Significance
Chronic recurrent multifocal osteomyelitis (CRMO) is an autoinflammatory bone disease that presents with bone destruction occurring primarily in children. In a mouse ENU mutagenesis screen, the Ali18 strain was isolated because of spontaneous inflammation in the joints and bones. Sequencing candidate genes in the Ali18 critical region identified a missense mutation in the C-terminal regulatory region of the Src family kinase (SFK) member, Fgr. Genome editing revealed Fgr dependency of the inflammatory phenotype in Ali18 mice. Further, whole exome sequencing in our CRMO cohort identified two patients with missense mutations in FGR. In vitro functional assays confirm altered protein function. This work identifies FGR as a CRMO susceptibility gene and suggests that targeting SFKs may be useful in its treatment.
Keywords: chronic recurrent multifocal osteomyelitis, autoinflammation, mouse model, tyrosine kinase, bone destruction
Abstract
Autoinflammatory syndromes are characterized by dysregulation of the innate immune response with subsequent episodes of acute spontaneous inflammation. Chronic recurrent multifocal osteomyelitis (CRMO) is an autoinflammatory bone disorder that presents with bone pain and localized swelling. Ali18 mice, isolated from a mutagenesis screen, exhibit a spontaneous inflammatory paw phenotype that includes sterile osteomyelitis and systemic reduced bone mineral density. To elucidate the molecular basis of the disease, positional cloning of the causative gene for Ali18 was attempted. Using a candidate gene approach, a missense mutation in the C-terminal region of Fgr, a member of Src family tyrosine kinases (SFKs), was identified. For functional confirmation, additional mutations at the N terminus of Fgr were introduced in Ali18 mice by CRISPR/Cas9-mediated genome editing. N-terminal deleterious mutations of Fgr abolished the inflammatory phenotype in Ali18 mice, but in-frame and missense mutations in the same region continue to exhibit the phenotype. The fact that Fgr null mutant mice are morphologically normal suggests that the inflammation in this model depends on Fgr products. Furthermore, the levels of C-terminal negative regulatory phosphorylation of FgrAli18 are distinctly reduced compared with that of wild-type Fgr. In addition, whole-exome sequencing of 99 CRMO patients including 88 trios (proband and parents) identified 13 patients with heterozygous coding sequence variants in FGR, including two missense mutant proteins that affect kinase activity. Our results strongly indicate that gain-of-function mutations in Fgr are involved in sterile osteomyelitis, and thus targeting SFKs using specific inhibitors may allow for efficient treatment of the disease.
Autoinflammatory syndromes are disorders of innate immunity characterized by episodes of seemingly unprovoked sterile inflammation without increased autoantibodies or involvement of self-reactive lymphocytes (1). Many autoinflammatory disorders have a monogenic basis, but for most, a combination of genetic and environmental factors contributes to disease susceptibility. Chronic recurrent multifocal osteomyelitis (CRMO), also known as chronic nonbacterial osteomyelitis (CNO), is an autoinflammatory bone disease which presents with bone pain and local swelling due to unifocal, or more often multifocal sites of sterile osteomyelitis (2–5). While the genes for two syndromic forms of CRMO (LPIN2 and IL1RN) are known (6, 7), the genetic etiology for nonsyndromic CNO/CRMO is poorly understood.
A forward genetics approach using animal models is a powerful tool for the molecular dissection of pathogenesis in inflammatory bone diseases (8). Examples include Pstpip2 (lupo and cmo mice) (9–11) and Zap70 (skg mice) (12), which were identified and well characterized without human disease data. Further, similar autoinflammatory phenotypes of Ali5 (13) and Ali14 (14) mice caused by missense mutations in phospholipase C gamma (Plcg2) are related to the novel human hereditary disease PLAID (PLCG2-associated antibody deficiency and immune dysregulation) (15, 16). However, most of the intracellular cascades which lead to autoinflammatory diseases are not well known.
The Ali18 mutant mouse strain was isolated in the Munich ENU mutagenesis project because of paw inflammation (Fig. 1A) (17). Ali18 mice show synovitis, sterile osteomyelitis, and systemic reduced bone mineral density, particularly in trabecular areas of long bones (17). Because these phenotypes are reconstituted by bone marrow transfer and are independent of mature lymphocytes (18), Ali18 mice are considered a mouse model of autoinflammatory bone disease. Although the Ali18 locus was mapped to mouse chromosome 4 by standard genetic mapping, complex modifier effects hinder its precise determination (19). In this study, positional candidate cloning identified Fgr, a member of Src family kinases, as the causative gene for Ali18. Further, two other missense mutations in FGR were found in our cohort of patients with CRMO.
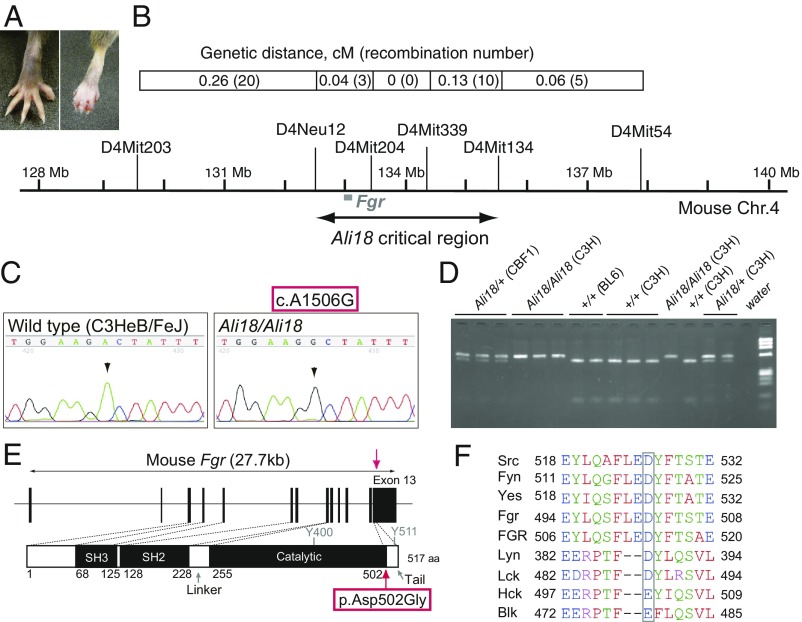
Fig. 1.
Positional candidate cloning of the Ali18 mutation. (A) Ali18/+ (Right) and wild-type C3HeB/FeJ (Left) hind paws. Ali18 mice show reddening and swelling in peripheral paws. (B) Genetic map and the critical interval of the Ali18 locus. The complex modifier effects from the C57BL/6J genetic background prevented further narrowing down of the region. (C) By Sanger sequencing of candidate genes in the region, a point mutation (c.1506A > G) in exon 13 of Fgr, a member of the SFKs, was detected. (D) Mbo II restriction enzyme digestion of PCR products spanning exon 13 correlated to the swollen paw phenotype. Genetic background is described as C3H (C3HeB/FeJ), BL6 (C57BL/6J), and CBF1 (F1 from C3H and BL6 crossing). Ali18 mice were originally derived from C3H parents. (E) Schematic diagram of the p.Asp502Gly (D502G) amino acid change induced by c.1506A > G. Y400 and Y511 indicate the autophosphorylation site and the C-terminal regulatory phosphorylation site, respectively. (F) Alignment of Src family tyrosine kinases. Square encompasses the amino acid residues exchanged by the c.1506A > G mutation.
Results
Ali18 Mice, Fine Mapping, and Candidate Resequencing.
By standard genetic mapping, we narrowed down the critical region to ∼3 Mb utilizing recombination between wild-type and heterozygous/homozygous genotypes (Fig. 1B and SI Appendix, Table S1). In the identified region, we focused on 16 candidate genes using a literature-based search engine, PosMed (20). By sequencing the exonic region of these genes by the Sanger method, we found a mutation, c.1506A > G, in the protein coding region of the Gardner-Rasheed feline sarcoma viral (v-Fgr) oncogene homolog (Fgr) gene (21) (Fig. 1C). Digestion of PCR products encompassing exon 13 of Fgr by Mbo II restriction enzyme, which recognizes the wild-type allele (5′-GAAGA-3′) but not c.1506A > G (5′-GAAGG-3′), produces longer DNA fragments in Ali18 mice (Fig. 1D). Genotype–phenotype correlations revealed that the mutation was not present in unaffected littermates or wild-type controls.
A Member of SFK as the Top Candidate for the Causative Gene.
Fgr is a member of the Src family kinases (SFKs), which share the SH2, SH3, and catalytic domains with high homology to other family members (22), and the c.1506A > G mutation causes an amino acid change (p.Asp502Gly) in the terminal end of the catalytic domain (Fig. 1E). Alignment of the amino acid sequences of Fgr with those of other SFKs indicates that the 502 aspartic acid is conserved among Src, Fyn, Yes, Lyn, and Lck (Fig. 1F). The corresponding amino acid in Hck and Blk is glutamic acid and has a negatively charged side chain as does aspartic acid. The aspartic acid of Fgr is also conserved in humans (Fig. 1F, FGR). We searched sequence variants among 36 mouse inbred strains in the “mousepost.be” database (23) and no variants were detected in the Fgr locus. The PROVEAN (Protein Variation Effect Analyzer) software (24) predicts that the amino acid substitution is deleterious (score = −6.440; cutoff = −2.5). In addition, we performed whole-genome sequencing by next generation sequencer (NGS) using genomic DNA from Ali18/+ and wild-type mice on the same genetic background, and Fgr c.1506A > G (IGV_2.3.94, mouse mm10, chr4: 133,000,294, SI Appendix, Fig. S1A) mutations were found in only Ali18/+ DNA as a heterozygous change (NGS reads, A:20 and G:24). Within the Ali18 critical region, we found three other candidate mutations (IGV_2.3.94, mouse mm10, chr4: 133,543,428; chr4: 133,705,306; chr4: 133,919,389, SI Appendix, Fig. S1 B–D) besides the Fgr coding mutation. However, all three mutations are located in noncoding regions.
Deficiency of Fgr Abolishes the Autoinflammatory Phenotype of Ali18 Mice.
To confirm whether the inflammatory phenotype of Ali18 mice is caused by the Fgr coding mutation, we used the prokaryotic antiviral system, CRISPR/Cas9, to induce additional loss-of-function mutations in the N-terminal region of Fgr besides p.Asp502Gly. Because Fgr knockout mice show no overt phenotype (25, 26), it is predicted that loss-of-function mutations in Fgr do not support the osteomyelitis phenotype in Ali18 mice. As shown in Fig. 2 A and B, two pX330 related constructs containing guide RNA around exon 3 of the Fgr gene (fgRNA1 and -2) were microinjected into Ali18/Ali18 fertilized eggs. Therefore, all of the Cas9-induced mutations are in the Ali18 haplotype containing the Fgr c.1506A > G/p.Asp502Gly mutation (Fig. 2A). In the founder (F0) generation, we sequenced the region flanking and including exon 3 which contains the transcriptional initiation site of Fgr. All F0 mice (nos. 405, 406, 407, 409, 410, and 411) without mutation around the Cas9-targeted PAM sites exhibit the arthritic phenotype (6/6). In contrast, half of the F0 mice harboring mutations around the PAM sites did not show the inflammatory paw phenotype (5/10).
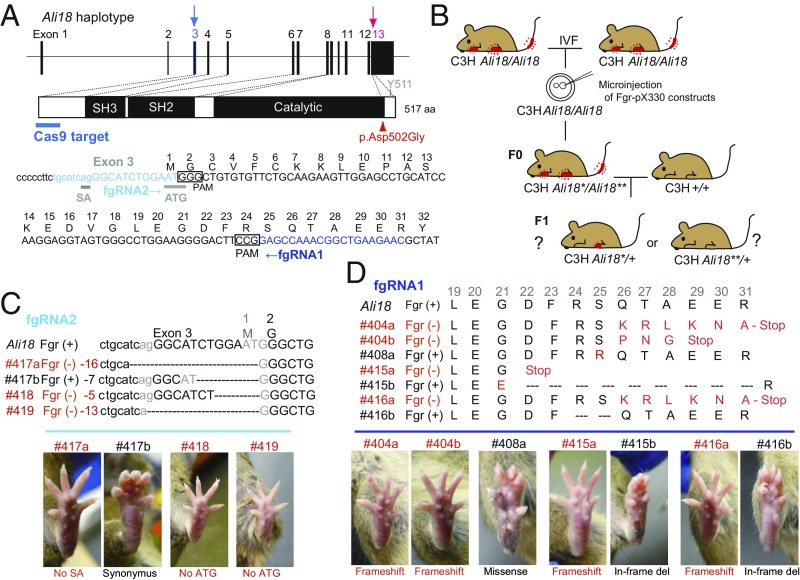
Fig. 2.
Disruption of the Fgr gene by genome editing alters the autoinflammatory phenotype in Ali18 mice. (A) DNA sequence of guide RNA (fgRNA1 and fgRNA2) and PAM around exon 3 of Fgr are indicated. The p.Asp502Gly mutation in exon 13 is also shown. Sanger sequencing of a PCR fragment around exon 3 and genotyping of p.Asp502Gly were done using genomic DNA from F0 and F1 mice. (B) Schematic strategy of genome editing in the Fgr locus of Ali18 mice. pX330-based constructs were microinjected into Ali18/Ali18 oocytes in C3H (C3HeB/FeJ) genetic background from in vitro fertilization. The founder mice (F0) derived from microinjection were bred with wild-type C3H mice to obtain F1 mice. (C and D) Correlation of Fgr genotypes and lower limb morphology of F1 mice. Loss-of-function mutations show no morphological abnormality (red font). In contrast, missense, in-frame deletion, and synonymous mutations exhibit autoinflammatory paws. SA, splice acceptor; ATG, the translational initiation site. See also SI Appendix, Table S2 for detail.
As it is expected that somatic and germ cells of the F0 mice are chimeric with various indel mutations around the PAM, we crossed F0 mice with wild-type C3HeB/FeJ mice to obtain F1 mice without chimerism (Fig. 2B). Twelve germline-transmitted lines were established from seven F0 mice with a mutation around PAM (SI Appendix, Table S2). Among all 12 lines established, 8 lines, which have deleterious mutations in the Fgr coding region, such as frameshift (#404a, #404b, #415a, and #416a) and deletion of translational initiation site (#418) and/or splicing acceptor (#417a and #419), abolish the inflamed paw phenotype of Ali18 mice (Fig. 2 C and D and SI Appendix, Figs. S2 and S3). In contrast, the other four lines with missense (#408 and #416b), in-frame (#415b), and synonymous (#417b) mutations of Fgr exhibit the inflammatory paw phenotype (Fig. 2 C and D and SI Appendix, Figs. S2 and S3 and Table S2).
Interestingly, #408a and #417b F1 mice showed different severity of paw swelling compared with original Ali18 mice. Ali18_#408a (p.Ser25Arg;Asp502Gly) (Fig. 2D and SI Appendix, Fig. S2C) mice show more severely inflamed paws, indicating that combination of the amino acid exchanges enhances paw swelling. The #417b (Fig. 2C and SI Appendix, Fig. S2I) line, which has an in-frame 5′ UTR deletion with a newly formed ATG codon in Fgr, showed swollen paws. However, not all mice of the #417b line showed a swollen paw phenotype (4/9, SI Appendix, Table S2 and Fig. S3). It is likely that the newly formed translational initiation site or deletion in the UTR region causes low transcriptional and/or translational efficiency of the Fgr protein. These results strongly support that the Fgr p.Asp502Gly mutation is responsible for autoinflammation in Ali18 mice.
Abnormal C-Terminal Phosphorylation of FgrAli18 Reveals Gain of Function.
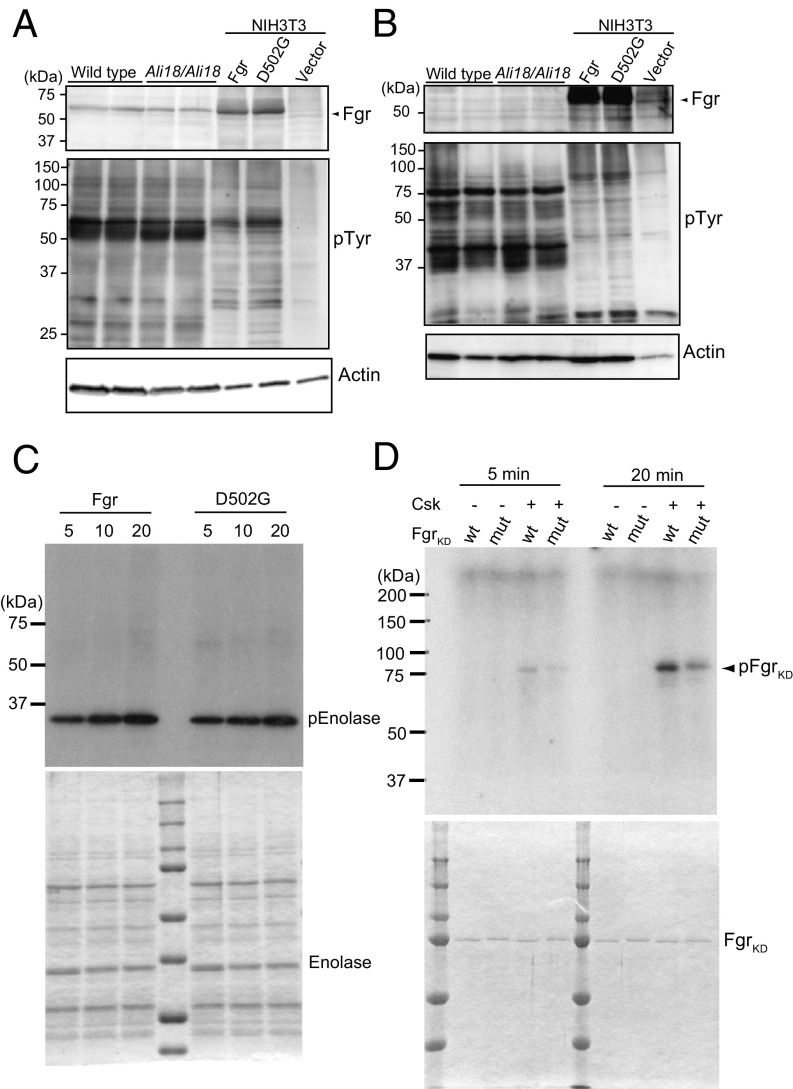
To elucidate how the p.Asp502Gly mutation affects Fgr function, we analyzed the tyrosine kinase activities of wild-type and p.Asp502Gly Fgr proteins by standard kinase assay using radioisotope-labeled ATP. Fig. 3C shows tyrosine kinase activity of in vitro translated Fgr proteins against affinity purified enolase as a substrate of SFKs. Unlike oncogenic mutations, the kinase activity of p.Asp502Gly Fgr was not dramatically changed. These results were also supported by Western blot analysis using antiphosphotyrosine in Ali18/Ali18 organs and Fgr p.Asp502Gly expressing cultured cells (Fig. 3 A and B). We then focused on efficiency of the C-terminal phosphorylation, because p.Asp502Gly is located close to this phosphorylation site. SFKs are normally inactive with the C-terminal phosphorylation by C-terminal Src kinase (Csk, another member of SFKs). To detect the C-terminal phosphorylation, a kinase-ablation mutation (KD, kinase dead) is necessary to avoid autophosphorylation of Src (27). Therefore, we used affinity purified Fgr protein with a p.Lys279Met (K279M) kinase dead mutation (FgrKD, WT) and FgrKD with the p.Asp502Gly mutation (FgrKDAsp502Gly, mut) as substrates for kinase assays by Csk. As shown in Fig. 3D, specifically phosphorylated products were detected in the samples containing Csk without autophosphorylation. Further, the efficiency of phosphorylation of FgrKD (WT) by Csk is approximately two times more than that of FgrKDAsp502Gly (mut) (relative activity ratio: 0.472 ± 0.072). The decrease of C-terminal phosphorylation indicates that FgrAsp502Gly is more active than wild-type protein. These results strongly suggest that p.Asp502Gly is a gain-of-function mutation of the Fgr tyrosine kinase.
Fig. 3.
Western blot analysis, tyrosine kinase assays, and phosphorylation of Fgr by C-terminal Src kinase (Csk). Cell lysate of spleen (A) and bone marrow cells (B) from wild-type and Ali18/Ali18 mice were used for Western blotting. As control experiments, transformants of wild-type and p.Asp502Gly (D502G) Fgr expression constructs in murine embryo-derived cultured fibroblast, NIH 3T3, cells were used. Empty vector was transfected as negative control. Anti-Fgr (Upper), anti-phosphotyrosine (Middle), and anti-actin (Lower, loading control) were used. No overt changes in Fgr protein levels of Ali18/Ali18 spleen (relative ratio: 0.900 ± 0.256, P = 0.538, t test) and NIH 3T3 cells (relative ratio: 0.982 ± 0.139, P = 0.833, t test) were detected. (C) In vitro translated wild-type and p.Asp502Gly Fgr proteins were used for kinase assay experiments. Recombinant enolase protein was used as SFK-specific substrate (Lower, loading control). No activity changes were detected in different reaction time. (D) The C-terminal phosphorylation of KD Fgr by Csk was measured. Fgr KD with p.Asp502Gly showed five and two times less phosphorylation levels in 5- and 20-min reaction time, respectively. The proteins used for kinase assays were fractionated by SDS/PAGE and stained by Coomassie Brilliant Blue. Experiments were independently triplicated.
FGR Coding Variants Were Detected in Autoinflammatory Bone Disease Patients.
As part of a human CRMO genetic study, whole-exome sequencing by NGS was performed on 99 affected individuals and their family members. The families included 88 family trios (affected and both unaffected parents) and 11 dyads (affected and one unaffected parent). FGR variants from affected subjects were queried, and 11 rare exonic variants in total were identified in 13 probands (SI Appendix, Table S3). These include two missense variants, p.Arg118Trp and p.Pro525Ser (Fig. 4H). For the patient with the p.Arg118Trp mutation, radiographs show osteolytic lesion with sclerosis and periosteal reaction in distal femur (Fig. 4 A and B) which demonstrated abnormal signal intensity and enhancement on postcontrast MRI compatible with CRMO; an additional MRI shows abnormal inflammation detected in the sacroiliac joint by short-tau inversion recovery (STIR) (Fig. 4C).
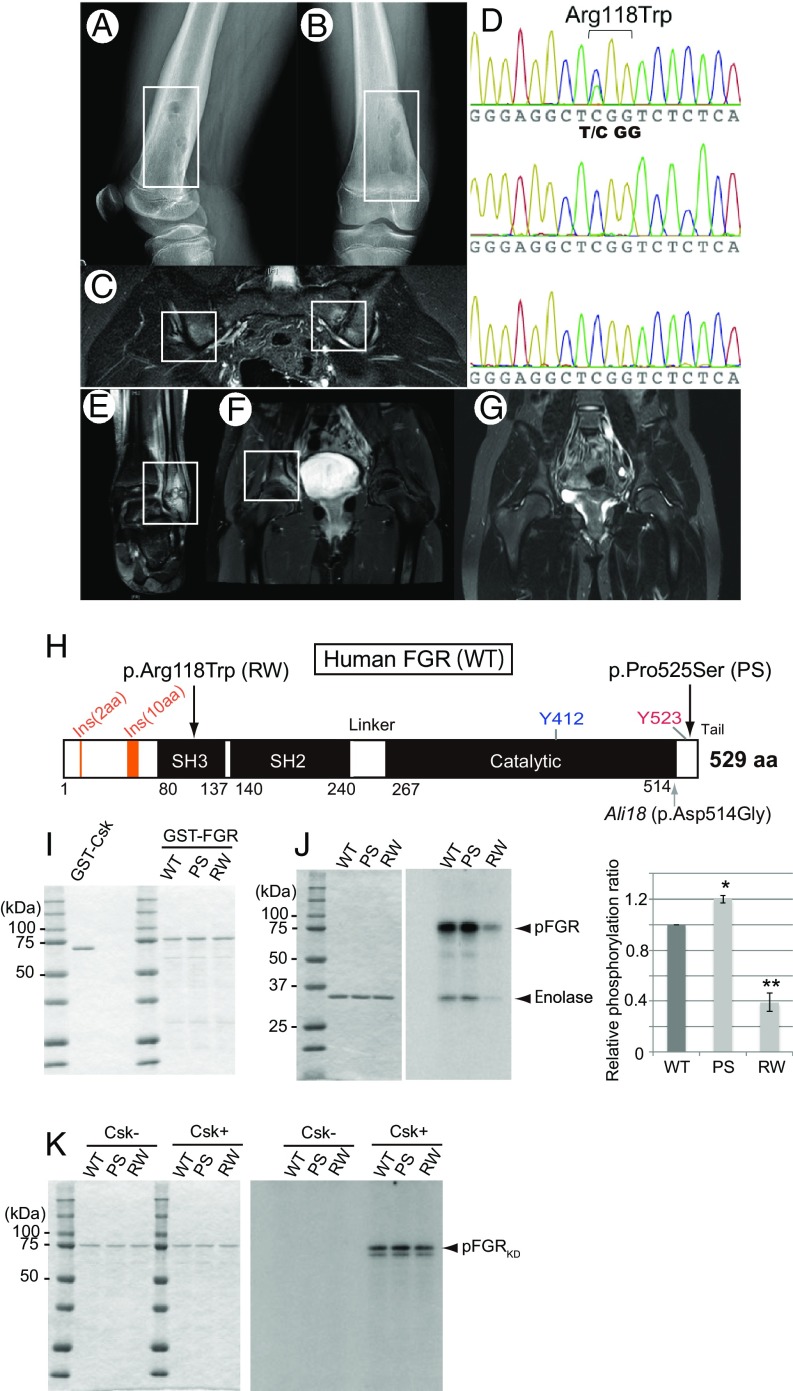
Fig. 4.
Missense mutations of FGR in CRMO and phosphorylation assays. (A and B) Radiograph of case 1 (p.Arg118Trp). Osteolytic lesions with sclerosis and periosteal elevation of the right distal femur are shown. (C) MRI of case 1. Increased STIR signal intensity on the iliac and sacral sides of the left sacroiliac joints is shown. (D) Sanger sequencing chromatogram of p.Arg118Trp in the proband (Top), father (Middle), and mother (Bottom). Proband harbors a de novo C > T mutation, which induces a p.Arg118Trp amino acid change in FGR. (E–G) MRI of case 2 (p.Pro525Ser). Abnormalities included increased signal intensity on STIR images in the left distal fibula (E) and the pelvis at the right acetabulum (F). (G) Repeat MRI 9 mo after naproxen therapy showing improvement in the left acetabular lesion. (H) Schematic diagram of amino acid substitution from the FGR mutations found in human CRMO (Top arrows) and the mouse Ali18 mutation (Lower arrow). (I) SDS/PAGE of affinity purified Csk and FGR used in kinase assays. (J) Kinase activity of FGR with CRMO variants. (J, Left) SDS/PAGE of affinity purified substrate, enolase, used in each assay. (J, Right) Phosphorylation intensity of enolase and FGR indicate enzyme activity. (K) Phosphorylation of FGRKD with CRMO variants by Csk. (Left) SDS/PAGE of affinity purified FGRKD of human CRMO variants as substrate used in each assay. (Right) FGRKD proteins were phosphorylated by Csk in equal intensity. Experiments were independently triplicated.
Genomic DNA from the patient and parents was validated by Sanger sequencing, and the chromatograms indicate a de novo mutation in the child with the p.Arg118Trp FGR mutation (Fig. 4D). A p.Pro525Ser variation is detected in another child for which DNA from only one parent was available, so it is not known if the variant is inherited or de novo. MRI abnormalities in the fibula of the child are shown in Fig. 4 E–G. Both variants are rare (SI Appendix, Table S3); the p.Arg118Trp (rs774209795) and p.Pro525Ser (rs143850913) variants have minor allele frequencies of 1.2 × 10−5 and 3.5 × 10−4, respectively, according to gnomAD (28). The PROVEAN software predicts that both amino acid substitutions are deleterious; the score of p.Arg118Trp is −5.015 (cutoff = −2.5); the score of p.Pro525Ser is −2.716 (cutoff = −2.5) and the other predictors including SIFT, Polyphen2, and CADD also predict pathogenicity (SI Appendix, Table S5). There were no mutations in the known CRMO susceptibility genes including FBLIM1, IL1RN, LIPIN2, or PSTPIP2 in these two patients (SI Appendix, Table S3). However, the same missense variant in FBLIM1 was found in two patients with FGR splice or synonymous mutations, and a missense mutation in LIPIN2 was detected in a patient with an FGR 5′ UTR mutation (SI Appendix, Tables S3 and S4). All three variants are in trans with the coding FGR variant based on parental genotypes. The FBLIM1 variant identified in two probands induces a p.Gly311Arg amino acid change and the LPIN2 variant induces a p.Cys874Phe change.
To elucidate functional abnormality of the variants found in CRMO patients, kinase assay experiments were performed using affinity purified mutant FGR proteins and enolase (Fig. 4 I and J). The p.Arg118Trp FGR protein (RW, Fig. 4H) show decreased phosphorylation compared with that of wild-type protein (WT) (relative activity ratio: 0.39 ± 0.12, P < 0.0001, t test, Fig. 4 I and J). As shown in SI Appendix, Figs. S4 and S5, the structural model of p.Arg118Trp may lead to disruption of SH3-kinase linker interaction which destabilizes the protein functions (see also SI Appendix, Results and Discussion). In contrast, p.Pro525Ser (PS, Fig. 4H) may inhibit the closed-inactive formation of FGR as predicted by the structural model (SI Appendix, Fig. S6). Actually, the kinase assay experiments of PS show increased levels of phosphorylation compared with WT (relative activity ratio: 1.20 ± 0.05, P = 0.015, t test, Fig. 4 I and J). Unlike the Ali18 mutation, the decreased levels of C-terminal phosphorylation of kinase dead RW and PS by Csk were not observed (Fig. 4K). However, both murine p.Arg106Trp (corresponding to PS) and p.Arg106Trp;p.Asp502Gly Fgr constructs showed an ∼10% decrease in C-terminal phosphorylation by Csk compared with the wild-type and p.Asp502Gly Fgr constructs, respectively (SI Appendix, Fig. S7).
Discussion
In this study, we identified Fgr as the causative gene for inflammatory disease in the Ali18 mutant mice by positional candidate cloning despite complex modifier effects from the genetic background. Further genetic studies by genome editing confirmed that multiple null alleles of Fgr disrupt the inflammatory paw phenotype in Ali18 mice. Previously, it was shown that the Ali18 phenotype occurs independently of the adaptive immune system (disease occurs in Ali18;Rag1−/− mice that lack B and T cells); further, granulocytes are increased in peripheral blood of Ali18 mice (18). Fgr is predominantly expressed in myeloid lineage cells such as granulocytes, monocytes, and dendritic cells (29). This expression pattern fits the cell populations implicated in the autoinflammatory phenotype of Ali18 mice. Because Fgr plays an important role in mast cell activation (30, 31) and neutrophil adhesion (29), these cell types are likely contributing independently, or in combination, to autoinflammation in Ali18 mice and is the subject of ongoing research.
In SFKs, the C-terminal region is important for inactivation of tyrosine kinase activity; therefore defects in the C-terminal region cause constitutive activation, which leads to cancerization (32, 33). Csk is another member of SFKs and specifically phosphorylates a C-terminal tyrosine of SFKs (34). Without C-terminal phosphorylation by Csk, SFKs cannot form a folded inactive conformation. For this phosphorylation, it is known that docking between the C-terminal region of SFKs and Csk is necessary (27). In our results, FgrAsp502Gly was less phosphorylated than wild-type Fgr by Csk. The corresponding amino acid substitution in Src (p.Asp518Ala) also shows decreased phosphorylation of the C-terminal region (27). Thus, it strongly suggests that constitutive activation of Fgr leads to the inflammatory phenotypes of Ali18 mice.
In the cmo (chronic multifocal osteomyelitis) mouse model, the proinflammatory cytokine IL1-β mediates autoinflammation but does not require the Nlrp3 inflammasome (35). In FgrAli18 mice, IL1β is not up-regulated in peripheral blood (18). Although Fgr is not known to activate the inflammasome directly, NEK7 serine-threonine kinase and two tyrosine kinases, Btk and Syk, control inflammasome activation by phosphorylation (36). Thus, the phosphorylation network is important for regulation of inflammasome activation in myeloid cells. Recently, it was reported that phosphorylation of CBL ubiquitin ligase at Y371 by SFKs negatively controls NLRP3 inflammasome activation (37). Further, Cbl ubiquitinates Fgr directly for its activation (38). Thus, gain of function of Fgr may lead to the activation loop of Fgr-Cbl which suppresses the NLRP3 inflammasome. These suggest that autoinflammation caused by Fgr activation occurs independent of Nlrp3 inflammasome activation.
In the cohort of patients with CRMO, we found two FGR coding variants, p.Arg118Trp and p.Pro525Ser, which are located in the SH3 domain and C-terminal tail, respectively. Since decreased C-terminal phosphorylation by Csk was not observed using human FGR constructs, it is likely that mouse and human mutations have different functional properties. Increased kinase activation was observed in p.Pro525Ser FGR. Pro525 is the only hydrophobic amino acid in the C terminus of FGR. In the inactive form of Src, the C-terminal region is bound to two hydrophobic pockets of the protein surface in the SH2 domain (39, 40). The substitution of the substitution of Pro525 for hydrophilic Ser could be repellent to the surface of the hydrophobic binding pocket. Because the surface region is conserved between Src and Fgr (39), p.Pro525Ser FGR probably leads to instability of the inactive form. Thus, the active form of FGR could dominate the signaling cascade in the patient. In contrast, the role of p.Arg118Trp in CRMO pathogenesis remains difficult to define. The murine equivalent p.Arg106Trp mutation showed a decrease in phosphorylation versus wild-type murine Fgr, yet in vitro functional studies of p.Arg118Trp in human, constructs showed decreased kinase activity. Since human FGR has additional amino acid residues at the N terminus (amino acid residues 14–15 and 49–58; Fig. 4H) compared with murine Fgr, we hypothesize that these structural differences in FGR influence C-terminal phosphorylation. As described in Results, Ali18_#408a (p.Ser25Arg;Asp502Gly) mice exhibited more severe inflammatory phenotypes; this genetic model supports the importance of C- and N-terminal interactions in Fgr function. While the de novo status of the p.Arg118Trp mutation strongly supports its pathogenicity, in the absence of additional human CRMO cases with the same mutation, Fgr p.Arg106Trp mutant mice or FGR p.Arg118Trp knockin mice are needed to better determine the role of this mutation in sterile bone inflammation.
The genetic etiology of most sporadic CRMO cases is not yet known and it is likely that variants at multiple loci, in addition to environmental factors, both contribute together to disease development. We queried sequence data from the 13 CRMO probands with FGR coding variants for additional protein coding changes in the other CRMO candidate genes. A p.Gly311Arg change in FBLIM1 was identified in two probands and a p.Cys874Phe change in LPIN2 was identified in one proband. The FBLIM1 variant is not rare, with a minor allele frequency of 0.0195 in gnomAD and is in the FERMT2-binding domain, not the filamin-binding domain as was the variant previously reported (41). Phosphorylation of FERMT2 by Src facilitates interaction of FERMT2 with FBLIM1, and this complex then is recruited to focal adhesions as part of integrin-mediated cellular signaling (42). The concurrent and compound heterozygous status of both FGR and FBLIM1 variants in CRMO probands is supportive of genetic interactions between the two genes and future experiments will verify if variants in FGR or FBLIM1 found in CRMO patients disrupt this feedback loop. Regarding the LPIN2 missense variant, to date there is no established interaction between LIPIN2 and FGR.
We experimentally showed that inactivation of Fgr by genome editing abolished autoinflammatory bone and joint disease in Fgr p.Asp502Gly mutant Ali18 mice. This suggests targeting FGR function would be of potential therapeutic benefit in humans with autoinflammatory phenotypes. Tyrosine kinase inhibitors effective against SFK, including PP1, PP2, and dasatinib (43, 44), are therapeutic candidates. In particular, dasatinib relieves bone pain in animal models of cancer-induced bone pain (45). Such small molecule inhibitors may be effective on other members of SFKs, by targeting the ATP binding domain; however, this increases the likelihood of side effects. Recently, however, the FGR-specific inhibitor, TL02-59, has been identified as a suppressor of acute myelogenous leukemia cell growth (46). Testing these therapies in Ali18 mice will help determine the effectiveness of SFK inhibitors as a potential therapeutic target for the treatment of CRMO and other inflammatory diseases.
In conclusion, we identified the tyrosine kinase FGR as a functional intracellular signaling molecule involved in the pathogenesis of autoinflammatory bone diseases. Dysregulation of active–inactive conformational changes in FGR protein leads to osteomyelitis in mice and humans. Thus, trial and development of SFK inhibitors using the animal model will provide insights into the potential for therapeutic options in the treatment of human CRMO.
Methods
Mice.
The Ali18 strain (C3HeB/FeJ-Mhdaali18) was established in the Munich mouse ENU mutagenesis project (47), and its character and maintenance have been described previously (17, 19). For genotyping of the Fgr locus of Ali18 mice, see SI Appendix, Methods. All animal care and experimental protocols using mice were conducted in accordance with the Tokai University Institutional Animal Care and Use Committee, and all animal research was approved by this committee before the experiments.
Positional Candidate Cloning.
Standard genetic mapping was used to narrow down the critical region (17, 19). In the region, we used a literature-based search engine, PosMed (20), to select candidate genes. Because bone marrow cells from Ali18 mice could reconstitute the inflammatory phenotype of Ali18 mice in irradiated wild-type mice (18), we used “bone marrow” as a keyword for search by PosMed. Sixteen genes were selected, and PCR primer pairs spanning the exonic region were designed by ExonPrimer (https://ihg.helmholtz-muenchen.de). The PCR products from wild-type and Ali18/Ali18 templates were then sequenced by standard Sanger sequencing.
Genome Editing of Ali18 Mice.
A standard DNA microinjection method into Ali18/Ali18 fertilized eggs was performed (48) using pX330-based constructs described in SI Appendix, Methods. To obtain Ali18/Ali18 fertilized eggs, in vitro fertilization with Ali18/Ali18 oocyte and sperm was done. The CRISPR/Cas9-mediated mutations of F0 and F1 mice were confirmed by Sanger sequencing. We only used F0 mice (Ali18/Ali18) with newly introduced mutations in exon 3 of Fgr for further mating with C3HeB/FeJ mice to obtain F1 mice (Ali18/+).
Western Blotting.
Western blotting was done by a standard procedure as previously described (49). Antibodies used in this study were as follows: anti-Fgr (M60) (sc-50338, Santa Cruz Biotechnology, Inc.), anti-phosphotyrosine (06–427, Upstate), and anti-actin (A5471, Sigma). For protein preparation from tissues and cultured cells, see SI Appendix, Methods.
Protein Purification for Kinase Assays.
The in vitro transcription/translation system was used to synthesize mouse Fgr protein (TNT Quick Systems, Promega). GST-Csk (mouse), GST-FgrKD (mouse), and GST-FGR and GST-FGRKD (human) were expressed in Escherichia coli BL21(DE3), purified by glutathione Sepharose 4B (GE Healthcare), and stored in 50 mM Tris⋅HCl, pH 8.0, at −80 °C. Purity of the purified proteins was examined by SDS-polyacrylamide gel electrophoresis and Coomassie Brilliant Blue (CBB) staining. Protein concentrations were determined by using a Pierce BCA Protein Assay Kit (Thermo Scientific). For the phosphorylation assay procedures, see SI Appendix, Methods.
Clinical Samples and Exome Sequencing.
All human research was approved by the University of Iowa Institutional Review Board. Written informed consent was obtained from all clinical study participants and written parental or guardian consent was obtained, because all participants were under the age of 18. Use of clinical samples, whole-exome sequencing, and data analysis is described previously in detail (41), although more recent Agilent exome platforms (V5 and V6+UTR) were used for 8/99 samples (V5) and 79/99 (V6+UTR) samples.
Supplementary Material
Acknowledgments
We thank Kumiko Ishikawa, Maya Tanigawa, Miyu Hirano, Miyuki Kitabayashi, Kumiko Sugizaki, and Hideo Tsukamoto for excellent technical assistance. We are also thankful to Sanae Ogiwara, Ayaka Nakamura, and Yutaka Ishikawa for mouse embryo manipulation. This work was supported in part by grants from Japan Society for the Promotion of Science (JSPS 19500370 and 22500392), Takeda Science Foundation, and Research Foundation of Ituu to K.A., German Federal Ministry of Education and Research (Infrafrontier Grant 01KX1012 to M.H.d.A.), the National Institutes of Health (National Institute of Arthritis, Musculoskeletal and Skin R01AR059703 to P.J.F. and A.G.B.). V.B.M. and A.G.B. are supported by NIH Grants (R01EY026682, R01EY024665, R01EY025225, R01EY024698, and R21A050437), the Doris Duke Charitable Foundation Grant 2013103, and Research to Prevent Blindness New York, NY. G.V. is supported by NIH Grants (F30EYE027986 and T32GM997337).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.R.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819825116/-/DCSupplemental.
References
- 1.Masters S. L., Simon A., Aksentijevich I., Kastner D. L., Horror autoinflammaticus: The molecular pathophysiology of autoinflammatory disease (*). Annu. Rev. Immunol. 27, 621–668 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jansson A., et al. , Classification of non-bacterial osteitis: Retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatology (Oxford) 46, 154–160 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Ferguson P. J., Sandu M., Current understanding of the pathogenesis and management of chronic recurrent multifocal osteomyelitis. Curr. Rheumatol. Rep. 14, 130–141 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann S. R., et al. , Chronic recurrent multifocal osteomyelitis (CRMO): Presentation, pathogenesis, and treatment. Curr. Osteoporos. Rep. 15, 542–554 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roderick M. R., Sen E. S., Ramanan A. V., Chronic recurrent multifocal osteomyelitis in children and adults: Current understanding and areas for development. Rheumatology (Oxford) 57, 41–48 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Ferguson P. J., et al. , Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome). J. Med. Genet. 42, 551–557 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksentijevich I., et al. , An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N. Engl. J. Med. 360, 2426–2437 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abe K., Yu P., Positional cloning in mice and its use for molecular dissection of inflammatory arthritis. Curr. Pharm. Biotechnol. 10, 252–260 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Grosse J., et al. , Mutation of mouse Mayp/Pstpip2 causes a macrophage autoinflammatory disease. Blood 107, 3350–3358 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd L., Grossmann M., Potter M., Shen-Ong G. L., Chronic multifocal osteomyelitis, a new recessive mutation on chromosome 18 of the mouse. Genomics 11, 794–798 (1991). [DOI] [PubMed] [Google Scholar]
- 11.Ferguson P. J., et al. , A missense mutation in pstpip2 is associated with the murine autoinflammatory disorder chronic multifocal osteomyelitis. Bone 38, 41–47 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi N., et al. , Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426, 454–460 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Yu P., et al. , Autoimmunity and inflammation due to a gain-of-function mutation in phospholipase C gamma 2 that specifically increases external Ca2+ entry. Immunity 22, 451–465 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Abe K., et al. , A novel N-ethyl-N-nitrosourea-induced mutation in phospholipase Cγ2 causes inflammatory arthritis, metabolic defects, and male infertility in vitro in a murine model. Arthritis Rheum. 63, 1301–1311 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Ombrello M. J., et al. , Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N. Engl. J. Med. 366, 330–338 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Q., et al. , A hypermorphic missense mutation in PLCG2, encoding phospholipase Cγ2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am. J. Hum. Genet. 91, 713–720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe K., Fuchs H., Lisse T., Hans W., Hrabe de Angelis M., New ENU-induced semidominant mutation, Ali18, causes inflammatory arthritis, dermatitis, and osteoporosis in the mouse. Mamm. Genome 17, 915–926 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Abe K., et al. , Novel lymphocyte-independent mechanisms to initiate inflammatory arthritis via bone marrow-derived cells of Ali18 mutant mice. Rheumatology (Oxford) 47, 292–300 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Abe K., et al. , Genome-wide search for genes that modulate inflammatory arthritis caused by Ali18 mutation in mice. Mamm. Genome 20, 152–161 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Yoshida Y., et al. , PosMed (Positional Medline): Prioritizing genes with an artificial neural network comprising medical documents to accelerate positional cloning. Nucleic Acids Res. 37, W147–W152 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naharro G., et al. , Molecular cloning of integrated Gardner-Rasheed feline sarcoma virus: Genetic structure of its cell-derived sequence differs from that of other tyrosine kinase-coding onc genes. J. Virol. 47, 611–619 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown M. T., Cooper J. A., Regulation, substrates and functions of src. Biochim. Biophys. Acta 1287, 121–149 (1996). [DOI] [PubMed] [Google Scholar]
- 23.Timmermans S., Van Montagu M., Libert C., Complete overview of protein-inactivating sequence variations in 36 sequenced mouse inbred strains. Proc. Natl. Acad. Sci. U.S.A. 114, 9158–9163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi Y., Sims G. E., Murphy S., Miller J. R., Chan A. P., Predicting the functional effect of amino acid substitutions and indels. PLoS One 7, e46688 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowell C. A., Soriano P., Varmus H. E., Functional overlap in the src gene family: Inactivation of hck and fgr impairs natural immunity. Genes Dev. 8, 387–398 (1994). [DOI] [PubMed] [Google Scholar]
- 26.Lowell C. A., Soriano P., Knockouts of Src-family kinases: Stiff bones, wimpy T cells, and bad memories. Genes Dev. 10, 1845–1857 (1996). [DOI] [PubMed] [Google Scholar]
- 27.Lee S., Ayrapetov M. K., Kemble D. J., Parang K., Sun G., Docking-based substrate recognition by the catalytic domain of a protein tyrosine kinase, C-terminal Src kinase (Csk). J. Biol. Chem. 281, 8183–8189 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Lek M., et al. ; Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowell C. A., Src-family kinases: Rheostats of immune cell signaling. Mol. Immunol. 41, 631–643 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Lee J. H., et al. , The Src family kinase Fgr is critical for activation of mast cells and IgE-mediated anaphylaxis in mice. J. Immunol. 187, 1807–1815 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki R., et al. , Molecular editing of cellular responses by the high-affinity receptor for IgE. Science 343, 1021–1025 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtneidge S. A., Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 4, 1471–1477 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper J. A., Gould K. L., Cartwright C. A., Hunter T., Tyr527 is phosphorylated in pp60c-src: Implications for regulation. Science 231, 1431–1434 (1986). [DOI] [PubMed] [Google Scholar]
- 34.Okada M., Regulation of the SRC family kinases by Csk. Int. J. Biol. Sci. 8, 1385–1397 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassel S. L., et al. , Inflammasome-independent IL-1β mediates autoinflammatory disease in Pstpip2-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 111, 1072–1077 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong T., Jiang W., Zhou R., Control of inflammasome activation by phosphorylation. Trends Biochem. Sci. 43, 685–699 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Chung I. C., et al. , Src-family kinase-Cbl axis negatively regulates NLRP3 inflammasome activation. Cell Death Dis. 9, 1109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melander F., Andersson T., Dib K., Fgr but not Syk tyrosine kinase is a target for beta 2 integrin-induced c-Cbl-mediated ubiquitination in adherent human neutrophils. Biochem. J. 370, 687–694 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waksman G., et al. , Crystal structure of the phosphotyrosine recognition domain SH2 of v-src complexed with tyrosine-phosphorylated peptides. Nature 358, 646–653 (1992). [DOI] [PubMed] [Google Scholar]
- 40.Waksman G., Shoelson S. E., Pant N., Cowburn D., Kuriyan J., Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: Crystal structures of the complexed and peptide-free forms. Cell 72, 779–790 (1993). [DOI] [PubMed] [Google Scholar]
- 41.Cox A. J., et al. , Recessive coding and regulatory mutations in FBLIM1 underlie the pathogenesis of chronic recurrent multifocal osteomyelitis (CRMO). PLoS One 12, e0169687 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z., et al. , Kindlin-2 phosphorylation by Src at Y193 enhances Src activity and is involved in Migfilin recruitment to the focal adhesions. FEBS Lett. 589, 2001–2010 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Hanke J. H., et al. , Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271, 695–701 (1996). [DOI] [PubMed] [Google Scholar]
- 44.O’Hare T., et al. , Inhibition of wild-type and mutant Bcr-Abl by AP23464, a potent ATP-based oncogenic protein kinase inhibitor: Implications for CML. Blood 104, 2532–2539 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Appel C. K., et al. , The Src family kinase inhibitor dasatinib delays pain-related behaviour and conserves bone in a rat model of cancer-induced bone pain. Sci. Rep. 7, 4792 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weir M. C., et al. , Selective inhibition of the myeloid Src-family kinase Fgr potently suppresses AML cell growth in vitro and in vivo. ACS Chem. Biol. 13, 1551–1559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hrabe de Angelis M., et al. , Genome-wide, large-scale production of mutant mice by ENU mutagenesis. Nat. Genet. 25, 444–447 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Hogan M., Beddingtom R., Constantini F., Lacy E., Manipulating the Mouse Embryo (Cold Spring Harbor Laboratory Press, New York, ed. 2, 1994). [Google Scholar]
- 49.Abe K., et al. , Novel ENU-induced mutation in Tbx6 causes dominant spondylocostal dysostosis-like vertebral malformations in the rat. PLoS One 10, e0130231 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.