Abstract
The prime task of nociceptors is the transformation of noxious stimuli into action potentials that are propagated along the neurites of nociceptive neurons from the periphery to the spinal cord. This function of nociceptors relies on the coordinated operation of a variety of ion channels. In this review, we summarize how members of nine different families of ion channels expressed in sensory neurons contribute to nociception. Furthermore, data on 35 different types of G protein coupled receptors are presented, activation of which controls the gating of the aforementioned ion channels. These receptors are not only targeted by more than 20 separate endogenous modulators, but can also be affected by pharmacotherapeutic agents. Thereby, this review provides information on how ion channel modulation via G protein coupled receptors in nociceptors can be exploited to provide improved analgesic therapy.
Keywords: nociceptor, inflammatory pain, G protein-coupled receptor, voltage-gated ion channel, TRP channel, K2P channel, Ca2+-activated Cl− channel
1. Introduction
Nociception refers to “neural processes of encoding and processing noxious stimuli” as defined by the International Association for the Study of Pain. Noxious stimuli are “actually or potentially tissue damaging events” that need to act on nociceptors in order to cause pain. Accordingly, nociceptors are viewed as “sensory receptors that are capable of transducing and encoding noxious stimuli”. As such, nociceptors are peripheral nerve endings of first order nociceptive neurons; these are part of the peripheral nervous system with neuronal cell bodies located mostly in dorsal root ganglia and with central neurites projecting to second order nociceptive neurons located in the dorsal horn of the spinal cord [1].
Noxious stimuli that impinge on nociceptors comprise mechanical forces, temperature changes (heat and cold), and chemical agents (e.g., protons and plant-derived irritants such as capsaicin, menthol, or isothiocyanates). Apart from acting directly on nociceptors, such injurious impact may lead to inflammation, as do infections. This pathologic response is characterized by the release of a plethora of mediators from various types of cells including, amongst others, macrophages, mast cells, immune cells, platelets and the nociceptive neurons themselves [2]. Together, these mediators are called inflammatory soup and lead to an increased responsiveness of nociceptive neurons. This latter mechanism is known as sensitization and forms the pathophysiological basis of allodynia and hyperalgesia: pain in response to a non-nociceptive stimulus and increased pain sensitivity, respectively [1].
Constituents of the inflammatory soup comprise protons, nucleotides and nucleosides, enzymes (proteases), fatty acid derivatives (prostanglandins), biogenic amines (histamine, noradrenaline, and serotonin), cytokines, chemokines, neurotrophins and other peptides (bradykinin, endothelin, and tachykinins) [3]. These multifarious endogenous agents influence nociceptor signaling through a variety of different receptors:
Protons act directly on ion channels that are members of either the TRP or the ASIC family [4,5].
ATP as a prototypic nucleotide may activate a subset of ligand-gated ion channels known as P2X receptors [6].
Cytokines such as various interleukins or tumor necrosis factors (TNFs) target different subtypes of cytokine receptors [7].
Neurotrophins, in particular nerve growth factor, bind to high affinity tyrosine receptor kinases (trks) and to the low affinity receptor p75 [8].
All others of the aforementioned inflammatory mediators and ATP elicit their actions on nociceptors via some type of G protein-coupled receptor (GPCR).
Hence, most of the influence of the inflammatory soup on nociceptors is mediated by GPCRs [3]. The common outcome of the separate actions of the single components contained in the inflammatory soup is sensitization of nociceptors, as mentioned above. The prime task of nociceptors is the transformation of noxious stimuli into action potentials that are propagated along the neurites of nociceptive neurons from the periphery to the spinal cord. Accordingly, sensitization means that this transformation of noxious stimuli into action potentials is facilitated, and this may occur through one of two possible mechanisms: reduction in the action potential threshold or increased responses to suprathreshold stimuli. In principle, these two pathophysiological alternatives underlie the clinical phenomena of allodynia and hyperalgesia, respectively [1]. Consequently, this review summarizes how activation of certain GPCRs can impinge on either of these two mechanisms underlying the sensitization of nociceptors.
Obviously, the transformation of noxious stimuli into action potentials relies on the coordinated operation of a variety of ion channels. Therefore, inflammatory mediators must ultimately act on the function of these ion channels to be able to sensitize nociceptors. In this regard, the present review summarizes signaling mechanisms that link an activation of GPCRs to changes in ion channel function in nociceptors.
When dealing with GPCRs expressed in peripheral nociceptive neurons, one must take into account that not all of them subserve stimulatory actions that in the end lead to sensitization. Several of these GPCRs mediate inhibitory effects which rather diminish than enhance neuronal excitability. For the sake of comprehensiveness, such inhibitory receptors are considered as well.
2. Ion Channels as Targets of GPCR Signaling in Peripheral Nociceptive Neurons
In this section, ion channel families are described in light of their roles in nociceptive neurons. In this respect, one can discern between ion channels that are directly involved in the sensation of noxious stimuli and those that are rather responsible for the ensuing generation and propagation of action potentials. The former group comprises TRP channels, ASICs, and mechanosensitive K and Piezo channels, whereas voltage-activated Na and Ca channels as well as various types of K channels belong to the latter. This basic characterization of each of these ion channel families is followed by a description of the mechanisms that link activation of various GPCRs to changes in functions of these ion channels.
2.1. TRP Channels Involved in Pain Sensation
Transient receptor potential (TRP) channels are expressed in a variety of tissues throughout the body, such as skin, kidney, bladder, vascular smooth muscle cells and the nervous system [9]. The TRP channel family consists of six sub-families: TRPA (Ankyrin), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystin), and TRPV (vanniloid), encoded by a total of 28 genes [10]. The latter five can be further divided into subtypes: TRPC1-7, TRPM1-8, TRPML1-3, TRPP1-3, and TRPV1-6 [11]. The broad variety of TRP channels allows sensing both noxious and innocuous signals [12]. Thus far, TRPV1-4 [12] and TRPM3 [13] channels have been implicated in the sensation of noxious heat. TRPA1, TRPC5 and TRPM8 channels have been suggested to detect noxious cold temperatures [14]. In addition, both TRPA1 and TRPV4 subtypes are thought to be involved in the detection of noxious mechanical stimuli [12], while TRPA1, TRPV1, TRPV3, TRPV4, TRPM8, and TRPC3 may contribute to the sensation of itch [15,16].
The aforementioned TRP channel subtypes are expressed in different types of peripheral sensory neurons such as dorsal root ganglion (DRG) and trigeminal ganglion (TG) neurons. TRPV1 channels and TRPM8 channels are mainly expressed on separate sets of neurons. TRPV1 channels can be found in C-fibers, whereas TRPM8 channels can be found on both fiber types transferring noxious signals (A- and C-fibers) [17]. Nevertheless, coexpression of TRPM8 and TRPV1 in one DRG neuron has been reported as well [18,19]. TRPA1 and TRPV1, in contrast, are mostly coexpressed in sensory neurons [17,19]. TRPV2 channels have been detected in A-fibers [12]. TRPV3 channels can be found on keratinocytes rather than sensory neurons [20], and TRPV4 channels are expressed in variety of tissues including the CNS and peripheral neurons [21]. TRPM3 channels are expressed in a large subset of DRG and trigeminal ganglion neurons. TRPM3 mRNA can be detected in approximately 80% of these sensory neurons at a level that is comparable to that of TRPA1 and TRPV1. However, only small-diameter neurons produce currents mediated by TRPM3 [22].
The role of TRPV1 channels as sensors of noxious heat is well established [23]. In general, TRP channels resemble the structure of voltage-gated K (K) channels. Each channel is made up of four subunits, each having six membrane spanning domains. Similar to K channels, transmembrane domains 5 and 6 comprise the channel pore, whereas transmembrane domains 1–4 resemble a voltage sensor. Both N- and C-termini are found on the intracellular side [14] and harbor a number of regulatory domains. Channel trafficking and assembly is regulated by six so-called ankyrin repeats located at the N-terminus [24,25]. TRPV1 channels are not just activated by noxious heat, but also by voltage, binding of vanilloids, such as capsaicin, or high concentrations of H ions [10]. As compared to K channels, TRPV1 channels display a rather weak voltage sensitivity [26], which can be explained by the fact that the voltage-sensing transmembrane domains 1–4 remain fairly static during activation [26,27,28]. In addition, transmembrane domain 4 of TRPV1 channels contains a lower number of positively charged amino acids as compared to K channels. Hence, an additional voltage-sensing segment might be required for TRP channels [14]. The gating in response to heat is regulated by the so-called TRP domain. However, this process remains incompletely understood [29]. The TRP domain spans 25 amino acid residues and is located immediately adjacent to transmembrane domain 6. It contains the TRP box, a stretch of conserved amino acid residues (WKFQR), which is a hallmark of TRP channels. The TRP domain is thought to be involved in a number of processes, like PIP binding or channel assembly, but the exact mechanism still needs to be fully elucidated [24]. As mentioned before, PIP is thought to regulate TRPV1 channel function, however it is still under debate if PIP is a positive or a negative regulator [30]. Cryo-EM studies in nanodiscs revealed the position of PIP in proximity to the vanilloid binding site. Binding of a vanilloid displaces a part of the PIP molecule, which reaches into the vanilloid binding pocket. The removal of the phosphoinositide is thought to lead to channel gating [26]. Such an effect would rather point towards a negative regulatory effect of PIP.
The threshold value for classifying a thermal response as noxious was determined to be 43 °C [2]. TRPV1 channels activate at temperatures that exceed 43 °C, TRPV2 activate at even higher temperatures (>52 °C), whereas TRPV3 and TRPV4 channels gate in a temperature range between 26 °C and 34 °C [20]. Similar to TRPV1 channels, heterologously expressed TRPM3 channels activate at a temperature exceeding 40 °C [22]. Interestingly, mice lacking TRPV1 channels display a delayed nocifensive response only at temperatures exceeding 50 °C [31,32]. However, as compared to TRPV1 channels, the role of the other TRPV channels linked to the detection of noxious signals remains incompletely understood [12]. The role of TRPV2 to TRPV4 channels in detecting noxious signals remains debated [13], since both TRPV2 knock-out [33] and TRPV3/TRPV4 double knock-out [34] animals retain normal thermal and mechanical sensation. The nocifensive response times of TRPM3 knock-out mice is prolonged at temperatures exceeding 52 °C [22]. Mice lacking both TRPV1 and TRPM3 show a significantly increased nocifensive response time already at 45 °C. However, some sensory neurons still produce currents in response to heat. Only a triple knock-out of TRPV1, TRPM3 and, interestingly, TRPA1 leads to a complete heat-insensitivity of sensory neurons. Furthermore, these mice were completely heat insensitive in behavioral tests [35].
The detection of both noxious and innocuous cold signals is suggested to involve TRPM8 and TRPA1 channels [23]. TRPM8 channels gate at temperatures below 25 °C [20]. Knock-out animals of TRPM8 channels lose the ability to detect cool temperatures, but retain the ability to detect noxious cold signals below 15 °C [13]. Hence, the role of TRPM8 channels as cold sensors is well established, but an additional set of ion channels needs to be involved in detecting noxious cold temperatures. TRPA1 channels are thought to be involved, but their role remains controversial [20]. Rodent TRPA1 channels were found to be gated by noxious cold temperatures, however, that function is lost in primate TRPA1 channels [36]. By contrast, human TRPA1 channels, reconstituted in lipid bilayers, were found to be activated by noxious cold temperatures [37]. In addition to these conflicting results, TRPA1 channels are usually expressed on the same set of neurons as TRPV1 channels, which appears counterintuitive [17]. Furthermore, animal studies involving TRPA1 knock-out mice point towards an insignificant role in the detection of noxious cold temperatures [20]. While their role in the detection of mechanical stimuli remains controversial as well, their contribution to the detection of noxious chemical signals is well established [12]. A large number of structurally unrelated electrophilic compounds can gate TRPA1 channels [13]. These compounds covalently modify one or more of the 31 cysteine residues, which causes channel opening [38].
GPCR Regulation of TRP Channels
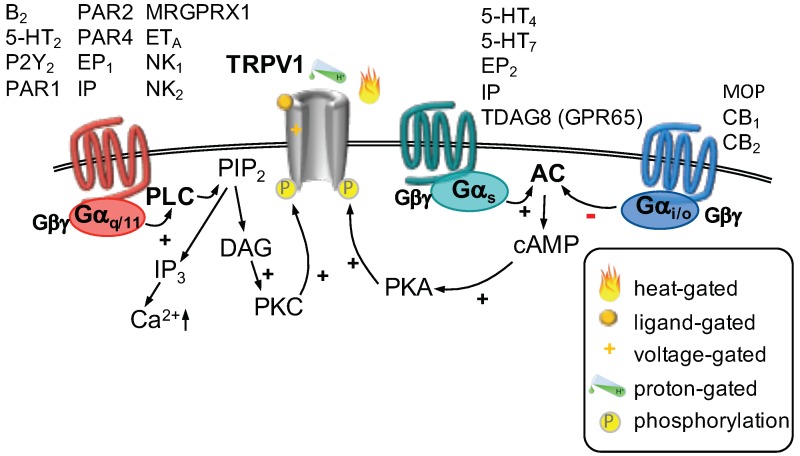
TRPV1 channels have been studied extensively for their modulation by GPCRs. Currents through TRPV1 channels are increased in response to inflammation, which mediates an enhanced depolarization and increased excitability [2]. The sensitization of TRPV1 channels can be mediated by both G- and G-coupled receptors. Stimulation of a G-coupled receptor leads to activation of phospholipase C (PLC), which hydrolyzes membrane bound phosphatitylinositol 1,4, bisphosphate (PIP) into soluble inositol 1,4,5 trisphosphate (IP) and membrane bound diacylglycerol (DAG, Figure 1). Subsequently, IP binds to IP receptors located at the membrane of the endoplasmic reticulum, which triggers the release of Ca. DAG in turn activates protein kinase C, which phosphorylates target proteins. Every step of this cascade can interfere with the function of TRPV1 channels [39]. Presence of PIP in the membrane is thought to decrease TRPV1 channel function by interfering with agonist binding [26]. If PIP is depleted from the membrane in response to PLC activation, TRPV1 activity may increase [30]. The exact role of PIP remains debated, as it was also shown to activate TRPV1 channels [30]. Activated PKC was shown to phosphorylate two serine residues at the C-terminus, which is thought to mediate sensitization [40]. A rise in cytosolic Ca is not considered to contribute to sensitization as it usually leads to rapid channel desensitization in response to prolonged activation [39]. A large number of inflammatory modulators was shown to increase TRPV1 channels via one of these mechanisms (Table 1).
Figure 1.
TRPV1 channels can be gated by different mechanisms (as indicated). Three major G-protein-dependent pathways modulate the function of TRPV1 channels. Activation of G-coupled receptors (left) leads to activation of phospholipase C (PLC), which hydrolyzes phosphatitylinositol 1,4, bisphosphate (PIP) into inositiol 1,4,5 trisphosphate (IP) and diacylglycerol (DAG). DAG activates protein kinase C (PKC), which phosphorylates TRPV1 channels, thereby increasing their function. Activation of a G-coupled receptor (center) stimulates adenylyl cyclase (AC), which produces cyclic adenosine monophosphate (cAMP). Subsequent activation of protein kinase A (PKA) leads to phosphorylation of TRPV1 channels and an increase in current. Stimulation of a G-coupled receptor (right) decreases AC activity. Therefore, less cAMP is formed, PKA is less active and hence TRPV1 channels are not phosphorylated which decreases their activity.
Table 1.
GPCRs modulating TRPV1 function.
| GPCR Ligand | Involved GPCR | Pathway | Effect on TRPV1 | Reference |
|---|---|---|---|---|
| Bradykinin | B | G-DAG-PKC | increased current | [2,41] |
| Serotonin | 5-HT | G-DAG-PKC | increased current | [42,43,44] |
| 5-HT | G-AC-PKA | increased current | [43] | |
| 5-HT | G-AC-PKA | increased current | [42] | |
| UTP | P2Y | G-DAG-PKC | increased current | [45,46,47] |
| BAM 8-22 | MRGPRX1 | G-DAG-PKC-PIP | increased current | [48] |
| Proteases | PAR2 | G-PKC | increased current | [49,50] |
| PAR1 | G-PKC | increased current | [51] | |
| PAR4 | G-PKC | increased current | [51] | |
| PGE | EP | G-PKC | increased current | [52] |
| EP | G-PKA | increased current | [52,53,54] | |
| PGI | IP | G-PKC | increased current | [52] |
| IP | G-PKA | increased current | [52] | |
| Endothelin-1 | ET | G -PKC | increased current | [55,56] |
| Substance P | NK | G -PKC | increased current | [57] |
| NK | G-PKC | increased current | [58] | |
| H | TDAG8 (GPR65) | G-PKA | increased current | [59] |
| Morphine | MOP | G-reduced AC | decreased current | [60,61] |
| Endocannabinoids | CB | G-reduced AC | decreased current | [62] |
| CB | G-reduced AC | decreased current | [63] |
AC, adenylyl cylcase; DAG, diacylglycerol; PKA, proteinkinase A; PKC, protein kinase C; BAM 8–22, bovine adrenal medulla peptide 8–22.
Activation of a G-coupled receptor stimulates the activity of adenylyl cyclase, which produces cyclic adenosine monophosphate (cAMP). This nucleotide is needed to activate protein kinase A (PKA), which then phosphorylates its target proteins. PKA-mediated phosphorylation of TRPV1 channels increases their sensitivity towards their agonists and reduces Ca-mediated desensitization [54]. Several inflammatory mediators were found to sensitize TRPV1 channels utilizing this pathway (Table 1).
By contrast, activation of a G-coupled receptor decreases the activity of adenylyl cyclase which reduces the abundance of cAMP and subsequent activation of PKA (Table 1). Indeed, activation of G-coupled cannabinoid [62,63] and -opioid (MOP) [60,61] receptors was shown to reduce currents of TRPV1 receptors, which is thought to contribute to the peripheral analgesic action of opioids [60,61] and cannabinoids [62,63].
In addition to the three major GPCR pathways, TRPV1 channels were shown to be sensitized by nerve growth factor (NGF), which requires the early activation of PI3 kinase and the presence of PKC and CamKII (Ca/calmodulin dependent protein kinase II) [64]. The inflammatory mediator histamine sensitizes TRPV1 channels via G-coupled H receptors. Instead of utilizing the signaling cascade described above, histamine-mediated sensitization requires activation of phospholipase A2 and lipoxigenases [65,66,67].
In sensory neurons, a variety of G-coupled receptors were found to inhibit currents through TRPM3 channels: including GABA receptors [68,69,70], -opioid receptors [68,69], somatostatin receptors [68,70], CB- [69], as well as, CB receptors [68], and neuropeptide Y receptors [69]. Likewise, low concentrations of noradrenaline reduce TRPM3 activity, hinting towards as mediating receptor. However, the adrenergic receptor involved was not further characterized [68]. Whether -opioid receptors also mediate a TRPM3 inhibition remains controversial: while deltorphin, a -selective peptide was able to reduce TRPM3 function [68], the small-molecule -selective agonist SB205607 was not [69]. Likewise, activation of G-coupled metabotropic glutamate receptors (mGluR) did not reduce TRPM3 function [69]. In a heterologous system, G-coupled M receptors were found to inhibit currents through TRPM3 channels [70]. However, in sensory neurons, activation of G-coupled mGluR only weakly inhibited TRPM3 [68]. The inhibition of TRPM3 in sensory neurons involves activation pertussis toxin (PTX)-sensitive G-coupled receptors [68,69,70]. The effect did not require signaling downstream of G activation, but relied on a direct interaction with the dimer [68,69,70].
TRPA1 channels are sensitized by G- and G-coupled receptors in sensory neurons. A G-mediated interaction has not been reported for sensory neurons. Activation of G-coupled PAR2 receptors increased currents mediated by TRPA1 receptors in dorsal root ganglion neurons. This interaction required the activation of PLC but none of the downstream products. Consequently, depletion of PIP from the membrane was shown to be sufficient for this interaction [12]. The inflammatory mediator bradykinin was shown to increase currents through TRPA1 channels in dorsal root ganglion neurons. This effect was mediated by G-coupled B receptors and required the activation of PLC. Interestingly, activation of PKA was further required for the interaction of B receptors and TRPA1 channels [71]. An interaction of G-coupled bradykinin B receptors with TRPA1 channels was reported in behavioral experiments. This interaction relied on activation of PLC and PKC [72]. Histamine was shown to cause nocifensive behavior in a TRPA1 dependent manner. It is thought to involve G-coupled H receptors and activation of PLC [73]. Adenosine, another component of the inflammatory soup, was found to sensitize esophageal C-fibers and increase TRPA1 currents via G-coupled A receptors. Activation of PKA is necessary for this interaction [74]. Electrophilic metabolites of prostaglandins, however, were demonstrated to activate TRPA1 channels directly [75,76].
The inflammatory mediators prostaglandin E2 (PGE), bradykinin and histamine were tested for their influence on TRPM8 channel function. As opposed to the previously described members of the TRP channel family, the activity of the cool sensor TRPM8 is reduced in the presence of bradykinin and PGE. However, application of histamine did not interfere with TRPM8 channel function [77]. The action of bradykinin required the mobilization of PKC [77,78], whereas PGE involved activation of PKA [77]. Bradykinin is assumed to act via G-coupled B receptors [79] and several modes of action have been suggested: it was found that depletion of PIP from the plasma membrane reduced heterologously expressed TRPM8 channel function [80]. However, these experiments were performed in absence of GPCRs and it remains to be established if PIP depletion is also sufficient to reduce TRPM8 channel function in a native cell system. PIP is hydrolyzed to form IP and DAG, which is required to activate PKC. PKC is thought to activate protein phosphatase I (PPI), which is suggested to dephosphorylate TRPM8 channels. The dephosphorylation of TRPM8 channels is proposed to finally inhibit TRPM8 channel function [79]. More recently, both bradykinin and histamine were found to inhibit TRPM8 channels via a direct interaction of G subunits with the channels. The inhibitory effect of both mediators did not require activation of PLC or any of the subsequent steps in the signaling cascade [81]. The receptor via which PGE exerts its effect has not been determined, however it was found that activation of a G-coupled receptor and subsequent PKA stimulation was required. Furthermore, the exact mechanism how PKA modulates TRPM8 channel function remains unknown [79].
2.2. Acid-Sensing Ion Channels
Acid-sensing ion channels (ASIC) represent one of many ion channel families that detect noxious chemical stimuli. Additional chemical sensors are TRP channels, namely TRPA1 and TRPV1, as well as ATP-gated P2X receptors [82]. As the name suggests, acid sensing ion channels are activated in low pH conditions [83]. Such acidic conditions occur during an inflammatory response, ischemia, or fatiguing exercise [82]. ASICs can be divided into three subtypes ASIC1 to ASIC3. ASIC1 and ASIC2 can even be further subclassified into two splice variants each (ASIC1a, ASIC1b; ASIC2a, ASIC2b) [84]. A fourth analog, sometimes referred to as ASIC4 [85], rather affects expression levels of ASIC1a and ASIC3, instead of producing proton-gated currents [84]. The EC for proton-mediated currents via ASIC1 and ASIC3 channels ranges between a pH of 6.2 to 6.8, whereas ASIC2 channels have an EC between pH 4.1 and 5 [84]. All forms of ASICs can be detected in somata and peripheral ends of sensory neurons [85]. ASIC1a and ASIC3 channels are preferentially expressed in small diameter DRG neurons which also express TRPV1 and most likely subserve nociceptive function [86]. A functional channel is composed of three subunits and all but one subunit can participate in both homo- and heteromeric channels. Only ASIC2b does not form functional homomeric channels [87]. One subunit consists of two transmembrane domains, having both the N- and C-termini at the intracellular side [88,89]. Most of the protein is located at the extracellular side forming the large extracellular domain (termed ECD). The structure of the extracellular domain was compared to a hand holding a ball, which explains the peculiar terminology for parts of the ECD, such as palm, knuckle, thumb, finger and -ball [90]. The ECD contains a number of regulatory domains: for example, an acidic pocket is formed by acidic amino acid residues at the subunit–subunit interface, which is involved in binding of H ions and subsequent gating [89]. ASIC channels follow a three-state kinetic model, from a closed to an activated to an inactivated state. The recovery from inactivation can only be achieved in high pH conditions [88] and this desensitized state is thought to be regulated by the thumb domain within the ECD [90].
Genetic studies have suggested that ASICs play a role in sensing mechanical signals, but the exact gating mechanism is unknown, and their role remains heavily debated [91].
GPCR Regulation of ASICs
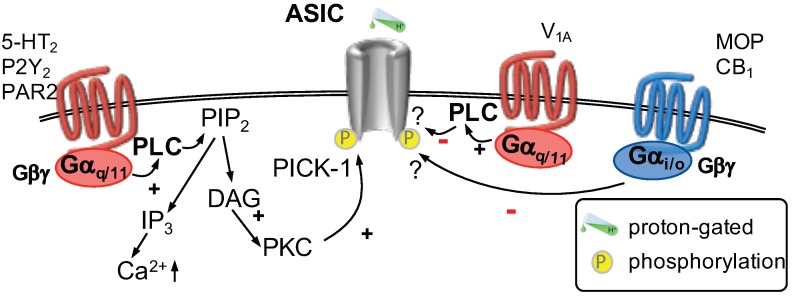
A few components of the inflammatory soup have been tested for their modulatory effect on acid-sensing ion channels: histamine was shown to selectively potentiate heterologously expressed ASIC1a channels. This process involved a direct action of histamine and did not require the presence of a GPCR [92]. The nucleotides UTP and ATP were shown to increase acid-induced currents (Figure 2) in rat dorsal root ganglion neurons as well as acid-induced membrane excitability. In this respect, UTP was found to act via G-coupled P2Y receptors and required the activation of PLC, subsequent stimulation of PKC and the presence of the anchoring protein PICK-1 (protein interacting with C-kinase 1) [93]. A similar effect can be observed when serotonin is applied: both ASIC-mediated currents and neuronal excitability are increased [94]. Serotonin was found to act via G-coupled 5-HT receptors [94] and required activation of PKC [94,95]. Two phosphorylation sites, one at the N-terminus and one at the C-terminus of ASIC3, need to be phosphorylated for the full effect. Again, PICK-1 is necessary for PKC-mediated phosphorylation of ASIC channels. This scaffold protein is thought to bind to ASIC2b subunits in heterotrimeric channels and to link PKC to the channel and to enable phosphorylation [95]. Another G-coupled receptor, PAR2, was found to increase ASIC-mediated currents in rat pulmonary sensory neurons. Interestingly, neither PLC nor PKC were required for the PAR2 mediated current increase but the pathway involved was not studied further [96]. Depending on the activation mechanism of PAR2, one may observe an increase of cytosolic Ca following the G-dependent activation of PLC and formation of IP. On the other hand, PAR2 activation was shown to signal also via G proteins, which activate Rho kinase and lead to ERK phosphorylation. Additionally, PAR2 activation may lead to -arrestin recruitment. Whether PAR2 activation may also decrease or increase cAMP levels remains controversial [97].
Figure 2.
Acid sensing ion channels (ASIC) are gated by increasing concentrations of H. Two G-protein pathways modulate the function of ASICs. Activation of G-coupled receptors (left) leads to activation of phospholipase C (PLC) which hydrolyzes phosphatitylinositol 1,4, bisphosphate (PIP) into inositiol 1,4,5 trisphosphate (IP) and diacylglycerol (DAG). DAG activates protein kinase C (PKC) which phosphorylates ASICs increasing their function. The interaction of PKC with ASICs requires the presence of PICK-1 (protein interacting with C-kinase 1). By contrast, activation of G-coupled V receptors (center) were shown to decrease ASIC-mediated currents via an unknown mechanism. Stimulation of G-coupled receptors (right) was shown to decrease currents through ASICs involving an undetermined mechanism.
By contrast, activation of G-coupled receptors was shown to decrease currents through ASIC channels. First, stimulation of cannabinoid CB receptors was found to reduce nocifensive behavior triggered by local acidosis which relies on an interaction between CB receptors and ASIC channels [98]. Second, activation of -opioid receptors was shown to decrease ASIC-mediated currents, neuronal excitability in dorsal root ganglion neurons and nocifensive behavior induced by local acidification [99]. In addition, nocifensive behavior provoked by mechanical stimuli [100] or local acidification [101] is reduced by local application of oxytocin. Oxytocin reduces ASIC-mediated currents in dorsal root ganglion neurons via activation of vasopressin V receptors. This effect was found to be dependent on G activation, but further steps of the cascade were not tested [101]. It remains to be determined if the observed differences in G-mediated effects on ASIC channels, for example, depend on the recruitment of PICK-1.
2.3. Mechanosensitive Channels in Pain Sensation
The variety of mechanical stimuli detected by so-called mechanosensors in the sensory nervous system ranges from light to noxious mechanical stimuli. These specialized neurons express mechanotransducer channels [102]. To sense noxious mechanical stimuli, high-threshold mechanosensors are required, which should express ion channels that open in response to strong mechanical stimuli and lead to a depolarization of these neurons [103]. Acid sensing ion channels (as described above) were suggested to act as mechanotransducer channels in C. elegans. However, heterologously expressed mammalian ASICs do not gate in response to mechanical stimuli. Hence, such depolarizing mechanotransducer channels in nociceptive neurons remain to be identified [103].
One family of ion channels that contributes to the sensation of noxious mechanical stimuli is the family of two-pore K channels (K) [103]. The family of K channels consist of 15 members, which usually provide so-called background or leak currents, which are the major contributors to the resting membrane potential [11]. Three members of the K family were found to be involved in sensing noxious mechanical stimuli: K2.1 (TREK1), K4.1 (TRAAK), and K10.1 (TREK2) [104]. A functional K channel is formed by two subunits consisting of four transmembrane segments each (TMS1–4). Both the N- and C-termini are located in the intracellular space and the linker regions between TMS1 and -2, as well as TMS3 and -4 are located inside the plasma membrane to form one selectivity filter each [105]. Accordingly, a total of eight transmembrane domains and four selectivity filter regions line the ion conduction pore. This structure of the pore is highly homologous to that of voltage-gated K channels [104]. In closed conformation, the ion conduction pathway is blocked by lipid acyl side chains and membrane stretch directly gates K channels [104]. K2.1 and K4.1 channels are strongly expressed in small-diameter DRG neurons and only weakly expressed in medium- and large-diameter DRG neurons, whereas K10.1 channels are exclusively expressed in small-diameter neurons [106]. Interestingly, knock-out of these channels leads to an increased nocifensive response to mechanical stimuli [107]. Since all these channels are selective K channels, opening of K channels leads to an efflux of K ions and subsequent hyperpolarization. It is thought that stretch-activation of K channels counteracts the activation of depolarizing mechanotransducer channels and thereby finetunes the mechanically induced nociceptive signal which is transferred to the brain [102]. Hence, it is clear that these three members of the K family contribute to the perception of noxious mechanical stimuli, but they cannot represent the primary depolarizing mechanotransducer channel [103]. Such a functional entity is rather provided by Piezo channels, in particular Piezo2, which contributes to mechanically activated currents in DRG neurons [108]. However, deletion of Piezo2 impairs touch, but sensitizes mechanical pain in mice [109]. Therefore, additional sensors of mechanical pain remain to be identified.
2.3.1. GPCR Regulation of Mechanosensitive Potassium Channels
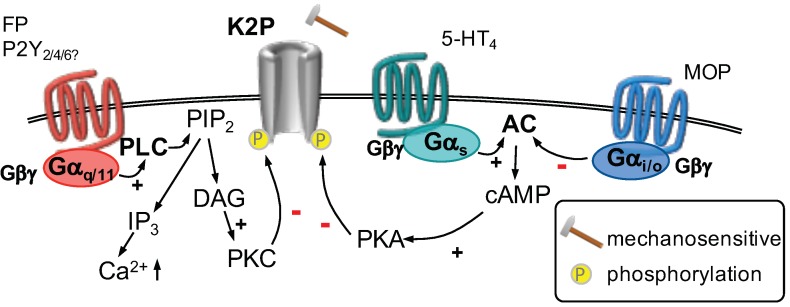
The function of mechanosensitive K channels can be adjusted by a number of modulators like arachidonic acid, polyunsaturated fatty acids, glutamate, noradrenaline, acetylcholine, TRH [105] or serotonin [107] (Figure 3). Arachidonic acid and polyunsaturated fatty acids activate these channels directly [105]. The other modulators influence K channel function via activation of GPCR pathways. All three major GPCR pathways affect K2.1 and K10.1 channel activity: phosphorylation of two different C-terminally located serine residues either by cAMP activated PKA or PKC leads to an inhibition of both subtypes. Phosphorylation of yet another serine residue by protein kinase G (PKG) on the other hand activates K2.1 and K10.1 channels. PKG is activated by an increase of cyclic guanosine monophosphate (cGMP) which in turn is formed by soluble guanylyl cyclase. Soluble guanylyl cyclase is directly activated by nitric oxide and does not involve activation of a GPCR [105]. In dorsal root ganglion neurons, prostaglandin F (PGF) was shown to decrease K mediated currents [106]. The exact coupling mechanism was not elucidated, however, PGF is the endogenous ligand for G-coupled FP prostanoid receptors [110] and it is likely that PKC activation is involved in this process. In addition, prostaglandin E (PGE)-induced nocifensive behavior was reduced in K10.1 knock-out mice, but the mechanism of action was not investigated [111].
Figure 3.
K channels are opened in response to mechanical stimuli. Stimulation of a G-coupled receptor (left) activates phospholipase C (PLC). Hydrolyzation of phosphatitylinositol 1,4, bisphosphate (PIP) forms inositiol 1,4,5 trisphosphate (IP) and diacylglycerol (DAG). DAG activates protein kinase C (PKC) which phosphorylates K channels decreasing their function. Activation of G-coupled receptors (center) leads to activation of adenylyl cyclase (AC) which produces cyclic adenosine monophosphate (cAMP). Thereafter, protein kinase A (PKA) is activated which phosphorylates K channels and decreases their currents. Stimulation of G-coupled receptors (right) decreases AC activity. Therefore, less cAMP is formed, PKA is less active and subsequently K channels are not phosphorylated, which increases their activity.
Activation of a K permeable ion channel leads to an efflux of K ions and a subsequent hyperpolarization. K channels are active at resting conditions and contribute to the formation of the resting membrane potential. Inhibition of these channels leads to a depolarization and a subsequent increase in excitability [107]. Other components of the inflammatory soup were examined for their effects on K channels: for example, serotonin was also found to inhibit K2.1 and K10.1 channels via activation of 5-HT receptors in a heterologous cell system [112]. These GPCRs are G-coupled and activate PKA, which is thought to mediate this effect [107]. Application of UTP, which may act through G-coupled P2Y, P2Y or P2Y receptors, leads to an inhibition of K channels in mammary epithelial cells [113]. It remains to be determined if these inflammatory mediators also interact with mechanosensitive K channels in dorsal root ganglion neurons.
On the other hand, activation -opioid receptors were found to increase K2.1 currents in hippocampal astrocytes [114], in a heterologous cell system [115], and in substantia gelatinosa neurons of the spinal cord. The latter mechanism is thought to be involved in the antinociceptive actions of opioids [116]. All opioid receptors are coupled to G G-proteins, which reduce the activity of adenylyl cyclase [110]. Subsequently, less cAMP is formed, which in turn leads to reduced PKA activity and less PKA-mediated phosphorylation of K channels [105]. Since opioid receptors are also expressed in peripheral sensory neurons [117], such an interaction between -opioid receptors and K channels might also exist in peripheral sensory neurons and contribute to opioid-mediated antinociception.
2.3.2. GPCR Regulation of Piezo Channels
Mechanically activated and rapidly adapting currents in DRG neurons are carried by Piezo2 channels [108] and get sensitized by the activation of B bradykinin receptors, an effect that appears to involve PKA as well as PKC [118]. Akin currents are enhanced in the presence of ATP and UTP which act most likely through an activation of P2Y receptors [119]. Likewise, mechanically activated and rapidly adapting currents in DRG neurons as well as currents in cells expressing recombinant Piezo2 channels are enhanced by intracellular GTP or GTPS which both lead to the activation of G proteins [120]. This confirms that the function of mechanosensitive Piezo2 channels can be enhanced by inflammatory mediators acting on GPCRs. Interestingly, Piezo2 channels can be inhibited by a depletion of membrane PIP, and this mechanism is believed to underlie the reduction of mechanically activated rapidly adapting currents in DRG neurons in response to an activation of TRPV1 by capsaicin [121]. Why such an effect cannot be observed in DRG neurons during the activation of Gq-linked GPCRs, such as B bradykinin and P2Y receptors [118,119], remains open for future investigation.
2.4. Calcium-Activated Chloride Channels in Pain Sensation
Calcium-activated chloride (CaCC) channels occur in a variety of tissues. They are widely expressed in the nervous system but also other tissues like vascular smooth muscle cells. In addition, CaCCs are used as a marker for stroma tumors in the gastrointestinal tract and were initially termed DOG1 [122]. Functionally described CaCCs were linked to transmembrane proteins of unknown function 16A and B (TMEM16) [123,124,125]. Thereafter, the term anoctamin was introduced to account for its predicted eight (lat. octo) membrane spanning domains and its function as an anion channel [122,126]. The family of TMEM16 proteins consists of ten members termed TMEM16A to TMEM16K (I is left out), which correspond to anoctamin 1 to anoctamin 10 (ANO1 to ANO10) [127]. Only ANO1 and ANO2 were unequivocally identified as mediating calcium-activated anion currents, the other members were either identified as calcium-activated lipid scramblases or as dual-function scramblases/ ion channels [128,129]. The subtypes ANO1, ANO2 and ANO3 were found to be expressed in sensory neurons [130,131]. ANO1 was only detected in TRPV1 positive neurons [132], whereas about 50% of ANO3 positive neurons were also TRPV1 positive [131]. Both ANO1 [133] and ANO3 [131] are involved in nociceptive behavior in mouse models of inflammatory pain. Cyro-EM- [134,135,136] and X-ray studies [137] revealed that TMEM16 analogs actually consist of ten transmembrane domains. TMEM16A forms homodimers [138,139], where transmembrane domains 3–7 of both subunits form one separate ion conduction pathway creating a two-pore anion channel [134,135,136]. Calcium, needed for gating, binds to two regions of negatively charged amino acid residues in transmembrane domains 6–8. Subsequently, transmembrane domain 6 is displaced, which ultimately opens the channel [134,135]. In the absence of Ca, CaCC function is not altered by changes in membrane voltage within physiological limits. Only voltages exceeding 100 mV may gate CaCCs directly if Ca is absent [140], presence of cytosolic Ca merely shifts the voltage-dependence to more physiological levels [141]. In sensory neurons, Ca can either rise in response to activation of a G-coupled receptor and subsequent release from intracellular stores [142], or due to an influx of extracellular Ca via Ca-permeable ion channels like TRPV1 channels [143], or to a lesser extend voltage-gated Ca channels [142].
Mice lacking TRPV1 receptors, the canonical heat sensor, retain some sensitivity to noxious heat and CaCCs were suggested to fulfill that task [122]. Indeed, heterologously expressed TMEM16A channels produced currents at temperatures that exceeded 44 °C [144] and tissue specific knock-out reduced mechanically and thermally induced nocifensive behavior [132,133].
GPCR Regulation of Calcium-Activated Chloride Channels
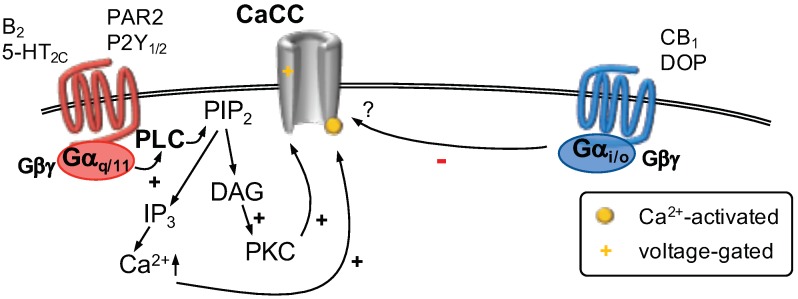
As described above, there are three possible sources for Ca to activate CaCCs. One of these possibilities is to activate a G-coupled receptor and the subsequent signaling cascade (Figure 4). In dorsal root ganglion neurons, the inflammatory mediator bradykinin was demonstrated to increase neuronal excitability via gating of CaCCs which leads to a depolarizing Cl efflux due to comparably high intracellular Cl concentrations. The induction of Cl currents through CaCCs by bradykinin required activation of PLC, formation of IP and an increase of cytosolic Ca levels [145]. In addition, it was also shown that activation of proteinase-activated receptor 2 (PAR2) induced currents through CaCCs in dorsal root ganglion neurons. This action is dependent on increasing levels of cytosolic Ca and a close proximity of IP receptors, which are located on the membrane of the endoplasmic reticulum, to ANO1 channels, which are located at the plasma membrane [142]. Furthermore, expression of both TMEM16A (ANO1) and PAR2 is induced in a model of neuropathic pain and both proteins are co-expressed in the same set of dorsal root ganglion neurons [146]. The inflammatory mediator serotonin was shown to induce currents mediated via CaCCs in dorsal root ganglion neurons. Even though all three types of 5-HT receptors are expressed on small-diameter dorsal root ganglion neurons, only activation of 5-HT receptors was able to induce such currents. Furthermore, the according increase in excitability also required activation of TRPV1 channels, which provides an additional Ca source [44]. Other possible mediators for sensitization include nucleotides: Both, UTP and ATP were found to interact with CaCCs via activation of G-coupled P2Y and P2Y receptors. However, this interaction was only investigated in kidney [147] and pancreatic cells [148]. It remains to be established if such an interaction also contributes to nucleotide-mediated sensitization of sensory neurons.
Figure 4.
Calcium-activated Cl channels (CaCC) are gated by increasing concentrations of cytosolic Ca and influenced by membrane voltage. The source of Ca may be Ca-permeable ion channels located in proximity to CaCCs (not shown) or Ca released from intracellular stores. Stimulation of G-coupled receptors (left) activates phospholipase C (PLC). The subsequent hydrolysis of phosphatitylinositol 1,4, bisphosphate (PIP) forms inositiol 1,4,5 trisphosphate (IP) and diacylglycerol (DAG). DAG activates protein kinase C (PKC) which influences CaCC function. IP binds to IP receptors located in the membrane of the endoplasmic reticulum (ER) which causes Ca release from the ER. Activation of G-coupled receptors (right) was shown to decrease CaCC currents via an unknown mechanism.
In animal experiments, it was determined that activation of G coupled cannabinoid CB receptors may contribute to peripheral antinociception via an interaction with CaCCs [149]. In addition, central antinociception via activation of G-coupled -opioid (DOP) receptors may involve an interaction with CaCCs [150]. However, it remains unclear how G-coupled receptors interfere with CaCC function.
2.5. Voltage-Gated Na Channels
Voltage-gated Na channels (Na) are crucial not just for excitable cells like central or peripheral neurons, skeletal or cardiac muscle cells, but also occur in immune cells, which are considered non-excitable [151]. In excitable cells, the principal role of Na channels is generating action potentials. Action potentials are generated, if a sufficient number of Na channels are activated in response to a local depolarization. As opposed to local depolarizations which can only spread over a few millimeters, action potentials can travel along several meters and thus transfer the information to the central nervous system. The stronger a local depolarization is, for example in response to a noxious stimulus, the more action potentials are triggered [2]. If Na channels are rendered non-functional, action potentials cannot be evoked and information transfer to the central nervous system is stopped [152,153,154,155]. This mechanism is highlighted by patients carrying loss-of-function mutations in their Na1.7 or Na1.9 genes, who experience insensitivity towards painful stimuli [156].
To date, nine pore-forming -subunits of Na channels are described, designated Na1.1 to Na1.9 [11]. Only Na1.6 to Na1.9 channels can be found in nociceptive neurons. A functional voltage-gated Na channel is formed by a pore-forming -subunit and one additional auxiliary -subunit, of which four (1 to 4) have been described. An -subunit is composed of 24 transmembrane segments, which can be grouped into four domains (DI–DIV) of six transmembrane segments each (S1–S6) [151]. The transmembrane segments S1–S4 of each domain form the voltage-sensor, whereas all four S5 and S6 segments contribute to the channel pore. The S4 segments are of particular interest as they harbor a number of positively charged amino acid residues. These residues move the entire S4 segment upwards upon depolarization and lead to the gating of these ion channels. This basic principle of activation is conserved among all members of voltage-gated ion channels [157]. Voltage-gated Na channels activate within a fraction of a millisecond and subsequently enter a fast-inactivated state. This inactivation is mediated by the intracellularly located DIII-DIV linker [151]. The subunits Na1.5, Na1.8 and Na1.9 have a low affinity for tetrodotoxin (TTX) as it ranges from 10 to 100 M. The affinity for all other subtypes ranges between 1 to 10 nM [11]. The former subtypes are therefore described as TTX-insensitive and this represents a simple experimental tool to distinguish between Nas relevant for nociception and those that are not relevant for nociception [151]. In addition to the previously described channelopathies leading to pain-insensitive patients, the importance of voltage-gated Na channels for nociception is highlighted by the fact that the most widely used local anesthetic drug, lidocaine, leads to a use-dependent block of these channels, which prevents the propagation of painful stimuli [151].
2.6. GPCR Regulation of Voltage-Gated Na Channels
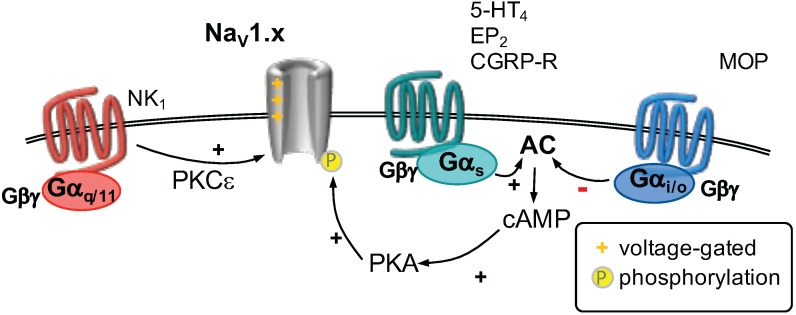
The activity of TTX-resistant voltage-gated Na channels is affected by activation of G coupled receptors (Figure 5). Direct activation of adenylyl cyclase by forskolin increases TTX-resistant Na currents in dorsal root ganglion neurons. Activation of adenylyl cyclase increases the formation of cAMP, which wash shown to be involved in this process [158]. In sensory neurons, the inflammatory mediators serotonin [158], PGE [159,160] and CGRP [161] were found increase TTX-resistant Na currents via a mechanism involving G-coupled 5-HT [162,163], EP [164], and CGRP [161] receptors, respectively. Application of substance P leads to an increase in neuronal excitability in small diameter dorsal root ganglion neurons [165]. Substance P activates neurokinin 1 (NK) receptors, amongst others [110]. These G- and G-coupled receptors were found to increase TTX-resistant Na currents in dorsal root ganglion neurons in a PKC dependent manner [166]. In addition, ATP, another component of the inflammatory soup, was also found to increase TTX-resistant Na currents in sensory neurons. By contrast, TTX-sensitive currents were not affected by application of ATP [167]. The underlying signaling cascade was not elucidated and hence it remains to be determined if, for example, G-coupled P2Y receptors could be involved.
Figure 5.
Na1.x channels are activated by depolarizing voltages (as indicated). Activation of G-coupled receptors stimulates adenylyl cyclase (AC) activity (center). Subsequently, cyclic adenosine monophosphate (cAMP) is formed, which activates protein kinase A (PKA). PKA-mediated phosphorylation of voltage-gated Na channels increases their currents. By contrast, activation of G-coupled receptors (right) decreases AC activity and counteracts G-mediated current increases. Activation of a G-receptor (left)was shown to increase Na-mediated currents involving protein kinase C (PKC).
On the other hand, activation of protease activated receptor 2 (PAR2) does not affect TTX-resistant Na currents in nociceptive neurons [168]. These GPCRs are coupled to heterotrimeric G-proteins and lead to release of Ca from intracellular stores as well as activation of PKC [142].
With respect to G-coupled receptors, only activation of -opioid receptors by the agonist DAMGO was investigated. In sensory neurons, application of DAMGO was able to prevent the PGE-mediated increase of TTX-resistant Na channels [169]. The involved pathway was not investigated, but is seems reasonable to assume an interference of the G-pathway with the G-mediated potentiation of these NaV channels.
2.7. Voltage-Gated Ca Channels
Neuronal calcium channels are protein complexes formed by a pore-forming 1 subunit, one and one 2 subunit, the latter regulating membrane trafficking and voltage dependence [170]. There are ten different 1 subunits that can be divided into three families (Ca1.x–3.x). Each of these families consists of several subtypes that differ in biophysical parameters, expression pattern and physiological function. In addition, there is a clear distinction in the voltage dependence of Ca1.x and Ca2.x when compared to Ca3.x. While the latter activates at quite hyperpolarized potentials (<−50 mV), thus being termed low voltage activated channel (LVA), the former need much higher depolarizations to be opened and are called high voltage activated channels (HVA). In DRG neurons, all three families of Cas are found [171] and all can be modulated by GPCRs to different extents. Depending on the type of GPCR, the channels are modulated via different pathways.
GPCR Regulation of Voltage-Gated Ca Channels
The most prominent and by far best studied mechanism is the so-called voltage-dependent inhibition (Figure 6). This is found in Ca2.x channels, where the G-protein G subunits can directly bind to the calcium channel 1 subunit. This binding event leads to a shift in the gating mode from “wiilling” to a “reluctant” one that manifests itself mainly in a marked slowing of activation [172,173,174]. The term “voltage dependent” refers to the fact that depolarization can relief the channels from inhibition and restore normal gating. As this type of inhibition is mostly exerted by G-coupled receptors it can be abolished by treating the cells with pertussis toxin (PTX) that ribosilates G-proteins and renders them inactive.
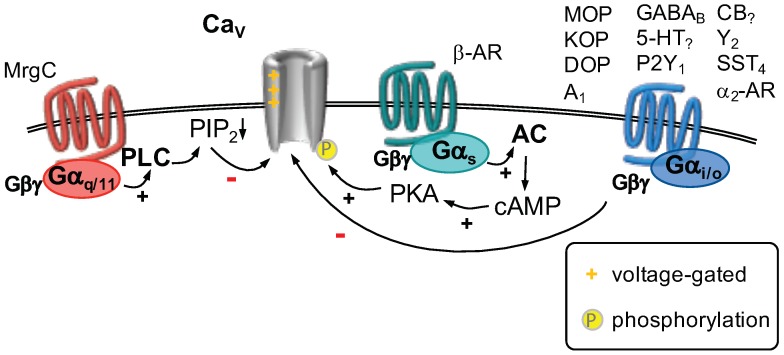
Figure 6.
Ca channels are activated by depolarizing voltages (as indicated). Activation of a G-coupled receptor (right) leads to a phenomenon called “voltage-dependent inhibition”. This involves direct binding of the G dimer to Ca2.x channels. Activation of G-coupled receptors (center) leads to activation of adenylyl cyclase (AC), which forms cAMP (cyclic adenosine monophosphate) to activate protein kinase A (PKA). PKA-mediated phosphorylation of Ca1.x channels was shown to increase their currents. Activation of a G-coupled receptor (left) stimulates phospholipase C (PLC), which hydrolyzes phosphatitylinositol 4,5 bisphosphate (PIP). Depletion of PIP is sufficient to decrease currents through Ca channels.
One of the best studied examples for voltage dependent calcium channel inhibition is the action of opioid receptors. All three types of opioid receptors, (MOP), (KOP) and (DOP), are found in DRG neurons, with the exact expression pattern depending on the cell type [175,176]. Initially, it was found that exposure of nociceptive neurons to opioids leads to a shortening of action potential durations [177,178,179,180,181]. It was demonstrated that application of [D-Ala2]-enkephalin (DADLE), an unspecific opioid receptor agonist, not only reduced action potential duration, but also diminished substance P release in these neurons [177]. As became clear later on, both effects were caused mainly by the inhibition of Ca channels [172,182]. The major target for opioid modulation are Ca2.x channels [183,184,185,186,187,188]. These channels are found at the presynapse and govern neurotransmitter release [170], thus inhibition of Ca2.x channels leads to reduced Ca influx and concomitantly reduced transmitter release from peripheral nociceptive neurons onto second-order neurons of the pain pathway located in the spinal dorsal horn. In line with the fact that opioid receptors couple predominantly to G G proteins [110], opioid induced calcium channel inhibition was found to be PTX sensitive [188], and voltage dependent [172]. These findings were corroborated by intracellular administration of G antiserum, that strongly reduced opioid receptor mediated I inhibition [189], unequivocally demonstrating the mechanism of action.
Besides opioid receptors, many other GPCRs expressed in DRG neurons were found to lead to a similar kind of calcium channel modulation. For example GABA [190], adenosine A [191], 5-HT [163,192,193,194,195], P2Y [196], cannabinoid [197], neuropeptide Y Y receptors [198,199,200], somatostatin SST [201] or adrenoceptors [202].
Besides the well-studied G mediated voltage dependent inhibition, other mechanisms have also been described [174]. Most prominently, phospholipase C (PLC) mediated PIP depletion can lead to inhibition of calcium channels [203,204,205]. Similar to K7 and K3 channels (see below), PIP stabilizes the open state of calcium channels. Thus, a reduction in membrane PIP leads to a voltage independent decrease in channel open probability.
While several reports about this kind of inhibition exist from sympathetic neurons [174], there are few data from nociceptive neurons. However, given the similarity of receptor and channel expression between sympathetic and sensory neurons, it is reasonable to assume that these findings will also hold true in DRG neurons. Only recently, the Mas-related G protein coupled receptor type C (MrgC) was found to inhibit high voltage-gated calcium channels in a PLC dependent manner [206].
Recently, a potentially novel form of calcium channel inhibition has been described. Huang et al. [207] found that GABA receptors not only inhibit HVA channels but also LVA channels. Inhibition of both channel types was PTX sensitive, however the LVA inhibition was strongly reduced by application of DTT, pointing to a novel mechanism that involves redox processes.
GPCRs cannot only inhibit Ca channels but they are also known to be able to facilitate their function. A classic example would be PKA which phosphorylates Ca1.x channels and increases their currents [208]. In line with this, a broadening of the action potential upon application of noradrenaline to DRG neurons was reported. This increase in action potential duration could finally be attributed to an increase in Ca1.x currents [202].
2.8. Voltage-Gated K Channels
2.8.1. K7 Channels
Amongst potassium channels, K channels constitute the most diverse group with 12 known families [209]. Literature abounds on how various K channels modulate nociception at different levels of the pain pathway [210]. The prototypical structural assembly of K channels involves six transmembrane segments of which the first four (S1-S4) constitute the voltage-sensing domain (VSD) while the S5 and S6 segments constitute the pore through which K ions pass [211,212]. Amongst others, the K7 channel family has received immense attention in lieu of its amenability by GPCR modulation [213,214], with five known members (K7.1– K7.5) encoded by KCNQ1-5 genes [209]. Four monomers come together in a homo- or heterotetrameric configuration in a subunit-specific way to yield a functional K7 channel [215]. The electrophysiological correlate of K7 channel activity is a slowly deactivating, non-inactivating current that has an activation threshold below −60 mV. This conductance is also known as M current as it was originally described as a current that is suppressed by an activation of muscarinic acetylcholine receptors [216]. In nociceptors, these channels contribute majorly to the resting membrane potential [217]. K7 channels are expressed in all functional parts of a first-order neuron which include free nerve endings, nodes of Ranvier, and the somata of dorsal root ganglion (DRG) neurons [218]. They regulate action potential (AP) firing, which is the basis for encoding of noxious stimuli in the pain pathway [219]. Enhancing K7 currents exerts analgesic effects since hyperpolarizing the resting membrane potential of nociceptors decreases neuronal excitability [220,221]. Similarly, inhibiting K7 currents is proalgesic since concomitant depolarization of the resting membrane potential enhances neuronal firing [222,223].
2.8.2. GPCR Regulation of K7 Channels
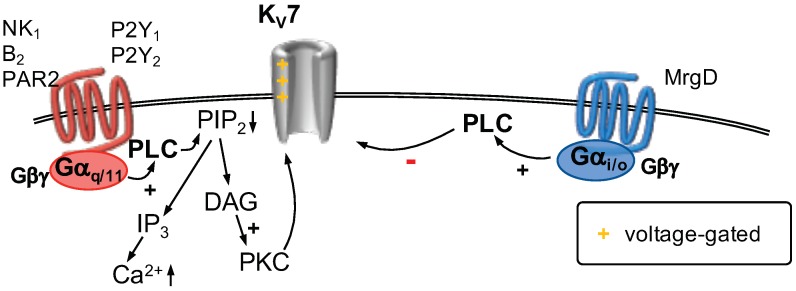
A plethora of neurotransmitters and neuropeptides modulate K7 channel function via GPCR signaling (Figure 7), specifically of the G class [224]. One of the early reports was of the nociception-relevant neuropeptide Substance P (SP) that inhibited K7 currents in bullfrog DRG neurons [225]. Subsequently it was revealed that neurokinin A (NKA inhibited currents through K7 channels in bullfrog DRG neuronsvia NK receptors which were coupled to PTX-insensitive G proteins [226], even though this receptor may impinge on the functions of K7 channels through G protein-independent mechanisms as well [227]. The activation of G-coupled receptors leads phospholipase C (PLC) to hydrolyze the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP) into inositol-1,4,5-trisphosphate (IP) and diacylglycerol (DAG) [228]. The function of K7 channels is governed by the presence of sufficient membrane PIP pools and depletion of membrane PIP levels leads K7 channels to close [229]. The proalgesic mediator bradykinin mediates inhibition of K7 currents via its actions on the B receptor, a G-coupled receptor [230]. The active nociception mediated by bradykinin, consequent to enhanced neuronal excitability can be attenuated by prior application of the K7 channel opener retigabine [145]. In addition to membrane PIP depletion, the inhibition of K7 channels via IP-mediated increase in intracellular Ca levels and subsequent binding to calmodulin is well known [231,232]. In sympathetic neurons, the governing factor for substrate (PIP)- versus product (Ca)-mediated inhibition consequent to application of bradykinin is contingent upon Ca availability and rate of PIP synthesis [233]. B receptors are closely opposed to the endoplasmic reticulum (ER) where IP can diffuse and consequently mobilize Ca reserves [216]. In DRG neurons, bradykinin primarily employs the Ca axis to inhibit K7 currents as evidenced by the fact that inhibition of Ca release from IP-sensitive stores with pharmacological tools as well as chelation of intracellular Ca prevents bradykinin-mediated inhibition of K7 channels, akin to direct activation of PLC [145]. One of the targets of the inflammatory soup is the protease-activated receptor 2 (PAR2), a G-coupled receptor expressed in nociceptors [49,234]. Activation of these receptors has an inhibitory impact on K7 currents leading to nociception which requires concurrent increase in cytosolic Ca levels in addition to depletion of PIP levels [235]. Another example is the modulation of excitability in nociceptors by nucleotides. The P2Y and P2Y receptors are G-coupled receptors [236]. Activation of these receptors by the nucleotides adenosine diphosphate (ADP), and 2-thio-uridine triphosphate (2-thio-UTP), respectively, leads to the inhibition of currents through K7 channels [47]. Moreover, the observed effects were prevented by the application of U73122, a PLC inhibitor, inhibition of CaATPases by thapsigargin, and chelation of intracellular Ca levels by BAPTA-AM [47].
Figure 7.
K7 channels are activated by depolarizing voltages (as indicated). G-coupled receptors (left) activate phospholipase C (PLC), which cleaves phosphatitylinositol 4,5, bisphosphate (PIP) into inositol 1,4,5 trisphosphate (IP) and diacylglycerol (DAG). Depletion of PIP from the plasma membrane is sufficient to decrease currents through K7 channels. In addition, Ca is released from the endoplasmic reticulum subsequent to formation of IP. Ca decreases K7 currents via an interaction with calmodulin. Activation of a G-coupled receptor (right) was shown to decrease K7 currents in a PLC-dependent manner.
The GPCRs encoded by Mas-related genes (Mrgs) are a more recently identified subset of GPCRs that are widely expressed in sensory neurons and implicated in the modulation of nociceptive information [237,238]. Specifically, the MrgD isoform is expressed in DRG neurons especially in non-peptidergic, small-diameter IB4-postive C-fiber somata [239,240]. Activation of endogenous MrgD with the agonist alanine results in the inhibition of K7 currents in DRG neurons, mainly employing a pertussis toxin-sensitive pathway implicating the involvement of G. Such an inhibition translates into enhanced neuronal firing in phasic DRG neurons, which classically shoot single single APs [241]. Recombinant cell-lines coexpressing MrgD receptors and K7.2/7.3 heteromers exhibit an inhibition of K7 currents upon stimulation with alanine, an effect that could be reversed partially by pharmacologically blocking G and reversed completely by PLC inhibition [241].
2.8.3. K1.4, K3.4 and K4 Channels
The K channels K1.4, K3.4 and K4 members contribute to the so-called transient A-current (I) [242,243], which plays a key role in regulating AP firing in DRG neurons [210,244]. The somata, axons and central terminals of DRG neurons that abut the spinal dorsal horn are enriched with K3.4 channels. The expression of K4.3 channels, on the other hand is restricted to the somata of non-peptidergic DRG neurons [245]. The rapidly inactivating K3.4 channel is a key player in AP repolarization in DRG neurons [246,247]. Activating PKC through physiological or pharmacological means leads to a decline in fast N-type inactivation in endogenously expressed K3.4 channels. This directly impacts the biophysical properties of the nociceptor: the AP gets narrowed while the AP repolarization is accelerated [248]. Specific siRNAs that selectively target K3.4 channel expression abolish the changes in AP waveform mediated by PKC activation [248]. In a rat model of cervical spinal cord injury (SCI), the surface expression of K3.4 channels was shown to be impaired; such dysregulation was associated with the failure of PKC to shorten the AP duration in DRG neurons [249]. Similarly, the phosphatase calcineurin (CaN) antagonizes PKC activity as revealed by a reduction in the inactivation of K3.4 channels upon pharmacological inhibition of the former [250].
2.8.4. GPCR Regulation of K1.4, K3.4 and K4 Channels
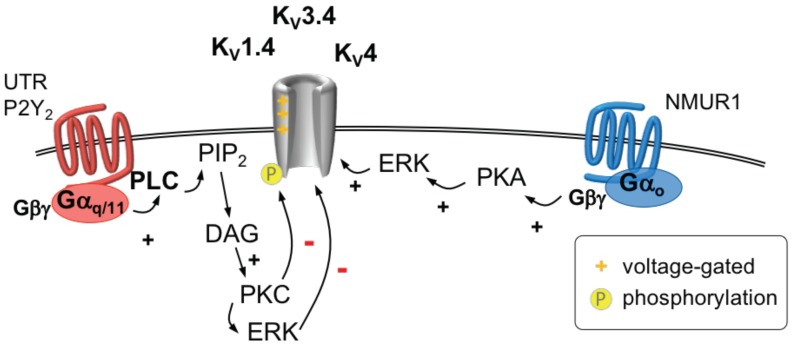
Neuromedin U (NMU) is a neuropeptide that decreases neuronal excitability in DRG neurons via the enhancement of I currents through its actions on the NMU type 1 receptor (NMUR1, Figure 8) [251]. NMUR1 couples to G proteins, PKA and the ERK pathway in a sequential manner [251]. On the other hand, the cyclic undecapeptide urotensin-II activates the urotensin-II receptor (UTR), which couples to G [252,253]. This leads to a reduction in I in a dose-dependent fashion in trigeminal ganglion neurons, mediated via the activation of PKC [254]. The concomitant recruitment of ERK signaling cascade culminates in an enhanced excitability of TG neurons [254]. In rat TG neurons, the K1.4, K3.4, K4.2 and K4.3 channels are co-expressed with P2Y receptors [255], which in turn couple to different G proteins [256]. The application of UTP, an agonist at the P2Y receptor inhibits I currents via the ERK pathway and enhances excitability in these neurons, an effect that can be reversed by the P2Y receptor antagonist suramin [255].
Figure 8.
The so-called A-current is mediated by voltage-activated K1.4, K3.4, and K4 channels. Activation of a G-coupled receptor (right) increases A-type currents via a mechanism involving the G dimer, protein kinase A (PKA) and extracellular signal-regulated kinase (ERK). Stimulation of G-coupled receptors (left) activates phospholipase C (PLC) leading to hydrolysis of phosphatityl 4,5 bisphosphate (PIP) to diacylglycerol (DAG) and IP. DAG activates protein kinase C (PKC), which phosphorylates A-type channels and thus inhibits these channels. ERK is activated in parallel which also phosphorylates A-type channels and thereby decreases their function.
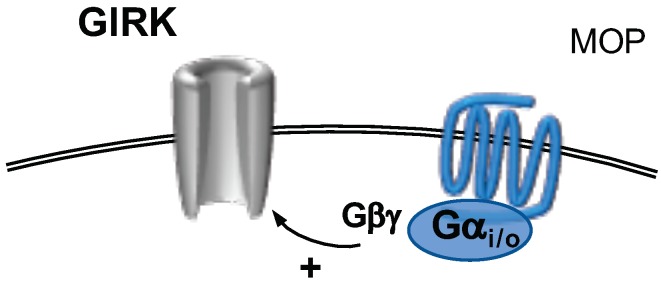
2.9. G-Protein Activated, Inwardly Rectifying Potassium Channels
G protein activated, inwardly rectifying potassium channels (GIRK) are homo- or heterotetrameric channels formed from four different subunits (K3.1–3.4) encoded by the genes KCNJ3, KCNJ6, KCNJ9 and KCNJ5, respectively [257,258]. They are activated by pertussis toxin sensitive G proteins via binding of dimers to the channel [257]. In addition, it has also been demonstrated that the G subunit can directly bind to the channels modulating their basal activity in the absence of GPCR activation [259]. Besides their G protein-mediated modulation, it has been demonstrated several times that K3 channels bind PIP and that this is necessary for their function [260,261].
GPCR Modulation of Girk Channels
Compared to their role in the CNS, data on their physiological role in DRG neurons is scarce [258]. In rat DRG neurons, co-localization of GIRK channels and -opioid receptors has been found [262]. The picture is complicated by the fact that, while all four subunits are expressed in rat and human DRG neurons [117,263], only low mRNA levels and a lack of immunostaining have been reported in mice [117]. This is corroborated by the fact that local application of DAMGO (Figure 9), a -opioid receptor agonist, is ineffective in inflammatory pain mouse models [117]. This lack of effect, however, could be overcome by expressing K3.2 in Na1.8 expressing nociceptive neurons [117], demonstrating the importance of K3 channels for peripheral analgesia. These findings are contrasted by the fact that GIRK currents could be induced in a small number of about 15–20% of mouse DRG neurons by application of DAMGO [264], rendering the interpretation of mouse data difficult.
Figure 9.
G-protein activated, inwardly rectifying K channels (GIRK) are activated subsequent to stimulation of G-coupled receptors. The dissociated G dimer binds directly to GIRK channels.
3. Conclusions
In this review, members of ten different ion channel families that are expressed in sensory neurons are described with respect to their contribution to nociception. Appropriate gating of these channels is subject to modulation by at least 35 different types of GPCRs, which are targeted by more than 20 separate endogenous modulators (Table 2). Thereby, GPCRs provide the largest superfamily of receptors, activation of which can mediate pro- as well as antinociceptive effects. Accordingly, these GPCRs are relevant as potential targets for analgesic drugs. However, only a few of them are currently exploited in analgesic therapy, such as opioid, cannabinoid, and CGRP receptors or prostanoid receptors as indirect targets of cyclooxygenase inhibitors. Therefore, this review should be viewed as incitement to further investigate how the modulation of ion channels via GPCRs might be tackled to provide novel pharmacotherapeutic agents for improved analgesic therapy.
Table 2.
Endogenous ligands for GPCRs modulating ion channel function.
| Endogenous Ligand | GPCR | ASIC | CaCC | Ca | GIRK | KP | K1.4 K3.4 K4 |
K7 | Na | TRPA1 TRPM3 TRPM8 TRPV1 |
Piezo |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine | A | Ca | |||||||||
| A | TRPA1 | ||||||||||
| Alanine | MrgD | K7 | |||||||||
| BAM 8-22 | MRGPRX1 | TRPV1 | |||||||||
| Bradykinin | B | TRPA1 | |||||||||
| B | CaCC | K7 | TRPA1 | Piezo2 | |||||||
| TRPM8 | |||||||||||
| TRPV1 | |||||||||||
| CGRP | CGRP-R | Na | |||||||||
| Endo- | CB | ASIC | CaCC | TRPV1 | |||||||
| cannabinoids | CB | TRPV1 | |||||||||
| CB | Ca | ||||||||||
| Endothelin 1 | ET | TRPV1 | |||||||||
| GABA | GABA | Ca | |||||||||
| H | TDAG8/ | TRPV1 | |||||||||
| (GPR65) | |||||||||||
| Histamine | H | TRPA1 | |||||||||
| Neuromedin U | NMUR1 | K1.4 | |||||||||
| K3.4 | |||||||||||
| K4 | |||||||||||
| Neuropeptide Y | Y | Ca | |||||||||
| Noradrenaline | Ca | ||||||||||
| Ca | |||||||||||
| Nucleotides | P2Y | CaCC | Ca | K7 | |||||||
| P2Y | ASIC | CaCC | K1.4 | K7 | Piezo2 | ||||||
| K3.4 | |||||||||||
| K4 | |||||||||||
| P2Y | KP | ||||||||||
| Opioids | MOP | ASIC | Ca | GIRK | KP | Na | TRPV1 | ||||
| DOP | CaCC | Ca | |||||||||
| KOP | Ca | ||||||||||
| Prostaglandins | EP | TRPV1 | |||||||||
| EP | Na | TRPV1 | |||||||||
| IP | TRPV1 | ||||||||||
| FP | KP | ||||||||||
| Proteases | PAR1 | TRPV1 | |||||||||
| PAR2 | ASIC | CaCC | TRPV1 | ||||||||
| PAR4 | TRPV1 | ||||||||||
| Serotonin | 5-HT | ASIC | CaCC | TRPV1 | |||||||
| 5-HT | KP | Na | TRPV1 | ||||||||
| 5-HT | TRPV1 | ||||||||||
| 5-HT | Ca | ||||||||||
| Somatostatin | SST | Ca | |||||||||
| Substance P/ | NK | K7 | Na | TRPV1 | |||||||
| neurokinin A | NK | TRPV1 | |||||||||
| Urotensin | UTR | K1.4 | |||||||||
| K3.4 | |||||||||||
| K4 | |||||||||||
| ? | MrgC | Ca |
ASIC, acid sensing ion channel; CaCC, Ca-activated Cl channel; Ca, voltage-gated Ca channel; GIRK, G-protein activated; inwardly rectifying K channel; KP, two-pore K channel; K, voltage-gated K channel; Na, voltage-gated Na channel; TRP, transient receptor potential channel; TRPA, ankyrin family; TRPM, melastatin family; TRPV, vanilloid family; BAM 8-22, bovine adrenal medulla peptide 8-22; CGRP, calcidonin-gene related peptide; CGRP-R, CGRP receptor; GABA, -amino butyric acid; ?, unknown.
Abbreviations
The following abbreviations are used in this manuscript:
| AR | adrenoceptor |
| AR | adrenoceptor |
| -OR | opioid receptor |
| 5-HT | 5-hydroxytryptamine receptor 2 or 4 |
| A | adenosine A receptor |
| AC | adenylyl cyclase |
| ADP | adenosine diphosphate |
| ANO1-10 | anoctamin 1 to 10 |
| AP | action potential |
| ASIC | acid sensing ion channel |
| ATP | adenosine triphosphate |
| B | bradykinin B receptor |
| BAPTA-AM | 1,2-Bis(2-aminophenoxy)ethane-N,N,N,N-tetraacetic acid tetrakis(acetoxymethyl ester) |
| Ca | voltage-gated Ca channel |
| CaCC | Ca-activated Cl channel |
| CamKII | Ca/calmodulin dependent protein kinase II |
| cAMP | cyclic adenosine monophosphate |
| CaN | calcineurin |
| CB | cannabinoid CB or CB receptor |
| cGMP | cyclic guanosine monophosphate |
| CGRP | calcitonin gene-related peptide |
| CGRP-R | calcitonin gene-related peptide receptor |
| CNS | central nervous system |
| Cryo-EM | cryogenic electron microscopy |
| DADLE | [D-Ala2]-enkephalin |
| DAG | diacylglycerol |
| DAMGO | [D-Ala, NMe-Phe, Gly-ol]-enkephalin |
| DI-IV | domain I to IV of Ca and Na channels |
| DOG1 | discovered on GIST 1 |
| DOP | opioid receptor |
| DRG | dorsal root ganglion |
| ECD | extracellular domain |
| EP | prostanoid EP or EP receptor |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| ET | endothelin ET receptor |
| FP | prostanoid FP receptor |
| G | G protein |
| G | G protein |
| G | G protein |
| G | G dimer |
| GABA | -aminobutyric acid receptor B |
| GIRK | G-protein activated, inwardly rectifying potassium channels |
| GIST | gastrointestinal stroma tumor |
| GPCR | G-protein coupled receptor |
| HVA | high voltage activated Ca channel |
| I | A-type K current |
| IP | prostanoid IP receptor |
| IP | inositol 1,4,5 trisphosphate |
| K | two-pore K channel |
| K | inwardly rectifying potassium channel |
| K | voltage-gated K |
| KCNJ | gene name potassium voltage-gated channel subfamily J |
| KCNQ | gene name potassium voltage-gated channel subfamily Q |
| KOP | opioid receptor |
| LVA | low voltage activated Ca channel |
| MOP | opioid receptor |
| MrgC | mas-related G protein coupled receptor type C |
| MrgD | mas-related G protein coupled receptor type D |
| mRNA | messenger ribonucleic acid |
| Na | voltage-gated Na channel |
| NGF | nerve growth factor |
| NK | tachynin NK or NK receptor |
| NKA | neurokinin A |
| NMU | neuromedin U |
| NMUR | NMU receptor |
| P2X | purinergic P2X receptor |
| P2Y | purinergic P2Y receptor |
| PAR2/ 4 | protease-activated receptor type 2 or type 4 |
| PGE | prostaglandin E |
| PGF | prostaglandin F |
| PICK-1 | protein interacting with C-kinase 1 |
| PIP | phosphatitylinositol 4,5 bisphosphate |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PKG | protein kinase G |
| PLA | phospholipase A |
| PLC | phospholipase C |
| PPI | protein phosphatase I |
| PTX | pertussis toxin |
| S1–4 | transmembrane segment 1–4 of voltage-gated channels |
| SCI | spinal cord injury |
| siRNA | small interfering RNA |
| SP | substance P |
| SST | somatostatin SST receptor |
| TG | trigeminal ganglion |
| TMEM16 | transmembrane protein of unknown function family 16 |
| TMS | transmembrane segment |
| TNF | tumor necrosis factor |
| TRAAK | TWIK-related arachidonic acid activated K channel = K4.1 |
| TREK1/2 | TWIK-related K channel 1 (K2.1) or TWIK-related K channel 2 (K10.1) |
| TRH | thyrotropin-releasing hormon |
| trk | tyrosine receptor kinase |
| TRPA1 | transient receptor potential channel ankyrin family |
| TRPM8 | transient receptor potential channel melastatin family |
| TRPV | transient receptor potential channel vanilloid family |
| TTX | tetrodotoxin |
| TWIK | tandem of pore domains in a weak inward rectifying K channel |
| UTP | uridine triphosphate |
| UTR | urotensin-II receptor |
| V | vasopressin V receptor |
| Y | neuropeptide Y Y receptor |
Author Contributions
I.S., S.R., K.S. and S.B. wrote the manuscript; I.S. performed artwork.
Funding
Work in the authors’ laboratory was supported by the doctoral program CCHD funded by the Austrian Science Fund (FWF, W1205) and the Medical University of Vienna as well as by the inter-university cluster project “Novel scaffolds for improved antiepileptic drugs” financed by the University of Vienna and the Medical University of Vienna.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Loeser J.D., Treede R.D. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008;137:473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Basbaum A.I., Bautista D.M., Scherrer G., Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hucho T., Levine J.D. Signaling pathways in sensitization: Toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Venkatachalam K., Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron A., Lingueglia E. Pharmacology of acid-sensing ion channels—Physiological and therapeutical perspectives. Neuropharmacology. 2015;94:19–35. doi: 10.1016/j.neuropharm.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 6.North R.A., Jarvis M.F. P2X receptors as drug targets. Mol. Pharmacol. 2013;83:759–769. doi: 10.1124/mol.112.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook A.D., Christensen A.D., Tewari D., McMahon S.B., Hamilton J.A. Immune Cytokines and Their Receptors in Inflammatory Pain. Trends Immunol. 2018;39:240–255. doi: 10.1016/j.it.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Denk F., Bennett D.L., McMahon S.B. Nerve Growth Factor and Pain Mechanisms. Annu. Rev. Neurosci. 2017;40:307–325. doi: 10.1146/annurev-neuro-072116-031121. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko Y., Szallasi A. Transient receptor potential (TRP) channels: A clinical perspective. Br. J. Pharmacol. 2014;171:2474–2507. doi: 10.1111/bph.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basso L., Altier C. Transient Receptor Potential Channels in neuropathic pain. Curr. Opin. Pharmacol. 2017;32:9–15. doi: 10.1016/j.coph.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Alexander S.P., Striessnig J., Kelly E., Marrion N.V., Peters J.A., Faccenda E., Harding S.D., Pawson A.J., Sharman J.L., Southan C., et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Voltage-gated ion channels. Br. J. Pharmacol. 2017;174:S160–S194. doi: 10.1111/bph.13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai Y. TRPs and pain. Semin. Immunopathol. 2016;38:277–291. doi: 10.1007/s00281-015-0526-0. [DOI] [PubMed] [Google Scholar]
- 13.Mickle A.D., Shepherd A.J., Mohapatra D.P. Sensory TRP channels: The key transducers of nociception and pain. Prog. Mol. Biol. Transl. Sci. 2015;131:73–118. doi: 10.1016/bs.pmbts.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinberg X., Lespay-Rebolledo C., Brauchi S. A structural view of ligand-dependent activation in thermoTRP channels. Front. Physiol. 2014;5:171. doi: 10.3389/fphys.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G., Wang K. The Ca2+-Permeable Cation Transient Receptor Potential TRPV3 Channel: An Emerging Pivotal Target for Itch and Skin Diseases. Mol. Pharmacol. 2017;92:193–200. doi: 10.1124/mol.116.107946. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X. Targeting TRP ion channels for itch relief. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015;388:389–399. doi: 10.1007/s00210-014-1068-z. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K., Fukuoka T., Obata K., Yamanaka H., Dai Y., Tokunaga A., Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 18.Okazawa M., Inoue W., Hori A., Hosokawa H., Matsumura K., Kobayashi S. Noxious heat receptors present in cold-sensory cells in rats. Neurosci. Lett. 2004;359:33–36. doi: 10.1016/j.neulet.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 19.Usoskin D., Furlan A., Islam S., Abdo H., Lönnerberg P., Lou D., Hjerling-Leffler J., Haeggström J., Kharchenko O., Kharchenko P.V., et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 20.Palkar R., Lippoldt E.K., McKemy D.D. The molecular and cellular basis of thermosensation in mammals. Curr. Opin. Neurobiol. 2015;34:14–19. doi: 10.1016/j.conb.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White J.P.M., Cibelli M., Urban L., Nilius B., McGeown J.G., Nagy I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol. Rev. 2016;96:911–973. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]
- 22.Vriens J., Owsianik G., Hofmann T., Philipp S.E., Stab J., Chen X., Benoit M., Xue F., Janssens A., Kerselaers S., et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 23.Laing R.J., Dhaka A. ThermoTRPs and Pain. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 2016;22:171–187. doi: 10.1177/1073858414567884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latorre R., Zaelzer C., Brauchi S. Structure-functional intimacies of transient receptor potential channels. Q. Rev. Biophys. 2009;42:201–246. doi: 10.1017/S0033583509990072. [DOI] [PubMed] [Google Scholar]
- 25.Jardín I., López J.J., Diez R., Sánchez-Collado J., Cantonero C., Albarrán L., Woodard G.E., Redondo P.C., Salido G.M., Smani T., et al. TRPs in Pain Sensation. Front. Physiol. 2017;8:392. doi: 10.3389/fphys.2017.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y., Cao E., Julius D., Cheng Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature. 2016;534:347–351. doi: 10.1038/nature17964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao E., Liao M., Cheng Y., Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao M., Cao E., Julius D., Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregorio-Teruel L., Valente P., Liu B., Fernández-Ballester G., Qin F., Ferrer-Montiel A. The Integrity of the TRP Domain Is Pivotal for Correct TRPV1 Channel Gating. Biophys. J. 2015;109:529–541. doi: 10.1016/j.bpj.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohacs T. Phosphoinositide regulation of TRPV1 revisited. Pflugers Arch. Eur. J. Physiol. 2015;467:1851–1869. doi: 10.1007/s00424-015-1695-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caterina M.J., Leffler A., Malmberg A.B., Martin W.J., Trafton J., Petersen-Zeitz K.R., Koltzenburg M., Basbaum A.I., Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 32.Davis J.B., Gray J., Gunthorpe M.J., Hatcher J.P., Davey P.T., Overend P., Harries M.H., Latcham J., Clapham C., Atkinson K., et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 33.Park U., Vastani N., Guan Y., Raja S.N., Koltzenburg M., Caterina M.J. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:11425–11436. doi: 10.1523/JNEUROSCI.1384-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang S.M., Li X., Yu Y., Wang J., Caterina M.J. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol. Pain. 2011;7:37. doi: 10.1186/1744-8069-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandewauw I., De Clercq K., Mulier M., Held K., Pinto S., Van Ranst N., Segal A., Voet T., Vennekens R., Zimmermann K., et al. A TRP channel trio mediates acute noxious heat sensing. Nature. 2018;555:662–666. doi: 10.1038/nature26137. [DOI] [PubMed] [Google Scholar]
- 36.Chen J., Kang D., Xu J., Lake M., Hogan J.O., Sun C., Walter K., Yao B., Kim D. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat. Commun. 2013;4:2501. doi: 10.1038/ncomms3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moparthi L., Survery S., Kreir M., Simonsen C., Kjellbom P., Högestätt E.D., Johanson U., Zygmunt P.M. Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc. Natl. Acad. Sci. USA. 2014;111:16901–16906. doi: 10.1073/pnas.1412689111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilius B., Appendino G., Owsianik G. The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflugers Arch. Eur. J. Physiol. 2012;464:425–458. doi: 10.1007/s00424-012-1158-z. [DOI] [PubMed] [Google Scholar]
- 39.Rohacs T. Regulation of transient receptor potential channels by the phospholipase C pathway. Adv. Biol. Regul. 2013;53:341–355. doi: 10.1016/j.jbior.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Julius D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 41.Tang H.B., Inoue A., Oshita K., Nakata Y. Sensitization of vanilloid receptor 1 induced by bradykinin via the activation of second messenger signaling cascades in rat primary afferent neurons. Eur. J. Pharmacol. 2004;498:37–43. doi: 10.1016/j.ejphar.2004.07.076. [DOI] [PubMed] [Google Scholar]
- 42.Ohta T., Ikemi Y., Murakami M., Imagawa T., Otsuguro K.I., Ito S. Potentiation of transient receptor potential V1 functions by the activation of metabotropic 5-HT receptors in rat primary sensory neurons. J. Physiol. 2006;576:809–822. doi: 10.1113/jphysiol.2006.112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugiuar T., Bielefeldt K., Gebhart G.F. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J. Neurosci. Off. J. Soc. Neurosci. 2004;24:9521–9530. doi: 10.1523/JNEUROSCI.2639-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salzer I., Gantumur E., Yousuf A., Boehm S. Control of sensory neuron excitability by serotonin involves 5HT2C receptors and Ca(2+)-activated chloride channels. Neuropharmacology. 2016;110:277–286. doi: 10.1016/j.neuropharm.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moriyama T., Iida T., Kobayashi K., Higashi T., Fukuoka T., Tsumura H., Leon C., Suzuki N., Inoue K., Gachet C., et al. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J. Neurosci. Off. J. Soc. Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malin S.A., Davis B.M., Koerber H.R., Reynolds I.J., Albers K.M., Molliver D.C. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain. 2008;138:484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yousuf A., Klinger F., Schicker K., Boehm S. Nucleotides control the excitability of sensory neurons via two P2Y receptors and a bifurcated signaling cascade. Pain. 2011;152:1899–1908. doi: 10.1016/j.pain.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solinski H.J., Zierler S., Gudermann T., Breit A. Human sensory neuron-specific Mas-related G protein-coupled receptors-X1 sensitize and directly activate transient receptor potential cation channel V1 via distinct signaling pathways. J. Biol. Chem. 2012;287:40956–40971. doi: 10.1074/jbc.M112.408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amadesi S., Nie J., Vergnolle N., Cottrell G.S., Grady E.F., Trevisani M., Manni C., Geppetti P., McRoberts J.A., Ennes H., et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J. Neurosci. Off. J. Soc. Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai Y., Moriyama T., Higashi T., Togashi K., Kobayashi K., Yamanaka H., Tominaga M., Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J. Neurosci. Off. J. Soc. Neurosci. 2004;24:4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vellani V., Kinsey A.M., Prandini M., Hechtfischer S.C., Reeh P., Magherini P.C., Giacomoni C., McNaughton P.A. Protease activated receptors 1 and 4 sensitize TRPV1 in nociceptive neurones. Mol. Pain. 2010;6:61. doi: 10.1186/1744-8069-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moriyama T., Higashi T., Togashi K., Iida T., Segi E., Sugimoto Y., Tominaga T., Narumiya S., Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schnizler K., Shutov L.P., Van Kanegan M.J., Merrill M.A., Nichols B., McKnight G.S., Strack S., Hell J.W., Usachev Y.M. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:4904–4917. doi: 10.1523/JNEUROSCI.0233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bao Y., Gao Y., Yang L., Kong X., Yu J., Hou W., Hua B. The mechanism of μ-opioid receptor (MOR)-TRPV1 crosstalk in TRPV1 activation involves morphine anti-nociception, tolerance and dependence. Channels. 2015;9:235–243. doi: 10.1080/19336950.2015.1069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plant T.D., Zöllner C., Mousa S.A., Oksche A. Endothelin-1 potentiates capsaicin-induced TRPV1 currents via the endothelin A receptor. Exp. Biol. Med. 2006;231:1161–1164. [PubMed] [Google Scholar]
- 56.Plant T.D., Zöllner C., Kepura F., Mousa S.S., Eichhorst J., Schaefer M., Furkert J., Stein C., Oksche A. Endothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase C. Mol. Pain. 2007;3:35. doi: 10.1186/1744-8069-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H., Cang C.L., Kawasaki Y., Liang L.L., Zhang Y.Q., Ji R.R., Zhao Z.Q. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: A novel pathway for heat hyperalgesia. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sculptoreanu A., Aura Kullmann F., de Groat W.C. Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in DRG neurons from adult rats. Eur. J. Neurosci. 2008;27:3171–3181. doi: 10.1111/j.1460-9568.2008.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y.J., Huang C.W., Lin C.S., Chang W.H., Sun W.H. Expression and function of proton-sensing G-protein-coupled receptors in inflammatory pain. Mol. Pain. 2009;5:39. doi: 10.1016/j.jpain.2009.01.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vetter I., Wyse B.D., Monteith G.R., Roberts-Thomson S.J., Cabot P.J. The mu opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol. Pain. 2006;2:22. doi: 10.1186/1744-8069-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Endres-Becker J., Heppenstall P.A., Mousa S.A., Labuz D., Oksche A., Schäfer M., Stein C., Zöllner C. Mu-opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain. Mol. Pharmacol. 2007;71:12–18. doi: 10.1124/mol.106.026740. [DOI] [PubMed] [Google Scholar]
- 62.Anand U., Otto W.R., Sanchez-Herrera D., Facer P., Yiangou Y., Korchev Y., Birch R., Benham C., Bountra C., Chessell I.P., et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 2008;138:667–680. doi: 10.1016/j.pain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 63.Sántha P., Jenes A., Somogyi C., Nagy I. The endogenous cannabinoid anandamide inhibits transient receptor potential vanilloid type 1 receptor-mediated currents in rat cultured primary sensory neurons. Acta Physiol. Hung. 2010;97:149–158. doi: 10.1556/APhysiol.97.2010.2.1. [DOI] [PubMed] [Google Scholar]
- 64.Bonnington J.K., McNaughton P.A. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J. Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim B.M., Lee S.H., Shim W.S., Oh U. Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/ TRPV1 pathway in rat sensory neurons. Neurosci. Lett. 2004;361:159–162. doi: 10.1016/j.neulet.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 66.Shim W.S., Tak M.H., Lee M.H., Kim M., Kim M., Koo J.Y., Lee C.H., Kim M., Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kajihara Y., Murakami M., Imagawa T., Otsuguro K., Ito S., Ohta T. Histamine potentiates acid-induced responses mediating transient receptor potential V1 in mouse primary sensory neurons. Neuroscience. 2010;166:292–304. doi: 10.1016/j.neuroscience.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Dembla S., Behrendt M., Mohr F., Goecke C., Sondermann J., Schneider F.M., Schmidt M., Stab J., Enzeroth R., Leitner M.G., et al. Anti-nociceptive action of peripheral mu-opioid receptors by G-beta-gamma protein-mediated inhibition of TRPM3 channels. eLife. 2017;6:1–32. doi: 10.7554/eLife.26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quallo T., Alkhatib O., Gentry C., Andersson D.A., Bevan S. G protein βγ subunits inhibit TRPM3 ion channels in sensory neurons. eLife. 2017;6:1–22. doi: 10.7554/eLife.26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Badheka D., Yudin Y., Borbiro I., Hartle C.M., Yazici A., Mirshahi T., Rohacs T. Inhibition of Transient Receptor Potential Melastatin 3 ion channels by G-protein βγ subunits. eLife. 2017;6:1–21. doi: 10.7554/eLife.26147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang S., Dai Y., Fukuoka T., Yamanaka H., Kobayashi K., Obata K., Cui X., Tominaga M., Noguchi K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: A molecular mechanism of inflammatory pain. Brain A J. Neurol. 2008;131:1241–1251. doi: 10.1093/brain/awn060. [DOI] [PubMed] [Google Scholar]
- 72.Meotti F.C., Figueiredo C.P., Manjavachi M., Calixto J.B. The transient receptor potential ankyrin-1 mediates mechanical hyperalgesia induced by the activation of B1 receptor in mice. Biochem. Pharmacol. 2017;125:75–83. doi: 10.1016/j.bcp.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Andrade E.L., Luiz A.P., Ferreira J., Calixto J.B. Pronociceptive response elicited by TRPA1 receptor activation in mice. Neuroscience. 2008;152:511–520. doi: 10.1016/j.neuroscience.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 74.Brozmanova M., Mazurova L., Ru F., Tatar M., Hu Y., Yu S., Kollarik M. Mechanisms of the adenosine A2A receptor-induced sensitization of esophageal C fibers. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310:G215–223. doi: 10.1152/ajpgi.00350.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor-Clark T.E., Undem B.J., Macglashan D.W., Ghatta S., Carr M.J., McAlexander M.A. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol. Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- 76.Weng Y., Batista-Schepman P.A., Barabas M.E., Harris E.Q., Dinsmore T.B., Kossyreva E.A., Foshage A.M., Wang M.H., Schwab M.J., Wang V.M., et al. Prostaglandin metabolite induces inhibition of TRPA1 and channel-dependent nociception. Mol. Pain. 2012;8:75. doi: 10.1186/1744-8069-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Linte R.M., Ciobanu C., Reid G., Babes A. Desensitization of cold- and menthol-sensitive rat dorsal root ganglion neurones by inflammatory mediators. Exp. Brain Res. 2007;178:89–98. doi: 10.1007/s00221-006-0712-3. [DOI] [PubMed] [Google Scholar]
- 78.Premkumar L.S., Raisinghani M., Pingle S.C., Long C., Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J. Neurosci. Off. J. Soc. Neurosci. 2005;25:11322–11329. doi: 10.1523/JNEUROSCI.3006-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Babes A., Ciobanu A.C., Neacsu C., Babes R.M. TRPM8, a sensor for mild cooling in mammalian sensory nerve endings. Curr. Pharm. Biotechnol. 2011;12:78–88. doi: 10.2174/138920111793937835. [DOI] [PubMed] [Google Scholar]
- 80.Yudin Y., Lukacs V., Cao C., Rohacs T. Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. J. Physiol. 2011;589:6007–6027. doi: 10.1113/jphysiol.2011.220228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang X., Mak S., Li L., Parra A., Denlinger B., Belmonte C., McNaughton P.A. Direct inhibition of the cold-activated TRPM8 ion channel by Gαq. Nat. Cell Biol. 2012;14:851–858. doi: 10.1038/ncb2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdelhamid R.E., Sluka K.A. ASICs Mediate Pain and Inflammation in Musculoskeletal Diseases. Physiology. 2015;30:449–459. doi: 10.1152/physiol.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hwang S.W., Oh U. Current concepts of nociception: Nociceptive molecular sensors in sensory neurons. Curr. Opin. Anaesthesiol. 2007;20:427–434. doi: 10.1097/ACO.0b013e3282eff91c. [DOI] [PubMed] [Google Scholar]
- 84.Alexander S.P., Peters J.A., Kelly E., Marrion N.V., Faccenda E., Harding S.D., Pawson A.J., Sharman J.L., Southan C., Davies J.A., et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Ligand-gated ion channels. Br. J. Pharmacol. 2017;174:S130–S159. doi: 10.1111/bph.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deval E., Lingueglia E. Acid-Sensing Ion Channels and nociception in the peripheral and central nervous systems. Neuropharmacology. 2015;94:49–57. doi: 10.1016/j.neuropharm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 86.Ugawa S., Ueda T., Yamamura H., Shimada S. In situ hybridization evidence for the coexistence of ASIC and TRPV1 within rat single sensory neurons. Brain Research. Mol. Brain Res. 2005;136:125–133. doi: 10.1016/j.molbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 87.Wemmie J.A., Taugher R.J., Kreple C.J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013;14:461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoder N., Gouaux E. Divalent cation and chloride ion sites of chicken acid sensing ion channel 1a elucidated by X-ray crystallography. PLoS ONE. 2018;13:e0202134. doi: 10.1371/journal.pone.0202134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rash L.D. Acid-Sensing Ion Channel Pharmacology, Past, Present, and Future …. Adv. Pharmacol. 2017;79:35–66. doi: 10.1016/bs.apha.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Krauson A.J., Carattino M.D. The Thumb Domain Mediates Acid-sensing Ion Channel Desensitization. J. Biol. Chem. 2016;291:11407–11419. doi: 10.1074/jbc.M115.702316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng Y.R., Jiang B.Y., Chen C.C. Acid-sensing ion channels: Dual function proteins for chemo-sensing and mechano-sensing. J. Biomed. Sci. 2018;25:46. doi: 10.1186/s12929-018-0448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagaeva E.I., Tikhonova T.B., Magazanik L.G., Tikhonov D.B. Histamine selectively potentiates acid-sensing ion channel 1a. Neurosci. Lett. 2016;632:136–140. doi: 10.1016/j.neulet.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 93.Ren C., Gan X., Wu J., Qiu C.Y., Hu W.P. Enhancement of acid-sensing ion channel activity by metabotropic P2Y UTP receptors in primary sensory neurons. Purinergic Signal. 2016;12:69–78. doi: 10.1007/s11302-015-9479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qiu F., Qiu C.Y., Liu Y.Q., Wu D., Li J.D., Hu W.P. Potentiation of acid-sensing ion channel activity by the activation of 5-HT2 receptors in rat dorsal root ganglion neurons. Neuropharmacology. 2012;63:494–500. doi: 10.1016/j.neuropharm.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 95.Deval E., Salinas M., Baron A., Lingueglia E., Lazdunski M. ASIC2b-dependent regulation of ASIC3, an essential acid-sensing ion channel subunit in sensory neurons via the partner protein PICK-1. J. Biol. Chem. 2004;279:19531–19539. doi: 10.1074/jbc.M313078200. [DOI] [PubMed] [Google Scholar]
- 96.Gu Q., Lee L.Y. Effect of protease-activated receptor 2 activation on single TRPV1 channel activities in rat vagal pulmonary sensory neurons. Exp. Physiol. 2009;94:928–936. doi: 10.1113/expphysiol.2009.047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao P., Metcalf M., Bunnett N.W. Biased signaling of protease-activated receptors. Front. Endocrinol. 2014;5:67. doi: 10.3389/fendo.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Y.Q., Qiu F., Qiu C.Y., Cai Q., Zou P., Wu H., Hu W.P. Cannabinoids inhibit acid-sensing ion channel currents in rat dorsal root ganglion neurons. PLoS ONE. 2012;7:e45531. doi: 10.1371/journal.pone.0045531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cai Q., Qiu C.Y., Qiu F., Liu T.T., Qu Z.W., Liu Y.M., Hu W.P. Morphine inhibits acid-sensing ion channel currents in rat dorsal root ganglion neurons. Brain Res. 2014;1554:12–20. doi: 10.1016/j.brainres.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 100.Kubo A., Shinoda M., Katagiri A., Takeda M., Suzuki T., Asaka J., Yeomans D.C., Iwata K. Oxytocin alleviates orofacial mechanical hypersensitivity associated with infraorbital nerve injury through vasopressin-1A receptors of the rat trigeminal ganglia. Pain. 2017;158:649–659. doi: 10.1097/j.pain.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 101.Qiu F., Qiu C.Y., Cai H., Liu T.T., Qu Z.W., Yang Z., Li J.D., Zhou Q.Y., Hu W.P. Oxytocin inhibits the activity of acid-sensing ion channels through the vasopressin, V1A receptor in primary sensory neurons. Br. J. Pharmacol. 2014;171:3065–3076. doi: 10.1111/bph.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Delmas P., Hao J., Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. 2011;12:139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- 103.Ranade S.S., Syeda R., Patapoutian A. Mechanically Activated Ion Channels. Neuron. 2015;87:1162–1179. doi: 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brohawn S.G. How ion channels sense mechanical force: Insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2. Ann. N. Y. Acad. Sci. 2015;1352:20–32. doi: 10.1111/nyas.12874. [DOI] [PubMed] [Google Scholar]
- 105.Enyedi P., Czirják G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol. Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 106.Viatchenko-Karpinski V., Ling J., Gu J.G. Characterization of temperature-sensitive leak K+ currents and expression of TRAAK, TREK-1, and TREK2 channels in dorsal root ganglion neurons of rats. Mol. Brain. 2018;11:40. doi: 10.1186/s13041-018-0384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li X.Y., Toyoda H. Role of leak potassium channels in pain signaling. Brain Res. Bull. 2015;119:73–79. doi: 10.1016/j.brainresbull.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 108.Coste B., Mathur J., Schmidt M., Earley T.J., Ranade S., Petrus M.J., Dubin A.E., Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang M., Wang Y., Geng J., Zhou S., Xiao B. Mechanically Activated Piezo Channels Mediate Touch and Suppress Acute Mechanical Pain Response in Mice. Cell Rep. 2019;26:1419–1431.e4. doi: 10.1016/j.celrep.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 110.Alexander S.P., Christopoulos A., Davenport A.P., Kelly E., Marrion N.V., Peters J.A., Faccenda E., Harding S.D., Pawson A.J., Sharman J.L., et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors. Br. J. Pharmacol. 2017;174:S17–S129. doi: 10.1111/bph.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pereira V., Busserolles J., Christin M., Devilliers M., Poupon L., Legha W., Alloui A., Aissouni Y., Bourinet E., Lesage F., et al. Role of the TREK2 potassium channel in cold and warm thermosensation and in pain perception. Pain. 2014;155:2534–2544. doi: 10.1016/j.pain.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 112.Patel A.J., Honoré E., Maingret F., Lesage F., Fink M., Duprat F., Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srisomboon Y., Zaidman N.A., Maniak P.J., Deachapunya C., O’Grady S.M. P2Y receptor regulation of K2P channels that facilitate K+ secretion by human mammary epithelial cells. Am. J. Physiol. Cell Physiol. 2018;314:C627–C639. doi: 10.1152/ajpcell.00342.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Woo D.H., Bae J.Y., Nam M.H., An H., Ju Y.H., Won J., Choi J.H., Hwang E.M., Han K.S., Bae Y.C., et al. Activation of Astrocytic μ-opioid Receptor Elicits Fast Glutamate Release Through TREK-1-Containing K2P Channel in Hippocampal Astrocytes. Front. Cell. Neurosci. 2018;12:319. doi: 10.3389/fncel.2018.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Devilliers M., Busserolles J., Lolignier S., Deval E., Pereira V., Alloui A., Christin M., Mazet B., Delmas P., Noel J., et al. Activation of TREK-1 by morphine results in analgesia without adverse side effects. Nat. Commun. 2013;4:2941. doi: 10.1038/ncomms3941. [DOI] [PubMed] [Google Scholar]
- 116.Cho P.S., Lee H.K., Lee S.H., Im J.Z., Jung S.J. DAMGO modulates two-pore domain K(+) channels in the substantia gelatinosa neurons of rat spinal cord. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2016;20:525–531. doi: 10.4196/kjpp.2016.20.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nockemann D., Rouault M., Labuz D., Hublitz P., McKnelly K., Reis F.C., Stein C., Heppenstall P.A. The K(+) channel GIRK2 is both necessary and sufficient for peripheral opioid-mediated analgesia. EMBO Mol. Med. 2013;5:1263–1277. doi: 10.1002/emmm.201201980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dubin A.E., Schmidt M., Mathur J., Petrus M.J., Xiao B., Coste B., Patapoutian A. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Rep. 2012;2:511–517. doi: 10.1016/j.celrep.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lechner S.G., Lewin G.R. Peripheral sensitisation of nociceptors via G-protein-dependent potentiation of mechanotransduction currents. J. Physiol. 2009;587:3493–3503. doi: 10.1113/jphysiol.2009.175059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jia Z., Ikeda R., Ling J., Gu J.G. GTP-dependent run-up of Piezo2-type mechanically activated currents in rat dorsal root ganglion neurons. Mol. Brain. 2013;6:57. doi: 10.1186/1756-6606-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Borbiro I., Badheka D., Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Sci. Signal. 2015;8:ra15. doi: 10.1126/scisignal.2005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Oh U., Jung J. Cellular functions of TMEM16/anoctamin. Pflugers Arch. Eur. J. Physiol. 2016;468:443–453. doi: 10.1007/s00424-016-1790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schroeder B.C., Cheng T., Jan Y.N., Jan L.Y. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L.J.V. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 125.Yang Y.D., Cho H., Koo J.Y., Tak M.H., Cho Y., Shim W.S., Park S.P., Lee J., Lee B., Kim B.M., et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 126.Falzone M.E., Malvezzi M., Lee B.C., Accardi A. Known structures and unknown mechanisms of TMEM16 scramblases and channels. J. Gen. Physiol. 2018;150:933–947. doi: 10.1085/jgp.201711957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huang F., Wong X., Jan L.Y. International Union of Basic and Clinical Pharmacology. LXXXV: calcium-activated chloride channels. Pharmacol. Rev. 2012;64:1–15. doi: 10.1124/pr.111.005009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suzuki J., Fujii T., Imao T., Ishihara K., Kuba H., Nagata S. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J. Biol. Chem. 2013;288:13305–13316. doi: 10.1074/jbc.M113.457937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Scudieri P., Caci E., Venturini A., Sondo E., Pianigiani G., Marchetti C., Ravazzolo R., Pagani F., Galietta L.J.V. Ion channel and lipid scramblase activity associated with expression of TMEM16F/ANO6 isoforms. J. Physiol. 2015;593:3829–3848. doi: 10.1113/JP270691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao L., Li L.I., Ma K.T., Wang Y., Li J., Shi W.Y., Zhu H.E., Zhang Z.S., Si J.Q. NSAIDs modulate GABA-activated currents via Ca2+-activated Cl- channels in rat dorsal root ganglion neurons. Exp. Ther. Med. 2016;11:1755–1761. doi: 10.3892/etm.2016.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang F., Wang X., Ostertag E.M., Nuwal T., Huang B., Jan Y.N., Basbaum A.I., Jan L.Y. TMEM16C facilitates Na(+)-activated K+ currents in rat sensory neurons and regulates pain processing. Nat. Neurosci. 2013;16:1284–1290. doi: 10.1038/nn.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cho H., Yang Y.D., Lee J., Lee B., Kim T., Jang Y., Back S.K., Na H.S., Harfe B.D., Wang F., et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 2012;15:1015–1021. doi: 10.1038/nn.3111. [DOI] [PubMed] [Google Scholar]
- 133.Lee B., Cho H., Jung J., Yang Y.D., Yang D.J., Oh U. Anoctamin 1 contributes to inflammatory and nerve-injury induced hypersensitivity. Mol. Pain. 2014;10:5. doi: 10.1186/1744-8069-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dang S., Feng S., Tien J., Peters C.J., Bulkley D., Lolicato M., Zhao J., Zuberbühler K., Ye W., Qi L., et al. Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature. 2017;552:426–429. doi: 10.1038/nature25024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paulino C., Kalienkova V., Lam A.K.M., Neldner Y., Dutzler R. Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature. 2017;552:421–425. doi: 10.1038/nature24652. [DOI] [PubMed] [Google Scholar]
- 136.Paulino C., Neldner Y., Lam A.K., Kalienkova V., Brunner J.D., Schenck S., Dutzler R. Structural basis for anion conduction in the calcium-activated chloride channel TMEM16A. eLife. 2017;6:1–23. doi: 10.7554/eLife.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brunner J.D., Lim N.K., Schenck S., Duerst A., Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature. 2014;516:207–212. doi: 10.1038/nature13984. [DOI] [PubMed] [Google Scholar]
- 138.Fallah G., Römer T., Detro-Dassen S., Braam U., Markwardt F., Schmalzing G. TMEM16A(a)/anoctamin-1 shares a homodimeric architecture with CLC chloride channels. Mol. Cell. Proteom. MCP. 2011;10:M110.004697. doi: 10.1074/mcp.M110.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sheridan J.T., Worthington E.N., Yu K., Gabriel S.E., Hartzell H.C., Tarran R. Characterization of the oligomeric structure of the Ca(2+)-activated Cl- channel Ano1/TMEM16A. J. Biol. Chem. 2011;286:1381–1388. doi: 10.1074/jbc.M110.174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xiao Q., Yu K., Perez-Cornejo P., Cui Y., Arreola J., Hartzell H.C. Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc. Natl. Acad. Sci. USA. 2011;108:8891–8896. doi: 10.1073/pnas.1102147108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ma K., Wang H., Yu J., Wei M., Xiao Q. New Insights on the Regulation of Ca2+ -Activated Chloride Channel TMEM16A. J. Cell. Physiol. 2017;232:707–716. doi: 10.1002/jcp.25621. [DOI] [PubMed] [Google Scholar]
- 142.Jin X., Shah S., Liu Y., Zhang H., Lees M., Fu Z., Lippiat J.D., Beech D.J., Sivaprasadarao A., Baldwin S.A., et al. Activation of the Cl- channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci. Signal. 2013;6:ra73. doi: 10.1126/scisignal.2004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takayama Y., Uta D., Furue H., Tominaga M. Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc. Natl. Acad. Sci. USA. 2015;112:5213–5218. doi: 10.1073/pnas.1421507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cho H., Oh U. Anoctamin 1 mediates thermal pain as a heat sensor. Curr. Neuropharmacol. 2013;11:641–651. doi: 10.2174/1570159X113119990038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu B., Linley J.E., Du X., Zhang X., Ooi L., Zhang H., Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J. Clin. Investig. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang Y., Wang H., Ke J., Wei Y., Ji H., Qian Z., Liu L., Tao J. Inhibition of A-Type K+ Channels by Urotensin-II Induces Sensory Neuronal Hyperexcitability Through the PKCα-ERK Pathway. Endocrinology. 2018;159:2253–2263. doi: 10.1210/en.2018-00108. [DOI] [PubMed] [Google Scholar]
- 147.Rajagopal M., Kathpalia P.P., Thomas S.V., Pao A.C. Activation of P2Y1 and P2Y2 receptors induces chloride secretion via calcium-activated chloride channels in kidney inner medullary collecting duct cells. Am. J. Physiology. Ren. Physiol. 2011;301:F544–553. doi: 10.1152/ajprenal.00709.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang J., Haanes K.A., Novak I. Purinergic regulation of CFTR and Ca(2+)-activated Cl(-) channels and K(+) channels in human pancreatic duct epithelium. Am. J. Physiology. Cell Physiol. 2013;304:C673–684. doi: 10.1152/ajpcell.00196.2012. [DOI] [PubMed] [Google Scholar]
- 149.Romero T.R.L., Pacheco D.D.F., Duarte I.D.G. Probable involvement of Ca(2+)-activated Cl(-) channels (CaCCs) in the activation of CB1 cannabinoid receptors. Life Sci. 2013;92:815–820. doi: 10.1016/j.lfs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 150.Pacheco D.d.F., Pacheco C.M.D.F., Duarte I.D.G. δ-Opioid receptor agonist SNC80 induces central antinociception mediated by Ca2+ -activated Cl- channels. J. Pharm. Pharmacol. 2012;64:1084–1089. doi: 10.1111/j.2042-7158.2012.01472.x. [DOI] [PubMed] [Google Scholar]
- 151.Cardoso F.C., Lewis R.J. Sodium channels and pain: From toxins to therapies. Br. J. Pharmacol. 2018;175:2138–2157. doi: 10.1111/bph.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hodgkin A.L., Huxley A.F. The components of membrane conductance in the giant axon of Loligo. J. Physiol. 1952;116:473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hodgkin A.L., Huxley A.F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J. Physiol. 1952;116:497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hodgkin A.L., Huxley A.F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 1952;116:449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hodgkin A.L., Huxley A.F., Katz B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J. Physiol. 1952;116:424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brouwer B.A., Merkies I.S.J., Gerrits M.M., Waxman S.G., Hoeijmakers J.G.J., Faber C.G. Painful neuropathies: The emerging role of sodium channelopathies. J. Peripher. Nerv. Syst. JPNS. 2014;19:53–65. doi: 10.1111/jns5.12071. [DOI] [PubMed] [Google Scholar]
- 157.Bezanilla F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008;9:323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- 158.Cardenas L.M., Cardenas C.G., Scroggs R.S. 5HT increases excitability of nociceptor-like rat dorsal root ganglion neurons via cAMP-coupled TTX-resistant Na(+) channels. J. Neurophysiol. 2001;86:241–248. doi: 10.1152/jn.2001.86.1.241. [DOI] [PubMed] [Google Scholar]
- 159.Gold M.S., Reichling D.B., Shuster M.J., Levine J.D. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc. Natl. Acad. Sci. USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.England S., Bevan S., Docherty R.J. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J. Physiol. 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Natura G., von Banchet G.S., Schaible H.G. Calcitonin gene-related peptide enhances TTX-resistant sodium currents in cultured dorsal root ganglion neurons from adult rats. Pain. 2005;116:194–204. doi: 10.1016/j.pain.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 162.Scroggs R.S. Serotonin upregulates low- and high-threshold tetrodotoxin-resistant sodium channels in the same subpopulation of rat nociceptors. Neuroscience. 2010;165:1293–1300. doi: 10.1016/j.neuroscience.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 163.Cardenas C.G., Del Mar L.P., Scroggs R.S. Two parallel signaling pathways couple 5HT1A receptors to N- and L-type calcium channels in C-like rat dorsal root ganglion cells. J. Neurophysiol. 1997;77:3284–3296. doi: 10.1152/jn.1997.77.6.3284. [DOI] [PubMed] [Google Scholar]
- 164.Matsumoto S., Ikeda M., Yoshida S., Tanimoto T., Takeda M., Nasu M. Prostaglandin E2-induced modification of tetrodotoxin-resistant Na+ currents involves activation of both EP2 and EP4 receptors in neonatal rat nodose ganglion neurones. Br. J. Pharmacol. 2005;145:503–513. doi: 10.1038/sj.bjp.0706212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moraes E.R., Kushmerick C., Naves L.A. Characteristics of dorsal root ganglia neurons sensitive to Substance P. Mol. Pain. 2014;10:73. doi: 10.1186/1744-8069-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cang C.L., Zhang H., Zhang Y.Q., Zhao Z.Q. PKCepsilon-dependent potentiation of TTX-resistant Nav1.8 current by neurokinin-1 receptor activation in rat dorsal root ganglion neurons. Mol. Pain. 2009;5:33. doi: 10.1186/1744-8069-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Song J.H., Shin Y.K., Lee C.S., Bang H., Park M. Effects of ATP on TTX-sensitive and TTX-resistant sodium currents in rat sensory neurons. Neuroreport. 2001;12:3659–3662. doi: 10.1097/00001756-200112040-00011. [DOI] [PubMed] [Google Scholar]
- 168.Kayssi A., Amadesi S., Bautista F., Bunnett N.W., Vanner S. Mechanisms of protease-activated receptor 2-evoked hyperexcitability of nociceptive neurons innervating the mouse colon. J. Physiol. 2007;580:977–991. doi: 10.1113/jphysiol.2006.126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gold M.S., Levine J.D. DAMGO inhibits prostaglandin E2-induced potentiation of a TTX-resistant Na+ current in rat sensory neurons in vitro. Neurosci. Lett. 1996;212:83–86. doi: 10.1016/0304-3940(96)12791-9. [DOI] [PubMed] [Google Scholar]
- 170.Catterall W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Acosta C.G., López H.S. delta opioid receptor modulation of several voltage-dependent Ca(2+) currents in rat sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 1999;19:8337–8348. doi: 10.1523/JNEUROSCI.19-19-08337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bean B.P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 173.Dolphin A.C. Beta subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 2003;35:599–620. doi: 10.1023/B:JOBB.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 174.Proft J., Weiss N. G protein regulation of neuronal calcium channels: Back to the future. Mol. Pharmacol. 2015;87:890–906. doi: 10.1124/mol.114.096008. [DOI] [PubMed] [Google Scholar]
- 175.Scherrer G., Imamachi N., Cao Y.Q., Contet C., Mennicken F., O’Donnell D., Kieffer B.L., Basbaum A.I. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Snyder L.M., Chiang M.C., Loeza-Alcocer E., Omori Y., Hachisuka J., Sheahan T.D., Gale J.R., Adelman P.C., Sypek E.I., Fulton S.A., et al. Kappa Opioid Receptor Distribution and Function in Primary Afferents. Neuron. 2018;99:1274–1288.e6. doi: 10.1016/j.neuron.2018.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mudge A.W., Leeman S.E., Fischbach G.D. Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc. Natl. Acad. Sci. USA. 1979;76:526–530. doi: 10.1073/pnas.76.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Werz M.A., Grega D.S., MacDonald R.L. Actions of mu, delta and kappa opioid agonists and antagonists on mouse primary afferent neurons in culture. J. Pharmacol. Exp. Ther. 1987;243:258–263. [PubMed] [Google Scholar]
- 179.Werz M.A., Macdonald R.L. Heterogeneous sensitivity of cultured dorsal root ganglion neurones to opioid peptides selective for mu- and delta-opiate receptors. Nature. 1982;299:730–733. doi: 10.1038/299730a0. [DOI] [PubMed] [Google Scholar]
- 180.Werz M.A., Macdonald R.L. Dynorphin reduces calcium-dependent action potential duration by decreasing voltage-dependent calcium conductance. Neurosci. Lett. 1984;46:185–190. doi: 10.1016/0304-3940(84)90439-7. [DOI] [PubMed] [Google Scholar]
- 181.Werz M.A., MacDonald R.L. Opioid peptides selective for mu- and delta-opiate receptors reduce calcium-dependent action potential duration by increasing potassium conductance. Neurosci. Lett. 1983;42:173–178. doi: 10.1016/0304-3940(83)90402-0. [DOI] [PubMed] [Google Scholar]
- 182.Macdonald R.L., Werz M.A. Dynorphin A decreases voltage-dependent calcium conductance of mouse dorsal root ganglion neurones. J. Physiol. 1986;377:237–249. doi: 10.1113/jphysiol.1986.sp016184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Andrade A., Denome S., Jiang Y.Q., Marangoudakis S., Lipscombe D. Opioid inhibition of N-type Ca2+ channels and spinal analgesia couple to alternative splicing. Nat. Neurosci. 2010;13:1249–1256. doi: 10.1038/nn.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gross R.A., Macdonald R.L. Dynorphin A selectively reduces a large transient (N-type) calcium current of mouse dorsal root ganglion neurons in cell culture. Proc. Natl. Acad. Sci. USA. 1987;84:5469–5473. doi: 10.1073/pnas.84.15.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Heinke B., Gingl E., Sandkühler J. Multiple targets of μ-opioid receptor-mediated presynaptic inhibition at primary afferent Aδ- and C-fibers. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:1313–1322. doi: 10.1523/JNEUROSCI.4060-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.King A.P., Hall K.E., Macdonald R.L. kappa- and mu-Opioid inhibition of N-type calcium currents is attenuated by 4beta-phorbol 12-myristate 13-acetate and protein kinase C in rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 1999;289:312–320. [PubMed] [Google Scholar]
- 187.Moises H.C., Rusin K.I., Macdonald R.L. Mu- and kappa-opioid receptors selectively reduce the same transient components of high-threshold calcium current in rat dorsal root ganglion sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 1994;14:5903–5916. doi: 10.1523/JNEUROSCI.14-10-05903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Su X., Wachtel R.E., Gebhart G.F. Inhibition of calcium currents in rat colon sensory neurons by K- but not mu- or delta-opioids. J. Neurophysiol. 1998;80:3112–3119. doi: 10.1152/jn.1998.80.6.3112. [DOI] [PubMed] [Google Scholar]
- 189.Wiley J.W., Moises H.C., Gross R.A., MacDonald R.L. Dynorphin A-mediated reduction in multiple calcium currents involves a G(o) alpha-subtype G protein in rat primary afferent neurons. J. Neurophysiol. 1997;77:1338–1348. doi: 10.1152/jn.1997.77.3.1338. [DOI] [PubMed] [Google Scholar]
- 190.Dolphin A.C., McGuirk S.M., Scott R.H. An investigation into the mechanisms of inhibition of calcium channel currents in cultured sensory neurones of the rat by guanine nucleotide analogues and (-)-baclofen. Br. J. Pharmacol. 1989;97:263–273. doi: 10.1111/j.1476-5381.1989.tb11950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dolphin A.C., Forda S.R., Scott R.H. Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. J. Physiol. 1986;373:47–61. doi: 10.1113/jphysiol.1986.sp016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cardenas C.G., Del Mar L.P., Scroggs R.S. Variation in serotonergic inhibition of calcium channel currents in four types of rat sensory neurons differentiated by membrane properties. J. Neurophysiol. 1995;74:1870–1879. doi: 10.1152/jn.1995.74.5.1870. [DOI] [PubMed] [Google Scholar]
- 193.Del Mar L.P., Cardenas C.G., Scroggs R.S. Serotonin inhibits high-threshold Ca2+ channel currents in capsaicin-sensitive acutely isolated adult rat DRG neurons. J. Neurophysiol. 1994;72:2551–2554. doi: 10.1152/jn.1994.72.5.2551. [DOI] [PubMed] [Google Scholar]
- 194.Dunlap K., Fischbach G.D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J. Physiol. 1981;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Holz G.G., Shefner S.A., Anderson E.G. Serotonin decreases the duration of action potentials recorded from tetraethylammonium-treated bullfrog dorsal root ganglion cells. J. Neurosci. Off. J. Soc. Neurosci. 1986;6:620–626. doi: 10.1523/JNEUROSCI.06-03-00620.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gerevich Z., Borvendeg S.J., Schröder W., Franke H., Wirkner K., Nörenberg W., Fürst S., Gillen C., Illes P. Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J. Neurosci. Off. J. Soc. Neurosci. 2004;24:797–807. doi: 10.1523/JNEUROSCI.4019-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ross R.A., Coutts A.A., McFarlane S.M., Anavi-Goffer S., Irving A.J., Pertwee R.G., MacEwan D.J., Scott R.H. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: Implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/S0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 198.Bleakman D., Colmers W.F., Fournier A., Miller R.J. Neuropeptide Y inhibits Ca2+ influx into cultured dorsal root ganglion neurones of the rat via a Y2 receptor. Br. J. Pharmacol. 1991;103:1781–1789. doi: 10.1111/j.1476-5381.1991.tb09863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ewald D.A., Matthies H.J., Perney T.M., Walker M.W., Miller R.J. The effect of down regulation of protein kinase C on the inhibitory modulation of dorsal root ganglion neuron Ca2+ currents by neuropeptide Y. J. Neurosci. Off. J. Soc. Neurosci. 1988;8:2447–2451. doi: 10.1523/JNEUROSCI.08-07-02447.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Walker M.W., Ewald D.A., Perney T.M., Miller R.J. Neuropeptide Y modulates neurotransmitter release and Ca2+ currents in rat sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 1988;8:2438–2446. doi: 10.1523/JNEUROSCI.08-07-02438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gorham L., Just S., Doods H. Somatostatin 4 receptor activation modulates G-protein coupled inward rectifying potassium channels and voltage stimulated calcium signals in dorsal root ganglion neurons. Eur. J. Pharmacol. 2014;736:101–106. doi: 10.1016/j.ejphar.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 202.Abdulla F.A., Smith P.A. Ectopic alpha2-adrenoceptors couple to N-type Ca2+ channels in axotomized rat sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 1997;17:1633–1641. doi: 10.1523/JNEUROSCI.17-05-01633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gamper N., Reznikov V., Yamada Y., Yang J., Shapiro M.S. Phosphatidylinositol [correction] 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J. Neurosci. Off. J. Soc. Neurosci. 2004;24:10980–10992. doi: 10.1523/JNEUROSCI.3869-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lechner S.G., Hussl S., Schicker K.W., Drobny H., Boehm S. Presynaptic inhibition via a phospholipase C- and phosphatidylinositol bisphosphate-dependent regulation of neuronal Ca2+ channels. Mol. Pharmacol. 2005;68:1387–1396. doi: 10.1124/mol.105.014886. [DOI] [PubMed] [Google Scholar]
- 205.Wu L., Bauer C.S., Zhen X.G., Xie C., Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature. 2002;419:947–952. doi: 10.1038/nature01118. [DOI] [PubMed] [Google Scholar]
- 206.Li Z., He S.Q., Tseng P.Y., Xu Q., Tiwari V., Yang F., Shu B., Zhang T., Tang Z., Raja S.N., et al. The inhibition of high-voltage-activated calcium current by activation of MrgC11 involves phospholipase C-dependent mechanisms. Neuroscience. 2015;300:393–403. doi: 10.1016/j.neuroscience.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Huang D., Huang S., Peers C., Du X., Zhang H., Gamper N. GABAB receptors inhibit low-voltage activated and high-voltage activated Ca(2+) channels in sensory neurons via distinct mechanisms. Biochem. Biophys. Res. Commun. 2015;465:188–193. doi: 10.1016/j.bbrc.2015.07.137. [DOI] [PubMed] [Google Scholar]
- 208.Hofmann F., Flockerzi V., Kahl S., Wegener J.W. L-type CaV1.2 calcium channels: From in vitro findings to in vivo function. Physiol. Rev. 2014;94:303–326. doi: 10.1152/physrev.00016.2013. [DOI] [PubMed] [Google Scholar]
- 209.Gutman G.A., Chandy K.G., Adelman J.P., Aiyar J., Bayliss D.A., Clapham D.E., Covarriubias M., Desir G.V., Furuichi K., Ganetzky B., et al. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: Potassium channels. Pharmacol. Rev. 2003;55:583–586. doi: 10.1124/pr.55.4.9. [DOI] [PubMed] [Google Scholar]
- 210.Tsantoulas C., McMahon S.B. Opening paths to novel analgesics: The role of potassium channels in chronic pain. Trends Neurosci. 2014;37:146–158. doi: 10.1016/j.tins.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Swartz K.J. Towards a structural view of gating in potassium channels. Nat. Reviews. Neurosci. 2004;5:905–916. doi: 10.1038/nrn1559. [DOI] [PubMed] [Google Scholar]
- 212.Kuang Q., Purhonen P., Hebert H. Structure of potassium channels. Cell. Mol. Life Sci. CMLS. 2015;72:3677–3693. doi: 10.1007/s00018-015-1948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Brown D.A., Passmore G.M. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Du X., Gao H., Jaffe D., Zhang H., Gamper N. M-type K+ channels in peripheral nociceptive pathways. Br. J. Pharmacol. 2018;175:2158–2172. doi: 10.1111/bph.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Howard R.J., Clark K.A., Holton J.M., Minor D.L. Structural insight into KCNQ (Kv7) channel assembly and channelopathy. Neuron. 2007;53:663–675. doi: 10.1016/j.neuron.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Delmas P., Brown D.A. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat. Rev. Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- 217.Du X., Hao H., Gigout S., Huang D., Yang Y., Li L., Wang C., Sundt D., Jaffe D.B., Zhang H., Gamper N. Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain. 2014;155:2306–2322. doi: 10.1016/j.pain.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rivera-Arconada I., Roza C., Lopez-Garcia J.A. Enhancing m currents: A way out for neuropathic pain? Front. Mol. Neurosci. 2009;2:10. doi: 10.3389/neuro.02.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Passmore G.M., Selyanko A.A., Mistry M., Al-Qatari M., Marsh S.J., Matthews E.A., Dickenson A.H., Brown T.A., Burbidge S.A., Main M., et al. KCNQ/M currents in sensory neurons: Significance for pain therapy. J. Neurosci. Off. J. Soc. Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zheng Y., Xu H., Zhan L., Zhou X., Chen X., Gao Z. Activation of peripheral KCNQ channels relieves gout pain. Pain. 2015;156:1025–1035. doi: 10.1097/j.pain.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ray S., Salzer I., Kronschläger M.T., Boehm S. The paracetamol metabolite N-acetylp-benzoquinone imine reduces excitability in first- and second-order neurons of the pain pathway through actions on KV7 channels. Pain. 2019;160:954–964. doi: 10.1097/j.pain.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zheng Q., Fang D., Liu M., Cai J., Wan Y., Han J.S., Xing G.G. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain. 2013;154:434–448. doi: 10.1016/j.pain.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 223.Yu T., Li L., Liu H., Li H., Liu Z., Li Z. KCNQ2/3/5 channels in dorsal root ganglion neurons can be therapeutic targets of neuropathic pain in diabetic rats. Mol. Pain. 2018;14:1744806918793229. doi: 10.1177/1744806918793229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Brown B.S., Yu S.P. Modulation and genetic identification of the M channel. Prog. Biophys. Mol. Biol. 2000;73:135–166. doi: 10.1016/S0079-6107(00)00004-3. [DOI] [PubMed] [Google Scholar]
- 225.Ishimatsu M. Substance P produces an inward current by suppressing voltage-dependent and -independent K+ currents in bullfrog primary afferent neurons. Neurosci. Res. 1994;19:9–20. doi: 10.1016/0168-0102(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 226.Akasu T., Ishimatsu M., Yamada K. Tachykinins cause inward current through NK1 receptors in bullfrog sensory neurons. Brain Res. 1996;713:160–167. doi: 10.1016/0006-8993(95)01506-X. [DOI] [PubMed] [Google Scholar]
- 227.Lin C.C.J., Chen W.N., Chen C.J., Lin Y.W., Zimmer A., Chen C.C. An antinociceptive role for substance P in acid-induced chronic muscle pain. Proc. Natl. Acad. Sci. USA. 2012;109:E76–E83. doi: 10.1073/pnas.1108903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Neves S.R., Ram P.T., Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 229.Suh B.C., Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/S0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- 230.Higashida H., Brown D.A. Two polyphosphatidylinositide metabolites control two K+ currents in a neuronal cell. Nature. 1986;323:333–335. doi: 10.1038/323333a0. [DOI] [PubMed] [Google Scholar]
- 231.Selyanko A.A., Brown D.A. Intracellular calcium directly inhibits potassium M channels in excised membrane patches from rat sympathetic neurons. Neuron. 1996;16:151–162. doi: 10.1016/S0896-6273(00)80032-X. [DOI] [PubMed] [Google Scholar]
- 232.Gamper N., Shapiro M.S. Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels. J. Gen. Physiol. 2003;122:17–31. doi: 10.1085/jgp.200208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Brown D.A., Hughes S.A., Marsh S.J., Tinker A. Regulation of M(Kv7.2/7.3) channels in neurons by PIP(2) and products of PIP(2) hydrolysis: Significance for receptor-mediated inhibition. J. Physiol. 2007;582:917–925. doi: 10.1113/jphysiol.2007.132498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Soh U.J., Dores M.R., Chen B., Trejo J. Signal transduction by protease-activated receptors. Br. J. Pharmacol. 2010;160:191–203. doi: 10.1111/j.1476-5381.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Linley J.E., Rose K., Patil M., Robertson B., Akopian A.N., Gamper N. Inhibition of M current in sensory neurons by exogenous proteases: A signaling pathway mediating inflammatory nociception. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:11240–11249. doi: 10.1523/JNEUROSCI.2297-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol. Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 237.Dong X., Han S., Zylka M.J., Simon M.I., Anderson D.J. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/S0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 238.Choi S.S., Lahn B.T. Adaptive evolution of MRG, a neuron-specific gene family implicated in nociception. Genome Res. 2003;13:2252–2259. doi: 10.1101/gr.1431603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang L., Taylor N., Xie Y., Ford R., Johnson J., Paulsen J.E., Bates B. Cloning and expression of MRG receptors in macaque, mouse, and human. Brain Res. Mol. Brain Res. 2005;133:187–197. doi: 10.1016/j.molbrainres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 240.Zylka M.J., Rice F.L., Anderson D.J. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 241.Crozier R.A., Ajit S.K., Kaftan E.J., Pausch M.H. MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:4492–4496. doi: 10.1523/JNEUROSCI.4932-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Baldwin T.J., Tsaur M.L., Lopez G.A., Jan Y.N., Jan L.Y. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 1991;7:471–483. doi: 10.1016/0896-6273(91)90299-F. [DOI] [PubMed] [Google Scholar]
- 243.Shibata R., Nakahira K., Shibasaki K., Wakazono Y., Imoto K., Ikenaka K. A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. J. Neurosci. Off. J. Soc. Neurosci. 2000;20:4145–4155. doi: 10.1523/JNEUROSCI.20-11-04145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Vydyanathan A., Wu Z.Z., Chen S.R., Pan H.L. A-type voltage-gated K+ currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons. J. Neurophysiol. 2005;93:3401–3409. doi: 10.1152/jn.01267.2004. [DOI] [PubMed] [Google Scholar]
- 245.Chien L.Y., Cheng J.K., Chu D., Cheng C.F., Tsaur M.L. Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:9855–9865. doi: 10.1523/JNEUROSCI.0604-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Liu P.W., Blair N.T., Bean B.P. Action Potential Broadening in Capsaicin-Sensitive DRG Neurons from Frequency-Dependent Reduction of Kv3 Current. J. Neurosci. Off. J. Soc. Neurosci. 2017;37:9705–9714. doi: 10.1523/JNEUROSCI.1703-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zemel B.M., Ritter D.M., Covarrubias M., Muqeem T. A-Type KV Channels in Dorsal Root Ganglion Neurons: Diversity, Function, and Dysfunction. Front. Mol. Neurosci. 2018;11:253. doi: 10.3389/fnmol.2018.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ritter D.M., Ho C., O’Leary M.E., Covarrubias M. Modulation of Kv3.4 channel N-type inactivation by protein kinase C shapes the action potential in dorsal root ganglion neurons. J. Physiol. 2012;590:145–161. doi: 10.1113/jphysiol.2011.218560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ritter D.M., Zemel B.M., Hala T.J., O’Leary M.E., Lepore A.C., Covarrubias M. Dysregulation of Kv3.4 channels in dorsal root ganglia following spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2015;35:1260–1273. doi: 10.1523/JNEUROSCI.1594-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zemel B.M., Muqeem T., Brown E.V., Goulão M., Urban M.W., Tymanskyj S.R., Lepore A.C., Covarrubias M. Calcineurin Dysregulation Underlies Spinal Cord Injury-Induced K+ Channel Dysfunction in DRG Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2017;37:8256–8272. doi: 10.1523/JNEUROSCI.0434-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhang Y., Jiang D., Zhang Y., Jiang X., Wang F., Tao J. Neuromedin U type 1 receptor stimulation of A-type K+ current requires the βγ subunits of Go protein, protein kinase A, and extracellular signal-regulated kinase 1/2 (ERK1/2) in sensory neurons. J. Biol. Chem. 2012;287:18562–18572. doi: 10.1074/jbc.M111.322271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Saetrum Opgaard O., Nothacker H., Ehlert F.J., Krause D.N. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur. J. Pharmacol. 2000;406:265–271. doi: 10.1016/S0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- 253.Maguire J.J., Davenport A.P. Is urotensin-II the new endothelin? Br. J. Pharmacol. 2002;137:579–588. doi: 10.1038/sj.bjp.0704924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhang M., Gao C.X., Wang Y.P., Ma K.T., Li L., Yin J.W., Dai Z.G., Wang S., Si J.Q. The association between the expression of PAR2 and TMEM16A and neuropathic pain. Mol. Med. Rep. 2018;17:3744–3750. doi: 10.3892/mmr.2017.8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li N., Lu Z.Y., Yu L.H., Burnstock G., Deng X.M., Ma B. Inhibition of G protein-coupled P2Y2 receptor induced analgesia in a rat model of trigeminal neuropathic pain. Mol. Pain. 2014;10:21. doi: 10.1186/1744-8069-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Jacobson K.A., Ivanov A.A., de Castro S., Harden T.K., Ko H. Development of selective agonists and antagonists of P2Y receptors. Purinergic Signal. 2009;5:75–89. doi: 10.1007/s11302-008-9106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Glaaser I.W., Slesinger P.A. Structural Insights into GIRK Channel Function. Int. Rev. Neurobiol. 2015;123:117–160. doi: 10.1016/bs.irn.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 258.Nagi K., Pineyro G. Kir3 channel signaling complexes: Focus on opioid receptor signaling. Front. Cell. Neurosci. 2014;8:186. doi: 10.3389/fncel.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Peleg S., Varon D., Ivanina T., Dessauer C.W., Dascal N. G(alpha)(i) controls the gating of the G protein-activated K(+) channel, GIRK. Neuron. 2002;33:87–99. doi: 10.1016/S0896-6273(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 260.Huang C.L., Feng S., Hilgemann D.W. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 261.Sui J.L., Petit-Jacques J., Logothetis D.E. Activation of the atrial KACh channel by the betagamma subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc. Natl. Acad. Sci. USA. 1998;95:1307–1312. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Khodorova A., Navarro B., Jouaville L.S., Murphy J.E., Rice F.L., Mazurkiewicz J.E., Long-Woodward D., Stoffel M., Strichartz G.R., Yukhananov R., et al. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat. Med. 2003;9:1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 263.Gao X.F., Zhang H.L., You Z.D., Lu C.L., He C. G protein-coupled inwardly rectifying potassium channels in dorsal root ganglion neurons. Acta Pharmacol. Sin. 2007;28:185–190. doi: 10.1111/j.1745-7254.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 264.Stötzner P., Spahn V., Celik M.Ö., Labuz D., Machelska H. Mu-Opioid Receptor Agonist Induces Kir3 Currents in Mouse Peripheral Sensory Neurons - Effects of Nerve Injury. Front. Pharmacol. 2018;9:1478. doi: 10.3389/fphar.2018.01478. [DOI] [PMC free article] [PubMed] [Google Scholar]