Significance
Endometriosis is a common gynecological disorder whose physiopathology is still unclear. Thyroid autoimmunity has long been associated with endometriosis, without a mechanistic explanation to link the two pathologies. First, we showed that thyroid hormone metabolism is altered in endometriotic cells. Our in vitro study further revealed the proliferative and prooxidative roles of thyroid hormones in endometriotic cells. Finally, we confirmed the aggravating role of thyroid hormones in endometriosis evolution in three different mouse models. In humans, thyroid disorders are associated with more severe forms of endometriosis. Thus, thyroid function and thyroid hormone medication should be taken into account for the care of women with endometriosis.
Keywords: thyroid, thyroid hormones, endometriosis, oxidative stress, autoimmunity
Abstract
Endometriosis is characterized by the presence of ectopic endometrial cells outside the uterine cavity. Thyroid autoimmunity has been associated with endometriosis. This work investigated the potential pathophysiological link between endometriosis and thyroid disorders. Transcripts and proteins involved in thyroid metabolism are dysregulated in eutopic and ectopic endometrium of endometriotic patients, leading to resistance of ectopic endometrium to triiodothyronine (T3) action and local accumulation of thyroxine (T4). Thyroid-stimulating hormone (TSH) acts as a proliferative and prooxidative hormone on all endometria of endometriosis patients and controls, whereas T3 and T4 act to specifically increase ectopic endometrial cell proliferation and reactive oxygen species (ROS) production. Mouse studies confirmed the data gained in vitro since endometriotic implants were found to be bigger when thyroid hormones increased. A retrospective analysis of endometriosis patients with or without a thyroid disorder revealed an increased chronic pelvic pain and disease score in endometriotic patients with a thyroid disorder.
Endometriosis is a benign gynecological disorder affecting up to 10% of women of reproductive age (1). Its characteristic feature is the presence of ectopic endometrium outside the uterine cavity (2), which is responsible for severe inflammation leading to pelvic pain and infertility (3, 4). The pathophysiology of endometriosis is still unclear, but the release of endometrial cells in the peritoneal cavity by retrograde menstrual reflux is probably the most commonly accepted theory to explain the presence of ectopic endometrium (5). However, menstrual reflux occurs in up to 90% of women, while endometriosis affects only around 10%. Thus, additional factors must explain endometriosis development (6, 7). Hormonal dysregulation with ectopic expression of aromatase leading to local production of estrogen, along with progesterone resistance of ectopic tissue, is a hallmark of endometriosis and contributes to lesion growth. Among inflammatory factors, some authors have suggested autoimmunity as a major factor in endometriosis development (8). Indeed, endometriosis recapitulates some characteristics of autoimmune disorders, such as chronic local inflammation associated with the presence of autoantibodies (Abs) (9, 10). Moreover, endometriosis is frequently associated with several other organ-specific autoimmune diseases (11). Among them, autoimmune thyroid disorder(s) (AITD) have long been highlighted in endometriosis patients (11–13) suggesting a pathogenic association between these two pathologies. Grave’s disease and Hashimoto’s thyroiditis are the two main clinical presentations (14), resulting from a breach of tolerance against thyroid-specific antigens. Hashimoto’s thyroiditis is a chronic lymphocytic thyroiditis with progressive destruction of the gland leading to hypothyroidism (15). Grave’s disease is characterized by the presence of anti–thyroid-stimulating hormone (TSH) receptor Abs causing hyperthyroidism (16). Many studies have shown an association between the presence of thyroid Abs and endometriosis (11–13, 17), with AITD leading either to hypothyroidism or hyperthyroidism (13). With endometriosis being characterized by a chronic inflammatory process, the high prevalence of AITD in endometriosis patients could be the result of the immune dysregulation observed in women with endometriosis (6, 8). On the other hand, although no pathophysiological link has been demonstrated so far, AITD and, more generally, thyroid dysfunction may also have consequences on endometriosis progression. Thyroid involvement in endometrium and ovary physiology has already been described (18, 19), and thyroid diseases are frequently associated with gynecological and obstetrical disorders such as infertility, miscarriages, or preterm births (20). Moreover, there are structural similarities and cross-reactions between TSH and the luteinizing hormone (LH), whose receptors are present on the uterus and ovaries and are implicated in endometriotic lesion growth (21). The aim of this study was to investigate the interplay between endometriosis and AITD and, more precisely, to explore the consequences of thyroid disorders on endometriosis evolution in vitro using endometrial and endometriotic primary cell lines and tissue and in vivo using relevant mouse models of thyroiditis and endometriosis. Finally, a retrospective analysis of clinical features in endometriosis patients with or without thyroid disorder was conducted.
Results
Transcripts and Proteins Involved in Thyroid Hormone Signaling and Metabolism Are Dysregulated in Eutopic and Ectopic Endometrium of Endometriotic Patients.
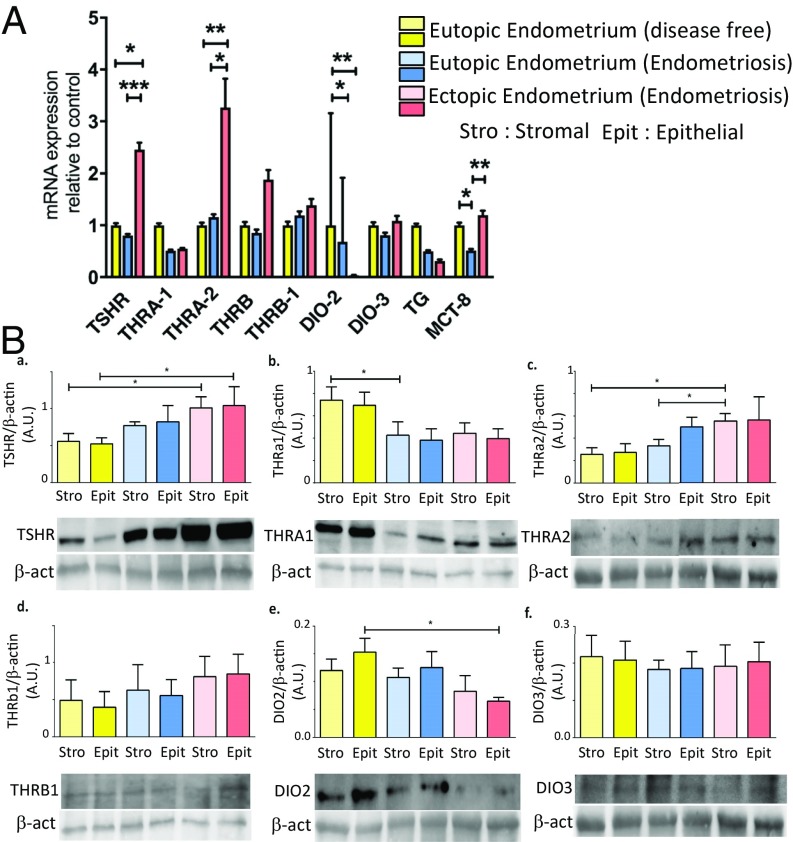
Clinical characteristics of the patients whose biopsies were used for the qRT-PCR analysis are summarized in SI Appendix, S1. We confirmed that TSHR, THRA1, THRA2, THRB, and THRB1 were expressed in human endometrium at the messenger RNA (mRNA) and protein levels (19, 22). We found an increased expression of TSHR in ectopic endometrium by a mean of 2.5-fold (P < 0.001) and of THRA2 by a mean of 3.3-fold compared with controls (P < 0.01). There was no difference for these transcripts in eutopic endometrium of endometriosis patients compared with control endometrium (Fig. 1, 1). An increased expression of LH receptors LHRa (LHCGR isoform a), LHRb, LHRc, and LHRd was found in ectopic endometrium by a mean of 8.2-fold (P < 0.01), 52.7-fold (P < 0.01), 19.2-fold (P < 0.01), and 11.8-fold (P < 0.01), respectively, compared with endometrium of endometriosis-free women (SI Appendix, S2). Western blot analysis confirmed qRT-PCR results, as TSHR (P < 0.05) and THRA2 (P < 0.05) proteins were increased in ectopic endometrium of endometriosis patients compared with the endometrium of endometriosis-free patients (Fig. 1, 2). THRA2 encodes a truncated isoform of thyroid receptor, which is thus unable to bind triiodothyronine (T3) and acts as a competitive inhibitor of transcriptional activity of T3 (23). DIO2 is an enzyme responsible for the deiodination of the prohormone thyroxine (T4) into the bioactive T3, while DIO3 is responsible for deiodination of T4 and T3 into triiodothyronine inverse and diiodothyronine, two inactive metabolites of T3 and T4. We found 31.2-fold and 21.3-fold decreased DIO2 expression in ectopic endometrium (P < 0.01) and eutopic endometrium (P < 0.05) from endometriotic patients compared with control endometrium, respectively. This decreased expression was confirmed by Western blot analysis, especially in epithelial cells of ectopic lesions compared with controls (P < 0.05) (Fig. 1, 2); meanwhile, no difference was found for DIO3 expression in qRT-PCR and Western blot analysis. Altogether, mRNA expression of THRA1, THRA2, DIO2, and DIO3 suggests that ectopic endometrium of endometriotic biopsies may display decreased production of T3 and may be resistant to nuclear action of T3, while accumulating T4 locally. This hypothesis was confirmed by the decreased production of T3 and increased in vitro production of T4 by endometriotic cells compared with control endometrial cells (SI Appendix, S3). Thyroglobulin (TG), a glycoprotein acting as a substrate for the synthesis of T3 and T4, is expressed in normal endometrium as previously described (22), but also, and at the same level, in the endometrium and lesions of women with endometriosis. MCT8 is a transporter of T3 and T4 that allows trafficking of these hormones into the cells. MCT8 expression was found to be decreased in eutopic endometrium of women with endometriosis compared with ectopic endometrium and control endometrium by 2.3-fold and twofold, respectively (P < 0.01) (Fig. 1).
Fig. 1.
Transcripts and proteins involved in thyroid hormone signaling and metabolism are dysregulated in eutopic and ectopic endometrium of endometriotic patients. (A) Relative levels of transcripts for TSHR, THRA1, THRA2, THRB, THRB1, DIO-2, DIO3, TG, and MCT8 were quantified by qRT-PCR for eutopic endometrium from endometriosis-free patients and from eutopic and ectopic endometrium from endometriosis patients. Expression levels are expressed in arbitrary units (A.U.) ± SEM. P values are indicated (Kruskal–Wallis and Dunn’s post hoc test). (B, a–f) Protein levels of TSHR, THRA1, THRA2, THRB1, DIO2, and DIO3 were assessed by Western blot analysis for stromal and epithelial cells derived from eutopic endometrium from controls and from eutopic endometrium and ectopic endometrium from endometriosis patients. Images of representative blots are displayed. THRA2 and DIO3 were measured on the same blot, so the β-actin is the same in both cases. It is also the case for TSHR and DIO2, as well as for THRA1 and THRB1. The graphs show relative expression of β-actin ± SEM (*P < 0.05; **P < 0.01; ***P < 0.001 measured by Mann–Whitney test).
TSH Acts as a Proliferative and Prooxidative Hormone on the Endometrium of Endometriotic Patients and Controls in Vitro.
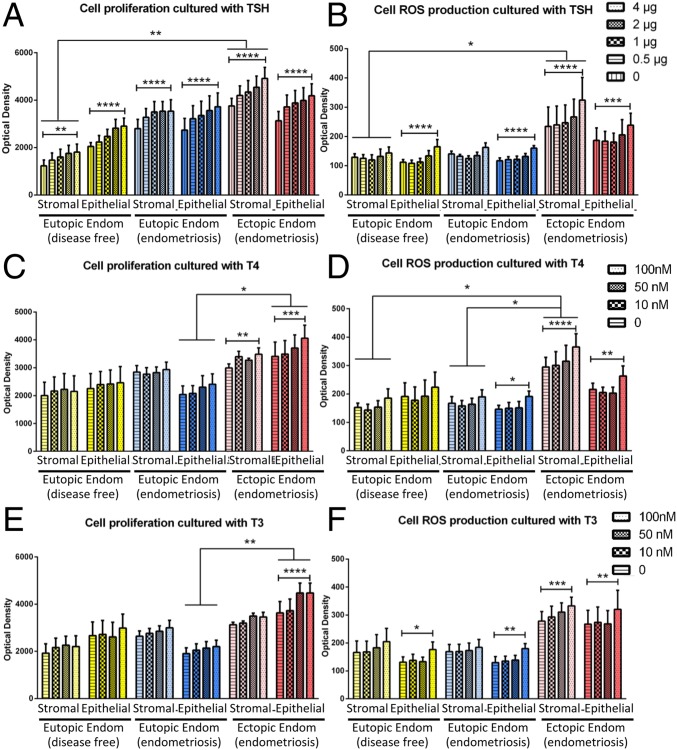
Clinical characteristics of the patients used for the in vitro study are summarized in SI Appendix, S4. The addition of TSH (4 μg) in the cell culture medium led to an increased proliferation by 30.1% for the epithelial cells (P < 0.0001) and 22.9% for the stromal cells (P < 0.01) of control endometrium, and by 15.6% on epithelial cells (P < 0.0001) and 7.80% on stromal cells (P < 0.0001) of eutopic endometrium of patients with endometriosis (Fig. 2A). Regarding ectopic endometrium of endometriotic patients, TSH increased cell proliferation by 12.6% for epithelial cells (P < 0.0001) and 17.10% for stromal cells (P < 0.0001). The increase of cell proliferation with TSH is dose-dependent, regardless of the cell type (Fig. 2A). However, the proliferation rates induced by TSH are similar in the different tissues, with no impact of the disease. Interestingly, TSH also had a prooxidative effect on endometrial cells, especially on epithelial cells (Fig. 2B). Compared with unstimulated epithelial cells, TSH (4 μg) induced reactive oxygen species (ROS) production with increases of 47.2%, 37.1%, and 27.7% on epithelial cells from control endometrium (P < 0.0001), eutopic endometrium (P < 0.0001), and ectopic endometrium (P < 0.001) from endometriosis patients, respectively. Ectopic stromal cells also increased their ROS production by 38.3% (P < 0.0001) when cultured with 4 μg of TSH, while control and eutopic stromal cells did not display any TSH-induced ROS increase.
Fig. 2.
ROS production and cell proliferation of endometrial and endometriotic cells cultured with TSH and thyroid hormones T3 and T4. Cell proliferation (A, C, and E) and ROS production (B, D, and F) of epithelial and stromal cells from eutopic endometrium of disease-free women and from eutopic and ectopic endometrium from endometriosis patients after treating cells with various amounts of TSH (A and B), T3 (C and D), and T4 (E and F). Results are expressed as mean ± SEM. (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 measured by two-way ANOVA and Tukey’s post hoc test). Endom, Endometrium.
Thyroid Hormones T3 and T4 Increase Ectopic Endometrial Cell Proliferation and ROS Production in Vitro.
Addition of 100 nM T4 increased in vitro proliferation by 19.1% in ectopic epithelial cells (P < 0.001) and by 16.4% in ectopic stromal cells (P < 0.01) compared with unstimulated cells. By contrast, eutopic endometrium epithelial and stromal cells from controls and patients do not proliferate more in the presence of 100 nM T4 (Fig. 2C). T4 at 100 nM also affected ROS production in ectopic cells, increasing by 21.9% and 23.8% in epithelial (P < 0.0001) and stromal (P < 0.01) cells, respectively (Fig. 2D). Interestingly, T3 at 100 nM increased the proliferation of ectopic epithelial cells by 23.2% (P < 0.0001) (Fig. 2E). T3 at 100 nM increased ROS production but affected both eutopic and ectopic epithelial cells from endometriotic patients. Compared with unstimulated cells, T3 increased ROS production by 19.6% for ectopic stromal cells (P < 0.001) and by 34.1%, 38.6%, and 19.8% for epithelial cells from the endometrium of disease-free women (P < 0.05), and from the eutopic (P < 0.01) and ectopic endometrium (P < 0.01) of women with endometriosis, respectively (Fig. 2F).
Endometriotic Implants Are Reduced in a Mouse Model of Toxic Thyroiditis.
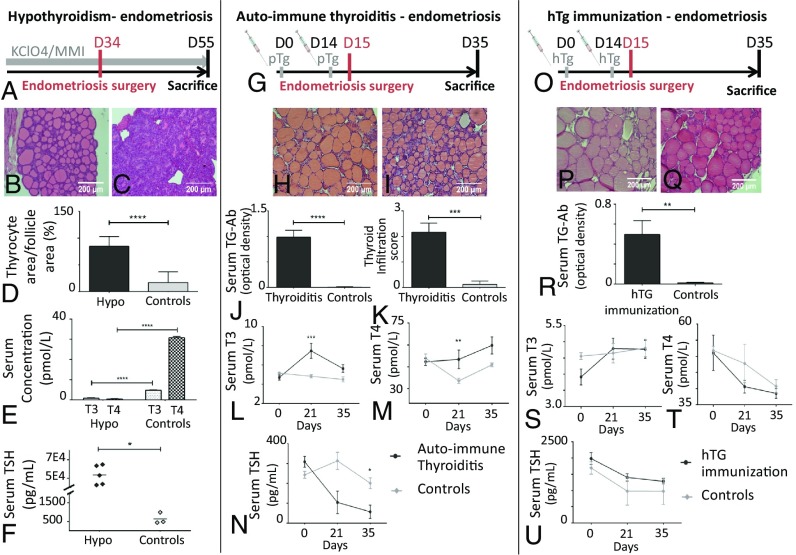
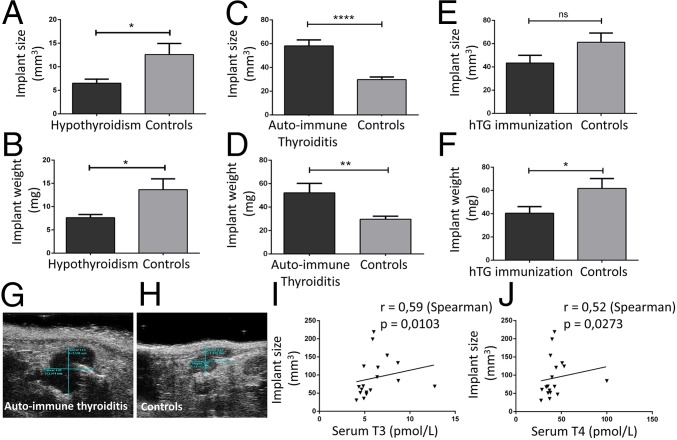
We showed that TSHR expression is increased in ectopic endometrium and TSH has proproliferative and prooxidative effects on endometrial cells. Thus, we first explored whether increased TSH during thyroiditis might be responsible for the increase in endometriotic implant growth in vivo. We used a mouse model of toxic hypothyroiditis induced by methimazole (MMI) treatment leading to hypothyroidism, in which we induced endometriosis by uterine horn surgical implantation (Fig. 3A). Histological examination of thyroid sections showed thyrocyte hypertrophy in MMI-treated mice (Fig. 3 B and C) with the thyrocyte area/follicle area ratio being significantly increased in treated mice (P < 0.0001) (Fig. 3D). T3 and T4 hormone levels were significantly decreased in the treated mice compared with controls [T3: 4.79 versus 0.85 pg/mL (P < 0.0001) and T4: 30.79 versus 0.53 pg/mL (P < 0.0001) in controls and treated mice, respectively] (Fig. 3E). Consistent with pituitary hypothalamic feedback and the decrease of thyroid hormone levels, TSH was greatly increased in the treated mice compared with the control mice [53,643 vs. 639 pg/mL (P < 0.05)] (Fig. 3F). After 8 wk of treatment, endometriosis was induced in mice. Twenty-one days after endometriosis induction, endometriotic implants of MMI-treated mice were 1.9-fold smaller (P < 0.05) and 1.8-fold lighter (P < 0.05) than those of control animals (Fig. 4 A and B). The results do not support the trophic effect of TSH on endometriotic implants but highlight a possible role of thyroid hormones in endometriosis evolution.
Fig. 3.
Description and validation of the three mouse models of thyroiditis coupled with endometriosis. The three models coupled with endometriosis were as follows: toxic hypothyroidism (Hypo) (A–F), autoimmune thyroiditis (G–N), and TG immunization with euthyroidism (O–U). (A, G, and O) Experimental setups for toxic hypothyroidism (A, C57Bl6 mice), for autoimmune thyroiditis (B, CBA mice), and for TG immunization with euthyroidism (O, BALB/c mice), all coupled with the endometriosis model (details are provided in Materials and Methods). Histological sections of thyroid stained with H&E showing normal thyrocytes in control mice (B, H, and P) and hTg-immunized mice (Q), thyrocyte hypertrophy in the hypothyroidism mouse model (B), and thyroid infiltration by mononuclear cells in the autoimmune thyroiditis mice (I). (D) Morphological parameter in thyroids: ratio of thyrocyte area on follicle area. Serum TG Ab detected with ELISA (J) and thyroid infiltration score (K) show thyroiditis hallmarks in pTg-immunized mice. (R) Serum TG Ab detected with ELISA showing high amounts of Abs in hTg-immunized mice. T3 and T4 serum concentration (E, L, M, S, and T) and TSH serum concentration (F, N, and U) show hypothyroidism (E and F), transient hyperthyroidism (L, M, and N), and euthyroidism (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 measured by Mann–Whitney test).
Fig. 4.
Effects of thyroiditis on endometriosis evolution. Implant size and weight of endometriotic implants of C57BL/6 mice (A and B, hypothyroidism model), CBA mice (C and D, autoimmune thyroiditis model), and BALB/c mice (E and F, hTG immunization model). The correlation between implant size and serum T3 concentration (I) and serum T4 concentration (J) in the autoimmune thyroiditis model (Spearman correlation) is shown. Ultrasonography images of endometriotic implants in autoimmune thyroiditis mice (G) and control mice (H) are shown. (*P < 0.05; **P < 0.01; ****P < 0.0001 measured by Mann–Whitney test). ns, nonsignificant.
Endometriotic Implants Proliferate in a Mouse Model of Experimental Autoimmune Thyroiditis.
To investigate the potential role of thyroid hormones in endometriosis evolution in vivo, we combined endometriosis with experimental autoimmune thyroiditis (EAT) in a mouse model (Fig. 3G). At the time of death, thyroid sections showed mononuclear cell infiltration in immunized mice (P < 0.001) (Fig. 3 H, I, and K). As shown in Fig. 3J, immunization induced the production of large amounts of anti-TG Abs in treated mice, while no such Abs were detectable in control mice (P < 0.0001). Reactivity of such Abs toward mouse thyroid has been evaluated in SI Appendix, S5. A significant increase in T3 and T4 levels was observed on day 21 in immunized mice compared with control mice (1.5-fold with P < 0.001 for T3 and 1.4-fold with P < 0.01 for T4) (Fig. 3 L and M). These increases are transient, as there are no differences anymore at day 35. As expected, TSH was much lower in the treated mice compared with control mice [57.0 versus 201 pg/mL (P < 0.05 at day 35)] (Fig. 3N). At the time of death at day 35, endometriotic implants were significantly larger [58.2 versus 29.8 mm3 (P < 0.0001)] and heavier [52.3 versus 29.7 mg (P < 0.01)] in porcine thyroglobulin (pTg)-immunized mice than in control mice (Fig. 4 C, D, G, and H). Interestingly, we found a positive linear relationship (P < 0.05) between implant size and concentration of both thyroid hormones T3 and T4 (Fig. 4 I and J). Thus, this experiment shows that thyroid hormone may promote endometriotic implant growth. Nevertheless, we could not exclude the potential role of thyroid autoimmunity and, more precisely, of anti-TG Abs on endometriotic lesion development.
Endometriotic Implant Growth Is Not Increased in a Mouse Model of TG Immunization with Euthyroidism.
To highlight the putative role of anti-TG Abs, a third mouse model was developed (24). BALB/c mice were immunized with human thyroglobulin (hTg) (Fig. 3O). This model is not associated with thyroid infiltration and thyroid destruction (Fig. 3 P and Q). Nevertheless, immunization with hTg led to the production of high levels of anti-TG Abs in the immunized mice, while no Abs were detected in the control mice (P < 0.01) (Fig. 3R). Reactivity of hTG Abs toward mouse thyroid has been evaluated in SI Appendix, S5. No significant differences in T3, T4, and TSH levels were observed between immunized and control mice (Fig. 3 S–U). Thus, this model allowed us to specifically study the role of anti-TG Abs on endometriotic implant evolution. Interestingly, at death, there was no difference between implant sizes of immunized and control mice (Fig. 4E), but implants of immunized mice were slightly lighter than those of the control mice [40.4 versus 61.84 mg, respectively (P < 0.05)] (Fig. 4F). Thus, thyroid autoimmunity and anti-TG Abs do not induce an increased growth of endometriotic implants.
Thyroid Dysfunction Favors Endometriosis Severity in Humans.
To support our in vitro and in vivo findings, we explored the relationship between thyroid disorder and endometriosis in terms of prevalence and clinical features. First, endometriosis patients did not present a higher prevalence of thyroid disorder than the general female population of childbearing age, and the patients with thyroid dysfunction did not have a higher prevalence of endometriosis. However, endometriosis patients with thyroid dysfunction displayed significantly more chronic pelvic pain than endometriosis patients without thyroid disorder (P = 0.006). This result was found in two independent cohorts of patients (Table 1 and SI Appendix, S6). Additionally, in the cohort that underwent surgery in the gynecological department, the American Society for Reproductive Medicine severity score was higher in patients with thyroid disorders (P = 0.008) than in patients with endometriosis only. In both cohorts, there was a trend toward a higher number of deep infiltrating lesions in patients with thyroid disorders (P = 0.078).
Table 1.
Clinical characteristics of endometriosis patients with or without thyroid disorders
| Patients’ Characteristics | EndoOnly (n = 569)* | EndoThyro (n = 32)† | P value |
| Age, y | 36.8 ± 0.2 | 38.6 ± 0.9 | 0.034 |
| BMI, kg/m2 | 22.1 ± 0.2 | 24.9 ± 0.8 | 0.000 |
| Family history of endo (n, %) | 84 (16) | 5 (16) | 0.973 |
| Previous endo surgery (n, %) | 164 (31) | 12 (38) | 0.412 |
| Gravidity ≥ 1 (n, %) | 140 (28) | 11 (38) | 0.235 |
| Painful symptoms (VAS score) | |||
| Dysmenorrhea | 7.1 ± 0.1 | 7 ± 0.4 | 0.469 |
| Deep dyspareunia | 4.2 ± 0.2 | 4.3 ± 0.6 | 0.464 |
| Noncyclical chronic pelvic pain | 2.9 ± 0.1 | 4.3 ± 0.6 | 0.006 |
| ASRM total score‡,§ | 26 ± 1.5 | 44.1 ± 11.8 | 0.008 |
| ASRM stage III/IV‡ (n, %) | 159 (53) | 10 (71) | 0.172 |
| Endometriosis phenotype (n, %) | 0.946 | ||
| SUP | 118 (26) | 9 (27) | |
| OMA | 64 (14) | 4 (12) | |
| DIE | 270 (60) | 20 (61) | |
| Mean number of DIE lesions | 2.9 ± 0.1 | 3.5 ± 0.5 | 0.078 |
ASRM, American Society for Reproductive Medicine; BMI, body mass index; endo, endometriosis; EndoOnly, endo without thyroid dysfunction; EndoThyro, endo + thyroid dysfunction; SUP, superficial endometriosis; OMA, endometrioma; DIE, deep infiltrating endometriosis; VAS, visual analog scale. Significant P values are shown in bold.
Patient cohort: 225 from the assisted reproduction department and 314 from the gynecology department.
Patient cohort: 18 from the assisted reproduction department and 14 from the gynecology department.
Only patients from the gynecology department.
ASRM total score is the score according to the ASRM classification (revised American Fertility Society classification, 1997).
Discussion
Endometriosis is a common gynecological disorder whose pathophysiology remains largely unknown (1). Several authors have highlighted the association between thyroid dysfunction and endometriosis, suggesting a potential link between the two pathologies (11–13, 25). AITD has been incriminated in endometriosis-associated infertility for many years (25), but, to our knowledge, the mechanistic explanation for the role of thyroid dysfunction on endometriosis evolution has not been elucidated yet. In this study, we report the expression of thyroid hormones, TSHR, and enzymes from the thyroid hormone metabolism in endometrium from healthy women. Our results confirmed those from Aghajanova et al. (19) and Catalano et al. (22), who found expression of all of the transcripts involved in thyroxin synthesis and activation in the endometrium. The latter group also reported a correlation between these transcripts and mifepristone intake, a progesterone antagonist (22). Because progesterone resistance is a hallmark of endometriosis, it can be hypothesized that progesterone resistance in endometriosis could be responsible for the dysregulation of thyroid transcripts in the endometrium of patients with endometriosis. Moreover, Aghajanova and Giudice (26) have also compared microarray analysis of mild versus severe endometriosis, and reported a potential involvement of thyroid hormone homeostasis and metabolism in the pathophysiology of endometriosis. Our results confirm this hypothesis. We showed that TSHR is overexpressed in ectopic endometrium, and could thus be sensitive to TSH variations. We also observed an increase of THRA2 transcript and protein on ectopic endometrium. THRA2 encodes a nuclear receptor that is unable to bind T3 and acts as a competitive inhibitor of transcriptional activity of T3 (23). Therefore, an increase of THRA2 may lead to a resistance to the nuclear action of T3 by the ectopic endometrium. Moreover, the decrease of DIO2 that catalyzes the transformation of T4 into bioactive T3 may lead to an accumulation of T4 in ectopic endometrium. These results suggest a profound modification of the thyroid metabolism that may favor a decrease in the biosynthesis of T3 and an accumulation of T4 in situ in ectopic endometrium as observed by us in the supernatant of endometriotic cells compared with endometrial control cells. Our results differ from those of Catalano et al. (22) since they found an increase in DIO2 expression and THRB but a decrease of THRA1/2 after mifepristone intake, which means that thyroid-related gene expression is not only regulated by progesterone in endometriosis. From this ex vivo study on thyroid transcripts in patients with endometriosis, an increase in TSH (hypothyroidism) or in thyroid hormone T4 (hyperthyroidism or T4 intake) could be hypothesized as a participating factor for endometriosis aggravation. Interestingly, it has already been shown that thyroid hormone, especially T4, correlated with polyp aggravation without mechanistical evidence (27). Cross-talk between thyroid hormones and estrogens has been suggested by the direct stimulation of estrogen receptors on endometrial cells by thyroid hormones, leading to cell proliferation (28). T4 action can be underestimated because of its ineffectiveness when not catalyzed into T3, the biologically active form of thyroid hormone. However, receptors on plasma cell membranes, such as integrin αVβ3, as well as receptors on intracellular organelles membranes (29) can directly bind to T4 (and T3). These nongenomic actions of thyroid hormones differ from the classical thyroid hormone signaling pathways and involve the phosphatidylinositol 3-kinase (PI3K) or mitogen-activated protein kinase pathways (29, 30). As a result, T3 and T4 are able to induce cell proliferation and neoangiogenesis via extracellular signal-related kinase (ERK) and PI3K/Akt activation (28, 31–34), two hallmarks of endometriosis physiopathology. We thus conducted in vitro studies to explore TSH, T3, and T4 action on endometrial and endometriotic cells from women with endometriosis and controls. We demonstrated that while TSH activates the proliferation of all endometriotic and control cells, T4 has a specific proliferative effect on epithelial and stromal ectopic endometrial cells, whereas T3 only acts on epithelial cells. In addition, thyroid hormones favor ROS production by ectopic endometrial cells. In addition to their participation in the inflammatory reaction that characterizes endometriosis, Ngô et al. (35) showed that ROS production by endometriotic cell induces their proliferation via ERK activation via either a direct or paracrine effect. A limitation of our study resides in the heterogeneity of the patients from which the samples were obtained (severity and type of endometriosis, treatment) for qRT-PCR and in vitro data. However, the strength of our data is further reinforced by Western blot analysis on endometriotic and control endometrial cell line samples from other patients.
The influence of thyroid function on endometriosis development was then evaluated in vivo in three mouse models. These models allowed us to study the impact of hypo-, hyper- and euthyroidism, as well as thyroid autoimmunity, on endometriosis evolution. Endometriosis is an inflammatory disease (1, 2, 6), characterized by an important leukocytic infiltration in the ectopic endometrium (36, 37). The presence of high levels of thyroid antigens like TG, thyroid hormones, or TSHR in endometriotic cells may favor their exposure to the immune system in an immunostimulant environment that could trigger the breach of tolerance and the autoimmune reaction toward the thyroid autoantigens. On the other hand, the local synthesis of thyroid hormones can activate neutrophils and macrophages to locally promote a proinflammatory environment (38, 39) and ROS production that may favor endometriotic cell proliferation (40). For these reasons, our endometriosis model is an autologous graft model of the uterine horn, which kept the mice immunocompetent (41). Our endometriosis model was further coupled with a hypothyroid model (40), an autoimmune thyroiditis with hyperthyroidism (42), and an autoimmune thyroid condition with euthyroidism (24). Our model of hyperthyroidism is an experimental model of autoimmune thyroiditis, mimicking Hashimoto’s disease, known to generate a hypothyroid condition (42). However, in the first stage of this disease, the thyroid gland is destroyed by a massive infiltration of mononuclear cells, leading to transitional hyperthyroidism, with an increase of thyroid hormones and a decrease of TSH (42). Thus, at this early stage, this model allowed us to study thyroid autoimmunity with the presence of anti-TG Abs, along with increased thyroid hormone, in the immunized mice. As endometriotic implants were bigger in the hyperthyroid condition, smaller in hypothyroid condition, and slightly smaller in thyroid autoimmunity with euthyroidism, we concluded that thyroid hormones, instead of TSH, may participate in endometriosis lesion growth. Thyroid dysfunction is often associated with autoimmunity in patients suffering from endometriosis (12, 25). Two of the murine models used were associated with anti-TG production, but only one was associated with disease progression, indicating that anti-TG Abs are not responsible for endometriotic lesion growth. These results do not completely rule out the role of Abs against other thyroid antigens like anti-thyroid peroxidase Abs, whose prevalence is highly increased in endometriosis patients (12). To evaluate whether our results could be translated to humans, we performed a retrospective analysis on endometriosis patients for whom we had access to extensive clinical data. We found that endometriosis patients suffering from thyroid disorders display more chronic pelvic pain, with a more severe clinical score, than patients without thyroid dysfunction. Most of the endometriosis patients with thyroid dysfunction suffered from Hashimoto thyroiditis and were treated accordingly with T4 supplementation. According to our data gained in our mouse model of Hashimoto thyroiditis, other conditions (treatment, period of hyperthyroidism preceding the hypothyroidism) may promote endometriosis development and/or evolution. In our cohort, only Hashimoto thyroiditis was associated with endometriosis, but an association with Grave’s disease has been described by others (13). Activating anti-TSHR Abs are the pathognomonic hallmark of Grave’s disease (43). Considering our in vitro results, we can hypothesize that activating anti-TSHR Abs might have a role in the proliferation and ROS production on endometriotic cells, thus aggravating endometriosis. Moreover, Grave’s disease is a hyperthyroid condition in which T3 and T4 hormones are elevated, so we can hypothesize that Grave’s disease could be a risk factor for endometriosis and/or may be associated with an aggravated form of the disease. In conclusion, if ectopic production of estrogen and progesterone resistance play a key role in lesion growth, we demonstrate herein the influence of the dysfunctions of thyroid hormone metabolism in endometriotic cells and the consequences on endometriosis progression in vitro and in vivo in relevant mouse models. As patients with Hashimoto thyroiditis present with a more severe form of endometriosis, thyroid function, T4/levothyroxine intake, and endometriosis progression should be carefully monitored in these patients.
Materials and Methods
Patients and Tissue Collection.
This study was approved by the local institutional review board (approval no. 05-2006, provided by the Comité de Protection des Personnes et des Biens dans la Recherche Biomédicale of Paris Cochin), and all participants gave written informed consent. Inclusion criteria are provided in SI Appendix, S7. Basic clinical characteristics of the patients were collected using a standardized questionnaire. Endometriosis was categorized and scored according to the revised American Fertility Society classification (44). Control patients displayed nonendometriotic ovarian cysts, tubal infertility, or uterine myoma and did not have macroscopic endometriotic lesions according to a thorough surgical examination of the abdominopelvic cavity. Patients with endometriosis provided eutopic and ectopic endometrium, and controls provided eutopic endometrium.
Real-Time RT-PCR.
After surgical resection, the samples (11 ectopic and 11 eutopic biopsies from endometriotic patients and 11 control endometrial biopsies) were immediately frozen in liquid nitrogen. qRT-PCR was performed using standard techniques (SI Appendix, S8 and S9).
Isolation and Culture of Endometrial and Endometriotic Cells.
Eight primary cell lines were obtained from biopsies of eutopic endometrium from eight disease-free women (control endometrial cells). Fifteen endometriosis patients provided their endometrial and/or endometriotic biopsies: Nine primary cell lines were extracted from eutopic endometrium, whereas 11 were extracted from biopsies of ectopic endometrium (endometriotic cells). Cell isolation from endometrial and endometriotic biopsies and primary endometrial and endometriotic cell cultures were prepared as previously described (35). All of the various tests were performed in triplicate. Tests on the primary cultures were obtained between 7 and 14 d after sample collection.
Western Blot Analysis.
TSHR, THRA1, THRA2, THRB1, DIO2, and DIO3 expression was assessed by Western blot (SI Appendix, S10) from at least four endometrial epithelial and stromal primary cell lines of endometriosis-free patients and four endometrial and endometriotic epithelial and stromal cell lines of endometriotic patients, obtained as explained above.
ROS Production and Endometrial and Endometriotic Cell in Vitro Proliferation Assay.
ROS production was assessed by the 2′,7′-dichlorodihydrofluorescein diacetate fluorescent assay. Cell proliferation was determined using the Uptiblue proliferation test. Cells were treated with 0, 0.5, 1, 2, or 4 μg/mL recombinant human TSH (0.9 mg of Thyrogen; Genzyme); 0, 10, 50, or 100 nM T4 (Sigma–Aldrich); or 0, 10, 50, or 100 nM T3 (Sigma–Aldrich). More details are provided in SI Appendix, S11 and S12.
Animal Models of Thyroiditis and Endometriosis.
Six-week-old C57BL/6, CBA/J, and BALB/c female mice were purchased from the Janvier Laboratory. The Institutional Review Board from Université Paris Descartes approved all experimental procedures and animal care (permit no. 2016040716219897). Three murine models have been developed: toxic hypothyroiditis induced in mice by MMI and potassium perchlorate (40), EAT induced by immunization with pTg (42), and thyroid immunization with euthyroidism induced by immunization with hTg (24). Each thyroid disorder model was coupled with an endometriosis model induced in immunocompetent mice by a syngeneic graft of uterine horns to generate endometriosis-like lesions as described (41) (SI Appendix, S13 and S14).
Screening of Mice Sera for Anti-TG Auto-Abs and Thyroid Function Tests.
Details are provided in SI Appendix, S15–S17.
Clinical Study of Relationship Between Thyroid Disorder and Endometriosis.
Details are provided in SI Appendix, S18 and S19.
Statistical Analysis.
All statistics and graphics were performed using GraphPad Prism 6 and appear in more detail in SI Appendix, S20. In all figures, error bars represent the SEM. A value of P < 0.05 was recognized as significant (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Supplementary Material
Acknowledgments
This work was supported by grants from the University Paris Descartes, INSERM, and the Fondation pour la Recherche Médicale.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820469116/-/DCSupplemental.
References
- 1.Bulun S. E. Endometriosis. N. Engl. J. Med. 360, 268–279 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Giudice L. C., Kao L. C., Endometriosis. Lancet 364, 1789–1799 (2004). [DOI] [PubMed] [Google Scholar]
- 3.de Ziegler D., Borghese B., Chapron C., Endometriosis and infertility: Pathophysiology and management. Lancet 376, 730–738 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Chapron C., et al. , Deeply infiltrating endometriosis: Pathogenetic implications of the anatomical distribution. Hum. Reprod. 21, 1839–1845 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Sampson J. A., Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 14, 422–469 (1927). [Google Scholar]
- 6.Ahn S. H., et al. , Pathophysiology and immune dysfunction in endometriosis. BioMed Res. Int. 2015, e795976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burney R. O., Giudice L. C., Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 98, 511–519 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenberg V. H., Zolti M., Soriano D., Is there an association between autoimmunity and endometriosis? Autoimmun. Rev. 11, 806–814 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Taylor P. V., et al. , Autoreactivity in women with endometriosis. Br. J. Obstet. Gynaecol. 98, 680–684 (1991). [DOI] [PubMed] [Google Scholar]
- 10.Hatayama H., et al. , Detection of antiendometrial antibodies in patients with endometriosis by cell ELISA. Am. J. Reprod. Immunol. 35, 118–122 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Sinaii N., Cleary S. D., Ballweg M. L., Nieman L. K., Stratton P., High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum. Reprod. 17, 2715–2724 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Poppe K., et al. , Thyroid dysfunction and autoimmunity in infertile women. Thyroid 12, 997–1001 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Yuk J.-S., et al. , Graves disease is associated with endometriosis: A 3-year population-based cross-sectional study. Medicine (Baltimore) 95, e2975 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fröhlich E., Wahl R., Thyroid autoimmunity: Role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front. Immunol. 8, 521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orgiazzi J., Thyroid autoimmunity. Presse Med. 41, e611–e625 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Stassi G., De Maria R., Autoimmune thyroid disease: New models of cell death in autoimmunity. Nat. Rev. Immunol. 2, 195–204 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Poppe K., Velkeniers B., Glinoer D., Thyroid disease and female reproduction. Clin. Endocrinol. (Oxf.) 66, 309–321 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Aghajanova L., et al. , Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod. Biomed. Online 18, 337–347 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Aghajanova L., et al. , Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil. Steril. 95, 230–237, 237.e1–237.e2 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Michalakis K. G., et al. , Subclinical elevations of thyroid-stimulating hormone and assisted reproductive technology outcomes. Fertil. Steril. 95, 2634–2637 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosugi S., Sugawa H., Mori T., TSH receptor and LH receptor, 1996. Endocr. J. 43, 595–604 (1996). [DOI] [PubMed] [Google Scholar]
- 22.Catalano S. D., et al. , Mifepristone induced progesterone withdrawal reveals novel regulatory pathways in human endometrium. Mol. Hum. Reprod. 13, 641–654 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Ortiga-Carvalho T. M., Sidhaye A. R., Wondisford F. E., Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat. Rev. Endocrinol. 10, 582–591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matalon S. T., et al. , The pathogenic role of anti-thyroglobulin antibody on pregnancy: Evidence from an active immunization model in mice. Hum. Reprod. 18, 1094–1099 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Poppe K., Velkeniers B., Female infertility and the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 18, 153–165 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Aghajanova L., Giudice L. C., Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod. Sci. 18, 229–251 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saccardi C., et al. , Endometrial polyps in women affected by levothyroxine-treated hypothyroidism—Histological features, immunohistochemical findings, and possible explanation of etiopathogenic mechanism: A pilot study. Biomed Res. Int. 2013, 503419 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mousa S. A., et al. , The proangiogenic action of thyroid hormone analogue GC-1 is initiated at an integrin. J. Cardiovasc. Pharmacol. 46, 356–360 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Flamant F., et al. , Thyroid hormone signaling pathways: Time for a more precise nomenclature. Endocrinology 158, 2052–2057 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhargava M., Lei J., Ingbar D. H., Nongenomic actions of L-thyroxine and 3,5,3′-triiodo-L-thyronine. Focus on “L-thyroxine vs. 3,5,3′-triiodo-L-thyronine and cell proliferation: Activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase”. Am. J. Physiol. Cell Physiol. 296, C977–C979 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scarlett A., et al. , Thyroid hormone stimulation of extracellular signal-regulated kinase and cell proliferation in human osteoblast-like cells is initiated at integrin alphaVbeta3. J. Endocrinol. 196, 509–517 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Lin H., et al. , Thyroid hormone induces activation of mitogen-activated protein kinase in cultured cells. Am. J. Physiol. 276, C1014–C1024 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Bergh J. J., et al. , Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146, 2864–2871 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Leconte M., et al. , The mTOR/AKT inhibitor temsirolimus prevents deep infiltrating endometriosis in mice. Am. J. Pathol. 179, 880–889 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngô C., et al. , Reactive oxygen species controls endometriosis progression. Am. J. Pathol. 175, 225–234 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul Dmowski W., Braun D. P., Immunology of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 18, 245–263 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Izumi G., et al. , Involvement of immune cells in the pathogenesis of endometriosis. J. Obstet. Gynaecol. Res. 44, 191–198 (2018). [DOI] [PubMed] [Google Scholar]
- 38.De Vito P., et al. , Thyroid hormones as modulators of immune activities at the cellular level. Thyroid 21, 879–890 (2011). [DOI] [PubMed] [Google Scholar]
- 39.van der Spek A. H., Fliers E., Boelen A., Thyroid hormone metabolism in innate immune cells. J. Endocrinol. 232, R67–R81 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Yi J., et al. , Decreased pain threshold and enhanced synaptic transmission in the anterior cingulate cortex of experimental hypothyroidism mice. Mol. Pain 10, 38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcellin L., et al. , Alteration of Nrf2 and glutamate cysteine ligase expression contribute to lesions growth and fibrogenesis in ectopic endometriosis. Free Radic. Biol. Med. 110, 1–10 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Charreire J., Immune mechanisms in autoimmune thyroiditis. Adv. Immunol. 46, 263–334 (1989). [DOI] [PubMed] [Google Scholar]
- 43.Michalek K., Morshed S. A., Latif R., Davies T. F., TSH receptor autoantibodies. Autoimmun. Rev. 9, 113–116 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brosens I. A., Cornillie F., Koninckx P., Vásquez G., Evolution of the Revised American Fertility Society classification of endometriosis. Fertil. Steril. 44, 714–716 (1985). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.