Abstract
We, like many others, wish to use modern molecular methods to alter neuronal functionality in primates. For us, this requires expression in a large proportion of the targeted cell population. Long generation times make germ line modification of limited use. The size and intricate primate brain anatomy poses additional challenges. We survey methods using lentiviruses and serotypes of adeno-associated viruses (AAVs) to introduce active molecular material into cortical and subcortical regions of old world monkey brains. Slow injections of AAV2 gives well-defined expression of neurons in the cortex surrounding the injection site. Somewhat surprisingly we find that in the monkey the use of cytomegalovirus (CMV) promoter in lentivirus primarily targets glial cells but few neurons. In contrast, with a synapsin promoter fragment the lentivirus expression is neuron specific at high transduction levels in all cortical layers. We also achieve specific targeting of tyrosine hydroxlase (TH) rich neurons in the locus coeruleus and substantia nigra with a lentvirus carrying a fragment of the TH promoter. Lentiviruses carrying neuron specific promoters are suitable for both cortical and subcortical injections even when injected quickly.
Keywords: old-world monkeys, adeno-associated virus, lentivirus, injection techniques, brain injections, tyrosine hydroxylase promoter
INTRODUCTION
Modern molecular methods allow the introduction of exogenous genetic material into cells with the goal of altering cellular function. A large number of experiments have focused on repairing cellular function with the goal of developing therapies (http://www.wiley.com/legacy/wileychi/genmed/clinical/) such as introducing a functional gene to replace a mutated gene 1, 2, down-regulating a disruptive gene 3, 4 or adding protective properties to cells affected by disease5.
For those interested in studying information processing in the nervous system a parallel effort is underway to introduce agents into developed neurons to allow control over neuronal activity via optical or chemical methods 6–8. In experimental settings such agents can provide a means to connect cellular mechanisms and activity to brain function 9–11 and in clinical settings could eventually offer an opportunity to ameliorate or even cure neuronal disorders attributed to failures in normal brain activity 12.
To repair cellular function it is often adequate or even desirable to target whole organs, such as the lung 13 or the whole brain 14. To manipulate neural activity selectively, it is necessary to deliver the genetic material to a specific cell population often into a particular local brain region with a high rate of transduction (penetrance). To reach this goal we must identify agents and techniques for controlling delivery and expression in the targeted brain region or specific neuron populations. To date some of the most successful experiments in this regard are those done in rodents making use of germline modification 10, 15–17. Confining expression to a specific area and cell type has been challenging in old world monkeys, the experimental animal whose brain structure is most similar to humans. In old-world monkeys germline modification is not practical because of long generation times. There are also limitations in the number of animals available for any single study.
Replication deficient viruses can be used to deliver molecularly active agents into developed central nervous system (CNS) neurons in vivo 18. The two most widely applied viruses in nonhuman primates, are adeno-associated virus (AAV)19 and lentivirus20. A single injection via convection enhanced delivery (CED) of AAV into striatum and thalamus is followed by transgene expression in a large volume of tissue 21, 22. However, in brain structures that are not spherical, such as the cortical sheet or in a deep nucleus, such as the substantia nigra, this single injection approach is not likely to work very well. Three recent reports using AAV to deliver optogenetic constructs into cortical areas of non-human primates highlight both the potential and the difficulties. All three had at least some success. However, populations showing expression were limited in idiosyncratic ways. AAV5 injected into motor cortex showed good penetrance (30–70%) in the deeper layers, and basically no expression in layers 1–3; there was no apparent behavioral effect even though there were easily observed alterations in neuronal activity when the material was activated23. AAV1 injections of an activating channel into primary visual cortex (V1) were followed by strong expression in layer 4B only; there was a light induced behavioral and electrophysiological effect 24. Single site AAV8 injection with an optogenetic construct into the superior colliculus (SC) was followed by expression in approximately 30% of SC neurons; this was high enough penetrance to yield modest light induced behavioral and electrophysiological effects 25. Han et al. 2011 used lentivirus transduction with a promoter specific to pyramidal cells but did not further report data on the extent of the expressing area 26.
Although the idiosyncrasies described above will be important for targeting specific layers of cortex, it would be good to have a means of expressing the desired gene product in most neurons throughout a targeted region. For our purpose, which is to control neuronal activity, it will be necessary to achieve uniform coverage for neurons in a specified region of the cortex or to target neuron populations in deeper regions of the brain. Here we report the results of a survey intended to identify a set of injection techniques and viral construct backbones that yield confined tissue coverage and high penetrance. Our experiments were not intended as an extensive set of parametric studies but rather they were an effort to develop techniques that provide the means of expressing molecular components to manipulate neuronal activity in rhesus monkeys. To this end, we used several AAV and lentivirus serotypes, different promoters and several variations in injection technique. In an example of how variably constructs can behave when packaged in different viruses, we find that expression from a cytomegalovirus (CMV) promoter is neuron specific in several AAV serotypes but when the CMV promoter is packaged into lentivirus the protein expression is found primarily in glial cells. In our efforts to improve the targeting in specific brain regions, we were able to use AAV2 effectively. The material selectively targeted neurons and the spread of the material was moderate making it easy to estimate how to keep it confined to the targeted tissue. Lentivirus with a synapsin promoter expresses in all layers of the cortex at high penetrance and a 3.1 kb fragment of the primate tyrosine hydroxylase (TH) promoter results in specific expression in noradrenergic neurons in the locus coeruleus and in dopaminergic neurons in the substantia nigra respectively.
RESULTS
Testing injection parameters for AAV virus serotypes in visual and motor cortices
To identify an AAV serotype, that would show high local neuron penetrance and achieve expression across a controllable extent of cortex, for example, within a single architectonic region, we surveyed several vectors made with different AAV serotypes expressing a GFP protein from a CMV promoter. The serotypes AAV1, AAV2, AAV6, AAV8, AAV9, AAV10Rh were used because they were available as stock vectors at the PENN Vector Core at the University of Pennsylvania Gene Therapy Program, several of which have been previously tested in rodents27. Virus titers were standardized to 10E13 particles/ml, with exception to AAV2, which was supplied at a titer of 5×10E12. We surveyed injection speeds from 100nl/min to 2000nl/min and volumes from 1ul to 5ul into motor cortex and visual cortex using a motorized pump. The syringes were held in a standard stereotaxic device mounted on a computer controlled pump. The needle was inserted quickly to penetrate the pia and then positioned to the desired injection depth.
There was an inverse relation between injection speed and virus spread. The slower the injection speed, the larger the volume of covered tissue relative to volume of virus injected. 500 nl/min and a volume of 2 ul of virus suspension per injection appear to be a good compromise between limiting the length of surgery time and cortical volume covered by virus expression from one injection. All serotypes with the exception of AAV8 showed a spread of at least 2 mm from the injection sites in both tissues using these parameters. AAV2 and AAV6 had a good combination of well-defined edges and neuronal selectivity. Spread from a single injection ranged from 2 – 4 mm. These agents make it possible to cover substantial and controllably sized areas by injecting multiple sites as we did for motor cortex (Fig. 1A – E, AAV6) and visual cortex (Fig.1F – J, AAV2) respectively. From gross examination, other serotypes seemed less suitable for neuron coverage of visual or motor cortex when compared to AAV2, therefore we did not characterize them in any detail. AAV9, and AAV10R showed a wide area coverage of up to 10 mm per injection. The spread from a single injection seemed too wide to keep expression within a confined cortical area. Also, the infection efficiency tapered off gradually from the injection site without a creating a clear boundary. AAV1 showed well-defined edges of expression and seemed to have reasonably uniform expression, but there was a substantial preference for cells lacking NeuN staining, likely glial cells (Fig S1 A,B). In our experiments, AAV8 did not spread well, yielding low area coverage (<2 mm) in motor and visual cortex. From this initial survey, we decided that AAV2 has the best promise to achieve reasonably uniform coverage in cortex. For all serotypes, with the exception of AAV8 we saw axonal labeling and transport of GFP to the axon terminals (see s Fig. S1C–F for examples).
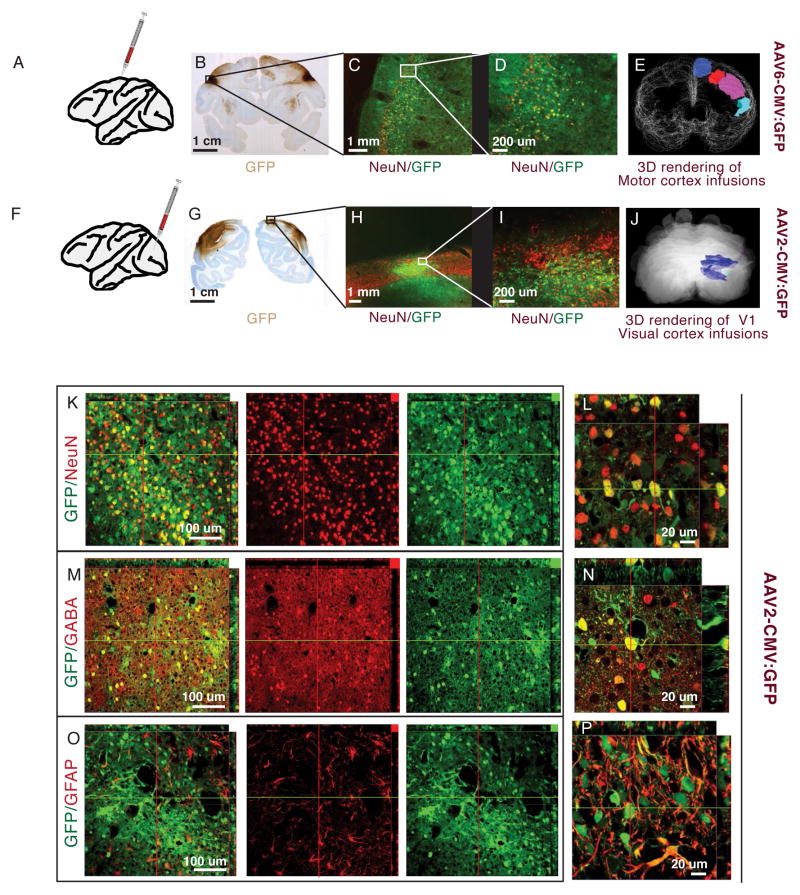
Figure 1. Comparing AAV serotypes in monkey cortex.
A–E. Injections into motor areas. A. Schematic representation of injection site. B. Coronal section from monkey injected with AAV6 on the left and AA1 on the right – visualized with DAB-HRP for GFP expression. C,D. Motor cortex of a section adjacent to B, visualized green for GFP and red for NeuN (Alexa 568). Native GFP expressed from AAV6 and NeuN. GFP expression overlaps with cortical layers, but some expression is visible in the white matter. Most GFP expressing cells co-localize with NeuN staining (yellow cells indicate co-expression). E. 3D rendering of HRP stained areas covered by AAV6 injections into the pre-motor cortex. F–J. Injections into visual cortex. F. Schematic representation of injection site. G. Coronal section from monkey injected with AAV-RH10R in the left V1 and AAV2 in the right V1. H, I. Native GFP expressed from AAV2 and NeuN visualized green for GFP and red for NeuN (Alexa 568). Most GFP expressing cells co-localize with NeuN staining (yellow cells) and are concentrated in cortical layers. J. 3D rendering of HRP stained areas covered by AAV2 injections into the visual cortex. K–P. Confocal slices of visual area injected with AAV2, visualized with green for GFP and red for Alexa 568 antibody staining. K, L. GFP expressing cells co-localize with NeuN (Alexa 568). M,N. GFP expression co-localizes with GABA positive (Alexa 568) interneurons. O,P. Only a small percentage of GFP expressing cells co-localize with GFAP positive glia.
Expression of the AAV2 serotype has been well characterized for injections into subcortical regions in the monkey brain 21, 28. Here we analyzed its cortical expression. The AAV2 expressing tissue was stained for the neuron marker NeuN to look for co-localization with GFP to assess neuronal expression 29, stained for GABA to assess expression in interneurons 30, and stained for GFAP to assess glial expression. As shown in Figure 1K–L, expression was high in neurons. In the example shown, injection of a 2ul virus suspension at 500nl/min of an AAV2 construct with GFP, we counted 483 NeuN positive cells of 500 GFP positive cells within 500um of the injection site at 41% penetrance (483 GFP expressing of 1186 total counted NeuN positive cells), including inhibitory neurons near the injections site (Fig 1M,N). Although there was some expression in glia (Figure 1O,P), expression levels were generally much lower than in neurons. For AAV2, like for the other serotypes surveyed, GFP expression in neurons varied widely from injection to injection, with the range of penetrance being consistent with that seen in other studies using AAV serotypes (30% to 70% within 500um) 23. Neuron specificity and penetrance of AAV6 in the cortex was similar to AAV2. In the motor cortex we counted 274 NeuN positive cells of 300 cells expressing GFP within 500 um of the injection site at 36% penetrance (292 GFP expressing of 808 total counted NeuN positive cells). However, in the regions surveyed, we did not find any advantage of using AAV6 over AAV2, and AAV2 has the slight advantage that it has been used in other non-human primate studies without any reports of adverse effects 21, 28. Our results do suggest that it should be possible to achieve reasonable levels with expression occurring primarily within neurons using of AAV2, at least in the visual and motor cortices.
Handheld versus pump driven injections of AAV and Lentivirus expressing ivermectin sensitive Chloride channels
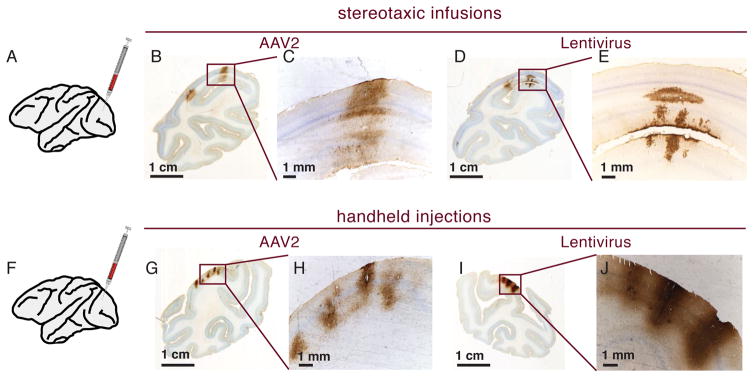
Because of the size, sheet-like structure and sulci, many cortical areas of the brain will require several injections to achieve uniform coverage. Stereotactically guided injections allow good control for speed and placement of injections on the lateral cortical surface but are time consuming to place. Hand held injections, in contrast, offer quick change of position from one injection site of to the next, especially for areas difficult to reach, such as rhinal cortex 31, where stereotaxic injections would require long penetrations of brain tissue. We compared handheld and stereotaxic injections for both AAV2 and lentiviral vectors in visual cortex. To test whether we could detect virally expressed membrane channels relevant for functional experiments, injection contained an equal mixture of viruses expressing fluorescent protein tagged GluCl alpha and/or beta subunits, previously used for neuronal silencing in rodents 32. Figure 2 shows the results of these injections using GFP antibody staining for detection. For pump driven stereotactic injections two injection tracks were placed 1.5 mm apart with an injection speed of 500 nl/min (Fig. 2A–E). For handheld injections the virus was delivered quickly by tapping the plunger to deliver approximately 1ul through a Hamilton needle into the cortex (Fig. 2F–J). The needle was then removed without delay and inserted into the next location for injection. This procedure was repeated several times to cover the intended cortical area in a grid-like pattern. As expected from the previous AAV survey, pump driven infusion yielded good spread of AAV2 virus. Two injections, placed 1.5 mm apart achieved uniform coverage (Fig. 2B,C), whereas with fast handheld injections AAV2 coverage was only a few hundred micra per injection (Fig. 2G–H). Perhaps surprisingly, lentivirus showed the opposite pattern. Pump driven injections at 500 nl/min resulted in only a small area of virus coverage of a few hundred micrometer (Fig. 2D–E), whereas it was clear from inspection that the handheld injections showed more uniform coverage with higher local expression levels (Fig. 2I–J). We did not find any obvious preference for specific cortical layers for either lentivirus or AAV2, as GFP expressing cells were found in all layers. Thus in our hands to achieve usable spread of lentivirus requires fast injection speeds. Handheld injections of lentivirus seem promising for covering large cortical areas, particular in regions that would be difficult to cover with stereotaxic injections.
Figure 2. Comparing injection parameters for AAV2 and Lentivirus in V1.
A. Schematic representation of stereotaxic infusions. B–C. Two overlapping injection of AAV2-GFP at 500nl/min. Injections resulted in contiguous coverage. D–E. Lentivirus-GFP injected at 500nl/min. Staining only covers a small area in the cortical layers. F. Schematic representation for handheld injection. G–H. Handheld injections with AAV2-GFP. Only a limited area around the injection sites express GFP. I–J. Handheld injections with Lentivirus-GFP. Injections result in better cortical coverage around the injection site than slow stereotaxic injections (compare with D,E).
Lentivirus expression from a CMV promoter is expressed in dopamine receptor D2 positive cells but shows strong glial cell preference
A promising application for molecular tools is to target specific cell types by either controlling expression or by targeting a functional cellular component. As an example for the latter, RNA interference by injection of dopamine receptor D2 antisense expressing DNA plasmid into rhinal cortex has been used to temporarily change a cognitive behavior in the monkey 31. We first established that we could visualize cellular Dopamine receptor staining on monkey brain sections (Fig. 3A–E, see also methods). Then, to test whether we can target DRD2 neurons with viral vector constructs, we injected AAV2 carrying a CFP tagged membrane receptor construct (AAV-CMV::GluCla-CFP) 32 into the left rhinal cortex (Fig. 3E–G) and a RNAi lentivirus construct with a GFP reporter (pGIPZ-CMV-GFP) into the right rhinal cortex (Fig. 3E; H–I) using the handheld injection method.
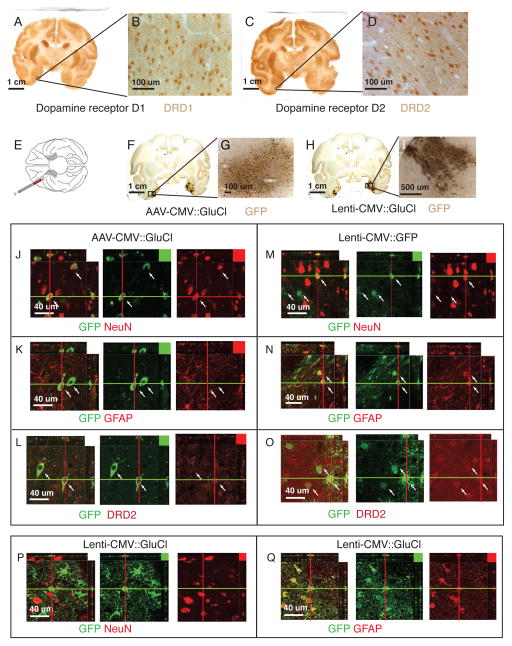
Figure 3. Expression of AAV2 and Lentivirus in the Rhinal Cortex.
A,B. Coronal sections stained for DRD1 visualized with DAB-HRP. Cellular staining is visible in areas consistent with known DRD1 expression 41. C,D. Coronal section stained for DRD2 visualized with DAB-HRP. Cellular staining is visible in areas consistent with known DRD2 expression 40. E. Schematic of ventral view of macaque brain with virus injections into rhinal cortex. F–I. Coronal sections stained for GFP. Left rhinal injected with AAV2-CMV::GluCl. Right rhinal injected with Lenti-CMV::shRNAi-GFP and Lenti-CMV::GluCl. J–Q. Confocal slices of AAV2-CMV::GluCl injected areas GFP is visualized in green (DyLight 488), other markers in red (Alexa 568). J. AAV expressed GFP co-localizes with NeuN neuron marker (red - cells indicated with white arrows). K. AAV2 expressed GFP does not co-localize with glia marker GFAP (red). L. AAV2 expressed GFP does co-localize with DRD2 antibody staining (red). M. Only a very small percentage of lentivirus GFP expressing cells overlap with NeuN expressing neurons (red). N. Lentivirus GFP is expressed in GFAP positive glia (red). O. Lentivirus GFP is expressed in cells stained with DRD2 antibody (red). P. Only few Lenti-GluCla-CFP expressing cells co-localize with NeuN expressing neurons (red). Q. The majority of Lenti-GluCla-CFP expressing cells overlap with GFAP positive glia (red).
Both, AAV2 and lentivirus expressed GFP derivatives showed partial overlap with DRD2 expressing cells in rhinal cortex (Figs. 3A–D; 3J; M). Based on visual inspection, AAV2 staining for GFP was generally observed in cells with stronger expression levels of DRD2, whereas lentivirus staining for GFP showed co-labeling in cells with lower but detectable levels of expression of DRD2. On further analysis we found that the AAV2 expression was almost exclusively neuronal (GluCl-CFP expression co-localized with the neuronal marker NeuN; Fig. 3H–I) whereas lentivirus expression from the pGIPZ-CMV-GFP construct expressed strongly in GFAP positive gilal cells and only in a very limited number of neuronal cells (Fig. K–N). To exclude the possibility that this bias is because of the pGIPZ backbone, we tested our Lenti-CMV:: GluCla-CFP construct, which has a different backbone and observed the same glial cell specificity (Fig. 3P–Q).
From these results it appears that packaging the CMV promoter into lentivirus conveys a strong bias toward infecting glial cells in monkeys independent of vector backbone.
Lentivirus expression from a synapsin promoter is specific to neurons in all 6 layers of the visual cortex
Lentivirus can carry a substantially larger packaging load than AAV 33, providing more flexibility for using larger promoter fragments or design of inducible constructs 3. It is also easier to produce in house to high grade in vivo titers 34. In earlier experiments, we showed that lentivirus spreads better in handheld injections than in slow stereotaxic infusions. However, stereotaxic injections have the potential to provide better control over speed and volume when covering cortical areas. Therefore, we modified the lentiviral expression vector to express GFP from a synapsin promoter fragment and tested micropump driven injections at a relatively high rate of 2 ul/min into the visual cortex to determine whether the synapsin promoter would convey neuronal specificity to the lentivirus constructs. Injections were set approximately 3 mm apart from each other.
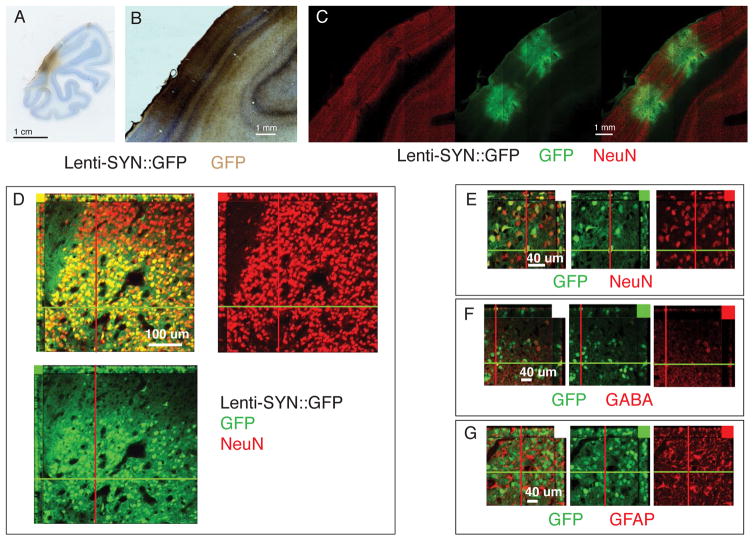
All neuron layers of V1 showed expression in an area of approximately 1mm around the needle track (Fig. 4A–C). Expressing cells were confined to a specific region around the injection site with the number of GFP positive cells falling sharply off outside this region (Fig. 4D). Out of 1362 NeuN positive cells counted on confocal sections within 750 um of one injections site 1178 were GFP positive, putting the penetrance at 86%. There were only 19 cells (1.3%) GFP positive cells within this region, which had no detectable NeuN expression (Fig. 4E). Whereas there was co-labeling of GFP with GABA positive interneurons (Fig. 4F), we did not find any evidence of GFP expression in Glia by staining sections with antibodies for the GFAP protein (Fig. 4G).
Figure 4. Lentivirus synapsin promoter expression in visual cortex.
A–C. Coronal sections of V1 injected with Lenti-SYN::GFP. A,B. GFP visualized with HRP-DAB staining. C. Tiled confocal scan of section stained for GFP and NeuN. Same section in all images with GFP visualized in green (DyLight 488) and NeuN in red (Alexa 568). D–E. Confocal slice to show dense expression of GFP in NeuN positive neurons. Note the sharp transition border of viral GFP expression. F. GFP does co-localized with GABA positive interneurons (red) G. GFP does not co-localized with GFAP positive glia (red).
Thus, injections of a construct using a backbone of lentivirus with the synapsin promoter leads to expression in a majority of neurons within 750 um radius of the injection site. Therefore, a stereotaxic injection matrix with a distance of 1.5mm at a speed of 2ul/min should be sufficient to achieve cortical coverage with a lentiviral synapsin construct.
Lentivirus expression from a tyrosine hydroxylase promoter is specific to norardenergic cell in the locus coeruleus and to dopaminergic cells in the substantia nigra
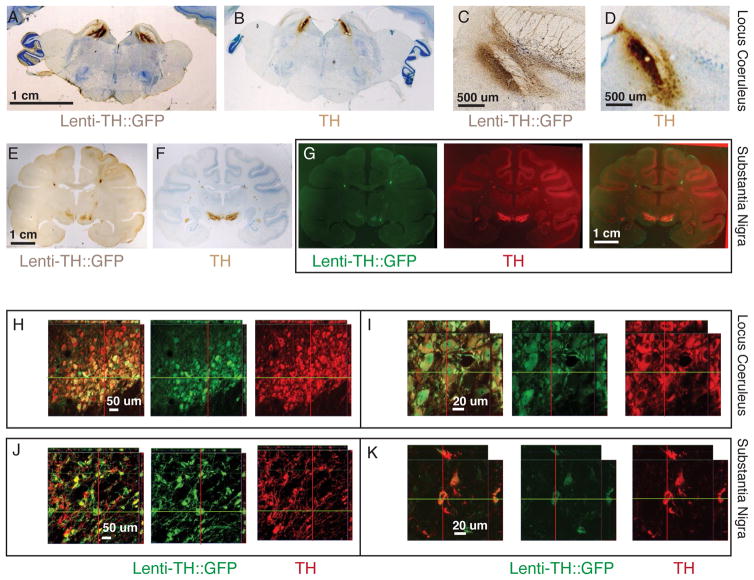
We injected a lentivirus containing a 3.1 kb proximal promoter fragment of the rhesus monkey tyrosine hydroxylase (TH) promoter driving expression of GFP bilaterally into the substantia nigra (SN) of two monkeys. In one of these monkeys we also injected the locus coeruleus (LC) on both sides. From histological analysis of whole brain sections we saw that expression of GFP was confined to the same general area as TH expression on consecutive sections for both LC (Fig. 5A–D) and SN (Fig. 5E–G). Using confocal microscopy to analyze the expression area on a cellular level on double stained sections (Fig. 5H–L), we found that the GFP expression was nearly exclusively confined to TH expressing neurons of LC (98 cells out of 102 counted GFP positive cells also expressed TH) and in SN, where 92 cells out of 95 counted GFP positive cells also expressed TH. Penetrance was as high as 90% in some regions of the LC (82 GFP positive of 93 counted TH positive cells). Penetrance was lower (<50%) in the SN, where our targeting of the injection sites was not centered as well in the SN in these experiments (55 GFP positive of 116 counted TH positive cells).
Figure 5. Lentivirus tyrosine hydroxylase promoter expression in locus coeruleus and substantia nigra.
A–D. Coronal section of monkey bilaterally injected into locus coeruleus. A,C. GFP expression detected with HRP-DAB covers most of LC. B,D. Adjacent sections of those shown in A,C but stained for TH expression (note staining in the same area as in A,C). E–I. Coronal sections of monkey bilaterally injected into substantia nigra. E,F. GFP expression detected with HRP-DAB is mostly restricted to SN. G. Scans pseudocolored with green for GFP and red for TH stain. Overlay of both sections in photoshop shows that expression overlaps. H–K. Confocal slices of injected areas. H,I. GFP and TH expression occurs in the same cell population. J,K. Most GFP expressing cells co-localize with TH positive cells.
DISCUSSION
To use molecular tools to modify neuronal function in large, complicated brains such those of primates and humans, it is going to be necessary to introduce the materials into mature neurons safely and efficiently. Germline modification, a method often used to gain specificity for these tools in rodents, is impractical in non-human primates, especially in old-world monkeys, which are expensive to breed, have long gestation times (on the order of 6 months), and slow development for reaching sexual and behavioral maturity (3–5 years). Currently, replication deficient viruses appear to be the most promising approach to carry the desired genes into mature neurons. We surveyed several virus serotypes, promoters and injection parameters to identify combinations with good transduction properties in the macaque brain. We identified some combinations that allow for either lentivirus or AAV serotypes to transduce neurons in delimited regions with high penetrance.
In our experiments in cortex coverage with lentivirus and AAV depended on injection speed in opposite ways. AAV spread in inverse relation to the injection speed. The slower the speed the larger the area covered with a volume injected. This may likely be due to backflow along the needle track at higher speeds 35. When Salegio et al. 2012 added a step in needle diameter 3 mm back from the tip of the cannula to prevent backflow (by simply slipping a tight fitting silicate tube onto the needle), they were able to inject AAV into the basal forebrain at much faster speeds than without a step and still had good spread of the virus 28. Our injection speeds were generally slower than Salegio et al. 2012. We injected into cortex where cells and extracellular matrix are much more densely packed. In contrast to AAV, lentivirus required faster injection speeds to achieve good spread. We believe that this may be due to the five-fold larger particle size than AAV, needing more pressure to spread through the densely packed tissue. It is possible that injection of lentivirus with step needles would allow for a better spread with lower speeds, by allowing pressure to build more slowly, but preventing the backflow along the needle track.
Several recent studies seem to show that some AAV serotypes have a preference for specific cortical layers. Diester et al. 2011 found opsin expression via AAV5 predominantly in the deeper cortical layers in the motor cortex23, while Jazayeri et al. 2012 saw expression of AAV1 only in a single cortical layer (4Cb) in striate cortex 24. In our hands, three AAV serotypes, AAV2 and AAV6 showed a natural preference for neurons over glial cells through the cortical layers.
AAV serotypes also showed considerable variability in the way that they spread from the injection site. This might be for a couple of reasons, including variable coat proteins and transsynaptic transport. For the AAV2 and AAV6 serotype, which we analyzed in more detail, we did not observe any indication for transsynaptic transport, as cellular expression of the injected material was restricted to a well-defined area around the injection site. Finally, AAV2 led to reasonable expression without obvious bias across the cortical layers. Other serotypes, such as AAV1, AAV9 and AAV RH10 resulted in a much more diffuse expression across a wide area away from the injection site. It is possible that the source of this diffuse expression occurred via retrograde or anterograde transsynaptic transport. For our purposes this was not be desirable, but for some experiments it may be of advantage to investigate these serotypes further.
AAV2 which we studied in more detail than other serotypes, showed good expression throughout all layers in both visual and motor cortices. Lentivirus containing the 500 bp synapsin promoter fragment also showed high penetrance and consistent expression in neurons of all cortical layers of the V1 visual cortex. Lentivirus expression fell off sharply 1 – 1.5 mm from the expression track, leading to gaps in expression when injections were 3 – 4 mm apart. With AAV a single track leads to wider expression. However, it appears from our results that lentivirus gives higher local penetrance, and with a grid of injections placed 1.5 mm apart there should be good contiguous expression of lentivirus in cortex.
We chose lentivirus for a more detailed characterization of cortical coverage and specific promoters, partly because of the ease of in house production and partly because the greater packaging size allowed us to test larger promoter fragments like the TH promoter. When we used a proximal 3.1 kb fragment of the primate TH promoter in lentivirus construct expression was limited to catecholaminergic neurons. By selecting the location where the virus was injected, either the substantia nigra or the locus coeruleus, this promoter fragment had expression limited in dopaminergic or noradrenergic neurons, respectively. This offers the possibility to study effects that occur with either activation or silencing of catecholaminergic neurons.
When packaged in a AAV2 capsid, a cytomegalovirus (CMV) promoter drives expression primarily in neurons. However, when similar CMV promoter constructs are packaged in lentivirus, they drove expression primarily in glial cells and only a limited number of neurons, possibly GABA positive interneurons. This limited neuronal expression with CMV promoter in lentivirus is probably not due to selective virus specificity, because when a construct using a 500 bp human synapsin promoter fragment was packed in lentivirus, expression occurred almost exclusively within neurons, but not within glial cells. In the rhinal cortex we see co-expression of lentivirus CMV expressed GFP with DRD2 antibody staining and with the glia marker GFAP but not with the neuronal marker NeuN. Visual inspection suggests that GFP expression appears to be limited to a population of weaker expressing DRD2 positive cells, which may indicate a weaker DRD2 expression in glia than in neurons of the rhinal cortex. These results show that there is clearly more non-human primate specific characterization necessary to gain a better understanding of virus specificity and promoter fragments necessary to target specific cell populations.
Non-human primate brain, with sulci and large sheet-like cortical areas, poses big challenges for getting uniform high penetrance injections of viral constructs. Because of this difference in structure and likely different patterns of selectivity in viral targeting, the data from rodent experiments are not sufficient in planning injections for functional experiments in old world monkeys. This makes surveys like ours a useful step toward gaining control of the tools for use in the primate brain. Even though this study is only a survey of various techniques, we hope it provides useful information for application and development of molecular approaches of neuronal manipulation in the monkey brain – which is essential if these techniques are ever to be used for selective experimental work in monkeys and therapeutic applications in the human.
Summary of Recommendations
We conducted this survey to determine the best method to achieve viral expression at high neuronal penetrance large but defined locations of the monkey brain. Below is a summary of parameters that we are currently are using based on our results:
AAV virus injections into the open cortex
The slower the better. We use 2 ul virus per site injected at 500 nl/min to balance injection time (which increases the length of surgery) with spread of virus per injection. To cover a large area of cortex, we recommend a 1.5 mm injection matrix. Currently, we are experimenting with interlaced injections using a 2 × 3 grid, where the needles are spaced 3 mm from each other to reduce potential for tissue damage and to reduce the overall time in surgery.
Lentivirus injections into the open cortex
We have good experience with either handheld or stereotaxic injections (1ul/min virus per injection). Injections are placed approximately 1.5 mm apart. For handheld injections a finger tap is used to advance the plunger of the Hamilton syringe. This injection technique is particularly useful for deep and convoluted areas, like rhinal cortex, where during surgery the lateral temporal cortex must be retracted to make the targeted area accessible.
Lentivirus injections into deep brain areas
For deep brain areas, like the SN and LC, we inject by placing the needles trough an implanted grid 2 mm apart from each other and let the needle rest for 10 minutes at the location of injection. For vertical spacing, we pull up the needle 2 mm before injecting again.
SUBJECTS AND METHODS
DNA constructs
For plasmids to produce Lenti-CMV::iCla-cerulean and Lenti-iClb-venus, the coding region of previously published GluCl constructs was re-optimized and point mutation corresponding to cerulean and venus were introduced into the CFP and YFP regions. Coding regions for the plasmids were synthesized by GeneArt® and cloned into the p156RRL-sinPPT-CMV-GFP-PRE/Nhe I plasmid (Addgene plasmid 17448) by replacing GFP. To create the DNA for Lenti-TH::GFP, 3.1 kb of the proximal portion of the rhesus monkey TH promoter (http://www.ncbi.nlm.nih.gov) was synthesized by GeneArt® and cloned into the p156RRL-sinPPT-CMV-GFP-PRE/Nhe I plasmid by replacing the CMV promoter. Similar, for Lenti-SYN::GFP, the proximal 500 bp of the human synapsin promoter, was synthesized by GeneArt® and cloned into the p156RRL-sinPPT-CMV-GFP-PRE/Nhe I plasmid by replacing the CMV promoter.
Virus preparation and storage
Lentivirus was produced at titers >10E9 as described by Han et al. 2010 34. In short, 293T cells (Lenti-X Invitrogen 632180) were transfected with the Lenti-backbone plasmid and packaging plasmids (Addgene). Supernatant was replaced by new medium after 24 hours with Ultraculture medium (Invitrogen ). Lentivirus was harvested by collecting the supernatant 48 hours and 72 hours after transfections and spinning it at 22,000 rpm (Beckman S28 rotor) trough a 20% sucrose cushion in PBS. Virus pellet was suspended by incubation in ice cold PBS for one hour, carefully mixed, aliquoted and frozen on dry ice for storage at −80C until needed for surgery. Titers were determined by preparing genomic DNA from 293T cells transduced with the Lentivirus and using qPCR to compare amplification of a 150 bp Lentivirus fragment with amplification of a comparable fragment of the endogenous human Vasopressin receptor gene (hVAR1). AAV vectors for the AAV serotype survey were stock vectors from the PENN vector core. AAV2-GluCla and AAV2-GluClb vectors 32 were produced by the UNC vector core at titers of 10E12 unites/ml. Viruses were shipped in aliquots on dry ice and stored at −80C until needed for surgery.
Subjects
We used 8 old-world non human primates for this study. 2 Macaca fascicularis (cynomolgus monkey) were used for the AAV serotype survey in the visual cortex. 6 Macaca mulatta were used for the other studies: Two for injections of AAV in to motor cortex, one for comparing stereotaxic vs. handheld injections of AAV and lentivirus in to the visual cortex, two for injections of AAV and lentivirus into the rhinal cortex, two for Lenti-TH injections into locus coeruleus and substantia nigra and one monkey for Lenti-synapsin injections into the visual cortex. All experimental procedures conformed to the Institute of Laboratory Animal Resources guide and were approved by the National Institute of Mental Health Animal Care and Use Committee.
Surgeries
Surgeries were performed under aseptic conditions in a fully equipped operating suite with veterinary supervision. Before surgery, animals were sedated with ketamine hydrochloride (10 mg/kg, i.m.); a surgical level of anesthesia was then induced and maintained with isoflurane gas (1–4% to effect). Body temperature, heart rate, blood pressure and expired CO2 were monitored throughout all surgical procedures. For all cortical injection procedures the cortex was exposed by removing a bone flap and reflection of the dura mater. Handheld injections into the rhinal cortex have been described in detail previously 36. All virus aliquots were allowed to thaw on ice for at least 15 minutes and diluted with sterile PBS if required by carefully mixing by tapping the tube. If necessary, the liquid was spun to the bottom of the tube with a small benchtop centrifuge. Virus was then carefully taken up into the needles by manually raising the plunger of a Hamilton syringe. For handheld injections, a 10 ul Hamilton syringe with a 30 gauge needle was inserted into the intended area of injection by one person and a second person pressed the plunger to approximately expulse 1ul, after which the needle was removed and inserted into the next location. For stereotaxic injections, the 10 ul Hamilton syringe was mounted into a motorized nano-injector (Havard). During penetration of the pia mater and the cortical tissue to reach the injection site, the injection speed was set to 100 nl/min to exert positive pressure to reduce to possibility of needle blockage. Once the target position was reached, the injection speed was adjusted to the desired injections rate. For injections into the substantia nigra and the locus coeruleus, the location was determined via MRI and mounting an injection chamber with grid as described previously 37, 38. Injections were placed at a 2mm distance from each other, either laterally via grid holes or vertically along the needle track. The needle was kept in place for 10 minutes after each injection before retraction.
Histology
Between 3 and 6 weeks following the injections, each monkey was deeply anesthetized with Beuthanasia solution and perfused with 1 liter of normal saline solution followed by 3 liters of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). Monkeys for the AAV serotype survey and handheld vs stereotaxic injections (Figs. 1 and 2) were euthanized between 3 and 4 weeks after injection, all other monkey were sacrificed between 5 and 6 weeks after virus injections. The brains were removed and cryoprotected through a series of glycerols in 0.1 M PBS 39. The brains were blocked in the coronal plane and then quickly frozen in −80 C isopentane. The brain was sectioned into 40-μm slices in the coronal plan and the sections collected in 10 series. For histochemistry sectioneds were blocked in 5% normal goat serum and 0.3% Triton-X 100 in TBS, incubated with primary antibody in blocking buffer, washed and, incubated with an Horse Radish peroxidase secondary antibody (Vector labs PI-1000) for 90 minutes and visualized with a DAB reaction and optionally counterstained with thionine. For DRD1 and DRD2 DAB histochemistry the reaction was enhanced with the Vector ABC kit (Vector labs VECTASTAIN ABC System). DRD1 and DRD2 staining throughout the brain is consistent with published expression for these receptors in the non-human primate 40, 41 (Fig. 3A–D). For immunofluorescence secondary rabbit and mouse antibodies were purchased from Molecular Probes, spectra as indicated in the figure legends. The secondary antibody for fluorescence detection of chick anti GFP was purchased from Abcam (DyLight 488 goat anti-Ch - ab96947). See supplemental table T1 for primary antibody specifications, dilutions and incubation times. For 3D reconstruction of AAV serotype survey, one series (1:10) of 40 um sections was stained for GFP with DAB, the stained areas were outlined at 10x magnification and reconstructed using the Neurolucida software (MBF Bioscience). Confocal images were examined using a Zeiss Axiovert 200M microscope and imaged at 20X and 63X using LSM 510 inverted scanning confocal instrument. 3D Z-stacks of approximately 1um depth individual sections were visualized using the Zeiss LSM Confocal software. All cell counts were performed on optical confocal slices to ensure co-localization of fluorescent staining.
Supplementary Material
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary information is available at Gene Therapy’s website
References
- 1.Byrne BJ, Falk DJ, Clement N, Mah CS. Gene therapy approaches for lysosomal storage disease: next-generation treatment. Human gene therapy. 2012;23(8):808–15. doi: 10.1089/hum.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prickett M, Jain M. Gene therapy in cystic fibrosis. Translational research : the journal of laboratory and clinical medicine. 2012 doi: 10.1016/j.trsl.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Gavrilov K, Saltzman WM. Therapeutic siRNA: principles, challenges, and strategies. The Yale journal of biology and medicine. 2012;85(2):187–200. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Rossi JJ. Current progress in the development of RNAi-based therapeutics for HIV–1. Gene therapy. 2011;18(12):1134–8. doi: 10.1038/gt.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poewe W, Mahlknecht P, Jankovic J. Emerging therapies for Parkinson’s disease. Current opinion in neurology. 2012;25(4):448–59. doi: 10.1097/WCO.0b013e3283542fde. [DOI] [PubMed] [Google Scholar]
- 6.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nature reviews Neuroscience. 2012;13(4):251–66. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrell MS, Roth BL. Pharmacosynthetics: Reimagining the pharmacogenetic approach. Brain research. 2012 doi: 10.1016/j.brainres.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro MG, Frazier SJ, Lester HA. Unparalleled control of neural activity using orthogonal pharmacogenetics. ACS chemical neuroscience. 2012;3(8):619–29. doi: 10.1021/cn300053q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2012 doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nature neuroscience. 2011;14(1):22–4. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488(7411):379–83. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annual review of neuroscience. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Podolska K, Stachurska A, Hajdukiewicz K, Malecki M. Gene therapy prospects--intranasal delivery of therapeutic genes. Advances in clinical and experimental medicine : official organ Wroclaw Medical University. 2012;21(4):525–34. [PubMed] [Google Scholar]
- 14.Dayton RD, Wang DB, Klein RL. The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert opinion on biological therapy. 2012;12(6):757–66. doi: 10.1517/14712598.2012.681463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2012 doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492(7429):428–32. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484(7394):381–5. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nayerossadat N, Maedeh T, Ali PA. Viral and nonviral delivery systems for gene delivery. Advanced biomedical research. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bankiewicz KS, Leff SE, Nagy D, Jungles S, Rokovich J, Spratt K, et al. Practical aspects of the development of ex vivo and in vivo gene therapy for Parkinson’s disease. Experimental neurology. 1997;144(1):147–56. doi: 10.1006/exnr.1996.6401. [DOI] [PubMed] [Google Scholar]
- 20.Dissen GA, Lomniczi A, Neff TL, Hobbs TR, Kohama SG, Kroenke CD, et al. In vivo manipulation of gene expression in non-human primates using lentiviral vectors as delivery vehicles. Methods. 2009;49(1):70–7. doi: 10.1016/j.ymeth.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanftner LM, Sommer JM, Suzuki BM, Smith PH, Vijay S, Vargas JA, et al. AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters. Experimental neurology. 2005;194(2):476–83. doi: 10.1016/j.expneurol.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su X, Kells AP, Salegio EA, Richardson RM, Hadaczek P, Beyer J, et al. Real-time MR imaging with Gadoteridol predicts distribution of transgenes after convection-enhanced delivery of AAV2 vectors. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(8):1490–5. doi: 10.1038/mt.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, et al. An optogenetic toolbox designed for primates. Nature neuroscience. 2011;14(3):387–97. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jazayeri M, Lindbloom-Brown Z, Horwitz GD. Saccadic eye movements evoked by optogenetic activation of primate V1. Nature neuroscience. 2012;15(10):1368–70. doi: 10.1038/nn.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavanaugh J, Monosov IE, McAlonan K, Berman R, Smith MK, Cao V, et al. Optogenetic inactivation modifies monkey visuomotor behavior. Neuron. 2012;76(5):901–7. doi: 10.1016/j.neuron.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Frontiers in systems neuroscience. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;13(3):528–37. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Salegio EA, Samaranch L, Kells AP, Forsayeth J, Bankiewicz K. Guided delivery of adeno-associated viral vectors into the primate brain. Advanced drug delivery reviews. 2012;64(7):598–604. doi: 10.1016/j.addr.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116(1):201–11. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 30.Ribak CE. Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase. Journal of neurocytology. 1978;7(4):461–78. doi: 10.1007/BF01173991. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Richmond BJ, Murray EA, Saunders RC, Steenrod S, Stubblefield BK, et al. DNA targeting of rhinal cortex D2 receptor protein reversibly blocks learning of cues that predict reward. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(33):12336–41. doi: 10.1073/pnas.0403639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, et al. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl-channel. Neuron. 2007;54(1):35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 33.Kumar M, Keller B, Makalou N, Sutton RE. Systematic determination of the packaging limit of lentiviral vectors. Human gene therapy. 2001;12(15):1893–905. doi: 10.1089/104303401753153947. [DOI] [PubMed] [Google Scholar]
- 34.Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62(2):191–8. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krauze MT, Saito R, Noble C, Tamas M, Bringas J, Park JW, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. Journal of neurosurgery. 2005;103(5):923–9. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turchi J, Saunders RC, Mishkin M. Effects of cholinergic deafferentation of the rhinal cortex on visual recognition memory in monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(6):2158–61. doi: 10.1073/pnas.0409708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravel S, Richmond BJ. Dopamine neuronal responses in monkeys performing visually cued reward schedules. The European journal of neuroscience. 2006;24(1):277–90. doi: 10.1111/j.1460-9568.2006.04905.x. [DOI] [PubMed] [Google Scholar]
- 38.Bouret S, Richmond BJ. Relation of locus coeruleus neurons in monkeys to Pavlovian and operant behaviors. Journal of neurophysiology. 2009;101(2):898–911. doi: 10.1152/jn.91048.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosene DL, Roy NJ, Davis BJ. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1986;34(10):1301–15. doi: 10.1177/34.10.3745909. [DOI] [PubMed] [Google Scholar]
- 40.Lidow MS, Goldman-Rakic PS, Rakic P, Innis RB. Dopamine D2 receptors in the cerebral cortex: distribution and pharmacological characterization with [3H]raclopride. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(16):6412–6. doi: 10.1073/pnas.86.16.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(12):5720–4. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.