Abstract
Background:
Invasive candidiasis (IC) is a major cause of morbimortality in children. Previous studies described the clinical characteristics and risk factors for this infection; however, limited data are available on the predictors of mortality in these patients. In this context, we evaluated the risk factors associated with death due to IC in a pediatric tertiary care hospital in South of Brazil.
Methods:
This is a retrospective, cross-sectional, observational, and analytical study of a series of pediatric patients with clinical and laboratory diagnosis of IC from March 2014 to September 2017. Univariate and multivariate analysis were performed to estimate the association between the characteristics of the patients and death.
Results:
A total of 94 cases of IC were included. The incidence was 1.13 cases per 1000 patients/d, with a mortality rate of 14%. There was a predominance of non-albicans Candida (71.3%) in IC cases and, although there is no species difference in mortality rates, biofilm formation was associated with increased mortality. Clinical characteristics such as male sex, stay in the intensive care unit, and thrombocytopenia; comorbidities such as cardiological disease and renal insufficiency; and risks such as mechanical ventilation and dialysis were associated with increased mortality.
Conclusion:
Data from this study suggest that biofilm formation by Candida sp. is associated with increased mortality, and this is the first study to correlate the male sex and cardiological disease as risk factors for death in pediatric IC patients.
Keywords: Candida, intensive care unit, invasive candidiasis, mortality, pediatric
1. Introduction
Fungi have recently emerged as a major cause of human diseases, and the genus Candida remains the most common cause of invasive fungal infections (IFIs) in hospitalized patients.[1,2] They are associated with several clinical manifestations, ranging from mucocutaneous infections to invasive diseases.[1,2] Currently, there are >150 known species of Candida and, although Candida albicans is mainly responsible for these infections, non-albicans species have also emerged as important nosocomial pathogens, with varied distribution in different geographic areas and the potential to develop antifungal resistance.[3–5] The morbidity and mortality associated with IFIs are substantial, and although children and adults are similarly vulnerable, there are important differences in the host responses, the capacity of immune reconstitution after chemotherapy, and comorbidities. All these factors affect the risk and outcomes of the IFIs, and therefore, account for the differences in their epidemiology between the adult and pediatric populations.[6–10]Candida species are a major contributor to morbidity and mortality in hospitalized children, but prognostic factors in these patients have not yet been elucidated.[7,11] Thus, the aim of this study is to evaluate the risk factors associated with death due to invasive candidiasis (IC) in pediatric patients.
2. Methods
2.1. Design of study, setting, and population
This is a retrospective, cross-sectional, observational, and analytical study of a series of pediatric patients with clinical and laboratory diagnosis of IC, at a pediatric tertiary care 372-bed hospital in south of Brazil. The Brazilian health system is formed by a public–private combination; the public component, the Unified Health System (Sistema Único de Saúde; SUS), is based on the principle that health is a right of the citizen and duty of the state, but due to the high demand, around half the population opts to pay private health plans.[12] Thus, the philanthropic hospital included in the study attends to patients of the public and private healthcare network, with 60% of their beds being allocated to the public health system and 40% to the private ones; in addition, its institutional policy ensures homogeneity and equally cares for all patients from both origins.
The study included hospitalized patients between 0 and 18 years of age who presented with IC from March 2014 to September 2017. All the included patients were hospitalized for >72 hours and tested positive for Candida spp. from a normally sterile body fluid or from a newly placed drain inserted into a normally sterile body site. Patients with urinary tract infections (UTIs) and long-term bladder catheters were included only after catheter removal and collection of new urine samples, which were probed and confirmed for UTIs (i.e., without suspicion of another site as a source of infection). Patients older than 18 years and those who did not meet the IC criteria were excluded from this study.
The Institutional Review Board (IRB) of the participating center (IRB #2.943.365) approved this study. Investigations were carried out by securing each patient's anonymity.
2.2. Clinical data
The following medical record data for all patients were collected: demographic data, hospital unit, clinical characteristics, site of infection, previous occurrence of bacteremia, and the persistence of infection in cases of candidemia. In addition, information regarding surgical procedures, the use of mechanical ventilation, parenteral nutrition, central venous catheter, and dialysis was also collected. Data were used to assess the risk factors associated with death due to IC in pediatric patients included in the study.
2.3. Laboratory data collection (microbiology)
Clinical samples from patients suspected of invasive infection were collected by appropriate aseptic procedures and sent to the microbiology laboratory for culture. All blood cultures were performed using a BD BACTEC 9120 Blood Culture System (Becton Dickinson, Franklin Lakes, NJ). Positive cultures for Candida spp. were identified by Vitek 2 Compact YST-ID card (BioMérieux, Durham, NC). From 2016, Candida species isolated were identified by both Vitek 2 Compact (BioMérieux, Marcy-l’Etoile, France) and Matrix Associated Laser Desorption-Ionization Time of Flight (MALDI-TOF/Vitek MS) (BioMérieux). If any discrepancy was observed between these 2 methodologies, the identification with an ID score >2.00 obtained by MALDI-TOF/Vitek MS was considered. Qualitative biofilm production was analyzed from 2016, using the tube method previously described by Christensen et al.[13–15]
2.4. Statistical analysis
We selected demographic data, hospital unit, clinical characteristics, and comorbidities previously studied by other authors as possible risk factors for IC or IC-related deaths, which could be evaluated in our pediatric population.[2,9,16–19] The data were expressed as minimum and maximum values, mean ± standard deviation (SD), or medians for continuous variables and as percentages for categorical variables. For continuous variables, Student t test or Wilcoxon test was used to compare 2 groups of normally distributed or nonparametric data, respectively. Categorical variables were compared by using Fisher exact test or the chi-square test. Univariate analysis was performed to determine the association between IC patient's characteristics (variables) and death. Associations with P < .05 were considered significant. Multivariate model selection started at univariate analysis; all factors with P < .2, were considered in the model, following which, a stepwise method was used to obtain the final model. The odds ratio (OR) obtained indicated the risk of one category in relation to another of the same variable. Confidence intervals (CIs) not including the value 1 with a P < .05 indicated a significant variable effect. A multivariate logistic regression model was used to investigate the multiple relationships between the risk factors for mortality among all variables. All statistical analyses were performed using R version 3.4.4, an open-source software environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria).[20]
3. Results
Based on the inclusion criteria, a total of 94 cases of IC were included in the study. The mean age of the included patients was 4.5 ± 5.6 years, and the median age was 1.4 years (ranging from 0 to 18 years). Among the 94 cases studied, 49 involved patients under 2 years of age, of which 4 were newborns. The incidence of IC was 1.13 cases/1000 patients/d and 4.1 cases/1000 hospital admissions. The main characteristics of the patients included in this study are summarized in Table 1.
Table 1.
Demographics and clinical characteristics of patients with invasive candidiasis.
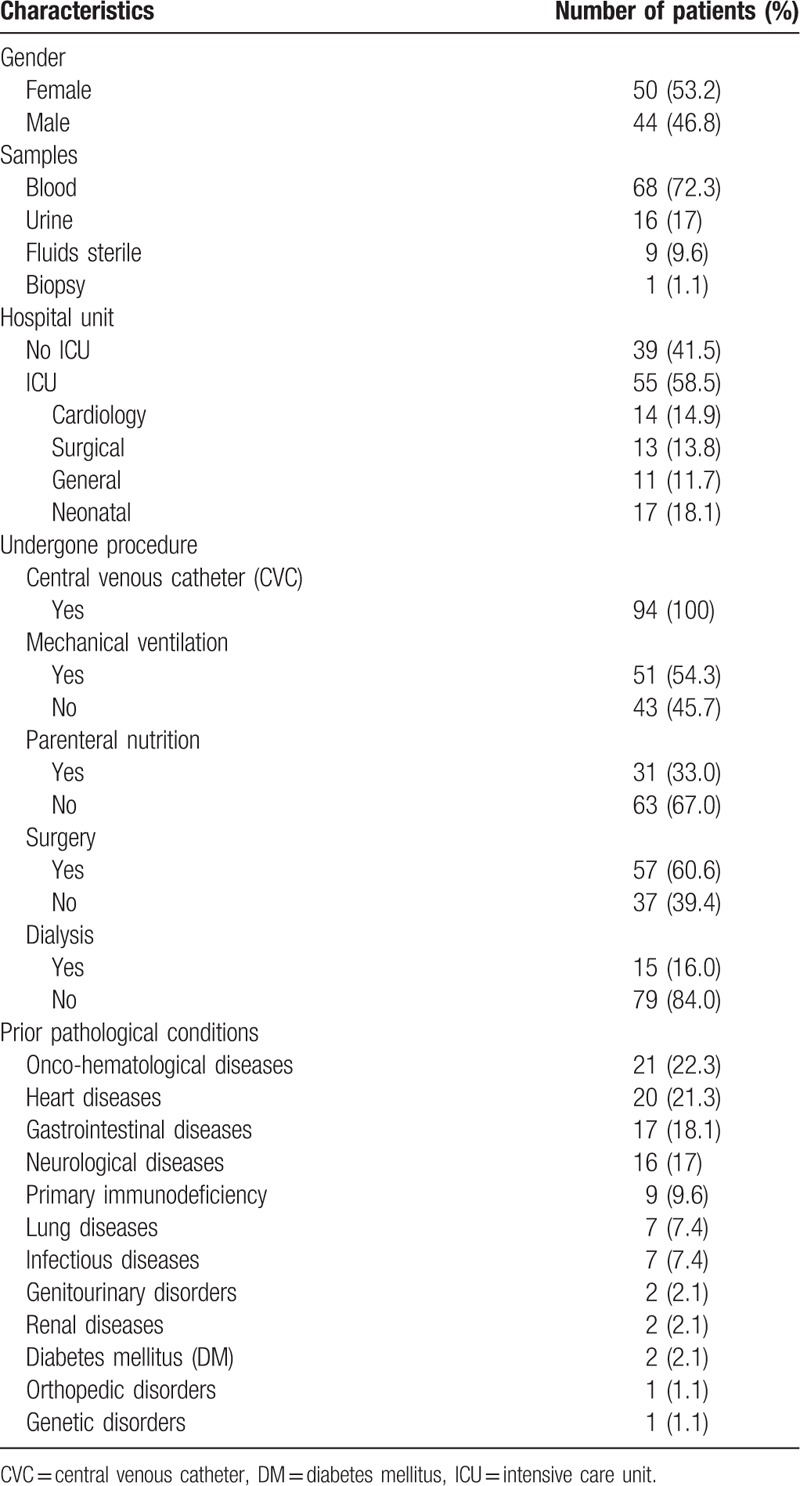
C. albicans and non-albicans species were isolated from 27 (28.7%) and 67 (71.3%) patients, respectively (Table 2). All isolates were identified by Vitek 2 Compact and 28 of them were also identified by MALDI-TOF/Vitek MS, and only 2 discrepancies were observed. Two strains of yeast previously identified by Vitek 2 Compact as Candida utilis and Candida famata were reidentified as Candida fabianii and Candida guilliermondii, respectively, by MALDI-TOF/Vitek MS. Table 2 summarizes the microbiological characteristics of the Candida species identified.
Table 2.
Distribution of Candida species in 94 episodes of invasive candidiasis.

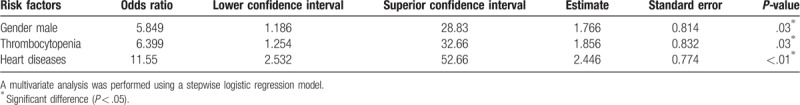
The mortality rate associated with IC was 14%. One of the 94 patients was excluded from the statistical analysis since there was not enough data to attribute death to the IC episode. The results of the univariate analysis of the factors associated with mortality among 93 patients with IC (80 survivors and 13 non-survivors) are presented in Table 3. Patients with thrombocytopenia, cardiological disease, renal insufficiency, mechanical ventilation, dialysis, and infections caused by Candida sp. biofilm production, as well as those admitted to the ICU, showed high rates of mortality. However, the multivariate analysis identified 3 factors that had significant association with death due to IC: male gender (OR 5.849; CI 1.186–28.83; P = .03), thrombocytopenia (OR 6.399; CI 1.254–32.66; P = .03), and cardiological disease (OR 11.55; CI 2.532–52.66; P < .01) (Table 4).
Table 3.
Univariate analysis for risk factors associated with mortality in patients with invasive candidiasis.
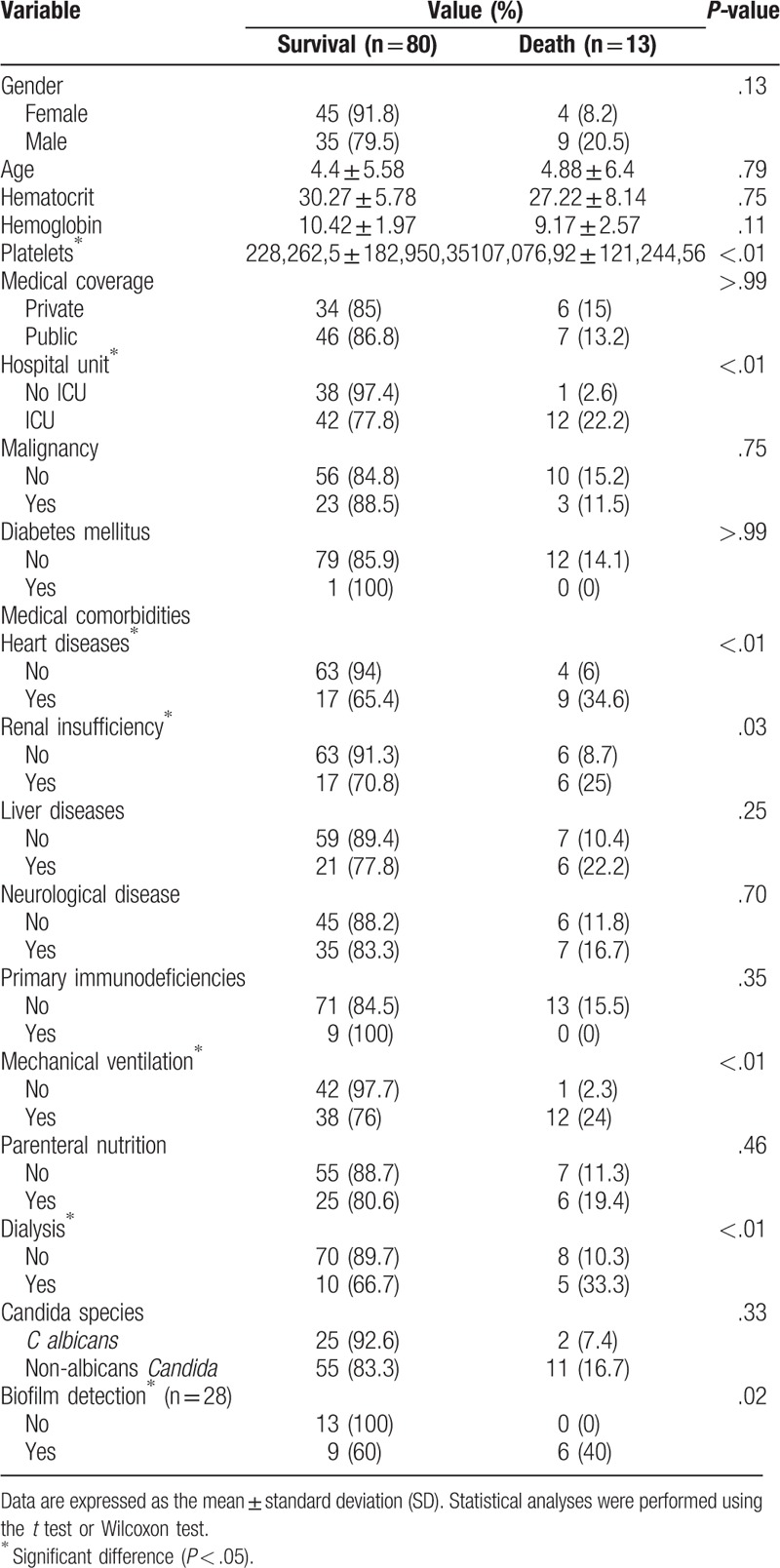
Table 4.
Multivariate analysis of risk factors associated with death in patients with invasive candidiasis.
4. Discussion
Although previous studies have reported some risk factors for IC in children and adults,[11,16–18,21,22] there are very few reports that evaluated the risk factors associated with death due to IC in the pediatric population. Here, we present a series of 94 pediatric patients with IC, evaluating the mortality rate and the involved risk factors.
Consistent with the current literature that reports mortality rates between 10% and 35% for pediatric patients with IC, we found a mortality rate of 14% (13/83), which reinforces the severe outcomes associated with these infections.[8,11,16,23,24] Most episodes of death (92.3%: 12/13) were recorded in the ICU, as already reported for adults and children; however, as previously reported for the adults,[8,25–27] there was a statistical difference between admission and non-admission to ICU as a risk factor for death associated with IC (P < .01).
Our analysis demonstrated that patients with thrombocytopenia had a higher mortality rate than those with normal platelet counts (P < .01). Though thrombocytopenia has previously been described as a risk factor for candidemia and IC in children and newborns, no specificity for the diagnosis or death has been found.[28–31] Although some crucial effects of platelets, such as the ability to attract cells of the immune system, activate the complement system, and release microbicidal proteins, have already been clarified, their involvement in the pathogenesis of IC and, therefore, the real significance of thrombocytopenia as a risk factor for infection and death remains unclear.[31]
Regarding the pathological conditions prior to IC, it was noted that patients with heart diseases and renal insufficiency were associated with increased mortality (P < .01 and .03, respectively). Recently, some publications have demonstrated an association between renal insufficiency and death in pediatric patients with IC.[16,32] However, this is the first report that describes the association between heart diseases and increased mortality. We found that 9/13 (69.2%) patients who succumbed to IC had heart disease. Previous studies have also reported IC-related mortality rates of 15% and 21% in adults and pediatric patients with heart disease, respectively. Based on all these findings, it is accepted that heart disease (especially congenital heart disease) is a risk factor for death in patients with IC. Therefore, greater efforts are required to prevent infections in patients in the ICU, especially after surgery.[33,34]
We found that the use of mechanical ventilation (P < .01) and dialysis (P = .03) were associated with increased mortality, in agreement with our previous publications.[16,26]
Although C. albicans is predominant in adult and pediatric populations, some authors describe the emergence and even inversion of this scenario by other non-albicans species.[17,35,36] We report a predominance of non-albicans species (71.2%) with Candida parapsilosis being the most common (35.1%), followed by C. albicans (28.8%) and Candida tropicalis (23.4%). The distribution of Candida spp. is important, as some species seem to be associated with relatively better outcomes. Some authors have reported that IC cases linked to C. parapsilosis are less aggressive than others linked to C. albicans[17,22];however, we did not find any difference in the mortality rates associated with C. albicans and non-albicans species. Our results are consistent with recent literature reports that describe C. parapsilosis and C. tropicalis among the most common non-albicans species in Latin America, Southern Europe, India, and Pakistan, while in the United States and Europe, Candida glabrata stands out among non-albicans species.[37]
Our results indicate that biofilm formation is associated with increased mortality (P = .02). Biofilm is a community of microorganisms that are irreversibly attached to living or nonliving surfaces, producing extracellular polymeric substances that provide a structural matrix.[38] The ability of Candida isolates to form biofilms varies by species and is considered an important virulence factor that could contribute to the development of antifungal resistance and persistence of infections.[39] However, the clinical significance of in vitro biofilm production by Candida spp. remains unclear. The in vitro detection of biofilm by laboratory techniques does not necessarily indicate the in vivo production.[38,40,41] We tested 23 isolates for biofilm production, of which 15 (65.2%) were found to be producers. The biofilm producer species included C. tropicalis (7; 46.6%), C. parapsilosis (6; 40%), Candida krusei (1; 6.7%), and C. fabianii (1; 6.7%). Although C. albicans is recognized as the main biofilm-producing species, other studies have demonstrated higher production by the non-albicans species, especially C. parapsilosis and C. tropicalis.[39–41]
Multivariate analysis showed that factors independently associated with death in pediatric IC included male sex (OR 5.849; CI 1.186–28.83; P = .03), thrombocytopenia (OR 6.399; CI 1.254–32.66; P = .03), and heart disease (OR 11.55; CI 2.532–52.66; P < .01). Infectious diseases rarely affect men and women equally, and evidence suggests that physiological sex differences are behind the differences in prevalence and mortality in many infectious diseases.[42] Some invasive fungal infections endemic to Brazil, such as paracoccidioidomycosis, cryptococcosis, aspergillosis, mucormycosis, and episodes of IC (including candidemia), have been found to be prevalent among men; in addition, male sex is a risk factor for IC in neonates.[19,27,43,44] However, for the first time, we have shown that male sex is a risk factor for death in pediatric IC patients in a population with homogeneous distribution between men and women.
Our study had a few limitations. It was a retrospective study performed in a single pediatric tertiary hospital; our epidemiology findings cannot be applied to all other health centers. Although the diagnosis of invasive candidiasis was judicious, some cases may have been erroneously classified, and some cases were lost during the study. Due to the great diversity of factors that could be evaluated in relation to the risk of IC-related death, not all were considered in the statistical analysis of this study, including prematurity and low birth weight. In addition, not all isolates of Candida sp. could be investigated with respect to biofilm production capacity, which precludes deep reflections of this finding in relation to the risk of death for the IC patient, although the results from the data on hand indicate a statistically significant finding.
In this study, variables were selected based on the previous evidence on risk factors for IC and mortality by IC, in addition to biological knowledge. However, if we consider the uncertainties generated by the statistical analyses in the scientific context in view of the current knowledge, it would be possible to come up with new approaches.[45] For example, using the Bonferroni correction considering the multiple comparisons made in this study, the significance criterion would change to P = .002 and P = .005 for the univariate and multivariate analysis, respectively, to maintain the overall type I (alpha) error probability previously considered (0.05). In this context, only heart disease would be the possible risk factor for death by IC; however, these types of correction increase the probability of type II (beta) error. Therefore, it is important that further studies on the subject in other clinical settings be developed in order to reinforce if the variables found in this study would also be determinant in death by IC.
In conclusion, data from this study emphasizes that mortality among pediatric patients with IC is around 15%. While we found that the mortality rates are not dependent on the Candida species, they could be directly related to biofilm formation. Additionally, we have identified for the first time that heart disease and male sex are possible risk factors for IC-related death in pediatric patients.
Acknowledgments
Editage provided English editing of the manuscript.
Author contributions
Fábio Araújo Motta and Libera Maria Dalla Costa designed the study.
Luiza Souza Rodrigues and Libera Maria Dalla Costa submitted the research to the Ethics and Research Committee for approval.
Fábio Araújo Motta performed clinical evaluations and selected patients for the study.
Gledson Luiz Picharski performed the statistical analyses.
Thaís Muniz Vasconcelos, Marinei Campos Riccieri, and Luiza Souza Rodrigues collected the demographic, clinical, and laboratory data.
Luiza Souza Rodrigues and Fábio Araújo Motta wrote the manuscript, and all authors made contributions. All authors reviewed and approved the final manuscript.
Conceptualization: Fabio Araujo Motta, Libera Maria Dalla-Costa.
Data curation: Gledson Luiz Picharski, Libera Maria Dalla-Costa.
Formal analysis: Gledson Luiz Picharski.
Investigation: Fabio Araujo Motta.
Methodology: Luiza Souza Rodrigues, Thaís Muniz Vasconcelos, Marinei Campos Riccieri.
Project administration: Luiza Souza Rodrigues, Fabio Araujo Motta, Libera Maria Dalla-Costa.
Supervision: Fabio Araujo Motta.
Validation: Gledson Luiz Picharski.
Writing – original draft: Luiza Souza Rodrigues, Libera Maria Dalla-Costa.
Writing – review & editing: Fabio Araujo Motta, Libera Maria Dalla-Costa.
Luiza Souza Rodrigues orcid: 0000-0002-9774-8650.
Footnotes
Abbreviations: CVC = central venous catheter, IC = invasive candidiasis, ICU = intensive care unit, IFIs = invasive fungal infections, IRB = Institutional Review Board, SD = standard deviation, UTIs = urinary tract infections.
This study had financial support in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
The authors report no conflicts of interest.
References
- [1].Sardi JC, Scorzoni L, Bernardi T, et al. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol 2013;62(pt 1):10–24. [DOI] [PubMed] [Google Scholar]
- [2].Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007;20:133–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 2014;20suppl:5–10. [DOI] [PubMed] [Google Scholar]
- [4].Colombo AL, Júnior JNA, Guinea J. Emerging multidrug-resistant Candida species. Curr Opin Infect Dis 2017;30:528–38. [DOI] [PubMed] [Google Scholar]
- [5].Jeffery-Smith A, Taori SK, Schelenz S, et al. Candida auris: a review of the literature. Clin Microbiol Rev 2017;31: pii: e00029-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colombo AL, Guimarães T, Camargo LF, et al. Brazilian guidelines for the management of candidiasis - a joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz J Infect Dis 2013;17:283–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lehrnbecher T, Groll AH. Invasive fungal infections in the pediatric population. Expert Rev Anti Infect Ther 2011;9:275–8. [DOI] [PubMed] [Google Scholar]
- [8].Steinbach WJ. Pediatric invasive Candidiasis: epidemiology and diagnosis in children. J Fungi (Basel) 2016;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag 2014;10:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Motta FA, Dalla-Costa LM, Muro MD, et al. Risk factors for candidemia mortality in hospitalized children. J Pediatr (Rio J) 2017;93:165–71. [DOI] [PubMed] [Google Scholar]
- [11].Pana ZD, Roilides E, Warris A, et al. Epidemiology of invasive fungal disease in children. J Pediatric Infect Dis Soc 2017;6suppl:S3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Paim J, Travassos C, Almeida C, et al. The Brazilian health system: history, advances, and challenges. Lancet 2011;377:1778–97. [DOI] [PubMed] [Google Scholar]
- [13].Christensen GD, Simpson WA, Bisno AL, et al. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun 1982;37:318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sida H, Shah P, Pethani J, et al. Study of biofilm formation as a virulence marker in Candida species isolated from various clinical specimens. Int J Med Sci Public Health 2016;5:842–6. [Google Scholar]
- [15].Hassan A, Usman J, Kaleem F, et al. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz J Infect Dis 2011;15:305–11. [PubMed] [Google Scholar]
- [16].Motta AL, Almeida GM, Almeida Júnior JN, et al. Candidemia epidemiology and susceptibility profile in the largest Brazilian teaching hospital complex. Braz J Infect Dis 2010;14:441–8. [PubMed] [Google Scholar]
- [17].Brissaud O, Guichoux J, Harambat J, et al. Invasive fungal disease in PICU: epidemiology and risk factors. Ann Intensive Care 2012;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zaoutis TE, Greves HM, Lautenbach E, et al. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J 2004;23:635–41. [DOI] [PubMed] [Google Scholar]
- [19].Benjamin DK, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006;117:84–92. [DOI] [PubMed] [Google Scholar]
- [20].Cox DR. The regression analysis of binary sequences. J R Stat Soc 1958;20:215–42. [Google Scholar]
- [21].Lai MY, Hsu JF, Chu SM, et al. Breakthrough candidemia in children: clinical and microbiological characteristics, therapeutic strategies and impact on outcomes. Future Microbiol 2017;12:695–705. [DOI] [PubMed] [Google Scholar]
- [22].Zaoutis T. Candidemia in children. Curr Med Res Opin 2010;26:1761–8. [DOI] [PubMed] [Google Scholar]
- [23].Tsai MH, Hsu JF, Chu SM, et al. Clinical and microbiological characteristics, and impact of therapeutic strategies on the outcomes of children with candidemia. Sci Rep 2017;7:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Leroy O, Gangneux JP, Montravers P, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005-2006). Crit Care Med 2009;37:1612–8. [DOI] [PubMed] [Google Scholar]
- [25].Akbar DH, Tahawi AT. Candidemia at a University Hospital: epidemiology, risk factors and predictors of mortality. Ann Saudi Med 2001;21:178–82. [DOI] [PubMed] [Google Scholar]
- [26].Celebi S, Hacimustafaoglu M, Ozdemir O, et al. Nosocomial candidaemia in children: results of a 9-year study. Mycoses 2008;51:248–57. [DOI] [PubMed] [Google Scholar]
- [27].Doi AM, Pignatari AC, Edmond MB, et al. Epidemiology and microbiologic characterization of nosocomial candidemia from a Brazilian National Surveillance Program. PLoS One 2016;11:e0146909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].King J, Pana ZD, Lehrnbecher T, et al. Recognition and clinical presentation of invasive fungal disease in neonates and children. J Pediatric Infect Dis Soc 2017;6suppl:S12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu M, Huang S, Guo L, et al. Clinical features and risk factors for blood stream infections of. Exp Ther Med 2015;10:1139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kelly MS, Benjamin DK, Smith PB. The epidemiology and diagnosis of invasive candidiasis among premature infants. Clin Perinatol 2015;42:105–17. viii–ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Speth C, Rambach G, Lass-Flörl C. Platelet immunology in fungal infections. Thromb Haemost 2014;112:632–9. [DOI] [PubMed] [Google Scholar]
- [32].Santolaya ME, Alvarado T, Queiroz-Telles F, et al. Active surveillance of candidemia in children from Latin America: a key requirement for improving disease outcome. Pediatr Infect Dis J 2014;33:e40–4. [DOI] [PubMed] [Google Scholar]
- [33].Ascher SB, Smith PB, Clark RH, et al. Sepsis in young infants with congenital heart disease. Early Hum Dev 2012;88suppl:S92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang Y, Guo F, Kang Y, et al. Epidemiology, clinical characteristics, and risk factors for mortality of early- and late-onset invasive candidiasis in intensive care units in China. Medicine (Baltimore) 2017;96:e7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Blyth CC, Chen SC, Slavin MA, et al. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics 2009;123:1360–8. [DOI] [PubMed] [Google Scholar]
- [36].Kang SJ, Kim SE, Kim UJ, et al. Clinical characteristics and risk factors for mortality in adult patients with persistent candidemia. J Infect 2017;75:246–53. [DOI] [PubMed] [Google Scholar]
- [37].Pappas PG, Lionakis MS, Arendrup MC, et al. Invasive candidiasis. Nat Rev Dis Primers 2018;4:18026. [DOI] [PubMed] [Google Scholar]
- [38].Cavalheiro M, Teixeira MC. Biofilms: threats, challenges, and promising strategies. Front Med (Lausanne) 2018;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li WS, Chen YC, Kuo SF, et al. The impact of biofilm formation on the persistence of candidemia. Front Microbiol 2018;9:1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pongrácz J, Benedek K, Juhász E, et al. In vitro biofilm production of Candida bloodstream isolates: any association with clinical characteristics? J Med Microbiol 2016;65:272–7. [DOI] [PubMed] [Google Scholar]
- [41].Tumbarello M, Fiori B, Trecarichi EM, et al. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One 2012;7:e33705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Guerra-Silveira F, Abad-Franch F. Sex bias in infectious disease epidemiology: patterns and processes. PLoS One 2013;8:e62390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Strollo S, Lionakis MS, Adjemian J, et al. Epidemiology of hospitalizations associated with invasive candidiasis, United States, 2002-2012. Emerg Infect Dis 2016;23:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bitar D, Lortholary O, Le Strat Y, et al. Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg Infect Dis 2014;20:1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “p < 0.05”. Am Stat 2019;73suppl:1–9. [Google Scholar]


