Abstract
A number of neuropsychiatric disorders, including Parkinson’s disease, schizophrenia, attention deficit hyperactivity disorder, and, to some extent, depression, involve dysregulation of the brain dopamine systems. The etiology of these diseases is multifactorial, involving genetic and environmental factors. Evidence suggests that inadequate levels of n-3 (omega-3) polyunsaturated fatty acids (PUFA) in the brain may represent a risk factor for these disorders. These fatty acids, which are derived from the diet, are a major component of neuronal membranes and are of particular importance in brain development and function. Low levels of n-3 PUFAs in the brain affect the brain dopamine systems and, when combined with appropriate genetic and other factors, increase the risk of developing these disorders and/or the severity of the disease. This article reviews the neurobiology of n-3 PUFAs and their effects on dopaminergic function. Clinical studies supporting their role in the etiologies of diseases involving the brain dopamine systems and the potential of n-3 PUFAs in the treatment of these disorders are discussed.
Keywords: dopamine, schizophrenia, attention deficit hyperactivity disorder, Parkinson’s disease, depression, omega-3, docosahexaenoic acid
1. Introduction
A growing body of literature supports the importance of polyunsaturated fatty acids (PUFA) in brain function. PUFAs in the n-3 and n-6 (omega-3 and omega-6) families play a number of important physiological roles as components of cell membranes, as signaling mediators, and as precursors of signaling mediators. The role of PUFAs, particularly n-3 PUFAs, in brain development is well established [1]. There is also increasing evidence that suboptimal levels of n-3 PUFAs, as a result of inadequate diet or metabolic deficiencies, appear to interact with genetic and environmental factors in the etiologies of a variety of neuropsychiatric disorders. This article reviews the literature on the roles of n-3 PUFAs in the modulation of brain dopamine systems. Evidence for the involvement of these fatty acids in neuropsychiatric disorders involving dopamine systems, such as Parkinson’s disease (PD), schizophrenia, and attention deficit hyperactivity disorder (ADHD) are discussed, as well the effects of n-3 PUFAs on dopaminergic alterations observed in depression. The potential of n-3 PUFAs, n-3 PUFA-derived mediators, or compounds that mimic their actions in the prevention and treatment of these disorders is also discussed.
2. N-3 PUFAs and t]he Brain
PUFAs are important dietary fats containing more than one double bond, and are named according to the number of carbons they contain, the number of double bonds, and the position of the first double bond from the methyl end. Long-chain PUFAs, which are at least 20 carbons in length, have important functional roles as components of membrane phospholipids and as signaling molecules in all tissues including the brain [2, 3]. Biologically important long-chain PUFAs include docosahexaenoic acid (DHA or 22:6n-3), a 22-carbon PUFA with six double bonds and the first double bond at the third carbon from the methyl end, is part of the n-3 class of PUFAs. DHA constitutes approximately 12–15% by weight of the total fatty acids in the human brain. The major species of n-6 PUFAs in brain is arachidonic acid (AA or 20:4n-6), which is 20 carbons in length, with four double bonds beginning with the sixth carbon from the methyl end, and which makes up 8–11% of the total fatty acids in the brain [4].
2.1. PUFA biosynthesis and brain accretion
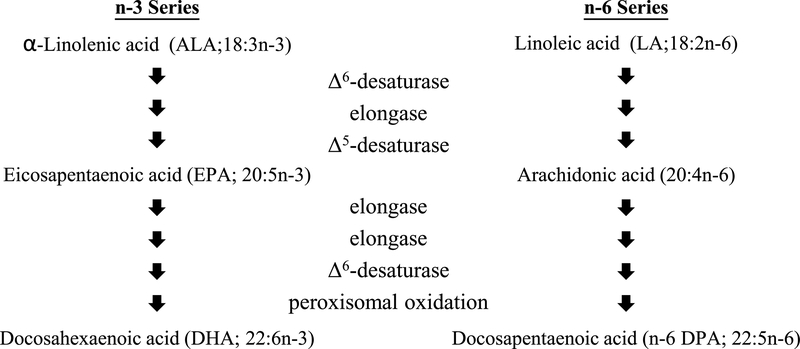
Mammals cannot synthesize long-chain n-3 and n-6 PUFAs de novo. Instead, DHA and AA must be consumed in the diet, or their essential fatty acid precursors α-linolenic acid (ALA or 18:3n-3) and linoleic acid (LA or 18:2n-6) must be provided. ALA is metabolized by desaturases and elongases to form DHA (Fig. 1). The same enzymes convert LA to AA, and ultimately to docosapentaenoic acid (n-6 DPA or 22:5n-6) [5]. Accordingly, the relative abundance of ALA and LA influences the amounts of DHA and AA produced [6].
Fig. 1.
Biosynthesis of n-3 and n-6 PUFA.
DHA plays an important role in brain growth and development. DHA is supplied from maternal sources to the fetus through the placenta, and to infants in breast milk. In humans, brain accumulation of DHA occurs primarily during late gestation and early childhood, with lifelong turnover [7, 8]. In rats, accumulation of DHA in the brain occurs in the last three days of gestation through weaning [9, 10]. Long-chain PUFAs are sometimes called “conditionally essential” because no gross deficiency disorders are known. However, visual and cognitive deficits in children fed a low n-3 diet, and conversely, benefits bestowed by supplementation with n-3 PUFAs during pregnancy have been reported [11–13].
2.2. Influence of diet and other factors on brain fatty acid composition
Inadequate brain accumulation of DHA during development, which can result from an n-3 PUFA-deficient diet or a metabolic deficit, causes the compensatory incorporation of n-6 DPA, an n-6 PUFA that is normally present in only trace amounts, thus qualitatively altering the composition of the fatty acids in the cell membranes [14, 15].
Diet can also affect tissue PUFA composition in adult animals. In mature animals, peripheral tissues are affected first, such that feeding adult rats an n-3-PUFA-deficient diet for 7 months decreased n-3 PUFA content of several organs, including liver, heart and testes, but not the brain [16]. However, in adult female rats or adult male mice, prolonged consumption of n-3 PUFA-deficient diets did decrease the percentage of DHA the brain [17, 18]. These observations suggest that regulation of brain fatty acid composition may differ between sexes and/or species.
Human dietary consumption of n-3 PUFAs varies widely. In contrast to some diets where the n-6:n-3 ratio can be as low as 2:1, Western diets are low in n-3 fatty acids, and have an n-6:n-3 ratio as high as 16.7:1 [19]. A high dietary n-6:n-3 ratio has been implicated in a variety of human diseases such as coronary artery disease, hypertension, diabetes, arthritis, osteoporosis, autoimmune disorders, cancer and psychiatric disorders, whereas low dietary n-6:n-3 or n-3 supplementation has beneficial effects [20–27]. Thus, there is high potential for individuals in Western countries to benefit from nutritional or pharmacological interventions targeting n-3 PUFAs or their derivatives.
Physiological states can also affect the PUFA status of the brain. For example, the demands of supplying DHA to offspring during pregnancy and lactation can deplete n-3 PUFAs in reproducing females. Although brain fatty acid composition after pregnancy has not been examined in humans, several studies found as much as a 50% decrease in maternal plasma DHA levels after pregnancy [28–30]. Studies in rats, where it is possible to exam the brain postpartum, indicate that if the diet contains inadequate n-3 PUFAs, the fatty acid compositions of both the brain and peripheral tissues are affected, and that gestating and nursing a single litter can result in a decrease in brain DHA content of as much as 25% [17, 31, 32].
Genetic variation in the ability to synthesize or use long-chain PUFAs may also affect brain PUFA status. Polymorphisms in genes such as the fatty acid desaturase (FADS) FADS1/FADS2 genes have been identified that cause reduced biosynthesis of long-chain PUFA [33, 34]. Other polymorphisms, such as the phospholipase A2G4A BanI polymorphism, and some SNPs of arachidonate 12-lipoxygenase (ALOX12), also affect utilization of long-chain PUFAs [35–38].
2.3. Roles of PUFAs in cell function
As elements of the cell membrane, long-chain PUFAs contribute to the membrane’s physicochemical properties, and thus impact the function of lipid rafts and membrane-bound proteins, such as receptors, transporters, and ion channels [6, 39, 40]. Notably, manipulation of membrane PUFA composition in vitro has been shown to alter the function and/or signaling of a variety of receptors, including dopaminergic, cholinergic, and GABAergic receptors, as well as the Na+/K+ ATPase [41–45].
AA is distributed evenly among cell types within the brain, and is an important signaling molecule in inflammatory cascades [46, 47]. It is preferentially cleaved from phospholipids in the brain by both cytosolic phospholipase A2 (cPLA2) and secretory phospholipase A2 (sPLA2). A portion of the released AA can by metabolized by cyclooxygenases (COX) and lipoxygenases (LOX) to form a class of compounds known as the eicosanoids, which include a variety of mediators of cellular activity, including prostaglandins, leukotrienes and lipoxins. Most AA-derived metabolites have pro-inflammatory functions, although AA contributes to mediators with a broad range of functions in signaling, memory, and learning modulation [48, 49].
DHA is highly enriched in neuronal and synaptic membranes, suggesting an important role in neural cell signaling [46]. It is incorporated preferentially into phosphatidylethanolamine and phosphatidylserine on the inner membrane layer of synapses, and its steric incompatibility with cholesterol drives the formation of either DHA- or cholesterol-rich lipid rafts. These rafts serve as protected microdomains and function in compartmentalization of various cell signaling molecules [46]. DHA also affects membrane fatty acid chain fluidity, ion permeability, elasticity, protein function, phase behavior, and fusion [50–52]. In addition, it is cleaved from the phospholipids by the inducible DHA-selective calcium-independent phospholipase A2 (iPLA2) [53]. Once unesterified, DHA acts as a ligand for a variety of receptors, such as the peroxisome-proliferator-activated receptor (PPAR), the retinoid X receptor (RXR) and the toll-like receptors (TLR), as well as being metabolized by COX and LOX to form docosanoids such as the resolvins, protectins, maresins, neuroprotectin D1 (NPD1), and the recently discovered electrophile oxo-derivatives (EFOXs) [49, 54–58]. The resolvins are a class of anti-inflammatory compounds produced by the COX-2 pathway in the presence of aspirin [59, 60]. NPD1 is a peptide that is formed by PLA2 lipoxygenase from free DHA, and has been shown to induce anti-apoptotic B-cell lymphoma-2 (Bcl-2) proteins, inhibit pro-apoptotic Bcl-2 proteins, and suppress inflammatory gene expression [61]. Like DHA, NPD1 affects membrane structure and stability, and alter the balance of n-3 to n-6 PUFAs in the membrane, which in turn influences the AA cascade by suppression of COX-2. Maresins, DHA-derived metabolic products with anti-inflammatory properties similar to the resolvins and NDP1, are produced by macrophages under inflammatory conditions [62]. COX-2 EFOX metabolites act as anti-apoptotic Nrf-2 activators, PPAR-γ agonists, and inhibitors of cytokine and nitric oxide (NO) production [56]. DHA also has the ability to inhibit TLR4, an important pro-inflammatory factor, and various nuclear receptor initiators of the nuclear factor kappa-light-chain-enhancer of activated B cells (Nf-κB)-mediated anti-inflammatory response [54, 63]. Accordingly, in clinical studies, n-3 fatty acid treatment decreased cytokine production, and COX-2 activity [54, 64]. Conversely, under oxidative stress, DHA is oxidized into neuroprostanes, a class of prostaglandin-like compounds formed without COX. These compounds trigger reactive oxygen species (ROS) formation at both sides of the phospholipid membrane [65]. DHA also activates syntaxin-3, a crucial factor in neuron growth and regeneration, which may contribute to DHA’s role in optimal brain growth and development [4]. Stimulation of the Src-mediated calcium-induced growth and phosphatidylinositol3 kinase (PI3K)-Akt-protein kinase C (PKC) cascades are also implicated in the beneficial effects of n-3 PUFAs in brain [66].
Although not as well studied as DHA, eicosapentaenoic acid (EPA or 20:3n-3), the 20-carbon n-3 precursor to DHA. Although the EPA incorporated into brain phospholipids represents only about 1–2% of total brain fatty acids [67], the fatty acid has a number of important biological activities. Like DHA, EPA forms eicosanoids, most of which are less inflammatory than their AA-derived counterparts, as well as anti-inflammatory mediators such at the E-series resolvins [68]. EPA competes with AA for the same enzymes, so the n-6:n-3 ratio influences EPA metabolite production. Under conditions of cell membrane damage (such as oxidative stress), abundant intracellular calcium and inflammatory stimuli, PLA2 and COX-2 transcription is upregulated, leading to increased production of AA metabolites [49]. It also inhibits COX-2 expression and also acts as an inhibitor of COX-2, activates the PI3-K-Akt pathway, stimulates myelinogenesis, and is a PPAR ligand [69–73].
2.4. Mechanisms by which n-3 PUFAs may influence development and maintenance of dopaminergic neurons
DHA is one of a number of ligands for the nuclear receptor RXR [55], and as such, dietary DHA intake may play a role in RXR regulation. The RXR family consists of three main isotypes: RXR-α, RXR-β and RXR-γ. RXR-α is expressed in liver, kidney, spleen, placenta, epidermis, and visceral tissue; RXR-β is expressed in nearly every body tissue; and RXR-γ is mostly in muscle and brain tissue. The physiological roles of RXR are complex and not completely understood, but RXR isoforms are known to be involved in muscle metabolism, insulin resistance, atherosclerosis and cholesterol metabolism, apoptosis, and a variety of differentiation processes, including neuronal development [74].
In the dopaminergic system, RXR is a crucial developmental and survival factor, and acts in conjunction with its NR4A1 partners nuclear receptor-related-1 protein (Nurr1) and nerve growth factor 1B (Nur77) [75]. RXR forms heterodimers with many other nuclear receptors, implicating it in multiple transcription pathways. In particular, RXR heterodimerizes with both Nurr1 and Nur77. Nurr1 is crucial to dopaminergic neuronal development, as well as regulation of the hypothalamic-pituitary-adrenal axis [76]. Nurr1 is expressed chiefly in the substantia nigra, ventral tegmental area, and limbic areas, where dopamine plays a vital role. Expression of these proteins peaks in the embryo, yet remains high in dopaminergic neurons throughout the lifespan, with Nurr1 expressed in 96% of adult substantia nigra neurons [77–79]. Nurr1 regulates the transcription of tyrosine hydroxylase and the dopamine transporter by binding to the nerve growth factor 1B response element (NBRE) sequence in the 5’-untranslated region, and binds to RXR. The resulting heterodimer plays an important role in the development of neurons [80, 81]. Dopaminergic terminal fields express both RXR-β and RXR-γ isoforms. Mice deficient in these receptors have impaired motor function, and decreased mRNA transcription of D1 and D2 receptors in the striatum [82]. Knockout RXR-γ animals have also been found to have abnormalities in synaptic plasticity and learning [74]. Although the specific role(s) of n-3 PUFAs in RXR-mediated effects on the dopaminergic systems remain to be elucidated, it is likely that these fatty acids influence dopaminergic neuronal development and survival through these mechanisms.
3. N-3 PUFAs and Parkinson’s disease
Parkinson’s disease is a progressive neurodegenerative disorder affecting 0.1–0.2% of the general population and 2% of people over 65 years of age [83]. Major clinical signs of PD are bradykinesia, resting tremor, rigidity, postural instability, and “masked” facial expression [84]. The neuropathology of PD is histologically characterized by loss of melanin-pigmented neurons in dopaminergic regions of the brain, most notably the substantia nigra pars compacta, and the presence of Lewy bodies containing abnormal α-synuclein aggregations. The etiology of the majority of cases is unknown, but a minority, especially early-onset cases, are attributable to familial mutations in genes such as Park 1, which encodes α-synuclein [85–90]. The variability in clinical presentation, progression of disease, and age of onset of the disease suggest a multifactorial etiology likely occurring as “multiple hits” in which perinatal anomalies and insults, genetic polymorphisms, and postnatal environmental factors lead to a reduction in dopamine neuron numbers or function, thus making an individual more susceptible to subsequent stressors, and therefore more likely to develop PD [91–93]. Several environmental toxins, notably metals and agricultural agents may contribute to incidence of PD, as well as certain viral infections. A number of cases were also caused by exposure to the nigrostriatal-selective neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [94–99]. Current treatments for PD include pharmacological management, primarily with drugs that mimic or augment dopaminergic neurotransmission [83]. Surgical treatment, deep-brain stimulation, and physical therapy are also used; however, there is no cure, and all current treatments eventually become ineffective at managing symptoms [100, 101]. Therefore, understanding the foundations of PD, including the effects of diet on the etiology and treatment of PD, may prove crucial to developing treatments to minimize suffering of PD patients.
Oxidative stress appears to be central to the pathogenesis of PD. The oxidative metabolism of dopamine can yield hydrogen peroxide and other ROS, which contribute to lipid peroxidation [102]. Increased concentrations of the lipid peroxidation products malondialdehyde and hydroperoxide have been found in the substantia nigra of PD patients [103]. Another cell-damaging product of lipid peroxidation, 4-hydroxynonenal, has also been detected in PD dopaminergic neurons [104]. The substantia nigra, even under ideal conditions, is highly prone to oxidative stress, being rich in both reactive dopamine and in peroxidation-prone PUFAs, which can lead to the formation of dopamine adducts. Importantly, an AA-derived adduct, hexanoyl dopamine, was found to be notably cytotoxic in human SH-SY5Y neuroblastoma cells expressing monoamine transporters [105, 106], suggesting that it, and perhaps other PUFA-dopamine products, may play an important role in the pathogenesis of PD.
3.1. Clinical studies
A few studies have examined the involvement of PUFAs in PD patients. The prospective cohort Rotterdam study evaluated 5,289 subjects age 55 and older to examine the relationship between intake of PUFAs and the incidence of PD. In that study, a high intake of PUFAs was associated with lower risk of PD [107]. Likewise, a case-control study of n-3 PUFA consumption in individuals with and without occupational exposure to pesticides found the incidence of PD to be inversely related to dietary n-3 PUFA content, and that higher consumption of n-3 PUFAs reduced the PD risk associated with pesticide exposure [108]. Another case-control study in a Japanese population, however, found that n-3 PUFA intake and dietary n-6:n-3 ratio did not affect risk of PD, although increased AA intake, interestingly, increased risk [109]. Finally, a randomized, double-blind, placebo-controlled clinical trial in PD patients found that treatment with flaxseed oil, a source of the n-3 PUFA ALA, plus vitamin E for 3 months improved the Unified Parkinson’s disease rating state score, as well as increased total antioxidant capacity and glutathione concentrations, and decreased high-sensitivity C-reactive protein levels [110]. Accordingly, most of these studies suggest that increased consumption of n-3 PUFAs may be beneficial for both the prevention and treatment of PD.
3.2. Preclinical studies
Animal studies also generally indicate beneficial effects of n-3 PUFAs in PD and point towards underlying mechanisms. Of note, consumption of adequate n-3 PUFAs appears to be important for the survival and health of dopaminergic neurons. Rats raised from conception on an n-3 PUFA-deficient diet had 33% fewer neurons in the substantia nigra and ventral tegmental area expressing the dopamine marker tyrosine hydroxylase at adulthood compared to controls [111]. Similarly, a study of rats raised from conception on a diet that resulted in a 65% decrease in brain n-3 PUFA content found a 40% decrease in the number of dopamine neurons in the substantia nigra, as well as increased lipid peroxidation and decreased catalase activity in both the substantia nigra and striatum [112]. These decreases in the number of dopaminergic neurons, as well as increased indices of oxidative stress, may increase susceptibility to other insults, consistent with the “multiple hit” hypothesis.
Studies in experimental models of PD also suggest that n-3 PUFAs may mitigate the effects of dopaminergic neurotoxins. In rat studies using the unilateral 6-hydroxydopamine (6-OHDA) lesion model of PD, various treatments with n-3 PUFAs reduced the effects of 6-OHDA, resulting in decreased agonist-induced rotation. In addition, the lesioned striata of the n-3 PUFA-treated animals had higher levels of tyrosine hydroxylase, dopamine, and synapsin-1 expression, and lower lipid peroxidation, nitrite levels, density of inducible nitric oxide synthase (iNOS)-immunoreactive cells, microglia and astrocyte reactivity compared to controls [113–115]. Likewise, n-3 PUFA treatments in MPTP-treated rats or mice improved motor performance, showed smaller reductions in MPTP-induced dopamine cell loss, striatal dopamine content, COX-2 activity, Nurr1 and dopamine transporter mRNA levels, and modulated Akt and Bcl-2 pathways [116–119]. DHA supplementation in an MPTP model also increased glial cell-derived neurotrophic factor (GDNF) and neurturin, both important trophic factors involved in dopaminergic neuron health [120]. Likewise, in MPTP-treated cynomolgus monkeys, DHA administration reduced levodopa-induced dyskinesias when given either before or after MPTP administration [121]. Interestingly, in the MPTP-probenecid mouse model, pretreatment with an ethyl-EPA-enriched diet decreased the MPTP-induced hypokinesia, but did not reduce dopaminergic cell loss [122]. Finally, administration of the DHA-derived resolvin D2 in rats treated with the dopaminergic neurotoxin lipopolysaccharide prevented the activation of the TLR4/Nf-κB pathway [123]. Similar neuroprotective effects have also been observed in primary mesencephalic primary cultures [124]. Taken together, evidence from these animal studies points to the ability of n-3 PUFAs to modulate factors involved in neuroprotection in PD models, suggesting that increased n-3 PUFA intake could prevent or attenuate the progress of PD in humans.
Other studies, however, found effects of PUFAs that may be detrimental in PD. The double bonds in PUFAs make them susceptible to lipid peroxidation in pro-oxidative environments, and several studies have implicated high n-3 PUFA levels with increased oxidative damage in PD and PD models. For example, the DHA-derived lipid peroxidation products neuroprostanes were increased in the brains of advanced PD patients [65]. Also, dopamine can react in vitro with fatty acid peroxides to form 6-OHDA, providing a potential mechanism for endogenous production of 6-OHDA in the pathogenesis of PD [125]. In one 6-OHDA study in mice, intraperitoneal injection of a DHA ethyl ester resulted in increased levels of lipid peroxidation in the striatum [126]. In A53T α-synuclein mutant mice, accumulation of both soluble and insoluble α-synuclein, neuritic injury, and astrocytosis were increased after DHA supplementation, whereas these effects were attenuated by decreased dietary DHA content[127]. In addition, an in vitro experiment with oligodendrocytes transfected with A53T α-synuclein that were supplemented with DHA for 3 days before subjection to oxidative stress showed enhanced aggregation of α-synuclein [128]. Furthermore, PUFAs in the brain can form neurotoxic adducts with dopamine [105]. Since the most toxic of these adducts was formed from an n-6 PUFA, the n-6:n-3 ratio in the diet may have an effect on development of these adducts and the damage they can cause.
Some of the effects of n-3 PUFAs in PD may result in activation of RXR and/or Nurr1. The apparent complexity of the relationships among the nuclear receptors involved in nigral neurogenesis make it difficult to isolate the effects of each, but it is apparent that these developmental and survival factors may play an important role in the susceptibility of adult neurons under conditions of neurodegenerative disease. For example, stimulation of RXR by synthetic RXR ligand LG100268 and RXR-Nurr1 ligand XCT0139508 in rat dopaminergic cell cultures protected against oxidative stress induced by 6-OHDA and hypoxia; however, RXR expression alone could not protect the cells when treated with kainic acid and hydrogen peroxide; rather, neuroprotection against these was selective to Nurr1-expressing cells, still implicating RXR as its dimerization partner [129]. Notably, when administered to rats treated with 6-OHDA, IRX4204, a selective RXR agonist, and bexarotene, a cancer drug that binds to Nurr1-RXR heterodimers, attenuated the behavioral and neurochemical deficits in that PD model [130, 131]. Bexarotene also restored signaling by Ret, the canonical GDNF receptor, in mesencephalic mouse neurons overexpressing α-synuclein, suggesting that activation of RXR-Nurr1 may counteract the effect α-synuclein in PD [132, 133]. Nevertheless, the specific involvement of RXR in the effects of n-3 PUFAs in PD remain to be definitively determined.
3.3. Implications for prevention and treatment
The potential for n-3 PUFAs to contribute to the processes involved in neuroprotection and neuronal repair, or alternately to the increased lipid peroxidation found in PD, suggest the possibility for a role of these fatty acids in both the prevention and treatment of the disease. Furthermore, n-3 PUFAs appear to influence survival and function of dopaminergic neurons during neurodevelopment as well as at adulthood, suggesting a variety of opportunities for intervention. However, while evidence for the role of n-3 PUFAs in PD remains promising, it is currently inconclusive, and additional clinical and preclinical studies are necessary to determine the effects of n-3 PUFAs in PD.
4. N-3 PUFAs and Schizophrenia
Schizophrenia is a complex disorder involving symptoms such as hallucinations, delusions, disorganized thought, blunted or inappropriate affect, social withdrawal, and cognitive dysfunction [134, 135]. The disease affects roughly 1% of the population worldwide. Its etiology is unclear but appears to involve the interaction of genetic and epigenetic factors that result in aberrant neurodevelopmental processes [136–139]. Several studies suggest the second trimester of gestation as the period during which incidents, such as maternal malnutrition or influenza infection, increase the risk of developing schizophrenia later in life [140–142]; however, events in later development may also contribute.
Although schizophrenia involves dysfunction of a variety of neurotransmitter systems, including the glutamatergic, serotonergic, and GABAergic systems, the brain dopamine systems play an important role [143, 144]. Of note, all clinically effective antipsychotic drugs are antagonists at the D2 dopamine receptor, and their affinity for the D2 receptor correlates with their clinical potency [145]. These, and other observations, led to the formulation of the “Dopamine Hypothesis of Schizophrenia”, which theorizes that the psychotic symptoms of schizophrenia result from hyperactivity of the mesolimbic dopamine pathway. Mesolimbic hyperactivity in schizophrenics has yet to be definitively demonstrated[143]; however, the assertion is supported by the effects of drugs, such as amphetamine, that can produce psychotic symptoms [146]. Likewise, increased density of striatal D2 receptors has been repeatedly observed in schizophrenics and does not appear to be an artifact of antipsychotic drug treatment [147]. In addition, schizophrenics exhibit hypofunction of the prefrontal cortex, which appears to involve decreased activity of mesocortical dopamine neurons [147–149], in particular reduced activity of the cortico-striatal-thalamic loop circuit, which, in normal subjects, is activated by salient stimuli [150]. Decreased expression of Nur77 and Nurr1 have also been reported in the dorsal lateral prefrontal cortex of schizophrenics [151].
4.1. Clinical studies
A number of clinical studies suggest the contribution of n-3 PUFAs in schizophrenia. Studies of erythrocytes and other peripheral tissues of schizophrenics demonstrate alterations in the relative abundance of a variety of long-chain PUFAs, and meta-analyses support decreased levels of DHA and AA in erythrocyte membranes of schizophrenics compared to controls [152, 153]. Furthermore, levels of n-3 PUFAs were inversely correlated with the severity of schizophrenic symptoms, particularly negative symptoms [154–157]. Similarly, in individuals at ultra-high risk of psychosis, low peripheral tissues levels or low dietary consumption of n-3 PUFAs, which can lead to low tissue levels, were associated with electroencephalographic (EEG) alterations indicating poorer alertness and vigilance and increased risk conversion into psychosis [158, 159].
In addition to the differences in the relative abundance of various fatty acids observed in peripheral tissues of individuals with schizophrenia, several alterations in PUFAs and PUFA-related mediators have been found in the brain. Notably, lower concentrations of fatty acids including DHA and AA were found in the orbitofrontal cortex of schizophrenics relative to controls [160, 161], although no differences in these fatty acids were found in the prefrontal cortex or the amygdala [162, 163]. Post-mortem brains of schizophrenics also exhibited higher levels of iPLA2, which cleaves DHA from the membrane, and Δ6 desaturase, an enzyme involved in PUFA biosynthesis [164–166]. Schizophrenia or schizophrenia-related behaviors were also associated with several PUFA-related genes such as Acyl-CoA synthetase medium-chain family member 1 (ASCM1), fatty acid binding protein 7 (Fabp7), the phospholipase A2G4A BanI polymorphism, and certain single nucleotide polymorphisms (SNP) of arachidonate 12-lipoxygenase (ALOX12) [35–38]. In addition, RXR, along with its dimerization partner Nur77, has been implicated in modulating a number of motor side effects of antipsychotic drugs, and decreased Nur77 has observed in post-mortem brains from schizophrenic patients [75], suggesting another potential mechanism by which n-3 PUFAs could play a role in schizophrenia.
A relatively small number of clinical trials have examined the efficacy of n-3 PUFA preparations in schizophrenia. Although one review of these clinical trials was unable to draw firm conclusions regarding the therapeutic utility of n-3 PUFA supplements in this disease [167], several studies, as well as a meta-analysis, indicate that while n-3 PUFA supplements may enhance the effects of antipsychotic drugs, the n-3 PUFA treatments were not sufficiently effective for use as monotherapy [168–172]. Another meta-analysis found that n-3 PUFAs appeared to be more efficacious during the prodrome and first episode, rather than in chronic patients or for preventing relapse [173]. Finally, a trial in individuals with subthreshold psychotic states found that n-3 PUFAs prevented progression to a psychotic disorder [174].
4.2. Preclinical studies
Depending on the specific manipulation and the point in development when it is made, changes in brain phospholipid fatty acid composition produce neurobiological effects that may be relevant schizophrenia [175], as well as other conditions such as ADHD (see below). In particular, feeding an n-3 PUFA-deficient diet for two generations, which produced adult rats with a 70% reduction of brain DHA content, produced a number of effects on the dopamine systems, many of which were similar to those observed in schizophrenics [176]. Dopaminergic alterations in the frontal cortices of these animals included reduced levels of dopamine-immunoreactive vesicles, the vesicular monoamine transporter VMAT2, and the D2 dopamine receptor [177–179], suggesting decreased activity of the mesocortical projection. Conversely, the treatment also resulted in an apparent net increase in activity of the mesolimbic projection as indicated by increased basal dopamine release and D2 dopamine receptors density in the nucleus accumbens, and increased tyrosine hydroxylase activity in the ventral tegmental area [180, 181]. In contrast, the nigrostriatal system of these rats did not appear to be affected [177, 182]. In another multigenerational rat model that reduced the concentration of DHA in brain phospholipids by about 80%, the decrease in brain DHA content resulted in different effects at adolescence and adulthood, with adolescent rats exhibiting increased expression of tyrosine hydroxylase in the dorsal striatum, and adults exhibiting decreased tyrosine hydroxylase levels and increased levels of VAMT2 [183]. Other studies used a model in which an n-3 PUFA-deficient diet was fed during prenatal and early postnatal development in a single generation, which increased the ratio of n-6 DPA:DHA in brain tissue phosphatidylcholine by 6.8-fold compared to controls. These studies found dopaminergic alterations such as decreased levels of tyrosine hydroxylase and VMAT2 in the hippocampus, and increased expression D1 and D2 receptors in the striatum and cortex [184, 185]. However, a pre- and postnatal diet-induced decrease in brain DHA of about 20%, resulted in no alterations in either the concentration of dopamine or the densities of D1 or D2 receptors in the nucleus accumbens, the frontal cortex, or the striatum [186]. These observations suggest that effects of variation in the availability of n-3 PUFAs on the brain dopaminergic systems varies depending on the magnitude and timing of the change in brain DHA status, and may thus, along with an individual’s genotype, influence that individual’s susceptibility to a particular neuropsychiatric disorder.
Similar to the variety of effects of n-3 PUFA manipulations on dopaminergic neurochemistry, the behavioral changes resulting from such treatments also differ between studies. Several investigations reported increased locomotor activity in rats raised on n-3 PUFA-deficient diets [183, 186–189], although the effects varied depending on magnitude of the decrease in brain DHA and the age of the rats [186, 187]. Likewise, compared to those fed a control diet, rhesus monkeys with long-term deficiency of n-3 PUFAs showed a higher level locomotor activity, as well as more stereotyped behavior [190]. However, another study of adult rats found that those with a 70% decrease in brain DHA displayed less exploratory behavior in a novel environment than controls [191]. Accordingly, changes in n-3 PUFA status affect the dopaminergic systems involved in motor function; however, the specific effects appear to differ depending on the specific treatment. Furthermore, a study in which brain DHA was restored by feeding a rats a DHA-supplemented diet at adulthood found that the developmental deficits in brain DHA content were fully reversible; however, only some of the effects of the dopamine-related behavioral effects of inadequate DHA accumulation during early development were reversed [188]. Thus, taken together, these observations suggest that developmental processes and brain DHA status interact to produce at least some of the observed effects.
N-3 PUFAs also affect behavior in several animal models of schizophrenia. In a study of amphetamine-induced behavioral impairment as a rat model of schizophrenia, treatment with DHA and EPA reduced the amphetamine-induced deficits in several behavioral tests and also decreased lipid peroxidation and cytokine release. In that study, the n-3 PUFAs were most effective when co-administered with the antipsychotic drug risperidone, thus supporting the use of n-3 PUFAs as an adjunct treatment [192]. In addition to motor function, sensorimotor gating, which is altered in schizophrenia [193], is affected after experimental manipulation of brain DHA in animal models. For example, in rats raised from conception on diets varying in n-3 PUFA content to produce varying concentrations of brain DHA, those with lowest brain DHA levels exhibited deficits in prepulse inhibition compared with those with the highest brain concentrations of DHA [194]. Likewise, in the ketamine model of schizophrenia, behaviors such as impaired social interactions, inhibition of startle response, and ketamine-induced increases in acetylcholinesterase were decreased by n-3 PUFAs [195–197]
In addition to their effects of dopaminergic function, n-3 PUFAs modulate glutamatergic neurotransmission, which is also aberrant in schizophrenia [198]. Most notably, in mice with reduced levels of NMDA receptors, n-3 PUFAs failed to attenuate any of the behavioral deficits resulting from NMDA receptor hypofunction; however, the NMDA receptor knock-down mice had elevated brain concentrations of n-6 PUFAs regardless of the n-3 PUFA content of their diet, and an n-3 PUFA-deficient diet increased their deficits in executive function [199]. Likewise, in an in vitro study, addition of free, but not membrane-bound, DHA to cultured rat astrocytes or rat brain membrane preparations, decreased glutamate uptake [200].
Finally, some antipsychotic drugs appear to affect n-3 PUFA homeostasis. Administration of risperidone to rats for 30 days resulted in higher erythrocyte and brain DHA concentrations compared to controls [201]. However, brain DHA status was not altered by treatment with haloperidol or clozapine for 21 days [67], suggesting either differential effects of these drugs or an inadequate treatment period or dose in the latter study.
4.3. Implications for prevention and treatment
Taken together, these clinical and preclinical studies suggest that inadequate availability of n-3 PUFAs during key periods of early brain development may lead to altered dopaminergic function consistent with at least some of the abnormalities observed in schizophrenia. Combined with observations of reduced n-3 PUFAs in the brains and other tissues of schizophrenics, this observation supports the need for adequate nutrition during fetal and neonatal life, which is already acknowledged as being important for optimal brain development. The potential for n-3 PUFAs or n-3 PUFA-derived mediators as treatments for schizophrenia is less clear. The few studies done to date suggest that at least some dopamine-related behavior deficits of developmental n-3 PUFA deficiency are not reversible. The relative lack of efficacy of n-3 PUFA treatments in patients with symptomatic schizophrenia also suggests that n-3 PUFAs may prove more useful as a preventative intervention, though use as a an adjunct to antipsychotic medications, particularly early in treatment appears to have some promise.
5. N-3 PUFAs and ADHD
ADHD is a clinically heterogeneous disorder characterized by inattention, hyperactivity, and impulsivity that affects 2–5% of the general population [202, 203]. It is five-times more prevalent in boys than girls [204]. Although initially described as a childhood disorder, attention deficits can continue into adulthood [205]. The heritability of ADHD is about 80%, indicating a strong genetic component; however, other causative factors are likely involved [202].
Although the underlying pathology of ADHD is not fully understood, it appears to involve dysregulation of the brain dopaminergic and/or noradrenergic systems [206]. Neuropsychological and neuroimaging studies in patients with ADHD indicate frontolimbic dysfunction involving brain regions such as the cortico-striato-thalamo-cortical loop [207–212]. The behavioral symptoms of ADHD are typically treated with psychostimulants, such as methylphenidate or amphetamine, which improve attention and decrease motor activity. These drugs increase synaptic availability of dopamine and norepinephrine and have been shown to enhance the inhibitory effects of frontal cortical activity on subcortical structures [213]. Taken together, these clinical observations and experimental results suggest that ADHD involves decreased activity of dopaminergic and/or noradrenergic projections from the ventral tegmental area, substantia nigra, and locus coeruleus that innervate the frontosubcortical brain regions. A role for dopamine in the pathogenesis of ADHD is further supported by animal and genetic studies. Rats with neonatal 6-OHDA lesions exhibit hyperactivity [214]. Mutant mice lacking the dopamine transporter also exhibit increased activity and are considered an animal model of the disease [215]. Genetic association studies in ADHD implicate polymorphisms of the D2 and D4 dopamine receptors, dopamine transporter, and dopamine β-hydroxylase genes [216–218]. These studies also implicate the D4 receptor gene in novelty seeking, which could contribute to the impulsivity observed in ADHD [219, 220].. Furthermore, a recent systematic review of brain imaging-genetics studies identified 62 candidate genes, most of which were dopamine-related [221]. A single nucleotide polymorphism FADS2, a desaturase enzyme involved in the biosynthesis of long-chain PUFAs, has also been associated with ADHD [222].
5.1. Clinical studies
At least a subset of children and adults with ADHD exhibited altered fatty acid compositions of plasma or erythrocytes compared to normal controls, including lower levels of DHA [223–226]. These findings were supported by meta-analyses that found either low n-3 PUFA levels or increased ratio of n-6:n-3 PUFAs in ADHD [227, 228]. Erythrocyte DHA levels, which appear to correlate with brain levels [229], were reduced to 70–85% of normal controls [223, 224, 230]. Moreover, patients with low plasma or erythrocyte levels of DHA and other long-chain PUFAs had significantly more ADHD-related behavioral symptoms than those with higher DHA levels [230]. In the studies that also examined dietary n-3 PUFA content, consumption by individuals with ADHD was considered adequate [231, 232]; however, another study found that children with ADHD had higher than normal exhalant levels of ethane, suggesting increased oxidative metabolism of n-3 PUFAs [233], which could necessitate increased dietary consumption of these fatty acids.
Clinical trials of fatty acid preparations in ADHD have yielded varying results. Treatment with n-3 PUFA preparations increased serum and/or erythrocyte EPA and DHA in adults and children with ADHD [228, 234, 235]. Furthermore, in children treated with n-3 or n-3/n-6 PUFA preparations, the resulting increases in plasma or erythrocyte EPA and n-6 DPA correlated with decreased ADHD symptoms [236, 237]. Although studies varied with respect to the PUFA preparations used, dose, and duration of treatment, a number of randomized, controlled trials with various n-3 or n-3/n-6 preparations in children with ADHD also reported improvement in symptoms [235, 238–244]. However, other randomized, controlled trials failed to find a beneficial effect of these treatments on ADHD symptoms in children [245–249], even in subjects in whom the treatment resulted in increased erythrocyte EPA and DHA concentrations [250]. Meta-analyses of the effects of n-3 PUFA treatments in ADHD have yielded varying results ranging from a small beneficial effect [227, 251, 252] to effective only on some symptoms in a subpopulation of patients [253], to negative [254, 255], to inconclusive [256]. Concomitant treatment with methylphenidate and either an n-3 or n-3/n-6 PUFA preparation was not superior to methylphenidate alone [257, 258].
5.2. Preclinical studies
As discussed above for schizophrenia, inadequate availability of n-3 PUFAs during development can result in neurochemical alterations consistent with frontocortical dopaminergic hypoactivity, as well increased locomotor and stereotyped activity (see 4.2). In addition, rats with reduced amounts of brain DHA during development exhibited impaired performance compared to controls on delayed matching-to-place in a water maze, in an active avoidance test, and in acquisition of olfactory discrimination [259–262]. Initiation of a diet low in ALA as late as one month of age, also decreased performance of rats in the Morris water maze [261]. Moreover, the spontaneously hypertensive rat (SHR), a putative model of ADHD [263], has lower brain DHA content than its less active progenitor strain, the Wystar Kyoto rat [264]. In the SHR rat model, locomotor activity was inversely related to the dietary n-3 PUFA content [265]. Lower n-3 PUFA consumption also resulted in higher brain total creatine levels which where correlated hyperactivity in a familiar environment [266]. In addition, feeding a diet enriched in n-3 PUFAs increased striatal turnover of dopamine and serotonin, increased attention, and decreased hyperactivity and impulsiveness of male, though not female, SHR rats [267]. Thus, lower brain DHA content during development appears to result in animals that exhibit increased activity, inattention, and impaired cognitive function similar to that associated with ADHD, as well as the frontocortical dopaminergic hypofunction associated with the disorder.
5.3. Implications for prevention and treatment
As with schizophrenia, the ADHD-like effects of inadequate availability of n-3 PUFAs during early brain development on behavior and dopaminergic function combined with the clinical observations of reduced levels of n-3 PUFAs in individuals with ADHD support a potential role for n-3 PUFAs or n-3 PUFA-derived mediators in the disorder. Again, this observation supports the need for adequate nutrition during fetal and neonatal life, which is already acknowledged as being important for brain development. Although the clinical trials with n-3 PUFAs in ADHD have been somewhat more promising than those in schizophrenia, the potential for n-3 PUFAs or n-3 PUFA-derived mediators as treatments for ADHD remains to be determined, and again likely depends to a great on extent on the reversibility of n-3 PUFA deficiency-induced effects that contribute to the disorder.
6. N-3 PUFAs and Dopaminergic Aspects of Depression
Depression is characterized by symptoms such as depressed mood, lack of interest, anhedonia, feelings of worthlessness or guilt, and suicidality [268]. It has a lifetime prevalence of about 20% and occurs roughly twice as often in women, with the postpartum period representing a time notable vulnerability for women [269–271]. Although the pathophysiological basis of the disease remains to be fully understood, genetic and environmental factors are involved [272, 273]. Extensive clinical and animal evidence implicates the serotonin system, brain-derived neurotrophic factor (BDNF) in the hippocampus, and the hypothalamic-pituitary-adrenal axis as key systems affected in disorder [273–278]. A number of studies have examined the potential role of altered brain n-3 PUFA status, particularly decreased DHA, in depressive illnesses. These studies show that brain n-3 PUFA status affects these mediators in a manner consistent with an etiologic role in the disease. Furthermore, clinical trials indicate the potential therapeutic benefit of n-3 PUFA supplements in major depression (for extensive review of effects on the serotonin system, brain-derived neurotrophic factor (BDNF) in the hippocampus, and the hypothalamic-pituitary-adrenal axis, and clinical trials see: [279–281]). In view of the focus of this review on the effects of PUFAs on the dopaminergic systems, only the effects of PUFAs specifically on dopaminergic alterations relevant to depression are discussed here.
Altered dopaminergic function appears to contribute to the pathogenesis of depression, particularly the symptoms of anhedonia and decreased motivation [282, 283]. Notably, decreased engagement in reward-oriented behaviors is observed in animals with decreased activity of the mesolimbic dopamine projection [282, 283]. Consistent with these behavioral observations, levels of D2 dopamine receptors or D2 receptor mRNA were found the ventral striata not only of depressed women, but in several rat depression models including the Wystar-Kyoto rat, the socially-isolated Flinders sensitive line rat, and chronic mild stress-induced anhedonia [284–288]. The density of striatal dopamine transporters was also lower in depressed patients as assessed by single-photon emission computed tomography (SPECT) [289]. In addition, cerebrospinal fluid concentrations of the dopamine metabolite homovanillic acid (HVA) were lower in depressed patients and suicide victims, and were associated with depressed mood and psychomotor retardation [290–292]. Depression is also common in Parkinson’s disease, often developing prior to the onset of motor symptoms [293–296], and depression-related behaviors, such as anhedonia and increased immobility in the forced swim test, have been observed in rodent models of the disease [297]. Furthermore, inhibition of dopamine re-uptake is a major mechanism of the antidepressant drug bupropion [298]. Taken together, these observations suggest a contributory role of altered dopaminergic function in at least a subset of depressed individuals.
6.1. Clinical studies
Although the effects of higher tissue and/or dietary levels of n-3 PUFAs have been studied in numerous human and animal studies relevant to depression including many clinical trials (for review see: [281]), only a few studies examined dopaminergic parameters. One clinical study examined serum concentrations of prolactin, an anterior pituitary hormone whose release is inhibited by dopamine and can thus serves as a marker of dopaminergic function. In depressed patients, plasma prolactin concentrations were inversely related to DHA levels, suggesting the association of decreased dopaminergic function with low DHA status [299].
6.2. Preclinical studies
Consistent with the clinical finding on dopamine in depression, adult rats fed diets that increased brain DHA content had higher dopamine concentrations [300]. In another study, postpartum female rats that were fed an n-3 PUFA-deficient diet during pregnancy and lactation, had a 25% decrease in brain DHA and lower expression of D2 dopamine receptors in the nucleus accumbens, similar to the decrease in D2 receptor density observed in depressed females and rat models of depression [301]. In that study, virgin female rats with decreased brain DHA levels also exhibited a smaller, nearly significant decrease in accumbal D2 receptors, suggesting that pregnancy and nursing augment the effects of DHA status on D2 receptor regulation. Likewise, the density of D2 receptors in the nucleus accumbens were lower in mutant mice lacking RXR-γ, a mediator of DHA signaling, which also exhibited the depressive behaviors of despair and anhedonia, along with changes in serotonin signaling [302].
6.3. Implications for prevention and treatment
The preponderance of the literature on the role of n-3 PUFAs in depression indicates that low dietary and tissue levels of n-3 PUFAs are consistently observed in the disorder, and that some experimental treatments that reduce n-3 PUFA levels cause a number of neurobiological effects similar to those observed in depression. Both the dopaminergic and non-dopaminergic effects (i.e., effects on the serotonin system, brain-derived neurotrophic factor (BDNF) in the hippocampus, and the hypothalamic-pituitary-adrenal axis) of n-3 PUFA deficiency support a role for n-3 PUFAs in depression in at least a subset of patients. The positive clinical trials of n-3 PUFA preparations in major depression support the involvement of brain n-3 PUFA status in the disorder, and suggest the benefit of these or related treatments in symptomatic patients. Interestingly, in contrast the effects of inadequate n-3 PUFAs during early brain development that resulted in dopaminergic alterations similar to those observed in schizophrenia, ADHD, or PD, depression-like neurobiological changes were observed most notable when n-3 PUFA status was altered in adult animals. This suggests that with respect to preventing depression, maintaining adequate n-3 PUFA levels throughout the lifespan may be critical. Thus, the potential of n-3 PUFA administration for prevention appears plausible, but must be tested in clinical trials. Furthermore, the preclinical data suggests that low levels of n-3 PUFAs may be contributory to anhedonic symptoms through effects on dopamine receptor expression, suggesting that n-3 PUFA treatment may prove useful in ameliorating depressive anhedonia and may thus be of particularly benefit to anhedonic patients. Future study must confirm this observation in depressed patients.
7. Conclusion
The clinical and preclinical studies reviewed here indicate that n-3 PUFAs, when combined with genetic and other environmental factors, likely contribute to the development and maintenance of brain dopamine systems and the etiologies of neuropsychiatric disorders involving those systems. The differing effects in conditions such as Parkinson’s disease, schizophrenia, ADHD, and depression likely result from the interaction of n-3 PUFA status with genetic and other factors to result in specific neurobiological alterations and symptoms. Furthermore, these studies suggest these disorders may be amenable to prevention and/or treatment with n-3 PUFAs, n-3 PUFA-derived mediators, or drugs that mimic their effects.
Future studies must fully elucidate the roles of long-chain PUFAs in the etiology of each of the neuropsychiatric disorders affecting the brain dopamine systems. In particular, how n-3 PUFA status interacts with genetic and environmental factors must be determined, as well as the interaction with developmental processes. In addition, the critical biological functions of the long-chain PUFAs, such as their role in membrane phospholipids, actions of long-chain PUFA-derived mediators, etc., in each of these disorders must be established. It is likely that these may differ between dopaminergic disorders. Furthermore, n-3 PUFAs may have both beneficial and detrimental actions in these conditions. Ultimately, the net effect in each disease must be determined as the definitive assessment of therapeutic potential and utility. In addition, although studies have shown that a DHA-enriched diet can restore brain DHA content in animals that have experienced a diet-induced loss of DHA from the brain [188, 303], the reversibility of each of neurobiological consequences of inadequate accumulation or a loss of n-3 PUFAs from brain must be determined, and may likely differ depending on the point in lifespan the deficit occurred. If such effects prove not to be fully reversible, this would indicate the critical importance of ensuring adequate DHA accumulation during development, as well as preventing a loss of brain DHA under physiological demands such as pregnancy. In contrast, effects that prove to be reversible would be anticipated to respond to treatments initiated after symptoms arise. Finally, should the data indicate the viability of using n-3 PUFAs, n-3 PUFA-derived mediators, or drugs that mimic their effects for the prevention or treatment of neuropsychiatric disorders involving the brain dopamine systems, the optimal agent, formulation, dose, and treatment duration must also to be determined.
Acknowledgements
Supported by NIH Grants NS067422 (BL), RR016475 (BL), HD02528 (BL), the University of Kansas Medical Center Research Institute Lied Endowment (BL), the Institute for Advancing Medical Innovation (MH-S), the Mabel A. Woodyard Fellowship in Neurodegenerative Disorders (MH-S), the University of Kansas Endowment (MH-S), and the University of Kansas Medical Center Institute for Neurological Discoveries (MH-S).
Abbreviations
- AA
arachidonic acid
- ALA
α-linolenic acid
- ADHD
attention deficit hyperactivity disorder
- ALOX12
arachidonate 12-lipoxygenase
- ASCM1
Acyl-CoA synthetase medium-chain family member 1
- Bcl-2
B-cell lymphoma-2
- BDNF
brain-derived neurotrophic factor
- cPLA2
cytosolic phospholipase A2
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- n-6 DPA
docosapentaenoic acid
- EEG
electroecephalograph
- EFOX
electrophile oxo-derivatives
- EPA
eicosapentaenoic acid
- Fabp7
fatty acid binding protein 7
- FADS
fatty acid desaturase
- GDNF
glial cell-derived neurotrophic factor
- iPLA2
calcium-independent phospholipase A2
- LA
linoleic acid
- LOX
lipoxygenase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- Nf-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NBRE
nerve growth factor 1B response element
- NMDA
n-methyl-d-aspartate
- NO
nitric oxide
- iNOS
inducible nitric oxide synthase
- NPD1
neuroprotectin D1
- NURR1
nuclear receptor-related-1 protein
- NURR77
nerve growth factor 1B
- 6-OHDA
6-hyrdoxydopamine
- PD
Parkinson’s disease
- PI3K
phosphatidylinositol-3 kinase
- PKC
protein kinase C
- PPAR
peroxisome-proliferator-activated receptor
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- SHR
spontaneously hypertensive rat
- SNP
single nucleotide polymorphism
- SPECT
Single-photon emission computed tomography
- RXR
retinoid X receptor
- sPLA2
secretory phospholipase A2
- TLR
toll-like receptor
Footnotes
Conflict of Interest
The authors have no conflicts of interest with respect to this work.
References
- [1].Sun GY, Simonyi A, Fritsche KL, Chuang DY, Hannink M, Gu Z, et al. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot Essent Fatty Acids 2017; S0952–3278: 30213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet 1998; 352: 688–91. [DOI] [PubMed] [Google Scholar]
- [3].Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol 2000; 42: 174–81. [DOI] [PubMed] [Google Scholar]
- [4].Whelan J. (n-6) and (n-3) Polyunsaturated fatty acids and the aging brain: food for thought. J Nutr 2008; 138: 2521–2. [DOI] [PubMed] [Google Scholar]
- [5].Dyall S, Michael-Titus A. Neurological benefits of omega-3 fatty acids. Neuromolecular Med 2008; 10: 219–35. [DOI] [PubMed] [Google Scholar]
- [6].Salem N Jr., Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001; 36: 945–59. [DOI] [PubMed] [Google Scholar]
- [7].Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirements. Early Hum Dev 1980; 4: 131–8. [DOI] [PubMed] [Google Scholar]
- [8].Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev 1980; 4: 121–9. [DOI] [PubMed] [Google Scholar]
- [9].Green P, Yavin E. Fatty acid composition of late embryonic and early postnatal rat brain. Lipids 1996; 31: 859–65. [DOI] [PubMed] [Google Scholar]
- [10].Kishimoto Y, Davies WE, Radin NS. Developing rat brain: changes in cholesterol, galactolipids, and the individual fatty acids of gangliosides and glycerophosphatides. J Lipid Res 1965; 6: 532–6. [PubMed] [Google Scholar]
- [11].Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Influence of long-chain polyunsaturated fatty acids on infant cognitive function. Lipids 1998; 33: 973–80. [DOI] [PubMed] [Google Scholar]
- [12].Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol 2000; 42: 174–81. [DOI] [PubMed] [Google Scholar]
- [13].McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids 2006; 75: 329–49. [DOI] [PubMed] [Google Scholar]
- [14].Galli C, Trzeciak H, Paoletti R. Effects of dietary fatty acids on the fatty acid composition of brain ethanolamine phosphoglyceride: reciprocal replacement of n-6 and n-3 polyunsaturated fatty acids. Biochim Biophys Acta 1971; 248: 449–54. [Google Scholar]
- [15].Ozias MK, Carlson SE, Levant B. Maternal parity and diet (n-3) polyunsaturated fatty acid concentration influence accretion of brain phospholipid docosahexaenoic acid in developing rats. J Nutr 2007; 137: 125–9. [DOI] [PubMed] [Google Scholar]
- [16].Bourre JM, Dumont OS, Piciotti MJ, Pascal GA, Durand GA. Dietary alpha-linolenic acid deficiency in adult rats for 7 months does not alter brain docosahexaenoic acid content, in contrast to liver, heart and testes. Biochim Biophys Acta 1992; 1124: 119–22. [DOI] [PubMed] [Google Scholar]
- [17].Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity interact to alter maternal rat brain phospholipid fatty acid composition. J Nutr 2006; 136: 2236–42. [DOI] [PubMed] [Google Scholar]
- [18].McNamara R,K, Sullivan J, Richtand NM, Jandacek R, Rider T, Tso P. Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult DBA/2J mice: Relationship with ventral striatum dopamine concentrations. Synapse 2008; 62: 725–35. [DOI] [PubMed] [Google Scholar]
- [19].Simopoulos AP. Essential fatty acids in health and chronic diseases. Forum Nutr 2003; 56: 67–70. [PubMed] [Google Scholar]
- [20].Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008; 233: 674–88. [DOI] [PubMed] [Google Scholar]
- [21].Fetterman JW Jr., Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm 2009; 66: 1169–79. [DOI] [PubMed] [Google Scholar]
- [22].Hartwich J, Malec M, Partyka L, Pérez-Martinez P, Marin C, López-Miranda J, et al. The effect of the plasma n-3/n-6 polyunsaturated fatty acid ratio on the dietary LDL phenotype transformation - insights from the LIPGENE study. Clin Nutr 2009; 28: 510–5. [DOI] [PubMed] [Google Scholar]
- [23].Haworth O, Buckley CD. Resolving the problem of persistence in the switch from acute to chronic inflammation. Proc Natl Acad Sci U S A 2007; 104: 20647–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol 2009; 54: 585–94. [DOI] [PubMed] [Google Scholar]
- [25].Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther 2014; 141: 272–82. [DOI] [PubMed] [Google Scholar]
- [26].Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015; 1851: 469–84. [DOI] [PubMed] [Google Scholar]
- [27].Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2017; 135: e867–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Otto SJ, Houwelingen AC, Antal M, Manninen A, Godfrey K, Lopez-Jaramillo P, et al. Maternal and neonatal essential fatty acid status in phospholipids: an international comparative study. Eur J Clin Nutr 1997; 51: 232–42. [DOI] [PubMed] [Google Scholar]
- [29].Holman RT, Johnson SB, Ogburn PL. Deficiency of essential fatty acids and membrane fluidity during pregnancy and lactation. Proc Natl Acad Sci U S A 1991; 88: 4835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Al MD, van Houwelingen AC, Kester AD, Hasaart TH, de Jong AE, Hornstra G. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br J Nutr 1995; 74: 55–68. [DOI] [PubMed] [Google Scholar]
- [31].Levant B, Radel JD, Carlson SE. Reduced brain DHA content after a single reproductive cycle in female rats fed a diet deficient in N-3 polyunsaturated fatty acids. Biol Psychiatry 2006; 60: 987–90. [DOI] [PubMed] [Google Scholar]
- [32].Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity affect liver and erythrocyte phospholipid fatty acid composition in female rats. J Nutr 2007; 137: 2425–30. [DOI] [PubMed] [Google Scholar]
- [33].Xie L, Innis SM. Association of fatty acid desaturase gene polymorphisms with blood lipid essential fatty acids and perinatal depression among Canadian women: a pilot study. J Nutrigenet Nutrigenomics 2009; 2: 243–50. [DOI] [PubMed] [Google Scholar]
- [34].Zhang JY, Kothapalli KS, Brenna JT. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr Opin Clin Nutr Metab Care 2016; 19: 103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kim T, Kim HJ, Park JK, Kim JW, Chung JH. Association between polymorphisms of arachidonate 12-lipoxygenase (ALOX12) and schizophrenia in a Korean population. Behav Brain Funct 2010; 6: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li W, Ji W, Li Z, He K, Wang Q, Chen J, et al. Genetic association of ACSM1 variation with schizophrenia and major depressive disorder in the Han Chinese population. Am J Med Genet B Neuropsychiatr Genet 2015; 168B: 144–9. [DOI] [PubMed] [Google Scholar]
- [37].Nadalin S, Rubesa G, Giacometti J, Vulin M, Tomljanovic D, Vranekovic J, et al. BanI polymorphism of cytosolic phospholipase A2 gene is associated with age at onset in male patients with schizophrenia and schizoaffective disorder. Prostaglandins Leukot Essent Fatty Acids 2008; 78: 351–60. [DOI] [PubMed] [Google Scholar]
- [38].Watanabe A, Toyota T, Owada Y, Hayashi T, Iwayama Y, Matsumata M, et al. Fabp7 maps to a quantitative trait locus for a schizophrenia endophenotype. PLoS Biol 2007; 5: e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev 2005; 45: 559–79. [DOI] [PubMed] [Google Scholar]
- [40].Shaikh SR, Kinnun JJ, Leng X, Williams JA, Wassall SR. How polyunsaturated fatty acids modify molecular organization in membranes: insight from NMR studies of model systems. Biochim Biophys Acta 2015; 1848: 211–9. [DOI] [PubMed] [Google Scholar]
- [41].Fong TM, McNamee MG. Correlation between acetylcholine receptor function and structural properties of membranes. Biochem J 1986; 25: 830–40. [DOI] [PubMed] [Google Scholar]
- [42].Lundbaek JA, Anderson OS. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J Gen Physiol 1994; 104: 645–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Malnoe A, Milon H, Reme C. Effect of in vivo modulation of membrane docosahexaenoic acid levels on the dopamine-dependent adenylate cyclase activity in rat retina. J Neurochem 1990; 55: 1480–5. [DOI] [PubMed] [Google Scholar]
- [44].Turner N, Else PL, Hulbert AJ. Docosahexaenoic acid (DHA) content of membranes determines molecular activity of the sodium pump: implications for disease states and metabolism. Naturwissenschaften 2003; 90: 521–3. [DOI] [PubMed] [Google Scholar]
- [45].Witt M-R, Nielsen M. Characterization of the influence of unsaturated free fatty acids on brain GABA/benzodiazepine receptor binding in vitro. J Neurochem 1994; 62: 1432–9. [DOI] [PubMed] [Google Scholar]
- [46].Farooqui A, Horrocks L, Farooqui T. Interactions between neural membrane glycerophospholipid and sphingolipid mediators: a recipe for neural cell survival or suicide. J Neurosci Res 2007; 85: 1834–50. [DOI] [PubMed] [Google Scholar]
- [47].Lee HJ, Bazinet RP, Rapoport SI, Bhattacharjee AK. Brain arachidonic acid cascade enzymes are upregulated in a rat model of unilateral Parkinson disease. Neurochem Res 2010; 35: 613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 2001; 294: 1871–5. [DOI] [PubMed] [Google Scholar]
- [49].Orr S, Bazinet R. The emerging role of docosahexaenoic acid in neuroinflammation. Curr Opin Investig Drugs 2008; 9: 735–43. [PubMed] [Google Scholar]
- [50].Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev 2005; 45: 559–79. [DOI] [PubMed] [Google Scholar]
- [51].Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids 2003; 126: 1–27. [DOI] [PubMed] [Google Scholar]
- [52].Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids 2008; 153: 57–63. [DOI] [PubMed] [Google Scholar]
- [53].Green JT, Orr SK, Bazinet RP. The emerging role of group VI calcium-independent phospholipase A2 in releasing docosahexaenoic acid from brain phospholipids. J Lipid Res 2008; 49: 939–44. [DOI] [PubMed] [Google Scholar]
- [54].Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, et al. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem 2003; 278: 37041–51. [DOI] [PubMed] [Google Scholar]
- [55].Lengqvist J, Mata De Urquiza A, Bergman A, Willson T, Sjövall J, Perlmann T, et al. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol Cell Proteomics 2004; 3: 692–703. [DOI] [PubMed] [Google Scholar]
- [56].Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol 2010; 6: 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Weylandt KH. Docosapentaenoic acid derived metabolites and mediators - The new world of lipid mediator medicine in a nutshell. Eur J Pharmacol 2016; 785: 108–15. [DOI] [PubMed] [Google Scholar]
- [58].Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta 2015; 1851: 397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sharon R, Bar-Joseph I, Mirick G, Serhan C, Selkoe D. Altered fatty acid composition of dopaminergic neurons expressing alpha-synuclein and human brains with alpha-synucleinopathies. J Biol Chem 2003; 278: 49874–81. [DOI] [PubMed] [Google Scholar]
- [60].Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat 2004; 73: 155–72. [DOI] [PubMed] [Google Scholar]
- [61].Lukiw WJ, Bazan NG. Docosahexaenoic acid and the aging brain. J Nutr 2008; 138: 2510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med 2009; 206: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol 2005; 174: 5390–7. [DOI] [PubMed] [Google Scholar]
- [64].Spector AA. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J Mol Neurosci 2001; 16: 159–65; discussion 215–21. [DOI] [PubMed] [Google Scholar]
- [65].Montine KS, Quinn JF, Zhang J, Fessel JP, Roberts LJ, Morrow JD, et al. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem Phys Lipids 2004; 128: 117–24. [DOI] [PubMed] [Google Scholar]
- [66].Hammamieh R, Chakraborty N, Gautam A, Miller SA, Muhie S, Meyerhoff J, et al. Transcriptomic analysis of the effects of a fish oil enriched diet on murine brains. PLoS One 2014; 9: e90425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Levant B, Crane JF, Carlson SE. Sub-chronic antipsychotic drug treatment does not alter brain phospholipid fatty acid composition in rats. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 728–32. [DOI] [PubMed] [Google Scholar]
- [68].Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chemical Rev 2011; 111: 5922–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Nieves D, Moreno JJ. Effect of arachidonic and eicosapentaenoic acid metabolism on RAW 264.7 macrophage proliferation. J Cell Physiol 2006; 208: 428–34. [DOI] [PubMed] [Google Scholar]
- [70].Su KP, Yang HT, Chang JP, Shih YH, Guu TW, Kumaran SS, et al. Eicosapentaenoic and docosahexaenoic acids have different effects on peripheral phospholipase A2 gene expressions in acute depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 2018; 80: 227–33. [DOI] [PubMed] [Google Scholar]
- [71].Salvati S, Natali F, Attorri L, Di Benedetto R, Leonardi F, Di Biase A, et al. Eicosapentaenoic acid stimulates the expression of myelin proteins in rat brain. J Neurosci Res 2008; 86: 776–84. [DOI] [PubMed] [Google Scholar]
- [72].Kawashima A, Harada T, Kami H, Yano T, Imada K, Mizuguchi K. Effects of eicosapentaenoic acid on synaptic plasticity, fatty acid profile and phosphoinositide 3-kinase signaling in rat hippocampus and differentiated PC12 cells. J Nutr Biochem 2010; 21: 268–77. [DOI] [PubMed] [Google Scholar]
- [73].Das UN. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis 2008; 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Szanto A, Narkar V, Shen Q, Uray IP, Davies PJ, Nagy L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ 2004; 11 Suppl 2: S126–43. [DOI] [PubMed] [Google Scholar]
- [75].Lévesque D, Rouillard C. Nur77 and retinoid X receptors: crucial factors in dopamine-related neuroadaptation. Trends Neurosci 2007; 30: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Murphy EP, Conneely OM. Neuroendocrine regulation of the hypothalamic pituitary adrenal axis by the nurr1/nur77 subfamily of nuclear receptors. Mol Endocrinol 1997; 11: 39–47. [DOI] [PubMed] [Google Scholar]
- [77].Bäckman C, Perlmann T, Wallén A, Hoffer B, Morales M. A selective group of dopaminergic neurons express Nurr1 in the adult mouse brain. Brain Res 1999; 851: 125–32. [DOI] [PubMed] [Google Scholar]
- [78].Le W, Conneely O, Zou L, He Y, Saucedo-Cardenas O, Jankovic J, et al. Selective agenesis of mesencephalic dopaminergic neurons in Nurr1-deficient mice. Exp Neurol 1999; 159: 451–8. [DOI] [PubMed] [Google Scholar]
- [79].Zetterström R, Solomin L, Jansson L, Hoffer B, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science 1997; 276: 248–50. [DOI] [PubMed] [Google Scholar]
- [80].Schimmel J, Crews L, Roffler-Tarlov S, Chikaraishi D. 4.5 kb of the rat tyrosine hydroxylase 5’ flanking sequence directs tissue specific expression during development and contains consensus sites for multiple transcription factors. Brain Res Mol Brain Res 1999; 74: 1–14. [DOI] [PubMed] [Google Scholar]
- [81].Sacchetti P, Dwornik H, Formstecher P, Rachez C, Lefebvre P. Requirements for heterodimerization between the orphan nuclear receptor Nurr1 and retinoid X receptors. J Biol Chem 2002; 277: 35088–96. [DOI] [PubMed] [Google Scholar]
- [82].Krezel W, Ghyselinck N, Samad T, Dupé V, Kastner P, Borrelli E, et al. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science 1998; 279: 863–7. [DOI] [PubMed] [Google Scholar]
- [83].Aminoff MJ. Pharmacologic management of Parkinsonism & othe movement disorders In: Katzybg BG, Trevor AJ, Eds. Basic and Clinical Pharmacology. 13th ed. New York: Lange; 2015; pp. 472–89. [Google Scholar]
- [84].Zhao Y, Wee H, Chan Y, Seah S, Au W, Lau P, et al. Progression of Parkinson’s disease as evaluated by Hoehn and Yahr stage transition times. Mov Disord 2010; 25: 702–8. [DOI] [PubMed] [Google Scholar]
- [85].Takahashi H, Ohama E, Suzuki S, Horikawa Y, Ishikawa A, Morita T, et al. Familial juvenile parkinsonism: clinical and pathologic study in a family. Neurology 1994; 44: 437–41. [DOI] [PubMed] [Google Scholar]
- [86].Levy OA, Malagelada C, Greene LA. Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis 2009; 14: 478–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997; 276: 2045–7. [DOI] [PubMed] [Google Scholar]
- [88].Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 1998; 18: 106–8. [DOI] [PubMed] [Google Scholar]
- [89].Bekris LM, Mata IF, Zabetian CP. The genetics of Parkinson disease. J Geriatr Psychiatry Neurol 2010; 23: 228–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Schapira AH. Etiology of Parkinson’s disease. Neurology 2006; 66: S10–23. [DOI] [PubMed] [Google Scholar]
- [91].Barlow B, Cory-Slechta D, Richfield E, Thiruchelvam M. The gestational environment and Parkinson’s disease: evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod Toxicol 2007; 23: 457–70. [DOI] [PubMed] [Google Scholar]
- [92].Carvey PM, Punati A, Newman MB. Progressive dopamine neuron loss in Parkinson’s disease: the multiple hit hypothesis. Cell Transplant 2006; 15: 239–50. [DOI] [PubMed] [Google Scholar]
- [93].Holmäng A Perinatal origin of adult disease. Scand Cardiovasc J 2001; 35: 178–85. [DOI] [PubMed] [Google Scholar]
- [94].Gorell JM, Rybicki BA, Cole Johnson C, Peterson EL. Occupational metal exposures and the risk of Parkinson’s disease. Neuroepidemiology 1999; 18: 303–8. [DOI] [PubMed] [Google Scholar]
- [95].Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983; 219: 979–80. [DOI] [PubMed] [Google Scholar]
- [96].McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, et al. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 2002; 10: 119–27. [DOI] [PubMed] [Google Scholar]
- [97].Barlow B, Richfield E, Cory-Slechta D, Thiruchelvam M. A fetal risk factor for Parkinson’s disease. Dev Neurosci 2004; 26: 11–23. [DOI] [PubMed] [Google Scholar]
- [98].Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson’s disease: a metaanalysis. Environ Res 2001; 86: 122–7. [DOI] [PubMed] [Google Scholar]
- [99].Jang H, Boltz DA, Webster RG, Smeyne RJ. Viral parkinsonism. Biochim Biophys Acta 2009; 1792: 714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bloem BR, de Vries NM, Ebersbach G. Nonpharmacological treatments for patients with Parkinson’s disease. Mov Disord 2015; 30: 1504–20. [DOI] [PubMed] [Google Scholar]
- [101].Rowland NC, Sammartino F, Lozano AM. Advances in surgery for movement disorders. Mov Disord 2017; 32: 5–10. [DOI] [PubMed] [Google Scholar]
- [102].Olanow CW. Oxidation reactions in Parkinson’s disease. Neurology 1990; 40: suppl 32–7; discussion 7–9. [PubMed] [Google Scholar]
- [103].Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem 1989; 52: 381–9. [DOI] [PubMed] [Google Scholar]
- [104].Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A 1996; 93: 2696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liu X, Yamada N, Maruyama W, Osawa T. Formation of dopamine adducts derived from brain polyunsaturated fatty acids: mechanism for Parkinson disease. J Biol Chem 2008; 283: 34887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Liu X, Yamada N, Osawa T. Amide-type adduct of dopamine - plausible cause of Parkinson diseases. Subcell Biochem 2014; 77: 49–60. [DOI] [PubMed] [Google Scholar]
- [107].de Lau LM, Bornebroek M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology 2005; 64: 2040–5. [DOI] [PubMed] [Google Scholar]
- [108].Kamel F, Goldman SM, Umbach DM, Chen H, Richardson G, Barber MR, et al. Dietary fat intake, pesticide use, and Parkinson’s disease. Parkinsonism Relat Disord 2014; 20: 82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Miyake Y, Sasaki S, Tanaka K, Fukushima W, Kiyohara C, Tsuboi Y, et al. Dietary fat intake and risk of Parkinson’s disease: a case-control study in Japan. J Neurol Sci 2010; 288: 117–22. [DOI] [PubMed] [Google Scholar]
- [110].Taghizadeh M, Tamtaji OR, Dadgostar E, Daneshvar Kakhaki R, Bahmani F, Abolhassani J, et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem Int 2017. [DOI] [PubMed] [Google Scholar]
- [111].Ahmad S, Park J, Radel J, Levant B. Reduced numbers of dopamine neurons in the substantia nigra pars compacta and ventral tegmental area of rats fed an n-3 polyunsaturated fatty acid-deficient diet: a stereological study. Neurosci Lett 2008; 438: 303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Cardoso HD, dos Santos Junior EF, de Santana DF, Goncalves-Pimentel C, Angelim MK, Isaac AR, et al. Omega-3 deficiency and neurodegeneration in the substantia nigra: involvement of increased nitric oxide production and reduced BDNF expression. Biochim Biophys Acta 2014; 1840: 1902–12. [DOI] [PubMed] [Google Scholar]
- [113].Cansev M, Ulus I, Wang L, Maher T, Wurtman R. Restorative effects of uridine plus docosahexaenoic acid in a rat model of Parkinson’s disease. Neurosci Res 2008; 62: 206–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Barros AS, Crispim RYG, Cavalcanti JU, Souza RB, Lemos JC, Cristino Filho G, et al. Impact of the Chronic Omega-3 Fatty Acids Supplementation in Hemiparkinsonism Model Induced by 6-Hydroxydopamine in Rats. Basic Clin Pharmacol Toxicol 2017; 120: 523–31. [DOI] [PubMed] [Google Scholar]
- [115].Mori MA, Delattre AM, Carabelli B, Pudell C, Bortolanza M, Staziaki PV, et al. Neuroprotective effect of omega-3 polyunsaturated fatty acids in the 6-OHDA model of Parkinson’s disease is mediated by a reduction of inducible nitric oxide synthase. Nutr Neurosci 2017; 1–11. [DOI] [PubMed] [Google Scholar]
- [116].Hacioglu G, Seval-Celik Y, Tanriover G, Ozsoy O, Saka-Topcuoglu E, Balkan S, et al. Docosahexaenoic acid provides protective mechanism in bilaterally MPTP-lesioned rat model of Parkinson’s disease. Folia Histochem Cytobiol 2012; 50: 228–38. [DOI] [PubMed] [Google Scholar]
- [117].Bousquet M, Saint-Pierre M, Julien C, Salem NJ, Cicchetti F, Calon F. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson’s disease. FASEB J 2008; 22: 1213–25. [DOI] [PubMed] [Google Scholar]
- [118].Ozsoy O, Seval-Celik Y, Hacioglu G, Yargicoglu P, Demir R, Agar A, et al. The influence and the mechanism of docosahexaenoic acid on a mouse model of Parkinson’s disease. Neurochem Int 2011; 59: 664–70. [DOI] [PubMed] [Google Scholar]
- [119].Coulombe K, Saint-Pierre M, Cisbani G, St-Amour I, Gibrat C, Giguere-Rancourt A, et al. Partial neurorescue effects of DHA following a 6-OHDA lesion of the mouse dopaminergic system. J Nutr Biochem 2016; 30: 133–42. [DOI] [PubMed] [Google Scholar]
- [120].Tanriover G, Seval-Celik Y, Ozsoy O, Akkoyunlu G, Savcioglu F, Hacioglu G, et al. The effects of docosahexaenoic acid on glial derived neurotrophic factor and neurturin in bilateral rat model of Parkinson’s disease. Folia Histochem Cytobiol 2010; 48: 434–41. [DOI] [PubMed] [Google Scholar]
- [121].Samadi P, Grégoire L, Rouillard C, Bédard P, Di Paolo T, Lévesque D. Docosahexaenoic acid reduces levodopa-induced dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkeys. Ann Neurol 2006; 59: 282–8. [DOI] [PubMed] [Google Scholar]
- [122].Luchtman DW, Meng Q, Song C. Ethyl-eicosapentaenoate (E-EPA) attenuates motor impairments and inflammation in the MPTP-probenecid mouse model of Parkinson’s disease. Behav Brain Res 2012; 226: 386–96. [DOI] [PubMed] [Google Scholar]
- [123].Tian Y, Zhang Y, Zhang R, Qiao S, Fan J. Resolvin D2 recovers neural injury by suppressing inflammatory mediators expression in lipopolysaccharide-induced Parkinson’s disease rat model. Biochem Biophys Res Commun 2015; 460: 799–805. [DOI] [PubMed] [Google Scholar]
- [124].Luchtman DW, Meng Q, Wang X, Shao D, Song C. omega-3 fatty acid eicosapentaenoic acid attenuates MPP+-induced neurodegeneration in fully differentiated human SH-SY5Y and primary mesencephalic cells. J Neurochem 2013; 124: 855–68. [DOI] [PubMed] [Google Scholar]
- [125].Pezzella A, d’Ischia M, Napolitano A, Misuraca G, Prota G. Iron-mediated generation of the neurotoxin 6-hydroxydopamine quinone by reaction of fatty acid hydroperoxides with dopamine: a possible contributory mechanism for neuronal degeneration in Parkinson’s disease. J Med Chem 1997; 40: 2211–6. [DOI] [PubMed] [Google Scholar]
- [126].Kabuto H, Amakawa M, Mankura M, Yamanushi T, Mori A. Docosahexaenoic acid ethyl ester enhances 6-hydroxydopamine-induced neuronal damage by induction of lipid peroxidation in mouse striatum. Neurochem Res 2009; 34: 1299–303. [DOI] [PubMed] [Google Scholar]
- [127].Yakunin E, Loeb V, Kisos H, Biala Y, Yehuda S, Yaari Y, et al. A-synuclein neuropathology is controlled by nuclear hormone receptors and enhanced by docosahexaenoic acid in a mouse model for Parkinson’s disease. Brain Pathol 2012; 22: 280–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Riedel M, Goldbaum O, Wille M, Richter-Landsberg C. Membrane Lipid Modification by Docosahexaenoic Acid (DHA) Promotes the Formation of alpha-Synuclein Inclusion Bodies Immunopositive for SUMO-1 in Oligodendroglial Cells After Oxidative Stress. J Mol Neurosci 2010. [DOI] [PubMed] [Google Scholar]
- [129].Friling S, Bergsland M, Kjellander S. Activation of Retinoid X Receptor increases dopamine cell survival in models for Parkinson’s disease. BMC Neurosci 2009; 10: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wang J, Bi W, Zhao W, Varghese M, Koch RJ, Walker RH, et al. Selective brain penetrable Nurr1 transactivator for treating Parkinson’s disease. Oncotarget 2016; 7: 7469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].McFarland K, Spalding TA, Hubbard D, Ma JN, Olsson R, Burstein ES. Low dose bexarotene treatment rescues dopamine neurons and restores behavioral function in models of Parkinson’s disease. ACS Chem Neurosci 2013; 4: 1430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Drinkut A, Tillack K, Meka DP, Schulz JB, Kugler S, Kramer ER. Ret is essential to mediate GDNF’s neuroprotective and neuroregenerative effect in a Parkinson disease mouse model. Cell Death Dis 2016; 7: e2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Volakakis N, Tiklova K, Decressac M, Papathanou M, Mattsson B, Gillberg L, et al. Nurr1 and Retinoid X Receptor Ligands Stimulate Ret Signaling in Dopamine Neurons and Can Alleviate alpha-Synuclein Disrupted Gene Expression. J Neurosci 2015; 35: 14370–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Briere R, Diop L, Gottberg E, Grondin L, Reader TA. Stereospecific binding of a new benzazepine, [3H]SCH23390, in cortex and neostriatum. Can J Physiol Pharmacol 1987; 65: 1507–11. [DOI] [PubMed] [Google Scholar]
- [135].Schizophrenia Dean L.. In: Pratt V, McLeod H, Dean L, Malheiro A, Rubinstein W, Eds. Medical Genetics Summaries. Bethesda (MD) 2012. [Google Scholar]
- [136].Bunney WE Jr., Bunney BG. Neurodevelopmental hypothesis of schizophrenia In: Charney DS, Neslter EJ, Bunney BS, Eds. Neurobiology of Mental Illness. Oxford: Oxford University Press; 1999; pp. 225–35. [Google Scholar]
- [137].Cariaga-Martinez A, Alelu-Paz R. Rethinking the Epigenetic Framework to Unravel the Molecular Pathology of Schizophrenia. Int J Mol Sci 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Lai CY, Scarr E, Udawela M, Everall I, Chen WJ, Dean B. Biomarkers in schizophrenia: A focus on blood based diagnostics and theranostics. World J Psychiatry 2016; 6: 102–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Fullard JF, Halene TB, Giambartolomei C, Haroutunian V, Akbarian S, Roussos P. Understanding the genetic liability to schizophrenia through the neuroepigenome. Schizophr Res 2016; 177: 115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mednick SA, Huttunen MO, Machon RA. Prenatal influenza infections and adult schizophrenia. Schizophr Bull 1994; 20: 263–7. [DOI] [PubMed] [Google Scholar]
- [141].Susser E, Neugebauer R, Hoek HBA, Lin S, Labowitz D, Gormna J. Schizophrenia after prenatal famine: further evidence. Arch Gen Psychiatry 1996; 53: 25–31. [DOI] [PubMed] [Google Scholar]
- [142].Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci 1994; 9: 357–81. [DOI] [PubMed] [Google Scholar]
- [143].Byne W, Kemether E, Jones L, Haroutunian V, Davis KL. The neurochemistry of schizophrenia In: Charney DS, Neslter EJ, Bunney BG, Eds. Neurobiology of Mental Illness. Oxford: Oxford University Press; 1999; pp. 236–45. [Google Scholar]
- [144].Dean B Neurochemistry of schizophrenia: the contribution of neuroimaging postmortem pathology and neurochemistry in schizophrenia. Curr Top Med Chem 2012; 12: 2375–92. [DOI] [PubMed] [Google Scholar]
- [145].Seeman P, Chau-Wong M, Tedesco J, Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci U S A 1975; 72: 4376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Meyer JM. Pharmacotherapy of Psychosis and Mania In: Brunton LL, B.A. C, Knollmann BC, Eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011; pp. 417–56. [Google Scholar]
- [147].Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991; 148: 1474–86. [DOI] [PubMed] [Google Scholar]
- [148].Weinberger DR. Schizophrenia and the frontal lobe. Trends Neurosci 1988; 11: 367–70. [DOI] [PubMed] [Google Scholar]
- [149].Pickar D, Breier A, Hsiao JK, Doran AR, Wolkowitz OM, Pato CN, et al. Cerebrospinal fluid and plasma monoamine metabolites and their relation to psychosis: implications for regional brain dysfunction in schizophrenia. Arch General Psychiatry 1990; 47: 641–8. [DOI] [PubMed] [Google Scholar]
- [150].Peters SK, Dunlop K, Downar J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front Syst Neurosci 2016; 10: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Corley SM, Tsai SY, Wilkins MR, Shannon Weickert C. Transcriptomic Analysis Shows Decreased Cortical Expression of NR4A1, NR4A2 and RXRB in Schizophrenia and Provides Evidence for Nuclear Receptor Dysregulation. PloS one 2016; 11: e0166944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Hoen WP, Lijmer JG, Duran M, Wanders RJ, van Beveren NJ, de Haan L. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry Res 2013; 207: 1–12. [DOI] [PubMed] [Google Scholar]
- [153].van der Kemp WJ, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. A meta-analysis of the polyunsaturated fatty acid composition of erythrocyte membranes in schizophrenia. Schizophr Res 2012; 141: 153–61. [DOI] [PubMed] [Google Scholar]
- [154].Bentsen H, Solberg DK, Refsum H, Bohmer T. Clinical and biochemical validation of two endophenotypes of schizophrenia defined by levels of polyunsaturated fatty acids in red blood cells. Prostaglandins, leukotrienes, and essential fatty acids 2012; 87: 35–41. [DOI] [PubMed] [Google Scholar]
- [155].Montesinos-Rueda L, Canete-Crespillo J, Palma-Sevillano C, Gine-Serven E. Erythrocyte membrane polyunsaturated fatty acid (pufa) levels in a sample of patients with schizophrenia and relation with clinical and progression variables. Actas Esp Psiquiatr 2015; 43: 170–6. [PubMed] [Google Scholar]
- [156].Peet M, Laugharne JDE, Mellor JE, Ramchand CN. Essential fatty acid deficiency in erythrocyte membranes from chronic schizophrenic patients, and the clinical effects of dietary supplementation. Prostaglandins Leukot Essent Fatty Acids 1996; 55: 71–5. [DOI] [PubMed] [Google Scholar]
- [157].Kim SW, Jhon M, Kim JM, Smesny S, Rice S, Berk M, et al. Relationship between Erythrocyte Fatty Acid Composition and Psychopathology in the Vienna Omega-3 Study. PloS one 2016; 11: e0151417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Lavoie S, Whitford TJ, Benninger F, Feucht M, Kim SW, Klier CM, et al. Correlates of electroencephalographic resting states and erythrocyte membrane docosahexaenoic and eicosapentaenoic acid levels in individuals at ultra-high risk of psychosis. Aust NZ J Psychiatr 2016; 50: 56–63. [DOI] [PubMed] [Google Scholar]
- [159].Pawelczyk T, Trafalska E, Kotlicka-Antczak M, Pawelczyk A. The association between polyunsaturated fatty acid consumption and the transition to psychosis in ultra-high risk individuals. Prostaglandins Leukot Essent Fatty Acids 2016; 108: 30–7. [DOI] [PubMed] [Google Scholar]
- [160].Hamazaki K, Hamazaki T, Inadera H. Abnormalities in the fatty acid composition of the postmortem entorhinal cortex of patients with schizophrenia, bipolar disorder, and major depressive disorder. Psychiatry Res 2013; 210: 346–50. [DOI] [PubMed] [Google Scholar]
- [161].McNamara RK, Jandacek R, Rider T, Tso P, Hahn CG, Richtand NM, et al. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medications. Schizophr Res 2007; 91: 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hamazaki K, Hamazaki T, Inadera H. Fatty acid composition in the postmortem amygdala of patients with schizophrenia, bipolar disorder, and major depressive disorder. J Psychiatr Res 2012; 46: 1024–8. [DOI] [PubMed] [Google Scholar]
- [163].Hamazaki K, Maekawa M, Toyota T, Dean B, Hamazaki T, Yoshikawa T. Fatty acid composition of the postmortem prefrontal cortex of patients with schizophrenia, bipolar disorder, and major depressive disorder. Psychiatry Res 2015; 227: 353–9. [DOI] [PubMed] [Google Scholar]
- [164].Liu Y, Jandacek R, Rider T, Tso P, McNamara RK. Elevated delta-6 desaturase (FADS2) expression in the postmortem prefrontal cortex of schizophrenic patients: relationship with fatty acid composition. Schizophr Res 2009; 109: 113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Rao JS, Kim HW, Harry GJ, Rapoport SI, Reese EA. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in the postmortem frontal cortex from schizophrenia patients. Schizophr Res 2013; 147: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Ross BM, Turenne S, Moszczynska A, Warsh JJ, Kish SJ. Differential alteration of phospholipase A2 activities in brain of patients with schizophrenia. Brain Res 1999; 821: 407–13. [DOI] [PubMed] [Google Scholar]
- [167].Politi P, Rocchetti M, Emanuele E, Rondanelli M, Barale F. Randomized placebo-controlled trials of omega-3 polyunsaturated fatty acids in psychiatric disorders: a review of the current literature. Curr Drug Discov Technol 2013; 10: 245–53. [DOI] [PubMed] [Google Scholar]
- [168].Emsley R, Chiliza B, Asmal L, du Plessis S, Phahladira L, van Niekerk E, et al. A randomized, controlled trial of omega-3 fatty acids plus an antioxidant for relapse prevention after antipsychotic discontinuation in first-episode schizophrenia. Schizophr Res 2014; 158: 230–5. [DOI] [PubMed] [Google Scholar]
- [169].Fusar-Poli P, Berger G. Eicosapentaenoic acid interventions in schizophrenia: meta-analysis of randomized, placebo-controlled studies. J Clin Psychopharmacol 2012; 32: 179–85. [DOI] [PubMed] [Google Scholar]
- [170].Jamilian H, Solhi H, Jamilian M. Randomized, placebo-controlled clinical trial of omega-3 as supplemental treatment in schizophrenia. Glob J Health Sci 2014; 6: 103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Joy CB, Mumby-Croft R, Joy LA. Polyunsaturated fatty acid supplementation for schizophrenia. Cochrane Database Syst Rev 2006; CD001257. [DOI] [PubMed] [Google Scholar]
- [172].Pawelczyk T, Grancow-Grabka M, Kotlicka-Antczak M, Trafalska E, Pawelczyk A. A randomized controlled study of the efficacy of six-month supplementation with concentrated fish oil rich in omega-3 polyunsaturated fatty acids in first episode schizophrenia. J Psychiatr Res 2016; 73: 34–44. [DOI] [PubMed] [Google Scholar]
- [173].Chen AT, Chibnall JT, Nasrallah HA. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: Possible stage-specific effects. Ann Clin Psychiatry 2015; 27: 289–96. [PubMed] [Google Scholar]
- [174].Amminger GP, Schafer MR, Schlogelhofer M, Klier CM, McGorry PD. Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat Commun 2015; 6: 7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Frontiers in psychiatry 2014; 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Chalon S Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids 2006; 75: 259–69. [DOI] [PubMed] [Google Scholar]
- [177].Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G. alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J Neurochem 1996; 66: 1582–91. [DOI] [PubMed] [Google Scholar]
- [178].Zimmer L, Delpal S, Guilloteau D, Aioun J, Durand G, Chalon S. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci Lett 2000; 284: 25–8. [DOI] [PubMed] [Google Scholar]
- [179].Zimmer L, Durand G, Guilloteau D, Chalon S. n-3 polyunsaturated fatty acid deficiency and dopamine metabolism in the rat frontal cortex. Lipids 1999; 34 Suppl: S251. [DOI] [PubMed] [Google Scholar]
- [180].Zimmer L, Delion S, Vancassel S, Durand G, Guilloteau D, Bodard S, et al. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. J Lipid Res 2000; 41: 32–40. [PubMed] [Google Scholar]
- [181].Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, et al. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. The American journal of clinical nutrition 2002; 75: 662–7. [DOI] [PubMed] [Google Scholar]
- [182].Kodas E, Page G, Zimmer L, Vancassel S, Guilloteau D, Durand G, et al. Neither the density nor function of striatal dopamine transporters were influenced by chronic n-3 polyunsaturated fatty acid deficiency in rodents. Neurosci Lett 2002; 321: 95–9. [DOI] [PubMed] [Google Scholar]
- [183].Bondi CO, Taha AY, Tock JL, Totah NK, Cheon Y, Torres GE, et al. Adolescent behavior and dopamine availability are uniquely sensitive to dietary omega-3 fatty acid deficiency. Biol Psychiatry 2014; 75: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Kuperstein F, Eilam R, Yavin E. Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. J Neurochem 2008; 106: 662–71. [DOI] [PubMed] [Google Scholar]
- [185].Kuperstein F, Yakubov E, Dinerman P, Gil S, Eylam R, Salem N Jr., et al. Overexpression of dopamine receptor genes and their products in the postnatal rat brain following maternal n-3 fatty acid dietary deficiency. J Neurochem 2005; 95: 1550–62. [DOI] [PubMed] [Google Scholar]
- [186].Levant B, Zarcone TJ, Fowler SC. Developmental effects of dietary n-3 fatty acids on activity and response to novelty. Physiol Behav 2010; 101: 176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Levant B, Ozias MK, Carlson SE. Sex-specific effects of brain LC-PUFA composition on locomotor activity in rats. Physiol Behav 2006; 89: 196–204. [DOI] [PubMed] [Google Scholar]
- [188].Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav Brain Res 2004; 152: 49–57. [DOI] [PubMed] [Google Scholar]
- [189].Vancassel S, Blondeau C, Lallemand S, Cador M, Linard A, Lavialle M, et al. Hyperactivity in the rat is associated with spontaneous low level of n-3 polyunsaturated fatty acids in the frontal cortex. Behav Brain Res 2007; 180: 119–26. [DOI] [PubMed] [Google Scholar]
- [190].Reisbick S, Neuringer M, Hasnain R, Connor WE. Home cage behavior of rhesus monkeys with long-term deficiency of omega-3 fatty acids. Physiol Behav 1994; 55: 231–9. [DOI] [PubMed] [Google Scholar]
- [191].Enslen M, Milon H, Malnoe A. Effect of low intake of n-3 fatty acids during development on brain phospholipid fatty acid composition and exploratory behavior in rats. Lipids 1991; 26: 203–8. [DOI] [PubMed] [Google Scholar]
- [192].El-Sayed El-Sisi A, Sokkar SS, El-Sayed El-Sayad M, Sayed Ramadan E, Osman EY. Celecoxib and omega-3 fatty acids alone and in combination with risperidone affect the behavior and brain biochemistry in amphetamine-induced model of schizophrenia. Biomed Pharmacother 2016; 82: 425–31. [DOI] [PubMed] [Google Scholar]
- [193].Geyer MA, Markou A. Animal models of psychiatric disorders In: Bloom FE, Kufer DJ, Eds. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995; pp. 787–98. [Google Scholar]
- [194].Fedorova I, Alvheim AR, Hussein N, Salem N Jr., Deficit in prepulse inhibition in mice caused by dietary n-3 fatty acid deficiency. Behav Neurosci 2009; 123: 1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Gama CS, Canever L, Panizzutti B, Gubert C, Stertz L, Massuda R, et al. Effects of omega-3 dietary supplement in prevention of positive, negative and cognitive symptoms: a study in adolescent rats with ketamine-induced model of schizophrenia. Schizophr Res 2012; 141: 162–7. [DOI] [PubMed] [Google Scholar]
- [196].Zugno AI, Chipindo H, Canever L, Budni J, Alves de Castro A, Bittencourt de Oliveira M, et al. Omega-3 fatty acids prevent the ketamine-induced increase in acetylcholinesterase activity in an animal model of schizophrenia. Life Sci 2015; 121: 65–9. [DOI] [PubMed] [Google Scholar]
- [197].Zugno AI, Chipindo HL, Volpato AM, Budni J, Steckert AV, de Oliveira MB, et al. Omega-3 prevents behavior response and brain oxidative damage in the ketamine model of schizophrenia. Neuroscience 2014; 259: 223–31. [DOI] [PubMed] [Google Scholar]
- [198].Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol 2015; 29: 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Islam R, Trepanier MO, Milenkovic M, Horsfall W, Salahpour A, Bazinet RP, et al. Vulnerability to omega-3 deprivation in a mouse model of NMDA receptor hypofunction. NPJ Schizophr 2017; 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Grintal B, Champeil-Potokar G, Lavialle M, Vancassel S, Breton S, Denis I. Inhibition of astroglial glutamate transport by polyunsaturated fatty acids: evidence for a signalling role of docosahexaenoic acid. Neurochem Int 2009; 54: 535–43. [DOI] [PubMed] [Google Scholar]
- [201].McNamara RK, Able JA, Jandacek R, Rider T, Tso P. Chronic risperidone treatment preferentially increases rat erythrocyte and prefrontal cortex omega-3 fatty acid composition: evidence for augmented biosynthesis. Schizophr Res 2009; 107: 150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Faracone SV, Biederman J. The neurobiology of attention deficit hyperactivity disorder In: Charney DS, Neslter EJ, Bunney BS, Eds. Neurobiology of Mental Illness. Oxford: Oxford University Press; 1999; pp. 788–809. [Google Scholar]
- [203].Zametkin AJ, Liotta W. The neurobiology of attention-deficit/hyperactivity disorder. The J Clinical Psychiatr 1998; 59: 17–23. [PubMed] [Google Scholar]
- [204].Szatmari P The epidemiology of attention-deficit hyperactivity disorders. Child Adolesc Psychiatr Clin North Am 1982; 1: 361–71. [Google Scholar]
- [205].Seidman LJ, Biederman J, Faracone SV, Milberger S, Norman D, Seivard K, et al. Effects of family history and comorbidity on the neuropsychological performance of children with ADHD: preliminary findings. J Am Acad Child Adolesc Psychiatry 1995; 34: 1015–24. [DOI] [PubMed] [Google Scholar]
- [206].Killeen PR. A decade of contributions to understanding and ameliorating attention deficit-hyperactivity disorder. Behav Brain Funct 2016; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Lou H, Henricksen L, Bruhn P. Focal cerebral dysfunction in developmental learning disabilities. Lancet 1991; 335: 8–11. [DOI] [PubMed] [Google Scholar]
- [208].Lou H, Henricksen L, Bruhn P, Borner H, Nielson J. Striatal dysfunction in attention deficit hyperkinetic disorder. Arch Neurol 1989; 46: 48–52. [DOI] [PubMed] [Google Scholar]
- [209].Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology 1997; 48: 589–601. [DOI] [PubMed] [Google Scholar]
- [210].Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50: 873–80. [DOI] [PubMed] [Google Scholar]
- [211].Friedman LA, Rapoport JL. Brain development in ADHD. Curr Opin Neurobiol 2015; 30: 106–11. [DOI] [PubMed] [Google Scholar]
- [212].Posner J, Park C, Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol Rev 2014; 24: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Zametkin AJ, Nordahi TE, Gross M, King C, Semple WE, Rumsey J, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990; 323: 1361–6. [DOI] [PubMed] [Google Scholar]
- [214].Shaywitz SE, Cohen DJ, Shaywitz BA. The biochemical basis of minimal brain dysfunction. J Pediatr 1978; 92: 179–87. [DOI] [PubMed] [Google Scholar]
- [215].Majzoub J, Muglia L. Knockout mice. N Engl J Med 1996; 334: 904–7. [DOI] [PubMed] [Google Scholar]
- [216].Comings DE, Comings BG, Muhlman D, Dietz G, Shahbahrami B, Tast D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. J Am Med Assoc 1991; 266: 1793–800. [PubMed] [Google Scholar]
- [217].Cook EH, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, et al. Association of attention deficit and the dopamine transporter gene. Am J Hum Genet 1995; 56: 993–8. [PMC free article] [PubMed] [Google Scholar]
- [218].LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, et al. Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatr 1996; 1: 128–31. [PubMed] [Google Scholar]
- [219].Benjamin J, Patterson C, Greenberg BD, Murphy DL, Hamer DH. Population and familial association between the D4 dopamine receptor gene and measures of novelty seeking. Nat Genet 1996; 12: 81–4. [DOI] [PubMed] [Google Scholar]
- [220].Ebstein RP, Novick O, Umansky RPB, Osher Y, Blame D, Bennett ER, et al. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality train of novelty seeking. Nat Genet 1986; 12: 78–80. [DOI] [PubMed] [Google Scholar]
- [221].Klein M, Onnink M, van Donkelaar M, Wolfers T, Harich B, Shi Y, et al. Brain imaging genetics in ADHD and beyond - mapping pathways from gene to disorder at different levels of complexity. Neurosci Biobehav Rev 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Brookes KJ, Chen W, Xu X, Taylor E, Asherson P. Association of fatty acid desaturase genes with attention-deficit/hyperactivity disorder. Biol Psychiatry 2006; 60: 1053–61. [DOI] [PubMed] [Google Scholar]
- [223].Mitchell EA, Aman MG, Turbott SH, Manku MS. Clinical characteristics and serum fatty acid levels in hyperactive children. Clin Pediatrics 1987; 26: 406–11. [DOI] [PubMed] [Google Scholar]
- [224].Stevens L, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr 1995; 62: 761–8. [DOI] [PubMed] [Google Scholar]
- [225].Young GS, Maharaj NJ, Conquer JA. Blood phospholipid fatty acid analysis of adults with and without attention deficit/hyperactivity disorder. Lipids 2004; 39: 117–23. [DOI] [PubMed] [Google Scholar]
- [226].Chen JR, Hsu SF, Hsu CD, Hwang LH, Yang SC. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J Nutr Biochem 2004; 15: 467–72. [DOI] [PubMed] [Google Scholar]
- [227].Hawkey E, Nigg JT. Omega-3 fatty acid and ADHD: blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev 2014; 34: 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].LaChance L, McKenzie K, Taylor VH, Vigod SN. Omega-6 to Omega-3 Fatty Acid Ratio in Patients with ADHD: A Meta-Analysis. J Can Acad Child Adolesc Psychiatry 2016; 25: 87–96. [PMC free article] [PubMed] [Google Scholar]
- [229].Yao J, Stanley JA, Reddy RD, Keshavan MS, Pettegrew JW. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipid metabolites. Biol Psychiatry 2002; 52: 823–30. [DOI] [PubMed] [Google Scholar]
- [230].Burgess JR, Stevens L, Zhang W, Peck L. Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am J Clin Nutr 2000; 71: 327–30. [DOI] [PubMed] [Google Scholar]
- [231].Colter AL, Cutler C, Meckling KA. Fatty acid status and behavioural symptoms of attention deficit hyperactivity disorder in adolescents: a case-control study. Nutr J 2008; 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Ng KH, Meyer BJ, Reece L, Sinn N. Dietary PUFA intakes in children with attention-deficit/hyperactivity disorder symptoms. Br J Nutr 2009; 102: 1635–41. [DOI] [PubMed] [Google Scholar]
- [233].Ross BM, McKenzie I, Glen I, Bennett CP. Increased levels of ethane, a non-invasive marker of n-3 fatty acid oxidation, in breath of children with attention deficit hyperactivity disorder. Nutr Neurosci 2003; 6: 277–81. [DOI] [PubMed] [Google Scholar]
- [234].Vaisman N, Kaysar N, Zaruk-Adasha Y, Pelled D, Brichon G, Zwingelstein G, et al. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: effect of dietary n-3 fatty acids containing phospholipids. Am J Clin Nutr 2008; 87: 1170–80. [DOI] [PubMed] [Google Scholar]
- [235].Belanger SA, Vanasse M, Spahis S, Sylvestre MP, Lippe S, L’Heureux F, et al. Omega-3 fatty acid treatment of children with attention-deficit hyperactivity disorder: A randomized, double-blind, placebo-controlled study. Paediatr Child Health 2009; 14: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Johnson M, Mansson JE, Ostlund S, Fransson G, Areskoug B, Hjalmarsson K, et al. Fatty acids in ADHD: plasma profiles in a placebo-controlled study of Omega 3/6 fatty acids in children and adolescents. Atten Defic Hyperact Disord 2012; 4: 199–204. [DOI] [PubMed] [Google Scholar]
- [237].Bos DJ, Oranje B, Veerhoek ES, Van Diepen RM, Weusten JM, Demmelmair H, et al. Reduced Symptoms of Inattention after Dietary Omega-3 Fatty Acid Supplementation in Boys with and without Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology 2015; 40: 2298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Richardson AJ, Puri BK. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog Neuro-psychopharmacol Biol Psychiatr 2002; 26: 233–9. [DOI] [PubMed] [Google Scholar]
- [239].Dashti N, Hekmat H, Soltani HR, Rahimdel A, Javaherchian M. Comparison of therapeutic effects of omega-3 and methylphenidate (ritalin((R))) in treating children with attention deficit hyperactivity disorder. Iran J Psychiatry Behav Sci 2014; 8: 7–11. [PMC free article] [PubMed] [Google Scholar]
- [240].Manor I, Magen A, Keidar D, Rosen S, Tasker H, Cohen T, et al. The effect of phosphatidylserine containing Omega3 fatty-acids on attention-deficit hyperactivity disorder symptoms in children: a double-blind placebo-controlled trial, followed by an open-label extension. Eur Psychiatry 2012; 27: 335–42. [DOI] [PubMed] [Google Scholar]
- [241].Kean JD, Sarris J, Scholey A, Silberstein R, Downey LA, Stough C. Reduced inattention and hyperactivity and improved cognition after marine oil extract (PCSO-524(R)) supplementation in children and adolescents with clinical and subclinical symptoms of attention-deficit hyperactivity disorder (ADHD): a randomised, double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2017; 234: 403–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Perera H, Jeewandara KC, Seneviratne S, Guruge C. Combined omega3 and omega6 supplementation in children with attention-deficit hyperactivity disorder (ADHD) refractory to methylphenidate treatment: a double-blind, placebo-controlled study. J Child Neurol 2012; 27: 747–53. [DOI] [PubMed] [Google Scholar]
- [243].Johnson M, Ostlund S, Fransson G, Kadesjo B, Gillberg C. Omega-3/omega-6 fatty acids for attention deficit hyperactivity disorder: a randomized placebo-controlled trial in children and adolescents. J Atten Disord 2009; 12: 394–401. [DOI] [PubMed] [Google Scholar]
- [244].Huss M, Volp A, Stauss-Grabo M. Supplementation of polyunsaturated fatty acids, magnesium and zinc in children seeking medical advice for attention-deficit/hyperactivity problems - an observational cohort study. Lipids Health Dis 2010; 9: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr 2001; 139: 189–96. [DOI] [PubMed] [Google Scholar]
- [246].Salehi B, Mohammadbeigi A, Sheykholeslam H, Moshiri E, Dorreh F. Omega-3 and Zinc supplementation as complementary therapies in children with attention-deficit/hyperactivity disorder. J Res Pharm Pract 2016; 5: 22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Milte CM, Parletta N, Buckley JD, Coates AM, Young RM, Howe PR. Increased Erythrocyte Eicosapentaenoic Acid and Docosahexaenoic Acid Are Associated With Improved Attention and Behavior in Children With ADHD in a Randomized Controlled Three-Way Crossover Trial. J Atten Disord 2015; 19: 954–64. [DOI] [PubMed] [Google Scholar]
- [248].Dubnov-Raz G, Khoury Z, Wright I, Raz R, Berger I. The effect of alpha-linolenic acid supplementation on ADHD symptoms in children: a randomized controlled double-blind study. Front Hum Neurosci 2014; 8: 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Matsudaira T, Gow RV, Kelly J, Murphy C, Potts L, Sumich A, et al. Biochemical and Psychological Effects of Omega-3/6 Supplements in Male Adolescents with Attention-Deficit/Hyperactivity Disorder: A Randomized, Placebo-Controlled, Clinical Trial. J Child Adolesc Psychopharmacol 2015; 25: 775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Widenhorn-Muller K, Schwanda S, Scholz E, Spitzer M, Bode H. Effect of supplementation with long-chain omega-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): a randomized placebo-controlled intervention trial. Prostaglandins Leukot Essent Fatty Acids 2014; 91: 49–60. [DOI] [PubMed] [Google Scholar]
- [251].Konigs A, Kiliaan AJ. Critical appraisal of omega-3 fatty acids in attention-deficit/hyperactivity disorder treatment. Neuropsychiatr Dis Treat 2016; 12: 1869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 2011; 50: 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P. The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behaviour and conduct problems in ADHD: A systematic review and meta-analysis. J Affect Disord 2016; 190: 474–82. [DOI] [PubMed] [Google Scholar]
- [254].Puri BK, Martins JG. Which polyunsaturated fatty acids are active in children with attention-deficit hyperactivity disorder receiving PUFA supplementation? A fatty acid validated meta-regression analysis of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids 2014; 90: 179–89. [DOI] [PubMed] [Google Scholar]
- [255].Gillies D, Sinn J, Lad SS, Leach MJ, Ross MJ. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev 2012; CD007986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Tan ML, Ho JJ, Teh KH. Polyunsaturated fatty acids (PUFAs) for children with specific learning disorders. Cochrane Database Syst Rev 2016; 9: CD009398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Behdani F, Hebrani P, Naseraee A, Haghighi MB, Akhavanrezayat A. Does omega-3 supplement enhance the therapeutic results of methylphenidate in attention deficit hyperactivity disorder patients? J Res Med Sci 2013; 18: 653–8. [PMC free article] [PubMed] [Google Scholar]
- [258].Barragan E, Breuer D, Dopfner M. Efficacy and Safety of Omega-3/6 Fatty Acids, Methylphenidate, and a Combined Treatment in Children With ADHD. J Atten Disord 2017; 21: 433–41. [DOI] [PubMed] [Google Scholar]
- [259].Wainwright PE. Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc 2002; 61: 61–9. [DOI] [PubMed] [Google Scholar]
- [260].Takeuchi T, Fukumoto Y, Harada E. Influence of a dietary n-3 fatty acid deficiency on the cerebral catecholamine contents, EEG and learning ability in rat. Behav Brain Res 2002; 131: 193–203. [DOI] [PubMed] [Google Scholar]
- [261].Moriguchi T, Sheaff-Greiner R, Salem N Jr., Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem 2000; 75: 2563–73. [DOI] [PubMed] [Google Scholar]
- [262].Sheaff-Greiner R, Moriguchi T, H A, Slotnik BM, Salem NJ. Rats with low levels of brain docosahexaenoic acid show impaired performance in olfactory-based and spatial learning tasks. Lipids 1999; 34: 239–43. [DOI] [PubMed] [Google Scholar]
- [263].Sagvolden T Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD). Neurosci Biobehav Rev 2000; 24: 31–9. [DOI] [PubMed] [Google Scholar]
- [264].Wei JW, Yang LM, Sun SH, Chiang CL. Phospholipids and fatty acid profile of brain synaptosomal membrane from normotensive and hypertensive rats. Int J Biochem 1987; 19: 1225–8. [DOI] [PubMed] [Google Scholar]
- [265].Hauser J, Makulska-Gertruda E, Reissmann A, Sontag TA, Tucha O, Lange KW. The effects of nutritional polyunsaturated fatty acids on locomotor activity in spontaneously hypertensive rats. Atten Defic Hyperact Disord 2014; 6: 61–5. [DOI] [PubMed] [Google Scholar]
- [266].Liso Navarro AA, Sikoglu EM, Heinze CR, Rogan RC, Russell VA, King JA, et al. Effect of diet on brain metabolites and behavior in spontaneously hypertensive rats. Behav Brain Res 2014; 270: 240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Dervola KS, Roberg BA, Woien G, Bogen IL, Sandvik TH, Sagvolden T, et al. Marine Omicron-3 polyunsaturated fatty acids induce sex-specific changes in reinforcer-controlled behaviour and neurotransmitter metabolism in a spontaneously hypertensive rat model of ADHD. Behav Brain Funct 2012; 8: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Nemeroff CB, Kelsey JE. Affective disorders In: Enna SJ, Coyle JT, Eds. Pharmacological Management of Neurological and Psychiatric Disorders. New York: McGraw Hill; 1998; pp. 95–136. [Google Scholar]
- [269].Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51: 8–19. [DOI] [PubMed] [Google Scholar]
- [270].Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord 2005; 87: 141–50. [DOI] [PubMed] [Google Scholar]
- [271].Burt VK, Stein K. Epidemiology of depression throughout the female life cycle. J Clin Psychiatry 2002; 63 Suppl 7: 9–15. [PubMed] [Google Scholar]
- [272].Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE 2004; 2004: re5. [DOI] [PubMed] [Google Scholar]
- [273].Dean J, Keshavan M. The neurobiology of depression: An integrated view. Asian J Psychiatr 2017; 27: 101–11. [DOI] [PubMed] [Google Scholar]
- [274].Duman RS. The neurochemistry of mood disorders: preclinical studies In: Charney DS, Nestler EJ, Eds. Neurobiology of Mental Illness. New York: Oxford University Press; 2004; pp. 421–339. [Google Scholar]
- [275].Garlow SJ, Musselman DL, Nemeroff CB. The neurochemistry of mood disorders: clinical studies In: Charney DS, Nestler EJ, Eds. Neurobiology of Mental Illness. New York: Oxford University Press; 2004; pp. 440–60. [Google Scholar]
- [276].Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res 2003; 37: 357–73. [DOI] [PubMed] [Google Scholar]
- [277].Schmidt H, Shelton R, Duman R. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 2011; 36: 2375–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am 1998; 21: 293–307. [DOI] [PubMed] [Google Scholar]
- [279].Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 2007; 64: 327–37. [DOI] [PubMed] [Google Scholar]
- [280].Gershon AA, Vishne T, Grunhaus L. Dopamine D2-like receptors and the antidepressant response. Biol Psychiatry 2007; 61: 145–53. [DOI] [PubMed] [Google Scholar]
- [281].Levant B Role of n-3 (omega-3) polyunsaturated fatty acids in postpartum depression: mechanisms and implications for prevention and treatment In: Hedge MV, A Z A, Adekar SP, Eds. Omega-3 Fatty Acids: Keys to Nutritional Health. Switzerland: Springer Science + Business Media; 2016; pp. 267–84. [Google Scholar]
- [282].Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry 2007; 12: 703, 67–75. [DOI] [PubMed] [Google Scholar]
- [283].Nestler EJ, Carlezon WA Jr., The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 2006; 59: 1151–9. [DOI] [PubMed] [Google Scholar]
- [284].Moses-Kolko EL, Price JC, Wisner KL, Hanusa BH, Meltzer CC, Berga SL, et al. Postpartum and depression status are associated with lower [[(1)(1)C]raclopride BP(ND) in reproductive-age women. Neuropsychopharmacology 2012; 37: 1422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Papp M, Klimek V, Willner P. Parallel changes in dopamine D2 receptor binding in limbic forebrain associated with chronic mild stress-induced anhedonia and its reversal by imipramine. Psychopharmacology 1994; 115: 441–6. [DOI] [PubMed] [Google Scholar]
- [286].Bjornebekk A, Mathe AA, Brene S. Isolated Flinders Sensitive Line rats have decreased dopamine D2 receptor mRNA. Neuroreport 2007; 18: 1039–43. [DOI] [PubMed] [Google Scholar]
- [287].Yaroslavsky I, Colletti M, Jiao X, Tejani-Butt S. Strain differences in the distribution of dopamine (DA-2 and DA-3) receptor sites in rat brain. Life Sci 2006; 79: 772–6. [DOI] [PubMed] [Google Scholar]
- [288].Kram ML, Kramer GL, Ronan PJ, Steciuk M, Petty F. Dopamine receptors and learned helplessness in the rat: an autoradiographic study. Prog Neuro-psychopharmacol Biol Psychiatry 2002; 26: 639–45. [DOI] [PubMed] [Google Scholar]
- [289].Camardese G, De Risio L, Di Nicola M, Pucci L, Cocciolillo F, Bria P, et al. Changes of dopamine transporter availability in depressed patients with and without anhedonia: a 123I-N-omega-fluoropropyl-carbomethoxy-3beta-(4-Iodophenyl)tropane SPECT study. Neuropsychobiology 2014; 70: 235–43. [DOI] [PubMed] [Google Scholar]
- [290].Post RM, Kotin J, Goodwin FK, Gordon E. Psychomotor activity and cerebrospinal fluid metabolites in affective illness. Am J Psychiatry 1973; 130: 67–72. [DOI] [PubMed] [Google Scholar]
- [291].Roy A, Karoum F, Pollack S. Marked reduction in indexes of dopamine transmission among patients with depression who attempted suicide. Arc Gen Psychiatry 1992; 49: 447–50. [DOI] [PubMed] [Google Scholar]
- [292].Brown AS, Gershon S. Dopamine and depression. J Neural Trans 1993; 91: 75–109. [DOI] [PubMed] [Google Scholar]
- [293].Van Praag HM, Korf J, Lakke J, Schut T. Dopamine metabolism in depression, psychoses, and Parkinson’s disease: the problem of specificity of biological variables in behavior disorders. Psychol Med 1975; 4: 21–9. [DOI] [PubMed] [Google Scholar]
- [294].Guze BH, Barrio JC. The etiology of depression in Parkinson’s disease patients. Psychosomatics 1991; 32: 390–4. [DOI] [PubMed] [Google Scholar]
- [295].Murphy DL. l-DOPA, behavioral activation and psychopathology. Res Publ Ass Res Nerv Ment Dis 1972; 50: 430–7. [PubMed] [Google Scholar]
- [296].Dooley M, Markham A. Pramipexole. A review of its use in the management of early and advanced Parkinson’s disease. Drugs Aging 1998; 12: 495–514. [DOI] [PubMed] [Google Scholar]
- [297].Magnard R, Vachez Y, Carcenac C, Krack P, David O, Savasta M, et al. What can rodent models tell us about apathy and associated neuropsychiatric symptoms in Parkinson’s disease? Transl Psychiatry 2016; 6: e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 1995; 56: 395–401. [PubMed] [Google Scholar]
- [299].Sublette ME, Galfalvy HC, Hibbeln JR, Keilp JG, Malone KM, Oquendo MA, et al. Polyunsaturated fatty acid associations with dopaminergic indices in major depressive disorder. Int J Neuropsychopharmacol 2014; 17: 383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Sugasini D, Lokesh BR. Rats given linseed oil in microemulsion forms enriches the brain synaptic membrane with docosahexaenoic acid and enhances the neurotransmitter levels in the brain. Nutr Neurosci 2014; 12: 87–96. [DOI] [PubMed] [Google Scholar]
- [301].Davis PF, Ozias MK, Carlson SE, Reed GA, Winter MK, McCarson KE, et al. Dopamine receptor alterations in female rats with diet-induced decreased brain docosahexaenoic acid (DHA): interactions with reproductive status. Nutr Neurosci 2010; 13: 161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Krzyzosiak A, Szyszka-Niagolov M, Wietrzych M, Gobaille S, Muramatsu S, Krezel W. Retinoid x receptor gamma control of affective behaviors involves dopaminergic signaling in mice. Neuron 2010; 66: 908–20. [DOI] [PubMed] [Google Scholar]
- [303].Levant B N-3 (omega-3) Fatty acids in postpartum depression: implications for prevention and treatment. Depress Res Treat 2011; 2011: 467349. [DOI] [PMC free article] [PubMed] [Google Scholar]