps as well, it acts as a competitive inhibitor of substrate binding and efflux. PAβN is not as effective when combined with tetracycline and carbenicillin, suggesting that these antibiotics have a binding site different from that of PAβN. There is not much scientific evidence about the mechanism of action of PAβN, but computational simulations with AcrB have predicted that it interacts with F135, F178, F615, F628, Q176 and E673 residues24. Although there is some degree of evidence that it additionally affects the outer membrane permeability, there is a requirement of more investigations to lay a strong claim19.
Verapamil is a small molecule that acts as an ion channel blocker and is used in the treatment of hypertension. Studies in Mycobacterium tuberculosis have shown that verapamil potentiates the activity of bedaquiline and ofloxacin25,26. Further studies have identified that verapamil inhibits the activity of MATE pumps. It has a low amount of toxicity towards bacterial cells not expressing MATE efflux pumps, suggesting specificity towards bacteria expressing these pumps and a competitive mode of inhibition. Crystallization studies confirmed that verapamil binds to the active site of the MATE efflux pumps in a manner similar to the substrates of the pump. Although verapamil interacted with two prototype MATE pumps, DinF and NorM, in a separate manner, the overall effect of inhibition of pump activity was the same27.
Another molecule conforming to this category, 1-(1-napthylmethyl)-piperazine (NMP), was derived from a parent molecule that was screened out of a synthetic library of compounds28. The library was assessed for potentiators of levofloxacin in E. coli cells overexpressing the efflux pumps AcrAB and AcrEF. NMP resulted in increased accumulation of levofloxacin in the cells, which enhanced its activity. NMP was also found to potentiate oxacillin, rifampin, chloramphenicol and clarithromycin and to lower extent fluoroquinolones, azithromycin, clindamycin, nitrofurantoin and doxycycline28. Mutagenesis using error-prone PCR resulted in AcrB mutants resistant to the potentiating activity of NMP. This resulted in the identification of core residues, G141, N282 and F610, which are crucial for NMP binding. NMP interacts with the F610, residue and causes conformational change in AcrB, resulting in inhibition in a non-competitive manner29. However, the molecule also has an antibacterial property at a concentration four-fold higher than what is used as an EPI, suggesting a secondary target for the molecule as well.
Types of EPIs based on their origin
Although many molecules have shown potential as EPIs, the mechanism of action is not known for a majority of them. Therefore, it becomes difficult to fit such molecules in a classification scheme based on their mode of action. To accommodate EPIs with no definite mode of action, the EPIs can be categorized based on their source. This leads to three broad categories that include EPIs derived from plant products, synthetic chemistry and microorganisms.
Plant-derived EPIs
Plant-derived phytochemicals include a wide variety of chemical adjuvants that synergistically enhance the efficacy of antibiotics up to several folds30. Major subclasses of plant-derived EPIs are enumerated as follows:
Plant alkaloids: Reserpine, an antipsychotic drug extracted from the roots of Rauwolfia serpentina, is a promising EPI that targets efflux pumps of the MFS and RND superfamily30. Reserpine is reported to potentiate antimicrobial activity of antibiotics by interacting directly with amino acid residues in the efflux transporter protein Bmr, which mediates tetracycline efflux in B. subtilis. In addition, reserpine has also been shown to reverse NorA-mediated resistance in S. aureus by enhancing the activity of norfloxacin up to four-fold31. The clinical application of reserpine with clinically used antibiotics, however, has not yet been achieved due to its nephrotoxic nature32.
Piperine (isolated from Piper nigram) is another alkaloid known to inhibit the human P-glycoprotein of ABC transporters via cytochrome P450-mediated pathways. The efflux pump inhibitory activity of both piperine and its derivative, piperidine, has also been reported against pathogenic bacteria including S. aureus and Mycobacteria spp.33. A study conducted in S. aureus showed that piperine enhances the accumulation of ciprofloxacin by inhibiting NorA efflux pump. In M. tuberculosis H37Rv and several clinical isolates, piperine has been reported to potentiate the activity of rifampicin by inhibiting an uncharacterized efflux pump – Rv1258c. In Mycobacterium smegmatis, piperine has been shown to decrease the MIC of ethidium bromide indicating its application as an EPI across bacterial genera34.
Flavonoids: Baicalein, a 5,6,7-trihydroflavone, is a weak antimicrobial flavone isolated from thyme leaves (Thymus vulgaris). It improves the susceptibility of clinical MRSA strain towards ciprofloxacin and β-lactam antibiotics including oxacillin, cefmetazole and ampicillin35,36. Baicalein is also reported to increase the potency of tetracycline in TetK-overexpressing Staphylococci by inhibiting the uptake of [3H] tetracycline36.
5’-methoxy-hydnocarpin, a flavolignan isolated from Berberis fremontii, has been reported to enhance the efficacy of several NorA substrates, including norfloxacin and berberine by inhibiting this proton pump. However, due to its toxic nature, its clinical success is doubtful37. Some of the other plant derived isoflavones (isolated from Lupinus argenteus) including genistein, orobol and biochanin A, have been reported to reduce the MIC of berberine and norfloxacin in clinical S. aureus and M. smegmatis by blocking the MDR efflux pumps38.
Polyphenols: Catechin gallates, a group of phenolic metabolites, have been reported to reverse the MRSA resistance. Catechin gallates such as epicatechin gallate and epigallocatechin gallate are weak inhibitors of NorA efflux pump, with epicatechin gallate being slightly more potent. Interestingly, both compounds have been reported to enhance the efflux at low concentrations39. It has been proposed that these molecules have two different binding sites on the NorA efflux transporter with different affinities. At low concentrations, catechins occupy high-affinity binding sites leading to increased efflux of NorA substrate. Their effect as EPI is observed only at a higher concentration.
Epigallocatechin gallate has also been reported to enhance the potency of tetracycline, erythromycin and ciprofloxacin in TetK-overexpressing Gram-positive Staphylococci and in Gram-negative Campylobacter spp. However, due to toxicity concerns associated with it, further in vivo and pre-clinical studies were not undertaken40.
Phenolic diterpenes: Phenolic diterpenes, such as carnosic acid and carnosol, isolated from herb Rosemary (Rosmarinus officinalis), have been reported as EPIs. These enhance the potency of antibiotics such as tetracycline and erythromycin against macrolide-resistant strain of S. aureus expressing the ABC transporter MsrA and TetK efflux pumps41.
Geraniol (monoterpenoid alcohol), isolated from Helichrysum italicum, has also been reported tomodulate drug resistance in several GNB species by targeting MDR efflux mechanisms. It decreases the MIC of chloramphenicol in Enterobacter aerogenes CM-64 strain that overexpresses the tripartite efflux pump, AcrAB-TolC42.
EPIs of synthetic origin
Apart from natural plant-derived products, screening of novel semi-synthetic or synthetic diversified chemical libraries is a useful way to identify potential EPIs. Many screening efforts have yielded results with varying amount of success. Such synthetic small molecule EPIs can be further classified as follows:
Peptidomimetic compounds: The dipeptide amide compound PAβN was one of the first EPIs discovered through chemical genetics approach. It has been reported to potentiate the activity of many antibiotics including fluoroquinolones, macrolides and chloramphenicol in GNB by inhibiting RND efflux pumps19,24. However, it had limited clinical potential due to toxicity towards mammalian cells. Although some synthetic derivatives with different basic properties such as reduced toxicity, enhanced stability, and better solubility were evaluated, none of the active analogues could significantly reduce the drawback of the parent molecule. Thus, PAβN and its novel derivatives are limited to use in laboratory as standards to determine the level of inhibitor-sensitive efflux for specific antibiotics in various bacterial pathogens43.
Quinoline derivatives: This novel class of compounds was discovered by using several screening approaches against clinical MDR bacterial strains. Quinoline derivatives such as pyridoquinolones can restore the activity of norfloxacin in E. aerogenes overexpressing the AcrAB-TolC efflux pump, by acting as competitive inhibitor of this RND pump44. Some other synthetic analogues such as 4-substituted thioalkyl, alkylamino and alkoxy quinolone have also been reported to enhance the activity of tetracyclines, norfloxacin and chloramphenicol in clinical isolates of K. pneumoniae and E. aerogenes45. A series of 2-phenyl-4(1H)-quinolone and 2-phenyl-4-hydroxyquinoline derivatives have been synthesized by modifying the flavone scaffold and these have been reported as potent inhibitors of NorA efflux pump in S. aureus46.
Arylpiperidines and aryl piperazine derivatives: Arylpiperidine and its derivatives such as 3-arylpiperidine have been reported to restore susceptibility to linezolid and enhance its accumulation in E. coli47. Another series of analogues, phenylpiperidines, which are selective serotonin re-uptake inhibitors, are known to inhibit the function of S. aureus MDR efflux pumps. These compounds also affect the activity of the AcrAB-TolC pump in E. coli partially but have no effect on the efflux activity of the P. aeruginosa RND efflux pumps such as MexAB-OprM or MexCD-OprJ48.
One of the leading arylpiperazine compounds, NMP, has been shown to restore the activity of RND pump substrates including levofloxacin and EtBr in E. coli-overexpressing AcrAB and AcrEF. However, due to serotonin re-uptake inhibitor property of arylpiperazines, these compounds are likely to be toxic to mammalian cells28.
Pyridopyrimidine and pyranopyridine derivatives: Pyridopyrimidine analogues D2 and D13-9001 have been reported as MexAB-OprM-specific pump inhibitor in MexABoverexpressing P. aeruginosa under both in vitro and in vivo conditions49. It has been proposed that D13-9001 is able to inhibit the efflux of antibiotics by binding to specific site in efflux pumps (AcrB in E. coli and MexB in P. aeruginosa). Further, the crystallographic data suggested that the hydrophobic tert-butyl thiazolyl aminocarboxyl moiety of D13-9001 binds tightly to the hydrophobic trap in deep substrate binding pocket of the pump and prevents the conformational changes that are needed for the proper activity of the pump. In addition, the hydrophilic component of D13-9001 is also reported to interact with the substrate binding channel of pump, thereby preventing the substrate binding to the pumps43.
MBX2319, a synthetic pyrazolopyridine, was screened as a potentiator of fluoroquinolones antibiotics from a library of small molecules. It enhances the efficacy of ciprofloxacin, levofloxacin and piperacillin up to eight-fold against E. coli AB115729. Further, MBX2319 also led to increased intracellular accumulation of Hoechst dye in wild type and AcrAB-TolC-overexpressing E. coli29. A detailed X-ray crystallographic study suggested that MBX2319 interacts with the hydrophobic trap of the AcrB pump with its pyridine ring predicted to form a ring stacking interaction with the amino acid residues43.
In addition, many synthetic/semisynthetic derivatives have been synthesized artificially that mainly target MDR efflux pump of both GPB and GNB (Table).
Table.
List of efflux pump inhibitors (EPIs) from various sources
| EPIs | Target efflux pump(s) | Bacterial strain(s) | Substrate(s) | References |
|---|---|---|---|---|
| Natural EPIs from plant sources | ||||
| Pheophorbide A | NorA, MexAB-OprM | Streptococcus aureus, Pseudomonas aeruginosa | Berberine, ciprofloxacin | 50 |
| 5′-MHC | NorA | S. aureus | Berberine | 37, 38 |
| Carnosic acid | MsrA | S. aureus | Erythromycin | 41 |
| Carnosol | MsrA, TetK | S. aureus | Tetracycline | 41 |
| Cathinone | acrAB-TolC |
Salmonella Typhimurium |
Ciprofloxacin | 51 |
| Theobromine | acrAB-TolC |
S. Typhimurium, Klebsiella pneumoniae |
Ciprofloxacin, tetracycline | 51 |
| Reserpine | NorA, TetK, MepA, Bmr |
S. aureus, Bacillus subtilis, Streptococcus pneumoniae |
Norfloxacin, ciprofloxacin, tetracycline | 30, 52 |
| ABC: Rv2936-Rv2937- Rv2938 (DrrABC) Rv0933 (PstB) Rv2686c-Rv2687c-Rv2688c RND: Rv0678, Rv1145, Rv1146, Rv2942 (mmpL7) MFS: Rv1410c (P55), Rv1877 Rv2846c SMR: Rv3065 (mmr) | Mycobacterium spp. | Ciprofloxacin, ofloxacin | 53 | |
| 4’,5’- O-dicaffeoylquinic acid | NorA | S. aureus | Berberine, norfloxacin | 54 |
| Curcumin | NorA | S. aureus | Norfloxacin, ciprofloxacin | 55 |
| Kaempferol | NorA | S. aureus | Norfloxacin, ciprofloxacin | 56 |
| N-trans-feruloyl 4’- O-methyldopamine | NorA | S. aureus | Norfloxacin, ciprofloxacin | 57 |
| Silibinin | NorA | S. aureus | Norfloxacin | 49 |
| Genistein, Isoflavone | NorA | S. aureus | Berberine | 38 |
| Artesunate | AcrAB-TolC | Escherichia coli | Penicillin G; ampicillin, cefazolin, cefuroxime, cefoperazone | 58 |
| Orizabins | NorA | S. aureus | Norfloxacin, berberine | 49 |
| Resin glycosides (Orizabins IX, Murucoidins, Stoloniferin) | NorA | S. aureus | Norfloxacin, ciprofloxacin | 59 |
| Citropten and furocoumarins | NorA, ErmA, ErmB | S. aureus | Norfloxacin, ciprofloxacin | 60 |
| Natural EPIs from plant sources | ||||
| Coumarins | NorA | S. aureus | Norfloxacin, ciprofloxacin | 61 |
| Crysoplenol and Crysoplenetin | NorA | S. aureus | Berberine, norfloxacin | 62 |
| Diosmetin | MsrA, NorA | S. aureus | Erythromycin, norfloxacin | 63 |
| Murucoidins | NorA | S. aureus | Norfloxacin | 59 |
| Chrysosplenol-D | NorA | S. aureus | Berberine | 62 |
| Phenylpropanoid | Rv1145, Rv1146 Rv1877, Rv2846c Rv3065(mmr) | Mycobacterium spp. | Et-Br | 64 |
| Compound 1 | NorA | S. aureus | Norfloxacin | 65 |
| Essential oils (Salvia species) | Tet (K) | Staphylococcus epidermidis | Tetracycline | 66 |
| Spectinamides | Rv1258c | Mycobacterium spp. | Clarithromycin, Doxycycline and Clindamycin | 67 |
| Diterpenes (ferruginol) | MsrA, TetK, NorA |
S. aureus, Mycobacterium spp. |
Tetracycline, erythromycin, norfloxacin isoniazid | 68 |
| Totarol | MsrA, TetK | S. aureus, Mycobacterium spp. | Erythromycin, isoniazid | 69 |
| Boeravinone B | NorA | S. aureus | Norfloxacin, ciprofloxacin | 70 |
| α-Terpinene | TetK | S. aureus | Tetracycline | 71 |
| Biochanin A | NorA | S. aureus | Berberine, norfloxacin | 38 |
| Cumin seed oil, cuminaldehyde | LmrS | S. aureus | Et-Br | 72 |
| Epigallocatechin gallate, Epicatechin gallate | TetK | S. aureus | Tetracycline | 39,40 |
| Galbanic acid | NorA | S. aureus | Norfloxacin, ciprofloxacin | 73 |
| Orobol | NorA | S. aureus | Berberine | 38 |
| Baicalein | NorA, TetK | S. aureus, E. coli | Ciprofloxacin, tetracycline | 35,36 |
| Tannic acid | TetK, NorA | S. aureus | Tetracycline, norfloxacin | 74 |
| Conessine | MexAB-OprM, AdeIJK | Pseudomonas aeruginosa, Acinetobacter baumannii | Cefotaxime, levofloxacin, tetracycline, novobiocin and rifampicin | 75,76 |
| Linoleic and oleic acids | MsrA | S. aureus | Erythromycin | 77 |
| Tiliroside, kaempferol-3-O-b-d- (6-E-p-coumaroyl)Glucopyranoside | NorA | S. aureus | Norfloxacin, ciprofloxacin | 78 |
| Natural EPIs from plant sources | ||||
| Capsaicin (8-methyl-N-vanillyl-6 nonenamide) | NorA | S. aureus | Norfloxacin, ciprofloxacin | 79 |
| Caeffeoylquinic acid | NorA | Enterococcus faecalis, S. aureus | Berberine | 54 |
| Piperine | NorA, MdeA, Rv1258c | S. aureus, Mycobacterium spp. | Norfloxacin, ciprofloxacin | 33,34 |
| Clerodane diterpene 16α-hydroxycleroda-3,13 (14)-Z-dien-15,16-olide | norA, norB, norC, mepA, mdeA | S. aureus | Norfloxacin, ciprofloxacin | 80 |
| Chalcone | NorA | S. aureus | Berberine, norfloxacin | 30 |
| Olaanolic acid, Ulvaol | NorA | S. aureus | Norfloxacin, oxacillin | 81 |
| Quercetin | Rv3065(mmr) | Mycobacterium spp. | - | 53 |
| Tetrandrine | Rv2459 (jefA), Rv3728 Rv3065(mmr) | Mycobacterium spp. | Isoniazid and ethambutol | 53 |
| Farnesol | - | Mycobacterium spp. | Et-Br | 53 |
| Synthetic EPIs (Chemically synthesized) | ||||
| 4-acetyl-3-(4-fluorophenyl) - 1-(p-tolyl)-5-methylpyrrole | NorA | S. aureus | Norfloxacin, ciprofloxacin | 82 |
| N-trans-3,4-O dimethylcaffeoyl Tryptamine | NorA | S. aureus | Norfloxacin, ciprofloxacin | 83 |
| 5,7 deoxyhydnocarpin-D (5,7-DHC-D) | NorA | S. aureus | Berberine | 31 |
| Chalcone and derivatives | NorA | S. aureus | Norfloxacin, ciprofloxacin | 84 |
| 4-phenoxy-4’- dimethylaminoethoxy chalcone, (4-DAEC) | NorA | S. aureus | Norfloxacin, ciprofloxacin | 57 |
| SK-20 and SK-56 (Piperine analogs) | NorA | S. aureus | Norfloxacin, ciprofloxacin | 33 |
| SLUPP-225, SLUPP-417 | AcrAB-TolC | E. coli | Novobiocin and erythromycin | 85 |
| PAβN | AdeFGH | A. baumannii | Trimethoprim, chloramphenicol and clindamycin | 86 |
| NMP (1-(1naphthylmethyl)-piperazine) | AdeABC, AcrAB, AcrEF | A. baumannii, E. coli, Enterobacter aerogenes, K. pneumonia | Levofloxacin | 28 |
| 5-MPC | NorA | S. aureus | Norfloxacin, ciprofloxacin | 83 |
| Verapamil | (efpA [Rv2846c], Rv1258c, jefA [Rv2459], and P55 [Rv1410c]) and (Rv1819c and pstB [Rv0933] | M. tuberculosis | Isoniazid | 25, 26, 53 |
| Piperazine Arylideneimidazolones | AcrAB Tol-C and AcrEF | E. coli | Fluoroquinolones | 87 |
| Synthetic EPIs (Chemically synthesized) | ||||
| Ethyl 6-amino-1 cyclopropyl- 7-[4-(hydroxyimino)-3-methyl-3,4,7,8- tetrahydro-2H-thiopyrano[3,2-c] pyridin-6 (5H)-yl]-8-methyl-4-oxo-1,4- dihydroquinoline-3-carboxylate (EDCQ) | NorA | S. aureus | Norfloxacin, ciprofloxacin | 83 |
| 10-(4-(-3-phenylureido)- benzylamino)-9-fluoro-3,7- dihydro-3-methyl-7-oxo-2H-[1,4]oxazino[2,3,4-ij] quinoline-6-carboxilic acid (Q6CA) | NorA, MepA | S. aureus | Norfloxacin, ciprofloxacin | 68 |
| Pyridoquinolines | AcrAB-TolC | E. aerogenes | Norfloxacin | 44 |
| 2-phenyl-4-hydroxyquinoline derivativesN, N-diethyl-2- {[2-(4-propoxyphenyl) quinolin-4-yl] oxy}-ethanamine hydrochloride (PPQE) | NorA | S. aureus | Norfloxacin, ciprofloxacin | 46 |
| 4-(2-piperidin-1-ylethoxy)- 2-(4 propoxyphenyl) quinoline (PPQ) | NorA | S. aureus | Norfloxacin, ciprofloxacin | 46 |
| 4-(2-(piperazin-1-yl) ethoxy)-2-(4-propoxyphenyl) quinolone - PQQ4R | AcrAB-TolC | E. coli | Ofloxacin, tetracycline | 88 |
| (Z)-5-(2,4-dimethoxybenzylidene)-3- (2-hydroxy-3-(isopropylamino) propyl) imidazolidine-2,4-dione | AcrAB-TolC | E. aerogenes | Chloramphenicol, nalidixic acid and sparfloxacin | 89 |
| 5-nitro-2-phenylindole, (INF 55, INF 240, INF 240, INF 271, INF 277) | NorA | S. aureus | Ciprofloxacin | 83 |
| [4-benzyloxy-2-(5-nitro-1H-2-yl)- phenyl]-methanol (BNPM) | NorA | S. aureus | Berberine, norfloxacin | 83 |
| 2-phenylbenzo[b] thiophene-3 carboxaldehyde (2-PTC) | NorA | S. aureus | Ciprofloxacin | 83 |
| 3-(3,4-dihydronapth-2-yl)-propenoic acid isobutyl amide (3-PIA) | NorA | S. aureus | Ciprofloxacin | 83 |
| 2-((2-(4-propoxyphenyl) quinolin-4-yl) oxy) alkylamines 1-4 | NorA | S. aureus | Ciprofloxacin | 46 |
| 13-cyclopentylthio-5-OH-TC (13-CPTC), semisynthetic tetracycline (TC) analogs | TetA or TetB | E. coli | Tetracycline | 90 |
| Cholecalciferol and alpha-tocopherol | TetK, MsrA | S. aureus | Erythromycin, tetracycline | 91 |
| Phe-Arg-β-naphthylamide (MC-207, 110) | MexAB-OprM | P. aeruginosa | Levofloxacin | 19 |
| Biricodar, G-918 | NorA | S. aureus, E. faecalis | FQs, Norfloxacin | 49 |
| Timcodar | - | S. aureus, Mycobacterium spp. | Norfloxacin, isoniazid, rifampicin | 49 |
| SILA 421 | mdr-1 | Mycobacterium spp. | - | 92 |
| Synthetic EPIs (Chemically synthesized) | ||||
| Phenothiazine and its derivatives (methylene blue, promethazine, chlorpromazine and thioridazine) | NorA, AcrB | S. aureus, E. coli | Norfloxacin, FQs | 49 |
| - | Burkholderia pseudomallei | Erythromycin, levofloxacin and azithromycin | ||
| Chlorpromazine | AcrB | S. enterica | Et-Br | 49 |
| phenyl-1,4-benzothiazine derivatives | NorA | S. aureus | Ciprofloxacin | 93 |
| Pyridoquinolines | AcrAB-ToIC | K. pneumonia, E. aerogenes | Tetracycline, norfloxacin, chloramphenicol | 44 |
| 2-(4-Propoxy-phenyl) quinolone derivatives | NorA | S. aureus | Ciprofloxacin | 46 |
| Valinomycin | Rv1410c (P55) | Mycobacterium spp. | Isoniazid | 57 |
| Pyridopyrimidine analogues (D13-9001, D2) | AcrB and MexB | E. coli, P. aeruginosa | FQs | 49 |
| Pyranopyridine derivatives (MBX2319) | AcrAB | E. coli | Ciprofloxacin | 29 |
| (E)-N-(3,4-difluorophenyl)- 2-(2-(3-(methylthio) phenylimino)-4-oxothiazolidin-5-yl | AbeM | A. baumannii | Norfloxacin, ciprofloxacin | 20 |
| DHA7, DHA 27 | AcrB | E. coli | FQs | 94 |
| Riparin-B | NorA | S. aureus | Ciprofloxacin, norfloxacin | 95 |
| Nerol, Dimethyl octanol and Estragole (monoterpenes) | NorA | S. aureus | Norfloxacin | 96 |
| PA EPA amides | NorA | S. aureus | Norfloxacin | 97 |
| 6-(aryl) alkoxypyridine-3-boronic acids, 6-(3-Phenylpropoxy) pyridine-3-boronic acid 3i and 6-(4-phenylbutoxy) pyridine-3-boronic acid 3j | NorA | S. aureus | Ciprofloxacin | 98 |
| Ginsenoside 20(S)-Rh2 (Rh2) | NorA | S. aureus | Ciprofloxacin | 99 |
| Pimozide (neuroleptic drug) | AcrAB-TolC | E. coli | Et-Br | 100 |
| Sertraline | AcrAB, AcrEF, MdtEF and MexAB | E. coli | Levofloxacin, tetracycline | 45 |
| EPIs from microbial sources | ||||
| EA-371α and EA-371δ | MexAB-OprM | P. aeruginosa | Levofloxacin | 101 |
PA, piperic acid; EPA, 4-ethylpiperic acid; DHA7, dihydroartemisinine 7; PaβN, Phenylalanine-arginine β-naphthylamide; 5′-MHC, 5’- methoxyhydnocarpin
EPIs derived from microbes
Although most of the EPIs have their origin in natural products or semi-synthetic/synthetic chemical libraries, a small fraction of EPIs has been reported to originate from microbes. EA-371α and EA-371d, first extracted from fermentation extract of Streptomyces spp., have been recognized as specific inhibitors of the MexAB-OprM pump in P. Aeruginosa101. The novel structure of these compounds offers an opportunity to the researchers to synthesize novel derivatives with increased potency, bioavailability and reduced toxicity. With the three-dimensional crystal structure of efflux pumps available, further computational studies could also be useful to identify the molecular interaction of these compounds with such MDR pumps.
Current challenges for EPIs as therapeutic agents
Even though EPIs have been in laboratory experimentation since the 1990s, these are one of the futuristic prospects in our struggle against antibiotic-resistant bacteria. However, the path leading to a successful commercial EPI has a lot of roadblocks. These challenges are diverse in nature ranging from scientific and academic to administrative and economic. A major hurdle in developing and marketing an EPI is its economic worth. Major players in the pharmaceutical sector tend to stay away from this field as EPI is ultimately a new chemical entity (NCE). The drug experts are well versed with the problems associated with NCE which is trumped by the idea of modifying the currently known antibiotics that, in turn, have a well-documented pharmacological profile and clinical data from numerous patient records102. Academicians have looked for EPIs from both natural and synthetic compounds, however, their commercial production has not been taken under consideration at the laboratory level. The naturally derived EPIs have a complex and bulky structure making it difficult to synthesize. While synthetic molecules are easier to synthesize, these often suffer from poor solubility, toxicity and problems with cell permeability. The discovery of NCE is a demanding process in terms of capital and time as well. A considerable effort is also lost in satisfying the regulatory conditions that are extremely stringent. This, combined with average economic returns, makes the discovery of EPIs, and NCE in general, a financially infeasible venture keeping most of the pharmaceutical companies away.
A therapy using EPIs would essentially be a combination therapy. This puts another challenge of compatibility of the EPI and the antibiotic partner. The pharmacokinetics of both the partners must complement each other for a successful therapeutic combination102. These considerations are often neglected in laboratory experiments, but these assume extreme importance from the clinical point of view. For example, the combination of verapamil, a Ca++ channel blocker, with clarithromycin, a macrolide antibiotic, has been observed to be fatal, with the US FDA issuing a strict warning103. The target of clarithromycin is a cytochrome that is responsible for metabolism of verapamil. The combined use of both the drugs could lead to accumulation of verapamil at extremely toxic levels leading to kidney failures, hypotension and death103.
A major challenge for EPIs as therapeutic agents itself lies with their targets. Efflux pumps are one of the mechanisms but not always the only mechanism of antibiotic resistance. In bacteria such as A. baumannii and P. aeruginosa, the fluoroquinolone resistance is often mediated by the efflux pumps as well as point mutations in the gyrase-coding genes104. The problem is compounded by co-expression of multiple pumps and substrate redundancy. This makes the EPI-antibiotic combinatorial therapy case-specific and casts doubts over the success at the community level.
While EPIs usually show promise with an antibiotic against the efflux pump, it is often seen that the same EPI does not potentiate the activity of other substrates of the same efflux pump. PAβN is effective at potentiating only a certain set of antibiotics while it does not really potentiate other substrate antibiotics of the pump MexAB19. Like PAβN, many EPIs are substrates of the pumps and act at a particular substrate-binding site. An indirect implication of this observation is that a high concentration of EPI would be required to ensure that these competitively prohibit the interaction of substrate antibiotics with the pump. Unfortunately, the fare well with antibiotics that are also the substrate of the pump but have a different substrate-binding site. This greatly narrows the spectrum of an EPI, making it highly specific for only a limited number of substrates. Although it is difficult to discover an NCE that inhibits the efflux of antibiotic from a pump, it is extremely hard to find an EPI that would inhibit multiple pumps across multiple bacterial species. Although some molecules have a common mechanism of inhibition, these have been found to inhibit animal efflux pumps as well, resulting in toxicity and unfavourable pharmacological profile102.
Other challenges that plague the success of EPIs stem from the lack of pre-clinical and clinical data. There is a limited amount of information on model organisms and patient data to support the activity of EPIs. More work at the pre-clinical and clinical level is required to take the EPI research to the next level102. No to low frequency of mutant generation is one of the advantages of using EPIs. However, random mutagenesis using PCR has resulted in efflux pump variants that retain their activity but are resistant to the action of EPI32. Although it seems a rare possibility, it cannot be denied that under an immense selection pressure, bacteria may develop such modifications that ultimately save them from the EPI-antibiotic combination therapy.
Future perspectives
Although the use of EPIs as therapeutic agents faces a lot of challenges, that should, in no way, undermine the importance and advantage they offer. In times where the antibacterial pipeline has almost dried out, EPIs provide a ray of hope by rejuvenating the activity of already available antibiotics. The use of EPIs obviates the discovery of new antibiotics, a strategy that saves a lot of time, effort and capital associated with discovery of a novel antibiotic. It allows the clinicians to exploit the already well-established pharmacological properties of known antibiotics. A very important implication of EPIs as therapeutic agents is the ability to reverse antibiotic resistance. It assumes great importance when we consider the fact that the current economic conditions also favour the large-scale production of already optimized and stockpiled antibiotics. Another striking advantage of using EPIs is the extremely low frequency of generation of resistant mutants. The combination of antibiotic and EPI is, therefore, effective in not only tackling the already resistant bacteria but also providing respite from the future problems of development of resistance.
Evaluating the potential of EPIs, it appears that although the use of EPIs is an attractive strategy, it is far from realization yet. There are many gaps that need to be plugged and a lot of distance to be covered. The technical downsides and limitations of the EPIs need urgent attention. More research is required to highlight the scientific and economic merit of EPIs. This would ultimately help in attracting the interest of pharmaceutical industries and more capital. To sum up, there is a considerable amount of effort currently underway at the bench level; however, it will take more consideration and effort before the EPIs can finally make it to the bedside.
Footnotes
Financial support & sponsorship: Authors thank the Indian Council of Medical Research, New Delhi for financial support (Research grant No. AMR/15/2011-ECD-I), which led to the discovery of IITR08027, a novel EPI.
Conflicts of Interest: None.
References
- 1.Lewis K. Antibiotics: Recover the lost art of drug discovery. Nature. 2012;485:439–40. doi: 10.1038/485439a. [DOI] [PubMed] [Google Scholar]
- 2.Ory EM, Yow EM. The use and abuse of the broad spectrum antibiotics. JAMA. 1963;185:273–9. doi: 10.1001/jama.1963.03060040057022. [DOI] [PubMed] [Google Scholar]
- 3.Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM., Jr Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864–73. doi: 10.1001/jama.2016.4151. [DOI] [PubMed] [Google Scholar]
- 4.Haque M, Sartelli M, McKimm J, Abu Bakar M. Health care-associated infections-an overview. Infect Drug Resist. 2018;11:2321–33. doi: 10.2147/IDR.S177247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventola CL. The antibiotic resistance crisis: Part 1: Causes and threats. P T. 2015;40:277–83. [PMC free article] [PubMed] [Google Scholar]
- 6.Falagas ME, Karageorgopoulos DE. Pandrug resistance (PDR), extensive drug resistance (XDR), and multidrug resistance (MDR) among Gram-negative bacilli: Need for international harmonization in terminology. Clin Infect Dis. 2008;46:1121–2. doi: 10.1086/528867. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman SJ, Outterson K. Introduction: What will it take to address the global threat of antibiotic resistance? J Law Med Ethics. 2015;43(Suppl 3):6–11. doi: 10.1111/jlme.12267. [DOI] [PubMed] [Google Scholar]
- 8.Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 9.Piddock LJ. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schindler BD, Kaatz GW. Multidrug efflux pumps of Gram-positive bacteria. Drug Resist Updat. 2016;27:1–3. doi: 10.1016/j.drup.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Li XZ, Plésiat P, Nikaido H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev. 2015;28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blair JM, Richmond GE, Piddock LJ. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol. 2014;9:1165–77. doi: 10.2217/fmb.14.66. [DOI] [PubMed] [Google Scholar]
- 13.Webber MA, Piddock LJ. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother. 2003;51:9–11. doi: 10.1093/jac/dkg050. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A, Sharma R, Bhattacharyya T, Bhando T, Pathania R. Fosfomycin resistance in Acinetobacter baumannii is mediated by efflux through a major facilitator superfamily (MFS) transporter-AbaF. J Antimicrob Chemother. 2017;72:68–74. doi: 10.1093/jac/dkw382. [DOI] [PubMed] [Google Scholar]
- 15.Costa SS, Ntokou E, Martins A, Viveiros M, Pournaras S, Couto I, et al. Identification of the plasmid-encoded QacA efflux pump gene in meticillin-resistant Staphylococcus aureus (MRSA) strain HPV107, a representative of the MRSA iberian clone. Int J Antimicrob Agents. 2010;36:557–61. doi: 10.1016/j.ijantimicag.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Santagati M, Iannelli F, Cascone C, Campanile F, Oggioni MR, Stefani S, et al. The novel conjugative transposon TN1207.3 carries the macrolide efflux gene mef(A) in Streptococcus pyogenes. Microb Drug Resist. 2003;9:243–7. doi: 10.1089/107662903322286445. [DOI] [PubMed] [Google Scholar]
- 17.Piddock LJ. Multidrug-resistance efflux pumps – Not just for resistance. Nat Rev Microbiol. 2006;4:629–36. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 18.Bhardwaj AK, Mohanty P. Bacterial efflux pumps involved in multidrug resistance and their inhibitors: Rejuvinating the antimicrobial chemotherapy. Recent Pat Antiinfect Drug Discov. 2012;7:73–89. doi: 10.2174/157489112799829710. [DOI] [PubMed] [Google Scholar]
- 19.Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M, et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: Novel agents for combination therapy. Antimicrob Agents Chemother. 2001;45:105–16. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharyya T, Sharma A, Akhter J, Pathania R. The small molecule IITR08027 restores the antibacterial activity of fluoroquinolones against multidrug-resistant Acinetobacter baumannii by efflux inhibition. Int J Antimicrob Agents. 2017;50:219–26. doi: 10.1016/j.ijantimicag.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Anoushiravani M, Falsafi T, Niknam V. Proton motive force-dependent efflux of tetracycline in clinical isolates of Helicobacter pylori. J Med Microbiol. 2009;58:1309–13. doi: 10.1099/jmm.0.010876-0. [DOI] [PubMed] [Google Scholar]
- 22.Fenosa A, Fusté E, Ruiz L, Veiga-Crespo P, Vinuesa T, Guallar V, et al. Role of tolC in Klebsiella oxytoca resistance to antibiotics. J Antimicrob Chemother. 2009;63:668–74. doi: 10.1093/jac/dkp027. [DOI] [PubMed] [Google Scholar]
- 23.Osei Sekyere J, Amoako DG. Carbonyl cyanide m-chlorophenylhydrazine (CCCP) reverses resistance to colistin, but not to carbapenems and tigecycline in multidrug-resistant Enterobacteriaceae. Front Microbiol. 2017;8:228. doi: 10.3389/fmicb.2017.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vargiu AV, Nikaido H. Multidrug binding properties of the AcrB efflux pump characterized by molecular dynamics simulations. Proc Natl Acad Sci. 2012;109:20637–42. doi: 10.1073/pnas.1218348109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Cohen KA, Winglee K, Maiga M, Diarra B, Bishai WR. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014;58:574–6. doi: 10.1128/AAC.01462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh M, Jadaun GP, Ramdas, Srivastava K, Chauhan V, Mishra R, et al. Effect of efflux pump inhibitors on drug susceptibility of ofloxacin resistant Mycobacterium tuberculosis isolates. Indian J Med Res. 2011;133:535–40. [PMC free article] [PubMed] [Google Scholar]
- 27.Radchenko M, Symersky J, Nie R, Lu M. Structural basis for the blockade of MATE multidrug efflux pumps. Nat Commun. 2015;6:7995. doi: 10.1038/ncomms8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohnert JA, Kern WV. Selected arylpiperazines are capable of reversing multidrug resistance in Escherichia coli overexpressing RND efflux pumps. Antimicrob Agents Chemother. 2005;49:849–52. doi: 10.1128/AAC.49.2.849-852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vargiu AV, Ruggerone P, Opperman TJ, Nguyen ST, Nikaido H. Molecular mechanism of MBX2319 inhibition of Escherichia coli AcrB multidrug efflux pump and comparison with other inhibitors. Antimicrob Agents Chemother. 2014;58:6224–34. doi: 10.1128/AAC.03283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stavri M, Piddock LJ, Gibbons S. Bacterial efflux pump inhibitors from natural sources. J Antimicrob Chemother. 2007;59:1247–60. doi: 10.1093/jac/dkl460. [DOI] [PubMed] [Google Scholar]
- 31.Gibbons S, Oluwatuyi M, Kaatz GW. A novel inhibitor of multidrug efflux pumps in Staphylococcus aureus. J Antimicrob Chemother. 2003;51:13–7. doi: 10.1093/jac/dkg044. [DOI] [PubMed] [Google Scholar]
- 32.Pfeifer HJ, Greenblatt DK, Koch-Wester J. Clinical toxicity of reserpine in hospitalized patients: A report from the Boston collaborative drug surveillance program. Am J Med Sci. 1976;271:269–76. doi: 10.1097/00000441-197605000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A, Khan IA, Koul S, Koul JL, Taneja SC, Ali I, et al. Novel structural analogues of piperine as inhibitors of the NorA efflux pump of Staphylococcus aureus. J Antimicrob Chemother. 2008;61:1270–6. doi: 10.1093/jac/dkn088. [DOI] [PubMed] [Google Scholar]
- 34.Sharma S, Kumar M, Sharma S, Nargotra A, Koul S, Khan IA. Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis. J Antimicrob Chemother. 2010;65:1694–701. doi: 10.1093/jac/dkq186. [DOI] [PubMed] [Google Scholar]
- 35.Chan BC, Ip M, Lau CB, Lui SL, Jolivalt C, Ganem-Elbaz C, et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J Ethnopharmacol. 2011;137:767–73. doi: 10.1016/j.jep.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 36.Fujita M, Shiota S, Kuroda T, Hatano T, Yoshida T, Mizushima T, et al. Remarkable synergies between baicalein and tetracycline, and baicalein and beta-lactams against methicillin-resistant Staphylococcus aureus. Microbiol Immunol. 2005;49:391–6. doi: 10.1111/j.1348-0421.2005.tb03732.x. [DOI] [PubMed] [Google Scholar]
- 37.Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5’-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci. 2000;97:1433–7. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morel C, Stermitz FR, Tegos G, Lewis K. Isoflavones as potentiators of antibacterial activity. J Agric Food Chem. 2003;51:5677–9. doi: 10.1021/jf0302714. [DOI] [PubMed] [Google Scholar]
- 39.Gibbons S, Moser E, Kaatz GW. Catechin gallates inhibit multidrug resistance (MDR) in Staphylococcus aureus. Planta Med. 2004;70:1240–2. doi: 10.1055/s-2004-835860. [DOI] [PubMed] [Google Scholar]
- 40.Sudano Roccaro A, Blanco AR, Giuliano F, Rusciano D, Enea V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob Agents Chemother. 2004;48:1968–73. doi: 10.1128/AAC.48.6.1968-1973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oluwatuyi M, Kaatz GW, Gibbons S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry. 2004;65:3249–54. doi: 10.1016/j.phytochem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Lorenzi V, Muselli A, Bernardini AF, Berti L, Pagès JM, Amaral L, et al. Geraniol restores antibiotic activities against multidrug-resistant isolates from Gram-negative species. Antimicrob Agents Chemother. 2009;53:2209–11. doi: 10.1128/AAC.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Opperman TJ, Nguyen ST. Recent advances toward a molecular mechanism of efflux pump inhibition. Front Microbiol. 2015;6:421. doi: 10.3389/fmicb.2015.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chevalier J, Atifi S, Eyraud A, Mahamoud A, Barbe J, Pagès JM. New pyridoquinoline derivatives as potential inhibitors of the fluoroquinolone efflux pump in resistant Enterobacter aerogenes strains. J Med Chem. 2001;44:4023–6. doi: 10.1021/jm010911z. [DOI] [PubMed] [Google Scholar]
- 45.Pradel E, Pagès JM. The AcrAB-tolC efflux pump contributes to multidrug resistance in the nosocomial pathogen Enterobacter aerogenes. Antimicrob Agents Chemother. 2002;46:2640–3. doi: 10.1128/AAC.46.8.2640-2643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabatini S, Gosetto F, Manfroni G, Tabarrini O, Kaatz GW, Patel D, et al. Evolution from a natural flavones nucleus to obtain 2-(4-propoxyphenyl)quinoline derivatives as potent inhibitors of the S. aureus NorA efflux pump. J Med Chem. 2011;54:5722–36. doi: 10.1021/jm200370y. [DOI] [PubMed] [Google Scholar]
- 47.Thorarensen A, Presley-Bodnar AL, Marotti KR, Boyle TP, Heckaman CL, Bohanon MJ, et al. 3-arylpiperidines as potentiators of existing antibacterial agents. Bioorg Med Chem Lett. 2001;11:1903–6. doi: 10.1016/s0960-894x(01)00330-4. [DOI] [PubMed] [Google Scholar]
- 48.Kaatz GW, Moudgal VV, Seo SM, Hansen JB, Kristiansen JE. Phenylpiperidine selective serotonin reuptake inhibitors interfere with multidrug efflux pump activity in Staphylococcus aureus. Int J Antimicrob Agents. 2003;22:254–61. doi: 10.1016/s0924-8579(03)00220-6. [DOI] [PubMed] [Google Scholar]
- 49.Mahmood HY, Jamshidi S, Sutton JM, Rahman KM. Current advances in developing inhibitors of bacterial multidrug efflux pumps. Curr Med Chem. 2016;23:1062–81. doi: 10.2174/0929867323666160304150522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zechini B, Versace I. Inhibitors of multidrug resistant efflux systems in bacteria. Recent Pat Antiinfect Drug Discov. 2009;4:37–50. doi: 10.2174/157489109787236256. [DOI] [PubMed] [Google Scholar]
- 51.Piddock LJ, Garvey MI, Rahman MM, Gibbons S. Natural and synthetic compounds such as trimethoprim behave as inhibitors of efflux in Gram-negative bacteria. J Antimicrob Chemother. 2010;65:1215–23. doi: 10.1093/jac/dkq079. [DOI] [PubMed] [Google Scholar]
- 52.Neyfakh AA, Bidnenko VE, Chen LB. Efflux-mediated multidrug resistance in Bacillus subtilis: Similarities and dissimilarities with the mammalian system. Proc Natl Acad Sci U S A. 1991;88:4781–5. doi: 10.1073/pnas.88.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song L, Wu X. Development of efflux pump inhibitors in antituberculosis therapy. Int J Antimicrob Agents. 2016;47:421–9. doi: 10.1016/j.ijantimicag.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Fiamegos YC, Kastritis PL, Exarchou V, Han H, Bonvin AM, Vervoort J, et al. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against Gram-positive pathogenic bacteria. PLoS One. 2011;6:e18127. doi: 10.1371/journal.pone.0018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joshi P, Singh S, Wani A, Sharma S, Jain SK, Singh B, et al. Osthol and curcumin as inhibitors of human Pgp and multidrug efflux pumps of Staphylococcus aureus: Reversing the resistance against frontline antibacterial drugs. Med Chem Commun. 2014;5:1540–7. [Google Scholar]
- 56.Holler JG, Christensen SB, Slotved HC, Rasmussen HB, Gúzman A, Olsen CE, et al. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a kaempferol rhamnoside isolated from Persea lingue nees. J Antimicrob Chemother. 2012;67:1138–44. doi: 10.1093/jac/dks005. [DOI] [PubMed] [Google Scholar]
- 57.Michalet S, Cartier G, David B, Mariotte AM, Dijoux-franca MG, Kaatz GW, et al. N-caffeoylphenalkylamide derivatives as bacterial efflux pump inhibitors. Bioorg Med Chem Lett. 2007;17:1755–8. doi: 10.1016/j.bmcl.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 58.Li B, Yao Q, Pan XC, Wang N, Zhang R, Li J, et al. Artesunate enhances the antibacterial effect of {beta|-lactam antibiotics against Escherichia coli by increasing antibiotic accumulation via inhibition of the multidrug efflux pump system AcrAB-TolC. J Antimicrob Chemother. 2011;66:769–77. doi: 10.1093/jac/dkr017. [DOI] [PubMed] [Google Scholar]
- 59.Chérigo L, Pereda-Miranda R, Fragoso-Serrano M, Jacobo-Herrera N, Kaatz GW, Gibbons S. Inhibitors of bacterial multidrug efflux pumps from the resin glycosides of Ipomoea murucoides. J Nat Prod. 2008;71:1037–45. doi: 10.1021/np800148w. [DOI] [PubMed] [Google Scholar]
- 60.Rana T, Singh S, Kaur N, Pathania K, Farooq U. A review on efflux pump inhibitors of medically important bacteria from plant sources. Int J Pharm Sci Rev Res. 2014;26:101–11. [Google Scholar]
- 61.Roy SK, Kumari N, Pahwa S, Agrahari UC, Bhutani KK, Jachak SM, et al. NorA efflux pump inhibitory activity of coumarins from Mesua ferrea. Fitoterapia. 2013;90:140–50. doi: 10.1016/j.fitote.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 62.Stermitz FR, Scriven LN, Tegos G, Lewis K. Two flavonols from Artemisa annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Med. 2002;68:1140–1. doi: 10.1055/s-2002-36347. [DOI] [PubMed] [Google Scholar]
- 63.Chan BC, Ip M, Gong H, Lui SL, See RH, Jolivalt C, et al. Synergistic effects of diosmetin with erythromycin against ABC transporter over-expressed methicillin-resistant Staphylococcus aureus (MRSA) RN4220/pUL5054 and inhibition of MRSA pyruvate kinase. Phytomedicine. 2013;20:611–4. doi: 10.1016/j.phymed.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 64.Roy SK, Pahwa S, Nandanwar H, Jachak SM. Phenylpropanoids of Alpinia galangal as efflux pump inhibitors in Mycobacterium smegmatis mc2 155. Fitoterapia. 2012;83:1248–55. doi: 10.1016/j.fitote.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 65.Shiu WK, Malkinson JP, Rahman MM, Curry J, Stapleton P, Gunaratnam M, et al. A new plant-derived antibacterial is an inhibitor of efflux pumps in Staphylococcus aureus. Int J Antimicrob Agents. 2013;42:513–8. doi: 10.1016/j.ijantimicag.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Chovanová R, Mezovská J, Vaverková Š, Mikulášová M. The inhibition the Tet(K) efflux pump of tetracycline resistant Staphylococcus epidermidis by essential oils from three Salvia species. Lett Appl Microbiol. 2015;61:58–62. doi: 10.1111/lam.12424. [DOI] [PubMed] [Google Scholar]
- 67.Bruhn DF, Scherman MS, Liu J, Scherbakov D, Meibohm B, Böttger EC, et al. In vitro and in vivo evaluation of synergism between anti-tubercular spectinamides and non-classical tuberculosis antibiotics. Sci Rep. 2015;5:13985. doi: 10.1038/srep13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith EC, Williamson EM, Wareham N, Kaatz GW, Gibbons S. Antibacterials and modulators of bacterial resistance from the immature cones of Chamaecyparis lawsoniana. Phytochemistry. 2007;68:210–7. doi: 10.1016/j.phytochem.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Smith EC, Kaatz GW, Seo SM, Wareham N, Williamson EM, Gibbons S. The phenolic diterpene totarol inhibits multidrug efflux pump activity in Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:4480–3. doi: 10.1128/AAC.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh S, Kalia NP, Joshi P, Kumar A, Sharma PR, Kumar A, et al. Boeravinone B, A novel dual inhibitor of NorA bacterial efflux pump of Staphylococcus aureus and human P-glycoprotein, reduces the biofilm formation and intracellular invasion of bacteria. Front Microbiol. 2017;8:1868. doi: 10.3389/fmicb.2017.01868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Limaverde PW, Campina FF, da Cunha FAB, Crispim FD, Figueredo FG, Lima LF, et al. Inhibition of the TetK efflux-pump by the essential oil of Chenopodium ambrosioides L. and α-terpinene against Staphylococcus aureus IS-58. Food Chem Toxicol. 2017;109:957–61. doi: 10.1016/j.fct.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 72.Kakarla P, Floyd J, Mukherjee M, Devireddy AR, Inupakutika MA, Ranweera I, et al. Inhibition of the multidrug efflux pump LmrS from Staphylococcus aureus by cumin spice Cuminum cyminum. Arch Microbiol. 2017;199:465–74. doi: 10.1007/s00203-016-1314-5. [DOI] [PubMed] [Google Scholar]
- 73.Fazly Bazzaz BS, Iranshahi M, Naderinasab M, Hajian S, Sabeti Z, Masumi E. Evaluation of the effects of galbanic acid from Ferula szowitsiana and conferol from F. badrakema, as modulators of multi-drug resistance in clinical isolates of Escherichia coli and Staphylococcus aureus. Res Pharm Sci. 2010;5:21–8. [PMC free article] [PubMed] [Google Scholar]
- 74.Tintino SR, Morais-Tintino CD, Campina FF, Costa MDS, Menezes IRA, de Matos YMLS, et al. Tannic acid affects the phenotype of Staphylococcus aureus resistant to tetracycline and erythromycin by inhibition of efflux pumps. Bioorg Chem. 2017;74:197–200. doi: 10.1016/j.bioorg.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 75.Siriyong T, Srimanote P, Chusri S, Yingyongnarongkul BE, Suaisom C, Tipmanee V, et al. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement Altern Med. 2017;17:405. doi: 10.1186/s12906-017-1913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siriyong T, Chusri S, Srimanote P, Tipmanee V, Voravuthikunchai SP. Holarrhena antidysenterica extract and its steroidal alkaloid, conessine, as resistance-modifying agents against extensively drug-resistant Acinetobacter baumannii. Microb Drug Resist. 2016;22:273–82. doi: 10.1089/mdr.2015.0194. [DOI] [PubMed] [Google Scholar]
- 77.Chan BC, Han XQ, Lui SL, Wong CW, Wang TB, Cheung DW, et al. Combating against methicillin-resistant Staphylococcus aureus – Two fatty acids from purslane (Portulaca oleracea L.) exhibit synergistic effects with erythromycin. J Pharm Pharmacol. 2015;67:107–16. doi: 10.1111/jphp.12315. [DOI] [PubMed] [Google Scholar]
- 78.Falcão-Silva VS, Silva DA, Souza Mde F, Siqueira-Junior JP. Modulation of drug resistance in Staphylococcus aureus by a kaempferol glycoside from Herissantia tiubae (Malvaceae) Phytother Res. 2009;23:1367–70. doi: 10.1002/ptr.2695. [DOI] [PubMed] [Google Scholar]
- 79.Kalia NP, Mahajan P, Mehra R, Nargotra A, Sharma JP, Koul S, et al. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. J Antimicrob Chemother. 2012;67:2401–8. doi: 10.1093/jac/dks232. [DOI] [PubMed] [Google Scholar]
- 80.Gupta VK, Tiwari N, Gupta P, Verma S, Pal A, Srivastava SK, et al. A clerodane diterpene from Polyalthia longifolia as a modifying agent of the resistance of methicillin resistant Staphylococcus aureus. Phytomedicine. 2016;23:654–61. doi: 10.1016/j.phymed.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Martins A, Vasas A, Viveiros M, Molnár J, Hohmann J, Amaral L, et al. Antibacterial properties of compounds isolated from Carpobrotus edulis. Int J Antimicrob Agents. 2011;37:438–44. doi: 10.1016/j.ijantimicag.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 82.Bharate JB, Singh S, Wani A, Sharma S, Joshi P, Khan IA, et al. Discovery of 4-acetyl-3-(4-fluorophenyl)-1-(p-tolyl)-5-methylpyrrole as a dual inhibitor of human P-glycoprotein and Staphylococcus aureus nor A efflux pump. Org Biomol Chem. 2015;13:5424–31. doi: 10.1039/c5ob00246j. [DOI] [PubMed] [Google Scholar]
- 83.Schindler BD, Jacinto P, Kaatz GW. Inhibition of drug efflux pumps in Staphylococcus aureus: Current status of potentiating existing antibiotics. Future Microbiol. 2013;8:491–507. doi: 10.2217/fmb.13.16. [DOI] [PubMed] [Google Scholar]
- 84.Holler JG, Slotved HC, Mølgaard P, Olsen CE, Christensen SB. Chalcone inhibitors of the NorA efflux pump in Staphylococcus aureus whole cells and enriched everted membrane vesicles. Bioorg Med Chem. 2012;20:4514–21. doi: 10.1016/j.bmc.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 85.Haynes KM, Abdali N, Jhawar V, Zgurskaya HI, Parks JM, Green AT, et al. Identification and structure-activity relationships of novel compounds that potentiate the activities of antibiotics in Escherichia coli. J Med Chem. 2017;60:6205–19. doi: 10.1021/acs.jmedchem.7b00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cortez-Cordova J, Kumar A. Activity of the efflux pump inhibitor phenylalanine-arginine β-naphthylamide against the AdeFGH pump of Acinetobacter baumannii. Int J Antimicrob Agents. 2011;37:420–4. doi: 10.1016/j.ijantimicag.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 87.Bohnert JA, Schuster S, Kern WV, Karcz T, Olejarz A, Kaczor A, et al. Novel piperazine arylideneimidazolones inhibit the AcrAB-tolC pump in Escherichia coli and simultaneously act as fluorescent membrane probes in a combined real-time influx and efflux assay. Antimicrob Agents Chemother. 2016;60:1974–83. doi: 10.1128/AAC.01995-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Machado D, Fernandes L, Costa SS, Cannalire R, Manfroni G, Tabarrini O, et al. Mode of action of the 2-phenylquinoline efflux inhibitor PQQ4R against Escherichia coli. Peer J. 2017;5:e3168. doi: 10.7717/peerj.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Handzlik J, Szymańska E, Alibert S, Chevalier J, Otrębska E, Pękala E, et al. Search for new tools to combat Gram-negative resistant bacteria among amine derivatives of 5-arylidenehydantoin. Bioorg Med Chem. 2013;21:135–45. doi: 10.1016/j.bmc.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 90.Nelson ML, Levy SB. Reversal of tetracycline resistance mediated by different bacterial tetracycline resistance determinants by an inhibitor of the Tet(B) antiport protein. Antimicrob Agents Chemother. 1999;43:1719–24. doi: 10.1128/aac.43.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tintino SR, Morais-Tintino CD, Campina FF, Pereira RL, Costa Mdo S, Braga MF, et al. Action of cholecalciferol and alpha-tocopherol on Staphylococcus aureus efflux pumps. EXCLI J. 2016;15:315–22. doi: 10.17179/excli2016-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simons SO, Kristiansen JE, Hajos G, van der Laan T, Molnár J, Boeree MJ, et al. Activity of the efflux pump inhibitor SILA 421 against drug-resistant tuberculosis. Int J Antimicrob Agents. 2013;41:488–9. doi: 10.1016/j.ijantimicag.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 93.Sabatini S, Kaatz GW, Rossolini GM, Brandini D, Fravolini A. From phenothiazine to 3-phenyl-1,4-benzothiazine derivatives as inhibitors of the Staphylococcus aureus NorA multidrug efflux pump. J Med Chem. 2008;51:4321–30. doi: 10.1021/jm701623q. [DOI] [PubMed] [Google Scholar]
- 94.Song Y, Qin R, Pan X, Ouyang Q, Liu T, Zhai Z, et al. Design of new antibacterial enhancers based on AcrB's structure and the evaluation of their antibacterial enhancement activity. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111934. pii: E1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Costa LM, de Macedo EV, Oliveira FA, Ferreira JH, Gutierrez SJ, Peláez WJ, et al. Inhibition of the NorA efflux pump of Staphylococcus aureus by synthetic riparins. J Appl Microbiol. 2016;121:1312–22. doi: 10.1111/jam.13258. [DOI] [PubMed] [Google Scholar]
- 96.Coêlho ML, Ferreira JH, de Siqueira Júnior JP, Kaatz GW, Barreto HM, de Carvalho Melo Cavalcante AA, et al. Inhibition of the NorA multi-drug transporter by oxygenated monoterpenes. Microb Pathog. 2016;99:173–7. doi: 10.1016/j.micpath.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 97.Wani NA, Singh S, Farooq S, Shankar S, Koul S, Khan IA, et al. Amino acid amides of piperic acid (PA) and 4-ethylpiperic acid (EPA) as NorA efflux pump inhibitors of Staphylococcus aureus. Bioorg Med Chem Lett. 2016;26:4174–8. doi: 10.1016/j.bmcl.2016.07.062. [DOI] [PubMed] [Google Scholar]
- 98.Fontaine F, Héquet A, Voisin-Chiret AS, Bouillon A, Lesnard A, Cresteil T, et al. Boronic species as promising inhibitors of the Staphylococcus aureus NorA efflux pump: Study of 6-substituted pyridine-3-boronic acid derivatives. Eur J Med Chem. 2015;95:185–98. doi: 10.1016/j.ejmech.2015.02.056. [DOI] [PubMed] [Google Scholar]
- 99.Zhang J, Sun Y, Wang Y, Lu M, He J, Liu J, et al. Non-antibiotic agent ginsenoside 20(S)-rh2 enhanced the antibacterial effects of ciprofloxacin in vitro and in vivo as a potential NorA inhibitor. Eur J Pharmacol. 2014;740:277–84. doi: 10.1016/j.ejphar.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 100.Bohnert JA, Schuster S, Kern WV. Pimozide inhibits the AcrAB-tolC efflux pump in Escherichia coli. Open Microbiol J. 2013;7:83–6. doi: 10.2174/1874285801307010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee MD, Galazzo JL, Staley AL, Lee JC, Warren MS, Fuernkranz H, et al. Microbial fermentation-derived inhibitors of efflux-pump-mediated drug resistance. Farmaco. 2001;56:81–5. doi: 10.1016/s0014-827x(01)01002-3. [DOI] [PubMed] [Google Scholar]
- 102.Lomovskaya O, Bostian KA. Practical applications and feasibility of efflux pump inhibitors in the clinic – A vision for applied use. Biochem Pharmacol. 2006;71:910–8. doi: 10.1016/j.bcp.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 103.Gandhi S, Fleet JL, Bailey DG, McArthur E, Wald R, Rehman F, et al. Calcium-channel blocker-clarithromycin drug interactions and acute kidney injury. JAMA. 2013;310:2544–53. doi: 10.1001/jama.2013.282426. [DOI] [PubMed] [Google Scholar]
- 104.Nakajima A, Sugimoto Y, Yoneyama H, Nakae T. High-level fluoroquinolone resistance in Pseudomonas aeruginosa due to interplay of the MexAB-oprM efflux pump and the DNA gyrase mutation. Microbiol Immunol. 2002;46:391–5. doi: 10.1111/j.1348-0421.2002.tb02711.x. [DOI] [PubMed] [Google Scholar]