Abstract
Background
Behavioral and psychological symptoms of dementia (BPSD) are prevalent in people with neurodegenerative diseases.
Purpose
In this scoping review the Kales, Gitlin and Lykestos framework is used to answer the question: What high quality evidence exists for the patient, caregiver and environmental determinants of five specific BPSD: aggression, agitation, apathy, depression and psychosis?
Method
An a priori review protocol was developed; 692 of 6013 articles retrieved in the search were deemed eligible for review. Gough’s Weight of Evidence Framework and the Cochrane Collaboration’s tool for assessing risk of bias were used. The findings from 56 high quality/low bias articles are summarized.
Discussion
Each symptom had its own set of determinants, but many were common across several symptoms: neurodegeneration, type of dementia, severity of cognitive impairments, and declining functional abilities, and to a lesser extent, caregiver burden and communication.
Conclusion
Research and policy implications are relevant to the National Plan to Address Alzheimer’s Disease.
Keywords: Behavioral and psychological, symptoms of dementia, Determinants, Scoping review
Introduction
Worldwide, 47.5 million people have dementia, a clinical syndrome caused by a number of neurodegenerative diseases (World Health Organization, 2016). The vast majority of these individuals exhibit behavioral and psychological symptoms of dementia (BPSD), which are distressing perceptions, thought content and mood, and behaviors such as aggression and apathy (Kales, Gitlin, Lyketsos, & Detroit Expert Panel on Assessment Management of Neuropsychiatric Symptoms of Dementia, 2014). BPSD result in precipitous declines in function, risk for physical abuse, poor quality of life, caregiver burden, and they account for more than one-third of all dementia-related costs (Adelman, Tmanova, Delgado, Dion, & Lachs, 2014; Herrmann et al., 2006; Toot, Swinson, Devine, Challis, & Orrell, 2017). Although national and international efforts have resulted in progress toward humanizing dementia care, BPSD continue to be treated mainly with antipsychotic drugs and other restraining methods that result in excess mortality, falls, and social isolation from the sedating effects of these drugs (Wunderlich & Kohler, 2000). This is in spite of Food and Drug Administration warnings about antipsychotic medications and consistent recommendations from experts in leading geriatric organizations that call for the use of nonpharmacologic approaches as the first line of treatment (American Geriatrics Society & American Association for Geriatric Psychiatry, 2003).
The effectiveness of any treatment for BPSD relies on accurate identification of the precipitating cause(s) or determinant(s) of the symptom, which then become the target of intervention (Kales, Gitlin, & Lyketsos, 2015). Neurodegeneration has been a primary target of pharmaceutical research, but there are other factors that contribute to BPSD (Casanova, Starkstein, & Jellinger, 2011). Nursing science has a rich history of conceptualizing BPSD as expressions of unmet needs within frameworks, where a number of pathophysiologic, psychological, and environmental determinants are hypothesized to underlie BPSD (Algase et al., 1996; Hall & Buckwalter, 1987). These frameworks have guided research but rarely have large effect sizes been demonstrated for the interventions derived from them (Livingston et al., 2014). Pharmaceutical treatments directed at pathophysiologic targets have also shown disappointing effects.
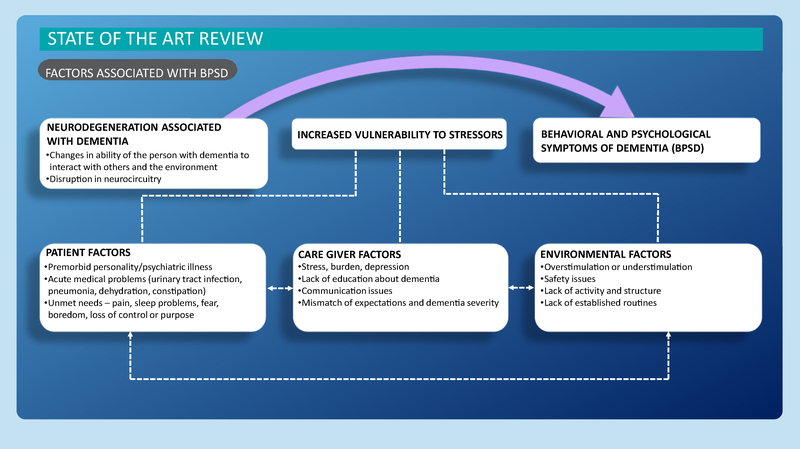
A recent framework for BPSD is that conceptualized by Kales et al. (2015). Figure 1 illustrates their model, which builds on prior frameworks and the advances made in neuroscience, genetics, and caregiving during the past 20 years. Importantly, this framework provides a more comprehensive range of potential patient, caregiver, and environmental determinants of BPSD than was available in the past. The identification of determinants of BPSD is needed for the development of efficacious person-centered interventions. Rather than mask BPSD, which may be the individual’s only method for communicating distress, interventions designed around evidence-based determinants target their root cause.
Figure 1 —
Theoretical framework. Reproduced from Kales et al. (2015) with permission from BMJ Publishing Group Ltd.
BPSD include many different symptoms often measured inconsistently and imprecisely as one construct. In the past, few systematic reviews have focused on individual clinical symptoms but more commonly their aggregate. Anticipating that the literature on individual symptoms would be complex and heterogeneous, we conducted a scoping review, as opposed to a systematic review. In this approach, the existing evidence base for individual symptoms is examined regardless of the heterogeneity of the literature, key determinants are identified, the quality of evidence is evaluated, and gaps in research are identified–all objectives of scoping reviews (Peters et al., 2015). Thus, the aim of this scoping review was to use the framework developed by Kales et al. (2015) to answer the question: What high-quality evidence exists for the patient, caregiver, and environmental determinants of five specific BPSD: aggression, agitation, apathy, depression, and psychosis?
This scoping review is the outcome of a presummit activity of the 2017 National Research Summit on Dementia Care: Care and Services for Persons with Dementia, Family Members and Caregivers (https://aspe.hhs.gov/report/national-plan-address-alzheimers-disease-2016-update). To that purpose, we conclude with a discussion of broad research and policy implications that are informed by the review and discussed at the National Summit.
Methods
An a priori scoping review protocol was developed by the team and included the aim of the project, definition of key concepts, and methods. The search strategy was designed to find published studies that reported on the association of patient, caregiver, and environmental determinants of BPSD. The categories of determinants we examined were those identified in the framework by Kales et al. (2015). Patient determinants were defined as individual characteristics that put the person at risk for BPSD. Caregiver determinants included characteristics of the caregiver and the quality of the dyadic relationship. Environmental determinants encompassed the qualities or characteristics of the physical and psychosocial environments. Caregiver and environmental determinants were viewed as precipitating causes of BPSD. To comprehensively assess the literature, specific determinants were not predefined for inclusion. Two of the authors (L.N.G. and H.C.K.) who developed the guiding framework provided examples of possible determinants that might be examined in the literature. A list of those examples is included in Appendix A.
Article Selection
All study designs as well as systematic reviews and meta-analyses were included. Editorials, commentaries, case studies, expert opinions, and studies that focused on participants with mild cognitive impairment exclusively or delirium were excluded. Also excluded were pharmacologic studies because of the vast amount of literature and the availability of existing systematic reviews in this area. The unpublished gray literature was not searched.
Inclusion/Exclusion Criteria
Predetermined inclusion and exclusion criteria were used to identify articles relevant to the research question. For the sample, studies enrolling adults 55 years or older, with dementia and BPSD, and who resided in any setting (community, assisted living, or nursing home) were included. The dementia diagnosis had to be established using a standard and valid approach such as a validated instrument for diagnosis, physician diagnosis, and/or autopsy confirmation. Questions about the validity of any dementia assessment tool were resolved by H.C.K., a geriatric psychiatrist.
Procedure
Systematic comprehensive searches were created and executed by a research librarian (A.K.). A combination of controlled vocabulary and text terms were searched in the titles and abstracts. Searches were limited to human studies, English language publications, and studies of middle-aged/aged adults 55+ years. Several basic searches were used to identify key terms and subject headings. Reviews and resources developed by the Cochrane Dementia and Cognitive Improvement Review Group were consulted to help develop the search strategy. The initial keywords and the full search strategy are provided in Appendix B.
MEDLINE/PubMed, PsycInfo, CINAHL, and Cochrane Library were searched from January 1, 1992 to March 1, 2016, a period judged to include the most important dementia work. The search was updated on April 1, 2016.
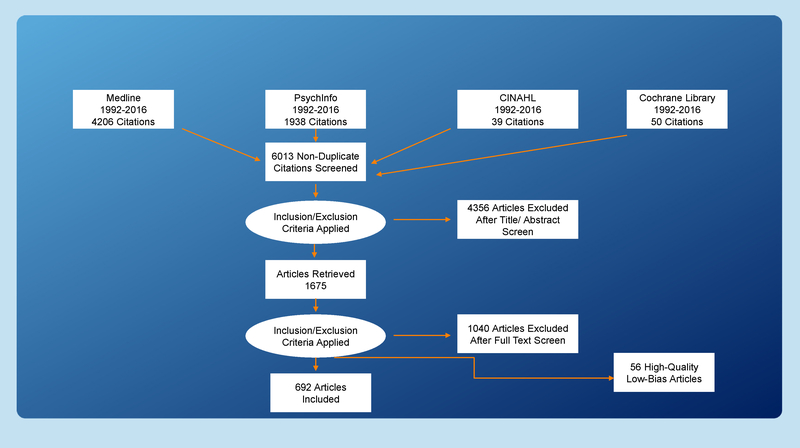
Search results were exported to EndNote (Clarivate Analytics, Philadelphia, PA). Before the initiation of screening, duplicate records were removed, resulting in 6,013 unique records. These articles were then exported to an excel file containing the extraction fields.
Quality Rating
Data extraction was done in two phases: a practical review and a methodological review (Fink, 2010). In the practical review, the title and abstract of each of the 6,013 articles retrieved in the search was reviewed independently for inclusion by a team of two reviewers. Disagreements were resolved by consensus and resulted in 1,675 articles selected for full review. In the full-text review, inclusion/exclusion criteria were applied again, yielding 692 articles eligible for methodological review. The 692 articles were then rated for quality using Gough’s Weight of Evidence Framework (low, medium, or high; Gough, 2007) and the Cochrane Collaboration’s tool for assessing risk of bias (low, unclear, or high; Zeng et al., 2015). A random sample of 10% of the articles selected for full review (n = 168) was independently rated by two raters for percentage agreement on inclusion/exclusion (79%), methodological quality (90%), and risk of bias (90%). Figure 2 is the flowchart of the article selection process.
Figure 2 —
Flowchart of article selection process.
For this article, the findings from 56 high-quality/ low-bias articles are summarized using a narrative report. Quantitative analyses were not conducted because of the diversity of instruments used to measure both symptoms and determinants across studies.
Findings
Aggression
Aggression is defined as destructive actions directed toward persons, objects, or self (Cohen-Mansfield, Marx, & Rosenthal, 1989). It occurs most often within the context of direct caregiving and is one of the most challenging aspects of dementia care. Aggression has a prevalence rate of about 18% (Eastley & Wilcock, 1997), is not as prevalent as apathy or agitation, but it tends to be persistent over time when present (Berger et al., 2005; Devanand et al., 1997).
Eight high-quality/low-bias studies addressing the determinants of aggression were found. They included seven correlational studies, three of which were longitudinal in design, and one systematic review of 18 studies. Aggression was measured using a number of different instruments, including the Neuropsychiatric Instrument (NPI; Cummings, 1997), aggression subscale of the Cohen-Mansfield Agitation Inventory (CMAI; Cohen-Mansfield et al., 1989), Behavioral Pathology in Alzheimer’s Disease Rating Scale (Reisberg, Auer, & Monteiro, 1996), Present Behavioral Examination (PBE; Hope & Fairburn, 1992), the Columbia Scale for Psychopathology in Alzheimer’s Disease (Devanand et al., 1992), and informant report of aggressive behaviors. Five patient (higher severity of dementia, male gender, reduced functional ability, sadness, and premorbid neuroticism) and one caregiver (greater caregiver burden) determinants were identified. No high-quality/low-bias evidence for environmental determinants was found.
Patient Determinants
Despite its persistence across the disease trajectory, the evidence for an association between aggression and cognitive ability is mixed. Two studies found either a weak (Fernandez-Martinez, Molano, Castro, & Zarranz, 2010) or no association (Eastley & Wilcock, 1997) between cognitive ability and aggression, whereas Devanand et al. (1997) found that during a 4-year period, subjects with lower cognitive ability had a higher probability of developing aggression in subsequent years. Another well-designed study showed that in a sample of 870 Korean participants, aggression increased as dementia severity increased in those with both early and late-onset Alzheimer’s disease (AD) (Park et al., 2015).
Gender was identified as a risk factor for aggression. Males and females were equally likely to be verbally abusive, but men were more likely to be physically assaultive (Eastley & Wilcock, 1997). In a longitudinal study with 3 years of follow-up data, male gender was an independent predictor for physical aggression at years 1 and 2, but not at year 3 (McShane, Keene, Fairburn, Jacoby, & Hope, 1998).
Eastley & Wilcock (1997) reported a prevalence rate of 18% for physical aggression and found that dyspraxia was associated with the behavior. Devanand et al. (1997) reported similar findings in their longitudinal study of participants with AD. In early AD, poorer baseline functional status was associated with physical aggression, and poorer functional status increased the transition probability of physical aggression during a 4-year period.
One study was found that examined the association of sadness and aggression. In 86 participants, cox regression analyses indicated that during a 4-year period, the onset of physical aggression was predicted by sad appearance on the PBE (McShane et al., 1998).
One high-quality systematic review examined the association between premorbid personality and aggression and included 18 studies (1996e2008) that met the inclusion criteria (Osborne, Simpson, & Stokes, 2010). Seven studies were deemed high quality. Overall, 72% of studies indicated a positive and significant association between premorbid personality and BPSD. The strongest evidence was for the association between premorbid neuroticism and aggression.
Finally, in a cross-sectional correlational study, discomfort was assessed in nursing home residents by registered nurses familiar with the participant (Pelletier & Landreville, 2007). After controlling for severity of dementia, gender, and disability, the investigators found no association between the presence of discomfort and physical aggression.
Caregiver Determinants
In a 2-year longitudinal study of clinic patients with mild-stage dementia, Berger et al. (2005) found that the association between caregiver burden and aggression increased significantly over time.
Summary
The evidence indicates that aggression, although not prevalent, is a persistent behavior. Male gender, caregiver burden, sadness, and loss of functional abilities, which necessitates more direct caregiving, are associated with physical aggression. Without effective intervention, aggressive behavior is likely to increase caregiver burden during direct caregiving and promote a cycle of reoccurring aggression. A positive association between premorbid neuroticism and aggression was also found, suggesting a heightened risk for aggression in the face of a lifetime of increased vulnerability to stress and negative relationships. Limitations across all studies were the reliance on retrospective informant report for the measure of aggression and the lack of consistent measures for aggression, making comparison of findings difficult.
Agitation
Agitation is defined as inappropriate verbal, vocal, or excessive motor activity that does not result from clearly identifiable daily needs (Cohen-Mansfield, 1996). It is common, persists over the disease trajectory, and can overlap with other neuropsychiatric symptoms such as aggression and wandering (Cummings et al., 1994, 2015). Agitation results in disability, decreased quality of life for individuals with dementia and their caregivers, increased cost of care, and may lead to early institutionalization.
There were 13 high-quality/low-bias articles that included agitation as a major outcome variable. Seven of the studies used a cross-sectional design, one was longitudinal, three were experimental, and two were systematic reviews. In the vast majority of the studies, the concept of agitation was operationalized using the CMAI or the Neuropsychiatric Inventory (CohenMansfield, 1996; Cummings et al., 1994). Seven patient determinants of agitation were found: younger age, male gender, type of dementia, dementia severity, premorbid personality traits, the presence of pain, and boredom. The one caregiver determinant of agitation was caregiver communication, and the two environmental determinants were music and balanced sensory stimulation.
Patient Determinants
Most high-quality studies focused on patient determinants of agitation. It was noted that younger age and younger age of dementia onset were positively associated with agitation (Proitsi et al., 2011). In addition, male gender was positively associated with agitation in the context of dementia (Proitsi et al., 2011). Two studies demonstrated that agitation is positively associated with AD in comparison to other forms of dementia (Di Paola et al., 2015; Leger & Banks, 2014), and one study showed that the prevalence and mean scores of agitation increased as the severity of dementia increased (Fernandez-Martinez et al., 2010). Two intervention studies demonstrated that responding to unmet needs, such as pain and boredom, is associated with less agitation among individuals with dementia (Kolanowski, Litaker, Buettner, Moeller, & Costa, 2011; Pelletier & Landreville, 2007). A systematic review found that premorbid personality traits were associated with agitation (Osborne et al., 2010). Specifically, in three of the 18 studies reviewed, neuroticism was positively associated with agitation, and in two studies, agreeableness was negatively associated with agitation.
There were mixed results related to the relationship between ApoE phenotype and agitation (Del Prete, Spaccavento, Craca, Fiore, & Angelelli, 2009; Scarmeas et al., 2002). One study found that there was no relationship between the ApoE phenotype and agitation among individuals with AD (Scarmeas et al., 2002), whereas an Italian study found that the ApoE4 phenotype was associated with excess motor activity among patients with AD (Del Prete et al., 2009). There were no significant findings related to agitation and the neuroanatomic structure of the corpus callosum (Di Paola et al., 2015) or specific cognitive domains, such as executive functioning, working memory, visual memory, and language (Koppel et al., 2012).
Caregiver Determinants
In a systematic review of 160 studies that examined nonpharmacologic care approaches for BPSD, interventions designed to improve caregiver communication resulted in less agitation but only among caregivers in nursing homes and not with family (Livingston et al., 2014).
Environmental Determinants
Kovach et al. (2004) found that an intervention designed to balance arousal states and provide individualized sensory stimulation resulted in a significant reduction in agitation among older adults with dementia. In addition, a systematic review also indicated that appropriate amounts of sensory stimulation and music also resulted in decreased agitation (Livingston et al., 2014). There is no evidence that aromatherapy (Burns et al., 2011), bright light therapy, or therapeutic touch result in less agitation among individuals with dementia (Livingston et al., 2014). Lin and colleagues found that acupressure and Montessori-based-activities decreased agitated behaviors to a greater extent than the presence of a visitor (Lin et al., 2009).
Summary
Most high-quality/low-bias studies focused on patient determinants of agitation. Although the evidence is limited, agitation appears to be positively associated with younger age and younger age of dementia onset, male gender, and AD. Agitation worsens as dementia progresses but does respond to interventions that address unmet needs. At the caregiver level, improved communication between the care recipient and caregiver was associated with less agitation. Environmental determinants such as balanced sensory stimulation and music were also found to significantly decrease agitation.
Unlike other neuropsychiatric symptoms, agitation in the context of dementia is a common and persistent symptom. Although there is some evidence that neural substrates underlie the type of agitated behaviors in dementia, no clear or consistent patterns have emerged. Agitation is a construct that includes many different symptoms, making it an imprecise target. Other major limitations were the reliance on retrospective informant report for the measure of agitation, and the lack of consistent measures, making comparison of findings across studies difficult.
Apathy
Apathy is defined as a loss of motivation that is accompanied by diminished self-initiated behavior, reduced goal-directed cognitive activity, and diminished emotion (Robert et al., 2009). Apathy is a common behavioral symptom negatively affecting patient outcomes including functional deterioration and even mortality (O’Connor et al., 2016; Vilalta-Franch, Calvo-Perxas, Garre-Olmo, Turro-Garriga, & Lopez-Pousa, 2013). Apathy is also a particularly troublesome behavior for caregivers. High levels of depression, burden, and stress have been reported in caregivers of apathetic persons across dementia types (Dauphinot et al., 2015; Feast, Moniz-Cook, Stoner, Charlesworth, & Orrell, 2016; Massimo et al., 2009).
Sixteen high-quality/low-bias studies addressing the determinants of apathy were found. These included three that were experimental in design, one longitudinal cohort study, 11 correlational studies, and one descriptive study. The operational definition of apathy varied by study. The most common instrument used to measure apathy was the subscale of the NPI (Cummings et al., 1994). Informant report was used most often to rate apathy, which is not surprising given that reduced insight often co-occurs with apathy (Eslinger, Moore, Antani, Anderson, & Grossman, 2012; Horning, Melrose, & Sultzer, 2014). Seven patient determinants (dementia type, severity of dementia, neuroanatomic structure, cerebrospinal biomarkers, genetics, gender, and the presence of other BPSDs) and one environmental determinant (nonpharmacologic intervention) were found. No high-quality evidence for any caregiver determinant was found.
Patient Determinants
Gender does not appear to be related to apathy (Proitsi et al., 2011). Although apathy is prevalent across dementia types, there is limited evidence in the four studies that were reviewed to suggest that apathy occurs more frequently in one dementia type. Leger and Banks (2014) found that apathy was more common in behavioral variant frontotemporal degeneration (bvFTD) than AD. Park et al., 2015 found that apathy was more common in early onset AD than late-onset AD. A study comparing AD and vascular dementia (VaD) found that apathy was more common in VaD, but the results were not statistically significant (Saz et al., 2009). Johnson, Watts, Chapin, Anderson, and Burns (2011) found that apathy was reported most frequently in dementia with Lewy bodies, but again the results were not statistically significant.
There is strong evidence that apathy is related to the severity of cognitive impairment in dementia. Apathy is associated with more severe cognitive impairment on Mini-Mental State Examination (Proitsi et al., 2011; Reyes et al., 2009) and dementia severity according to Clinical Dementia Rating Scale (Fernandez-Martinez et al., 2010). Kuzis, Sabe, Tiberti, Dorrego, and Starkstein (1999) examined specific cognitive deficits in persons with AD and found that apathy is associated with more severe frontal lobe-related cognitive deficits.
Apathy also appears to be associated with the presence of other BPSD (Reyes et al., 2009). In addition, baseline apathy and antidepressant use is associated with increasing apathy over time in AD (Donovan et al., 2014).
The most commonly studied patient determinant was biologic factors, which appear to be associated with apathy. For example, neuroanatomic changes in gray matter and white matter were found to be associated with apathy (Donovan et al., 2014; Macfarlane et al., 2015; Reyes et al., 2009); however, apathy was not associated with reduced white matter integrity in the corpus callosum in AD (Di Paola et al., 2015). Apathy appears to be associated with genetic factors such as APOE-e4 in AD (Del Prete et al., 2009; Park et al., 2015) and c9ORF72 in FTD patients (Snowden et al., 2012). Other biologic factors such as cerebral spinal fluid biomarkers in AD patients do not appear to be associated with apathy (Donovan et al., 2014).
Environmental Determinants
Three studies evaluated environmental factors, specifically nonpharmacologic interventions and apathy. AD patients participating in cognitive stimulation (Niu, Tan, Guan, Zhang, & Wang, 2010), therapeutic conversation (Tappen & Williams, 2009), or activities tailored to personality and physical ability (Kolanowski et al., 2011) showed decreased apathy.
Summary
Most high-quality/low-bias studies focused on patient determinants of apathy, particularly biologic factors; however, a few environmental factors, such as non-pharmacologic interventions, can reduce apathy. There is strong evidence that apathy is associated with neurodegeneration. Relatively few studies examined apathy across neurodegenerative conditions, which make it difficult to assess whether underlying neural mechanisms associated with apathy are shared across the neurodegenerative disease spectrum. Other major limitations were the lack of consistency in definition and instrumentation used to measure apathy.
Depression
Along with dementia, depression is one of the most common psychiatric syndromes experienced by the older population. According to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) (DSM-IV-TR), major depression is defined as depressed mood or loss of interest in pleasure and activity that is present most days and persists beyond 2 weeks. A diagnosis of depression is made when five or more characteristic symptoms are present: sleep disturbance, changes in appetite/weight, reduced energy and physical activity, altered cognition and decision-making ability, problems concentrating, suicide ideation, and/or feelings of worthlessness or guilt (American Psychiatric Association, 2000). Depression is known to significantly impact quality of life and frequently affects people with dementia at all stages including mild cognitive impairment (Enache, Winblad, & Aarsland, 2011). Although depression affects people with all types of dementia, depression is more prevalent in VaD (50%) compared with AD (20%; Byers & Yaffe, 2011).
The review of the determinants for depressive symptoms yielded the largest number of articles examining determinants for BPSD. A total of 35 high-quality/low-bias studies were found. These included eight studies that were experimental in design, 25 correlational studies, one longitudinal cohort study, and one systematic review of 18 studies. The operational definition of depression used by these studies ranged widely. A total of 12 different measures of depressive symptoms were used. The most commonly used measures were the NPI mood subscale (Cummings et al., 1994), the Geriatric Depression Scale (Yesavage et al., 1982), and the Cornell scale for dementia and depression (Alexopoulos, Abrams, Young, & Shamoian, 1988). Although the vast majority of the measures were completed via self-report, proxy report, or observation; three studies used the DSM-IV criteria for major depressive disorder as the operational definition of depression. Because of the heterogeneity of measures used, the term depressive symptoms is used below to discuss the findings.
A large number of patient determinants were found, including female gender, Chinese American ethnicity, lower level of education, younger age of onset of dementia, multiple genetic factors, multiple biologic changes in the brain, multiple neurologic diagnoses, cardiovascular comorbidities, withdrawal from neuroleptic medication, cognitive impairment, specific aspects of cognition, the presence of other BPSD symptoms, premorbid personality, functional impairment, and lack of engagement in exercise and interests. Several caregiver-related determinants were found, including greater informal caregiver burden, caregiver-focused interventions (e.g., cognitive stimulation, caregiver interactional skills, and acupressure), as well as environmental determinants (system-level collaborative care management model interventions and country of residence).
Patient Determinants
Although some demographic factors, such as female gender (Arlt et al., 2013; Caraci et al., 2012; Del Prete et al., 2009; Delano-Wood et al., 2008; Proitsi et al., 2011; Scarmeas et al., 2002; Slifer, Martin, Gilbert, Haines, & Pericak-Vance, 2009), Chinese American ethnicity (Chao et al., 2014), lower level of education (Chao et al., 2014), and younger age of onset of dementia (Proitsi et al., 2011) were found to be related with the development of depressive symptoms in dementia, the most common patient determinant examined was in the area of genetic risk. Genetic risk factors that were found to be associated with depressive symptoms in dementia were APOE-e4 allele carriers (Slifer et al., 2009) and monoamine oxidase A variable number tandem repeat (Arlt et al., 2013), proteins (cyclic adenosine monophosphate-response element-binding protein; Platenik et al., 2014), enzymes (glycogen synthase kinase 3 beta; Platenik et al., 2014), and transforming growth factor B1 (Caraci et al., 2012). Biologic changes in the brain were also associated with depressive symptoms, including changes in the corpus callosum (Di Paola et al., 2015), tau-negative pathology (Leger & Banks, 2014), and the presence of Lewy bodies in the amygdala (Lopez, Becker, Sweet, Martin-Sanchez, & Hamilton, 2006). Multiple neurologic diagnoses (Calleo, Amspoker, Marsh, & Kunik, 2015), VaD (Johnson et al., 2011), and early-onset AD (Park et al., 2015) as the primary cause of dementia were also associated with higher incidence of depressive symptoms compared with AD, Parkinson’s disease, and late-onset AD (Johnson et al., 2011; Park et al., 2015).
The relationship between cardiovascular comorbidities and depressive symptoms is mixed in that cerebral small vessel disease (Ogawa et al., 2013) and spontaneous cerebral emboli (Purandare et al., 2006) were associated with depressive symptoms, whereas cardiovascular morbidities (hypertension, diabetes, history of myocardial infarction, history stroke, and hyperlipidemia) were not associated with depressive symptoms (Chao et al., 2014). Use of placebo compared with withdrawal from neuroleptic medication was associated with worse outcomes with respect to mood (Ballard et al., 2004). The relationship between cognitive impairment and depressive symptoms was also mixed with reported positive association (Conde-Sala et al., 2016; Devanand et al., 1997; Fernandez-Martinez et al., 2010; Koppel et al., 2012; Proitsi et al., 2011) and lack of association (Kuzis et al., 1999; Marin et al., 1997). With regard to specific aspects of cognition, executive function, working memory and visual memory, and anosognosia were associated with decreased depressive symptoms (Conde-Sala et al., 2016; Koppel et al., 2012). In addition, the relationship between the presence of other BPSD symptoms and depressive symptoms was mixed in that the presence of overall BPSD symptoms was not associated with depressive symptoms (Kandiah, Chander, Zhang, & Yee, 2014), whereas severity of baseline depressive symptoms (Marin et al., 1997) and psychosis (Proitsi et al., 2011) were associated with depressive symptoms. Premorbid personality (i.e., premorbid neuroticism; Osborne et al., 2010), severity of functional impairment (Chao et al., 2014), and lack of engagement in exercise and interests were found positively associated with depressive symptoms (Regan, Katona, Walker, & Livingston, 2005).
Caregiver Determinants
One study found that high levels of informal caregiver burden were related to patient depressive symptoms (Berger et al., 2005). Randomized clinical trial (RCT) intervention studies focused on both formal and informal caregivers as interventionists. Three RCT studies found a positive effect on patient depressive symptoms by using interventions focused on the enhancement of caregiver interactional skills. These skills were geared toward teaching family caregivers how to engage in problem solving (Kiosses et al., 2015), enhance pleasant events (Teri, Logsdon, Uomoto, & McCurry, 1997), and use therapeutic communication strategies (Tappen & Williams, 2009). A positive effect on patient depressive symptoms was also found when formal caregivers delivered cognitive stimulation (Niu et al., 2010; Onor et al., 2007) or acupressure points on the ear (Rodriguez-Mansilla et al., 2015).
Environmental Determinants
Two studies examined environmental determinants of depressive symptoms. One RCT focused on a systems level collaborative care management model intervention. The investigators found that, although the intervention had a significant impact on many aspects of health care utilization and behavioral symptoms, patient depressive symptoms were not affected (Callahan et al., 2006). Investigators in the second study used a large sample of persons living with dementia in the United Kingdom, Ireland, and Greece and found that persons residing in the United Kingdom had higher depressive symptom levels than those residing in other countries. The findings brought up potentially intriguing questions about population-based heterogeneity in the expression of depressive symptoms as a potential determinant of these symptoms in people living with dementia (Proitsi et al., 2011).
Summary
Depression is one of the most prevalent BPSD and significantly affects quality of life. Patient determinants compromised the largest number of determinants for depressive symptoms. Although some demographic characteristics were associated with depressive symptoms, multiple genetic factors and biologic changes in the brain were identified. Multiple dementia diagnoses as the primary cause of BPSD were also consistently associated with a higher incidence of depressive symptoms, whereas the relationship between the presence of other BPSD symptoms and depressive symptoms was mixed. Specific cognitive abilities (e.g., executive function, working memory and visual memory, and anosognosia) were consistently associated with lower depressive symptoms, whereas the relationship between global cognitive impairment and depressive symptomatology was mixed. Cardiovascular comorbidities also had a mixed association with depressive symptoms.
Comparatively, fewer studies examined caregiver and environmental determinants. At the caregiver level, several interventions such as enhancement of caregiver interactional skills or caregiver-delivered cognitive stimulation and acupressure points on the ear were found to reduce depressive symptoms. At the environmental level, a systems level collaborative care management model intervention was not associated with depressive symptoms, whereas country of residence in several European countries was associated with depressive symptoms.
Major limitations across all studies were the use of varied measures for depression, from symptom measures to formal diagnostic procedures/criteria, and the use of samples with great heterogeneity around level of depressive symptoms ranging from subsyndromal to major depressive disorder. These limitations made the comparison of findings across studies difficult.
Psychosis
Psychosis in dementia is defined as the presence of delusions, hallucinations, or persistent misinterpretations (Leroi, Voulgari, Breitner, & Lyketsos, 2003). Delusions are strongly held false and irrational beliefs that are unaccepted by a patient’s culture, whereas hallucinations are false sensory perceptions, such as seeing, hearing, feeling, or smelling something that is not present (Holroyd, 2000). Specific misinterpretations of reality that occur in dementia have also been described and include misrepresentations such as believing that one’s home is not their home or that loved ones are imposters (Burns & Philpot, 1987). Psychosis occurs commonly in dementia and occurs at various stages of illness (Leroi et al., 2003).
Among the articles reviewed, 20 high-quality/low-biased studies were identified that addressed determinants of psychosis/psychotic behaviors in dementia. These included one longitudinal descriptive study, 18 correlational studies (4 longitudinal), and one systematic review. Psychosis was measured using clinical assessments and several validated measures–most commonly, the NPI. Patient determinants were the most frequently identified: neurodegeneration, genetics, comorbidities, premorbid personality, functional abilities, demographics, and cognitive abilities. One study addressed caregiver burden as a determinant of psychosis.
Patient Determinants
Most studies explored specific neurodegenerative changes as determinants of psychosis in dementia including examination of neurofibrillary tangles (Farber et al., 2000), regional brain metabolism (Koppel et al., 2014), white matter hyperintensities (Kandiah et al., 2014), cerebral perfusion (Nomura et al., 2012), muscarinic receptor density (Lai et al., 2001), and changes in 5-hydroxytryptamine levels (Marcos et al., 2008).
Findings from these studies suggest that different dementia etiologies may produce different types of psychotic symptoms reflective of distinct neuropathologic changes and/or differential neuronal network involvement. Specifically, patients with AD have been found to have more delusions and hallucinations than those with FTD (Leger & Banks, 2014), and patients who are tau negative and have bvFTD have been shown to experience more delusions (Leger & Banks, 2014). Evidence from three studies suggest a positive relationship between psychosis and dementia severity (Devanand et al., 1997; Farber et al., 2000; Fernandez-Martinez et al., 2010)–with one study reporting a 2.3-fold greater density of neurofibrillary tangles in individuals with psychosis (Farber et al., 2000). One study found an association of psychosis to small vessel disease (Ogawa et al., 2013).
Other studies were unable to ascertain any specific determinants for psychosis (Johnson et al., 2011; Marin et al., 1997; Osborne et al., 2010). Negative associations between premorbid personality and apathy (Osborne et al., 2010) and disturbances in mood and agitation and apathy (Marin et al., 1997) were found. One study found that rigidity in AD is independent of psychosis (Sweet et al., 1998).
Neurodegenerative determinants of psychosis also suggest that specific delusions may be related to changes in distinct neural networks. For example, one study found hyperperfusion of the bilateral precuneous, left insula, and right thalamus to be associated with the commonly reported delusions of home not being home, abandonment, and others not being who they claim to be. On the other hand, hypoperfusion of the right inferior temporal gyrus, inferior temporal gyrus, and hyperperfusion of the middle frontal gyrus, insula, and posterior cingulate gyrus were associated with the specific delusions of abandonment and jealousy (Nomura et al., 2012). Another study found that paranoid delusions may require sparing of cognitive functions and may be favored by frontal lobe dysfunction (Quaranta et al., 2015). Although only two studies examined genetic determinants, both found the presence of the APO-E4 allele to increase risk for delusions, particularly in those with late-onset dementia (Park et al., 2015; Scarmeas et al., 2002).
Several studies, but not all, that examined specific cognitive domains as determinants of psychosis found that psychosis increased with greater cognitive impairment (Koppel et al., 2012, 2014; Proitsi et al., 2011), and one study found visuospatial impairment to be linked to greater visual hallucinations (Quaranta et al., 2015).
Caregiver Determinants
One high-quality/low-bias study addressed caregiver determinants of psychosis (Berger et al., 2005). This longitudinal study did not find a significant association between caregiver depression or caregiver burden and psychosis (Berger et al., 2005). No studies were found that examined environmental determinants of psychosis.
Summary
Evidence to date suggests that psychosis in dementia is worsened by dementia severity (Farber et al., 2000; Fernandez-Martinez et al., 2010), greater functional and cognitive impairment (Koppel et al., 2014; Proitsi et al., 2011), and the presence of the APO-E4 allele (Park et al., 2015). Underlying neuropathologic and cognitive deficits may be related to the development of specific types of psychotic delusions or hallucinations (Nomura et al., 2012; Quaranta et al., 2015). The evidence remains limited by lack of diverse samples and in most cases, lack of validation across samples. Synthesis of these findings is also hindered by lack of consistent measurement and classification of psychotic symptoms.
Discussion and Recommendations
This scoping review is one of the first to describe the existing evidence base for the determinants of specific BPSD, and it provides a broad map for future research studies, including other systematic reviews. The review verified that there is a large and complex literature on the determinants of BPSD, but most of it is not high quality. In the low-quality studies, there was a lack of methodological rigor in the approach and/or a risk of bias. In the high-quality/low-bias studies reviewed, a number of determinants were identified, but no one determinant was more important than others. Although each symptom had its own set of determinants, a number of determinants were common across several symptoms: neurodegeneration, type of dementia, severity of cognitive impairments and declining functional abilities, and to a lesser extent, caregiver burden, communication skills, and tailored activities. Across BPSD, patient determinants had a greater amount of evidence than other factors because many are biologic drug targets with strong funding sources. There was very little high-quality/low-bias research for caregiver and environmental determinants; depression was the only symptom that had studies in all three determinant categories. Also noted was a preponderance of cross-sectional studies and little longitudinal data on specific symptoms. These are important gaps in research that, if filled, would provide much needed data for the design of interventions. Well-designed longitudinal studies would also improve our understanding of when during the illness trajectory interventions are most likely to have their greatest effect.
Limitations in the current evidence base for determinants of specific symptoms can be summarized as lack of consistent measures for specific symptoms and determinants, lack of longitudinal data, and lack of well-characterized samples. There are also limitations of this review: our inclusion criteria limited citations to those in English only, the gray literature was not searched, the dates for article inclusion were limited to those between 1992 and 2016, and the determinants identified in this review are likely not the only determinants associated with symptom expression in people living with dementia.
Despite these limitations, there are a number of research and policy implications that are informed by this scoping review. To begin, BPSD is an umbrella term for a variety of specific symptoms. There is widespread heterogeneity in symptom conceptualization and measurement. In many studies, symptoms were measured with instruments that aggregate different symptoms. This approach to measurement dilutes the ability to see an association between a specific determinant and a specific symptom. Across the literature reviewed, a need for more precision in defining, and greater consistency in measuring, symptoms was noted.
Individual symptoms vary over time and possibly by type of dementia. The relationship between a specific determinant and a symptom may not progress in a linear fashion or persist between assessment points. These findings underscore the need for longitudinal designs that capture changing patterns over time. There is also a need for more well-characterized samples that meet criteria for specific types of neurodegeneration. Without greater specificity as to type of dementia, it is difficult to understand the neuroanatomic associations with specific symptoms. Also important is the need for rigorous qualitative work that improves our understanding of symptoms and their determinants from the perspective of the person living with dementia and their caregivers.
The incorporation of biomarkers and neuroimaging techniques in treatment studies has potential to help elucidate the mechanisms by which some interventions produce their effects, and there is evidence that this is becoming a standard methodological approach. Heterogeneity in types of imaging analyses, however, continue to make it difficult to compare findings across studies.
Because this scoping review is a pre-2017 National Research Summit on Dementia Care activity, important policy implications can be gleaned in the areas of research, services, and measurement. First, given the universality of behavioral symptoms and that their occurrence traverses disease trajectory and etiologies, research on this issue is imperative. More funds must be allocated to build the science of BPSD in several key areas: their role in disease progression; the underlying determinants and mechanisms of behavioral domains; measurement development to capture with greater precision in the characteristics of behaviors and the context in which they occur; development, evaluation, and implementation of interventions/strategies/ programs and approaches preclinical to end of life that can prevent or mitigate behavioral symptoms; and the cost effectiveness of efficacious intervention.
Second, the review reaffirms the importance of all health and human service professionals as well as members in the service sector to understand that behavioral symptoms are a key clinical feature of dementia and may be triggered by factors that are modifiable in the environment. This understanding is fundamental to the use of a range of nonpharmacologic strategies that seek to address the patient, caregiver, and environmental determinants that may trigger or contribute to symptom occurrence. Given their universality and the importance of assessment and treatment to determine underlying contributors, the assessment and overall management of BPSD should be reimbursed by insurers and the Centers for Medicare and Medicaid Services in any clinical and or social service setting. Support for research that examines the potential benefits of such reimbursement is needed.
Yet another policy implication linked to the gap in measurement is the need to derive and implement quality indicators in clinical practice. Two existing quality measurement sets have been developed, both of which include the assessment and treatment of BPSD as one of their core indicators of effective dementia care (see the American Academy of Neurology, http://www.ncbi.nim.nih.gov/pubmed/24397784; and International Consortium for Health Outcomes Measurement Standard set for dementia at http://www.ichom.org/medical-conditions/dementia/).
Another way to understand the policy implications of this review is through the five goals outlined in the National Plan to Address Alzheimer’s Disease (https://aspe.hhs.gov/report/national-plan-address-alzheimersdisease-2016-update), each of which speak to the need to invest in research to determine if early detection and treatment may help to slow disease progression (goal 1), improve care quality (goal 2), enhance education and skills of caregivers to address BPSD (goal 3), enhance public awareness of BPSD (goal 4), and improve better identification and tracking to evaluate disease and treatment impacts (goal 5).
Conclusion
BPSDs are universally experienced by people living with dementia. They contribute to poor quality of life, more rapid cognitive and physical decline, caregiver burden, and high costs of care. The treatment of these symptoms involves targeting their cause(s) or determinant(s). In this scoping review, we summarized the high-quality/low-bias research for the determinants of five specific symptoms: aggression, agitation, apathy, depression, and psychosis. Common determinants across several symptoms included neurodegeneration, type of dementia, severity of cognitive impairments, declining functional abilities, and to a lesser extent, caregiver burden, communication, and tailored activities. This review adds to the literature because it identifies many gaps in the literature that must be addressed to improve the quality of dementia care. Furthermore, it provides some evidence to guide the development of interventions that allay the expression of BPSD. Finally, this review provides policymakers, who are entrusted with the responsible allocation of public resources, with guidance on how those resources should be directed to support needed research for the well-being of people living with dementia.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
This scientific work is a presummit activity that will inform the 2017 Research Summit on Dementia Care: Care and Services for Persons with Dementia, Family Members and Caregivers. Members of the Advisory Council on Alzheimer’s Research, Care and Services recognize the value of activities such as this.
Appendix A. Determinants of BPSD
Patient determinants include individual characteristics that may put the person at risk for BPSD: neurodegeneration, genetics, acute medical conditions, medications, unmet physical & psychosocial needs, comorbidities (including psychiatric illnesses & mood disorders), premorbid personality/roles/occupation, gender, sociability, functional abilities, and cognitive abilities (by discrete domains).
Caregiver determinants include characteristics of the caregiver and the quality of the dyadic relationship that may precipitate BPSD: stress, resilience, readiness for care, style of caregiving, lack of education (including mismatch of abilities and expectations), communication (including dyadic interaction), health (mental and physical), Socioeconomic status, cultural influences, competing demands, quality of relationship between caregiver and care recipient, values and beliefs, role, staff autonomy (ability to make decisions), and staff capacity.
Environmental determinants include environmental aspects/qualities/characteristics that may precipitate BPSD individually or in at-risk individuals: physical (i.e., lighting, noise, temperature) and social (i.e., crowding, activities available) characteristics, organizational policies, social policies, social networks, resources, and leadership support.
Appendix B. Initial Keywords and Full Search Strategy
Initial keywords included: Behavioral and psychological disorders: BPSD OR Behavioral Symptoms OR Psychological symptoms OR Neuropsychiatric Symptoms OR Aggression OR Agitation OR Anxiety OR Apathy OR Psychotic Disorders OR Mood Disorders OR Affective Disorders OR Irritable Mood; Dementia: dementia OR Alzheimer disease OR Lewy Bodies OR Alzheimers OR Aphasia OR Creutzfeldt-Jakob Syndrome OR Huntington OR Lewy Body OR Lewy Bodies OR Kluver-Bucy.
Search Strategy: ((((((((((((((((((((BPSD[Title/Abstract]) OR Behavioral Symptoms[MeSH Terms]) OR (“Behavioral [Title/Abstract] AND psychological symptoms”[Title/Abstract])) OR “Neuropsychiatric Symptoms”[Title/Abstract]) OR Aggression[MeSH Terms]) OR Aggression [Title/Abstract]) OR Agitation[Title/Abstract]) OR Anxiety [MeSH Terms]) OR Anxiety[Title/Abstract]) OR Apathy [MeSH Terms]) OR Apathy[Title/Abstract]) OR depression [MeSH Terms]) OR depression[Title/Abstract]) OR Depressive Disorder[MeSH Terms]) OR Psychotic Disorders[MeSH Terms]) OR Mood Disorders[MeSH Terms]) OR Affective Disorders, Psychotic[MeSH Terms]) OR Irritable Mood[MeSH Terms])) AND ((((((((((((((((dementia[MeSH Major Topic]) OR Alzheimer disease[MeSH Major Topic]) OR Lewy Bodies[MeSH Major Topic]) OR Alzheimer’s [Title/Abstract]) OR Alzheimers[Title/Abstract]) OR Alzheimer[Title/Abstract]) OR Aphasia[MeSH Major Topic]) OR Aphasia[Title/Abstract]) OR Creutzfeldt-Jakob Syndrome[MeSH Major Topic]) OR “Creutzfeldt-Jakob”[Title/ Abstract]) ORHuntington Disease[MeSH MajorTopic])OR Huntington Disease[Title/Abstract]) OR Lewy Body[Title/ Abstract]) OR Lewy Bodies[Title/Abstract]) OR KluverBucy[Title/Abstract]) OR dementia[Title/Abstract])) AND (((((((((((((((((Randomized Controlled Trial[Publication Type]) OR observational study[Publication Type]) OR retrospective study[MeSH Terms]) OR prospective study [MeSH Terms]) OR cohort studies[MeSH Terms]) OR meta-analysis[Publication Type]) OR qualitative research [MeSH Terms]) OR random allocation[MeSH Terms]) OR follow-up studies[MeSH Terms]) OR epidemiologic studies[MeSH Terms]) OR evaluation studies[Publication Type]) OR research design[MeSH Terms]) OR multicenter study[Publication Type]) OR clinical trial[Publication Type]) OR controlled clinical trial[Publication Type]) OR “Systematic Review”[Title/Abstract]) OR evidence based medicine[MeSH Terms])
Middle aged + aged: 55+ years.
Human.
English.
REFERENCES
- Adelman RD, Tmanova LL, Delgado D, Dion S, & Lachs MS (2014). Caregiver burden: A clinical review. JAMA, 311(10), 1052–1060. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Abrams RC, Young RC, & Shamoian CA (1988). Cornell Scale for Depression in Dementia. Biological Psychiatry, 23(3), 271–284. [DOI] [PubMed] [Google Scholar]
- Algase DL, Beck C, Kolanowski A, Whall A, Berent S, & Richards K (1996). Need-driven dementia-compromised behavior: An alternative view of disruptive behavior. American Journal of Alzheimer’s Disease and Other Dementias, 11(6), 10–19. [Google Scholar]
- American Geriatrics Society & American Association for Geriatric Psychiatry. (2003). The American Geriatrics Society and American Association for Geriatric Psychiatry recommendations for policies in support of quality mental health care in U.S. nursing homes. Journal of the American Geriatrics Society, 51(9), 1299–1304. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders. Text Revision (DSM-IV-TRTM) (4th ed.) Washington, DC: American Psychiatric Association. [Google Scholar]
- Arlt S, Demiralay C, Tharun B, Geisel O, Storm N, Eichenlaub M, ..., Jahn H (2013). Genetic risk factors for depression in Alzheimer’s disease patients. Current Alzheimer Research, 10(1), 72–81,* Denotes article used in review.
- Ballard CG, Thomas A, Fossey J, Lee L, Jacoby R, Lana MM, …, O’Brien JT (2004). A 3-month, randomized, placebo-controlled, neuroleptic discontinuation study in 100 people with dementia: The Neuropsychiatric Inventory median cutoff is a predictor of clinical outcome. Journal of Clinical Psychiatry, 65(1), 114–119,* Denotes article used in review.
- Berger G, Bernhardt T, Weimer E, Peters J, Kratzsch T, & Frolich L (2005). Longitudinal study on the relationship between symptomatology of dementia and levels of subjective burden and depression among family caregivers in memory clinic patients. Journal of Geriatric Psychiatry and Neurology, 18(3), 119–128,* Denotes article used in review.
- Burns A, Perry E, Holmes C, Francis P, Morris J, Howes MJ, …, Ballard C (2011). A double-blind placebo-controlled randomized trial of Melissa officinalis oil and donepezil for the treatment of agitation in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 31(2), 158–164,* Denotes article used in review.
- Burns A, & Philpot M (1987). Capgras’ syndrome in a patient with dementia. The British Journal of Psychiatry, 150, 876–877. [DOI] [PubMed] [Google Scholar]
- Byers AL, & Yaffe K (2011). Depression and risk of developing dementia. Nature Reviews: Neurology, 7(6), 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, …, Hendrie HC (2006). Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: A randomized controlled trial. JAMA, 295(18), 2148–2157,* Denotes article used in review.
- Calleo J, Amspoker AB, Marsh L, & Kunik ME (2015). Mental health diagnoses and health care utilization in persons with dementia, Parkinson’s disease, and stroke. Journal of Neuropsychiatry and Clinical Neurosciences, 27(2), e117–e121,* Denotes article used in review.
- Caraci F, Bosco P, Signorelli M, Spada RS, Cosentino FI, Toscano G, …, Ferri R (2012). The CC genotype of transforming growth factor-beta1 increases the risk of late-onset Alzheimer’s disease and is associated with AD-related depression. European Neuropsychopharmacology, 22(4), 281–289,* Denotes article used in review.
- Casanova MF, Starkstein SE, & Jellinger KA (2011). Clinicopathological correlates of behavioral and psychological symptoms of dementia. Acta Neuropathologica, 122(2), 117–135. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Matthews BR, Yokoyama JS, Lai NB, Ong H, Tse M, …, Rosen HJ (2014). Depressive symptoms in Chinese-American subjects with cognitive impairment. American Journal of Geriatric Psychiatry, 22(7), 642–652,* Denotes article used in review.
- Cohen-Mansfield J (1996). Assessment of agitation. International Psychogeriatrics, 8(2), 233–245. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Marx MS, & Rosenthal AS (1989). A description of agitation in a nursing home. Journal of Gerontology, 44(3), M77–M84. [DOI] [PubMed] [Google Scholar]
- Conde-Sala JL, Turro-Garriga O, Pinan-Hernandez S, Portellano-Ortiz C, Vinas-Diez V, Gascon-Bayarri J, & Rene-Ramirez R (2016). Effects of anosognosia and neuropsychiatric symptoms on the quality of life of patients with Alzheimer’s disease: A 24-month follow-up study. International Journal of Geriatric Psychiatry, 31(2), 109–119,* Denotes article used in review.
- Cummings JL (1997). The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology, 48(5 Suppl. 6), S10–S16. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, & Gornbein J (1994). The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology, 44(12), 2308–2314. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mintzer J, Brodaty H, Sano M, Banerjee S, Devanand D, …, International Psychogeriatric Association. (2015). Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. International Psychogeriatrics, 27(01), 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinot V, Delphin-Combe F, Mouchoux C, Dorey A, Bathsavanis A, Makaroff Z, …, Krolak-Salmon P (2015). Risk factors of caregiver burden among patients with Alzheimer’s disease or related disorders: A cross-sectional study. Journal of Alzheimer’s Disease, 44(3), 907–916. [DOI] [PubMed] [Google Scholar]
- Del Prete M, Spaccavento S, Craca A, Fiore P, & Angelelli P (2009). Neuropsychiatric symptoms and the APOE genotype in Alzheimer’s disease. Neurological Sciences, 30(5), 367–373,* Denotes article used in review.
- Delano-Wood L, Houston WS, Emond JA, Marchant NL, Salmon DP, Jeste DV, …, Bondi MW (2008). APOE genotype predicts depression in women with Alzheimer’s disease: A retrospective study. International Journal of Geriatric Psychiatry, 23(6), 632–636,* Denotes article used in review.
- Devanand DP, Jacobs DM, Tang M-X, Del CastilloCastaneda C, Sano M, Marder K, …, Albert M (1997). The course of psychopathologic features in mild to moderate Alzheimer disease. Archives of General Psychiatry, 54(3), 257–263,* Denotes article used in review.
- Devanand DP, Miller L, Richards M, Marder K, Bell K, Mayeux R, & Stern Y (1992). The Columbia University Scale for Psychopathology in Alzheimer’s disease. Archives of Neurology, 49(4), 371–376. [DOI] [PubMed] [Google Scholar]
- Di Paola M, Phillips O, Orfei MD, Piras F, Cacciari C, Caltagirone C, & Spalletta G (2015). Corpus callosum structure is topographically correlated with the early course of cognition and depression in Alzheimer’s disease. Journal of Alzheimer’s Disease, 45(4), 1097–1108,* Denotes article used in review.
- Donovan NJ, Wadsworth LP, Lorius N, Locascio JJ, Rentz DM, Johnson KA, …, Alzheimer Disease Neuroimaging Initiative. (2014). Regional cortical thinning predicts worsening apathy and hallucinations across the Alzheimer disease spectrum. The American Journal of Geriatric Psychiatry, 22(11), 1168–1179,* Denotes article used in review.
- Eastley R, & Wilcock GK (1997). Prevalence and correlates of aggressive behaviours occurring in patients with Alzheimer’s disease. International Journal of Geriatric Psychiatry, 12(4), 484–487,* Denotes article used in review.
- Enache D, Winblad B, & Aarsland D (2011). Depression in dementia: Epidemiology, mechanisms, and treatment. Current Opinion in Psychiatry, 24(6), 461–472. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Antani S, Anderson C, & Grossman M (2012). Apathy in frontotemporal dementia: Behavioral and neuroimaging correlates. Behavioural Neurology, 25(2), 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber NB, Rubin EH, Newcomer JW, Kinscherf DA, Miller JP, Morris JC, …, McKeel DW Jr. (2000). Increased neocortical neurofibrillary tangle density in subjects with Alzheimer disease and psychosis. Archives of General Psychiatry, 57(12), 1165–1173,* Denotes article used in review.
- Feast A, Moniz-Cook E, Stoner C, Charlesworth G, & Orrell M (2016). A systematic review of the relationship between behavioral and psychological symptoms (BPSD) and caregiver well-being. International Psychogeriatrics, 28(11), 1761–1774. [DOI] [PubMed] [Google Scholar]
- Fernandez-Martinez M, Molano A, Castro J, & Zarranz JJ (2010). Prevalence of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s disease, and its relationship with cognitive impairment. Current Alzheimer Research, 7(6), 517–526,* Denotes article used in review.
- Fink A (2010). Conducting research literature reviews (3rd ed.) Los Angeles, CA: Sage Publications. [Google Scholar]
- Gough D (2007). Weight of evidence: A framework for the appraisal of the quality and relevance of evidence. Research Papers in Education, 22(2), 213–228. [Google Scholar]
- Hall GR, & Buckwalter KC (1987). Progressively lowered stress threshold: A conceptual model for care of adults with Alzheimer’s disease. Archives of Psychiatric Nursing, 1(6), 399–406. [PubMed] [Google Scholar]
- Herrmann N, Lanctot KL, Sambrook R, Lesnikova N, Hebert R, McCracken P, …, Nguyen E (2006). The contribution of neuropsychiatric symptoms to the cost of dementia care. International Journal of Geriatric Psychiatry, 21(10), 972–976. [DOI] [PubMed] [Google Scholar]
- Holroyd S (2000). Hallucinations and delusions in dementia. International Psychogeriatrics, 12(S1), 113–117. [Google Scholar]
- Hope T, & Fairburn CG (1992). The Present Behavioural Examination (PBE): The development of an interview to measure current behavioural abnormalities. Psychological Medicine, 22(1), 223–230. [DOI] [PubMed] [Google Scholar]
- Horning SM, Melrose R, & Sultzer D (2014). Insight in Alzheimer’s disease and its relation to psychiatric and behavioral disturbances. International Journal of Geriatric Psychiatry, 29(1), 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DK, Watts AS, Chapin BA, Anderson R, & Burns JM (2011). Neuropsychiatric profiles in dementia. Alzheimer Disease and Associated Disorders, 25(4), 326,* Denotes article used in review.
- Kales HC, Gitlin LN, & Lyketsos CG (2015). Assessment and management of behavioral and psychological symptoms of dementia. BMJ, 350, h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales HC, Gitlin LN, Lyketsos CG, & Detroit Expert Panel on Assessment Management of Neuropsychiatric Symptoms of Dementia. (2014). Management of neuropsychiatric symptoms of dementia in clinical settings: Recommendations from a multidisciplinary expert panel. Journal of the American Geriatric Society, 62(4), 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandiah N, Chander R, Zhang A, & Yee CC (2014). Cerebral white matter disease is independently associated with BPSD in Alzheimer’s disease. Journal of the Neurological Sciences, 337(1e2), 162–166,* Denotes article used in review.
- Kiosses DN, Ravdin LD, Gross JJ, Raue P, Kotbi N, & Alexopoulos GS (2015). Problem adaptation therapy for older adults with major depression and cognitive impairment: A randomized clinical trial. JAMA Psychiatry, 72(1), 22–30,* Denotes article used in review.
- Kolanowski A, Litaker M, Buettner L, Moeller J, & Costa P (2011). A randomized clinical trial of theory based activities for the behavioral symptoms of dementia in nursing home residents. Journal of the American Geriatrics Society, 59(6), 1032–1041,* Denotes article used in review.
- Koppel J, Goldberg TE, Gordon ML, Huey E, Davies P, Keehlisen L, …, Greenwald BS (2012). Relationships between behavioral syndromes and cognitive domains in Alzheimer disease: The impact of mood and psychosis. The American Journal of Geriatric Psychiatry, 20(11), 994–1000,* Denotes article used in review.
- Koppel J, Sunday S, Goldberg TE, Davies P, Christen E, Greenwald BS, & Alzheimer’s Disease Neuroimaging Initiative. (2014). Psychosis in Alzheimer’s disease is associated with frontal metabolic impairment and accelerated decline in working memory: Findings from the Alzheimer’s Disease Neuroimaging Initiative. American Journal of Geriatric Psychiatry, 22(7), 698–707,* Denotes article used in review.
- Kovach CR, Taneli Y, Dohearty P, Schlidt AM, Cashin S, & Silva-Smith AL (2004). Effect of the BACE intervention on agitation of people with dementia. The Gerontologist, 44(6), 797–806,* Denotes article used in review.
- Kuzis G, Sabe L, Tiberti C, Dorrego F, & Starkstein S (1999). Neuropsychological correlates of apathy and depression in patients with dementia. Neurology, 52(7), 1403–1407,* Denotes article used in review.
- Lai MK, Lai OF, Keene J, Esiri MM, Francis PT, Hope T, & Chen CP (2001). Psychosis of Alzheimer’s disease is associated with elevated muscarinic M2 binding in the cortex. Neurology, 57(5), 805–811,* Denotes article used in review.
- Leger GC, & Banks SJ (2014). Neuropsychiatric symptom profile differs based on pathology in patients with clinically diagnosed behavioral variant frontotemporal dementia. Dementia and Geriatric Cognitive Disorders, 37(1–2), 104–112,* Denotes article used in review.
- Leroi I, Voulgari A, Breitner JC, & Lyketsos CG (2003). The epidemiology of psychosis in dementia. American Journal of Geriatric Psychiatry, 11(1), 83–91,* Denotes article used in review.
- Lin LC, Yang MH, Kao CC, Wu SC, Tang SH, & Lin JG (2009). Using acupressure and Montessori-based activities to decrease agitation for residents with dementia: A cross-over trial. Journal of the American Geriatrics Society, 57(6), 1022–1029,* Denotes article used in review.
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, …, Cooper C (2014). A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technology Assessment, 18(39), 1–226,* Denotes article used in review.
- Lopez OL, Becker JT, Sweet RA, Martin-Sanchez FJ, & Hamilton RL (2006). Lewy bodies in the amygdala increase risk for major depression in subjects with Alzheimer disease. Neurology, 67(4), 660–665,* Denotes article used in review.
- Macfarlane MD, Jakabek D, Walterfang M, Vestberg S, Velakoulis D, Wilkes FA, …, Santillo AF (2015). Striatal atrophy in the behavioural variant of frontotemporal dementia: Correlation with diagnosis, negative symptoms and disease severity. PLoS One, 10(6), e0129692,* Denotes article used in review.
- Marcos B, Garcia-Alloza M, Gil-Bea FJ, Chuang TT, Francis PT, Chen CP, …, Ramirez MJ (2008). Involvement of an altered 5-HT -{6} receptor function in behavioral symptoms of Alzheimer’s disease. Journal of Alzheimer’s Disease, 14(1), 43–50,* Denotes article used in review.
- Marin DB, Green CR, Schmeidler J, Harvey PD, Lawlor BA, Ryan TM, …, Mohs RC (1997). Noncognitive disturbances in Alzheimer’s disease: Frequency, longitudinal course, and relationship to cognitive symptoms. Journal of the American Geriatrics Society, 45(11), 1331–1338,* Denotes article used in review.
- Massimo L, Powers C, Moore P, Vesely L, Avants B, Gee J, …, Grossman M (2009). Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dementia and Geriatric Cognitive Disorders, 27(1), 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane R, Keene J, Fairburn C, Jacoby R, & Hope T (1998). Psychiatric symptoms in patients with dementia predict the later development of behavioural abnormalities. Psychological Medicine, 28(5), 1119–1127,* Denotes article used in review.
- Niu YX, Tan JP, Guan JQ, Zhang ZQ, & Wang LN (2010). Cognitive stimulation therapy in the treatment of neuropsychiatric symptoms in Alzheimer’s disease: A randomized controlled trial. Clinical Rehabilitation, 24(12), 1102–1111,* Denotes article used in review.
- Nomura K, Kazui H, Wada T, Sugiyama H, Yamamoto D, Yoshiyama K, …, Takeda M (2012). Classification of delusions in Alzheimer’s disease and their neural correlates. Psychogeriatrics, 12(3), 200–210,* Denotes article used in review.
- O’Connor CM, Clemson L, Hornberger M, Leyton CE, Hodges JR, Piguet O, & Mioshi E (2016). Longitudinal change in everyday function and behavioral symptoms in frontotemporal dementia. Neurology. Clinical Practice, 6(5), 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Hashimoto M, Yatabe Y, Kaneda K, Honda K, Yuuki S, …, Ikeda M (2013). Association of cerebral small vessel disease with delusions in patients with Alzheimer’s disease. International Journal of Geriatric Psychiatry, 28(1), 18–25,* Denotes article used in review.
- Onor ML, Trevisiol M, Negro C, Signorini A, Saina M, & Aguglia E (2007). Impact of a multimodal rehabilitative intervention on demented patients and their caregivers. American Journal of Alzheimer’s Disease and Other Dementias, 22(4), 261–272,* Denotes article used in review.
- Osborne H, Simpson J, & Stokes G (2010). The relationship between pre-morbid personality and challenging behaviour in people with dementia: A systematic review. Aging & Mental Health, 14(5), 503–515,* Denotes article used in review.
- Park HK, Choi SH, Park SA, Kim HJ, Lee Y, Han SH, …, Lee JH (2015). Cognitive profiles and neuropsychiatric symptoms in Korean early-onset Alzheimer’s disease patients: A CREDOS study. Journal of Alzheimer’s Disease, 44(2), 661–673,* Denotes article used in review.
- Pelletier I, & Landreville P (2007). Discomfort and agitation in older adults with dementia. BMC Geriatrics, 7(1), 27,* Denotes article used in review.
- Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, & Soares CB (2015). Guidance for conducting systematic scoping reviews. International Journal of Evidence-Based Healthcare, 13(3), 141–146. [DOI] [PubMed] [Google Scholar]
- Platenik J, Fisar Z, Buchal R, Jirak R, Kitzlerova E, Zverova M, & Raboch J (2014). GSK3beta, CREB, and BDNF in peripheral blood of patients with Alzheimer’s disease and depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 50, 83–93,* Denotes article used in review.
- Proitsi P, Hamilton G, Tsolaki M, Lupton M, Daniilidou M, Hollingworth P, …, McGuinness B (2011). A multiple indicators multiple causes (MIMIC) model of behavioural and psychological symptoms in dementia (BPSD). Neurobiology of Aging, 32(3), 434–442,* Denotes article used in review.
- Purandare N, Voshaar RC, Hardicre J, Byrne J, McCollum C, & Burns A (2006). Cerebral emboli and depressive symptoms in dementia. The British Journal of Psychiatry, 189, 260–263,* Denotes article used in review.
- Quaranta D, Vita MG, Bizzarro A, Masullo C, Piccininni C, Gainotti G, & Marra C (2015). Cognitive and behavioral determinants of psychotic symptoms in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 39(3–4), 194–206,* Denotes article used in review.
- Regan C, Katona C, Walker Z, & Livingston G (2005). Relationship of exercise and other risk factors to depression of Alzheimer’s disease: The LASER-AD study. International Journal of Geriatric Psychiatry, 20(3), 261–268,* Denotes article used in review.
- Reisberg B, Auer SR, & Monteiro IM (1996). Behavioral pathology in Alzheimer’s disease (BEHAVE-AD) rating scale. International Psychogeriatrics, 8(Suppl. 3), 301–308, discussion 351–354. [DOI] [PubMed] [Google Scholar]
- Reyes S, Viswanathan A, Godin O, Dufouil C, Benisty S, Hernandez K, …, Chabriat H (2009). Apathy: A major symptom in CADASIL. Neurology, 72(10), 905–910,* Denotes article used in review.
- Robert P, Onyike CU, Leentjens AF, Dujardin K, Aalten P, Starkstein S, …, Byrne J (2009). Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. European Psychiatry, 24(2), 98–104. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mansilla J, Gonzalez Lopez-Arza MV, Varela-Donoso E, Montanero-Fernandez J, Gonzalez Sanchez B, & Garrido-Ardila EM (2015). The effects of ear acupressure, massage therapy and no therapy on symptoms of dementia: A randomized controlled trial. Clinical Rehabilitation, 29(7), 683–693,* Denotes article used in review.
- Saz P, Lopez-Anton R, Dewey ME, Ventura T, Martin A, Marcos G, …, Lobo A (2009). Prevalence and implications of psychopathological non-cognitive symptoms in dementia. Acta Psychiatrica Scandinavica, 119(2), 107–116,* Denotes article used in review.
- Scarmeas N, Brandt J, Albert M, Devanand D, Marder K, Bell K, …, Stern Y (2002). Association between the APOE genotype and psychopathologic symptoms in Alzheimer’s disease. Neurology, 58(8), 1182–1188,* Denotes article used in review.
- Slifer MA, Martin ER, Gilbert JR, Haines JL, & Pericak-Vance MA (2009). Resolving the relationship between ApolipoproteinE and depression. Neuroscience Letters, 455(2), 116–119,* Denotes article used in review.
- Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM, …, Pickering-Brown SM (2012). Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain, 135(Pt. 3), 693–708,* Denotes article used in review.
- Sweet RA, Akil M, Mulsant BH, Ulrich R, Pasternak RE, & Zubenko GS (1998). Determinants of spontaneous extrapyramidal symptoms in elderly psychiatric inpatients diagnosed with Alzheimer’s disease, major depressive disorder, or psychotic disorders. Journal of Neuropsychiatry and Clinical Neurosciences, 10(1), 68–77,* Denotes article used in review.
- Tappen RM, & Williams CL (2009). Therapeutic conversation to improve mood in nursing home residents with Alzheimer’s disease. Research in Gerontological Nursing, 2(4), 267–275,* Denotes article used in review.
- Teri L, Logsdon RG, Uomoto J, & McCurry SM (1997). Behavioral treatment of depression in dementia patients: A controlled clinical trial. The Journals of Gerontology. Series B: Psychological Sciences and Social Sciences, 52(4), P159–P166,* Denotes article used in review.
- Toot S, Swinson T, Devine M, Challis D, & Orrell M (2017). Causes of nursing home placement for older people with dementia: A systematic review and meta-analysis. International Psychogeriatrics, 29(2), 195–208. [DOI] [PubMed] [Google Scholar]
- Vilalta-Franch J, Calvo-Perxas L, Garre-Olmo J, TurroGarriga O, & Lopez-Pousa S (2013). Apathy syndrome in Alzheimer’s disease epidemiology: Prevalence, incidence, persistence, and risk and mortality factors. Journal of Alzheimer’s Disease, 33(2), 535–543. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2016). Dementia. Retrieved from http://www.who.int/mediacentre/factsheets/fs362/en/
- Wunderlich G, & Kohler P (2000). Improving the quality of long-term care. Washington, DC: Retrieved from http://www.nap.edu/openbook.php?record_id=9611 [PubMed]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, & Leirer VO (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, …, Du L (2015). The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. Journal of Evidence-Based Medicine, 8(1), 2–10. [DOI] [PubMed] [Google Scholar]