Abstract
Background
Survival and health status (e.g., symptoms and quality of life) are key outcomes in clinical trials of heart failure treatment. However, health status can only be recorded on survivors, potentially biasing treatment effect estimates when there is differential survival across treatment groups. Joint modeling of survival and health status can address this bias.
Methods and Results
We analyzed patient-level data from the PARTNER 1B trial of transcatheter aortic valve replacement (TAVR) versus standard care. Health status was quantified with the Kansas City Cardiomyopathy Questionnaire (KCCQ) at randomization, 1, 6, and 12 months. We compared hazard ratios for survival and mean differences in KCCQ scores at 12 months using several models: the original growth curve model for KCCQ scores (ignoring death), separate Bayesian models for survival and KCCQ scores, and a Bayesian joint longitudinal-survival model fit to either 12 or 30 months of survival follow-up. The benefit of TAVR on 12-month KCCQ scores was greatest in the joint model fit to all survival data (mean difference = 33.7 points; 95% CrI: 24.2, 42.4), followed by the joint model fit to 12 months of survival follow-up (32.3 points; 95% CrI: 22.5, 41.5), a Bayesian model without integrating death (30.4 points; 95% CrI: 21.4, 39.3), and the original growth curve model (26.0 points; 95% CI: 18.7, 33.3). At 12 months, the survival benefit of TAVR was also greater in the joint model (hazard ratio = 0.50; 95% CrI: 0.32, 0.73) than in the non-joint Bayesian model (0.54; 95% CrI: 0.37, 0.75) or the original Kaplan-Meier estimate (0.55; 95% CI: 0.40, 0.74).
Conclusions
In patients with severe symptomatic aortic stenosis and prohibitive surgical risk, the estimated benefits of TAVR on survival and health status compared with standard care were greater in joint Bayesian models than other approaches.
Patients with heart disease seek treatments to extend survival and optimize their health status; their symptoms, function, and quality of life.1, 2 Accordingly, many clinical trials include patient-reported outcomes alongside mortality to measure the impact of treatment on patients’ health status. Most trials have reported mortality and health status results independently, such that the health status outcomes are reported only in surviving patients who are able to complete the health status questionnaires. Although this approach has appeal due to its simplicity, it is likely to provide biased estimates of the treatment effect that is most relevant to patients and providers. Specifically, we are interested in counterfactual health status at a specific point in time for an average individual under each treatment if they survived to that time. To that end, we aim to account for informatively missing data due to death. Contrary to a summary of health status among survivors, this endpoint is likely to be what physicians and patients most care about when weighing treatment decisions. An example illustrates this point: suppose treatment and control have no effect on an individual’s quality of life, but treatment keeps everyone alive while control kills people with very low quality of life. Looking at observed averages we would conclude that control is better in terms of quality of life (though worse in terms of survival). Weighing this evidence with her physician, a patient who really values having a high quality of life may conclude that she should be on control, though this is clearly a bad choice. The obvious problem is that the quality of life among survivors is not really what matters, and averages of observed quality of life (among survivors) are biased by the counterfactual effect that is really of interest. When outcomes are correlated, joint modeling provides a way to estimate the endpoint of interest with less bias than separate analyses, while also potentially increasing the precision of both health status and survival effect estimates.3–7
While a number of potential strategies have been proposed for this purpose,8–11 there has been growing interest in the use of Bayesian models to jointly model survival and longitudinal health status outcomes, largely driven by successful applications in oncology and HIV research.12–16 A joint modeling approach can enable survival data to inform estimates of longitudinal health status trajectories, thereby partially addressing the problem of informatively missing health status data by estimating a counterfactual average health status trajectory had all subjects survived. Furthermore, the precision of treatment effect estimates on both survival and health status may be better than separate or sequential estimates.15 Joint modeling is possible in a frequentist paradigm, but we present it in a Bayesian framework here. The Bayesian version has several advantages in that uncertainty summaries (e.g. posterior intervals) are non-asymptotic, model assessment is simplified, and priors can be used to incorporate historical data or regularize models that have many free parameters. 17, 18 To date, Bayesian joint modeling approaches have mostly focused on improving the power of clinical trials by incorporating patients’ health status into the survival estimates, as opposed to using the survival data to improve the health status estimates.12, 15 To clarify the impact of such approaches on the estimates of health status, we applied joint modeling to a cardiovascular clinical trial in which the experimental treatment had an impact on both survival and health status. Specifically, we applied a Bayesian joint model of survival and health status to describe the outcomes of patients with severe, symptomatic aortic stenosis and extreme surgical risk who were not candidates for surgery and were randomized to either transcatheter aortic valve replacement (TAVR) or standard therapy (including a combination of medical management and balloon valvuloplasty) in the Placement of Aortic Transcatheter Valves PARTNER 1B trial.19, 20 We compared these estimates with alternative modeling approaches including the original analysis and a more standard Bayesian model that didn’t jointly model the outcomes. These analyses can highlight the potential benefits of a joint-modeling analytic approach using a Bayesian framework to provide a deeper understanding of the benefits of TAVR on patients’ health status and survival.
METHODS
The PARTNER 1B trial data used in this analysis is not publicly available. Stan code for our joint models is printed in the supplementary appendix (Appendix C). Complete R code and original Stan files are available online as a zip file.
PARTNER 1B Trial Data
The PARTNER 1B trial was a randomized, open-label trial designed to test TAVR in patients with severe, symptomatic aortic stenosis for whom surgical aortic valve replacement (SAVR) was considered to present a prohibitive risk for surgery. Eligible patients were randomized to TAVR (performed by a transfemoral approach using the balloon expandable Sapien valve) or standard care, consisting of drug therapy and/or balloon valvuloplasty. As previously described, the primary results of the PARTNER 1B trial demonstrated that TAVR led to substantial improvements in both survival19 and health status.20 In the Reynolds et al paper reporting the health status results of the PARTNER 1B trial, longitudinal random-effect growth curve models were used to estimate health status trajectories while adjusting for covariates including prespecified patient characteristics and functions of follow-up time.20 We performed secondary analyses of the patient-level data from this study to examine the impact of a Bayesian joint modeling approach on both outcomes. The study was approved by IRB at each site, and all patients provided informed consent. The Saint Luke’s Health System’s Institutional Review Board reviewed the project and determined this secondary analysis not to be human subjects research.
Outcomes
Health status was evaluated using the Kansas City Cardiomyopathy Questionnaire (KCCQ),21 a 23-item, disease-specific questionnaire that quantifies patients’ symptoms, physical and social limitations, self-efficacy, and quality of life due to heart failure.21–24 The KCCQ has undergone extensive reliability and validity testing in various heart failure populations,21 including those with severe aortic stenosis.22 Each domain is converted to a range of 0–100, where higher scores indicate fewer symptoms, less functional limitation, and better quality of life. The Symptom, Physical Limitation, Social Limitation and Quality of Life scales can be combined to form the KCCQ overall summary scale (KCCQ-OS), which also ranges from 0 to 100, with higher scores indicating better health status. Lower KCCQ scores have been associated with increased risk of death, including in patients undergoing TAVR.6, 25 Changes of 5, 10, and 20 points correspond to small, moderate or large clinical improvements, respectively.23 The KCCQ was administered prior to randomization and at 1, 6 and 12 months later, providing a longitudinal trajectory summarizing surviving patients’ health status over time. Mortality was measured as death from any cause. At the time of database lock for the quality of life study, all patients had been followed for survival for at least 1 year and up to 30 months.
Joint Modeling Approach
To address informatively missing health status data due to death, we specified a piecewise linear model for the mean health status trajectories with intercepts at baseline and 1 month and with a linear slope between months 1 and 12. This model (fully specified in Appendix A) was chosen based on observing a large improvement in health status immediately after TAVR or standard therapy, and an approximately linear trend afterwards. Treatment effects were parameterized with two coefficients, one for the difference between treatment and standard care at 1 month and a second for the difference in post-1-month slopes. Baseline average scores were constrained to be the same in the two treatment groups, which was felt to be reasonable owing to randomization and was empirically verified in the study sample. The errors were assumed to be Gaussian and individuals had 3 Gaussian random effects: a baseline intercept, a 1-month intercept, and a post-1-month slope.
Survival times were modeled using a Weibull distribution, with a single shape parameter and a regression model for the scale parameter. The regression model included an indicator of treatment group and two of the individual-level random effects: the 1-month intercept and the post-1-month slope of KCCQ scores. The coefficient on the treatment indicator quantifies the treatment effect in the survival sub-model. The coefficients on the individual-level random effects quantify the relationship between the health status trajectories and hazard of death. Positive coefficients on the random effects would indicate that higher health status scores at 1 month and more positive slopes are associated with shorter survival. In this joint model, survival times also influence the individual-level random effects, which helps account for censoring of health status by death. We specified weakly informative priors following best practices in Bayesian data analysis.17
To fit the model to the KCCQ-OS data, we first transformed the scores to make them suitable for a Gaussian model. We trimmed scores of 0 and 100 to 1 and 99, then divided by 100 and applied a probit transformation. Given that the KCCQ-OS does not have a score of 1 or 99, the trimmed scores remained the lowest and highest possible. The resulting transformed KCCQ-OS scores were approximately normally distributed (see Figure S1 in Appendix B). All data analyses were done using R software, version 3.3.1. Model code was written in Stan for Hamiltonian Monte Carlo (HMC) sampling, and fit with the ‘rstan’ package and is provided in Appendix C.26 The modeling of means using frequentist t-tests and regression estimates generate confidence intervals (CI) around the point estimates, whereas the Bayesian analyses generate credible intervals (CrI). We assessed convergence of our HMC chains using R-hat statistics, where an R-hat below 1.1 indicated reasonably good convergence, and by visually examining trace plots.17
We compared multiple approaches to illuminate features of the analyses that influence the estimates of health status outcomes. These comparators include the original estimates of health status outcomes, which were based on longitudinal growth curve models that ignored death,20 and the Bayesian model of health status data we specified with no sharing of information through joint random effects (termed the ‘separate’ Bayesian analysis). Furthermore, we included two implementations of our joint Bayesian model: one using survival data only through to 12 months to match the primary survival analysis19 and one using up to 30 months of survival data to examine whether including all available data can further improve joint model fitting and inferences. To determine the extent to which survival data actually informed health status estimates in our joint modeling procedure, we examined the survival coefficients of the random 1-month intercept and post-1-month slope random effects. As they are complicated and mostly of technical interest, the results for the joint random effects are presented in Appendix D.
RESULTS
Patient Population
Table 1 displays baseline patient characteristics and observed outcomes of the 358 randomized patients. The population was elderly, with a mean age of 83.2 ± 8.4 years, 46.4% were male, and 91.3% were white. By design, the population was at high risk for SAVR (mean STS score of 11.5), and patients had high rates of cerebrovascular disease and chronic obstructive pulmonary disease.
Table 1:
Baseline Characteristics and 1-Year Outcomes
| Variable | TAVR (N = 179) | Medical Therapy (N = 179) |
|---|---|---|
| Age, years ± SD | 83.1 ± 8.6 | 83.2 ± 8.3 |
| Male sex, % | 45.8 | 46.9 |
| White race, % | 92.2 | 90.5 |
| COPD, % | 41.3 | 52.5 |
| Cerebrovascular Disease, % | 27.4 | 27.4 |
| STS Mortality Risk Score ± SD | 11.2 ± 5.8 | 11.9 ± 4.8 |
| KCCQ-OS | ||
| Baseline ± SD | 36.2 ± 20.5 | 34.4 ± 20.1 |
| 1-Month ± SD | 61.6 ± 26.2 | 49.2 ± 24.3 |
| 6-Month ± SD | 70.7 ± 23.0 | 50.5 ± 26.1 |
| 12-Month ± SD | 69.4 ± 25.4 | 47.0 ± 24.6 |
| 1-Year Survival (95% CI) | 0.69 (0.63, 0.76) | 0.49 (0.42, 0.57) |
SD = standard deviation; STS = Society of Thoracic Surgeons; COPD = chronic obstructive pulmonary disease; CI = confidence interval.
At 1 month, the raw data indicated significantly more mean KCCQ-OS improvement in the TAVR group than in the standard care group. People in the standard care group had worse KCCQ-OS scores at 12 months than at 1 month, indicating a decline in health status over time. In contrast, patients treated with TAVR experienced improved health status, on average, throughout the year of observation. 1-year survival was also significantly worse in the standard care group than in the TAVR group (49% vs 69%; p<0.01).
Impact of Joint Modeling on Health Status Inference
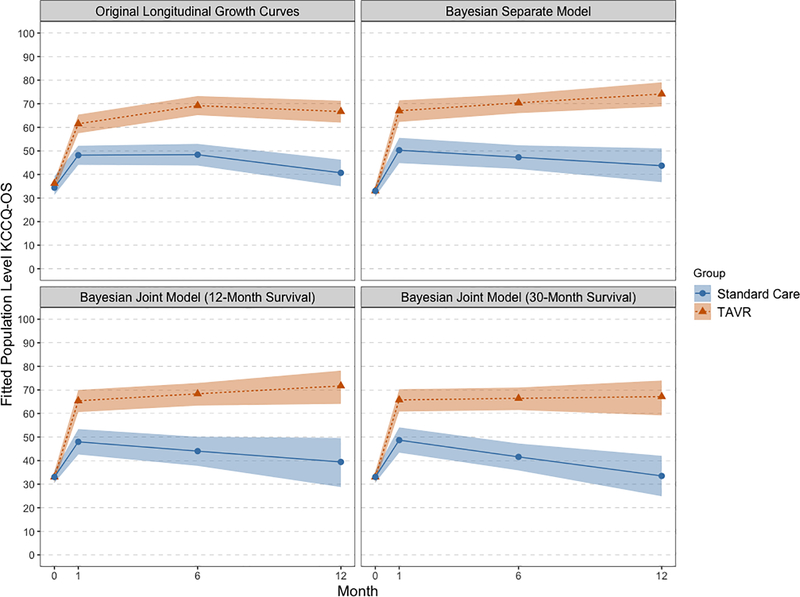
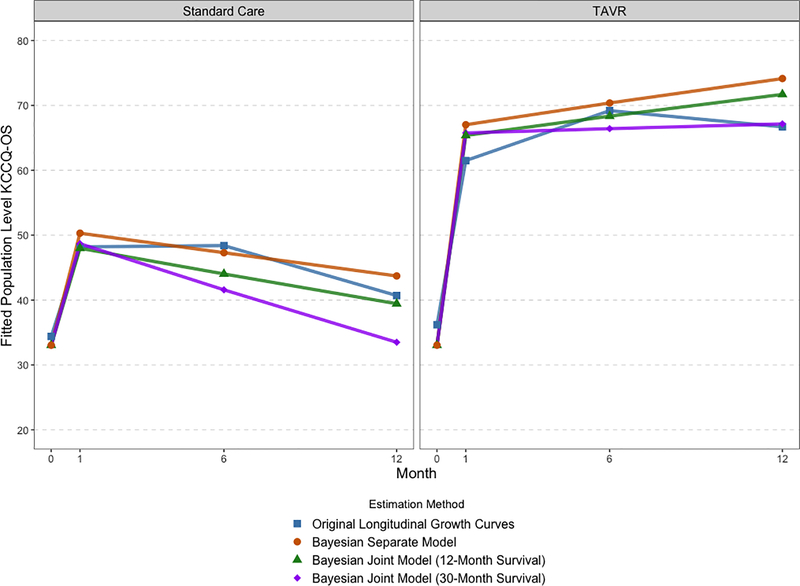
Figures 1 and 2 plot the KCCQ-OS results in two distinct ways. Figure 1 plots the estimates from different methods overlaid on each other to facilitate comparison, with separate panels for treatment group. Figure 2 plots each method in a separate panel and includes 95% CIs or CrIs for each estimated trajectory. These plots show that both joint approaches estimated lower health status in the standard care group than either the longitudinal growth curves or the separate Bayesian model. Furthermore, all 3 Bayesian models estimated slightly higher health status in the TAVR group at 12-months, though as we accounted for increasing amounts of survival data the health status trajectories in TAVR-treated patients slightly decreased. Including survival data in the joint model changed the estimated trajectories for the standard care group: the estimate for standard care 12-month KCCQ-OS was 33 from the full survival joint model compared with 44 from the separate Bayesian model and 41 from the longitudinal growth curve model. In terms of inference, the joint methods exhibited wider credible intervals than either the growth curves (which estimated frequentist confidence intervals) or the separate Bayesian model. However, including more survival data led to tighter credible intervals, as shown in the comparison of plots 2C and 2D.
Figure 1: Estimated KCCQ-OS score trajectories for TAVR and standard care according to the original longitudinal growth curves and Bayesian models.
Estimated population KCCQ-OS trajectories. Different estimation methods are overlaid and confidence/credible interval ribbons are suppressed for clarity.
Figure 2: Estimated KCCQ-OS scores for each estimation method with confidence/credible interval ribbons.
Estimated population level KCCQ-OS scores from baseline (0-months) to 12-month. Panels show (from left to right, top to bottom) longitudinal growth curve model, Bayesian piecewise linear model fit with no joint parameters, Bayesian piecewise linear model with joint random effects utilizing 12-months of survival data, and Bayesian piecewise linear model with joint random effects utilizing the full 30-months of survival data. Sold lines with points are joint model estimates, ribbons are 95% credible intervals. Standard care group is in red, TAVR is blue.
These differences were also apparent in the effect estimates, defined as the difference in estimated KCCQ scores between TAVR and standard therapy health status at each timepoint (Table 2). The joint Bayesian methods estimated a larger treatment benefit than either the original growth curve model or the separate Bayesian model. In addition, using the full 30-months of survival follow-up data led to a slightly larger KCCQ effect estimate than using only 12 months of survival data.
Table 2:
Estimated Health Status Benefits
| Original Longitudinal Growth Curves (95% CI) | Separate Bayesian Model (95% CrI) | Joint Model with 12-Month Survival Data (95% CrI) | Joint Model with 30-Month Survival Data (95% CrI) | |
|---|---|---|---|---|
| 1-Month KCCQ-OS Effect | 13.3 (7.6, 19.0) | 16.7 (9.6, 23.7) | 17.4 (9.9, 24.4) | 17.1 (9.8, 24.0) |
| 6-Month KCCQ-OS Effect | 20.8 (14.7, 27.0) | 23.1 (16.3, 29.7) | 24.3 (17.5, 30.9) | 24.8 (18.0, 31.5) |
| 12-Month KCCQ-OS Effect | 26.0 (18.7, 33.3) | 30.4 (21.4, 39.3) | 32.3 (22.5, 41.5) | 33.7 (24.2, 42.4) |
KCCQ-OS Effect = Difference in Estimated Mean KCCQ-OS between the TAVR and standard of care groups. CI = confidence interval, CrI = credible interval.
Impact of Joint Modeling on Survival Inferences
The hazard ratio of TAVR is 0.50 (95% CrI: 0.32, 0.73) from the joint model, which indicates a nominally larger treatment benefit compared with the original finding of a hazard ratio of 0.55 (95% CI: 0.40, 0.74), or compared to a Bayesian Weibull model with no joint parameters (0.54; 95% CrI: 0.37, 0.75).
DISCUSSION
As patient-centered outcomes assume increasing importance in both clinical trials and shared decision-making, there is a need for analytic strategies to obtain estimates of the health status benefits when there is informatively missing data, such as censoring by death. This is particularly important in conditions where mortality is common, including heart failure and severe aortic stenosis. In this study, we jointly modeled survival and health status using a Bayesian framework to estimate the impact of mortality on the longitudinal health status benefit of TAVR and to refine the estimates of the survival benefit from TAVR.
We compared our findings to those based on longitudinal growth curve models reported in Reynolds et al (2011).20 As in this paper, the longitudinal endpoints of interest were counterfactual health status trajectories, and not observed averages among survivors. That analysis assumed that the informative missingness could be handled by covariate adjustment. We took a different approach that did not make this assumption, though it did require other assumptions about the form of the model, which we discuss below. We focused our attention on applying joint modeling to the PARTNER 1B data and comparing substantive inferences to past analyses. We refer the interested reader to our bibliography for more details on the benefits and drawbacks of joint models from a technical perspective. 12–16, 18, 27–29
Integrating mortality and health status demonstrated increased treatment benefits of TAVR compared with medical therapy on both outcomes. Thus, the true benefits of TAVR (in terms of both health status and survival) may have been underestimated by the original analyses, which ignored informative censoring and treated each endpoint separately. These findings illustrate the potential value of joint modeling to enrich a discussion between patients and providers regarding the anticipated health status patients could expect to experience at a specific time (e.g. 1 year) should they live that long. Without such an approach, current estimates of health status have likely over-estimated the health status that a patient can expect to experience, due to the fact that sicker patients (i.e. those with worse heath status) are more likely to die over the course of a trial. This is especially problematic when different treatments imply different survival rates, as effect estimates based on observed longitudinal data are biased by the differential survival.
Our work extends prior research on the use of joint models to analyze clinical trials. To date, joint modeling has been largely restricted to clinical trials in oncology and HIV/AIDS. While this approach may reflect a greater focus in those fields on analyzing treatments in terms of both survival and quality of life, joint modeling has also been used to improve the precision of survival hazard estimates using longitudinal biomarkers or patient-reported outcomes.12, 14–16 In contrast, cardiovascular trials have traditionally focused on survival or other discrete events, and have only recently begun to emphasize health status outcomes, which have typically been analyzed separately and either reported among survivors or modeled using observed covariates.20 Indeed, to our knowledge, this is the first application of joint modeling of health status and survival data in a cardiovascular clinical trial.
In particular, applying a joint Bayesian model can enable more accurate assessment of treatment benefits among patients with a highly mortal cardiovascular condition. Previous treatment estimates derived from surviving patients describe the average health status of survivors. However, since patients with worse health status are also less likely to survive, this analytic approach may underestimate the true health status benefit of treatment. In contrast, by explicitly considering the interrelationship between survival and health status, the joint modeling approach provides an estimate of the health status benefit that individual patients would be expected to achieve had they survived to 12 months. We believe that this is the type of information that a prospective patient would want to know when trying to decide whether to undergo a major procedure.
In terms of 12-month survival, the joint model assuming a Weibull distribution estimated slightly better survival compared with the Kaplan-Meier estimate, especially in the TAVR group. Indeed, the hazard of death for TAVR treatment was 50% lower than standard therapy, which was slightly larger than the originally reported treatment effect of a 45% reduction in mortality.19
Limitations
This study should be interpreted in the context of several potential limitations. First, given the study design, there were relatively few health status measurements per person (no more than 4). In using 3 parameters (a baseline intercept, a 1-month intercept, and a post 1-month slope) to define each person’s recovery, the model fit many parameters to relatively sparse longitudinal data. In the future, collection of more longitudinal measurements per subject could allow for more flexible models and lead to improved precision. Modeling a bounded health-status outcome was also challenging. We opted to transform the KCCQ-OS scale to fit a Gaussian model and then back-transform for inference. Our Bayesian approach facilitated this, as we could transform posterior draws and still ensure correct inference. However other modeling choices are defensible, and the treatment effect estimates may be sensitive to the parametric form of the model and data transformations. For future studies, alternative approaches, including a Beta or zero-augmented Beta distribution to model the outcome on its original scale, should be considered.13 Moreover, we had to make additional assumptions about the data in order to use parametric sub-models for the longitudinal trajectories and survival, e.g. that post-1-month health status trajectories were approximately linear and survival could be modeled as a Weibull. These assumptions were necessary to fit a joint model that could account for differential survival, despite having limited longitudinal health status measurements. If more data points were available, these assumptions could be relaxed by fitting more flexible sub-models. In addition, while the PARTNER 1B trial was an excellent study with which to explore joint modeling methods given the profound influence of TAVR on both survival and health status, the impact of a joint modeling approach in less effective treatments needs to be explored. Finally, these methods remain unfamiliar to clinicians. While we have attempted to provide intuitive explanations for our approach and for the interpretation of our results, this work represents an early application of joint modeling in cardiovascular disease. Greater experience with these methods and alternative explanations may improve the ability to convey the results of analyses that integrate survival and health status.
Conclusions
In summary, this study provides the first example in the cardiovascular literature of integrating survival and health status outcomes in a randomized clinical trial using a joint Bayesian framework. We found that in patients with severe, symptomatic aortic stenosis and extreme surgical risk who were randomized to either TAVR or standard medical therapy, the estimated benefits of TAVR on both survival and health status were greater than those observed in analyses that considered those outcomes separately. Since death informatively censors health status observations, this approach may provide a better means of integrating these two clinically important outcomes than assuming that health status scores are representative of the overall population of patients in a clinical trial after adjusting for observed covariates. It would be reasonable for future studies to consider incorporating these methods to generate a more accurate estimate of the survival and health status benefits of cardiovascular therapies.
Supplementary Material
Acknowledgments
DISCLOSURES
Dr. Spertus owns the copyright to the KCCQ. Drs. Cohen and Leon are supported by grants from Edwards Life Sciences, Medtronic, Boston Scientific, and Abbott. Dr. Cohen also receives consulting income from Medtronic. Dr. Arnold is supported by a grant from the National Institutes of Health (K23 HL116799). Jacob Spertus is supported by the National Science Foundation Graduate Research Fellowship. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
REFERENCES
- 1.Rumsfeld JS, Alexander KP, Goff DC Jr., Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat-Jacobson D, Zerwic JJ, American Heart Association Council on Quality of C, Outcomes Research CoC, Stroke Nursing CoE, Prevention CoPVD and Stroke C. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–49. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 3.Bell ML and Fairclough DL. Practical and statistical issues in missing data for longitudinal patient-reported outcomes. Stat Methods Med Res. 2014;23:440–59. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, Peterson ED, Masoudi FA, Krumholz HM, Havranek EP, Conard MW, Williams RE and Cardiovascular Outcomes Research C. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47:752–6. [DOI] [PubMed] [Google Scholar]
- 5.Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P and Spertus JA. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115:1975–81. [DOI] [PubMed] [Google Scholar]
- 6.Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG and Spertus JA. Association of Serial Kansas City Cardiomyopathy Questionnaire Assessments With Death and Hospitalization in Patients With Heart Failure With Preserved and Reduced Ejection Fraction: A Secondary Analysis of 2 Randomized Clinical Trials. JAMA Cardiol. 2017;2:1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soto GE, Jones P, Weintraub WS, Krumholz HM and Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110:546–51. [DOI] [PubMed] [Google Scholar]
- 8.Fielding S, Ogbuagu A, Sivasubramaniam S, MacLennan G and Ramsay CR. Reporting and dealing with missing quality of life data in RCTs: has the picture changed in the last decade? Quality of Life Research. 2016;25:2977–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grady KL, Jones PG, Cristian-Andrei A, Naftel DC, Myers S, Dew MA, Idrissi K, Weidner G, Wissman SA, Kirklin JK and Spertus JA. Causes and Consequences of Missing Health-Related Quality of Life Assessments in Patients Who Undergo Mechanical Circulatory Support Implantation: Insights From INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). Circ Cardiovasc Qual Outcomes. 2017;10:e003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sales AE, Plomondon ME, Magid DJ, Spertus JA and Rumsfeld JS. Assessing response bias from missing quality of life data: the Heckman method. Health Qual Life Outcomes. 2004;2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zwinderman AH. Statistical Analysis of Longitudinal Quality of Life Data with Missing Measurements. Quality of Life Research. 1992;1:219–224. [DOI] [PubMed] [Google Scholar]
- 12.Gould AL, Boye ME, Crowther MJ, Ibrahim JG, Quartey G, Micallef S and Bois FY. Joint modeling of survival and longitudinal non-survival data: current methods and issues. report of the DIA Bayesian joint modeling working group. Stat Med. 2015;34:2181–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatfield LA, Boye ME, Hackshaw MD and Carlin BP. Multilevel Bayesian Models for Survival Times and Longitudinal Patient-Reported Outcomes With Many Zeros. Journal of the American Statistical Association. 2012;107:875–885. [Google Scholar]
- 14.Ibrahim JG, Chen MH and Sinha D. Bayesian semiparametric models for survival data with a cure fraction. Biometrics. 2001;57:383–8. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim JG, Chu H and Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol. 2010;28:2796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsiatis A and Davidian M. Joint Modeling of Longitudinal and Time-to-event Data: An Overview. Statistica Sinica. 2004;14:809–834. [Google Scholar]
- 17.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A and Rubin DB. Bayesian Data Analysis, Third Edition: CRC Press, Taylor & Francis Group, LLC; 2013. [Google Scholar]
- 18.Ibrahim JG, Chen MH and Sinha D. Bayesian Survival Analysis. New York: Springer Science+Business Media; 2001. [Google Scholar]
- 19.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D and Pocock S. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. New England Journal of Medicine. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, Webb JG, Babaliaros VC, Bowers BS, Fearon WF, Herrmann HC, Kapadia S, Kodali SK, Makkar RR, Pichard AD and Cohen DJ. Health-Related Quality of Life After Transcatheter Aortic Valve Replacement in Inoperable Patients With Severe Aortic Stenosis. Circulation. 2011;124:1964–72. [DOI] [PubMed] [Google Scholar]
- 21.Green CP, Porter CB, Bresnahan DR and Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 22.Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG and Cohen DJ. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61–7. [DOI] [PubMed] [Google Scholar]
- 23.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS and Cardiovascular Outcomes Research C. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–15. [DOI] [PubMed] [Google Scholar]
- 24.Spertus JA and Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold SV, Spertus JA, Vemulapalli S, Dai D, O’Brien SM, Baron SJ, Kirtane AJ, Mack MJ, Green P, Reynolds MR, Rumsfeld JS and Cohen DJ. Association of Patient-Reported Health Status With Long-Term Mortality After Transcatheter Aortic Valve Replacement: Report From the STS/ACC TVT Registry. Circ Cardiovasc Interv. 2015;8:e002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stan Development Team. RStan: the R interface to Stan. R package version 2.17.3. 2018.
- 27.Dejardin D, Lesaffre E and Verbeke G. Joint modeling of progression-free survival and death in advanced cancer clinical trials. Stat Med. 2010;29:1724–34. [DOI] [PubMed] [Google Scholar]
- 28.Ratcliffe SJ, Guo W and Ten Have TR. Joint modeling of longitudinal and survival data via a common frailty. Biometrics. 2004;60:892–9. [DOI] [PubMed] [Google Scholar]
- 29.Hatfield LA, Hodges JS and Carlin BP. Joint models: When are treatment estimates improved? Statistics and its Interface. 2014;7:439–453. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.