Abstract
Neurons and glia experience rapid fluctuations in transmembrane solute and water fluxes during normal brain activity. Cell volume must be regulated under these conditions to maintain optimal neural function. Almost nothing is known, however, about how brain cells respond to volume challenges induced by changes in transmembrane solute flux. As such, we characterized the volume-regulatory mechanisms of cultured cortical neurons swollen by veratridine-stimulated Na+ influx. Exposure of cortical neurons to 100 μm veratridine for 10–15 min caused a 1.8- to 2-fold increase in cell volume that persisted for at least 90 min. This volume increase was blocked by extracellular Na+ removal or by exposure to 5 μm tetrodotoxin, indicating that swelling is a result of Na+ entry via Na+ channels. Treatment of cells with veratridine together with various NMDA receptor antagonists had no effect on the magnitude of swelling. NMDA receptor antagonist-treated cells, however, underwent nearly complete volume recovery within 50–70 min after veratridine exposure. This recovery suggests that NMDA receptor activation disrupts neuronal osmoregulatory pathways. Volume regulation was blocked by Ba2+, quinidine, or 5-nitro-2-(3-phenylpropylamino) benzoic acid, indicating that swelling activates volume regulatory K+ and Cl− channels. Veratridine also caused a rapid, transient increase in intracellular Ca2+. Extracellular Ca2+ removal or intracellular Ca2+ chelation prevented or dramatically reduced veratridine-induced increases in intracellular Ca2+ and completely blocked volume recovery. These findings indicate that increases in Ca2+ during cell swelling induced by Na+ influx are required for activation of neuronal volume-regulatory pathways.
Keywords: osmoregulation, edema, excitotoxicity, MK-801
Changes in transmembrane solute and water transport have the potential to significantly disrupt cellular ionic and osmotic homeostasis. In many cells and tissues, alterations in transmembrane transport occur under normal physiological conditions. For example, in certain secretory and reabsorptive epithelia, rates of transepithelial solute and fluid movement can change abruptly and continuously as the physiological and hormonal status of the animal changes (Foskett and Melvin, 1989; Takemura et al., 1991). Epithelial cell volume and ionic composition are regulated closely in the face of these potentially disruptive changes in membrane transport. As rates of solute flux into or out of a cell via a specific transport pathway change, the rates of solute flux through other transport pathways are adjusted automatically so that cell volume and ionic composition are maintained (Schultz, 1981; Diamond, 1982; Schultz and Hudson, 1990).
Neural activity has been shown to be associated with significant changes in neuronal and glial cell volume. For example, transient shrinkage of the extracellular space, presumably a result of cell swelling, occurs during evoked activity in the sensorimotor cortex of cats (Dietzel et al., 1980). Serve et al. (1988) demonstrated that continuous electrical stimulation of motor neurons from isolated frog spinal cord causes transient cell shrinkage of 3–10%. Depolarizing pulses induce apparent swelling of cortical neurons in guinea pig brain slices (Lipton, 1973). Ransom et al. (1985) observed that direct electrical stimulation induced swelling in the rat optic nerve, andMcBain et al. (1990) observed that continuous synaptic activity caused changes in the electrophysiological properties of rat hippocampal slices consistent with cell swelling. So-called “intrinsic signals” in the brain are thought to be a result of activity-dependent swelling of glial cells (Lieke et al., 1989).
Little is known about how cells in the CNS maintain ionic and osmotic homeostasis during neural activity. We have begun to investigate this problem using primary cultures of rat cortical neurons. When Na+ influx through voltage-dependent Na+channels is stimulated by veratridine, cortical neurons swell 1.8- to 2-fold and remain swollen for prolonged periods. If the cells are treated with NMDA antagonists, however, a rapid and complete regulatory volume decrease (RVD) is observed. These results demonstrate that cortical neurons possess powerful volume-regulatory mechanisms that function to preserve cell volume during fluctuations in transmembrane solute transport. Excessive stimulation of NMDA receptors seems to disrupt these volume-regulatory processes.
MATERIALS AND METHODS
Cell culture. Astrocyte-poor, neuron-rich dissociated cell cultures derived from rat embryonic cerebral cortex were prepared as described previously (Rosenberg, 1991; Harris and Rosenberg, 1993). Briefly, tissue from the cerebral cortex was removed from Sprague Dawley rat fetuses at day 16. The tissue was dissociated using 0.027% trypsin, and dissociated cells were plated on poly-l-lysine-coated glass coverslips. Cells were grown in 8 parts DMEM containing 2 mm glutamine (Sigma, St. Louis, MO), 1 part Ham’s F-12 (Sigma), and 1 part heat-inactivated calf serum (Hyclone, Logan, UT) (DHS), with penicillin-streptomycin. After 4 d of growth in this medium, cells were exposed to 5 μmcytosine arabinoside for 48 hr to inhibit astrocyte proliferation. On day 7, the culture medium was removed and replaced with a medium containing 90% MEM, 10% NuSerum IV (Collaborative Biomedical Products, Bedford, MA), 10 μg/ml catalase (Sigma), 1 mg/ml superoxide dismutase (Boehringer Mannheim, Indianapolis, IN), 2 mmglutamine, 11 mm glucose, and 9.3 mmNaHCO3. Cells were cultured for 3–6 weeks before use. The medium was not changed in these cultures, and the culture dishes were placed on water-soaked filter paper pads to retard loss of water from the culture medium (Rosenberg, 1991).
Cell volume measurements. All experiments were performed using a HEPES-buffered saline (HBS) containing 130 mm NaCl, 5.4 mm KCl, 0.8 mm MgCl2, 1.8 mm CaCl2, 20 mm HEPES, and 15 mm glucose, pH 7.4. Neurons were imaged using methods described previously (Strange and Spring, 1986). Briefly, neurons grown on coverslips were placed in 35 mm diameter tissue culture dishes. These dishes were prepared for use by drilling a 17 mm diameter hole in the bottom of the dish. A 25 mm diameter coverslip was cemented over the bottom of the hole, creating a shallow well. The culture dish was mounted on the stage of an inverted microscope (Nikon Diaphot; Nikon Microscope, Garden City, NY) equipped with differential interference contrast (DIC) optics, a Zeiss Neofluar X63 (1.25 numerical aperture) oil-immersion objective lens with a 500 μm working distance and a Leitz X32 (0.4 numerical aperture) objective-condenser lens with a 6.6 mm working distance. Images of neurons were recorded on laser disk (model TQ-2028F; Panasonic, Secaucus, NJ) using a Dage video camera (model NC-65, Dage-MTI, Michigan City, IN).
The cross-sectional area (CSA) of the soma of single neurons was quantified by digitizing video images recorded on video disk with the use of an image-processing computer board (model AFG, Imaging Technology, Woburn, MA) with 512 × 480 × 8 bit resolution and an 80386 PC-compatible computer (model 325D, Dell Computer, Austin, TX). Digitized images were displayed on a 14 inch color video monitor (model PVM-1342Q Sony Trinitron, Tokyo, Japan). Cell borders were traced on the monitor with the use of a mouse and a computer-generated cursor. The CSA of the traced regions was determined by image analysis software (Optimas; Bioscan, Edmonds, WA). Each image was traced twice, and the values were averaged. The image acquisition and analysis system allows detection of changes in CSA with an accuracy of ±2-3%.
Measurement of intracellular calcium. Neurons grown on coverslips were exposed for 45–55 min at room temperature to HBS containing 1% BSA plus fura-2 AM (Molecular Probes, Eugene, OR), added from a 1 mm stock solution in DMSO. Coverslips were mounted in a perfusion chamber that permitted complete exchange of the extracellular medium within 3–5 sec.
Cells were imaged as described above with the use of a Zeiss Achroplan X63 (0.9 numerical aperture) water-immersion objective lens and a Zeiss Axiovert 35 inverted microscope. Excitation light was provided by a short-arc xenon lamp. Light intensity was reduced by passage through a 0.6 neutral density filter (Omega Optical, Brattleboro, VT) and then filtered at wavelengths of 340 or 380 nm by two bandpass filters (Omega Optical) mounted on a filter wheel equipped with a high-speed shutter (model Lambda 10; Sutter Instruments, Nevato, CA). Both the filter wheel and shutter were controlled by the imaging software and an IBM-compatible 80486 33 MHz computer (Universal Imaging, West Chester, PA).
Emitted fluorescence was detected with a KS-1381 microchannel plate image intensifier (Video Scope International, Washington, DC) connected to a CCD camera (model 200; Video Scope International). Analog signals were digitized by a Matrox MVP/AT image-processing board (Matrox Electronics Systems, Dorvall, Quebec, Canada). Fluorescence ratios (340:380 nm emissions) were calculated using Image-1/FL software (Universal Imaging). Values for the 340:380 ratio were obtained from the neuronal cell body; a typical experiment included analysis of four to eight cell bodies in a single field of view.
It was not possible to perform an intracellular calibration of fura-2 AM because cells rapidly detached from the growth substrate during the calibration procedure. Instead, we used the following in vitro calibration method. Solutions containing 25–35 μm fura-2 AM/free acid (Molecular Probes), 150 mm KCl, 10 mm HEPES, pH 7.0, 1 mmEGTA, and either 1 mm CaCl2 (“saturating” Ca2+) or 0 Ca2+ were sandwiched between glass coverslips that were mounted on the microscope stage. Fluorescence emissions at 340 and 380 nm were collected as described above. Conversion of measured 340:380 ratios to intracellular Ca2+concentrations was performed using the following relationship (Grynkiewicz et al., 1985):
| Equation 1 |
where [Ca2+]cell is the intracellular concentration of free calcium; R is the cellular 340:380 emission ratio; Rmax is the 340:380 ratio of a solution containing sufficient Ca2+ to saturate fura-2 AM;Rmin is the ratio of a solution containing 0 Ca2+; Fmin is the fluorescence emission at 380 nm of a solution containing 0 Ca2+;Fmax is the fluorescence emission at 380 nm of a solution containing saturating Ca2+; andKd is the in vitro dissociation constant for fura-2 AM (assumed to be 224 nm; Grynkiewicz et al., 1985).
Chemicals. Dizocilipine (MK-801), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), (±)2-amino-5-phosphonopentanoic acid (AP-5), and 7-chloro-kynurenate (7-CK) were purchased from Research Biochemicals (Natick, MA), veratridine and tertaethylammonium chloride (TEA) from Aldrich Chemical Company (Milwaukee, WI), 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) from Biomol Research Laboratories (Plymouth Meeting, PA), tetrodotoxin (TTX) and quinidine from Sigma, and BAPTA-AM from Molecular Probes. Chemicals were dissolved in water, 95% ethanol, or DMSO. When ethanol or DMSO was used as a solvent for a given reagent, appropriate control experiments were conducted using vehicle alone. Ethanol and DMSO concentrations were always ≤0.2%.
Cells were loaded with BAPTA by exposing them to HBS containing 30 μm BAPTA-AM for 45 min. BAPTA-AM was present in the medium throughout the entire experiment.
Statistical analysis. Data are expressed as mean ± SE. Student’s two-way t test for independent means was performed. Statistical significance was defined as p ≤ 0.05. Unless stated otherwise, n is reported as number of experiments; number of cells.
RESULTS
Validation of cell volume measurement technique
To estimate cell volume changes, somal cross-sectional areas were converted to relative cell volumes using Equation 2: Relative Cell Volume = (Experimental CSA/Control CSA)3/2 (2)
This approach assumes that the soma swells and shrinks in a symmetrical manner as if it were a sphere. We validated this assumption by measuring cell volume changes directly using optical sectioning techniques (Strange and Spring, 1986). Briefly, the microscope was focused on the bottom of a single neuronal soma. A video image was then recorded and the microscope was step focused through the cell via a computer-controlled stepping motor attached to the fine focus drive. At each 1.2 μm of focal displacement, a video image was recorded until the top of the cell was reached. At a later time, the CSA of each image was measured and cell volume was calculated as described previously (Persson and Spring, 1982).
Cell volume changes were measured by optical sectioning in neurons 5 min after exposure to a hypertonic (400 mOsm) or hypotonic (212 mOsm) solution, and 16 min after exposure to 100 μmveratridine. In addition, a single image was chosen randomly from each pair (i.e., control and experimental) of optical sections, and the cell volume change was calculated according to Equation 2. As shown in Table1, there was no significant difference between cell volume changes measured directly by optical sectioning and those calculated from CSA measurements. These results demonstrate that the neuronal soma swells and shrinks as if it were a sphere. Therefore, in all subsequent experiments, cell volume changes were calculated according to Equation 2 with the use of CSA measurements obtained from images of neuronal soma recorded at a single focal plane.
Table 1.
Comparison of relative cell volume determined directly by optical sectioning and calculated from CSA measurements of soma imaged at a single focal plane
| Experiment | Relative cell volume calculated from CSA measurements | Relative cell volume measured by optical sectioning |
|---|---|---|
| Veratridine-induced swelling1-a (n = 4;4) | 1.44 ± 0.13 | 1.44 ± 0.16 p < 1.0 |
| Hypotonic1-b | 1.24 ± 0.03 | 1.27 ± 0.04 |
| (n = 5;5) | p < 0.58 | |
| Hypertonic1-b | 0.82 ± 0.03 | 0.83 ± 0.03 |
| (n = 4;4) | p < 0.87 |
Values are mean ± SE.
Images were recorded before and 16 min after addition of 100 μm veratridine.
Images were recorded before and 5 min after exposure to hypotonic (212 mOsm) or hypertonic (400 mOsm) saline.
Veratridine causes neuronal swelling by stimulation of net Na+ influx
As shown in Figure 1, exposure of cortical neurons in astrocyte-poor cultures to 100 μm veratridine induced rapid and dramatic cell swelling. The mean ± SE maximal volume increase induced by veratridine was 1.86 ± 0.1 (n= 12;17). Peak cell swelling typically was reached 10–15 min after addition of veratridine to the bath. Of the 17 neurons examined, 12 remained swollen throughout the duration of the experiment. Five of the neurons examined showed what seemed to be partial volume recovery. These neurons returned to within 7–67% of their control volume 70 min after the peak veratridine-induced swelling was reached. Overall, there was no statistically significant difference (p< 0.41) between the peak volume increase induced by veratridine and the volume observed 70 min after addition of the drug to the bath.
Fig. 1.
Effect of 100 μm veratridine exposure on neuronal somal volume (n = 12;17). Veratridine (VT) was added as indicated by thearrow. Values are mean ± SE.
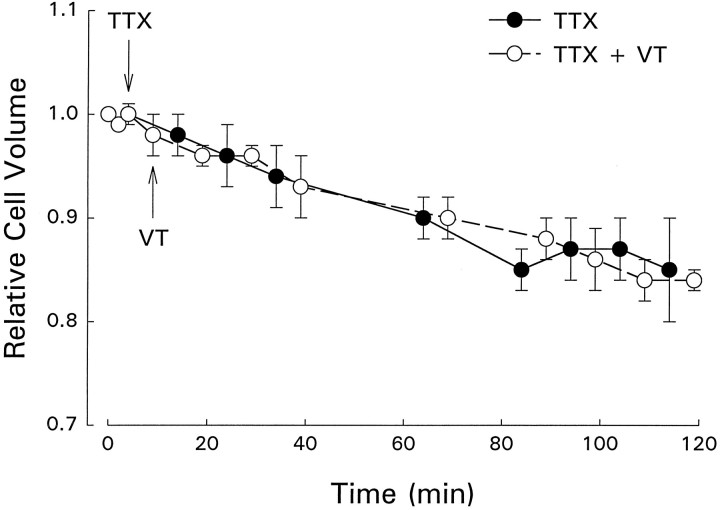
Veratridine prevents inactivation of voltage-dependent Na+channels, suggesting that the observed cell swelling was a result of stimulation of net Na+ influx. To test this idea directly, we exposed neurons to 5 μm tetrodotoxin alone and in combination with veratridine. As shown in Figure 2, TTX induced a mean ± SE cell shrinkage of 15 ± 5% (n = 3;3). The rate of cell volume decrease was slow; maximal shrinkage was observed 50–60 min after addition of TTX to the bath (Fig. 2). Neurons treated with TTX did not swell in the presence of veratridine (Fig. 2). The pattern of cell volume change in the presence of TTX and veratridine was similar to that observed with TTX alone (Fig. 2).
Fig. 2.
Effect of 5 μm TTX in the presence or absence of 100 μm veratridine (VT) on neuronal somal volume. TTX and VT were added as indicated by the arrows. Values are mean ± SE (TTX, n = 3;3; TTX + VT,n = 3;3).
If veratridine-induced cell swelling is a result of net Na+influx, it should also be blocked by removal of Na+ from the bath. As shown in Figure 3, replacement of bath Na+ with N-methyl-d-glucamine (NMDG) caused neurons to shrink 28 ± 7% (mean ± SE;n = 3;3). Veratridine-induced cell swelling was blocked completely in Na+-free medium (Fig. 3). The patterns of volume change observed in the presence and absence of veratridine were similar (Fig. 3). Taken together, the data described above indicate that veratridine-induced cell swelling is a result of stimulation of net Na+ influx. The most likely route of entry is via TTX-sensitive Na+ channels.
Fig. 3.
Effect of extracellular Na+ removal in the presence or absence of 100 μm veratridine (VT) on neuronal somal volume. Sodium was replaced by NMDG and veratridine was added as indicated by thearrows. Values are mean ± SE (0 Na+,n = 2;3; 0 Na+ + VT,n = 3;3).
The slow cell shrinkage observed with TTX exposure (Fig. 2) indicates that basal Na+ channel activity provides a pathway for Na+ influx into the cell. Under steady-state conditions, Na+ influx and efflux are balanced and cell volume remains constant. When the channels are blocked by TTX, however, net Na+ efflux occurs. Anions accompany Na+ lost from the cell, osmotically obliged water follows, and the cells shrink.
Removal of extracellular Na+ also caused cell shrinkage. Both the rate and the extent of shrinkage, however, were substantially larger than those observed with TTX (compare Figs. 2 and 3). Substitution of extracellular Na+ with impermeant cations such as NMDG reverses the normally inwardly directed Na+gradient. This reversal in turn causes Na+ loss via reversal of numerous Na+-dependent transport systems.
Veratridine-swollen neurons undergo RVD when treated with NMDA receptor antagonists
High levels of extracellular glutamate cause cell swelling and excitotoxic injury to neurons (Choi et al., 1987; Ramnath et al., 1992). Because glutamate is expected to be released from neurons in response to veratridine-induced membrane depolarization, we examined the effects of various NMDA receptor antagonists on cell swelling. As shown in Figure 4, pretreatment of neurons with 10 μm MK-801, a noncompetitive NMDA receptor antagonist, had no effect on the extent of veratridine-induced cell swelling. The mean ± SE peak swelling in the presence of MK-801 was 1.80 ± 0.07, which was not significantly different (p < 0.65) from that observed with veratridine alone (see Fig. 1). Cells exposed to 10 μm MK-801, however, underwent substantial RVD. The mean ± SE relative cell volume 70 min after peak swelling was reached was 1.03 ± 0.07 (n = 13;16). This value was significantly different (p < 0.0003) from that observed with veratridine alone. Of the 16 neurons examined in these experiments, all underwent significant volume recovery. RVD with MK-801 was seen at concentrations as low as 100 nm (Table 2). DIC images illustrating the effects of MK-801 (10 μm) on neuronal volume at various times after the addition of veratridine to the bath are shown in Figure 5.
Fig. 4.
Effect of 10 μm MK-801 on veratridine-induced cell swelling. Cells were pretreated with MK-801 for 5 min before 100 μm veratridine (VT) was added to the bath (arrow). Values are mean ± SE (n = 13;16).
Table 2.
Effect of MK-801 concentration on mean relative cell volume at peak veratridine-induced swelling and after long-term veratridine exposure
| Experiment | Relative cell volume 15–20 min after veratridine exposure | Relative cell volume 85–90 min after veratridine exposure |
|---|---|---|
| MK-801 (1 μm) | 1.62 ± 0.17 | 0.92 ± 0.06 |
| (n = 2;3) | p < 0.06 | |
| MK-801 (100 nm) | 1.68 ± 0.13 | 0.91 ± 0.06 |
| (n = 4;4) | p < 0.006 | |
| MK-801 (10 nm) | 1.84 ± 0.04 | 1.77 ± 0.13 |
| (n = 2;3) | p < 0.64 |
Cultures were equilibrated with MK-801 for 5 min before addition of 100 μm veratridine to the bath. Values are mean ± SE.
Fig. 5.
Representative DIC images of veratridine (VERAT)-treated neurons in the presence or absence of 10 μm MK-801. Cells were exposed to 100 μm veratridine at 0 min. In the absence of MK-801, neurons remain swollen and take on a refractile appearance. Cells treated with MK-801, however, undergo a complete volume recovery and have a normal appearance.
RVD was observed when cells were treated with competitive and other noncompetitive NMDA receptor antagonists. Shown in Table3 are the relative cell volumes at peak swelling compared with cell volumes measured after long-term exposure to veratridine and the NMDA antagonists 7-CK and AP-5, and CNQX (with no added glycine). A significant difference between mean peak volume and the volume measured 70 min after peak swelling was seen with all three antagonists.
Table 3.
Effect of NMDA antagonists on mean relative cell volume at peak veratridine-induced swelling and after long-term veratridine exposure
| Experiment | Relative cell volume 15–20 min after veratridine exposure | Relative cell volume 85–90 min after veratridine exposure | Neurons undergoing RVD (%) |
|---|---|---|---|
| 100 μm 7-CK | 1.78 ± 0.03 | 1.15 ± 0.06 | 100 |
| (n = 5;5) | p < 0.0001 | ||
| 5 mm AP-5 | 1.84 ± 0.18 | 1.25 ± 0.11 | 100 |
| (n = 4;5) | p < 0.03 | ||
| 100 μm CNQX (no added glycine) | 1.65 ± 0.14 | 0.95 ± 0.09 | 100 |
| (n = 5;5) | p < 0.006 |
Cultures were preincubated with NMDA antagonists for 5 min before the addition of 100 μm veratridine to the bath. Values are mean ± SE.
We also examined the effects of inhibition of non-NMDA receptors on veratridine-induced cell swelling. At a concentration of 100 μm, CNQX inhibits both the NMDA and non-NMDA classes of ionotropic glutamate receptors. NMDA receptor inhibition occurs by binding of CNQX to the receptor glycine site. This antagonism can be overcome, however, with 1 mm glycine (Yamada et al., 1989;Koh and Choi, 1991). We therefore exposed neurons to 100 μm CNQX in the presence of 1 mm glycine to selectively inhibit non-NMDA receptors. As shown in Figure6, cells treated with CNQX plus glycine remained swollen after veratridine exposure. Complete RVD was observed, however, when cells were exposed to CNQX in the absence of glycine (Table 3).
Fig. 6.
Effect of 100 μm CNQX and 1 mm glycine on veratridine-induced cell swelling. Values are mean ± SE (n = 8;13). Cells were pretreated with CNQX and glycine for 5 min before 100 μm veratridine (VT) was added to the bath (arrow).
RVD is elicited by delayed exposure of neurons to MK-801
In the studies described above, MK-801 was present in the bathing medium when the cells were exposed to veratridine. We therefore determined whether RVD could be elicited by delayed exposure to MK-801. Neurons were first exposed to veratridine alone to induce cell swelling. After 25 or 55 min of veratridine exposure, 10 μm MK-801 was added to the bathing medium. As shown in Figure 7, RVD occurred normally when MK-801 was added 25 min after induction of cell swelling. Volume regulation did not occur when MK-801 was added at later times (Fig. 7).
Fig. 7.
Effect of delayed treatment with 10 μm MK-801 on somal volume of veratridine-treated neurons. MK-801 was added 25 min (filled circles,n = 3;3) or 55 min (open circles,n = 6;6) after the addition of 100 μmveratridine to the bath (arrow). Values are mean ± SE.
MK-801-stimulated RVD is inhibited by K+ and anion channel blockers
Regulatory volume decrease in most mammalian cell types is mediated by activation of K+ and anion channels (Chamberlin and Strange, 1989; Hoffmann and Simonsen, 1989; Hallows and Knauf, 1994). As shown in Figure 8, MK-801-stimulated RVD was inhibited nearly completely by exposure of the cells to 50 μm NPPB, a known anion channel blocker. Volume regulation was also inhibited by treatment of cells with the K+channel blockers 1 mm quinidine and 5 mmBa2+ (Fig. 8), but not by 10 or 25 mm TEA (data not shown). In the presence of NPPB, quinidine, or Ba2+, there was no significant difference (p < 0.23) between the peak swelling volume and the cell volume measured 70 min after peak swelling was attained.
Fig. 8.
Effect of K+ and Cl−channel blockers on MK-801-elicited RVD. RVD is blocked by 50 μm NPPB (n = 7;7), 5 mmBa2+ (n = 6;7), or 1 mmquinidine (n = 9;9). Values are mean ± SE. Cells were pretreated with 10 μm MK-801 for 5 min before 100 μm veratridine (VT) was added to the bath (arrow). K+ and Cl−channel blockers were added 20 min after veratridine.
Role of intracellular Ca2+ in regulating MK-801-elicited RVD
Increases in intracellular Ca2+ are thought to regulate RVD transport pathways activated by hypotonic swelling in various cell types such as astrocytes (O’Connor and Kimelberg, 1993;Bender and Norenberg, 1994), frog urinary bladder cells (Davis and Finn, 1987), proximal tubule cells (Suzuki et al., 1990), and osteosarcoma cells (Yamaguchi et al., 1989). We therefore examined the role of intracellular Ca2+ in controlling MK-801-elicited RVD in response to veratridine-induced cell swelling. As shown in Figure 9, fura-2 AM measurements revealed that veratridine exposure caused a very rapid increase in intracellular Ca2+. Calcium concentration rose from resting levels of 15–30 nm to 600–800 nm 30 sec after addition of veratridine to the bath. This increase was followed by a rapid decline in Ca2+ levels. Thirty minutes after veratridine exposure, intracellular Ca2+ concentration was ∼150 nm.
Fig. 9.
Veratridine-induced changes in intracellular Ca2+ concentration. Fura-2 AM emission ratios were measured in neuronal cell bodies. Veratridine (VT) was added to the bath as indicated by the arrow. Cells were treated with 100 μm veratridine alone (solid points) or 100 μm veratridine plus 10 μm MK-801 (open points). MK-801 was added to the bath 5 min before veratridine addition. Values are mean ± SE (VT, n = 5;26; VT + MK-801,n = 5;30).
The veratridine-induced increase in intracellular Ca2+ was similar when cells were first treated with MK-801. In the MK-801-treated cells, however, there was a more rapid and extensive fall in intracellular Ca2+ levels. The mean intracellular Ca2+ concentration 30 min after veratridine exposure was ∼45 nm. This value was significantly different (p < 0.0001) from that observed in cells treated with veratridine alone.
MK-801 was also capable of enhancing the rate of reduction of intracellular Ca2+ when added after veratridine. As shown in Figure 10, intracellular Ca2+ levels were nearly stable or declining slowly at rates of 1–2 nm/min ∼25 and ∼60 min after veratridine exposure. Addition of 10 μm MK-801 to that bath at these times increased the rate of intracellular Ca2+ decline to ∼28 nm/min.
Fig. 10.
Effect of delayed addition of MK-801 on intracellular Ca2+ levels. Cells were exposed to 100 μm veratridine for ∼25 and ∼60 min before addition of 10 μm MK-801 to the bath (arrows). Values are mean ± SE (left, n = 3–5;13–27; right, n = 3;25).
If intracellular Ca2+ plays a role in regulating the RVD transport pathways, prevention of the Ca2+ increase should inhibit volume recovery. As shown in Figure 11, removal of extracellular Ca2+ blocked the MK-801-elicited RVD response and completely prevented the veratridine-induced increase in intracellular Ca2+. Loading the cells with 30 μm BAPTA, an intracellular Ca2+ chelator, dramatically reduced the Ca2+ increase and blocked MK-801-elicited RVD. Taken together, data in Figures 9 and 11 indicate that elevation of intracellular Ca2+ levels is required for activation of RVD transport pathways.
Fig. 11.
A, Effect of Ca2+-free medium and intracellular BAPTA loading on MK-801-induced RVD. Values are mean ± SE (Ca2+ removal, n = 7;8; BAPTA loading, n = 4;5). B,Effect of Ca2+-free medium and intracellular BAPTA loading on intracellular Ca2+ levels. Values are mean ± SE (Ca2+ removal, n = 1;9; BAPTA loading,n = 2;11). For the experiments shown in both panels, cells were pretreated with 10 μm MK-801 for 5 min before addition of 100 μm veratridine (VT) to the bath (arrows).
DISCUSSION
Volume recovery in neurons after isotonic swelling
Cell volume can be altered by changes in extracellular osmolality (anisotonic volume change) or intracellular solute content (isotonic volume change). Brain cells are normally protected from anisotonic volume perturbations by precise renal control of plasma osmolality. Various forms of brain injury, however, can lead to profound isotonic swelling in both glia and neurons (Kimelberg, 1995). In addition, normal neural activity can cause isotonic cell swelling or shrinkage (Dietzel et al., 1980; Ransom et al., 1985; Serve et al., 1988; Lieke et al., 1989; McBain et al., 1990). Cell volume perturbations induced by neural activity must be corrected rapidly to maintain brain function.
Extensive studies of volume regulation after anisotonic swelling have been performed in astrocytes (for review, see Kimelberg and Goderie, 1988; Schousboe and Pasantes-Morales, 1992; Kimelberg, 1995). There has been comparatively little work, however, on the volume-regulatory physiology of neurons. Almost nothing is known about how neurons respond to isotonic volume changes. We have begun to investigate this problem by exposing neurons to veratridine, which prevents inactivation of voltage-gated Na+ channels. Veratridine induces a nearly twofold increase in the volume of cortical neurons (Fig. 1). This swelling is a result of the influx of Na+ into the cell. The most likely major route of entry is through TTX-sensitive Na+ channels (Figs. 2, 3). Anions enter the cell through undefined pathways, and osmotically obliged water follows passively.
Our results are comparable to those obtained by Lipton (1973) nearly 25 years ago in guinea pig cerebral cortical brain slices. Lipton monitored decreases in tissue reflectance as a measure of cell swelling. Exposure of brain slices to veratridine caused a decrease in tissue reflectance that was blocked by TTX and by replacement of extracellular Cl− with glucuronate. Decreases in reflectance were also observed in response to depolarizing pulses (Lipton, 1973). This finding, together with the observed effects of veratridine and TTX, suggests that the reflectance changes were a result of swelling of cortical neurons.
In our studies, we observed that neurons exposed to veratridine alone remain swollen for at least 90 min (Fig. 1). When pretreated with MK-801, as well as other noncompetitive and competitive NMDA antagonists, however, the cells underwent RVD (Figs. 4, 6; Table 3). The ability of 7-CK, AP-5, and CNQX (with no added glycine) to elicit RVD indicates that the observed effects were not a result of nonspecific actions of MK-801. Instead, they seem to be the result of selective inhibition of NMDA receptors.
The NMDA receptor antagonist MK-801 was capable of eliciting RVD when administered at times after veratridine exposure. It is of interest that this effect exhibited a distinct temporal sensitivity. When administered 25 min after veratridine, MK-801 induced a complete RVD response. No RVD was seen, however, when the drug was given 60 min after veratridine (Fig. 7). This result suggests that prolonged activation of NMDA receptors somehow disrupts neuronal osmoregulatory capabilities (discussed below).
MK-801-elicited RVD is blocked by conventional K+ and Cl− channel inhibitors, which suggests that it is mediated by swelling-activated anion and cation channels (Fig. 8). Mechanisms of RVD similar to those described in this paper have been observed in hypotonically swollen astrocytes (Medrano and Gruenstein, 1993;O’Connor and Kimelberg, 1993; Vitarella et al., 1994) and PC12 cells (Delpire et al., 1991). The efflux of organic osmolytes such as taurine, glutamate, aspartate, and myo-inositol has also been proposed to play an important role in RVD in cultured astrocytes (Kimelberg et al., 1990a; Pasantes-Morales et al., 1993a; Moran et al., 1994; Vitarella et al., 1994), cultured neurons (Schousboe and Pasantes-Morales, 1992; Pasantes-Morales et al., 1993a,b), and the intact brain (Gullans and Verbalis, 1993). In both astrocytes and neurons, taurine efflux can be elicited by either hypotonic or high K+-induced isotonic swelling (Martin et al., 1990;Kimelberg et al., 1990; Schousboe and Pasantes-Morales, 1992). Further studies are needed to determine whether organic osmolytes participate in MK-801-elicited RVD in cortical neurons and to define the functional properties of the RVD K+ and Cl− channels. In addition, it will be important to carry out studies in the presence of CO2/HCO3. The experiments described in this paper were performed in HEPES-buffered saline. It is possible that under more physiological conditions, other transport processes may contribute to both veratridine-induced swelling and MK-801-elicited RVD.
Role of Ca2+ in MK-801-elicited RVD
Veratridine exposure induced a rapid and dramatic increase in intracellular Ca2+ (Fig. 9). The increase was blocked by extracellular Ca2+ removal (Fig. 11), which indicated that the rise is a result of enhanced Ca2+ influx. Calcium influx is most likely mediated by voltage-dependent Ca2+channels activated in response to veratridine-induced membrane depolarization. Changes in both the rate and direction of Na+–Ca2+ exchange may also contribute to the rise in intracellular Ca2+ levels. Veratridine-induced membrane depolarization and elevation of intracellular Na+will inhibit and likely reverse the Na+–Ca2+exchanger.
The veratridine-induced rise in intracellular Ca2+ is required for activation of MK-801-elicited RVD transport pathways. Removal of extracellular Ca2+ or intracellular Ca2+ chelation with BAPTA completely inhibited RVD (Fig.11). Additional studies are required to assess the mechanisms by which intracellular Ca2+ controls neuronal RVD pathways.
Changes in intracellular Ca2+ have been implicated in the control of volume-regulatory pathways in a variety of cell types (for review, see McCarty and O’Neil, 1992). For example, detailed studies by O’Connor and Kimelberg (1993) have shown that RVD in cortical astrocytes after hypotonic swelling is Ca2+ dependent. Astrocyte swelling causes an increase in intracellular Ca2+that seems to be brought about by both increased influx through L-type Ca2+ channels and release from intracellular stores. Removal of extracellular Ca2+ inhibits RVD and swelling-activated K+ and Cl− efflux. Similar findings have been made by Bender and Norenberg (1994).
Possible mechanisms by which NMDA receptor inhibition induces RVD
The observation that RVD requires inhibition of NMDA receptors is novel. Fast synaptic transmission in the CNS is mediated largely by depolarization-induced release of glutamate at excitatory synapses. Once released, glutamate binds to NMDA and non-NMDA receptor types. NMDA receptors are linked to voltage-sensitive, high conductance cation channels that are permeable to both Na+ and Ca2+ (Mayer and Westbrook, 1987). Normally, glutamate released into synapses is removed rapidly by neuronal and glial uptake mechanisms (Kanner and Schuldiner, 1987). These uptake mechanisms presumably fail during hypoxia, ischemia, and related insults, however, causing glutamate to accumulate in the extracellular space (Attwell et al., 1993). Activation of NMDA receptor-linked cation channels leads to an increase in intracellular Ca2+, which may cause further glutamate release (Choi, 1994).
Excessive activation of glutamate receptors causes irreversible injury leading to neuronal death, a process termed “excitotoxicity” (Choi, 1994). The first stage of excitoxic injury involves an influx of Na+, Cl−, and water into neurons, resulting in extensive cell swelling. Neuronal injury and death still occur even if cell swelling is prevented or reversed and are dependent on the presence of extracellular Ca2+ (Choi, 1988, 1994,1995).
Veratridine-induced membrane depolarization seems to cause glutamate release with subsequent activation of NMDA receptors (Rothman, 1985;Choi et al., 1988; Schramm et al., 1990; Ramnath et al., 1992). As shown in the present study, activation of these receptors inhibits neuronal volume regulation. The inhibition of volume control could occur by several mechanisms. One possibility is that activation of NMDA receptor-linked cation channels may simply provide another pathway for solute movement into the cell and increase the rate of net solute uptake. If the rate of volume-regulatory solute efflux does not exceed solute entry, RVD will not occur. This explanation seems unlikely, however. Veratridine induced similar degrees of cell swelling in the presence or absence of MK-801 (Figs. 1, 4), which suggests that NMDA receptor-linked cation channels do not contribute significantly to the net solute influx.
NMDA receptor activation may inhibit neuronal volume-regulatory pathways via elevation of intracellular Ca2+. On the surface, such a possibility seems somewhat paradoxical. As discussed above, an increase in intracellular Ca2+ is required for activation of RVD pathways (Fig. 11). How could RVD be both stimulated and inhibited by Ca2+? One possibility is that there is a “threshold” level of Ca2+ needed for activation of RVD pathways. Elevation of Ca2+ above this level, brought about by stimulation of NMDA receptors, might be toxic and disrupt osmoregulatory mechanisms. Evidence for a Ca2+ threshold is lacking, however. Intracellular Ca2+ levels rose at similar rates and to similar extents in cells treated with veratridine alone or veratridine plus MK-801 (Fig. 9). There was, however, a difference in both the rate and degree of Ca2+ recovery in the two groups of cells. In cells treated with veratridine only, intracellular Ca2+ levels fell more slowly (Fig. 9). Thirty minutes after treatment with veratridine alone, intracellular Ca2+ was ∼150 nm. In contrast, cell Ca2+ was ∼45 nm in cells exposed to both veratridine and MK-801. The prolonged elevation of Ca2+ in the veratridine-treated neurons may activate pathways that disrupt volume-regulatory mechanisms.
If prolonged elevation of Ca2+ does indeed disrupt RVD pathways, it is unlikely that this effect is simply a result of a Ca2+-mediated killing of the cells. As shown in Figures 7and 10, MK-801 was not capable of eliciting RVD when administered 55–60 min after veratridine exposure, but it did cause a rapid fall in intracellular Ca2+ levels. The fall must be a result of active Ca2+ extrusion and/or uptake into intracellular stores because Ca2+ influx through NMDA receptor-linked cation channels is blocked. This rapid fall in Ca2+indicates that the cells are still viable.
Whatever the mechanism responsible, our findings indicate that an early manifestation of excitotoxicity is loss of neuronal osmoregulatory capabilities. It is intriguing to speculate that some of the protective effects of drugs such as MK-801 may be mediated through their ability to prevent disruption of osmoregulatory pathways. Investigations designed to examine this possibility are clearly warranted.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants NS30591 and DK45628 to K.S. and NS31353, NS26830, and NS32570 to P.A.R.; by a Mental Retardation Center Core Grant to Children’s Hospital; and by NIH Grant DK49222 and National Science Foundation Grant IBN-9407997 to S.H.W. K.S. and P.A.R. are Established Investigators of the American Heart Association.
Correspondence should be addressed to Dr. Kevin Strange, Children’s Hospital, Enders 12, 320 Longwood Avenue, Boston, MA 02115.
REFERENCES
- 1.Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- 2.Bender AS, Norenberg MD. Calcium dependence of hypoosmotically induced potassium release in cultured astrocytes. J Neurosci. 1994;14:4237–4243. doi: 10.1523/JNEUROSCI.14-07-04237.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamberlin ME, Strange K. Anisosmotic cell volume regulation: a comparative view. Am J Physiol. 1989;257:C159–C173. doi: 10.1152/ajpcell.1989.257.2.C159. [DOI] [PubMed] [Google Scholar]
- 4.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 5.Choi DW. Glutamate receptors and the induction of excitotoxic neuronal death. Prog Brain Res. 1994;100:47–51. doi: 10.1016/s0079-6123(08)60767-0. [DOI] [PubMed] [Google Scholar]
- 6.Choi DW. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 7.Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi DW, Koh JY, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis CW, Finn AL. Interactions of sodium transport, cell volume, and calcium in frog urinary bladder. J Gen Physiol. 1987;89:687–702. doi: 10.1085/jgp.89.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delpire E, Cornet M, Gilles R. Volume regulation in rat pheochromocytoma cultured cells submitted to hypoosmotic conditions. Arch Int Physiol Biochim Biophys. 1991;99:71–76. doi: 10.3109/13813459109145906. [DOI] [PubMed] [Google Scholar]
- 11.Diamond JM. Transcellular cross-talk between epithelial cell membranes. Nature. 1982;300:683–685. doi: 10.1038/300683a0. [DOI] [PubMed] [Google Scholar]
- 12.Dietzel I, Heinemann U, Hofmeier G, Lux HD. Transient changes in the size of the extracellular space in the sensorimotor cortex of cats in relation to stimulus-induced changes in potassium concentration. Exp Brain Res. 1980;40:432–439. doi: 10.1007/BF00236151. [DOI] [PubMed] [Google Scholar]
- 13.Foskett JK, Melvin JE. Activation of salivary secretion: coupling of cell volume and Ca2+i in single cells. Science. 1989;244:1582–1585. doi: 10.1126/science.2500708. [DOI] [PubMed] [Google Scholar]
- 14.Grynkiewicz G, Poenie M, Tsein RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 15.Gullans S, Verbalis J. Control of brain volume during hyperosmolar and hypoosmolar conditions. Annu Rev Med. 1993;44:289–301. doi: 10.1146/annurev.me.44.020193.001445. [DOI] [PubMed] [Google Scholar]
- 16.Hallows KR, Knauf PA. Principles of cell volume regulation. In: Strange K, editor. Cellular and molecular physiology of cell volume regulation. CRC; Boca Raton, FL: 1994. pp. 3–29. [Google Scholar]
- 17.Harris KM, Rosenberg PA. Localization of synapses in rat cortical cultures. Neuroscience. 1993;53:495–508. doi: 10.1016/0306-4522(93)90214-z. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann EK, Simonsen LO. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989;69:315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- 19.Kanner BI, Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22:1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- 20.Kimelberg HK. Current concepts of brain edema. J Neurosurg. 1995;83:1051–1059. doi: 10.3171/jns.1995.83.6.1051. [DOI] [PubMed] [Google Scholar]
- 21.Kimelberg HK, Goderie SK. Volume regulation after swelling in primary astrocyte cultures. In: Norenberg MD, Hertz L, editors. Biochemical pathology of astrocytes. Liss; New York: 1988. pp. 299–311. [Google Scholar]
- 22.Kimelberg K, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh JY, Choi DW. Selective blockade of non-NMDA receptors does not block rapidly triggered glutamate-induced neuronal death. Brain Res. 1991;548:318–321. doi: 10.1016/0006-8993(91)91140-v. [DOI] [PubMed] [Google Scholar]
- 24.Lieke EE, Frostig RD, Arieli A, Ts’o DY, Hildesheim R, Grinvald A. Optical imaging of cortical activity: real-time imaging using extrinsic dye-signals and high resolution imaging based on slow intrinsic-signals. Annu Rev Physiol. 1989;51:543–559. doi: 10.1146/annurev.ph.51.030189.002551. [DOI] [PubMed] [Google Scholar]
- 25.Lipton P. Effects of membrane depolarization on light scattering by cerebral cortical slices. J Physiol (Lond) 1973;231:365–383. doi: 10.1113/jphysiol.1973.sp010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin DL, Madelian V, Seligmann B, Shain W. The role of osmotic pressure and membrane potential in K+-stimulated taurine release from cultured astrocytes and LRM55 cells. J Neurosci. 1990;10:571–577. doi: 10.1523/JNEUROSCI.10-02-00571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 28.McBain CJ, Traynelis SF, Dingledine R. Regional variation of extracellular space in the hippocampus. Science. 1990;249:674–677. doi: 10.1126/science.2382142. [DOI] [PubMed] [Google Scholar]
- 29.McCarty NA, O’Neil RG. Calcium signaling in cell volume regulation. Physiol Rev. 1992;72:1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- 30.Medrano S, Gruenstein E. Mechanisms of regulatory volume decrease in UC-11MG human astrocytoma cells. Am J Physiol. 1993;264:C1201–C1209. doi: 10.1152/ajpcell.1993.264.5.C1201. [DOI] [PubMed] [Google Scholar]
- 31.Moran J, Maar TE, Pasantes-Morales H. Impaired cell volume regulation in taurine deficient cultured astrocytes. Neurochem Res. 1994;19:415–420. doi: 10.1007/BF00967318. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor ER, Kimelberg HK. Role of calcium in astrocyte volume regulation and in the release of ions and amino acids. J Neurosci. 1993;13:2638–2650. doi: 10.1523/JNEUROSCI.13-06-02638.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasantes-Morales H, Alavez S, Sanchez Olea R, Moran J. Contribution of organic and inorganic osmolytes to volume regulation in rat brain cells in culture. Neurochem Res. 1993a;18:445–452. doi: 10.1007/BF00967248. [DOI] [PubMed] [Google Scholar]
- 34.Pasantes-Morales H, Maar TE, Moran J. Cell volume regulation in cultured cerebellar granule neurons. J Neurosci Res. 1993b;34:219–224. doi: 10.1002/jnr.490340209. [DOI] [PubMed] [Google Scholar]
- 35.Persson BE, Spring KR. Gallbladder epithelial cell hydraulic water permeability and volume regulation. J Gen Physiol. 1982;79:481–505. doi: 10.1085/jgp.79.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramnath RR, Strange K, Rosenberg PA. Neuronal injury evoked by depolarizing agents in rat cortical cultures. Neuroscience. 1992;51:931–939. doi: 10.1016/0306-4522(92)90530-f. [DOI] [PubMed] [Google Scholar]
- 37.Ransom BR, Yamate CL, Connors BW. Activity-dependent shrinkage of extracellular space in rat optic nerve: a developmental study. J Neurosci. 1985;5:532–535. doi: 10.1523/JNEUROSCI.05-02-00532.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg PA. Accumulation of extracellular glutamate and neuronal death in astrocyte-poor cortical cultures exposed to glutamine. Glia. 1991;4:91–100. doi: 10.1002/glia.440040111. [DOI] [PubMed] [Google Scholar]
- 39.Rothman SM. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985;5:1483–1489. doi: 10.1523/JNEUROSCI.05-06-01483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schousboe A, Pasantes-Morales H. Role of taurine in neural cell volume regulation. Can J Physiol Pharmacol [Suppl] 1992;70:S356–S361. doi: 10.1139/y92-283. [DOI] [PubMed] [Google Scholar]
- 41.Schramm M, Eimerl S, Costa E. Serum and depolarizing agents cause acute neurotoxicity in cultured cerebellar granule cells: role of the glutamate receptor responsive to N-methyl-d-aspartate. Proc Natl Acad Sci USA. 1990;87:1193–1197. doi: 10.1073/pnas.87.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schultz SG. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by “flush-through”. Am J Physiol. 1981;241:F579–F590. doi: 10.1152/ajprenal.1981.241.6.F579. [DOI] [PubMed] [Google Scholar]
- 43.Schultz SG, Hudson RL. How do sodium-absorbing cells do their job and survive? News Physiol Sci. 1990;1:185–188. [Google Scholar]
- 44.Serve G, Endres W, Grafe P. Continuous electrophysiological measurements of changes in cell volume of motoneurons in the isolated frog spinal cord. Pflügers Arch. 1988;411:410–415. doi: 10.1007/BF00587720. [DOI] [PubMed] [Google Scholar]
- 45.Strange K, Spring KR. Methods for imaging renal tubule cells. Kidney Int. 1986;30:192–200. doi: 10.1038/ki.1986.171. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki M, Kawahara K, Ogawa A, Morita T, Kawaguchi Y, Kurihara S, Sakai O. [Ca2+]i rises via G protein during regulatory volume decreases in rabbit proximal tubule cells. Am J Physiol. 1990;258:F690–F696. doi: 10.1152/ajprenal.1990.258.3.F690. [DOI] [PubMed] [Google Scholar]
- 47.Takemura T, Sato F, Saga K, Suzuki Y, Sato K. Intracellular ion concentrations and cell volume during cholinergic stimulation of eccrine secretory coil cells. J Membr Biol. 1991;119:211–219. doi: 10.1007/BF01868726. [DOI] [PubMed] [Google Scholar]
- 48.Vitarella D, DiRisio DJ, Kimelberg HK, Aschner M. Potassium and taurine release are highly correlated with regulatory volume decrease in neonatal primary rat astrocyte cultures. J Neurochem. 1994;63:1143–1149. doi: 10.1046/j.1471-4159.1994.63031143.x. [DOI] [PubMed] [Google Scholar]
- 49.Yamada KA, Dubinsky JM, Rothman SM. Quantitative physiological characterization of a quinoxalinedione non-NMDA receptor antagonist. J Neurosci. 1989;9:3230–3236. doi: 10.1523/JNEUROSCI.09-09-03230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi DT, Green J, Kleeman CR, Muallem S. Characterization of volume-sensitive, calcium-permeating pathways in the osteosarcoma cell line UMR-106-01. J Biol Chem. 1989;264:4383–4390. [PubMed] [Google Scholar]