Abstract
There has been an increasing appreciation of the role of vascular contributions to cognitive impairment and dementia (VCID) associated with old age. Strong preclinical and translational evidence links age-related dysfunction and structural alterations of the cerebral arteries, arterioles, and capillaries to the pathogenesis of many types of dementia in the elderly, including Alzheimer’s disease. The low-pressure, low-velocity, and large-volume venous circulation of the brain also plays critical roles in the maintenance of homeostasis in the central nervous system. Despite its physiological importance, the role of age-related alterations of the brain venous circulation in the pathogenesis of vascular cognitive impairment and dementia is much less understood. This overview discusses the role of cerebral veins in the pathogenesis of VCID. Pathophysiological consequences of age-related dysregulation of the cerebral venous circulation are explored, including blood-brain barrier disruption, neuroinflammation, exacerbation of neurodegeneration, development of cerebral microhemorrhages of venous origin, altered production of cerebrospinal fluid, impaired function of the glymphatics system, dysregulation of cerebral blood flow, and ischemic neuronal dysfunction and damage. Understanding the age-related functional and phenotypic alterations of the cerebral venous circulation is critical for developing new preventive, diagnostic, and therapeutic approaches to preserve brain health in older individuals.
Keywords: cerebral circulation, senescence, vascular cognitive impairment, vascular contributions to cognitive impairment and dementia, vein
INTRODUCTION
Recent advances in cerebrovascular pathophysiology and vascular aging research highlight the significance of vascular contributions to cognitive impairment and dementia (VCID) associated with old age (79, 176, 189). VCID encompasses all types of vascular pathology-related cognitive decline, the most common being cerebral small vessel disease. Vascular cognitive impairment and dementia are now recognized as the second most common cause of cognitive decline in older individuals often overlapping with Alzheimer’s disease. There is increasing recognition that vascular mechanisms contributing to cognitive impairment are potentially reversible and that treatments and interventions that preserve cerebrovascular health may help to prevent cognitive decline, even of the Alzheimer’s type. The prevalence of vascular cognitive impairment and dementia is strongly age related (63). Accordingly, there is ever-growing evidence that age-related structural and functional alterations of large arteries, arterioles, and capillaries lead to dysregulation of cerebral blood flow (CBF) and ischemia, blood-brain barrier disruption, impaired clearance of metabolic byproducts, increased neuroinflammation, and impaired paracrine regulation of the function of neighboring cells (e.g., neuronal stem cells), all of which act synergistically to impair brain function. There is also strong evidence demonstrating the contribution of age-related alterations in arteriolar microvessels and capillaries to Alzheimer's disease (164, 176).
The low-pressure, low-velocity, and large-volume venous circulation of the brain plays critical roles in the maintenance of homeostasis in the central nervous system. Despite its physiological importance, the role of age-related alterations of the brain venous circulation in the pathogenesis of vascular cognitive impairment and dementia and Alzheimer's disease is much less understood. In this review, the effect of aging on the functional and structural integrity of the brain venous circulation is considered in terms of potential mechanisms involved in the pathogenesis of neurodegeneration and cognitive decline.
ANATOMY AND PHYSIOLOGY OF CEREBRAL VENOUS CIRCULATION AND CEREBROSPINAL FLUID DYNAMICS
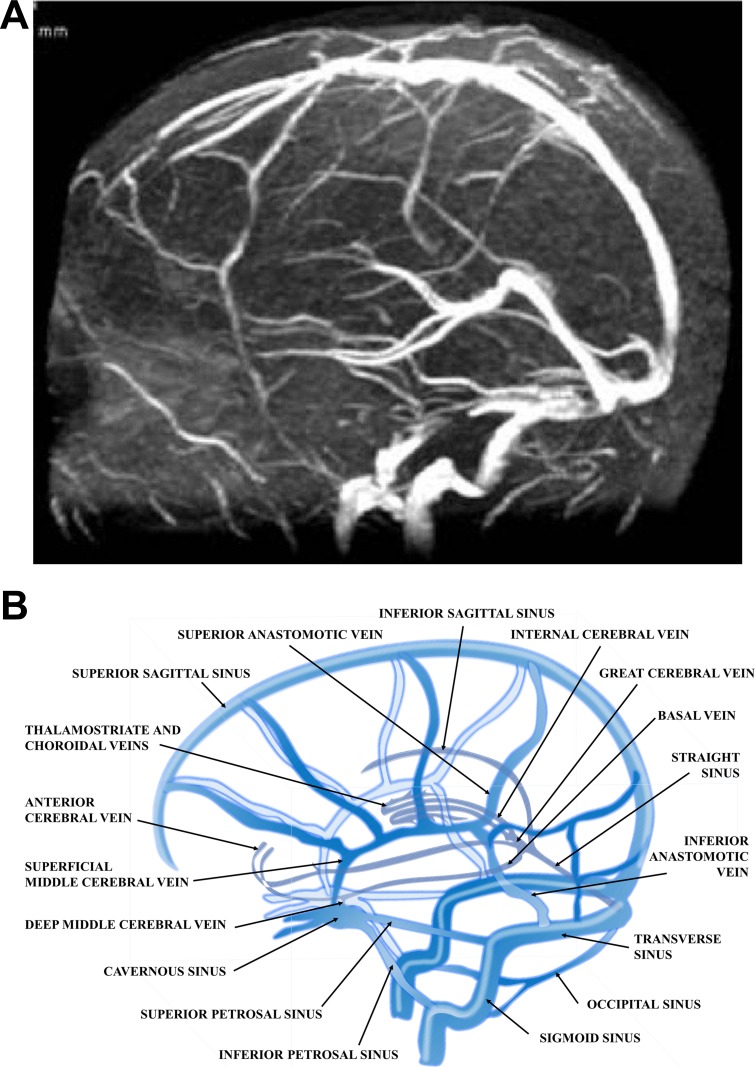
The venous circulation of the brain consists of two main systems, the superficial (cortical) and the deep venous system (Fig. 1). The superficial venous system drains the cortex and superficial white matter mainly collected by dural sinuses, the superior sagittal sinus, and the cavernous sinus. In addition to direct contacts to sinuses, venous blood also reaches the sinuses through bridging veins between the superficial venous network and the superior sagittal sinus running in the subdural space. The deep venous system, which consists of the internal cerebral veins, veins of Rosenthal and Galen, and their collaterals, drains the deep white matter, the lateral ventricle, third ventricle, and basal cistern via the straight sinus. Ultimately the venous blood of the superficial and deep system drains through the sigmoid sinus to the veins of the neck, most importantly to the jugular veins (105, 236). The intracerebral veins possess no valves, and their walls are extremely thin (and vulnerable) due to the absence of a well-developed smooth muscle layer (65, 105).
Fig. 1.
Venous drainage of the central nervous system. A: venous-phase cerebral angiogram showing most of the cerebral venous structures. Image is from Coutinho et al. (49). B: normal anatomy of the cerebral venous system.
The internal jugular veins are considered to be the main pathways of cerebral venous drainage. However, angiographic and anatomical evidence demonstrate that a wide anatomical variability exists that results in varying contributions of jugular and nonjugular venous drainage (64). Furthermore, there is significant postural dependency of the cerebral venous outflow (203). In the supine position, there is a predominance of the jugular veins in cerebrovenous drainage. In contrast, in the erect position, the vertebral venous system represents a major outflow pathway (203). For example, the inner jugular vein diameter increases and the distensibility decreases while reclining attributable to the intraluminal pressure elevation, forming most of the cerebral venous drainage in the majority of normal subjects (~70%), called jugular drainers. In the remaining 30% of the normal population, draining occurs via the vertebral veins, deep neck veins, or via the intraspinal venous system. Reduced diameter and high distensibility of the inner jugular vein could be observed in the erect body position when the intraluminal venous pressure is low because blood flow to the atrium is facilitated by gravity, and most of the cerebral venous drainage is ensured by the vertebral venous system (27, 64).
To understand brain pathologies of venous origin, it is crucial to understand to cross talk between CBF and cerebrospinal fluid (CSF) and thus the interaction between the venous system and the CSF. According to the classical theory, CSF is produced by the choroid plexus and flows through the ventricles, cisterns, and subarachnoid space to ultimately be absorbed into the venous blood by the arachnoid villi (19, 33). The pressure gradient needed for this absorption between the two spaces is 5–7 mmHg. Thus any increase in the venous sinus pressure may significantly affect CSF absorption (21). In addition to the unidirectional flow of CSF from the site of production to the site of absorption, it also exhibits pulsatility (19, 212), as the fluid compartment of CSF serves as a Windkessel, dampening the arterial pulse wave entering the skull. Because the brain requires nonpulsatile and continuous flow, the Windkessel effect is a critical function of both the CSF and cerebral venous fluid compartments. The Monro-Kellie doctrine describes the principle of homeostatic intracerebral volume regulation, which stipulates that the total volume of the parenchyma, CSF, and blood remains constant. Accordingly, because blood and CSF are not compressible, the arterial pulsation results in CSF shift through foramen magnum or a compression of the veins. Thus any change in the venous circulation, namely pressure overload, backward flow, or even a change in the compliance of the neck and brain veins, not only affects the drainage of the brain, but also may lead to alterations in CSF homeostasis (19).
STRUCTURAL AND FUNCTIONAL ALTERATIONS AFFECTING THE CEREBRAL VENOUS CIRCULATION IN AGING
Structural/Morphological Alterations and Altered Distensibility of Cerebral Veins in Aging
Aging is known to alter the structure of cerebral capillaries, promoting structural abnormalities of the basement membrane, increasing perivascular collagen deposits, and leading to basement membrane thickening (69). Age-related increased collagenosis also occurs in cerebral veins and venules (34, 130) attributable to increased expression and deposition of collagen subtypes I and III in the vascular wall. This age-related remodeling of the venous wall is thought to maintain venous tensile strength in response to pathological penetration of the increased arterial pulse wave (attributable to stiffening of large conduit arteries) through the capillary network into the venous system in aging (172). Venous collagenosis was reported to be increased in brains with manifest leukoaraiosis (34), suggesting that pathological remodeling of the venous wall may contribute to white matter lesions both in normal aging and in Alzheimer’s disease (104).
Arteriolar tortuosity is a frequent age-related vascular pathology in the white matter that often associates with leukoaraiosis (34). Tortuous vessels are often surrounded by enlarged perivascular spaces corresponding to “État criblé” (also known as status cribrosum) (34). Histopathological examination of postmortem brains of older individuals and advanced imaging modalities in vivo (e.g., ultra-high field time-of-flight MR angiography and susceptibility-weighted imaging) reveal that venules also often exhibit age-related increased tortuosity (100, 148) (Fig. 2A). Recent studies provide preliminary evidence that venular tortuosity may be an early neuroimaging marker of small vessel disease and may correlate with white matter hyperintensities (WMHs) and/or cerebral microhemorrhages (148). A recent study comparing deep medullary veins visualized on 7T-MRI revealed that patients with early Alzheimer's disease also exhibit increased venular tortuosity (32). It should be noted that the existing imaging studies reporting venular tortuosity in aging patients and/or in patients with Alzheimer's disease are cross sectional in nature; thus its remains to be determined whether venular tortuosity is a progressive condition.
Fig. 2.
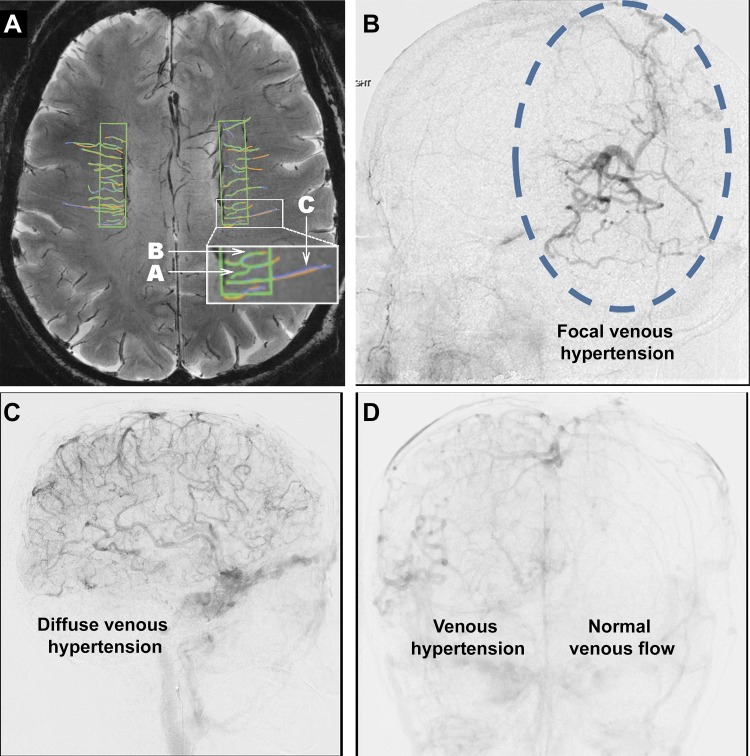
Cerebral venous tortuosity, a potential radiological sign of increased venous pressure. A: detection of tortuosity in small intracerebral venules on susceptibility-weighted image (SWI; the MRI was taken without the use of any contrast agents at 7T). B indicates a tortuous venule, whereas C marks a straight venule. A is an example of a venule that would not be included in the statistical evaluation of venous tortuosity because it was traced by only 1 of the 3 raters. [Republished with permission of American Society of Neuroradiology, from “In vivo imaging of venous side cerebral small-vessel disease in older adults: an MRI method at 7T,” Shaaban CE, Aizenstein HJ, Jorgensen DR, MacCloud RL, Meckes NA, Erickson KI, Glynn NW, Mettenburg J, Guralnik J, Newman AB, Ibrahim TS, Laurienti PJ, Vallejo AN, Rosano C; LIFE Study Group, AJNR Am J Neuroradiol 38: 1923–1928, 2017; permission conveyed through Copyright Clearance Center, Inc.] B–D: examples of focal (B) and diffuse (C) tortuosity in cortical veins on lateral views of the venous phase of cerebral angiograms in patients with venous congestion related to intracranial arteriovenous fistulas. D: frontal projection from cerebral angiogram showing venous tortuosity attributable to a dural arteriovenous fistula. Images were kindly provided by Dr. Ali Shaibani (Department of Neuroradiology, Northwestern University).
The mechanisms underlying increased venous tortuosity are multifaceted and, on the basis of analog mechanisms manifested in the peripheral circulation, likely include increased cerebral venular pressure (similar to the hemodynamic environment promoting varicose vein formation in the lower extremities) (112), altered elasticity of the vascular wall, degenerative changes of the smooth muscle and endothelial cells, and pathological remodeling of the extracellular matrix and basal membrane (100). Age-related mechanisms that promote adverse remodeling of the venular wall include impaired expression of angiogenic and growth factors (e.g., VEGF), cellular senescence, oxidative stress, and dysregulation of matrix metalloproteinases (100). Interestingly, pharmacological depletion of mural cells using a platelet-derived growth factor receptor-β antagonist was reported to increase venular tortuosity in animal models (111), mimicking the aging phenotype. It is likely that cerebral venous tortuosity correlates with the presence of retinal tortuous veins attributable to shared etiology (83). In that regard, it is interesting that increased tortuosity of retinal venules was shown to predict Alzheimer's disease (39). The critical role for increased venous pressure in the genesis of venous tortuosity is supported by the findings that patients with venous congestion related to an intracranial dural arteriovenous fistula also exhibit tortuous, engorged pial veins clearly visible on angiograms (222). Examples of focal and diffuse tortuosity in cerebral veins attributable to intracranial arteriovenous fistulas are shown in Fig. 2, B–D.
Age-related alterations of the bridging veins, which connect the superficial venous network to dural sinuses, play a central role in traumatic brain injury-related subdural bleedings in the elderly (84). Because of brain atrophy and subsequent expansion of the subdural space, increased mechanical tension is imposed on the bridging veins in the elderly (84, 229). This increased mechanical burden combined with the age-related decline in elasticity of venous wall predispose the bridging veins to rupture in response to even minor brain trauma, resulting in increased incidence of bleedings into the subdural space in older adults (84, 229).
The venous wall is significantly more distensible compared with the arterial wall, which has important physiological relevance. The distensibility of the internal jugular vein is a major determinant of cerebral venous drainage and keeps cerebral venous pressure within normal values. There is strong evidence that aging reduces the distensibility of the upper limb venous system by 38% when determined by plethysmography (75). Aging also decreases jugular vein distensibility by 68% (measured by ultrasonography) in the supine position (although this effect may have postural dependency) (27).
It is likely that, because of an age-related reduction in distensibility, the inner jugular vein loses its compensatory ability to increase transmural pressure and thereby predisposes the cerebral venous system to venous hypertension. It is also likely that the effects of aging on the venous wall biomechanics are multifaceted and include age-related pathological remodeling of the venous wall, including changes in the extracellular matrix and the medial layer. Age-related changes of the biomechanical properties of jugular venous walls are also defined by aging-induced changes in venous tone, intra- and extraluminal pressure, and the relationship to surrounding tissues. The age-related changes in the multilevel control of venous biomechanics likely include alterations in intrinsic local myogenic and humoral mechanisms, as well as extrinsic systemic hormonal and nervous influences (129).
Hemodynamic factors (changes in pressure and flow) together with inflammatory processes contribute to the age-related changes in the biomechanical properties of the jugular veins, which further promote age-related progression of venous dysfunction (127). Several age-associated pathological conditions, including chronic heart failure, pulmonary hypertension, and chronic obstructive pulmonary disease, can elevate central venous pressure in the elderly and thereby alter biomechanical properties of the veins. In addition, sex, obesity, and sedentary lifestyle may importantly modulate the effects of aging on biomechanical properties of the cerebral venous system (8, 9, 121), similar to peripheral veins (71, 119). Studies on 70 adult Caucasian twins from the Italian twin registry demonstrate that hereditary factors are responsible for 30–70% of the biomechanical properties of internal jugular veins (170). Longitudinal studies should elucidate how genetic factors determine successful venous aging and predispose to exacerbated pathological remodeling of the cerebral venous system in aging.
Numerous malformations can also affect the venous circulation of the neck in older subjects, leading to impaired venous drainage in the brain (236). Causes of narrowing or occlusion can be intraluminal, such as septa, flaps, or abnormal valves, or extraluminal, such as any anatomical or pathological mass compressing the vessel.
Abnormal remodeling and increased stiffness of the venous wall and/or increases in venous pressure may impair the Windkessel effect of the venous circulation (19, 212). As the venous circulation plays a bigger role in the Windkessel effect and dampening of arterial pulsatility with aging, any age-related alteration that affects the venous system will exert a significant impact on penetration of the arterial pressure wave into the brain (19). Increased pulse pressure can reach the venous side through the arterial tree because of the lack of proper myogenic autoregulatory protection in the proximal cerebral resistance arteries (174, 176, 178). In addition, increased arterial pulsation can be transmitted to venous pulsation indirectly through the CSF. In the presence of age-related alterations of CSF circulation, when compliance of the CSF compartment is decreased, less dampened pulse waves can reach the venules and veins. The age-related increased pulse pressure attributable to arterial stiffening and the lack of arterial myogenic protection together with the decreased Windkessel function of the CSF compartment impose mechanical stress on the venous wall in aged individuals. There is also a cross talk between the different types of venous abnormalities, as structural and morphological changes may lead to hemodynamic consequences. For example, pressure elevation in the sagittal sinus will also increase pressure in the cortical veins, making them more stiff and resistant against compression, compromising the Windkessel effect (19).
Previous preclinical studies have characterized age-related degenerative changes and pathological remodeling in venous valves (87), which likely contribute to venous valve insufficiency associated with old age. Clinical studies confirm that aging is associated with pathological remodeling of venous valves in the peripheral venous circulation, which likely impairs valve function (146, 205). On the basis of our understanding of the pathogenesis of chronic venous insufficiency in the peripheral circulation, it is likely that age-related changes in cerebral venous valves contribute to valvular incompetence (204), leading to venous reflux and cerebral venous hypertension.
Jugular Venous Reflux and Increased Cerebral Venous Pressure
Increased cerebral venous pressure is likely to contribute to pathological processes that play a significant role in development of brain pathologies in older individuals, including microhemorrhages, blood-brain barrier disruption, and perivascular inflammation (125, 191). When venous hypertension occurs in the superior sagittal sinus, CSF absorption is also impaired, leading to altered CSF outflow. Factors that contribute to increased cerebral venous pressure include penetration of arterial hypertension to the venous circulation, venous drainage impairment, presence of an arteriovenous fistula, and retrograde transmission of increased central venous pressure to the cerebral venous circulation.
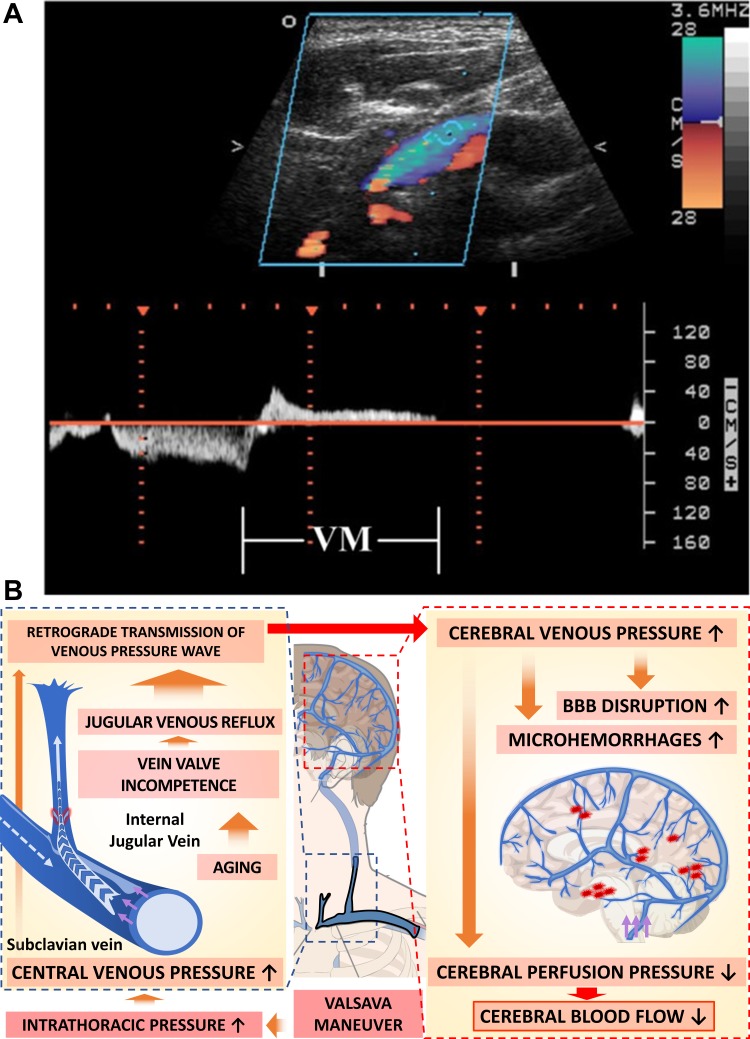
Jugular venous reflux is a potentially important clinically hemodynamic abnormality (Fig. 3). It can be caused, for example, by physiological compression of the brachiocephalic vein that leads to stagnation or reversal of the internal jugular vein flow, promoting increased cerebral venous pressure. The pressure gradient determines the flow in the veins, and a missing or damaged internal jugular vein valve can easily lead to retrograde flow (45).
Fig. 3.
Pathophysiology of jugular venous reflux. A: retrograde flow detected by color duplex and in the Doppler spectrum during Valsalva maneuver (VM) reveals jugular venous reflux in an older individual (mean age of study participants: 74 ± 12 yr). Image is from Chung et al. (43). B: scheme depicting the role of jugular venous reflux in transmission of increased central venous pressure to the cerebral veins and its consequences in older adults. Jugular venous reflux occurs when increased intrathoracic pressure elevates central venous pressure, and the resulting venous pressure wave is beyond the competence of internal jugular venous valves. VM-induced jugular venous reflux may retrogradely transmit venous hypertension into cerebral venous system, decreasing cerebral perfusion pressure and consequently reducing cerebral blood flow and causing damage to the thin-walled venules, including cerebral microhemorrhages of venous origin and blood-brain barrier (BBB) disruption.
The internal jugular vein valve, which is the only venous valve situated in the venous circulation between the heart and the brain, is critical for the prevention of retrograde flow of venous blood. Despite their clinical significance, the presence and function of the valves in the internal jugular veins are often overlooked (62, 115). Anatomical evidence obtained in human cadavers suggests that internal jugular vein valve is frequently incompetent. With an incompetent internal jugular vein valve, any increase in intrathoracic pressure (e.g., during Valsalva maneuver) could result in jugular venous reflux (236). The incidence of jugular venous reflux has been reported to increase with age (92, 96, 103, 106, 109, 184), likely attributable to age-related degenerative changes in the venous valves. Interestingly, there are studies extant reporting that incidence of jugular valve incompetence can reach ~30 to 90% in the general population (2, 204). Jugular valve insufficiency and the resulting retrograde jugular venous flow and back transmission of central venous pressure likely contribute to various brain pathologies. Jugular venous reflux was shown to associate with intracranial structural changes in patients with mild cognitive impairment and Alzheimer's disease (22, 24). It has been suggested that jugular venous reflux retrogradely transmits increased venous pressure into the brain, promoting edema as well as a wide range of microvascular pathologies associated with increased venular pressure. There are case reports extant showing that age-related jugular valve incompetence in association with the physical exertion during sexual intercourse possibly can lead to intracerebral hemorrhage of venous origin (4).
There are case reports extant suggesting that, during central venous catheter placement, venous valves located in the right internal jugular vein may be damaged (72, 225). Because the internal jugular valve is often located in the retroclavicular space, the ultrasound assessment of this valve can be difficult. Valve cusps are thin structures, and forceful attempts to force a catheter through them may result in valve damage.
Age-Related Phenotypic and Genotypic Changes in Endothelial Cells
Despite their common developmental origins, endothelial cells in the arterial and venous circulatory system are not identical (61). Functionally, one of the key roles of arteriolar endothelial cells is the regulation of vascular tone and thereby blood flow and the production of a number of trophic factors, paracrine mediators, and gaseotransmitters. On the other hand, postcapillary venular endothelial cells are the primary site of leukocyte trafficking and stem cell extravasation. Interestingly, recent studies report that cerebral arteries and veins differentially exhibit an endothelial glycocalyx (e.g., in mice, cerebral arteries and capillaries have an intact endothelial glycocalyx, but veins and venules do not) (231), which may have relevance for regulation of inflammatory processes. Among the endothelial glycocalyx constituents, syndecan-1 is a main component, and it is also predominantly expressed in arterial endothelial cells compared with venous endothelial cells in the brain (86). Despite the pathophysiological importance of the venous endothelium, the role of age-related functional changes in the venous endothelial cells has not been explored.
There is ample evidence that aging is associated with critical phenotypic alterations in the endothelial cells in the arterial circulation attributable to age-related changes in their gene expression profile (16, 52, 56, 58, 166, 183, 187a, 188, 192, 193). Studies on endothelial cells from diverse vascular beds suggest that aging promotes proinflammatory, pro-oxidative, and prosenescence changes in endothelial gene expression in the arterial circulation, altering the cytokine and chemokine secretory profile of endothelial cells (51, 57, 58, 73), dysregulating mitochondrial biogenesis (187a), altering transport, barrier, and vasomotor function and free radical production (56, 166, 178, 182), impairing angiogenic capacity (16, 51, 192, 193), and facilitating endothelial-leukocyte interactions (51, 53, 185, 188). It can be hypothesized that, if aging is associated with gene expression changes in endothelial cells in the venous circulation, which are similar to those in endothelial cells in the arterial circulation, then these changes may significantly contribute to pathological processes promoting neuroinflammation and impaired tissue homeostasis.
Venous endothelial cells express unique molecular markers (86), including Endomucin (Emcn) (232), Ephrin type-B receptor 4 (EphB4), Lefty-1, Lefty-2, Neuropilin-2, and Flt4 (61). Preliminary analysis did not reveal significant age-related changes in the gene expression profile of these markers in the mouse brain. Further studies are warranted to isolate endothelial cells from the venous circulation based on these surface markers and analyze age-related changes in functionally relevant genes (e.g., inflammation-related gene expression, including adhesion molecules and chemokines). A recent breakthrough study published a single-cell RNA sequencing data set that defines arterial, capillary, and venous endothelial cells in mouse brain (86). This study identified >180 genes that are enriched (over 2-fold) in venous endothelial cells vs. capillary endothelial cells (including Gm5127, Wnt5a, Ptgs2, Cfb, and Cfh). Interestingly, among them Vcam1 and Icam1 show an ~54-fold and ~4-fold enrichment, respectively, in venous endothelial cells vs. capillary endothelial cells, which corresponds to the primary role of the postcapillary venules in leukocyte transmigration. Using single-cell RNA sequencing, future studies should compare age-related changes in these subsets of endothelial cells.
POTENTIAL ROLE OF PATHOLOGICAL ALTERATIONS OF THE CEREBRAL VENOUS CIRCULATION IN NEURODEGENERATIVE DISEASES AND COGNITIVE DECLINE
The links among alterations in the cerebral venous circulation, neurogenerative diseases, and cognitive impairment are not well understood. Here we provide a review of existing data supporting a potentially important role for venous dysfunction in different brain pathological conditions. Some speculations are also offered as to the mechanisms by which alterations of the cerebral venous circulation may contribute to the pathogenesis of age-related cerebral diseases and cognitive decline.
Chronic Cerebrospinal Venous Insufficiency: Lessons from Studies on Multiple Sclerosis
Originally Zamboni et al. (234) proposed the hypothesis that cerebrospinal venous insufficiency, caused by intraluminal stenotic malformations in the internal jugular and azygos veins with insufficient opening of collaterals leads to impaired venous drainage of the brain and thereby contributes to the pathogenesis of multiple sclerosis (MS). This hypothesis was built on the findings of early studies that MS lesions have venous involvement (59, 139, 140). According to Zamboni's hypothesis, cerebrospinal venous insufficiency is similar to chronic venous disorders of the lower extremity in that the altered hemodynamic environment in the venous circulation promotes chronic inflammation, iron deposition, and tissue injury (233). In support of this hypothesis, Zamboni and coworkers reported that, in patients with MS, there are alterations in venous drainage of the brain (234). However, these results proved to be controversial because of the lack of scientific rigor (e.g., proper controls) (135) and could not be reproduced by other investigators (47, 142, 180), mainly attributable to the high degree of variability and nonspecificity of the diagnostic ultrasound criteria (reflux, internal jugular vein stenosis, absent flow detectable by Doppler ultrasonography in the internal jugular or vertebral veins, reversed postural flow in the internal jugular vein) that define cerebrospinal venous insufficiency. Other investigators proposed that pulse-wave encephalopathy may be an initiating step in the pathogenesis of MS (101). In support of this hypothesis, increases in the Virchow-Robin space, decreased intracranial compliance, and higher microvascular pulsatility have been reported in patients with MS (101). Given the critical role of the venous circulation in inflammatory processes (e.g., endothelium-leukocyte interactions), control of the blood-brain barrier, microglia activation, and microvascular injury, further studies are warranted to better elucidate the role of hemodynamic factors in general and the venous pathologies in particular in the pathogenesis of various forms of neurodegenerative diseases.
Leukoaraiosis/WMHs
Leukoaraiosis is a radiological finding showing damage in the white matter regions of the brain near the lateral ventricles on CT scans (showing up as hypodense periventricular white matter lesions) or on T2/FLAIR MRI sequences (WMHs; Fig. 4A). Its clinical significance stems from the association of leukoaraiosis with vascular dementia, gait disturbances, and stroke (133). WMHs have been detected in patients with Alzheimer's disease (94, 102, 124) or at risk for developing Alzheimer's disease (46). The present view is that WMHs are early and independent predictors of Alzheimer's disease (131). WMH burden is even more significant in patients with vascular cognitive impairment (6, 30). There are numerous studies attempting to define the neuropathological correlates of WMHs (reviewed recently in Ref. 100), which include a variety of small vessel pathologies (accentuation of perivascular Virchow-Robin spaces, sclerotic vessels, microvascular amyloidosis, arteriolosclerosis, hyalinosis, and collagenosis), gliosis, periventricular necrosis, axonal degeneration, and reactive astrocytosis. Global WMHs are hypoperfused compared with normal white matter, suggesting that ischemia may play a role in their pathogenesis (124).
Fig. 4.
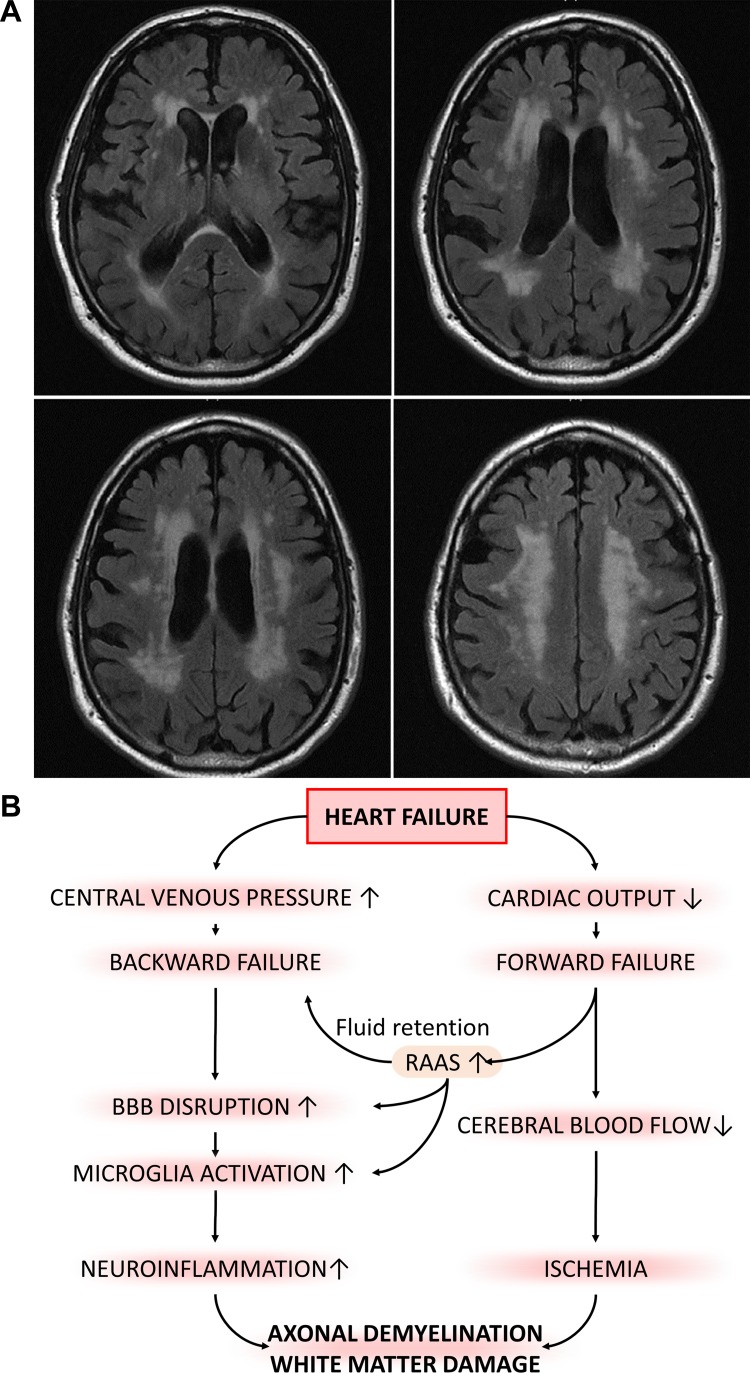
Potential contribution of elevated venous pressure to increased incidence of white matter hyperintensities (WMHs) in elderly patients with heart failure. In older adults, heart failure was shown to associate with WMHs. A: to illustrate this point, this MRI scan shows extensive periventricular WMHs on FLAIR sequences in a 64-yr-old man with heart failure associated with mild cognitive impairment. B: scheme depicting putative synergistic effects of heart failure-induced chronic hypoperfusion of the brain and increased venous pressure, which may exacerbate white matter injury, promoting cognitive impairment in elderly patients with heart failure. BBB, blood-brain barrier; RAAS, renin-angiotensin-aldosterone system.
Important for the present overview is the observation that leukoaraiosis is associated with a number of venous abnormalities, including periventricular venous collagenosis (130) and venular tortuosity. Jugular venous reflux and increased cerebral venous pressure were suggested to contribute to the pathogenesis of leukoaraisosis (44). Chronic cerebral venous hypertension likely decreases CBF, promoting local ischemia in the white matter (44), disrupts the blood-brain barrier, promoting perivascular inflammation, and exacerbates pathological remodeling of the cerebral venules. Progressive injury of the ischemic periventricular white matter is likely exacerbated by damage to the blood-brain barrier, accumulation of toxic metabolites and proinflammatory plasma constituents, the heightened inflammatory status of microglia and astrocytes, and/or by the presence of amyloid deposits (102). Higher venous pressure, remodeling of the veins, and/or jugular venous reflux may lead to impaired Windkessel effect and increased pulsatility in the capillary bed. Higher pulsatility per se may contribute to the pathogenesis of leukoaraiosis (18).
Normal Pressure Hydrocephalus
Normal pressure hydrocephalus (NPH) results from an abnormal accumulation of CSF in the ventricles of the brain, leading to ventriculomegaly (67). The enlarged ventricles exert increased pressure on the adjacent cortical tissue, impairing brain function and resulting in gait disturbance, dementia, and urinary incontinence (67). Patients with NPH are known to have an accumulation of CSF and dilation of the ventricles without an intracranial pressure elevation, which is canonically explained by lower absorption of CSF by the arachnoid villi to the venous circulation (152). An alternative hypothesis focuses on the decreased venous compliance in patients with NPH (17). In patients with NPH, intracranial venous flow and pressure are abnormal (110), and it is believed that an elevation of venous pressure contributes significantly to the neuronal damage and dysfunction associated with NPH (17). Venous hypertension may, not only promote pulse-wave encephalopathy, but also impair reabsorption of CSF through the arachnoid villi, leading to abnormal accumulation of CSF (152). Interestingly, there is a higher prevalence of cardiovascular diseases in patients with NPH, suggesting that the pathogenesis of NPH and cardiovascular disease may be linked (66), for example, by increasing jugular venous pressure.
Role of the Venous Circulation in the Pathogenesis of Cerebral Microhemorrhages
Cerebral microhemorrhages (CMHs, also known as “cerebral microbleeds”) (191), which are associated with rupture of small intracerebral vessels, are highly prevalent in patients 65 and older (191). CMHs have been defined as multiple small (<5 to 10 mm in diameter) round or oval hypointense lesions on T2*-weighted gradient-recall echo (T2*-GRE) MRI sequences, which correspond to focal, persisting hemosiderin depositions in microglia. There is strong evidence from population-based cross-sectional studies that CMHs contribute significantly to cognitive decline (37, 89, 138, 191, 206, 219, 220, 224, 227, 228).
Although many CMHs likely originate from small arterioles (the main risk factors being aging, hypertension, and amyloid deposition), there is increasing evidence that rupture of small veins and venules as well as capillaries can also result in CMHs (161, 191). In support of this concept, recent evidence shows that development of CMHs can be causally linked to the performance of Valsalva maneuvers (195). The Valsalva maneuver (defined as a forced expiratory blow against a closed glottis) is common in many everyday activities that involve moderate exertion, including weight lifting, blowing air into inflatable devices or musical instruments (e.g., playing the oboe), intense coughing, vomiting, nose blowing, and strain during defecation or sexual intercourse (195). Intrathoracic pressure in these conditions may increase well over >150–200 mmHg (149), which is transmitted to the venous circulation, resulting in a substantial elevation in central venous pressure (226).
If in older individuals the internal jugular vein valves are incompetent, then they would enable retrograde transmission of increased venous pressure to the cerebral venous system during the Valsalva maneuver (42, 70, 235, 236). It is likely that, when in elderly patients venous pressure exceeds the threshold for structural injury in thin-walled cerebral venules, multifocal venous CMHs ensue. In support of this concept, there is direct evidence that retinal hemorrhages of venous origin can be also generated by Valsalva maneuvers (3, 38). Preclinical studies on mouse models with surgical jugular vein occlusion (14) confirm that elevation of venous pressure in the cerebral circulation promotes the development of CMHs (G. A. Fulop and Z. Ungvari, unpublished observation). Furthermore, when retrograde penetration of a venous pressure wave into the cerebral venous circulation is mimicked experimentally by injecting a carmine-gelatin solution under high pressure into the vein of Galen in human cadavers, multifocal venous ruptures develop. These studies suggest that pressure-induced venous ruptures are predominantly localized to the region of the lateral ventricle, where there are connections between medullary and subependymal veins (147). It is likely that these vessels are more prone to rupture because of their branching pattern, anatomy, and the structural characteristics of their walls.
Another line of evidence supporting an important role of venous aging in the pathogenesis of intracerebral hemorrhages comes from studies on arteriovenous malformations (AVMs) (134). AVMs are congenital vascular lesions (incidence: ~1 per 100,000 persons). The risk for AVM rupture significantly increases with age (50, 107), suggesting that with advanced aging the fragility of the venous wall increases. We posit that age-related structural changes in the venular wall also promote venular fragility, contributing to the pathogenesis of CMHs of venous origin in older individuals.
Role of the Venous Circulation in the Pathogenesis of Cerebral Microinfarcts
Cerebral microinfarcts are small (0.05–3 mm in diameter) ischemic lesions that are prevalent in the aged human brain (10, 11, 35, 85). The association between the number of cerebral microinfarcts and cognitive decline has been established by population-based radiological and pathological studies and confirmed by histopathological examinations (77, 154, 155, 209, 210). Present thinking suggests that cerebral microinfarcts develop because of critical ischemia induced by the occlusion of arteriolar microvessels, which supply the respective small volume of brain tissue. Recent experimental studies demonstrated that the occlusion of small venules can also lead to the genesis of cerebral microinfarcts, which appear identical to those resulting from arteriole occlusion (85). On the basis of these observations, it has been proposed that cerebral venule pathology may also contribute to the pathogenesis of cerebral microinfarcts in older adults (85). Future clinical and experimental studies are warranted to establish the association between microinfarcts and venular pathology.
Glymphatic Circulation
The brain parenchyma does not contain lymphatic vessels. Instead, in the central nervous system, the “glymphatic” system (paravascular system) functions as a waste-clearance pathway (98). The glymphatic system consists of a para-arterial influx route for CSF to enter the brain parenchyma through the Virchow-Robin space and a paravenous efflux route. The inner wall of the perivascular space containing the flowing paravascular fluid is the outer wall of the vessels, and the outer wall of perivascular space is covered by astrocytic endfeet. According to the presently accepted hypothesis, circulation of the CSF in the paravascular system and the exchange of solutes between CSF and interstitial fluid are driven primarily by arterial pulsation (29). Clearance of soluble proteins and metabolic byproducts from the brain parenchyma is accomplished through convective bulk flow of interstitial fluid, facilitated by aquaporin 4 channels located on the astrocytic endfeet (26) (although there are important theoretical and experimental studies extant questioning the exact role of these mechanisms) (99, 153). There is experimental evidence that amyloid-β injected directly to the brain parenchyma is cleared through the glymphatic system along the large veins (91). It is believed that the glymphatic system drains into lymphatic vessels in the meninges (123). It can be speculated that altered cerebral venous circulation may affect this clearance mechanism indirectly in older individuals. Because the interstitial fluid-CSF mixture is partly collected through the arachnoid villi, the elevated venous pressure could adversely impact its reabsorption.
Cognitive Dysfunction in Heart Failure
Chronic heart failure is a significant health problem in the Western world that affects ≥10% of persons 70 yr of age or older (116). Cognitive impairment is an important complication of heart failure among elderly people (20, 90, 223), with an incidence ranging from 25% to 80% (116). Heart failure exerts multifaceted effects on the cerebral circulation (97). On the one hand, it attenuates CBF by decreasing cardiac output (forward failure) (41, 48, 82, 122, 145), lowering blood pressure, and impairing cerebrovascular reactivity, all of which have direct negative effects on brain function. Cerebrovascular autoregulation, which maintains CBF constant despite changes in blood pressure in healthy subjects, is abnormal in patients with heart failure, predisposing these patients to ischemic neuronal injury (36, 76). On the other hand, heart failure also causes systemic venous congestion (backward failure), which associates with increased jugular venous pressure and possibly reflux (218). There is evidence suggesting that patients with heart failure exhibit WMHs (5), supporting the hypothesis that increased cerebral venous pressure is causally linked to the pathogenesis of leukoaraiosis (Fig. 4, A and B). Backward failure also likely leads to higher capillary pulsatility and decreased waste clearance through the perivascular glymphatic system.
PERSPECTIVES
Although in the past two decades significant progress has been achieved in understanding age-related alterations in vascular function and phenotype in the arterial circulation, research efforts should persist in this direction to investigate similar alterations in the venous circulation. New investigations are needed to elucidate the mechanism by which age-related alterations in the venous circulation may lead to blood-brain barrier disruption, neuroinflammation, dysregulation of CBF, and local ischemia, promoting white matter injury and exacerbating VCI. Understanding the interaction of processes of aging and chronic diseases (e.g., hypertension, metabolic diseases) in the context of venous pathologies contributing to VCI should be a high priority. Studies on the venous circulation in both animal models of aging and accelerated vascular aging are warranted (162, 164). In addition, animal models to study the pathophysiological roles of increased cerebral venous pressure are needed. Potentially useful models include the mouse model proposed by Auletta et al. (14), which involves surgical occlusion of the jugular veins in mice. The authors have characterized the model, which can be adapted to study consequences of cerebral venous hypertension as they relate to blood-brain barrier disruption, dysregulation of CBF, WMHs, pathogenesis of cerebral microhemorrhages of venous origin, cognitive impairment, and gait disturbances. Furthermore, better alignment of preclinical studies on venous aging and human investigations is needed.
Critical areas of research, on the basis of recent achievements in the biology of aging, should focus on the role of known cellular and molecular mechanisms of aging in pathological alterations of the venous circulation. New studies investigating the role of sterile (221) and pathogen-induced vascular inflammation in the venous circulation are warranted. Importantly, in the United States, over 90% of adults 80 yr of age or older have persistent human cytomegalovirus (CMV) infection (1, 93, 114, 132, 157, 160). CMV replicates in the vascular endothelial cells, including venous endothelial cells, during the entire life of the host following initial infection. Severity of CMV infection (assessed on the basis of circulating antibody titers) was shown to predict increased incidence of frailty and risk of mortality in older adults (214). There is also evidence linking CMV infection to sinus vein thrombosis (150). Additional studies investigating the pathogenic role of CMV-induced alterations in venous endothelial cells as they relate to heightened inflammatory status, structural remodeling, microhemorrhages, and Alzheimer's pathology are needed. Additional important areas of research should focus on age-related changes in extracellular matrix (126, 187, 194, 200), oxidative stress (56, 60, 108, 168, 187), mechanisms involved in altered cellular stress resilience (108, 168, 184a, 185, 186, 202), pathways involved in cellular senescence (40, 136, 144, 177, 190, 213, 230), the pathogenic role of the renin-angiotensin system (55, 159, 181, 215–217), altered nutrient-sensing pathways (7, 113, 173, 201), epigenetic factors (80, 199), and neuroendocrine mechanisms of aging (12, 13, 25, 68, 137, 141, 171, 211), including IGF-1 deficiency (15, 74, 156, 163, 165, 167, 175, 179). Recent studies (118, 158) identified mammalian target of rapamycin as a critical factor contributing to cerebral vascular damage and dysfunction in Alzheimer’s disease models (117, 118, 207, 208) and in models of VCI (95). It has to be determined how the pharmacological targeting of this pathway may affect the cerebral venous circulation. Innovative strategies need to be developed to improve the health of the venous circulation, including novel pharmacological strategies (28, 53, 54, 118, 143, 158, 166) and modification of lifestyle and dietary factors (78, 81, 88, 128, 211).
To better understand the relationships between functional alterations of venous circulation, prospective clinical studies, similar to the Heart-Brain Study in the Netherlands (90), are needed. The Heart-Brain Study investigates the link between the hemodynamic status of the heart and the brain and cognitive impairment in patients with heart failure. Specifically, the Heart-Brain Study hypothesizes that the impaired hemodynamic status of the heart and the consequential brain hypoperfusion are important determinants of VCI. Because backward failure and alterations of the venous circulation likely affect the brain, if would be advantageous to also include in the study design end points that reflect jugular venous reflux and cerebral venous pressure/microvascular damage. Using additional sensitive assays to detect markers of neuroinflammation (151) and behavioral consequences (23, 31, 120, 169, 211) would benefit both clinical and preclinical studies as well.
GRANTS
We acknowledge support from the National Institute on Aging-funded Geroscience Training Program in Oklahoma (T32-AG-052363). This work was supported by grants from the American Heart Association (S. Tarantini), the Oklahoma Center for the Advancement of Science and Technology (to A. Csiszar, A. Yabluchanskiy, Z. Ungvari), the National Institute on Aging (R01-AG055395, R01-AG047879, R01-AG038747), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS100782, R01-NS056218), the Department of Veterans Affairs (Merit Number 1I01CX000340), a Pilot Grant from the Stephenson Cancer Center funded by the National Cancer Institute Cancer Center Support Grant P30CA225520 awarded to the University of Oklahoma Stephenson Cancer Center, the Oklahoma Shared Clinical and Translational Resources (OSCTR) program funded by the National Institute of General Medical Sciences (U54GM104938, to A. Yabluchanskiy), the Presbyterian Health Foundation (to Z. Ungvari, A. Csiszar, A. Yabluchanskiy, C. I. Prodan), the European Union-funded grants EFOP-3.6.1-16-2016-00008, 20765-3/2018/FEKUTSTRAT, EFOP-3.6.2.-16-2017-00008, GINOP-2.3.2-15-2016-00048, and GINOP-2.3.3-15-2016-00032, the National Research, Development and Innovation Office (NKFI-FK123798), the Hungarian Academy of Sciences (Bolyai Research Scholarship BO/00634/15), and the ÚNKP-18-4-PTE-6 New National Excellence Program of the Ministry of Human Capacities (to P. Toth).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.B., F.S., and Z.U. conceived and designed research; G.A.F., S.T., A.Y., C.I.P., P.B., E.F., P.T., F.S., A.C., and Z.U. prepared figures; P.B., performed experiments; P.B. and F.S. analyzed data; P.B. and F.S. interpreted results of experiments; G.A.F., S.T., A.Y., C.I.P., P.B., E.F., P.T., F.S., A.C., and Z.U. drafted manuscript; G.A.F., S.T., A.Y., A.M., C.I.P., T.K., T.C., A.L., P.B., E.F., P.T., F.S., A.C., and Z.U. edited and revised manuscript; G.A.F., S.T., A.Y., A.M., C.I.P., T.K., T.C., A.L., P.B., E.F., P.T., F.S., A.C., and Z.U. approved final version of manuscript.
REFERENCES
- 1.Aiello AE, Chiu YL, Frasca D. How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms? Geroscience 39: 261–271, 2017. doi: 10.1007/s11357-017-9983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkawi NM, Agosti C, Borroni B, Rozzini L, Magoni M, Vignolo LA, Padovani A. Jugular valve incompetence: a study using air contrast ultrasonography on a general population. J Ultrasound Med 21: 747–751, 2002. doi: 10.7863/jum.2002.21.7.747. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mujaini AS, Montana CC. Valsalva retinopathy in pregnancy: a case report. J Med Case Reports 2: 101, 2008. doi: 10.1186/1752-1947-2-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albano B, Gandolfo C, Del Sette M. Post-coital intra-cerebral venous hemorrhage in a 78-year-old man with jugular valve incompetence: a case report. J Med Case Reports 4: 225, 2010. doi: 10.1186/1752-1947-4-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alosco ML, Brickman AM, Spitznagel MB, Griffith EY, Narkhede A, Raz N, Cohen R, Sweet LH, Hughes J, Rosneck J, Gunstad J. Independent and interactive effects of blood pressure and cardiac function on brain volume and white matter hyperintensities in heart failure. J Am Soc Hypertens 7: 336–343, 2013. doi: 10.1016/j.jash.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altamura C, Scrascia F, Quattrocchi CC, Errante Y, Gangemi E, Curcio G, Ursini F, Silvestrini M, Maggio P, Beomonte Zobel B, Rossini PM, Pasqualetti P, Falsetti L, Vernieri F. Regional MRI diffusion, white-matter hyperintensities, and cognitive function in Alzheimer’s disease and vascular dementia. J Clin Neurol 12: 201–208, 2016. doi: 10.3988/jcn.2016.12.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An JY, Quarles EK, Mekvanich S, Kang A, Liu A, Santos D, Miller RA, Rabinovitch PS, Cox TC, Kaeberlein M. Rapamycin treatment attenuates age-associated periodontitis in mice. Geroscience 39: 457–463, 2017. doi: 10.1007/s11357-017-9994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbeille P, Fomina G, Roumy J, Alferova I, Tobal N, Herault S. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights. Eur J Appl Physiol 86: 157–168, 2001. doi: 10.1007/s004210100473. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong PJ, Sutherland R, Scott DH. The effect of position and different manoeuvres on internal jugular vein diameter size. Acta Anaesthesiol Scand 38: 229–231, 1994. doi: 10.1111/j.1399-6576.1994.tb03879.x. [DOI] [PubMed] [Google Scholar]
- 10.Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA. The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol 27: 77–85, 2017. doi: 10.1111/bpa.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke 42: 722–727, 2011. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience 39: 129–145, 2017. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atwood CS, Hayashi K, Meethal SV, Gonzales T, Bowen RL. Does the degree of endocrine dyscrasia post-reproduction dictate post-reproductive lifespan? Lessons from semelparous and iteroparous species. Geroscience 39: 103–116, 2017. doi: 10.1007/s11357-016-9955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auletta L, Greco A, Albanese S, Meomartino L, Salvatore M, Mancini M. Original research: feasibility and safety of two surgical techniques for the development of an animal model of jugular vein occlusion. Exp Biol Med (Maywood) 242: 22–28, 2017. doi: 10.1177/1535370216657446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci 67A: 313–329, 2012. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banki E, Sosnowska D, Tucsek Z, Gautam T, Toth P, Tarantini S, Tamas A, Helyes Z, Reglodi D, Sonntag WE, Csiszar A, Ungvari Z. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci 70: 665–674, 2015. doi: 10.1093/gerona/glu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateman GA. The pathophysiology of idiopathic normal pressure hydrocephalus: cerebral ischemia or altered venous hemodynamics? AJNR Am J Neuroradiol 29: 198–203, 2008. doi: 10.3174/ajnr.A0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bateman GA. Pulse-wave encephalopathy: a comparative study of the hydrodynamics of leukoaraiosis and normal-pressure hydrocephalus. Neuroradiology 44: 740–748, 2002. doi: 10.1007/s00234-002-0812-0. [DOI] [PubMed] [Google Scholar]
- 19.Bateman GA, Levi CR, Schofield P, Wang Y, Lovett EC. The venous manifestations of pulse wave encephalopathy: windkessel dysfunction in normal aging and senile dementia. Neuroradiology 50: 491–497, 2008. doi: 10.1007/s00234-008-0374-x. [DOI] [PubMed] [Google Scholar]
- 20.Beer C, Ebenezer E, Fenner S, Lautenschlager NT, Arnolda L, Flicker L, Almeida OP. Contributors to cognitive impairment in congestive heart failure: a pilot case-control study. Intern Med J 39: 600–605, 2009. doi: 10.1111/j.1445-5994.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- 21.Beggs CB. Venous hemodynamics in neurological disorders: an analytical review with hydrodynamic analysis. BMC Med 11: 142, 2013. doi: 10.1186/1741-7015-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beggs C, Chung CP, Bergsland N, Wang PN, Shepherd S, Cheng CY, Dwyer MG, Hu HH, Zivadinov R. Jugular venous reflux and brain parenchyma volumes in elderly patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol 13: 157, 2013. doi: 10.1186/1471-2377-13-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belghali M, Chastan N, Cignetti F, Davenne D, Decker LM. Loss of gait control assessed by cognitive-motor dual-tasks: pros and cons in detecting people at risk of developing Alzheimer’s and Parkinson’s diseases. Geroscience 39: 305–329, 2017. doi: 10.1007/s11357-017-9977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belov P, Magnano C, Krawiecki J, Hagemeier J, Bergsland N, Beggs C, Zivadinov R. Age-related brain atrophy may be mitigated by internal jugular vein enlargement in male individuals without neurologic disease. Phlebology 32: 125–134, 2017. doi: 10.1177/0268355516633610. [DOI] [PubMed] [Google Scholar]
- 25.Bennis MT, Schneider A, Victoria B, Do A, Wiesenborn DS, Spinel L, Gesing A, Kopchick JJ, Siddiqi SA, Masternak MM. The role of transplanted visceral fat from the long-lived growth hormone receptor knockout mice on insulin signaling. Geroscience 39: 51–59, 2017. doi: 10.1007/s11357-017-9957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benveniste H, Lee H, Volkow ND. The glymphatic pathway: waste removal from the CNS via cerebrospinal fluid transport. Neuroscientist 23: 454–465, 2017. doi: 10.1177/1073858417691030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bérczi V, Molnár AA, Apor A, Kovács V, Ruzics C, Várallyay C, Hüttl K, Monos E, Nádasy GL. Non-invasive assessment of human large vein diameter, capacity, distensibility and ellipticity in situ: dependence on anatomical location, age, body position and pressure. Eur J Appl Physiol 95: 283–289, 2005. doi: 10.1007/s00421-005-0002-y. [DOI] [PubMed] [Google Scholar]
- 28.Bernier M, Wahl D, Ali A, Allard J, Faulkner S, Wnorowski A, Sanghvi M, Moaddel R, Alfaras I, Mattison JA, Tarantini S, Tucsek Z, Ungvari Z, Csiszar A, Pearson KJ, de Cabo R. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany NY) 8: 899–916, 2016. doi: 10.18632/aging.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilston LE, Fletcher DF, Brodbelt AR, Stoodley MA. Arterial pulsation-driven cerebrospinal fluid flow in the perivascular space: a computational model. Comput Methods Biomech Biomed Engin 6: 235–241, 2003. doi: 10.1080/10255840310001606116. [DOI] [PubMed] [Google Scholar]
- 30.Ble A, Ranzini M, Zurlo A, Menozzi L, Atti AR, Munari MR, Volpato S, Scaramelli G, Fellin R, Zuliani G. Leukoaraiosis is associated with functional impairment in older patients with Alzheimer’s disease but not vascular dementia. J Nutr Health Aging 10: 31–35, 2006. [PubMed] [Google Scholar]
- 31.Blodgett JM, Theou O, Howlett SE, Rockwood K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. Geroscience 39: 447–455, 2017. doi: 10.1007/s11357-017-9993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouvy WH, Kuijf HJ, Zwanenburg JJ, Koek HL, Kappelle LJ, Luijten PR, Ikram MK, Biessels GJ; Utrecht Vascular Cognitive Impairment (VCI) Study group . Abnormalities of cerebral deep medullary veins on 7 Tesla MRI in amnestic mild cognitive impairment and early Alzheimer’s disease: a pilot study. J Alzheimers Dis 57: 705–710, 2017. doi: 10.3233/JAD-160952. [DOI] [PubMed] [Google Scholar]
- 33.Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11: 10, 2014. doi: 10.1186/2045-8118-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci 203-204: 159–163, 2002. doi: 10.1016/S0022-510X(02)00283-6. [DOI] [PubMed] [Google Scholar]
- 35.Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab 32: 425–436, 2012. doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caldas JR, Panerai RB, Haunton VJ, Almeida JP, Ferreira GS, Camara L, Nogueira RC, Bor-Seng-Shu E, Oliveira ML, Groehs RR, Ferreira-Santos L, Teixeira MJ, Galas FR, Robinson TG, Jatene FB, Hajjar LA. Cerebral blood flow autoregulation in ischemic heart failure. Am J Physiol Regul Integr Comp Physiol 312: R108–R113, 2017. doi: 10.1152/ajpregu.00361.2016. [DOI] [PubMed] [Google Scholar]
- 37.Chai C, Wang Z, Fan L, Zhang M, Chu Z, Zuo C, Liu L, Mark Haacke E, Guo W, Shen W, Xia S. Increased number and distribution of cerebral microbleeds is a risk factor for cognitive dysfunction in hemodialysis patients: a longitudinal study. Medicine (Baltimore) 95: e2974, 2016. doi: 10.1097/MD.0000000000002974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapman-Davies A, Lazarevic A. Valsalva maculopathy. Clin Exp Optom 85: 42–45, 2002. doi: 10.1111/j.1444-0938.2002.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 39.Cheung CY, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, Catindig JA, Venketasubramanian N, Yap P, Seow D, Chen CP, Wong TY. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers Dement 10: 135–142, 2014. doi: 10.1016/j.jalz.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354: 472–477, 2016. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi BR, Kim JS, Yang YJ, Park KM, Lee CW, Kim YH, Hong MK, Song JK, Park SW, Park SJ, Kim JJ. Factors associated with decreased cerebral blood flow in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol 97: 1365–1369, 2006. doi: 10.1016/j.amjcard.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 42.Chung CP, Beggs C, Wang PN, Bergsland N, Shepherd S, Cheng CY, Ramasamy DP, Dwyer MG, Hu HH, Zivadinov R. Jugular venous reflux and white matter abnormalities in Alzheimer’s disease: a pilot study. J Alzheimers Dis 39: 601–609, 2014. doi: 10.3233/JAD-131112. [DOI] [PubMed] [Google Scholar]
- 43.Chung CP, Cheng CY, Zivadinov R, Chen WC, Sheng WY, Lee YC, Hu HH, Hsu HY, Yang KY. Jugular venous reflux and plasma endothelin-1 are associated with cough syncope: a case control pilot study. BMC Neurol 13: 9, 2013. doi: 10.1186/1471-2377-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung CP, Hu HH. Pathogenesis of leukoaraiosis: role of jugular venous reflux. Med Hypotheses 75: 85–90, 2010. doi: 10.1016/j.mehy.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 45.Chung CP, Lin YJ, Chao AC, Lin SJ, Chen YY, Wang YJ, Hu HH. Jugular venous hemodynamic changes with aging. Ultrasound Med Biol 36: 1776–1782, 2010. doi: 10.1016/j.ultrasmedbio.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Coffman JA, Torello MW, Bornstein RA, Chakeres D, Burns E, Nasrallah HA. Leukoaraiosis in asymptomatic adult offspring of individuals with Alzheimer’s disease. Biol Psychiatry 27: 1244–1248, 1990. doi: 10.1016/0006-3223(90)90422-X. [DOI] [PubMed] [Google Scholar]
- 47.Comi G, Battaglia MA, Bertolotto A, Del Sette M, Ghezzi A, Malferrari G, Salvetti M, Sormani MP, Tesio L, Stolz E, Mancardi G. Italian multicentre observational study of the prevalence of CCSVI in multiple sclerosis (CoSMo study): rationale, design, and methodology. Neurol Sci 34: 1297–1307, 2013. doi: 10.1007/s10072-012-1269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cornwell WK 3rd, Levine BD. Patients with heart failure with reduced ejection fraction have exaggerated reductions in cerebral blood flow during upright posture. JACC Heart Fail 3: 176–179, 2015. doi: 10.1016/j.jchf.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Coutinho E, Silva AM, Freitas C, Santos E. Graves’ disease presenting as pseudotumor cerebri: a case report. J Med Case Reports 5: 68, 2011. doi: 10.1186/1752-1947-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crawford PM, West CR, Chadwick DW, Shaw MD. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry 49: 1–10, 1986. doi: 10.1136/jnnp.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol 307: H292–H306, 2014. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev 130: 518–527, 2009. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-α treatment in aging. Am J Pathol 170: 388–698, 2007. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci 67: 811–820, 2012. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Csiszar A, Tarantini S, Fülöp GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience 39: 359–372, 2017. doi: 10.1007/s11357-017-9991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. doi: 10.1161/01.RES.0000020401.61826.EA. [DOI] [PubMed] [Google Scholar]
- 57.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J 17: 1183–1185, 2003. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 58.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 59.Dawson JW. XVIII.—The histology of disseminated sclerosis. Trans R Soc Edinb 50: 517–740, 1916. doi: 10.1017/S0080456800027174. [DOI] [Google Scholar]
- 60.Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A. A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. Geroscience 39: 187–198, 2017. doi: 10.1007/s11357-017-9975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.dela Paz NG, D’Amore PA. Arterial versus venous endothelial cells. Cell Tissue Res 335: 5–16, 2009. doi: 10.1007/s00441-008-0706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dhanger S, Vaidiyanathan B, Tripathy DK. Internal jugular venous valve: Well known but mostly neglected. Indian J Anaesth 60: 602–603, 2016. doi: 10.4103/0019-5049.187813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dichgans M, Leys D. Vascular cognitive impairment. Circ Res 120: 573–591, 2017. doi: 10.1161/CIRCRESAHA.116.308426. [DOI] [PubMed] [Google Scholar]
- 64.Doepp F, Schreiber SJ, von Münster T, Rademacher J, Klingebiel R, Valdueza JM. How does the blood leave the brain? A systematic ultrasound analysis of cerebral venous drainage patterns. Neuroradiology 46: 565–570, 2004. doi: 10.1007/s00234-004-1213-3. [DOI] [PubMed] [Google Scholar]
- 65.Egemen E, Solaroglu I. Anatomy of Cerebral Veins and Dural Sinuses. In: Primer on Cerebrovascular Diseases, 2nd ed.,edited by Caplan LR, Leary MC, Thomas AJ, Zhang JH, Biller J, Lo EH, and Yenari M. London: Academic, 2017. [Google Scholar]
- 66.Eide PK, Pripp AH. Increased prevalence of cardiovascular disease in idiopathic normal pressure hydrocephalus patients compared to a population-based cohort from the HUNT3 survey. Fluids Barriers CNS 11: 19, 2014. doi: 10.1186/2045-8118-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Espay AJ, Da Prat GA, Dwivedi AK, Rodriguez-Porcel F, Vaughan JE, Rosso M, Devoto JL, Duker AP, Masellis M, Smith CD, Mandybur GT, Merola A, Lang AE. Deconstructing normal pressure hydrocephalus: ventriculomegaly as early sign of neurodegeneration. Ann Neurol 82: 503–513, 2017. doi: 10.1002/ana.25046. [DOI] [PubMed] [Google Scholar]
- 68.Fang Y, McFadden S, Darcy J, Hill CM, Huber JA, Verhulst S, Kopchick JJ, Miller RA, Sun LY, Bartke A. Differential effects of early-life nutrient restriction in long-lived GHR-KO and normal mice. Geroscience 39: 347–356, 2017. doi: 10.1007/s11357-017-9978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64: 575–611, 2001. doi: 10.1016/S0301-0082(00)00068-X. [DOI] [PubMed] [Google Scholar]
- 70.Fisher J, Vaghaiwalla F, Tsitlik J, Levin H, Brinker J, Weisfeldt M, Yin F. Determinants and clinical significance of jugular venous valve competence. Circulation 65: 188–196, 1982. doi: 10.1161/01.CIR.65.1.188. [DOI] [PubMed] [Google Scholar]
- 71.Fronek A, Criqui MH, Denenberg J, Langer RD. Common femoral vein dimensions and hemodynamics including Valsalva response as a function of sex, age, and ethnicity in a population study. J Vasc Surg 33: 1050–1056, 2001. doi: 10.1067/mva.2001.113496. [DOI] [PubMed] [Google Scholar]
- 72.Fukazawa K, Aguina L, Pretto EA Jr. Internal jugular valve and central catheter placement. Anesthesiology 112: 979, 2010. doi: 10.1097/ALN.0b013e3181d436de. [DOI] [PubMed] [Google Scholar]
- 73.Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience 40: 513–521, 2018. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fulop GA, Ramirez-Perez FI, Kiss T, Tarantini S, Valcarcel Ares MN, Toth P, Yabluchanskiy A, Conley SM, Ballabh P, Martinez-Lemus LA, Ungvari Z, Csiszar A. IGF-1 deficiency promotes pathological remodeling of cerebral arteries: a potential mechanism contributing to the pathogenesis of intracerebral hemorrhages in aging. J Gerontol A Biol Sci Med Sci 74: 446–454, 2019. doi: 10.1093/gerona/gly144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gascho JA, Fanelli C, Zelis R. Aging reduces venous distensibility and the venodilatory response to nitroglycerin in normal subjects. Am J Cardiol 63: 1267–1270, 1989. doi: 10.1016/0002-9149(89)90188-4. [DOI] [PubMed] [Google Scholar]
- 76.Georgiadis D, Sievert M, Cencetti S, Uhlmann F, Krivokuca M, Zierz S, Werdan K. Cerebrovascular reactivity is impaired in patients with cardiac failure. Eur Heart J 21: 407–413, 2000. doi: 10.1053/euhj.1999.1742. [DOI] [PubMed] [Google Scholar]
- 77.Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kövari E. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain 130: 2830–2836, 2007. doi: 10.1093/brain/awm228. [DOI] [PubMed] [Google Scholar]
- 78.Gopinath B, Flood VM, Wang JJ, Rochtchina E, Wong TY, Mitchell P. Is quality of diet associated with the microvasculature? An analysis of diet quality and retinal vascular calibre in older adults. Br J Nutr 110: 739–746, 2013. doi: 10.1017/S0007114512005491. [DOI] [PubMed] [Google Scholar]
- 79.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713, 2011. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grant CD, Jafari N, Hou L, Li Y, Stewart JD, Zhang G, Lamichhane A, Manson JE, Baccarelli AA, Whitsel EA, Conneely KN. A longitudinal study of DNA methylation as a potential mediator of age-related diabetes risk. Geroscience 39: 475–489, 2017. doi: 10.1007/s11357-017-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grimmig B, Kim SH, Nash K, Bickford PC, Douglas Shytle R. Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience 39: 19–32, 2017. doi: 10.1007/s11357-017-9958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, Aldershvile J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke 32: 2530–2533, 2001. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- 83.Han HC. Twisted blood vessels: symptoms, etiology and biomechanical mechanisms. J Vasc Res 49: 185–197, 2012. doi: 10.1159/000335123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanif S, Abodunde O, Ali Z, Pidgeon C. Age related outcome in acute subdural haematoma following traumatic head injury. Ir Med J 102: 255–257, 2009. [PubMed] [Google Scholar]
- 85.Hartmann DA, Hyacinth HI, Liao FF, Shih AY. Does pathology of small venules contribute to cerebral microinfarcts and dementia? J Neurochem 144: 517–526, 2018. doi: 10.1111/jnc.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He L, Vanlandewijck M, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Segerstolpe Å, Liu J, Gustafsson S, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci Data 5: 180160, 2018. doi: 10.1038/sdata.2018.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hemmeryckx B, Emmerechts J, Bovill EG, Hoylaerts MF, Lijnen HR. Effect of ageing on the murine venous circulation. Histochem Cell Biol 137: 537–546, 2012. doi: 10.1007/s00418-012-0913-8. [DOI] [PubMed] [Google Scholar]
- 88.Hernandez JP, Franke WD. Effects of a 6-mo endurance-training program on venous compliance and maximal lower body negative pressure in older men and women. J Appl Physiol (1985) 99: 1070–1077, 2005. doi: 10.1152/japplphysiol.01169.2004. [DOI] [PubMed] [Google Scholar]
- 89.Hilal S, Saini M, Tan CS, Catindig JA, Koay WI, Niessen WJ, Vrooman HA, Wong TY, Chen C, Ikram MK, Venketasubramanian N. Cerebral microbleeds and cognition: the epidemiology of dementia in Singapore study. Alzheimer Dis Assoc Disord 28: 106–112, 2014. doi: 10.1097/WAD.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 90.Hooghiemstra AM, Bertens AS, Leeuwis AE, Bron EE, Bots ML, Brunner-La Rocca HP, de Craen AJ, van der Geest RJ, Greving JP, Kappelle LJ, Niessen WJ, van Oostenbrugge RJ, van Osch MJP, de Roos A, van Rossum AC, Biessels GJ, van Buchem MA, Daemen MJ, van der Flier WM; Heart-Brain Connection Consortium . The missing link in the pathophysiology of vascular cognitive impairment: design of the Heart-Brain Study. Cerebrovasc Dis Extra 7: 140–152, 2017. doi: 10.1159/000480738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4: 147ra111, 2012. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inano S, Itoh D, Takao H, Hayashi N, Mori H, Kunimatsu A, Abe O, Aoki S, Ohtomo K. High signal intensity in the dural sinuses on 3D-TOF MR angiography at 3.0 T. Clin Imaging 34: 332–336, 2010. doi: 10.1016/j.clinimag.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 93.Jackson SE, Redeker A, Arens R, van Baarle D, van den Berg SPH, Benedict CA, Čičin-Šain L, Hill AB, Wills MR. CMV immune evasion and manipulation of the immune system with aging. Geroscience 39: 273–291, 2017. doi: 10.1007/s11357-017-9986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jacobs HI, Clerx L, Gronenschild EH, Aalten P, Verhey FR. White matter hyperintensities are positively associated with cortical thickness in Alzheimer’s disease. J Alzheimers Dis 39: 409–422, 2014. doi: 10.3233/JAD-131232. [DOI] [PubMed] [Google Scholar]
- 95.Jahrling JB, Lin AL, DeRosa N, Hussong SA, Van Skike CE, Girotti M, Javors M, Zhao Q, Maslin LA, Asmis R, Galvan V. mTOR drives cerebral blood flow and memory deficits in LDLR-/- mice modeling atherosclerosis and vascular cognitive impairment. J Cereb Blood Flow Metab 38: 58–74, 2018. doi: 10.1177/0271678X17705973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jang J, Kim BS, Kim BY, Choi HS, Jung SL, Ahn KJ, Byun JY. Reflux venous flow in dural sinus and internal jugular vein on 3D time-of-flight MR angiography. Neuroradiology 55: 1205–1211, 2013. doi: 10.1007/s00234-013-1239-5. [DOI] [PubMed] [Google Scholar]
- 97.Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O’Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation 122: 690–697, 2010. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res 40: 2583–2599, 2015. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jin BJ, Smith AJ, Verkman AS. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol 148: 489–501, 2016. doi: 10.1085/jgp.201611684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jorgensen DR, Shaaban CE, Wiley CA, Gianaros PJ, Mettenburg J, Rosano C. A population neuroscience approach to the study of cerebral small vessel disease in midlife and late life: an invited review. Am J Physiol Heart Circ Physiol 314: H1117–H1136, 2018. doi: 10.1152/ajpheart.00535.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Juurlink BH. Is there a pulse wave encephalopathy component to multiple sclerosis? Curr Neurovasc Res 12: 199–209, 2015. doi: 10.2174/1567202612666150311113205. [DOI] [PubMed] [Google Scholar]
- 102.Kandel BM, Avants BB, Gee JC, McMillan CT, Erus G, Doshi J, Davatzikos C, Wolk DA. White matter hyperintensities are more highly associated with preclinical Alzheimer’s disease than imaging and cognitive markers of neurodegeneration. Alzheimers Dement (Amst) 4: 18–27, 2016. doi: 10.1016/j.dadm.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kang Y, Kim E, Kim JH, Choi BS, Jung C, Bae YJ, Lee KM, Lee DH. Time of flight MR angiography assessment casts doubt on the association between transient global amnesia and intracranial jugular venous reflux. Eur Radiol 25: 703–709, 2015. doi: 10.1007/s00330-014-3448-7. [DOI] [PubMed] [Google Scholar]
- 104.Keith J, Gao FQ, Noor R, Kiss A, Balasubramaniam G, Au K, Rogaeva E, Masellis M, Black SE. Collagenosis of the deep medullary veins: an underrecognized pathologic correlate of white matter hyperintensities and periventricular infarction? J Neuropathol Exp Neurol 76: 299–312, 2017. doi: 10.1093/jnen/nlx009. [DOI] [PubMed] [Google Scholar]
- 105.Kılıç T, Akakin A. Anatomy of cerebral veins and sinuses. Front Neurol Neurosci 23: 4–15, 2008. doi: 10.1159/000111256. [DOI] [PubMed] [Google Scholar]
- 106.Kim E, Kim JH, Choi BS, Jung C, Lee DH. MRI and MR angiography findings to differentiate jugular venous reflux from cavernous dural arteriovenous fistula. AJR Am J Roentgenol 202: 839–846, 2014. doi: 10.2214/AJR.13.11048. [DOI] [PubMed] [Google Scholar]
- 107.Kim H, Al-Shahi Salman R, McCulloch CE, Stapf C, Young WL; MARS Coinvestigators . Untreated brain arteriovenous malformation: patient-level meta-analysis of hemorrhage predictors. Neurology 83: 590–597, 2014. doi: 10.1212/WNL.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Konopka AR, Laurin JL, Musci RV, Wolff CA, Reid JJ, Biela LM, Zhang Q, Peelor FF III, Melby CL, Hamilton KL, Miller BF. Influence of Nrf2 activators on subcellular skeletal muscle protein and DNA synthesis rates after 6 weeks of milk protein feeding in older adults. Geroscience 39: 175–186, 2017. doi: 10.1007/s11357-017-9968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kudo K, Terae S, Ishii A, Omatsu T, Asano T, Tha KK, Miyasaka K. Physiologic change in flow velocity and direction of dural venous sinuses with respiration: MR venography and flow analysis. AJNR Am J Neuroradiol 25: 551–557, 2004. [PMC free article] [PubMed] [Google Scholar]
- 110.Kuriyama N, Tokuda T, Yamada K, Akazawa K, Hosoda M, Sakai K, Watanabe Y, Nakagawa M. Flow velocity of the superior sagittal sinus is reduced in patients with idiopathic normal pressure hydrocephalus. J Neuroimaging 21: 365–369, 2011. doi: 10.1111/j.1552-6569.2011.00592.x. [DOI] [PubMed] [Google Scholar]
- 111.Lai AY, Dorr A, Thomason LA, Koletar MM, Sled JG, Stefanovic B, McLaurin J. Venular degeneration leads to vascular dysfunction in a transgenic model of Alzheimer’s disease. Brain 138: 1046–1058, 2015. doi: 10.1093/brain/awv023. [DOI] [PubMed] [Google Scholar]
- 112.Lee AY, Han B, Lamm SD, Fierro CA, Han HC. Effects of elastin degradation and surrounding matrix support on artery stability. Am J Physiol Heart Circ Physiol 302: H873–H884, 2012. doi: 10.1152/ajpheart.00463.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee MB, Carr DT, Kiflezghi MG, Zhao YT, Kim DB, Thon S, Moore MD, Li MAK, Kaeberlein M. A system to identify inhibitors of mTOR signaling using high-resolution growth analysis in Saccharomyces cerevisiae. Geroscience 39: 419–428, 2017. doi: 10.1007/s11357-017-9988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leng SX, Kamil J, Purdy JG, Lemmermann NA, Reddehase MJ, Goodrum FD. Recent advances in CMV tropism, latency, and diagnosis during aging. Geroscience 39: 251–259, 2017. doi: 10.1007/s11357-017-9985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lepori D, Capasso P, Fournier D, Genton CY, Schnyder P. High-resolution ultrasound evaluation of internal jugular venous valves. Eur Radiol 9: 1222–1226, 1999. doi: 10.1007/s003300050822. [DOI] [PubMed] [Google Scholar]
- 116.Leto L, Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol 11: 316–328, 2014. doi: 10.11909/j.issn.1671-5411.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin AL, Jahrling JB, Zhang W, DeRosa N, Bakshi V, Romero P, Galvan V, Richardson A. Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer’s disease. J Cereb Blood Flow Metab 37: 217–226, 2017. doi: 10.1177/0271678X15621575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih YY, Muir E, Solano Fonseca R, Strong R, Richardson AG, Lechleiter JD, Fox PT, Galvan V. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab 33: 1412–1421, 2013. doi: 10.1038/jcbfm.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lindenberger M, Länne T. Decreased capillary filtration but maintained venous compliance in the lower limb of aging women. Am J Physiol Heart Circ Physiol 293: H3568–H3574, 2007. doi: 10.1152/ajpheart.00725.2007. [DOI] [PubMed] [Google Scholar]
- 120.Liu X, Bhatt T, Wang S, Yang F, Pai YC. Retention of the “first-trial effect” in gait-slip among community-living older adults. Geroscience 39: 93–102, 2017. doi: 10.1007/s11357-017-9963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lobato EB, Florete OG Jr, Paige GB, Morey TE. Cross-sectional area and intravascular pressure of the right internal jugular vein during anesthesia: effects of Trendelenburg position, positive intrathoracic pressure, and hepatic compression. J Clin Anesth 10: 1–5, 1998. doi: 10.1016/S0952-8180(97)00189-X. [DOI] [PubMed] [Google Scholar]
- 122.Loncar G, Bozic B, Lepic T, Dimkovic S, Prodanovic N, Radojicic Z, Cvorovic V, Markovic N, Brajovic M, Despotovic N, Putnikovic B, Popovic-Brkic V. Relationship of reduced cerebral blood flow and heart failure severity in elderly males. Aging Male 14: 59–65, 2011. doi: 10.3109/13685538.2010.511326. [DOI] [PubMed] [Google Scholar]
- 123.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341, 2015. [Erratum in: Nature 533: 278, 2016.] doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Makedonov I, Black SE, MacIntosh BJ. Cerebral small vessel disease in aging and Alzheimer’s disease: a comparative study using MRI and SPECT. Eur J Neurol 20: 243–250, 2013. doi: 10.1111/j.1468-1331.2012.03785.x. [DOI] [PubMed] [Google Scholar]
- 125.Mayhan WG, Heistad DD. Role of veins and cerebral venous pressure in disruption of the blood-brain barrier. Circ Res 59: 216–220, 1986. doi: 10.1161/01.RES.59.2.216. [DOI] [PubMed] [Google Scholar]
- 126.Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience 39: 7–18, 2017. doi: 10.1007/s11357-017-9959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Molnár AA, Apor A, Kristóf V, Nádasy GL, Préda I, Hüttl K, Acsády G, Monos E, Bérczi V. Generalized changes in venous distensibility in postthrombotic patients. Thromb Res 117: 639–645, 2006. doi: 10.1016/j.thromres.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 128.Monahan KD, Dinenno FA, Seals DR, Halliwill JR. Smaller age-associated reductions in leg venous compliance in endurance exercise-trained men. Am J Physiol Heart Circ Physiol 281: H1267–H1273, 2001. doi: 10.1152/ajpheart.2001.281.3.H1267. [DOI] [PubMed] [Google Scholar]
- 129.Monos E, Bérczi V, Nádasy G. Local control of veins: biomechanical, metabolic, and humoral aspects. Physiol Rev 75: 611–666, 1995. doi: 10.1152/physrev.1995.75.3.611. [DOI] [PubMed] [Google Scholar]
- 130.Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis: association with leukoaraiosis. Radiology 194: 469–476, 1995. doi: 10.1148/radiology.194.2.7824728. [DOI] [PubMed] [Google Scholar]
- 131.Mortamais M, Artero S, Ritchie K. White matter hyperintensities as early and independent predictors of Alzheimer’s disease risk. J Alzheimers Dis 42, Suppl 4: S393–S400, 2014. doi: 10.3233/JAD-141473. [DOI] [PubMed] [Google Scholar]
- 132.Nikolich-Žugich J, van Lier RA. Cytomegalovirus (CMV) research in immune senescence comes of age: overview of the 6th International Workshop on CMV and Immunosenescence. Geroscience 39: 245–249, 2017. doi: 10.1007/s11357-017-9984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O’Sullivan M. Leukoaraiosis. Pract Neurol 8: 26–38, 2008. doi: 10.1136/jnnp.2007.139428. [DOI] [PubMed] [Google Scholar]
- 134.Pabaney AH, Reinard KA, Kole MK, Seyfried DM, Malik GM. Management of arteriovenous malformations in the elderly: a single-center case series and analysis of outcomes. J Neurosurg 125: 145–151, 2016. doi: 10.3171/2015.6.JNS15293. [DOI] [PubMed] [Google Scholar]
- 135.Paul F, Wattjes MP. Chronic cerebrospinal venous insufficiency in multiple sclerosis: the final curtain. Lancet 383: 106–108, 2014. doi: 10.1016/S0140-6736(13)61912-1. [DOI] [PubMed] [Google Scholar]
- 136.Perrott KM, Wiley CD, Desprez PY, Campisi J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience 39: 161–173, 2017. doi: 10.1007/s11357-017-9970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE, Csiszar A, Ungvari Z. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience 39: 147–160, 2017. doi: 10.1007/s11357-017-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM, Vernooij MW. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology 78: 326–333, 2012. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 139.Putnam TJ. Evidences of vascular occlusion in multiple sclerosis and “encephalomyelitis”. Arch Neurol Psychiatry 37: 1298–1321, 1937. doi: 10.1001/archneurpsyc.1937.02260180078006. [DOI] [Google Scholar]
- 140.Putnam TJ, Adler A. Vascular architecture of the lesions of multiple sclerosis. Arch Neurol Psychiatry 38: 1–15, 1937. doi: 10.1001/archneurpsyc.1937.02260190011001. [DOI] [Google Scholar]
- 141.Rais M, Wilson RM, Urbanski HF, Messaoudi I. Androgen supplementation improves some but not all aspects of immune senescence in aged male macaques. Geroscience 39: 373–384, 2017. doi: 10.1007/s11357-017-9979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rodger IW, Dilar D, Dwyer J, Bienenstock J, Coret A, Coret-Simon J, Foster G, Franchetto A, Franic S, Goldsmith CH, Koff D, Konyer NB, Levine M, McDonald E, Noseworthy MD, Paulseth J, Ribeiro L, Sayles MJ, Thabane L. Evidence against the involvement of chronic cerebrospinal venous abnormalities in multiple sclerosis. A case-control study. PLoS One 8: e72495, 2013. doi: 10.1371/journal.pone.0072495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15: 973–977, 2016. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol 313: H890–H895, 2017. doi: 10.1152/ajpheart.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Roy B, Woo MA, Wang DJ, Fonarow GC, Harper RM, Kumar R. Reduced regional cerebral blood flow in patients with heart failure. Eur J Heart Fail 19: 1294–1302, 2017. doi: 10.1002/ejhf.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saphir O, Lev M. The venous valve in the aged. Am Heart J 44: 843–850, 1952. doi: 10.1016/0002-8703(52)90130-0. [DOI] [PubMed] [Google Scholar]
- 147.Schlesinger B. The venous drainage of the brain, with special reference to the galenic system. Brain 62: 274–291, 1939. doi: 10.1093/brain/62.3.274. [DOI] [Google Scholar]
- 148.Shaaban CE, Aizenstein HJ, Jorgensen DR, MacCloud RL, Meckes NA, Erickson KI, Glynn NW, Mettenburg J, Guralnik J, Newman AB, Ibrahim TS, Laurienti PJ, Vallejo AN, Rosano C; LIFE Study Group . In vivo imaging of venous side cerebral small-vessel disease in older adults: an MRI method at 7T. AJNR Am J Neuroradiol 38: 1923–1928, 2017. doi: 10.3174/ajnr.A5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sharpey-Schafer EP. The mechanism of syncope after coughing. BMJ 2: 860–863, 1953. doi: 10.1136/bmj.2.4841.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sherman S, Justo D, Engel T, Yossepowitch O, Bregman N, Gadoth A, Paran Y. Cytomegalovirus-associated cerebral sinus vein thrombosis. J Med Virol 84: 1934–1936, 2012. doi: 10.1002/jmv.23424. [DOI] [PubMed] [Google Scholar]
- 151.Shobin E, Bowley MP, Estrada LI, Heyworth NC, Orczykowski ME, Eldridge SA, Calderazzo SM, Mortazavi F, Moore TL, Rosene DL. Microglia activation and phagocytosis: relationship with aging and cognitive impairment in the rhesus monkey. Geroscience 39: 199–220, 2017. doi: 10.1007/s11357-017-9965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shprecher D, Schwalb J, Kurlan R. Normal pressure hydrocephalus: diagnosis and treatment. Curr Neurol Neurosci Rep 8: 371–376, 2008. doi: 10.1007/s11910-008-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife 6: e27679, 2017. doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol 11: 272–282, 2012. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 62: 406–413, 2007. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 156.Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci 5: 27, 2013. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Souquette A, Frere J, Smithey M, Sauce D, Thomas PG. A constant companion: immune recognition and response to cytomegalovirus with aging and implications for immune fitness. Geroscience 39: 293–303, 2017. doi: 10.1007/s11357-017-9982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One 5: e9979, 2010. [Correction in: PLoS One 2011: 6, 2011.] doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol 24: 1397–1402, 2004. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 160.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis 43: 1143–1151, 2006. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 161.Sumbria RK, Grigoryan MM, Vasilevko V, Krasieva TB, Scadeng M, Dvornikova AK, Paganini-Hill A, Kim R, Cribbs DH, Fisher MJ. A murine model of inflammation-induced cerebral microbleeds. J Neuroinflammation 13: 218, 2016. doi: 10.1186/s12974-016-0693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tarantini S, Fulop GA, Kiss T, Farkas E, Zölei-Szénási D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience 39: 465–473, 2017. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, Sonntag WE, Ungvari Z, Csiszar A. IGF-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation: implications for the developmental origins of health and disease hypothesis. Age (Dordr) 38: 239–258, 2016. doi: 10.1007/s11357-016-9943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tarantini S, Tran CH, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol 94: 52–58, 2017. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 38: 273–289, 2016. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 17: e12731, 2018. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell 16: 469–479, 2017. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, Gautam T, Zhang XA, Sonntag WE, de Cabo R, Farkas E, Elliott MH, Kinter MT, Deak F, Ungvari Z, Csiszar A. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci 73: 853–863, 2018. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tarantini S, Yabluchanksiy A, Fülöp GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience 39: 601–614, 2017. [Erratum in: Geroscience 40: 219, 2018.] doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tarnoki AD, Molnar AA, Tarnoki DL, Littvay L, Medda E, Fagnani C, Arnolfi A, Farina F, Baracchini C, Meneghetti G, Pucci G, Schillaci G, Stazi MA, Nadasy GL. Heritability of the dimensions, compliance and distensibility of the human internal jugular vein wall. PLoS One 13: e0192948, 2018. [Erratum in: PLoS One13: e0197526, 2018.] doi: 10.1371/journal.pone.0192948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tenk J, Rostás I, Füredi N, Mikó A, Solymár M, Soós S, Gaszner B, Feller D, Székely M, Pétervári E, Balaskó M. Age-related changes in central effects of corticotropin-releasing factor (CRF) suggest a role for this mediator in aging anorexia and cachexia. Geroscience 39: 61–72, 2017. doi: 10.1007/s11357-017-9962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thorin-Trescases N, de Montgolfier O, Pinçon A, Raignault A, Caland L, Labbé P, Thorin E. Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol Heart Circ Physiol 314: H1214–H1224, 2018. doi: 10.1152/ajpheart.00637.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tian Q, Gromov P, Clement JH, Wang Y, Riemann M, Weih F, Sun XX, Dai MS, Fedorov LM. RHEB1 insufficiency in aged male mice is associated with stress-induced seizures. Geroscience 39: 557–570, 2017. doi: 10.1007/s11357-017-9997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol 305: H1698–H1708, 2013. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell 14: 1034–1044, 2015. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 312: H1–H20, 2017. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell 14: 400–408, 2015. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab 34: 1887–1897, 2014. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Traboulsee AL, Knox KB, Machan L, Zhao Y, Yee I, Rauscher A, Klass D, Szkup P, Otani R, Kopriva D, Lala S, Li DK, Sadovnick D. Prevalence of extracranial venous narrowing on catheter venography in people with multiple sclerosis, their siblings, and unrelated healthy controls: a blinded, case-control study. Lancet 383: 138–145, 2014. doi: 10.1016/S0140-6736(13)61747-X. [DOI] [PubMed] [Google Scholar]
- 181.Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, Yabluchanskiy A, Fülöp G, Gautam T, Orock A, Csiszar A, Deak F, Ungvari Z. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience 39: 385–406, 2017. doi: 10.1007/s11357-017-9981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 69: 1212–1226, 2014. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci 69: 1339–1352, 2014. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Uchino A, Nomiyama K, Takase Y, Nakazono T, Tominaga Y, Imaizumi T, Kudo S. Retrograde flow in the dural sinuses detected by three-dimensional time-of-flight MR angiography. Neuroradiology 49: 211–215, 2007. doi: 10.1007/s00234-006-0186-9. [DOI] [PubMed] [Google Scholar]
- 184a.Ungvari Z, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol 300: H1133–H1140, 2011. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta EG, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-kappaB activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci 66A: 866–875, 2011. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol 301: H363–H372, 2011. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 65A: 1028–1041, 2010. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187a.Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 188.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 189.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res 123: 849–867, 2018. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fülöp GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience 39: 33–42, 2017. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol 312: H1128–H1143, 2017. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol 15: 555–565, 2018. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, Sonntag WE, Csiszar A. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci 68: 877–891, 2013. doi: 10.1093/gerona/gls242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ungvari Z, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Fülöp GA, Kiss T, Csiszar A. Connective tissue growth factor (CTGF) in age-related vascular pathologies. Geroscience 39: 491–498, 2017. doi: 10.1007/s11357-017-9995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ungvari Z, Yabluchanskiy A, Tarantini S, Toth P, Kirkpatrick AC, Csiszar A, Prodan CI. Repeated Valsalva maneuvers promote symptomatic manifestations of cerebral microhemorrhages: implications for the pathogenesis of vascular cognitive impairment in older adults. Geroscience 40: 485–496, 2018. doi: 10.1007/s11357-018-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Unnikrishnan A, Jackson J, Matyi SA, Hadad N, Wronowski B, Georgescu C, Garrett KP, Wren JD, Freeman WM, Richardson A. Role of DNA methylation in the dietary restriction mediated cellular memory. Geroscience 39: 331–345, 2017. doi: 10.1007/s11357-017-9976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DE, Kaeberlein M. Asymptomatic heart valve dysfunction in healthy middle-aged companion dogs and its implications for cardiac aging. Geroscience 39: 43–50, 2017. doi: 10.1007/s11357-016-9956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DEL, Kaeberlein M. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience 39: 117–127, 2017. doi: 10.1007/s11357-017-9972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci 67: 821–829, 2012. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Valdueza JM, von Münster T, Hoffman O, Schreiber S, Einhäupl KM. Postural dependency of the cerebral venous outflow. Lancet 355: 200–201, 2000. doi: 10.1016/S0140-6736(99)04804-7. [DOI] [PubMed] [Google Scholar]
- 204.Valecchi D, Bacci D, Gulisano M, Sgambati E, Sibilio M, Lipomas M, Macchi C. Internal jugular vein valves: an assessment of prevalence, morphology and competence by color Doppler echography in 240 healthy subjects. Ital J Anat Embryol 115: 185–189, 2010. [PubMed] [Google Scholar]
- 205.van Langevelde K, Srámek A, Rosendaal FR. The effect of aging on venous valves. Arterioscler Thromb Vasc Biol 30: 2075–2080, 2010. doi: 10.1161/ATVBAHA.110.209049. [DOI] [PubMed] [Google Scholar]
- 206.van Norden AG, van den Berg HA, de Laat KF, Gons RA, van Dijk EJ, de Leeuw FE. Frontal and temporal microbleeds are related to cognitive function: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study. Stroke 42: 3382–3386, 2011. doi: 10.1161/STROKEAHA.111.629634. [DOI] [PubMed] [Google Scholar]
- 207.van Skike CE, Galvan V. A perfect sTORm: the role of the mammalian target of rapamycin (mTOR) in cerebrovascular dysfunction of Alzheimer’s disease: a mini-review. Gerontology 64: 205–211, 2018. doi: 10.1159/000485381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari ZI, Lechleiter JD, Galvan V. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol 314: H693–H703, 2018. doi: 10.1152/ajpheart.00570.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.van Veluw SJ, Hilal S, Kuijf HJ, Ikram MK, Xin X, Yeow TB, Venketasubramanian N, Biessels GJ, Chen C. Cortical microinfarcts on 3T MRI: clinical correlates in memory-clinic patients. Alzheimers Dement 11: 1500–1509, 2015. doi: 10.1016/j.jalz.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 210.van Veluw SJ, Shih AY, Smith EE, Chen C, Schneider JA, Wardlaw JM, Greenberg SM, Biessels GJ. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol 16: 730–740, 2017. doi: 10.1016/S1474-4422(17)30196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vedovelli K, Giacobbo BL, Corrêa MS, Wieck A, Argimon IIL, Bromberg E. Multimodal physical activity increases brain-derived neurotrophic factor levels and improves cognition in institutionalized older women. Geroscience 39: 407–417, 2017. doi: 10.1007/s11357-017-9987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wagshul ME, Eide PK, Madsen JR. The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 8: 5, 2011. doi: 10.1186/2045-8118-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang CY, Kim HH, Hiroi Y, Sawada N, Salomone S, Benjamin LE, Walsh K, Moskowitz MA, Liao JK. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal 2: ra11, 2009. doi: 10.1126/scisignal.2000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol 171: 1144–1152, 2010. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension 41: 1308–1316, 2003. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 216.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50: 219–227, 2007. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 217.Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol 167: 1429–1442, 2005. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Watson RD, Gibbs CR, Lip GY. ABC of heart failure. Clinical features and complications. BMJ 320: 236–239, 2000. doi: 10.1136/bmj.320.7229.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, Brown MM, Jäger HR. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain 127: 2265–2275, 2004. doi: 10.1093/brain/awh253. [DOI] [PubMed] [Google Scholar]
- 220.Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci 299: 131–135, 2010. doi: 10.1016/j.jns.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 221.Wilhelm I, Nyúl-Tóth Á, Kozma M, Farkas AE, Krizbai IA. Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am J Physiol Heart Circ Physiol 313: H1000–H1012, 2017. doi: 10.1152/ajpheart.00106.2017. [DOI] [PubMed] [Google Scholar]
- 222.Willinsky R, Goyal M, terBrugge K, Montanera W. Tortuous, engorged pial veins in intracranial dural arteriovenous fistulas: correlations with presentation, location, and MR findings in 122 patients. AJNR Am J Neuroradiol 20: 1031–1036, 1999. [PMC free article] [PubMed] [Google Scholar]
- 223.Woo MA, Kumar R, Macey PM, Fonarow GC, Harper RM. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail 15: 214–223, 2009. doi: 10.1016/j.cardfail.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wu R, Feng C, Zhao Y, Jin AP, Fang M, Liu X. A meta-analysis of association between cerebral microbleeds and cognitive impairment. Med Sci Monit 20: 2189–2198, 2014. doi: 10.12659/MSM.891004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wu X, Studer W, Erb T, Skarvan K, Seeberger MD. Competence of the internal jugular vein valve is damaged by cannulation and catheterization of the internal jugular vein. Anesthesiology 93: 319–324, 2000. doi: 10.1097/00000542-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 226.Wysoki MG, Covey A, Pollak J, Rosenblatt M, Aruny J, Denbow N. Evaluation of various maneuvers for prevention of air embolism during central venous catheter placement. J Vasc Interv Radiol 12: 764–766, 2001. doi: 10.1016/S1051-0443(07)61451-1. [DOI] [PubMed] [Google Scholar]
- 227.Yakushiji Y, Noguchi T, Charidimou A, Eriguchi M, Nishihara M, Hara M, Nanri Y, Horikawa E, Nishiyama M, Werring DJ, Hara H. Basal ganglia cerebral microbleeds and global cognitive function: the Kashima Scan Study. J Stroke Cerebrovasc Dis 24: 431–439, 2015. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 228.Yakushiji Y, Werring DJ. Cerebrovascular disease: lobar cerebral microbleeds signal early cognitive impairment. Nat Rev Neurol 12: 680–682, 2016. doi: 10.1038/nrneurol.2016.179. [DOI] [PubMed] [Google Scholar]
- 229.Yang AI, Balser DS, Mikheev A, Offen S, Huang JH, Babb J, Rusinek H, Samadani U. Cerebral atrophy is associated with development of chronic subdural haematoma. Brain Inj 26: 1731–1736, 2012. doi: 10.3109/02699052.2012.698364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yepuri G, Velagapudi S, Xiong Y, Rajapakse AG, Montani JP, Ming XF, Yang Z. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell 11: 1005–1016, 2012. doi: 10.1111/acel.12001. [DOI] [PubMed] [Google Scholar]
- 231.Yoon JH, Lee ES, Jeong Y. In vivo imaging of the cerebral endothelial glycocalyx in mice. J Vasc Res 54: 59–67, 2017. doi: 10.1159/000457799. [DOI] [PubMed] [Google Scholar]
- 232.Zahr A, Alcaide P, Yang J, Jones A, Gregory M, dela Paz NG, Patel-Hett S, Nevers T, Koirala A, Luscinskas FW, Saint-Geniez M, Ksander B, D’Amore PA, Argüeso P. Endomucin prevents leukocyte-endothelial cell adhesion and has a critical role under resting and inflammatory conditions. Nat Commun 7: 10363, 2016. doi: 10.1038/ncomms10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zamboni P. The big idea: iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J R Soc Med 99: 589–593, 2006. doi: 10.1177/014107680609901122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zamboni P, Galeotti R, Menegatti E, Malagoni AM, Tacconi G, Dall’Ara S, Bartolomei I, Salvi F. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 80: 392–399, 2009. doi: 10.1136/jnnp.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zivadinov R. Is there a link between the extracranial venous system and central nervous system pathology? BMC Med 11: 259, 2013. doi: 10.1186/1741-7015-11-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zivadinov R, Chung CP. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med 11: 260, 2013. doi: 10.1186/1741-7015-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]