Abstract
Emerging infectious diseases are an increasingly important threat to wildlife conservation, with amphibian chytridiomycosis, caused by Batrachochytrium dendrobatidis, the disease most commonly associated with species declines and extinctions. However, some amphibians can be infected with B. dendrobatidis in the absence of disease and can act as reservoirs of the pathogen. We surveyed robber frogs (Eleutherodactylus spp.), potential B. dendrobatidis reservoir species, at three sites on Montserrat, 2011–2013, and on Dominica in 2014, to identify seasonal patterns in B. dendrobatidis infection prevalence and load (B. dendrobatidis genomic equivalents). On Montserrat there was significant seasonality in B. dendrobatidis prevalence and B. dendrobatidis load, both of which were correlated with temperature but not rainfall. B. dendrobatidis prevalence reached 35% in the cooler, drier months but was repeatedly undetectable during the warmer, wetter months. Also, B. dendrobatidis prevalence significantly decreased from 53.2% when the pathogen emerged on Montserrat in 2009 to a maximum 34.8% by 2011, after which it remained stable. On Dominica, where B. dendrobatidis emerged seven years prior to Montserrat, the same seasonal pattern was recorded but at lower prevalence, possibly indicating long-term decline. Understanding the dynamics of disease threats such as chytridiomycosis is key to planning conservation measures. For example, reintroductions of chytridiomycosis-threatened species could be timed to coincide with periods of low B. dendrobatidis infection risk, increasing potential for reintroduction success.
Keywords: Chytridiomycosis, Wildlife disease, Amphibians, Pathogen reservoirs, Disease dynamics, Conservation, Caribbean herpetology
Introduction
Emerging infectious diseases are a growing threat to wildlife conservation, causing species declines and extinctions globally (Aguirre & Tabor, 2008; Daszak, Cunningham & Hyatt, 2000). Traditional epidemiological theory suggests that a disease is unlikely to cause extinction: when a pathogen causes a host decline and host population density falls below a threshold at which further transmission and resulting mortality is limited (Anderson & May, 1979; Daszak et al., 1999). However, transmission dynamics are altered in favour of the likelihood of extinction when pathogens are able to persist in environmental or species reservoirs (Brunner et al., 2004; De Castro & Bolker, 2005; McCallum, 2012; Mitchell et al., 2008). Neutralisation of threats has long been considered an essential precursor to conservation interventions such as reintroduction (Caughley, 1994). Therefore, an inability to eradicate pathogens that persist in reservoirs poses a major problem to conservation managers, who must find alternative methods to mitigate disease impact (Harding, Griffiths & Pavajeau, 2015). Understanding infection dynamics in reservoir species, including how this varies through time and with environmental conditions, is a first step towards designing such mitigation strategies.
Amphibian chytridiomycosis, caused by infection with the chytrid fungus Batrachochytrium dendrobatidis, threatens hundreds of species of amphibian (Fisher, Garner & Walker, 2009; Scheele et al., 2019). Whilst lethal in a wide range of species, B. dendrobatidis does not cause disease in all amphibian species it infects (Gervasi et al., 2013; Stockwell, Clulow & Mahony, 2010). These reservoirs can lead to continued exposure of susceptible hosts even when these hosts are present in low numbers. Indeed, chytridiomycosis-mediated declines of the Corroboree frog (Pseudophryne pengilleyi) in south-eastern Australia continue in the presence of a B. dendrobatidis reservoir species, where they would have otherwise ceased (Scheele et al., 2017). The possibility that B. dendrobatidis can persist in the environment outside of a host (Johnson & Speare, 2003, 2005; Walker et al., 2007) or in non-amphibian hosts (McMahon et al., 2013), thus providing multiple potential reservoirs, has not been ruled out, although evidence for this is not robust.
The risk of infection with B. dendrobatidis has been shown to vary seasonally (Berger et al., 2004; Ruggeri et al., 2015) and may be driven predominantly by variation in ambient temperature (Forrest & Schlaepfer, 2011; Whitfield et al., 2012) and rainfall (Holmes, McLaren & Wilson, 2014; Terrell et al., 2014; Longo, Burrowes & Joglar, 2010). Mechanisms for temperature as a driver are well described. Frogs have been shown to exhibit temperature-dependent immunity (Raffel et al., 2006; Rowley & Alford, 2013), the antimicrobial activity of frog skin microbiota is temperature dependent (Daskin et al., 2014; Longo et al., 2015), and B. dendrobatidis is sensitive to high temperatures and desiccation (Johnson et al., 2003; Piotrowski, Annis & Longcore, 2004). How changes in precipitation act on B. dendrobatidis infection prevalence is less clear. Longo, Burrowes & Joglar (2010) suggest the aggregation of individuals in moist refugia during droughts increases infection transmission rate, increasing infection prevalence. Others have found increased precipitation to be a driver of increased infection prevalence (Ruggeri et al., 2018), presumably due to increased persistence of B. dendrobatidis in moist environments (James et al., 2015). There is, however, no consensus on the relative importance of these drivers and they appear to differ between sites and species.
In this study, we investigate temporal variation in the prevalence and load of B. dendrobatidis infection in robber frogs (Eleutherodactylus spp.) on Montserrat and Dominica in the eastern Caribbean, the only two islands on which the Critically Endangered mountain chicken (Leptodactylus fallax) is found (IUCN SSC Amphibian Specialist Group, 2017). The mountain chicken is a giant frog which has suffered catastrophic population declines and near-extinction due to chytridiomycosis (Hudson et al., 2016a). The native robber frog, Eleutherodactylus martinicensis, is the only amphibian sympatric to the mountain chicken on Dominica. On Montserrat, the robber frog, E. johnstonei, is the predominant sympatric amphibian; although the introduced cane toad (Rhinella marina) is also found on this island. E. johnstonei is thought to have originated in the Antilles but it has invaded much of the remainder of the Caribbean; it has been described as a possible native of Montserrat (Kaiser, 1997). Despite some uncertainty about the possible presence of E. johnstonei on Dominica (Kaiser, 1992), recent surveys indicate that only E. martinicensis is sympatric with the mountain chicken on this island (Cunningham et al., 2008). Each of these Eleutherodactylus spp. are direct developers and are common and widespread on their respective islands. Alongside the preliminary surveys included in the current study which identified B. dendrobatidis infections to be widespread in the eleutherodactylid frogs on both islands (see Results and Discussion), Eleutherodactylus spp. on other Caribbean islands have been shown to be carriers of B. dendrobatidis, often in the absence of chytridiomycosis (Burrowes et al., 2017; Longo & Burrowes, 2010). Thus, even though the islands are small, the eradication of B. dendrobatidis would likely be extremely challenging, if not impossible. To determine the seasonal and spatial variation in B. dendrobatidis infection in Eleutherodactylus spp., and to inform mountain chicken conservation management, such as the spatiotemporal requirement for future interventions to mitigate B. dendrobatidis infection, repeat multi-year surveys of robber frogs were conducted on both islands. In this study, we test the hypothesis that B. dendrobatidis infection prevalence and load in Eleutherodactylus spp. vary seasonally, dependent on local environmental conditions, and determine whether there is inter-site variation which could inform future reintroductions of mountain chickens.
Materials and Methods
Montserrat is a British Overseas Territory in the Lesser Antilles island chain in the Eastern Caribbean (16.45°N, 62.15°W). It is a small island, 102 km2, of which the southern half is in an exclusion zone due to volcanic activity. Fieldwork was conducted at three sites on the unrestricted part of the island within or near the Centre Hills Protected Area: Fairy Walk (FW, 16.752°N, −62.176°W, 600 m asl) and Sweetwater Ghaut (SWG, 16.782°N, −62.185°W, 600 m asl) on the east coast, and Collins Ghaut (CG, 16.779°N, −62.193°W, 500 m asl) in the north of the Centre Hills. These sites were selected as they comprised: (1) the last remaining site containing mountain chickens (FW), (2) a site being considered for mountain chicken reintroductions (SWG), and (3) a site outside the historical range of the mountain chicken (CG). Each of the sites was surveyed up to once per month between Feb 2011 and Nov 2013.
Dominica is also in the Lesser Antilles, is south of Montserrat (15.42, −61.35) and is larger, at 750 km2, and more mountainous. Here, surveys were conducted approximately every two months throughout 2014 at three sites: Wallhouse (WH, 15.280°N, −61.370°W, 100 m asl), Colihaut (CH, 15.489°N, −61.455°W, 100 m asl) and Soufriere (SF, 15.242°N, −61.349°W, 150 m asl). These sites were selected as they are three of the last remaining sites of extant mountain chicken populations on Dominica following the emergence of B. dendrobatidis (Hudson et al., 2016a).
The climate in the Lesser Antilles is characterised by two seasons (Oppel et al., 2014). The relatively warm and wet season occurs from Jun to Nov, with average temperatures of 25–32 °C and rainfall of 200–280 mm/month (Fig. S1). The relatively cool and dry season occurs from Dec to May, with average temperatures of 22–28 °C and rainfall of 60–120 mm/month (Fig. S1).
Field methods
For each survey, 60 robber frogs were caught and skin-swabbed to estimate the B. dendrobatidis infection prevalence at each site. A total of 60 frogs provided a compromise between the precision of the prevalence estimate achieved, which increases with sample size (DiGiacomo & Koepsell, 1986), and the time required to catch and sample frogs. A team of between three and five people exhaustively sampled robber frogs within a 20 m radius of a chosen ‘station’ centred at the start of an established transect at the site. If the first station did not yield 60 frogs, the team moved 50 m along the transect and repeated the process for up to three stations. Each site was therefore up to 40 m wide and 140 m in length. We used a new pair of disposable latex gloves for each frog handled to prevent cross-contamination of B. dendrobatidis or B. dendrobatidis DNA between animals.
On capture, each frog was swabbed five times on the ventral abdomen, hind legs and feet with a rayon-tipped swab (MW100; Medical Wire & Co., Corsham, UK), as described by Hyatt et al. (2007). Frogs were examined for signs of chytridiomycosis, specifically red ventral skin, lethargy, muscle tremors and skin sloughing. After swabbing, each tree frog was held separately until all captures were completed to ensure no recaptures occurred. Each frog was then released as close to the individual’s capture site as possible. Swabs were stored refrigerated prior to analysis.
Two temperature data loggers (iButton® DS1922L-F5; Maxim, Sunnyvale, CA, US) were placed at ground level in a shaded area on either side of the SWG transect on Montserrat and two at each of the sites in Dominica throughout the surveys to record the air temperature at hourly intervals. As part of a parallel study, temperature data loggers were also placed at both CG and FW on Montserrat between Jun and Sep 2012. Post hoc comparison showed only minor differences in temperature patterns and variances between the Montserrat sites (Fig. S2), therefore temperature data from SWG was used to represent all sites on this island. No analyses were conducted using the Dominica data because of the low B. dendrobatidis infection rates found during the study and therefore low variation in prevalence between months.
Rainfall data were available for Montserrat only. These were routinely collected by the Montserrat Utilities department. We obtained rainfall data from the nearest gauge to each study site: Blakes FIFA (16.783979N, −62.185641W) for SWG, Ginger Ground (16.772867N, −62.214672W) for CG and New Windward (16.765874N, −62.168201W) for FW.
The capture, handling and swabbing of robber frogs was conducted in collaboration with the Montserrat Department of Environment and the Dominica Forestry Department, who permitted the work on their respective islands. This study was approved by the Zoological Society of London’s Ethics Committee (project refs: WLE/0362 and WLE/0568).
Laboratory methods
DNA was extracted from each swab using methods modified from Hyatt et al. (2007). Briefly, the tip of the swab was removed using a sterile blade and placed in a sterile Eppendorf tube. Then, 60 μl of PrepMan Ultra (Applied Biosystems, Foster City, CA, USA) was added along with 30 to 40 mg of 0.5 mm zirconium/silica beads. The sample was homogenised for 45 s in a TissueLyser 2 (Qiagen, Ltd., Manchester, U.K.). After briefly centrifuging (one min at 4,000×g rpm in a benchtop centrifuge), the homogenisation and centrifugation steps were repeated. The sample was then placed in a heat block at 100 °C for 10 min, cooled for two min, then centrifuged at 4,000×g rpm for three min. As much supernatant (extracted DNA) as possible was recovered and stored at –20 °C prior to analysis.
The extracted DNA was diluted one in 10 in laboratory grade distilled water and the amount of B. dendrobatidis DNA present was quantified using a B. dendrobatidis-specific Taqman real-time PCR, as described by Boyle et al. (2004) modified by the inclusion of bovine serum albumin to reduce PCR inhibition (Garland et al., 2010). Samples were run in duplicate, including a negative control (containing laboratory grade distilled water) and four positive controls (100, 10, 1, 0.1 B. dendrobatidis genome equivalents) in duplicate on each plate. Positive controls were derived from a B. dendrobatidis Global Pandemic Lineage isolate (ref. IA2003 43) cultured from a dead Alytes obstetricans metamorph collected from Ibon Acherito, Spain. A sample was considered positive if PCR amplification occurred in both duplicates with a mean quantity of ≥0.1 genome equivalents. If a single positive was obtained, the sample was re-run in duplicate up to three times until a consensus between the duplicates was reached. If there was no consensus on the third occasion, the sample was considered negative.
Data analysis
The B. dendrobatidis infection prevalence was calculated for each survey occasion as the number of frogs testing positive for B. dendrobatidis DNA divided by the number of frogs sampled. The binomial 95% confidence intervals (CI) around this prevalence were calculated using Quantitative Parasitology software (Rózsa, Reiczigel & Majoros, 2000). The data for each month were included in the analysis only when all three sites had been sampled in the same month to ensure the amount of data was not biased to any site.
Uneven time intervals between sampling occasions meant time series analysis could not be used to decompose the seasonality and trend without interpolation over gaps which would have resulted in artificial smoothing. Each sampling occasion (separated by a minimum of 30 days) was, therefore, treated as independent. As the Montserrat population of E. johnstonei is very large, the recapture rate of individuals was likely negligible-to-low, so an assumption of independence was likely fulfilled. Due to the inability to decompose long term trends, attempts to detect changes in B. dendrobatidis infection prevalence and load were tested by comparing the peak value for each year. Prevalence estimate comparisons were made using the Chi-squared test. Comparison of B. dendrobatidis infection loads was performed using Kruskal–Wallis tests when comparing multiple occasions, and Mann–Whitney U, for comparison of two occasions. To test for differences in seasonal B. dendrobatidis infection prevalence, the highest and lowest prevalences were compared in each year for each site using a Chi-squared test.
For the analyses of the Montserrat data, air temperature was described as the mean temperature from the two dataloggers at SWG in the 30 days prior to each sampling occasion. Mean temperature was used instead of minimum or maximum as all three showed very similar patterns and the mean appeared less susceptible to temporary extremes through, for example, contact with rainwater. Monthly rainfall for each site was calculated as the total rainfall occurring in the 30 days prior to each sampling occasion from which mean daily rainfall was calculated. In vitro, B. dendrobatidis has a lifecycle from zoospore to zoosporangium of approximately 5 days at 22 °C (Berger et al., 2005), therefore environmental influences on B. dendrobatidis prevalence should be detectable over a 30 day period (Kriger & Hero, 2006).
Logistic regression was used to examine the relationship between environmental variables, site and the likelihood of B. dendrobatidis infection on Montserrat. A quasi-binomial model was fitted to compensate for over-dispersion. Likelihood ratio tests were performed to determine the importance of each variable. Multicollinearity among all explanatory variables was examined prior to inclusion in the regression analysis, using variance inflation factors (VIF; Zuur et al., 2009). Because VIF values were between 1.012 and 1.402, all variables were included in the subsequent analyses.
Linear regression was used to test for a relationship between the environmental variables and infection loads of B. dendrobatidis-positive E. johnstonei on Montserrat. Infection loads varied over several orders of magnitude and resulted in heavily skewed, non-normal model residuals and so were log-transformed prior to analysis. Tukey’s HSD was used to perform post-hoc analyses of inter-site differences. VIF values were between 1.024 and 1.281 and so all variables were included in the subsequent analyses.
In each of the regressions, the following explanatory variables were tested: mean daily rainfall in the 30 days prior to the survey, the mean temperature in the 30 days prior to the survey, site, and an interaction between rainfall and temperature.
Unless stated otherwise, all analyses were carried out in R Core Team (2017).
Results
Montserrat
Batrachochytrium dendrobatidis DNA was detected from 12.8% of the 3,674 robber frogs skin-swabbed, 2011–2013. The maximum prevalence recorded was 34.8% (95% CI [24.1–47.0]) which was in CG in December 2011 (Fig. 1). The maximum annual prevalence across all the Montserrat sites did not decrease over the duration of the study (Kruskall–Wallis; CG: Chi-sq (2) = 2.900, p = 0.235; FW: Chi-sq (2) = 0.972, p = 0.615; SWG: Chi-sq (2) = 3.729, p = 0.155; Table S1. Throughout the study, evidence of chytridiomycosis was not detected in any robber frog.
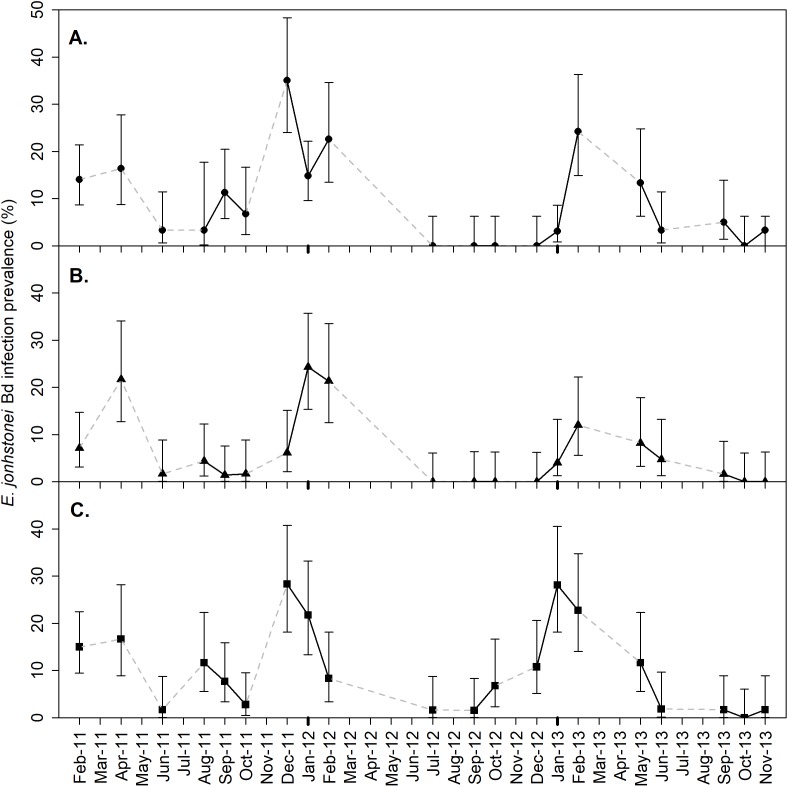
Figure 1. Eleutherodactylus johnstonei Batrachochytrium dendrobatidis infection prevalence for (A) Collins Ghaut (CG), (B) Sweetwater Ghaut (SWG) and (C) Fairy Walk (FW), on Montserrat.
Each point represents the percentage of animals testing positive for Batrachochytrium dendrobatidis DNA through qPCR of a skin swab during a single survey of approximately 60 eleutherodactylid frogs. Solid, black, lines indicate connections between sampling occasions in successive months and broken, grey, lines indicate connections between sampling occasions separated by more than one month. 95% binomial confidence intervals are presented based on a sample size of approximately 60 frogs.
The prevalence of B. dendrobatidis infection in robber frogs varied significantly across seasons, with the greatest infection prevalence consistently occurring during the cooler, drier season (Nov to May) in each of the 3 years of the study (Fig. 1). Within each year there was a significant difference between the high (dry/cool season) and low (warm/wet season) prevalences (Table 1). Infection prevalence, 2011–2013, was as low as 0% on 12 occasions (CG: 5, FW: 1, SWG: 6). A prevalence estimate of 0% is not sufficient evidence to conclude the absence of B. dendrobatidis infection from the population, as a prevalence of <5% would fall below the 95% CI for detection with a sample size of 60 (DiGiacomo & Koepsell, 1986). The fastest increase in prevalence in consecutive months was recorded between Dec 2011 and Jan 2012 at SWG, where it increased from 6.2% (95% CI [2.1–15.4]) to 24.3% (95% CI [15.3–35.7]) within 30 days. The fastest decrease in prevalence across consecutive months was recorded in the same months at CG, decreasing from 35% (95% CI [24.0–48.3]) to 15% (95% CI [9.6–22.2]).
Table 1. The annual maximum and minimum Batrachochytrium dendrobatidis prevalence detected in E. johnstonei at (A) Collins Ghaut, (B) Sweetwater Ghaut and (C) Fairy Walk, on Montserrat.
| Site | Year | High month | High prev. | 95% CI | Low month | Low prev. | 95% CI | Chi-sq | df | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| FW | 2011 | Dec | 28.3 | [18.2–40.8] | Jun | 1.7 | [0.09–8.73] | 16.732 | 1 | <0.001 |
| FW | 2012 | Jan | 21.7 | [13.4–33.2] | Sep | 1.6 | [0.09–8.33] | 12.772 | 1 | <0.001 |
| FW | 2013 | Jan | 28.1 | [18.2–40.6] | Oct | 0.0 | [0.00–6.30] | 19.741 | 1 | <0.001 |
| SWG | 2011 | Apr | 26.7 | [16.4–39.1] | Sep | 1.4 | [0.08–7.61] | 18.104 | 1 | <0.001 |
| SWG | 2012 | Jan | 25.7 | [16.7–37.1] | Sep | 0.0 | [0.00–6.40] | 17.951 | 1 | <0.001 |
| SWG | 2013 | Feb | 11.9 | [5.6–22.1] | Oct | 0.0 | [0.00–6.10] | 7.769 | 1 | 0.005 |
| CG | 2011 | Dec | 34.8 | [24.1–47.0] | Jun | 3.3 | [0.60–11.4] | 19.627 | 1 | <0.001 |
| CG | 2012 | Feb | 22.6 | [13.5–34.6] | Sep | 0.0 | [0.00–6.30] | 15.305 | 1 | <0.001 |
| CG | 2013 | Feb | 24.2 | [14.9–36.3] | Oct | 0.0 | [0.00–6.30] | 16.661 | 1 | <0.001 |
Note:
The Chi-squared statistics are derived from a comparison of the highest and lowest prevalence detected within a site within each year.
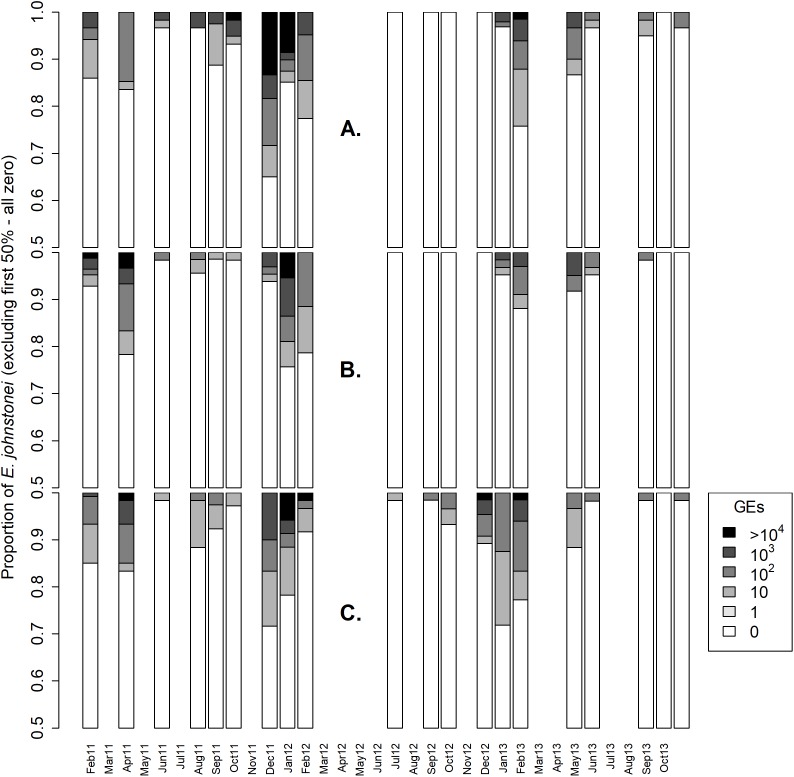
The B. dendrobatidis infection loads recorded on infected frogs were stable throughout 2011–2013 (Fig. 2), with no significant difference between the median infection loads in the peak month at any of the sites (CG: Chi-sq (2) = 1.114, p = 0.573; FW: Chi-sq (2) = 3.748, p = 0.154; SWG: Chi-sq (2) = 0.044, p = 0.978). Dry season B. dendrobatidis infection loads were significantly higher than in the wet season in each year of the study (Mann–Whitney U; 2011 p < 0.001, 2012 p < 0.001, 2013 p < 0.001).
Figure 2. Eleutherodactylus johnstonei Batrachochytrium dendrobatidis infection loads for (A) Collins Ghaut (CG), (B) Sweetwater Ghaut (SWG) and (C) Fairy Walk (FW), on Montserrat.
The proportion of eleutherodactylid frogs, on each sampling occasion, recorded at each of six Batrachochytrium dendrobatidis infection load bands as measured in genome equivalents (GEs) from skin swabs and qPCR. The y-axis begins at 0.5 to enhance viewing as at least 50% of animals tested negative for Batrachochytrium dendrobatidis DNA on every occasion. Missing bars indicates that no survey took place.
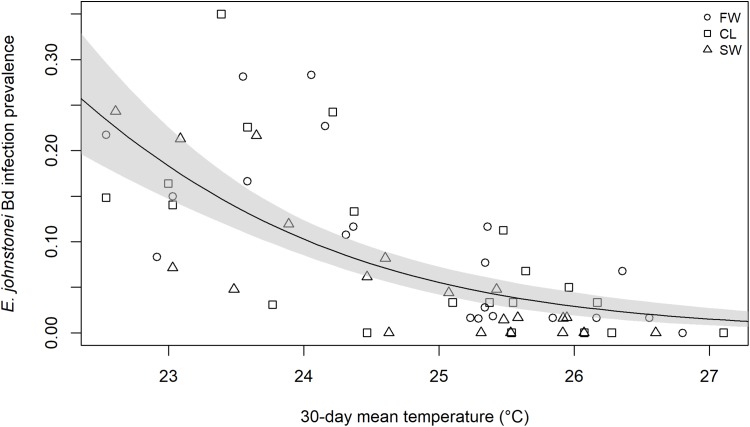
Over the course of the study, the air temperature ranged from 18.2 to 34.2 °C, with a mean of 24.8 °C (SE = 1.7), which is within the optimal temperature range (17–25 °C) for the growth of B. dendrobatidis (Longcore, Pessier & Nichols, 1999; Piotrowski, Annis & Longcore, 2004). The likelihood of B. dendrobatidis infection in E. johnstonei was inversely related to mean 30-day air temperature (OR = 0.464, 95% CI [0.377–0.570], p <0.001) (Fig. 3): 30 of 32 sampling sessions where this was over 25 °C had a prevalence of less than 10% whereas 16 of 27 sampling sessions with a mean 30-day air temperature below 25 °C had a prevalence greater than 10% (Fig. 3). Prevalence greater than 20% was only recorded when the mean 30-day air temperature was below 24.5 °C; 30% prevalence or more only occurred when the mean 30-day air temperature was below 23.5 °C.
Figure 3. Logistic model of relationship between 30-day mean temperature, site and likelihood of E. johnstonei infection with Batrachochytrium dendrobatidis on Montserrat.
This is a quasi-binomial logistic regression of Batrachochytrium dendrobatidis infection prevalence against the mean temperature across the 30 days prior to the survey. Each point represents the proportion of animals testing positive for Batrachochytrium dendrobatidis DNA through qPCR of skin swabs of a single survey of approximately 60 eleutherodactylid frogs.
The 30-day mean daily rainfall (mm) was not found to be a significant predictor of the likelihood of E. johnstonei being infected with B. dendrobatidis (p = 0.053).
There was no significant difference in B. dendrobatidis infection prevalence between the sites (p = 0.136), and no significant interaction between rainfall and temperature (p = 0.137).
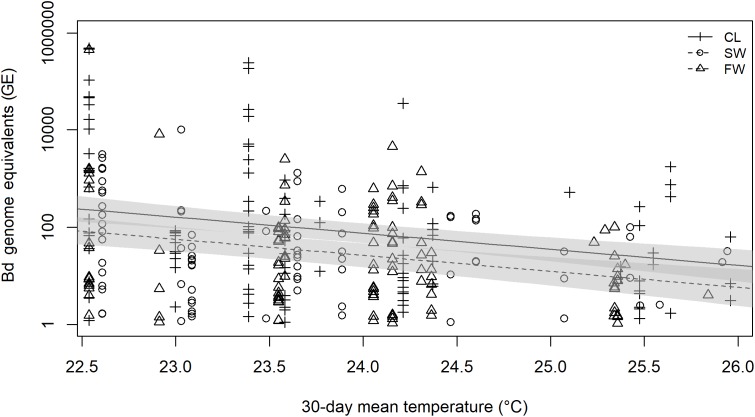
Mean 30-day air temperature was inversely related to the B. dendrobatidis infection load of infected robber frogs (beta on log scale = −0.7439, SE = 0.14, p < 0.001) (Fig. 4). Also, infection load of infected frogs differed significantly between sites, with infected frogs at FW and SWG having significantly lower infection loads than those at CG (SWG beta = −1.040, SE = 0.369, p = 0.014; FW beta = −1.092, SE = 0.331, p = 0.003) (Fig. 4). There was no difference in infection load between FW and SWG (beta = 0.052, SE = 0.369, p = 0.989). The final model accounted for c. 12% of the variation in infection loads of infected frogs (R2 = 0.1185). The 30-day mean daily rainfall was not found to be a significant predictor of B. dendrobatidis infection load (p = 0.457).
Figure 4. Linear model prediction of relationship between 30-day mean temperature, site and Batrachochytrium dendrobatidis infection load of infected E. johnstonei on Montserrat.
Batrachochytrium dendrobatidis genome equivalents (GEs) were log transformed prior to analysis as they spanned several orders of magnitude and resulted in heavily skewed, non-normal model residuals. A total of 95% CI are plotted for CL and SWG model predictions. No model prediction is plotted for FW as it was very similar to the prediction for SWG.
Dominica
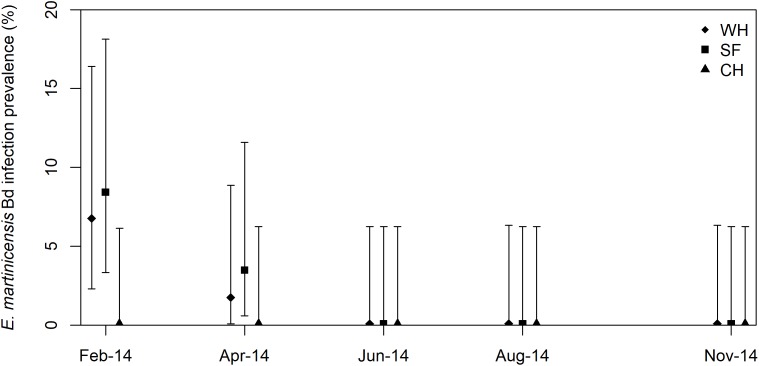
On Dominica, B. dendrobatidis was detected from 1.3% of the 900 robber frogs skin-swabbed during 2014. Robber frogs testing positive for B. dendrobatidis infection were only identified in SF and WH during February and April, with none detected during the rest of the year (Fig. 5). No B. dendrobatidis-positive samples were detected at any time at CH. At SF and WH, the infection prevalence was significantly higher in February than at any other sampling period in 2014 (Chi-sq = 5.217, df = 1, p = 0.022), with the highest recorded prevalence occurring at SF (8.3%, (95% CI [3.4–18.1]), Fig. 5).
Figure 5. Eleutherodactylus martinicensis Batrachochytrium dendrobatidis infection prevalence for Colihaut (CH), Soufriere (SF) and Wallhouse (WH) on Dominica.
Each point represents the percentage of animals testing positive for B. dendroabatidis DNA during a single survey of approximately 60 eleutherodactylid frogs. 95% binomial confidence intervals are presented based on a sample size of 60 frogs at each occasion.
Over the study period the temperature ranged from 18.6 to 39.1 °C with a mean of 25.5 °C (SE = 1.9) which is within the range of tolerable temperatures for B. dendrobatidis. At 39.1 °C, however, the maximum temperature was much higher than the maximum thermal tolerance of B. dendrobatidis (Young, Berger & Speare, 2007) and these authors postulated that B. dendrobatidis might not survive outside the hose if temperatures exceed 25 °C for an extended period of time. As the number of B. dendrobatidis-positive robber frogs in Dominica was so low, no analysis was undertaken to examine any relationship with environmental variables, however the timing of peak prevalence matched that on Montserrat.
Discussion
Amphibian chytridiomycosis threatens many hundreds of species world-wide aided by the persistence of infection in unaffected (or less affected) reservoir hosts (Brannelly et al., 2018; Scheele et al., 2017). Our results show that there is significant seasonality in B. dendrobatidis infection prevalence in an important reservoir species of robber frog on Montserrat with strong evidence of an inverse relationship with temperature. Rainfall, however, was not found to be a significant predictor of B. dendrobatidis infection prevalence. There were no significant differences in the likelihood of robber frogs being infected with B. dendrobatidis between sites. For B. dendrobatidis infection loads, temperature was an important predictor, and there was significant variation between sites. Fewer data collected over a shorter time period were available from Dominica precluding detailed analysis of possible factors driving B. dendrobatidis epidemiology, however a similar pattern of seasonal variation was observed on both islands.
It has previously been hypothesised that rainfall and B. dendrobatidis prevalence are positively correlated (Kriger, 2009), but on Montserrat we found that this was not the case. We did, however, find evidence of decreased B. dendrobatidis infection prevalence and decreased B. dendrobatidis infection load during the warmer, wetter months. The seasonal variation in B. dendrobatidis infection observed during the current study reflects the findings in other parts of the Caribbean and in South America where chytridiomycosis-driven mortality is greatest during the cooler, drier seasons (Longo et al., 2013; Ruggeri et al., 2015). In this study, however, lack of rainfall was not found to be a significant predictor of B. dendrobatidis infection prevalence. Although B. dendrobatidis transmission, which is via motile zoospores, is dependent on water (Berger et al., 2005; Kriger, 2009), behavioural adaptations of frogs to dry conditions, particularly congregating in damp refugia, could result in this apparent paradox (Burrowes, Joglar & Green, 2004; Longo, Burrowes & Joglar, 2010). Apparently-healthy E. johnstonei on Montserrat and E. marticinensis on Dominica have been observed aggregating on the forest floor during the dry season, possibly in search of water (L. Martin, C. Fenton, S.-L. Adams, 2009–2016, personal observations) with robber frogs being found in remnant pools and moist refugia. In addition to these behaviours increasing contact rates amongst robber frogs, they can increase contact rates with, and infection probability to/from, sympatric species of amphibian (Fig. 6). This aggregation in damp refugia has been reported elsewhere (Roznik & Alford, 2015) and has been shown experimentally to lead to increased B. dendrobatidis prevalence (Longo, Burrowes & Joglar, 2010).
Figure 6. An observation, in the wild, of natural direct contact between a robber frog (Eleutherodactylus johnstonei) sat on the back of a mountain chicken (Leptodactylus fallax).
This photo was taken at Sweetwater Ghaut in 2008. Credit: Gerardo Garcia.
While the amount of B. dendrobatidis DNA detected in swabs was inversely correlated with temperature, there was no relationship between rainfall and B. dendrobatidis infection load. Rather than being a result of the degree of extrinsic exposure to B. dendrobatidis, the variation in B. dendrobatidis load is more likely to reflect the growth and spread of B. dendrobatidis within the individual (Woodhams et al., 2008). Thus infection load will be influenced by factors such as individual immunological and behavioural differences, degree of skin microbiota antifungal activity (Daskin et al., 2014) and intrinsic B. dendrobatidis growth rate. Many of these, including B. dendrobatidis growth rate, are influenced by temperature (Johnson et al., 2003; Rowley & Alford, 2013). There is, however, likely to be more error in the measurements of load compared to prevalence, and this higher degree of error might obscure relationships with the measured variables.
Similar surveys for B. dendrobatidis infection in robber frogs were conducted by us in areas of Montserrat in 2009 and in WH in Dominica in December 2011. The 2009 Montserrat survey was conducted within a year of first B. dendrobatidis detection on that island and during the peak of epizootic mortality of the mountain chicken due to chytridiomycosis on Montserrat (Hudson et al., 2016a). At this time, B. dendrobatidis infection prevalence in E. johnstonei was 53.2% (95% CI [40.3–65.4]), which is significantly higher (Chi-sq = 4.387, df = 1, p = 0.036) than any prevalence detected during the 2011–2013 surveys (maximum 34.8% (95% CI [24.1–47.0])). The observed reduction in prevalence from the epizootic stage to the enzootic stage reflects similar patterns recorded in amphibian assemblages in Queensland, Australia (McDonald et al., 2005; Retallick, McCallum & Speare, 2004) and Panama (Brem & Lips, 2008). There are likely multiple mechanisms for this pattern including increased immune response to infection with B. dendrobatidis (McDonald et al., 2005), or a reduction in the number of susceptible hosts following chytridiomycosis driven declines resulting in reduced contact rates (De Castro & Bolker, 2005).
The introduction and initial epizootic phase of B. dendrobatidis in Dominica, however, occurred in 2002–2003 (Hudson et al., 2016a), but the 2011 survey of robber frogs on Dominica detected an infection prevalence of 39.3% (95% CI [27.8–52.5]), which is significantly higher (Chi-sq = 15.963, df = 1, p < 0.001) than the highest prevalence (of 8.3% (95% CI [3.4–18.1])) detected at any time or location on Dominica in 2014. It is possible that the survey, which was prompted by a localised detection of chytridiomycosis-related mortality in a remnant mountain chicken population, represented a localised epizootic in a previously B. dendrobatidis-negative population. It is also possible that we detected such a localised population of B. dendrobatidis-free robber frogs at the CH site in 2014, although B. dendrobatidis infected mountain chickens had been previously detected at this site (M.A. Hudson, M. Sulton, A. Blackman, 2014–2017, personal observations).
Taking into account the seven year difference in the timing of emergence of chytridiomycosis on the islands (Dominica 2002, Montserrat 2009: Hudson et al., 2016a), and assuming the B. dendrobatidis infection prevalence was similar on both islands at the height of the epizootic phase, the low prevalence recorded across Dominica in 2014 might represent a long-term decline in B. dendrobatidis prevalence in the reservoir host population. We did not, however, identify a downward trend in maximum annual prevalence at any site on Montserrat, but three years is a relatively short study period and we may have failed to detect a longer-term underlying trend if it was present. Alternatively, lower prevalence rates on Dominica than on Montserrat might be due to environmental differences between these islands. Specifically, the maximum ambient temperature on Dominica was well above the 30 °C tolerance threshold for B. dendrobatidis survival (Young, Berger & Speare, 2007) and 5 °C greater than the maximum recorded on Montserrat (Dominica mean = 25.5 °C, SE = 1.9, range = 18.6–39.1 °C, Montserrat mean = 24.8 °C, SE = 1.7, range = 18.2–34.2 °C). Under these conditions, it is likely that B. dendrobatidis could survive the warmest periods of the year only in low temperature microcosms, increasing the likelihood of a decline in the population density of the pathogen. Finally, as different species of robber frog were sampled on Montserrat and Dominica, it is also possible that the difference in B. dendrobatidis infection prevalence was the result of some intrinsic variation in traits between the species. The high prevalence detected on Dominica in 2011, however, suggests that this is not the case.
Although no robber frogs were seen to be exhibiting signs of chytridiomycosis on either island, due to their high population size, small body size, cryptic colouration and dense forest habitat, there is a very low chance of detecting sick or dead robber frogs should they be present. Behavioural changes due to chytridiomycosis may further reduce detection of affected individuals (Jennelle et al., 2007; Murray et al., 2009) meaning the impacts of B. dendrobatidis might be missed. A small number of robber frogs on Montserrat were found to have very high B. dendrobatidis-infection loads, with swabs from nine frogs having over 100,000 genome equivalents (Fig. 4). Despite these high loads, there has not been a noticeable decrease in the number of robber frogs on Montserrat (or Dominica), or increase in the time taken to capture 60 frogs (L. Martin, C. Fenton, M.A. Hudson, 2009–2014, personal observations). Chytridiomycosis has been implicated in the decline of closely related Eleutherodactylus spp. on Puerto Rico (Longo, Burrowes & Joglar, 2010; Longo et al., 2013) and, whilst this might not be considered a conservation issue with regards to the potentially invasive E. johnstonei, it could be of concern for E. amplinympha, an Endangered species endemic to Dominica which occurs only at high elevations, and therefore at cooler temperatures which are within the optimal range for B. dendrobatidis growth.
Understanding the epidemiological patterns of B. dendrobatidis infection in reservoir hosts is key to decision making for amphibian conservation (Brannelly et al., 2018). For example, between July and November on Montserrat there is a four-month period during which time the mean temperature exceeds 25 °C and when B. dendrobatidis prevalence drops below 10% in the robber frogs (Fig. 1; Fig. S1). This might provide time within which reintroduced mountain chickens, a species of frog endemic to Montserrat and Dominica and which was decimated by chytridiomycosis, could adapt to the environment before being challenged with B. dendrobatidis infection as has been seen in other species reintroductions in the face of disease (Viggers, Lindenmayer & Spratt, 1993). The high risk periods of the year were also well defined and predictable, thus enabling the targeting of seasonal treatments to reduce the impact of B. dendrobatidis on susceptible species, such as in-situ anti-fungal treatments (Hudson et al., 2016b).
Conclusions
The long-term monitoring of trends in B. dendrobatidis prevalence is required to understand the drivers of seasonal variation in B. dendrobatidis infection risk. In addition to improving our understanding of the ecology of B. dendrobatidis, the long-term monitoring of B. dendrobatidis infection prevalence is key to informing mitigation measures such as in-situ treatment of susceptible amphibians, environmental management to reduce exposure to infection and the timing of reintroductions. In this study, we have obtained an indication that B. dendrobatidis infection prevalence decreases in reservoir hosts following epizootic mortality due to chytridiomycosis in disease-susceptible hosts; in this case, the mountain chicken (Hudson et al., 2016a). Further investigations are required to determine if this decline is linear and if it persists over the longer term. This is an important finding as a reduction in B. dendrobatidis prevalence in surviving reservoir hosts might reduce the infection pressure on surviving or reintroduced disease-susceptible individuals through reduced likelihood of contact with the fungus. Additional long-term monitoring will also help elucidate site-specific drivers of infection risk, enabling population monitoring, disease surveillance and further mitigation efforts (e.g. in-situ treatment; Hudson et al., 2016b) for conservation purposes to be targeted during high risk periods.
Supplemental Information
There are only limited differences between the sites, therefore SWG temperature data were used to represent every site for our analyses.
Black line represents temperature, and grey bars represent rainfall. These variables were significantly positively correlated (Pearson’s correlation: t = 2.795, df = 58, p-value = 0.007), showing that cooler temperatures are associated with periods of low rainfall.
Chi-squared statistics are derived from a Kruskall-Wallis comparison of the highest prevalence detected each year, for each site. Site acronyms are defined in the methods section.
Raw data from surveys of eleutherodactylid frogs on Montserrat. Each row represents a survey of approximately 60 frogs. Data presented are the date the sampling was undertaken, the B. dendrobatidis infection prevalence estimate and the environmental data associated with that sampling occassion.
Raw data from surveys of eleutherodactylid frogs on Montserrat. Each row represents an individual frog captured and swabbed during a survey. Data presented are the B. dendrobatidis qPCR result (positive / negative for B. dendrobatidis DNA), the B. dendrobatidis infection load (GEs), the date the animal was captured and swabbed and the environmental data associated with that sampling occassion.
Acknowledgments
The authors would like to thank Roxanne Gardiner for assistance in molecular diagnostics, the Montserrat Forestry Team for assistance in the fieldwork conducted on Montserrat and Rhayim Honore for assistance in the fieldwork conducted on Dominica. Andrew A. Cunningham and Richard P. Young have been allocated joint senior authors on this manuscript as they worked equally and in concert to develop the concept of the study.
Funding Statement
This work was funded by Darwin Grant 18018 and The Balcombe Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Michael A. Hudson conceived and designed the experiments, performed the experiments, analysed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Richard A. Griffiths contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Lloyd Martin performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Calvin Fenton performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Sarah-Louise Adams performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Alex Blackman performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Machel Sulton performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Matthew W. Perkins performed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Javier Lopez performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Gerardo Garcia performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Benjamin Tapley performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Richard P. Young conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Andrew A. Cunningham conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e. approving body and any reference numbers):
This study was approved by the Zoological Society of London’s Ethics Committee (project refs: WLE/0362 and WLE/0568).
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The Montserrat Department of Environment and the Dominica Forestry, Wildlife and Parks Division permitted and, in collaboration with the study partners, conducted this work on their respective islands. Both government partners are represented by authors in this article.
Data Availability
The following information was supplied regarding data availability:
Raw data is available in the Supplemental Files.
References
- Aguirre & Tabor (2008).Aguirre AA, Tabor GM. Global factors driving emerging infectious diseases. Annals of the New York Academy of Sciences. 2008;1149(1):1–3. doi: 10.1196/annals.1428.052. [DOI] [PubMed] [Google Scholar]
- Anderson & May (1979).Anderson RM, May RM. Population biology of infectious diseases: part I. Nature. 1979;280(5721):316–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- Berger et al. (2005).Berger L, Hyatt AD, Speare R, Longcore JE. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms. 2005;68:51–63. doi: 10.3354/dao068051. [DOI] [PubMed] [Google Scholar]
- Berger et al. (2004).Berger L, Speare R, Hines HB, Marantelli G, Hyatt AD, McDonald KR, Skerratt LF, Olsen V, Clarke JM, Gillespie G, Mahony M, Sheppard N, Williams C, Tyler MJ. Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Australian Veterinary Journal. 2004;82(7):434–439. doi: 10.1111/j.1751-0813.2004.tb11137.x. [DOI] [PubMed] [Google Scholar]
- Boyle et al. (2004).Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Diseases of Aquatic Organisms. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- Brannelly et al. (2018).Brannelly LA, Webb RJ, Hunter DA, Clemann N, Howard K, Skerratt LF, Berger L, Scheele BC. Non-declining amphibians can be important reservoir hosts for amphibian chytrid fungus. Animal Conservation. 2018;21(2):91–101. doi: 10.1111/acv.12380. [DOI] [Google Scholar]
- Brem & Lips (2008).Brem FMR, Lips KR. Batrachochytrium dendrobatidis infection patterns among Panamanian amphibian species, habitats and elevations during epizootic and enzootic stages. Diseases of Aquatic Organisms. 2008;81:189–202. doi: 10.3354/dao01960. [DOI] [PubMed] [Google Scholar]
- Brunner et al. (2004).Brunner JL, Schock DM, Davidson EW, Collins JP. Intraspecific reservoirs: complex life history and the persistence of a lethal ranavirus. Ecology. 2004;85(2):560–566. doi: 10.1890/02-0374. [DOI] [Google Scholar]
- Burrowes, Joglar & Green (2004).Burrowes PA, Joglar RL, Green DE. Potential causes for amphibian declines in Puerto Rico. Herpetologica. 2004;60(2):141–154. doi: 10.1655/03-50. [DOI] [Google Scholar]
- Burrowes et al. (2017).Burrowes PA, Martes MC, Torres-Ríos M, Longo AV. Arboreality predicts Batrachochytrium dendrobatidis infection level in tropical direct-developing frogs. Journal of Natural History. 2017;51(11–12):643–656. doi: 10.1080/00222933.2017.1297504. [DOI] [Google Scholar]
- Caughley (1994).Caughley G. Directions in conservation biology. Journal of Animal Ecology. 1994;63(2):215–244. doi: 10.2307/5542. [DOI] [Google Scholar]
- Cunningham et al. (2008).Cunningham AA, Lawson B, Burton M, Thomas R. Darwin initiative final report: addressing a threat to Caribbean amphibians: capacity building in Dominica (No. 13032) 2008. Institute of Zoology, Zoological Society of London.
- Daskin et al. (2014).Daskin JH, Bell SC, Schwarzkopf L, Alford RA. Cool temperatures reduce antifungal activity of symbiotic bacteria of threatened amphibians—implications for disease management and patterns of decline. PLOS ONE. 2014;9(6):e100378. doi: 10.1371/journal.pone.0100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak et al. (1999).Daszak P, Berger L, Cunningham AA, Hyatt AD, Green DE, Speare R. Emerging infectious diseases and amphibian population declines. Emerging Infectious Diseases. 1999;5(6):735–748. doi: 10.3201/eid0506.990601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak, Cunningham & Hyatt (2000).Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science. 2000;287(5452):443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- De Castro & Bolker (2005).De Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecology Letters. 2005;8(1):117–126. doi: 10.1111/j.1461-0248.2004.00693.x. [DOI] [Google Scholar]
- DiGiacomo & Koepsell (1986).DiGiacomo RF, Koepsell TD. Sampling for detection of infection or disease in animal populations. Journal of the American Veterinary Medical Association. 1986;189(1):22–23. [PubMed] [Google Scholar]
- Fisher, Garner & Walker (2009).Fisher MC, Garner TWJ, Walker SF. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annual Review of Microbiology. 2009;63(1):291–310. doi: 10.1146/annurev.micro.091208.073435. [DOI] [PubMed] [Google Scholar]
- Forrest & Schlaepfer (2011).Forrest MJ, Schlaepfer MA. Nothing a hot bath won’t cure: infection rates of amphibian chytrid fungus correlate negatively with water temperature under natural field settings. PLOS ONE. 2011;6(12):e28444. doi: 10.1371/journal.pone.0028444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland et al. (2010).Garland S, Baker A, Phillott AD, Skerratt LF. BSA reduces inhibition in a TaqMan® assay for the detection of Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms. 2010;92(3):113–116. doi: 10.3354/dao02053. [DOI] [PubMed] [Google Scholar]
- Gervasi et al. (2013).Gervasi S, Gondhalekar C, Olson DH, Blaustein AR. Host identity matters in the amphibian-Batrachochytrium dendrobatidis system: fine-scale patterns of variation in responses to a multi-host pathogen. PLOS ONE. 2013;8(1):e54490. doi: 10.1371/journal.pone.0054490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, Griffiths & Pavajeau (2015).Harding G, Griffiths RA, Pavajeau L. Developments in amphibian captive breeding and reintroduction programs. Conservation Biology. 2015;30(2):340–349. doi: 10.1111/cobi.12612. [DOI] [PubMed] [Google Scholar]
- Holmes, McLaren & Wilson (2014).Holmes I, McLaren K, Wilson B. Precipitation constrains amphibian chytrid fungus infection rates in a terrestrial frog assemblage in Jamaica, West Indies. Biotropica. 2014;46(2):219–228. doi: 10.1111/btp.12093. [DOI] [Google Scholar]
- Hudson et al. (2016a).Hudson MA, Young RP, Jackson JD, Orozco-terWengel P, Martin L, James A, Sulton M, Garcia G, Griffiths RA, Thomas R, Magin C, Bruford MW, Cunningham AA. Dynamics and genetics of a disease-driven species decline to near extinction: lessons for conservation. Scientific Reports. 2016a;6(1):30772. doi: 10.1038/srep30772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson et al. (2016b).Hudson MA, Young RP, Lopez J, Martin L, Fenton C, McCrea R, Griffiths RA, Adams S-L, Gray G, Garcia G, Cunningham AA. In-situ itraconazole treatment improves survival rate during an amphibian chytridiomycosis epidemic. Biological Conservation. 2016b;195:37–45. doi: 10.1016/j.biocon.2015.12.041. [DOI] [Google Scholar]
- Hyatt et al. (2007).Hyatt A, Boyle Dg, Olsen V, Boyle Db, Berger L, Obendorf D, Dalton A, Kriger K, Hero M, Hines H, Phillott R, Campbell R, Marantelli G, Gleason F, Colling A. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms. 2007;73:175–192. doi: 10.3354/dao073175. [DOI] [PubMed] [Google Scholar]
- IUCN SSC Amphibian Specialist Group (2017).IUCN SSC Amphibian Specialist Group Leptodactylus fallax. The IUCN Red List of Threatened Species. 2017;2017:e.T57125A3055585. doi: 10.2305/IUCN.UK.2017-3.RLTS.T57125A3055585.en. [DOI] [Google Scholar]
- James et al. (2015).James TY, Toledo LF, Rödder D, da Silva Leite D, Belasen AM, Betancourt-Román CM, Jenkinson TS, Soto-Azat C, Lambertini C, Longo AV, Ruggeri J, Collins JP, Burrowes PA, Lips KR, Zamudio KR, Longcore JE. Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: lessons from the first 15 years of amphibian chytridiomycosis research. Ecology and Evolution. 2015;5(18):4079–4097. doi: 10.1002/ece3.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennelle et al. (2007).Jennelle CS, Cooch EG, Conroy MJ, Senar JC. State-specific detection probabilities and disease prevalence. Ecological Applications. 2007;17(1):154–167. doi: 10.1890/1051-0761(2007)017[0154:sdpadp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Johnson et al. (2003).Johnson ML, Berger L, Philips L, Speare R. Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms. 2003;57:255–260. doi: 10.3354/dao057255. [DOI] [PubMed] [Google Scholar]
- Johnson & Speare (2003).Johnson ML, Speare R. Survival of Batrachochytrium dendrobatidis in water: quarantine and disease control implications. Emerging Infectious Diseases. 2003;9(8):922–925. doi: 10.3201/eid0908.030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson & Speare (2005).Johnson ML, Speare R. Possible modes of dissemination of the amphibian chytrid Batrachochytrium dendrobatidis in the environment. Diseases of Aquatic Organisms. 2005;65:181–186. doi: 10.3354/dao065181. [DOI] [PubMed] [Google Scholar]
- Kaiser (1992).Kaiser H. The trade-mediated introduction of Eleutherodactylus martinicensis (Anura: Leptodactylidae) on St. Barthélémy, French Antilles, and its implications for lesser Antillean biogeography. Journal of Herpetology. 1992;26(3):264–273. doi: 10.2307/1564880. [DOI] [Google Scholar]
- Kaiser (1997).Kaiser H. Origins and introductions of the Caribbean frog, Eleutherodactylus johnstonei (Leptodactylidae): management and conservation concerns. Biodiversity & Conservation. 1997;6(10):1391–1407. [Google Scholar]
- Kriger (2009).Kriger KM. Lack of evidence for the drought-linked chytridiomycosis hypothesis. Journal of Wildlife Diseases. 2009;45(2):537–541. doi: 10.7589/0090-3558-45.2.537. [DOI] [PubMed] [Google Scholar]
- Kriger & Hero (2006).Kriger KM, Hero J-M. Survivorship in wild frogs infected with chytridiomycosis. EcoHealth. 2006;3(3):171–177. doi: 10.1007/s10393-006-0027-7. [DOI] [Google Scholar]
- Longcore, Pessier & Nichols (1999).Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 1999;91(2):219–227. doi: 10.2307/3761366. [DOI] [Google Scholar]
- Longo & Burrowes (2010).Longo AV, Burrowes PA. Persistence with chytridiomycosis does not assure survival of direct-developing frogs. EcoHealth. 2010;7(2):185–195. doi: 10.1007/s10393-010-0327-9. [DOI] [PubMed] [Google Scholar]
- Longo, Burrowes & Joglar (2010).Longo AV, Burrowes PA, Joglar RL. Seasonality of Batrachochytrium dendrobatidis infection in direct-developing frogs suggests a mechanism for persistence. Diseases of Aquatic Organisms. 2010;92(3):253–260. doi: 10.3354/dao02054. [DOI] [PubMed] [Google Scholar]
- Longo et al. (2013).Longo AV, Ossiboff RJ, Zamudio KR, Burrowes PA. Lability in host defenses: terrestrial frogs die from chytridiomycosis under enzootic conditions. Journal of Wildlife Diseases. 2013;49(1):197–199. doi: 10.7589/2012-05-129. [DOI] [PubMed] [Google Scholar]
- Longo et al. (2015).Longo AV, Savage AE, Hewson I, Zamudio KR. Seasonal and ontogenetic variation of skin microbial communities and relationships to natural disease dynamics in declining amphibians. Royal Society Open Science. 2015;2:140377. doi: 10.1098/rsos.140377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum (2012).McCallum H. Disease and the dynamics of extinction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367(1604):2828–2839. doi: 10.1098/rstb.2012.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald et al. (2005).McDonald KR, Méndez D, Müller R, Freeman AB, Speare R. Decline in the prevalence of chytridiomycosis in frog populations in North Queensland, Australia. Pacific Conservation Biology. 2005;11(2):114–120. doi: 10.1071/pc050114. [DOI] [Google Scholar]
- McMahon et al. (2013).McMahon TA, Brannelly LA, Chatfield MWH, Johnson PTJ, Joseph MB, McKenzie VJ, Richards-Zawacki CL, Venesky MD, Rohr JR. Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in the absence of infection. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(1):210–215. doi: 10.1073/pnas.1200592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell et al. (2008).Mitchell KM, Churcher TS, Garner TWJ, Fisher MC. Persistence of the emerging pathogen Batrachochytrium dendrobatidis outside the amphibian host greatly increases the probability of host extinction. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1632):329–334. doi: 10.1098/rspb.2007.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray et al. (2009).Murray KA, Skerratt LF, Speare R, McCallum H. Impact and dynamics of disease in species threatened by the amphibian chytrid fungus, Batrachochytrium dendrobatidis. Conservation Biology. 2009;23(5):1242–1252. doi: 10.1111/j.1523-1739.2009.01211.x. [DOI] [PubMed] [Google Scholar]
- Oppel et al. (2014).Oppel S, Hilton G, Ratcliffe N, Fenton C, Daley J, Gray G, Vickery J, Gibbons D. Assessing population viability while accounting for demographic and environmental uncertainty. Ecology. 2014;95(7):1809–1818. doi: 10.1890/13-0733.1. [DOI] [PubMed] [Google Scholar]
- Piotrowski, Annis & Longcore (2004).Piotrowski JS, Annis SL, Longcore JE. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia. 2004;96:9–15. doi: 10.1080/15572536.2005.11832990. [DOI] [PubMed] [Google Scholar]
- R Core Team (2017).R Core Team . R: a language and environment for statistical computing. Vienna: The R Foundation for Statistical Computing; 2017. [Google Scholar]
- Raffel et al. (2006).Raffel TR, Rohr JR, Kiesecker JM, Hudson PJ. Negative effects of changing temperature on amphibian immunity under field conditions. Functional Ecology. 2006;20(5):819–828. doi: 10.1111/j.1365-2435.2006.01159.x. [DOI] [Google Scholar]
- Retallick, McCallum & Speare (2004).Retallick RWR, McCallum H, Speare R. Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLOS Biology. 2004;2(11):e351. doi: 10.1371/journal.pbio.0020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley & Alford (2013).Rowley JJL, Alford RA. Hot bodies protect amphibians against chytrid infection in nature. Scientific Reports. 2013;3(1):1515. doi: 10.1038/srep01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roznik & Alford (2015).Roznik EA, Alford RA. Seasonal ecology and behavior of an endangered rainforest frog (Litoria rheocola) threatened by disease. PLOS ONE. 2015;10(5):e0127851. doi: 10.1371/journal.pone.0127851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rózsa, Reiczigel & Majoros (2000).Rózsa L, Reiczigel J, Majoros G. Quantifying parasites in samples of hosts. Journal of Parasitology. 2000;86(2):228–232. doi: 10.1645/0022-3395(2000)086[0228:qpisoh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ruggeri et al. (2018).Ruggeri J, Carvalho-E-Silva SP, James TY, Toledo LF. Amphibian chytrid infection is influenced by rainfall seasonality and water availability. Diseases of Aquatic Organisms. 2018;127(2):107–115. doi: 10.3354/dao03191. [DOI] [PubMed] [Google Scholar]
- Ruggeri et al. (2015).Ruggeri J, Longo AV, Gaiarsa MP, Alencar LRV, Lambertini C, Leite DS, Carvalho-e-Silva SP, Zamudio KR, Toledo LF, Martins M. Seasonal variation in population abundance and chytrid infection in stream-dwelling frogs of the Brazilian Atlantic forest. PLOS ONE. 2015;10(7):e0130554. doi: 10.1371/journal.pone.0130554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele et al. (2017).Scheele BC, Hunter DA, Brannelly LA, Skerratt LF, Driscoll DA. Reservoir-host amplification of disease impact in an endangered amphibian. Conservation Biology. 2017;31(3):592–600. doi: 10.1111/cobi.12830. [DOI] [PubMed] [Google Scholar]
- Scheele et al. (2019).Scheele BC, Pasmans F, Skerratt LF, Berger L, Martel A, Beukema W, Acevedo AA, Burrowes PA, Carvalho T, Catenazzi A, De la Riva I, Fisher MC, Flechas SV, Foster CN, Frías-Álvarez P, Garner TWJ, Gratwicke B, Guayasamin JM, Hirschfeld M, Kolby JE, Kosch TA, La Marca E, Lindenmayer DB, Lips KR, Longo AV, Maneyro R, McDonald CA, Mendelson J, 3rd, Palacios-Rodriguez P, Parra-Olea G, Richards-Zawacki CL, Rödel MO, Rovito SM, Soto-Azat C, Toledo LF, Voyles J, Weldon C, Whitfield SM, Wilkinson M, Zamudio KR, Canessa S. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science. 2019;363(6434):1459–1463. doi: 10.1126/science.aav0379. [DOI] [PubMed] [Google Scholar]
- Stockwell, Clulow & Mahony (2010).Stockwell MP, Clulow J, Mahony MJ. Host species determines whether infection load increases beyond disease-causing thresholds following exposure to the amphibian chytrid fungus. Animal Conservation. 2010;13:62–71. doi: 10.1111/j.1469-1795.2010.00407.x. [DOI] [Google Scholar]
- Terrell et al. (2014).Terrell VCK, Engbrecht NJ, Pessier AP, Lannoo MJ. Drought reduces chytrid fungus (Batrachochytrium dendrobatidis) infection intensity and mortality but not prevalence in adult crawfish frogs (Lithobates areolatus) Journal of Wildlife Diseases. 2014;50(1):56–62. doi: 10.7589/2013-01-016. [DOI] [PubMed] [Google Scholar]
- Viggers, Lindenmayer & Spratt (1993).Viggers K, Lindenmayer D, Spratt D. The importance of disease in reintroduction programmes. Wildlife Research. 1993;20(5):687–698. doi: 10.1071/wr9930687. [DOI] [Google Scholar]
- Walker et al. (2007).Walker SF, Salas MB, Jenkins D, Garner TWJ, Cunningham AA, Hyatt AD, Bosch J, Fisher MC. Environmental detection of Batrachochytrium dendrobatidis in a temperate climate. Diseases of Aquatic Organisms. 2007;77:105–112. doi: 10.3354/dao01850. [DOI] [PubMed] [Google Scholar]
- Whitfield et al. (2012).Whitfield SM, Kerby J, Gentry LR, Donnelly MA. Temporal variation in infection prevalence by the amphibian chytrid fungus in three species of frogs at La Selva, Costa Rica. Biotropica. 2012;44(6):779–784. doi: 10.1111/j.1744-7429.2012.00872.x. [DOI] [Google Scholar]
- Woodhams et al. (2008).Woodhams DC, Alford RA, Briggs CJ, Johnson M, Rollins-Smith LA. Life-history trade-offs influence disease in changing climates: strategies of an amphibian pathogen. Ecology. 2008;89(6):1627–1639. doi: 10.1890/06-1842.1. [DOI] [PubMed] [Google Scholar]
- Young, Berger & Speare (2007).Young S, Berger L, Speare R. Amphibian chytridiomycosis: strategies for captive management and conservation. International Zoo Yearbook. 2007;41(1):85–95. doi: 10.1111/j.1748-1090.2007.00010.x. [DOI] [Google Scholar]
- Zuur et al. (2009).Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R, statistics for biology and health. New York: Springer-Verlag; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
There are only limited differences between the sites, therefore SWG temperature data were used to represent every site for our analyses.
Black line represents temperature, and grey bars represent rainfall. These variables were significantly positively correlated (Pearson’s correlation: t = 2.795, df = 58, p-value = 0.007), showing that cooler temperatures are associated with periods of low rainfall.
Chi-squared statistics are derived from a Kruskall-Wallis comparison of the highest prevalence detected each year, for each site. Site acronyms are defined in the methods section.
Raw data from surveys of eleutherodactylid frogs on Montserrat. Each row represents a survey of approximately 60 frogs. Data presented are the date the sampling was undertaken, the B. dendrobatidis infection prevalence estimate and the environmental data associated with that sampling occassion.
Raw data from surveys of eleutherodactylid frogs on Montserrat. Each row represents an individual frog captured and swabbed during a survey. Data presented are the B. dendrobatidis qPCR result (positive / negative for B. dendrobatidis DNA), the B. dendrobatidis infection load (GEs), the date the animal was captured and swabbed and the environmental data associated with that sampling occassion.
Data Availability Statement
The following information was supplied regarding data availability:
Raw data is available in the Supplemental Files.