Abstract
Neurotransmitter release from frog motor nerve terminals is strongly modulated by change in muscle length. Over the physiological range, there is an ∼10% increase in spontaneous and evoked release per 1% muscle stretch. Because many muscle fibers do not receive suprathreshold synaptic inputs at rest length, this stretch-induced enhancement of release constitutes a strong peripheral amplifier of the spinal stretch reflex. The stretch modulation of release is inhibited by peptides that block integrin binding of natural ligands. The modulation varies linearly with length, with a delay of no more than ∼1-2 msec and is maintained constant at the new length. Moreover, the stretch modulation persists in a zero Ca2+ Ringer and, hence, is not dependent on Ca2+ influx through stretch activated channels. Eliminating transmembrane Ca2+gradients and buffering intraterminal Ca2+ to approximately normal resting levels does not eliminate the modulation, suggesting that it is not the result of release of Ca2+ from internal stores. Finally, changes in temperature have no detectable effect on the kinetics of stretch-induced changes in endplate potential (EPP) amplitude or miniature EPP (mEPP) frequency. We conclude, therefore, that stretch does not act via second messenger pathways or a chemical modification of molecules involved in the release pathway. Instead, there is direct mechanical modulation of release. We postulate that tension on integrins in the presynaptic membrane is transduced mechanically into changes in the position or conformation of one or more molecules involved in neurotransmitter release, altering sensitivity to Ca2+ or the equilibrium for a critical reaction leading to vesicle fusion.
Keywords: synaptic plasticity, EPPs, mEPPs, muscle length, neuromuscular junction, mechanotransduction, RGD, muscle stretch
Transmitter release from frog motor nerve terminals is powerfully regulated by muscle length (Fatt and Katz, 1952; Kuffler, 1952). A muscle stretch of 5–10% can cause more than a doubling in endplate potential (EPP) amplitude or miniature EPP (mEPP) frequency, with no change in mEPP amplitude (Hutter and Trautwein, 1956; Turkanis, 1973). Over the physiological range (up to 10–15% stretch), the enhancement is approximately linear with length, is maintained constant at a new length, and is readily reversible (Hutter and Trautwein, 1956; Turkanis, 1973). In contrast to the enhancement of mEPP frequency as a result of K+-induced depolarization, the stretch enhancement is not affected by high Mg2+ in the Ringer (Hutter and Trautwein, 1956); hence, it is not a result of a stretch-induced depolarization of the terminal. Moreover, the increase in mEPP frequency is not suppressed in a Ringer containing 10 mm Mg2+ and 0.1 mmCa2+, suggesting to Turkanis (1973) that it is not dependent on Ca2+ influx. However, this conclusion predated the discovery of stretch-activated channels (SACs) (Yang and Sachs, 1990; French, 1992; Martinac, 1993; Sackin, 1995), some of which might be Ca2+-permeable and not blocked by Mg2+.
We have reported recently that the effects of stretch on neurotransmitter release can be suppressed by exposure to peptides containing the tripeptide arginine–glycine–aspartic acid (RGD), which interferes with binding of integrins to their native ligands (Chen and Grinnell, 1995). Changes in external binding of ligands by integrins can trigger profound changes within cells, such as activation of phospholipase C; production of DAG and IP3; stimulation of Ca2+ influx and Ca2+ release from internal stores; cytoplasmic alkylization; activation of protein kinase C; induction of NO synthase; induction of immediate early gene expression; and initiation of tyrosine phosphorylation cascades that have strong influences on cell shape, motility, and cytoskeletal organization (see, for example, Schwartz et al., 1991; Fujimoto et al., 1991; Damsky and Werb, 1992; Hynes, 1992; Kornberg and Juliano, 1992; Cybulsky et al., 1993; Schwartz, 1993; Miyamoto et al., 1995; Schaller et al., 1995). By their connections both to the ECM and to the semirigid cytoskeleton, integrins are also capable of transducing mechanical signals in both directions across the cell membrane (Ingber, 1991; Wang et al., 1993), potentially causing important biochemical changes, e.g., by bringing kinases closer to their substrates or changing reaction equilibria by altering protein conformation or stress on noncovalent bonds (Damsky and Werb, 1992; Miyamoto et al., 1995; Ingber, 1996).
In this paper, we present additional evidence implicating integrins in the enhancement, and describe experiments designed to determine, in a generic sense, the mechanism(s) of enhancement. Our data suggest that interference with integrin bonds eliminates much or all of the phenomenon, that the enhancement does not require Ca2+influx or release of Ca2+ from internal stores (although some basal level of intraterminal Ca2+ is necessary), and that the modulation does not depend on stretch-mediated biochemical processes such as phosphorylation/dephosphorylation reactions. Instead, this seems to be an entirely mechanical form of modulation, altering the probability of a diffusion-limited process that strongly influences release. To the best of our knowledge, this represents the fastest, most powerful form of integrin-mediated mechanical modulation of cellular physiology reported to date.
MATERIALS AND METHODS
Preparations and solutions
Rana pipiens.Rana pipiens (2.5–3 inches, nose to anus) were anesthetized by immersion in 0.1% tricaine methanesulfonate (Sigma), double pithed, and the sartorius nerve-muscle preparation was dissected out. At rest length (with the hind limbs extended backward at a 45° angle, the knees bent, and the distal part of the leg pointed directly backward), the sarcomere spacing is ∼2.25 μm, which was used as the criterion for rest length.
Muscles were held in a bathing chamber accessible to both dissecting and compound microscope examination, fixed at either end to moveable arms (see below). The nerve was pulled into a suction electrode for stimulation at different lengths or phases of a lengthening–shortening cycle. The bath temperature was controlled by a Peltier device affixed to the metal plate, into which the preparation chamber fitted. The temperature could be varied in a controllable way between ∼10° and 23°.
Normal frog Ringer (NFR) contained (in mm): 116 NaCl, 1 NaHC03, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 Na-HEPES, and 5 HEPES, pH 7.3. “Zero Ca2+ Ringer” contained (in mm): 2 MgCl2, 1 EGTA, 116 NaCl, 2 KCl, 1 NaHCO3, 5 Na-HEPES, and 5 HEPES, pH 7.3. When recording mEPPs, usually 1 μg/ml neostigmine is added to the Ringer. Recordings at full quantal content were made with 3–6 μmd-tubocurarine chloride (curare) in the NFR, or after 0.5 hr treatment with μ-conotoxin, which blocks Na+ channels in muscle but not in nerve (Robitaille and Charlton, 1992; Cruz et al., 1985). Reduced quantal content EPPs were obtained by lowering the Ca2+:Mg2+ ratio in the Ringer, usually to 0.56 mm Ca2+, 4 mm Mg2+(“low Ca2+ Ringer”).
Terminal loading with Ca2+ buffers. In several experiments, nerve terminals were loaded with BAPTA or dimethyl (DM)-BAPTA. With techniques developed by Robitaille and Charlton (1992), preparations were incubated for 60–90 min at room temperature with 10–25 μm of the −AM form of the buffer in NFR, made up from a stock solution of 1–5 mm in DMSO, with 0.02% w/w pluronic acid (Molecular Probes) to facilitate loading of the buffer. Twenty micromolar TPEN [tetrakis(2-pyridylmethyl)ethylenediamine] (Molecular Probes) was added to chelate heavy metals. Controls were treated with the same final concentrations of DMSO, pluronic acid, and TPEN as experimentals. The −AM buffer enters the terminals, where it becomes trapped and concentrated, typically into the mm range, as cytoplasmic esterases convert it into the active (Ca2+-binding) membrane impermeant form.
Recording and data analysis
Intracellular recordings were made with microelectrodes of 20–60 MΩ resistance, filled either with 3 m KCl or with 0.6 m K2SO4 + 5 mm KCl (D’Alonzo and Grinnell, 1985). For almost all recordings, to maintain penetrations during changes in muscle length, “floating” electrodes were used. These are made by breaking off the shank and tip of a standard sharp microelectrode containing an internal filament (Garner Glass Company, KG-33), allowing it to be filled by capillarity and backfilling. This reduced electrode is then affixed on the end of a long (7 cm), partly coiled, flexible 100 μm diameter silver electrode wire connected to the input stage of an AM-2 Physiological Amplifier (Biodyne, Mission Viejo, CA). The analog output of the amplifier was digitized with a Labmaster DMA analog/digital 12 bit interface (Scientific Solutions, Solon, OH) and stored on the hard disk of a Dell 386 PC.
Stimulation of the preparation and recording of data, as well as subsequent analysis, was carried out using pClamp version 5.5 software (Axon Instruments, Foster City, CA). EPPs were stored and analyzed in blocks of 25 (for each different stretch state of the muscle) using the pClamp modules “Clampex” and “Clampan.” MEPPs were detected using an Axon Instruments A2020A Event Detector and recorded and analyzed for frequency in blocks of 25–100 (depending on mEPP frequency) using the modules “Fetchex” and “Fetchan.” Data were also recorded by FM tape recorder (Store 4D; Lockheed Electronics Co., Inc., Plainfield, NJ), and by chart recorder (Lineacorder Mark VII WR3101, Western Graphtec, Irvine, CA). We found that using paired muscles from the same preparation as controls reduced variability in the results. Data are given as mean ± SE, and significance values were determined by Student’s t test.
Imposition of stretch. For measurements of the kinetics of stretch modulation of release, we used an analog servomotor-drive apparatus in which the two ends of the sartorius were attached to the two writing arms of an MFE 1200 chart recorder that had been removed from the writing assembly with their motors and mounted next to the bath. Mirror image electrical signals from the computer moved the two arms by equal amounts in opposite directions. This greatly reduced the longitudinal displacement of the floating electrode, making it easier to maintain good penetrations. These writing arms are strong enough to impose stretch controlled accurately in magnitude (up to 4 mm) and rate (up to 1 mm/5 msec).
Direct calibration of muscle length was done with an optical technique. For these measurements, muscles were pinned at one end and attached to one of the moving arms at the other. The bottom of the bath was covered with an opaque layer of aluminum foil, into which was cut a uniform narrow slit, immediately to one side of the muscle, through which light was passed. A small infrared phototransistor was located above the slit to measure the amount of light passing through it. A small piece of aluminum foil was affixed at the mid-region of the muscle, projecting over the slit. As the muscle was stretched, an increasing fraction of the light was occluded. This procedure was not done in every muscle, but measurements in several muscles showed almost no variability and established that the muscle length closely paralleled the movement of the stretching arms. Multiple records were averaged to reduce noise in the phototransistor circuit, resulting in the traces shown in Figures 4and 5. Stimuli to the nerve could be applied at any desired time before, during, or after a stretch. The sartorius muscles in the frogs we used were all ∼30 mm long. In most experiments, stretches of 1 and/or 2 mm were imposed, corresponding to increases in length of 3% and 6%.
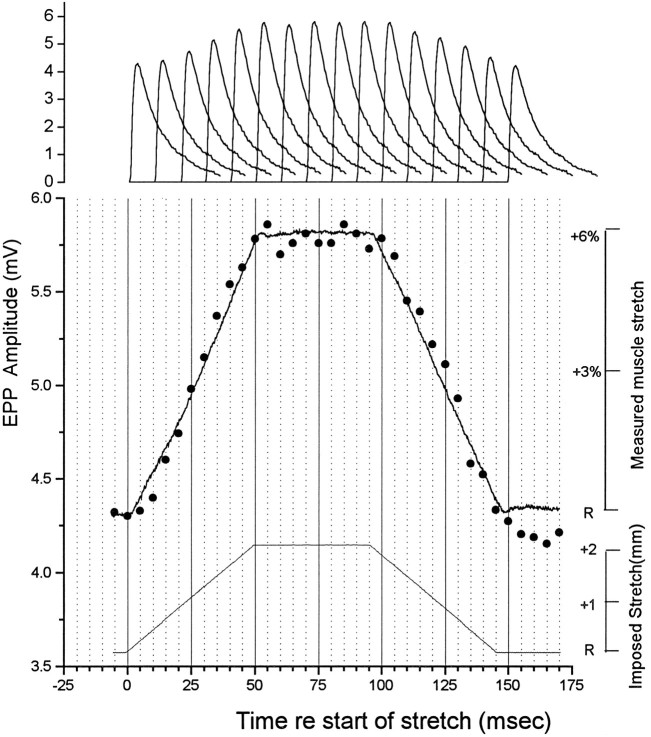
Fig. 4.
Kinetics of stretch enhancement of EPP amplitude in a representative sartorius junction in low Ca2+ Ringer. The muscle was attached at either end to a pair of arms that moved simultaneously in opposite directions in response to a command signal from the computer, beginning to lengthen at 0 msec, reaching 2 mm (6%) stretch at 50 msec, holding that length for 45 msec, and shortening in another 50 msec. The actual measured change in length of the muscle (noisy solid line) and corresponding EPP amplitudes at 36 time points during the cycle (filled circles) are shown in the middle records, and sample EPPs taken at different times are shown at the top. Each point is the average of 50 or more EPPs, each evoked in a single lengthening–shortening cycle.
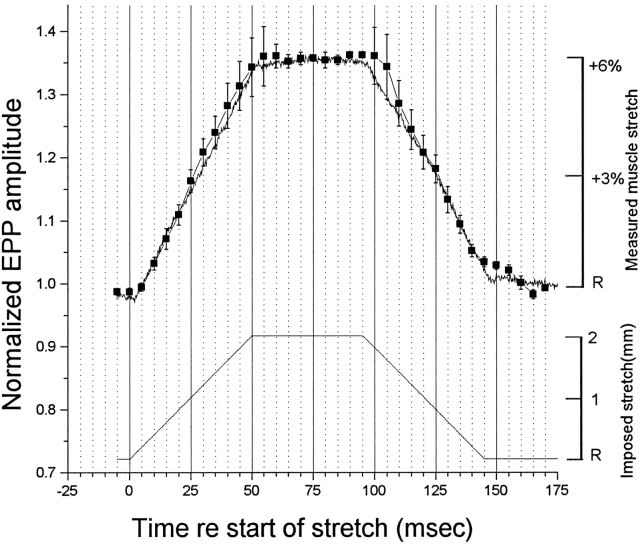
Fig. 5.
Kinetics of stretch enhancement of EPP amplitude. Average of six experiments like that shown in Figure 4. Note the relatively large arrow bars at the peak of the lengthening phase and the beginning of shortening. Muscle length is shown by the noisy line, where the noise arose primarily in the light source used to measure length (see Materials and Methods). Except at the beginning of the shortening phase, there was virtually no delay between change in muscle length and change in release efficacy.
Integrin-blocking reagents. The hexapeptide GRGDSP (RGD, Peninsula Labs) was used as an agent that is known in many other systems to block integrin binding to natural ligands (Pierschbacher and Ruoslahti, 1987, Albeda and Buck, 1990, Hynes, 1992), whereas GRGESP (RGE) was used as the inactive control.
RESULTS
Magnitude and linearity of enhancement of release by stretch
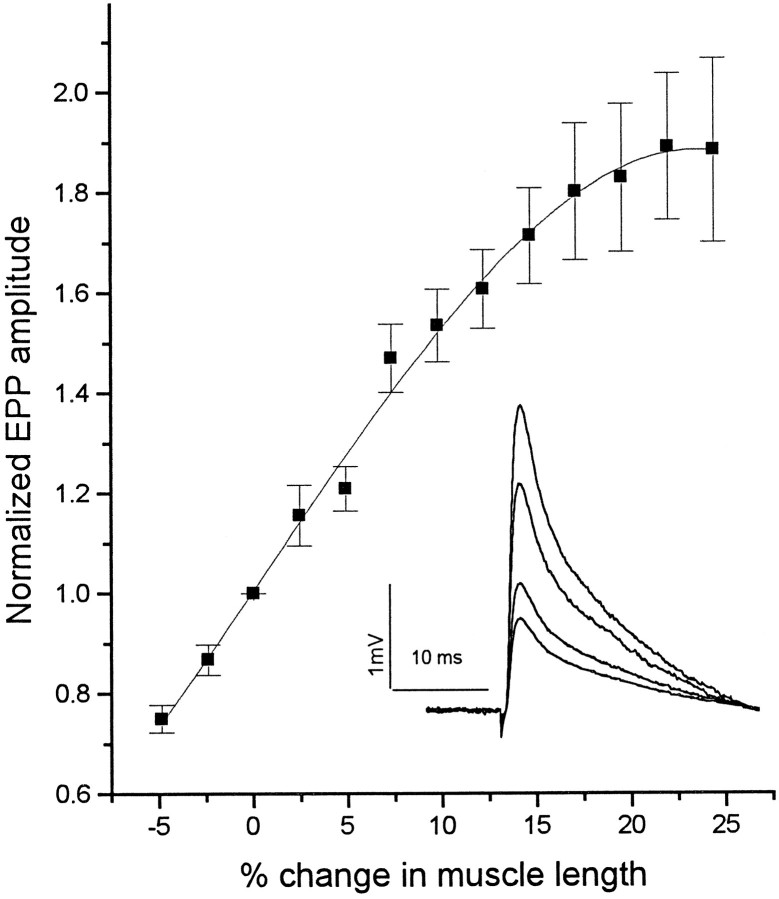
For the population of sartorius junctions as a whole, a 3% stretch caused a 32.6 ± 1.6% (n = 315) increase in mEPP frequency; a 6% stretch caused a 68.9 ± 2.9 (n = 287) increase (see Figs. 7 and 8). EPP amplitudes are also sharply enhanced by stretch. In a Ringer containing 0.56 mm Ca2+ and 4 mm Mg2+, in which EPP quantal contents were reduced below threshold for action potential generation (below ∼10), the EPP amplitude changed by an average 31 ± 3.9% (n = 32) at 3% stretch and 63 ± 5.9% (n = 27) at 6% stretch. The inset in Figure 1 shows EPPs recorded from a sensitive preparation at 95, 100, 110, and 120% of rest length. The enhancement was 76% at 10% stretch and 243% at 20% stretch. As Figure 1 shows, the enhancement is nearly linear in magnitude from ∼5-10% below rest length to 15–20% stretch. The enhancement tended to plateau above 15–20% stretch. Most of our experiments used stretches of 6% or less.
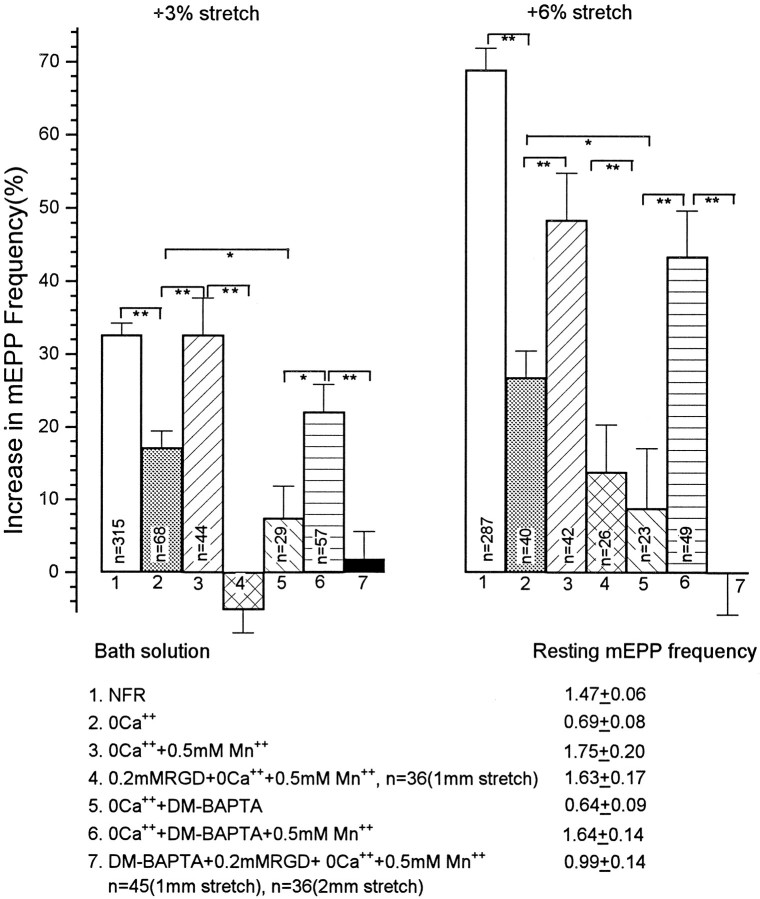
Fig. 7.
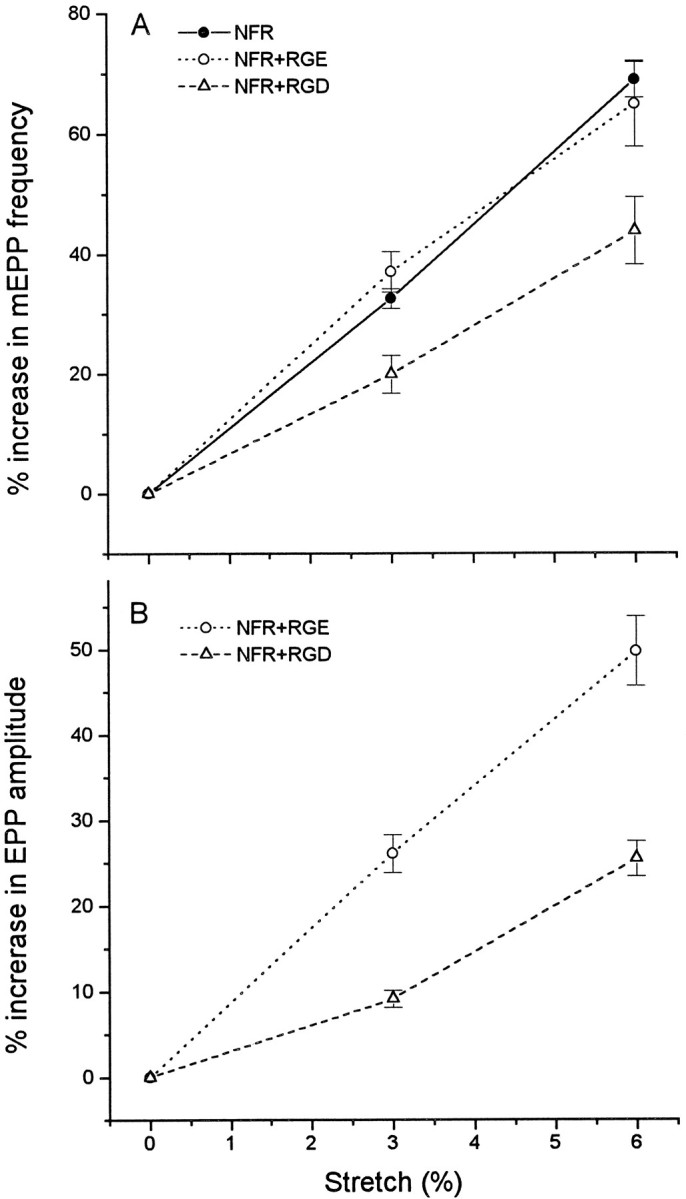
Suppression of the stretch enhancement by RGD peptides. The graphs show that enhancement of mEPP frequency and EPP amplitude was approximately linear with length and that 0.2 mm RGD, but not the inactive control RGE, suppressed the effect. EPPs were recorded in 3–6 μm curare.
Fig. 8.
Effects of manipulations affecting Ca2+ levels on the magnitude of the increase in mEPP frequency with stretch. Zero Ca2+ Ringer reduced the enhancement by ∼50% (column 2), but the enhancement was still highly significant. Mn2+ (0.5 mm) restored much of the enhancement (column 3), which was then essentially totally blocked by 0.2 mm RGD, applied both before and after addition of Mn2+ (column 4). Loading of terminals with a strong Ca2+buffer by exposure to DM-BAPTA-AM in zero Ca2+ Ringer strongly suppressed the enhancement (column 5), but again 0.5 mm Mn2+ mostly restored it (column 6); 0.2 mm RGD, applied both in the zero Ca2+ Ringer and during subsequent addition of Mn2+, totally eliminated the enhancement (column 7). These data show that Ca2+ influx is not necessary for the enhancement and that the enhancement can be totally eliminated by agents that interfere with integrin binding. Absolute mEPP frequencies at rest length are also indicated for each condition, and asterisks indicate the probability of the indicated matches (*p < 0.05, **p < 0.005).
Fig. 1.
Average change in EPP amplitude as a function of stretch of the sartorius muscle. Mean ± SE for six junctions.Inset, EPPs obtained from a sensitive preparation at 95, 100, 110, and 120% of rest length. All in low Ca2+ Ringer containing 0.56 mm Ca2+ and 4 mmMg2+.
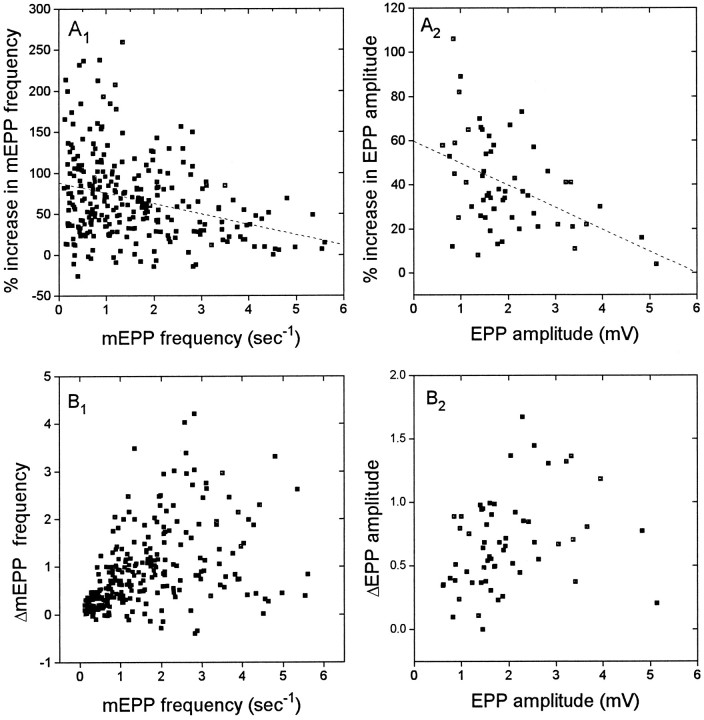
There is considerable variability in the sensitivity of different junctions to stretch. The basis for this variability is not known. Figure 2A shows plots of the percentage increase in mEPP frequency and EPP amplitude as a function of the values at rest length. There is clearly tremendous variability in the degree of enhancement for junctions of any given resting mEPP frequency or EPP amplitude. For the population as a whole, there was a mean inverse relationship between the percent enhancement and the synaptic efficacy at rest length. Because EPP amplitude is primarily a function of Ca2+ influx, and mEPP frequency also is significantly dependent on external Ca2+ (Grinnell and Pawson, 1989), this inverse relationship might be interpreted as indicating that stretch adds a certain amount of free Ca2+within the terminal and that this increment is decreasingly important as resting synaptic strength increases. However, as Figure2B shows, there was a positive relationship between the absolute stretch enhancement and the rest length synaptic efficacy, at least for the weaker junctions (mEPP frequency up to ∼2 Hz and EPP amplitudes up to ∼2 mV). For stronger junctions this positive relationship disappeared, and there was no apparent correlation between resting mEPP frequency or EPP amplitude and the degree of stretch enhancement.
Fig. 2.
Variability and sensitivity of different junctions to 6% muscle stretch. A1 andA2, scattergraphs showing percentage increase in mEPP frequency and EPP amplitude for different junctions, plotted against their release at rest length. The vast majority of junctions showed an increase in release with stretch, but it varied between no change and a two- to threefold increase. The dashed lines represent linear best fits. B1 andB2, absolute values of change in mEPP frequency and EPP amplitude in the same junctions. Note that there was little or no correlation between degree of enhancement and resting release levels in the stronger junctions. EPP recordings were in 3 μmcurare.
In contrast to the variability observed among different junctions, the stretch enhancement of release at any particular junction was essentially the same through repeated cycles of lengthening and shortening. Moreover, the enhancement was extremely stable, showing no evident adaptation with time for as long as the new length was maintained.
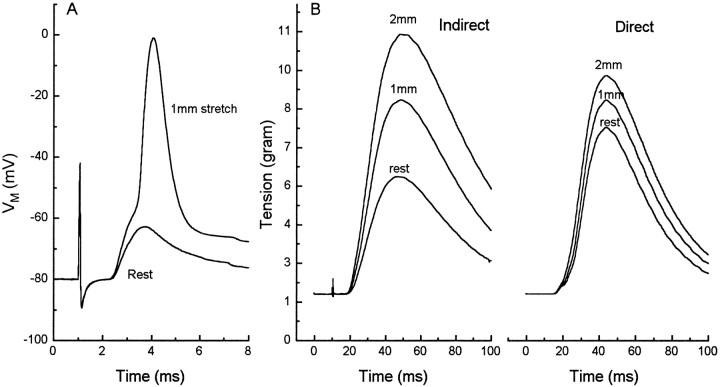
The stretch modulation of release is of obvious functional importance to frogs, serving as an amplifier of the spinal stretch reflex. An estimated 30% of the muscle fibers in the frog sartorius do not receive synaptic inputs that are suprathreshold for muscle activation at rest length (Kuffler, 1952; Grinnell and Herrera, 1980; Grinnell and Trussell, 1983). A small stretch, caused by contraction of antagonist muscles, can increase evoked release enough to elevate the EPP above threshold in many of these fibers, increasing the force of contraction when axons to the muscle are excited. As Figure 3illustrates, in NFR a 1 mm (3%) stretch can elevate an EPP above threshold, and 3 and 6% stretches can greatly enhance the magnitude of a twitch.
Fig. 3.
Effects of stretch on response in NFR.A, intracellular records from an unblocked junction in which stretch of the muscle elicited only an EPP at rest length but a full action potential after 1 mm (3%) muscle stretch.B, Records of twitch tension at rest length and after 1 and 2 mm (6%) stretch in a 30 mm sartorius in response to nerve stimulation (left) and direct muscle stimulation (right). The increase in tension with stretch in the directly stimulated muscle is predicted from the length–tension relationship of individual fibers. The much greater enhancement with indirect stimulation reflects the recruitment of large populations of fibers that were not excited above threshold at rest length. That indirect stimulation produced a larger twitch than direct stimulation at 6% stretch suggests that the direct stimulus did not evoke action potentials in all fibers in the muscle.
Kinetics of stretch enhancement
A major factor in developing an hypothesis to explain the stretch enhancement is the kinetics of the phenomenon, i.e., the rate of change in release efficacy with change in length. Figure 4shows the time course of change in EPP amplitude at a single junction when the muscle was stretched by 2 mm (6%) in 50 msec, held at the new length for 45 msec, and then returned to rest length in 50 msec (see Materials and Methods). Low Ca2+ Ringer, containing 0.56 mm Ca2+ and 4 mm Mg2+, was used to reduce quantal content, eliminating muscle contraction. Every point is the average of 50 or more EPPs, each in response to a single stimulus given during a separate cycle of lengthening and shortening. Thus, the data for this figure came from a series of ∼1800 separate cycles of lengthening and shortening, presented at a rate of 0.5 Hz. The direct measurement of muscle length shown in Figure4 was done with an optical technique (see Materials and Methods) after microelectrode recording. As Figure 4 shows, the muscle stretch near the point of physiological recording quite accurately reflected the time course of the command signals to the mechanical arm, but with a delay of ∼2-3 msec. The changes in release efficacy paralleled the stretch and shortening with remarkable accuracy and stability, with virtually no delay as the length was changed.
Figure 5 shows the average of six such experiments, normalized for 100% of the full response to 6% stretch, where rest length was taken as the mean of several responses before the onset of stretch and 100% enhancement is the average of all of the EPPs recorded at 6% stretch. Again, there was no significant deviation of the enhancement from a linear, virtually instantaneous dependence on muscle length, with the possible exception of the beginning of the shortening phase, where enhancement slightly lagged the shortening of the muscle, and at the end of the shortening phase, when slight enhancement persisted for a few milliseconds. We do not know the explanation for these deviations. In general, however, there seemed to be virtually no delay between change in length and change in release efficacy. Clearly the rate of development and decay of the enhancement required no more than 1–2 msec, if that, at a rate of change in length of 2 mm in 50 msec.
Another set of measurements confirmed that there is virtually no delay between stretch and enhancement. Although the requirement that the floating electrode stay in the muscle fiber throughout the experiment precluded the ideal experiment, i.e., abruptly changing length and measuring the rate of change in EPP amplitude, it was possible in some experiments to maintain good penetrations at lengthening rates up to 2 mm/20 msec. Figure 6A shows the average of five such experiments, normalized to the amplitude achieved at the end of a 50 msec stretch, with each point the average of five trials per experiment. The amplitude of an EPP elicited immediately at the point of completing the 2 mm stretch command was ∼3% less when the stretch was done in 20 msec than when it was done in 50 msec. This might suggest that at the faster rate of lengthening, there is a greater delay in development of enhancement of release. However, as Figure 6B shows, this shortfall can easily be explained by a difference in actual muscle length when the terminal was stimulated. At a lengthening rate of 2 mm/20 msec, the stretch of the muscle lagged behind the computer command significantly more than at 2 mm/50 msec. Thus, if the nerve was stimulated at the time of first reaching the peak of the command voltage, causing action potential invasion of the terminal 1–2 msec later, the actual muscle (and terminal) length would have been less in the case of the 20 msec stretch than the 50 msec stretch. Indeed, the degree of stretch of the muscle was ∼10% less at 20 msec in the rapid stretch than at 50 msec in the slower stretch, so one might have predicted an even larger difference in enhancement. We conclude that there was no greater delay in development of enhancement at a faster stretch rate and that the modulation of evoked release follows muscle length with essentially no delay. There is no time for complicated chemistry.
Fig. 6.
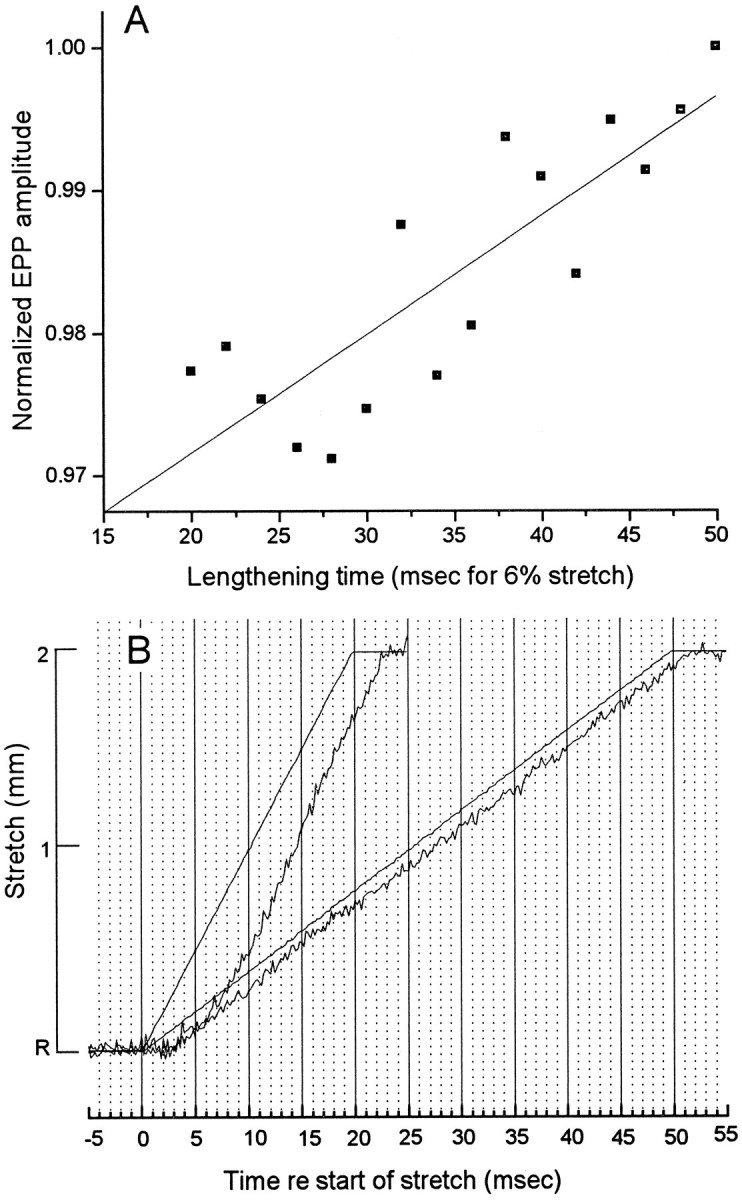
A, EPP amplitudes in low Ca2+ Ringer evoked by stimulation at the moment of reaching the 2 mm stretch command voltage, when the stretch was accomplished at different rates between 2 mm/20 msec and 2 mm/50 msec. The enhancement after the most rapid lengthening (20 msec) was ∼3% less than after a lengthening that took 50 msec. B, Optical measurement of muscle length during 2 mm stretches in 20 and 50 msec. The greater lag between command voltage and actual muscle stretch at 2 mm/20 msec can explain the slightly reduced enhancement. The muscle length was 30 mm, hence 2 mm represented 6% stretch.
Integrins and mechanical coupling of terminal to ECM/muscle
An early hypothesis to explain the stretch effect was the possibility that stretching the nerve terminal straightened out folds in the presynaptic membrane, exposing more release sites (Hutter and Trautwein, 1956). Subsequent studies of the freeze-fracture ultrastructure of frog motor nerve terminals (Heuser et al., 1974;Peper et al., 1974; Heuser and Reese, 1981; Propst and Ko, 1987;Meriney et al., 1996; P. A. Pawson, B. Wolowske, and A. D. Grinnell, unpublished observations) have failed to reveal significant membrane folding, especially near active zones, and it is unlikely that this model could explain the magnitude of enhancement (10% increase for a 1% change in length). Instead, it seems likely that there are mechanical connections between the nerve terminal and the muscle fiber or basal lamina that, in exerting tension on the terminal, somehow modulate transmitter release. There is no paucity of mechanical connections. The terminal is heavily invested in connective tissue and tightly covered (and partially enwrapped) by Schwann cells that are also attached via the matrix to the muscle cell. The rapidity and strength of the modulation suggest, however, that a mechanical stimulus is somehow delivered to the nerve terminal close to the release sites. Integrins, which are widespread, versatile cell membrane adhesion molecules with well established transduction capabilities, clearly play a role in delivering the mechanical stimulus (Chen and Grinnell, 1995).
Integrin–ligand bonds require divalent cations for stabilization and can be blocked by extrinsic RGD-containing peptides. Figure7 shows the effect of RGD on the stretch enhancement of mEPP frequency and EPP amplitude in NFR. Preparations were incubated for 1 hr in NFR containing 0.2 mm RGD or the control peptide, RGE, before commencing recording. RGD did not affect mEPP frequency or EPP amplitude at rest length but caused a 33–46% reduction in the enhancement of mEPP frequency and a 50–65% decrease in the stretch enhancement of EPP amplitude, compared with preparations treated with RGE or with no peptide. RGD does not affect the increase in mEPP frequency produced by terminal depolarization. Changing from NFR to Ringer containing 6 mm K+ produced a 55 ± 13% increase in mEPP frequency in the presence of 0.2 mm RGD (n = 65), a 49 ± 10% increase in its absence (n = 45).
The effectiveness of RGD in suppressing the stretch enhancement is strong evidence for the involvement of integrins in the phenomenon. Moreover, a polyclonal antibody directed against the extracellular domain of Xenopus β1 integrin subunit, kindly provided by Dr. D. DeSimone (University of Virginia), also partially inhibited the enhancement (Chen and Grinnell, 1995). However, under the conditions outlined above, neither RGD nor the antibody completely suppressed the stretch effect, leaving open the possibility that nonintegrin pathways also contribute. It should be noted, on the other hand, that RGD, like other function-blocking peptides, normally does not cause a complete block of integrin-binding interactions (Gartner and Bennett, 1985). Especially in a preparation in which integrins are already bound to their native ligands, it should not be expected that extrinsic RGD peptides could entirely displace the natural ligands. It is noteworthy, therefore, that other conditions were found in which RGD could, in fact, totally eliminate the stretch enhancement (see sections D and E in Results).
D. Lack of dependence of the effect on stretch-induced Ca2+ influx
The stretch modulation of release is not blocked by Mg2+ (Turkanis, 1973) or by ω-conotoxin GVIA (Chen and Grinnell, 1995), which blocks N-type voltage sensitive Ca2+channels and neuromuscular transmission in frogs (Kerr and Yoshikami, 1984). It is still possible, however, that stretch opens channels (SACs) that are Ca2+-permeable and not blocked by Mg2+. The most rigorous test of whether Ca2+influx is necessary for stretch enhancement is to eliminate all Ca2+ from the external medium. In EGTA-buffered zero Ca2+ Ringer, frog muscle fibers tend to twitch spontaneously. This twitching can be prevented, however, by treating the muscle for 25 min with 25 μm μ-conotoxin, which blocks Na+ channels in muscle but not in nerve (Cruz et al., 1985; Robitaille and Charlton, 1992). After this treatment and 1 hr further incubation in zero Ca2+ Ringer, EPPs were gone and resting mEPP frequency was decreased from a mean of 1.47 to 0.69 Hz, but there was still a clear stretch enhancement of mEPP frequency [by 17.9% at 1 mm and 28.4% at 2 mm stretch (see Fig.8, column 2)]. Thus, Ca2+influx, through SACs or any other kind of channel, is not required for the stretch modulation of spontaneous release.
The ∼50% reduction in stretch enhancement in zero Ca2+Ringer might reflect the loss of a component that is dependent on Ca2+ influx, or it might be the result of destabilization of integrin bonds by the withdrawal of Ca2+. We believe that the latter is the important factor because addition of 0.5 mm Mn2+, a good stabilizer of integrin bonds as well as an effective Ca2+ channel blocker (Gailit and Ruoslahti, 1988; Kirchhofer et al., 1990; Grinnell and Backman, 1991), restored the stretch enhancement to almost its full magnitude (Fig. 8,column 3). Moreover, under these conditions, in preparations pretreated with zero Ca2+ and RGD to weaken binding to natural ligands before addition of Mn2+, RGD totally blocked the stretch enhancement (Fig. 8, column 4), suggesting that all of the enhancement is mediated via integrins. The greater effectiveness of RGD in inhibiting the integrin-mediated phenomenon in a Mn2+-stabilized preparation is consistent with observations that Mn2+ increases the affinity of integrins for RGD at the expense of natural ligands (Gailit and Ruoslahti, 1988; Kirchhofer et al., 1990).
E. Does stretch act via an elevation in intraterminal Ca2+?
Although modulation of mEPP frequency does not require stretch-induced Ca2+ influx, [Ca2+]i is important. If terminals were loaded with BAPTA or DM-BAPTA by immersion in the −AM form of the buffer (see Materials and Methods), the stretch effect in zero Ca2+ Ringer was severely reduced. Loading of terminals with DM-BAPTA, a fast, high-affinity Ca2+ buffer, reduced resting mEPP frequency to an average 0.46 Hz, and reduced the stretch enhancement to 7.5% at 1 mm (n = 29) and 8.8% at 2 mm stretch (n = 23) (Fig. 8, column 5). It was of interest that 0.5 mm Mn2+ added to the zero Ca2+ Ringer raised the resting mEPP frequency in the DM-BAPTA-loaded terminals to a mean of 1.64 Hz and increased the stretch enhancement to 22.1 ± 3.7% at 1 mm (n = 57) and 43.4 ± 6.3% at 2 mm (n = 49; Fig. 8,column 6). Again, after pretreatment with zero Ca2+ Ringer and RGD, this Mn2+-supported enhancement was totally blocked by 0.2 mm RGD (Fig. 8,column 7).
The stretch enhancement of EPP amplitude was also sensitive to internal Ca2+ buffering. Loading of terminals by 1 hr incubation in BAPTA-AM did not affect the mean rest amplitude of EPPs in a 0.56 mm Ca2+, 4 mm Mg2+Ringer, but did reduce the stretch enhancement by 60–70%, from 31 ± 3.9% (n = 32) to 11.3 ± 4.1% (n = 22) at 1 mm (p < .001) and from 63 ± 5.9% (n = 27) to 24.6 ± 3.8% (n = 28) at 2 mm stretch (p < 10−5).
Binding of integrins can activate second messenger cascades that lead to release of Ca2+ from internal stores (see Introduction). It seems unlikely that such a process could act fast enough to explain stretch-induced changes in evoked release, but they could easily explain the increase in mEPP frequency. In an attempt to determine whether stretch acts by causing an increment in [Ca2+]i, we have attempted to “clamp” [Ca2+]i at a fixed level, while eliminating the transmembrane electrochemical gradient for Ca2+. The terminal was loaded with BAPTA by immersion for 60–90 min in 25 μm BAPTA-AM in NFR. Although the effectiveKD of Ca2+ binding is dependent on ionic strength and the specific intracellular environment, it is ∼100 nm (Tsien, 1980), close to the probable normal level. The effectiveness of BAPTA loading can be tested by determining the effect of K+ depolarization on mEPP frequency. Where normally 10 mm K+ increased mEPP frequency by a factor of 5.04 ± 2.02 (n = 10), after BAPTA loading the increase was only 1.32 ± 0.63X (n = 9).
Because the exogenous buffer has a KD near or slightly above the normal [Ca2+]i, the free Ca2+ level will still be determined in part by the intrinsic buffers that have a higher Ca2+ affinity, and small changes might be quite significant against a low background (hence, capable of modulating release). Therefore, two additional manipulations were done to minimize such changes. Thapsigargin (10 μm), a membrane-permeant reagent that blocks endoplasmic reticulum Ca2+-ATPase, was used to unload the primary internal stores of Ca2+ rapidly and irreversibly (Thastrup et al., 1989). Thapsigargin caused a transient increase in mEPP frequency that returned to original levels within 15–20 min. This treatment thus removed a major potential source of release of Ca2+ from internal stores. Finally, wishing to fix intraterminal [Ca2+]i at ∼100 nm, we immersed the preparation in an EGTA-buffered Ringer containing 1 nm free Ca2+, which would be approximately in electrochemical equilibrium with the assumed intraterminal [Ca2+]i of ∼100 nm at a membrane potential of about −70 mV. To facilitate free exchange of Ca2+ across the membrane, the Ca2+ ionophore A23187 (2 μm), diluted from a 10 mm solution in DMSO, was used in several experiments (Duncan and Statham, 1977; Rehder et al., 1992). We saw no evident difference between experiments done with and without A23187. Under these conditions, the mEPP frequency was maintained slightly above its normal level (mean, 1.73 Hz; n = 32), and there was still a large stretch enhancement (Fig. 9). Although it remains theoretically possible that stretch causes the release of Ca2+ from a thapsigargin-resistant compartment in a sharply confined space near the release sites, it seems much more likely that stretch enhancement does not involve an increment in intraterminal Ca2+.
Fig. 9.
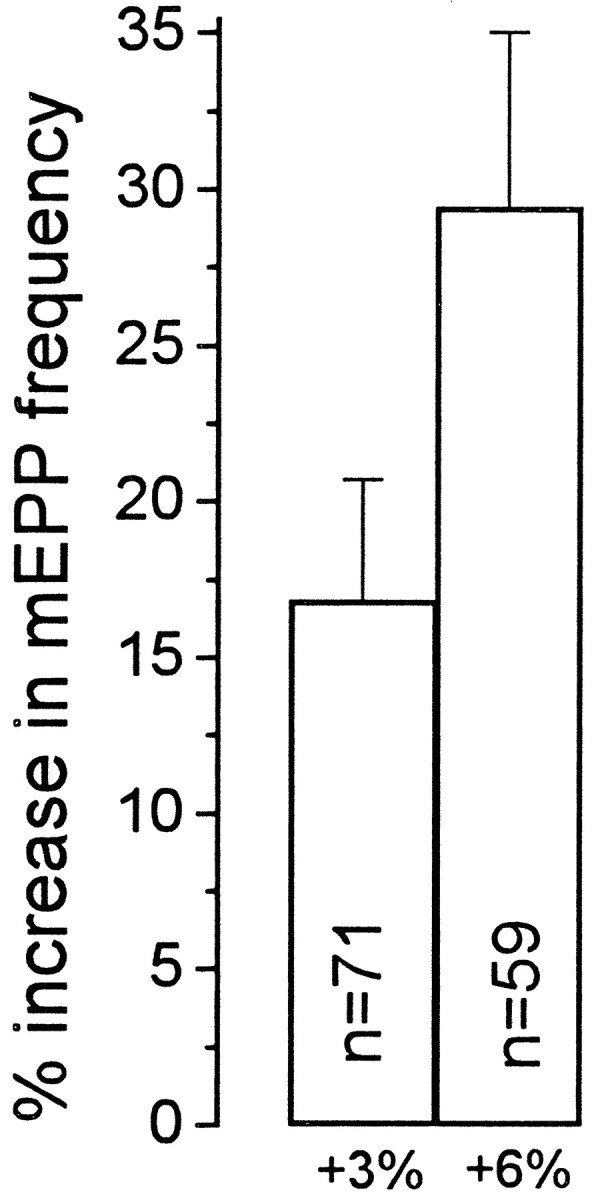
Stretch enhancement of release with 3 and 6% stretch in preparations in which [Ca2+]iwas “clamped” at ∼100 nm by loading the terminal with BAPTA, disabling the main intrinsic Ca2+ buffers with 10 μm thapsigargin, and reducing or eliminating the electrochemical gradient for Ca2+ across the terminal membrane by immersion in a Ringer containing 1 nmCa2+ and 2 μm of the Ca2+ionophore A23187. Under these conditions, stretch enhancement of release was still about one-half that found in NFR and very close to that obtained in zero Ca2+ Ringer (see Fig. 8).
F. Temperature coefficient of the stretch enhancement
If stretch does not elevate [Ca2+]i, the implication is that the enhancement is a result of either a change in the Ca2+ affinity of a Ca2+-sensing molecule necessary for vesicle docking or release, or a change in theKm of a reaction subsequent to Ca2+binding in the release process. We know almost nothing about such reactions, although it is clear that the Ca2+-sensing process can be bypassed under certain conditions, e.g., by α-latrotoxin, which somehow induces massive release when it binds to its receptor neurexins, which are associated with synaptotagmin, syntaxin, and Ca2+ channels in a “synaptosecretosome” (Rosenthal et al., 1990; O’Connor et al., 1993). Two generic hypotheses can be proposed: first, that stretch changes Ca2+ binding properties or critical reaction kinetics by a metabolic modification of some molecule(s), e.g., by a phosphorylation or dephosphorylation; and second, that the modulation of release probability is accomplished by a purely mechanical alteration in the position or conformation of one or more molecules, changing the equilibrium for a critical reaction. Any process fitting the first hypothesis would be expected to have a Q10 of 2 or higher, whereas a purely mechanical modification of molecular conformation or position, affecting a diffusion-limited process, would be expected to have a Q10 closer to 1.
Both the absolute EPP amplitude and mEPP frequency change markedly with temperature. Figure 10A shows the time course of change in EPP amplitude with 2 mm stretch and shortening in a representative preparation at 22.2° and after 1 hr equilibration at 13°C. The absolute EPP amplitude was decreased by nearly 50% at the lower temperature, consistent with the finding that quantal size decreases with temperature (Adams, 1989), but the percentage change in release with the change in length was actually somewhat greater. When the change with length is normalized (Fig. 10B), it is clear that the time course of the change in amplitude was essentially indistinguishable at the two temperatures during both lengthening and shortening phases (Fig. 10B).
Fig. 10.
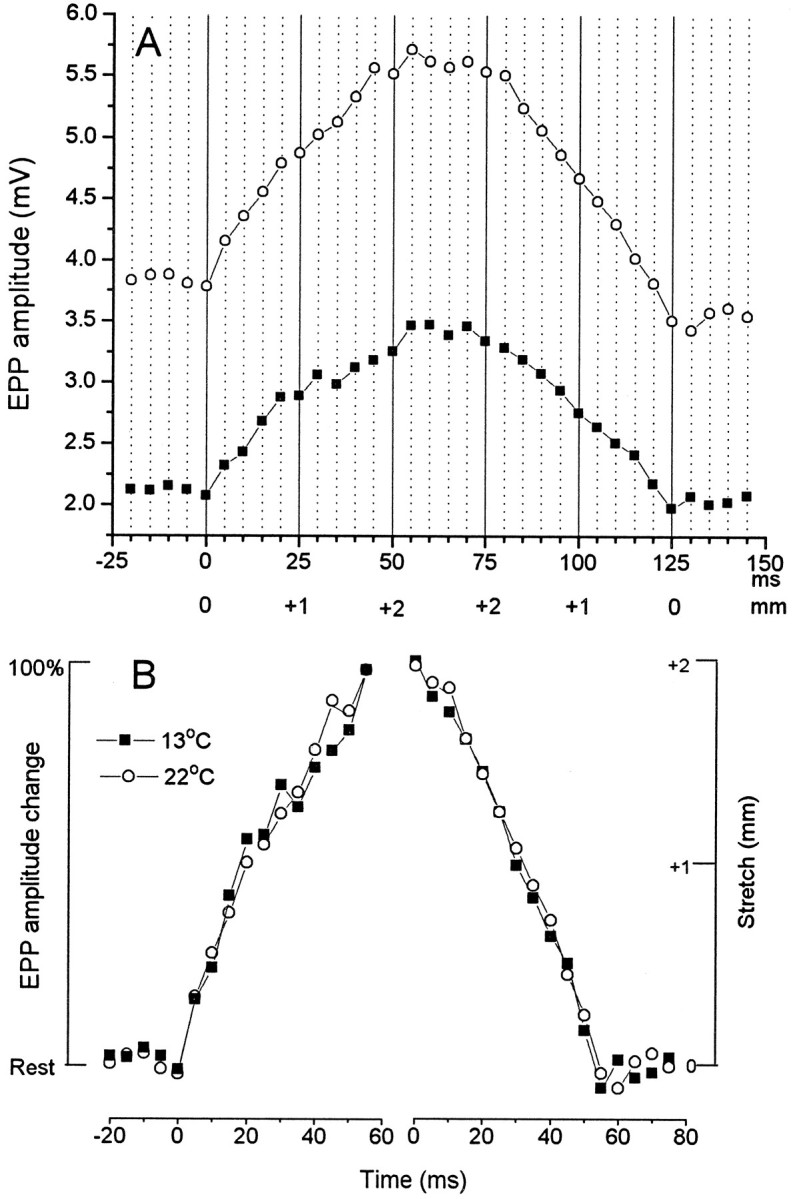
Effects of change in temperature on stretch enhancement of the EPP in low Ca2+ Ringer.A, Average of records from five lengthening–shortening cycles at a single representative junction at 22.2° and at 13°, showing the change in absolute EPP amplitude with stretch and shortening [using a stretching regime similar to that of Figs. 4 and 5but with stretch maintained at +2 mm (6%) for only 25 msec]. The absolute amplitude was much decreased at 13°, but the percentage change with stretch was actually increased at the lower temperature.B, Normalized plots of the lengthening and shortening phases at the two temperatures. The kinetics of the change in amplitude with change in length were essentially unaffected by temperature in this range.
MEPP frequency typically dropped by 50% or more when a preparation was cooled from 22–23° to 10–13°, but again the effects of stretch were undiminished. As Figure 11 shows for a representative preparation, the percentage changes in mEPP frequency were somewhat larger at the lower temperature, even though the absolute levels were depressed. When the absolute frequency at 10° was increased by depolarizing with 10 mm K+, raising the level approximately to that in NFR at 23°, the absolute changes in frequency with length at 10° and 23° were almost exactly the same. Thus, both the dynamics of the modulation and the percentage change in release effectiveness with stretch are undiminished by cooling within the range tested, and the Q10 values of these phenomena are close to 1.
Fig. 11.
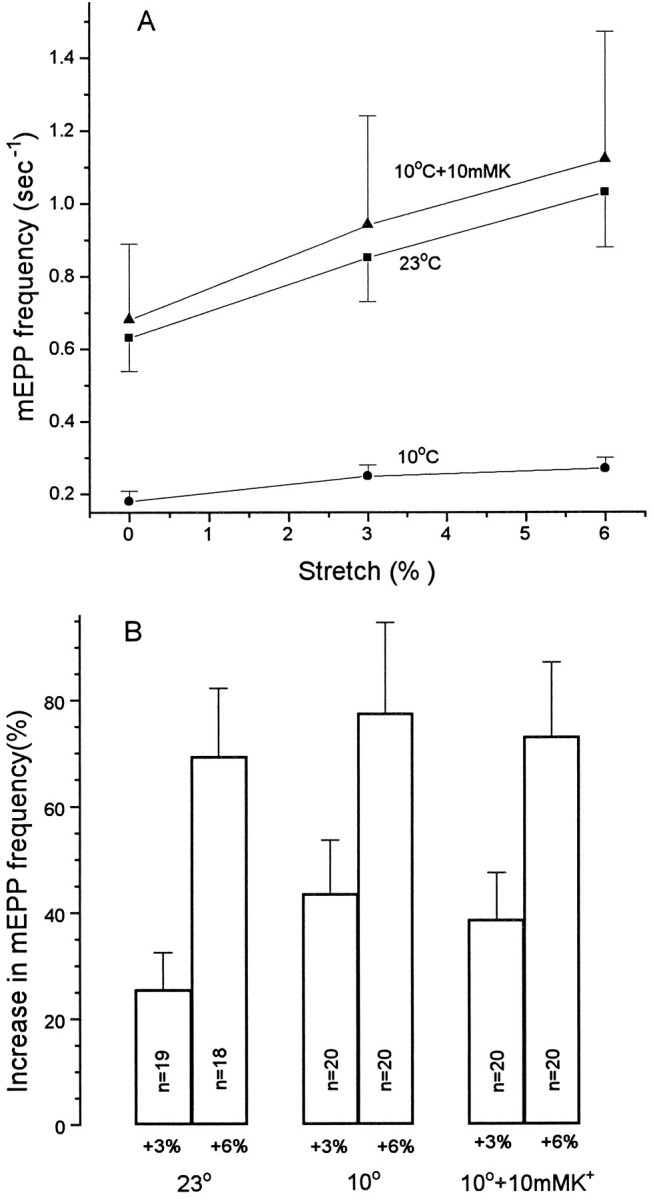
Effects of temperature on sensitivity of mEPP frequency to stretch. A, mEPP frequency as a function of muscle length at 10° and 23° in NFR, and at 10° in NFR plus 10 mm K+. B, Percentage change in mEPP frequency at 3 and 6% mm stretch under each of these conditions. Cooling reduced the resting mEPP frequency but had little effect on the enhancement by stretch. When the resting mEPP frequency was increased by K+ depolarization to approximately the level in NFR at 23°, the effect of stretch was indistinguishable from that at 23°C.
DISCUSSION
Stretch is a powerful modulator of both evoked and spontaneous transmitter release at the frog neuromuscular junction. In vivo, as the hind limb is flexed and the sartorius is stretched by activation of opposing muscle groups, action potentials in sartorius nerve terminals would be expected to elicit larger EPPs and generate action potentials in a higher percentage of muscle fibers, increasing the force of sartorius contraction. A similar enhancement of release obtains in the cutaneous pectoris (A. Kashani and A. D. Grinnell, unpublished observations) and is probably characteristic of most frog muscles.
We consider it probable that enhancement of spontaneous and evoked release are both manifestations of the same change in the release machinery caused by the level of tension on integrins in the membrane. However, important properties of the stretch enhancement can be studied only in one or the other phenomenon. The rapid kinetics of the modulation can be tested carefully only with EPPs, for example, and might not apply to mEPPs. Conversely, a lack of dependence of modulation on Ca2+ influx can be demonstrated only for mEPPs because EPPs require some Ca2+ in the external medium. Stretch-activated channels could still be involved in the modulation of EPP amplitude. On the other hand, the similar nonadapting nature of the change in EPP amplitude and mEPP frequency at a new length, the similar magnitude and linearity of the changes, and the low Q10 of both are consistent with the idea that they result from the same mechanism. Both show a similar susceptibility to blockage by RGD peptides, and hence involve integrins at some step in the process. We have no data incompatible with the conclusion that they are modulated by the same mechanism, but it is essential to keep in mind that this is not necessarily the case, especially given evidence that spontaneous and evoked quantal release can behave differently with different manipulations (Barrett and Magleby, 1976; Andreu and Barrett, 1980; Narita et al., 1990b; Umbach et al., 1990; Zengel and Sosa, 1994).
The modulation of release at frog neuromuscular junctions is extraordinarily rapid, linear with change in length, and nonadapting, all properties that argue against the involvement of second messenger pathways. External Ca2+ is not needed for stretch modulation of mEPP frequency, ruling out changes in Ca2+influx through voltage- or stretch-sensitive channels. However, it is more difficult to exclude the possibility that stretch causes a release of Ca2+ from internal stores, especially in view of the finding that loading of terminals with a strong Ca2+ buffer suppresses the enhancement. Integrin binding is capable of activating phospholipase C, releasing DAG and IP3 (Cybulsky et al., 1990; McNamee et al., 1993; Schwartz, 1993), which can sharply elevate [Ca2+]i. In most cases this is much too slow a process to explain the stretch enhancement of EPP amplitude, but in a tightly confined space this might not be the case. In olfactory receptors, for example, IP3 can rise in concentration within 25 msec after the activation of receptors (Restrepo et al., 1993). Another candidate pathway would be through mobilization of cyclic ADP-ribose, thought to be the endogenous activator of Ca2+-induced Ca2+ release (Galione, 1994), primarily from the endoplasmic reticulum (Etcheberrigaray et al., 1991). However, even if mobilization of IP3 or cyclic ADP-ribose could elevate [Ca2+]i quickly enough to explain the change in release, there are so many potent buffering and pumping systems regulating internal [Ca2+]i that a nonadapting change seems highly improbable.
It is of interest that 0.5 mm Mn2+ could almost fully restore the stretch enhancement in zero Ca2+ Ringer. In addition to blocking Ca2+ channels (Hagiwara and Takahashi, 1967), Mn2+ increases resting mEPP frequency (see Fig. 8) but does not support evoked release. The mechanism of this enhancement of mEPP frequency is not known, although it is possible that Mn2+ permeates through voltage-sensitive Ca2+ channels and displaces Ca2+ from internal stores (Kita et al., 1981; Misler and Falke, 1987; Narita et al., 1990a). It is noteworthy that Mn2+ also increased the resting mEPP frequency and sharply enhanced the stretch effect in preparations that had been loaded with DM-BAPTA (see Fig. 8), which would be expected to buffer any internal changes in free Ca2+. However, by analogy with the divalent cation binding properties of the BAPTA derivative fluo-3, which binds Mn2+∼70 times more strongly than Ca2+ (Minta et al., 1989), if a significant amount of Mn2+ did permeate the membrane, it might displace Ca2+ from BAPTA, elevating intraterminal Ca2+. Ca2+ release from internal stores under these conditions might be largely unaffected by the presence of BAPTA. On the other hand, even in a BAPTA-loaded, Mn2+-treated preparation, 0.2 mm RGD completely suppressed the stretch effect and reduced the resting mEPP frequency, arguing that a major function of Mn2+ is stabilization of integrin–ECM bonds. This finding suggests several conclusions: that integrin binding is a critical link for essentially 100% of the stretch effect; that stretch does not work primarily by elevating [Ca2+]i(although a moderate level of internal free Ca2+ may be necessary); and that the enhancement of resting mEPP frequency by Mn2+ may be a result, at least in part, of effects on the integrin-mediated pathway that helps regulate mEPP frequency.
A stretch-induced chemical modification of one or more intermediaries in the release pathway, e.g., by phosphorylation or dephosphorylation, also is unlikely. Reactions leading to phosphodiesterase activation, cGMP hydrolysis, and Na+ channel modulation occur within tens of milliseconds in photoreceptors (Detwiler and Gray-Keller, 1992), but this is a rapidly adapting process and it is highly unlikely that the phenomenon could reverse as quickly as it develops. Instead, the development and the decay of the stretch modulation occur with a delay of no more than 1–2 msec, perhaps virtually instantaneously. This rules out stretch modulation of any second messenger or enzymatic pathway.
The very low Q10 (close to 1 for both EPP and mEPP enhancement) also militates against any biochemical reaction being the direct result of stretch. Instead, lengthening of muscle seems to cause a change in release probability by mechanically altering theKm of a diffusion-limited process.
The nature of this mechanical process is still unknown and must be the subject of future research. It is highly unlikely that either the volume or the surface area of the terminal changes significantly during stretch, so the effects would be on shape and perhaps on local membrane conformation near points of attachment to the ECM or other connective tissue. Integrins are important anchoring molecules, connecting the cytoskeleton to the ECM. Because of the semirigidity of the cytoskeleton and its role in organizing arrays of associated molecules (Ingber, 1996), tension on integrins could transmit mechanical stimuli virtually instantaneously to other regions of the cell, potentially causing a change in the proximity or reactivity of molecules that interact in the process of vesicle docking and fusion. If this is the case, it represents a particularly rapid, powerful form of mechanical modulation of intracellular physiological processes.
It is important, however, to emphasize the specificity of this mechanical pathway and the role of integrins in it. After RGD treatment in a Mn2+-treated preparation has eliminated the stretch enhancement, most of the effects of stretch on terminal structure would seem to be unchanged. Muscle stretch still causes terminals to elongate, with whatever changes in cytoskeletal organization are associated with this elongation; mechanical stress on the membrane persists at sites of non-integrin-mediated connective tissue connections to the terminal, and transmitter release is normal, but the stretch enhancement of release is eliminated. We do not yet know the nature of the critical connections between integrins and the ECM, nor do we have information regarding the chain of connections between integrins and the release apparatus inside the terminal. The data indicate that there are such connections, however, with a powerful effect on release. These connections must somehow be fitted into molecular models of vesicle docking and fusion.
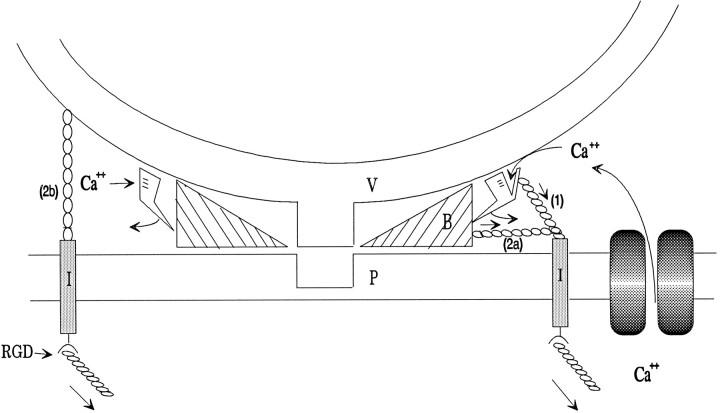
We propose that the integrin-mediated pathway is working in one of two general ways, indicated schematically in Figure 12. Tension on integrins might, via an unknown chain of intraterminal connections: (1) change the Ca2+-binding affinity of the Ca2+ sensing molecules responsible for triggering the molecular machinery that causes (or disinhibits) vesicle fusion with the plasma membrane; or (2) transduce mechanical force directly onto molecules or structures affecting the probability of vesicle fusion, either (2a) by facilitating the reactions leading to vesicle fusion (or helping displace molecules that block fusion), or (2b) by directly increasing the likelihood of lipid/lipid interactions between the plasma and vesicle membranes, e.g., by pulling vesicles closer to the plasma membrane, or, via localized stress on the plasma membrane lipid, somehow facilitating fusion.
Fig. 12.
A schematic model suggesting, generically, how extracellularly applied tension on integrins might increase the probability of vesicle fusion. The fusion process is pictured as resulting when a docked vesicle (V) is allowed to be pulled into contact with the plasma membrane (P) at a specific site. This process is normally triggered by Ca2+interaction with a Ca2+-sensing molecule that can, through an unknown number of steps, activate the fusion process, perhaps by displacing a molecule (B) that physically blocks contact of the two membranes. Tension on integrins (I) might alter the Ca2+ binding affinity of the Ca2+ sensor (1) or physically exert force contributing to the removal of the block of fusion (2a) or pulling of the vesicle down toward fusion-promoting molecules in the plasma membrane (2b). Another interpretation of alternative (2b) might be an increased probability of vesicle–plasma membrane fusion, the result somehow of a direct effect of mechanical stress on the conformation of the plasma membrane lipid. RGD inhibits the modulation by blocking integrin binding to ligands in the ECM.
It would be surprising if frog neuromuscular junctions were the only synapses in which integrins helped regulate transmitter release. Given their transduction capabilities and the wide variety of physiological changes that they can trigger in cells, integrins might play different but important roles in central nervous system synapses, e.g., in response to subtle osmotic changes associated with use. It is of interest that interference with integrin binding by RGD peptides inhibits long-term potentiation in the hippocampus (Staubli et al., 1990), and a Drosophila mutant, volado, which lacks an α1 integrin subunit, suffers an olfactory learning deficit (R. L. Davis, X-R. Zhu, and K. H. Wu, unpublished observations).
Footnotes
We are grateful to Dr. D. DeSimone for providing the anti-Xenopus integrin β1 antibodies that helped convince us, early in this research, that integrins were involved; to Dr. E. Stefani for helpful suggestions; and to Dr. B. Yazejian, Brad Smith, and Amir Kashani for help in various phases of the research.
Correspondence should be addressed to Dr. Alan D. Grinnell, Department of Physiology, JLNRC, and Ahmanson Laboratory of Neurobiology, UCLA School of Medicine, Los Angeles, CA 90095.
REFERENCES
- 1.Adams BA. Temperature and synaptic efficacy in frog skeletal muscle. J Physiol (Lond) 1989;408:443–455. doi: 10.1113/jphysiol.1989.sp017469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreu R, Barrett EF. Calcium dependence of evoked transmitter release at very low quantal contents at the frog neuromuscular junction. J Physiol (Lond) 1980;308:79–97. doi: 10.1113/jphysiol.1980.sp013463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett EF, Magleby KL. Physiology of cholinergic transmission. In: Goldberg AM, Hanin I, editors. Biology of cholinergic function. Raven; New York: 1976. pp. 29–100. [Google Scholar]
- 4.Chen BM, Grinnell AD. Integrins and modulation of transmitter release from motor nerve terminals by stretch. Science. 1995;269:1578–1580. doi: 10.1126/science.7667637. [DOI] [PubMed] [Google Scholar]
- 5.Cruz LJ, Gray WR, Olivera BM, Zeikus RD, Kerr L, Yoshikami D, Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985;260:9280–9288. [PubMed] [Google Scholar]
- 6.Cybulsky AV, Carbonetto S, Cyr MD, McTavish AJ, Huang Q. Extracellular matrix-stimulated phospholipase activation is mediated by β1-integrin. Am J Physiol. 1993;264:C323–C332. doi: 10.1152/ajpcell.1993.264.2.C323. [DOI] [PubMed] [Google Scholar]
- 7.D’Alonzo AJ, Grinnell AD. Profiles of evoked transmitter release along the length of frog motor nerve terminals. J Physiol (Lond) 1985;359:235–258. doi: 10.1113/jphysiol.1985.sp015583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damsky CH, Werb Z. Signal transduction by integrin receptors for extracellular matrix: cooperative processing of extracellular information. Curr Opin Cell Biol. 1992;4:772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 9.Detwiler PB, Gray-Keller MP. Some unresolved issues in the physiology and biochemistry of phototransduction. Curr Opin Neurobiol. 1992;2:433–438. doi: 10.1016/0959-4388(92)90176-l. [DOI] [PubMed] [Google Scholar]
- 10.Duncan CJ, Statham HE. Interacting effects of temperature and extracellular calcium on the spontaneous release of transmitter at the frog neuromuscular junction. J Physiol (Lond) 1977;268:319–333. doi: 10.1113/jphysiol.1977.sp011859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etcheberrigaray R, Fiedler JL, Pollard HB, Rojas E. Endoplasmic reticulum as a source of Ca2+ in neurotransmitter secretion. Ann NY Acad Sci. 1991;635:90–99. doi: 10.1111/j.1749-6632.1991.tb36484.x. [DOI] [PubMed] [Google Scholar]
- 12.Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol (Lond) 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 13.French AS. Mechanotransduction. Annu Rev Physiol. 1992;54:135–152. doi: 10.1146/annurev.ph.54.030192.001031. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto T, Fujimura K, Kuramoto A. Electrophysiological evidence that glycoprotein IIb–IIIa complex is involved in calcium channel activation on human platelet plasma membrane. J Biol Chem. 1991;266:16370–16375. [PubMed] [Google Scholar]
- 15.Gailit J, Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988;263:12927–12932. [PubMed] [Google Scholar]
- 16.Galione A. Cyclic ADP-ribose, the ADP-ribosyl cyclase pathway and calcium signalling. Mol Cell Endocrinol. 1994;98:125–131. doi: 10.1016/0303-7207(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 17.Gartner TK, Bennett JS. The tetrapeptide analogue of the cell attachment site of fibronectin inhibits platelet aggregation and fibrinogen binding to activated platelets. J Biol Chem. 1985;260:891–894. [PubMed] [Google Scholar]
- 18.Grinnell F, Backman R. Role of integrin receptors in manganese-dependent BHK cell spreading on albumin-coated substrata. Exp Cell Res. 1991;195:218–223. doi: 10.1016/0014-4827(91)90520-5. [DOI] [PubMed] [Google Scholar]
- 19.Grinnell AD, Herrera AA. Physiological regulation of synaptic effectiveness at frog neuromuscular junctions. J Physiol (Lond) 1980;307:301–317. doi: 10.1113/jphysiol.1980.sp013436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grinnell AD, Pawson PA. Dependence of spontaneous release at frog junctions on synaptic strength, external calcium, and terminal length. J Physiol (Lond) 1989;418:397–410. doi: 10.1113/jphysiol.1989.sp017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grinnell AD, Trussell L. Synaptic strength as a function of motor unit size in the normal frog sartorius. J Physiol (Lond) 1983;338:221–243. doi: 10.1113/jphysiol.1983.sp014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagiwara S, Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle fiber membrane. J Gen Physiol. 1967;50:583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heuser JE, Reese TS. Structural changes after transmitter release at the frog neuromuscular junction. J Cell Biol. 1981;88:564–580. doi: 10.1083/jcb.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heuser JE, Reese TS, Landis DMD. Functional changes in frog neuromuscular junctions studies with freeze-fracture. J Neurocytol. 1974;3:109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- 25.Hutter OF, Trautwein W. Neuromuscular facilitation by stretch of motor nerve endings. J Physiol (Lond) 1956;133:610–625. doi: 10.1113/jphysiol.1956.sp005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 27.Ingber DE. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- 28.Ingber DE (1996) Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol, in press. [DOI] [PubMed]
- 29.Kerr LM, Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature. 1984;308:282–284. doi: 10.1038/308282a0. [DOI] [PubMed] [Google Scholar]
- 30.Kirchhofer D, Gailit J, Ruoslahti E, Grzesiak J, Pierschbacher MD. Cation-dependent changes in the binding specificity of the platelet receptor GPIIb/IIIa. J Biol Chem. 1990;265:18525–18530. [PubMed] [Google Scholar]
- 31.Kita H, Narita K, Van der Kloot W. Tetanic stimulation increases the frequency of miniature end-plate potentials at the frog neuromuscular junction in Mn2+-, Co2+-, and Ni2+-saline solutions. Brain Res. 1981;205:111–121. doi: 10.1016/0006-8993(81)90723-x. [DOI] [PubMed] [Google Scholar]
- 32.Kornberg L, Juliano RL. Signal transduction from the extracellular matrix: the integrin-tyrosine kinase connection. Trends Neurosci. 1992;13:93–95. doi: 10.1016/0165-6147(92)90034-4. [DOI] [PubMed] [Google Scholar]
- 33.Kuffler SW. Incomplete neuromuscular transmission in the twitch system of frog’s skeletal muscles. Fed Proc. 1952;11:87. [Google Scholar]
- 34.Martinac B. Mechanosensitive ion channels: biophysics and physiology. In: Jackson M, editor. Thermodynamics of cell surface receptors. CRC; Boca Raton, FL: 1993. pp. 327–351. [Google Scholar]
- 35.McNamee HP, Ingber DE, Schwartz MA. Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J Cell Biol. 1993;121:673–678. doi: 10.1083/jcb.121.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meriney SD, Wolowske B, Ezzatti E, Grinnell AD. Low calcium-induced disruption of active zone structure and function at the frog neuromuscular junction. Synapse. 1996;24:1–11. doi: 10.1002/(SICI)1098-2396(199609)24:1<1::AID-SYN1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 37.Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- 38.Misler S, Falke LC. Dependence on multivalent cations of quantal release of transmitter induced by black widow spider venom. Am J Physiol. 1987;253:C469–C476. doi: 10.1152/ajpcell.1987.253.3.C469. [DOI] [PubMed] [Google Scholar]
- 39.Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 40.Narita K, Kawasaki F, Kita H. Mn2+ and Mg2+ influxes through Ca2+ channels of motor nerve terminals are prevented by verapamil in frogs. Brain Res. 1990a;510:289–295. doi: 10.1016/0006-8993(90)91379-u. [DOI] [PubMed] [Google Scholar]
- 41.Narita K, Kawasaki F, Kita H. Spontaneous and evoked transmitter releases after concanavalin A treatment are affected differently by hypertonic low calcium solutions at frog neuromuscular junction. Brain Res. 1990b;512:33–39. doi: 10.1016/0006-8993(90)91166-e. [DOI] [PubMed] [Google Scholar]
- 42.O’Connor VM, Shamotienko O, Grishin E, Betz H. On the structure of the “synaptosecretosome.” Evidence for a neurexin/synaptotagmin/syntaxin/Ca2+ channel complex. FEBS Lett. 1993;326:255–260. doi: 10.1016/0014-5793(93)81802-7. [DOI] [PubMed] [Google Scholar]
- 43.Peper K, Dryer F, Sandri C, Akert K, Moor H. Structure and ultrastructure of frog motor endplate. Cell Tissue Res. 1974;149:437–455. doi: 10.1007/BF00223024. [DOI] [PubMed] [Google Scholar]
- 44.Pierschbacher MD, Ruoslahti E. Influence of stereochemistry of the sequence Arg–Gly–Asp–Xaa on binding specificity in cell adhesion. J Biol Chem. 1987;36:17294–17298. [PubMed] [Google Scholar]
- 45.Propst JW, Ko CP. Correlations between active zone ultrastructure and synaptic function studied with freeze-fracture of physiologically identified neuromuscular junctions. J Neurosci. 1987;7:3654–3664. doi: 10.1523/JNEUROSCI.07-11-03654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rehder V, Jensen JR, Kater SB. The initial stages of neural regeneration are dependent upon intracellular calcium levels. Neuroscience. 1992;51:565–574. doi: 10.1016/0306-4522(92)90296-e. [DOI] [PubMed] [Google Scholar]
- 47.Restrepo D, Boekhoff I, Breer H. Rapid kinetic measurements of second messenger formation in olfactory cilia from channel catfish. Am J Physiol. 1993;264:C906–C911. doi: 10.1152/ajpcell.1993.264.4.C906. [DOI] [PubMed] [Google Scholar]
- 48.Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenthal L, Zacchetti D, Madeddu L, Meldolesi J. Mode of action of α-latrotoxin: role of divalent cations in Ca2+-dependent and Ca2+-independent effects mediated by the toxin. Mol Pharmacol. 1990;38:917–923. [PubMed] [Google Scholar]
- 50.Sackin H. Mechanosensitive channels. Annu Rev Physiol. 1995;57:333–353. doi: 10.1146/annurev.ph.57.030195.002001. [DOI] [PubMed] [Google Scholar]
- 51.Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking β integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz MA. Spreading of human endothelial cells on fibronectin or vitronectin triggers elevation of intracellular free calcium. J Cell Biol. 1993;120:1003–1010. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz MA, Ingber DE, Lawrence M, Springer TA, Lechene C. Multiple integrins share the ability to induce elevation of intracellular pH. Exp Cell Res. 1991;195:533–535. doi: 10.1016/0014-4827(91)90407-l. [DOI] [PubMed] [Google Scholar]
- 54.Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drbak BK, Bjerrum PJ, Christensen SB, Hanley MR. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989;27:17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- 55.Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 56.Turkanis SA. Effects of muscle stretch on transmitter release at end-plates of rat diaphragm and frog sartorius muscle. J Physiol (Lond) 1973;230:391–403. doi: 10.1113/jphysiol.1973.sp010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Umbach JA, Zinsmaier KE, Eberle KK, Buchner E, Benzer S, Gundersen CB. Presynaptic dysfunction in Drosophila csp mutants. Neuron. 1994;13:899–907. doi: 10.1016/0896-6273(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 58.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 59.Yang XC, Sachs F. Characterization of stretch-activated ion channels in Xenopus oocytes. J Physiol (Lond) 1990;431:103–122. doi: 10.1113/jphysiol.1990.sp018322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zengel JE, Sosa MA. Changes in mEPP frequency during depression of evoked release at the frog neuromuscular junction. J Physiol (Lond) 1994;477:267–277. doi: 10.1113/jphysiol.1994.sp020189. [DOI] [PMC free article] [PubMed] [Google Scholar]