Abstract
Host-guest motifs are likely the most recognizable manifestation of supramolecular chemistry. These complexes are characterized by the organization of small molecules on the basis of preferential association of a guest within the portal of a host. In the context of their therapeutic use, the primary application of these complexes has been as excipients which enhance the solubility or improve the stability of drug formulations, primarily in a vial. However, there may be opportunities to go significantly beyond such a role and leverage key features of the affinity, specificity, and dynamics of the interaction itself toward “smarter” therapeutic designs. One approach in this regard would seek stimuli-responsive host-guest recognition, wherein a complex forms in a manner that is sensitive to, or can be governed by, externally applied triggers, disease-specific proteins and analytes, or the presence of a competing guest. This review will highlight the general and phenomenological design considerations governing host-guest recognition and the specific types of chemistry which have been used and are available for different applications. Finally, a discussion of the molecular engineering and design approaches which enable sensitivity to a variety of different stimuli are highlighted. Ultimately, these molecular-scale approaches offer an assortment of new chemistry and material design tools toward improving precision in drug delivery.
Keywords: Crown ether, porphyrin, calixarenes, pillararenes, cyclodextrin, cucurbituril, rotaxane
1. Introduction
The design of efficient, effective, and safe therapeutics remains a present challenge in addressing numerous diseases and afflictions. In spite of significant efforts in drug discovery and development, over half of proposed therapeutics fail in the course of clinical trials due to reasons which include a lack of therapeutic efficacy and unacceptable safety 1,2. As such, it remains difficult to predict the success of new therapeutic entities in spite of in vitro target validation, translational studies, and small-scale clinical evaluation. Side-effects from off-target activity or co-morbidities often limit the dose of a drug which can be administered, narrowing the therapeutic index to the point where therapy is no longer feasible. As the adage in pharmaceutics often goes, the dose makes the poison. It is increasingly appreciated that drug formulation and delivery methods play a very large role in both the therapeutic effectiveness and safety of a pharmaceutical agent 3. By varying the method of delivery, drug pharmacokinetics, bioavailability, distribution, metabolism, clearance, and toxicity can all be impacted 4. As such, an increased focus on formulation approaches and drug delivery devices may be key to converting active therapeutic entities into clinically deployed drugs.
One commonly explored route toward achieving more refined drug delivery is through the use of stimuli-responsive triggers to bias drug biodistribution with spatiotemporal control such that a drug acts both when it is needed and at the site where it is needed 5-7. By this design, a priori knowledge of the location of need for a therapeutic might be coupled to regionally controlled application of a stimulus such as light, pulsed ultrasound, or a magnetic field. Alternatively, the process could be made more autonomous by integrating an ability to respond to physiologic indicators of disease, such as changes in pH, increased redox activity, or elevated enzyme levels. In this way, it may be possible to broaden the therapeutic index by ensuring more of an administered drug reaches its target, thus lowering ED50, while simultaneously sequestering drug activity systemically, thus increasing the LD50. Achieving more effective therapy while reducing the risks for dose-limiting side-effects is an important development in improving pharmaceutical practice.
Supramolecular interactions afford many useful tools for the design of new biomaterials and drug delivery devices 8-14. The earliest uses of supramolecular macrocycles in drug delivery were in the context of excipients which functioned primarily by improving the solubility and formulation stability of a diverse array of hydrophobic drugs and increasing cell permeability for charged species 15-17. In addition, there are examples using inclusion within supramolecular macrocycles as a method to mitigate the toxicity of a drug 18. These uses are typically characterized by equilibrium-governed interactions between a drug guest and a hydrophilic macrocycle host. Related approaches have extended to the stabilization and improved solubility of protein therapeutics, wherein macrocycles may serve to sequester hydrophobic domains and inhibit their aggregation in formulation 19,20. Host-guest interactions are inherently dynamic and concentration-dependent, meaning interactions which successfully solubilize or stabilize drugs in a concentrated vial on a shelf would be expected to dissociate instantly and practically irreversibly once introduced into the diluting and competitive physiologic environment. Opportunities to increase the kinetic barrier to dissociation or slow the dynamics through increased affinity may afford interesting new applications for the application of drugs in the body.
An assortment of supramolecular systems with therapeutic objectives has been thoroughly reviewed in recent years 8-14. However, one area where host-guest technologies may advance beyond simply formulation excipients would align with the general goals of other stimuli-responsive therapeutics. Specifically, strategies which link complex formation, including its affinity and dynamics, to the existence of an applied stimulus or environmental cue are being actively explored toward “smart” and autonomous therapies with spatiotemporal precision (Fig. 1). Specific efforts to design and enable stimuli-responsive host-guest systems are a much more limited subset of work in the general application of supramolecular macrocycles for therapeutic purposes, and as such form the basis for this review.
Figure 1.
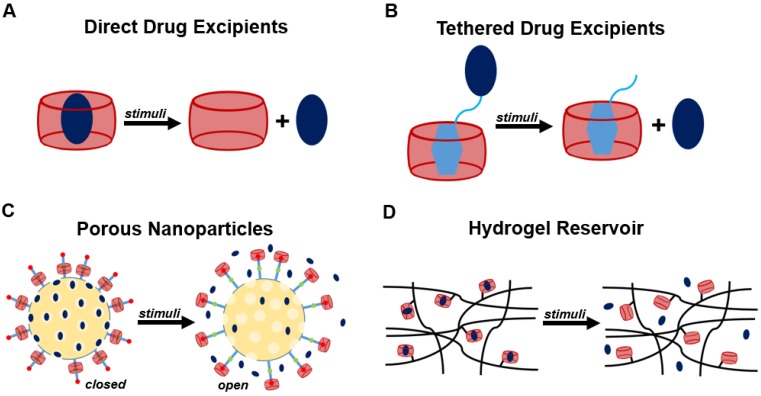
Overview of common approaches in designing stimuli-responsive host-guest systems. (A) Drug encapsulation is the most basic approach used in host-guest drug delivery applications. (B) The panel of drugs available for use in host-guest systems may be greatly expanded in the tethering of guest motifs to drugs of interest by labile bonds. (C) Porous nanoparticles have been used to encapsulate drugs, and these can be further equipped using host-guest chemistry to sterically block the release of cargo from the nanoparticle. Cargo is released as a specific stimuli shifts the host-guest binding away from the nanoparticle surface or displaces the macrocycle entirely. (D) Host motifs can also be incorporated into oligomeric or polymeric building blocks to enable the formation of material or hydrogel drug depots for the localized release of drug.
2. General Molecular Design Considerations in Host-Guest Systems
To evaluate the suitability of using host-guest supramolecular macrocycles as part of a new therapy, one key is to understand specific design principles coupled with their underlying molecular and thermodynamic origins. Before a supramolecular macrocycle may be tailored for a specific therapeutic application, it is imperative that benefits and limitations of macrocycle strategies more broadly, and host-specific constraints specifically, are understood. A subset of these general considerations that are widely applicable to all host-guest systems are offered here. This is intended as a primer for discussion of the available macrocycle chemistries that follows.
2.1 Affinity and Dynamics
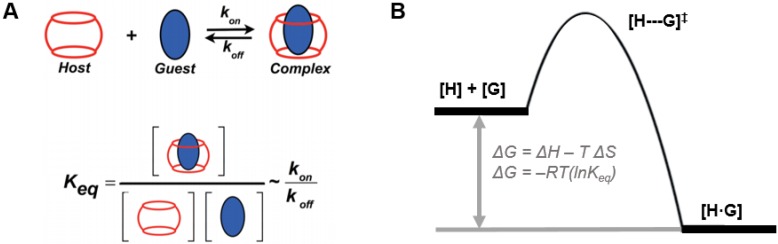
One reason to use a macrocyclic host in designing a therapy may be to afford specific affinity for a drug or payload of interest as a guest within the host. In another use, affinity may offer an opportunity to prepare modular constructs, for example by using guest-appended drugs or targeting groups to facilitate “mix-and-match” functionality in an engineered system. Regardless of the motivation, an appreciation for both the affinity and dynamics of the chosen interaction is necessary to understand whether a complex will remain stable once introduced into the diluted conditions of the body and for how long these entities remain associated once a complex is formed. Equilibrium binding affinity, often abbreviated as Keq or Ka, is a quantity defined by a ratio of concentrations of the formed complex to the individual substituents at equilibrium (Fig. 2A), as follows:
Figure 2.
General design considerations in host-guest complex formation. (A) The affinity and dynamics of guest recognition by host macrocycles can be defined at equilibrium as a function of the concentrations of each species, or alternatively is proportional to the rates of complex formation and dissolution. Figure used with permission from 21, Copyright 2019 American Chemical Society. (B) These interactions are furthermore defined by the thermodynamics.
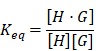 |
where [H] is the molar concentration of the host molecule, [G] is the molar concentration of the guest molecule, and [H·G] is the molar concentration of the host-guest complex. The resulting value for Keq is then expressed in units of M-1, and larger values of Keq indicate a higher binding affinity between host and guest. It is noted that this expression for Keq as written is defined in the context of a heterodimeric host-guest motif. The reaction scheme would thus be altered for the small subset of macrocycles which can simultaneously include two guests within their portal to form ternary complexes (i.e., yielding Keq with units of M-2). In order to appreciate the rates of complex formation and dissolution in a heterodimeric interaction as well as the lifetime of these interactions, it is often more helpful to express Keq in terms of a ratio of these rates, as follows:
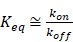 |
where kon and koff are the competing kinetic rates of association and dissociation, respectively, of the host-guest complex. The macrocyclic host-guest systems discussed in the context of this review primarily involve the recognition of small molecules, and in this case kon is often approximated to occur at the diffusion limit of ~108 M-1 s-1. With this reasonable assumption for kon, one can then estimate koff based on a measured value of Keq. For example, a complex with Keq ~105 M-1 may be approximated to have a koff on the order of 103 s-1, meaning a host-guest complex may form and break on the order of 1000 times per second. On the other hand, certain host-guest motifs may afford significantly higher affinity, with some examples demonstrated with Keq ~1012 M-1; in this case, once formed, a host-guest motif may be stable on the order of hours or more on average.
One of the factors dictating the magnitude of affinity is host-guest complementarity. This concept encompasses a classical “lock-and-key” model of non-covalent binding, wherein the alignment of supramolecular surface interactions on the inner cavity and portals of the host are compared to the surface interactions of the guest. These interactions include any combination (favorable or unfavorable) of electrostatics, dipole moments, van der Waals forces, and/or hydrophobic effects. For example, ferrocene-methylamine derivatives with a single positively-charged amine group form host-guest complexes with both cyclodextrin and cucurbituril macrocycles. As will be discussed, both of these macrocycles have electron-rich portals surrounding a hydrophobic cavity. The result is inclusion of the hydrophobic ferrocene moiety being favored within the hydrophobic cavity of both macrocycles, and the alignment of the electron-poor amine with the electron-rich portals serving to strengthen this interaction. However, ferrocene-methylamine derivatives have been observed to bind cucurbiturils with Keq values approximately 7 orders of magnitude higher compared to the same guests binding to cyclodextrin 22. As such, while host-guest complementarity is arguably the most important driving force for host-guest complexation, the accumulation of effects arising from macrocycle geometry and other intermolecular forces can lead to significant differences in the overall affinity.
Accordingly, the affinity of a host-guest interaction dictates in large part the stability and duration of complex formation and may contribute to the suitability of a certain macrocyclic host for a particular application. For example, in strategies using macrocyclic hosts for increased solubility in formulation or enhanced shelf-life, complexes which rapidly dissociate will do so immediately and irreversibly once in the body to enable the free drug to act rapidly. Conversely, a carrier intended for use in long-circulating applications may benefit from higher affinity, and thereby longer-lasting, host-guest complexation. Furthermore, affinity may translate to the bulk properties of hydrogels prepared from host-guest supramolecular motifs and govern the rate of controlled release of encapsulated macromolecules 23. In understanding the underlying principles governing affinity, it furthermore is possible to design stimuli-responsive interactions wherein specific conditions or applied stimuli lead to an interaction being weakened to release an encapsulated payload on demand.
2.2 Geometry
Each macrocycle discussed in this review has a distinct geometry that affects its ability to serve as a host for an assortment of guests. In the case of planar macrocycles (i.e., crown ethers, porphyrins), host molecules interact with a guest in relation to its cross-section, while the cryptands and cavitands include three-dimensional shape/volume effects in their inclusion of guests. The cone-like geometries of the calixarenes and cyclodextrins result in two different portal diameters, ultimately favoring guest molecules with a more cone-like topology. The base structure of pillararenes are columnar in shape, with both portal diameters equal to that of the cavity, though appended functional groups may be included to alter the geometry or taper of the overall compound. Lastly, cucurbiturils have a shape resembling a compressed sphere leading to an equatorial diameter that is greater than those of its portals and thus favoring guest molecules with spherical topology appended on opposing sides with sterically narrow functional groups.
Similar to the alignment of favorable surface interactions in facilitating binding, the alignment of flexible and rigid components between the host and guest can dictate features of the interaction. Several reports have supported a pattern in which rigid hosts such as calixarenes, pillararenes, cyclodextrins, and cucurbiturils most often favor rigid guests within their cavities, while flexible hosts such as crown ethers, cryptands, and some rotaxanes favor binding to flexible guests. This general observation has been supported by computational simulations to isolate and study the effect of rigidity vs. flexibility in host-guest systems 24. By keeping interaction potentials constant, it was demonstrated that the relative rigidity/flexibility of the host influenced its binding to guests with similar rigidity/flexibility profiles, with the highest binding affinities resulting from host-guest systems occupying similar rigidity/flexibility regimes.
Additionally, constrictive binding effects may be observed in macrocyclic hosts that possess a cavity with a diameter larger than one or both of its associated portals; this effect is particularly pronounced within cucurbiturils 24,25. In such systems, it may initially appear counterintuitive that a guest molecule would pass through a portal aperture smaller in diameter than the guest itself. However, it has been shown that macrocycle portals experience certain fluctuations which offer momentary elongations in the portals to enable larger guests to be included. This feature may be significant in the case where a large guest is to be displaced by a different guest with higher binding affinity, such as in the use of a competitor to enable temporal control of drug release. Although the relative Keq values of the guests inform the preferred distribution of the complexes at equilibrium, constrictive binding effects may lead to slower release rates of the first guest than would be expected from more simple estimations of koff discussed previously.
2.3 Thermodynamics
Host-guest binding can also be understood according to the enthalpy and entropy associated with the process of forming a complex (Fig. 2B). A major driving force for host-guest complex formation arises from gains in solvent entropy which align with the well-known hydrophobic effect, as frustrated water molecules required to solvate an often non-polar or hydrophobic guest become free to tumble in tetrahedral coordination with the bulk solvent after a host-guest complex is formed. For the participants in the complex, if host-guest complementarity is well-aligned, complexation of host and guest is enthalpically favored. However, the formation of an assembled complex from two dispersed molecules is inherently unfavorable in terms of their entropy. This balance of these favorable and unfavorable thermodynamic drivers gives rise to an understanding of the enthalpy-entropy compensation effect. Plots of this effect typically yield linear relationships between the gain of enthalpy and loss of entropy 26, though cucurbituril macrocycles have been shown not to conform to this linear trend 27. While the enthalpy change is favorable upon guest binding, an observation uncommon for other macrocycles is that binding to certain guests within cucurbiturils leads to favorable entropic changes for the host. This has been largely attributed to high-energy water molecules within the hydrophobic cucurbituril cavity. When a cucurbituril is fully solvated in water, the ureido carbonyl-lined portals establish enthalpically favorable hydrogen bonding with surrounding water molecules. However, cucurbiturils have unfavorable entropic effects on the bulk water displaced by their dissolution and significantly disrupt the hydrogen bonding network of the water solvent, incurring an energetic penalty both in the immediately adjacent bulk solvent and in the water molecules encapsulated within the macrocyclic cavity 28. When binding to a high affinity guest molecule, both traditional (entropy-driven) and non-traditional (enthalpy-driven) hydrophobic effects then contribute to breaking this typically linear trend in the enthalpy-entropy compensation. It has been shown that water molecules within the cavity are energetically frustrated due to their constricted volume, leading to increased solvent density and reduced hydrogen-bond counts compared to that of the bulk solvent. Thus, when these frustrated water molecules are expelled from the cavity upon guest binding, favorable enthalpic and entropic effects support high affinity guest complexation 29.
2.4 Biomedical Versatility
Incorporating supramolecular macrocycle hosts into drug delivery platforms affords several possible benefits depending on the macrocycle chosen. These include the following features: i) The host-guest pair is typically modular and though each macrocycle possesses distinct properties and geometries which dictate which molecules can act as guests, this still leaves the possibility for large libraries of guest molecules to enable mix-and-match combinations with a chosen host. Such modularity may be particularly appealing in the context of personalized medicine, where the same host-based drug delivery technology could be utilized in the delivery of a number of different drugs alone or in combination. ii.) The use of host-guest systems ensures predictable and reproducible approaches to drug formulation. Whereas other carriers of hydrophobic drugs exhibit dispersity in size and drug loading, the defined stoichiometry of the host-guest motif limits variability in the formulation. This contributes to predictable solubility, precise dosing, and assured pharmacokinetics, all of which would be expected to streamline development and improve the likelihood for predictable therapeutic performance. iii.) Many macrocycles are produced through relatively simple procedures with inexpensive starting materials. Cyclodextrin can be produced by the barrel from a starch feedstock using an enzymatic process, while many others are prepared from simple acid- or base-catalyzed condensation polymerization reactions of basic monomers. These procedures are typically easily scaled, offering accessible routes for industrial production. iv.) Synthetic macrocycles have typically shown predictable toxicological and safety profiles in vivo 30,31. A feature of many synthetic macrocycles is their chemical stability, meaning these should resist degradation within the body, reducing the risk of harmful degradation byproducts. Additionally, due to their typical size on the order of 1 kDa and aqueous solubility, most macrocycles by themselves would be expected to easily clear from circulation by renal mechanisms 32. Application-specific designs will be elaborated on in the course of this review. Considering initial toxicological studies and the numerous demonstrations of function in vitro, as well as emergent work to demonstrate function of some systems in vivo, the prospects for clinical implementation of these technologies appears promising. However, limited clinical progress has thus far been made.
3. Specific Host Macrocycle Chemistries Available in Therapeutic Design
While many of the features discussed previously apply broadly to virtually all macrocycle chemistries, there are other features and properties specific to each class of macrocycles which might contribute to improved functionality for a specific application. As such, there are many choices when it comes to macrocycles that may be considered in the context of their use in stimuli-responsive drug delivery, (Fig. 3A) and many of these different classes of macrocycles are discussed in greater detail here.
Figure 3.
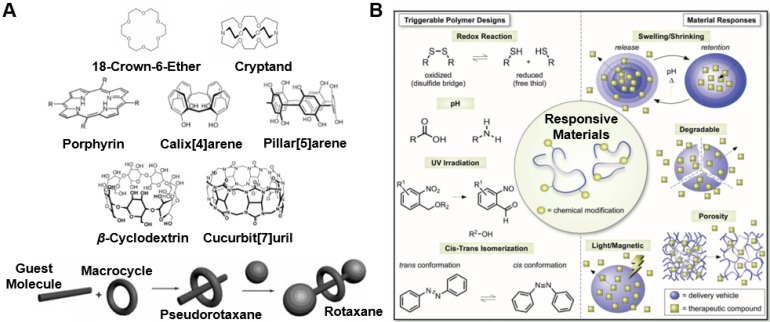
Examples of (A) common macrocycle structures discussed in this review. Adapted with permission from references 8,16,29. Copyright 2017 Royal Society of Chemistry; Copyright 2012 Royal Society of Chemistry; Copyright 2014 John Wiley and Sons, respectively. (B) Common triggers for stimuli-responsive materials. Figure adapted with permission from reference 3. Copyright 2018 John Wiley and Sons.
3.1 Crown Ethers and Cryptands
Crown ethers, sometimes referred to as coronands, are structurally simple macrocycles that can be engineered for host-guest complexation 33. These molecules are formed through cyclization of polyether chains, composed of three or more oxygen atoms with two or more carbon atoms between each oxygen 16. These oxygen atoms act as multi-dentate ligands to facilitate interactions with guest molecules, with the oxygen atoms enabling hydrogen-bonding, complementary polarity, or partial negative charges 34,35. The most common crown ethers include 12-crown-4, 15-crown-5, and 18-crown-6 ether, where the first number corresponds to the total number of atoms within the ring and the latter number signifies the number of these atoms which are oxygens. These flexible hosts are best known for their binding to various metal cations. Crown ethers can also act as ionophores and have inherent toxicity in the context of anti-cancer, anti-bacterial, and anti-parasitic therapies 36.
Cryptands constitute a related family of three-dimensional host molecules built from a crown ether framework. By substituting an oxygen atom in the cycle with a nitrogen atom, bridging units can be built around the central cavity. These additional steric constraints on the cavity allow for higher binding affinity to guests and, by engineering additional substitutions at the nitrogen atoms, offer a wider range of control over both the binding and release of guest molecules 33,34. The ongoing challenge for both crown ethers and cryptands are their relatively simple structure and subsequent poor affinity for anionic species, which are incompatible with their electron-rich electrostatic mode of binding, as well as to molecular guests larger than a typical metal ion.
3.2 Porphyrins
Porphyrins are heme-like tetrapyrrole structures capable of multi-dentate binding of guest molecules via hydrogen bonding and dipole moments. These rigid, highly conjugated, planar structures are abundant in nature, underlying the function of hemoglobins, cytochromes, and chlorophylls. The simple, symmetric, and non-modified core of the porphyrin ring, a cyclized tetrapyrrole, can be synthesized through a number of routes 37. Most commonly, monopyrroles can be polymerized and cyclized through an acid-catalyzed condensation reaction with aldehydes,38 or through the synthesis of dipyrroles followed by their dimerization to form closed porphyrin rings 39,40. A less restrictive synthetic route enables the cyclization of any tetrapyrrole, modified or not 41,42. One challenge is that the macrocycle product is often hydrophobic, and an array of functionalizations have been appended to the central porphyrin ring to improve water solubility, prevent porphyrin aggregation, and improve bioavailability 37,43,44. Interestingly, porphyrins are also inherently light-reactive, generating reactive oxygen species (ROS) when excited at specific wavelengths. This allows the porphyrin ring to act as both a host molecule and as a drug molecule on its own in the context of photodynamic therapy (PDT) 44-47. The light-responsiveness of the porphyrin ring can be tuned through appended functional groups and extension of the conjugated π-system, but porphyrin-based PDT often exhibits relatively long drug-to-light intervals which requires the photosensitizer to be administered 24-72 hours prior to light irradiation and the patient quarantined from direct light in the interim 48. Additionally, non-modified porphyrin has a relatively low molar absorption coefficient, requiring high-intensity light to sufficiently activate ROS generation which may lead to long-term photosensitivity and limit the penetration depth for therapeutic activation 45. It is noted that while small, planar porphyrins often act as host macrocycles to metal cations within nature, theranostic applications rely more heavily on photodynamic therapy effects of the macrocycle itself. As such, while not a traditional host, porphyrin applications have been included within this review due to their common similarities to related host-guest systems like crown ethers as well as their important historical role as an early macrocycle used in theranostic applications.
3.3 Calix[n]arenes
The first of the cavitand species discussed within this review, calix[n]arenes (CAs) are a family of macrocycles synthesized by base-catalyzed condensation of phenol monomers with aldehydes 49. Each monomer is connected to adjacent monomers by a methylene bridge in the meta-2,6 position, forming the distinct, rigid cone shape of the central cavity 50. Calix[n]arenes composed of n = 4, 5, 6, 7, 8, and 9 phenol monomers are the most commonly synthesized, though species of an even n (i.e., CA4, CA6, and CA8) are reportedly easier the make and/or purify and thus are more widely studied 51. The phenol subunits contribute to a hydrophobic central cavity with an electron-rich portal having partial negative charge. To counteract the hydrophobic nature of the hydrocarbon rings, a wide variety of modifications in the para-1,4 positions have been studied, selectively tailoring either or both of the portals with pendant functional groups; notably, sulfonation on oxygen atoms of the larger portal are quite common. This promotes increased water solubility, improved biocompatibility, and reduced cytotoxicity toward the development of CA-based drug delivery platforms 51-53. It should also be noted that CAs can be incorporated into photodynamic therapies, similar to porphyrins, and possess a shorter drug-to-light interval of only 15 minutes; however, challenges such as long-lasting photosensitivity are still reported by some patients 54.
3.4 Pillar[n]arenes
Pillar[n]arene (PA) structures are similar to that of calix[n]arenes, with the notable difference being that each monomer is connected in the para-2,5 positions rather than the calixarene-based meta-2,6 positions 55. This single-atom shift along the six-membered hydrocarbon ring leads to a rigid columnar or pillar-like geometry rather than the cone-like shape adopted by calixarenes; the phenol monomers maintain the macrocycle properties of a hydrophobic cavity and an electron-rich portal 56. Available pillar[n]arene (PA[n]) analogues are composed of n = 5-15 phenol subunits 56. The symmetrical portals of pillararenes should facilitate high-affinity binding compared to conical calixarenes, but the symmetry of pillararenes also results in more challenging synthetic procedures which can be low-yielding and entail cumbersome purification 56. Fortunately, pillararene subunits are also easily modified; leading to an array of possible appended functional groups to tune these macrocycles for a specific application 57.
3.5 Cyclodextrins
The cyclodextrin (CD) family of macrocycles has been, without question, the most used macrocycle in the context of biomedical and pharmaceutical applications. Most uses for CDs leverage their ability to greatly enhance the solubility, stability, and bioavailability of hydrophobic drug guests; their composition from natural sugar monomers also offers excellent biocompatibility 58. Whereas other macrocycles are named on the basis of their number of monomers, CDs are named using greek characters, with the four most common cyclodextrin analogues, α-, β-, γ-, and δ-CD, composed of 6, 7, 8, and 9 glucose monomers, respectively. These macrocycles are synthesized by the cyclization of glucose polysaccharides through intramolecular glycosylation via α(1→4) linkages 59. These linkages result in macrocycles with rigid, conical geometry, a hydrophobic core, electron-rich portals, and a hydrophilic exterior. Their relative ubiquity in pharmaceutical and biomedical practice has resulted in many great literature resources; readers are encouraged to reference a particularly excellent review detailing the discovery, development, and use of cyclodextrins 60.
3.6 Cucurbiturils
Cucurbiturils (CB[n]) are a family of supramolecular macrocycles synthesized by the cyclic polymerization of glycoluril monomers via acid-catalyzed condensation reaction with formaldehyde 61-63. The most common cucurbit[n]uril species are composed of n = 5, 6, 7, 8, and 10 glycoluril monomers. Although cucurbiturils have remarkable stability in a range of chemical environments, only those from an odd number of glycoluril monomers (i.e., CB5 and CB7) have the requisite water solubility in the dispersed state for most biological applications 28. The stability of these macrocycles presents a synthetic challenge in including site-specific modifications to enable their attachment to devices or drug carriers 64,65. CB species have been shown to have suitable biocompatibility, supporting their use in drug delivery and other biologically-applied platforms 66,67. Additionally, CBs possess a unique ability to bind certain guests with very high affinity, with the highest binding affinity (Keq = 7.2 x 1017 M-1 in D2O) ever reported arising from host-guest complexation between CB7 and a diamantane quaternary diammonium ion derivative 68,69. The reader is encouraged to consult a comprehensive review of the design and use of cucurbituril species for a variety of applications 70.
3.7 Designer Synthetic Macrocycles
The above macrocycles are all synthesized through the cyclic polymerization of a foundational monomer. Moving away from this paradigm, cyclization of a wide variety of motifs allows the development of designer macrocycles for specialized functionality. These structures are often based on aryl groups cyclized via short hydrophilic linkers, maintaining the common hydrophobic core and peripheral charge/polarity observed in other macrocycle classes 8. This customizable architecture can be fine-tuned to bind with high affinity and selectivity to specific guest molecules, such as hydrophobic theranostic agents, or may exhibit inherent theranostic activity through the macrocycle alone 71,72.
3.8 Rotaxanes
The concept of a rotaxane encompasses mechanically interlocked macrocycle(s) threaded through the cavity by a strand serving as a guest molecule. Rotaxanes can be assembled using several of the macrocycle species discussed, including crown ethers, calixarenes, or cyclodextrins, which are then threaded by another molecule or oligomer and trapped by the use of two sterically limiting capping groups 34,73,74. The resulting trapped architecture can alter the properties of the 'guest' molecule, which may include increased solubility, decreased aggregation, enhanced fluorescence, and improved cellular targeting which may be conferred by the macrocycle or its appended functional groups 74. Rotaxane architectures also enable a variety of complex, trigger-responsive cascades and reversible ON/OFF gating compared to other stimuli-responsive macrocycles 75,76.
4. Integrating Stimuli-Responsive Function in the Application of Host-Guest Therapeutics
There have been numerous efforts to prepare stimuli-responsive drug carriers (Fig. 3B), including several efforts designed to respond to disease-relevant stimuli 5-7. In terms of supramolecular materials, common stimuli alter material swelling or promote bond rupture through hydrolysis or enzymatic action 77. For host-guest systems particularly, the ability to precisely control the formation of a complex and link complex formation to biologically relevant or biologically compatible triggers has obvious application in improving therapeutic precision. In some cases, stimuli induce reversible changes in host-guest complex formation, while in other cases stimuli may promote irreversible degradation to prevent reversibility. Given the known benefit of most macrocycles arising from their chemical stability, creating stimuli-responsive complexes typically requires that the guest component undergo stimuli-triggered changes that impact its ability to bind to the host macrocycle. The type of macrocycle selected the platform to which the macrocycle is conjugated, and the intended therapeutic target all factor into the design of an effective therapy. Additionally, host-guest complex affinity plays a key role in stimuli-responsive triggers; affinity too low may lead to unwanted leaking or premature release of a therapy, whereas affinity too high may make it difficult to trigger a release event or slow the process sufficiently so as to prevent a therapeutic concentration from being reached. As such, the general and macrocycle-specific design criteria discussed previously inform the design of new stimuli-responsive therapeutics based on host-guest motifs.
4.1 Host-Guest Therapeutics Responsive to Externally Applied Stimuli
A variety of applied stimuli, which have commonly included light, ultrasound, and magnetic fields, have been investigated as stimuli to facilitate therapeutic deployment due to their ease of application and minimally invasive nature. Furthermore, the general equipment required to apply such stimuli is, for the most part, widely distributed and used in the course of routine medical practice. As such, this broad category of applied stimuli has been broadly explored, with some examples specific to host-guest chemistry presented here.
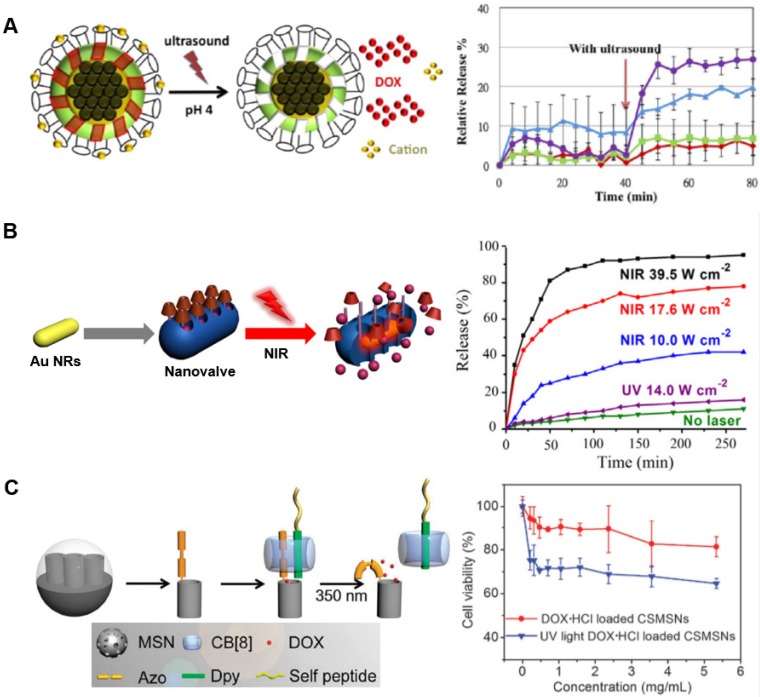
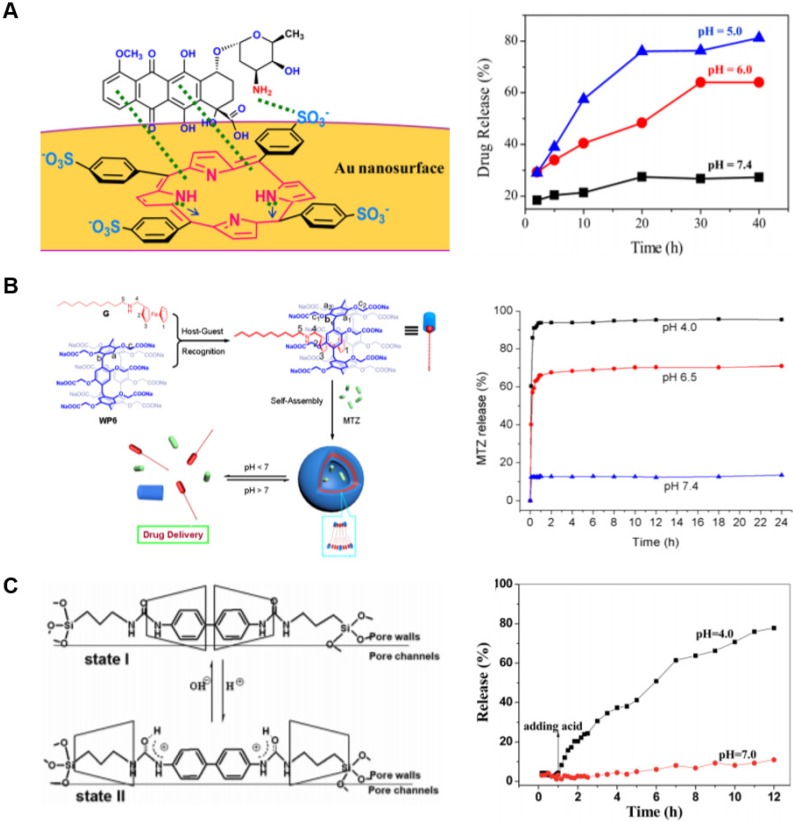
In an example combining both ultrasound and magnetism within the same therapeutic platform, recent efforts have focused on core-shell nanoparticles composed of a superparamagnetic iron oxide core covered in a mesoporous silica shell 35. Mesoporous silica nanoparticles (MSNs) have seen increased application due to their ease of surface functionalization, thermal stability, tunable and predictable pore sizes, ability to store drugs within its porosity, and exceptional biocompatibility 78-85. These silica pores, loaded with the anticancer drug doxorubicin, can be capped with crown ether macrocycles and sealed by host-guest complexation of crown ether moieties and either Na+ or Cs+ ions; Keq for this interaction is on the order of 105 M-1. The iron oxide core enabled these nanoparticles to be targeted within the body by an applied magnetic field and, once accumulated, the release of a bolus dose of drug payload was triggered by an ultrasound blast (Fig. 4A) 35. This stimulus did not harm surrounding tissue, but was strong enough to disturb the host-guest complexation between the crown ether macrocycles and the gatekeeping cations.
Figure 4.
Examples of drug release by externally applied stimuli. (A) Iron oxide core nanoparticles were coated with mesoporous silica and capped with cations bound to surface-functionalized crown ether macrocycles. Encapsulated drugs were released when the host-guest system was disrupted by ultrasonic waves. Adapted with permission from reference 35. Copyright 2013 American Chemical Society. (B) Gold nanorods were coated with mesoporous silica and capped with calix[4]arene host-guest assemblies. With increasing intensities of NIR light, the rate of drug release could be controlled. Figure used with permission from reference 85. Copyright 2015 Elsevier. (C) Mesoporous silica nanoparticles were surface-functionalized with azobenzene moieties, which allowed for ternary complex formation with cucurbit[8]uril and a peptide-conjugated dipyridyl. UV light induced conformational change in the azobenzene, releasing encapsulated drug. Adapted with permission from reference 89. Copyright 2015 Royal Society of Chemistry.
Porphyrins are particularly intriguing in the context of light-triggered therapies. Not only are porphyrins capable of participating in host-guest complexation, but these macrocycles are also photoactivatable, releasing reactive oxygen species (ROS) after exposure to certain wavelengths of light depending on the particular conjugated system 48. Many therapies have been developed on the basis of this principle, where porphyrins are conjugated to tumor targeting and/or cell penetrating peptides for their trafficking into subcellular space. Upon light exposure, these systems degrade and release ROS, inducing apoptosis in tumors 43-45. This approach affords notable safety advantages over typical chemotherapeutic approaches and quick clearance from the body. Porphyrins can furthermore be linked to assemblies that release a selection of pre-loaded drugs. For instance, porphyrin rings have been conjugated to lipids which self-assemble into liposomes. These membrane-embedded porphyrins were designed to be sensitive to both photodynamic therapy and sonodynamic therapy; using near-infrared (NIR) and/or low intensity focused ultrasound waves, the porphyrins were activated, generated ROS, induced lipid peroxidation to inhibit the self-assembly of the lipid monomers, and released encapsulated drug molecules 86.
In another example based on light-triggered self-assembled nanoparticles, water-soluble p-sulfonatocalix[4]arene was used to form host-guest complexes with a 9-alkoxy-substituted anthracene, a hydrophobic photosensitizing drug. After complexation with CA4, the complex experienced calixarene-induced aggregation, forming nanoparticles with improved solubility, bioavailability, and light sensitivity compared to the unbound anthracene. Upon photoirradiation, the complexed anthracene photolysed to form anthraquinone, the active therapeutic form of the administered drug 87.
Calix[4]arene was also used in developing a drug delivery platform based on the mesoporous silica material described above. In this example (Fig. 4B), the mesoporous silica layer was used to cover a gold nanorod and surface-functionalized with a choline derivative. Water-soluble CA4 has a Keq of approximately 104 M-1 in binding to choline derivatives, and was used in this system as a capping agent after the silica pores were pre-loaded with drug. Interestingly, this system was designed to leverage surface plasmonic heating of the internal gold nanorod instead of a more typical light-cleavable unit. By photoirradiation, the gold nanorod increased in temperature up to ~45°C. This heating effect had been previously used in hyperthermia-based therapies, but in this design heating instead serves to displace the choline-derived guest from the CA4 macrocycle, which induced complex dissociation and allowed the pre-loaded drug to freely diffuse from the silica nanopores 85,88.
There have also been examples of functionalized pillararenes used for stimuli-responsive drug delivery, as discussed in a recent review 90. In one interesting example, pillar[5]arene was used as a capping agent on surface functionalized mesoporous silica nanoparticles, similar to some of the other described uses of macrocycles. In this case, both choline-based and pyridinium-based moieties were conjugated to the surface of the silica nanoparticles. Two different pillararene species were developed to fine-tune the system for drug delivery under various conditions. Pillar[5]arenes were functionalized with either phosphonate groups or carboxylate groups around its symmetric portals. The phosphonated PA5 was observed to have higher binding affinity to the surface-functionalized nanoparticles, which inhibited premature release of drug relative to its carboxylated analogue. Using this system, the authors also explored the use of acidic pH, coordination with Zn2+ ions, and competitive binding with the higher affinity guest methyl viologen; these general strategies for drug release are discussed in subsequent sections. By incorporating a gold nanorod within the mesoporous silica nanoparticle, the system became light-responsive and released the pillararene species from the host-guest complex through the photothermal effect of gold under NIR light 91.
Fully soluble light-responsive drug delivery platforms using pillararenes have also been developed. The anti-cancer drug chlorambucil typically has very poor water solubility and limited bioavailability. By modifying chlorambucil with photo-cleavable 1-pyrenemethanol, the molecule became a favorable guest for water-soluble pillar[6]arene with carboxylate-modified portals. The host-guest complexation improved the water solubility of the pro-drug relative to the unmodified chlorambucil, which would be projected to improve the chemotherapeutic bioavailability. Once exposed to UV irradiation, the photo-cleavable pyrene functional group is removed. The remaining chlorambucil did not have substantial affinity for PA5, which resulted in dissociation of the drug and its delivery to nearby cells 92.
Mesoporous silica nanoparticles can also be surface-functionalized with stimuli-responsive moieties that act as guests with macrocycles. In several examples, different cyclodextrins have been used to complex with azobenzene-based moieties functionalized on the surface of mesoporous silica nanoparticles. In one such example, gold nanorods covered in mesoporous silica were loaded with a model drug cargo and sealed within these pores by host-guest complexation of trans-azobenzene with α-cyclodextrin. Upon photoirradiation, the azobenzene underwent a trans- to cis- conformational transition which reduced the host-guest complex affinity to dissociate the α-CD and release a trapped drug. This was evaluated in zebrafish embryos using UV/vis traceable model drugs and demonstrated spatially controlled drug release with applied light 93. A similar system was developed using azobenzene-functionalized mesoporous silica nanoparticles and β-CD which also showed photo-controllable drug release from the silica pores 94.
There are other examples of work that has combined multiple macrocycles into the same drug delivery platform, wherein each macrocycle may contribute different features to the system. In one example, a central porphyrin ring was covalently linked to one, two, or four surrounding cyclodextrins, using both β- and γ-CD. Both CD species bind paxlitaxel with Keq values on the order of 102 M-1. Individually, this binding affinity is not sufficient to counteract the diffusion/competition effects in its application as a therapy. However, when two or more CDs are conjugated on the same central porphyrin ring, the cooperative binding of paxlitaxel between multiple CDs is sufficient to maintain complex formation in vivo while improving solubility and bioavailability of both the porphyrin and anti-cancer drug. When photo-irradiated, the porphyrin degraded to release ROS which weakened the CD-paclitaxel host-guest complex and promoted drug release 95.
Cucurbiturils have also been explored for use in light-responsive platforms. In one study (Fig. 4C), mesoporous silica nanoparticles were surface functionalized with azobenzene groups; 'self' peptides known for their ability to protect assemblies from clearance by the native immune system were also conjugated to 4,4'-dipyridyl moieties. A ternary complex leveraging cucurbit[8]uril was created by binding to both the azobenzene and the dipyridyl groups. This effectively connected the 'self' peptides to the surface of nanoparticles loaded with doxorubicin. Upon UV irradiation, the azobenzene group isomerized from trans- to cis- which disrupted the ternary host-guest assembly and released the 'self' peptide and CB8 from the surface of the nanoparticle with subsequent release of the encapsulated doxorubicin 89. In another application, a hydrophobic electron-rich anthracene was conjugated to hydrophilic electron-poor pyridinium via an alkyl spacer. When complexed with CB7, only the pyridinium was encapsulated within the host-guest complex, facilitating the formation of spherical nanoparticles. However, when complexed with CB8, the entire anthracene-pyridinium molecule was encapsulated within the macrocyclic cavity which induced the formation of nanorods. It was subsequently shown that CB7 formed host-guest complexes with binding affinity near 105 M-1, whereas CB8 formed host-guest complexes with binding affinity near 107 M-1. The report further investigated the use of these assemblies in photodynamic therapy using UV irradiation of the nanoassemblies to degrade the anthracene-based guest into anthraquinone, inducing cytotoxicity, as well as 1-(4-hydroxybutyl)-pyridinium which remains complexed within CB7. These nanoassemblies could be further used for drug delivery applications as well as photodynamic therapy 96.
Designer synthetic macrocycles have also been used in the context of drug delivery in conjunction with external stimuli. Tetraphenylethylene macrocycles are well-studied, particularly in their assembly and crystal structures, due their aggregation-induced emission behavior. This macrocycle can also act as a host in binding the anti-cancer drug procarbazine. By precipitation of the host-guest complex through solvent-exchange, regularly sized spherical nanoparticles were formed. When exposed to ultrasonic waves, the spherical nanoparticles re-arranged into a bird nest-like assembly of nanorods, releasing the encapsulated drug 72,97.
Rotaxanes have been paired with many light-responsive moieties 98. One example of a rotaxane system for light-responsive drug delivery was developed from surface-functionalized mesoporous silica nanoparticles with an oligopeptide and light-responsive fumaramide moiety. This surface functionalization was threaded through a tetralactame rotaxane. Under normal conditions, the rotaxane macrocycle favors binding to the oligomer chain over the cis fumaramide conformation. However, when triggered by focused light exposure, the fumaramide photo-isomerized from its cis- to trans- conformation, which has favorable binding to the macrocycle compared to the oligomer. This offered a stable ON/OFF gate for opening and closing of silica pores loaded with drug or dye cargo 75.
4.2 pH-Responsive Host-Guest Drug Delivery
The design of therapeutic materials which respond to changes in pH, particularly acidification that arises upon endosomal processing of internalized carriers or disease-associated acidification of local tissue environments, is a prevailing approach in creating stimuli-responsive drug carriers 99. The mechanisms by which this effect may be leveraged in creating pH-responsive host-guest complexes are varied. For example, an acidic microenvironment could be useful in facilitating accelerated hydrolysis of labile linkages used to attach a drug to a guest. Alternatively, the increase in H+ concentration may weaken hydrogen bonding and alter electrostatic interactions which underlie affinity in many host-guest systems, thereby serving to weaken this affinity and promote complex dissociation.
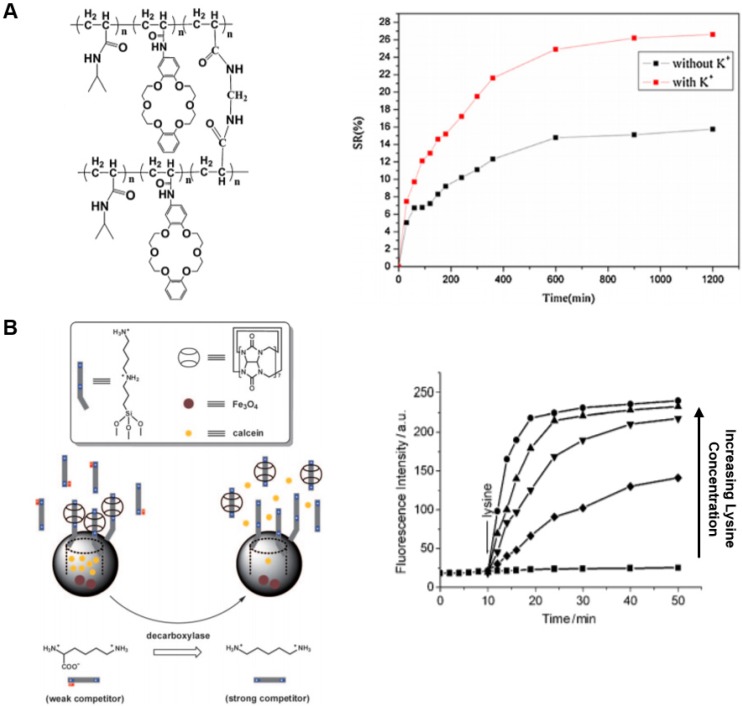
In one example where pH reduction was used to promote drug release, another platform based on a superparamagnetic iron oxide core with a mesoporous silica shell loaded with drug was used. Three crown ether moieties were conjugated together using a benzene ring to form a crown ether triad, which then bound to the surface of the nanoparticles to act as a capping agent inhibiting drug release by coordinating with the electron-poor iron surface. Within acidic environments, such as that found in tumors, elevated H+ displaced the macrocycle from the iron by interacting with the crown ether oxygens, prompting drug release from the nanoparticle. The iron core of the nanoparticle also allowed for magnetic targeting within the body as well as synergistic localized hyperthermia treatments by alternating magnetic currents 100. Another example demonstrated the use of a hydrogel prepared from conjugating a crown ether host to a guest moiety to form A-B diblock monomers. These monomers self-assemble to form both a pH- and temperature-responsive hydrogel which can be loaded with drug and released upon acidification. Similar to the mechanism above, the increased H+ concentration acts by competitive binding to crown ether oxygens, inhibiting binding to the conjugated guest and dissociating the gel 101.
Porphyrins, due to their planar structure, also coordinate the surface of gold nanoparticles through binding of pyrrolic nitrogens. Modifying the porphyrin ring with sulfonatophenyl groups facilitates binding to drugs on the other side of their portal; an example bound porphyrin simultaneously to both a gold surface and the anti-cancer drug doxorubicin (Fig. 5A). In an acidic environment, the hydrogen bonding of the porphyrin to both the gold surface and drug are weakened, dissociating the ternary complex and releasing active drug 102.
Figure 5.
Examples of drug release by pH change. (A) Porphyrin was used to noncovalently attach the anti-cancer drug doxorubicin to gold nanoparticles. The complex dissociates under acidic conditions as a result of competitive interactions with H+ ions. Figure adapted and used according to terms of use of 102. Copyright 2018 American Chemical Society. (B) Pillar[6]arene was used to form supramolecular amphiphiles with ferrocene-derived guests, which self-assembled into pH-responsive vesicles. Adapted with permission from reference 103. Copyright 2013 American Chemical Society. (C) A rotaxane system was designed as a mechanical gate across pores on the surface of porous silica. Acidic environments opened the gate by separating cyclodextrin macrocycles blocking the pore and allowing drug release. Adapted with permission from reference 104. Copyright 2016 Royal Society of Chemistry.
One useful feature of porphyrins is their suitability as components of metal-organic frameworks (MOFs). MOFs have gained attention in recent years as materials for drug delivery due to their tunable size, porosity, composition, functionality, loading capacity, and biocompatibility 105. One porphyrin-based MOF, PCN-221, was developed and evaluated for stimuli-responsive drug delivery. The pore size of this MOF allowed entrapment of the chemotherapeutic and immunosuppressant drug methotrexate. While this platform showed slow release of drug from the MOF under normal conditions, the drug release was dramatically accelerated under acidic conditions 106. A similar porphyrin-based MOF was used to encapsulate and improve the stability and bioavailability of tumor-associated antigens, which were loaded within MOFs that formed nanoparticles by lattice coordination of porphyrin and europium (Eu3+) cations. Under acidic conditions, the porphyrin-Eu chelation was disturbed, which dissociated cross-links in the MOF and released the encapsulated drug both in vitro and in vivo 107.
In order to introduce pH-responsive properties, amphoteric calix[8]arene was synthesized so that each macrocycle had both a positively-charged and a negatively-charged portal. This property enabled the macrocycles to assemble with aligned portals, forming an extended cavity which could accommodate small aggregates of the antibiotic drug ciprofloxacin. When pH was adjusted above 8.0 or below 6.0, the CA8 assemblies lost their amphoteric nature and ability to self-assemble via portal charge complementarity, which dispersed the aggregates and released the encapsulated drug108.
Calixarene macrocycles have also been integrated into a pH-responsive system based on mesoporous silica nanoparticles pre-loaded with drug and surface-functionalized with alkylammonium chains. This design allowed host-guest complexation with both sulfonatocalix[4]arene and sulfonatocalix[6]arene. Under acidic environments, the affinity of the calixarenes for the alkylammonium chain was reduced, which dissociated the calixarene capping agent and promoted drug release. This study further showed that CA6 had a higher binding affinity for the alkylammonium chain guest than CA4. This increased affinity resulted in a reduced ability for the CA6-based system to release drug at physiologically-relevant pH. To counteract this effect, the group designed a redox-responsive disulfide bond within the guest, which resulted in the host-guest complex remaining intact while still allowing drug to be released upon exposure to increased glutathione levels in the cytoplasm of cancer cells 109.
To introduce pillararenes into a pH-responsive platform for drug delivery, a ferrocene guest was conjugated to a hydrocarbon chain and, separately, a pillar[6]arene was symmetrically appended with carboxylate functional groups (Fig. 5B). Under neutral to basic conditions, the PA6 had enhanced water solubility and formed host-guest complexes with the ferrocene. This host-guest complex led to the formation of amphiphiles which self-assembled into vesicles and could be used to encapsulate the model drug mitoxantrone. Under acidic conditions, dissociation of the host-guest complex led to breakdown of the self-assembled vesicles and drug release. Mitoxantrone also had improved bioavailability when encapsulated in these vesicles 103. In another example, pillar[5]arene was synthesized with 5 tryptophan groups conjugated to each portal to improve the solubility of the macrocycle in water. A pyridinium-based guest was then conjugated to modified galactose to enable targeting of cancer cells, and was shown to form a host-guest complex with the Trp-decorated PA5 with Keq on the order of 105 M-1. This complex also self-assembled into a vesicle for drug encapsulation; in this case, the hydrophobic anti-cancer drug doxorubicin was chosen as model cargo. Display of modified galactose on their surface led to targeting and internalization by cancer cells. In the course of internalization, the acidic endosomal pH promoted dissociation of the host-guest complex to release the encapsulated drug. The incorporated tryptophan units on the portals of pillararene were also observed to intercalate DNA, resulting in a synergistic effect between the modified pillararene and the newly-released doxorubicin 110.
It should be noted that pillararenes have also been incorporated into MOF-based drug delivery technologies. In these systems, design concepts from both the above MOF examples and mesoporous silica nanoparticles technologies were combined. In one demonstration of this idea, magnetic iron oxide core nanoparticles were encapsulated within a UiO-66 Zr MOF which was subsequently surface-functionalized with units which bound carboxylated pillar[6]arene. The porous MOF was pre-loaded with a model drug cargo before using PA6 as a capping agent. These nanoparticles could be targeted in vivo using external magnetic forces, while cargo release under acidic conditions weakened the interaction between PA6 and surface-functionalized stalks 111. In a related report, the UiO-66 MOF was used to coat polypyrrole nanoparticles, effectively exchanging magnetic targeting capabilities for photothermal utility. To counteract the loss of active magnetic targeting, folic acid motifs were conjugated to the nanoparticle surface and used as active chemical targeting groups. Cargo was encapsulated into the MOF coating using a similar pillar[6]arene interaction as the iron oxide nanoparticles. The reported in vivo trials using combined pH-dependent drug release and photothermal therapy showed significant reduction in tumor volume relative to single-therapy regimes 112.
In other efforts toward pH-responsive drug delivery, acetylated α-CD was aggregated in the presence of hydrophobic drugs using oil-in-water emulsion solvent evaporation techniques, which formed drug-loaded CD-based nanoparticles. When internalized by cells, intracellular lysosomal acidity led to hydrolysis of the pendant acetyl groups on the CD, inducing nanoparticle dissociation and subsequent drug release 113. Interestingly, a follow-up report provided evidence of this platform circumventing multi-drug resistance pathways for paclitaxel, docetaxel, cis-diamminedichloroplatinum, camptothecin, and doxorubicin. The improved efficacy of these drugs against resistant cell lines was attributed to the cumulative sensitisation effects of α-CD that results from inhibiting P-glycoprotein expression, depleting intracellular ATP concentrations, and reducing PgP ATPase activity 114. An analogous study was reported using β-cyclodextrin conjugated to dextran, a cancer targeting agent. Benzimidazole-modified poly(ε-caprolactone) was used to form a host-guest complex under normal physiological conditions. These complexes formed amphiphiles that spontaneously self-assembled into micelles, into which hydrophobic drugs were loaded. When internalized via endolysosomal pathways, a pH of 6.0 or less would then decrease the association affinity between the β-CD and benzimidazole, leading to complex dissociation and release of micelle-encapsulated drug 115.
Mono-functionalized cucurbit[7]uril was modified with a biotin motif which facilitated its selective internalization into cancer cells 116. Based on previous reports, it was known that the anti-cancer drug oxaliplatin bound as a guest with CB7 macrocycles with reasonable affinity, which improved the solubility and stability of the drug while reducing its unwanted toxicity 117,118. However, once internalized within cancer cells by acidic endolysosomal means, the drug was released to induce cytotoxicity. Amending this host-guest complex with a targeting motif was expected to improve the bioavailability and selective therapeutic effect of the drug while further reducing unwanted toxicity. Interestingly, this report further explored the requirements for binding affinity between host and guest in this system, and identified several other drugs which bound as a guest in this platform, including camptothecin, irinotecan, temezolomide, albendazole, and tamoxifen 116. Additionally, several examples of nanoparticles capable of drug delivery via pH-responsive cucurbituril host-guest interactions have been developed. In one iteration of this technology, mesoporous silica nanoparticles were surface-functionalized with bis-ammonium units, facilitating pH-dependent binding of cucurbit[6]uril. Under acidic to neutral conditions, the electron-poor dialkylammonium favored interaction with the electron-rich portals of CB6; upon exposure to basic conditions, the dialkylammonium motif was deprotonated, weakening host-guest affinity. Following dissociation of the CB6 from the nanoparticle surface, pre-loaded cargo was released from the nanoparticle 119. Increasing the complexity of molecular design, mesoporous silica nanoparticles were instead surface-functionalized with a chain of spaced diammonium motifs capped with an anilinium group. Under neutral conditions, CB6 readily bound near the surface of the nanoparticle, sterically blocking release of pre-loaded cargo from the nanoparticle. When exposed to acidic conditions, the macrocycle favored interactions with the anilinium group further away from the nanoparticle surface, releasing encapsulated drugs; engineering the pKa of the anilinium group enabled fine-tuning of the pH needed for drug release. Alternatively, exposure to basic conditions deprotonated all amine functional groups and induced dissociation of the macrocycle from the nanoparticle surface 120. In a third report, these two iterations of controlled release technology were both used to encapsulate cargo within a mesoporous silica nanoparticle. However, this report also included the incorporation of azobenzene motifs within the silica pores to act as nano-impellers; when exposed to a wavelength of light that is absorbed by both the cis- and trans- conformation of azobenzenes, the continuous exchange of conformations induced a wagging motion to help expel encapsulated cargo. This technology leveraged both non-neutral pH and light to release encapsulated molecules, offering an example of a molecularly engineered AND logic gate 121.
Lastly, rotaxanes have also been designed as a component of pH-responsive drug delivery systems. In one example, periodic mesoporous organosilicas were developed and functionalized with symmetric chains across the silica pores (Fig. 5C). These chains were composed of a central biphenyl group between two ureido groups, which was nested between two propyl groups, and completed with siloxane stoppers on each end. Two β-CDs were threaded along each of these chains spanning the silica pores. Under neutral pH, the hydrophobicity of the biphenyl group brought the hydrophobic cavities of the cyclodextrin macrocycles together. Under acidic pH, the ureido groups were protonated to favor interactions with the cyclodextrin portals which forced the two macrocycles from their central position on the thread to the outer positions near the siloxane caps. This supramolecular interaction acts as a mechanical gate; with the CDs no longer sterically hindering the silica pores, the loaded drugs were free to diffuse into the local environment. Once removed from the acidic conditions, the cyclodextrin macrocycles returned to binding of the central biphenyl group, which is a more favored host-guest complex under neutral pH 104.
In a second example, a designer synthetic macrocycle was developed to improve the performance of a pH-sensitive croconaine dye. By encapsulating the dye within a tetralactam macrocycle, a stable rotaxane was formed with the croconaine dye as guest. When this theranostic technology was delivered into mice through liposomal administration, the host-guest system offered enhanced photothermal therapeutic and photoacoustic imaging capabilities, particularly within acidic environments such as those associated with cancer, infection, inflammation, or fibrosis. This dye system also exhibited strong NIR light absorbance with little production of ROS or dye photobleaching, stable ratiometric absorption that was unaffected by irradiation, and the ability to fine-tuned the pKa to match acidic physiological pH 122.
4.3 Enzymatic Triggers of Host-Guest Chemistry
The microenvironments of many diseased tissues, as well as intracellular and sub-cellular compartments, are often characterized by increased presence of a variety of enzymes which may offer useful triggers for drug release in stimuli-responsive platforms. Particularly, proteases such as esterase or urease are overexpressed in tissues in conjunction with various diseases, notably cancer. As such, drug release may be facilitated by the incorporation of known substrate groups or sequences to be cleaved and facilitate drug release. This approach often requires a more complex molecular design than is needed for ionic and/or electrostatic interactions. The more complicated guest designs in turn make this triggering approach less amenable for use with simple macrocycles, such as crown ethers.
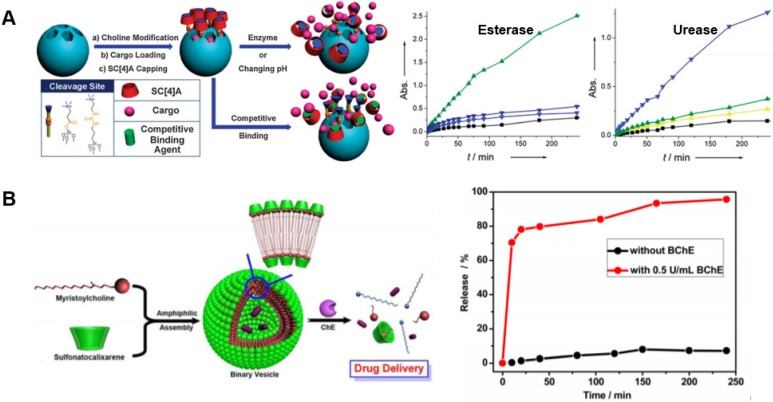
Building on light-responsive calixarene platforms, an enzyme-responsive approach was prepared from mesoporous silica nanoparticles which were surface-functionalized by ester-based or urea-based tethers to choline-like chains (Fig. 6A). These choline-like chains acted as guests to enable the use of water-soluble p-sulfonatocalix[4]arene macrocycles as capping groups. Upon exposure to esterase or urease enzymes, the host-guest complex did not dissociate but instead was cleaved entirely from the silica surface to release pre-loaded model cargo from pores of the nanoparticles 85,123. In a related approach, p-sulfonatocalix[4]arene was used to form host-guest complexes with a natural enzyme-cleavable myristoylcholine guest (Fig. 6B). These complexes formed amphiphiles which self-assembled into vesicles that were used to encapsulate a drug payload. The cholinesterase enzymes, acetylcholinesterase and butyrylcholinesterase, are overexpressed in the neural microenvironment in Alzheimer's. Upon action of cholinesterase enzymes, the myristoylcholine was cleaved into myristic acid and choline. Since neither of these natural products favor host-guest complexation with calix[4]arene, the vesicle disassembled to release the encapsulated drug 124.
Figure 6.
Examples of enzyme-responsive drug release. (A) Mesoporous silica nanoparticles were surface-functionalized with choline-like chains via ester- or urea-based tethers and capped with sulfonatocalix[4]arene. Encapsulated cargo was released upon exposure to esterase or urease enzymes. Adapted with permission from reference 123. Copyright 2013 Royal Society of Chemistry. (B) A vesicle self-assembled from amphiphiles produced by the host-guest association of myristoylcholine and sulfonatocalix[4]arene. Encapsulated drugs were released upon exposure to butyrylcholinesterase, which degraded the myristoylcholine guest molecule and dissociated the amphiphilic complex. Adapted with permission from reference 124. Copyright 2012 American Chemical Society.
There also have been many demonstrated uses integrating enzyme-responsive properties into cyclodextrin platforms 125. As an example, β-CD was conjugated through its hydroxyl groups to a copolymer containing maleic anhydride. Another copolymer was synthesized from the same material but appended with paclitaxel, forming a complementary strand for host-guest interactions. By affixing the β-CD host and drug guest onto polymers, the polyvalent construct exhibited an effective affinity that was four orders of magnitude higher than the monovalent host-guest interaction. As CD and paclitaxel were conjugated via ester linkages, their attachment was sensitive to esterase activity. The assemblies entered cancer cells and, once inside, esterases ruptured the bonds between the individual polymer with the host and drug leading to release of free paclitaxel, with therapeutic effects observed both in vitro and in vivo 126.
Rotaxanes have been used for many examples of enzyme-responsive drug delivery systems, and offer a robust tool for sequestering or deactivating cytotoxic drugs until exposed to an enzyme of choice 127. In one example, anti-cancer drugs were sequestered within the pores of a mesoporous silica nanoparticle. The silica surface was functionalized with alkoxysilane chains, threaded through α-CD macrocycles, and capped with peptide linker which included a substrate for the cathepsin B protease as well as a cell-penetrating peptide and a tumor-targeting peptide. Once this multifunctional peptide specifically targeted and penetrated the membrane of a cancer cell, the overexpression of cathepsin-B within endosomes and lysosomes would then cleave its substrate on the linker. Cleavage of the peptide released the α-CD macrocycle stopper, triggering the release of loaded doxorubicin which induced apoptosis within cancer cells 128. Another example of surface-functionalized mesoporous silica nanoparticles used diethylene glycol chains terminated with alkynes to thread α-CD. The terminal alkyne was then reacted with a benzoquinone stopper to form a rotaxane. The NAD(P)H:quinone oxidoreductase 1 enzyme induced the reductive activation of the benzoquinone to form hydroquinone, which introduced a self-immolative bond to cleave the capping agent from the tether and release the encapsulated drug. This system exhibited evidence of efficient drug release in vitro, and further demonstrated a reduction in toxicity and improved compatibility for the complete system compared to the free drugs 129. In a final example, mesoporous silica nanoparticles were surface-functionalized with triethylene glycol chains, threaded through α-CD, and capped with a motif which could be cleaved by exposure to porcine liver esterase. When exposed to the model enzyme, the engineered capping agent was cleaved from the triethylene glycol chains and the macrocycle diffused from the nanoparticle surface to release encapsulated cargo 130.
One final example illustrated an enzyme-triggered pre-programed cascade which incorporated many different responsive components that were designed to be sequentially activated. In this case, the anti-cancer drug paclitaxel was conjugated to a bulky, hydrophilic stopper via an ester linkage and threaded through a modified rotaxane macrocycle. Once internalized within cancer cells, the β-galactosidase enzyme cleaved a galactoside group conjugated to the rotaxane macrocycle. The products of this enzyme-catalyzed cleavage included a self-immolative nitro-benzyloxycarbonyl linker, which forced the stabilizing rotaxane molecule to undergo a ring-opening process and released the modified paclitaxel thread into solution. Finally, an esterase enzyme cleaved the appended stopper to release the authentic drug and activate its therapeutic effect 76.
4.4 Redox-Responsive Host-Guest Systems
Abnormal reduction-oxidation (redox) conditions are often also a hallmark of diseased tissues, offering another trigger for stimuli-responsive drug delivery platforms. Endogenous reactive molecules, including reducing agents like glutathione (GSH) and oxidizing agents like hydrogen peroxide (H2O2), afford opportunities to interface with redox-responsive chemical groups. The incorporation of redox-sensitive linkers, such as disulfide bonds, enables greater selectivity of drug delivery platforms for specific disease sites. As such, a design approach has been explored broadly to use a variety of labile or reversible chemical moieties to facilitate host-guest macrocyclic systems with redox sensitivity.
The light-responsive degradation of porphyrins results in the production of ROS which can be used in conjunction with redox-sensitive triggers as a component of a drug delivery platform. As such, porphyrins have been incorporated into nanoparticles prepared from components which contain a disulfide bond. Upon photo-irradiation, the porphyrin releases ROS which serve to disrupt these disulfide bonds and release drugs conjugated to or encapsulated within the nanoparticles 131,132. In one particular example, a porphyrin macrocycle was conjugated directly to the anti-cancer drug paclitaxel via a disulfide bond. The macrocycle-drug conjugates self-assembled into nanoparticles which were highly stable under normal physiological conditions, but dispersed quickly upon photo-irradiation as the porphyrin rings degraded to produce ROS and cleave the disulfide drug tethers 133. Porphyrin-based materials have also been used as a component of different nanoparticle-based drug encapsulation approaches. In one example, a fluorescently modified porphyrin was conjugated to a group containing short PEG chains and three hydrophobic 2,4-dinitrobenzenesulfonyl moieties which are sensitive to the reducing action of glutathione (GSH) and act to quench adjacently conjugated fluorophores. This combination of hydrophobic and hydrophilic side-chains resulted in amphiphilic character of the porphyrin-based system, spontaneously forming nanovesicles loaded with doxorubicin. The design of this system leverages elevated GSH concentrations within many cancer cells. Once the nanovesicles were internalized by cells and exposed to intracellular GSH, the 2,4-dinitrobenzenesulfonyl moieties were cleaved from the porphyrin leading to dissociation of the nanovesicle, release of the porphyrin (for potential photodynamic therapy), as well as release of the encapsulated doxorubicin. Studies in vitro demonstrated the same cytotoxicity for this platform as observed for free doxorubicin, but the platform may afford added benefit in reducing the off-target effects of the chemotherapeutic drug 134. A similar system has also been developed based on porphyrin macrocycles conjugated to hyperbranched polyglycerol nanoparticles through tethers containing disulfide bonds as a GSH-sensitive trigger to selectively release the porphyrins into cancer cells for photodynamic therapy 135.
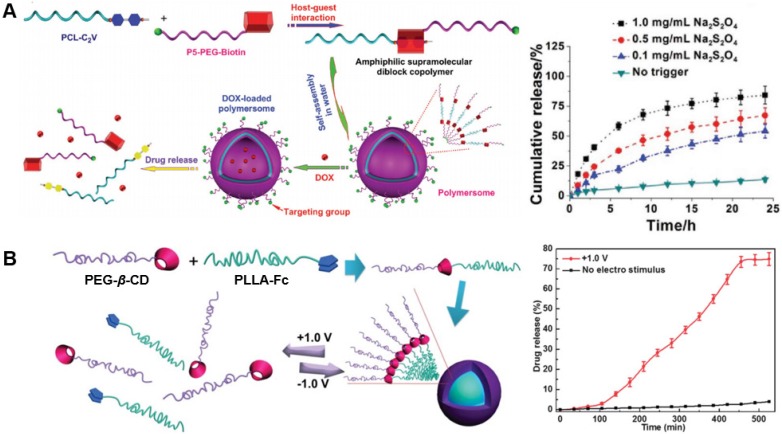
Toward redox-responsive systems based on pillararenes, one example has used pillar[5]arene appended with biotin-conjugated PEG polymer, which formed a host-guest complex upon mixing with a secondary polymer appended with viologen guests (Fig. 7A). The host-guest complexation of these two components resulted in self-assembly and formation of polymersomes that could encapsulate doxorubicin. The inclusion of biotin allowed for targeting the biotin receptor overexpressed on certain cancer cells, while limiting drug toxicity by minimizing uptake into healthy cells in vitro. The redox conditions within the tumor cells hindered the electrostatic interactions between the viologen and pillararene, disassembling the polymersome and releasing the encapsulated drug 136. In another report, pillar[5]arene was appended with biotin-conjugated PEG and the viologen group was conjugated to a brush copolymer. Host-guest complexation of these two molecules formed supramolecular nanoparticles. The inclusion of biotin as a targeting motif also improved cancer cell selectivity, but this design further allowed for the incorporation of specialized fluorescent and quenching groups to enable continuous tracking of location and drug release using imaging 137.
Figure 7.
Examples of redox-responsive drug release. (A) Modification of both a viologen guest motif and a pillar[5]arene host resulted in a supramolecular amphiphile which self-assembles into vesicles. Control of encapsulated drug release was mediated by varying concentrations of redox triggers. Figure adapted with permission from reference 136. Copyright 2016 John Wiley and Sons. (B) A similar vesicle system made of self-assembling supramolecular amphiphiles was developed from host-guest complexation between polymers modified with β-cyclodextrin or a ferrocene guest, with drug release controlled by voltage-controlled redox of the ferrocene guest chemistry. Adapted with permission from reference 138. Copyright 2014 Royal Society of Chemistry.
In another example of redox-responsive pillararenes, a water-soluble carboxy-modified pillar[5]arene was used to bind lysine modified via a disulfide bond with a long hydrophobic tail. The bound pillararene improved solubility of the modified lysine, forming an amphiphilic complex and leading to vesicle assembly. These nanostructures were used to encapsulate mitoxantrone until exposed to the acidic pH or intracellular GSH found in cancer cells. Once internalized by cancer cells, these mechanisms led to cleavage of the hydrophobic tail from the lysine-pillararene complex, disrupting vesicle self-assembly which released the encapsulated chemotherapeutic 139. In a different approach, pillar[5]arene portals were conjugated directly to positively-charged ferrocenium functional groups. These modified macrocycles spontaneously self-assembled into cationic vesicles to encapsulate both doxorubicin and therapeutic siRNA. When exposed to the reductive action of intracellular GSH, the ferrocenium groups were oxidized to neutral ferrocene groups, which disrupted the vesicle and released the encapsulated therapeutics 140.
Many drug carriers have been designed based on redox-responsive cyclodextrin 125,141-143. Three representative designs leveraging redox-responsive cyclodextrin are highlighted here. In the first, β-CD was conjugated to cross-linked low molecular weight polyethylenimine and also attached to a peptide targeting fibroblast growth factor receptors on the surface of cancer cells in a gene delivery platform. A complementary molecule was designed by conjugating PEG to an adamantyl group. When combined, the β-CD and adamantyl group host-guest complex resulted in a macromolecular polycation that was then combined with DNA and condensed into nanoparticles as a vector for gene therapy in vitro and in vivo. The DNA payload was protected from degradation until it was released through intracellular reduction in cancer cells 144. Another platform conjugated β-CD to PEG, while a ferrocene moiety was conjugated to poly(L-lactide) (Fig. 7B). When combined through the formation of host-guest complexes, these two components formed a supramolecular block copolymer that self-assembled into micelles for drug encapsulation. Rather than relying on intracellular redox conditions to reduce the ferrocene guest and decrease its affinity, this platform used externally-applied voltages (+1.0V) to disrupt the assembly and release encapsulated drug 138. A third approach to redox-responsive drug delivery relied on another redox-based trigger, H2O2, in conjunction with mesoporous silica nanoparticles surface-functionalized with ferrocene and capped with β-CD-modified gold nanoparticles. A model cargo loaded within the silica nanopores was used to track and quantify drug release over time. Upon exposure to disease-relevant concentrations of H2O2, the ferrocene adopted a positive charge which dramatically reduced its affinity for the β-CD. This dissociated the gold nanoparticles from the silica nanoparticles and released the encapsulated cargo 145.
Cucurbit[7]uril has also been incorporated into redox-responsive platforms, including an example wherein mesoporous silica nanoparticles were surface-functionalized with poly(glycidyl methacrylate)-based chains conjugated via disulfide bonds. CB7 formed high-affinity complexes with diamine groups added to the terminus of each tethered chain, and binds two chains simultaneously through ion-dipole interactions between the electron-rich CB7 portals and the electron-poor amine groups. CB7 thus acted as a capping agent to seal doxorubicin within the pores of silica. When exposed to intracellular GSH concentrations within cancer cells, the disulfide bonds which tethered the capping CB7-poly(glycidyl methacrylate) host-guest complexes to the surface of the MSNs were cleaved to begin drug release 146. However, it should be noted that the redox conditions did not directly dissociate the CB host-guest complex. While CB7 and CB8 form host-guest complexes with redox-responsive groups, such as viologens and ferrocenes, challenges remain in using these as redox-responsive triggering events in therapeutic design. For example, host-guest affinity interactions of CB7 and ferrocene-based derivatives often have remarkably high binding affinity in the range of 109 M-1 or greater, which might make dissociation too slow for practical application in drug delivery 147. Additionally, CBs can act to block the effect of some redox triggers, further inhibiting the use of redox-sensitive triggers to release guest molecules from cucurbituril macrocycles for drug delivery applications 148.
Redox-responsive triggers have also been incorporated into rotaxanes in conjunction with mesoporous silica nanoparticles surface-functionalized with guest threading using the macrocyclic ring cyclobis-(paraquat-p-phenylene) and capped with tetrathiafulvalene (TTF) to form a stable rotaxane. In the closed position, loaded drugs were sterically trapped within the silica pores. When exposed to ascorbic acid, the TTF2+ was reduced to neutral TTF, increasing the affinity between the macrocycle and the capping agent, which removed the steric barrier and promoted release of the encapsulated drug. By design, the macrocycle also quenches the fluorescence of the threaded guest, and when displaced by a redox trigger the fluorescence increased 3-fold which offered a fluorescence read-out for drug release 149.
4.5 Competitive Guest Exchange as Trigger
Tunable host-guest affinity affords a unique approach toward spatiotemporal control in drug delivery. Specifically, systems may be designed to leverage competitive guest exchange wherein the host-guest interaction facilitating drug loading or particle self-assembly can be displaced upon exposure to a stronger guest. This design approach requires a fine-tuned balance of the affinity and dynamics of a series of host-guest interactions. The opportunities to control such a design using natively occuring or endogenous guests is similarly limited, and as such this general approach, when applied to drug delivery, necessitates some precision in ensuring the displacing guest is present at the site where drug action is desired. The guest in this case may furthermore be considered as a drug in its own right, and may introduce additional regulatory hurdles to ensure its safety and efficacy.
A simple example of this design concept was demonstrated by incorporating crown ether units onto a polymer, poly(N-isopropylacrylamide), which forms a temperature-responsive hydrogel (Fig. 8A). By introducing the crown ether onto the polymer, the swelling of the hydrogel was reduced. This approach could offer potential for the release of drugs or other payloads encapsulated within the hydrogel formulation, wherein upon exposure to K+ ions, a common guest of crown ethers, the hydrogel would swell to increase the payload release rate 150.
Figure 8.
Examples of drug release by competitive guest displacement. (A) Crown ether motifs incorporated within a hydrogel dictated material swelling in a manner that could be controlled by the addition of K+ guests for the macrocycle. The addition of this competitive guest increased material swelling which would lead to drug release. Figure adapted with permission from reference 150. Copyright 2013 Elsevier. (B) Mesoporous silica nanoparticles were surface-functionalized with a guest motif having moderate binding affinity to cucurbit[7]uril. As a competitive lysine guest was added in the presence of decarboxylase enzyme, the macrocycle preferentially bound the enzymatically processed lysine, which displaced the macrocycle and released drug from within the silica nanopores. Figure adapted with permission from reference 151. Copyright 2011 John Wiley and Sons.
Toward the use of competitive affinity as a trigger in calixarenes and pillararenes, a platform capable of using either macrocycle species for drug delivery has been reported. In this system, mesoporous silica nanoparticles were surface-functionalized with choline-derived guest motifs. The nanoparticles were loaded with both drug and/or dye, and capped with either calix[4]arene or pillar[5]arene. Both macrocycles displayed Keq values on the order of 104 - 105 M-1, with pillararene having the slightly higher binding affinity; both had a higher binding affinity to native acetylcholine species. When exposed to models of the elevated concentration of acetylcholine, such as is observed in the cholinergic synapses of Parkinson's disease, the native acetylcholine displaced the choline-derivatives from the cavities of the macrocycles to remove the macrocycles from the surface of the particle and promote drug/dye release. Additionally, this study showed that by increasing concentrations of acetylcholine, the rate and amount of drug released could be increased for each macrocycle. However, the affinity of the macrocycle was also important, as the higher affinity pillararene released fewer drugs than did the calixarene capping agent at the same acetylcholine concentrations. As such, macrocycle affinity could also be used for concentration-dependent payload release, which offered an additional element of control in this system 152.
Among the first uses of competitive affinity to facilitate triggered drug release reported over thirty years ago used β-CD to improve the solubility and enhance the therapeutic effect of the anti-nausea drug cinnarizine when co-administered with a competitive phenylalanine guest 32,153,154. More recent work focused on using the concept of competitive guest binding by forming host-guest complexes between β-CD and the anti-cancer drug 2,2'-bibenzimidazole, which formed small multi-complex aggregates in aqueous solution. The cationic surfactant, cetyltrimethylammonium bromide, was then used as a competitive binding trigger to disrupt these aggregates and release the bound drug. The study also modified this same drug which resulted in higher binding affinity to β-CD and made the aggregates less sensitive to the surfactant, thus requiring higher concentrations to release comparable amounts of drug 155. Other work has designed vesicles for drug delivery that rely on competitive guest binding to release encapsulated drugs. The amino acid tyrosine binds to α-CD, β-CD, and γ-CD to form small amphiphilic complexes which self-assemble into bi-layered vesicles for drug encapsulation. Upon exposure to higher affinity guests including 1-hydroxyadamantane or Cu2+ ions, the tyrosine guest was efficiently replaced, which disassembled the vesicle and released the encapsulated drug molecules 156.
Competitive guest displacement has also been used in cucurbituril-based platforms, with one example exploring the use of gold nanoparticles surface-functionalized with a diaminohexane moiety that is a moderate guest for CB7. This cationic surface coating makes the nanoparticles cytotoxic, and CB7 was used as a capping agent to inhibit cytotoxicity by concealing these cytotoxic surface groups. When the nanoparticles were exposed to 1-adamantylamine, which is a high-affinity guest for CB7, the in vitro cytotoxicity of the nanoparticles could be activated within living cells in situ 157.
An interesting design combined both enzymatic activity and competitive guest binding within a platform of superparamagnetic iron oxide-embedded mesoporous silica nanoparticles that were surface-functionalized with 1,4-butanediamine as a moderate guest for CB7 (Fig. 8B). This platform was designed to leverage the increase in enzymatic decarboxylation of amino acids within cancer tissue, such as lysine decarboxylation to yield the high affinity CB7 guest cadaverine. Thus, these nanoparticles were designed to target sites of tumors through externally applied magnetic fields to then release encapsulated cargo once the nanoparticle-associated CB7 is freed by binding to this modified amino acid in the tumor microenvironment as it associates with the higher affinity guests 151.
Rotaxanes are especially interesting in the context of using competitive affinity as an activating trigger. Strictly defined, the macrocycle component of a rotaxane is threaded and capped on both ends. As such, rotaxanes are not readily capable of disruption by a competing guest since the secondary guest is unable to bind to the mechanically locked macrocycle. However, pseudo-rotaxanes which are uncapped may be designed to facilitate a competition-based mode of release. One example employed a dibenzo[24]crown-8 macrocycle threaded around dialkylammonium ions on the surface of porous silica nanoparticles. The pseudo-rotaxane design resulted in the macrocycle being loosely bound to the dialkylammonium chains and capping the release of a model cargo from within the silica pores. When exposed to the higher affinity fluorodialkylammonium cations, the pseudo-rotaxane was disrupted on the nanoparticle surface and the loaded drug was released. Importantly, to more thoroughly explore the effect of relative affinity of the competing guest, this same system was explored with various cations, demonstrating that under the same salt concentrations a reduction in drug release was observed when cations of decreasing affinity were used 158.
5. Conclusions
Many variations of supramolecular systems have been developed, but examples where these macrocycles have been used in the context of their application to new therapeutics or diagnostics are thus far limited. In discussing the subset of these engineered systems which exhibit stimuli-responsive features aligning with therapeutic deployment, function has often been demonstrated in a test tube or with cultured cells in vitro. Indeed, limited examples exist at this time which has used these systems in vivo. Inspirational examples using supramolecular macrocycles for drug delivery, in a context beyond that of a formulation excipient, have advanced to the clinical setting. Notably, CRLX-101 and CALAA-01 have both entered Phase II trials,159-162 highlighting the promise of using host-guest supramolecular recognition in the clinical treatment of disease. It is noted that these approaches do not feature stimuli-responsive properties in tuning drug release. As such, including stimuli-responsive functionality into the design of host-guest interactions offers a new approach to facilitate more precise or controlled therapy, thereby addressing the concerns that arise from dose-limiting side-effects, poor bioavailability, or limited biodistribution to sites of need. With an appreciation of the governing design parameters of host-guest chemistry, and the suite of different macrocycles available for inclusion in a design, features such as recognition affinity or complex equilibrium kinetics may be tuned to alter recognition of these systems. One common motif that emerges in exemplifying this use is that of a macrocycle-capped porous particle. Depending on the complexity of engineering such an approach, one might envision a multi-stimuli cascade as a route to further improve selectivity for the particular site of action of a drug. The use of host-guest fusion in creating self-assembling amphiphiles offers another route to integrate stimuli-responsive function into liposomes and micelles, among the most commonly used forms of drug carriers. Other common motifs include hydrogels, which could be viewed in the context of injectable drug-releasing depots, which may be engineered to respond to particular stimuli by making an encapsulated drug locally or systemically bioavailable 163. A final motif entails the use of stable complexes between a macrocyclic host and either a drug or guest-modified prodrug. Depending on the affinity and dynamics of such a complex, this approach may be viewed in its application as similar to standard small molecule pharmaceutical practice, with the drug becoming activated by complex dissociation or rupture of a pendant guest modifier. In all, the design space for stimuli-responsive host-guest chemistry is vast and there is great promise in using technologies of this type to design new therapies.
References
- 1.Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS. Failure of Investigational Drugs in Late-Stage Clinical Development and Publication of Trial Results. JAMA Intern Med. 2016;176:1826–1833. doi: 10.1001/jamainternmed.2016.6008. [DOI] [PubMed] [Google Scholar]
- 2.Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp Clin Trials Commun. 2018;11:156–164. doi: 10.1016/j.conctc.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenton OS, Olafson KN, Pillai PS, Mitchell MJ, Langer R. Advances in Biomaterials for Drug Delivery. Adv Mater; 2018. e1705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tibbitt MW, Dahlman JE, Langer R. Emerging Frontiers in Drug Delivery. J Am Chem Soc. 2016;138:704–717. doi: 10.1021/jacs.5b09974. [DOI] [PubMed] [Google Scholar]
- 5.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 6.Meng F, Zhong Z, Feijen J. Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules. 2009;10:197–209. doi: 10.1021/bm801127d. [DOI] [PubMed] [Google Scholar]
- 7.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Nalluri SKM, Stoddart JF. Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes. Chem Soc Rev. 2017;46:2459–2478. doi: 10.1039/c7cs00185a. [DOI] [PubMed] [Google Scholar]
- 9.Webber MJ, Langer R. Drug delivery by supramolecular design. Chem Soc Rev. 2017;46:6600–6620. doi: 10.1039/c7cs00391a. [DOI] [PubMed] [Google Scholar]
- 10.Dong R, Zhou Y, Huang X, Zhu X, Lu Y, Shen J. Functional Supramolecular Polymers for Biomedical Applications. Adv Mater. 2014;27:498–526. doi: 10.1002/adma.201402975. [DOI] [PubMed] [Google Scholar]
- 11.Webber MJ, Appel EA, Meijer EW, Langer R. Supramolecular biomaterials. Nat Mater. 2016;15:13–26. doi: 10.1038/nmat4474. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Yu G, Huang F. Supramolecular chemotherapy based on host-guest molecular recognition: a novel strategy in the battle against cancer with a bright future. Chem Soc Rev. 2017;46:7021–7053. doi: 10.1039/c6cs00898d. [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Shangguan L, Shi B, Yu G, Huang F. Recent progress in macrocyclic amphiphiles and macrocyclic host-based supra-amphiphiles [Internet] Materials Chemistry Frontiers; 2018. pp. 2152-2174. doi:10.1039/c8qm00314a. [Google Scholar]
- 14.Yu G, Jie K, Huang F. Supramolecular Amphiphiles Based on Host-Guest Molecular Recognition Motifs. Chem Rev. 2015;115:7240–7303. doi: 10.1021/cr5005315. [DOI] [PubMed] [Google Scholar]
- 15.Yudin AK. Macrocycles: lessons from the distant past, recent developments, and future directions. Chem Sci. 2015;6:30–49. doi: 10.1039/c4sc03089c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng B, Wang F, Dong S, Huang F. Supramolecular polymers constructed by crown ether-based molecular recognition. Chem Soc Rev. 2012;41:1621–1636. doi: 10.1039/c1cs15220c. [DOI] [PubMed] [Google Scholar]
- 17.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 18.Guimaraes PPG, Tan M, Tammela T, Wu K, Chung A, Oberli M. et al. Potent in vivo lung cancer Wnt signaling inhibition via cyclodextrin-LGK974 inclusion complexes. J Control Release. 2018;290:75–87. doi: 10.1016/j.jconrel.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewster ME, Hora MS, Simpkins JW, Bodor N. Use of 2-hydroxypropyl-beta-cyclodextrin as a solubilizing and stabilizing excipient for protein drugs. Pharm Res. 1991;8:792–795. doi: 10.1023/a:1015870521744. [DOI] [PubMed] [Google Scholar]
- 20.Webber MJ, Appel EA, Vinciguerra B, Cortinas AB, Thapa LS, Jhunjhunwala S. et al. Supramolecular PEGylation of biopharmaceuticals. Proc Natl Acad Sci U S A. 2016;113:14189–14194. doi: 10.1073/pnas.1616639113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantooth SM, Munoz-Robles BG, Webber MJ. Dynamic Hydrogels from Host-Guest Supramolecular Interactions. Macromol Biosci. 2019;19:e1800281. doi: 10.1002/mabi.201800281. [DOI] [PubMed] [Google Scholar]
- 22.Jeon WS, Moon K, Park SH, Chun H, Ko YH, Lee JY. et al. Complexation of ferrocene derivatives by the cucurbit[7]uril host: a comparative study of the cucurbituril and cyclodextrin host families. J Am Chem Soc. 2005;127:12984–12989. doi: 10.1021/ja052912c. [DOI] [PubMed] [Google Scholar]
- 23.Zou L, Braegelman AS, Webber MJ. Dynamic Supramolecular Hydrogels Spanning an Unprecedented Range of Host-Guest Affinity. ACS Appl Mater Interfaces. 2019 doi: 10.1021/acsami.8b22151. doi:10.1021/acsami.8b22151. [DOI] [PubMed] [Google Scholar]
- 24.Forrey C, Douglas JF, Gilson MK. The Fundamental Role of Flexibility on the Strength of Molecular Binding. Soft Matter. 2012;8:6385–6392. doi: 10.1039/C2SM25160D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pluth MD, Raymond KN. Reversible guest exchange mechanisms in supramolecular host-guest assemblies. Chem Soc Rev. 2007;36:161–171. doi: 10.1039/b603168b. [DOI] [PubMed] [Google Scholar]
- 26.Chodera JD, Mobley DL. Entropy-enthalpy compensation: role and ramifications in biomolecular ligand recognition and design. Annu Rev Biophys. 2013;42:121–142. doi: 10.1146/annurev-biophys-083012-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rekharsky MV, Mori T, Yang C, Ko YH, Selvapalam N, Kim H. et al. A synthetic host-guest system achieves avidin-biotin affinity by overcoming enthalpy-entropy compensation. Proc Natl Acad Sci U S A. 2007;104:20737–20742. doi: 10.1073/pnas.0706407105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malaspina T, Fileti E, Chaban VV. Peculiar Aqueous Solubility Trend in Cucurbiturils Unraveled by Atomistic Simulations. J Phys Chem B. 2016;120:7511–7516. doi: 10.1021/acs.jpcb.6b05425. [DOI] [PubMed] [Google Scholar]
- 29.Biedermann F, Nau WM, Schneider H-J. The hydrophobic effect revisited-studies with supramolecular complexes imply high-energy water as a noncovalent driving force. Angew Chem Int Ed Engl. 2014;53:11158–11171. doi: 10.1002/anie.201310958. [DOI] [PubMed] [Google Scholar]
- 30.Hettiarachchi G, Nguyen D, Wu J, Lucas D, Ma D, Isaacs L. et al. Toxicology and drug delivery by cucurbit[n]uril type molecular containers. PLoS One. 2010;5:e10514. doi: 10.1371/journal.pone.0010514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oun R, Floriano RS, Isaacs L, Rowan EG, Wheate NJ. The ex vivo neurotoxic, myotoxic and cardiotoxic activity of cucurbituril-based macrocyclic drug delivery vehicles. Toxicol Res. 2014;3:447–455. doi: 10.1039/C4TX00082J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stella VJ, Rao VM, Zannou EA, Zia V V. Mechanisms of drug release from cyclodextrin complexes. Adv Drug Deliv Rev. 1999;36:3–16. doi: 10.1016/s0169-409x(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 33.Brachvogel R-C, Hampel F, von Delius M. Self-assembly of dynamic orthoester cryptates. Nat Commun. 2015;6:7129. doi: 10.1038/ncomms8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, Yan X, Huang F, Niu Z, Gibson HW. Stimuli-responsive host-guest systems based on the recognition of cryptands by organic guests. Acc Chem Res. 2014;47:1995–2005. doi: 10.1021/ar500046r. [DOI] [PubMed] [Google Scholar]
- 35.Lee S-F, Zhu X-M, Wang Y-XJ, Xuan S-H, You Q, Chan W-H. et al. Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl Mater Interfaces. 2013;5:1566–1574. doi: 10.1021/am4004705. [DOI] [PubMed] [Google Scholar]
- 36.Morrison PWJ, Porfiryeva NN, Chahal S, Salakhov IA, Lacourt C, Semina II. et al. Crown Ethers: Novel Permeability Enhancers for Ocular Drug Delivery? Mol Pharm. 2017;14:3528–3538. doi: 10.1021/acs.molpharmaceut.7b00556. [DOI] [PubMed] [Google Scholar]
- 37.Vicente M, Smith K. Syntheses and Functionalizations of Porphyrin Macrocycles. Curr Org Synth. 2014;11:3–28. doi: 10.2174/15701794113106660083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothemund P. FORMATION OF PORPHYRINS FROM PYRROLE AND ALDEHYDES. J Am Chem Soc. 1935;57:2010–2011. [Google Scholar]
- 39.Arsenault GP, Bullock E, MacDonald SF. Pyrromethanes and Porphyrins Therefrom1. J Am Chem Soc. 1960;82:4384–4389. [Google Scholar]
- 40.Woodward RB. Totalsynthese des Chlorophylls. Angew Chem Int Ed Engl. 1960;72:651–662. [Google Scholar]
- 41.Johnson AW, Kay IT. 468. The formation of porphyrins by the cyclisation of bilenes. J Chem Soc; 1961. p. 2418. [Google Scholar]
- 42.Smith KM, Minnetian OM. Novel porphyrins from copper(II)-mediated cyclizations of 1',8'-dimethyl-A,C-biladiene salts: mechanism of the cyclization reaction. J Org Chem. 1985;50:2073–2080. [Google Scholar]
- 43.Ma D, Liu Z-H, Zheng Q-Q, Zhou X-Y, Zhang Y, Shi Y-F. et al. Star-shaped polymer consisting of a porphyrin core and poly(L-lysine) dendron arms: synthesis, drug delivery, and in vitro chemo/photodynamic therapy. Macromol Rapid Commun. 2013;34:548–552. doi: 10.1002/marc.201200742. [DOI] [PubMed] [Google Scholar]
- 44.Dondi R, Yaghini E, Tewari KM, Wang L, Giuntini F, Loizidou M. et al. Flexible synthesis of cationic peptide-porphyrin derivatives for light-triggered drug delivery and photodynamic therapy. Org Biomol Chem. 2016;14:11488–11501. doi: 10.1039/c6ob02135b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kou J, Dou D, Yang L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget; 2017. p. 8. doi:10.18632/oncotarget.20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mauriello-Jimenez C, Croissant J, Maynadier M, Cattoën X, Man MWC, Vergnaud J. et al. Porphyrin-functionalized mesoporous organosilica nanoparticles for two-photon imaging of cancer cells and drug delivery. J Mater Chem B Mater Biol Med. 2015;3:3681–3684. doi: 10.1039/c5tb00315f. [DOI] [PubMed] [Google Scholar]
- 47.Huang H, Song W, Rieffel J, Lovell JF. Emerging applications of porphyrins in photomedicine. Front Phys; 2015. p. 3. doi:10.3389/fphy.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horiuchi H, Sakai A, Akiyama S, Ikeda R, Ito S, Furuya M. et al. Extension of π-system of silylated porphyrin derivative for photodynamic therapy. J Photochem Photobiol A Chem. 2017;339:19–24. [Google Scholar]
- 49.Gutsche CD, -G. Lin L. ChemInform Abstract: Calixarenes. Part 12. The Synthesis of Functionalized Calixarenes. Chemischer Informationsdienst; 1986. p. 17. doi:10.1002/chin.198631207. [Google Scholar]
- 50.Agrawal YK, Pancholi JP, Vyas JM. ChemInform Abstract: Design and Synthesis of Calixarene. ChemInform. 2010;41:no–no. [Google Scholar]
- 51.Baldini L, Casnati A, Sansone F, Ungaro R. Calixarene-based multivalent ligands. Chem Soc Rev. 2007;36:254–266. doi: 10.1039/b603082n. [DOI] [PubMed] [Google Scholar]
- 52.Makha M, Raston CL. Direct synthesis of calixarenes with extended arms: p-phenylcalix[4,5,6,8]arenes and their water-soluble sulfonated derivatives. Tetrahedron Lett. 2001;42:6215–6217. [Google Scholar]
- 53.Ukhatskaya EV, Kurkov SV, Matthews SE, Loftsson T. Encapsulation of drug molecules into calix[n]arene nanobaskets. role of aminocalix[n]arenes in biopharmaceutical field. J Pharm Sci. 2013;102:3485–3512. doi: 10.1002/jps.23681. [DOI] [PubMed] [Google Scholar]
- 54.Abd Hamid S, Hamid SA, Bunnori NM, Adekunle IA, Ali Y. Applications of calixarenes in cancer chemotherapy: facts and perspectives. Drug Des Devel Ther; 2015. p. 2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogoshi T, Yamagishi T-A. New Synthetic Host Pillararenes: Their Synthesis and Application to Supramolecular Materials. Bull Chem Soc Jpn. 2013;86:312–332. [Google Scholar]
- 56.Yang K, Pei Y, Wen J, Pei Z. Recent advances in pillar[n]arenes: synthesis and applications based on host-guest interactions. Chem Commun. 2016;52:9316–9326. doi: 10.1039/c6cc03641d. [DOI] [PubMed] [Google Scholar]
- 57.Ogoshi T, Yamagishi T-A, Nakamoto Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem Rev. 2016;116:7937–8002. doi: 10.1021/acs.chemrev.5b00765. [DOI] [PubMed] [Google Scholar]
- 58.Gidwani B, Vyas A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed Res Int. 2015;2015:1–15. doi: 10.1155/2015/198268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wakao M, Fukase K, Kusumoto S. Chemical synthesis of cyclodextrins by using intramolecular glycosylation. J Org Chem. 2002;67:8182–8190. doi: 10.1021/jo025887r. [DOI] [PubMed] [Google Scholar]
- 60.Crini G. Review: a history of cyclodextrins. Chem Rev. 2014;114:10940–10975. doi: 10.1021/cr500081p. [DOI] [PubMed] [Google Scholar]
- 61.Day A, Arnold AP, Blanch RJ, Snushall B. Controlling factors in the synthesis of cucurbituril and its homologues. J Org Chem. 2001;66:8094–8100. doi: 10.1021/jo015897c. [DOI] [PubMed] [Google Scholar]
- 62.Isaacs L. The Mechanism of Cucurbituril Formation. Isr J Chem. 2011;51:578–591. [Google Scholar]
- 63.Assaf KI, Nau WM. Cucurbiturils: from synthesis to high-affinity binding and catalysis. Chem Soc Rev. 2015;44:394–418. doi: 10.1039/c4cs00273c. [DOI] [PubMed] [Google Scholar]
- 64.Lucas D, Minami T, Iannuzzi G, Cao L, Wittenberg JB, Anzenbacher P Jr. et al. Templated synthesis of glycoluril hexamer and monofunctionalized cucurbit[6]uril derivatives. J Am Chem Soc. 2011;133:17966–17976. doi: 10.1021/ja208229d. [DOI] [PubMed] [Google Scholar]
- 65.Vinciguerra B, Cao L, Cannon JR, Zavalij PY, Fenselau C, Isaacs L. Synthesis and self-assembly processes of monofunctionalized cucurbit[7]uril. J Am Chem Soc. 2012;134:13133–13140. doi: 10.1021/ja3058502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu L. Controlled release from cucurbituril. J Incl Phenom Macrocycl Chem. 2016;87:1–12. [Google Scholar]
- 67.Wheate NJ, Limantoro C. Cucurbit[n]urils as excipients in pharmaceutical dosage forms. Supramol Chem. 2016;28:849–856. [Google Scholar]
- 68.Shetty D, Khedkar JK, Park KM, Kim K. Can we beat the biotin-avidin pair?: cucurbit[7]uril-based ultrahigh affinity host-guest complexes and their applications. Chem Soc Rev. 2015;44:8747–8761. doi: 10.1039/c5cs00631g. [DOI] [PubMed] [Google Scholar]
- 69.Cao L, Śekutor M, Zavalij PY, Mlinarić-Majerski K, Glaser R, Isaacs L. Cucurbit[7]uril⋅guest pair with an attomolar dissociation constant. Angew Chem Int Ed Engl. 2014;53:988–993. doi: 10.1002/anie.201309635. [DOI] [PubMed] [Google Scholar]
- 70.Barrow SJ, Kasera S, Rowland MJ, del Barrio J, Scherman OA. Cucurbituril-Based Molecular Recognition. Chem Rev. 2015;115:12320–12406. doi: 10.1021/acs.chemrev.5b00341. [DOI] [PubMed] [Google Scholar]
- 71.Wu J-R, Yang Y-W. New opportunities in synthetic macrocyclic arenes. Chem Commun. 2019;55:1533–1543. doi: 10.1039/c8cc09374a. [DOI] [PubMed] [Google Scholar]
- 72.Feng H-T, Yuan Y-X, Xiong J-B, Zheng Y-S, Tang BZ. Macrocycles and cages based on tetraphenylethylene with aggregation-induced emission effect. Chem Soc Rev. 2018;47:7452–7476. doi: 10.1039/c8cs00444g. [DOI] [PubMed] [Google Scholar]
- 73.Faiz JA, Heitz V, Sauvage J-P. Design and synthesis of porphyrin-containing catenanes and rotaxanes. Chem Soc Rev. 2009;38:422–442. doi: 10.1039/b710908n. [DOI] [PubMed] [Google Scholar]
- 74.Pairault N, Barat R, Tranoy-Opalinski I, Renoux B, Thomas M, Papot S. Rotaxane-based architectures for biological applications. C R Chim. 2016;19:103–112. [Google Scholar]
- 75.Martinez-Cuezva A, Valero-Moya S, Alajarin M, Berna J. Light-responsive peptide [2]rotaxanes as gatekeepers of mechanised nanocontainers. Chem Commun. 2015;51:14501–14504. doi: 10.1039/c5cc04365d. [DOI] [PubMed] [Google Scholar]
- 76.Barat R, Legigan T, Tranoy-Opalinski I, Renoux B, Péraudeau E, Clarhaut J. et al. A mechanically interlocked molecular system programmed for the delivery of an anticancer drug. Chem Sci. 2015;6:2608–2613. doi: 10.1039/c5sc00648a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Webber MJ. Engineering responsive supramolecular biomaterials: Toward smart therapeutics. Bioeng Transl Med. 2016;1:252–266. doi: 10.1002/btm2.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mekaru H, Lu J, Tamanoi F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv Drug Deliv Rev. 2015;95:40–49. doi: 10.1016/j.addr.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song Y, Li Y, Xu Q, Liu Z. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: advances, challenges, and outlook. Int J Nanomedicine. 2017;12:87–110. doi: 10.2147/IJN.S117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ambrogio MW, Thomas CR, Zhao Y-L, Zink JI, Stoddart JF. Mechanized silica nanoparticles: a new frontier in theranostic nanomedicine. Acc Chem Res. 2011;44:903–913. doi: 10.1021/ar200018x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41:2590–2605. doi: 10.1039/c1cs15246g. [DOI] [PubMed] [Google Scholar]
- 82.Song N, Yang Y-W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Chem Soc Rev. 2015;44:3474–3504. doi: 10.1039/c5cs00243e. [DOI] [PubMed] [Google Scholar]
- 83.Aznar E, Oroval M, Pascual L, Murguía JR, Martínez-Máñez R, Sancenón F. Gated Materials for On-Command Release of Guest Molecules. Chem Rev. 2016;116:561–718. doi: 10.1021/acs.chemrev.5b00456. [DOI] [PubMed] [Google Scholar]
- 84.Lou X-Y, Li Y-P, Yang Y-W. Gated Materials: Installing Macrocyclic Arenes-Based Supramolecular Nanovalves on Porous Nanomaterials for Controlled Cargo Release. Biotechnol J. 2019;14:e1800354. doi: 10.1002/biot.201800354. [DOI] [PubMed] [Google Scholar]
- 85.Zhou Y, Li H, Yang Y-W. Controlled drug delivery systems based on calixarenes [Internet] Chinese Chemical Letters; 2015. pp. 825-828. doi:10.1016/j.cclet.2015.01.038. [Google Scholar]
- 86.Wang X, Yan F, Liu X, Wang P, Shao S, Sun Y. et al. Enhanced drug delivery using sonoactivatable liposomes with membrane-embedded porphyrins. J Control Release. 2018;286:358–368. doi: 10.1016/j.jconrel.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y-X, Zhang Y-M, Liu Y. Photolysis of an amphiphilic assembly by calixarene-induced aggregation. J Am Chem Soc. 2015;137:4543–4549. doi: 10.1021/jacs.5b01566. [DOI] [PubMed] [Google Scholar]
- 88.Li H, Tan L-L, Jia P, Li Q-L, Sun Y-L, Zhang J, Near-infrared light-responsive supramolecular nanovalve based on mesoporous silica-coated gold nanorods [Internet] Chemical Science; 2014. p. 2804. doi:10.1039/c4sc00198b. [Google Scholar]
- 89.Ma N, Wang W-J, Chen S, Wang X-S, Wang X-Q, Wang S-B. et al. Cucurbit[8]uril-mediated supramolecular photoswitching for self-preservation of mesoporous silica nanoparticle delivery system. Chem Commun. 2015;51:12970–12973. doi: 10.1039/c5cc04631a. [DOI] [PubMed] [Google Scholar]
- 90.Zhang H, Liu Z, Zhao Y. Pillararene-based self-assembled amphiphiles. Chem Soc Rev. 2018;47:5491–5528. doi: 10.1039/c8cs00037a. [DOI] [PubMed] [Google Scholar]
- 91.Huang X, Wu S, Ke X, Li X, Du X. Phosphonated Pillar[5]arene-Valved Mesoporous Silica Drug Delivery Systems. ACS Appl Mater Interfaces. 2017;9:19638–19645. doi: 10.1021/acsami.7b04015. [DOI] [PubMed] [Google Scholar]
- 92.Yu G, Yu W, Mao Z, Gao C, Huang F. A pillararene-based ternary drug-delivery system with photocontrolled anticancer drug release. Small. 2015;11:919–925. doi: 10.1002/smll.201402236. [DOI] [PubMed] [Google Scholar]
- 93.Li M, Yan H, Teh C, Korzh V, Zhao Y. NIR-triggered drug release from switchable rotaxane-functionalized silica-covered Au nanorods. Chem Commun. 2014;50:9745–9748. doi: 10.1039/c4cc02966f. [DOI] [PubMed] [Google Scholar]
- 94.Wang D, Wu S. Red-Light-Responsive Supramolecular Valves for Photocontrolled Drug Release from Mesoporous Nanoparticles. Langmuir. 2016;32:632–636. doi: 10.1021/acs.langmuir.5b04399. [DOI] [PubMed] [Google Scholar]
- 95.Králová J, Kejík Z, Bríza T, Poucková P, Král A, Martásek P. et al. Porphyrin-cyclodextrin conjugates as a nanosystem for versatile drug delivery and multimodal cancer therapy. J Med Chem. 2010;53:128–138. doi: 10.1021/jm9007278. [DOI] [PubMed] [Google Scholar]
- 96.Assaf KI, Alnajjar MA, Nau WM. Supramolecular assemblies through host-guest complexation between cucurbiturils and an amphiphilic guest molecule. Chem Commun. 2018;54:1734–1737. doi: 10.1039/c7cc09519h. [DOI] [PubMed] [Google Scholar]
- 97.Song S, Zheng Y-S. Hollow spheres self-assembled by a tetraphenylethylene macrocycle and their transformation to bird nests under ultrasound. Org Lett. 2013;15:820–823. doi: 10.1021/ol3035005. [DOI] [PubMed] [Google Scholar]
- 98.Jia S, Fong W-K, Graham B, Boyd BJ. Photoswitchable Molecules in Long-Wavelength Light-Responsive Drug Delivery: From Molecular Design to Applications. Chem Mater. 2018;30:2873–2887. [Google Scholar]
- 99.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery☆. Adv Drug Deliv Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 100.Santha Moorthy M, Moorthy MS, Bharathiraja S, Manivasagan P, Lee KD, Oh J. Crown ether triad modified core-shell magnetic mesoporous silica nanocarrier for pH-responsive drug delivery and magnetic hyperthermia applications. New J Chem. 2017;41:10935–10947. [Google Scholar]
- 101.Dong S, Luo Y, Yan X, Zheng B, Ding X, Yu Y. et al. A Dual-Responsive Supramolecular Polymer Gel Formed by Crown Ether Based Molecular Recognition. Angew Chem Int Ed. 2011;50:1905–1909. doi: 10.1002/anie.201006999. [DOI] [PubMed] [Google Scholar]
- 102.Bera K, Maiti S, Maity M, Mandal C, Maiti NC. Porphyrin-Gold Nanomaterial for Efficient Drug Delivery to Cancerous Cells. ACS Omega. 2018;3:4602–4619. doi: 10.1021/acsomega.8b00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duan Q, Cao Y, Li Y, Hu X, Xiao T, Lin C. et al. pH-responsive supramolecular vesicles based on water-soluble pillar[6]arene and ferrocene derivative for drug delivery. J Am Chem Soc. 2013;135:10542–10549. doi: 10.1021/ja405014r. [DOI] [PubMed] [Google Scholar]
- 104.Gao M, Han S, Hu Y, Dynes JJ, Liu X, Wang D. A pH-driven molecular shuttle based on rotaxane-bridged periodic mesoporous organosilicas with responsive release of guests. RSC Adv. 2016;6:27922–27932. [Google Scholar]
- 105.Wu M-X, Yang Y-W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv Mater; 2017. p. 29. doi:10.1002/adma.201606134. [DOI] [PubMed] [Google Scholar]
- 106.Lin W, Hu Q, Jiang K, Yang Y, Yang Y, Cui Y. et al. A porphyrin-based metal-organic framework as a pH-responsive drug carrier. J Solid State Chem. 2016;237:307–312. [Google Scholar]
- 107.Duan F, Feng X, Yang X, Sun W, Jin Y, Liu H. et al. A simple and powerful co-delivery system based on pH-responsive metal-organic frameworks for enhanced cancer immunotherapy. Biomaterials. 2017;122:23–33. doi: 10.1016/j.biomaterials.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 108.Xue Y, Guan Y, Zheng A, Xiao H. Amphoteric calix[8]arene-based complex for pH-triggered drug delivery. Colloids Surf B Biointerfaces. 2013;101:55–60. doi: 10.1016/j.colsurfb.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 109.Zhou T, Song N, Xu S-H, Dong B, Yang Y-W. Dual-Responsive Mechanized Mesoporous Silica Nanoparticles Based on Sulfonatocalixarene Supramolecular Switches. Chemphyschem. 2016;17:1840–1845. doi: 10.1002/cphc.201500726. [DOI] [PubMed] [Google Scholar]
- 110.Yang K, Chang Y, Wen J, Lu Y, Pei Y, Cao S. et al. Supramolecular Vesicles Based on Complex of Trp-Modified Pillar[5]arene and Galactose Derivative for Synergistic and Targeted Drug Delivery. Chem Mater. 2016;28:1990–1993. [Google Scholar]
- 111.Wu M-X, Gao J, Wang F, Yang J, Song N, Jin X. et al. Multistimuli Responsive Core-Shell Nanoplatform Constructed from Fe O @MOF Equipped with Pillar[6]arene Nanovalves. Small. 2018;14:e1704440. doi: 10.1002/smll.201704440. [DOI] [PubMed] [Google Scholar]
- 112.Wu M-X, Yan H-J, Gao J, Cheng Y, Yang J, Wu J-R. et al. Multifunctional Supramolecular Materials Constructed from Polypyrrole@UiO-66 Nanohybrids and Pillararene Nanovalves for Targeted Chemophotothermal Therapy. ACS Appl Mater Interfaces. 2018;10:34655–34663. doi: 10.1021/acsami.8b13758. [DOI] [PubMed] [Google Scholar]
- 113.He H, Chen S, Zhou J, Dou Y, Song L, Che L. et al. Cyclodextrin-derived pH-responsive nanoparticles for delivery of paclitaxel. Biomaterials. 2013;34:5344–5358. doi: 10.1016/j.biomaterials.2013.03.068. [DOI] [PubMed] [Google Scholar]
- 114.Shi Q, Zhang L, Liu M, Zhang X, Zhang X, Xu X. et al. Reversion of multidrug resistance by a pH-responsive cyclodextrin-derived nanomedicine in drug resistant cancer cells. Biomaterials. 2015;67:169–182. doi: 10.1016/j.biomaterials.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Z, Ding J, Chen X, Xiao C, He C, Zhuang X. et al. Intracellular pH-sensitive supramolecular amphiphiles based on host-guest recognition between benzimidazole and β-cyclodextrin as potential drug delivery vehicles. Polym Chem. 2013;4:3265. [Google Scholar]
- 116.Cao L, Hettiarachchi G, Briken V, Isaacs L. Cucurbit[7]uril containers for targeted delivery of oxaliplatin to cancer cells. Angew Chem Int Ed Engl. 2013;52:12033–12037. doi: 10.1002/anie.201305061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jeon YJ, Kim S-Y, Ko YH, Sakamoto S, Yamaguchi K, Kim K. Novel molecular drug carrier: encapsulation of oxaliplatin in cucurbit[7]uril and its effects on stability and reactivity of the drug. Org Biomol Chem. 2005;3:2122–2125. doi: 10.1039/b504487a. [DOI] [PubMed] [Google Scholar]
- 118.Wheate NJ, Buck DP, Day AI, Collins JG. Cucurbit[n]uril binding of platinum anticancer complexes. Dalton Trans; 2006. pp. 451–458. [DOI] [PubMed] [Google Scholar]
- 119.Angelos S, Yang Y-W, Patel K, Stoddart JF, Zink JI. pH-responsive supramolecular nanovalves based on cucurbit[6]uril pseudorotaxanes. Angew Chem Int Ed Engl. 2008;47:2222–2226. doi: 10.1002/anie.200705211. [DOI] [PubMed] [Google Scholar]
- 120.Angelos S, Khashab NM, Yang Y-W, Trabolsi A, Khatib HA, Stoddart JF. et al. pH clock-operated mechanized nanoparticles. J Am Chem Soc. 2009;131:12912–12914. doi: 10.1021/ja9010157. [DOI] [PubMed] [Google Scholar]
- 121.Angelos S, Yang Y-W, Khashab NM, Stoddart JF, Zink JI. Dual-controlled nanoparticles exhibiting AND logic. J Am Chem Soc. 2009;131:11344–11346. doi: 10.1021/ja9042752. [DOI] [PubMed] [Google Scholar]
- 122.Guha S, Shaw GK, Mitcham TM, Bouchard RR, Smith BD. Croconaine rotaxane for acid activated photothermal heating and ratiometric photoacoustic imaging of acidic pH. Chem Commun. 2016;52:120–123. doi: 10.1039/c5cc08317f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun Y-L, Zhou Y, Li Q-L, Yang Y-W. Enzyme-responsive supramolecular nanovalves crafted by mesoporous silica nanoparticles and choline-sulfonatocalix[4]arene [2]pseudorotaxanes for controlled cargo release. Chem Commun. 2013;49:9033–9035. doi: 10.1039/c3cc45216f. [DOI] [PubMed] [Google Scholar]
- 124.Guo D-S, Wang K, Wang Y-X, Liu Y. Cholinesterase-responsive supramolecular vesicle. J Am Chem Soc. 2012;134:10244–10250. doi: 10.1021/ja303280r. [DOI] [PubMed] [Google Scholar]
- 125.Dan Z, Cao H, He X, Zeng L, Zou L, Shen Q. et al. Biological stimuli-responsive cyclodextrin-based host-guest nanosystems for cancer therapy. Int J Pharm. 2015;483:63–68. doi: 10.1016/j.ijpharm.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 126.Namgung R, Mi Lee Y, Kim J, Jang Y, Lee B-H, Kim I-S. et al. Poly-cyclodextrin and poly-paclitaxel nano-assembly for anticancer therapy. Nat Commun. 2014;5:3702. doi: 10.1038/ncomms4702. [DOI] [PubMed] [Google Scholar]
- 127.Zhang J, Ma PX. Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev. 2013;65:1215–1233. doi: 10.1016/j.addr.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng Y-J, Luo G-F, Zhu J-Y, Xu X-D, Zeng X, Cheng D-B. et al. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl Mater Interfaces. 2015;7:9078–9087. doi: 10.1021/acsami.5b00752. [DOI] [PubMed] [Google Scholar]
- 129.Gayam SR, Venkatesan P, Sung Y-M, Sung S-Y, Hu S-H, Hsu H-Y. et al. An NAD(P)H:quinone oxidoreductase 1 (NQO1) enzyme responsive nanocarrier based on mesoporous silica nanoparticles for tumor targeted drug delivery in vitro and in vivo. Nanoscale. 2016;8:12307–12317. doi: 10.1039/c6nr03525f. [DOI] [PubMed] [Google Scholar]
- 130.Patel K, Angelos S, Dichtel WR, Coskun A, Yang Y-W, Zink JI. et al. Enzyme-responsive snap-top covered silica nanocontainers. J Am Chem Soc. 2008;130:2382–2383. doi: 10.1021/ja0772086. [DOI] [PubMed] [Google Scholar]
- 131.Liu F, Zhang Y, Pan X, Xu L, Xue Y, Zhang W. Doxorubicin-loaded redox-responsive amphiphilic dendritic porphyrin conjugates for chemotherapy and photodynamic therapy. RSC Adv. 2016;6:57552–57562. [Google Scholar]
- 132.Li D, Bu Y, Zhang L, Wang X, Yang Y, Zhuang Y. et al. Facile Construction of pH- and Redox-Responsive Micelles from a Biodegradable Poly(β-hydroxyl amine) for Drug Delivery. Biomacromolecules. 2016;17:291–300. doi: 10.1021/acs.biomac.5b01394. [DOI] [PubMed] [Google Scholar]
- 133.Zheng X, Li Z, Chen L, Xie Z, Jing X. Self-Assembly of Porphyrin-Paclitaxel Conjugates Into Nanomedicines: Enhanced Cytotoxicity due to Endosomal Escape. Chem Asian J. 2016;11:1780–1784. doi: 10.1002/asia.201600423. [DOI] [PubMed] [Google Scholar]
- 134.Xu X-D, Zhao L, Qu Q, Wang J-G, Shi H, Zhao Y. Imaging-Guided Drug Release from Glutathione-Responsive Supramolecular Porphysome Nanovesicles. ACS Appl Mater Interfaces. 2015;7:17371–17380. doi: 10.1021/acsami.5b06026. [DOI] [PubMed] [Google Scholar]
- 135.Staegemann MH, Gräfe S, Gitter B, Achazi K, Quaas E, Haag R. et al. Hyperbranched Polyglycerol Loaded with (Zinc-)Porphyrins: Photosensitizer Release Under Reductive and Acidic Conditions for Improved Photodynamic Therapy. Biomacromolecules. 2018;19:222–238. doi: 10.1021/acs.biomac.7b01485. [DOI] [PubMed] [Google Scholar]
- 136.Yu G, Yu W, Shao L, Zhang Z, Chi X, Mao Z. et al. Fabrication of a Targeted Drug Delivery System from a Pillar[5]arene-Based Supramolecular Diblock Copolymeric Amphiphile for Effective Cancer Therapy. Adv Funct Mater. 2016;26:8999–9008. [Google Scholar]
- 137.Yu G, Zhao R, Wu D, Zhang F, Shao L, Zhou J. et al. Pillar[5]arene-based amphiphilic supramolecular brush copolymer: fabrication, controllable self-assembly and application in self-imaging targeted drug delivery. Polym Chem. 2016;7:6178–6188. doi: 10.1039/C6PY01402J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peng L, Feng A, Zhang H, Wang H, Jian C, Liu B. et al. Voltage-responsive micelles based on the assembly of two biocompatible homopolymers. Polym Chem. 2014;5:1751–1759. [Google Scholar]
- 139.Wu X, Li Y, Lin C, Hu X-Y, Wang L. GSH- and pH-responsive drug delivery system constructed by water-soluble pillar[5]arene and lysine derivative for controllable drug release. Chem Commun. 2015;51:6832–6835. doi: 10.1039/c5cc01393c. [DOI] [PubMed] [Google Scholar]
- 140.Chang Y, Yang K, Wei P, Huang S, Pei Y, Zhao W. et al. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: a redox-responsive system for drug/siRNA co-delivery. Angew Chem Int Ed Engl. 2014;53:13126–13130. doi: 10.1002/anie.201407272. [DOI] [PubMed] [Google Scholar]
- 141.Peng L, Liu S, Feng A, Yuan J. Polymeric Nanocarriers Based on Cyclodextrins for Drug Delivery: Host-Guest Interaction as Stimuli Responsive Linker. Mol Pharm. 2017;14:2475–2486. doi: 10.1021/acs.molpharmaceut.7b00160. [DOI] [PubMed] [Google Scholar]
- 142.Luo Z, Cai K, Hu Y, Li J, Ding X, Zhang B. et al. Redox-responsive molecular nanoreservoirs for controlled intracellular anticancer drug delivery based on magnetic nanoparticles. Adv Mater. 2012;24:431–435. doi: 10.1002/adma.201103458. [DOI] [PubMed] [Google Scholar]
- 143.Zuo C, Dai X, Zhao S, Liu X, Ding S, Ma L. et al. Fabrication of Dual-Redox Responsive Supramolecular Copolymers Using a Reducible β-Cyclodextran-Ferrocene Double-Head Unit. ACS Macro Lett. 2016;5:873–878. doi: 10.1021/acsmacrolett.6b00450. [DOI] [PubMed] [Google Scholar]
- 144.Ping Y, Hu Q, Tang G, Li J. FGFR-targeted gene delivery mediated by supramolecular assembly between β-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials. 2013;34:6482–6494. doi: 10.1016/j.biomaterials.2013.03.071. [DOI] [PubMed] [Google Scholar]
- 145.Qu H, Yang L, Yu J, Dong T, Rong M, Zhang J. et al. A redox responsive controlled release system using mesoporous silica nanoparticles capped with Au nanoparticles. RSC Adv. 2017;7:35704–35710. [Google Scholar]
- 146.Li Q-L, Xu S-H, Zhou H, Wang X, Dong B, Gao H. et al. pH and Glutathione Dual-Responsive Dynamic Cross-Linked Supramolecular Network on Mesoporous Silica Nanoparticles for Controlled Anticancer Drug Release. ACS Appl Mater Interfaces. 2015;7:28656–28664. doi: 10.1021/acsami.5b10534. [DOI] [PubMed] [Google Scholar]
- 147.Peng L, Feng A, Huo M, Yuan J. Ferrocene-based supramolecular structures and their applications in electrochemical responsive systems. Chem Commun. 2014;50:13005–13014. doi: 10.1039/c4cc05192k. [DOI] [PubMed] [Google Scholar]
- 148.Kaifer AE, Li W, Yi S. Cucurbiturils as Versatile Receptors for Redox Active Substrates. Isr J Chem. 2011;51:496–505. [Google Scholar]
- 149.Nguyen TD, Liu Y, Saha S, Leung KC-F, Stoddart JF, Zink JI. Design and optimization of molecular nanovalves based on redox-switchable bistable rotaxanes. J Am Chem Soc. 2007;129:626–634. doi: 10.1021/ja065485r. [DOI] [PubMed] [Google Scholar]
- 150.Liu X-W, Zhu S, Wu S-R, Wang P, Han G-Z. Response behavior of ion-sensitive hydrogel based on crown ether. Colloids Surf A Physicochem Eng Asp. 2013;417:140–145. [Google Scholar]
- 151.Liu J, Du X, Zhang X. Enzyme-inspired controlled release of cucurbit[7]uril nanovalves by using magnetic mesoporous silica. Chemistry. 2011;17:810–815. doi: 10.1002/chem.201002899. [DOI] [PubMed] [Google Scholar]
- 152.Zhou Y, Tan L-L, Li Q-L, Qiu X-L, Qi A-D, Tao Y. et al. Acetylcholine-triggered cargo release from supramolecular nanovalves based on different macrocyclic receptors. Chemistry. 2014;20:2998–3004. doi: 10.1002/chem.201304864. [DOI] [PubMed] [Google Scholar]
- 153.Tokumura T, Nanba M, Tsushima Y, Tatsuishi K, Kayano M, Machida Y. et al. Enhancement of bioavailability of cinnarizine from its beta-cyclodextrin complex on oral administration with DL-phenylalanine as a competing agent. J Pharm Sci. 1986;75:391–394. doi: 10.1002/jps.2600750415. [DOI] [PubMed] [Google Scholar]
- 154.Tokumura T, Tsushima Y, Kayano M, Machida Y, Nagai T. Enhancement of bioavailability of cinnarizine from its beta-cyclodextrin complex on oral administration with DL-phenylalanine as a competing agent. J Pharm Sci. 1985;74:496–497. doi: 10.1002/jps.2600740428. [DOI] [PubMed] [Google Scholar]
- 155.Kashapov RR, Mamedov VA, Zhukova NA, Kadirov MK, Nizameev IR, Zakharova LY. et al. Controlling the binding of hydrophobic drugs with supramolecular assemblies of β-cyclodextrin. Colloids Surf A Physicochem Eng Asp. 2017;527:55–62. [Google Scholar]
- 156.Ma M, Xu S, Xing P, Li S, Chu X, Hao A. A multistimuli-responsive supramolecular vesicle constructed by cyclodextrins and tyrosine. Colloid Polym Sci. 2014;293:891–900. [Google Scholar]
- 157.Kim C, Agasti SS, Zhu Z, Isaacs L, Rotello VM. Recognition-mediated activation of therapeutic gold nanoparticles inside living cells. Nat Chem. 2010;2:962–966. doi: 10.1038/nchem.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Leung KC-F, -F. Leung KC, Nguyen TD, Fraser Stoddart J, Zink JI. Supramolecular Nanovalves Controlled by Proton Abstraction and Competitive Binding. Chem Mater. 2006;18:5919–5928. [Google Scholar]
- 159.Clark AJ, Wiley DT, Zuckerman JE, Webster P, Chao J, Lin J, CRLX101 nanoparticles localize in human tumors and not in adjacent, nonneoplastic tissue after intravenous dosing [Internet] Proceedings of the National Academy of Sciences; 2016. pp. 3850-3854. doi:10.1073/pnas.1603018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Voss MH, Hussain A, Vogelzang N, Lee JL, Keam B, Rha SY. et al. A randomized phase II trial of CRLX101 in combination with bevacizumab versus standard of care in patients with advanced renal cell carcinoma. Ann Oncol. 2017;28:2754–2760. doi: 10.1093/annonc/mdx493. [DOI] [PubMed] [Google Scholar]
- 161.Zuckerman JE, Gritli I, Tolcher A, Heidel JD, Lim D, Morgan R. et al. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc Natl Acad Sci U S A. 2014;111:11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee S-S. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol Ther Nucleic Acids. 2017;8:132–143. doi: 10.1016/j.omtn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sahoo JK, VandenBerg MA, Webber MJ. Injectable network biomaterials via molecular or colloidal self-assembly. Adv Drug Deliv Rev. 2018;127:185–207. doi: 10.1016/j.addr.2017.11.005. [DOI] [PubMed] [Google Scholar]