Abstract
We ectopically expressed the transcription factor Pitx2a, one of the Pitx2 isoforms, in HeLa cells by using a tetracycline-inducible expression system and examined whether Pitx2a was capable of modulating Rho GTPase signaling and altering the cell's cytoskeleton. Ectopic expression of Pitx2a induced actin-myosin reorganization, leading to increased cell spreading, suppression of cell migration, and the strengthening of cell-cell adhesion, marked by the accumulation and localization of β-catenin and N-cadherin to the sites of cell-cell contacts. Moreover, Pitx2a expression resulted in activation of the Rho GTPases Rac1 and RhoA, and the dominant negative Rac1 mutant N17Rac1 inhibited cell spreading and disrupted localization of β-catenin to the sites of cell-cell contacts. Both reorganization of actin-myosin and cell spreading require phosphatidylinositol 3-kinase activity, which is also necessary for activation of the Rho GTPase proteins. Pitx2a induced the expression of Trio, a guanine nucleotide exchange factor for Rac1 and RhoA, which preceded cell spreading, and the expression of Trio protein was down-regulated after the changes in cell spreading and cell morphology were initiated. In addition, Pitx2a also induces cell cycle arrest at G0/G1, most likely due to the accumulation of the tumor suppressor proteins p53 and p21. Our data indicate that the transcriptional activities initiated in the nucleus by Pitx2a result in profound changes in HeLa cell morphology, migration, and proliferation.
INTRODUCTION
Pitx2, a bicoid-type homeodomain transcription factor, has been implicated as one of the genes responsible for Rieger's syndrome in humans (Semina et al., 1996; Alward, 2000; Amendt et al., 2000). Rieger's syndrome is an autosomal-dominant genetic disorder characterized by ocular, craniofacial, and umbilical abnormalities with occasional defects in cardiac, limb, and pituitary development (Alward, 2000; Amendt et al., 2000). As one of the downstream targets for Sonic Hedgehog and Nodal, Pitx2 also plays a crucial role in determining left-right asymmetry during organogenesis in mice (Meno et al., 1998; Piedra et al., 1998; Ryan et al., 1998; Yoshioka et al., 1998), chickens (Logan et al., 1998; Piedra et al., 1998; Ryan et al., 1998; Yoshioka et al., 1998; St Amand et al., 2000), frogs (Ryan et al., 1998; Campione et al., 1999), and zebrafish (Campione et al., 1999). Knockout experiments further confirm that mice lacking Pitx2 show right pulmonary isomerism and defects in cardiac, ocular, tooth, and pituitary development (Gage et al., 1999; Kitamura et al., 1999; Lin et al., 1999; Lu et al., 1999). It is thought that cell proliferation, cell death, and cell motility, as well as changes in instructive signals for cell fate in local areas during organogenesis, are involved in directing asymmetric development of specific organs (Lin et al., 1999; Logan et al., 1998; Capdevila et al., 2000). Pitx2 was also isolated as a downstream target for the human acute leukemia ALL1 gene, the homolog of Drosophila trithorax. Loss-of-function of the ALL1 gene has been implicated in the development of human acute leukemia associated with abnormalities at 11q23 (Croce, 1999). Pitx2 is expressed in normal human bone marrow and leukemic cell lines with a normal ALL1 allele, but is not expressed in the leukemic cell lines in which ALL1 is rearranged (Arakawa et al., 1998). It is, therefore, reasonable to speculate that Pitx2 is likely to be involved in the regulation of cell differentiation and cell proliferation.
As a transcription factor, Pitx2 should activate and/or repress the transcription of its target genes to execute specific cellular functions. Pitx2 has been shown to bind to consensus and nonconsensus binding sites for bicoid-type homeodomain transcription factors and to transactivate promoters containing bicoid-specific binding sites (Amendt et al., 1998, 1999; Dave et al., 2000; Zhao et al., 2000; Hjalt et al., 2001). The lysine residue (K) at amino acid 50 in the homeodomain of Pitx2 is critical for its binding to the bicoid consensus site (TAATCC). In contrast, bicoid-related homeodomain proteins with a glutamine (Q) at position 50 in the homeodomain sequence will bind to a different consensus site (TAATGG). There are three isoforms of Pitx2 (a, b, and c) that result from alternative pre-mRNA splicing. Differential function of the different Pitx2 isoforms during organogenesis has been suggested recently in frogs (Schweickert et al., 2000), chickens (Yu et al., 2001), and zebrafish (Essner et al., 2000). However, the exact cellular function of Pitx2 still remains elusive.
Therefore, we initiated this project by asking what kinds of effects ectopic expression of Pitx2a in cultured HeLa cells would have on a number of important cellular functions, specifically actin-myosin cytoskeletal organization, cell motility, and cell proliferation. Our purpose was to trace the effects of Pitx2a on a cellular level in an effort to identify the various signal transduction mechanisms brought into play after its expression. HeLa cells that stably express mouse Pitx2a isoforms were generated by using the tetracycline-inducible expression system. We report herein that ectopic expression of Pitx2a in HeLa cells activates the Rho GTPase proteins Rac1 and RhoA, leading to marked changes in cell morphology, cell-cell contacts, cell motility, and the actin-myosin organization. We also demonstrate that the activation of the Rho GTPase proteins by Pitx2a requires phosphatidylinositol 3-kinase activity. In addition, ectopic expression of Pitx2a in HeLa cells leads to inhibition of cell proliferation and arrest of the cell cycle at G0/G1, most likely due to the accumulation of the gene products from the tumor suppressor gene p53 and its downstream target protein, p21. The overall effect is to convert this HeLa cell line into a stable line displaying a less malignant phenotype.
MATERIALS AND METHODS
Plasmids and Cell Culture
The mouse Pitx2a cDNA was amplified from embryonic day 12.5 total RNA derived from mouse head tissue by reverse transcription-polymerase chain reaction (RT-PCR) (Gage and Camper, 1997). The cDNA fragment was cloned into the HindIII/BamHI sites of pTRE-GFP (Wei and Adelstein, 2000), generating pTRE-GFP-Pitx2a. The K50R and K50Q variants of Pitx2a were generated using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instruction. All constructs were confirmed by nucleotide sequencing. Myc-tagged L63RhoA, N19RhoA, V12Rac1, and N17Rac1 were kindly provided by Dr. Alan Hall (University College, London, England).
The HeLa Tet-On cells (CLONTECH, Palo Alto, CA) were cultured in DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) in the presence of geneticin (100 μg/ml; Invitrogen). The expression plasmids were transfected into HeLa Tet-On cells by using an Effectene transfection kit (QIAGEN, Valencia, CA) according to the manufacturer's instruction. The transgenes were induced to express by addition of 0.5 μg/ml doxycycline (Dox) (Sigma, St. Louis, MO). The transfected cells were selected in 200 μg/ml hygromycin B (Invitrogen) and 200 μg/ml geneticin (Invitrogen) for 10–14 d. The resulting colonies were screened by fluorescent microscopy, after the addition of Dox for 24 h. The colonies with strong fluorescence in the nucleus were transferred to the medium without Dox. The stable cell lines were maintained in DMEM supplemented with 10% FBS in the presence of hygromycin B (100 μg/ml; Invitrogen) and geneticin (100 μg/ml; Invitrogen). Y27632 (a gift from Dr. Masafumi Arita, Yoshitomi Pharmaceutical Industries, Iruma-shi, Japan), LY294002 (Upstate Biotechnology, Lake Placid, NY), and PD98059 (Upstate Biotechnology) were dissolved in dimethyl sulfoxide and added to the culture at final concentrations indicated in the text
Immunofluorescence
Cells were grown on collagen-coated coverslips for the periods of time indicated, fixed in 3.7% paraformaldehyde for 15 min, permeabilized in 0.5% Triton X-100 for 10 min, blocked in 1% bovine serum albumin (BSA) for 1 h at 23°C, incubated with primary antibodies for 3 h at 23°C or overnight at 4°C, followed by incubation with secondary antibodies for 1 h at 23°C. Affinity-purified polyclonal rabbit antibodies against a carboxyl-terminal sequence of nonmuscle myosin heavy chain II-A (NMHC II-A; 1:1000) were previously described by Phillips et al. (1995). Affinity-purified polyclonal rabbit antibodies against phosphorylated myosin light chain (1:50) were a gift from Dr. Fumio Matsumura (Rutgers University, New Brunswick, NJ). Monoclonal antibodies to Myc (9E10; 1:500) and polyclonal antibodies to β-catenin (1:250) and hemagglutinin (HA) (1:200) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibodies to β-catenin (1:500), N-cadherin (1:500), and E-cadherin (1:500) were purchased from Zymed Laboratories (South San Francisco, CA). The secondary antibodies Alexa 594 goat antimouse IgG (1:1000), Alexa 594 goat antirabbit IgG (1:1000), and Alexa 350 goat antimouse IgG (1:500) were from Molecular Probes (Eugene, OR). Actin filaments were visualized by incubation with rhodamine-phalloidin (1:1000; Molecular Probes) for 1 h at 23°C. The coverslips were mounted using a Prolong antifade kit (Molecular Probes). The images were collected using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY).
Immunoblot Analysis
Total cell proteins from different stable cell lines were separated by SDS-6% or 4–20% PAGE, transferred to an Immobilon-P transfer membrane (Millipore, Bedford, MA), blocked with 5% nonfat milk for 1 h at 23°C, incubated with primary antibodies overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:5000; Santa Cruz Biotechnology) for 1 h at 23°C. The following primary antibodies were used: affinity-purified polyclonal rabbit antibodies against human Pitx2 (1:1000) as described by Hjalt et al. (2000); polyclonal antibodies to green fluorescent protein (GFP) (1:1000; CLONTECH); monoclonal antibodies to β-catenin (1:5000), N-cadherin (1:5000), and E-cadherin (1:1000; Zymed Laboratories); polyclonal goat antibodies to Trio (1:200) and monoclonal antibodies against p53 (1:1000) and RhoA (1:200; Santa Cruz Biotechnology); monoclonal antibodies to p21 (1:1000) and Rac1 (1:1000; Upstate Biotechnology); and monoclonal antibodies to β-tubulin (1:5000; Sigma). The blots were visualized by Renaissance Western Blot, and Western Blot Plus Chemiluminescence Reagent (PerkinElmer Life Sciences, Boston, MA).
Rac1 and RhoA Activity Assay
The stable cell lines were cultured with or without Dox for the periods of time as indicated, washed with phosphate-buffered saline, and then lysed in RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 2.5% Na-deoxycholate, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM Na3VO4, 1 mM NaF). The same amount of total protein from clarified lysate was incubated with GST-PBD (p21-binding domain of human PAK-1) or GST-RBD (Rho binding domain of rhotekin) to precipitate GTP-bound Rac1 and GTP-bound RhoA, respectively, according to the manufacturer's instructions (Upstate Biotechnology). Precipitated GTP-bound Rac1 or RhoA were resolved on a 4–20% SDS-PAGE and immunoblotted using monoclonal antibodies specific for Rac1 (1:1000; Upstate Biotechnology) and RhoA (1:200; Santa Cruz Biotechnology). Six percent of the cell lysate were also resolved in a 4–20% SDS-PAGE and immunoblotted to measure the total amount of Rac1 or RhoA.
Cell Migration Assay
Pitx2a cells were seeded in collagen-coated 60-mm dishes and cultured with or without Dox for 72 h. A wound was introduced in the central area of the confluent culture by using a pipette tip. The wound was incubated for a further 15 min or 12 h. The cells were fixed and stained with rhodamine-phalloidin to visualize actin filaments. Images were visualized with a Zeiss LSM 510 confocal microscope (Carl Zeiss) as well as with an Olympus 1X70 microscope with a 20× objective and digital images were obtained with WinView/32 software (Princeton Instruments, Trenton, NJ) and a PentaMax KDK-1400 charge-coupled device camera (Princeton Instruments).
Cell migration assays were also performed using a transwell Boyden chamber containing polycarbonate membrane inserts with 8-μm pores (Corning Glassworks, Corning, NY). The undersides of the membranes were either not coated or were coated with fibronectin (50 μg/ml) or collagen (100 μg/ml) for 3 h at 37°C and then blocked with 1% BSA in DMEM for 1 h at 37°C. Then 0.5 ml of 1% BSA was added to the lower chamber. For the uncoated inserts, 0.5 ml of DMEM with 10% FBS was added to the lower chamber. The Pitx2a cells cultured with or without Dox for 3 d were trypsinized, resuspended in DMEM with 1% BSA, and allowed to migrate to the undersides of the membranes for 4 h at 37°C. Membranes were fixed in 3.7% paraformaldehyde for 10 min. Cells remaining on the upper sides of the membranes were removed using a cotton swab. The migrating cells were stained with Coomassie Brilliant Blue G (Sigma) and counted.
Flow Cytometric Analysis
Flow cytometric analysis was carried out as previously described (Zhang et al., 2001). To arrest the cells in the G2-M phase, nocodazole (Sigma) was added to the culture at the final concentration of 0.3 μg/ml and incubated for a further 16 h and all the cells (including floating and attached cells) were collected for flow cytometric analysis.
RESULTS
Overexpression of Pitx2a Results in Changes in Cell Morphology
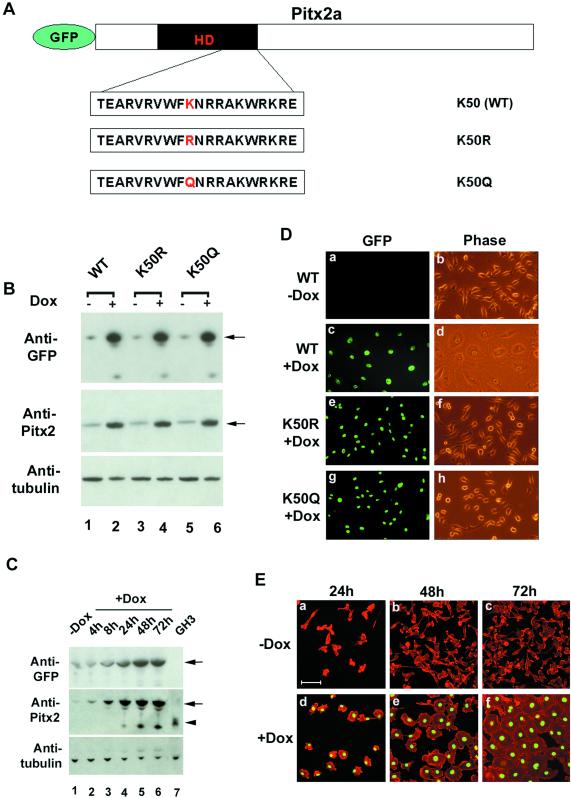
To test the effects of overexpression of Pitx2a in HeLa cells with respect to the actin-myosin cytoskeleton, we stably expressed mouse Pitx2a-GFP fusion protein (both wild-type and mutants of Pitx2a; Figure 1A) by using a tetracycline-inducible expression system that confers a high level of Pitx2a-GFP fusion protein expression in the presence of Dox (Figure 1B). In the absence of Dox, extremely small amounts of protein were expressed with no discernible effect. Figure 1C shows that these HeLa cells do not express endogenous human Pitx2 (Figure 1C, lane 1) and that the expression level of exogenous mouse Pitx2a in these cells was somewhat higher than that of endogenous Pitx2a shown for the rat pituitary cell line GH3, but was comparable (compare Figure 1C, lane 7, arrowhead, with lane 6, arrow, middle gel). Overexpression of wild-type Pitx2a (K50) in HeLa cells, in the presence of Dox, induced cell spreading and the formation of organized cell-cell contacts (Figures 1Dd and 1Ef and 2A). In contrast, Pitx2a cells cultured without Dox, like parental HeLa cells, showed unorganized cell-cell contacts, less cell spreading (Figure 1Db and 1Ec), and cells grew to overlap each other when they were plated at high density (our unpublished data). A significant amount of Pitx2a protein began to be detected by immunoblot analysis 8 h after addition of Dox (Figure 1C, lane 3), but there was no obvious change in cell spreading and cell morphology. By 24 h after Dox addition, high levels of Pitx2a protein were detected by Western blot (Figure 1C, lane 4) and obvious cell spreading was observed (Figure 1Ed). By 48 and 72 h, the cells expressing Pitx2a continued to show cell membrane protrusion and cell spreading until they made organized cell-cell contacts (Figure 1Ee,f; see below). The effects of Dox were reversible. After Dox was removed from the culture media, Pitx2a protein decreased to background levels in 3 d and the phenotype induced by overexpression of Pitx2a reverted to that of the parental HeLa cells in 6 d (our unpublished data).
Figure 1.
Overexpression of Pitx2a in HeLa cells induces cell spreading. (A) Schematic diagram of GFP-Pitx2a fusion DNA fragment used to generate the inducible cell lines. The amino acids of the third helix of the Pitx2a bicoid homeodomain (HD) are shown. The lysine (K) at position 50 of the wild-type (K50) Pitx2 homeodomain was either mutated to arginine (K50R) or to glutamine (K50Q). (B) Immunoblot analysis of GFP-Pitx2a fusion proteins in K50- (WT), K50R-, and K50Q-inducible cell lines cultured with (+) or without (−) Dox for 3 d, by using antibodies specific for GFP or Pitx2a. Tubulin serves as a control for sample loading. (C) Immunoblot analysis of GFP-Pitx2a fusion protein in the K50-inducible cell line −Dox for 3 d or +Dox for 4, 8, 24, 48, and 72 h. The GH3 cell line serves as a positive control for Pitx2 expression (lane 7). The fastest migrating band in lanes 4–6 is most likely a degradation product of GFP-Pitx2a. Note that Pitx2a migrates more slowly in lanes 1–6 compared with lane 7 due to GFP. (D) K50- (WT) (a–d), K50R- (e and f), and K50Q (g and h)-inducible cell lines were cultured without (a and b) or with (c–h) Dox for 3 d and visualized by immunofluorescence (GFP) and phase microscopy. (E) K50-inducible cells were cultured without (a–c) or with (d–f) Dox for 24 h (a and d), 48 h (b and e), and 72 h (c and f), followed by staining of actin with rhodamine-phalloidin (red). Bar, 100 μm. Green in D and E is due to GFP-Pitx2a fusion protein.
It has been shown that the residue at position 50 of the homeodomain of bicoid-related transcription factors is critical for differential DNA binding (Hanes and Brent, 1989; Treisman et al., 1989, 1992) and this residue can either be a lysine (known as the K50 class, such as Pitx2) or glutamine (known as the Q50 class, such as Ftz). We next asked whether this lysine residue is required for induction of cell spreading and the morphological changes shown herein after Pitx2a expression in HeLa cells. The relevant lysine residue was mutated to either arginine (K50R) or glutamine (K50Q) and stably expressed in HeLa cells by using the tetracycline-inducible expression system (Figure 1, A and B). The K50R and K50Q mutants did not cause changes in cell spreading and morphology (Figures 1Df,h and 2Af,h), suggesting that the cellular phenotype induced by overexpression of Pitx2a requires the Pitx2a homeodomain with lysine at position 50.
Overexpression of Pitx2a Induces Actin-Myosin Cytoskeletal Reorganization
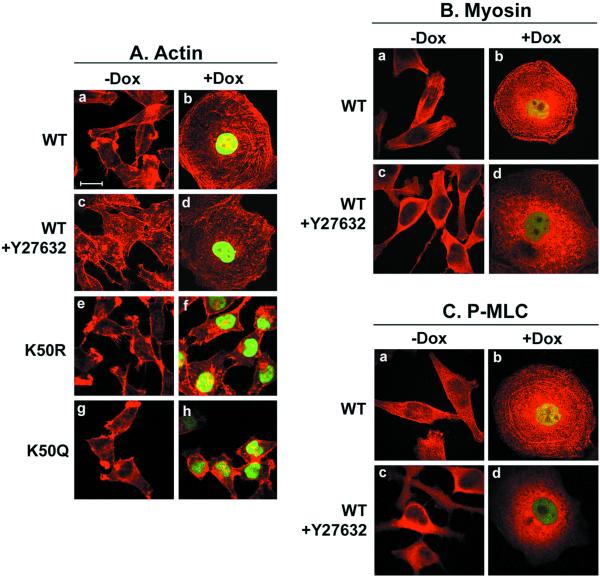
The dramatic change in cell morphology induced by the overexpression of Pitx2a led us to analyze the actin-myosin cytoskeletal system in greater detail. The parental HeLa Tet-On cells are not enriched in actin stress fibers, but they have obvious cortical actin filaments, similar to those observed in cells cultured in the absence of Dox (Figure 2Aa). Expression of Pitx2a in HeLa cells increased actin filament formation (Figure 2Ab). We and others (Nobes and Hall, 1995; Schmidt et al., 1997; Wei and Adelstein, 2000) have previously shown that expression of dominant active RhoA (L63RhoA) results in increased stress fiber formation. Therefore, it was of interest to see whether inhibition of the Rho signaling network would have an effect on the +Dox phenotype. Figure 2Ad shows that actin filaments were significantly disrupted after treatment with 20 μM Rho kinase inhibitor Y27632, suggesting that RhoA signaling was activated in HeLa cells expressing Pitx2a. In contrast to the expression of wild-type Pitx2a, expression of the K50R and K50Q mutant forms had no obvious effect on the formation of actin stress fibers (compare Figure 2Af,h with 2Ab).
The HeLa cells used in this study only express NMHC II-A (or MYH9) and not NMHC II-B (or MYH10). Overexpression of Pitx2a does not change the expression level of endogenous NMHC II-A (our unpublished data), but significantly increases myosin filament formation (Figure 2Bb).
The Rho kinase inhibitor Y27632 also markedly inhibited formation of myosin filaments induced by Pitx2a (Figure 2Bd), further confirming the importance of RhoA in the induction of actin-myosin filament formation by Pitx2a. Phosphorylation of the 20-kDa myosin light chain (MLC20) through RhoA/Rho kinase signaling results in the formation of actin-myosin filaments (Van Aelst and D'Souza-Schorey, 1997; Kaibuchi et al., 1999). Immunofluorescence studies with an antibody specific for phosphorylated serine-19 in MLC20 showed a similar pattern to that of myosin II-A staining (compare Figure 2Bb with 2Cb), suggesting that MLC20 is phosphorylated during actin-myosin reorganization induced by the overexpression of Pitx2a. The filamentous staining pattern of phosphorylated MLC20 was markedly decreased after treatment with the Rho kinase inhibitor Y27632 (Figure 2Cd), indicating that RhoA/Rho kinase signaling is involved in stress fiber formation induced by the overexpression of Pitx2a.
Overexpression of Pitx2a Activates the Rho GTPases Rac1 and RhoA
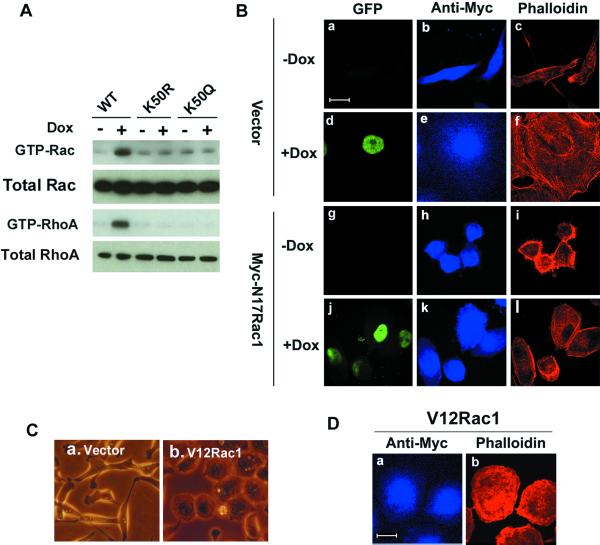
The ability of the Rho kinase inhibitor to decrease stress fiber formation suggested that the Rho GTPases, including Rac1 and RhoA, may play a role in regulating cell spreading and actin-myosin organization (Ridley et al., 1992; Nobes and Hall, 1995; Van Aelst and D'Souza-Schorey, 1997; Clark et al., 1998; Hall, 1998; Price et al., 1998; van Leeuwen et al., 1999; Berrier et al., 2000). We, therefore, investigated a possible role for the Rho GTPases in the development of the phenotype induced by overexpression of Pitx2a in HeLa cells. To assay the endogenous Rac1 and RhoA activity in these cells, we performed a GST-pulldown assay (see MATERIALS AND METHODS). Figure 3A shows that the activity of Rac1 and RhoA was increased in cells cultured in the presence of Dox and that expression of the K50R and K50Q mutants of Pitx2a had no effect on Rac1 and RhoA activity.
Figure 3.
Pitx2a induces cell spreading through activation of Rac1. (A) Activation of Rac1 and RhoA by Pitx2a. The inducible cell lines were cultured with (+) or without (−) Dox for 3 d and the GTP-bound GTPase proteins were isolated as described in MATERIALS AND METHODS and blotted with antibodies specific for Rac1 and RhoA. An equal amount of whole cell lysate was also blotted to evaluate total GTPase proteins. (B) Cell spreading and stress fiber formation induced by Pitx2a were inhibited by dominant negative Rac1 mutant (N17Rac1). K50-transfected cells were transfected with vector (a–f) or N17Rac1 (g–l) and cultured with or without Dox for a total of 72 h. Actin filaments were visualized by staining with Alexa 594 phalloidin (red). Vector or N17Rac1 were visualized by staining with anti-myc antibody, followed by Alexa 350 antimouse IgG (blue). (C) HeLa cell spreading induced by dominant active Rac1 mutant (V12Rac1). HeLa cells were transfected with vector (a) or V12Rac1 (b) and phase images were taken 24 h after transfection. (D) Cells from C,b were fixed and stained with Alexa 594 phalloidin and anti-myc antibody to visualize the actin filaments (red) and V12Rac1 (blue), respectively. Bar, 20 μm.
If the Rho GTPases are involved in the regulation of the cell phenotype induced by the expression of Pitx2a, dominant negative mutants of the Rho GTPases should be able to block the cell phenotype. Therefore, we transiently transfected Pitx2a cells with dominant negative mutants of the Rho GTPase proteins N17Rac1 and N19RhoA. The dominant negative Rac1 mutant N17Rac1 significantly inhibited cell spreading induced by Pitx2a (compare Figure 3Bf with 3Bl). Furthermore, the actin filaments in Pitx2a cells transfected with N17Rac1 and cultured in the presence of Dox (Figure 3Bl) were mainly cortical in location, similar to those in HeLa cells transfected with empty vector and cultured without Dox (compare Figure 3Bl with 3Bc). This suggests that the increased stress fiber formation in HeLa cells expressing Pitx2a (Figure 3Bf) might have resulted from the activation of RhoA by Rac1. In contrast, the dominant negative construct N19RhoA was not able to block cell spreading induced by Pitx2a (our unpublished data).
Previous work has shown that RhoA induces stress fiber formation through activation of Rho kinase (Chrzanowska-Wodnicka and Burridge, 1996; Kaibuchi et al., 1999). Indeed, stress fiber formation induced by Pitx2a was blocked by the Rho kinase inhibitor Y27632 (Figure 2Ad and 2Bd). However, Y27632 did not significantly inhibit cell spreading induced by Pitx2a, further confirming that the activation of Rac1, but not RhoA, was critical for the cell spreading induced by Pitx2a. We, therefore, expressed a dominant active mutant of Rac1 in these HeLa cells to see whether active Rac1 alone was capable of inducing cell spreading in the absence of Pitx2a expression. Figure 3Cb and 3Db show that expression of dominant active Rac1 significantly induces cell spreading in HeLa cells, further confirming the importance of the activation of Rac1 signaling for cell spreading induced by the expression of Pitx2a.
Cell Spreading Induced by Pitx2a Requires Activity of PI 3-Kinase
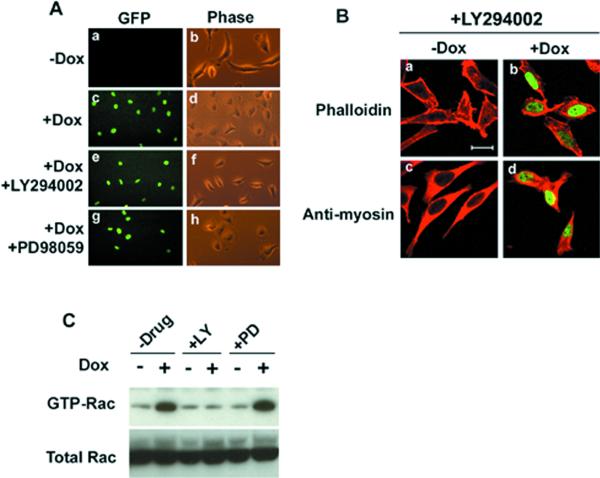
Phosphatidylinositol 3-kinase (PI3K) has been implicated in the regulation of the Rho GTPase activity and subsequent modulation of the actin cytoskeletal organization (Reif et al., 1996; Rodriguez-Viciana et al., 1997). Therefore, we examined whether PI3K could act as an upstream signal for the Rho GTPases involved in regulation of cell spreading and actin-myosin cytoskeletal reorganization induced by Pitx2a. We used the PI3K inhibitor LY294002 to test this possibility. HeLa cells transfected with Pitx2a were cultured with Dox for 24 h and then switched to a medium containing both 20 μM LY294002 and Dox for another 24 h. As shown in Figure 4Af, LY294002 significantly inhibited cell spreading. In contrast, the mitogen-activated protein kinase kinase-1 (MEK1) inhibitors 20 μM PD98059 and 10 μM U0126 had no significant effect on cell spreading induced by Pitx2a (Figure 4Ah; our unpublished data). Furthermore, the formation of actin-myosin filaments induced by Pitx2a was also inhibited by LY294002 (compare Figures 4Bb,d with 2Ab and 2Bb).
Figure 4.
PI3K activity is required for the cell spreading induced by Pitx2a. (A) LY294002 inhibits the cell spreading induced by Pitx2a. After K50-transfected cells were cultured without (a and b) or with (c–h) Dox for 24 h, no inhibitor (a–d), 20 μM LY294002 (e and f), or 20 μM PD98059 (g and h) was added and the cells were cultured for another 24 h. (B) LY294002 inhibits the actin-myosin filament reorganization induced by Pitx2a. Cells were treated (+Dox) and not treated (−Dox) with Dox for 24 h and then incubated with LY294002 for 24 h (Dox remaining in b and d). They were then fixed and stained with phalloidin or anti-myosin antibody to visualize the actin filaments (red, a and b) and myosin filaments (red, c and d), respectively. (C) LY294002 blocks activation of Rac1 by Pitx2a. K50-transfected cells from A were lysed and GTP-bound (active) Rac1 was isolated and blotted with antibody specific for Rac1 (see MATERIALS AND METHODS). Green in A and B is due to GFP-Pitx2a fusion proteins. Bar, 20 μm (B).
To substantiate a role for PI3K after Pitx2a expression, we used a GST-pulldown assay to detect a change in Rac1 activity after treatment of HeLa cells with Dox in the presence and absence of the PI3K inhibitor. As shown in Figure 4C, the PI3K inhibitor LY294002, but not the MEK1 inhibitor PD98059, significantly inhibited the activation of Rac1 by Pitx2a, confirming that the increase in GTP-Rac1 activity after Pitx2a expression was mediated by PI3K.
Cadherin-mediated Cell-Cell Contacts Are Induced by Overexpression of Pitx2a
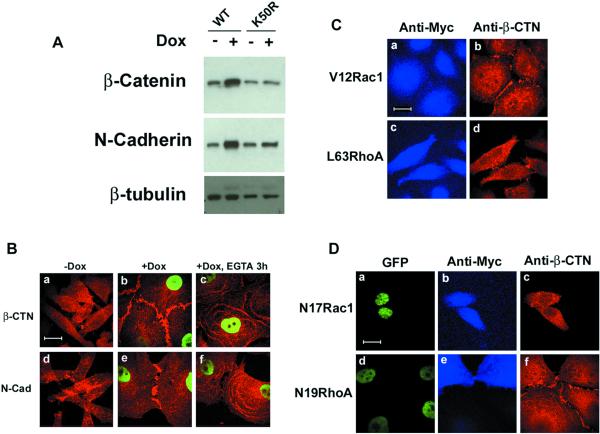
In addition to cell spreading, the most striking phenotype induced by Pitx2a expression was organized cell-cell interactions. HeLa Tet-On cells do not express E-cadherin, but do express N-cadherin, P-cadherin, α-catenin, β-catenin, and γ-catenin (Figure 5A; our unpublished data). Of these molecules, only β-catenin and N-cadherin were up-regulated by overexpression of Pitx2a (Figure 5A). As shown, both proteins localized to the sites of cell-cell contacts (Figure 5Bb,e). This cell-cell interaction is calcium-dependent because depletion of calcium in the media with EGTA led to diffuse distribution of β-catenin and N-cadherin in the cytoplasm (Figure 5Bc,f). P-Cadherin was also localized to the sites of cell-cell contacts (our unpublished data). Overexpression of the K50R mutant of Pitx2a did not up-regulate the expression of β-catenin and N-cadherin (Figure 5A) and did not alter the distribution of β-catenin and N-cadherin (our unpublished data).
Figure 5.
Pitx2a induces cadherin-mediated cell-cell contacts. (A) Western blot analysis of β-catenin and N-cadherin expression in K50 (WT) or K50R-transfected cells, cultured with (+) or without (−) Dox for 3 d. Note that K50 (lanes 1 and 2), but not K50R (lanes 3 and 4), Pitx2a up-regulates the expression of β-catenin and N-cadherin. (B) β-Catenin and N-cadherin localize to sites of cell-cell contacts. K50-transfected cells were cultured without (a and d) or with (b, c, e, and f) Dox for 3 d and stained with antibodies specific for β-catenin (red, a–c) or N-cadherin (red, d–f), followed by Alexa 594 anti-mouse IgG. In c and f, cells were incubated with EGTA (2 μM) for 3 h before being processed for immunofluorescence study. (C) V12Rac1, but not L63RhoA, induces localization of β-catenin to the sites of cell-cell contacts. HeLa cells were transfected with dominant active Rac1 (V12Rac1, a and b) or dominant active RhoA (L63RhoA, c and d) and stained with antibodies specific for myc (blue, a and c) or β-catenin (red, b and d). (D) N17Rac1, but not N19RhoA, inhibits the localization of β-catenin to the sites of cell-cell contacts induced by Pitx2a. K50-transfected cells cultured with Dox were transfected with the dominant negative Rac1 mutant (N17Rac1, a–c) or dominant negative RhoA mutant (N19RhoA, d–f) and stained with antibodies specific for myc (blue, b and e) or β-catenin (red, c and f). Bar, 20 μm.
Because Rac1 and RhoA have been implicated in the regulation of the formation of cell-cell contacts (Braga et al., 1997; Takaishi et al., 1997; Jou et al., 1998; Jou and Nelson, 1998; Stoffler et al., 1998), we investigated the role of Rho GTPase proteins in Pitx2a-induced cell-cell contacts. Expression of the dominant active Rac1 mutant (V12Rac1), but not the dominant active RhoA mutant (L63RhoA), was sufficient for β-catenin to localize to the sites of cell-cell contacts in these HeLa cells (Figure 5Cb,d). However, staining for β-catenin at the sites of cell-cell contacts in Pitx2a-expressing cells was more pronounced (compare Figure 5Bb with 5Cb), consistent with our results that β-catenin and N-cadherin are up-regulated by Pitx2a (Figure 5A). As expected, expression of the dominant negative Rac1 mutant (N17Rac1) inhibited localization of β-catenin to the sites of cell-cell contacts (Figure 5Dc). However, the dominant negative RhoA mutant (N19RhoA) did not significantly change the distribution of β-catenin (Figure 5Df).
Overexpression of Pitx2a Results in Inhibition of Cell Movement
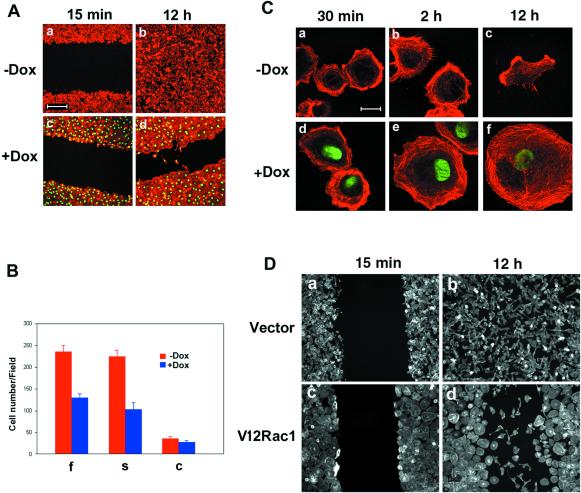
The Rho GTPase proteins have been shown to be implicated in the regulation of cell motility by modulating the actin-myosin cytoskeleton (Hordijk et al., 1997; Keely et al., 1997; Nobes and Hall, 1999; Evers et al., 2000). As demonstrated above, because the Rho GTPase proteins were activated by the expression of Pitx2a, we performed wound-healing assays to test whether overexpression of Pitx2a in HeLa cells has any effect on cell motility. After HeLa cells were cultured with or without Dox for 3 d, a wound was introduced using a pipette tip and wound healing was observed for 12 h. HeLa cells, when cultured without Dox, were able to move to the center of the wound (Figure 6Aa,b). In contrast, Pitx2a-expressing cells did not move to the center of the wound by 12 h (Figure 6Ac,d). These results indicated that overexpression of Pitx2a inhibited cell movement. In addition, the ability of cells to migrate toward fibronectin, collagen, or serum was also evaluated using a transwell Boyden chamber. As quantitated in Figure 6B, cell movement toward fibronectin (f) or serum (s) was inhibited by overexpression of Pitx2a (+Dox) compared with HeLa cells that were not expressing Pitx2a (−Dox). There was less of a difference in cell movement toward collagen (c).
Figure 6.
Overexpression of Pitx2a in HeLa cells inhibits cell movement. (A) Inhibition of cell migration by Pitx2a in wound healing assay. After K50 transfection, cells were cultured with or without Dox for 3 d and wound healing was observed for 15 min (a and c) or 12 h (b and d) after generation. (B) Inhibition of cell migration by Pitx2a in an assay with transwell Boyden chambers. After culture with or without Dox for 3 d, K50-transfected cells were trypsinized and cell migration toward fibronectin (f), serum (s), or collagen (c) was assayed for 4 h. The bars indicate the number of cells migrating through the membrane. (C) After culture with or without Dox for 3 d, K50-transfected cells were trypsinized and replated on a fibronectin surface for 30 min (a and d), 2 h (b and e), and 12 h (c and f). (D) Expression of the dominant active Rac1 mutant V12Rac1 in HeLa cells inhibits cell migration. HeLa cells were transfected with vector or V12Rac1 for 24 h, followed by a wound-healing assay for 15 min (a and c) or 12 h (b and d). Bar, 200 μm (A) and 20 μm (C).
To gain insight into the mechanism by which cell motility was affected by expression of Pitx2a, Pitx2a-expressing cells were trypsinized and replated on a fibronectin surface in a serum-free medium after being cultured with or without Dox for 3 d. Two hours after replating, cells cultured with Dox began to show more cell spreading (Figure 6Ce). By 12 h, there was not only a significant difference in cell spreading between cells cultured with or without Dox, but there was also an obvious difference in cell morphology. Cells cultured with Dox show cell membrane protrusion in every direction around the cells and the cells are not polarized (Figure 6Cf). In contrast, cells cultured without Dox are less spread and are polarized (Figure 6Cc), indicating that the cells were more motile than those cultured with Dox. In addition, the cells cultured with Dox show more actin filaments (Figure 6Ce), consistent with the idea that cells became stationary upon the bundling of actin-myosin filaments (Chrzanowska-Wodnicka and Burridge, 1996; Burridge, 1999).
Previous work has shown that Rac1/Cdc42 activity increased cell migration in a number of cell lines, such as rat embryonic fibroblast and T47D cells (Keely et al., 1997; Nobes and Hall, 1999). In contrast, there is also evidence demonstrating that activation of Rac1 suppresses epithelial cell migration (Hordijk et al., 1997; Sander et al., 1999). Our results show that overexpression of Pitx2a in these HeLa cells resulted in activation of Rac1, leading to changes in cell spreading and cell morphology as well as inhibition of cell motility. As demonstrated in Figure 3, C and D, expression of a dominant active Rac1 mutant (V12Rac1) in these cells led to the loss of a polarized morphology, indicating that cell migration might be affected by Rac1 in HeLa cells. Therefore, a wound-healing assay was performed to test this possibility. As shown in Figure 6D, expression of dominant active V12Rac1 alone (in the absence of Pitx2a) significantly inhibits cell migration. Of note is that the cells that did move to the center of the wound were polarized and did not express V12Rac1 (Figure 6Dd). In contrast, cells expressing V12Rac1, similar to cells expressing Pitx2a, were not polarized and did not move to the center of the wound (compare Figure 6Dd with 6Ad).
Increased Expression of a Guanine Nucleotide Exchange Factor, Trio, by Pitx2a
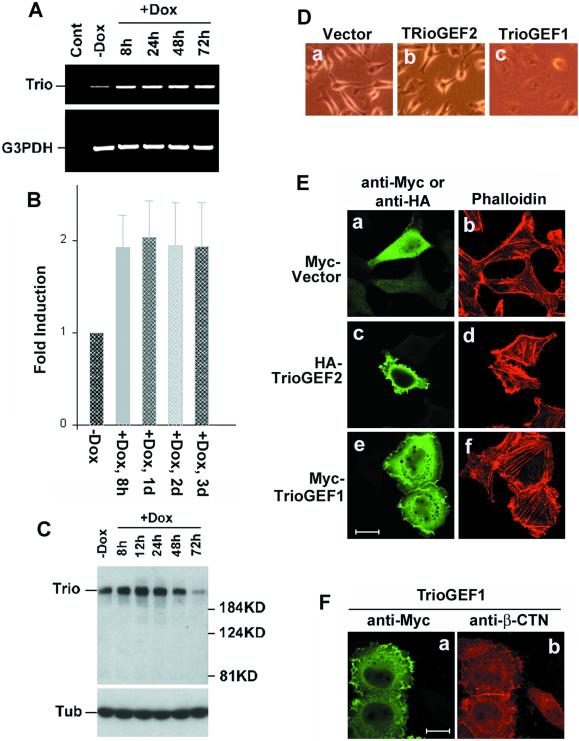
The guanine nucleotide exchange factors (GEFs) have been shown to be major activators of the Rho GTPases Rac1 and RhoA (Van Aelst and D'Souza-Schorey, 1997; Hall, 1998). Therefore, several GEFs were screened by RT-PCR to check their expression level in Pitx2a cells after the addition of Dox. One of them, Trio, was found to be up-regulated by overexpression of Pitx2a. Trio contains two GEF domains, GEF-D1 and GEF-D2, which can activate Rac1 and RhoA, respectively (Debant et al., 1996). Trio mRNA was increased approximately twofold at least 8 h after addition of Dox (Figure 7, A and B), suggesting that Trio might be directly induced by Pitx2a. The RT-PCR product was confirmed to be Trio by nucleotide sequencing. Interestingly, Trio protein begins to be down-regulated after 48 h in the presence of Dox and almost disappears after 72 h in the presence of Dox (Figure 7C). The beginning of the Trio down-regulation is coincident with the changes in cell morphology induced by Pitx2a, suggesting that the Pitx2a-induced cell phenotype can initiate a negative feedback loop to down-regulate expression of Trio protein.
Figure 7.
Up-regulation of Trio by Pitx2a. (A) RT-PCR analysis of Trio expression in Pitx2a cells after addition of Dox for different periods of time. A 600-bp DNA fragment from Trio was amplified. Cont, no reverse transcriptase. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was amplified as a control. (B) Quantitation of the data from three independent RT-PCR experiments. (C) Immunoblot analysis of Trio expression after the expression of Pitx2a in HeLa cells by using an antibody specific for Trio. (D) Phase images of HeLa Tet-On cells transfected with empty vector (a), TrioGEF2 (b), or TrioGEF1 (c). Note that expression of TrioGEF1 induces cell spreading and cell morphological changes, but not TrioGEF2. (E) HeLa Tet-On cells were transiently transfected with empty vector (a and b) or plasmids encoding HA-TrioGEF2 (c and d) or Myc-TrioGEF1 (e and f). Twenty-four hours after transfection, cells were fixed and stained with phalloidin (red) and antibodies specific for Myc-tagged or HA-tagged (green) protein. Note that TrioGEF1 induced cell spreading and morphological changes (e and f), but not TrioGEF2. (F) TrioGEF1 induced β-catenin accumulation at the sites of cell-cell contacts. Bar, 20 μm.
To further confirm the involvement of Trio in the induction of the cell phenotype, HeLa Tet-On cells were transfected with plasmids encoding TrioGEF1 or TrioGEF2, which have GEF activity toward Rac1 and RhoA, respectively (Bellanger et al., 1998, 2000; Blangy et al., 2000). Expression of TrioGEF1, but not TrioGEF2, induced similar cell morphological changes to those seen after Pitx2a expression, as shown in Figure 7, D and E. In addition, expression of TrioGEF1 also induced the formation of cell-cell contacts and accumulation of β-catenin to the sites of cell-cell interactions (Figure 7F).
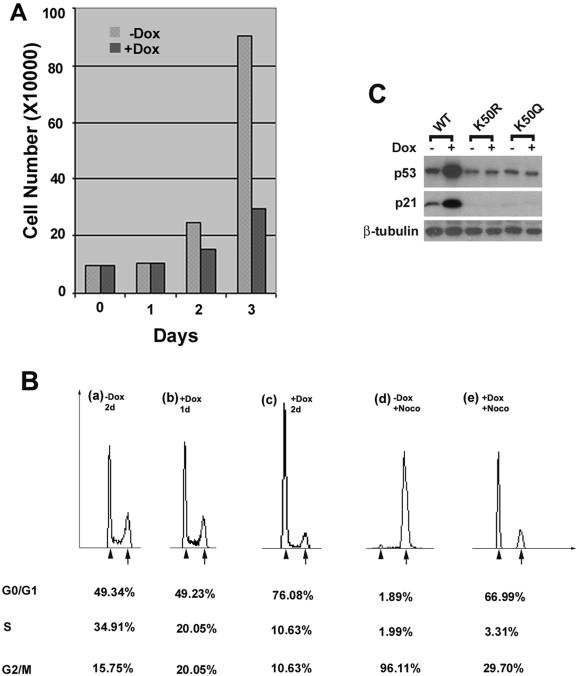
Overexpression of Pitx2a Leads to Inhibition of Cell Proliferation and Cell Cycle Progression
Pitx2 has also been isolated as a downstream target for the human acute leukemia ALL1 gene (Arakawa et al., 1998), indicating that Pitx2 might be involved in the regulation of cell proliferation and/or differentiation. Therefore, we also investigated whether overexpression of Pitx2a in HeLa cells has an effect on cell proliferation. Treatment of K50-transfected cells with Dox for 3 d inhibited cell proliferation by >50% (Figure 8A). In contrast, expression of K50R or K50Q Pitx2a mutants did not have a significant effect on cell proliferation (our unpublished data). Analysis of the cell cycle by flow cytometry shows that after 2-d culture in the presence of Dox, ∼76% of the cells were in G0/G1 (Figure 8Bc), compared with 49% of the cells that were untreated (Figure 8Ba). This suggests that overexpression of Pitx2a in HeLa cells induces an arrest of the cell cycle at G0/G1. To confirm this finding, the cells were treated with nocodazole for 16 h, to arrest the cells in the G2-M phase after being cultured for 24 h in the presence or absence of Dox and then the cells were analyzed by flow cytometry. Whereas 96% of the untreated HeLa cells (i.e., without Dox, but treated with nocodazole) arrested in G2-M (Figure 8Bd), only 30% of the Dox-treated cells arrested at this step (Figure 8Be). This result strongly suggests that overexpression of Pitx2a causes cells to arrest at G0/G1. These effects were also reversible as flow cytometric analysis showed that the cell cycle profile reverts to normal 6 d after removal of Dox and trypan blue staining confirmed that these cells were still viable (our unpublished data).
Figure 8.
Overexpression of Pitx2a results in the accumulation of p53 and p21, leading to cell cycle arrest. (A) Inhibition of cell proliferation by Pitx2a. K50-transfected cells were cultured with or without Dox for 1, 2, or 3 d and the cells were counted. (B) Cell cycle arrest induced by Pitx2a. After culture without Dox for 2 d (a), with Dox for 1 d (b) or 2 d (c), and with or without Dox for 2 d plus nocodazole for 16 h (d and e), Pitx2a cells were fixed, stained with propidium iodide, and analyzed by flow cytometry. Arrowhead and arrow indicate 2N and 4N nuclei, respectively. (C) Immunoblot analysis for p53 and p21 expression in K50- (WT), K50R-, and K50Q-transfected cells cultured with or without Dox. Equal loading of the samples was evaluated by blotting with antibody for β-tubulin.
To understand the mechanism responsible for cell cycle arrest induced by the overexpression of Pitx2a, we used antibodies specific for some of the cell cycle regulatory proteins to quantitate their expression. We found that p53 protein accumulated in HeLa cells overexpressing Pitx2a (Figure 8C). p21 was also up-regulated by Pitx2a in these cells, consistent with previous evidence that p21 is a downstream target for p53 and is transcriptionally activated by p53 (Levine, 1997). In contrast, neither the K50R nor K50Q mutants of Pitx2a was capable of inducing the accumulation of p53 and p21 proteins (Figure 8C). These results are also consistent with previous reports demonstrating that accumulation of the p53 and p21 proteins is able to arrest the cell cycle at G1 (Levine, 1997).
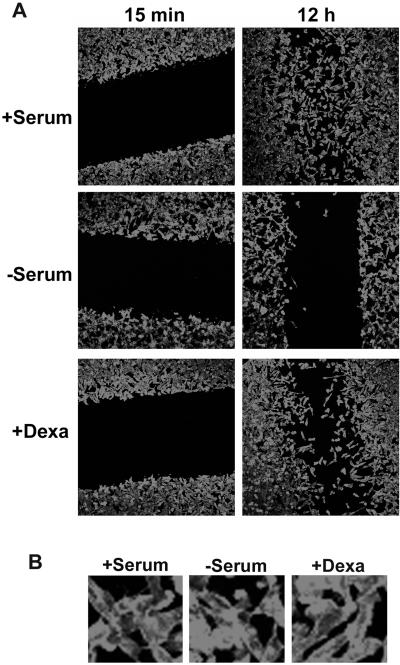
Because cell cycle arrest at G0/G1 is known to cause many secondary consequences, we therefore asked whether G0/G1 arrest, induced by means other than Pitx2a, is able to generate phenotypic changes similar to those induced by the expression of Pitx2a in HeLa cells. When HeLa Tet-On cells were arrested at G0/G1 by serum starvation or dexamethasone treatment for 24 or 36 h, we did not observe the increased cell spreading and morphological changes seen in Pitx2a cells cultured with Dox (Figure 9B). However, both serum starvation and dexamethasone treatment significantly inhibited cell migration in a wound-healing assay, as shown in Figure 9A. These results suggest that the decrease in cell migration we observed in HeLa cells may be a less specific consequence due to G0/G1 arrest, although Pitx2a may also play a role. On the other hand, the changes in cell morphology and cell-cell interaction appear to be a more specific consequence of Pitx2a expression.
Figure 9.
Cell cycle arrest at G0/G1 by serum starvation or dexamethasone inhibit cell migration. (A) After HeLa Tet-On cells were cultured with +serum, −serum, or the addition of 1 μM dexamethasone (+Dexa) for 24 h, a wound was introduced and the cells were continued in culture under the same conditions for another 15 min or 12 h. Cells were fixed and stained with phalloidin. (B) Enlarged images from panels above (12 h) showing no change in cell spreading and morphology.
DISCUSSION
The Rho GTPase proteins, including RhoA, Rac1, and Cdc42, play an essential role in the regulation of the actin-myosin cytoskeleton organization, which, in turn, defines cell morphology, cell-cell interaction, and cell migration (Van Aelst and D'Souza-Schorey, 1997; Hall, 1998; Kaibuchi et al., 1999; Evers et al., 2000). RhoA induces the phosphorylation of myosin light chains by activating its downstream effector Rho kinase, leading to the formation of stress fibers. Rac1 regulates lamellipodium formation and membrane ruffling. Cdc42 mediates filopodium formation. High Rac1 activity in cells leads to an epithelial-like morphology, whereas high RhoA activity is involved in maintaining a fibroblast-like morphology (Sander et al., 1999; Evers et al., 2000; Zondag et al., 2000). Using a culture cell system, we provide evidence showing that the bicoid-type homeodomain transcription factor Pitx2a is capable of activating the Rho GTPase proteins, leading to marked changes in cell morphology and cell migration. Although both Rac1 and RhoA are activated in HeLa cells expressing Pitx2a, our results indicate that it is the activation of Rac1 that is essential for the phenotypic changes. Activation of RhoA and the induction of stress fiber formation are most likely secondary to the activation of Rac1, consistent with previous reports with Swiss 3T3 cells, in which expression of active Rac1 resulted in the activation of RhoA and the formation of stress fibers (Nobes and Hall, 1995). In contrast, there is also evidence demonstrating that Rac1 can down-regulate the activity of RhoA in NIH3T3 cells and still induce an epithelial-like morphology and suppress cell migration (Sander et al., 1999). It is likely that Rac1 activity is dominant over RhoA activity in HeLa cells expressing Pitx2a, because expression of the dominant active form of Rac1 (V12Rac1) alone inhibited HeLa cell migration and resulted in an epithelial-like morphology. Consistent with this argument, expression of a constitutively activated form of RhoA (L63RhoA) induced formation of thick actin filaments and blocked the cell spreading induced by Pitx2a in HeLa cells (our unpublished data). These results are in agreement with the notion that the balance between the activities of Rac1 and RhoA is critical for determining cell morphology (Sander et al., 1999; Zondag et al., 2000).
In addition to the induction of Rho GTPase protein activation, Pitx2a also causes accumulation of p53 and p21 proteins, leading to cell cycle arrest and the inhibition of cell proliferation in HeLa cells. Like most of the cervical carcinoma cell lines, HeLa cells carry wild-type p53 and Rb genes. However, the expression of E6 and E7 proteins from high-risk human papillomaviruses, which are integrated in the genomes of HeLa cells, leads to the ubiquitination and degradation of p53 and Rb proteins (Villa, 1997; Thomas et al., 1999; Francis et al., 2000; Goodwin and DiMaio, 2000; Hietanen et al., 2000). Therefore, there is only a very low level of p53 and Rb protein in HeLa cells. The accumulation of p53 protein induced by Pitx2a is most likely due to increased protein stability and Pitx2a probably does not affect the transcription of p53, because p53 mRNA levels were unchanged (our unpublished data). Cell cycle arrest is a hallmark biological function of p53 in response to DNA damage or oncogenic activation, suggesting that the accumulated p53 protein in these HeLa cells, induced by Pitx2a, is responsible for inhibiting cell cycle progression. p21 has been shown to be directly up-regulated by p53 and to mediate cell cycle arrest induced by p53 (Levine, 1997). Therefore, it is likely that the up-regulation of p21 we observed is due to the accumulation of p53 protein. However, we cannot rule out the possibility that p21 was up-regulated by other signaling pathways, too.
How does Pitx2a induce the activation of the Rho GTPase proteins and cause the accumulation of p53 and p21 proteins? Our data indicate that PI3K activity is necessary for the activation of Rac1 by Pitx2a, leading to changes in cell morphology and actin cytoskeleton organization. Evidence suggests that PI3K plays an important role in regulating activity of GEFs, such as Vav, Sos-1, and Tiam1, which, in turn, activate Rac1 (Han et al., 1998; Sander et al., 1998; Bar-Sagi and Hall, 2000; Bustelo, 2000). However, PI3K is not required for the accumulation of the p53 protein because p53 accumulation was not blocked by the inhibitor LY294002 (our unpublished data). Interestingly, up-regulation of p21 was blocked by LY294002 (our unpublished data), suggesting that PI3K may play a role in the regulation of p21 expression induced by Pitx2a. Another plausible upstream component is Ras, because Ras signaling has been shown not only to induce the accumulation of the p53 protein but also to activate Rac1 signaling (Levine, 1997; Sherr, 1998; Bar-Sagi and Hall, 2000; Scita et al., 2000). However, endogenous Ras activity did not increase and the MEK1 inhibitor PD98059 did not block the accumulation of p53 and p21 proteins (our unpublished data), suggesting that Ras signaling is not involved in the activation of Rac signaling and the accumulation of p53 and p21 induced by Pitx2a. We presently do not know how Pitx2a induces the accumulation of p53 and p21.
As potent regulators of the actin cytoskeleton organization, which, in turn, defines cell morphology and cell migration, Rho GTPases have been implicated as mediators of tissue morphogenesis during embryonic development of Drosophila and Xenopus (Magie et al., 1999; Wunnenberg-Stapleton et al., 1999; Settleman, 2000). The activities of the Rho GTPases were regulated by the following three signaling components: 1) GEFs, which catalyze the exchange of GDP for GTP and activate the Rho GTPases; 2) GTPase-activating proteins, which catalyze the hydrolysis of GTP to GDP and inactivate the Rho GTPases; and 3) guanine nucleotide dissociation inhibitors, which can inhibit both the exchange of GTP and the hydrolysis of bound GTP. More than 20 members of GEFs have been isolated and among these GEFs, Tiam-1 and Vav drew our attention. Expression of Tiam-1 in epithelial cells activates Rac1 and suppresses cell migration (Hordijk et al., 1997). Vav can activate both Rac1 and RhoA and the expression of Vav induces actin cytoskeleton reorganization (Han et al., 1998; Liu and Burridge, 2000). The biological functions of both Tiam-1 and Vav require PI3K activity (Han et al., 1998; Sander et al., 1998). However, the expression level of Tiam-1 and Vav did not change after addition of Dox (our unpublished data), suggesting that Tiam-1 and Vav might not be involved in the activation of Rac1 induced by Pitx2a. On the other hand, Trio, another member of the GEFs, was up-regulated by Pitx2a. Both Trio mRNA and protein increased as early as 8 h after addition of Dox, suggesting that the induction of Trio was most likely due to direct transactivation by Pitx2a. Of note, the expression of Trio protein gradually decreased 48 h after addition of Dox and almost disappeared after 72 h in the presence of Dox, suggesting that a negative feedback loop was generated to suppress the expression of Trio protein after the development of cell phenotype. The importance of Trio homologs in Drosophila and Caenorhabditis elegans has been well documented. UNC-73, the C. elegans homolog of Trio, has been demonstrated to be important for cell migration and exon guidance, acting cell autonomously to regulate actin dynamics during cell and growth cone migration (Steven et al., 1998). More recently, UNC-73/Trio was shown to participate in a signaling system that orients and polarizes the migrating neuroblast in a left/right asymmetrical manner during development in C. elegans (Honigberg and Kenyon, 2000). Trio-deficient mouse embryos showed abnormal development of skeletal muscles as well as aberrant organization in several regions within the brain (O'Brien et al., 2000). Ectopic expression of Trio in cultured cells activates the Rho GTPases Rac1 and RhoA, leading to changes in actin cytoskeletal organization, cell migration and cell growth (Bellanger et al., 1998, 2000; Blangy et al., 2000).
The importance of Pitx2 during organogenesis, especially of ocular, pituitary, tooth, and heart tissue, has been well documented. The data described in this article suggest that ectopic expression of Pitx2a in cultured HeLa cells is capable of modulating both the Rac1 and p53 pathways, leading to major changes in cell morphology and the inhibition of cell cycle progression. These results indicate that the transcriptional activity of Pitx2a is capable of initiating nucleus-to-cytoplasm signals that have a profound effect on cell shape, migration, and proliferation. It has been reported that the nuclear protein Rb can influence Ras activity in both mammalian cells and nematodes (Lu and Horvitz, 1998; Lee et al., 1999). Recently, Eid et al. (2000) reported that complexes containing p300 and the nuclear oncoprotein SYT induced nucleus-to-cytoplasm signals that promote cell adhesion to a fibronectin matrix. The transcription factor Snail can act as a repressor of E-cadherin gene expression, inducing an epithelial-to-mesenchymal transition in Madin-Darby canine kidney cells (Batlle et al., 2000; Cano et al., 2000). Therefore, nucleus-to-cytoplasm signal transduction plays an important role in regulating cell morphology and cell proliferation. These findings represent the first steps on a cellular level toward an understanding of how Pitx2a might regulate organogenesis in vertebrates.
Figure 2.
Actin-myosin reorganization results from the overexpression of Pitx2a. All inducible cell lines were cultured without (−Dox) or with (+Dox) Dox for 3 d before being fixed and processed for immunofluorescence staining. (A) Actin filament formation induced by Pitx2a. Cells were stained with Alexa 594 phalloidin to visualize the actin filaments (red). In c and d, Pitx2a-inducible cells were treated with 20 μM Rho kinase inhibitor Y27632 for 60 min before being fixed and stained. (B) Myosin filament formation induced by Pitx2a. HeLa cells were fixed and stained with an antibody specific for NMHC II-A or MYH9, followed by Alexa 594 antirabbit IgG (red). In c and d, K50-transfected cells were treated with 20 μM Y27632 for 60 min before being processed for immunofluorescence study. (C) Phosphorylation of myosin light chains detected by a phospho-myosin light chain-specific antibody (P-MLC). K50-transfected cells were fixed and stained with an antibody specific for P-MLC, followed by Alexa 594 antirabbit IgG (red). In c and d, cells were treated with Y27632 for 60 min before being processed for immunofluorescence study. Bar, 20 μm.
ACKNOWLEDGMENTS
We thank Dr. Christian A. Combs (National Heart, Lung, and Blood Institute Light Microscopy Facility) for help and advice on the use of the confocal microscope, Dr. Shuling Zhang (National Cancer Institute) for help with flow cytometry, Drs. T.A. Hjalt and J.C. Murray for antibodies to Pitx2, Dr. A. Blangy for providing plasmids expressing TrioGEF1 and TrioGEF2, members of the Laboratory of Molecular Cardiology for useful discussions and criticisms, and Catherine S. Magruder for editorial assistance.
Footnotes
Online version of this article contains video material. Online version is available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–07-0358. Article and publication date are at www.molbiolcell.org/cgi/10.1091/mbc.01–07-0358.
REFERENCES
- Alward WL. Axenfeld-Rieger syndrome in the age of molecular genetics. Am J Ophthalmol. 2000;130:107–115. doi: 10.1016/s0002-9394(00)00525-0. [DOI] [PubMed] [Google Scholar]
- Amendt BA, Semina EV, Alward WL. Rieger syndrome: a clinical, molecular, and biochemical analysis. Cell Mol Life Sci. 2000;57:1652–1666. doi: 10.1007/PL00000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendt BA, Sutherland LB, Russo AF. Multifunctional role of the Pitx2 homeodomain protein C-terminal tail. Mol Cell Biol. 1999;19:7001–7010. doi: 10.1128/mcb.19.10.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendt BA, Sutherland LB, Semina EV, Russo AF. The molecular basis of Rieger syndrome. Analysis of Pitx2 homeodomain protein activities. J Biol Chem. 1998;273:20066–20072. doi: 10.1074/jbc.273.32.20066. [DOI] [PubMed] [Google Scholar]
- Arakawa H, et al. Identification and characterization of the ARP1 gene, a target for the human acute leukemia ALL1 gene. Proc Natl Acad Sci USA. 1998;95:4573–4578. doi: 10.1073/pnas.95.8.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia DH. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumor cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Bellanger JM, Astier C, Sardet C, Ohta Y, Stossel TP, Debant A. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat Cell Biol. 2000;2:888–892. doi: 10.1038/35046533. [DOI] [PubMed] [Google Scholar]
- Bellanger JM, Lazaro JB, Diriong S, Fernandez A, Lamb N, Debant A. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene. 1998;16:147–152. doi: 10.1038/sj.onc.1201532. [DOI] [PubMed] [Google Scholar]
- Berrier AL, Mastrangelo AM, Downward J, Ginsberg M, LaFlamme SE. Activated R-ras, Rac1, PI 3-kinase, and PKCepsilon can each restore cell spreading inhibited by isolated integrin beta1 cytoplasmic domains. J Cell Biol. 2000;151:1549–1560. doi: 10.1083/jcb.151.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouviere C, Fort P. Trio GEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci. 2000;113:729–739. doi: 10.1242/jcs.113.4.729. [DOI] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Crosstalk between Rac and Rho. Science. 1999;283:2028–2029. doi: 10.1126/science.283.5410.2028. [DOI] [PubMed] [Google Scholar]
- Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campione M, et al. The homeobox gene Pitx2: mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development. 1999;126:1225–1234. doi: 10.1242/dev.126.6.1225. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Vogan KJ, Tabin CJ, Izpisua-Belmonte JC. Mechanisms of left-right determination in vertebrates. Cell. 2000;101:9–21. doi: 10.1016/S0092-8674(00)80619-4. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;33:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce CM. Role of TCL1 and ALL1 in human leukemias and development. Cancer Res. 1999;59:s1778–s1783. [PubMed] [Google Scholar]
- Dave V, Zhao C, Yang F, Tung CS, Ma J. Reprogrammable recognition codes in bicoid homeodomain-DNA interaction. Mol Cell Biol. 2000;20:7673–7684. doi: 10.1128/mcb.20.20.7673-7684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debant A, Serra-Pages C, Seipel K, O'Brien S, Tang M, Park SH, Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci USA. 1996;93:5466–5471. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid JE, Kung AL, Scully R, Livingston DM. p300 interacts with the nuclear proto-oncoprotein SYT as part of the active control of cell adhesion. Cell. 2000;102:839–848. doi: 10.1016/s0092-8674(00)00072-6. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Branford WW, Zhang J, Yost HJ. Mesendoderm and left-right brain, heart and gut development are differentially regulated by pitx2 isoforms. Development. 2000;127:1081–1093. doi: 10.1242/dev.127.5.1081. [DOI] [PubMed] [Google Scholar]
- Evers EE, Zondag GC, Malliri A, Price LS, ten Klooster JP, van der Kammen RA, Collard JG. Rho family proteins in cell adhesion and cell migration. Eur J Cancer. 2000;36:1269–1274. doi: 10.1016/s0959-8049(00)00091-5. [DOI] [PubMed] [Google Scholar]
- Francis DA, Schmid SI, Howley PM. Repression of the integrated papillomavirus E6/E7 promoter is required for growth suppression of cervical cancer cells. J Virol. 2000;74:2679–2686. doi: 10.1128/jvi.74.6.2679-2686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Camper SA. Pituitary homeobox 2, a novel member of the bicoid-related family of homeobox genes, is a potential regulator of anterior structure formation. Hum Mol Genet. 1997;6:457–464. doi: 10.1093/hmg/6.3.457. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Goodwin EC, DiMaio D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc Natl Acad Sci USA. 2000;97:12513–12518. doi: 10.1073/pnas.97.23.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Hanes SD, Brent R. DNA specificity of the bicoid activator protein is determined by homeodomain recognition helix residue 9. Cell. 1989;57:1275–1283. doi: 10.1016/0092-8674(89)90063-9. [DOI] [PubMed] [Google Scholar]
- Hietanen S, Lain S, Krausz E, Blattner C, Lane DP. Activation of p53 in cervical carcinoma cells by small molecules. Proc Natl Acad Sci USA. 2000;97:8501–8506. doi: 10.1073/pnas.97.15.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalt TA, Amendt BA, Murray JC. PITX2 regulates procollagen lysyl hydroxylase (PLOD) gene expression: implications for the pathology of Rieger syndrome. J Cell Biol. 2001;152:545–552. doi: 10.1083/jcb.152.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalt TA, Semina EV, Amendt BA, Murray JC. The Pitx2 protein in mouse development. Dev Dyn. 2000;218:195–200. doi: 10.1002/(SICI)1097-0177(200005)218:1<195::AID-DVDY17>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Honigberg L, Kenyon C. Establishment of left/right asymmetry in neuroblast migration by UNC-40/DCC, UNC-73/Trio and DPY-19 proteins in C. elegans. Development. 2000;127:4655–4668. doi: 10.1242/dev.127.21.4655. [DOI] [PubMed] [Google Scholar]
- Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Jou TS, Nelson WJ. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- Kitamura K, et al. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 1999;126:5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- Lee KY, Ladha MH, McMahon C, Ewen ME. The retinoblastoma protein is linked to the activation of Ras. Mol Cell Biol. 1999;19:7724–7732. doi: 10.1128/mcb.19.11.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O'Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- Liu BP, Burridge K. Vav2 activates Rac1, Cdc42 and RhoA downstream from growth factor receptors but not beta1 integrins. Mol Cell Biol. 2000;20:7160–7169. doi: 10.1128/mcb.20.19.7160-7169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M, Pagan-Westphal SM, Smith DM, Paganessi L, Tabin CJ. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left-right asymmetric signals. Cell. 1998;94:307–317. doi: 10.1016/s0092-8674(00)81474-9. [DOI] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Lu X, Horvitz HR. lin-35 and lin-53, two genes that antagonize a C. elegans Ras pathway, encode proteins similar to Rb and its binding protein RbAp48. Cell. 1998;95:981–991. doi: 10.1016/s0092-8674(00)81722-5. [DOI] [PubMed] [Google Scholar]
- Magie CR, Meyer MR, Gorsuch MS, Parkhurst SM. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development. 1999;126:5353–5364. doi: 10.1242/dev.126.23.5353. [DOI] [PubMed] [Google Scholar]
- Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H, Hamada H. lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell. 1998;94:287–297. doi: 10.1016/s0092-8674(00)81472-5. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien SP, Seipel K, Medley QG, Bronson R, Segal R, Streuli M. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc Natl Acad Sci USA. 2000;97:12074–12078. doi: 10.1073/pnas.97.22.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CL, Yamakawa K, Adelstein RS. Cloning of the cDNA encoding human nonmuscle myosin heavy chain-B and analysis of human tissues with isoform-specific antibodies. J Muscle Res Cell Motil. 1995;16:379–389. doi: 10.1007/BF00114503. [DOI] [PubMed] [Google Scholar]
- Piedra ME, Icardo JM, Albajar M, Rodriguez-Rey JC, Ros MA. Pitx2 participates in the late phase of the pathway controlling left-right asymmetry. Cell. 1998;94:319–324. doi: 10.1016/s0092-8674(00)81475-0. [DOI] [PubMed] [Google Scholar]
- Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif K, Nobes CD, Thomas G, Hall A, Cantrell DA. Phosphatidylinositol 3-kinase signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Ryan AK, et al. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature. 1998;394:545–551. doi: 10.1038/29004. [DOI] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander EE, van Delft S, ten Klooster JP, Reid T, van der Kammen RA, Michiels F, Collard JG. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- Schweickert A, Campione M, Steinbeisser H, Blum M. Pitx2 isoforms: involvement of Pitx2c but not Pitx2a or Pitx2b in vertebrate left-right asymmetry. Mech Dev. 2000;90:41–51. doi: 10.1016/s0925-4773(99)00227-0. [DOI] [PubMed] [Google Scholar]
- Scita G, Tenca P, Frittoli E, Tocchetti A, Innocenti M, Giardina G, Di Fiore PP. Signaling from Ras to Rac and beyond: not just a matter of GEFs. EMBO J. 2000;19:2393–2398. doi: 10.1093/emboj/19.11.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semina EV, et al. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- Settleman J. Getting in shape with Rho. Nat Cell Biol. 2000;2:E7–E9. doi: 10.1038/71390. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 1998;12:2984–2991. doi: 10.1101/gad.12.19.2984. [DOI] [PubMed] [Google Scholar]
- St Amand TR, Zhang Y, Semina EV, Zhao X, Hu Y, Nguyen L, Murray JC, Chen Y. Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev Biol. 2000;217:323–332. doi: 10.1006/dbio.1999.9547. [DOI] [PubMed] [Google Scholar]
- Steven R, Kubiseski TJ, Zheng H, Kulkarni S, Mancillas J, Ruiz MA, Hogue CW, Pawson T, Culotti J. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998;92:785–795. doi: 10.1016/s0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- Stoffler HE, Honnert U, Bauer CA, Hofer D, Schwarz H, Muller RT, Drenckhahn D, Bahler M. Targeting of the myosin-I myr 3 to intercellular adherens type junctions induced by dominant active Cdc42 in HeLa cells. J Cell Sci. 1998;111:2779–2788. doi: 10.1242/jcs.111.18.2779. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Pim D, Banks L. The role of the E6–p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–7700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- Treisman J, Gonczy P, Vashishtha M, Harris E, Desplan C. A single amino acid can determine the DNA binding specificity of homeodomain proteins. Cell. 1989;59:553–562. doi: 10.1016/0092-8674(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Treisman J, Harris E, Wilson D, Desplan C. The homeodomain: a new face for the helix-turn-helix? Bioessays. 1992;14:145–150. doi: 10.1002/bies.950140302. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FN, van Delft S, Kain HE, van der Kammen RA, Collard JG. Rac regulates phosphorylation of the myosin-II heavy chain, actomyosin disassembly and cell spreading. Nat Cell Biol. 1999;1:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- Villa LL. Human papillomaviruses and cervical cancer. Adv Cancer Res. 1997;71:321–341. doi: 10.1016/s0065-230x(08)60102-5. [DOI] [PubMed] [Google Scholar]
- Wei Q, Adelstein RS. Conditional expression of a truncated fragment of nonmuscle myosin II-A alters cell shape but not cytokinesis in HeLa cells. Mol Biol Cell. 2000;11:3617–3627. doi: 10.1091/mbc.11.10.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunnenberg-Stapleton K, Blitz IL, Hashimoto C, Cho KW. Involvement of the small GTPases XRhoA and XRnd1 in cell adhesion and head formation in early Xenopus development. Development. 1999;126:5339–5351. doi: 10.1242/dev.126.23.5339. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, et al. Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left-right asymmetry. Cell. 1998;94:299–305. doi: 10.1016/s0092-8674(00)81473-7. [DOI] [PubMed] [Google Scholar]
- Yu X, St Amand TR, Wang S, Li G, Zhang Y, Hu YP, Nguyen L, Qiu MS, Chen YP. Differential expression and functional analysis of Pitx2 isoforms in regulation of heart looping in the chick. Development. 2001;128:1005–1013. doi: 10.1242/dev.128.6.1005. [DOI] [PubMed] [Google Scholar]
- Zhang SL, DuBois W, Ramsay ES, Bliskovski V, Morse III HC, Taddesse-Heath L, Vass WC, DePinho RA, Mock BA. Efficiency alleles of the Pctr1 modifier locus for plasmacytoma susceptibility. Mol Cell Biol. 2001;21:310–318. doi: 10.1128/MCB.21.1.310-318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Dave V, Yang F, Scarborough T, Ma J. Target selectivity of bicoid is dependent on nonconsensus site recognition and protein-protein interaction. Mol Cell Biol. 2000;20:8112–8123. doi: 10.1128/mcb.20.21.8112-8123.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondag GC, Evers EE, ten Klooster JP, Janssen L, van der Kammen RA, Collard JG. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity, and epithelial-mesenchymal transition. J Cell Biol. 2000;149:775–782. doi: 10.1083/jcb.149.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]