Short abstract
Objective
Complement C1q tumor necrosis factor-related proteins (CTRPs), belonging to the CTRP superfamily, are extensively involved in regulating metabolism and the immune-inflammatory response. The inflammatory process is linked to the pathogenesis of coronary artery disease (CAD). Here, we investigated the association of serum levels of CTRP1 with CAD.
Methods
Study participants were divided into two groups according to the results of coronary angiography: a control group (n = 63) and a CAD group (n = 76). The concentrations of serum CTRP1 and inflammatory cytokines were determined by enzyme-linked immunosorbent assay. Further analysis of CTRP1 levels in individuals with different severities of CAD was conducted. The CAD severity was assessed by Gensini score.
Results
Serum levels of CTRP1 were significantly higher in CAD patients than in controls (17.24 ± 1.07 versus 9.31 ± 0.56 ng/mL), and CTRP1 levels increased with increasing severity of CAD. CTRP1 levels were positively correlated with concentrations of tumor necrosis factor-α and interleukin-6. Multiple logistic regression analysis showed that CTRP1 was significantly associated with CAD.
Conclusions
Our data showed close associations of serum CTRP1 levels with the prevalence and severity of CAD, indicating that CTRP1 can be regarded as a novel and valuable biomarker for CAD.
Keywords: Complement C1q tumor necrosis factor-related protein 1, coronary artery disease, Gensini score, inflammatory cytokines, inflammation, biomarker, adipokine
Introduction
Obesity is a pandemic social problem that causes numerous metabolic disorders, including type 2 diabetes mellitus and coronary artery disease (CAD).1–4 Obesity is an independent risk factor for the development and progression of CAD.5 CAD is one of the most common types of cardiovascular disease, with high mortality and morbidity worldwide.6,7 There is accumulating evidence that adipose tissue-derived hormones (adipokines) are implicated in the pathophysiology of CAD.8–10 Multiple pro-inflammatory adipokines, including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), are elevated in obesity and associated with accelerated progression of CAD. Furthermore, certain anti-inflammatory adipokines, including adiponectin (APN), are down-regulated under obese conditions, further contributing to the pathogenesis of CAD.11–13
The complement C1q tumor necrosis factor-related protein (CTRPs) superfamily is a newly discovered paralog of APN; currently, 15 family members (CTRP1–CTRP15) have been identified.14,15 Studies have shown that members of the CTRP family are involved in the regulation of glucose metabolism, lipid metabolism, and inflammation.16 CTRP1 is a novel adipokine that is mainly expressed in adipose-derived cells and adipocytes. High levels of circulating CTRP1 are associated with metabolic syndrome, congestive heart disease, and hypertension.17–19 A recent experiment found that CTRP1 and APN had similar effects on hyperglycemia, insulin resistance, and obesity. Recently, a study by Lu et al.20 reported a decrease in atherogenesis and vascular inflammation in CTRP1-deficient apolipoprotein E knockout (apoE−/−) mice, and intraperitoneal injection of CTRP1 promoted atherogenesis in apoE−/− mice. These data suggest that CTRP1 is associated with obesity-related metabolic and cardiovascular diseases. In this study, we investigated the serum levels of CTRP1 in patients with CAD and the associations with related inflammatory cytokines.
Material and methods
Study participants
This case-control study was conducted on 139 subjects who underwent coronary angiography at Ruijin Hospital between 2013 and 2015. CAD was diagnosed by a cardiologist based on coronary angiography results. Coronary angiography was performed using the standard Judkins method; a diagnosis of CAD was defined as stenosis of at least one major coronary artery (including left main coronary artery, anterior descending coronary artery, circumflex branch, and right coronary artery) lumen of ≥50%. The coronary angiography records of the patients were examined and their Gensini scores (GS) were calculated. We excluded patients with acute myocardial infarction, congestive heart failure, thrombotic diseases, type I diabetes, severe hepatic or renal dysfunction, infections, hemodialysis, or malignancy. The subjects were divided into a control group (n = 63) and CAD group (n = 76). The CAD group was further grouped into three subgroups by their GS: mild CAD group (GS < 20, n = 25), moderate CAD group (20 ≤ GS ≤40, n = 26), and severe CAD group (GS > 40, n = 25).
All participants gave written informed consent. This study was approved by the ethics committee of Ruijin Hospital.
Laboratory methods
Blood samples were collected from all study participants after overnight fasting and centrifuged at 1200 × g at 4°C after being allowed to clot in the test tube. The supernatant was extracted and collected in an Eppendorf tube after 10 minutes. Serum levels of CTRP1, TNF-α, IL-6, and interleukin-8 (IL-8) were measured by enzyme-linked immunosorbent assay using a commercially available ELISA kit (Senxiong Biotech Industry Company, Shanghai, China). Total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and fasting blood glucose (FBG) were determined by standard assays. Blood pressure (BP) was measured by a standard sphygmomanometer after at least 10 minutes’ rest in a seated position. Body mass index (BMI) was calculated as the ratio of weight (kg) to squared height (m2)
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA). Continuous variables were presented as means ± standard errors (SE). Differences in measurement data were compared by using a t-test between groups. One-way analysis of variance was used to analyze the difference between multiple groups. If intergroup difference was shown in the analysis of variance, Bonferroni’s method was used to assess pairwise comparisons between two groups with specific differences. Count data were presented as frequencies and analyzed using the chi-squared test. Pearson analysis was used for correlation analysis. Logistic regression analysis was used to identify associations between CAD and other variables. A value of p < 0.05 was considered statistically significant.
Results
Clinical characteristics
Clinical characteristics of control subjects and CAD patients are listed in Table 1. Patients with CAD had significantly higher levels of BMI, systolic blood pressure (SBP), TG, and FBG than control subjects. Plasma CTRP1, TNF-α, IL-6, and IL-8 levels were markedly higher in CAD patients than in controls (Figure 1). HDL-C level was lower in the CAD group than in the control group. However, age, sex, frequency of smokers, diastolic blood pressure (DBP), TC, and LDL-C did not differ between the two groups (Table 1).
Table 1.
Clinical characteristics of the study population.
| Characteristics | Control(n = 63) | CAD(n = 76) | p-value |
|---|---|---|---|
| Sex [male (%)] | 38 (60.3) | 55 (72.4) | 0.150 |
| Age (years) | 63.83 ± 1.28 | 66.47 ± 1.28 | 0.148 |
| BMI (kg/m2) | 23.01 ± 0.30 | 25.12 ± 0.32 | <0.001 |
| Smoking (%) | 8 (12.7) | 12 (15.8) | 0.637 |
| SBP (mm Hg) | 127.02 ± 2.41 | 135.12 ± 2.20 | <0.05 |
| DBP (mm Hg) | 74.70 ± 1.29 | 75.82 ± 1.20 | 0.527 |
| TC (mmol/L) | 4.29 ± 0.15 | 4.26 ± 0.14 | 0.900 |
| TG (mmol/L) | 1.37 ± 0.91 | 2.26 ± 0.40 | <0.05 |
| LDL-C (mmol/L) | 2.66 ± 0.12 | 2.95 ± 0.13 | 0.107 |
| HDL-C (mmol/L) | 1.17 ± 0.36 | 1.04 ± 0.34 | <0.01 |
| FBG (mmol/L) | 5.70 ± 0.19 | 7.16 ± 0.40 | <0.01 |
| TNF-α (pg/mL) | 33.80 ± 1.43 | 38.16 ± 1.52 | <0.05 |
| IL-6 (pg/mL) | 22.56 ± 0.53 | 25.29 ± 0.80 | <0.01 |
| IL-8 (pg/mL) | 20.31 ± 0.89 | 25.58 ± 2.01 | <0.05 |
| CTRP1 (ng/mL) | 9.31 ± 0.56 | 17.24 ± 1.07 | <0.001 |
Data are presented as mean ± SE. CAD, coronary heart disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; FBG, fasting blood glucose; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-8, interleukin-8; CTRP1, C1q/TNF-related protein-1.
Figure 1.
Serum levels (means ± standard errors) of CTRP1, TNF-α, IL-6, and IL-8. (a) Serum levels of CTRP1 were higher in patients with CAD compared with the control group (***p < 0.001). (b) Serum levels of TNF-α, IL-6, and IL-8 were higher in CAD patients than that in control subjects (*p < 0.05, **p < 0.01). CTRP1, C1q/TNF-related protein-1; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-8, interleukin-8; and CAD, coronary artery disease.
Association of serum CTRP1 levels with CAD severity
To determine the correlation between increased levels of CTRP1 and the severity of CAD, we divided the CAD patients into three subgroups according to their GS: mild (GS < 20), moderate (20 ≤ GS ≤40), and severe (GS >40). We compared CTRP1 levels in these different subgroups (Figure 2a). The CTRP1 levels in the severe CAD group were significantly higher than those in the mild and moderate CAD groups (p < 0.001 and p < 0.01, respectively). However, the difference was not significant between mild and moderate CAD groups. Pearson correlation analysis showed that serum CTRP1 levels were positively correlated with GS in the overall CAD group (r = 0.482, p < 0.001) (Figure 2b).
Figure 2.
(a) Levels (means ± standard errors) of CTRP1 in patients with mild, moderate, and severe CAD (***p < 0.001, **p < 0.01). (b) Correlation between serum CTRP1 level and Gensini score in the CAD group. CTRP1, C1q/TNF-related protein-1; CAD, coronary artery disease.
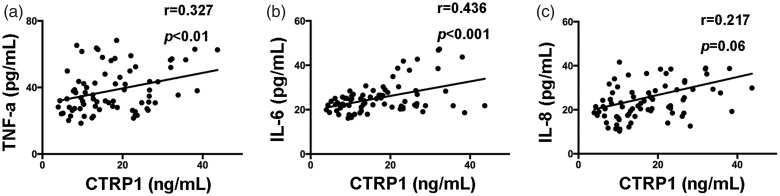
Correlation between CTRP1 and serum pro-inflammatory cytokines
We further assessed the relationship between serum CTRP1 levels and serum pro-inflammatory cytokines (TNF-α, IL-6, and IL-8) in the CAD group. Serum CTRP1 levels were correlated positively with TNF-α and IL-6 in CAD patients (Figure 3a, 3b). In addition, serum CTRP1 levels tended to correlate positively with IL-8 level (Figure 3c).
Figure 3.
Correlations (r) between CTRP1 levels and serum cytokine levels in CAD group (a) TNF-α, (b) IL-6, and (c) IL-8. CTRP1, C1q/TNF-related protein-1; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-8, interleukin-8; CAD, coronary artery disease.
Association of CTRP1 levels with CAD
To determine the association between CTRP1 and CAD, single and multiple logistic regression analyses were performed between control and CAD groups. Age, BMI, SBP, DBP, TG, TC, HDL-C, LDL-C, FBG, TNF-α, IL-6, IL-8, and CTRP1 were regarded as independent variables. In the single logistic regression analysis, BMI, SBP, TG, HDL-C, FBG, TNF-α, IL-6, IL-8, and CTRP1 were significantly associated with CAD (Table 2). Multiple regression analysis showed that BMI, FBG, and CTRP1 were significantly associated with CAD.
Table 2.
Association with coronary artery disease (CAD).
|
Single |
Multiple |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Age (years) | 1.024 (0.992–1.057) | 0.150 | ||
| BMI (kg/m2) | 1.397 (1.187–1.644) | <0.001 | 1.323 (1.057–1.657) | <0.05 |
| SBP (mm Hg) | 1.023 (1.004–1.042) | <0.05 | ||
| DBP (mm Hg) | 1.011 (0.978–1.044) | 0.524 | ||
| TC (mmol/L) | 0.982 (0.743–1.298) | 0.899 | ||
| TG (mmol/L) | 2.030 (1.287–3.202) | <0.01 | ||
| LDL-C (mmol/L) | 1.335 (0.933–1.909) | 0.114 | ||
| HDL-C (mmol/L) | 0.208 (0.061–0.703) | <0.05 | ||
| FBG (mmol/L) | 1.309 (1.074–1.596) | <0.01 | 1.220 (1.007–1.478) | <0.05 |
| TNF-α (pg/mL) | 1.029 (1.001–1.058) | <0.05 | ||
| IL-6 (pg/mL) | 1.093 (1.020–1.072) | <0.05 | ||
| IL-8 (pg/mL) | 1.064 (1.015–1.114) | <0.01 | ||
| CTRP1 (ng/mL) | 1.186 (1.107–1.272) | <0.001 | 1.210 (1.102–1.330) | <0.001 |
CI, confidence interval; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; FBG, fasting blood glucose; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-8, interleukin-8; CTRP1, C1q/TNF-related protein-1.
Discussion
Our study showed that serum levels of CTRP1, TNF-α, IL-6, and IL-8 were increased in patients with CAD compared with controls, and CTRP1 levels trended upward with increasing severity of CAD. In CAD patients, serum levels of CTRP1 were positively correlated with concentrations of TNF-α and IL-6. Multiple logistic regression analysis showed that BMI, FBG, and CTRP1 levels were dependent risk factors for CAD.
CTRP1 is a newly discovered adipokine belonging to the CTRP family and it shares several biochemical features with APN. Experimental studies indicate the important role of APN in cardiometabolic disorders. However, the functional role of CTRP1 remains poorly understood in cardiovascular disease. Peterson et al.21 demonstrated that CTRP1 is a novel metabolic regulator that enhances fatty acid oxidation in muscle via AMP-activated protein kinase (AMPK) pathways. It has been shown that CTRP1 improves insulin sensitivity and enhances glucose tolerance.22,23 Injection of recombinant CTRP1 into mice significantly lowered blood glucose, and CTRP1 knockout mice had glucose and lipid metabolic disorders.24,25 It has been reported that CTRP1 modulates the level of CYP11B2 and aldosterone release through the mitogen-activated protein kinase (MAPK) signaling pathway, indicating an important role of CTRP1 in the cardiovascular system.18 In our study, we found that CTRP1 levels were significantly higher in CAD patients than in controls. Also, CTRP1 levels increased with increasing severity of CAD. These findings are consistent with earlier published studies. For instance, Chalupova et al.22 found that CTRP1 is associated with metabolic syndrome, and Yuasa et al.26 indicated a close association between circulating CTRP1 levels and CAD in men.
Evidence from several recent studies indicates that the involvement of CTRP1 in coronary atherosclerosis could be associated with low-grade chronic inflammation. TNF-α, IL-6, and IL-1β are considered the molecular links and inflammatory mediators between inflammation and obesity.27 Kim et al.28 found elevated mRNA expression of CTRP1 in adipose tissue of Zucker diabetic fatty rats (fa/fa), and CTRP1 gene expression was found to be mediated by TNF-α and IL-1β after LPS stimulation. However, the role of CTRP1 in cardiovascular disease remains controversial. The study of Yuasa et al. reported that CTRP1 protects the heart from ischemia/reperfusion (I/R) injury by reducing cardiomyocyte apoptosis and inflammatory response.29 Kanemura et al.30 demonstrated that systemic administration of Ad-CTRP1 to wild-type mice reduced neointimal thickening after arterial injury. In our study, serum levels of TNF-α, IL-6, and IL-8 were significantly higher in CAD patients than in controls, which was in accordance with previous studies.18,22 Also, TNF-α and IL-6 were positively correlated with CTRP1. These data jointly suggest that CTRP1 is an adipokine associated with the inflammatory reaction in the pathogenesis of CAD.
Because inflammation and obesity-linked metabolic disorders might also contribute to elevated levels of CTRP1, we used multiple logistic regression analysis to test whether our findings were influenced by those factors. Our results showed that BMI, FBG, and CTRP1 were independent risk factors for CAD. Although metabolic disorders induced by CAD affected circulating CTRP1 to some extent, other underlying factors might be involved in the increased expression of CTRP1.
Our study has several limitations that must be considered. First, the sample size was relatively small. Second, this was a cross-sectional study, which precludes drawing inferences about causality. Also, some CAD patients were on cholesterol-lowering statin medications, which could have affected the results. Future studies conducted on a larger population that exclude potential confounding factors such as medication treatment history are needed.
In conclusion, our data demonstrated an association between higher circulating levels of CTRP1 with increased risk of CAD, suggesting that CTRP1 might serve as an indicator of CAD prevalence and progression. Furthermore, TNF-α and IL-6 were positively correlated with CTRP1. Further studies are required to fully dissect the roles of CTRP1 in CAD.
Acknowledgements
We acknowledge with gratitude all collaborating researchers for assistance in collecting information during this study. We also thank the participants in this study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research was supported by grants from the Common Diseases Program Suitable for Technology & Development and Promotion & Application fund (No. 16CR4013A).
References
- 1.Wang Y, Rimm EB, Stampfer MJ, et al. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 2005; 81: 555–563. DOI: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 2.Matsuzawa Y. Therapy insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med 2006; 3: 35–42. DOI: 10.1038/ncpcardio0380. [DOI] [PubMed] [Google Scholar]
- 3.Canoy D. Coronary heart disease and body fat distribution. Curr Atheroscler Rep 2010; 12: 125–133. DOI: 10.1007/s11883-010-0092-9. [DOI] [PubMed] [Google Scholar]
- 4.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887. DOI: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 5.Ades PA, Savage PD. Obesity in coronary heart disease: an unaddressed behavioral risk factor. Prev Med 2017; 104: 117–119. DOI: 10.1016/j.ypmed.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997; 349: 1436–1442. DOI: 10.1016/s0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. DOI: 10.1016/s0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol 2013; 216: T17–T36. DOI: 10.1530/joe-12-0232. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11: 85–97. DOI: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol 2014; 63: 250–259. DOI: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kern PA, Saghizadeh M, Ong JM, et al. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest 1995; 95: 2111–2119. DOI: 10.1172/jci117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259: 87–91. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi K, Shibata R, Murohara T, et al. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab 2014; 25: 348–355. DOI: 10.1016/j.tem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Seldin MM, Peterson JM, Byerly MS, et al. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 2012; 287: 11968–11980. DOI: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong GW, Wang J, Hug C, et al. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A 2004; 101: 10302–10307. DOI: 10.1073/pnas.0403760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seldin MM, Tan SY, Wong GW. Metabolic function of the CTRP family of hormones. Rev Endocr Metab Disord 2014; 15: 111–123. DOI: 10.1007/s11154-013-9255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xin Y, Lyu X, Wang C, et al. Elevated circulating levels of CTRP1, a novel adipokine, in diabetic patients. Endocr J 2014; 61: 841–847. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Liu S, Zhang RY, et al. Association between C1q/TNF-related protein-1 levels in human plasma and epicardial adipose tissues and congestive heart failure. Cell Physiol Biochem 2017; 42: 2130–2143. DOI: 10.1159/000479915. [DOI] [PubMed] [Google Scholar]
- 19.Jeon JH, Kim KY, Kim JH, et al. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J 2008; 22: 1502–1511. DOI: 10.1096/fj.07-9412com. [DOI] [PubMed] [Google Scholar]
- 20.Lu L, Zhang RY, Wang XQ, et al. C1q/TNF-related protein-1: an adipokine marking and promoting atherosclerosis. Eur Heart J 2016; 37: 1762–1771. DOI: 10.1093/eurheartj/ehv649. [DOI] [PubMed] [Google Scholar]
- 21.Peterson JM, Aja S, Wei Z, et al. CTRP1 protein enhances fatty acid oxidation via AMP-activated protein kinase (AMPK) activation and acetyl-CoA carboxylase (ACC) inhibition. J Biol Chem 2012; 287: 1576–1587. DOI: 10.1074/jbc.M111.278333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalupova L, Zakovska A, Adamcova K. Development of a novel enzyme-linked immunosorbent assay (ELISA) for measurement of serum CTRP1: a pilot study: measurement of serum CTRP1 in healthy donors and patients with metabolic syndrome. Clin Biochem 2013; 46: 73–78. DOI: 10.1016/j.clinbiochem.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Han S, Park JS, Lee S, et al. CTRP1 protects against diet-induced hyperglycemia by enhancing glycolysis and fatty acid oxidation. J Nutr Biochem 2016; 27: 43–52. DOI: 10.1016/j.jnutbio.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, et al. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 2008; 416: 161–177. DOI: 10.1042/bj20081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez S, Lei X, Petersen PS, et al. Loss of CTRP1 disrupts glucose and lipid homeostasis. Am J Physiol Endocrinol Metab 2016; 311: E678–E697. DOI: 10.1152/ajpendo.00087.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuasa D, Ohashi K, Shibata R, et al. Association of circulating C1q/TNF-related protein 1 levels with coronary artery disease in men. PloS One 2014; 9: e99846. DOI: 10.1371/journal.pone.0099846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006; 6: 772–783. DOI: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 28.Kim KY, Kim HY, Kim JH, et al. Tumor necrosis factor-alpha and interleukin-1beta increases CTRP1 expression in adipose tissue. FEBS Lett 2006; 580: 3953–3960. DOI: 10.1016/j.febslet.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 29.Yuasa D, Ohashi K, Shibata R, et al. C1q/TNF-related protein-1 functions to protect against acute ischemic injury in the heart. FASEB J 2016; 30: 1065–1075. DOI: 10.1096/fj.15-279885. [DOI] [PubMed] [Google Scholar]
- 30.Kanemura N, Shibata R, Ohashi K, et al. C1q/TNF-related protein 1 prevents neointimal formation after arterial injury. Atherosclerosis 2017; 257: 138–145. DOI: 10.1016/j.atherosclerosis.2017.01.014. [DOI] [PubMed] [Google Scholar]