Abstract
Objective
To determine whether reported therapeutic interventions for arthrogenic muscle inhibition (AMI) in patients with ACL injuries, following ACL reconstruction, or in laboratory studies of AMI, are effective in improving quadriceps activation failure when compared with standard therapy in control groups.
Design
A scoping review of the efficacy of interventions was conducted in accordance with the methodological framework of Arksey and O’Malley and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Search terms included ‘arthrogenic muscle inhibition’, ‘quadriceps activation following knee injuries’, ‘anterior cruciate’ or ‘knee’ combined with ‘quadriceps activation’, ‘quadriceps inhibition’, ‘corticomotor’, ‘arthrogenic’, ‘brain activation’ and ‘neuroplasticity’. Articles were evaluated for risk of bias using the PEDro (Physiotherapy Evidence Database) criteria. The overall quality of evidence for each intervention was assessed using Grading of Recommendations Assessment, Development and Evaluation (GRADE).
Data sources
PubMed, EMBASE and Cumulative Index to Nursing and Allied Health Literature databases.
Eligibility criteria for selecting studies
Isolated case reports and articles reporting outcomes in patients with chronic disease or major trauma were excluded. All other original research articles were included.
Results
780 potential articles were identified. 20 met the inclusion criteria. These studies provided a moderate quality of evidence to support the efficacy of cryotherapy and physical exercises in the management of AMI. There was low-quality evidence for efficacy of neuromuscular electrical stimulation and transcutaneous electrical nerve stimulation, and very low-quality evidence for efficacy of ultrasound and vibration.
Conclusions
This scoping review demonstrated moderate-quality evidence for the efficacy of cryotherapy and physical exercises in improving quadriceps activation failure after ACL injury and reconstruction. These therapeutic modalities are therefore recommended in the management of AMI.
Keywords: knee acl, quadriceps, hamstring, neuromuscular, rehabilitation
Introduction
Quadriceps weakness is a frequently observed barrier to effective rehabilitation following ACL injury and reconstruction.1 2 It may lead to a wide range of important consequences, including extension deficit,3 gait abnormality,4 quadriceps atrophy,1 5 6 poor function,6 dynamic instability,7 persistent knee pain and early osteoarthritis.1 8
Quadriceps activation failure after ACL reconstruction (ACLR) is not simply an isolated local phenomenon related to atrophy. Many authors describe its synchronous occurrence in both reconstructed and contralateral limbs.9 This has been attributed to arthrogenic muscle inhibition (AMI), a process in which quadriceps activation failure is caused by neural inhibition. Mechanisms for this inhibition include alteration in muscle resting motor thresholds, changes in the discharge of articular sensory receptors, altered spinal reflex excitability (affecting the group I non-reciprocal (Ib) inhibitory pathway, the flexion reflex and the gamma loop)10 and abnormal cortical activity (intracortical inhibition and a requirement for greater frontal cortex theta power in basic movement and joint position sense tasks).11 12
Recently, several clinical studies have suggested specific treatment modalities for AMI.13–34 Most of the therapeutic interventions for AMI aim to alter motor excitability using disinhibitory mechanisms.35 These improve voluntary quadriceps activation by targeting either joint mechanoreceptors, the peripheral nervous system around the joint (mainly group III and IV afferent nerves) or the central nervous system directly.10 35 The aim of this scoping review was to determine the strength of evidence supporting the use of common therapeutic interventions for AMI in patients with ACL injuries, following ACLR, or in laboratory studies of AMI.
Methods
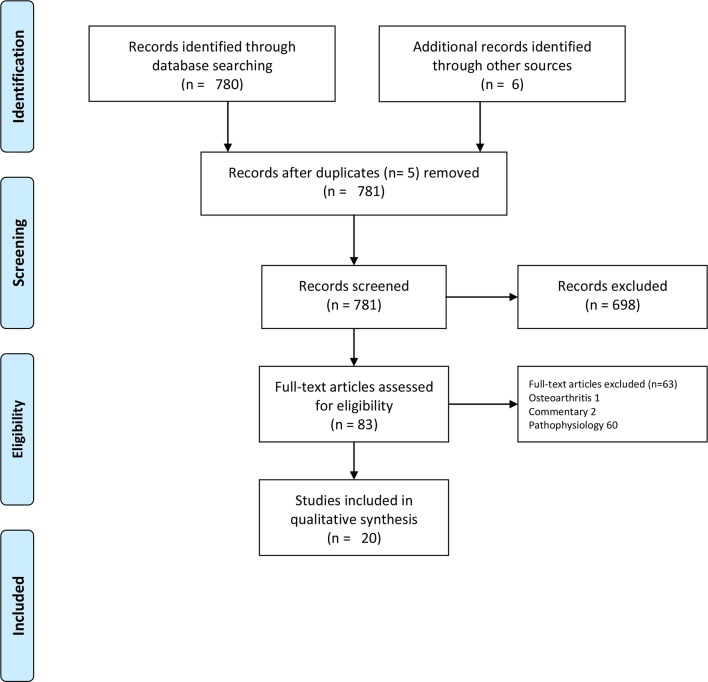
We conducted a scoping review as this approach is superior to a systematic review in addressing an exploratory research question.36 37 We followed the five-stage methodological framework of Arksey and O’Malley36 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 checklist (see figure 1). The study protocol was registered with PROSPERO (International prospective register of systematic reviews) database (trial registration number: CRD42017067499).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Stage 1: identifying the research question
Our research question was ‘What is the strength of evidence supporting the use of common therapeutic interventions for AMI in patients with ACL injuries, following ACL reconstruction, or in laboratory studies of AMI?’
Stage 2: identifying relevant studies
The literature search used subject mapping and keywords and is presented in online supplementary appendix 1. The search strategy was applied to the PubMed, EMBASE and Cumulative Index to Nursing and Allied Health Literature databases by two authors independently on 12 March 2017. The same authors also independently performed all aspects of the study selection.
bjsports-2017-098401supp001.docx (57.8KB, docx)
Stage 3: study selection
We included primary research studies that evaluated the efficacy of therapeutic interventions for AMI. The main focus of the review was on AMI in patients following ACL injury or reconstruction. Due to the fact that treatment of AMI is an emerging concept with a small evidence base, it was deemed appropriate to use a scoping review methodology and include studies that intended to evaluate the efficacy of therapeutic interventions for the same pathological processes in selected, relevant, alternative settings. Therefore, studies including young patients with artificially induced knee effusions and other relevant acute knee pathologies (restricted to other knee ligament injuries, meniscal pathology and patellofemoral instability) were also included. Only studies published in the English language were included.38 39
We excluded isolated case reports and articles which included patients with chronic conditions (eg, osteoarthritis) or major trauma (fracture, multiligament injury, neurovascular injury). Any disagreement between reviewers regarding study eligibility were resolved through discussion.
Each article was reviewed for relevance, and the references of the included articles were examined to identify further eligible studies.
Stage 4: charting the data
Data extraction and risk of bias assessment were performed independently by two investigators. A template was used for data extraction that included study design, participants, inclusion/exclusion criteria, intervention investigated, comparators, outcome measures (quadriceps activation/strength including central activation ratio (CAR), peak torque, maximal voluntary isometric contraction (MVIC), H-reflex amplitude, knee flexion angle symmetry and muscle fibre conduction velocity), main findings, conclusion and level of evidence.
Stage 5: collating, summarising and reporting the results
Due to heterogeneity among studies with respect to the populations, interventions and outcomes studied, it was not possible to pool data. Instead, we collated efficacy data with respect to the outcome measures defined in stage 4 and synthesised a narrative summary of the evidence for each intervention.
To determine the strength of evidence, the following steps were undertaken. All included articles were individually evaluated for risk of bias using the Physiotherapy Evidence Database (PEDro) criteria.40 41 The level of evidence for individual studies was assessed according to the Oxford Centre for Evidence-based Medicine.42 The overall quality of evidence for each therapeutic intervention was assessed using the GRADE working group grades of evidence.43 Details of how the GRADE guidelines were applied are included in online supplementary appendix 2. Effect sizes were calculated using the methodology of Thalheimer and Cook.44
bjsports-2017-098401supp002.docx (14.3KB, docx)
Results
The search strategy yielded 780 articles. The references of these articles were then reviewed and a further six eligible studies were included. Five studies were removed as they were duplicates. After application of eligibility criteria, a total of 20 relevant articles were identified. The date of publication of the included studies ranged from 1990 to 2017.
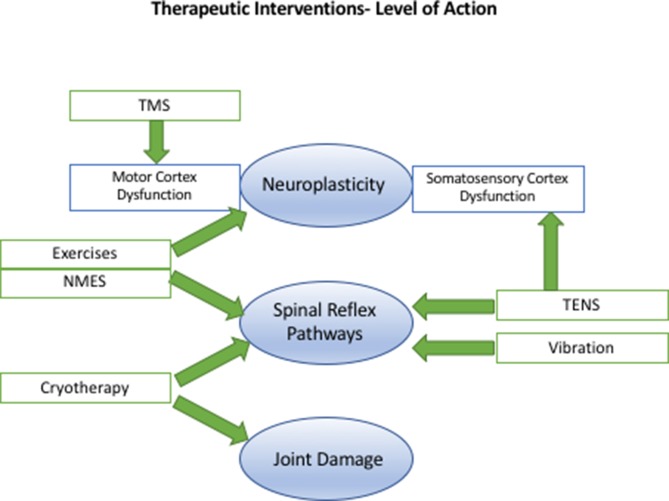
Table 1 summarises the study characteristics, level of evidence of the included studies, outcomes, effect sizes and the GRADE recommendation for each intervention. Table 2 demonstrates how the PEDro scores were calculated for each study. Table 3 demonstrates how the GRADE recommendations for each intervention were determined. The mechanism and level of action of the therapeutic interventions studied are summarised in figure 2.
Table 1.
Summary of included studies
| Study | Study design, level of evidence (CEBM) | Participants, n, mean age, sex | Injury | Intervention | Outcome | Effect size (Cohen’s d) |
Relative size |
| Cryotherapy | |||||||
| Hopkins et al 13 | RCT, 1b | n=30 (30 AKE), age=22, 11F/19M | AKE | 3 groups (cryotherapy, TENS and control). Cryotherapy group had 2 plastic bags containing 1.5 L of partially crushed ice placed directly on the knee for 30 min. A typical TENS protocol was used. The treatment session lasted 30 min. |
H-reflex (at 45 min). | 3.21 | Huge. |
| Rice et al 14 | RCT, 1b | n=15 (15 AKE), age=35, 5F/10M | AKE | 2 groups: cryotherapy and control. The cryotherapy group had ice around their knee joint for a 20 min period. The control group did not receive the cryotherapy intervention and remained seated for 20 min before performing postintervention measurements. |
MFCV. MVIC. |
1.62 1.21 |
Huge. Very large. |
| Hart et al 15 | RCT, 1b | n=30 (ACLR), age=27, 20F/10M | AMI (CAR<90%) post-ACLR | 3 groups: 1. 20 min of knee joint cryotherapy. 2. An hour of therapeutic rehabilitation exercises. 3. Cryotherapy followed by exercises. The patients attended 4 supervised visits over a 2-week period. |
MVIC: 1. Cryotherapy + exercise. 2. Cryotherapy alone. 3. Exercise alone. |
1.4 0.58 0.3 |
Very large. Medium. Small. |
| Kuenze et al 16 | Case series, 4 | n=20 (10 ACLR, 10 healthy), age=22, 9F/1M | Post-ACLR | The intervention included cryotherapy application to the knee joint followed by lower extremity muscle stretching, progressive strengthening exercises and balance training. | MVIC. CAR. |
0.34 1.22 |
Small. Very large. |
| GRADE=moderate | |||||||
| Exercise | |||||||
| Lowe and Dong26 | Case–control, 3b | n=18 (9 ACLR, 9 healthy), age=20, 11M/7F | AMI post-ACLR | Hamstring fatigue was induced by instructing participants to perform squats until rating of perceived exertion was 15 out of 20 (or ‘hard’) and their heart rate was approximately 150 beats/min. | CAR. | 1.27 | Very large. |
| Kuenze et al 16 | Case series, 4 | n=20 (10 ACLR, 10 healthy), age=22, 9F/1M | Post-ACLR | The intervention included cryotherapy application to the knee joint followed by lower extremity muscle stretching, progressive strengthening exercises and balance training. | MVIC. CAR. |
0.34 1.22 |
Small. Very large. |
| Hart et al 15 | RCT, 1b | n=30 (ACLR), age=27, 20F/10M | AMI (CAR<90%) post-ACLR | 3 groups: 1. 20 min of knee joint cryotherapy. 2. An hour of therapeutic rehabilitation exercises. 3. Cryotherapy followed by exercises. |
MVIC: 1. Cryotherapy + exercise. 2. Cryotherapy alone. 3. Exercise alone. |
1.4 0.58 0.3 |
Very large. Medium. Small. |
| Lepley et al 17 | Prospective cohort, 2b | n=46 (36 ACLR/10 healthy), age=22, 16F/33M | Post-ACLR | 4 treatment groups: 1. NMES and eccentric exercise. 2. Eccentrics-only. 3. NMES-only. 4. Standard of care. NMES and eccentrics received a combined NMES and eccentric protocol postreconstruction. |
MVIC: 1. NMES + eccentric. 2. Eccentrics-only. 3. NMES-only. |
1.05 1.25 0.03 |
Large. Very large. Negligible. |
| GRADE=moderate | |||||||
| NMES | |||||||
| Lepley et al 18 | Prospective cohort, 2b | n=46 (36 ACLR/10 healthy), age=22, 16F/33M | Post-ACLR | Healthy controls and 4 treatment groups: 1. NMES + eccentric exercise. 2. Eccentrics-only. 3. NMES-only. 4. Standard of care. NMES and eccentrics received a combined NMES and eccentric protocol postreconstruction. |
LSI: 1. NMES + eccentric. 2. Eccentrics-only. 3. NMES-only. |
0.43 0.3 0.16 |
Medium. Small. Small. |
| Lepley et al 17 | Prospective cohort, 2b | n=46 (36 ACLR/10 healthy), age=22, 16F/33M | Post-ACLR | 4 treatment groups: 1. NMES and eccentric exercise. 2. Eccentrics-only. 3. NMES-only. 4. Standard of care. NMES and eccentrics received a combined NMES and eccentric protocol postreconstruction. |
MVIC: 1. NMES + eccentric. 2. Eccentrics-only. 3. NMES-only. |
1.05 1.25 0.03 |
Large. Very large Negligible. |
| Glaviano et al 27 | RCT, 2b | n=18(18 knee pain, CAR<90), age=24, 8F/10M | AMI (CAR<90%) + knee pain | The treatment intervention was a 15 min patterned electrical neuromuscular stimulation, applied to the quadriceps and hamstring muscles. | MVIC. | No effect. | |
| GRADE=low | |||||||
| TENS | |||||||
| Son et al 19 | RCT, 1b | n=30 (30 AKE), age=23, 5F/10M | AKE | TENS or placebo treatment was administered to each group for 20 min, following infusion of hypertonic saline. | MVIC. | 1.34 | Very large. |
| Konishi et al 20 | RCT cross-over, 2b | n=12 (12 healthy), age=22, 12M | Vibration-induced quads activation failure | A cross-over design that involved 2 sessions for each participant was used. For up to 30 s before and then during the MVC, the participants were randomly assigned to receive TENS applied to the skin covering the knee joint or no TENS. | MVIC. | 0.76 | Large. |
| Hopkins et al 13 | RCT, 1b | n=30 (30 AKE), age=22, 11F/19M | AKE | 3 groups (cryotherapy, TENS and control). Cryotherapy group had 2 plastic bags containing 1.5 L of partially crushed ice placed directly on the knee for 30 min. A typical TENS protocol was used. The treatment session lasted 30 min. |
H-reflex (at 45 min). | 1.23 | Very large. |
| Hart et al 21 | RCT, 2b | n=30 (30 ACL), age=32, 10F/20M | ACL injury | All patients attended 4 sessions of supervised quadriceps strengthening exercises over 2 weeks prior to surgery. Patients were randomly allocated to 3 groups: 1. Exercises alone. 2. Exercise while wearing a sensory TENS device on the knee joint. 3. 20 min of knee joint cryotherapy immediately prior to each daily exercise session. |
MVIC: 1. Exercise only. 2. Exercise + TENS. 3. Exercise + cryotherapy. |
No effect over exercise. | |
| GRADE=low | |||||||
| Vibration | |||||||
| Pamukoff et al 22 | RCT, 1b | n=20 (20 ACLR), age=21, 14F/6M | Post-ACLR | 3 groups: LMV, WBV or control (sham). A custom-made LMV stimulator was affixed to the quadriceps tendon. During the WBV condition, subjects stood in an identical position as in the LMV intervention on a vibrating platform that provided the same stimulus (2 g of acceleration at a frequency of 30 Hz). |
AMT: WBV. LMV. CAR: WBV. LMV. MVIC: WBV. LMV. |
1.82 1.42 0.82 0.80 0.56 0.44 |
Huge. Very large. Large. Large. Medium. Medium. |
| Blackburn et al 23 | RCT, 1b | n=45 (45 AKE), age=21, 28F/17M | AKE | 3 groups: WBV, LMV and control. After intra-articular injection of 60 mL of saline, the WBV and LMV groups were then exposed to vibratory stimuli previously demonstrated to facilitate quadriceps function, and the control group performed these same procedures without vibratory stimuli. |
The CAR and MVIC improved in the WBV and LMV groups (p<0.05) immediately postintervention, but not in the control group. | NA (no SD provided). | |
| GRADE=very low | |||||||
| Ultrasound | |||||||
| Norte et al 24 | RCT, 1b | n=30 (30 knee injury), age=23, 15M/15F | Knee injury with AMI (CAR<90), (22/30 ACL) | 2 groups: ultrasound and control (sham). An ultrasound or sham treatment was applied to the anteromedial knee. The transducer was manually moved at an estimated rate of 4 cm/s over an area delineated by a custom template twice the size of the transducer surface area for a duration of 17 min. |
H-reflex (20 min postintervention). | 0.58 | Medium. |
| GRADE=very low | |||||||
| TMS | |||||||
| Gibbons et al 28 | RCT, 1b | n=20 (20 partial meniscectomy), age=38, 6F/14M | Partial meniscectomy with AMI (CAR<85) | 2 groups: TMS and control. Participants in the experimental group received TMS over the motor cortex that was contralateral to the involved leg and performed 3 maximal quadriceps contractions with the involved leg. |
No significant difference in CAR or MVIC was seen between groups (p=NS). | No evidence for TMS over control. | |
| No evidence for efficacy | |||||||
| Taping/Brace | |||||||
| Kim et al 29 | RCT, 2b | n=16 (16 knee injury), age=24, 7F/9M | Knee injury with AMI (CAR<90) | 2 groups: Kinesio taping and sham. H-reflex of the vastus medialis and quadriceps. CAR and MVIC were measured before taping and 20 min after tape was applied over the rectus femoris. All outcomes were measured again after tape was removed when participants returned to the laboratory 24–48 hours after taping. |
No significant difference between groups in H-reflex, CAR or MVIC (p=NS). | No evidence for use of Kinesio taping. | |
| Oliveira et al 30 | RCT, 1b | n=47 (47 ACLR), age=29, 47M | Post-ACLR | 2 groups: control, placebo and Kinesio taping. Kinesio taping group participants were submitted to Kinesio taping on the femoral quadriceps of the affected limb, while placebo group subjects used the same procedure without the tension proposed by the method. The control group remained at rest for 10 min. | None of the variables analysed showed significant intergroup or intragroup differences (p=NS). | No evidence for use of Kinesio taping. | |
| Davis et al 31 | Cross-over, 4 | n=14 (14 ACLR), age=23, 9F/5M | Post-ACLR | 3 groups: brace, sleeve or nothing. Participants performed a standardised aerobic exercise protocol on a treadmill. |
No differences were seen between bracing conditions after aerobic exercise (p=NS). | No evidence for use of bracing. | |
| No evidence for efficacy | |||||||
| Other | |||||||
| Drover et al 32 | Case series, 4 | n=9 (9 AKP), age=26, 5F/4M | AKP | The treatment intervention included the treatment protocols described in the ART lower extremity manual for the patella tendon, vastus medialis, vastus intermedius, vastus lateralis and rectus femoris. | Knee extensor strength (MVIT) and knee extensor inhibition were not significantly different. | No evidence for use of ART. | |
| Ageberg et al 33 | RCT, 2b | n=39 (39 ACL), age=24, 29F/20M | Post-ACLR | 2 groups: local cutaneous application of anaesthetic (EMLA) or placebo cream. 50 g of EMLA, or placebo, was applied on the leg 10 cm above and 10 cm below the centre of patella, leaving the area around the knee without cream. | No statistically significant differences were in the EMLA group or in the placebo group. | No evidence for use of temporary cutaneous anaesthesia. | |
| Warner et al 34 | RCT, 2b | n=12 (12 knee injury), age=26, 4F/8M | Knee injury with AMI (CAR<90) | 3 groups:
|
No significant difference in either CAR or MVIT (p=NS). | No evidence for use of superficial heat. | |
| No evidence for efficacy | |||||||
ACLR, ACL reconstruction; AKE, artificial knee effusion; AKP, anterior knee pain; AMI, arthrogenic muscle inhibition; AMT, active motor threshold; ART, active release technique; CAR, central activation ratio; F, female; LMV, local muscle vibration; M, male; MFCV, muscle fibre conduction velocity; MVC and MVIT, MVIC EMLA (Eutectic Mixture of Local Anesthetics); MVIC, maximal voluntary isometric contraction; NA, not available; NMES, neuromuscular electrical stimulation; RCT, randomised controlled trial; TENS, transcutaneous electrical nerve stimulation; TMS, transcranial magnetic stimulation; WBV, whole body vibration.
Table 2.
Assessment of Physiotherapy Evidence Database (PEDro) criteria
| Study | 1 Eligibility | 2 Randomized | 3 Concealed | 4 Baseline | 5 Blinding subjects | 6 Blinding therapists | 7 Blinding assessors | 8 Outcomes >85% | 9 Intention to treat analysis | 10 Between group comparisons | 11 Measures of variability | Score |
| Hopkins et al
13
2002 |
1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Rice et al
14
2009 |
1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Hart et al
15
2014 |
1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Kuenze et al
16
2017 |
1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Lowe and Dong26
2017 |
1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Hart et al
21
2014 |
1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Lepley et al
17
2015 |
1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Lepley et al
18
2015 |
1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Glaviano et al
27
2014 |
1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9 |
| Son et al
19
2016 |
1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9 |
| Konishi et al
20
2017 |
1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Pamukoff et al
22
2016 |
1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Blackburn et al
23
2014 |
1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| Norte et al
24
2015 |
1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9 |
| Gibbons et al
28
2010 |
1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Kim et al
29
2017 |
1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Oliveira et al
30
2016 |
1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Davis et al
31
2011 |
1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| Drover et al
32
2004 |
1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
| Ageberg et al
33
2012 |
1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Warner et al
34
2013 |
1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
Table 3.
GRADE table
| Risk of bias | Inconsistency | Indirectness | Imprecision | GRADE score | |
| Cryotherapy | Negligible | Negligible | Serious* | Negligible | Moderate |
| Exercise | Serious† | Negligible | Negligible | Negligible | Moderate |
| NMES | Serious‡ | Serious§ | Negligible | Negligible | Low |
| TENS | Negligible | Serious§ | Serious¶ | Negligible | Low |
| Vibration | Negligible | Negligible | Serious** | Very serious†† | Very low |
| Ultrasound | Negligible | Negligible‡‡ | Serious§§ | Very serious¶¶ | Very low |
GRADE calculation.
Risk of bias: PEDro <6, decrease one grade; PEDro <4, decrease two grades.
Inconsistency: Heterogeneity of results (wide variance of effect sizes), decrease one grade.
Indirectness: Population of study is not ACLR, decrease one grade.
Imprecision: Lower threshold of 95% CI reduces effect to negligible, decrease one grade; lower threshold of 95% CI would alter conclusion or not provided, decrease two grades.
*Indirectness of evidence (only one trial in ACLR patients with AMI).
†PEDro score of 5 for three of the four trials.
‡PEDro score of 5 for two of the three trials.
§Heterogeneity of results.
¶Indirectness of evidence (effect only seen in laboratory trials).
**Indirectness of evidence (one of the two trials was a laboratory test).
††Imprecision (CIs or SDs not provided in Blackburn et al 23 study).
‡‡Note: only one study.
§§Indirectness of evidence (knee injury population, not specifically ACL).
¶¶Imprecision (wide CIs, lower limit of effect size is negative).
ACLR, ACL reconstruction; AMI, arthrogenic muscle inhibition; NMES, neuromuscular electrical stimulation; PEDro, Physiotherapy Evidence Database; TENS, transcutaneous electrical nerve stimulation.
Figure 2.
Therapeutic interventions for arthrogenic muscle inhibition and their level of action. NMES, neuromuscular electrical stimulation; TENS, transcutaneous electrical nerve stimulation; TMS, transcranial magnetic stimulation.
Cryotherapy
Four studies evaluated the efficacy of cryotherapy in AMI. This included three randomised controlled trials (RCTs). All studies showed that cryotherapy improved the features of AMI; effect sizes were very large. Specifically, three of the studies demonstrated that cryotherapy was associated with significant improvement in quadriceps strength (measured by maximal voluntary isometric contraction).14–16 Additionally, Hopkins et al 13 demonstrated a significant improvement in quadriceps motor neuron pool recruitment (measured by the H-reflex). Note that two of these studies did not specifically evaluate patients following ACLR—they were laboratory studies of patients with artificially created knee effusions.13 14
Using the GRADE approach, there was moderate-quality evidence for the efficacy of cryotherapy in the treatment of AMI.
Exercise
Four studies evaluating the efficacy of exercise therapy in patients with AMI after ACLR were identified. These included the cryotherapy-based studies from Hart et al and Kuenze et al,15 16 which also made an adjunctive use of exercise. All of the included studies demonstrated that exercise therapy was associated with a significant improvement in quadriceps activation (MVIC and CAR).15–17 26
Three of the exercise programmes consisted of traditional open chain exercises with resistance, and progressive closed chain strengthening exercises of quadriceps and hamstring muscles.15–17 Resistance exercises included quadriceps sets, straight leg raises with hip abduction/adduction and progression to free-standing quarter squats, wall squats, hamstring curls, hip flexion/extension and leg press. Flexibility was attained using hamstring, quadriceps and calf stretching exercises. The fourth study examined the effect of a hamstring fatigue exercise protocol on patients with AMI following ACLR, in a case–control study.26 Hamstring fatigue was induced by participants performing squats to a height of approximately 0.45 m from the ground at the rate of one squat every 2 s. The quadriceps CAR of the ACLR group was significantly higher when evaluated after hamstring fatigue exercises (mean 96.0%, SD 7.6%) versus prefatigue (mean 81.2%, SD 15.8%; p=0.01).
GRADE assessment revealed moderate-quality evidence for the efficacy of exercise in the treatment of AMI.
Neuromuscular electrical stimulation
Two studies evaluated the same cohort of non-randomised patients who were allocated to four different rehabilitation groups.17 18 The authors reported that the group with combined neuromuscular electrical stimulation (NMES) and eccentric exercise demonstrated restored biomechanical limb symmetry that most closely resembled a control group of healthy individuals.18 However, there was no advantage with respect to quadriceps strength and activation.17 Furthermore, eccentric exercise alone recovered quadriceps strength (MVIC) better than individuals who only received NMES therapy, or the standard care following ACLR.17
Patterned electrical neuromuscular stimulation is a form of NMES which is proposed to mimic muscle-firing patterns of healthy individuals. A randomised trial failed to demonstrate any difference in knee extension torque or quadriceps activation, compared with a sham treatment.27
GRADE assessment revealed low-quality evidence for the efficacy of NMES in the treatment of AMI.
Transcutaneous electrical nerve stimulation
In the only randomised clinical study of transcutaneous electrical nerve stimulation (TENS) in patients with ACL rupture, there was no difference in isometric strength and quadriceps CAR among three groups (exercise only, exercise and TENS device, and 20 min of cryotherapy immediately prior to each daily exercise session).21 While all groups demonstrated a significant improvement in quadriceps strength, and effect sizes suggested potential clinical benefit to patients with AMI, the disinhibitory modalities were no better than exercise alone.
Three randomised trials, however, have shown some effect of TENS in improving AMI in the laboratory setting.13 19 20 Two trials showed a significant improvement compared with controls in the quadriceps muscle strength (MVIC),19 20 and the third showed it effectively disinhibited the quadriceps motor neuron pool (measured by H-reflex).13 Although TENS disinhibited the quadriceps motor neuron pool during the treatment, its beneficial effects were lost 30 min after cessation of treatment. In contrast, the beneficial effects of cryotherapy continued up to the final measurement of the H-reflex at 60 min post-treatment.
GRADE assessment revealed low-quality evidence for the efficacy of TENS in the treatment of AMI.
Vibration
Pamukoff et al 22 evaluated the role of vibration therapy. The authors randomised ACL reconstructed patients to three groups (whole body vibration (WBV), local muscle vibration (LMV) and control). There was a statistically significant increase in CAR (+4.9%) following WBV and LMV (+2.7%). There was also a reduction in quadriceps active motor threshold following WBV (−3.1%) and LMV (−2.9%), suggesting that the interventions increase corticomotor excitability. In a laboratory study, Blackburn et al 23 also identified that quadriceps CAR improved in WBV (11.4%) and LMV (7.3%) groups, but not in controls. However, we contend that these small changes are of limited clinical significance, particularly given that the mean time since ACLR was over 50 months and the patients did not have proof of AMI. Furthermore, these studies only evaluated the immediate effects of WBV and LMV.
GRADE assessment revealed very low-quality evidence for the efficacy of vibration in the treatment of AMI.
Ultrasound
An RCT evaluated patients with an intra-articular knee injury (22 of 30 were ACL injuries) and quadriceps CAR<90%.24 Non-thermal ultrasound (active) or sham treatment was applied to the knee for a duration of 17 min. The investigators observed increased quadriceps motor neuron pool excitability after ultrasound application compared with the sham group (14%–19% increase in the H-reflex amplitude, p=0.015).
GRADE assessment revealed very low-quality evidence for the efficacy of ultrasound in the treatment of AMI.
Transcranial magnetic stimulation
Only one trial was identified: an RCT (n=20) evaluated transcranial magnetic stimulation (TMS) in patients who had a partial meniscectomy and ongoing quadriceps weakness (CAR<85%).28 No significant difference in CAR or MVIC was seen compared with the control group that had no treatment. This systematic review did not identify any evidence supporting the use of TMS in the treatment of AMI.
Taping and bracing
Two RCTs assessed the effects of taping on quadriceps muscle performance.29 30 One assessed patients following ACLR,30 and the other assessed patients with a history of knee pathology and quadriceps CAR<90%.29 Neither study demonstrated a benefit over placebo for any outcome measure. A trial of 14 patients following ACLR randomised patients to receive either a knee brace, neoprene sleeve or no brace.31 No differences were observed between brace and no-brace conditions after aerobic exercise.
The available evidence does not support taping or bracing in AMI.
Other
A small case series of nine patients with anterior knee pain evaluated active release technique protocols.32 The active release technique is a system of soft tissue manipulation, purported to relieve tissue tension via the removal of fibrosis and adhesions. There was no effect in reducing quadriceps inhibition or increasing quadriceps strength. Another study found application of local anaesthetic cream had no effect in improving the sensorimotor function of the knee in subjects with ACL injury.33 An RCT of 12 patients with a history of knee-joint pathology and quadriceps CAR of <90% evaluated superficial heat application and found no effect on quadriceps function.34
The available evidence does not support these treatments in AMI.
Discussion
AMI is responsible for a considerable morbidity after ACLR.1 3 5–8 The main findings of this systematic review are that there is moderate-quality evidence to support the efficacy of cryotherapy and physical exercise therapy (open and closed chain resistance training, with hamstring fatigue exercises) in patients with AMI after ACL injury or reconstruction.
It is important to understand the pathophysiology of AMI to appropriately target therapeutic interventions. Several studies report an association between hamstring overactivity and dyskinesia with quadriceps weakness in AMI.45–47 This has been attributed to the flexion reflex spinal pathway, which produces a pattern of flexor facilitation and extensor inhibition. It is reported that greater hamstring coactivation is associated with significantly worse knee function.48 It is therefore unsurprising that hamstring fatigue exercises in patients with AMI following ACLR have been reported to be associated with a significant increase in quadriceps strength.26 In addition, all of the studies included in this review that evaluated physical therapy exercises (including open chain exercises with resistance, progressive closed chain quadriceps and hamstring strengthening exercises) in patients following ACLR, or artificially created knee effusion, demonstrated significantly improved quadriceps function (MVIC or CAR), which may indicate restoration of more optimal quadriceps neuromuscular function.14
All of the included studies on cryotherapy also demonstrated significantly improved quadriceps strength,13–16 and one study demonstrated reversed decline in motor recruitment (measured by H-reflex),13 in patients with AMI. Cryotherapy may reduce the discharge of sensory receptors and slow articular nerve conduction, thus decreasing transmission of the afferent impulses that contribute to deleterious spinal reflexive excitability.14
In this review, TENS13 19–21 and NMES17 18 27 had a low level quality of evidence to support their efficacy.19 TENS demonstrated large effect sizes in laboratory trials of artificial knee effusions. Although the single clinical trial on ACLR patients demonstrated no advantage over cryotherapy or exercise therapy alone, treatment with TENS demonstrated effect sizes that suggest potential benefits to patients with AMI.21 However, all patients in the TENS group also underwent exercise therapy, and therefore the role of TENS could not be evaluated in isolation. The three clinical trials identified in this study showed small to negligible effect sizes.17 18 27 The clinical results for both NMES and TENS preclude recommendation of these modalities in the management of AMI.
In this scoping review ultrasound therapy24 and vibration22 23 demonstrated very low-quality evidence for efficacy. Although two clinical studies (one vibration and one ultrasound) in ACLR patients demonstrated small statistically significant improvements (in CAR and H-reflex, respectively), these were of questionable clinical importance. Currently these modalities cannot be recommended in the management of AMI.
Other therapies including TMS,28 taping,29 30 bracing,31 application of heat34 and soft tissue release strategies32 were of no clinical benefit in the management of AMI.
Limitations
We note that some of the included trials were laboratory studies, and these cannot necessarily be extrapolated to the clinical scenario of AMI that occurs after ACL injury. The study methodology has attempted to account for this when awarding the GRADE level of evidence, with a decrease in the score by one level for studies that did not directly assess ACL-injured or reconstructed knees. Another limitation was that the studies were heterogeneous in design and limited by small patient numbers. The quality assessment of the trials using the PEDro scale ranged from 3 to 9 out of a maximum of 10. Lower quality studies were not excluded due to the relatively small number of clinical trials identified. Only English-language articles were included.
Conclusion
This scoping review demonstrated moderate-quality evidence for the efficacy of cryotherapy and physical exercises in improving quadriceps activation failure after ACL injury and reconstruction. These therapeutic modalities are therefore recommended in the management of AMI.
What is already known?
Lack of knee joint extension and impaired contraction of the quadriceps femoris muscle following ACL reconstruction is known as arthrogenic muscle inhibition (AMI).
AMI is associated with gait abnormality, long-term quadriceps atrophy, poor function, dynamic instability, persistent knee pain and early osteoarthritis.
What are the new findings?
Cryotherapy and physical exercises should form the mainstays of management of AMI.
Exercise should include traditional quadriceps and hamstring muscles open chain exercises with resistance, progressive closed chain strengthening exercises, as well as hamstring fatiguing exercises.
There is low-level evidence to support neuromuscular electrical stimulation and transcutaneous electrical nerve stimulation.
There is very low evidence to support ultrasound therapy and vibration.
Taping, bracing, application of heat and soft tissue release strategies demonstrated minimal or no benefit in the management of AMI.
Acknowledgments
None
Footnotes
Contributors: All authors have given final approval of the submitted manuscript and their agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have made substantial contributions to the design of the work and manuscript writing. Conceptualisation of the work was by MT, BS-C and AS. The acquisition, analysis and interpretation of data were performed by WB, AS and AB. All authors were involved in drafting the work or revising it critically for important intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: BS-C, AS and MT are all paid consultants for Arthrex. BS-C also receives royalties and research support from Arthrex.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Amin S, Baker K, Niu J, et al. . Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum 2009;60:189–98. 10.1002/art.24182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thomas AC, Wojtys EM, Brandon C, et al. . Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J Sci Med Sport 2016;19:7–11. 10.1016/j.jsams.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinto FG, Thaunat M, Daggett M, et al. . Hamstring contracture after acl reconstruction is associated with an increased risk of cyclops syndrome. Orthop J Sports Med 2017;5 10.1177/2325967116684121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewek M, Rudolph K, Axe M, et al. . The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech 2002;17:56–63. 10.1016/S0268-0033(01)00097-3 [DOI] [PubMed] [Google Scholar]
- 5. Konishi Y, Oda T, Tsukazaki S, et al. . Relationship between quadriceps femoris muscle volume and muscle torque at least 18 months after anterior cruciate ligament reconstruction. Scand J Med Sci Sports 2012;22:791–6. 10.1111/j.1600-0838.2011.01332.x [DOI] [PubMed] [Google Scholar]
- 6. Lindström M, Strandberg S, Wredmark T, et al. . Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports 2013;23:431–42. 10.1111/j.1600-0838.2011.01417.x [DOI] [PubMed] [Google Scholar]
- 7. Felson DT, Niu J, McClennan C, et al. . Knee buckling: prevalence, risk factors, and associated limitations in function. Ann Intern Med 2007;147:534–40. 10.7326/0003-4819-147-8-200710160-00005 [DOI] [PubMed] [Google Scholar]
- 8. Segal NA, Glass NA, Torner J, et al. . Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage 2010;18:769–75. 10.1016/j.joca.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Urbach D, Nebelung W, Weiler HT, et al. . Bilateral deficit of voluntary quadriceps muscle activation after unilateral ACL tear. Med Sci Sports Exerc 1999;31:1691–6. 10.1097/00005768-199912000-00001 [DOI] [PubMed] [Google Scholar]
- 10. Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum 2010;40:250–66. 10.1016/j.semarthrit.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 11. Baumeister J, Reinecke K, Schubert M, et al. . Altered electrocortical brain activity after ACL reconstruction during force control. J Orthop Res 2011;29:1383–9. 10.1002/jor.21380 [DOI] [PubMed] [Google Scholar]
- 12. Baumeister J, Reinecke K, Weiss M. Changed cortical activity after anterior cruciate ligament reconstruction in a joint position paradigm: an EEG study. Scand J Med Sci Sports 2008;18:473–84. 10.1111/j.1600-0838.2007.00702.x [DOI] [PubMed] [Google Scholar]
- 13. Hopkins J, Ingersoll CD, Edwards J, et al. . Cryotherapy and transcutaneous electric neuromuscular stimulation decrease arthrogenic muscle inhibition of the vastus medialis after knee joint effusion. J Athl Train 2002;37:25–31. [PMC free article] [PubMed] [Google Scholar]
- 14. Rice D, McNair PJ, Dalbeth N. Effects of cryotherapy on arthrogenic muscle inhibition using an experimental model of knee swelling. Arthritis Rheum 2009;61:78–83. 10.1002/art.24168 [DOI] [PubMed] [Google Scholar]
- 15. Hart JM, Kuenze CM, Diduch DR, et al. . Quadriceps muscle function after rehabilitation with cryotherapy in patients with anterior cruciate ligament reconstruction. J Athl Train 2014;49:733–9. 10.4085/1062-6050-49.3.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuenze C, Eltoukhy M, Kelly A, et al. . Impact of quadriceps strengthening on response to fatiguing exercise following ACL reconstruction. J Sci Med Sport 2017;20:6–11. 10.1016/j.jsams.2016.04.015 [DOI] [PubMed] [Google Scholar]
- 17. Lepley LK, Wojtys EM, Palmieri-Smith RM. Combination of eccentric exercise and neuromuscular electrical stimulation to improve quadriceps function post-ACL reconstruction. Knee 2015;22:270–7. 10.1016/j.knee.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lepley LK, Wojtys EM, Palmieri-Smith RM. Combination of eccentric exercise and neuromuscular electrical stimulation to improve biomechanical limb symmetry after anterior cruciate ligament reconstruction. Clin Biomech 2015;30:738–47. 10.1016/j.clinbiomech.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Son SJ, Kim H, Seeley MK, et al. . Effects of transcutaneous electrical nerve stimulation on quadriceps function in individuals with experimental knee pain. Scand J Med Sci Sports 2016;26:1080–90. 10.1111/sms.12539 [DOI] [PubMed] [Google Scholar]
- 20. Konishi Y, McNair PJ, Rice DA. TENS Alleviates muscle weakness attributable to attenuation of ia afferents. Int J Sports Med 2017;38:253–7. 10.1055/s-0042-118183 [DOI] [PubMed] [Google Scholar]
- 21. Hart JM, Kuenze CM, Pietrosimone BG, et al. . Quadriceps function in anterior cruciate ligament-deficient knees exercising with transcutaneous electrical nerve stimulation and cryotherapy: a randomized controlled study. Clin Rehabil 2012;26:974–81. 10.1177/0269215512438272 [DOI] [PubMed] [Google Scholar]
- 22. Pamukoff DN, Pietrosimone B, Lewek MD, et al. . Whole-body and local muscle vibration immediately improve quadriceps function in individuals with anterior cruciate ligament reconstruction. Arch Phys Med Rehabil 2016;97:1121–9. 10.1016/j.apmr.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 23. Blackburn JT, Pamukoff DN, Sakr M, et al. . Whole body and local muscle vibration reduce artificially induced quadriceps arthrogenic inhibition. Arch Phys Med Rehabil 2014;95:2021–8. 10.1016/j.apmr.2014.07.393 [DOI] [PubMed] [Google Scholar]
- 24. Norte GE, Saliba SA, Hart JM. Immediate effects of therapeutic ultrasound on quadriceps spinal reflex excitability in patients with knee injury. Arch Phys Med Rehabil 2015;96:1591–8. 10.1016/j.apmr.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 25. Delaloye JR, Murar J, Sánchez MG, et al. . How to rapidly abolish knee extension deficit after injury or surgery: A practice-changing video pearl from the scientific anterior cruciate ligament network international (SANTI) study group. Arthrosc Tech 2018;7:e601–e605. 10.1016/j.eats.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lowe T, Dong XN. The use of hamstring fatigue to reduce quadriceps inhibition after anterior cruciate ligament reconstruction. Percept Mot Skills 2018;125 10.1177/0031512517735744 [DOI] [PubMed] [Google Scholar]
- 27. Glaviano NR, Langston WT, Hart JM, et al. . Influence of patterned electrical neuromuscular stimulation on quadriceps activation in individuals with knee joint injury. Int J Sports Phys Ther 2014;9:915–23. [PMC free article] [PubMed] [Google Scholar]
- 28. Gibbons CE, Pietrosimone BG, Hart JM, et al. . Transcranial magnetic stimulation and volitional quadriceps activation. J Athl Train 2010;45:570–9. 10.4085/1062-6050-45.6.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim KM, Davis B, Hertel J, et al. . Effects of Kinesio taping in patients with quadriceps inhibition: A randomized, single-blinded study. Phys Ther Sport 2017;24:67–73. 10.1016/j.ptsp.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 30. Oliveira AK, Borges DT, Lins CA, et al. . Immediate effects of kinesio taping on neuromuscular performance of quadriceps and balance in individuals submitted to anterior cruciate ligament reconstruction: A randomized clinical trial. J Sci Med Sport 2016;19:2–6. 10.1016/j.jsams.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 31. Davis AG, Pietrosimone BG, Ingersoll CD, et al. . Quadriceps function after exercise in patients with anterior cruciate ligament-reconstructed knees wearing knee braces. J Athl Train 2011;46:615–20. 10.4085/1062-6050-46.6.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drover JM, Forand DR, Herzog W. Influence of active release technique on quadriceps inhibition and strength: a pilot study. J Manipulative Physiol Ther 2004;27:408–13. 10.1016/j.jmpt.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 33. Ageberg E, Björkman A, Rosén B, et al. . Principles of brain plasticity in improving sensorimotor function of the knee and leg in patients with anterior cruciate ligament injury: a double-blind randomized exploratory trial. BMC Musculoskelet Disord 2012;13:68 10.1186/1471-2474-13-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Warner B, Kim KM, Hart JM, et al. . Lack of effect of superficial heat to the knee on quadriceps function in individuals with quadriceps inhibition. J Sport Rehabil 2013;22:93–9. 10.1123/jsr.22.2.93 [DOI] [PubMed] [Google Scholar]
- 35. Harkey MS, Gribble PA, Pietrosimone BG. Disinhibitory interventions and voluntary quadriceps activation: a systematic review. J Athl Train 2014;49:411–21. 10.4085/1062-6050-49.1.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arksey H, O’Malley L, O’’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 37. Colquhoun HL, Levac D, O’Brien KK, et al. . Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol 2014;67:1291–4. 10.1016/j.jclinepi.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 38. Morrison A, Moulton K, Clark M, et al. . English-language restriction when conducting systematic review-based metaanalyses: Systematic review of published studies. Ottawa: Canadian Agency for Drugs and Technologies in Health, 2009. [Google Scholar]
- 39. Moher D, Pham B, Lawson ML, et al. . The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess 2003;7:1–90. 10.3310/hta7410 [DOI] [PubMed] [Google Scholar]
- 40. de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 2009;55:129–33. 10.1016/S0004-9514(09)70043-1 [DOI] [PubMed] [Google Scholar]
- 41. Maher CG, Sherrington C, Herbert RD, et al. . Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003;83:713–21. [PubMed] [Google Scholar]
- 42. Oxford Centre for Evidence-based Medicine - Levels of Evidence. 2009. CEBM 2009.
- 43. Guyatt GH, Oxman AD, Vist GE, et al. . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thalheimer W, Cook S. How to calculate effect sizes from published research articles: a simplified methodology. http://www.bwgriffin.com/gsu/courses/edur9131/content/Effect_Sizes_pdf5.pdf (accessed 28 Aug 2018).
- 45. Telianidis S, Perraton L, Clark RA, et al. . Diminished sub-maximal quadriceps force control in anterior cruciate ligament reconstructed patients is related to quadriceps and hamstring muscle dyskinesia. J Electromyogr Kinesiol 2014;24:513–9. 10.1016/j.jelekin.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 46. Alkjær T, Simonsen EB, Magnusson SP, et al. . Antagonist muscle moment is increased in ACL deficient subjects during maximal dynamic knee extension. Knee 2012;19:633–9. 10.1016/j.knee.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 47. Kellis E, Mademli L, Patikas D, et al. . Neuromuscular interactions around the knee in children, adults and elderly. World J Orthop 2014;5:469–85. 10.5312/wjo.v5.i4.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perraton L, Clark R, Crossley K, et al. . Impaired voluntary quadriceps force control following anterior cruciate ligament reconstruction: relationship with knee function. Knee Surg Sports Traumatol Arthrosc 2017. 25 10.1007/s00167-015-3937-5 [DOI] [PubMed] [Google Scholar]
- 49. Bremner CB, Holcomb WR, Brown CD, et al. . The Effectiveness of Neuromuscular Electrical Stimulation in Improving Voluntary Activation of the Quadriceps: A Critically Appraised Topic. J Sport Rehabil 2017. 26 10.1123/jsr.2015-0100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2017-098401supp001.docx (57.8KB, docx)
bjsports-2017-098401supp002.docx (14.3KB, docx)