Abstract
Background:
Autologous hepatocyte transplantation after ex vivo gene therapy is an alternative to liver transplantation for metabolic liver disease. Here we evaluate ex vivo gene therapy followed by transplantation of single-cell or spheroid hepatocytes.
Methods:
Pig and mouse hepatocytes were isolated, labeled with zirconium-89 and returned to the liver as single cells or spheroids. Biodistribution was evaluated through positron emission tomography-computed tomography. Fumarylacetoacetate hydrolase–deficient pig hepatocytes were isolated and transduced with a lentiviral vector containing the Fah gene. Animals received portal vein infusion of single-cell or spheroid autologous hepatocytes after ex vivo gene delivery. Portal pressures were measured and ultrasound was used to evaluate for thrombus. Differences in engraftment and expansion of ex vivo corrected single-cell or spheroid hepatocytes were followed through histologic analysis and animals’ ability to thrive off 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione.
Results:
Positron emission tomography-computed tomography imaging showed spheroid hepatocytes with increased heterogeneity in biodistribution as compared with single cells, which spread more uniformly throughout the liver. Animals receiving spheroids experienced higher mean changes in portal pressure than animals receiving single cells (P < .01). Additionally, two animals from the spheroid group developed portal vein thrombi that required systemic anticoagulation. Immunohistochemical analysis of spheroid- and single-cell–transplanted animals showed similar engraftment and expansion rates of fumarylacetoacetate hydrolase–positive hepatocytes in the liver, correlating with similar weight stabilization curves.
Conclusion:
Ex vivo gene correction of autologous hepatocytes in fumarylacetoacetate hydrolase–deficient pigs can be performed using hepatocyte spheroids or single-cell hepatocytes, with spheroids showing a more heterogeneous distribution within the liver and higher risks for portal vein thrombosis and increased portal pressures.
Hereditary tyrosinemia type 1 (HT1) is the most severe form of the tyrosine metabolism disorders. It is an autosomal recessive disease caused by a deficiency of the fumarylacetoacetate hydrolase (FAH) enzyme that leads to a buildup of toxic metabolites in the liver.1 These metabolites cause oxidative damage in hepatocytes, resulting in fibrosis, cirrhosis, high rates of hepatocellular carcinoma (HCC), liver failure, and death if untreated.2,3 Even with appropriate pharmacologic treatment with 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), an inhibitor of the tyrosine catabolic pathway upstream of the metabolic defect,4 HT1 remains a chronic disorder with long-term complications. Progression of fibrosis, cirrhosis, and development of HCC can occur in patients despite appropriate NTBC administration.5 The only therapeutic option for patients refractory to NTBC treatment or who develop HCC is liver transplantation.6 Furthermore, studies have shown that HT1 patients on chronic NTBC therapy have progressive neurocognitive decline with worsening executive function and social cognition skills when compared with healthy controls.7
Hepatocyte transplantation and gene therapy offer an alternative and possible cure for HT1. Our group has previously demonstrated that ex vivo gene transfer using a lentiviral vector to deliver a functional Fah cDNA into hepatocytes is able to correct the metabolic disorder in both mouse and pig models of HT1.8 This procedure involves a partial hepatectomy with subsequent primary hepatocyte isolation. These hepatocytes are then targeted ex vivo in culture by an integrating lentiviral vector carrying the functional Fah gene and, once corrected, the cells are transplanted back into the donor animal via the portal vein.
In our previous studies, ex vivo corrected hepatocytes were transplanted orthotopically via the portal vein, using single-cell hepatocyte suspension. A limitation of primary hepatocytes, however, is that their functionality declines rapidly after 24 hours in vitro under standard culture conditions.9 Spheroids are three-dimensional (3D), multicellular aggregates of hepatocytes that form spontaneously through cell-to-cell adhesion under rocked suspension culture conditions.10,11 As spheroids form, hepatocyte polarization develops, leading to the formation of bile canaliculi.12 The spheroidal structure protects the hepatocytes from apoptosis, allowing for them to remain viable in suspension for more than a week13 while they retain phenotypic stability with high levels of liver-specific functions, such as cytochrome P450 activity, urea synthesis, and albumin production.14 However, it is un-known whether these spheroids can engraft and proliferate in vivo. If spheroid engraftment is safe, these cells may allow for transplantation of higher cell densities per volume infused and for a higher efficiency of vector transduction, because transduction could occur over a longer period of time due to better durability in culture than single-cell suspension. Finally, spheroids may allow for multiple doses to be administered during several days, greatly increasing the cell mass transplanted. In this study, we examined the feasibility of intraportal transplantation of ex vivo corrected spheroid hepatocytes as an alternative to hepatocytes in single-cell suspension.
Materials and Methods
Animals
All animal procedures were performed in compliance with Mayo Clinic’s Institutional Animal Care and Use Committee regulations and all animals received humane care. For bio-distribution experiments, female Fox Chase SCID beige mice (Charles River Laboratories International, Inc., Wilmington, MA) and male heterozygous Fah+/− pigs were used. Fah−/− pigs were produced in a 50% Large White and 50% Landrace pig, as previously described,15,16 and were used in equal numbers for ex vivo gene therapy experiments. NTBC mixed in food was administered at a dose of 1 mg/kg/day with a maximum of 25 mg/day. All animals remained on NTBC until the time of transplantation, after which NTBC administration was discontinued to stimulate expansion of the corrected cells. In the absence of NTBC, the buildup of toxic metabolites in FAH-negative hepatocytes causes widespread apoptosis and cell death, while FAH-positive hepatocytes proliferate to repopulate the liver. Cycling of NTBC, using previously established protocols,8 prevents acute liver failure while stimulating liver repopulation by corrected cells until a time when the mass of FAH-positive hepatocytes became sufficient for phenotypic improvement. After hepatocyte transplantation, all animals were monitored daily for loss of appetite or any other clinical signs of morbidity. Animals were weighed daily for the first 2 weeks postoperatively, and weekly thereafter. If loss of appetite, weight loss, or any other signs of morbidity occurred, NTBC treatment was reinitiated for 7 days. Animals were cycled on and off NTBC in this fashion to stimulate expansion of corrected FAH-positive cells.
Liver resection and hepatocyte isolation
Six-week-old pigs (10–19 kg) underwent a laparoscopic partial hepatectomy involving the left lateral lobe under inhaled general anesthesia with 1%–3% isoflurane. Resection volumes represented 15%–20% of the total liver mass. An upper midline incision was made for placement of a 12-mm port, using an open Hasson technique, through which a 5-mm laparoscope (Stryker, Kalamazoo, MI) was passed. The abdomen was insufflated with CO2 to provide adequate visualization, and two additional 5-mm ports were placed. The liver vasculature was identified and isolated, at which point the parenchymal transection was performed, using an Endo GIA stapler with a 45-mm vascular load (Covidien, Dublin, Ireland). When parenchymal resection was complete, the liver section was retrieved using an Endo Catch bag (Covidien), and adequate homeostasis was ensured before port removal and incision closure. This liver section was then perfused ex vivo through the portal vein with a two-step perfusion system to isolate hepatocytes as previously described.17
Biodistribution of radiolabeled hepatocytes
For biodistribution experiments, hepatocytes in single-cell or spheroid form were radiolabeled in suspension with synthon 89 Zr-DBN at 27°C for 45 minutes in Hank’s Buffered Salt Solution as previously described.18–20
Hepatocyte transduction
Hepatocytes were transduced in suspension at a multiplicity of infection of 20 transduction units with a second-generation lentiviral vector carrying the porcine Fah cDNA under control of the human thyroxine-binding globulin promoter and two copies of the human α1-microglobulin/bikunin enhancer (Fig. 1, A). Hepatocytes from pigs randomized to receive single-cell suspension were transduced in suspension for 2 hours before transplantation. The hepatocyte medium and resuspension technique used have been previously described.8 Hepatocytes from pigs randomized to receive hepatocyte spheroids were transduced in suspension for 18–24 hours, while spheroid formation was taking place by means of a rocking technique.14 Spheroid viability was assessed through inverted epifluorescent microscopy (Axiovert 135 TV, Carl Zeiss Inc., Thornwood, NY) using the Fluoroquench fluorescent viability stain (One Lambda, Canoga Park, CA), as previously described.21 Spheroid diameter was measured by use of a Multisizer3 with 560 μm aperture (Beckman Coulter, Fullerton, CA). Spheroid versus single-cell hepatocyte ex vivo transduction efficiency was assessed through transduction with a lentiviral vector expressing green fluorescent protein (GFP) at multiplicities of infection of 0, 500, and 200 0 lentiviral particles per cell. GFP-positive cells were then quantified by flow cytometry at 96 hours post-transduction.
Fig. 1.
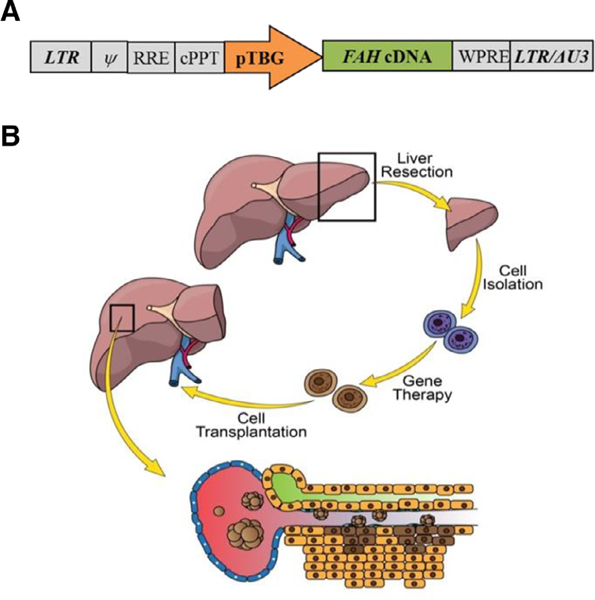
Second-generation lentiviral vector and experimental process involved in ex vivo gene therapy. (A) The porcine Fah cDNA is under control of the human TBG promoter. LTR, long terminal repeat; Ψ, psi packaging sequence; RRE, rev-responsive element; cPPT, central polypurine tract; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element; LTR/∆U3, 3´ long terminal repeat with deletion in U3 region. (B) Four steps are involved in this process: (1) partial hepatectomy, (2) primary hepatocyte isolation through collagenase perfusion, (3) ex vivo gene therapy with a lentiviral vector carrying the porcine Fah gene, and (4) retransplantation of autologous hepatocytes in single cell or spheroid form.
Hepatocyte transplantation in mice
In mice, cell transplants were performed intrasplenically at 12 weeks of age. Intrasplenic injections were performed through an open technique, where a small incision was made in the left upper quadrant of the abdomen. The spleen was visualized by partial evacuation from the abdomen, and cells were delivered directly into the splenic parenchyma via a 27- or 28-gauge 0.5-inch needle. Additionally, a control group of mice underwent intraperitoneal injections of spheroid suspension hepatocytes percutaneously into the right lower quadrant of the abdomen.
Hepatocyte transplantation in pigs
Fah+/− and Fah−/− pigs were randomized to receive autologous transplantation of either single-cell hepatocytes or spheroid hepatocytes through ultrasound-guided percutaneous portal vein infusion. Single-cell animals were kept under general anesthesia postoperatively until the time of transplantation, approximately 4 hours later; spheroid animals were recovered postoperatively and re-anesthetized for transplantation the next day. A schematic representation of the experimental process is presented in Fig. 1, B. The portal vein was identified using a 2–5 MegaHz transducer (Fujifilm SonoSite, Inc., Bothell, WA), and an 18-gauge 5-inch needle was directed toward the main portal vein before its bifurcation for manual infusion of hepatocytes. Portal pressures were monitored continuously during transplantation, and infusion was paused if pressures increased more than 8 mmHg above the upper limit of normal, defined as 10 mmHg. When portal pressures did not return to baseline after pausing infusion for 5 minutes, the injection was discontinued and the actual doses administered are reported. Heparinization of the cell solution was performed at 70 U/kg of recipient weight, as has been previously described for islet cell transplantation protocols.22 Ultrasound was used to evaluate for the presence of thrombotic events after transplantation.
Positron emission tomography–computed tomography imaging and analysis
In mice, imaging was performed on the Inveon MicroPET-CT (Siemens Medical Solutions USA, Inc., Malvern, PA) at 2, 24, and 48 hours post-transplantation. Computed tomography (CT) was performed at 80 kEv, 500 uA, with 250 ms/projection, 180 projections, bin 4. The effective pixel size was 94.59 um. Positron emission tomography (PET) was performed at 10-minute acquisition, OSEM2D reconstruction with Fourier rebinning, 4 iterations. In pigs, imaging was performed on the high-resolution GE Discovery 690 ADC PET/CT System (GE Healthcare, Chicago, IL) at 6, 30, and 66 hours post-transplantation. CT was performed at 120 kV and 150 mA, with tube rotation of 0.5 s and pitch of 0.516. PET was performed as a two-bed acquisition with 10 minutes per bed and 17 slice overlap, resulting in a 27-cm axial field of view. All post-reconstruction analysis was performed using PMOD software (PMOD Technologies LLC, Zürich, Switzerland). Activities were decay-corrected and normalized to standardized uptake value (SUV) units.
Histopathologic analysis
Tissue samples were fixed in 10% neutral buffered formalin (Azer Scientific, Morgantown, PA), paraffin embedded and sectioned. Hematoxylin and eosin (H&E) and Masson’s trichrome staining were performed by means of standard protocols. Immunohistochemistry for FAH was performed as previously described.23
Statistical analysis
Numerical data are expressed as mean (± standard deviation). The Mann-Whitney U test was used to analyze differences in continuous variables between spheroid and single-cell groups. Statistical analyses were performed with GraphPad Prism software v 7 (GraphPad Software, San Diego, CA).
Results
Spheroids show a higher efficiency of vector transduction than single-cell hepatocytes
To evaluate transduction efficiencies in spheroid hepatocytes compared with single-cell hepatocytes, an in vitro experiment was performed where primary pig hepatocytes in both forms were transduced with a lentiviral vector–expressing GFP at various multiplicities of infection. Percentage of GFP-positive cells detected by flow cytometry 96 hours after transduction was significantly higher in spheroid than in single-cell groups (Fig. 2, C).
Fig. 2.
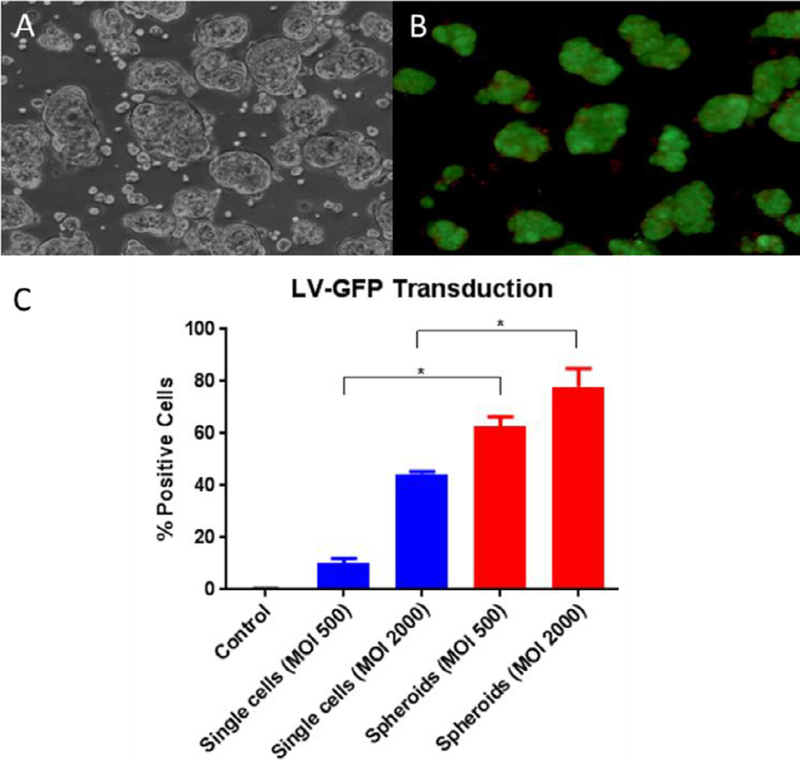
Morphology and viability of spheroids, and differences in transduction efficiency between single-cell and spheroid hepatocytes. (A) Light microscopy image of spheroids. (B) Inverted fluorescence microscopy image of spheroid hepatocytes, using the Fluoroquench viability stain before transplantation in pig 897; live cells (green) and necrotic cells (orange). (C) Ex vivo transduction of primary pig single-cell hepatocytes and hepatocyte spheroids with LV-GFP at MOls of 500 and 2000 LPs per cell and quantification of GFP-positive cells detected by flow cytometry 96 hours after transduction. Data are means ± SD ( n=3 replicates for single cells, 2 replicates for spheroids). *P < .01.
Hepatocyte spheroids engraft in the liver in mice
To compare engraftment of spheroid hepatocytes with single-cell hepatocytes, SCID beige mice were randomized to receive intrasplenic injection of single-cell hepatocyte suspension (n = 4) or spheroid hepatocyte suspension (n = 4). Hepatocytes were obtained from a wild-type pig harvest and were radiolabeled with 89 Zr (half-life 78.4 hours).24 The radiolabeling efficiency was ~20%. The radioactivity concentration in both single-cell and spheroid forms was ~0.1 MBq/106 cells. Each mouse was injected with approximately 665,0 0 0 cells. MicroPET-CT imaging at 2, 24, and 48 hours post-transplantation showed migration and engraftment in the liver of a majority of intrasplenically injected cells in both single-cell and spheroid groups. However, it also showed a higher percentage of the initial dose remaining in the liver in the single-cell mice compared with spheroid mice (Fig. 3, A), which approached significance (P = . 06). Other main sites of biodistribution were spleen and, to a lesser degree, intestine. In addition to higher liver positivity in the single-cell group, biodistribution of cells within the liver was visibly different between single-cell (Fig. 3, B) and spheroid (Fig. 3, C) mice. Intermouse variability was higher in the spheroid group, with a more heterogeneous distribution of cells when compared with single-cell mice (Figs. 3, C and D, Supplemental Fig 1). Three-dimensional video reconstructions of these images can be found in the supplemental materials.
Fig. 3.
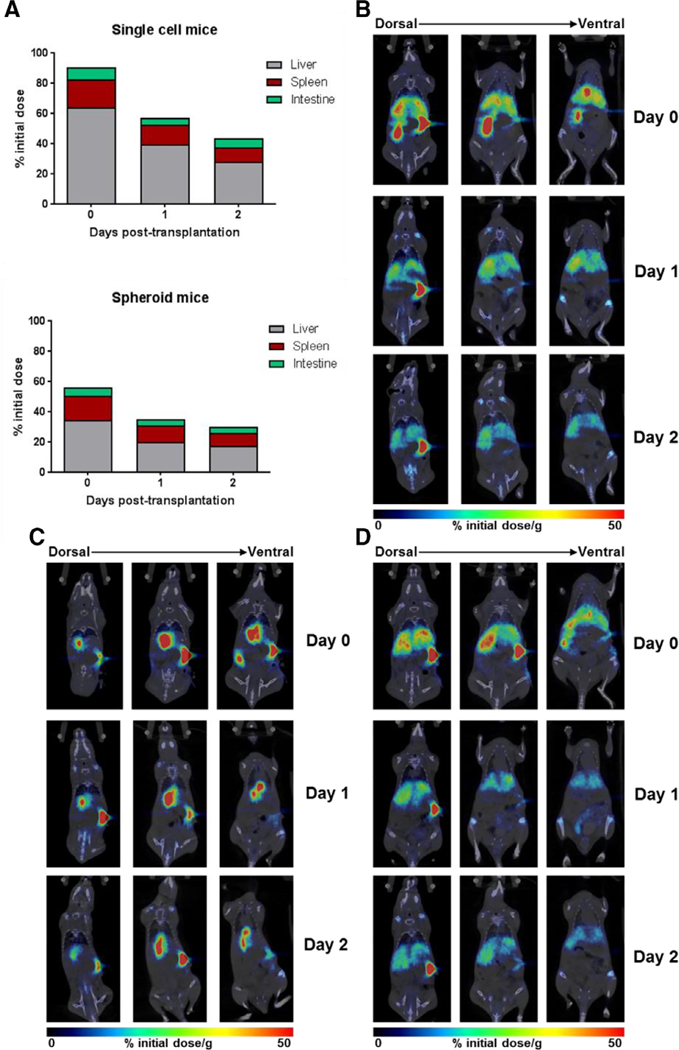
MicroPET-CT images of 89Zr-labeled single-cell and spheroid hepatocytes at 2, 24, and 48 hours post-transplantation in mice. (A) Percent of initial administered dose present in liver, spleen, and intestine at the 2-, 24-, and 48-hour time points in spheroid- and single cell-transplanted mice. (B) Coronal images of a representative single-cell-transplanted mouse, showing near homogenous distribution. (C and D) Coronal images of two representative spheroid-transplanted mice, showing intragroup differences in cell biodistribution.
Hepatocyte spheroids engraft in the liver in pigs
Spheroid hepatocyte transplantation was then compared with single-cell transplantation in wild-type pigs. After laparoscopic liver resection and ex vivo hepatocyte isolation, autologous hepatocytes were transplanted as single-cell suspension (n = 1) or spheroid suspension (n = 1) using ultrasound-guided percutaneous portal vein infusion. Each pig was injected with 4.18 × 108 radiolabeled cells (~0.1 MBq/106 cells, 8.36 × 105 cells/mL) in saline. No complication was noted in either procedure. PET-CT imaging at 6, 30, and 66 hours post-transplantation showed no difference in total radioactivity levels in the liver (8.50, 4.56, 3.91 versus 7.34, 4.90, 4.39 SUV; P > .99), spleen (1.29, 0.93, 0.39 versus 0.87, 0.32, 0.27 SUV; P = . 25), stomach (2.20, 0.64, 0.32 versus 5.03, 1.82, 1.40 SUV; P = . 75), or intestine (1.19, 0.79, 0.19 versus 1.24, 0.35, 0.15 SUV; P = .25) between the single-cell and spheroid animals (Fig. 4, A). There was no evidence of radiotracer present in the lungs or other organs. In both pigs, cells were efficiently cleared out of the spleen and stomach, with significant levels of radioactivity remaining only in the liver at 66 hours. However, PET-CT shows significant heterogeneity in the distribution pattern of the spheroid hepatocytes when compared with the single-cell suspension animal (Figs. 4, B and C). Axial PET-CT images from the spheroid transplant demonstrate a high signal intensity in the central segments of the liver with minimal distribution in the lateral segments. ln contrast, distribution of single-cell hepatocytes appears relatively homogeneous within the liver.
Fig. 4.
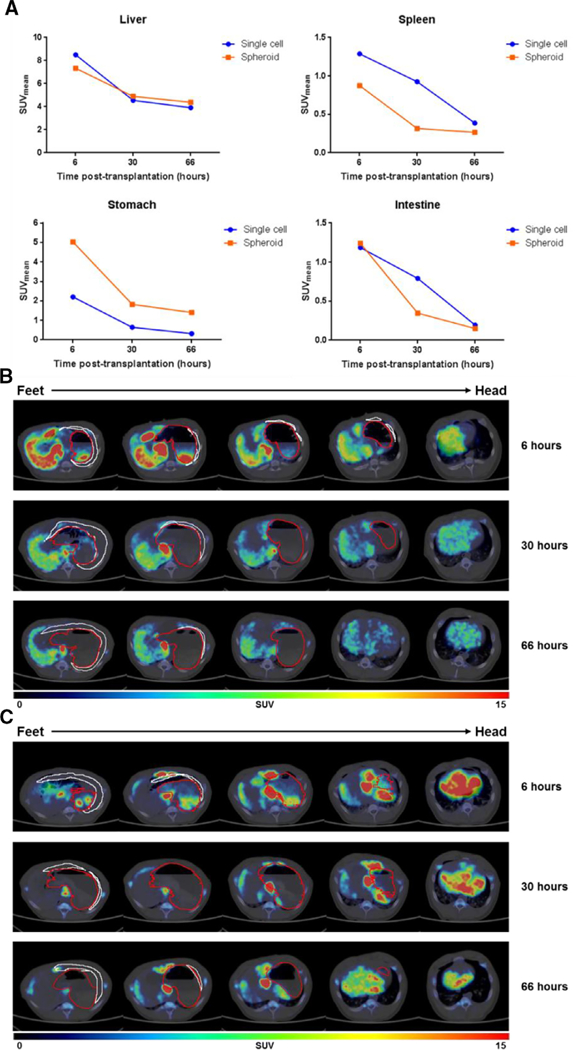
PET-CT images of 89Zr-labeled single-cell and spheroid hepatocytes at 6, 30, and 66 hours post-transplantation in pig. (A) Quantification of liver, spleen, stomach, and intestine standardized uptake values (SUV). (B and C) Axial views of five sequential PET-CT cuts in 19.6 mm slices at t 6, 30, and 66 hours after single-cell hepatocyte (B) or spheroid hepatocyte (C) transplantation in the pig. Stomach and spleen contours (outlined in red and white, respectively).
Hepatocyte spheroids are able to engraft and expand in a pig model of human HT1 after ex vivo lentiviral gene transfer
Autologous transplantation of spheroid hepatocytes was compared with single-cell suspension after ex vivo gene delivery of the porcine Fah gene in 6 Fah−/− pigs. Harvest results and dose of cells injected into each pig are presented in the Table 1. Pooled mean diameter of transplanted spheroids was 76.65 ± 18.97 μm. Portal pressures were monitored during intraportal infusion: single-cell transplanted animals experienced a mean increase in portal pressure of 2.53 ± 2.51 mmHg and animals transplanted with spheroid hepatocytes experienced a significantly higher mean increase in portal pressure of 10.98 ± 0.31 mmHg (P < .01; Fig. 5, A). ln all spheroid animals, injections were discontinued because of rises in portal pressure. Furthermore, two spheroid pigs developed portal vein thrombi (Fig. 5, B). These animals were treated with enoxaparin at the therapeutic dose of 1 mg/kg twice daily for 7 days, at which point ultrasound showed complete resolution of the thrombi. ln the third spheroid animal, systemic anticoagulation was performed with an 80 U/kg bolus of intravenous heparin immediately before transplantation, and no significant thrombotic complications were noted but significant changes in portal venous pressures were observed once again.
Table 1.
Summary of cell transplantations
| Pig lD | Single cell versus spheroid |
Weight at transplant (kg) |
Cells harvested (g) |
Viability (%) | Cells transplanted (g) |
Cells transplanted (× 106 ) |
Cells transplanted (× 106 / kg) |
|---|---|---|---|---|---|---|---|
| 889 | Single cell | 16.4 | 7.4 | 89 | 7.4 | 720 | 44 |
| 896 | Single cell | 18.2 | 4.8 | 52 | 4.8 | 250 | 14 |
| 960 | Single cell | 10.6 | 14.4 | 97 | 11.6 | 2,250 | 212 |
| Single cell mean ± SD | 15.1 ± 4.0 | 8.9 ± 5.0 | 79.3 ± 24.0 | 7.9 ± 3.4 | 1,100 ± 1,0 0 0 | 90 ± 110 | |
| 888 | Spheroid | 17.4 | 23.9 | 90 | 10.8 | 1,870 | 107 |
| 897 | Spheroid | 16.4 | 19.3 | 92 | 9.1 | 1,960 | 120 |
| 958 | Spheroid | 16.4 | 12.8 | 93 | 10.1 | 1,340 | 82 |
| Spheroid mean ± SD | 16.7 ± 0.6 | 18.7 ± 5.6 | 91.7 ± 1.5 | 10.0 ± 0.9 | 1,700 ± 300 | 100 ± 20 | |
Fig. 5.
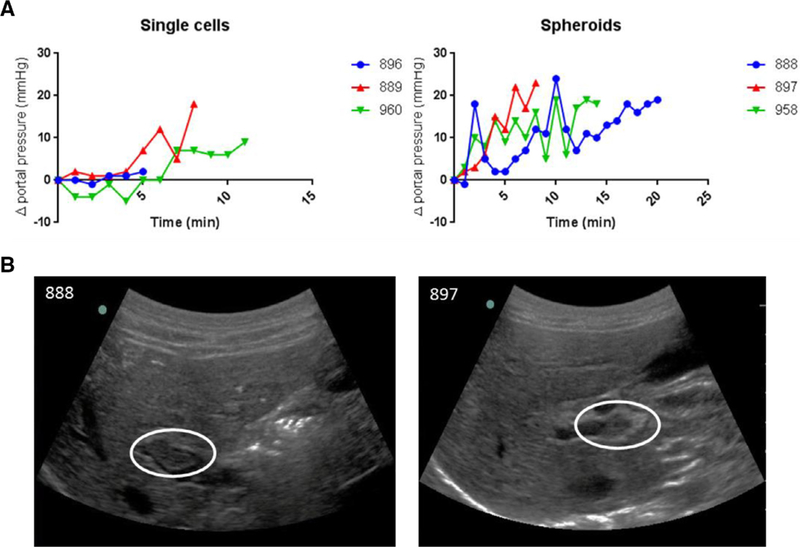
Safety data on percutaneous portal vein infusion of spheroid hepatocytes. (A) Average portal pressures during injection in single-cell and spheroid animals. (B) Ultrasound images showing the presence of thrombotic events in two spheroid hepatocyte transplanted pigs (white ovals). Pig 888, near-occlusive thrombus noted in the right anterior portal vein shortly after the start of the infusion. Pig 897, occlusive thrombus noted in the right main portal vein at the end of the infusion.
All animals remained on NTBC until the time of transplantation, at which point NTBC administration was discontinued to stimulate expansion of the transplanted FAH-positive hepatocytes. Animals were then cycled on and off NTBC based on weight and clinical parameters until weight stabilization occurred (Fig. 6, A). Only one single-cell animal (896) was kept as a control for the spheroid group. At 6 months post-transplantation, laparoscopic liver biopsies were performed on one single-cell (896) and one spheroid (888) animal. At 9–10 months post-transplantation, all animals were killed. Terminal histology showed robust FAH expression with multiple FAH-positive nodules occupying the three remaining liver lobes in all pigs, demonstrating expansion clusters of engrafted cells. Additionally, no evidence of any major pathology was found by H&E or Masson’s Trichrome staining (Fig. 6, B; Supplemental Fig. 2).
Fig. 6.
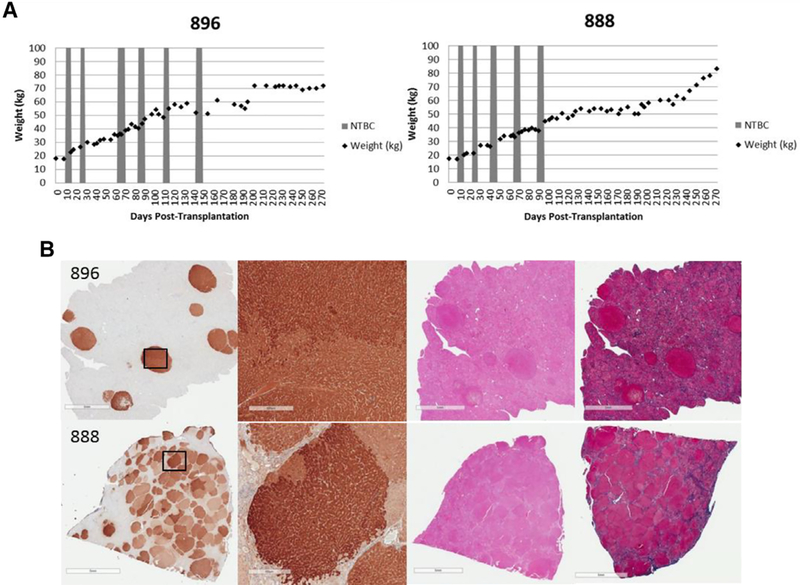
Weight gain and corresponding histology. (A) Weight stabilization of pigs 896 (single cell) and 888 (spheroid), demonstrating similar growth and NTBC-independence patterns. (B) Representative liver tissues stained for FAH from pigs 896 (top) and 888 (bottom ) at 9–10 months post-transplantation, showing near-complete liver repopulation with FAH-positive hepatocytes in pig 888. H&E-stained and Masson’s trichrome-stained serial liver sections are also presented. Scale bars, 5 mm (low magnification) and 500 pm (high magnification).
Discussion
Primary hepatocytes are difficult to maintain in vitro because of dedifferentiation and loss of proliferative capacity.25,26 Therefore, the clinical use of hepatocyte transplantation is still significantly limited by loss of viability and metabolic function in these cells.27,28 Spheroids, 3D hepatocyte aggregates, may circumvent some of these issues because of their improved longevity and phenotypic durability when compared with single-cell hepatocytes.13,14 Spheroid-like structures have been used experimentally to enhance cell transplantation in a number of studies. They have been demonstrated to improve transplanted cell survival and engraftment in both insulin-secreting islet cell and human cardiac progenitor cell transplantation.29,30 In another study, hepatocyte spheroids were created through a different method and transfected with non-viral vectors to show that subcutaneous transplantation of these spheroids resulted in longer-term transgene expression and preservation of proper functionality.31,32 These data, together with the higher lentiviral vector transduction efficiency we show in our rocker-formed spheroids, as compared with single-cell hepatocytes, make spheroids an interesting candidate for intraportal hepatocyte transplantation worth further development.
Our biodistribution studies showed initial presence of both single-cell hepatocytes and hepatocyte spheroids in the liver, spleen, and digestive tract, with cells being progressively cleared out of the spleen and digestive tract until only the liver remained positive. These results are consistent with those of other studies involving intrasplenic transplantation of cells in mice, where cells were found in the liver, spleen, stomach, and large intestine post-transplantation.33 Biodistribution of single-cell hepatocytes transplanted through portal vein infusion has been evaluated in humans with pediatric ornithine transcarbamoylase deficiency patients.34 In this study, a predominant hepatic distribution with an average liver-to-spleen ratio of 9.5 to 1 and no significant pulmonary radiotracer activity was found. Our results, obtained using a novel radiotracer and high-resolution imaging technology, are consistent with these data in both single-cell- and spheroid-transplanted pigs. Pulmonary translocation of hepatocytes after transplantation has been described in several studies,35,36 but we found no evidence of radiotracer in the pulmonary fields, possibly because of careful monitoring and prevention of portal hypertension in our models. Although there was no significant difference in overall biodistribution in terms of total cells in the liver between single-cell hepatocytes and hepatocyte spheroids, we found dissimilarities in distribution of cells within the liver between the two groups, with spheroids presenting a more uneven, irregular pattern. Heterogeneity in distribution of intraportally transplanted single-cell hepatocytes within the liver has been previously described.37 In this previous study, heterogeneity was because of differential intrahepatic portal venous blood flow. Heterogeneity observed in our study is likely because of spheroid size, where steric restrictions through the distal portal ramifications might limit transplant engraftment.
The thrombogenic potential of spheroids presents an important safety issue. It has been shown in pigs that portal pressures increase linearly with cell load and that, even with single-cell hepatocyte infusion, thrombi are formed in segmental portal branches.35 These concerns are magnified with the use of spheroids. In our study, portal pressures during infusion were significantly higher with spheroid than with single-cell suspensions, and both of the spheroid-transplanted animals that did not receive prophylactic heparin developed thrombotic complications requiring subsequent systemic anticoagulation. Safety issues, however, could be overcome by modification of infusion parameters and anticoagulation protocols. Use of a gravityfed bag system instead of the syringe method could allow for a controlled rate of infusion through a natural reduction in flow after any increase in portal pressure.38 Prophylactic systemic anticoagulation in our third pig appeared to prevent thrombus formation during spheroid infusion, although any interpretation must be contextualized as a single observation. The Edmonton Protocol for islet transplantation in humans includes a therapeutic heparin infusion, as well as low molecular weight heparin for a week after transplantation.22,39 Even so, partial-branch venous occlusion does occasionally occur and is managed with further anticoagulation and Doppler ultrasound follow-up without any further complications, as was the case in our study.
Another potential means to decrease the risk of thrombotic complications after hepatocyte transplantation while improving cell engraftment is to provoke sinusoidal vasodilation.40 Average human hepatic sinusoid diameter is under 10 μm,41 and average human hepatocyte diameter is just above 20 μm.42 By comparison, our average hepatocyte spheroid diameter was more than 70 μm. Therefore, sinusoidal vasodilation may be helpful for both single-cell and spheroid hepatocyte transplantation, but even with improved vasodilation we would expect spheroids to cause some degree of portal venous obstruction and lead to thrombotic events in a dose-dependent and rate-infusion–dependent manner. Finally, the remaining option to avoid the thrombogenic potential of spheroids that should be explored in future experiments would be to trypsinize these aggregates into single-cell hepatocytes before transplantation. By doing this, we would be taking advantage of the benefits spheroids offer in culture and completely bypassing the safety concerns associated to spheroid transplantation, at the cost of a certain percentage of viability loss.
Despite safety issues and differences in distribution between single-cell hepatocytes and spheroid hepatocytes, we have demonstrated that spheroid transplantation with hepatocytes after ex vivo gene therapy can effectively correct the metabolic deficiency in the clinically relevant pig model of HT1. Spheroids demonstrated the ability to engraft, proliferate, and eventually repopulate Fah−/− livers, making this method a viable alternative to single-cell hepa- tocyte transplantation for the treatment of metabolic liver disease. Furthermore, conclusions drawn from this study are applicable to allogeneic hepatocyte transplantation for the treatment of other liver diseases.
This study has several limitations. First, only two experimental animals were used to evaluate large-scale biodistribution, and six experimental animals were used to evaluate phenotypic HT1 correction after ex vivo gene therapy, of which only three received spheroids. Therefore, this study is not powered to establish superiority of one approach over the other, but to establish spheroid hepatocytes as a valid alternative to single-cell hepatocytes for transplantation after ex vivo gene therapy. More rigorous development and testing of transplantation protocols, including infusion methods and anticoagulation practices, are indicated. In addition, even with the use of spheroids, the main limitation to hepatocyte transplantation for metabolic liver disease remains: cell engraftment and proliferation, especially in diseases in which, unlike in HT1, a selective advantage does not exist for the transplanted cell to proliferate. Repopulation can be partially increased through several liver preconditioning methods before hepatocyte transplantation.43 These methods include partial hepatectomy, which has been trialed in humans,44,45 as well as liver irradiation and portal vein embolization, which have yielded promising results in non-human primate models.46,47 However, this problem will only be fully overcome with the ability to produce large quantities of human hepatocytes for transplant.
In conclusion, we have shown spheroid hepatocytes to be a relevant alternative to single-cell hepatocytes for transplantation after ex vivo gene therapy. Hepatocytes cultured to generate spheroids demonstrated a significantly higher efficiency of vector transduction and a comparable overall volume of cells within the liver after transplantation, despite showing a more heterogeneous biodistribution than single-cell hepatocytes. Importantly, spheroid transplantation resulted in successful engraftment and in vivo expansion of corrected autologous hepatocytes, and treatment of the clinically relevant large animal model of human HT1.
Supplementary Video 1. 3D render movie of microPET-CT images of 89 Zr-labeled single cell hepatocytes at 2 h post-transplantation in mice.
Supplementary Material
Acknowledgments
We thank D. Meixner for portal vein cannulation, T. Decklever for small animal PET imaging support, and T. Wyman and L. Filzen for large animal PET imaging support, L. Gross for histology support. We thank Children’s Hospital of Minnesota Foundation for financial support.
✰This work was supported by the Children’s Hospital of Minnesota Foundation.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.surg.2018.04.012.
References
- 1.Lindblad B, Lindstedt S , Steen G. On the enzymic defects in hereditary tyrosine- mia. Proc Natl Acad Sci U S A. 1977;74:4641–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grompe M The pathophysiology and treatment of hereditary tyrosinemia type 1. Semin Liver Dis. 2001;21:563–571. [DOI] [PubMed] [Google Scholar]
- 3.Endo F, Sun MS. Tyrosinaemia type I and apoptosis of hepatocytes and renal tubular cells. J Inherit Metab Dis. 2002;25:227–234. [DOI] [PubMed] [Google Scholar]
- 4.Lindstedt S Holme E Lock EA Hjalmarson O Strandvik B Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet. 1992;340:813–817. [DOI] [PubMed] [Google Scholar]
- 5.Mayorandan S Meyer U Gokcay G Segarra NG de Baulny HO van Spronsen F et al. Cross-sectional study of 168 patients with hepatorenal tyrosinaemia and implications for clinical practice. Orphanet J Rare Dis. 2014;9:107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Laet C, Dionisi-Vici C, Leonard JV, McKiernan P, Mitchell G, Monti L, et al. Recommendations for the management of tyrosinaemia type 1 Orphanet J Rare Dis 2013. ; 8 : 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Ginkel WG. Jahja R Huijbregts SC Daly A MacDonald A De Laet C et al. Neurocognitive outcome in tyrosinemia type 1 patients compared to healthy controls. Orphanet J Rare Dis. 2016;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickey RD, Mao SA, Glorioso J, Elgilani F , Amiot B, et al. Curative ex vivo liver-directed gene therapy in a pig model of hereditary tyrosinemia type 1. Sci Transl Med. 2016;8 349ra399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elaut G , Henkens T, Papeleu P, Snykers S, Vinken M, Vanhaecke T, et al. Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr Drug Metab. 2006;7:629–660. [DOI] [PubMed] [Google Scholar]
- 10.Landry J, Bernier D, Ouellet C, Goyette R , Marcea N. Spheroidal aggregate culture of rat liver cells: Histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyberg SL, Hardin J, Amiot B, Argikar UA, Remmel RP, Rinaldo P. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transpl. 2005;11:901–910 . [DOI] [PubMed] [Google Scholar]
- 12.Abu-Absi SF, Friend JR, Hansen LK, Hu WS. Structural polarity and functional bile canaliculi in rat hepatocyte spheroids. Exp Cell Res. 2002;274:56–67. [DOI] [PubMed] [Google Scholar]
- 13.Luebke-Wheeler JL, Nedredal G, Yee L, Amiot BP, Nyberg SL E-cadherin protects primary hepatocyte spheroids from cell death by a caspase-independent mechanism. Cell Transplant. 2009;18:1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brophy CM, Luebke-Wheeler JL, Amiot BP, Khan H, Remmel RP, Rinaldo P et al. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology. 2009;49:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickey RD, Lillegard JB, Fisher JE, McKenzie TJ, Hofherr SE, Finegold MJ et al. Efficient production of Fah-null heterozygote pigs by chimeric adeno-associated virus-mediated gene knockout and somatic cell nuclear transfer. Hepatology. 2011;54:1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickey RD, Mao SA, Glorioso J, Lillegard JB, Fisher JE, Amiot B, et al. Fumarylace-toacetate hydrolase deficient pigs are a novel large animal model of metabolic liver disease. Stem Cell Res. 2014;13:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sielaff TD, Hu MY, Rao S, Groehler K, Olson D, Mann HJ, et al. A technique for porcine hepatocyte harvest and description of differentiated metabolic functions in static culture. Transplantation. 1995;59:1459–1463. [DOI] [PubMed] [Google Scholar]
- 18.Pandey MK, Bansal A, Engelbrecht HP, Byrne JF, Packard AB, DeGrado TR Improved production and processing of 89 Zr using a solution target. Nucl Med Biol. 2016;43:97–100 . [DOI] [PubMed] [Google Scholar]
- 19.Pandey MK. Engelbrecht HP, Byrne JP, Packard AB, DeGrado TR Production of 89Zr via the 89Y(p,n)89Zr reaction in aqueous solution: Effect of solution composition on in-target chemistry. Nucl Med Biol. 2014;41:309–316. [DOI] [PubMed] [Google Scholar]
- 20.Bansal A, Pandey MK. Demirhan YE, Nesbitt JJ, Crespo-Diaz RJ, Terzic A et al. Novel (89)Zr cell labeling approach for PET-based cell trafficking studies. EJNMMI Res. 2015;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lillegard JB, Fisher JE, Nedredal G, Luebke-Wheeler J, Bao J, Wang W, et al. Normal atmospheric oxygen tension and the use of antioxidants improve hepatocyte spheroid viability and function. J Cell Physiol . 2011;226:2987–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro AM. Islet transplantation in type 1 diabetes: Ongoing challenges, refined procedures, and long-term outcome. Rev Diabet Stud. 2012;9:385–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickey RD, Mao SA, Amiot B, Suksanpaisan L, Miller A, Nace R, et al. Noninvasive 3-dimensional imaging of liver regeneration in a mouse model of hereditary tyrosinemia type 1 using the sodium iodide symporter gene. Liver Transpl. 2015;21:442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y Hong H, Cai W, PET tracers based on Zirconium-89. Curr Radiopharm. 2011;4:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauschke VM. Vorrink SU, Moro SM, Rezayee F, Nordling Å, Hendriks DF, et al. Massive rearrangements of cellular MicroRNA signatures are key drivers of hepatocyte dedifferentiation. Hepatology. 2016;64:1743–1756. [DOI] [PubMed] [Google Scholar]
- 26.Ramboer E De Craene B, De Kock J, Vanhaecke T, Berx G, Rogiers V et al. Strategies for immortalization of primary hepatocytes. J Hepatol. 2014;61:925–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes RD. Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012;93:342–347. [DOI] [PubMed] [Google Scholar]
- 28.Gramignoli R, Vosough M, Kannisto K, Srinivasan RC, Strom SC. Clinical hepatocyte transplantation: Practical limits and possible solutions. Eur Surg Res. 2015;54:162–177 . [DOI] [PubMed] [Google Scholar]
- 29.Kusamori K Nishikawa M, Mizuno N, Nishikawa T, Masuzawa A, Shimizu K. et al. Transplantation of insulin-secreting multicellular spheroids for the treatment of type 1 diabetes in mice. J Control Release. 2014;173:119–124. [DOI] [PubMed] [Google Scholar]
- 30.Oltolina F, Zamperone A, Colangelo D. Gregoletto L, Reano S, Pietronave S, et al. Human cardiac progenitor spheroids exhibit enhanced engraftment potential. PLoS One. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchida S, Itaka K, Nomoto T, Endo T, Matsumoto Y, Ishii T, et al. An injectable spheroid system with genetic modification for cell transplantation therapy. Biomaterials. 2014;35:2499–2506. [DOI] [PubMed] [Google Scholar]
- 32.Itaka K Uchida S, Matsui A, Yanagihara K, Ikegami M, Endo T, et al. Gene transfection toward spheroid cells on micropatterned culture plates for genetically- modified cell transplantation. J Uis Exp. 2015;101:e52384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagamoto Y Takayama K, Ohashi K, Okamoto R, Sakurai F, Tachibana M, et al. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64:1068–1075. [DOI] [PubMed] [Google Scholar]
- 34.Bohnen NI, Charron M, Reyes J, Rubinstein W, Strom SC, Swanson D, et al. Use of indium-111-labeled hepatocytes to determine the biodistribution of transplanted hepatocytes through portal vein infusion. Clin Nucl Med. 20 0 0;25:447–450. [DOI] [PubMed] [Google Scholar]
- 35.Muraca M , Charron M, Reyes J, Rubinstein W, Strom SC, Swanson D, et al. Intraportal hepatocyte transplantation in the pig: Hemodynamic and histopathological study. Transplantation. 2002;73:890–896. [DOI] [PubMed] [Google Scholar]
- 36.Shigeta T, Hsu HC, Enosawa S, Matsuno N, Kasahara M, Matsunari H, et al. Transgenic pig expressing the red fluorescent protein kusabira-orange as a novel tool for preclinical studies on hepatocyte transplantation. Transplant Proc. 2013;45:1808–1810. [DOI] [PubMed] [Google Scholar]
- 37.Timm F Vollmar B, Heterogeneity of the intrahepatic portal venous blood flow: Impact on hepatocyte transplantation. Microvasc Res. 2013;86:34–41. [DOI] [PubMed] [Google Scholar]
- 38.Baidal DA. Froud T, Ferreira JV, Khan A, Alejandro R, Ricordi C. The bag method for islet cell infusion. Cell Transplant. 2003;12:809–813. [DOI] [PubMed] [Google Scholar]
- 39.Koh A, Senior P, Salam A, Kin T, Imes S, et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010;89:465–471. [DOI] [PubMed] [Google Scholar]
- 40.Slehria S, Rajvanshi P, Ito Y, Sokhi RP , Bhargava KK, Palestro CJ, et al. Hepatic sinusoidal vasodilators improve transplanted cell engraftment and ameliorate microcirculatory perturbations in the liver. Hepatology . 2002;35:1320–1328. [DOI] [PubMed] [Google Scholar]
- 41.Wake K Sato T, “The sinusoid” in the liver: Lessons learned from the original definition by Charles Sedgwick Minot (1900). Anat Rec (Hoboken). 2015;298:2071–2080. [DOI] [PubMed] [Google Scholar]
- 42.Yoshizato K Growth potential of adult hepatocytes in mammals: Highly replicative small hepatocytes with liver progenitor-like traits. Dev Growth Differ. 2007;49:171–184. [DOI] [PubMed] [Google Scholar]
- 43.Puppi J Strom SC, Hughes RD, Bansal S, Castell JV, Dagher I, et al. Improving the techniques for human hepatocyte transplantation: Report from a consensus meeting in London. Cell Transplant. 2012;21:1–10. [DOI] [PubMed] [Google Scholar]
- 44.Grossman M, et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995;1:1148–1154 . [DOI] [PubMed] [Google Scholar]
- 45.Jorns C Nowak G, Nemeth A, Zemack H, Mörk LM, Johansson H, et al. De Novo Donor-Specific HLA Antibody Formation in two patients with Crigler-Najjar Syndrome Type I following human hepatocyte transplantation with partial hepatectomy preconditioning. Am J Transplant. 2016;16:1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamanouchi K Zhou H, Roy-Chowdhury N, Macaluso F, Liu L, Yamamoto T, et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology. 2009;49:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dagher I et al. Efficient hepatocyte engraftment and long-term transgene expression after reversible portal embolization in nonhuman primates. Hepatology. 2009;49:950–959 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


