Abstract
This study addresses the binding of ions and the permeation of substrates during function of the GABA transporter GAT1. GAT1 was expressed in Xenopus oocytes and studied electrophysiologically as well as with [3H]GABA flux; GAT1 was also expressed in mammalian cells and studied with [3H]GABA and [3H]tiagabine binding. Voltage jumps, Na+ and Cl− concentration jumps, and exposure to high-affinity blockers (NO-05-711 and SKF-100330A) all produce capacitive charge movements. Occlusive interactions among these three types of perturbations show that they all measure the same population of charges. The concentration dependences of the charge movements reveal (1) that two Na+ ions interact with the transporter even in the absence of GABA, and (2) that Cl−facilitates the binding of Na+. Comparison between the charge movements and the transport-associated current shows that this initial Na+-transporter interaction limits the overall transport rate when [GABA] is saturating. However, two classes of manipulation—treatment with high-affinity uptake blockers and the W68L mutation—“lock” Na+ onto the transporter by slowing or preventing the subsequent events that release the substrates to the intracellular medium. The Na+ substitutes Li+and Cs+ do not support charge movements, but they can permeate the transporter in an uncoupled manner. Our results (1) support the hypothesis that efficient removal of synaptic transmitter by the GABA transporter GAT1 depends on the previous binding of Na+ and Cl−, and (2) indicate the important role of the conserved putative transmembrane domain 1 in interactions with the permeant substrates.
Keywords: sodium, chloride, GAT1, GABA, transporter, Xenopus oocyte
This paper addresses the mechanism of GABA transport by GAT1 (Guastella et al., 1990) and the implications for efficient synaptic transmission. Within a few milliseconds after release, the transmitter is removed from the extracellular space. As a result, receptors begin to deactivate and transmitter cannot diffuse to neighboring synapses (Isaacson et al., 1993; Lester et al., 1994). The highly efficient enzyme acetylcholinesterase removes the transmitter at nicotinic synapses, but at all other chemical synapses, ion-coupled transporters play this role despite turnover rates approximately three orders of magnitude lower than that of acetylcholinesterase and slower than the decay rate of the synaptic event itself (Lester et al., 1994;Wadiche et al., 1995).
The transport process is driven by the electrochemical potential of Na+ and Cl− (Kanner and Schuldiner, 1987;Lester et al., 1994): two Na+ ions and one Cl−ion are transported for each GABA molecule. The cotransported ions must bind to the transporter at some step during the cycle. Previous experiments suggest that the ions can bind in the absence of GABA but, interestingly, the interaction between Na+ and the transporter requires several hundred milliseconds—many orders of magnitude longer than required for Na+ permeation through an ion channel (Mager et al., 1993; Cammack et al., 1994). The present study attempts to understand the molecular nature of this interaction. We address the specific suggestion that the binding of Na+and Cl− converts the transporter to a state with higher affinity for GABA (tens of μm). This process allows the transporter to initiate the steps leading to release of GABA into the cytoplasm. Although we do not directly address the functional importance of these mechanistic findings for synaptic transmission, we extend earlier suggestions that a high-affinity binding state would allow the transmitter to sequester the GABA that appears in the synaptic cleft, preventing further receptor activation (Cammack et al., 1994; Tong and Jahr, 1994; Wadiche et al., 1995).
We have studied the transporter with electrophysiological measurements that illuminate individual steps in the transport cycle. Although one cannot yet resolve the binding of one or two ions to a single transporter protein, synchronized binding to many transporters generates a measurable current. We synchronize the binding process by rapidly changing the concentration of [Na+] or [Cl−], the concentration of a high-affinity blocker, or the membrane potential (Mager et al., 1993; Galli et al., 1995; Wadiche et al., 1995). We correlate our data with steady-state measurements of [3H]GABA uptake and of the currents associated with the entire transport cycle. We also study the poorly functional mu- tant W68L.
Classical results show that only Na+ among monovalent cations supports GABA flux (Kanner and Schuldiner, 1987; Lester et al., 1994). Yet experience with other cation-binding proteins, such as ion channels, leads one to expect that other monovalent cations would bind, although presumably not as well as Na+ or with different consequences. We find that Cs+ and Li+ permeate through the transporter. However, in contrast to transport with Na+, these monovalent cations do not pause while awaiting GABA; they permeate in its absence. This uncoupled flux of Cs+ or Li+ again emphasizes the unique nature of the Na+–transporter interaction that prepares the transporter to accept GABA.
MATERIALS AND METHODS
Oocytes. Wild-type (WT) GAT1 and W68L (Kleinberger-Doron and Kanner, 1994) were transferred to pAMV-PA (Nowak et al., 1995), which is optimized for oocyte expression. RNA was synthesized in vitro with T7 polymerase and injected into stage VI Xenopus oocytes (Quick and Lester, 1994). For rapid solution changes, oocytes were held by pins in a chamber (volume, 100 μl) the volume of which was changed with a time constant of 700 msec under the control of electrically operated valves and studied with a two-electrode voltage-clamp circuit (Mager et al., 1993). We found that oocytes expressing both WT and W68L GAT1 had increased linear capacitive transients (time constant < 7 msec), presumably because of increased membrane area, as observed for the β2 subunit of voltage-gated Na+ channels (Isom et al., 1995). These transients have not been included in our analyses. Normal Ringer solution contains 96 mm NaCl, 2 mm KCl, 1 mm MgCl2, and 5 mm HEPES. Equimolar substitutions with other ions were made as described in the text.
GABA uptake in oocytes was measured by a 1 min incubation in 200 μl of Ringer solution containing 100 μm GABA. Oocytes were washed, dissolved in 1% SDS, and counted using liquid scintillation spectrometry.
Mammalian cells. Cultured HeLa cells (Keynan et al., 1992) were infected with recombinant vaccinia virus vTF7-3 (Fuerst et al., 1986), which encodes the T7 RNA polymerase, and subsequently transfected with pT7-GAT1 or the mutated transporter W68L (Kleinberger-Doron and Kanner, 1994). [3H]GABA uptake was measured in 35 mm dishes by a 10 min incubation in 42 nmGABA (Keynan et al., 1992), followed by a wash with 2 ml of Ringer solution.
For binding of [3H]tiagabine, cells were scraped from six 35 mm wells in 2.0 ml of ice-cold 150 mm LiCl containing 10 mm HEPES-Tris, pH 6; all further steps of the membrane preparation were done at 4°C. A few grains of DNase were added, and the cell suspension was disrupted using a Polytron homogenizer with two exposures for 15 sec at setting 5. Cell extracts were pelleted and resuspended in 9 ml of 1 m LiCl or NaCl containing 10 mm Tris-HEPES, pH 6.8. Ten microliters of a 10% ethanol solution containing 2.5 pmol of [H3]tiagabine (39.8 Ci/mmol, gift of Dr. Peter Suzdak, Novo Nordisk) were added to 190 μl of membrane preparation containing 40–50 μg of protein. After 30 min at 30°C, the reactions were terminated on 1 ml spin columns containing Sephadex-G-50-80 (Pharmacia, Uppsala, Sweden) pre-swollen with 1 m LiCl containing 10 mm HEPES-Tris, pH 6.8 (Radian and Kanner, 1985). The recovered radioactivity was counted using liquid scintillation spectrometry. In the presence of Na+, membranes from cells expressing the WT transporter attained equilibrium [3H]tiagabine binding over a time course of several minutes, at a level of ∼1 pmol/mg protein. In the absence of Na+, ligand binding was lower (∼0.25 pmol/mg protein), reached equilibrium in <10 sec, and resembled levels for membranes prepared from HeLa cells transfected with the vector pBluescript (without the GAT1 cDNA insert) alone, regardless of the presence of Na+. The Na+-independent binding, therefore, represents nonspecific binding to the membranes and was been subtracted from the value obtained in the presence of Na+. Excess unlabeled GABA (10 mm) reduced Na+-dependent tiagabine binding in the wild-type and the W68L mutant to the nonspecific levels.
RESULTS
Charge movements linked to Na+ binding
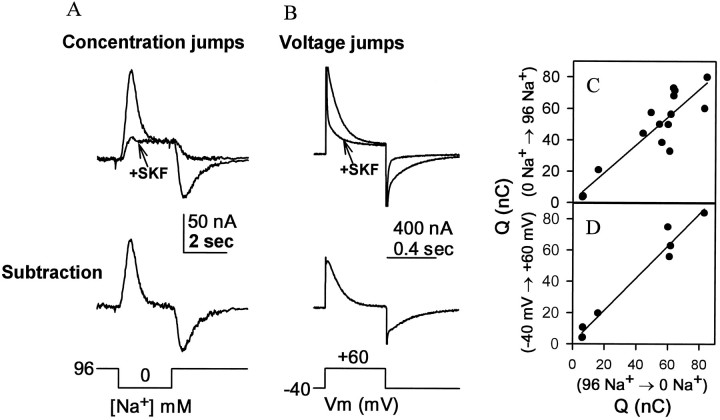
The GABA transporter GAT1 was expressed in Xenopusoocytes by cytoplasmic injection of cRNA. In the experiment shown in Figure 1A, an oocyte expressing GAT1 generated a transient outward current when the bathing solution with normal Na+ (96 mm) was switched to one containing no Na+, replaced byN-methyl-d-glucamine (NMDG). A similar but inward transient current developed after re-addition of Na+. These transient currents were absent in the presence of the GABA uptake blocker SKF-100330A, as shown in Figure1A, which allows a subtraction procedure that isolates the transient currents (Fig. 1A,bottom panel). The transient currents were also absent in noninjected or water-injected oocytes (data not shown).
Fig. 1.
Na+ concentration jumps and voltage jumps in a single oocyte expressing GAT1. A, The membrane potential was held at −40 mV during perfusion with an NaCl Ringer solution. At the indicated times, the perfusion was changed to NMDG-Cl Ringer solution and returned to NaCl Ringer solution.B, After an additional 5 sec, the membrane potential was stepped to +60 mV and back to −40 mV. The oocyte was then perfused with a Na+ Ringer solution containing 30 μmSKF-100330A, and the protocol was repeated. The records in the presence of SKF-100330A have been subtracted from those in the absence of the drug so as to isolate GAT1 currents. C, Comparison of charge movements for the Na+ concentration jumps (96→0 vs 0→96 mm Na+) at a holding potential of −40 mV. D, Charge movements during concentration jumps (96→0 mm Na+) versus voltage jumps (−40→+60 mV).
The charge movement during the outward current is calculated as the time integral of the transient current. This charge movement was nearly equal and opposite to that during the inward current (average ratio of 0.885 for the 14 oocytes in Fig. 1C). Thus, these transient currents are capacitive, rather than resistive; they result from ion binding and dissociation rather than from ion permeation. The small deviation from a ratio of 1 may be attributable to resistive leakage currents (Cammack et al., 1994).
The interaction between ions and GAT1 can also be synchronized by jumping the membrane potential (Fig. 1B) (Loo et al., 1993; Mager et al., 1993). We compared charge movements during the concentration-jump and voltage-jump procedures for synchronizing charge movement. For both measurements, the experiment began with the membrane clamped to −40 mV in normal Na+ Ringer solution. Either a jump to zero Na+ (Fig. 1A) or a jump to very positive voltage (+60 mV in the experiment of Fig.1B) is expected to remove Na+ from the transporters. In agreement with this view, the capacitive charge movements were equal for these two types of perturbation (average ratio of 1.01 for the 7 oocytes plotted in Fig. 1D). Thus, it is likely that a common molecular event is responsible for Na+binding and release during the concentration-jump and voltage-jump procedures.
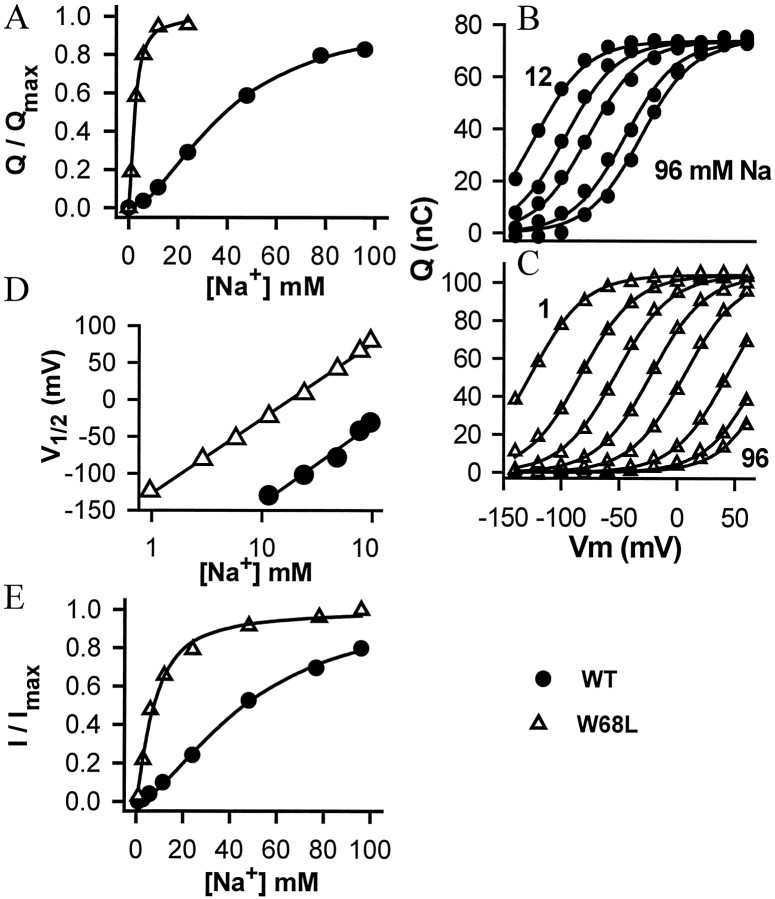
It is already thought that two Na+ ions accompany the transport of a single GABA molecule (Kanner and Schuldiner, 1987). [Na+] dependence of the concentration-jump charge movements displayed a Hill coefficient (nH) near 2 (Fig. 2A), similar to the Hill coefficient of the transport-associated current (Mager, 1993). Therefore, we conclude that the charge movement arises either from a highly cooperative binding of two Na+ ions within the membrane dielectric or from independent binding followed by a conformational change that moves charge(s) on the transporter within the membrane. More rapid Na+ concentration jumps (Cammack et al., 1994) may eventually allow a decision between these two choices, but it is already clear that even this early event in the transport cycle is much more likely to occur in the presence of two bound Na+ ions than in the presence of a single Na+ ion. We suggest that this early event is the step, or one of the steps, that prepares the transporter to accept GABA.
Fig. 2.
Effect of [Na+] and membrane potential on charge movements. A, Effect of [Na+] on concentration-jump charge movements for the WT (•) or W68L (▵). Membrane potential was held at −80 mV, and [Na+] was increased in steps. The normalized charge movement is plotted as a function of [Na+]. The original data were fit to the Hill equation with Qmaxof 60 ± 8 and 99 ± 27 nC, an EC50 of 43 ± 6 and 2.7 ± 0.5 mm, and Hill coefficientn of 1.8 ± 0.14 and 1.7 ± 0.13 for the WT (4 oocytes) and W68L (5 oocytes), respectively. B,C, Effect of membrane potential and [Na+] on the voltage-jump charge movements for oocytes expressing the WT transporter (B) and W68L mutant (C). For each oocyte, charge movement was measured during voltage jumps from a holding potential of −40 mV to various voltages between −140 and +60 mV in various [Na+]. Charge movements were fit to a Boltzmann distribution and shifted vertically so that the midpoints of the curves at varying [Na+] lay on the same horizontal line. [Na+] was 12, 24, 48, 77, and 96 mm inB and 1, 3, 6, 12, 24, 48, 77, and 96 mm inC. D, Fitted values forV1/2 are plotted versus [Na+]. Data points are averaged from 3 oocytes for each transporter (SEM are smaller than the size of the symbols). Lines are linear regression, corresponding to V1/2 changes of 106 ± 5 and 101 ± 3 mV for the WT (•) and W68L (▵), respectively, for a 10-fold change in [Na+]. E, Dose–response relations for the transport-associated current at −80 mV. The data were fit to the Hill equation as follows. For the WT, EC50 = 44 ± 1.8 mm,nH = 1.8 ± 0.15; for the mutant, EC50 = 6.76 ± 1.5 mm,nH = 1.63 ± 0.07 (300 μmand 3 mm GABA for WT and W68L, respectively; 3 oocytes tested for each transporter).
The W68L mutant: charge movements
The mutant W68L displays poor transport function (Kleinberger-Doron and Kanner, 1994); our analysis of transport function for this mutant will be presented in detail below. At this point, we compare the first step in transport between W68L and WT transporters by comparing the charge movements during Na+-concentration jumps. Strikingly, W68L charge movements occur at Na+ concentrations much lower than for WT; as shown in Figure 2A, the EC50 was 2.7 mm versus 43 mm, respectively. On the other hand, [Na+] dependence of the concentration-jump charge movements displayed similar maximal charge movements (Qmax) and Hill coefficients. Thus, a major difference between WT and W68L is that the two Na+ ions bind more tightly to the latter. We have found that the W68S mutant also displays tighter Na+ binding (data not shown).
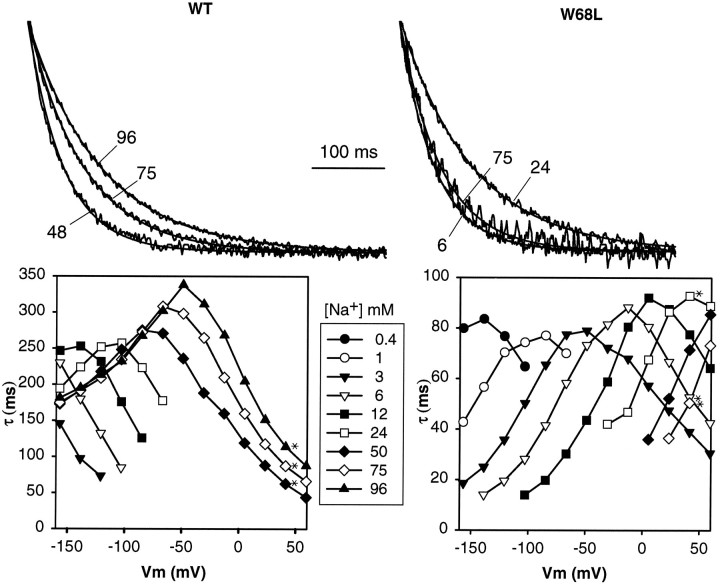
We compared the kinetics of voltage-jump charge movements for the WT and W68L transporters (Fig. 3) and found that the charge movements occur severalfold faster for the W68L transporter. This difference persists even when the kinetics are corrected for [Na+] dependence. For instance, at −140 mV charge movements were half-complete at 12 mm for the WT and at 1 mm for W68L, and the corresponding time constants were 252 ± 11 and 56 ± 5 msec, respectively (mean ± SEM,n = 9 and 4, respectively). Thus, the inefficient transport for the W68L mutant cannot be explained by an abnormally slow interaction between Na+ and the transporter; if anything, this process (or the subsequent conformational change) proceeds more quickly for the mutant.
Fig. 3.
Kinetics of voltage-jump relaxations for WT and W68L transporters. Top panels, Voltage-clamp currents for jumps from −40 to +40 mV in various [Na+] were measured ([Na+] in mm are shown for each trace); GAT1 currents were isolated by subtraction of traces in the presence of 10 μm NO-05-711. Single-exponential fits have been superimposed on the traces. Bottom panels, Voltage and [Na+] concentration of the time constants.Asterisks denote data from traces in the top panels.
Interestingly, the data show that the time constants of charge movements show a maximum as a function of membrane potential. Comparison of Figures 2 and 3 shows that these maxima occur at approximately the midpoint of the charge–voltage relation.
A formal description of the charge movements
We have measured the charge movements as they depend on both Na+ (Fig. 2A) and membrane potential (Fig. 2B,C) for both the WT and W68L transporter. At this point, it is appropriate to present a formal synthesis of these measurements. A modified Hill equation (Eq. 1) presents the effect of [Na+] and the membrane potential (V) on Na+ binding to the transporter (Fig.2B–D):
| Equation 1 |
In the above equation,Q(V,Na) represents the fraction of transporters without bound Na+, q is the elementary charge, δ is the electrical distance at which the two Na+ bind, and KNa is a zero-voltage intrinsic dissociation constant of the transporter for Na+. Detailed physical interpretations of this equation are given in Discussion. For the present, we point out the simple interpretation that the charge movements occur if and only if two Na+ ions bind simultaneously at the same electrical distance; however, basically similar formalisms would allow for the possibilities either (1) that some charge movement can occur in the absence of Na+binding or (2) that the two ions bind at different electrical distances.
The only parameter in Eq. 1 that differs significantly for WT and W68L transporters is KNa (518 and 30 mm, respectively; 3 oocytes in each case). The calculated electrical distances, δ, were 0.63 ± 0.03 and 0.67 ± 0.02 for the WT and the mutant, respectively; nH ranged from 1.66 to 1.45 (WT and W68L, respectively). Eq. 1 is also a Boltzmann relation (Mager et al., 1993) with a midpoint potential ofV1/2 = kT/qδ ln ([Na]/KNa) and slopes = kT/nHqδ. It is equivalent, therefore, to conclude that Na+ binds more tightly to the mutant at all potentials or that its binding is shifted to more positive potentials.
The W68L mutant: substrate translocation
To extend the contrast between the WT and W68L transporters, we measured GABA uptake and transport-associated current and compared these parameters with the level of transporter expression as measured either by binding of radiolabeled ligand or by charge movements. Binding of the GABA analog [3H]tiagabine was measured on membrane vesicle preparations from transfected HeLa cells and was comparable with levels with WT membranes, although [3H]tiagabine concentration dependence was not systematically studied. However, the mutant transporters were severely impaired in [3H]GABA transport when measured at 42 nm GABA (<5% of WT value). A similar picture emerged from the oocyte experiments. The number of transporters per oocyte, measured as Qmax in Eq. 1, was similar for the WT and W68L transporter (80 ± 5 vs 105 ± 6 nC, 11 and 9 oocytes, respectively). Yet oocytes expressing W68L showed very small transport-associated current (∼10% of WT at 100 μmGABA) as well as low [3H]GABA transport (3.5% of WT at 100 μm GABA).
Both the oocyte and the mammalian cell experiments thus show that W68L expresses at roughly WT levels, as measured by [3H]tiagabine binding or charge movements, but functions poorly. Measurements of transport-associated current in oocytes expressing the WT and W68L transporters provide additional functional kinetic parameters of the complete transport cycle. W68L showed a 16-fold higher EC50 for GABA compared with the WT [298 ± 17 μm (n = 4) vs 18.6 ± 0.9 μm (n = 5), respectively, −80 mV]. Dose–response relations for the effect of [Na+] on the transport-associated current (Fig. 2E) showed for the WT an EC50 of 44 ± 1.8 mm,nH = 1.8 ± 0.15 and for the mutant an EC50 of 6.76 ± 1.5 mm,nH = 1.63 ± 0.07 (300 μm and 3 mm GABA for WT and W68L, respectively; −80 mV; 3 oocytes tested for each trans- porter). Thus, the shift to a higher affinity for Na+, revealed by the concentration- and voltage jumps, is also a characteristic of the complete transport cycle.
A measure of the turnover rate is the ratio between the transport-associated current and the charge movement for an individual oocyte (Mager et al., 1993). This parameter was 15-fold lower for W68L than for the WT [0.56 ± 0.01 sec−1(n = 6) vs 8.4 ± 0.4 sec−1(n = 8), respectively, −80 mV]. Furthermore, for W68L some of this small Na+ flux is not directly coupled to GABA flux, because the ratio of total charge transported to [3H]GABA uptake (Kavanaugh et al., 1992) was 5.6 ± 0.7 versus 2.0 ± 0.1 for W68L and WT, respectively (100 μm GABA; 3 batches of oocytes; 5 oocytes per measurement). Taking these corrections into account, we calculate that the turnover rate for GABA is ∼4 sec−1 for the WT and ∼0.1 sec−1 for W68L at −80 mV and normal Ringer solution.
The tighter and faster binding of Na+, looser binding of GABA, and lower turnover rate for W68L all suggest that this mutant is blocked at a point in the transport cycle after the binding of Na+ and before that of GABA. Analysis of the W68L mutation, therefore, supports the view that Na+ binds before GABA. In this case, however, the Na+–transporter interaction does not limit the rate of transport. Because W68L binds GABA poorly, its bound Na+ remains for extended periods facing the extracellular solution or sequestered within the transporter, and the substrates are only slowly released to the intracellular side.
The Na+ dependence for charge movements and for steady-state currents are similar
According to our hypothesis, the major rate-limiting requirement for Na+ occurs before the binding of GABA. We believe that the charge movements reflect this process. As a consequence, one expects the external Na+ concentration to affect the entire transport cycle only through its effect on the charge movements. The entire transport cycle is monitored via transport-associated currents in the presence of GABA. In agreement with the hypothesis, Figure 2,A and E, shows that the charge movements and transport-associated currents for the WT transporter have nearly equal EC50 values (43 and 44 mm, respectively) and Hill coefficients (1.63 and 1.8, respectively) under conditions of saturat- ing GABA.
Although the W68L mutant displays much lower EC50 values, the values for the charge movements and transport-associated currents are still comparable with each other (6.8 and 2.7 mm, respectively). Possibly, these two values would be even closer without the complications of very small transport-associated currents, finite intracellular [Na+], and lack of GABA saturation even at concentrations of 3 mm. It is already clear, however, that the Na+–transporter interaction dominates transport under appropriate conditions for the W68L transporter as well as for the WT .
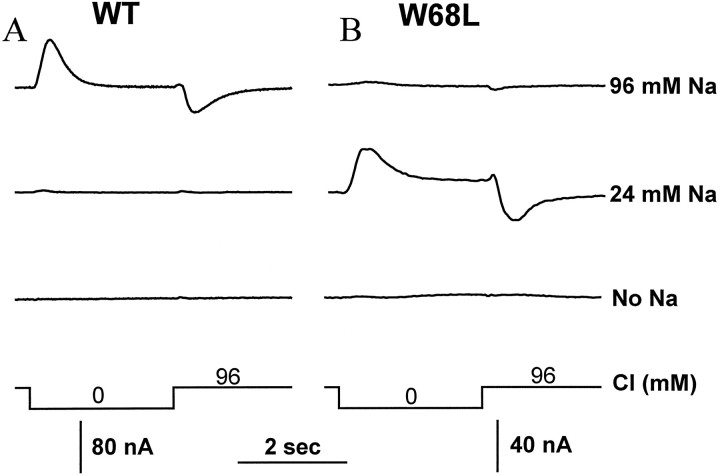
Cl− increases affinity for Na+
Cl− is required for transport by members of the GAT1 superfamily and is cotransported with Na+ and the organic substrate (Keynan and Kanner, 1988; Lester et al., 1994). For the WT transporter, in the presence of 96 mm Na+, the removal and addition of external Cl− generated outward and inward transient currents, respectively (Fig.4A,B). This is opposite to the expected direction for membrane exit and entry of an anion. In the absence of Na+, Cl−concentration jumps generated no transient currents. Evidently, Cl− facilitates Na+ binding (or the subsequent conformational change), because removal of Cl− results in at least partial reversal of this step. Charge movement during Na+-concentration jumps was also recorded in zero external Cl−. This charge movement was reduced by 66 ± 3% compared with that observed at 96 mm Cl (wild-type;n = 5; −60 mV). Thus, Cl− seems to facilitate Na+ binding to the transporter, but Cl− is not absolutely required.
Fig. 4.
Cl− concentration jumps. Membrane potential was held at −60 mV at various [Na+]; for the indicated time, Cl− in the perfusion medium was replaced by acetate. For the WT (A), the removal of Cl− in the presence of 96 mm Cl−results in an outward current, and the addition of Cl−results in an inward current. At 24 mm [Na+], only small currents developed. In the absence of Na+, Cl− concentration jumps evoked no charge movement. For the W68L mutant (B), Cl− concentration jumps were smaller in 96 mm Na+ than in 24 mm Na+. No transient current developed in the absence of Na+.
For the W68L transporter, larger transient currents developed in 24 mm Na+ than in 96 mmNa+ during Cl−-concentration jumps (Fig.4B). The W68L transporter has a high affinity for Na+; therefore, at 96 mm Na+, the transporter is fully occupied by Na+ even in the absence of Cl−. However, at lower [Na+], the decrease in affinity for Na+ attributable to the removal of Cl− affects the Na+ occupancy of the transporter.
These data show that Cl−-concentration jumps fail to generate charge movements in the absence of Na+. We therefore reexamined voltage-jump relaxations in the presence and absence of Cl−. Omitting Cl− produces shifts along the voltage axis in the Q versus V relation (Mager et al., 1993), as though KNa changes in Eq. 1, but Qmax changed by <3% for both wild-type (n = 6, 120 mm Na+) and W68L (n = 9, 16 mm Na+). This result may arise because Cl− cannot bind in the absence of Na+ and/or because the charge movements do not involve a movement of Cl− within the membrane dielectric. Together with the data that transport is enhanced by Cl−, these experiments support the hypothesis that Cl− and Na+ binding prepares the transporter to bind GABA.
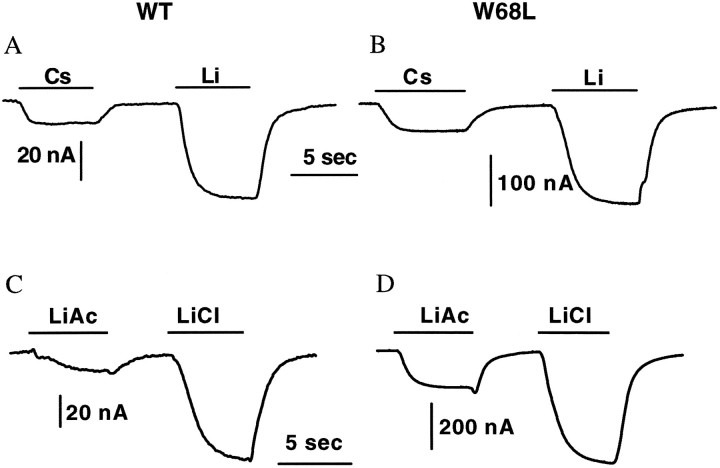
Li+ and Cs+ induce channel-like currents
GABA transport by GAT1 is absolutely dependent on the presence of Na+ and cannot be driven by other metal or organic ions (Kanner and Schuldiner, 1987). Yet Figure 5 shows that we found electrophysiological signals attributable to interactions between Cs+ or Li+ and GAT1. In the absence of GABA and Na+, steady inward currents developed when either Cs+ or Li+ was applied to oocytes (Fig.5A,B). Similar signals were not observed in noninjected oocytes. The Li+ currents were fourfold larger for W68L than for the WT transporter (157 ± 7 and 41 ± 8 nA, respectively) despite the similar charge movements for Na+-concentration jumps. This shows again that W68L seems to favor uncoupled fluxes of Li+ or Cs+. When the concentration jumps were performed between Na+ (rather than NMDG) and Li+ or Cs+, the records showed both binding current and permeation current (data not shown). LiCl produced 5.8 ± 0.6 and 2.4 ± 0.2 times larger steady-state current than did LiAc for the WT and W68L, respectively (8 and 7 cells, respectively; Fig. 5C,D), again suggesting that Cl− generally enhances the interaction between cations and the transporter.
Fig. 5.
Cs+ and Li+ leakage current. Holding potential, −60 mV. A,B, Oocytes were first superfused with NMDG · Cl Ringer. At the indicated time, the perfusion was changed to CsCl or LiCl Ringer. Cs+ and Li+ generated a steady inward current. C, D, Effect of Cl− on the Li+ leakage current. Oocytes were first superfused with NMDG · Cl Ringer. At the indicated time, the perfusion was changed to either LiCl or Li acetate (LiAc) Ringer. For all panels, GAT1-specific current was isolated as follows. After each series of changes pictured, oocytes were perfused for 10 sec with 30 μm SKF-100330A in 96 mm NaCl to block GAT1 currents. The ionic concentration changes were then repeated. The SKF-blocked record was then subtracted from the original record. The subtraction procedure yielded flat traces in similar protocols applied to uninjected oocytes.
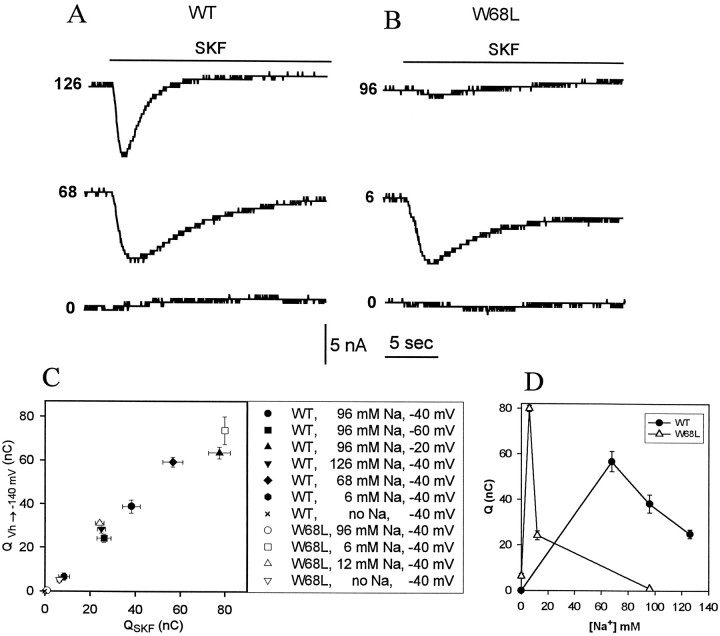
Tiagabine analogs induce a Na+“lock-in” current
The GABA uptake inhibitors SKF-89976A (Mager et al., 1993), NO-05-711 (Novo Pharmaceutical; data not shown) and SKF-100330A (Fig.1) all eliminate both concentration-jump and voltage-jump charge movements. This effect could arise either because the drug prevents Na+ binding or because it leads to Na+occlusion by the transporter. Figure 6 shows that in the presence of Na+, application of SKF-100330A stimulates inward current. We now present evidence that this current is attributable to Na+ binding induced by the inhibitor, lending support to an occlusion mechanism. The current depended on the presence of Na+ (Fig.6A,B). However, at higher [Na+], the charge movements (integral of the current over time) decreased for the WT (126 mm Na+) and almost disappeared for the mutant (96 mm Na+). Thus, the inhibitor generates no current if it binds to transporters that are already occupied by Na+. Furthermore, the charge movement induced by application of the inhibitor is equal to the charge movement induced by voltage jumps to high negative potential (−140 mV, Fig. 6C) or by concentration jumps to higher [Na+] (data not shown). For each of three procedures (increased [Na+], hyperpolarization, and application of inhibitor), therefore, charge movements occur by increasing Na+ occupancy of the transporter.
Fig. 6.
Inhibitor-induced Na+ “lock-in” current. A, B, Oocytes were perfused with a Ringer solution containing the specified [Na+] at a holding potential of −60 mV. At the indicated time, SKF-00330A (30 μm) was added to the perfusion medium. C, Comparison of charge movements for inhibitor applications as inA and B (x-axis) and for voltage jumps from the holding potential to −140 mV (y-axis). Open and closed symbols each represent an oocyte expressing WT or W68L GAT1, respectively. D, Effect of [Na+] on the charge movements obtained by integrating the traces in Aand B.
DISCUSSION
The data strongly support the idea that, in normal physiological solution, GAT1 is fully occupied by two Na+ ions and one Cl− ion and that this occupation prepares the transporter to bind GABA immediately, with the result that transport is initiated. The charge movements during concentration- and voltage-jump experiments show that WT GAT1 binds two Na+ ions and undergoes a subsequent event, probably a conformational change. These Na+-dependent charge movements occur at lower [Na+] if Cl− is present, and vice-versa, as though Na+ and Cl− bind to a common state associated with completion of the charge movements. When GABA is present, these ion–transporter interactions continue to dominate the entire transport cycle, as shown by the similar Na+dependence of the charge movements and the transport-associated currents (EC50 of 43 and 44 mm, respectively), by their similar Hill coefficients (1.66 and 1.8, respectively), and by their similar voltage dependence (equivalent charges of 1.04 and 0.89, respectively; Mager et al., 1993). Thus, the transport process has already undergone its rate-limiting steps when a GABA molecule binds (Cammack et al., 1994).
The transient currents in response to concentration jumps are linked to ion binding and dissociation
In response to rapid application of Na+, Cl−, or SKF-100330A, oocytes expressing the GABA transporter generate transient inward current. We believe that these transient currents occur at the GABA transporter because no similar transient current appeared in uninjected oocytes and because the current was blocked by various specific, high-affinity uptake inhibitors. We also have several reasons to believe that these transient currents are attributable to ion binding at the transporter (and within the membrane) rather than to complete permeation through the membrane. Equal and opposite charge movements (integral of the transient current over time) occurred when [Na+] or [Cl−] was reduced back to the initial concentration (Figs. 1C, 4A). Such charge conservation, which is formally modeled as a capacitance, is a typical characteristic of a reversible binding/dissociation reaction within the membrane dielectric, or of a conformational change in a membrane protein, but not of ion permeation. Most important, the charge movement for concentration jumps was nearly equal to that for voltage jumps under conditions designed to produce similar endpoints of ionic concentration and membrane potential (Fig. 1D). Previous studies show that the voltage-jump charge movements are also capacitive currents, closely related to binding and dissociation (Mager et al., 1993).
We wish to point out that we have noted briefer (time constant < 6 msec) components of voltage-jump relaxations at GAT1 (S. Mager and H. Lester, unpublished observations). These components may correspond to the voltage-dependent capacitances observed by Lu et al. (1996) and presumably arise from events faster than those considered here.
Molecular nature of the event that produces charge movements
The charge movements depend on [Na+] with a Hill coefficient near 2 (Fig. 2A), providing the strongest evidence yet that these charge movements are much more likely to occur in the presence of two bound Na+ ions than in the presence of a single bound Na+ ion. What is the event that produces charge movement? In one plausible mechanistic view, the two Na+ ions reach a binding site in the transporter that is functionally within the membrane dielectric, followed by a conformational change that greatly increases binding affinity but does not itself move charge. In a second plausible view, the Na+ions do not initially bind in a voltage-dependent manner; instead, the conformational change is the voltage-dependent step because it moves charge within the membrane electric field. Within the context of either interpretation, Eq. 1 implies rather tight coupling between the binding and the conformational change, in the sense that binding occurs with higher affinity as the conformational change becomes more probable, and vice-versa. Because of this tight coupling, the overall binding of Na+ would be expected to display a Hill coefficient of 2 even in the absence of cooperative interactions during the microscopic binding events. We suggest, therefore, that a conformational change occurs even before GABA binds, and that this conformational change prepares the transporter to bind GABA with high affinity.
We are impressed by the parallel between the charge movements and the conformational changes thought to govern ion channel gating. Both events occur on time scales of tens to hundreds of milliseconds. Rates for both events display a maximum with voltage, as though transitions are governed by forward and reverse rate constants with opposite signs of voltage dependence (in further support for such a model, the minimum rate occurs at the midpoint of the charge–voltage relation). Both events can be shifted in voltage dependence, but not in overall amplitude, by changes in ionic concentrations. These similarities would be expected from theories such as the alternating-access models of transporter function. On the other hand, the conformational change need not be global; recent simulations suggest that small changes in substrate interactions within a channel-like lumen can explain coupled transport (Su et al., 1996). Our data contrast with results for the Na/K pump, in which the slow charge movements recorded for Na+ transport show a monotonic increase in rate with hyperpolarization, and the actual electrogenic events are much faster (submicrosecond) ion-binding reactions (Hilgemann, 1994).
It is at first surprising that Cl− jumps produce charge movements opposite to the direction expected for an anion moving within the membrane dielectric. We conclude that Cl− favors the binding of Na+, directly at the Na+ binding site(s), allosterically by modifying the Na+ binding site, or allosterically by facilitating the conformational change that produces the charge movements. The latter possibility is quite attractive because the actual amplitude of charge moved, as measured byQmax, does not change in zero Cl−, although it occurs at different voltages. Cl− also binds at the intracellular face of the transporter (Lu et al., 1996).
Inhibitor-induced current
That an inward charge movement results from application of a high-affinity transport inhibitor (Fig. 6) provides direct support for suggestions that nonsubstrate GABA inhibitors, as well as some monoamine transporter inhibitors, act by analogy with transition-state analog inhibitors of enzymes: they lock the transport protein at an intermediate step in the transport cycle (Rudnick and Clark, 1993;Lester et al., 1994). Another analogy concerns the action of cardiac glycosides on the Na+ pump: they lock in two Na+ ions (Jorgensen and Andersen, 1988; Sturmer and Apell, 1992). In support of the “lock-in” idea, direct binding experiments show that a tiagabine analog binds more tightly to GABA transporters in the presence of Na+ (Braestrup et al., 1990). In our view, tiagabine and its analogs bind at least partially to the GABA binding site itself, after the conformational change associated with charge transfer. By freezing the transporter at this point, tiagabine and its analogs apparently prevent Na+ from dissociating to the intracellular solution, thereby increasing its affinity. Importantly, there were equal charge movements for inhibitor application and for voltage jumps that completely forced Na+ onto the transporter, suggesting that the inhibitor locks both Na+ions into their normal binding sites. Because tiagabine and its analogs produce neurotransmitter transporters with tightly bound Na+, these inhibitors may prove important in future experiments that seek to reveal structural properties of transporters at atomic scale.
Channel-like mode
The comparatively large currents produced by Cs+ and Li+ are analogous to uncoupled Cs+ and Li+ currents at other neurotransmitter transporters (Lester et al., 1994; Mager et al., 1994). A partial explanation for the lack of coupled transport by Cs+ and Li+ is that these two ions permeate through the transporter but do not require the binding of GABA. Uncoupled ion fluxes attributable to intracellular Cs+ solutions may also explain that leakage currents were high, that concentration-jump charge movements were not reversible, and that Na+ concentration jumps produced no charge movements in the study by Cammack et al. (1994). The Cs+ and Li+ currents represent a contrast to the action of tiagabine analogs, which allow no current at all. At this point, we do not know whether the Cs+ and Li+ currents are comprised of single-ion events or of clusters comparable in size with single-channel events, but single-channel events do underlie some leakage currents at both GAT1 (Cammack and Schwartz, 1996) and the mammalian 5-HT transporter (Lin et al., 1995).
The W68L mutation
Trp68 is in the first putative transmembrane domain, which is strikingly well conserved among members of the Na+/Cl−-coupled neurotransmitter transporter superfamily. It appears that both WT and W68L transporters can bind Na+ and Cl− and can undergo the conformational change that produces charge movements. The Na+–transporter interaction and the Cl−–transporter interaction dominate the entire transport cycle, for W68L as well as for the WT. Only the WT, however, can efficiently undergo the subsequent events—presumably including conformational changes—that release the substrates to the intracellular solution. Alternatively, the intracellular [Na+] may exceed the dissociation constant for the interaction between intracellular Na+ and W68L, so that Na+ is not efficiently released to the cytoplasm. Although we are not yet ready to choose among models that specify the bindings, dissociations, and conformational changes during transport (Su et al., 1996), we can certainly point out that poor balance among these processes leads to inefficient transport.
It would not be surprising if efficient transport requires that π electrons of aromatic groups (Trp68 in this case) interact with permeant cations and/or with cationic moieties of the organic substrate (Dougherty and Stauffer, 1990; Kleinberger-Doron and Kanner, 1994). The combination of high-resolution physiological measurements and site-directed mutagenesis, which has been fruitfully applied to ion channels in the past, can now be applied for identification of key amino acid residues and domains within several families of transporters. These families include neurotransmitter transporters as studied here and by others (Lu and Hilgemann, 1995; Wadiche et al., 1995), ion antiporters (Hilgemann et al., 1991), and ATPase pumps (Gadsby et al., 1993; Hilgemann, 1994; Holmgren and Rakowski, 1994; Lu and Hilgemann, 1995).
Transport function and synaptic transmission
GAT1 and its homologs play a key role in shaping the time course and spatial extent of synaptic transmission, apparently by removing GABA within a few milliseconds after its release from the presynaptic terminal (for review, see Lester et al., 1994). The data presented here provide additional support for the hypothesis that the cotransported ions bind before GABA, inducing a state that then accepts GABA (Kanner, 1987; Cammack et al., 1994; Tong and Jahr, 1994; Wadiche et al., 1995). From the dependence of charge movements on voltage and [Na+] (Figs. 2, 3) (Mager et al., 1993), one may conclude that the transporters undergo this conformational change with a time constant of ∼250 msec (−80 mV, 96 mm NaCl, 20°C) and eventually reach a steady-state distribution of >90% in the GABA-receptive state. However, because these ion-binding steps and the conformational change occur in the absence of GABA, the transporter can then respond quickly to the appearance of GABA by binding the transmitter, removing it from the receptors and preventing their reactivation. Of course, individual transporter molecules would require another ∼250 msec before binding another GABA molecule; synaptic transmission at intervals less than this might not be effectively shaped by transporters.
A key unknown fact that bears on our hypothesis is the density of transporter molecules near a GABA synapse. If this value is comparable with that of acetylcholinesterase molecules at a nicotinic synapse (>2000 molecules/μm2) or of GAT1 molecules in an expressing oocyte (>10,000 molecules/μm2), then transporters at a noncholinergic synapse can be as effective as acetylcholinesterase despite the much lower turnover rate of transporters.
Several other neurotransmitter transporters display functional characteristics analogous to those of GAT1. The similarities include Na+ specificity, Cl− dependence, charge movements (Galli et al., 1995; Wadiche et al., 1995), and positive interactions between the binding of Na+ and of high-affinity blockers (Rudnick and Clark, 1993). Our conceptual framework, therefore, may explain transmitter removal at various chemical synapses.
Footnotes
This work was supported by grants from the National Institute of Neurological Diseases and Stroke and the U.S./Israel Binational Science Foundation, and by fellowships from the Lester Deutsch Foundation and the Muscular Dystrophy Association (S.M.). We thank G. Rudnick for help with the [3H]tiagabine binding assay, C. Armstrong and A. Finkelstein for discussion of charge movements and membrane dielectrics, and J. Li and M. Nowak for comments on this manuscript.
Correspondence should be addressed to Henry A. Lester, Division of Biology 156-29, California Institute of Technology, Pasadena, CA 91125.
REFERENCES
- 1.Braestrup C, Nielsen EB, Sonnewald U, Knutsen LJS, Andersen KE, Jansen JA, Frederiksen K, Andersen PH, Mortensen A, Suzdak PD. (R )- N -[4,4-bis(3-methyl-2-thienyl)butyl-3- n -1-yl]nipecotic acid binds with high affinity to the brain γ-aminobutyric acid uptake carrier. J Neurochem. 1990;54:639–647. doi: 10.1111/j.1471-4159.1990.tb01919.x. [DOI] [PubMed] [Google Scholar]
- 2.Cammack JN, Schwartz EA. Channel behavior in a GABA transporter. Proc Natl Acad Sci USA. 1996;93:723–727. doi: 10.1073/pnas.93.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cammack JN, Rakhilin SV, Schwartz EA. A GABA transporter operates asymmetrically and with variable stoichiometry. Neuron. 1994;13:949–960. doi: 10.1016/0896-6273(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 4.Dougherty DA, Stauffer DA (1990) Acetylcholine binding by a synthetic receptor: implications for biological recognition. Science 250: 1558–1560. [DOI] [PubMed]
- 5.Fuerst TR, Niles EG, Studier W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadsby DC, Rakowski RF, Weer PD. Extracellular access to the Na/K pump: pathway similar to ion channel. Science. 1993;260:100–103. doi: 10.1126/science.7682009. [DOI] [PubMed] [Google Scholar]
- 7.Galli A, DeFelice LJ, Duke B-J, Moore KR, Blakely RD. Sodium-dependent norepinephrine-induced currents in norepinephrine-transporter-transfected HEK-293 cells blocked by cocaine and antidepressants. J Exp Biol. 1995;198:2197–2212. doi: 10.1242/jeb.198.10.2197. [DOI] [PubMed] [Google Scholar]
- 8.Guastella JG, Nelson N, Nelson H, Czyzyk L, Keynan S, Midel MC, Davidson N, Lester H, Kanner B. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- 9.Hilgemann DW. Channel-like function of the Na/K pump probed at microsecond resolution in giant membrane patches. Science. 1994;263:1429–1432. doi: 10.1126/science.8128223. [DOI] [PubMed] [Google Scholar]
- 10.Hilgemann DW, Nicoll DA, Philipson KD. Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature. 1991;352:715–718. doi: 10.1038/352715a0. [DOI] [PubMed] [Google Scholar]
- 11.Holmgren M, Rakowski RF. Pre-steady-state transient currents mediated by the Na/K pump in internally perfused Xenopus oocytes. Biophys J. 1994;66:912–922. doi: 10.1016/s0006-3495(94)80867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 13.Isom LL, Ragsdale DS, Dejongh KS, Westenbroek RE, Reber BFX, Scheuer T, Catterall WA. Structure and function of the β2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 1995;83:433–442. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen PL, Andersen JP. Structural basis for E1–E2 conformational transitions in Na,K-pump and Ca-pump proteins. J Membr Biol. 1988;103:95–120. doi: 10.1007/BF01870942. [DOI] [PubMed] [Google Scholar]
- 15.Kanner BI, Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22:1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- 16.Kavanaugh MP, Arriza JL, North RA, Amara SG. Electrogenic uptake of γ-aminobutyric-acid by a cloned transporter expressed in Xenopus oocytes. J Biol Chem. 1992;267:22007–22009. [PubMed] [Google Scholar]
- 17.Keynan S, Kanner BI. γ-Aminobutyric acid transport in reconstituted preparations from rat brain: coupled sodium and chloride fluxes. Biochemistry. 1988;27:12–17. doi: 10.1021/bi00401a003. [DOI] [PubMed] [Google Scholar]
- 18.Keynan S, Suh Y-J, Kanner BI, Rudnick G. Expression of a cloned γ-aminobutyric acid transporter in mammalian cells. Biochemistry. 1992;31:1974–1979. doi: 10.1021/bi00122a011. [DOI] [PubMed] [Google Scholar]
- 19.Kleinberger-Doron N, Kanner BI. Identification of tryptophan residues critical for the function and targeting of the γ-aminobutyric acid transporter (subtype A). J Biol Chem. 1994;269:3063–3067. [PubMed] [Google Scholar]
- 20.Lauger P. Sinauer; Sunderland, MA: 1991. Electrogenic ion pumps. . [Google Scholar]
- 21.Lester HA, Mager S, Quick MW, Corey JL. Permeation properties of neurotransmitter transporters. Annu Rev Pharmacol Toxicol. 1994;34:219–249. doi: 10.1146/annurev.pa.34.040194.001251. [DOI] [PubMed] [Google Scholar]
- 22.Lin F, Lester HA, Mager S. Single-channel studies of the serotonin transporter: (a) different conducting states and (b) an amino acid in the permeation pathway. Soc Neurosci Abstr. 1995;21:A781. [Google Scholar]
- 23.Loo DDF, Hazama A, Supplisson S, Turk E, Wright EM. Relaxation kinetics of the Na+/glucose cotransporter. Proc Natl Acad Sci USA. 1993;90:5767–5771. doi: 10.1073/pnas.90.12.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C-C, Hilgemann DW. Fast capacitance measurements in the study of membrane transporter electrogenicity in giant membrane patches. Biophys J. 1995;68:A411. [Google Scholar]
- 25.Lu C-C, Kabakov A, Markin VS, Mager S, Frazier A, Hilgemann DW. Membrane transport mechanisms probed by capacitance measurements with megahertz voltage clamp. Proc Natl Acad Sci USA. 1996;92:11220–11224. doi: 10.1073/pnas.92.24.11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mager S, Naeve J, Quick M, Guastella J, Davidson N, Lester HA. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993;10:177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- 27.Mager S, Min C, Henry DJ, Chavkin C, Hoffman BJ, Davidson N, Lester HA. Conducting states of a mammalian serotonin transporter. Neuron. 1994;12:845–859. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 28.Nowak MW, Kearney PC, Sampson JR, Saks ME, Labarca CG, Silverman SK, Zhong W, Thorson J, Abelson JN, Davidson N, Schultz PG, Dougherty DA, Lester HA. Nicotinic receptor binding site probed with unnatural amino acid incorporation in intact cells. Science. 1995;268:439–441. doi: 10.1126/science.7716551. [DOI] [PubMed] [Google Scholar]
- 29.Quick MW, Lester HA. Methods for expression of excitability proteins in Xenopus oocytes. In: Narahashi T, editor. Ion channels of excitable cells. Academic; San Diego: 1994. pp. 261–279. [Google Scholar]
- 30.Radian R, Kanner BI. Reconstitution and purification of the sodium- and chloride-coupled γ-aminobutyric acid transporter from rat brain. J Biol Chem. 1985;260:11859–11865. [PubMed] [Google Scholar]
- 31.Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- 32.Sturmer W, Apell HJ. Fluorescence study on cardiac glycoside binding to the Na/K pump: ouabain binding is associated with movement of electrical charge. FEBS Lett. 1992;300:1–4. doi: 10.1016/0014-5793(92)80151-6. [DOI] [PubMed] [Google Scholar]
- 33.Su A, Mager S, Mayo SL, Lester HA. A multi-substrate single-file model for ion-coupled transporters. Biophys J. 1996;70:762–777. doi: 10.1016/S0006-3495(96)79616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong G, Jahr CE. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 35.Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]