Abstract
Controlled structure, tunable porosity, and readily chemical functionalizability make metal-organic frameworks (MOFs) a powerful biomedical tool. Nanoscale MOF particles have been increasingly studied as drug carriers, bioimaging agents, and therapeutic agents due to their excellent physiochemical properties. In this review, we start with MOF as a nanocarrier for drug delivery, covering therapeutic MOF agents followed by a comprehensive discussion of surface bioengineering of MOF for improved biostability, biocompatibility, and targeted delivery. Finally, we detail the challenges and prospects of the future of MOF research for biomedical applications.
Keywords: Metal-organic frameworks, drug delivery, photodynamic therapy, surface engineering
Introduction
Metal-organic frameworks (MOFs), assembled from metal ions and organic linkers via coordination chemistry, have wide potentials in catalysis 1-5, energy 6-8, and biomedical applications 9, 10. As a new type of porous crystalline material, MOF building blocks can themselves be functional, which is different from the other nanomaterials. In particular, the tunable porosity, controlled structure, and readily chemical functionalizability of MOFs make them good examples as nanocarriers in biomedical applications 11. From bulk phase to nanoscale phase, the discovery of abundant applicable properties of MOFs has led to new applications in biomedicine, especially at nanoscale size. During the past few years, preparation of various uniform nanoscale MOFs has provided a significant platform to explore structure-orientated functions of MOFs 12. From nanocarriers to nanocargoes, MOFs have been able to make themselves a functional entity by controlling their assembling units. As a consequence, multifunctional MOFs have been extensively studied via direct synthesis or post-synthesis modification for biomedical applications. With a permanently porous structure, fluorescent dyes, small drug molecules, and even protein can be loaded into MOFs for targeted imaging and delivery by tuning the pore sizes 13. Synergistic therapy is believed to be a promising way to enhance tumor therapy efficacy. On-demand drug delivery, such as immunotherapy by loading immune checkpoint inhibitors, photodynamic therapy by conjugating photosensitizer, and photothermal therapy by combining with photothermal agents, and radio therapy 14-18 has been demonstrated to significantly enhance the therapeutic outcomes.
Recently, efforts have been devoted to demonstrating that nanoscale MOFs have great potential in preclinical applications. The goal of this review is to provide an overview of surface functionalization of MOFs for nanomedicine and cancer therapy. Here, we shall highlight the recent progress of MOF as a theranostic platform, including drug delivery, bioimaging, and smart MOF-based nanomedicine for enhanced tumor therapy. In contrast to other interesting reviews which cover a comprehensive survey of all MOF nanoparticles 9, 10, 19, 20, we highlight the surface modification-based biofunctionalization approaches of nanoscale MOFs. Factors that affect the drug delivery in terms of loading efficiency and stimulus-responsive release of the drugs will be discussed. In particular, the challenges and perspectives of MOFs to realize targeted delivery, enhanced therapeutics, and final clinical translation will also be discussed.
MOF loading with small molecules and proteins
Although various types of MOFs have been reported, MOFs that have nanoscale size showed significant potential in tumor therapy applications 16, 21-24. The most popular MOF therapeutic agents are Zr-based MOF series, porphyrinic MOF series, zeolitic imidazolate frameworks (ZIF) series, and Fe-based MOF series which typically have excellent aqueous stability. Merits of MOF can be concluded as follows: (1) Permanent porous crystal structure. Compared with traditional inorganic colloidal nanoparticles which usually carry cargo via covalent or noncovalent surface conjugation, MOFs have a much higher cargo loading efficiency due to their porous structure. In addition, cargo loading can be realized directly either through a one-pot synthesis or post-synthesis diffusion. (2) Tunable size of the pores. The framework originates from the coordination of building units metal ions and organic linkers. The length of the organic linker and the way of coordination determine the size of the pore. Basically, the longer the linker, the larger the size of the pore. The loading cargo can range from small molecules to proteins. (3) High multifunctional efficiency. With a minimized functional units and short processing steps, MOFs can realize much higher functional efficiency than other traditional nanomaterials.
Due to their facile production at low cost, MOFs are attracting many researchers to explore their novel biochemical properties for nanomedical applications 25. Typically, Zr-based MOF nanoparticles can be obtained by mixing a certain ratio of Zr source and organic linker in DMF and incubated for several hours at slightly elevated temperature 22. Compared with the synthesis of traditional inorganic colloidal nanoparticles, which requires hydrophobic organic solvents and high temperature to achieve good quality 26-29, the preparation of nanoscale MOFs usually does not need ultrahigh temperature or tedious organic synthesis. With this benefit of preparation, one can easily make various MOF nanoparticles for further biochemical studies.
Early biomedical studies of MOF mainly focused on drug delivery using MOF as a carrier 13. Drug delivery efficiency is a key factor for improving therapeutic effects 30. Most drug molecules are hydrophobic and cannot be delivered to the physiological environment directly. Conventionally, bioconjugation of the hydrophobic drugs to inorganic nanomaterials was studied as a major way for targeted delivery 31-34. Nanocarriers such as polymer micelles 35-37 and liposomes 38-41, which have a higher delivery efficiency than inorganic bioconjugation techniques, were also developed for drug delivery. Both nanomaterial-based bioconjugation and liposome carriers rely on enhanced permeability and retention effects to deliver drug molecules to the target tissue 42-44. For example, common organic linkers such as carboxylic acid, amine, and thiol have been applied to modify the surface of inorganic colloidal nanoparticles for further surface engineering through 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide hydrochloride (EDC)/ N-hydroxysuccinimide (NHS), NHS ester, and thiol-ene reactions. These crosslinkers provided very convenient platforms for conjugation of small molecules, polymers, peptides, enzymes, and proteins. Hollow liposome with membrane structure similar to cells with relative higher loading ability than inorganic nanoparticle conjugation is a very nice carrier. However, the stability of inorganic nanomaterials and liposomes is a very significant obstacle limiting the therapeutic efficacy. Poor colloidal stability of inorganic nanomaterials with a large size typically creates serious aggregation under physiological conditions and mostly accumulated in lung and liver thus lowering delivery efficiency. The phosphor lipid structure of liposomes with relatively high loading capability also have low physiological stability and can be easily diffused to another cell with similar membrane structure during circulation. So, MOF was believed to be a promising drug carrier when nanoscale MOF appeared.
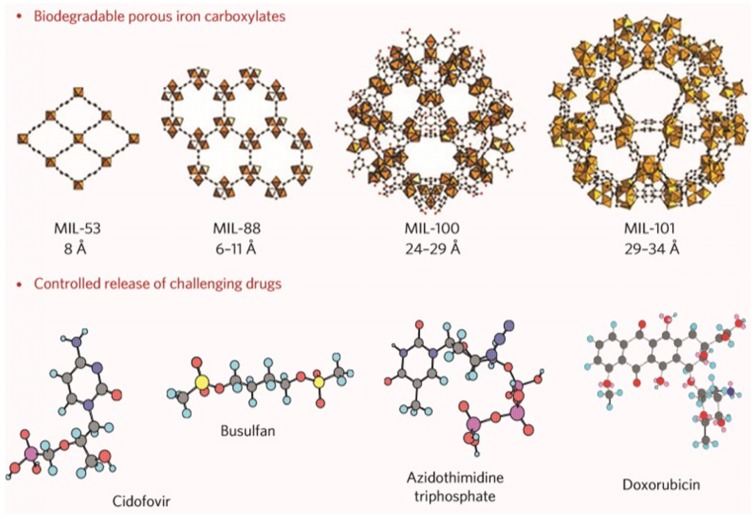
Previously, iron carboxylate MOFs have been demonstrated as biocompatible, degradable, and flexible drug carriers that can deliver various drugs that are not easily loaded using existing nanocarriers 13, 45, 46. As shown in Figure 1, the flexibility of iron carboxylate MOFs offered the opportunity to encapsulate not only the drugs from small molecules to relatively big molecules such as doxorubicin, but also the drugs from hydrophobic to hydrophilic. The biocompatibility and degradability of iron carboxylate MOFs solved the side effect issues that most other nanocarriers have. Besides single drug delivery, MOFs also provide a platform for co-delivery to enhance therapeutic efficacy through a synergistic effect 30, 47. Due to its porous structure, cargos such as small drug molecules and fluorescent dyes can be loaded into MOF structure on one hand. On the other hand, surface conjugation provides another opportunity to carry cargos of interest for a synergistic effect. For example, cisplatin prodrug and siRNA were co-loaded to an Universitetet i Oslo (UiO) MOF nanoparticle. A 12 wt % loading capability of cisplatin prodrug was achieved. SiRNA was bound to the surface of UiO MOF nanoparticles through multiple coordination between the phosphate backbone of SiRNA and Zr sites at the surface of MOF. This resulting co-delivery of cisplatin prodrug and siRNA significantly enhanced the in vitro chemotherapeutic efficacy.
Figure 1.
Porous iron carboxylate MOFs for dry delivery. Reprinted with permission from ref. 13. Copyright (2010) Nature Publishing Group.
Drug loading via physical adsorption by immersing the prepared MOF nanocarriers into cargo-containing solutions typically apply to the case when the size of cargo is smaller than the size of the pore of MOF nanoparticles. In other words, the size of pore determines whether the guest molecule can gain access to the pore of the MOF or not. Basically, the pore size of MOF and the size of the loading molecule have to be known. Physical adsorption on the surface of MOF may be obtained when the size of loading molecule is bigger than the pore size of MOF. To solve this size-dependent loading limitation, one-pot synthesis of cargo-loaded MOF nanoparticles has been developed 48-50. For example, ZIFs have very small pores. Small molecules such as fluorescein and camptothecin cannot be diffused into the pore of ZIF nanoparticles and insert into the ZIF structure. With this facile one-pot synthesis, larger sized guest molecules that can be diffused into MOF can be encapsulated into the inner side of ZIF nanoparticles for efficient target delivery without premature release.
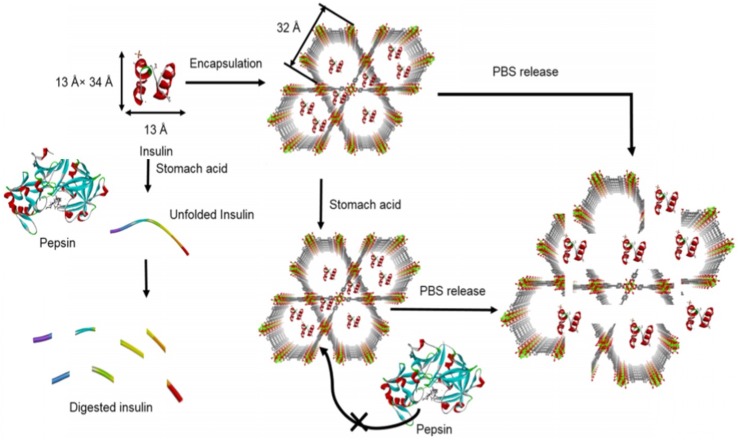
Compared with small molecule delivery, large molecule delivery such as peptides and proteins are encountering more challenges due to their size, surface charge, and component effects. First, direct conjugation of proteins to nanomaterials through covalent bonding typically yields a very low loading efficiency 34, 51-53. Surface area determines the conjugation ability of inorganic colloidal nanoparticles. Second, the poor biological stability of protein-inorganic conjugates also significantly decreases the delivery efficiency. Third, the surface charge of the protein may hinder the cellular internalization. However, these three obstacles can be overcome by using MOF as a protein carrier 54, 55. Physical adsorption by immersing the prepared MOF with large pore size into a guest protein-containing solution can significantly improve the protein loading efficiency. In addition, storing the protein inside the MOF structure can protect the protein from enzymatic degradation during transportation 56, 57. In most cases, enzymes which have a large molecular weight and may decompose loading cargo in normal conditions will not be able to access loading cargo substrate when stored inside the porous MOF carrier. So, typically MOF can not only carry the loading cargos to target sites but also protect them from decomposing during transportation. Most importantly, the intracellular uptake of protein can be controlled by further surface modification of MOF, such as controlling the size and adjusting the surface charge. For example, insulin, the most important protein drug for the treatment of type I diabetes (Figure 2), cannot be directly applied by oral delivery because of extremely poor bioavailability and a low diffusion rate through the mucus layer. In the stomach acid environment, free insulin can be denatured by strong acid and digested by pepsin. However, when using MOF as a carrier for oral delivery of insulin, ultra-stable MOF in stomach acid environment can maintain the integrity of insulin while simultaneously excluding pepsin from getting access to the insulin, thus limiting its proteolysis 56. This MOF carrier for insulin delivery provided insights to guide future protein and enzyme delivery. Further surface modification such as targeting molecule and aptamer may help realize a targeted delivery.
Figure 2.
Free insulin loading to MOF NU-1000 and releasing in the presence of phosphate buffer saline. Pepsin which can digest insulin cannot access to the insulin that was stored in the porous MOF NU-1000 because of the large size of pepsin. Reprinted (adapted) with permission from ref. 56. Copyright (2018) American Chemical Society.
Recently, MOFs with interconnected hierarchical mesoporous channels have been created as enzyme carriers for cell-free synthetic biology 58, 59. Lactate dehydrogenase was encapsulated in the large pores of MOFs to access nicotinamide adenine dinucleotide coenzymes for an in situ coenzyme regeneration. Although enzymes or proteins can be absorbed into the porous MOF and the pore size of MOF can be controlled by adjusting the length of organic linkers, tedious synthesis of organic linkers for MOF has limited the preparation of MOF with large pore size to load proteins with high molecular weight. One-pot synthesis has been demonstrated to be able to encapsulate small molecules to ZIF and its small pore size. It can also be generalized for protein encapsulation 60.
Recently, the Willner group encapsulated both insulin and glucose oxidase into ZIF-8 nanoparticles to construct a smart sense-and-treat carrier 61. In this smart sense-and-treat carrier, both insulin and glucose oxidase were encapsulated into ZIF-8 MOF particles. Insulin which can lower the blood glucose level has been applied to the treatment of type I diabetes. However, the usage of insulin may lead to hypoglycemia when using not properly. With glucose oxidase as a sensor, it can convert glucose to gluconic acid and lower the pH of the local environment. As a pH-sensitive carrier, lower pH can decompose the ZIF-8 carrier, thus releasing the loaded insulin to balance the blood glucose level. On the other hand, the lower blood glucose level balanced by insulin can also balance the pH of the local environment, thus balancing the release of insulin. So, this smart glucose-responsive insulin release has the potential to decrease the risk of hypoglycemia.
Biocatalytic cascades driven by multienzyme-encapsulated MOFs via one-pot synthesis was also reported 62. A model with three different enzymes β-galactosidase, glucose oxidase, and horseradish peroxidase was loaded into ZIF-8 nanoparticles. In the first step, β-galactosidase can convert lactose to glucose to provide a substrate for the second step. Subsequently, glucose oxidase can convert glucose and oxygen to gluconic acid and hydrogen peroxide which is a substrate for the third step. Finally, horseradish peroxidase can take advantage of hydrogen peroxide to convert amplex red to resorufin which is a fluorescent signal. Compared with the mixture of enzymatic catalysts in solution, a significant enhancement of catalytic cascades activity was obtained with this multienzyme-integrated MOF.
MOFs as smart drug carriers
Although the high loading capacity, low cytotoxicity, and effective cell and tissue permeation make MOFs excellent drug carriers, one of the significant issues of MOF as nanocarrier is the premature drug release. To solve this issue, stimuli-responsive drug release strategies have been designed. Typical stimuli such as pH, glutathione (GSH), ATP, and enzyme have been studied for controlled drug release 63-67. An acidic environment in tumor tissue makes pH one of the most widely investigated stimulus for targeted and controlled drug release. ZIF-8 takes advantage of the pH sensitivity to realize a pH-responsive drug delivery. In addition, other “smart” designs to lower the local pH and stimulate the drug release also have been reported 61. For example, glucose oxidase and insulin integrated ZIF-8 can be triggered by glucose for insulin delivery. Nucleic acid with acidic pH sensitivity was modified on the MOF nanoparticles surface as a “lock” to control the drug release 68. In a neutral pH environment, nucleic acids lock the drug inside the porous MOF nanoparticles. When the nucleic acid-modified MOF nanoparticles were transported to an acidic environment, such as pH=5.5, the nucleic acid would open the “lock” and slowly release the drug.
Compared with normal cells, the high intracellular concentration of GSH in cancer cells make GSH the second most important stimulus for controlled drug release 69-71. Disulfide bond-containing molecules with GSH responsive properties have been widely studied not only in polymer-based drug delivery but also in inorganic nanoparticle-based drug release via surface functionalization 65. In the case of MOF nanoparticles, disulfide bond-containing molecules or polymers were modified on the surface to block the premature drug release. Upon transporting to cancer cells with a high GSH level, the disulfide bond would be reduced and release the drug molecules. Using acidic pH and GSH as the stimuli-responsive drug delivery usually achieve targeted release and higher cancer therapy efficacy.
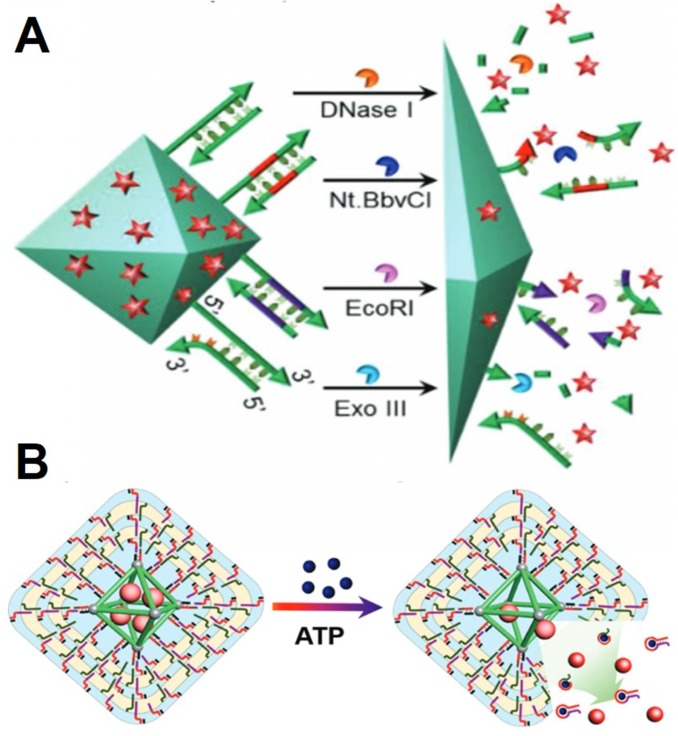
Adenosine triphosphate (ATP) is a very important complex organic chemical that living cells use it to provide energy to drive further many biological processes. Mitochondria, a double-layered membrane organelle in living cells is called an “energy factory” to generate energy. High concentration of ATP in the mitochondria of the cells also can contribute to stimuli-responsive drug delivery and intracellular imaging (Figure 3B) 64, 72, 73. For example, encapsulation-leading fluorescence off of Rhodamine B in ZIF-90 provides a platform for intracellular ATP imaging based on the ATP triggered disassembly. The fluorescence of Rhodamine B was significantly suppressed after encapsulated into ZIF-90. However, the competitive coordination between the metal node of ZIF-90 and ATP can disassemble the structure of ZIF-90, thus releasing the Rhodamine B from the ZIF-90 nanoparticles. The dynamic images of mitochondria ATP in live cells have been observed through this stimuli-responsive system. Furthermore, an ATP-responsive ZIF-90 platform for cytosolic protein delivery and clustered regularly interspaced short palindromic repeats-associated protein 9 (CRISPR/Cas9) genome editing was developed using a similar system. CRISPR/Cas9 has been demonstrated as a very promising genomic editing tool. With the one-pot synthesis method, the protein CRISPR/Cas9 was encapsulated into ZIF-90 without changing the function of CRISPR/Cas9. Upon delivering the CRISPR/Ca9 encapsulated ZIF-90, the high concentration of intracellular ATP will promote the disassembly of ZIF-90 to release CRISPR/Ca9. With this ATP-responsive delivery system, the genome editing protein CRISPR/Cas9 effectively knocked out the expression of the green fluorescent protein in HeLa cells. Furthermore, cytotoxic RNase A-encapsulated ZIF-90 significantly prohibited cancer cell growth.
Figure 3.
A) Cargo release from the duplex-capped MOF with different stimuli such as the DNase I, the nicking enzyme (Nt.BbvCI), the endonuclease (EcoRI), and the exonuclease (Exo III) as biocatalysts. Reprinted with permission from ref. 66 Copyright (2018) WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. B) ATP triggered drug release. Reprinted with permission from ref. 63 Copyright (2017) WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
The enzyme plays a very significant role in the balance of biological systems. Each enzyme has a specific function and can catalyze its substrate only under certain conditions. Oligonucleotides act as substrates for different enzymes, such as DNase I, endonuclease, and exonuclease III (Figure 3A) 66. DNA functionalization on the surface of colloidal nanoparticles has been well studied. Both single stranded and double stranded DNA can be functionalized on the surface of MOFs for enzyme-responsive drug delivery. For example, the camptothecin-loaded and tailored hairpin DNA strands-capped MOF showed selective cytotoxicity toward MDA-MB-231 cancer cells that had a high expression of exonuclease III. Low apoptosis to epithelial MCF-10A breast cells which has low expression of exonuclease III was also observed.
MOFs as photodynamic therapeutic agents
Photodynamic therapy, as a noninvasive treatment, has attracted tremendous interest owing to its fewer harmful side effects 74, 75. However, the integration of photosensitizer to nanomaterials is often limited because of low loading efficiency, poor stability, and increased cytotoxicity. Recently, the preparation of photosensitizer-based MOF overcame the limitations of photosensitizers 76, 77, such as aggregation, self-quenching, and uncontrollable in vivo administration. With the uniform and well-defined porous crystalline structure, porphyrinic MOF allows 3O2 and 1O2 to diffuse freely in and out of the framework 77, 78. In the past decade, various porphyrin and derivative linkers have been synthesized to prepare MOFs. The robust chemical structure and natural biological functions of porphyrins help preserve the functionality of porphyrins after coordinating with metal ions to form a MOF.
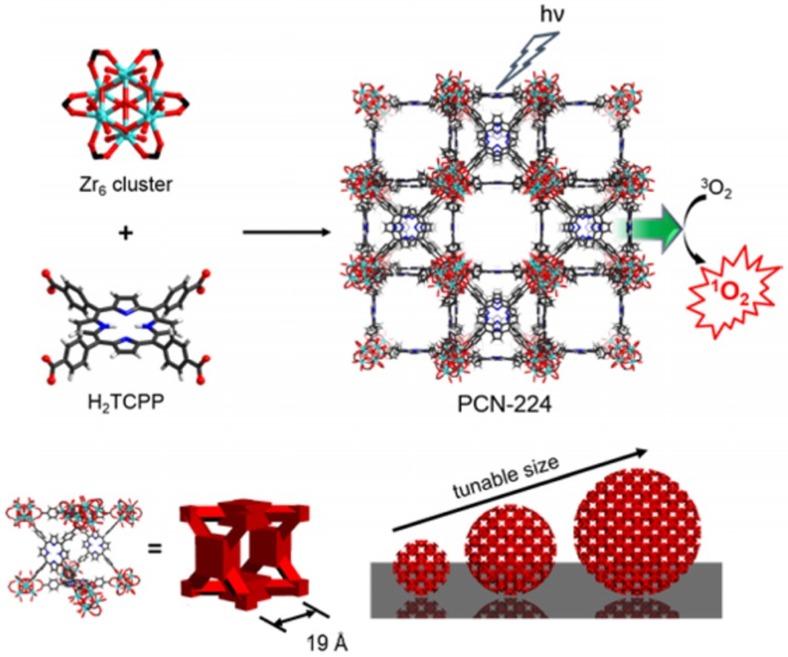
In 2014, the first nanoscale MOF used for photodynamic therapy was reported by the Lin group 79. A Hf-based MOF (Hf-MOF) nanoplate with 100 nm diameter and 10 nm thickness was prepared using the solvothermal method through coordination between Hf and 5,15-di(pbenzoato)-porphyrin. This Hf-MOF showed at least a two-fold increase in 1O2 generation compared to free porphyrin. In 2016, the Zhou group developed a size-controlled synthesis of Zr-based porphyrinic MOF (Zr-MOF) for targeted photodynamic therapy(Figure 4) 76. A broad size range of Zr-MOFs with precise control was prepared for size-dependent cellular uptake and photodynamic therapy. Intracellular cytotoxicity studies indicated that the 90 nm Zr-MOF nanoparticles showed the best photodynamic therapy efficacy, suggesting a promising photodynamic therapy candidate. Later, various metal-based porphyrinic MOFs were prepared for synergistic tumor therapy, such as photodynamic-photothermal therapy, photodynamic-radio therapy, and photodynamic-immune therapy 70, 80-83.
Figure 4.
Size-controlled synthesis of porphyrinic Zr-MOF (PCN-224) for photodynamic therapy. Reprinted (adapted) with permission from ref. 76. Copyright (2016) American Chemical Society.
Core-shell NP@MOF structure provides a multifunctional platform to extend the bioapplications of MOF in bioimaging, nanomedicine, and cancer therapy 83-87. The Huo group overcame the challenge and successfully grew ZIF-8 on various colloidal nanoparticles. The structure of ZIF-8 did not change after encapsulating colloidal nanoparticles. However, collective properties such as photoluminescent, catalytic, and magnetic properties were obtained with the heterogenous MOF structures 87. Photodynamic therapy using NIR light (980 nm) was achieved when upconversion nanoparticles @ MOF (UCNP@MOF) dimer was constructed through a fluorescence resonance energy transfer (FRET) strategy. The emission of UCNP with 980 nm light excitation at 650 nm can be adsorbed by porphyrinic MOF, thus generating toxic singlet oxygen for cancer cell therapy 83.
In the case of photodynamic-photothermal therapy, photothermal agent Au nanorods (AuNR) were used for photothermal therapy under the irradiation of NIR light 82. Porphyrinic MOFs were used as singlet oxygen generator for photodynamic therapy. Nanoscale core-shell AuNR@MOF nanoparticles were prepared by growing a layer of porphyrinic MOF on the surface of Au nanorod. This core-shell AuNR@MOF provides a dual-therapy model for tumor inhibition. The synergistic function from NIR light 808 nm for Au nanorods to generate heat and 660 nm for porphyrinic MOF to generate singlet oxygen significantly enhanced the therapy efficacy both in vitro and in vivo.
Radiotherapy has been commonly applied to tumor therapy owing to its ability to control cancer cell growth. However, high dose of radiation typically causes a serious side effect. Heavy metals such as Au, Hf, and Ru are common radiosensitizers to enhance radiotherapy efficacy. For example, Au nanoparticles accumulated in the tumor site could enhance the radiotherapy 88. Hf-based MOF has been demonstrated as an efficient agent for radiotherapy. The innovative combination of radiotherapy and radiodynamic therapy also has been demonstrated to significantly suppress tumor growth with a low dose of radiation 89. Recently, the Lin group developed a Hf-DBB-Ru [DBB-Ru = bis(2,2'-bipyridine) (5,5'-di(4-benzoato)-2,2'-bipyridine) ruthenium (II) chloride] nanoscale MOF for a combined radiotherapy and radiodynamic therapy (RT-RDT) 72. With nanoscale Hf-DBB-Ru MOF as a carrier, both Hf and Ru were used as a radiosensitizer to enhance the radiotherapy efficacy. Upon irradiating with X-ray, hydroxyl radical and singlet oxygen can be generated by this Hf-DBB-Ru MOF nanoparticle. In vitro and in vivo study indicated that the mitochondria-targeted RT-RDT can depolarize the membrane of mitochondrial to initiate the apoptosis of cancer cells, thus significantly inhibit the tumor growth in mouse models.
Immunotherapy which activating or suppressing the immune system to treat cancers has attracted intensive interest in the past decades. Current immunotherapy methods such as non-specific immunotherapies, oncolytic virus therapy, monoclonal antibodies, and tumor-agnostic therapies, T-cell therapy, and cancer vaccines typically work by suppressing the cancer cells growth, stopping cancer cells from spreading, and helping the immune system to fight cancer cells. Recently, MOFs have been used to enhance checkpoint blockade immunotherapy 14, 17, 90, 91. By incorporating radiosensitizers into MOF, enhanced radiotherapy was achieved to potentiate checkpoint blockade immunotherapy. In addition, combining anti-programmed death-ligand 1 antibody with MOF-mediated low-dose radiotherapy, the obvious abscopal effect was observed from a distant tumor. So, the local radiotherapy can trigger a local immune response by releasing immunostimulating signals to increase T cell infiltration to the tumor 92-94. Later, combined low-dose X-ray radiotherapy and radiodynamic therapy using nanoscale MOF were also demonstrated to enhance the checkpoint blockade immunotherapy 14.
Surface engineering of MOFs
Surface functionalization of nanomaterials has always been very significant for biochemical applications, such as analytical detection, bioimaging, and cancer therapy 51, 52, 95. The controlled manipulation of the external surface of MOFs to fit specific requirements and achieve the desired function is of paramount importance as it determines the overall performance of MOF nanoparticles 15, 96-98. For example, PEGylation was typically used to improve the colloidal stability of inorganic nanoparticles 99-101. Covalently anchoring a fluorophore on the surface of the nanoparticles can be used for bioimaging. Surface functionalization of a targeting molecule, such as a peptide or aptamer, can realize target binding or targeted delivery 76, 81, 102. Grafting functional polymers on the surface of the nanoparticles can achieve some stimuli-responsive properties. As a promising nanocarrier, surface functionalization of MOFs without changing their framework and porosity is also significant for the required biomedical applications. There are two popular post-synthesis incorporation ways to bioengineer the surface of MOFs 103-105. Since MOFs are made of organic linkers and metal ions via coordination bonds, the first way is to modify an anchor on the organic linker before the synthesis of the MOF and then covalently conjugate the target molecule with the anchor on the surface of the as-prepared MOF 104, 106, 107. The second method is to coordinate the target molecule on the surface of MOF directly in which the chelation between metal ions and target molecule acts as a bridge for the surface functionalization of the MOF 57, 105, 108.
The first example of anchor modification on an organic linker is the UiO-66 to UiO-66-N3 nanoparticles. The organic linker of UiO-66, benzene-1,4-dicarboxylic acid does not have any anchor and the resulting UiO-66 cannot be functionalized through a covalent anchor 104. In the case of UiO-66-N3, the azide group in 2-azido-1,4-benzene dicarboxylic acid can react with the alkane group via click reactions, modifying the target molecule on the MOF surface. Any alkane terminal ligands can be functionalized on the surface of MOF with controlled loading through this click reaction. In addition, a dibenzylcyclooctyne terminal DNA sequence can be conjugated on the surface of UiO-66-N3 nanoparticles.
Surface defects of nanoparticles are very common during synthesis. The unsaturated coordinative metal sites on the surface of MOFs provide opportunities for target molecules to bind to MOF nanoparticles through coordination. So far, different functional group-terminal ligands, such as carboxylate, phosphonate, histidine, and phenyl groups, have been reported to achieve incorporation 105, 106, 109. As basic coordination, various metal-carboxylate bindings have been used to form different MOFs in an organic solvent. Naturally, carboxylate containing ligands can bind the unsaturated metal sites, thus functionalizing MOF. Binding affinity is a significant way to evaluate the binding strength between metal and ligand and it varies between different metals and ligands 105. In the case of Zr-based MOFs, both carboxylate and phosphonate can coordinate with Zr, thus capping the ligand on the surface of the Zr-based MOF. However, the binding affinity between Zr and phosphonate is stronger than that between Zr and carboxylate. The Farha group have demonstrated that both carboxylate- and phosphonate-terminal ligands can be incorporated on the surface of NU-1000 94. However, extra phosphonate-terminal ligands can decompose the structure of NU-1000, while carboxylate-terminal ligands cannot.
Histidine which can be readily integrated into proteins or peptides significantly extended the scope for targeting molecule-functionalized MOFs. The Lächelt group reported a coordinative incorporation of oligohistidine-tags with MOFs 109. Despite different metal components, MIL-88A, HKUST-1, and Zr-fum exhibited considerable His-tag binding. Fluorescent models including His-carboxyfluorescein, His-green fluorescein protein, and His-ATTO 647N labeled human transferrin were selected to test the coordinative binding and cellular internalization using flow cytometry and confocal laser scanning microscopy. The His-tags binding to MOF demonstrated a general functionalization method of MOF with potential for protein and drug delivery. However, the limited histidine group in peptide and proteins my limit the application of this general surface functionalization strategy due to relatively weak binding affinity between histidine and the metal node of MOF nanoparticles.
Lipid coating to MOF is a facile method to functionalize MOF without changing its structural integrity and porosity 103, 110. The lipid ligand 1,2-dioleoyl-sn-glycero-3-phosphate has been used to transfer MOF from aqueous phase to the organic phase by facile surface encapsulation. Similarly, bilayer lipid-coated MOF was developed to study the intracellular release of loading dye with 1,2-dioleoyl-snglycero-3-phosphocholine. This bilayer lipid not only effectively stores the dye molecules inside the porous scaffold of MOF but also enables a high cellular uptake of MOF nanoparticles. Compared with artificial lipid layers, exosomes which have very similar membrane structures to cell membranes, are typically used for communication purposes by cells. They have the potential to form a protective coating on nanoparticles to bypass the immune system for longer circulation time for full biocompatibility. The Wuttke group overcame the challenge and successfully coated exosomes on the surface of MIL-88A using a fusion method 111. A slow calcein release was observed with exosome-coated calcein-loaded MIL-88A nanoparticles in HeLa cells, indicating that exosome coating is a very promising drug delivery system. The combination of exosome and MOF solved the premature release issue and improved the biocompatibility of MOF nanoparticles.
Phenolic group-terminated ligand can also form a stable coordination to directly modify MOF nanoparticles 95. Various metals such as Zr, Cr, Fe, Co, Cu, Zn, Al, In, and Eu have been demonstrated to chelate with phenolic group. The stable coordination can be attributed to a 5-member ring formed between metal ion and phenolic group. MOF nanoparticles includingUiO-66, ZIF, HKUST, and MIL-101 were transferred to organic phase from aqueous phase with phenolic lipid. This phenolic group provides a versatile platform for MOF surface functionalization.
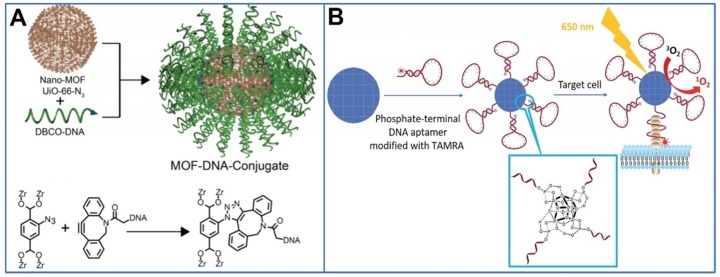
The three-dimensional oligonucleotides are of tremendous importance and have a wide application in biodetection, targeted binding, and genomic editing 112-116. The study of chemical interface properties between MOFs and nucleic acids render a potential application of MOFs for analytical detection, bioimaging, drug delivery, and cancer therapy 96, 98. A nucleic acid-MOF conjugate (Figure 5A) was constructed through a covalent click reaction with azido-anchored UiO-66 and dibenzylcyclooctyne functionalized DNA 104. Because of the natural phosphate backbone structure of DNA, phosphate-terminal DNA was later found to be able to be directly integrated onto the surface of MOFs (Figure 5B) 108, 117. With DNA capping on the surface, the colloidal stability and biocompatibility of MOF nanoparticles have been significantly improved. Aptamers are RNA and DNA oligonucleotides that bind their targets with high affinity and selectivity 118. Targeted imaging and drug delivery were achieved by incorporating aptamers onto the surface of MOF nanoparticles.
Figure 5.
A) Nucleic acid-MOF conjugation through covalent click reaction. Reprinted (adapted) with permission from ref. 93. Copyright (2014) American Chemical Society. B) Direct phosphate-terminal DNA conjugation to MOF. Reprinted (adapted) with permission from ref. 106. Copyright (2018), Royal Society of Chemistry.
Challenges and perspectives
The well-defined porous crystalline MOF has been demonstrated as a promising platform for drug delivery, bioimaging, and tumor therapy 16, 20, 24. The preparation of various nanoscale MOF particles with facile cargo loading renders a wide range of biomedical applications. Surface engineering of MOFs for targeted stimuli-responsive drug delivery significantly enhances the tumor therapy efficacy. Despite the considerable progress, biomedical applications of MOFs still face many challenges.
First, the poor stability of MOFs in physiological conditions has significantly limited its biomedical applications. Zn-carboxylate MOFs are very unstable in aqueous solution because of low coordinative affinity. Zr-based MOF nanoparticles are very sensitive to phosphate containing buffers such as PBS and RPMI cell culture medium which have a high concentration of phosphate ion owing to a stronger binding affinity between phosphate ion and Zr ion 56-57. Colloidal stability in aqueous solution due to large size (100 ~ 500 nm) of MOF also should be improved by surface functionalization. PEGylation or other hydrophilic ligand encapsulation is necessary to improve the colloidal stability of MOF nanoparticles for physiological studies 88-90. Without solving the biostability of MOFs under physiological condition, any other biomedical applications of MOFs will be futile.
Second, therapeutic proteins can be exploited to produce potentially highly specific drugs, thus curing the disease without the conventional drugs 54. The delivery of proteins without disrupting its bioavailability and activity depends on the delivery methods, and are affected by size, surface charge, and hydrophilicity 55. Porous MOFs typically have pore/channel size about 1 to 3 nm. Small molecules and peptide/protein with a small molecular weight (< 7 kD) do not have a problem being loaded into the MOF particles 56-57. However, the proteins with a large molecular weight (> 10 kD) typically need large pores/channels in order to be loaded into the MOF. Although the MOF particles with large pore/channel size need tedious work to synthesize their organic linkers, it is worth it for developing MOF particle systems with large pore/channel size for therapeutic protein delivery.
The final biomedical goal of MOF nanoparticles is a clinical application. The side effects or toxicity of MOF nanoparticles is the most significant factor to determine whether MOF nanoparticles can be applied to clinical research or not. Toxicity of nanomaterials is concentration dependent. Coordinating unit metal ions or organic linkers with minimal toxicity should be considered when constructing MOF particles as drug carriers or therapeutic agents 13. Controlled structure, tunable porosity, and readily chemical functionalizability make MOF a powerful biomedical tool for us to take advantage for biomedical applications. Bioengineering of MOF for nanomedicine is an interdisciplinary study. Future continued efforts need to focus on the biostability, biocompatibility, practicability, and efficacy to realize the full clinical applications of MOFs.
Acknowledgments
This work supported by the intramural research program of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH).
References
- 1.Guo Z, Xiao C, Maligal-Ganesh RV, Zhou L, Goh TW, Li X. et al. Pt nanoclusters confined within metal-organic framework cavities for chemoselective cinnamaldehyde hydrogenation. ACS Catal. 2014;4:1340–8. [Google Scholar]
- 2.Li Z, Peters AW, Platero-Prats AE, Liu J, Kung C-W, Noh H. et al. Fine-Tuning the Activity of Metal-Organic Framework-Supported Cobalt Catalysts for the Oxidative Dehydrogenation of Propane. J Am Chem Soc. 2017;139:15251–8. doi: 10.1021/jacs.7b09365. [DOI] [PubMed] [Google Scholar]
- 3.Zhao M, Yuan K, Wang Y, Li G, Guo J, Gu L. et al. Metal-organic frameworks as selectivity regulators for hydrogenation reactions. Nature. 2016;539:76. doi: 10.1038/nature19763. [DOI] [PubMed] [Google Scholar]
- 4.Li P, Moon S-Y, Guelta MA, Lin L, Gómez-Gualdrón DA, Snurr RQ. et al. Nanosizing a metal-organic framework enzyme carrier for accelerating nerve agent hydrolysis. ACS Nano. 2016;10:9174–82. doi: 10.1021/acsnano.6b04996. [DOI] [PubMed] [Google Scholar]
- 5.Shi W, Zhao X, Feng J, Liu J, Yang G, Wang G. et al. An Efficient, Visible-Light-Driven, Hydrogen Evolution Catalyst NiS/ZnxCd1-xS Nanocrystal Derived from a Metal-Organic Framework. Angew Chem Int Ed Engl. 2018;57:9790–4. doi: 10.1002/anie.201805425. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa H, Cordova KE, O'Keeffe M, Yaghi OM. The chemistry and applications of metal-organic frameworks. Science. 2013;341:1230444. doi: 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- 7.Schoedel A, Ji Z, Yaghi OM. The role of metal-organic frameworks in a carbon-neutral energy cycle. Nat Energy. 2016;1:16034. [Google Scholar]
- 8.Yuan D, Zhao D, Sun D, Zhou HC. An Isoreticular Series of Metal-Organic Frameworks with Dendritic Hexacarboxylate Ligands and Exceptionally High Gas-Uptake Capacity. Angew Chem Int Ed Engl. 2010;122:5485–9. doi: 10.1002/anie.201001009. [DOI] [PubMed] [Google Scholar]
- 9.Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P. et al. Metal-organic frameworks in biomedicine. Chem Rev. 2011;112:1232–68. doi: 10.1021/cr200256v. [DOI] [PubMed] [Google Scholar]
- 10.Simon-Yarza T, Mielcarek A, Couvreur P, Serre C. Nanoparticles of Metal-Organic Frameworks: On the Road to In Vivo Efficacy in Biomedicine. Adv Mater. 2018;30:1707365. doi: 10.1002/adma.201707365. [DOI] [PubMed] [Google Scholar]
- 11.Freund R, Lächelt U, Gruber T, Rühle B, Wuttke S. Multifunctional efficiency: extending the concept of atom economy to functional nanomaterials. ACS Nano. 2018;12:2094–105. doi: 10.1021/acsnano.8b00932. [DOI] [PubMed] [Google Scholar]
- 12.Cai W, Chu CC, Liu G, Wáng YXJ. Metal-organic framework-based nanomedicine platforms for drug delivery and molecular imaging. Small. 2015;11:4806–22. doi: 10.1002/smll.201500802. [DOI] [PubMed] [Google Scholar]
- 13.Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T. et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2010;9:172. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- 14.Lu K, He C, Guo N, Chan C, Ni K, Lan G, Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Engineering; 2018. p. 2. 600-10. [DOI] [PubMed] [Google Scholar]
- 15.Cai W, Gao H, Chu C, Wang X, Wang J, Zhang P. et al. Engineering Phototheranostic Nanoscale Metal-Organic Frameworks for Multimodal Imaging-Guided Cancer Therapy. ACS Appl Mater Interfaces. 2017;9:2040–51. doi: 10.1021/acsami.6b11579. [DOI] [PubMed] [Google Scholar]
- 16.He Z, Dai Y, Li X, Guo D, Liu Y, Huang X. et al. Hybrid Nanomedicine Fabricated from Photosensitizer-Terminated Metal-Organic Framework Nanoparticles for Photodynamic Therapy and Hypoxia-Activated Cascade Chemotherapy. Small. 2019;15:1804131. doi: 10.1002/smll.201804131. [DOI] [PubMed] [Google Scholar]
- 17.Lan G, Ni K, Xu Z, Veroneau SS, Song Y, Lin W. Nanoscale Metal-Organic Framework Overcomes Hypoxia for Photodynamic Therapy Primed Cancer Immunotherapy. J Am Chem Soc. 2018;140:5670–3. doi: 10.1021/jacs.8b01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Yang Y, Zhu W, Yi X, Dong Z, Xu X. et al. Nanoscale metal- organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials. 2016;97:1–9. doi: 10.1016/j.biomaterials.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Wuttke S, Lismont M, Escudero A, Rungtaweevoranit B, Parak WJ. Positioning metal-organic framework nanoparticles within the context of drug delivery-a comparison with mesoporous silica nanoparticles and dendrimers. Biomaterials. 2017;123:172–83. doi: 10.1016/j.biomaterials.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Wu MX, Yang YW. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv Mater. 2017;29:1606134. doi: 10.1002/adma.201606134. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, McGuirk CM, d'Aquino A, Mason JA, Mirkin CA. Metal-Organic Framework Nanoparticles. Adv Mater. 2018; 1800. 202. [DOI] [PubMed] [Google Scholar]
- 22.Wang XG, Cheng Q, Yu Y, Zhang XZ. Controlled Nucleation and Controlled Growth for Size Predicable Synthesis of Nanoscale Metal-Organic Frameworks (MOFs): A General and Scalable Approach. Angew Chem Int Ed Engl. 2018;57:7836–40. doi: 10.1002/anie.201803766. [DOI] [PubMed] [Google Scholar]
- 23.Li B, Wang X, Chen L, Zhou Y, Dang W, Chang J. et al. Ultrathin Cu-TCPP MOF nanosheets: a new theragnostic nanoplatform with magnetic resonance/near-infrared thermal imaging for synergistic phototherapy of cancers. Theranostics. 2018;8:4086–96. doi: 10.7150/thno.25433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Zhou J, Shi R, Wu H, Chen R, Duan B. et al. Biodegradable Core-shell Dual-Metal-Organic-Frameworks Nanotheranostic Agent for Multiple Imaging Guided Combination Cancer Therapy. Theranostics. 2017;7:4605–17. doi: 10.7150/thno.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sindoro M, Yanai N, Jee A-Y, Granick S. Colloidal-sized metal-organic frameworks: synthesis and applications. Acc Chem Res. 2013;47:459–69. doi: 10.1021/ar400151n. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Hou W, Sun H, Cui C, Zhang L, Jiang Y. et al. Thiol-ene click chemistry: a biocompatible way for orthogonal bioconjugation of colloidal nanoparticles. Chem Sci. 2017;8:6182–7. doi: 10.1039/c7sc01447c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Purich DL, Wu C, Wu Y, Chen T, Cui C. et al. Ionic functionalization of hydrophobic colloidal nanoparticles to form ionic nanoparticles with enzymelike properties. J Am Chem Soc. 2015;137:14952–8. doi: 10.1021/jacs.5b08533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch J, Zhuang J, Wang T, LaMontagne D, Wu H, Cao YC. Gas-bubble effects on the formation of colloidal iron oxide nanocrystals. J Am Chem Soc. 2011;133:12664–74. doi: 10.1021/ja2032597. [DOI] [PubMed] [Google Scholar]
- 29.Park J, An K, Hwang Y, Park J-G, Noh H-J, Kim J-Y. et al. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat Mater. 2004;3:891–5. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- 30.Chen Q, Xu M, Zheng W, Xu T, Deng H, Liu J. Se/Ru-Decorated Porous Metal-Organic Framework Nanoparticles for The Delivery of Pooled siRNAs to Reversing Multidrug Resistance in Taxol-Resistant Breast Cancer Cells. ACS App Mater Interfaces. 2017;9:6712–24. doi: 10.1021/acsami.6b12792. [DOI] [PubMed] [Google Scholar]
- 31.Biju V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem Soc Rev. 2014;43:744–64. doi: 10.1039/c3cs60273g. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Zhang W, Zhu G, Xie J, Chen X. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017;2:17024. doi: 10.1038/natrevmats.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howes PD, Chandrawati R, Stevens MM. Colloidal nanoparticles as advanced biological sensors. Science. 2014;346:1247390. doi: 10.1126/science.1247390. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Chen T, Wu C, Qiu L, Hu R, Li J. et al. Facile surface functionalization of hydrophobic magnetic nanoparticles. J Am Chem Soc. 2014;136:12552–5. doi: 10.1021/ja5060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad Z, Shah A, Siddiq M, Kraatz H-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014;4:17028–38. [Google Scholar]
- 36.Zhang Y, Huang Y, Li S. Polymeric micelles: nanocarriers for cancer-targeted drug delivery. AAPS PharmSciTech. 2014;15:862–71. doi: 10.1208/s12249-014-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Movassaghian S, Merkel OM, Torchilin VP. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:691–707. doi: 10.1002/wnan.1332. [DOI] [PubMed] [Google Scholar]
- 38.Fouladi F, Steffen KJ, Mallik S. Enzyme-responsive liposomes for the delivery of anticancer drugs. Bioconjug Chem. 2017;28:857–68. doi: 10.1021/acs.bioconjchem.6b00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen TX, Huang L, Gauthier M, Yang G, Wang Q. Recent advances in liposome surface modification for oral drug delivery. Nanomedicine. 2016;11:1169–85. doi: 10.2217/nnm.16.9. [DOI] [PubMed] [Google Scholar]
- 40.Rengan AK, Bukhari AB, Pradhan A, Malhotra R, Banerjee R, Srivastava R. et al. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett. 2015;15:842–8. doi: 10.1021/nl5045378. [DOI] [PubMed] [Google Scholar]
- 41.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chertok B, Moffat BA, David AE, Yu F, Bergemann C, Ross BD. et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29:487–96. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–51. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z, Tian R, Wu J, Fan Q, Yung BC, Niu G. et al. Impact of semiconducting perylene diimide nanoparticle size on lymph node mapping and cancer imaging. ACS Nano. 2017;11:4247–55. doi: 10.1021/acsnano.7b01261. [DOI] [PubMed] [Google Scholar]
- 45.Baati T, Njim L, Neffati F, Kerkeni A, Bouttemi M, Gref R. et al. In depth analysis of the in vivo toxicity of nanoparticles of porous iron (III) metal-organic frameworks. Chem Sci. 2013;4:1597–607. [Google Scholar]
- 46.Simon-Yarza T, Baati T, Neffati F, Njim L, Couvreur P, Serre C. et al. In vivo behavior of MIL-100 nanoparticles at early times after intravenous administration. Int J Pharm. 2016;511:1042–7. doi: 10.1016/j.ijpharm.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 47.He C, Lu K, Liu D, Lin W. Nanoscale metal-organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J Am Chem Soc. 2014;136:5181–4. doi: 10.1021/ja4098862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng H, Zhang Y, Liu L, Wan W, Guo P, Nyström AM. et al. One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J Am Chem Soc. 2016;138:962–8. doi: 10.1021/jacs.5b11720. [DOI] [PubMed] [Google Scholar]
- 49.Zhuang J, Kuo C-H, Chou L-Y, Liu D-Y, Weerapana E, Tsung C-K. Optimized metal-organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8:2812–9. doi: 10.1021/nn406590q. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Tong R, Shi Z, Yang B, Liu H, Ding S. et al. MOF Nanoparticles with Encapsulated Autophagy Inhibitor in Controlled Drug Delivery System for Antitumor. ACS Appl Mater Interfaces. 2018;10:2328–37. doi: 10.1021/acsami.7b16522. [DOI] [PubMed] [Google Scholar]
- 51.Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5:1909–17. doi: 10.1158/1535-7163.MCT-06-0141. [DOI] [PubMed] [Google Scholar]
- 52.Kango S, Kalia S, Celli A, Njuguna J, Habibi Y, Kumar R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—a review. Prog in Polym Sci. 2013;38:1232–61. [Google Scholar]
- 53.Xu ZP, Zeng QH, Lu GQ, Yu AB. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem Eng Sci. 2006;61:1027–40. [Google Scholar]
- 54.Kato S, Otake K-i, Chen H, Akpinar I, Buru CT, Islamoglu T. et al. Zirconium-Based Metal-Organic Frameworks for the Removal of Protein-Bound Uremic Toxin from Human Serum Albumin. J Am Chem Soc. 2019;141:2568–76. doi: 10.1021/jacs.8b12525. [DOI] [PubMed] [Google Scholar]
- 55.Lian X, Fang Y, Joseph E, Wang Q, Li J, Banerjee S. et al. Enzyme-MOF (metal-organic framework) composites. Chem Soc Rev. 2017;46:3386–401. doi: 10.1039/c7cs00058h. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Li P, Modica JA, Drout RJ, Farha OK. Acid-Resistant Mesoporous Metal-Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release. J Am Chem Soc. 2018;140:5678–81. doi: 10.1021/jacs.8b02089. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Chen Y, Wang S, Li P, Mirkin CA, Farha OK. DNA-Functionalized Metal-Organic Framework Nanoparticles for Intracellular Delivery of Proteins. J Am Chem Soc. 2019;141:2215–9. doi: 10.1021/jacs.8b12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li P, Modica JA, Howarth AJ, Vargas E, Moghadam PZ, Snurr RQ. et al. Toward design rules for enzyme immobilization in hierarchical mesoporous metal-organic frameworks. Chem. 2016;1:154–69. [Google Scholar]
- 59.Li P, Chen Q, Wang TC, Vermeulen NA, Mehdi BL, Dohnalkova A. et al. Hierarchically Engineered Mesoporous Metal-Organic Frameworks toward Cell-free Immobilized Enzyme Systems. Chem. 2018;4:1022–34. [Google Scholar]
- 60.Duan Y, Ye F, Huang Y, Qin Y, He C, Zhao S. One-pot synthesis of a metal-organic framework-based drug carrier for intelligent glucose-responsive insulin delivery. Chem Commun. 2018;54:5377–80. doi: 10.1039/c8cc02708k. [DOI] [PubMed] [Google Scholar]
- 61.Chen W-H, Luo G-F, Vázquez-González M, Cazelles R, Sohn YS, Nechushtai R. et al. Glucose-Responsive Metal-Organic-Framework Nanoparticles Act as “Smart” Sense-and-Treat Carriers. ACS Nano. 2018;12:7538–45. doi: 10.1021/acsnano.8b03417. [DOI] [PubMed] [Google Scholar]
- 62.Chen W-H, Vazquez-Gonzalez M, Zoabi A, Abu-Reziq R, Willner I. Biocatalytic cascades driven by enzymes encapsulated in metal-organic framework nanoparticles. Nat Catal. 2018;1:689–95. [Google Scholar]
- 63.Chen W-H, Yu X, Cecconello A, Sohn YS, Nechushtai R, Willner I. Stimuli-responsive nucleic acid-functionalized metal-organic framework nanoparticles using pH-and metal-ion-dependent DNAzymes as locks. Chem Sci. 2017;8:5769–80. doi: 10.1039/c7sc01765k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen WH, Yu X, Liao WC, Sohn YS, Cecconello A, Kozell A. et al. ATP-Responsive Aptamer-Based Metal-Organic Framework Nanoparticles (NMOFs) for the Controlled Release of Loads and Drugs. Adv Funct Mater. 2017;27:1702102. [Google Scholar]
- 65.Wang X-G, Dong Z-Y, Cheng H, Wan S-S, Chen W-H, Zou M-Z. et al. A multifunctional metal-organic framework based tumor targeting drug delivery system for cancer therapy. Nanoscale. 2015;7:16061–70. doi: 10.1039/c5nr04045k. [DOI] [PubMed] [Google Scholar]
- 66.Chen WH, Luo GF, Sohn YS, Nechushtai R, Willner I. Enzyme-Driven Release of Loads from Nucleic Acid-Capped Metal-Organic Framework Nanoparticles. Adv Funct Mater. 2019;29:1805341. [Google Scholar]
- 67.Ma Y, Li X, Li A, Yang P, Zhang C, Tang B. H2S-Activable MOF Nanoparticle Photosensitizer for Effective Photodynamic Therapy against Cancer with Controllable Singlet-Oxygen Release. Angew Chem Int Ed Engl. 2017;56:13752–6. doi: 10.1002/anie.201708005. [DOI] [PubMed] [Google Scholar]
- 68.Chen WH, Liao WC, Sohn YS, Fadeev M, Cecconello A, Nechushtai R. et al. Stimuli-Responsive Nucleic Acid-Based Polyacrylamide Hydrogel-Coated Metal-Organic Framework Nanoparticles for Controlled Drug Release. Adv Funct Mater. 2018;28:1705137. [Google Scholar]
- 69.Lin LS, Song J, Song L, Ke K, Liu Y, Zhou Z. et al. Simultaneous Fenton-like Ion Delivery and Glutathione Depletion by MnO2-Based Nanoagent to Enhance Chemodynamic Therapy. Angew Chem Int Ed Engl. 2018;130:4996–5000. doi: 10.1002/anie.201712027. [DOI] [PubMed] [Google Scholar]
- 70.Zhang W, Lu J, Gao X, Li P, Zhang W, Ma Y. et al. Enhanced Photodynamic Therapy by Reduced Levels of Intracellular Glutathione Obtained By Employing a Nano-MOF with CuII as the Active Center. Angew Chem Int Ed Engl. 2018;130:4985–90. doi: 10.1002/anie.201710800. [DOI] [PubMed] [Google Scholar]
- 71.Fan H, Yan G, Zhao Z, Hu X, Zhang W, Liu H. et al. A smart photosensitizer-manganese dioxide nanosystem for enhanced photodynamic therapy by reducing glutathione levels in cancer cells. Angew Chem Int Ed Engl. 2016;55:5477–82. doi: 10.1002/anie.201510748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng J, Wang K, Wang M, Yu P, Mao L. Mitochondria targeted nanoscale zeolitic imidazole framework-90 for ATP imaging in live cells. J Am Chem Soc. 2017;139:5877–82. doi: 10.1021/jacs.7b01229. [DOI] [PubMed] [Google Scholar]
- 73.Yang X, Tang Q, Jiang Y, Zhang M, Wang M, Mao L. Nanoscale ATP-responsive Zeolitic Imidazole Framework-90 as a General Platform for Cytosolic Protein Delivery and Genome Editing. J Am Chem Soc. 2019;141:3782–6. doi: 10.1021/jacs.8b11996. [DOI] [PubMed] [Google Scholar]
- 74.Fan W, Huang P, Chen X. Overcoming the Achilles' heel of photodynamic therapy. Chem Soc Rev. 2016;45:6488–519. doi: 10.1039/c6cs00616g. [DOI] [PubMed] [Google Scholar]
- 75.Lin J, Wang S, Huang P, Wang Z, Chen S, Niu G. et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano. 2013;7:5320–9. doi: 10.1021/nn4011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park J, Jiang Q, Feng D, Mao L, Zhou H-C. Size-controlled synthesis of porphyrinic metal-organic framework and functionalization for targeted photodynamic therapy. J Am Chem Soc. 2016;138:3518–25. doi: 10.1021/jacs.6b00007. [DOI] [PubMed] [Google Scholar]
- 77.Lismont M, Dreesen L, Wuttke S. Metal-Organic Framework Nanoparticles in Photodynamic Therapy: Current Status and Perspectives. Adv Funct Mater. 2017;27:1606314. [Google Scholar]
- 78.Liu Y, Sun H, Yang L, Zhu X, Wang X, Liang J. et al. Chelation-assisted assembly of multidentate colloidal nanoparticles into metal-organic nanoparticles. Nanoscale. 2018;10:21369–73. doi: 10.1039/c8nr06262e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu K, He C, Lin W. Nanoscale metal-organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J Am Chem Soc. 2014;136:16712–5. doi: 10.1021/ja508679h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu K, He C, Guo N, Chan C, Ni K, Weichselbaum RR. et al. Chlorin-based nanoscale metal-organic framework systemically rejects colorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J Am Chem Soc. 2016;138:12502–10. doi: 10.1021/jacs.6b06663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meng H-M, Hu X-X, Kong G-Z, Yang C, Fu T, Li Z-H. et al. Aptamer-functionalized nanoscale metal-organic frameworks for targeted photodynamic therapy. Theranostics. 2018;8:4332–44. doi: 10.7150/thno.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeng JY, Zhang MK, Peng MY, Gong D, Zhang XZ. Porphyrinic Metal-Organic Frameworks Coated Gold Nanorods as a Versatile Nanoplatform for Combined Photodynamic/Photothermal/Chemotherapy of Tumor. Adv Funct Mater. 2018;28:1705451. [Google Scholar]
- 83.Li Y, Di Z, Gao J, Cheng P, Di C, Zhang G. et al. Heterodimers Made of Upconversion Nanoparticles and Metal-Organic Frameworks. J Am Chem Soc. 2017;139:13804–10. doi: 10.1021/jacs.7b07302. [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Tang J, He L, Liu Y, Liu Y, Chen C. et al. Core-Shell Upconversion Nanoparticle@ Metal-Organic Framework Nanoprobes for Luminescent/Magnetic Dual-Mode Targeted Imaging. Adv Mater. 2015;27:4075–80. doi: 10.1002/adma.201501779. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y, He L, Pang K, Liu W, Tian Y, Chang L. et al. Core-Shell Noble-Metal@ Zeolitic-Imidazolate-Framework Nanocarriers with High Cancer Treatment Efficiency in Vitro. J Mater Chem B. 2019;7:1050–5. doi: 10.1039/c8tb03318h. [DOI] [PubMed] [Google Scholar]
- 86.Liu D, Wan J, Pang G, Tang Z. Hollow Metal-Organic-Framework Micro/Nanostructures and their Derivatives: Emerging Multifunctional Materials. Adv Mater. 2018; 1803. 291. [DOI] [PubMed] [Google Scholar]
- 87.Lu G, Li S, Guo Z, Farha OK, Hauser BG, Qi X. et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat Chem. 2012;4:310–6. doi: 10.1038/nchem.1272. [DOI] [PubMed] [Google Scholar]
- 88.Zhou Z, Chan A, Wang Z, Huang X, Yu G, Jacobson O. et al. Synchronous Chemoradiation Nanovesicles by X-Ray Triggered Cascade of Drug Release. Angew Chem Int Ed Engl. 2018;130:8599–603. doi: 10.1002/anie.201802351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu J, Gao J, Wei Q. Combination of photodynamic therapy with radiotherapy for cancer treatment. J Nanomater; 2016. p. 8507924. [Google Scholar]
- 90.Duan X, Chan C, Lin W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew Chem Int Ed Engl. 2019;58:670–80. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ni K, Lan G, Chan C, Quigley B, Lu K, Aung T. et al. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat Commun. 2018;9:2351. doi: 10.1038/s41467-018-04703-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 94.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41:503–10. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu B, Ma M, Zacher D, Bétard A, Yusenko K, Metzler-Nolte N. et al. Chemistry of SURMOFs: Layer-selective installation of functional groups and post-synthetic covalent modification probed by fluorescence microscopy. J Am Chem Soc. 2011;133:1734–7. doi: 10.1021/ja1109826. [DOI] [PubMed] [Google Scholar]
- 96.Huang X, He Z, Guo D, Liu Y, Song J, Yung BC. et al. “Three-in-one” Nanohybrids as Synergistic Nanoquenchers to Enhance No-Wash Fluorescence Biosensors for Ratiometric Detection of Cancer Biomarkers. Theranostics. 2018;8:3461–73. doi: 10.7150/thno.25179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu D, Huxford RC, Lin W. Phosphorescent nanoscale coordination polymers as contrast agents for optical imaging. Angew Chem Int Ed Engl. 2011;50:3696–700. doi: 10.1002/anie.201008277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao M, Wang Y, Ma Q, Huang Y, Zhang X, Ping J. et al. Ultrathin 2D metal-organic framework nanosheets. Adv Mater. 2015;27:7372–8. doi: 10.1002/adma.201503648. [DOI] [PubMed] [Google Scholar]
- 99.Cauda V, Argyo C, Bein T. Impact of different PEGylation patterns on the long-term bio-stability of colloidal mesoporous silica nanoparticles. J Mater Chem. 2010;20:8693–9. [Google Scholar]
- 100.Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2012;64:246–55. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 101.Shi Z, Chen X, Zhang L, Ding S, Wang X, Lei Q. et al. FA-PEG decorated MOF nanoparticles as a targeted drug delivery system for controlled release of an autophagy inhibitor. Biomater Sci. 2018;6:2582–90. doi: 10.1039/c8bm00625c. [DOI] [PubMed] [Google Scholar]
- 102.Wuttke S, Zimpel A, Bein T, Braig S, Stoiber K, Vollmar A. et al. Validating Metal-Organic Framework Nanoparticles for Their Nanosafety in Diverse Biomedical Applications. Adv Healtc Mater. 2017;6:1600818. doi: 10.1002/adhm.201600818. [DOI] [PubMed] [Google Scholar]
- 103.Wang S, Morris W, Liu Y, McGuirk CM, Zhou Y, Hupp JT. et al. Surface-Specific Functionalization of Nanoscale Metal-Organic Frameworks. Angew Chem Int Ed Engl. 2015;54:14738–42. doi: 10.1002/anie.201506888. [DOI] [PubMed] [Google Scholar]
- 104.Morris W, Briley WE, Auyeung E, Cabezas MD, Mirkin CA. Nucleic Acid-Metal Organic Framework (MOF) Nanoparticle Conjugates. J Am Chem Soc. 2014;136:7261–4. doi: 10.1021/ja503215w. [DOI] [PubMed] [Google Scholar]
- 105.Deria P, Bury W, Hod I, Kung C-W, Karagiaridi O, Hupp JT. et al. MOF functionalization via solvent-assisted ligand incorporation: Phosphonates vs carboxylates. Inorg Chem. 2015;54:2185–92. doi: 10.1021/ic502639v. [DOI] [PubMed] [Google Scholar]
- 106.Zhu W, Xiang G, Shang J, Guo J, Motevalli B, Durfee P. et al. Versatile Surface Functionalization of Metal-Organic Frameworks through Direct Metal Coordination with a Phenolic Lipid Enables Diverse Applications. Adv Funct Mater. 2018;28:1705274. [Google Scholar]
- 107.Yi X-C, Xi F-G, Qi Y, Gao E-Q. Synthesis and click modification of an azido-functionalized Zr (iv) metal-organic framework and a catalytic study. RSC Adv. 2015;5:893–900. [Google Scholar]
- 108.Wang S, McGuirk CM, Ross MB, Wang S, Chen P, Xing H. et al. General and Direct Method for Preparing Oligonucleotide-Functionalized Metal-Organic Framework Nanoparticles. J Am Chem Soc. 2017;139:9827–30. doi: 10.1021/jacs.7b05633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Röder R, Preiß T, Hirschle P, Steinborn B, Zimpel A, Höhn M. et al. Multifunctional nanoparticles by coordinative self-assembly of His-tagged units with metal-organic frameworks. J Am Chem Soc. 2017;139:2359–68. doi: 10.1021/jacs.6b11934. [DOI] [PubMed] [Google Scholar]
- 110.Wuttke S, Braig S, Preiß T, Zimpel A, Sicklinger J, Bellomo C. et al. MOF nanoparticles coated by lipid bilayers and their uptake by cancer cells. Chem Commun. 2015;51:15752–5. doi: 10.1039/c5cc06767g. [DOI] [PubMed] [Google Scholar]
- 111.Illes B, Hirschle P, Barnert S, Cauda V, Wuttke S, Engelke H. Exosome-Coated Metal-Organic Framework Nanoparticles: An Efficient Drug Delivery Platform. Chem Mater. 2017;29:8042–6. [Google Scholar]
- 112.Lyu Y, Chen G, Shangguan D, Zhang L, Wan S, Wu Y. et al. Generating cell targeting aptamers for nanotheranostics using cell-SELEX. Theranostics. 2016;6:1440–52. doi: 10.7150/thno.15666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tan W, Donovan MJ, Jiang J. Aptamers from cell-based selection for bioanalytical applications. Chem Rev. 2013;113:2842–62. doi: 10.1021/cr300468w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu G, Zhang H, Jacobson O, Wang Z, Chen H, Yang X. et al. Combinatorial screening of DNA aptamers for molecular imaging of HER2 in cancer. Bioconjug Chem. 2017;28:1068–75. doi: 10.1021/acs.bioconjchem.6b00746. [DOI] [PubMed] [Google Scholar]
- 115.Zhu G, Liu Y, Yang X, Kim Y-H, Zhang H, Jia R. et al. DNA-inorganic hybrid nanovaccine for cancer immunotherapy. Nanoscale. 2016;8:6684–92. doi: 10.1039/c5nr08821f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu G, Mei L, Vishwasrao HD, Jacobson O, Wang Z, Liu Y. et al. Intertwining DNA-RNA nanocapsules loaded with tumor neoantigens as synergistic nanovaccines for cancer immunotherapy. Nat Commun. 2017;8:1482. doi: 10.1038/s41467-017-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y, Hou W, Xia L, Cui C, Wan S, Jiang Y. et al. ZrMOF nanoparticles as quenchers to conjugate DNA aptamers for target-induced bioimaging and photodynamic therapy. Chem Sci. 2018;9:7505–9. doi: 10.1039/c8sc02210k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu G, Chen X. Aptamer-based targeted therapy. Adv Drug Deliv Rev. 2018;134:65–78. doi: 10.1016/j.addr.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]