Abstract
Objectives
There are positive relationships between physical and cognitive function in older adulthood; however, the strength of these relationships are inconsistent across studies. Although novel statistical tools provide flexibility to explore age-related differences in relationship magnitude, such methods have not been implemented in gerontological research. This study applied such methods to examine variations in relationship magnitude between physical function and cognition in healthy older adults (N = 2,783).
Method
Time-varying effects modeling (TVEM) is an extension of regression that models changes in relationships as a function of time-varying metrics like age. TVEM was used to examine if physical function (Turn 360, grip strength) predicted cognitive performance (memory, processing speed/attention, and reasoning) similarly across adults aged 65–90.
Results
All associations between Turn 360 and all cognitive domains were significant and positive; however, speed of processing had significant magnitude variation across age such that the young-old and the old-old demonstrated the strongest relationships. Associations between grip strength and all cognitive domains significantly strengthened with increased age.
Discussion
Results suggest that depending on the sample age, there may be inconsistencies in the relationships between physical and cognitive performance. Future research should explore these relationships longitudinally to better elucidate discrepant findings.
Keywords: Balance, Cognitive aging, Strength, Time-varying effects modeling
Physical function, conceptualized as the ability to carry out physical activities necessary for daily living (Painter, Stewart, & Carey, 1999), is an important component of older adults’ health and well-being. Poor physical function is consistently associated with adverse outcomes, including diminished cognitive performance and accelerated cognitive decline, although the exact temporal nature and magnitude of these relationships remains unclear (Alfaro-Acha et al., 2006; Anstey, Lord, & Williams, 1997; Atkinson et al., 2007, 2010; Blankevoort et al., 2013; Buchman, Boyle, Leurgans, Barnes, & Bennett, 2011; Clouston et al., 2013; Desjardins-Crépeau et al., 2014; Emery, Finkel, & Pedersen, 2012; Gale, Allerhand, Aihie Sayer, Cooper, & Deary, 2014; Infurna, Gerstorf, Ryan, & Smith, 2011; Krall, Carlson, Fried, & Xue, 2014; van Iersel, Kessels, Bloem, Verbeek, & Olde Rikkert, 2008). There are at least two likely explanations for these results. The first is that often only one measure per physical or cognitive construct is used (Clouston et al., 2013), which may not accurately reflect the intended construct. The second reason is that the association strengths vary as a function of age in a way that violates assumptions of linearity necessary for more traditional regression methodologies (Blankevoort et al., 2013; Clouston et al., 2013). To address these limitations, the current study uses a novel statistical approach to begin disentangling the interrelatedness of multiple physical and cognitive function measures across the older adult developmental continuum.
Generally, there is a positive relationship between physical and cognitive function (Alfaro-Acha et al., 2006; Anstey et al., 1997; Blankevoort et al., 2013; Nieto, Albert, Morrow, & Saxton, 2008; Voelcker-Rehage, Goode, & Staudinger, 2010). However, work examining differential relationships by specific physical and cognitive function domains are limited. Most frequently, studies implement one measure of physical or cognitive function. The few studies that use multiple cognitive measures tend to find differential associations between physical and cognitive function. For example, both simple and complex physical function measures positively predict dementia status (Blankevoort et al., 2013; Clouston et al., 2013), but they do not consistently predict processing speed, memory, or executive function domains (Blankevoort et al., 2013; Clouston et al., 2013). While complex physical function is associated with processing speed (Demnitz et al., 2016; Rosano et al., 2005), simple physical function is associated with executive function (Blankevoort et al., 2013). Additionally, neither simple nor complex physical function domains consistently predict memory (Blankevoort et al., 2013). These differential and sometimes inconsistent relationships demonstrate the necessity of incorporating a wider range of measures to elucidate these complex relationships rather than relying on single global measures of physical and cognitive functioning or dementia status (e.g., Mini-Mental State Examination [MMSE]). The current study will address this gap by including multiple measures of physical function and composite scores representing three cognitive domains.
Physical function has been shown to deteriorate with advancing age, with accelerated performance degradation among the oldest-old (Payette et al., 2011). Similar patterns of age-related cognitive decline have been found, with more rapid decline observed near death (i.e., terminal drop; Gerstorf, Ram, Hoppmann, Willis, & Schaie, 2011). Hence, the relationship between cognitive and physical performance may not be accurately reflected by estimates obtained in widely implemented statistical analyses that assume a constant linear relationship between physical function and cognition throughout adulthood. Recent evidence indicates that constant linear associations cannot be assumed. In adults aged 65 and older, decrements in grip strength were associated with decreases in cognitive performance, but this relationship was not observed in middle-aged adults (ages 40–64; Sternäng et al., 2015). Additionally, accelerated decline in grip strength was associated with accelerated decline in spatial ability and semantic, episodic, and short-term memory, but not working memory or motor and perceptual speed (Praetorius Björk, Johansson, & Hassing, 2016). That is, as one was closer to death, steeper declines in spatial ability and semantic, episodic, and short-term memory were associated with steeper declines in grip strength.
We addressed limitations of previous work by investigating the stability of relationships between multiple measures of physical function and cognition as a function of age using the time-varying effects modeling (TVEM). This method is novel to gerontology but has been applied to adolescent risk-taking behaviors and interventions (Evans-Polce, Maggs, Staff, & Lanza, 2017; Lanza, Vasilenko, Dziak, & Butera, 2015; Mason et al., 2015). In short, TVEM allows researchers to examine whether relationships between predictor variables and outcome variables are stable over time or whether there are differences in relationship magnitude over time, including cross-sectional age (Evans-Polce et al., 2017). The method is an extension of linear regression models; however, relationships can be more flexibly modeled as TVEM does not impose parametric function (e.g., linear or quadratic) restrictions. This flexibility is particularly ideal in exploratory work and can inform whether higher-order parametric functions are necessary to appropriately model a phenomenon of interest. Traditionally, TVEM is used in the context of intensive longitudinal data, but it can also be used with cross-sectional data when variability in the time-varying metric of interest (i.e., age) and the sample size at each time point (i.e., age) provides sufficient power to properly recover relationships. For this study, the rationale is that age is a time metric that varies between individuals, thus it is possible to examine differences in associations across the age continuum.
Additionally, cross-sectional TVEM analyses may provide more useful information compared to models that include age as a moderator or quadratic term since interactions also assume a linear pattern. For instance, age2 models a nonlinear relationship, but the assumption is that the relationship magnitude differs in a constant and linear function. It also assumes symmetry around an inflection (or centering) point even when this may not be tenable or theoretically meaningful (Ghisletta, Cantoni, & Jacot, 2015). Although the traditional parametric assumption is useful for conceptual and statistical parsimony, it fails to capture the likely richness of the relationships between two variables. Thus, common statistical techniques relying on assumptions of time-stable relationships may not sufficiently account for ebbs and surges in the strength of such associations across the older adulthood continuum (Clouston et al., 2013). This novel statistical method will extend the current body of literature by exploring age-dependent relationship magnitudes, and may lead to a better understanding of discrepant findings, as well as inform future studies.
Aims and Hypotheses
The current study examined changes in the magnitude of associations across the older adult age continuum without the linearity assumption imposed by traditional regression analysis. The goal was to examine the age-varying relationships between physical function (i.e., lower limb extremity/balance and grip strength) and cognitive domains examined in previous work (Anstey et al., 1997; Blankevoort et al., 2013; Rosano et al., 2005), specifically memory, processing speed/attention, and reasoning. We also directly compared results across measures of physical function to ascertain whether the relationship magnitudes were similar, that is, did lower limb extremity/balance and grip strength have similar relationship magnitudes to each other across older adulthood. An additional goal was to qualitatively compare the TVEM results against competing traditional regression models with either age * physical function or age2 interaction effects.
We hypothesized that the relationships between both physical function measures and all cognitive domains would strengthen in magnitude with increasing age as previous work suggested that the relationship between physical and cognitive function in older ages is stronger than in the young-old (Payette et al., 2011; Sternäng et al., 2015). We also hypothesized that lower limb extremity/balance would be more strongly correlated with complex cognitive domains compared to grip strength, particularly in the young- and middle-old while grip strength would be more strongly correlated with complex cognitive tasks only in the oldest-old (Stijntjes et al., 2016). Lastly, we also expected the TVEM models would recover significant relationships better than traditional interaction effects; however, there were no a priori hypotheses about the ages at which the differential prediction would occur.
Method
Participant Characteristics
The study consisted of secondary data analyses using the baseline Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE, ClinicalTrials.gov Identifier NCT00298558) study data (Jobe et al., 2001). Briefly, ACTIVE was a multisite, randomized, controlled, single-blinded study designed to examine the effects of three cognitive training interventions (memory, processing speed/attention, and reasoning) on cognitive function and health outcomes in healthy ambulatory older adults. Individuals were excluded from the study if they: (a) were less than 65 years old at screening, (b) scored 23 or less on the MMSE, (c) required extensive assistance with dressing, bathing, or personal hygiene, (d) had medical conditions likely predisposing participants to immediate functional decline (e.g., recent stroke) or likely to result in mortality within 2 years, (e) had severe sensory losses, (f) had communicative difficulties so severe that participation would be problematic, (g) had recent cognitive training, or (h) were unavailable for testing during the testing and training periods of the study. Sample sizes differed for each model, as participants did not perform the grip strength task if they reported arthritis, tendonitis, wrist pain, or hand or arm surgery in the previous 3 months. Nineteen participants were missing values for both physical function measures and were dropped from the analyses, leaving a full analytic sample of 2,783 participants. Descriptive statistics for all measures are in Table 1 and extensive details regarding ACTIVE can be found elsewhere (Jobe et al., 2001).
Table 1.
Sample Demographics for Analytic Sample (N = 2,783)
| Variable | M (SD) or n (%) | Range | n |
|---|---|---|---|
| Male sex, n (%) | 672 (24.15%) | — | 2,783 |
| White race, n (%) | 2,025 (72.76%) | — | 2,783 |
| Education, M (SD) | 13.53 (2.70) | 4–20 | 2,781 |
| Self-reported general health | 3.37 (0.88) | 1–5 | 2,734 |
| Age, M (SD) | 73.60 (5.87) | 65–94 | 2,783 |
| Turn 360, M (SD) | 6.94 (2.08) | 1.0–31.5 | 2,736 |
| Grip strength, M (SD) | 24.11 (8.30) | 1.5–63.0 | 2,413 |
| Memory composite, M (SD) | 0.01 (0.91) | −3.90–2.06 | 2,767 |
| AVLT, M (SD) | 48.53 (10.56) | 0–73 | 2,767 |
| HVLT, M (SD) | 26.08 (5.531) | 1–73 | 2,783 |
| Processing speed/attention composite, M (SD) | 0.01 (0.89) | −2.12–1.48 | 2,772 |
| UFOV2, M (SD) | 133.19 (125.43) | 16–500 | 2,772 |
| UFOV3, M (SD) | 320.66 (134.21) | 43–500 | 2,772 |
| Reasoning composite, M (SD) | 0.01 (0.90) | −1.86–3.58 | 2,772 |
| Word series, M (SD) | 9.52 (4.90) | 0–30 | 2,776 |
| Letter series, M (SD) | 10.05 (5.60) | 0–15 | 2,772 |
| Letter sets, M (SD) | 5.75 (2.81) | 0–15 | 2,772 |
Note: SD = standard deviation; AVLT = Rey Auditory Verbal Learning Test; HVLT = Hopkins Verbal Learning Test; UFOV = Useful Field of View. AVLT and HVLT created the memory composite, UFOV2 and UFOV3 created the processing speed/attention composite, and word series, letter series, and letter sets created the reasoning composite.
Measures
Covariates
Sex (female = 0, male = 1), race (non-White = 0, White = 1), years of education (0–20, indicating no formal education to a doctoral degree), and self-rated general health served as covariates. Of the 2,783 participants in the analytic sample, the mean age was 73.60 (SD = 5.87), 75.85% were female, and 72.76% were White. Over 89.26% of the sample had at least 12 years of education. Health was assessed with a single-item question asking, “In general, would you say your health is excellent, very good, good, fair, or poor?” with higher scores indicated better health; 84.53% reported good to excellent health (M = 3.37, SD = .88).
Physical function
Lower limb function and balance was assessed with the two trials of the Turn 360 test (Steinhagen-Thiessen & Borchelt, 1999). Participants were asked to turn in a complete circle as quickly and safely as possible while testers recorded the number of steps required to complete each turn. The vast majority of participants (98%) were able to complete the task independently without an assistive device. The Pearson’s correlation between trials 1 and 2 was .86. Composite scores were calculated by averaging the z-scores of the two trials; if only one trial was administered, the single standardized score was used. The composite scores were then reverse-coded so higher scores indicated better performance.
Grip strength was measured using the validated Jamar hydraulic hand dynamometer (Al Snih, Markides, Ray, Ostir, & Goodwin, 2002; Lafayette Instruments, Lafayette, IN). Two trials were obtained for the participant’s dominant hand and recorded in kilograms (kg). Participants did not complete this measure if they reported worsening wrist pain, arthritis or tendonitis in the wrist, or surgery on the hand or arm during the previous 3 months. The Pearson’s correlation between trials 1 and 2 was .95. Composite scores were calculated by averaging the z-scores for both trials; the single trial standardized score was used if only one trial was administered. Higher scores indicated better performance.
Cognition
Composite scores for memory, processing speed/attention, and reasoning were created by averaging the z-scores of each assessment within the respective domain. Memory was assessed using the Rey Auditory Verbal Learning Test, or AVLT (Rey, 1941), and the Hopkins Verbal Learning Test, or HVLT (Brandt, 1991) which are standardized paper-and-pencil instruments with higher scores indicating better performance. The AVLT asks participants to recall a list of 15 words within 2 min across seven separate trials. The HVLT asks participants to recall lists of 12 words within 2 min over three trials. The tests yielded a Cronbach’s alpha of .80.
Processing speed/attention was measured using the computerized Useful Field of View (UFOV), subtests 2 and 3 (Edwards et al., 2005). UFOV2 assessed divided attention and required participants to identify a central object (truck or car) and the location of a second object on the periphery. UFOV3 assessed selective attention and repeated subtest 2; however, the central and peripheral objects were located within a field of distractor triangles to increase the task difficulty. The presentation time (ms) required to correctly complete each task with a 75% accuracy rate was recorded. Scores were reverse-coded after the composite creation so that higher scores indicated better performance. The tests yielded a Cronbach’s alpha of .73.
Reasoning performance was assessed with the word series (Gonda & Schaie, 1985), letter series (Thurstone & Thurstone, 1949), and letter sets (Ekstrom, French, Harman, & Derman, 1976) tasks with higher scores indicating better performance. These are standardized, timed paper-and-pencil instruments, with higher scores indicating better performance. In word series, participants were shown a series of days of the week or months of the year and asked to select the next week or month in the series. In letter series, participants saw strings of letters and were asked which letter would come next in the series. There were 30 series in the letter series test. In letter sets, participants had five sets of letters with four letters in each set. Four of the five sets followed some rule, and the participants had to correctly identify which four-letter set did not follow the rule. There were 15 total sets in this test. The tests yielded a Cronbach’s alpha of .88.
Analytic Strategy
Participants aged 90 years and older were collapsed for analyses (n = 16) due to insufficient coverage (i.e., power) and to aid with interpretability (see Supplementary Figure 1). Data were analyzed using SAS 9.4 and the TVEM macro 2.1.1 for continuous data (“normal” macro, available from https://methodology.psu.edu/downloads/tvem). All TVEM models used the p-spline estimation method and were adjusted for sex, race, education, and health. Physical function measures were treated as time-varying predictors of cognitive performance. That is, the relationship between the physical function measure and the cognitive outcome variable was allowed to vary across the time metric (i.e., age) and was not constrained to be equal across all ages. Instead of traditional point estimates, the output for these variables was graphically represented (see Figures 1 and 2). The solid black line was the estimated coefficient. Positive relationships were represented by a confidence band above zero, and the relationship strength was indicated by the coefficient value. Higher absolute values indicated stronger relationships. The light gray band was the 95% confidence band. It is important to note the band width was not constrained to be equal across the time continuum. In traditional regression, the 95% confidence interval is assumed to be constant across all values. Instances where the 95% confidence band “fans out” were likely due to few data points. For instance, this data set had fewer adults at the extreme end of the age range (at least 90 years old, n = 16), so the confidence band was wider. There were no specific p-values reported for the time-varying variables beyond the significance value set at p < .05. Although there were no output statistics to compare within-group differences, one way to ascertain if the relationship magnitude was similar for different ages was to compare the confidence bands. If the confidence band for one age was included in the confidence band of the second age, the relationship magnitude would be considered equal. Lastly, time-invariant predictor variables should be interpreted as unstandardized regression estimates (b).
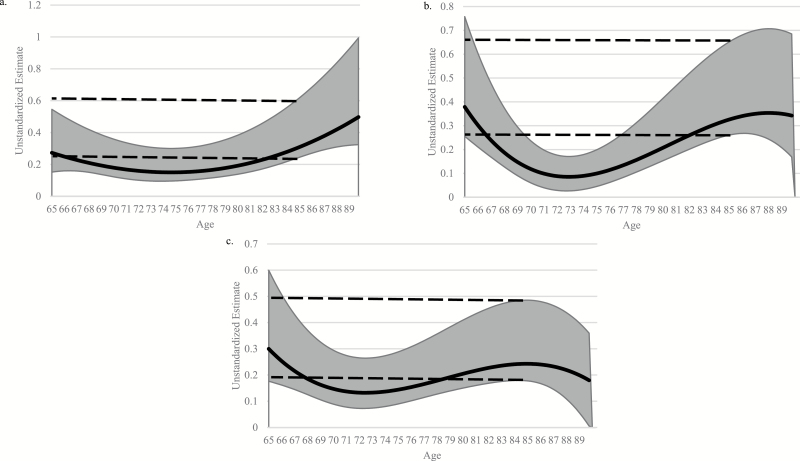
Figure 1.
Coefficient curve of Turn 360 on (a) memory composite score, (b) processing speed/attention composite score, and (c) reasoning composite score. Note: Models were adjusted for sex, race, education, and self-rated health. The solid line was the coefficient estimate, and the light gray band was the 95% confidence band. Higher coefficient scores indicated a higher relationship magnitude. A coefficient band intersecting 0.0 indicated a nonsignificant (p > .05) relationship. Analytic sample sizes for each model were (a) n = 2,672, (b) n = 2,676, and (c) n = 2,676. The dashed lines represented the 95% confidence band for 85-year-olds. When any part of the gray band fell between the dashed lines, this indicated that for the corresponding age, the estimate was not significantly different than for 85-year-old participants.
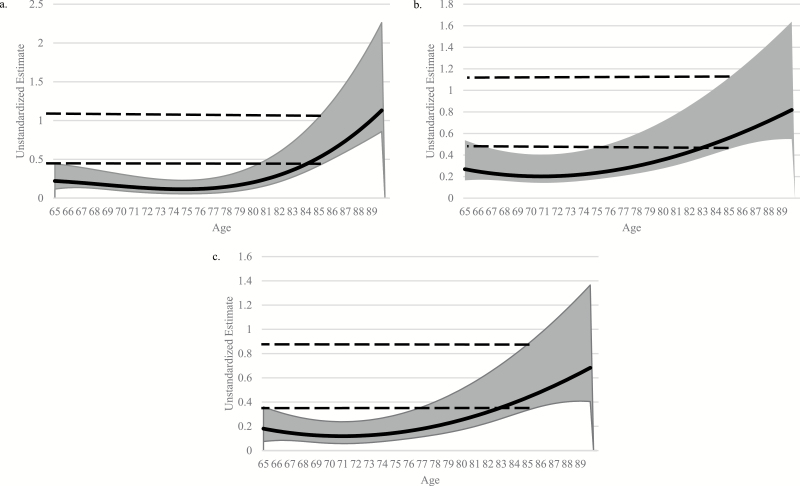
Figure 2.
Coefficient curve of grip strength on (a) memory composite score, (b) processing speed/attention composite score, and (c) reasoning composite score. Note: Models were adjusted for sex, race, education, and self-rated health. The solid line was the coefficient estimate, and the light gray band was the 95% confidence band. Higher coefficient scores indicated a higher relationship magnitude. A coefficient band intersecting 0.0 indicated a nonsignificant (p > .05) relationship. Analytic sample sizes for each model were (a) n = 2,364, (b) n = 2,367, and (c) n = 2,367. The dashed lines represented the 95% confidence band for 85-year-olds. When any part of the gray band fell between the dashed lines, this indicates that for the corresponding age, the estimate is not significantly different than for 85-year-old participants.
Multiple regression models with either age * physical function or age2 were analyzed to qualitatively compare against the TVEM approach. The same analytic sample was used (N = 2,783), and all models were conducted with SAS 9.4 using the PROC REG procedure. Sex, race, education, self-rated health, and age or physical function main effects were entered as covariates in simultaneous regression models. Significance for all models was set to p < .05.
Results
Turn 360 and Cognition
Memory
Better Turn 360 significantly predicted better memory performance across all ages (see Figure 1a) in TVEM. The magnitude of the relationship was slightly curvilinear, suggesting that the relationship between Turn 360 and memory is slightly stronger for adults 65–70 and 80+ than for older adults around 70–80 years of age. However, the relationship magnitude did not vary substantially (p > .05) since the confidence bands fell within each other across most ages. The relationship between Turn 360 and memory was stronger for older adults age 85 than age 75 (p < .05), but the general trend indicated little variation across older adulthood. Male sex (b = −.56, p < .001) was associated with worse performance, and White race (b = .24, p < .001) was associated with better performance. Education and self-rated health did not significantly predict memory performance (ps > .05).
In the age * Turn 360 linear regression model, there was no significant interaction effect (b < .01, p > .05) on memory. Age2 significantly predicted memory such that higher age was associated with a weaker effect of age on memory (b < −.01, p < .001). Better Turn 360 predicted better memory as well (b = .10, p < .001). For both interaction models, males performed significantly worse, whereas White race, having higher education, and having higher self-rated health were associated with better memory performance (ps < .001; Tables 2 and 3).
Table 2.
Results of Linear Regression Models: Unstandardized Estimates, or b, (Standard Error) of Age * Physical Function Interaction on Cognition
| Memory | Processing speed/attention | Reasoning | |
|---|---|---|---|
| Turn 360 | |||
| Male sex | −.60 (.03)*** | −.04 (.03) | −.08 (.03)* |
| White race | .39 (.03)*** | .27 (.03)*** | .55 (.03)*** |
| Education | .07 (.01)*** | .05 (.01)*** | .12 (.01)*** |
| Self-rated health | .12 (.02)*** | .11 (.02)*** | .15 (.02)*** |
| Age | −.05 (<.01)*** | −.06 (<.01)*** | −.04 (<.01)*** |
| Turn 360 | .09 (.02)*** | .06 (.02)** | .07 (.02)*** |
| Age * Turn 360 | <.01 (<.01) | <.01 (<.01) | <−.01 (<.01) |
| Grip strength | |||
| Male sex | −.72 (.05)*** | −.23 (.05)*** | −.13 (.04)** |
| White race | .38 (.04)*** | .32 (.04)*** | .56 (.03)*** |
| Education | .07 (.01)*** | .05 (.01)*** | .12 (.01)*** |
| Self-rated health | .14 (.02)*** | .11 (.02)*** | .15 (.02)*** |
| Age | −.04 (<.01)*** | −.06 (<.01)*** | −.04 (<.01)*** |
| Grip strength | .08 (.02)*** | .13 (.02)*** | .04 (.02)* |
| Age * grip strength | <.01 (<.01) | <.01 (<.01) | <.01 (<.01) |
Note: ***p < .001. **p < .01. *p < .05.
Table 3.
Results of Linear Regression Models: Unstandardized Estimates, or b, (Standard Error) of Age2 on Cognition
| Memory | Processing speed/attention | Reasoning | |
|---|---|---|---|
| Turn 360 | |||
| Male sex | −.60 (.03)*** | −.04 (.04) | −.08 (.03)* |
| White race | .38 (.03)*** | .27 (.03)*** | .55 (.03)*** |
| Education | .07 (.01)*** | .05 (.01)*** | .12 (<.01)*** |
| Self-rated health | .12 (.02)*** | .11 (.02)*** | .15 (.02)*** |
| Age | −.04 (<.01)*** | −.06 (<.01)*** | −.04 (<.01)*** |
| Age2 | <−.01 (<.01)*** | <−.01 (<.01)** | <−.01 (<.01) |
| Turn 360 | .10 (.02)*** | .07 (.02)*** | .07 (.01)*** |
| Grip strength | |||
| Male sex | −.71 (.05)*** | −.22 (.05)*** | −.13 (.05)** |
| White race | .38 (.04)*** | .31 (.04)*** | .56 (.03)*** |
| Education | .07 (.01)*** | .05 (.01)*** | .12 (.01)*** |
| Self-rated health | .14 (.02)*** | .11 (.02)*** | .16 (.02)*** |
| Age | −.04 (<.01)*** | −.05 (<.01)*** | −.04 (<.01)*** |
| Age2 | <−.01 (<.01)*** | <−.01 (<.01)* | <−.01 (<.01) |
| Grip strength | .07 (.02)** | .13 (.02)*** | .04 (.02) |
Note: ***p < .001. **p < .01. *p < .05.
Processing speed/attention
Better Turn 360 significantly predicted better processing speed/attention performance across the full sample (see Figure 1b) in TVEM. The relationship was curvilinear, suggesting stronger relationships for adults at the younger (ages 65 to 69) and older (ages 80 to 90+) ends of the age spectrum than for adults between the ages of 70 and 79. For example, at age 90, the coefficient between Turn 360 and processing speed/attention was .34. However, the coefficient was .11 at age 75. The relationship magnitudes for ages 65 to 69 and 80 to 90+ were substantially larger (p < .05) since they did not encompass the confidence bands of adults aged 70–79. White race (b = .12, p = .001) was associated with better processing speed/attention performance. Sex, education, and self-reported general health did not significantly predict processing speed/attention performance (ps > .05).
In the age * Turn 360 linear regression model, there was no significant interaction effect (b < .01, p > .05) on processing speed/attention. Age2 significantly predicted processing speed/attention such that higher age was associated with a weaker effect of age on processing speed/attention (b = .07, p < .001). There were no sex differences for either interaction models (ps > .05). White race, having higher education, and having higher self-rated health were associated with better processing speed/attention performance (ps < .001; Tables 2 and 3).
Reasoning
Better Turn 360 performance predicted significantly better reasoning performance between the ages of 65 and 90 (p < .05) and remained stable in magnitude (see Figure 1c) in TVEM. Although there appeared to be a curvilinear relationship, the confidence bands demonstrated nonsignificant differences in relationship magnitude. White race (b = .37, p < .001) and poorer general health (b = −.07, p < .001) were associated with better reasoning performance. Sex and education did not significantly predict reasoning performance (ps > .05).
In the age * Turn 360 interaction model, there was no significant interaction effect (b < −.01, p > .05) on reasoning. Age2 did not significantly predict reasoning (b < −.01, p > .05). In this model, better Turn 360 predicted better reasoning (b = .07, p < .001). For both interaction models, males performed significantly worse (ps < .05), whereas White race, having higher education, and having higher self-rated health were associated with better reasoning performance (ps < .001; Tables 2 and 3).
Grip Strength and Cognition
Memory
Stronger grip was significantly associated with better memory performance across all ages (see Figure 2a) in TVEM. This relationship was relatively stable from ages 65 to 80, and increased in magnitude after the age of 80. Male sex (b = −.85, p < .001) was associated with worse memory performance, and White race (b = .25, p < .001) was associated with better memory performance. Education and self-reported general health did not significantly predict memory performance (ps > .05).
In the age * grip strength linear regression model, there was no significant interaction effect (b < .01, p > .05) on memory. Age2 significantly predicted memory such that higher age was associated with a weaker effect of age on memory (b < −.01, p < .001). Better grip strength predicted better memory as well (b = .07, p < .01). For both interaction models, males performed significantly worse, whereas White race, having higher education (ps < .001), and having higher self-rated health were associated with better memory performance (ps < .001; Tables 2 and 3).
Processing speed/attention
Stronger grip was significantly related to better processing speed/attention performance across all ages (see Figure 2b) in TVEM. The magnitude of the relationship remained stable from ages 65 to 80, and became stronger after the age of 80. Male sex (b = −.41, p < .001) was associated with worse processing speed/attention performance, while White race (b = .19, p < .001) was associated with better processing speed/attention performance. Education and self-reported general health did not significantly predict processing speed/attention performance (ps > .05).
In the age * grip strength linear regression model, there was no significant interaction effect (b < .01, p > .05) on processing speed/attention. Age2 significantly processing speed/attention such that higher age was associated with a weaker effect of age on processing speed/attention (b < −.01, p < .05). In addition, better grip strength predicted better processing speed/attention (b = .13, p < .001). For both interaction models, males performed significantly worse, whereas White race, having higher education, and having higher self-rated health (ps < .001) were associated with better processing speed/attention performance (ps < .001; Tables 2 and 3).
Reasoning
Stronger grip was significantly associated with better reasoning performance across all ages (see Figure 2c) in TVEM. Similar to patterns of associations between memory and grip strength and memory and processing speed/attention, this relationship remained stable until around age 85, at which point it significantly strengthened in magnitude. Male sex (b = −.27, p < .001) was associated with poorer reasoning, while White race (b = .40, p < .001) and reporting poorer general health (b = −.06, p = .001) were associated with better reasoning performance. Education did not significantly predict reasoning performance (p > .05).
In the age * grip strength linear regression model, there was no significant interaction effect (b < .01, p > .05) on reasoning. Additionally, age2 (b < −.01, p > .05) and grip strength (b = .04, p > .05) did not significantly predict reasoning. For both regression models, males performed significantly worse (ps < .05), whereas White race, having higher education, and having higher self-rated health were associated with better reasoning performance (ps < .001; Tables 2 and 3).
Comparison of Turn 360 and Grip Strength on Relationship Magnitude
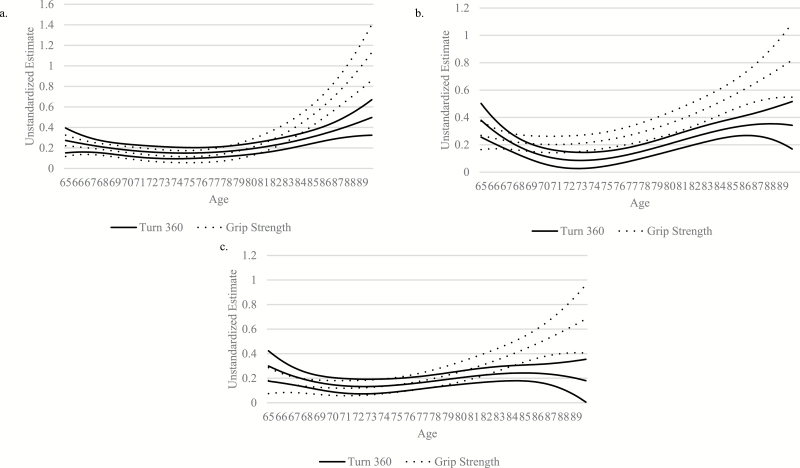
Results of the separate TVEM analyses were replotted on the same figure to allow direct comparison of relationship magnitudes between Turn 360 and grip strength on each of the cognitive domains. Relationship magnitudes were similar between Turn 360 and grip strength with memory until age 85, at which point grip strength was a significantly better predictor of memory than Turn 360 (Figure 3a). Turn 360 and grip strength similarly predicted processing speed/attention until age 73, at which point grip strength became a significantly better predictor of processing speed/attention than Turn 360 (Figure 3b). Lastly, Turn 360 and grip strength did not differentially predict reasoning until age 85, at which point grip strength became a significantly better predictor of reasoning than Turn 360 (Figure 3c).
Figure 3.
Turn 360 and grip strength model comparison for (a) memory, (b) processing speed/attention, and (c) reasoning. Note: Models were adjusted for sex, race, education, and self-rated health. The solid line was the coefficient estimate and 95% confidence band for Turn 360, while the dotted line was the coefficient estimate and 95% confidence band for grip strength. Higher coefficient scores indicated a higher relationship magnitude. A coefficient band intersecting 0.0 indicated a nonsignificant (p > .05) relationship. When the coefficient curves of one physical function measure overlapped the coefficient curves of the other physical function measure, this indicated both measures had similar estimates for the corresponding age. Turn 360 and grip strength similarly predicted memory until age 85, at which point grip strength was a significantly better predictor. Turn 360 and grip strength similarly predicted processing speed/attention performance until 73, at which point grip strength was a marginally better predictor. Turn 360 and grip strength similarly predicted reasoning performance until 85, at which point grip strength was a significantly better predictor.
Discussion
The influence of age is a critical consideration in empirical studies across numerous disciplines including the biological, psychological, developmental, and sociological sciences. This is particularly true when investigating cognition in older adulthood. Historically, age effects have been examined analytically by including age as grouping (Al Snih et al., 2002; Sternäng et al., 2015), control (Alfaro-Acha et al., 2006), or moderating variables (Blankevoort et al., 2013). Regardless of one’s conceptualization of age, these methods assume that age-related influences on physical and cognitive function are consistent, although recent work found this assumption may not be tenable (Clouston et al., 2013; Praetorius Björk et al., 2016; Sternäng et al., 2015). Evidence has suggested a seemingly undeniable, and likely dynamic, link between physical and cognitive function in older adulthood, but disparate findings across studies have not provided a clear understanding of the exact nature of that relationship’s magnitude. Results of the present study using a newer statistical technique, TVEM, indicate that this lack of clarity may be partly due to the way age has been commonly treated in statistical analyses. The magnitude of relationships between physical and cognitive function varied as a function of age across two measures of physical function and three domains of cognitive function. Additionally, the specific age variability patterns in relationship magnitudes differed according to the physical function measures and cognitive domains examined. For both memory and reasoning, Turn 360 and grip strength had statistically equivalent estimates; however, grip strength became a significantly better predictor for older adults 85–90 (Figure 3a and c). For processing speed/attention, Turn 360 and grip strength had statistically equivalent estimates until 73, at which point grip strength became a significantly better predictor than Turn 360 (Figure 3b).
There were generally curvilinear relationship magnitudes in the age-varying differences between Turn 360 and the cognitive domains. However, processing speed/attention was the only cognitive domain where the curvilinear relationship was significant indicating that Turn 360 was a stronger predictor of processing speed/attention for the young- and oldest-old than for middle-old (i.e., 70–80). This supports and extends previous work demonstrating relationships between measures of fluid intelligence, including processing speed/attention, and complex physical function tasks like walking speed (Clouston et al., 2013; Voelcker-Rehage et al., 2010). While the relationship remained significant across all ages in this sample, the demonstrable differences in relationship magnitude warrant future exploration to elucidate possible mechanisms. In the young-old, there is no candidate mechanism for the stronger relationship magnitude; however, there may be an unobserved third variable explaining the relationship, such as greater white matter integrity (Kerchner et al., 2012; Lu et al., 2011; Stewart, Tran, & Cramer, 2014). In the middle-old, there may be increased health and musculoskeletal difficulties affecting complex lower limb function that do not affect cognitive function (Payette et al., 2011); however, the inverse may be also true (Blankevoort et al., 2013). As a result, there is a short-term decoupling in the physical-cognitive relationship. Future work should examine possible explanations for the relationship magnitude shift to ascertain if this is a true phenomenon or a violation of the age convergence assumption (Sliwinski, Hoffman, & Hofer, 2010). For the processing speed/attention results, it may be that the oldest-old in this sample performed well because the unhealthy oldest-old would have been ineligible for the study. There was no evidence of floor effects for the oldest-old (data not shown), suggesting that these participants may be unusually healthy compared to those who were ineligible to participate. It may also be that the relationship magnitude is strengthened in the oldest-old due to terminal drops in both physical and cognitive function (Hajjar et al., 2009), although this observation has been limited to executive function and not extended to other domains such as processing speed/attention. This explanation also assumes the oldest-old are closer to death than the young- and middle-old. The relationships between Turn 360 and memory and reasoning in this study replicate previous work largely demonstrating significant relationships between complex physical function and cognitive domain performance in older adults (Blankevoort et al., 2013).
In comparison, associations between grip strength and performance in memory, processing speed/attention, and reasoning strengthened around age 85 and continued to increase in magnitude. These results suggest that grip strength and cognitive function are more tightly interwoven in the oldest-old than the young-old. This may lend additional explanations for inconsistencies in previous work. For example, Kuh and colleagues (2009) found that grip strength performance poorly predicted midlife cognitive performance, but better balance performance consistently predicted better cognitive performance. The current results reinforce and broaden these findings to a healthy older age group; present results suggest the young-old may physically function more similarly to middle adulthood than the middle- and oldest-old for simple physical function measures like grip strength. One potential reason for this apparent disconnect is that dynamic balance measures such as Turn 360 require the integration and coordination of multiple body systems (e.g., sensory, motor, and cognitive). Conversely, the grip strength assessment requires only hand grasping, which is a more fundamental motor movement. These relationships between Turn 360 and cognitive domains were consistent even when removing all participants who had missing grip strength scores (data not shown). For middle-aged adults and the young-old, complex physical tasks may be more appropriate for examining associated cognitive outcomes. For the middle-old and oldest-old, simpler physical function tasks may be more appropriate due to central nervous system degradations (Baltes & Lindenberger, 1997) or neural dedifferentiation (Heuninckx, Wenderoth, Debaere, Peeters, & Swinnen, 2005). For these older groups, complex physical function measures may be too complex, regardless of cognitive function (Kuh et al., 2009), but our data did not find evidence for floor effects (data not shown).
These results do not find support for a common cause since the pattern of results were not consistent across the two physical function domains, so future research is warranted to explore mechanisms for the differential relationships between physical and cognitive function domains. Although recent work posits that neurological mechanisms such as neural dedifferentiation (Sleimen-Malkoun, Temprado, & Hong, 2014) or myelin integrity (Lu et al., 2011) may explain the interrelatedness between physical and cognitive functions, a multitude of environmental factors affect physical and cognitive health (Thorpe et al., 2008). As such, simplifying the aging process to one or few mechanisms may be overly parsimonious. Future work should consider the impact of environmental factors and health on the physical-cognitive function link and whether those environmental factors are consistent across different domains.
In terms of the covariates, race was the most consistent predictor of cognitive performance across all three domains. These results are similar to other findings from the ACTIVE study (Tennstedt & Unverzagt, 2013). It is important to note that although there were significant baseline differences in cognitive performance by race, previous longitudinal examinations using ACTIVE demonstrated that the rate of change did not significantly differ between racial groups. This indicates that baseline effects in these analyses may not significantly affect cognitive aging trajectories for this sample (Tennstedt & Unverzagt, 2013). Surprisingly, poorer self-reported general health was associated with better reasoning performance. Self-reported health is associated with objective health outcomes (Wu et al., 2013), so it is unclear why this relationship was in an unexpected direction. The corresponding unstandardized estimates were small relative to the scale, so this may reflect statistically but not practically significant differences. Because the models reflect the unique contribution of self-reported general health after controlling for objective physical function, this may be indicative of a suppression effect. A bivariate Pearson’s correlation between self-reported general health and the reasoning composite score was in the expected direction (r = .30, p < .001). This suggests that the self-reported measure may be influenced by other constructs like self-efficacy or neuroticism. Previous work examining self-reported versus objective measures of cognitive performance have found poor concordance (Meltzer et al., 2017), so the same reporting bias may affect self-reported health scores.
There are a few limitations in the current analyses worth noting. These results are only generalizable to healthy older adults without dementia (i.e., MMSE ≥ 23), so it remains unclear how the time-varying relationship would change in more impaired individuals. Although our sample racial breakdown is representative of the larger U.S. racial makeup, it is also important to replicate these findings with an overrepresented non-White sample since the effect of race on the physical-cognitive function relationship is complex and warrants further examination (Ross, Sprague, Phillips, O’Connor, & Dodson, 2016; Smith-Ray, Makowski-Woidan, & Hughes, 2014). That is, the age-varying relationship may differ as a function of race and environmental factors such as sociodemographic status, so future work should utilize TVEM to examine whether the coefficient curves across racial groups mimic a similar pattern to each other. Thus, a larger non-White sample is necessary to ensure adequate sample sizes to estimate separate coefficient curves per racial group. Relatedly, these results may be cohort-specific since early life experiences, like early-life adversity or education, are related to physical (Birnie et al., 2011; Kasper et al., 2008) and cognitive function (Barnes et al., 2012; Wilson et al., 2009). These results should be replicated in future cohorts to ascertain whether they are generalizable age-related differences.
A second limitation is low statistical power due to small sample size at the upper bounds of the age range (i.e., 90+, n = 16). Because of the small sample size at this age, we were unable to reliably estimate relationship magnitudes without collapsing these participants across age. Despite this, the results suggest there may be relationships in the oldest-old worth examining. Thus, the field would benefit from future work examining these relationships using larger samples including the oldest-old adults. Finally, cross-sectional data were used. Since age is conceptualized as an indicator of time (i.e., time since birth) and varies in this sample, it is appropriate to use cross-sectional data (Evans-Polce et al., 2017). However, TVEM is unable to separate age, period, and cohort effects with cross-sectional data. It is important to note that like with other cross-sectional analyses, one can only discuss age differences and should not extrapolate findings to age-related changes. Future work should examine how these relationships may vary as function of time rather than as a function of cross-sectional age.
Despite these limitations of the current data set, there are strengths worth noting. This study utilized several objective measures of physical and cognitive function, addressing limitations previously noted about the importance of replication in several domains (Clouston et al., 2013). This is important because physical or cognitive function measures may not behave similarly to each other, and understanding the impact of a measure on the ability to predict cognitive performance is critical. For example, the relationship between grip strength and reasoning is significant, but the relationship will look weaker if the sample consists of only adults aged 65–75 compared to adults aged 80–90. The study also included a large geographically and racially diverse sample of healthy older adults. This is important for two reasons: (a) TVEM requires large sample sizes in order to converge or make estimations with high confidence, and (b) this allows us to understand general trends in the relationship between physical and cognitive function. These results suggest the age-varying trends may be similar for older adults across racial groups, but future research is necessary to explore the complex impact of race on physical and cognitive aging.
One last major strength is the ability to uncover age-related relationships that traditional age-moderated regression modeling would not capture. For example, traditional regression models revealed no significant interactions between age and either physical function measure (Table 2) for any cognitive domain, and found no relationships between age2 and reasoning (Table 3). In comparison, TVEM uncovered significantly larger relationship magnitudes for grip strength in the oldest-old. This demonstrates the utility of TVEM as an exploratory tool to capture the richness of physical-cognitive function relationships in older adulthood compared to other methods.
Taken together, these results suggest that there is a dynamic relationship between physical function and cognitive performance, and that TVEM is an appropriate method to begin to understand the way this relationship unfolds over age groups. This method is also a useful tool to determine which measurements of physical or cognitive function are most appropriate for the specific sample. Lastly, this method is helpful to determine if higher-order parametric functions should be included in order to better capture the true relationship between phenomena of interest. For example, it may be appropriate to use age2 as a covariate when examining the relationship between Turn 360 and processing speed/attention. Future work should continue to explore these relationships using longitudinal data with larger oldest-old and racially diverse samples.
Funding
This work was supported by cooperative agreements (U01AG14260; U01AG14263; U01AG14276; U01AG14282; U01AG14289; U01NR04507; U01NR04508) from the National Institute on Aging and the National Institute of Nursing Research, National Institutes of Health. B. N. Sprague received additional support by the Joseph and Jean Britton Distinguished Graduate Fellowship through The College of Health and Human Development and The Center for Healthy Aging, The Pennsylvania State University.
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
Special thanks to Dr. Stephanie Lanza for her helpful feedback and guidance on TVEM.
References
- Al Snih S. Markides K. S. Ray L. Ostir G. V. & Goodwin J. S (2002). Handgrip strength and mortality in older Mexican Americans. Journal of the American Geriatrics Society, 50, 1250–1256. doi:10.1046/j.1532-5415.2002.50312.x [DOI] [PubMed] [Google Scholar]
- Alfaro-Acha A. Al Snih S. Raji M. A. Kuo Y. F. Markides K. S. & Ottenbacher K. J (2006). Handgrip strength and cognitive decline in older Mexican Americans. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 61, 859–865. doi:10.1093/gerona/61.8.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey K. J. Lord S. R. & Williams P (1997). Strength in the lower limbs, visual contrast sensitivity, and simple reaction time predict cognition in older women. Psychology and Aging, 12, 137–144. doi:10.1037/0882-7974.12.1.137 [DOI] [PubMed] [Google Scholar]
- Atkinson H. H. Rapp S. R. Williamson J. D. Lovato J. Absher J. R. Gass M. … Espeland M. A (2010). The relationship between cognitive function and physical performance in older women: Results from the women’s health initiative memory study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 65, 300–306. doi:10.1093/gerona/glp149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson H. H. Rosano C. Simonsick E. M. Williamson J. D. Davis C. Ambrosius W. T., … Kritchevsky S. B; Health ABC Study (2007). Cognitive function, gait speed decline, and comorbidities: The health, aging and body composition study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 62, 844–850. doi:10.1093/gerona/62.8.844 [DOI] [PubMed] [Google Scholar]
- Baltes P. B., & Lindenberger U (1997). Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging?Psychology and Aging, 12, 12–21. doi:10.1037/0882-7974.12.1.12 [DOI] [PubMed] [Google Scholar]
- Barnes L. L. Wilson R. S. Everson-Rose S. A. Hayward M. D. Evans D. A. & Mendes de Leon C. F (2012). Effects of early-life adversity on cognitive decline in older African Americans and whites. Neurology, 79, 2321–2327. doi:10.1212/WNL.0b013e318278b607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie K. Martin R. M. Gallacher J. Bayer A. Gunnell D. Ebrahim S. & Ben-Shlomo Y (2011). Socio-economic disadvantage from childhood to adulthood and locomotor function in old age: A lifecourse analysis of the Boyd Orr and Caerphilly prospective studies. Journal of Epidemiology and Community Health, 65, 1014–1023. doi:10.1136/jech.2009.103648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankevoort C. G. Scherder E. J. Wieling M. B. Hortobágyi T. Brouwer W. H. Geuze R. H. & van Heuvelen M. J (2013). Physical predictors of cognitive performance in healthy older adults: A cross-sectional analysis. PLoS One, 8, e70799. doi:10.1371/journal.pone.0070799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. (1991). The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. The Clinical Nueropsychologist, 5, 125–142. doi:10.1080/13854041908403297 [Google Scholar]
- Buchman A. S. Boyle P. A. Leurgans S. E. Barnes L. L. & Bennett D. A (2011). Cognitive function is associated with the development of mobility impairments in community-dwelling elders. The American Journal of Geriatric Psychiatry, 19, 571–580. doi:10.1097/JGP.0b013e3181ef7a2e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouston S. A. Brewster P. Kuh D. Richards M. Cooper R. Hardy R. … Hofer S. M (2013). The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiologic Reviews, 35, 33–50. doi:10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demnitz N. Esser P. Dawes H. Valkanova V. Johansen-Berg H. Ebmeier K. P. & Sexton C (2016). A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait & Posture, 50, 164–174. doi:10.1016/j.gaitpost.2016.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins-Crépeau L. Berryman N. Vu T. T. Villalpando J. M. Kergoat M. J. Li K. Z. … Bherer L (2014). Physical functioning is associated with processing speed and executive functions in community-dwelling older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69, 837–844. doi:10.1093/geronb/gbu036 [DOI] [PubMed] [Google Scholar]
- Edwards J. D. Vance D. E. Wadley V. G. Cissell G. M. Roenker D. L. & Ball K. K (2005). Reliability and validity of useful field of view test scores as administered by personal computer. Journal of Clinical and Experimental Neuropsychology, 27, 529–543. doi:10.1080/13803390490515432 [DOI] [PubMed] [Google Scholar]
- Ekstrom R. B., French J. W., Harman H., & Derman D (1976). Kit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Service. [Google Scholar]
- Emery C. F. Finkel D. & Pedersen N. L (2012). Pulmonary function as a cause of cognitive aging. Psychological Science, 23, 1024–1032. doi:10.1177/0956797612439422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Polce R. J. Maggs J. L. Staff J. & Lanza S. T (2017). The age-varying association of student status with excessive alcohol use: Ages 18 to 30 years. Alcoholism, Clinical and Experimental Research, 41, 407–413. doi:10.1111/acer.13294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C. R., Allerhand M., Aihie Sayer A., Cooper C., & Deary I. L (2014). The dynamic relationship between cognitive function and walking speed: The English Longitudinal Study of Ageing. Age, 2014, 1–11. doi:10.1007/s11357-014-9682-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D. Ram N. Hoppmann C. Willis S. L. & Schaie K. W (2011). Cohort differences in cognitive aging and terminal decline in the Seattle Longitudinal Study. Developmental Psychology, 47, 1026–1041. doi:10.1037/a0023426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletta P., Cantoni E., & Jacot N (2015). Nonlinear growth curve models. In Stemmler M., Eye A. V., & Wiedermann W. (Eds.), Dependent data in social sciences research (pp. 47–66). Switzerland: Springer. [Google Scholar]
- Gonda J., & Schaie K. W (1985). Schaie-Thurstone Mental Abilities Test: Word Series Test. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Hajjar I. Yang F. Sorond F. Jones R. N. Milberg W. Cupples L. A. & Lipsitz L. A (2009). A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: Relationship to blood pressure and other cardiovascular risks. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 64, 994–1001. doi:10.1093/gerona/glp075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S. Wenderoth N. Debaere F. Peeters R. & Swinnen S. P (2005). Neural basis of aging: The penetration of cognition into action control. The Journal of Neuroscience, 25, 6787–6796. doi:10.1523/JNEUROSCI.1263-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infurna F. J., Gerstorf D., Ryan L. H., & Smith J (2011). Dynamic links between memory and functional limitations in old age: Longitudinal evidence for age-based structural dynamics from the AHEAD study. Psychology and Aging, 26, 546–558. doi:10.1037/a0023023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe J. B. Smith D. M. Ball K. Tennstedt S. L. Marsiske M. Willis S. L. … Kleinman K (2001). ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials, 22, 453–479. doi:10.1016/S0197-2456(01)00139-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper J. D. Ensminger M. E. Green K. M. Fothergill K. E. Juon H. S. Robertson J. & Thorpe R. J (2008). Effects of poverty and family stress over three decades on the functional status of older African American women. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 63, S201–S210. doi:10.1093/geronb/63.4.S201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner G. A., Racine C. A., Hale S., Wilheim R., Laluz V., Miller B. L., & Kramer J. H (2012). Cognitive processing speed in older adults: Relationship with white matter integrity. PLoS One, 7, e50425. doi:10.1371/journal.pone.0050425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall J. R. Carlson M. C. Fried L. P. & Xue Q. L (2014). Examining the dynamic, bidirectional associations between cognitive and physical functioning in older adults. American Journal of Epidemiology, 180, 838–846. doi:10.1093/aje/kwu198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D. Cooper R. Hardy R. Guralnik J. & Richards M; Musculoskeletal Study Team (2009). Lifetime cognitive performance is associated with midlife physical performance in a prospective national birth cohort study. Psychosomatic Medicine, 71, 38–48. doi:10.1097/PSY.0b013e31818a1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza S. T. Vasilenko S. A. Dziak J. J. & Butera N. M (2015). Trends among U.S. high school seniors in recent marijuana use and associations with other substances: 1976-2013. The Journal of Adolescent Health, 57, 198–204. doi:10.1016/j.jadohealth.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P. H. Lee G. J. Raven E. P. Tingus K. Khoo T. Thompson P. M. & Bartzokis G (2011). Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. Journal of Clinical and Experimental Neuropsychology, 33, 1059–1068. doi:10.1080/13803395.2011.595397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M. Mennis J. Way T. Lanza S. Russell M. & Zaharakis N (2015). Time-varying effects of a text-based smoking cessation intervention for urban adolescents. Drug and Alcohol Dependence, 157, 99–105. doi:10.1016/j.drugalcdep.2015.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer E. P., Kapoor A., Fogel J., Elbulok-Charcape M., Roth R. M., Katz M. J., … Rabin L. A (2017). Association of psychological, cognitive, and functional variables with self-reported executive functioning in a sample of nondemented community-dwelling older adults. Applied Neuropsychology: Adult, 24, 364–375. doi:10.1080/23279095.2016.1185428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M. L. Albert S. M. Morrow L. A. & Saxton J (2008). Cognitive status and physical function in older african americans. Journal of the American Geriatrics Society, 56, 2014–2019. doi:10.1111/j.1532-5415.2008.01938.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter P., Stewart A. L., & Carey S (1999). Physical functioning: Definitions, measurement, and expectations. Advances in Renal Replacement Therapy, 6, 110–123. doi:10.1016/S1073-4449(99)70028-2 [DOI] [PubMed] [Google Scholar]
- Payette H. Gueye N. R. Gaudreau P. Morais J. A. Shatenstein B. & Gray-Donald K (2011). Trajectories of physical function decline and psychological functioning: The Quebec longitudinal study on nutrition and successful aging (NuAge). The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(Suppl. 1), i82–i90. doi:10.1093/geronb/gbq085 [DOI] [PubMed] [Google Scholar]
- Praetorius Björk M. Johansson B. & Hassing L. B (2016). I forgot when I lost my grip-strong associations between cognition and grip strength in level of performance and change across time in relation to impending death. Neurobiology of Aging, 38, 68–72. doi:10.1016/j.neurobiolaging.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. (1941). L’examen clinique en psychologie. Archives de Psychologie, 28, 21. [Google Scholar]
- Rosano C., Simonsick E. M., Harris T. B., Kritchevsky S. B., Brach J., Visser M., … Newman A. B (2005). Association between physical and cognitive function in healthy elderly: The Health, Aging, and Body Composition Study. Neuroepidemiology, 24, 8–14. doi:10.1159/00081043 [DOI] [PubMed] [Google Scholar]
- Ross L. A., Sprague B. N., Phillips C. B., O’Connor M. L., & Dodson J. E (2016). The impact of three cognitive training interventions on older adults’ physical functioning across 5 years. Journal of Aging and Health. Advance online publication. doi:10.1177/0898264316682916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleimen-Malkoun R. Temprado J. J. & Hong S. L (2014). Aging induced loss of complexity and dedifferentiation: Consequences for coordination dynamics within and between brain, muscular and behavioral levels. Frontiers in Aging Neuroscience, 6, 140. doi:10.3389/fnagi.2014.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski M. Hoffman L. & Hofer S. M (2010). Evaluating convergence of within-person change and between-person age differences in age-heterogeneous longitudinal studies. Research in Human Development, 7, 45–60. doi:10.1080/15427600903578169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Ray R. L., Makowski-Woidan B., & Hughes S. L (2014). A randomized trial to measure the impact of a community-based cognitive training intervention on balance and gait in cognitively intact black older adults. Health Education & Behavior, 41(Suppl. 1), 62S–69S. doi:10.1177/1090198114537068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen-Thiessen E., & Borchelt M (1999). Morbidity, medication, and functional limitations in very old age. In Baltes P. B. & Mayer K. U. (Eds.), The Berlin Aging Study: Aging from 70 to 100 (pp. 131–166). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Sternäng O. Reynolds C. A. Finkel D. Ernsth-Bravell M. Pedersen N. L. & Dahl Aslan A. K (2015). Grip strength and cognitive abilities: Associations in old age. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 71, 841–848. doi:10.1093/geronb/gbv017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. C. Tran X. & Cramer S. C (2014). Age-related variability in performance of a motor action selection task is related to differences in brain function and structure among older adults. Neuroimage, 86, 326–334. doi:10.1016/j.neuroimage.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stijntjes M., Aartsen M. J., Taekema D. G., Gussekloo J., Huisman M., Meskers C. G., … Maier A. B (2016). Temporal relationship between cognitive and physical performance in middle-aged to oldest old people. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 00, 1–7. doi:10.1093/gerona/glw133 [DOI] [PubMed] [Google Scholar]
- Tennstedt S. L. & Unverzagt F. W (2013). The ACTIVE study: Study overview and major findings. Journal of Aging and Health, 25, 3S–20S. doi:10.1177/0898264313518133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe R. J. Jr., Kasper J. D. Szanton S. L. Frick K. D. Fried L. P. & Simonsick E. M (2008). Relationship of race and poverty to lower extremity function and decline: Findings from the Women’s Health and Aging Study. Social Science & Medicine, 66, 811–821. doi:10.1016/j.socscimed.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone L. L., & Thurstone T. G (1949). Examiner Manual for the SRA Primary Mental Abilities Test (Form 10-14). Chicago, IL: Science Research Associates. [Google Scholar]
- van Iersel M. B. Kessels R. P. Bloem B. R. Verbeek A. L. & Olde Rikkert M. G (2008). Executive functions are associated with gait and balance in community-living elderly people. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 63, 1344–1349. doi:10.1093/gerona/63.12.1344 [DOI] [PubMed] [Google Scholar]
- Voelcker-Rehage C. Godde B. & Staudinger U. M (2010). Physical and motor fitness are both related to cognition in old age. The European Journal of Neuroscience, 31, 167–176. doi:10.1111/j.1460-9568.2009.07014.x [DOI] [PubMed] [Google Scholar]
- Wilson R. S. Hebert L. E. Scherr P. A. Barnes L. L. Mendes de Leon C. F. & Evans D. A (2009). Educational attainment and cognitive decline in old age. Neurology, 72, 460–465. doi:10.1212/01.wnl.0000341782.71418.6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Wang R., Zhao Y., Ma X., Wu M., Yan X., & He J (2013). The relationship between self-rated health and objective health status: A population-based study. BMC Public Health, 13, 320. doi:10.1186/1471-2458-13-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.