Abstract
Introduction:
Conventional video-EEG monitoring (cvEEG) is required to diagnose seizures accurately in neonates. This tool is resource-intense and has limited availability in many centers. Seizure prediction models could help allocate resources by improving efficiency in which cvEEG is used to detect subclinical seizures. The aim of this retrospective study was to create a neonate-specific seizure prediction model using clinical characteristics and EEG background findings.
Methods:
We conducted a 3-year retrospective study of all consecutive neonates who underwent cvEEG at a tertiary care pediatric hospital. Variables including age, EEG indication, high risk clinical characteristics, and EEG background informed seizure prediction models based on a multivariable logistic regression model. A Cox proportional hazard regression model was used to construct time to first EEG seizure.
Results:
Prediction models with clinical variables or background EEG features alone vs. combined clinical and background EEG features were created from 210 neonates who met inclusion criteria. The combined clinical and EEG model had a higher area under the curve for combined sensitivity and specificity to 83.0% when compared to the clinical model (76.4%) or EEG model (66.2%). The same trend of higher sensitivity of the combined model was found for time to seizure outcome.
Conclusion:
While both clinical and EEG background features were predictive of neonatal seizures, the combination improved overall prediction of seizure occurrence and prediction of time to first seizure as compared to prediction models based solely on clinical or EEG features alone. With prospective validation, this model may improve efficiency of patient-oriented EEG monitoring.
Keywords: cvEEG, ICU EEG, neonatal epilepsy, neonatal intensive care unit, neonatal seizures, seizure prediction
Introduction
Continuous video-electroencephalographic monitoring (cvEEG) is critical for accurate diagnosis of seizures and encephalopathy in critically ill neonates. It is estimated that 10–50% of patients undergoing clinically indicated cvEEG in the pediatric ICU have electrographic seizures, depending on patient characteristics and inclusion criteria.1–8 Of these electrographic seizures, 14–43% qualify as electrographic status epilepticus, and up to 85% do not have any clinical signs.1,9,10 In neonates, these numbers are even higher, with up to 90% presenting as electrographic only seizures (subclinical) or having subtle clinical signs.11 Neonatal seizures and status epilepticus are associated with significant neurologic disability, post-neonatal epilepsy, and in-hospital mortality, underscoring the importance of accurate detection and treatment.10,12–14 Recent studies suggest that high seizure burden in neonates is independently associated with increased morbidity and mortality.6,15 Without the use of cvEEG, neonatal subclinical seizures and status epilepticus would go undetected, and hence untreated.
Despite the importance of cvEEG for accurate seizure diagnosis in neonates, the expertise, labor and resource-intense nature needed for neonatal cEEG prevents widespread use.16 Centers with limited resources face challenges in offering continuous EEG monitoring even to high risk patients, and may rely on short duration EEG studies to detect electrographic seizures. Seizure prediction models may improve allocation of resources, by identifying critically ill neonates at highest risk of electrographic seizures, and determine the duration of cvEEG monitoring needed to detect seizures. While such models currently exist for older children, specific models for neonates are not available.7
The aim of this retrospective study was to create a neonate-specific seizure prediction model using clinical and EEG background characteristics. We also aimed to determine the risk of seizure at specific times during the entire EEG monitoring period for a particular neonate, depending on their clinical and EEG background findings compared with the at-risk neonates.
Methods
We conducted a retrospective study of all consecutive critically ill patients <44 weeks’ postconceptional age between January 1st 2011 and January 1st 2014, who underwent continuous video-EEG (cvEEG) defined as conventional video-EEG monitoring for >3 hours, in the surgical, cardiac, and neonatal intensive care units at a tertiary care pediatric hospital. At our institution cvEEG is requested routinely for neonates undergoing hypothermia in addition to suspected subclinical or clinical seizures. Only the first episode of cvEEG monitoring for each neonate was included. This study was approved by the Institutional Review Board at Boston Children’s Hospital.
Patient Characteristics
Clinical characteristics, including age, sex, indication for EEG, and etiology of and therapies for critical illness, were obtained from the electronic medical record. We defined subpopulations of neonates by specific high-risk etiologies and therapies (Table 1).
Table 1:
Clinical characteristics of subjects and incidence of seizures
| Clinical characteristics | Number (% total) (n=210) |
Number (%) with seizures |
|---|---|---|
| Male | ||
| Term (>37 weeks’ gestation) | 115 (55%) | 48/115 (42%) |
| Preterm (<37 weeks’ gestation) | 158 (75%) 52 (25%) |
61/158 (39%) 12/52 (23%) |
| Etiologies and therapies * | ||
| Hypoxic ischemic encephalopathy(HIE) | 48 (23%) | 17/48 (35%) |
| Therapeutic hypothermia (TH) | 33 (16%) | 11/33 (33%) |
| Congenital heart disease (CHD) | 31 (15%) | 9/31 (28%) |
| Extracorporeal membrane oxygenation (ECMO) | 24 (11%) | 9/24 (38%) |
| Cardiac arrest (CA) | 19 (9%) | 7/19 (37%) |
| Stroke | 41 (19.5%) | 22/41 (54%) |
| Genetic | 20 (10%) | 3/20 (15%) |
| Metabolic | 10 (5%) | 4/10 (40%) |
| Infectious | 6 (1%) | 3/6 (50%) |
| EEG indication* | ||
| Suspected clinical seizure | 163 (78%) | 64/163 (39%) |
| Encephalopathy | 70 (33%) | 21/70 (30%) |
EEG data
Continuous video-EEG monitoring (cvEEG) was performed using the standard 10–20 EEG system of electrode placement, according to the American Clinical Neurophysiology Society’s (ACNS) guideline on Continuous EEG Monitoring in Neonates17. Electrographic neonatal seizures were defined per ACNS standardized EEG terminology18. Presence of continuity and degree of discontinuity (i.e. duration of inter-burst interval), variability, reactivity, and synchrony were determined from daily EEG reports. At our institution, neonatal EEG reports are created once daily and include presence or absence of sleep wake cycling in addition to description of electrographic patterns as they correlate to behavioral states. For discontinuous periods, the typical interburst interval duration is described and documented. Term newborn studies with prominent interburst intervals greater than 6 seconds and interburst interval amplitudes < 25 micro volts are considered excessively discontinuous. Excessive sharp waves are quantified based on frequency as being excessive in periods where the record is continuous.
Prediction Model Datasets
Clinical features used for the seizure prediction models included sex and post-conceptional age (i.e., corrected for prematurity), EEG indication, and disorders and therapies associated with a high risk for seizures (Table 1). The EEG background used in our model was defined as background description from clinical reports from EEG start to the following morning. The EEG background was classified as normal, excessively discontinuous, burst suppression, depressed/undifferentiated, or electrocerebral silence. This classification varied according to expected findings for postconceptional age at the time of cvEEG, i.e. duration of interburst interval defined for preterm compared with term neonates, tracé discontinue versus tracé alternant to describe quiet sleep patterns. In addition, EEG background was assessed for the presence or absence of excessive multifocal sharp wave transients and presence or absence of focal attenuation. The time to first electrographic seizure identified after onset of cvEEG monitoring was analyzed and incorporated into the model.
Statistical Approach
We summarized the main features with descriptive statistics, and developed prediction models using logistic regression for dichotomous outcome of seizure occurrence. Additionally, we utilized Cox’s proportional hazard regression for the time to event outcome of time to first detected seizure. We evaluated the proportional hazard assumption using residual graphs and by testing the significance of interaction terms between each predictor and event time. There were no major departures from the proportional hazards assumptions. The model parameters were chosen based on stepwise selection using minimization of the Akaike Information Criterion (AIC). The AIC selects models with a good fit, but penalizes the number of parameters and therefore, reduces the risk of overfitting. The optimal threshold for the final prediction models are based on the maximum of Youden’s Index (sensitivity+specificity-1). All the analysis was performed using CRAN R software version 3.4.3.
Results
Demographics and Clinical Features
Of the 210 neonates included in our analysis, 95 neonates were female, 158 were term-born and 52 were preterm, with a median gestational age was 39 weeks (IQR 38.4–40.2) for term and 35 weeks (IQR 29–36) for preterm neonates. The distribution of high risk etiologies and therapies among subjects is shown in Table 1, with overlap among these etiologies and therapies shown in Supplementary Table 1. The median age at cvEEG initiation was 3.95 days (IQR 0.95–13.5).
Electrographic seizures (ES) were detected in 73 neonates (35%); 12 (12/73, 16%) were preterm while 49 (48/73, 66%) were term. Twenty-eight neonates (38%) had only subclinical seizures, 8 (11%) had only electro-clinical seizures, while the remainder had a mix of both types. The rate of electrographic seizures by high risk group and EEG indication are shown in Table 1.
Univariate Predictors of Seizure Occurrence (Table 2)
Table 2:
Individual predictors of seizure occurrence and time to seizure
| Presence of Seizure | Time to First Seizure | ||||||
|---|---|---|---|---|---|---|---|
| Proportions (N = 210) |
OR | 95% (LCL, UCL) |
p-value | HR | 95% (LCL, UCL) |
p-value | |
| Clinical Features | |||||||
| Male vs. Female | 0.42/0.26 | 2.01 | (1.12, 3.64) | 0.021 | 1.78 | (1.10, 2.90) | 0.018 |
| Preterm vs. Term | 0.23/0.39 | 0.48 | (0.22, 0.96) | 0.044 | 0.58 | (0.31, 1.07) | 0.082 |
| EEG Indication | |||||||
| Suspected seizure or both indications vs. Encephalopathy | 0.39/0.19 | 2.73 | (1.28, 6.36) | 0.012 | 2.46 | (1.22, 4.95) | 0.011 |
| Encephalopathy or both indications vs. Suspected seizure alone | 0.30/0.37 | 0.73 | (0.38, 1.33) | 0.301 | 0.72 | (0.43, 1.19) | 0.197 |
| High Risk Characteristics | |||||||
| Hypoxic ischemic encephalopathy (HIE) vs. No HIE | 0.35/0.34 | 1.04 | (0.52, 2.02) | 0.913 | 0.97 | (0.57, 1.68) | 0.934 |
| Therapeutic Hypothermia (TH) vs. No TH | 0.33/0.35 | 0.93 | (0.41, 2.01) | 0.851 | 0.86 | (0.45, 1.64) | 0.650 |
| ECMO vs. Patients not on ECMO | 0.37/0.34 | 1.14 | (0.46, 2.72) | 0.765 | 1.03 | (0.51, 2.07) | 0.926 |
| Cardiac Arrest (CA) vs. No CA | 0.39/0.35 | 1.10 | (0.39, 2.88) | 0.842 | 0.97 | (0.44, 2.12) | 0.940 |
| CHD vs. No CHD | 0.29/0.36 | 0.74 | (0.31, 1.65) | 0.470 | 0.74 | (0.37, 1.48) | 0.389 |
| EEG Background Features | |||||||
| Excessive discontinuity vs. Normal | 0.46/0.11 | 7.10 | (3.34, 16.62) | <0.001 | 5.17 | (2.53, 10.57) | <0.001 |
| Depressed and undifferentiated vs. Normal | 0.77/0.11 | 27.78 | (7.09, 142.84) | <0.001 | 11.42 | (4.63, 28.14) | <0.001 |
| Burst suppression vs. Normal | 0.70/0.11 | 19.45 | (4.58, 103.85) | <0.001 | 10.26 | (3.81, 27.68) | <0.001 |
| Extremely low voltage/isoelectric vs. Normal | 0.33/0.11 | 4.17 | (0.18, 47.98) | 0.263 | 4.99 | (0.63, 39.55) | 0.127 |
| MFS vs. Absence of MFS | 0.40/0.27 | 1.78 | (0.99, 3.25) | 0.054 | 1.58 | (0.98, 2.57) | 0.062 |
| Focal vs. Absence of focal findings | 0.39/0.34 | 1.24 | (0.49, 2.97) | 0.642 | 1.19 | (0.59, 2.38) | 0.632 |
| Sleep wake cycling | 0.25/0.54 | 0.29 | (0.16, 0.53) | <0.001 | 0.39 | (0.25, 0.63) | <0.001 |
Male neonates had a higher risk of seizure (Odds Ratio, OR = 2.01, 95% Confidence Interval (CI) 1.12–3.64, p=0.021) compared with females, while preterm neonates had a lower risk (OR = 0.48, 95% CI 0.22–0.96, p=0.044) compared to term neonates. Neonates monitored for suspected clinical seizure were at higher risk of having seizure detected on EEG, compared with neonates monitored for only encephalopathy (OR = 2.73, 95% CI 1.28–6.36, p= 0.012). Other risk factors, such as HIE, CHD or ECMO, did not show a statistically increased risk of seizure occurrence by univariate analysis.
EEG background features were evaluated with regard to probability of electrographic seizure occurrence. The background was normal in 84 of 210 (40%), excessively discontinuous in 100(48%), burst suppression in 10 (5%), attenuated and featureless in 13(6%), and met criteria for electrocerebral inactivity in 3 (1%). Excess multifocal sharp waves were seen in 119 (57%), and patients had focal attenuation in 23 (11%). An excessively discontinuous (OR 7.10, 95% CI 3.34–16.62, p<0.001), burst suppression background (OR 19.45, 95% CI 4.58–103.85, p<0.001) or depressed/undifferentiated (OR 27.78, 95% CI 7.09–142.84, p<0.001), in comparison to the normal EEG background, were all significant risk factors for seizure. The presence of sleep wake cycling (OR 0.29, 95% CI 0.16–0.53, p<0.001) and a normal EEG background was strongly associated with a low likelihood of developing ES (OR 0.12, 95% CI 0.05–0.25, p<0.0001); only two term neonates with a normal EEG background had seizures (2.4%). Both neonates presented with focal clonic (electroclinical) seizures within the first hour of EEG recording; one had HSV encephalitis with evidence of multifocal areas of restricted diffusion and the other had a left MCA stroke. The presence of excessive multifocal sharp wave transients (OR 1.78, 95% CI 0.99– 3.25, p=0.05) was a marginally significant predictor of seizure occurrence, while the presence of focal attenuation (OR 1.24, 95% CI 0.49–2.97, p=0.6) was not a significant predictor of seizures. Four neonates with excessive multifocal sharp wave transients and otherwise normal background developed seizures (5%), as did one neonate with only focal attenuation (1%).
Univariate Predictors of Time to First Detected Seizure (Table 2)
The median duration of cvEEG monitoring was 33.4 hours (IQR 17.8–57.3 hours). Of the 73 patients with ES, the median time to first seizure from the start of cvEEG monitoring was 1.6 hours (IQR 40 minutes - 3.75 hours). In order to capture 99% of seizures in our population, neonates would need to be monitored for 25 hours.
The time to seizure risk was higher in neonates with indications of suspected clinical seizure with/without encephalopathy (Hazard Ratio, HR = 2.46, 95% CI 1.22–4.95, p=0.01) when compared to patients with encephalopathy alone. Neonates with EEG indication of encephalopathy (HR = 0.72, 95% CI 0.43–1.19, p=0.197) did not have a significant association with time to seizure when compared to neonates with a suspected clinical seizure with/without encephalopathy. With regard to EEG background, neonates with excessively discontinuous EEG had higher risk compared to neonates with normal EEG (HR 5.17, 95% CI 2.54–10.57, p<0.001). Neonates with burst suppression and with depressed and undifferentiated EEG were at higher risk with HR 10.26, 95% CI 3.81–27.68, p<0.001 and HR 11.42, 95% CI 4.63–28.14, p<0.001, respectively.
Seizure Prediction Models
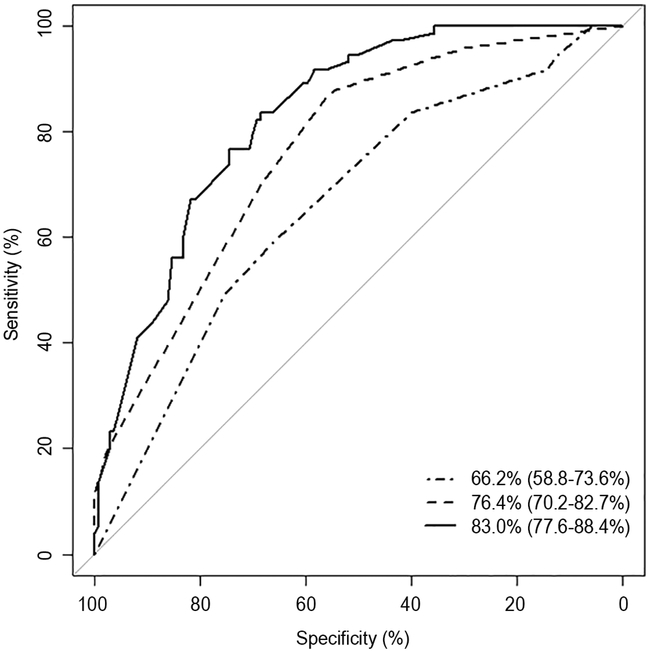
Three seizure prediction models were created: 1A-clinical variables alone, 1B- EEG features alone, 1C- clinical and EEG combined, with details of each model shown in Table 3. The performance of the seizure prediction models in terms of AUC for the ROC is 66.2% with 95%, CI of 58.8%−73.6% with clinical variables only, and 76.4% (95% CI 70.2–82.7%) with EEG variables alone. The combination of clinical variables with EEG features yielded better prediction with an AUC of 83.0% (95% CI 77.6–88.4%). The ROCs of seizure prediction Models 1A-C are shown in Figure 1.
Table 3:
Seizure prediction models
| Model 1A | Model 1B | Model 1C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std Err | OR | p-value | Estimate | Std Err | OR | p-value | Estimate | Std Err | OR | p-value | |
| Intercept | −1.708 | (0.437) | 0.181 | <0.001 | −2.474 | (0.417) | 0.084 | <0.001 | −3.392 | (0.763) | 0.018 | <0.001 |
| Male vs. Female | 0.687 | (0.308) | 1.988 | 0.026 | 0.771 | (0.363) | 2.140 | 0.034 | ||||
| Preterm vs. Term | −0.679 | (0.377) | 0.507 | 0.072 | −0.916 | (0.466) | 0.480 | 0.050 | ||||
| Suspected seizure or both indications vs. Encephalopathy | 1.034 | (0.412) | 2.812 | 0.012 | 1.443 | (0.478) | 4.171 | 0.003 | ||||
| Excessive discontinuity vs. Normal | 1.843 | (0.411) | 6.317 | <0.001 | 1.719 | (0.447) | 6.971 | <0.001 | ||||
| Depressed and undifferentiated vs. Normal | 3.501 | (0.764) | 33.159 | <0.001 | 3.581 | (0.900) | 57.432 | <0.001 | ||||
| Burst suppression vs. Normal | 3.081 | (0.788) | 21.774 | <0.001 | 2.878 | (0.886) | 29.140 | <0.001 | ||||
| Extremely low voltage/isoelectric vs. Normal | 1.781 | (1.294) | 5.936 | 0.169 | 1.382 | (1.455) | 7.562 | 0.339 | ||||
| MFS vs. No | 0.655 | (0.364) | 1.925 | 0.072 | 0.699 | (0.393) | 1.991 | 0.075 | ||||
| Sleep wake cycling | −0.682 | (0.415) | 0.415 | 0.100 | ||||||||
| AUC | 66.2% (58.8-73.6%) | 76.4% (70.2-82.7%) | 83.0% (77.6-88.4%) | |||||||||
| Optimal ES threshold* | −0.670 | −0.670 | −1.123 | |||||||||
| Sensitivity | 58.90 | 86.30 | 83.56 | |||||||||
| Specificity | 66.42 | 56.20 | 68.61 | |||||||||
| Positive predictive values | 48.31 | 51.22 | 58.65 | |||||||||
| Negative predictive values | 75.21 | 88.51 | 88.68 | |||||||||
MFS-multifocal sharp transients
Figure 1: AUC curves for seizure prediction.
(A) Sensitivity and specificity of Model 1A-C
Figure 1 displays the area under the curve for each seizure prediction model with respective confidence intervals. The dot/dash line represents Model 1A (the clinical model alone), the dash lines represent Model 1B (EEG model alone) while the black line represents the Model 1C (combined EEG and clinical model). The x axis represents the specificity of each model. The y axis represents the sensitivity of each model.
The threshold −0.67 was selected as the coordinates of the ROC curve based on the Youden’s Index, which provides a sensitivity of 58.9 and specificity of 66.4 for clinical variables alone, a sensitivity of 86.3 and specificity of 56.2 with EEG features alone, and a sensitivity of 83.6 and specificity of 68.6 for the combined model. The negative and positive predictive values were 75.2 (NPV) & 48.3(PPV) for the clinical model (Model 1A), 88.5(NPV) & 51.2(PPV) for the EEG model (Model 1B), and 88.7(NPV) & 58.7(PPV) for the combined model (Model 1C) (see Table 3).
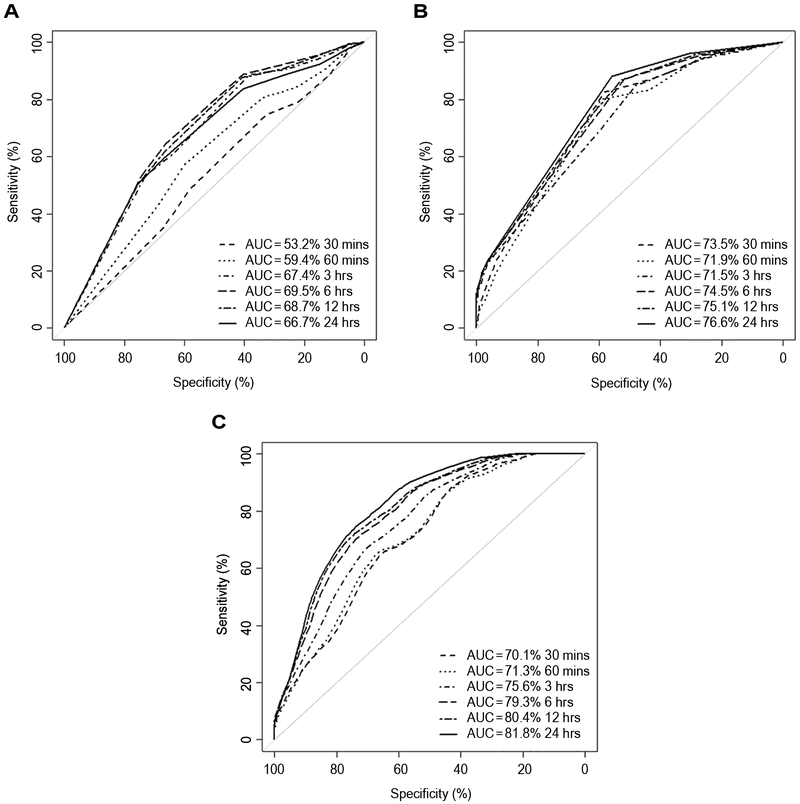
A prediction model for time to seizure was built using Cox’s proportional hazard regression models. Similar to seizure risk, this was incorporated into a clinical-only model (2A), EEG-only model (2B), and a combined clinical and EEG model (2C). These models determined the relative risk of an individual having a seizure at set time-points from the start of cvEEG monitoring. The time dependent ROC curves for Models 2A-C are presented in Figure 2 for specified time-points 19. The optimal threshold criterion (maximum sum of sensitivity and specificity), can be readily applied to ROCs at each time point to determine the thresholds and the corresponding sensitivity and specificity of prediction at each defined time-point. Model 2B with only EEG features outperforms Model 2A with clinical variables only in terms of sensitivity (average sensitivity of 85.9 for 2B vs. 52.3 for 2A). Model 2C (clinical and EEG combined) showed higher specificity compared to both models (Model 2C average specificity 70.32 vs. 55.2 for Model 2A or 54.4 for Model 2B) but had lower sensitivity compared to Model 2B (Average sensitivity 69.6 for Model 2C vs. 85.9 for Model 2B) (Figure 2). This trend is also seen at the specific chosen time points used in the models shown in Table 4.
Figure 2: AUC curves for time to seizure models.
(A) Model 2A
(B) Model 2B
(C) Model 2C
This figure represents the area under curve and sensitivity and specificity at different clinical time points for Model 2A (A) (clinical model alone), Model 2B (B) (EEG model alone) and Model 2C (C) clinical and EEG model combined. Time points are depicted by the different line types as described in the figure. The dash line represents the AUC at 30 minutes, dot line is the AUC at 60 minutes, dot/dash is the AUC at 3 hours, thick dash line is the AUC at 6 hours, dot/dash is the AUC at 12 hours, and the black line represents the AUC at 24 hours. The x axis represents the specificity and the y axis represents the sensitivity of each model at individual time points.
Table 4:
Prediction model of time to 1st seizure
| Model 2A | Model 2B | Model 2C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std Err | HR | p-value | Estimate | Std Err | HR | p-value | Estimate | Std Err | HR | p-value | |
| Male vs. Female | 0.556 | (0.248) | 1.743 | 0.025 | 0.424 | (0.255) | 1.528 | 0.096 | ||||
| Preterm vs. Term | −0.483 | (0.317) | 0.617 | 0.128 | −0.572 | (0.342) | 0.565 | 0.095 | ||||
| Suspected seizure or both indications vs. Encephalopathy | 0.917 | (0.357) | 2.503 | 0.010 | 1.039 | (0.371) | 2.828 | 0.005 | ||||
| Hypothermia vs. No hypothermia | −0.756 | (0.370) | 0.469 | 0.041 | ||||||||
| CHD vs. No CHD | −0.635 | (0.379) | 0.530 | 0.093 | ||||||||
| Excessive discontinuity vs. Normal | 1.523 | (0.369) | 4.588 | <0.001 | 1.539 | (0.393) | 4.659 | <0.001 | ||||
| Depressed and undifferentiated vs. Normal | 2.633 | (0.474) | 13.912 | <0.001 | 2.761 | (0.501) | 15.813 | <0.001 | ||||
| Burst suppression vs. Normal | 2.370 | (0.507) | 10.694 | <0.001 | 2.296 | (0.576) | 9.938 | <0.001 | ||||
| Extremely low voltage/ isoelectric vs. Normal | 1.899 | (1.068) | 6.681 | 0.076 | 2.041 | (1.147) | 7.699 | 0.075 | ||||
| MFS vs. No | 0.546 | (0.279) | 1.726 | 0.050 | 0.470 | (0.275) | 1.600 | 0.087 | ||||
| Sleep wake cycling | −0.461 | (0.279) | 0.631 | 0.098 | ||||||||
| AUC at 30 Minutes | 53.2% | 73.5% | 71.6% | |||||||||
| Optimal ES threshold* | −0.341 | 0.186 | 0.755 | |||||||||
| Sensitivity | 74.72 | 82.59 | 69.63 | |||||||||
| Specificity | 32.71 | 58.28 | 65.85 | |||||||||
| AUC at 60 Minutes | 59.4% | 71.9% | 72.8% | |||||||||
| Optimal ES threshold* | 0.020 | 0.186 | 0.755 | |||||||||
| Sensitivity | 57.14 | 79.89 | 69.04 | |||||||||
| Specificity | 60.05 | 59.39 | 67.40 | |||||||||
| AUC at 3 hours | 67.4% | 71.5% | 74.8% | |||||||||
| Optimal ES threshold* | −0.341 | −0.792 | 0.755 | |||||||||
| Sensitivity | 88.67 | 84.77 | 64.25 | |||||||||
| Specificity | 38.46 | 48.26 | 70.63 | |||||||||
| AUC at 6 hours | 69.5% | 74.5% | 79.1% | |||||||||
| Optimal ES threshold* | 0.021 | −0.792 | 0.719 | |||||||||
| Sensitivity | 57.99 | 87.33 | 69.41 | |||||||||
| Specificity | 66.53 | 51.74 | 73.22 | |||||||||
| AUC at 12 hours | 68.7% | 75.1% | 80.4% | |||||||||
| Optimal ES threshold* | 0.021 | −0.792 | 0.504 | |||||||||
| Sensitivity | 64.20 | 86.72 | 70.79 | |||||||||
| Specificity | 66.49 | 52.79 | 74.88 | |||||||||
| AUC at 24 hours | 66.7% | 76.6% | 81.1% | |||||||||
| Optimal ES threshold* | 0.021 | −0.792 | 0.232 | |||||||||
| Sensitivity | 59.38 | 88.06 | 74.87 | |||||||||
| Specificity | 66.97 | 55.81 | 69.94 | |||||||||
Threshold based on maximum of Youden’s Index (sum of sensitivity + specificity −1), Model 2A incorporates only clinical variables, Model 2B incorporates only EEG variable and Model 2C includes both clinical and EEG variables.
Discussion
There are three key findings from this single center, retrospective study of prediction of neonatal seizures using clinical and/or EEG variables. First, our data support the ACNS guideline recommending use of prolonged cvEEG in at risk neonates for at least 24 hours, because of the high incidence of seizures. Second, EEG background features are an important tool for prediction of electrographic seizures, since background features significantly improve prediction compared with models that use only clinical characteristics of the neonates. Third, seizure prediction models that take into account clinical and EEG features can be used in different ways to optimize efficiency regarding the duration of cvEEG monitoring necessary to detect seizures.
A high incidence of seizures in critically ill neonates, particularly electrographic-only (subclinical) seizures, has been reported previously,15,20,21 and our data add to this growing body of literature that supports the ACNS guideline recommending prolonged cvEEG in high risk neonates.17 Our data showed a particularly high risk of seizures among neonates with HIE, cardiac arrest, congenital heart disease and those treated with ECMO (28–38% with seizures). The majority of our patients had at least some subclinical seizures (some had only subclinical seizures), which may occur because of electroclinical dissociation (“uncoupling”),22 frequent use of sedative and paralytic medications in critically ill neonates masking outward clinical signs of seizures, and the often brief duration and subtle manifestations of neonatal seizures. In addition, our data supported the ACNS guideline’s recommendation for a minimum duration of 24 hours of cvEEG, since almost all neonates in our study developed seizures within 24 hours of cvEEG start.
Our study demonstrates in particular the importance of using initial EEG background features for seizure prediction, as our data showed that these features significantly improved seizure prediction over clinical variables alone. The use of EEG background as a tool for seizure prediction has also been assessed in previous work. Laroia et al. (1998) showed that a normal or mildly immature background predicted the absence of seizures in the following 18–24 hours with a sensitivity of 96% and specificity of 81%.23 Our data were similar to prior studies reporting a low seizure risk with a normal background pattern, as only 2 of our patients with a normal EEG background developed seizures. Glass et al. (2014) showed that a moderate or severely abnormal background was statistically superior for seizure prediction in term neonates with hypoxic-ischemic encephalopathy treated with hypothermia than clinical features alone.24 Our data expanded these findings, specifically excessively discontinuous, attenuated/featureless, and burst suppression background patterns were all associated with a risk of electrographic seizures, with the highest risk in neonates with attenuated featureless background (OR 28, p<0.001). Further, our results add to the existing literature as EEG background classification can be used for both, term and preterm neonates, and for a broad range of acute disorders to improve seizure prediction. Finally, a recent publication has shown good inter-rater agreement (K=0.7), p< 0.001) when using a similar five category EEG background classification (normal, excessively discontinuous, burst suppression, status epilepticus, or electro-cerebral inactivity) for term neonates with HIE,25 supporting the use of such an EEG classification scheme as a reliable means to improve seizure prediction.
Although EEG background classification improved seizure prediction, specific clinical variables added higher sensitivity and specificity for seizure prediction, and specific variables included gestational age and sex. There was a higher risk of seizures in term compared with preterm neonates. This supports a recent study by Lloyd et al. (2017) that showed that just 5% of consecutive preterm neonates had electrographic seizures, when monitored as soon as possible after birth for up to 72 hours with cvEEG.26 A higher probability for seizures was found in male neonates, which could potentially be related to a longer period of a depolarizing GABAergic response in males, demonstrated in rodent studies.27 Further, suspected seizure as indication for cvEEG was associated with a significantly higher risk of seizure than encephalopathy alone, which may have implications for choosing a prediction model that further refines the risk of seizure.
The unique value of our approach is that the seizure prediction models we created can improve efficiency of cvEEG monitoring strategies in the NICU. These models take a novel approach to assessment of seizure risk related to specific neonatal risk factors. While Model 1 can aide in the initial decision to start and / or continue cEEG, Model 2 allows for reassessment of the need for continued cEEG at shorter time intervals throughout the monitoring session. Estimates of seizure thresholds at time points of 1 hour, 3 hours, 6 hours, 12 hours and 24 hours have been calculated, to allow for reevaluation of clinical and EEG features to determine if the patient’s total score is moving away from or toward the threshold at these time points (see Table 4). This takes into consideration the possibility that the EEG background can change with time and uses this information to improve efficiency by either discontinuing cvEEG if the EEG is improving, or continuing if the background worsens. Various clinical scenarios may predispose to using a model (Model 2B) that is more sensitive and less specific at each time point (i.e. neonates with HIE at high risk of seizure) while other scenarios might be better suited to a model with more balanced sensitivity and specificity (e.g., full term neonate with a suspected seizure and a normal background after 6 hours of cvEEG). While our data support the ACNS guideline that neonates should be monitored for a minimum of 24 hours, we recognize that in certain clinical scenarios (e.g., where the EEG background is normal or mildly abnormal), our results show that cvEEG may be discontinued sooner because of low predicted risk of seizure occurrence. Studies in older pediatric ICU patients suggest that the decision to discontinue monitoring prior to 24 hours of cvEEG is reasonable when the EEG background is normal.28 In contrast, studies of neonatal hypoxic-ischemic encephalopathy have shown that some neonates have their first seizure after 24 hours of age or start of cvEEG.12 Similarly, some reports of high risk groups such as children with cardiac arrest treated with hypothermia show that seizures may occur late during late stages of hypothermia treatment and especially during rewarming.29,30 Data from all these studies should be taken into consideration when determining duration of cvEEG for specific patient populations. This model has promise as a useful clinical tool, however, prospective validation is necessary prior to clinical application. While the negative predictive value is high ranging from 75–89, the positive predictive value is low, with a range of 48–59. This suggests that our model identifies many patients who won’t develop seizures. While the overall sensitivity and negative predictive values are high in the EEG (Model 1B) and combined model (Model 1C), it should be noted that there is variability in the sensitivity and specificity at different time points in the prediction models that incorporate time. This suggests that some patients at continued risk of seizure may be missed by early discontinuation of cvEEG, depending on which model is chosen. As an example, when comparing Model 2B to Model 2C at 3 hours, the sensitivity is 85 and 64 respectively, showing the importance of choosing the model that best fits the patient’s characteristics and cvEEG monitoring goals.
Results need to be interpreted in the setting of data acquisition. First, the EEG background data were collected from EEG reports, so were not read by a single neurophysiologist, although high inter-rater agreement on background classification mitigates this limitation. Second, our study included neonates from a single pediatric tertiary care center who may have a higher risk of seizures than other neonatal populations. This is a limitation in terms of generalizability to other centers, although these seizure prediction models can be tailored to a given hospital’s population that takes into account characteristics of their population. Also, we included only neonates with a clinically indicated EEG, i.e. a population already at risk for seizures, so our results do not apply to all neonates in NICUs. In addition, our center uses a full 10–20 EEG montage, so centers using a reduced neonatal montage or aEEG may have a lower rate of seizure detection, altering the characteristics of the prediction models. Since our center does follow the ACNS guideline, our prediction models do apply to at risk neonates defined by that guideline. Finally, sedative and narcotic medications were not analyzed in these models, but could have affected both EEG background and seizure occurrence.
Conclusion
The decision to monitor critically ill neonates and the duration of monitoring is often dependent on individual practice and available resources,16 instead of being guided by evidence-based data. Data from our study support the use of readily available clinical features and initial background EEG features to identify neonates at high risk of seizures and the duration of cvEEG needed to detect seizures. Our findings support the ACNS clinical guideline for prolonged (24 hours) cvEEG in neonates at risk of seizures.17 After prospective validation, this model may be used to identify patients at higher or lower risk for seizure in whom the duration of monitoring may be modified accordingly.
Acknowledgments
This work was supported in part by the Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center (BCH IDDRC, NIH 1U54HD090255).
Disclosure of conflict of interests:
Arnold J. Sansevere is supported by an Eleanor and Miles Shore Fellowship for Scholars in Medicine.
Kush Kapur reports no disclosures.
Iván Sánchez Fernández is funded by the Epilepsy Research Fund and was funded by a grant for the study of Epileptic Encephalopathies from Fundación Alfonso Martín Escudero and from the HHV6 foundation.
Tobias Loddenkemper serves on the Council (and as President) of the American Clinical Neurophysiology Society, on the American Board of Clinical Neurophysiology, as committee chair at the American Epilepsy Society (Special Interest Group and Investigator Workshop Committees), as founder and consortium PI of the pediatric status epilepticus research group, as an Associate Editor for Seizure, and as an Associate Editor for Wyllie’s Treatment of Epilepsy 6th edition and 7th editions. He is part of pending patent applications to detect and predict seizures and to diagnose epilepsy. He receives research support from the NIH, PCORI, Epilepsy Research Fund, the Epilepsy Foundation of America, the Epilepsy Therapy Project, the Pediatric Epilepsy Research Foundation, and received research grants from Lundbeck, Eisai, Upsher-Smith, Mallinckrodt, Sage, and Pfizer. He serves as a consultant for Zogenix, Engage, Amzell, Upsher Smith, Eisai, and Sunovion. He performs video electroencephalogram long-term and ICU monitoring, electroencephalograms, and other electrophysiological studies at Boston Children’s Hospital and affiliated hospitals and bills for these procedures and he evaluates pediatric neurology patients and bills for clinical care. He has received speaker honorariums from national societies including the AAN, AES and ACNS, and for grand rounds at various academic centers. His wife, Dr. Karen Stannard, is a pediatric neurologist and she performs video electroencephalogram long-term and ICU monitoring, electroencephalograms, and other electrophysiological studies and bills for these procedures and she evaluates pediatric neurology patients and bills for clinical care.
Jurriaan Peters reports no disclosures related to the manuscript.
Janet Soul is supported by NIH grants R01NS066929, 5R01HD076258, 1R01EB017337, 1R21HD083956, and U54 HD090255 and grants from the Mooney Family Initiative for Translation and Clinical Studies in Rare Diseases and Patient Centered Outcomes Research Institute.
References
- 1.Abend NS, Dlugos DJ, Clancy RR. A review of long-term EEG monitoring in critically ill children with hypoxic-ischemic encephalopathy, congenital heart disease, ECMO, and stroke. J Clin Neurophysiol 2013;30(2):134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intensive care medicine. 2012:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greiner HM, Holland K, Leach JL, Horn PS, Hershey AD, Rose DF. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics. 2012;129(3):e748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76(12):1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tay SK, Hirsch LJ, Leary L, Jette N, Wittman J, Akman CI. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia. 2006;47(9):1504–1509. [DOI] [PubMed] [Google Scholar]
- 6.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014:awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang A, Arndt DH, Berg RA, et al. Development and validation of a seizure prediction model in critically ill children. Seizure. 2015;25:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clancy RR, Sharif U, Ichord R, et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia. 2005;46(1):84–90. [DOI] [PubMed] [Google Scholar]
- 9.Wusthoff CJ. Diagnosing neonatal seizures and status epilepticus. Journal of Clinical Neurophysiology. 2013;30(2):115–121. [DOI] [PubMed] [Google Scholar]
- 10.McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55(4):506–514. [DOI] [PubMed] [Google Scholar]
- 11.Wusthoff CJ. Diagnosing neonatal seizures and status epilepticus. J Clin Neurophysiol 2013;30(2):115–121. [DOI] [PubMed] [Google Scholar]
- 12.Shah DK, Wusthoff CJ, Clarke P, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed 2014;99(3):F219–224. [DOI] [PubMed] [Google Scholar]
- 13.Pavlidis E, Spagnoli C, Pelosi A, Mazzotta S, Pisani F. Neonatal status epilepticus: Differences between preterm and term newborns. European Journal of Paediatric Neurology. 2015;19(3):314–319. [DOI] [PubMed] [Google Scholar]
- 14.Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. The Journal of pediatrics. 2009;155(3):318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary Profile of Seizures in Neonates: A Prospective Cohort Study. J Pediatr 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG monitoring: current resources and practice in the United States and Canada. J Clin Neurophysiol 2013;30(2):156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s guideline on continuous electroencephalography monitoring in neonates. Journal of Clinical Neurophysiology. 2011;28(6):611–617. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol 2013;30(2):161–173. [DOI] [PubMed] [Google Scholar]
- 19.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000:337–344. [DOI] [PubMed] [Google Scholar]
- 20.Boylan GB, Kharoshankaya L, Wusthoff CJ. Seizures and hypothermia: importance of electroencephalographic monitoring and considerations for treatment. Semin Fetal Neonatal Med 2015;20(2):103–108. [DOI] [PubMed] [Google Scholar]
- 21.Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29(3):256–261. [DOI] [PubMed] [Google Scholar]
- 22.Scher MS, Alvin J, Gaus L, Minnigh B, Painter MJ. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol 2003;28(4):277–280. [DOI] [PubMed] [Google Scholar]
- 23.Laroia N, Guillet R, Burchfiel J, McBride MC. EEG background as predictor of electrographic seizures in high-risk neonates. Epilepsia. 1998;39(5):545–551. [DOI] [PubMed] [Google Scholar]
- 24.Glass HC, Wusthoff CJ, Shellhaas RA, et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology. 2014;82(14):1239–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wusthoff CJ, Sullivan J, Glass HC, et al. Interrater agreement in the interpretation of neonatal electroencephalography in hypoxic-ischemic encephalopathy. Epilepsia. 2017;58(3):429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd RO, O’Toole JM, Pavlidis E, Filan PM, Boylan GB. Electrographic Seizures during the Early Postnatal Period in Preterm Infants. J Pediatr 2017;187:18–25 e12. [DOI] [PubMed] [Google Scholar]
- 27.Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res 2008;80(2–3):99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sansevere AJ, Duncan ED, Libenson MH, Loddenkemper T, Pearl PL, Tasker RC. Continuous EEG in Pediatric Critical Care: Yield and Efficiency of Seizure Detection. J Clin Neurophysiol 2017. [DOI] [PubMed] [Google Scholar]
- 29.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72(22):1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng HH, Rajagopal SK, Sansevere AJ, et al. Post-arrest therapeutic hypothermia in pediatric patients with congenital heart disease. Resuscitation. 2018;126:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]