Controlling for the overlapping and competing risks of lower gestational age and birth weight demonstrates they have independent effects on different outcomes in HLHS.
Abstract
BACKGROUND:
Preterm delivery and low birth weight (LBW) are generally associated with worse outcomes in hypoplastic left heart syndrome (HLHS), but an individual preterm or small neonate may do well. We sought to explore the interactions between gestational age, birth weight, and birth weight for gestational age with intermediate outcomes in HLHS.
METHODS:
We analyzed survival, growth, neurodevelopment, length of stay, and complications to age 6 years in subjects with HLHS from the Single Ventricle Reconstruction trial. Univariate and multivariable survival and regression analyses examined the effects and interactions of LBW (<2500 g), weight for gestational age, and gestational age category.
RESULTS:
Early-term delivery (n = 234) was more common than term (n = 219) delivery. Small for gestational age (SGA) was present in 41% of subjects, but only 14% had LBW. Preterm, compared with term, delivery was associated with an increased risk of death or transplant at age 6 years (all: hazard ratio = 2.58, confidence interval = 1.43–4.67; Norwood survivors: hazard ratio = 1.96, confidence interval = 1.10–3.49) independent of LBW and weight for gestational age. Preterm delivery, early-term delivery, LBW, and SGA were each associated with lower weight at 6 years. Neurodevelopmental outcomes were worst in the LBW cohort.
CONCLUSIONS:
Preterm delivery in HLHS was associated with worse survival, even beyond Norwood hospitalization. LBW, SGA, and early-term delivery were associated with worse growth but not survival. LBW was associated with worse neurodevelopment, despite similar length of stay and complications. These data suggest that preterm birth and LBW (although often concomitant) are not equivalent, impacting clinical outcomes through mechanisms independent of perioperative course complexity.
What’s Known on This Subject:
As surgical and perioperative techniques improve, more high-risk neonates are surviving surgical palliation for hypoplastic left heart syndrome. On a population level, neonates born prematurely and with low birth weights (LBWs) have worse outcomes after surgical palliation.
What This Study Adds:
Only preterm delivery was associated with an increased mortality risk to 6 years, whereas LBW was associated with worse neurodevelopment. Preterm delivery, early-term delivery, LBW, and small for gestational age were all associated with lower weight at 6 years, compared with their reference groups.
Despite remarkable improvements in the treatment of this previously uniformly fatal disease, hypoplastic left heart syndrome (HLHS) continues to carry significant morbidity and mortality. Comorbid diagnoses can drastically increase the risk of poor outcomes in children undergoing single ventricle palliation. Prematurity (<37 weeks’ gestation) and low birth weight (LBW) (<2500 g) are 2 such diagnoses,1–4 but the individual preterm or small infant may do well.5 Similarly, although early-term delivery (37–38 6/7 weeks’ gestation) is associated with higher in-hospital mortality and complication rates and longer hospital length of stay (LOS) after neonatal surgery in a large cohort,6 it is unknown whether these risks persist long-term for the individual infant that survives the Norwood procedure. To our knowledge, no detailed analyses of the outcomes of the preterm or early-term infants who survived the Norwood procedure have been published. Premature and small neonates are often excluded from outcome analyses, and the few studies in which the outcomes in preterm infants are analyzed are limited by failure to control for covariates that have proven to be important distinguishing factors in neonatal intensive care outcomes, such as birth weight, growth restriction, and sex.7,8
Although gestational age, birth weight, and birth weight for gestational age are related risk factors, they may be associated with various outcomes in different ways. Furthermore, the independence of each of the 3 risk factors on Norwood outcomes is unknown. We performed a secondary analysis of the 6-year follow-up in the Pediatric Heart Network’s Single Ventricle Reconstruction (SVR) Trial to test the hypothesis that there are significant differences and interactions between the overlapping and competing risks of gestational age, birth weight, and birth weight for gestational age of infants with HLHS. In previous reports from the SVR cohort, it was shown that preterm birth was an independent risk factor for preoperative death9 as well as intermediate term mortality and transplant after the Norwood procedure.1 Our aim in this study was to assess the association and interaction of various categories of gestational age, birth weight, and birth weight for gestational age with outcomes up to 6 years of age.
Methods
Subjects
The study design, descriptive characterization of the cohort, and primary results of the SVR Trial and the SVR Extension Study have previously been published.10–12 In brief, of the 555 infants enrolled with a diagnosis of HLHS or related single morphologic right ventricle anomaly between May 2005 and July 2008, 549 infants were managed to age 6 years after undergoing a Norwood procedure with random assignment to either a right ventricle–to–pulmonary artery shunt (RVPAS) or modified Blalock-Taussig shunt (BTS). Those missing gestational age or birth weight data were excluded. In this analysis, shunt type was categorized by shunt in place when leaving the operating room. The protocol was approved by each center’s institutional review board, and written informed consent was obtained.
Definitions
Based on gestational age at delivery, we defined 4 gestational age categories: preterm (<37 weeks), early term (37–38 6/7 weeks), term (39–40 6/7 weeks), and late term (≥41 weeks). LBW was defined as birth weight <2500 g and “not LBW” as ≥2500 g.
Weight for gestational age was calculated on the basis of previously published normal birth weights13 and stratified as small for gestational age (SGA) for <10th percentile, appropriate for gestational age (AGA) for 10th to 90th percentile, and large for gestational age (LGA) for >90th percentile. Ponderal index was used to categorize asymmetry in anthropometry and calculated as weight divided by height cubed.
Collection of Outcome Data
Vital status was collected annually by review of medical records, annual clinic visits, telephone interviews, and/or the death index. Annual complications, interventions, and hospital days were collected both as part of the medical history from the parent or guardian and obtained from the medical record. Weight and height measurements were obtained from the medical record and translated to z scores on the basis of World Health Organization standards.14
Questionnaires regarding neurodevelopment, behavior, and quality of life (QoL) were mailed to families annually. For this study, we analyzed the results at 6 years of age from 3 instruments: (1) the 5 summary domains of the Vineland-II; (2) the 13 subdomains of the Behavior Assessment System for Children, Second Edition (BASC-2); and (3) the 10 subdomains of the Pediatric Quality of Life Inventory (PEDS-QL).15–17
Statistical Approach
Transplant-free survival was analyzed to determine associations with gestational age, birth weight, and birth weight for gestational age groups by using Cox proportional hazards regression, adjusting for significant associations with sex, shunt type, ponderal index, and other covariates (Supplemental Table 3). Because of violation of the proportional hazards assumption on the effect of shunt type and other covariates, these comparisons were split into <5 months of age and ≥5 months (mean age at stage II). Interactions of gestational age, birth weight, and birth weight for gestational age groups with each other and with sex, shunt type, and ponderal index were also considered. Only predictors, covariates, and interactions that were significantly related to the outcome were included in the final models. Predictors were not included in the same model if their Spearman correlation was >0.5. Similar regression modeling was performed to assess anthropometry, neurodevelopment, and QoL. In models for anthropometry, neurodevelopment, and QoL, we used the variables listed in Supplemental Table 3. Socioeconomic status was determined by using a census-based score from the subject’s census block tract at the time of random assignment.18
Cox competing risks models were used to test for associations between the predictors of gestational age, birth weight, and birth weight for gestational age with the outcomes of LOS for each of the 3 surgical stages (considered as time to event of discharge), complications, and interventions. Death before discharge, complication, or intervention was considered a competing risk. In the models, we controlled for significant effects of shunt type, sex, and their interactions. Numbers of complications and interventions were compared between groups by using Wilcoxon rank-sum tests.
All analyses were conducted by using SAS v9.4 (SAS Institute, Inc, Cary, NC). Statistical significance was tested at level 0.05. Because of the challenges of multiple testing, focus was placed on identifying general patterns across related outcomes.
Results
Demographics and Categorization
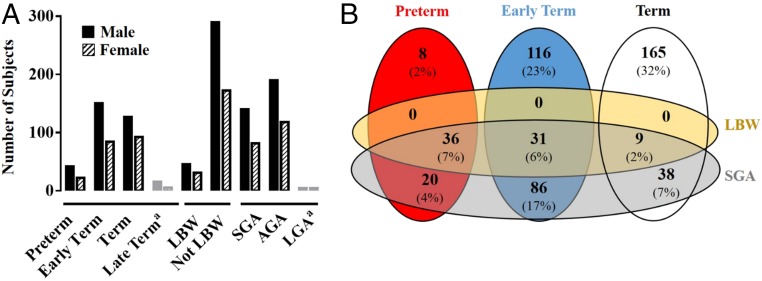
Of the 549 subjects in the analytic cohort, 12 did not have gestational age recorded. Of the 537 subjects eligible for this analysis, 64 (12%) were preterm, 234 (44%) were early-term, and 219 (41%) were term deliveries (Fig 1). Because late-term birth was infrequent (n = 20, 4%), these children were excluded from further analyses. LBW was infrequent (n = 76, 14%), but 221 (41%) subjects were SGA. Only 8 infants were LGA (1%) and were excluded from further analyses. There was a slight male predominance across categories of gestational age and weight for gestational age (58%–65%, Fig 1A). The intersection and overlap of SGA and LBW with each other and the gestational age categories are shown in Fig 1B.
FIGURE 1.
Cohort distribution. Number of subjects (n = 537) in each gestational age and birth weight category are shown according to sex (A). aLate-term delivery and LGA infants were excluded from analyses because of the small numbers. Intersection of gestational age category with LBW and SGA, in the cohort excluding LGA and late-term infants (n = 509), demonstrated all individuals who were categorized as LBW were also SGA, but not all SGA were LBW. There were LBW and SGA individuals distributed across all gestational age categories (B).
Transplant-Free Survival
Gestational Age
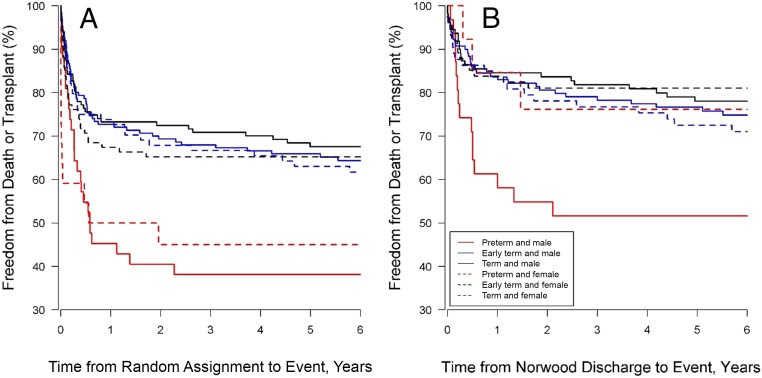
Preterm but not early-term delivery was associated with worse transplant-free survival (hazard ratio [HR]: 2.58; confidence interval [CI]: 1.43–4.67; Table 1, Fig 2). Increased risk for death or transplant among those born preterm persisted even after survival to discharge after the Norwood procedure (HR: 1.96; CI: 1.10–3.49, Fig 2). There was no significant interaction between gestational age category and sex. In Cox multivariable regression models, other than the predictor variables of interest, factors associated with survival included cardiac anatomy, shunt type, and presence of a recognized syndrome or extracardiac abnormality (Table 1). Spearman correlations between term groups and LBW, and term groups and SGA, were 0.37 and 0.42, respectively.
TABLE 1.
Multivariable Models for Survival
| Characteristics | HR (95% CI) | P |
|---|---|---|
| (A) Enrollment to age 6 y | ||
| Term group | ||
| Early term versus term | 1.35 (0.85–2.14) | .2 |
| Preterm versus term | 2.58 (1.43–4.67) | <.01 |
| Shunt type | ||
| RVPAS versus BTS <5 mo | 0.47 (0.27–0.82) | .01 |
| RVPAS versus BTS ≥5 mo | 1.16 (0.60–2.22) | .66 |
| Syndrome | ||
| Extra cardiac abnormality | 1.24 (0.76–2.02) | .4 |
| Syndrome | 2.56 (1.37–4.76) | <.01 |
| Anatomic subtype | ||
| AS/MS and related diagnoses <5 mo | 0.35 (0.16–0.80) | .01 |
| AS/MS and related diagnoses ≥5 mo | 0.12 (0.02–0.66) | .02 |
| Obstructed pulmonary veins | 3.57 (1.52–8.39) | <.01 |
| (B) Norwood discharge to age 6 y | ||
| Term group | ||
| Early term versus term | 1.25 (0.83–1.90) | .29 |
| Preterm versus term | 1.96 (1.10–3.49) | .02 |
| Shunt type | ||
| RVPAS versus BTS <5 mo | 0.34 (0.19–0.60) | <.01 |
| RVPAS versus BTS ≥5 mo | 1.69 (0.94–3.03) | .08 |
| Anatomic subtype | ||
| AS/MS and related diagnoses <5 mo | 0.32 (0.13–0.80) | .02 |
| AS/MS and related diagnoses ≥5 mo | 0.08 (0.01–0.52) | .01 |
| TAPVR | 3.46 (1.36–8.80) | .01 |
Final multivariable Cox model of freedom from transplant or death is shown from enrollment to 6 y for the entire cohort (A) and Norwood discharge to 6 y for those that survived to Norwood discharge (B). Only predictors, covariates, and interactions that were significantly related to the outcome were included in final models. AS, aortic stenosis; MS, mitral stenosis; TAPVR, total anomalous pulmonary venous return.
FIGURE 2.
Transplant-free survival. Freedom from transplant or death is shown from enrollment to 6 years for the entire cohort (A) and from Norwood discharge to 6 years for those who survived to Norwood discharge (B).
Weight Categories
LBW and SGA were not independently associated with worse survival. Moreover, there were no significant interactions between preterm birth, LBW, SGA, shunt type, or sex. LBW was associated with worse survival when preterm birth was not included in the model (HR: 1.73; CI: 1.23–2.44), but its effect was no longer statistically significant after accounting for preterm birth. A lower ponderal index (indicative of greater asymmetric growth) in SGA infants was weakly associated with an increased risk of death or transplant (interaction P value = .04, HR = 0.93 for each increase of 1 in ponderal index; CI = 0.86–1.02).
Anthropometry
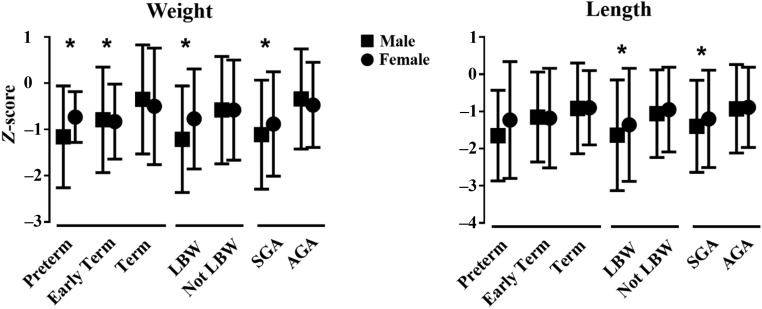
Preterm delivery, early-term delivery, LBW, and SGA were all associated with lower weight-for-age z-scores at 6 years of age compared with their reference group (term, not LBW, and AGA, respectively, Fig 3). In multivariable modeling, only SGA remained independently predictive (slope = −0.63 ± 0.15, P < .001), with additional significant effects of white race (slope = −0.45 ± 0.19, P = .02) and total hospital LOS over all surgeries (slope = −0.004 ± 0.002/day, P = .01) predicting lower z scores. LBW and SGA were also associated with lower length-for-age z scores (Fig 3). For both weight and length, worse anthropometry appeared to be driven by male infants, although sex did not have a statistically significant interaction across all groups. Shunt type did not affect growth or demonstrate any interactions with gestational age or birth weight categories.
FIGURE 3.
Growth. Weight- and length-for-age z scores at age 6 years are plotted by gestational age and birth weight (mean ± SD). * P ≤ .05, compared with reference group for male and female combined.
Neurodevelopment and QoL
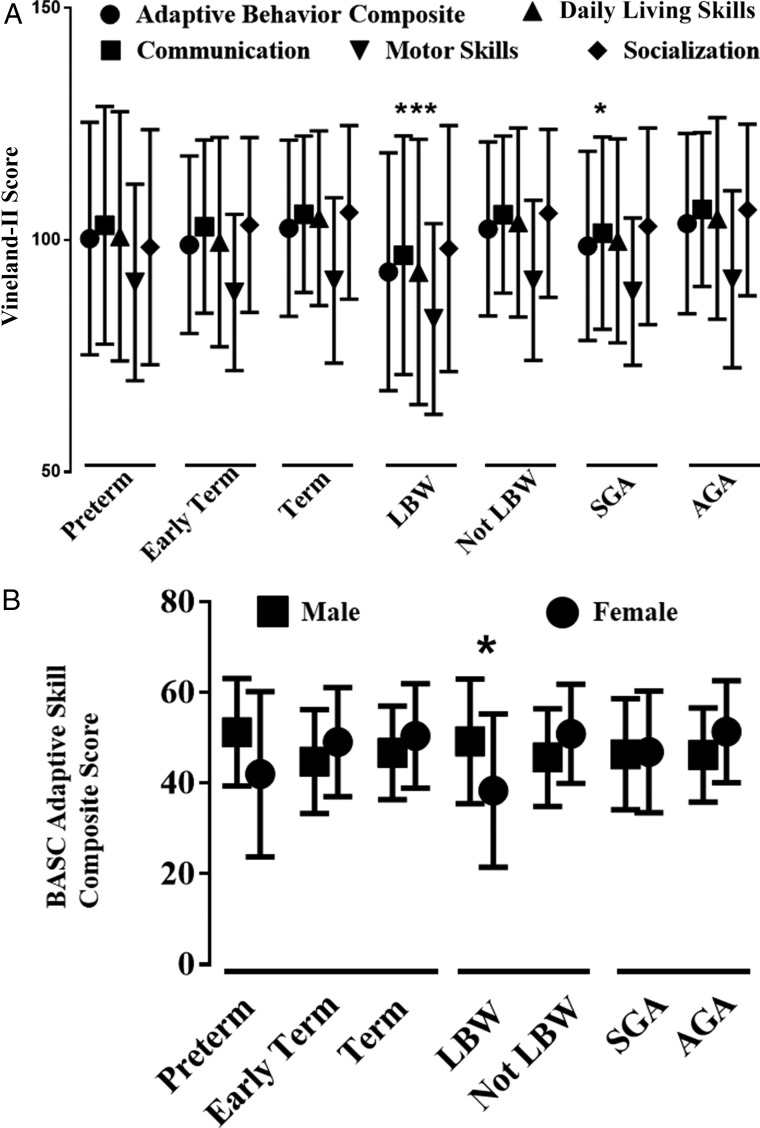
Gestational age did not appear to impact scores on tests of neurodevelopment and QoL for subjects who survived to 6 years of age. Specifically, there were no differences found in the results of the Vineland-II (Fig 4A), BASC-2, and PEDS-QL at age 6 years among the preterm, early-term, and term groups. LBW was associated with worse scores in multiple domains of the survey measures (Fig 4A, Table 2), including 3 of the 5 summary scores in the Vineland-II. Motor skills scores in the Vineland-II appeared consistently low across all categories. For comparisons within the study cohort, however, only LBW infants were significantly different from their reference group, not LBW (Fig 4A). Sex and LBW had significant interactions in multiple domains of the Vineland-II, BASC-2, and PEDS-QL. All of the BASC-2 scores were poorer in LBW females with the exception of the anxiety score, which was worse in LBW males (Table 2). The sex-specific differences for the BASC-2 Adaptive Skill composite score are shown in Fig 4B.
FIGURE 4.
Neurodevelopmental outcomes. Vineland-II subdomain scores (mean ± SD) are shown for each gestational age and birth weight category (A). From the many significant subdomain interactions in Table 2, 1 example of a significant interaction in the BASC Adaptive Skills composite score (mean ± SD) between birth weight and sex is shown (B). * P ≤ .05, compared with reference group.
TABLE 2.
Interactions Between Birth Weight Categories, Gestational Age, and Sex in Neurodevelopment Subdomains
| Significant Subdomains With a Worse Outcome |
| LBW |
| BASC: Atypicality |
| BASC: Functional Communication |
| Vineland-II: Communication |
| Vineland-II: Daily Living Skills |
| Vineland-II: Motor Skills |
| SGA |
| PEDS-QL: Treatment Anxiety |
| Vineland-II: Communication |
| Preterm |
| None |
| Early term |
| None |
| Significant Interactions With a Worse Outcome |
| LBW, female |
| BASC Adaptive Skills Composite |
| BASC: Activities of Daily Living |
| BASC: Adaptability |
| BASC: Attention |
| BASC: Social Skills |
| PEDS-QL: Physical Functioning |
| PEDS-QL: Cognitive Problems |
| PEDS-QL: Communication |
| Vineland-II: Communication |
| Vineland-II: Daily Living Skills |
| Vineland-II: Socialization |
| LBW, BTS |
| BASC: Behavioral Symptoms Index |
| BASC: Aggression |
| BASC: Hyperactivity |
| PEDS-QL: School Functioning |
| Preterm, male |
| BASC: Hyperactivity |
| Preterm, BTS |
| None |
| SGA, male |
| PEDS-QL: Perceived Physical Appearance |
| SGA, BTS |
| PEDS-QL: Perceived Physical Appearance |
The total number of significantly worse scores for each gestational age and birth weight category compared with their reference group (reference groups: term, not LBW, AGA) across all of the subdomains in the Vineland-II (5), BASC (13), and PEDS-QL (10). All significant interactions between gestational age or birth weight category and sex or shunt type that were associated with a worse outcome are listed.
Significant interactions were also found between shunt type and LBW. For all significant interactions, LBW had a greater association with worse test scores in children who had a BTS rather than RVPAS. Few interactions were found between gestational age or weight for gestational age and sex or shunt type (Table 2). In addition to the gestational age, birth weight, and weight for gestational age categories, male sex, extracorporeal membrane oxygenation (ECMO), seizures, vision problems, socioeconomic status, gastric feeding tube, and higher number of total complications were independent risk factors for worse assessment on the Vineland-II, BASC-2, and PEDS-QL in the final multivariable models (selected domains shown in Supplemental Table 4). The highest R2 values for these models was 0.34.
Hospital LOS, Complications, and Unplanned Interventions
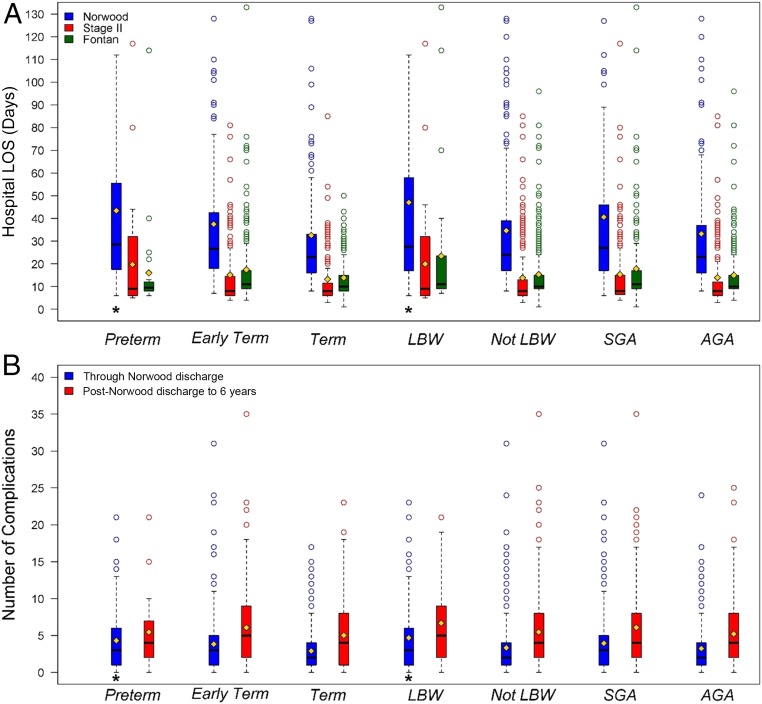
For subjects who survived to discharge after the Norwood surgery, preterm birth, LBW, and SGA were each associated with longer hospital LOS (median difference: 3.5–5 days, Fig 5A). In Cox multivariable regression models, there was an increased risk for longer LOS for preterm versus term infants (HR: 1.79; CI: 1.28–2.44; P < .01), LBW versus not LBW infants (HR: 1.64; CI: 1.23–2.17; P < .01), and SGA versus AGA infants (HR: 1.34; CI: 1.11–1.61; P < .01) but not early-term versus term infants (HR: 1.05; CI: 0.86–1.28). The multivariable models for LOS revealed no significant interactions with shunt type or sex. For subjects who survived to discharge, preterm birth, early-term birth, and LBW were associated with more complications through Norwood discharge compared with their reference groups (Fig 5B). No differences were seen in LOS or complication number surrounding stage II and Fontan procedures. Without shunt type in the multivariable model, having a shorter time to first complication was more likely in SGA versus AGA subjects (P = .045). When shunt type was included in the multivariable model, SGA was no longer significant (HR: 1.20; CI: 0.97–1.49) because shorter time to first complication was less likely in the RVPAS group (HR: 0.80; CI: 0.65–0.99; P = .04). Freedom from interventions was higher in the BTS group compared with the RVPAS group, but gestational age and birth weight groups had no effect on intervention rate.
FIGURE 5.
LOS and complications. LOS distribution for those that survived to hospital discharge after the Norwood (blue), stage II (red), and Fontan (green) surgeries (A). Outliers with hospital stay >130 days were not included in the graph. The mean (yellow diamond), median (bar), and distribution for the number of complications for survivors up to Norwood discharge (blue) and from Norwood discharge to 6 years (red) are shown (B). * P ≤ .05, compared with reference group, with Cox competing risk for the LOS and Wilcoxon rank test for complications.
Discussion
In this secondary analysis of 6-year outcomes in subjects with HLHS enrolled in the SVR studies, we found that for subjects born small and before 39 weeks, associations with outcomes are divergent depending on the outcome of interest. Although prematurity was associated with a continued risk of increased mortality even after survival to Norwood discharge, LBW had the strongest association with poorer neurodevelopmental outcomes. Preterm birth, early-term birth, LBW, and SGA were all associated with poorer growth, longer LOS, and higher complication rates. These findings suggest that mechanisms causing delivery at lower gestational age or birth weight lead to worse outcomes by divergent means.
It is not surprising that neonates born small and before 39 weeks’ gestation with HLHS have widely divergent outcomes. Drivers of preterm delivery and intrauterine growth (and resultant birth weight) in the absence of congenital heart disease are numerous, complex, and interrelated with a wide range of etiologies, including infectious, social, environmental, cardiovascular, metabolic, hematologic, genetic, and age-related risk factors.19–21 Various risk factors may also be associated with diverse outcomes in a sex-specific manner.22,23 It is important to distinguish between anthropometry in small neonates that is appropriate for the predicted growth potential and growth that is restricted because of an impaired intrauterine environment, but accurately defining growth restriction in the HLHS population is difficult. In addition to the usual determinants of growth potential based on parental size and genetics, newborns with HLHS are typically below average in birth weight and length and disproportionately further below average in head circumference.24–26 Our findings are consistent with these studies because nearly half our cohort met criteria for SGA.
Despite the problems in classifying growth in the fetus with HLHS, the importance of elucidating different mechanisms for divergent growth should not be overlooked as evidence increases to support the theory that an adverse prenatal environment imperils future cardiovascular outcomes.27 In HLHS, impaired placental function, estimated by umbilical artery Doppler flow, is associated with asymmetric and worse growth,28 and a composite variable for impaired maternal-fetal environment has been associated with worse survival.29 Supporting the importance of an adverse prenatal environment, a weak association between lower ponderal index and worse survival in the SGA group was demonstrated in our results. In contrast with other growth-restricted populations,30 however, we found no regression to the mean for weight or length in LBW and SGA subjects over a 6-year course and no independent association between birth weight category and survival. The 6-year findings fail to adequately answer whether the lower weight and length-for-age z scores represent a failure to achieve growth potential or an adequate achievement of a lower potential. In previous studies in which infants with HLHS were involved, copy number variants were associated with lower length-for-age z scores, suggesting that the growth potential may be lower and driven by genetics.31 Therefore, it is important to consider that although growth is a key outcome in HLHS, it may not be modifiable but, rather, be a surrogate for other risks.32,33
Similarly, the impact of genetic and in utero factors versus postnatal clinical care and complications on neurodevelopment remains uncertain. Despite similar LOS and complication rates in the preterm, LBW, and SGA groups, the LBW group demonstrated a preponderance of poorer performance in neurodevelopmental surveys at 6 years. There were also significant interactions between the birth weight category and both sex and shunt type for the LBW category of infants. We speculate that a LBW phenotype represents a more severe global insult in females compared with males, indicating a genetic contribution to the risk of a poor outcome34,35 or a sex-specific difference in the impaired maternal-fetal environment.36 The associations with LBW and BTS with worse neurodevelopmental outcomes, although physiologically plausible based on the lower diastolic blood pressure, were statistically significant for only a few tests and conflict with previous reports in which cerebral perfusion was similar by shunt type.37 This comparable cerebral perfusion between shunt types, however, was demonstrated in a cohort without LBW. The susceptibility to small physiologic changes may be more pronounced in the LBW population already at its limits of reserve in compensatory mechanisms to preserve cerebral nutrient delivery in the setting of a history of placental insufficiency.38 In our study, we did not find the association between early-term delivery and worse neurodevelopmental outcomes described by other investigators.39 The difference between our findings and previous reports may be explained by different ages at testing, type II error, or a masking of small difference in trends by the large variation in scores in the HLHS population.
As with any study that attempts to associate preoperative variables with later outcomes, the variation within a small cohort is an important limitation. The interquartile ranges, outliers, and standard deviations in the 6-year outcomes demonstrate a wide scatter. Only a small proportion of the outcome variance is explained by the multitude of variables accounted for in this prospectively followed cohort, suggesting we are unable to control for many of the factors that may impact the testing of our primary hypothesis. Regardless of these limitations, however, our study contributes to understanding the complex process of predicting outcomes for infants with HLHS faced with the additional risk factors of being born early and/or small, and may aid in counseling families. These data also suggest that modeling risk for longer-term outcomes in children surviving neonatal surgery is complex, and the variability in early-life events should be considered during both general pediatric and pediatric cardiac follow-up.
Conclusions
Preterm birth and lower birth weights, although often concomitant, are not equivalent, impacting clinical outcomes after surgical palliation for HLHS through mechanisms independent of perioperative course complexity. Preterm delivery, but not SGA or LBW, was independently associated with decreased transplant-free survival to 6 years of age, even when infants survived the Norwood hospitalization. Although gestational age and weight for gestational age categories were associated with lower weight and height at 6 years, they had little association with neurodevelopment. LBW appeared to have the greatest association with worse neurodevelopment. There were significant interactions between LBW and sex and shunt type, with worse neurodevelopmental outcomes in LBW females and LBW infants randomly assigned to a BTS. With these data, we provide additional insight into the complex pathology contributing to heterogeneous outcomes in HLHS. Our findings suggest the drivers of risk for preterm and small neonates may diverge depending on the outcome of interest, are not overcome by the successes associated with neonatal survival, and should be explored in other diagnoses.
Glossary
- AGA
appropriate for gestational age
- BASC-2
Behavior Assessment System for Children, Second Edition
- BTS
modified Blalock-Taussig shunt
- ECMO
extracorporeal membrane oxygenation
- HLHS
hypoplastic left heart syndrome
- HR
hazard ratio
- LBW
low birth weight
- LGA
large for gestational age
- LOS
length of stay
- PEDS-QL
Pediatric Quality of Life Inventory
- QoL
quality of life
- RVPAS
right ventricle–to–pulmonary artery shunt
- SGA
small for gestational age
- SVR
Single Ventricle Reconstruction
Footnotes
Mr Miller and Dr Minich conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; Ms Hu and Dr Tractenberg devised the statistical analysis plan, performed all statistical analyses, and reviewed and revised the manuscript; Drs Ghanayem, Newburger, McCrindle, DeWitt, Cnota, Wolf, Votava-Smith, Fifer, Shah, Graham, Pizarro, Jacobs, and Miller and Ms Pemberton and Lambert participated regularly in working committee conference calls critically reviewing the analyses in a stepwise approach and interpreting the results, and critically reviewed and revised all drafts of the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov/ct2/show/NCT00115934 (identifier NCT00115934).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The study was supported by grants (HL068270, HL068290, HL 109673, HL109737, HL109741, HL109741, HL109743, HL109777, HL109778, HL109781, HL109816, HL109818) from the National Heart, Lung, and Blood Institute, National Institutes of Health. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Tweddell JS, Sleeper LA, Ohye RG, et al. ; Pediatric Heart Network Investigators . Intermediate-term mortality and cardiac transplantation in infants with single-ventricle lesions: risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012;144(1):152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bové T, François K, De Groote K, et al. . Outcome analysis of major cardiac operations in low weight neonates. Ann Thorac Surg. 2004;78(1):181–187 [DOI] [PubMed] [Google Scholar]
- 3.Curzon CL, Milford-Beland S, Li JS, et al. . Cardiac surgery in infants with low birth weight is associated with increased mortality: analysis of the Society of Thoracic Surgeons Congenital Heart Database. J Thorac Cardiovasc Surg. 2008;135(3):546–551 [DOI] [PubMed] [Google Scholar]
- 4.Oppido G, Pace Napoleone C, Formigari R, et al. . Outcome of cardiac surgery in low birth weight and premature infants. Eur J Cardiothorac Surg. 2004;26(1):44–53 [DOI] [PubMed] [Google Scholar]
- 5.Ghanayem NS, Hoffman GM, Mussatto KA, et al. . Perioperative monitoring in high-risk infants after stage 1 palliation of univentricular congenital heart disease. J Thorac Cardiovasc Surg. 2010;140(4):857–863 [DOI] [PubMed] [Google Scholar]
- 6.Costello JM, Pasquali SK, Jacobs JP, et al. . Gestational age at birth and outcomes after neonatal cardiac surgery: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation. 2014;129(24):2511–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shim SY, Cho SJ, Kong KA, Park EA. Gestational age-specific sex difference in mortality and morbidities of preterm infants: a nationwide study. Sci Rep. 2017;7(1):6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, McKay K, Rosenkrantz TS, Hussain N. Comparisons of mortality and pre-discharge respiratory outcomes in small-for-gestational-age and appropriate-for-gestational-age premature infants. BMC Pediatr. 2004;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atz AM, Travison TG, Williams IA, et al. ; Pediatric Heart Network Investigators . Prenatal diagnosis and risk factors for preoperative death in neonates with single right ventricle and systemic outflow obstruction: screening data from the Pediatric Heart Network Single Ventricle Reconstruction Trial(∗). J Thorac Cardiovasc Surg. 2010;140(6):1245–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newburger JW, Sleeper LA, Gaynor JW, et al. ; Pediatric Heart Network Investigators . Transplant-free survival and interventions at 6 years in the SVR trial. Circulation. 2018;137(21):2246–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohye RG, Sleeper LA, Mahony L, et al. ; Pediatric Heart Network Investigators . Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362(21):1980–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohye RG, Gaynor JW, Ghanayem NS, et al. ; Pediatric Heart Network Investigators . Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136(4):968–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2). Available at: www.pediatrics.org/cgi/content/full/125/2/e214 [DOI] [PubMed] [Google Scholar]
- 14.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85 [DOI] [PubMed] [Google Scholar]
- 15.Sparrow DC, Balla D, Cicchetti DV. Vineland Adaptive Behavior Scales: Survey Forms Manual. 2nd ed. Minneapolis, MN: NCS Pearson; 2005 [Google Scholar]
- 16.Reynolds CRKR. Behavior Assessment System for Children. 2nd ed. Circle Pines, MN: American Guidance Service, Inc; 2004 [Google Scholar]
- 17.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812 [DOI] [PubMed] [Google Scholar]
- 18.Diez Roux AV, Merkin SS, Arnett D, et al. . Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106 [DOI] [PubMed] [Google Scholar]
- 19.Behrman RE, Butler AS, eds. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press (US); 2007 [PubMed] [Google Scholar]
- 20.Johnson CD, Jones S, Paranjothy S. Reducing low birth weight: prioritizing action to address modifiable risk factors. J Public Health (Oxf). 2017;39(1):122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99(3):490–496 [DOI] [PubMed] [Google Scholar]
- 22.Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction. 2013;145(1):R1–R13 [DOI] [PubMed] [Google Scholar]
- 23.Al-Qaraghouli M, Fang YMV. Effect of fetal sex on maternal and obstetric outcomes. Front Pediatr. 2017;5:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenthal GL. Patterns of prenatal growth among infants with cardiovascular malformations: possible fetal hemodynamic effects. Am J Epidemiol. 1996;143(5):505–513 [DOI] [PubMed] [Google Scholar]
- 25.Shillingford AJ, Ittenbach RF, Marino BS, et al. . Aortic morphometry and microcephaly in hypoplastic left heart syndrome. Cardiol Young. 2007;17(2):189–195 [DOI] [PubMed] [Google Scholar]
- 26.Miller TA, Zak V, Shrader P, et al. ; Pediatric Heart Network Investigators . Growth asymmetry, head circumference, and neurodevelopmental outcomes in infants with single ventricles. J Pediatr. 2016;168:220–225.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demicheva E, Crispi F. Long-term follow-up of intrauterine growth restriction: cardiovascular disorders. Fetal Diagn Ther. 2014;36(2):143–153 [DOI] [PubMed] [Google Scholar]
- 28.Miller TA, Joss-Moore L, Menon SC, Weng C, Puchalski MD. Umbilical artery systolic to diastolic ratio is associated with growth and myocardial performance in infants with hypoplastic left heart syndrome. Prenat Diagn. 2014;34(2):128–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaynor JW, Parry S, Moldenhauer JS, et al. . The impact of the maternal-foetal environment on outcomes of surgery for congenital heart disease in neonates. Eur J Cardiothorac Surg. 2018;54(2):348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crume TL, Scherzinger A, Stamm E, et al. . The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: the EPOCH study. Obesity (Silver Spring). 2014;22(2):608–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carey AS, Liang L, Edwards J, et al. . Effect of copy number variants on outcomes for infants with single ventricle heart defects. Circ Cardiovasc Genet. 2013;6(5):444–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burch PT, Ravishankar C, Newburger JW, et al. ; Pediatric Heart Network Investigators . Assessment of growth 6 years after the Norwood procedure. J Pediatr. 2017;180:270–274.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller TA. Growth in congenital heart disease: outcome or predictor? J Am Heart Assoc. 2018;7(17):e010262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rollins CK, Newburger JW, Roberts AE. Genetic contribution to neurodevelopmental outcomes in congenital heart disease: are some patients predetermined to have developmental delay? Curr Opin Pediatr. 2017;29(5):529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell MW, Chung WK, Kaltman JR, Miller TA. Advances in the understanding of the genetic determinants of congenital heart disease and their impact on clinical outcomes. J Am Heart Assoc. 2018;7(6):e006906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prior T, Wild M, Mullins E, Bennett P, Kumar S. Sex specific differences in fetal middle cerebral artery and umbilical venous Doppler. PLoS One. 2013;8(2):e56933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kussman BD, Gauvreau K, DiNardo JA, et al. . Cerebral perfusion and oxygenation after the Norwood procedure: comparison of right ventricle-pulmonary artery conduit with modified Blalock-Taussig shunt. J Thorac Cardiovasc Surg. 2007;133(3):648–655 [DOI] [PubMed] [Google Scholar]
- 38.Ishii H, Takami T, Fujioka T, et al. . Comparison of changes in cerebral and systemic perfusion between appropriate- and small-for-gestational-age infants during the first three days after birth. Brain Dev. 2014;36(5):380–387 [DOI] [PubMed] [Google Scholar]
- 39.Calderon J, Stopp C, Wypij D, et al. . Early-term birth in single-ventricle congenital heart disease after the fontan procedure: neurodevelopmental and psychiatric outcomes. J Pediatr. 2016;179:96–103 [DOI] [PubMed] [Google Scholar]