Summary
The expression of insecticidal proteins under constitutive promoters in transgenic plants is fraught with problems like developmental abnormalities, yield drag, expression in unwanted tissues, and seasonal changes in expression. RbPCD1pro, a rapid, early acting wound‐inducible promoter from rose that is activated within 5 min of wounding, was isolated and characterized. Wounding increased transcript levels up to 150 and 500 folds within 5 and 20 min coupled with high translation as seen by histochemical GUS enzyme activity within 5–20 min. RbPCD1pro was activated by both sucking and chewing insects and showed wound‐inducible expression in various aerial tissues of plants representing commercially important dicot and monocot families. The promoter showed no expression in any vegetative tissue except upon wounding. Functionality of RbPCD1pro was tested by its ability to drive expression of the insecticidal protein gene cryIAc in transgenic Arabidopsis and tomato. Strong wound‐inducible CryIAc expression was observed in both plants that increased 100–350 fold (Arabidopsis) and 280–600 fold (tomato) over the unwounded background within 5 min and over 1000–1600 fold within 20 min. The unwounded background level was just 3–6% of the CaMV35S promoter while wound‐induced expression was 5–27 folds higher than the best CaMV35S line in just 5 min and 80‐fold higher in 20 min. Transgenic plants showed strong resistance even to larger fourth instar larvae of H. armigera and no abnormalities in development and general plant growth. This is one of the earliest acting promoters with wide biotechnological application across monocot and dicot plants.
Keywords: insect resistance, constitutive promoter, GUS, jasmonic acid, Bacillus thuringiensis, tomato
Introduction
The large scale destruction of crop plants by various chewing and sucking insects has necessitated the development of chemical as well as biotechnological means to prevent damage. One of the most prevalent environment‐friendly biotechnological approaches for protection against Lepidopteran insects has been the development of transgenic plants expressing the cryIAc gene and its variants from Bacillus thuringiensis. These insecticidal protein genes have most commonly been expressed under strong ubiquitously expressing promoters like CaMV35S, ubiquitin, actin, rbcS etc. and prevent insect attack by maintaining continuous high level expression of the toxin protein (Cao et al., 2002; Nayak et al., 1997; Tang et al., 2006; Tu et al., 2000; Ye et al., 2001; Ye et al., 2009; Zhao et al., 2014).
Despite their success, constitutive promoters are not the best choice for driving insecticidal toxin gene expression for several reasons: (i) they ensure continuous expression of the toxic protein in most tissues, even in the absence of the insect, thus entailing a huge metabolic cost on the plant that may affect yield (Breitler et al., 2004; Gurr and Rushton, 2005; Kim et al., 2008; Xia et al., 2010). (ii) expression of the toxic protein affects plant development with many transgenic plants showing abnormalities in certain developmental aspects or failing to survive the initial stages of tissue culture (Bano‐Maqbool et al., 1998; Barton et al., 1987; Breitler et al., 2004; Diehn et al., 1996; Koul et al., 2014; Kranthi et al., 2005; Rawat et al., 2011; Rocher et al., 1998; Sachs et al., 1998). (iii) many constitutive promoters like the CaMV35S or the maize ubiquitin promoter are not truly constitutive since they show developmental and seasonal changes and expression may taper down towards flowering or in certain tissues making these susceptible to attack (Kranthi et al., 2005; Wu et al., 2002). (iv) continuous expression of the protein increases fears of the possibility of rapid development of resistance due to selection pressure on the insects (v) Expression of insecticidal proteins in seeds and embryos of rice or other edible plants (Breitler et al., 2001; Wu et al., 2002) may act as a psychological deterrent for consumers.
Inducible promoters, especially wound‐inducible promoters, provide an attractive alternative to constitutive promoters because of their ability to reduce unnecessary background expression of the toxic protein and prevent possible abnormalities from their expression. Much research has led to isolation of several wound‐inducible promoters from different plants. These include the AoPR1 promoter from PR1 gene of Asparagus officinalis (Warner et al., 1993), the mpiC1 promoter from a maize protease inhibitor gene (Cordero et al., 1994), the fib gene promoter from bell paper (Chen et al., 1998), the win3.12 gene promoter from Populus (Hollick and Gordon, 1995; Yevtushenko et al., 2004), the Shpx6b peroxidase promoter from the forage legume, Stylosanthes humilis, (Perera and Jones, 2004), the OsDof1 promoter of rice (Park et al., 2014) and others. A few of these such as AoPR1 and mpiC1 have been used for expression of CryIAc‐type proteins or protease inhibitors with varying success (Girijashankar et al., 2005; Gulbitti‐Onarici et al., 2009). A drawback with some of these has been the relative delay in their induction (from a few hours to even days for maximum activation), the reduced level of transgene induction or restricted specificity in heterologous systems (Wilmink et al., 1995; Zhang et al., 2013) thereby limiting their application for general use.
An early acting promoter, activated transcriptionally and translationally within minutes of wounding, has the potential to stall insect attack beyond a few nibbles and should ideally be effective not only against newly hatched larvae but also against larger larve of the 3rd and 4th instar stages and should stay silent in the absence of insect attack. Here, a strong wound‐inducible promoter, activated early within 5–20 min of mechanical and insect wounding is described. It is responsive to both chewing and sucking insects and functions in different tissues in several commercially important families. Functional validation through expression of the CryIAc protein in Arabidopsis and tomato shows its potential for use in several plants against insects.
Results
Characterization of the wound‐inducible RbPCD1 promoter
The wound‐inducible nature of RbPCD1 promoter was observed during study of abscission‐related cis elements in the promoter of a petal abscission up‐regulated gene of rose (Amar Pal Singh, 2011). A 523 nt region, upstream of the initiation codon, governing wound‐inducible GUS expression (Patent No 3866/DEL/2014 filed and WO2016103279A1) was explored in further detail in Arabidopsis and other plants.
A time‐course analysis of wound induction of the RbPCD1 promoter was carried out using leaves of more than five independent homozygous transgenic Arabidopsis lines expressing RbPCD1pro::GUS. Intact leaves were wounded by rapid punctures and kept for 5 and 20 min. This was followed by color development in the presence of cycloheximide to ensure that the observed color only represented the protein synthesized during the 5–20 min time course of wounding and not later during color development. Histochemical GUS assay of wounded leaves showed an intense blue color around the damaged area as well as the petiole tip from where leaves were detached (Figure 1a). No color was seen in the unwounded regions.
Figure 1.
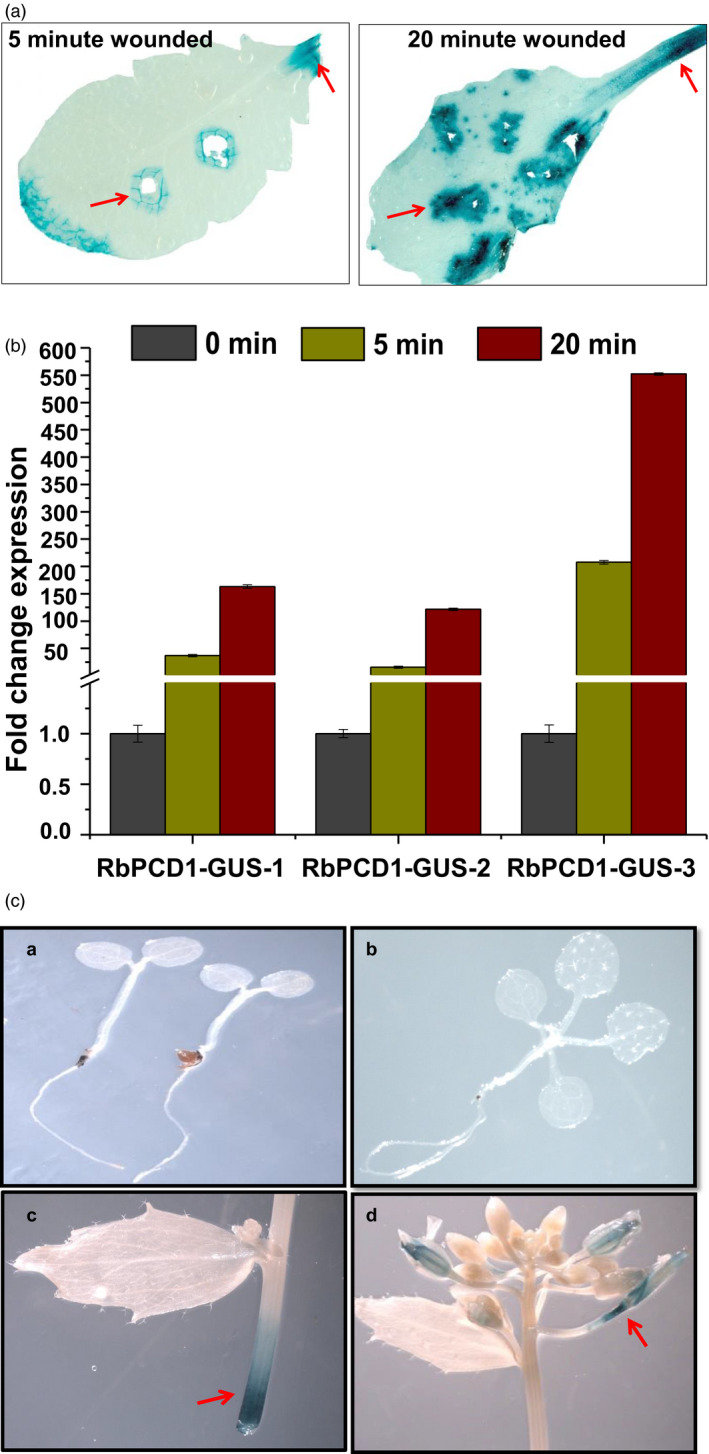
Wound‐inducible expression of GUS under the RbPCD1 promoter in transgenic Arabidopsis plants. (a) Histochemical GUS staining of transgenic Arabidopsis leaves expressing RbPCD1pro:: GUS at 5 and 20 min after mechanical wounding. Arrows indicate the site of mechanical wounding or damage. GUS analysis was carried out in presence of cycloheximide. (b) qRT‐PCR analysis of relative transcript levels of GUS after mechanical wounding in transgenic Arabidopsis expressing RbPCD1pro:: GUS . Analysis was carried out in three independent transgenic lines. AtUBIQUITIN10 was used as internal control. (c) Histochemical analysis of GUS activity in transgenic Arabidopsis expressing RbPCD1‐pro::GUS in various stages of plant development. (a) Two leaf stage (b) Four leaf stage (c) Excised stem (d) Flowering stage.
For further validation of the wound responsiveness of RbPCD1pro, a time course analysis of transcript accumulation of GUS mRNA in response to mechanical wounding was performed at 5/20 min after wounding. A rapid increase in GUS transcript levels, ranging from 50 to 150 folds within 5 min and from 150 to 550 folds within 20 min of wounding (compared to control), was observed in all lines suggesting that the promoter responded strongly to mechanical wounding (Figure 1b).
In order to examine the spatiotemporal expression patterns of RbPCD1pro::GUS, whole transgenic plants were histochemically stained at different developmental stages from seedling to flowering. No detectable GUS expression was seen in any plant part in the absence of wounding regardless of the stage of development except in the inflorescence and abscission zones (Figure 1c). The promoter was activated upon wounding at all stages (except senescent leaves) and in all tissues except root where no GUS expression could be observed even upon wounding (data not shown).
RbPCD1pro is induced by both chewing and sucking insects and activated in dicots and monocots
The RbPCD1 promoter was next tested for its ability to respond to insect wounds. Leaves of independent transgenic Arabidopsis plants harbouring RbPCD1pro::GUS were fed upon by Helicoverpa armigera, a chewing insect, or exposed to the aphid, Myzus persicae, a sucking pest. A strong blue color was observed around the area damaged by both insects (Figure 2a) showing that the promoter responded to wounding by both chewing and sucking pests.
Figure 2.
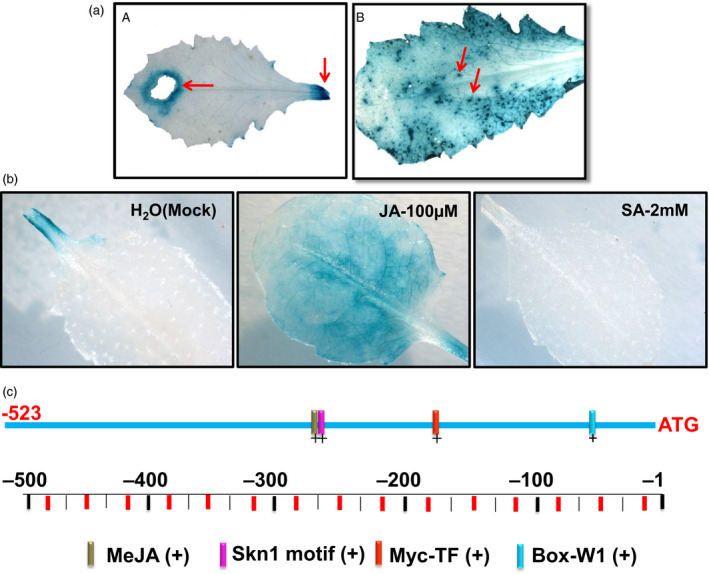
In silico analysis of the RbPCD1 promoter and its response to insect wounding and different hormones. (a) Activation of RbPCD1pro::GUS by insect wounding. Histochemical GUS staining of transgenic Arabidopsis leaves was carried out after exposure to the chewing insect, Helicoverpa armigera (A), and the sucking pest Myzus persicae (B). The arrow in the left panel indicates the site of insect bite while the blue dots in the lower panels indicate sites of feeding by the aphids. (b) Expression of RbPCD1pro::GUS in response to Jasmonic acid and salicylic acid in transgenic Arabidopsis. Leaves were sprayed with JA (100 μm)/SA (2 mm)/water, kept for 3 h and GUS expression checked. (c) In silico analysis of putative cis elements in the 523 nt region of the RbPCD1 promoter by PLACE.
The promoter was next tested for wound response in different plants of economic importance that included dicots like chickpea, cotton, tobacco, rose, and a monocot Gladiolus by transgenic means (chickpea) or by agro‐inoculation (all other plants). Strong wound‐inducible GUS expression, only at the site of wounding, was seen in transgenic chickpea leaves as well as agroinjected cotton sepals, rose petals, Gladiolus tepals, and tobacco leaves (Figure S1) indicating that the promoter could respond to wound signals in a variety of plants belonging to different families.
The RbPCD1 promoter is responsive to JA but suppressed by SA
Wound responses are known to be regulated by both JA and SA, with JA activating wound responses and SA suppressing JA effects (Koornneef et al., 2008). To check this, a study of RbPCD1pro induction by JA and SA (in the absence of wounding) was performed. As shown (Figure 2b), JA treatment could induce RbPCD1pro, albeit weakly, compared to that observed by wounding. Interestingly, SA seemed to suppress the promoter since no GUS expression could be seen even at the point of petiole excision.
An in silico analysis of the 523 nt region of RbPCD1 promoter was performed using PLACE (http://www.dna.affrc.go.jp/PLACE/signalscan.html). The analysis revealed several putative cis‐acting regulatory elements such as a Myc‐TF binding site, a JA responsive element, W‐boxes for WRKY transcription factor binding and others (Figure 2c).
RbPCD1pro drives cryIAc expression in a strong wound‐inducible manner and protects transgenic Arabidopsis plants from insect larvae
In order to test the comparative efficacy and functionality of the RbPCD1 promoter, constructs expressing cryIAc under the RbPCD1 and CaMV35S promoters were introduced into Arabidopsis (both promoters) and tomato (only RbPCD1pro) as described in methods. Three independent T3 generation lines of transgenic Arabidopsis, homozygous for the transgene (AtPCDCry 3‐3‐1, AtPCDCry 4‐2‐1, and AtPCDCry 6‐1‐2), and tomato (SlPCDCry 3, SlPCDCry 9, and SlPCDCry10, T1 generation) were studied for wound‐inducible protein production and insect bioassay and also monitored for possible abnormalities in leaf shape, size, and plant growth.
For transgenic Arabidopsis, a quantitative estimate of the CryIAc protein produced in response to varying puncture wounds (2, 4, and 12 wounds) was first obtained in leaves on transgenic plants after 5 and 20 min. As shown (Figure 3A), basal level expression of CryIAc in CaMV35Spro plants ranged from 8.9 to 24.8 ng/disc corresponding to 0.05 (lowest) to 0.14 ng/mm2 (highest expressing plant). The basal level in transgenic RbPCD1pro::cryIAc lines was 0.005, 0.01, and 0.011 ng/mm2 in AtPCDCry lines 3‐3‐1, 4‐2‐1, and 6‐1‐2, respectively (Figure 3A). This was just about 3%–6% of the best CaMV35S line (0.14 ng/mm2). However, within 5 min of wounding, CryIAc expression increased to 0.628, 0.971, and 3.83 ng/mm2 in AtPCDCry lines 3‐3‐1, 4‐2‐1, and 6‐1‐2, respectively. This represented an increase in 4.5–27 folds over the highest expressing CaMV35S line and about 100–350 folds over the unwounded background. By 20 min, the expression increased further by 11–82 folds (1.572, 5.83, and 11.442 ng/mm2 in AtPCDCry lines 3‐3‐1, 4‐2‐1, and 6‐1‐2, respectively), over the CaMV35S line and 315–1040 folds over the unwounded background. An increase in the number of wounds from 2 to 4 or 12 led to a higher accumulation of protein although the increase was not linear (Figure 3a). A longer period of wounding from 5 to 20 min led to a much greater increase in CryIAc accumulation, the difference being about threefold between 5 and 20 min. Thus, the RbPCD1 promoter could maintain the toxic CryIAc protein at a much lower basal level than the CaMV35S promoter but at a much higher induced level (up to 80 folds higher) compared to the best CaMV35S line and 100–1000 folds higher than the unwounded background level within 5–20 min. Since experiments were carried out in T3 generation plants, it also indicated that the wound‐inducible expression was maintained at least up to the third generation.
Figure 3.
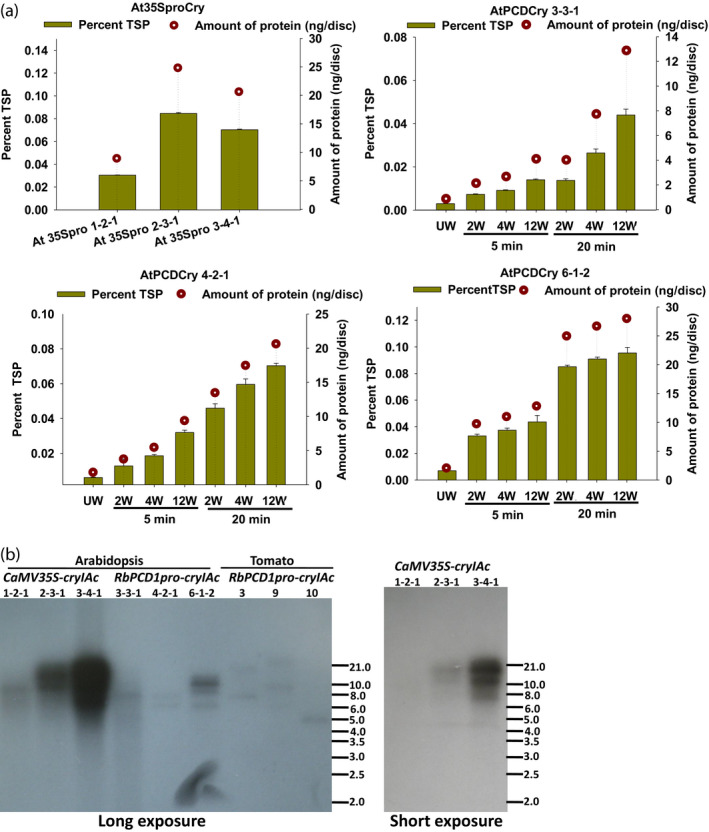
Copy number and expression of cryIAc under the CaMV35S and the RbPCD1 promoters in different transgenic Arabidopsis lines. (a) Estimation of CryIAc in different transgenic lines under the CaMV35S and RbPCD1 promoters. For CaMV35S lines, expression was estimated in leaf discs (177 mm2) by DAS‐ELISA using CryIAc specific antibodies. For wound‐inducible expression in RbPCD1pro::cryIAc plants, leaves were wounded with various punctures (2, 4, and 12), kept for 5 or 20 min on the plant and leaf discs excised from the plant. Green bars represent %TSP on left Y‐axis and circles represent amounts in ng/disc (177 mm2 surface area) on right Y‐axis. UW‐unwounded, W‐ wounded. (b) Southern blot analysis for copy number estimation of cryIAc in transgenic Arabidopsis and tomato lines. DNA from all independent transgenic plants expressing cryIAc under the CaMV35S (Arabidopsis) and RbPCD1 (Arabidopsis and tomato) promoters was digested with HindIII. Blotting and hybridization was carried out as described in methods using 32PdATP‐labeled cryIAc (that lacks an internal HindIII site). Each band represents an independent insertion event.
To ensure that the differences in expression between CaMV35Spro and RbPCD1pro plants were not due to copy number differences, a Southern blot analysis of the lines was carried out. As shown (Figure 3B), the number of cryIAc copies ranged from 1 to 3 in the CaMV35S lines (1 in 1‐2‐1, 2 in 2‐3‐1 and 3 in 3‐4‐1) and from 1 to 4 (1 in 3‐3‐1, 2 in 4‐2‐1 and 4 in 6‐1‐2) in the transgenic Arabidopsis RbPCD1pro lines indicating that copy number was not responsible for the differences in expression between CaMV35pro and RbPCD1pro lines.
To ascertain the efficacy of the CryIAc protein produced under the wound‐inducible RbPCD1pro, an insect bioassay was performed on transgenic RbPCD1pro::cryIAc plants. Both, detached leaf and whole plant insect bioassays, were conducted on Helicoverpa armigera larvae at four developmental stages (1st–4th instar) in independent sets of experiments.
For detached leaf bioassays, plants of 10 transgenic lines (of 14) were randomly selected for neonate first instar larvae studies. All the 10 RbPCD1pro::cryIAc lines showed greater than 60% larval mortality after 24 h of bioassay and >80% mortality within 48 h of feeding the leaves (Figure 4a). In two transgenic AtPCDCry lines, 4‐2‐1 and 6‐1‐2, 90%–100% larval mortality was observed within 24 h of exposure while seven plants killed all larvae within 48 h. The results showed that the transgenic lines, despite having low basal levels of CryIAc (3%–6% of CaMV35Spro lines), induced it to sufficiently high enough levels upon wounding to kill all Helicoverpa larvae and confer complete protection. No significant mortality was observed in larvae fed on nontransgenic leaves which were severely damaged by larval feeding (Figure 4b).
Figure 4.
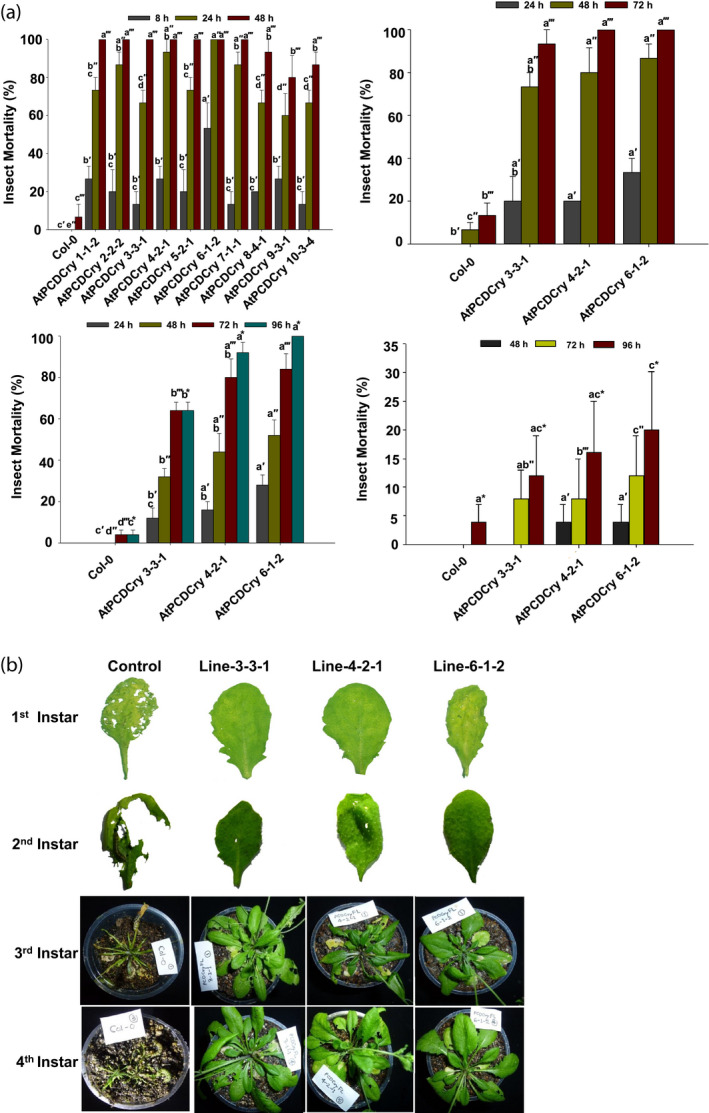
Efficacy of transgenic Arabidopsis plants expressing cryIAc under the RbPCD1 promoter against H. armigera larvae at various developmental stages. (a) Time course of mortality of various instar larvae fed on leaves of different RbPCD1pro::cryIAc transgenic Arabidopsis lines as a percent of the total. Mortality values were analysed by one‐way ANOVA and compared using Duncan's Multiple Range Test (DMRT). Values on the bar carrying different letters are significantly different (α = 0.05). (b) Resistance of transgenic RbPCD1pro::cryIAc Arabidopsis plants against H. armigera larvae in various developmental stages as studied by leaf damage in detached (first and second instar larvae) and whole plant (third and fourth instar larvae) insect assay in three independent transgenic lines.
Three transgenic lines (3, 4 and 6) that conferred 90%–100% mortality of neonate larvae within 24 h were selected for further bioassays with more advanced larval stages. As shown in Figure 4a, mortality of 2nd instar larvae on detached leaves of these lines was between 56%–80% within 48 h and reached 100% within 72 h of feeding. When studied with the 3rd instar larvae, these became inactive within 48 h of feeding on transgenic leaves and died between 72 h and 96 h. Whole plant bioassay from these selected transgenic lines showed 90%–100% mortality of H. armigera larvae after 96 h (Figure 4a). The amount of transgenic leaves consumed by larvae was very small compared to control plant leaves but sufficient to kill the 3rd instar larvae.
Unlike other larval stages, mortality of 4th instar larvae on transgenic leaves was much reduced by 5 days but was associated with weight reduction. Compared to control leaves, where a 304% increase in larval weight was observed in 5 days, a significant reduction in larval weight ranging from 7.6% to 44% of the initial larval weight was observed (Table 1). Even after 5 days, the transgenic plants showed little damage since the larvae, despite their size, refrained from feeding any further. They remained motionless for most part and died after 6–7 days. The results indicated that wound‐inducible expression of CryIAc in transgenic Arabidopsis was sufficient to confer protection even against larger larvae.
Table 1.
Percent weight reduction of 4th instar larvae fed on Arabidopsis
| Line | Larval weight (Mean ± SE) | Percent weight gained/lost (against initial larval weight) after 5 days feeding |
|---|---|---|
| Control | 204.53 ± 4.07a | 304% |
| AtPCDCry 3‐3‐1 | 62.23 ± 1.69b | −7.6% |
| AtPCDCry 4‐2‐1 | 48.91 ± 2.19c | −27.4% |
| AtPCDCry 6‐1‐2 | 37.66 ± 1.89d | −44% |
Each value shows the mean weight of 25 larvae after 5 days of feeding. Average larval weight and percent weight reduction were analysed by one‐way ANOVA and compared using Duncan's Multiple Range Test (DMRT). Values in the column carrying different letters are significantly different. Initial average larval weight was 67.27 ± 1.25 mg.
Importantly, unlike previous reports, none of the transgenic lines expressing RbPCD1pro::CryIAc showed any abnormality in vegetative or reproductive growth (Figure S3) compared to control.
Transgenic tomato expressing cryIAc under the wound‐inducible RbPCD1pro protect plants from insect larvae
The efficacy of RbPCD1pro to drive cryIAc expression was also tested in a commercially important Indian tomato, Pusa Early Dwarf. Three independent transgenic lines SlPCDCry 3, SlPCDCry 9, and SlPCDCry10, were selected for detailed studies. However, unlike in Arabidopsis, no plants expressing cryIAc under the CaMV35S promoter could be developed despite several efforts.
An estimation of the background and wound‐induced CryIAc protein produced in transgenic tomato leaves was performed using DAS‐ELISA as in case of Arabidopsis. As shown (Figure 5a), the transgenic tomato leaves had CryIAc background (unwounded) levels of 0.0042 ng/mm2 (SlPCDCry 3), 0.0054 ng/mm2 (SlPCDCry 9) and 0.0016 ng/mm2 (SlPCDCry10). Wounding caused a rapid increase in the CryIAc protein level with expression ranging from 0.981 to 1.995 ng/mm2 after 5 min of wounding in leaves. This was in the range observed for Arabidopsis and represented an increase in 284–613 fold over the unwounded background. The expression increased further to 2.57–3.66 ng/mm2 by 20 min, an increase in 680–1610 fold over the unwounded background. No CryIAc protein was detected in nontransgenic plants used as a negative control.
Figure 5.
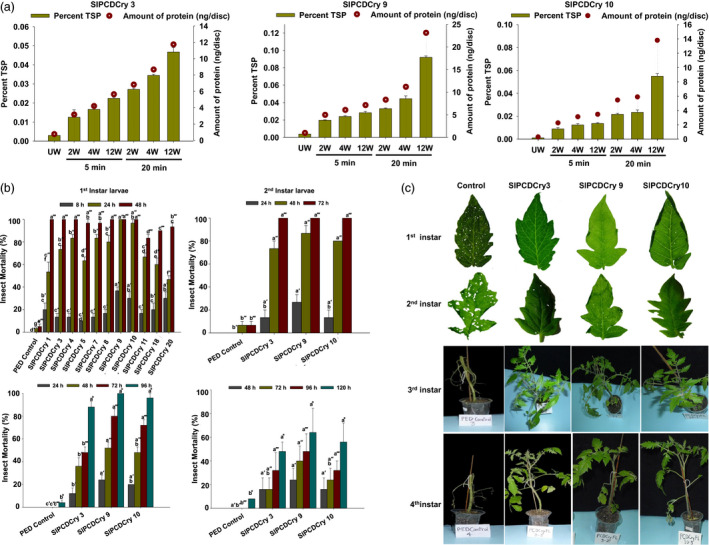
Wound‐inducible CryIAc estimation and efficacy of transgenic tomato plants expressing cryIAc under the RbPCD1 promoter against H. armigera larvae at various developmental stages. (a) Wound‐inducible expression of CryIAc toxin under the control of RbPCD1 promoter in different transgenic tomato lines. Expression was estimated in leaves of various transgenic lines as described in Figure 3a. Green bars represents %TSP on left Y‐axis and circles represent amounts in ng/disc (177 mm2 surface area) on right Y‐axis. UW‐unwounded, W‐ wounded. (b) Time course of mortality of various instar larvae fed on leaves of different RbPCD1pro::cryIAc transgenic tomato lines as a percent of the total. Mortality values were analysed by one‐way ANOVA and compared using Duncan's Multiple Range Test (DMRT). Values on the bar carrying different letters are significantly different (α = 0.05). (c) Resistance of transgenic RbPCD1pro::cryIAc tomato plants against H. armigera larvae in various developmental stages as studied by leaf damage in detached (neonate first and second instar larvae) and whole plant (third and fourth instar larvae) insect assay in three independent transgenic lines.
Insect bioassays were also performed to ascertain the toxicity of transgenic RbPCD1pro::cryIAc tomato plants towards H. armigera larvae at different stages using untransformed plants as control. Eleven transgenic plants (out of 22) from the T0 generation were randomly selected for insect bioassay on detached leaves using first instar neonate larvae. After 24 h of feeding, larval mortality in majority of transgenic lines was between 46%–96%, and reached 100% within 48 h of feeding (Figure 5b). The transgenic lines, SlPCDCry 9 and SlPCDCry 10 showed 97%–100% mortality within 24 h of exposure while six others displayed 100% insect mortality within 48 h of feeding. No mortality was observed in larvae feeding on nontransgenic leaves which showed far greater damage compared to transgenic leaves where hardly any damage was seen (Figure 5c).
The transgenic lines 3, 9, and 10, that conferred 100% mortality on neonate larvae within 48 h were selected for further insect bioassay with more advanced larval stages in the T1 generation. The selected transgenic lines displayed larval mortality of 70%–85% on 2nd instar larvae within 48 h and complete mortality within 72 h. These larvae caused severe damage on leaves of untransformed control plants but little or no damage on transgenic plant leaves (Figure 5b,c). Third instar larvae survived longer with larval mortality of 48%–80% after 72 h of feeding on transgenic plants and complete mortality only after 96 h (Figure 5b,c). The percentage of larval mortality of 4th instar larvae on transgenic plants after 5 days was lower and ranged between 28% and 32% (Figure 5b). Instead, a greater effect on weight reduction was observed in these larvae (Table 2). Compared to larvae fed on control leaves, where a 317% increase in larval weight was observed in 5 days, a significant reduction in larval weight, ranging from 3% to 35%, was observed compared to the initial larval weight (Table 2). As in case of Arabidopsis, the 4th instar larvae remained motionless for most part of the feeding trial and died after 6–7 days. The amount of transgenic leaves consumed by larvae even during their survival was very low unlike in controls where almost the complete plant was eaten by 4th instar larvae by the 3rd day.
Table 2.
Percent weight reduction of 4th instar larvae fed on transgenic tomato
| Line | Larval weight (Mean ± SE) | Percent weight gained/lost (against initial larval weight) after 5 days feeding |
|---|---|---|
| Control | 219.63 ± 4.71a | 317% |
| SlPCDCry 3 | 66.99 ± 1.34b | −3% |
| Sl PCDCry 9 | 44.67 ± 1.32d | −35% |
| SlPCDCry 10 | 58.61 ± 1.28b | −15% |
Each value shows the mean weight of 25 larvae after 5 days of feeding. Average larval weight and percent weight reduction were analysed by one‐way ANOVA and compared using Duncan's Multiple Range Test (DMRT). Values in the column carrying different letters are significantly different. Initial average weight of larvae was 68.97 ± 0.88 mg.
The insect bioassay showed that 1st and 2nd instar larvae were more severely affected than older instars (3rd and 4th) as far as mortality was concerned although most of the 3rd and 4th instar larvae failed to damage the leaves. The results indicated that wound‐inducible expression of CryIAc in transgenic tomato conferred complete protection against the insects.
Importantly, no abnormalities were observed in transgenic plants during the entire course of plant growth. General plant growth and height, flowering time, fruit size and ripening pattern were not affected (Figure 6a,b).
Figure 6.

Phenotypes of transgenic tomato plants expressing cryIAc under the wound inducible RbPCD1 promoter. (a) Whole plant growth after 3 months. (b) Fruit shape and size.
Collectively, these results showed improved efficacy of CryIAc when expressed under the wound‐inducible RbPCD1 promoter, heightened protection even against larger fourth instar larvae, reduced background levels compared to the CaMV35S promoter and no toxicity to plant growth and development unlike previous reports.
Discussion
The efficacy of any transgene is governed largely by the promoter driving its expression. While constitutive promoters like CaMV35S, ubiquitin, actin, rbcS have been successful for driving high level expression of transgenes for abiotic and biotic stress tolerance, these are often associated deleterious effects or a yield drag in the absence of the stress (Bano‐Maqbool et al., 1998; Breitler et al., 2004; Gurr and Rushton, 2005; Kim et al., 2008; Koul et al., 2014; Rawat et al., 2011; Xia et al., 2010). Wound‐inducible promoters can effectively overcome these problems, provided these are strong and early acting. However, previous reports showed that the wound‐inducible AoPR1 promoter of Asparagus officinalis (Warner et al., 1993), when used to express cryIAc in tobacco plants, accumulated the insecticidal protein only between 6 h and 72 h postwounding (Gulbitti‐Onarici et al., 2009). Similarly, the bell paper fib gene promoter, the Stylosanthes Shpx6b peroxidase promoter, and the Populus win3.12 promoter showed wound‐inducible expression only 20–24 h after wounding (Chen et al., 1998; Hollick and Gordon, 1995; Perera and Jones, 2004; Yevtushenko et al., 2004). The wound‐inducible maize protease inhibitor mpiC1 promoter when used to express a synthetic cry1Ac toxin in sorghum showed low accumulation of toxic protein only 12 h postwounding (Girijashankar et al., 2005). The rice OsDof1 promoter in contrast, showed wound‐inducible GUS expression within minutes of wounding in transgenic Arabidopsis and rice (Park et al., 2014). However, since the GUS histochemical reaction did not include cycloheximide, one cannot rule out GUS expression during the overnight incubation for color development in these studies. Many of these promoters are specific to a plant and cannot be used in others e.g. the WIP1 promoter from maize conferred wound response in rice but not in tobacco (Zhang et al., 2013). Often dicot promoters show reduced efficiency in monocots and vice versa (Christensen et al., 1992; Wilmink et al., 1995) restricting their use for transgene expression. An alternate strategy for insect resistance is high level expression of Cry toxins in plastids which also has an added advantage of transgene containment due to the maternal inheritance of the plastid (De Cosa et al., 2001; Kota et al., 1999). However, chloroplast transformation is restricted to a few plants due to a high level of standardization required.
In comparison, the RbPCD1 promoter confers a strong early acting wound‐inducible expression in several different plants. Detailed analysis in Arabidopsis and tomato showed induction, GUS transcript accumulation and GUS activity within 5–20 min of mechanical wounding in the transgenic Arabidopsis leaves (Figure 1). A quantitative measure of GUS transcript level using qRT‐PCR showed an induction of 50–150 fold after 5 min and 150–550 fold after 20 min of wounding. This transcriptional increase was also associated with high levels of GUS protein as reflected from the histochemical results indicating that both transcription and translation were responsible for the rapid GUS increase under the RbPCD1 promoter. The use of cycloheximide during color development ensured that all activity estimated during color development represented GUS protein produced only within the 5–20 min period of wounding. The activation of the promoter at the transcriptional level as well as translational level within 5 min of wounding makes it one of the fastest promoters to be studied so far.
Wound induction was also seen in transgenic chickpea leaves as well as agro‐infiltrated rose petals, Gladiolus tepals, cotton sepals, and tobacco leaves (Figure S1). This indicates that wound‐inducible cis elements within this region are recognized by the wound machinery across a wide range of plants that include both monocots and dicots and across a wide range of tissues such as leaves, sepals, and petals. This is important since it potentially extends the applicability of the promoter across monocots and dicots and in commercially important families such as Brassicaceae, Solanaceae, Leguminaceae, Rosaceae. The promoter is also activated in response to attack by sucking pests such as aphids and by chewing insects like Helicoverpa specifically at the points of insect damage (Figure 2A) indicating its potential to target both sucking and chewing type insects using suitable insecticidal toxins against each. Its activation by JA might explain its activation in a wide range of plants where JA functions as a primary hormone for wound induction and herbivory (Erb et al., 2012). The RbPCD1 promoter was shown to be rapidly induced by mechanical wounding in all aerial parts of Arabidopsis. At no stage of development was expression seen in the absence of wounding except in some inflorescence parts. This suggests that the RbPCD1 promoter could be used in a developmental stage‐independent manner unlike the CaMV35S promoter which, despite its constitutive nature, has been reported to show reduced expression at the time of flowering in cotton plants leading to susceptibility to insect attack at that stage (Kranthi et al., 2005).
Constitutive promoters drive gene expression independently of external stimuli leading to accumulation of high levels of toxic protein at all stages of development. This has been shown to be responsible for abnormalities in development in transgenic cotton (Rawat et al., 2011) and tomato plants (Koul et al., 2014, 2015) expressing the Bt toxins and their variants. The possible lethality to plants expressing high levels of the toxin under the CaMV35S promoter invariably leads to selection of plants expressing the Bt toxin at lesser amounts than is desirable for effective protection against insect attack (Rawat et al., 2011). In the present study, no transgenic plants expressing cryIAc under the CaMV35S promoter could be raised in spite of screening four times as many plants as for the RbPCD1 promoter. Strong constitutive expression of a toxic protein, even in the absence of its need, creates a high metabolic load on plants. This can lead to diversion of essential resources towards toxin production and affect other processes – developmental as well as adaptive. For instance, one of the first responses upon abiotic and biotic stress signalling is to reduce normal growth processes and divert resources towards fighting the stress. The opposite actions of stress hormones like JA/SA/ethylene/ABA versus growth hormones like auxin/GA often leads to either suppression of growth pathways during stress or of stress pathways during growth (Groszmann et al., 2015; Machado et al., 2013; Pandey et al., 2017). In such conditions, the presence of a strong constitutive promoter like CaMV35S that continuously synthesizes an insecticidal toxin protein regardless of a plants’ needs, may compromise defense or abiotic stress responses by reducing the resources available for fighting these, thereby increasing susceptibility to the stresses. In the absence of the stress, it may lead to a yield drag (Bano‐Maqbool et al., 1998; Breitler et al., 2004; Kim et al., 2008; Xia et al., 2010). In contrast, a wound‐inducible promoter would remain silent during normal developmental processes and during responses to any stress (other than that which causes wounding) and would thus not interfere with these responses. This is borne out from the present studies through expression of RbPCD1pro::cryIAc in two different transgenic plants, Arabidopsis and tomato, where expression of the CryIAc toxin was dependent on wounding. The base level of CryIAc protein in transgenic plants was less than a fifth of the best CaMV35S plants while induced levels of the CryIAc protein were 5–80 times higher than the CaMV35S lines per unit area and more than 300–1600 times higher than the unwounded background depending on the time. The time period of activation was early enough to prevent insect damage on leaves beyond the first few nibbles by small larvae and strong enough to produce the toxic Bt protein at levels that caused 100% mortality even up to third instar larvae within that period. Interestingly, even the normally voracious fourth instar larvae failed to eat the leaves and remained motionless. This indicates that the amount of protein produced upon wounding was toxic even to the much larger fourth instar larvae. Importantly, the expression of toxic gene under this promoter allowed plant growth without any abnormality at any stage of development in Arabidopsis as well as tomato unlike previous reports.
In conclusion, these studies demonstrate the efficacy of RbPCD1pro as one of the earliest acting wound‐inducible promoters in plants with widespread wound‐inducible expression in different aerial tissues and potential applicability across several dicot and monocot families compared to commonly used constitutive promoters.
Experimental procedures
The wound‐inducible RbPCD1 promoter was isolated from Rosa bourboniana during an abscission‐related study. Arabidopsis thaliana, ecotype Columbia (Col‐0) and tomato (Solanum lycopersicon var. Pusa Early Dwarf) were used for validation of the efficacy of wound‐inducible promoter. All the plants were grown in culture room/glasshouse at ~23°C with a 16/8 h light/dark period, at ~78% relative humidity.
Isolation of RbPCD1 promoter
The RbPCD1 promoter (sequence provided in Patent No 3866/DEL/2014 filed and WO2016103279A1) was isolated from a rose genome walking library as a 523 nt region upstream of the RbPCD1 translation initiation codon, identified during a petal abscission study (Tripathi, 2006). It was cloned in pBI101 and used for GUS expression studies (Figure S2A) in Arabidopsis and other plants.
Real‐time PCR to study wound‐induced GUS expression was carried out with primers GUSF‐SQ (AAAGGTTGGGCAGGCCAGCG) and GUSR‐SQ (GGCGTATAGCCGCCCTGATG C) on an ABI Prism 7000 machine (Applied Biosystems, Foster City, CA) using Power‐up SYBR Green mastermix in three biological and technical replicates. The analysed data were the mean of biological and technical triplicates. Relative gene expression was calculated using the method (Livak and Schmittgen, 2001) and normalized against AtUBIQUITIN10 amplified with the primers AtUbiq10‐F (GGCCTTGTATAATCCCTGATGAATAAG) and AtUbiq10‐R (AAAGAGATAACAGGAACGGAAACATAG).
Wounding and agro‐infiltration of plants
Transgenic plant leaves were wounded by puncturing with forceps (wound area 1 mm2) and kept on the plant for 5 and 20 min followed by color development as described (Gattolin et al., 2006). Cycloheximide (1.8 mm) was included in the color development reaction as a protein translation inhibitor to ensure that the GUS protein was synthesized only during the 5–20 min time course of wounding and not later during the development of color. For JA (100 μm) and SA (2 mm) treatments, excised leaves of 30‐day‐old transgenic plants were incubated with the hormones or mock (water or 0.1% ethanol) for 3 h before color development.
Agro‐inoculation of plants such as rose, cotton, tobacco, and gladiolus was carried out as described by Tripathi et al. (2009). Thereafter, tissues were kept on the plant for 2 days and analysed for GUS expression after wounding. Agrobacterial suspension containing pBI101 (no promoter) and pBI121 (GUS driven by CaMV35S promoter) were injected as above and used as negative and positive controls, respectively.
Generation of the RbPCD1pro::cry1Ac fusion construct
To study the ability of the RbPCD1 promoter to drive cry1Ac expression in a wound‐specific manner, the 523 nt region of RbPCD1pro was amplified using the primer PCDpro‐WlcryF (GGATCCTAACCGCTAGGCAGTGAGC) in combination with PCDpro‐WIcryR (AAGCT TCTTCTTCTCTGTTACCTGAAA). Amplified fragments were cloned in pTZ57R/T, sequence confirmed and the fragment excised from the vector using HindIII. The excised RbPCD1pro was used to replace the CaMV35S promoter from its source vector in the expression cassette 35Spro::cry1Ac (Koul et al., 2015) to obtain RbPCD1pro::cry1Ac (Figure S2B). The vectors carrying cryIAc under the wound‐inducible RbPCD1 and the CaMV35S promoters were introduced into Agrobacterium GV3101 by the freeze‐thaw method (Hofgen and Willmitzer, 1988). Arabidopsis was transformed with these constructs by the floral dip method (Clough and Bent, 1998) and tomato via Agrobacterium‐mediated transformation (McCormick et al., 1986). Transformants, selected on 50 μg/mL kanamycin, were confirmed by PCR. Progeny of at least three independent lines each of transgenic Arabidopsis, (AtPCDCry 3‐3‐1, 4‐2‐1, and 6‐1‐2, T3 generation) and tomato, (SlPCDCry 3, SlPCDCry 9, and SlPCDCry10, T1 generation) were studied in detail.
Southern analysis of transgenic plants
DNA from transgenic Arabidopsis and tomato plants expressing cryIAc under the CaMV35S and RbPCD1 promoters was isolated as described (Asha et al., 2007). About 25–30 μg DNA was restriction‐digested with HindIII, electrophoresed on a 0.8% TAE‐agarose gel and transferred to a Hybond‐N nylon membrane by vacuum transfer on a Vacugene blot (Pharmacia) as described (Sambrook et al., 1989). A 3.3 kb region of the cryIAc gene encompassing the regions between 1–1305, 1305–2509, and 2381–3342 nt in three fragments was radio‐labelled by PCR with 32PdCTP and used as a probe. Hybridization was performed as described (Sane et al., 1994) and the membrane exposed to X‐ray film for 3–7 days as per intensity of signal.
Insect bioassay
The efficacy of transgenic Arabidopsis and tomato plants expressing RbPCD1pro::cry1Ac was checked against different larval stages of Helicoverpa armigera by no choice insect bioassays using detached leaves and whole plants of 45‐day‐old transgenic Arabidopsis (T1 and T3 generation) and 2‐month‐old tomato (T0 and T1 generation) plants. Untransformed plants were used as controls. Leaves of independent transgenic lines were placed in boxes with a moist blotting paper and 10 larvae each of first or second instar placed on these. Mouths of boxes were covered with moist muslin cloth to maintain humidity and kept under 16/8 h light/dark regime at 25°C. Each experiment (repeated thrice) contained five replicates of leaves of independently grown transgenic lines.
Whole plant insect assay was performed independently with third and fourth instar larvae (five plants/line). Five larvae were released on the leaves of control and transgenic plants and enclosed within a plastic bag. Insects were allowed to feed on plants for four (Arabidopsis) or 5 days (tomato) by which time control plants were damaged considerably. Percent mortality, calculated as the number of dead insects/total number of insects ×100, was evaluated thereafter. Surviving larvae were collected and differences in weight compared to those fed on un‐transformed Arabidopsis/tomato plants recorded. All experiments were repeated thrice.
For studies with the sucking pest, transgenic Arabidopsis plants expressing RbPCD1pro‐GUS were exposed to insects of Myzus persicae, collected from a culture room with Arabidopsis plants showing chance infection with these insects. About 8–10 insects were allowed to feed on leaves of the transgenic plants. After 2 days, leaves of infested plants were tested for GUS expression.
Quantitative estimation of recombinant Cry1Ac protein in plants
Intact leaves of transgenic plants were wounded rapidly with a pair of forceps (wound area of 1 mm2) and leaves left on plant for 5/20 min after wounding. Thereafter leaf discs encompassing the wounded region were cut using a chopper (1.5 cm diameter) and immediately frozen in liquid N2 along with control (unwounded) leaf discs separately. Protein was isolated from the discs, homogenized in ice‐cold protein extraction buffer (100 mm Tris‐HCl, pH 8.0, 500 mm NaCl, 5 mm DTT, 2 mm PMSF, and 5 mm EDTA) and centrifuged at 12 000 g (10 min, 4°C). Total soluble protein (TSP) was determined as per Lowry et al. (1951). Quantitative estimation of Cry1Ac protein was performed using double antibody sandwich enzyme‐linked immunosorbent assay (DAS‐ELISA) on an antibody‐coated 96‐well microtiter plate (Peroxidase label, Agdia, Elkhart, IN) with the kit‐provided Cry1Ac from Bacillus thuringinesis azawaii as positive control and cell free extract of nontransformed plant as negative control. For quantification, a standard curve of varying amounts of quantified CryIAc protein (expressed from the source cDNA and obtained from Dr PK Singh, CSIR‐NBRI) was prepared. Quantification of the plant‐expressed protein from RbPCD1pro::cryIAc or CaMV35Spro::cryIAc plants was carried out against this reference. The Cry1Ac protein produced per wound (1 mm2) under RbPCD1pro was estimated by subtracting the background of CryIAc under unwounded conditions in the leaf disc. This was compared with protein produced under the constitutive CaMV35Spro within the disc (177 mm2 area) calculated as below:
Amount of CryIAc in unwounded transgenic leaf disc (177 mm2) = x ng
Background level of CryIAc expressed in RbPCD1pro::cryIAc plant/mm2 = x/177
Amount of CryIAc in transgenic leaf disc with 2 wounds (1 mm2 each) = y ng
Amount of CryIAc per wound (per mm2) = (y−x)/2
Amount of CryIAc in a CaMV35Spro::cryIAc leaf disc = z ng
Amount of CryIAc expressed in CaMV35Spro::cryIAc plant/mm2 = z/177
Statistical analysis
The data were statistically analysed by applying One way‐ ANOVA (P < 0.05) and means were compared using Duncan's Multiple Range Test (DMRT) by using SPSS software.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Figure S1 Wound‐induced activation of RbPCD1pro::GUS in different plants.
Figure S2a Schematic representation of the RbPCD1pro::GUS expression cassette in pBI101 backbone used for plant transformation.
Figure S2b Schematic representation of the RbPCD1pro::cry1Ac expression cassette in pBI101 backbone used for plant transformation.
Figure S3 Growth phenotypes of transgenic Arabidopsis plants expressing cryIAc under the RbPCD1 promoter.
Acknowledgements
We thank Dr PK Singh (Dept of Genetics and Plant Molecular Biology, CSIR‐NBRI) for the gift of the cryIAc gene and protein and Dr Priya Singh for help with the studies. We also thank Mr SMH Abidi and Mr Rakesh Srivastava for rearing the insects and help with insect bioassays and Mr Ram Awadh for care of the transgenic tomato plants. The Council of Scientific and Industrial Research (CSIR), New Delhi, Govt of India funded the work under the projects NWP‐03 and BSC0107 and supported SPP and APS with Senior Research Fellowships.
References
- Asha, Sane, V.A. , Sane, A.P. and Nath, P. (2007) Multiple forms of banana α‐expansin genes express during fruit ripening and development. Postharvest Biol. Technol. 45, 184–192. [Google Scholar]
- Bano‐Maqbool, S. , Husnain, T. , Riazuddin, S. , Masson, L. and Christou, P. (1998) Effective control of yellow stem borer and rice leaf folder in transgenic rice indica varieties Basmati 370 and M7 using the novel‐endotoxin cry2A Bacillus thuringiensis gene. Mol. Breed. 4, 501–507. [Google Scholar]
- Barton, K.A. , Whiteley, H.R. and Yang, N.S. (1987) Bacillus thuringiensis δ‐endotoxin expressed in transgenic Nicotiana tabaccum provides resistance to lepidopteran insects. Plant Physiol. 85, 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitler, J.C. , Cordero, M.J. , Royer, M. , Meynard, D. , San Segundo, B. and Guiderdoni, E. (2001) The −689/+197 region of the maize protease inhibitor gene directs high level, wound inducible expression of the cry1B gene which protects transgenic rice plants from stemborer attack. Mol. Breed. 7, 259–274. [Google Scholar]
- Breitler, J.C. , Vassal, J.M. , Catala, M.D.M. , Meynard, D. , Marfa, V. , Mele, E. , Royer, M. et al. (2004) Bt rice harbouring cry genes controlled by a constitutive or wound‐inducible promoter: protection and transgene expression under Mediterranean field conditions. Plant Biotech. J. 2, 417–430. [DOI] [PubMed] [Google Scholar]
- Cao, J. , Zhao, J.Z. , Tang, J.D. , Shelton, A.M. and Earle, E.D. (2002) Broccoli plants with pyramided cry1Ac and cry1C Bt genes control diamondback moths resistant to Cry1A and Cry1C proteins. Theor. Appl. Genet. 105, 258–264. [DOI] [PubMed] [Google Scholar]
- Chen, H.C. , Klein, A. , Xiang, M. , Ralph, A.B. and Marcel, K. (1998) Drought and wound‐induced expression in leaves of a gene encoding a chromoplast carotenoid‐associated protein. Plant J. 14, 317–326. [Google Scholar]
- Christensen, A.H. , Sharrock, R.A. and Quail, P.H. (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol. 18, 675–689. [DOI] [PubMed] [Google Scholar]
- Clough, S.J. and Bent, A.F. (1998) Floral dip: a simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana . Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Cordero, M.J. , Raventos, D. and San Segundo, B. (1994) Expression of a maize proteinase inhibitor is induced in response to wounding and fungal infection: systemic wound‐response of a monocot gene. Plant J. 6, 141–150. [DOI] [PubMed] [Google Scholar]
- De Cosa, B. , Moar, W. , Lee, S.B. , Miller, M. and Daniell, H. (2001) Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat. Biotechnol. 19, 71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehn, S.H. , De Rocher, E.J. and Green, P.J. (1996) Problems that can limit the expression of foreign genes in plants: lessons to be learned from B.t. toxin genes. In Genetic Engineering ( Setlow, J.K. , ed.), Vol 18, Boston: Springer. [DOI] [PubMed] [Google Scholar]
- Erb, M. , Meldau, S. and Howe, G.A. (2012) Role of phytohormones in insect‐specific plant reactions. Trends Plant Sci. 17, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattolin, S. , Alandete‐Saez, M. , Elliott, K. , Gonzalez‐Carranza, Z. , Naomab, E. , Powell, C. and Roberts, J.A. (2006) Spatial and temporal expression of response regulatorARR22 and ARR24 in Arabidopsis thaliana . J. Exp. Bot. 57, 4225–4233. [DOI] [PubMed] [Google Scholar]
- Girijashankar, V. , Sharma, H.C. , Sharma, K.K. , Swathisree, V. , Prasad, L.S. , Bhat, B.V. , Royer, M. et al. (2005) Development of transgenic sorghum for insect resistance against the spotted stem borer (Chilo partellus). Plant Cell Rep. 24, 513–522. [DOI] [PubMed] [Google Scholar]
- Groszmann, M. , Gonzalez‐Bayon, R. , Lyons, R.L. , Greaves, I.K. , Kazan, K. , Peacock, W.J. and Dennis, E.S. (2015) Hormone‐regulated defense and stress response networks contribute to heterosis in Arabidopsis F1 hybrids. Proc. Natl Acad. Sci. USA, 112, E6397–E6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbitti‐Onarici, S. , Zaidi, A.M. , Taga, I. , Ozcan, S. and Altosaar, I. (2009) Expression of Cry1Ac in transgenic tobacco plants under the control of a wound‐inducible promoter (AoPR1) isolated from Asparagus officinalis to control Heliothis virescens and Manduca sexta . Mol. Biotechnol. 42, 341–349. [DOI] [PubMed] [Google Scholar]
- Gurr, S. and Rushton, P. (2005) Engineering plants with increased disease resistance: how are we going to express it? Trends Biotechnol. 23, 283–290. [DOI] [PubMed] [Google Scholar]
- Hofgen, R. and Willmitzer, L. (1988) Storage for competent cells for Agrobacterium tumefaciens . Nucleic Acids Res. 16, 9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollick, J.B. and Gordon, M.P. (1995) Transgenic analysis of a hybrid poplar wound‐inducible promoter reveals developmental patterns of expression similar to that of storage protein genes. Plant Physiol. 109, 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.J. , Kim, C.J. , Li, W.N. , Kim, T.Y. , Li, Y.S. , Zaidi, M.A. and Altosaar, I. (2008) Inheritance and field performance of transgenic Korean Bt rice lines resistant to rice yellow stem borer. Euphytica, 164, 829–839. [Google Scholar]
- Koornneef, A. , Leon‐Reyes, A. , Ritsema, T. , Verhage, A. , Den Otter, F.C. , Van Loon, L.C. and Pieterse, C.M.J. (2008) Kinetics of salicylate‐mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol. 147, 1358–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota, M. , Daniell, H. , Varma, S. , Garczynski, S.F. , Gould, F. and Moar, W.J. (1999) Overexpression of the Bacillus thuringiensis (Bt) Cry2Aa2 protein in chloroplasts confers resistance to plants against susceptible and Bt‐resistant insects. Proc. Natl Acad. Sci. USA, 96, 1840–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul, B. , Srivastava, S. , Sanyal, I. , Tripathi, B.N. , Sharma, V. and Amla, D.V. (2014) Transgenic tomato line expressing modified Bacillus thuringiensis cry1Ab gene showing complete resistance to two lepidopteran pests. SpringerPlus, 3, 84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul, B. , Yadav, R. , Sanyal, I. and Amla, D.V. (2015) Comparative performance of modified full‐length and truncated Bacillus thuringiensis‐cry1Ac genes in transgenic tomato. SpringerPlus, 4, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranthi, K.R. , Naidu, S. , Dhawad, C.S. , Tatwawadi, A. , Mate, K. , Patil, E. , Bharose, A.A. et al. (2005) Temporal and intra‐plant variability of Cry1Ac expression in Bt‐cotton and its influence on the survival of the cotton bollworm, Helicoverpa armigera (Hübner) (Noctuidae: Lepidoptera). Curr. Sci. 89, 291–298. [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2‐ΔΔCt method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lowry, O.H. , Rosebrough, N.J. , Farr, A.L. and Randall, R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- Machado, R.A.R. , Ferrieri, A.P. , Robert, C.A.M. , Glauser, G. , Kallenbach, M. , Baldwin, I.T. and Erb, M. (2013) Leaf‐herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signalling. New Phytol. 200, 1234–1246. [DOI] [PubMed] [Google Scholar]
- McCormick, S. , Niedermeyer, J. , Fry, J. , Barnason, A. , Horsch, R. and Fraley, R. (1986) Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens . Plant Cell Rep. 5, 81–84. [DOI] [PubMed] [Google Scholar]
- Nayak, P. , Basu, D. , Das, S. , Basu, A. , Ghosh, D. , Ramakrishnan, N.A. , Ghosh, M. et al. (1997) Transgenic elite indica rice plants expressing CryIAc δ endotoxin of Bacillus thuringiensis are resistant against yellow stem borer (Scirophaga incertulas). Proc. Natl Acad. Sci. USA, 94, 2111–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, S.P. , Srivastava, S. , Goel, R. , Lakhwani, D. , Singh, P. , Asif, M.H. and Sane, A.P. (2017) Simulated herbivory in chickpea causes rapid changes in defense pathways and hormonal transcription networks of JA/ethylene/GA/auxin within minutes of wounding. Sci. Rep. 7, 44729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S.H. , Lim, H. , Hyun, S.J. , Yun, D.W. , Yoon, U.H. , Ji, H. , Kim, T. et al. (2014) Wound‐inducible expression of the OsDof1 gene promoter in a Ds insertion mutant and transgenic plants. Plant Biotechnol. Rep. 8, 305–313. [Google Scholar]
- Perera, M.R. and Jones, M.G.K. (2004) Expression of the peroxidase gene promoter (Shpx6b) from Stylosanthes humilis in transgenic plants during insect attack. Entomol. Exp. Appl. 111, 165–171. [Google Scholar]
- Rawat, P. , Singh, A.K. , Ray, K. , Chaudhary, B. , Kumar, S. , Gautam, T. , Kanoria, S. , et al. (2011) Detrimental effect of expression of Bt endotoxin Cry1Ac on in vitro regeneration, in vivo growth and development of tobacco and cotton transgenics. J. Biosci. 36, 363‐376. [DOI] [PubMed] [Google Scholar]
- Rocher, E.J.D. , Vargo‐Gogola, T.C. , Diehn, S.H. and Green, P.J. (1998) Direct evidence for rapid degradation of Bacillus thuringiensis toxin mRNA as a cause of poor expression in plants. Plant Physiol. 117, 1445–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs, E.S. , Benedic, J.H. , Stelly, D.M. , Taylor, J.F. , Altman, D.W. , Berberich, S.A. and Davis, S.K. (1998) Expression and segregation of genes encoding Cry1Ac insecticidal proteins in cotton. Crop Sci. 38, 1–11. [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd ed. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Sane, A.P. , Nath, P. and Sane, P.V. (1994) Mitochondrial ATP synthase genes may be implicated in cytoplasmic male sterility in Sorghum bicolor. J. Biosci. 19, 43–55. [Google Scholar]
- Singh, A.P. (2011) Functional studies of genes and promoters involved in petal abscission in rose (R. bourboniana). Thesis submitted to the Department of Biochemistry, University of Lucknow, India. [Google Scholar]
- Tang, W. , Chen, H. , Xu, C. , Li, X. , Lin, Y. and Zhang, Q. (2006) Development of insect‐resistant transgenic indica rice with a synthetic cry1C* gene. Mol. Breeding, 18, 1–10. [Google Scholar]
- Tripathi, S.K. (2006) Isolation and characterization of petal abscission related genes in rose. Thesis submitted to the Department of Botany, University of Lucknow, India. [Google Scholar]
- Tripathi, S.K. , Singh, A.P. , Sane, A.P. and Nath, P. (2009) Transcriptional activation of a 37 kDa ethylene responsive cysteine protease gene, RbCP1, is associated with protein degradation during petal abscission in rose. J. Exp. Bot. 60, 2035–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, J. , Zhang, G. , Datta, K. , Xu, C. , He, Y. , Zhang, Q. , Khush, G.S. et al. (2000) Field performance of transgenic elite commercial hybrid rice expressing Bacillus thuringiensis δ‐endotoxin. Nat. Biotechnol. 18, 1101–1104. [DOI] [PubMed] [Google Scholar]
- Warner, S.A. , Scott, R. and Draper, J. (1993) Isolation of an Asparagus intracellular PR gene (AoPR1) wound‐responsive promoter by the inverse polymerase chain reaction and its characterization in transgenic tobacco. Plant J. 3, 191–201. [DOI] [PubMed] [Google Scholar]
- Wilmink, A. , van de Ven, B.C. and Dons, J.J. (1995) Activity of constitutive promoters in various species from the Liliaceae. Plant Mol. Biol. 28, 949–955. [DOI] [PubMed] [Google Scholar]
- Wu, G. , Cui, H. , Ye, G. , Xia, Y. , Sardana, R. , Cheng, X. , Li, Y. et al. (2002) Inheritance and expression of the cry1Ab gene in Bt (Bacillus thuringiensis) transgenic rice. Theor. Appl. Genet. 104, 727–734. [DOI] [PubMed] [Google Scholar]
- Xia, H. , Chen, L. , Wang, F. and Lu, B. (2010) Yield benefit and underlying cost of insect‐resistance transgenic rice: implication in breeding and deploying transgenic crops. Field. Crop. Res. 118, 215–220. [Google Scholar]
- Ye, G.Y. , Shu, Q.Y. , Ao, H.W. , Cui, H.R. , Cheng, X.Y. , Hu, C. , Xia, Y.W. et al. (2001) Field evaluation of resistance of transgenic rice containing a synthetic cry1Ab gene from Bacillus thuringiensis Berliner to two stem borers. J. Econ. Entomol. 94, 271–276. [DOI] [PubMed] [Google Scholar]
- Ye, R. , Huang, H. , Yang, Z. , Chen, T. , Liu, L. , Li, X. , Chen, H. , et al. (2009) Development of insect‐resistant transgenic rice with Cry1C*‐free endosperm. Pest Manag Sci. 65, 1015‐1020. [DOI] [PubMed] [Google Scholar]
- Yevtushenko, D.P. , Sidorov, V.A. , Romero, R. , Kay, W.W. and Misra, S. (2004) Wound‐inducible promoter from poplar is responsive to fungal infection in transgenic potato. Plant Sci. 167, 715–724. [Google Scholar]
- Zhang, S. , Lian, Y. , Liu, Y. , Wang, X. , Liu, Y. and Wang, G. (2013) Characterization of a maize Wip1 promoter in transgenic plants. Int. J. Mol. Sci. 14, 23872–23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q. , Liu, M. , Tan, M. , Gao, J. and Shen, Z. (2014) Expression of Cry1Ab and Cry2Ab by a polycistronic transgene with a self‐cleavage peptide in rice. PLoS ONE, 9(10), e110006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Wound‐induced activation of RbPCD1pro::GUS in different plants.
Figure S2a Schematic representation of the RbPCD1pro::GUS expression cassette in pBI101 backbone used for plant transformation.
Figure S2b Schematic representation of the RbPCD1pro::cry1Ac expression cassette in pBI101 backbone used for plant transformation.
Figure S3 Growth phenotypes of transgenic Arabidopsis plants expressing cryIAc under the RbPCD1 promoter.


